Method for testing vascellum esoderma inhibin bioactivity
A technology of vascular endothelium and detection method, which is applied in the field of biological product activity detection, can solve problems such as difficult operation, unclear method description, large experimental error, etc., achieve specificity and repeatability, less human influence factors, and overcome repetition poor sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]Use 30mM acetic acid-sodium acetate buffer system with pH5.5±0.5 to perform ultrafiltration dialysis on the purified recombinant human endostatin protein solution, and prepare 90.9ml recombinant human endostatin solution with a concentration of 9.9mg / ml , add 60ml of 20% mannitol, add 4.20ml of acetic acid-sodium acetate buffer solution of about 1.5M pH5.5, and add water for injection to 300ml. Sterilize and filter through a 0.22μm microporous membrane, pack into vials, the liquid level is 1-1.5cm higher than the bottom of the bottle, add butyl rubber stopper, place the liquid in a freeze-drying box, and the temperature of the product drops to - 40°C, keep it for 3-4 hours, vacuumize, heat the partition, raise the temperature of the product to -20°C, keep it for 8 hours, continue heating to raise the temperature to 25°C, keep it for 6 hours, until the vacuum degree does not change much , take it out after vacuum pressing, and roll the cap. Preparation specifications: re...
Embodiment 2
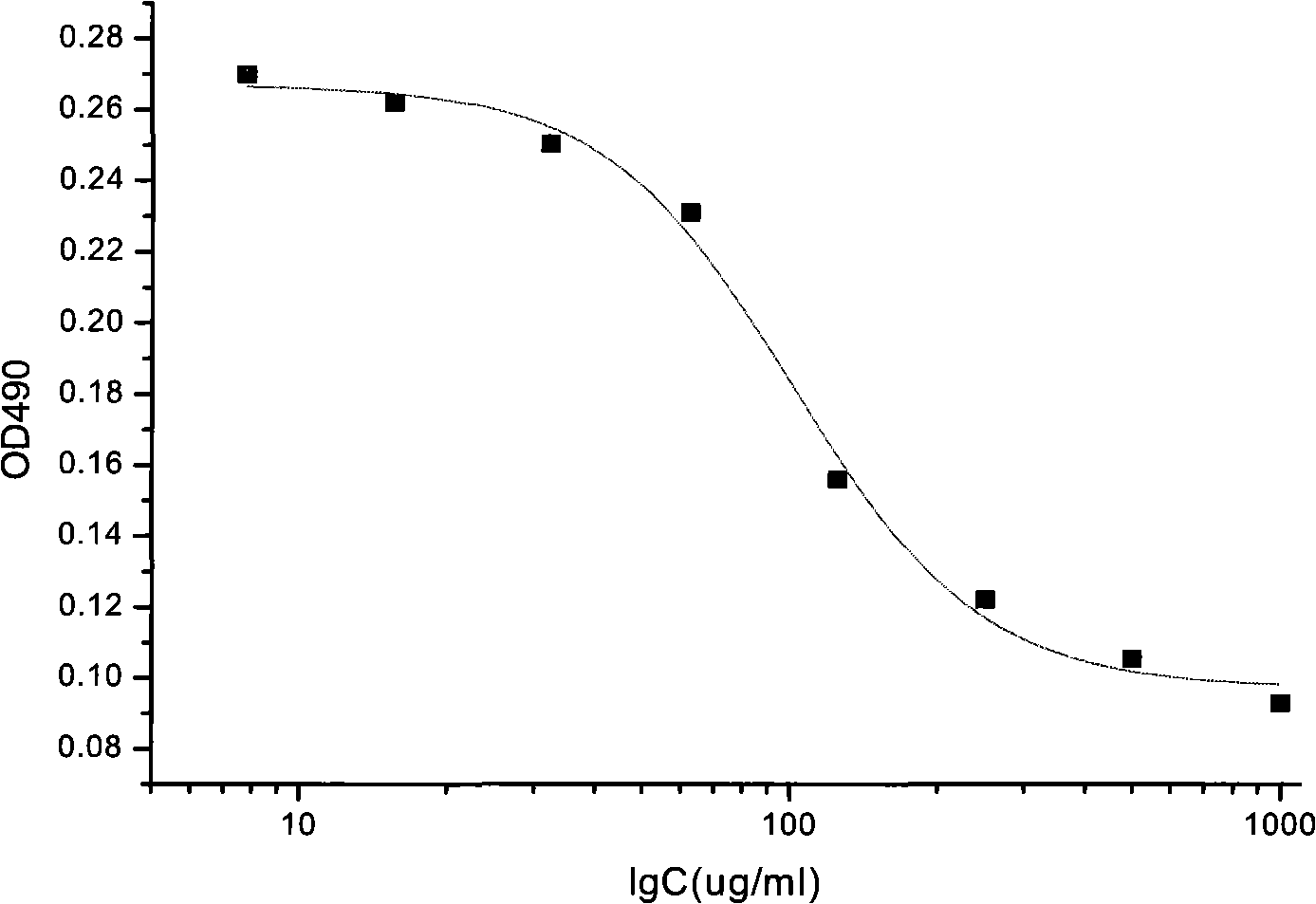
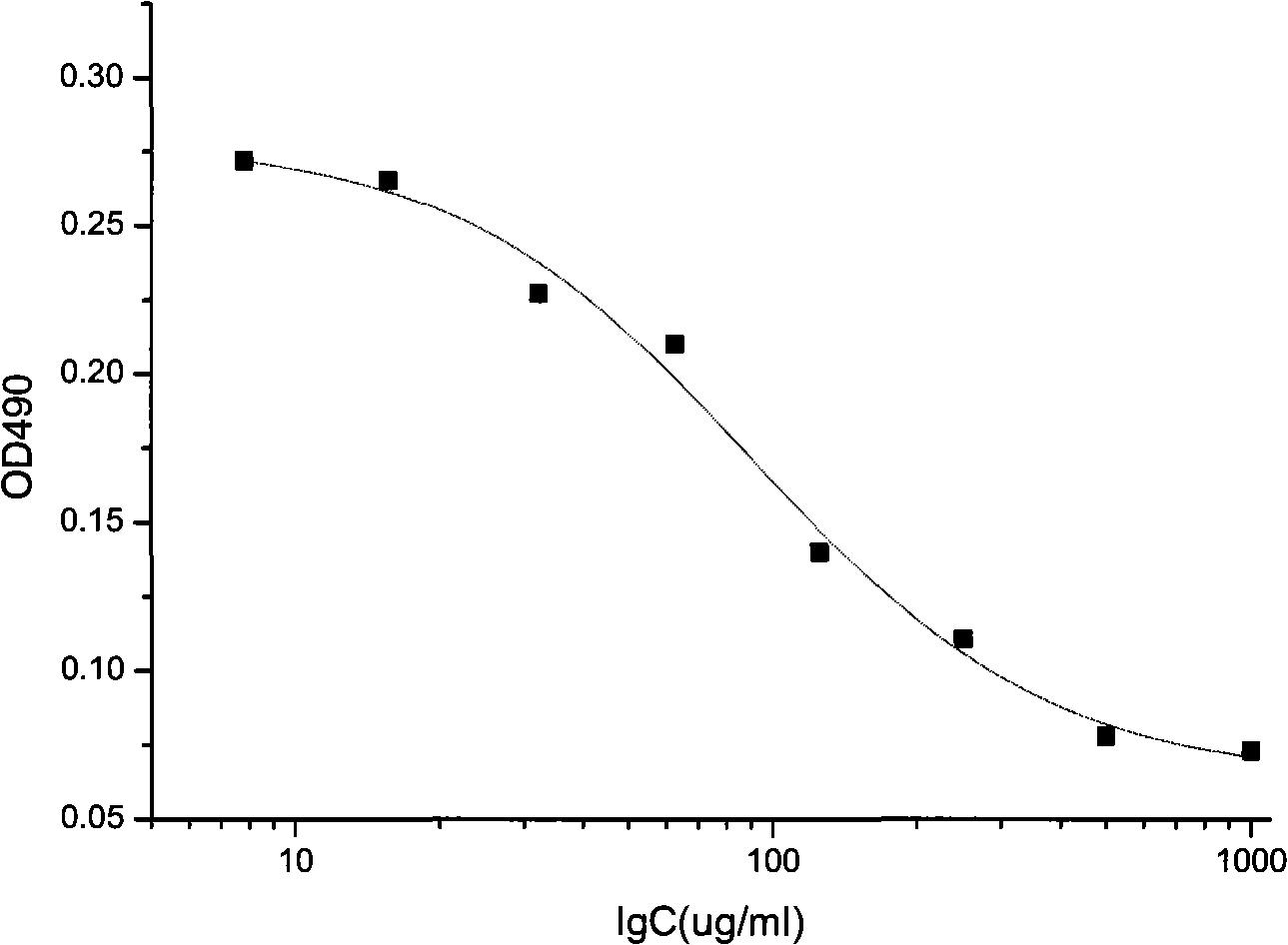
[0037] Example 2 sample detection
[0038] 2.1 Detection steps
[0039] 2.1.1 Cell culture: HUVEC cells were purchased from Sciencell, and the corresponding medium was also purchased from the company. According to the instructions, add FBS (fetal bovine serum in Chinese), ECGS (a mixture containing various factors that promote the growth of endothelial cells extracted from the bovine pituitary gland), P / OSolution (a mixture of penicillin and streptomycin) to the ECM medium , at 37°C, 5% CO 2 HUVEC cells were cultured in an incubator and subcultured to the fifth generation. After the cells were in good condition and entered the logarithmic growth phase, they were ready to be inoculated.
