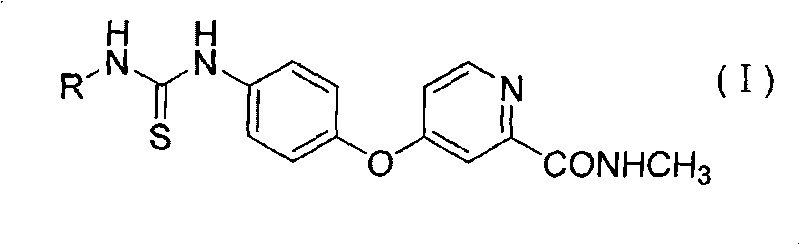
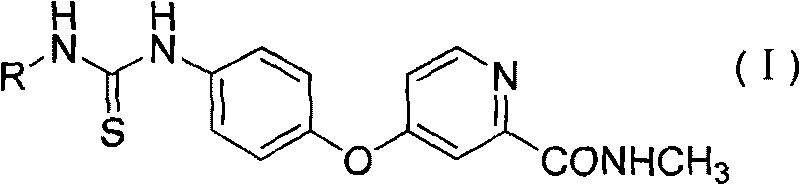
Aryl thiourea and preparation method and application thereof
An aryl thiourea and aryl technology, which is used in the synthesis and inhibition of VEGFR-2, new aryl thiourea compounds, and the field of anti-tumor drugs, can solve the problem of unpredictable thiourea compounds with VEGFR kinase inhibitory activity and its application. Drug safety performance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Synthesis of Intermediate III
[0052] step 1:
[0053] In a 250mL three-neck flask, replace the air with nitrogen, and 2 Add 36mLSOCl under protection 2 , carefully drop into 1.2mL of dry DMF, control the temperature at 42°C, stir for 10min, then slowly add 12g of 2-pyridinecarboxylic acid (completely added within 30min), then raise the temperature to 72°C, stir for 16h, cool to room temperature, add 100mL of toluene , concentrated to 40mL under reduced pressure, then added 60mL of toluene and concentrated to 40mL, repeated the above process once, a small amount of red solid appeared, filtered with suction, washed the solid with 50mL of toluene, placed the filtrate in a 250mL flask, and slowly added 40mL of methanol in an ice-water bath , stirred for 10 minutes, removed the ice bath, stirred at room temperature for 45 minutes, then placed in an ice-water bath for 5 minutes, added 40 mL of diethyl ether, a white precipitate precipitated, filtered, washed the...
Embodiment 2
[0058] The synthesis of embodiment 2 intermediate II isothiocyanates
[0059] method 1:
[0060] Add 1.85g of p-methoxyaniline, 6.3mL of triethylamine and 20mL of toluene into a 100mL single-necked bottle, and add 2.7mL of CS dropwise within 20min while stirring at room temperature 2 After the addition, the reaction at room temperature was continued until a large amount of solid precipitated. Cool the above reaction solution under an ice-water bath, slowly add 15 mL of toluene solution containing 1.48 g of trimeric phosgene (BTC) dropwise, continue to react at room temperature for about 4 hours after the addition, filter, wash the filter residue twice with toluene, and distill the filtrate under reduced pressure to obtain The crude product was separated by silica gel column chromatography (pure PE) to obtain 1.86 g of colorless liquid II-1 with a yield of 75.1%. 1 HNMR (600MHz, CDCl3): δ3.79(s, 3H), 6.83-6.84(d, 2H), 7.13-7.15(d, 2H).
[0061] Method 2:
[0062] Add 0.89g ...
Embodiment 3
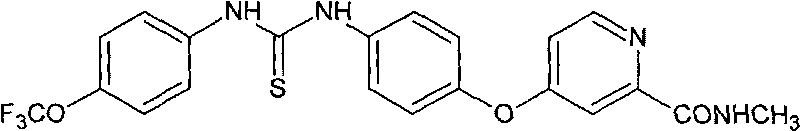
[0063] Example 3 Preparation of Compound 1
[0064]
[0065] In a 25mL single-necked bottle, 73mg of intermediate III was dissolved in 5mL of DMF, and 71mg of p-trifluoromethoxybenzene isothiocyanate dissolved in 5mL of DMF was added dropwise under cooling and stirring in an ice-water bath. After completion, extracted with ethyl acetate, washed with saturated brine, anhydrous Na 2 SO 4 After drying, it was separated by silica gel column chromatography (PE:EA=2:1 (v / v)) to obtain 76 mg of light gray powder with a yield of 52.8%. 1H NMR (600MHz, DMSO-d6): δ2.96-2.97(d, 3H), 7.01-7.02(m, 1H), 7.05-7.06(d, 2H), 7.18-7.19(d, 2H), 7.42- 7.45(t, 4H), 7.62(d, 1H), 8.11-8.12(br d, 1H), 8.39-8.40(d, 1H), 8.64(s, 1H), 8.70(s, 1H).
[0066] MS (ESI): 461.06 (M-H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com