[0040] 2.1.2 Inoculation: Digest the cells with 0.25% trypsin, centrifuge at 1000rpm for 5 minutes, discard the supernatant, resuspend with medium, and count live cells with a hemocytometer under a microscope. Adjust the cell density to 5000 cells / ml, and add 160 μl of cell suspension to ea...
Embodiment 3
[0066] Repeatability evaluation of embodiment 3 detection method
[0067] The repeatability evaluation of the detection method was evaluated by intra-assay repeatability and inter-assay repeatability. Intra-batch repeatability: refers to the results of a sample being tested 5 times in parallel on the same 96-well plate on the same day; inter-batch repeatability: refers to the results of a sample being tested 5 times on different days.
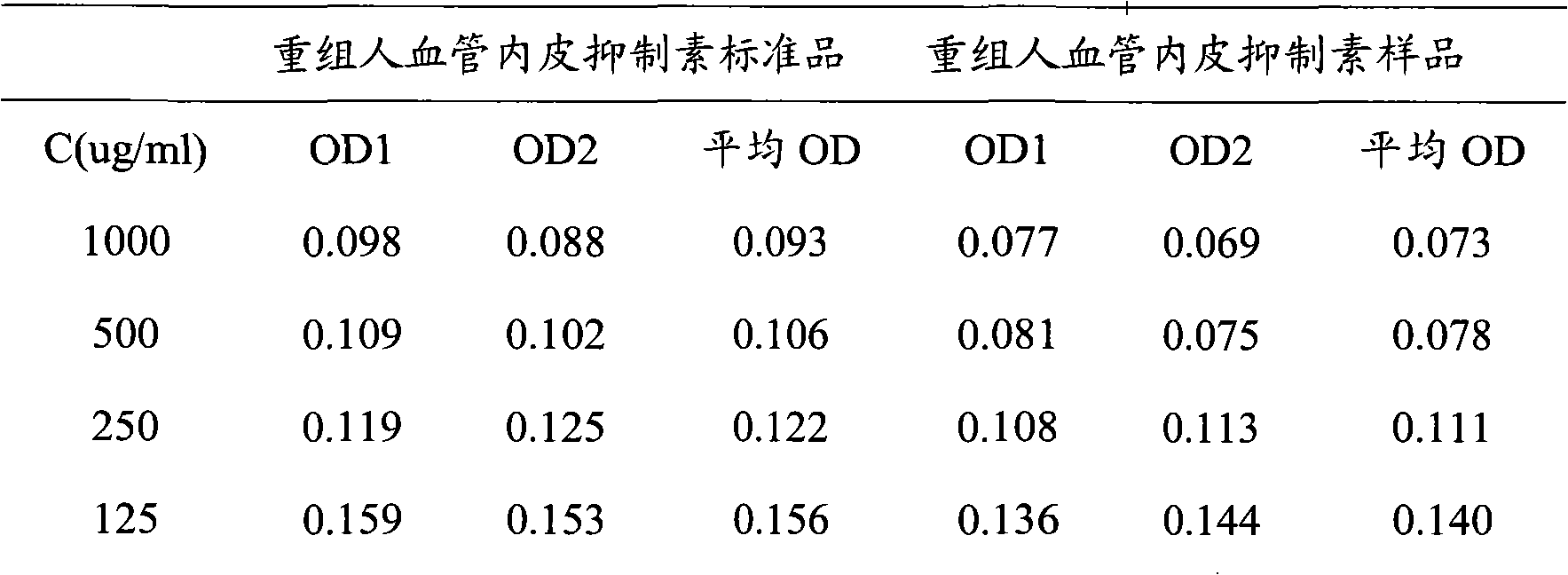
[0068] The results showed that the half-effective concentration of recombinant human endostatin samples (EC 50 ) within the batch relative standard deviation (RSD) was 37.8% (see Table 3), recombinant human vascular endostatin half-effective concentration (EC 50 ) between batches relative standard deviation (RSD) was 45.0% (see Table 4). The intra-batch and inter-batch recombinant human endostatin samples had good repeatability, and the error was controlled below 45.0%.
[0069] Table 3 Intra-assay repeatability detection of biological activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com