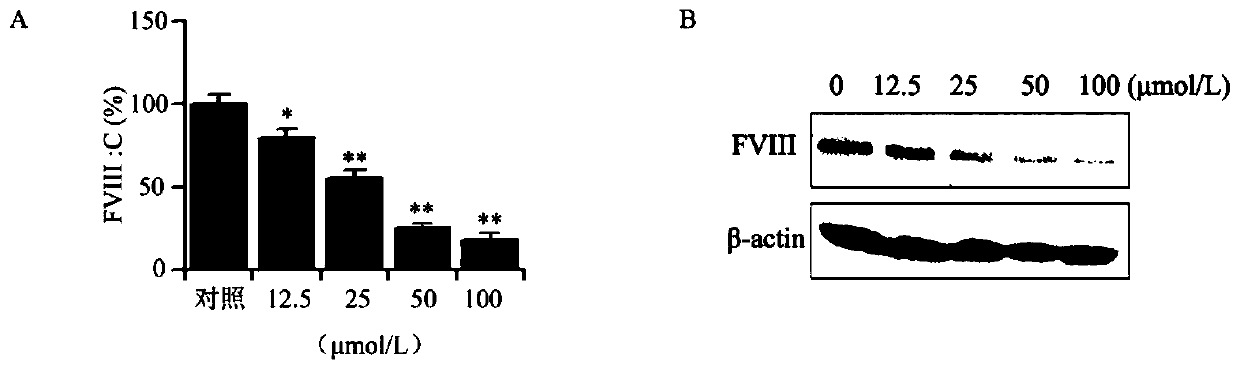
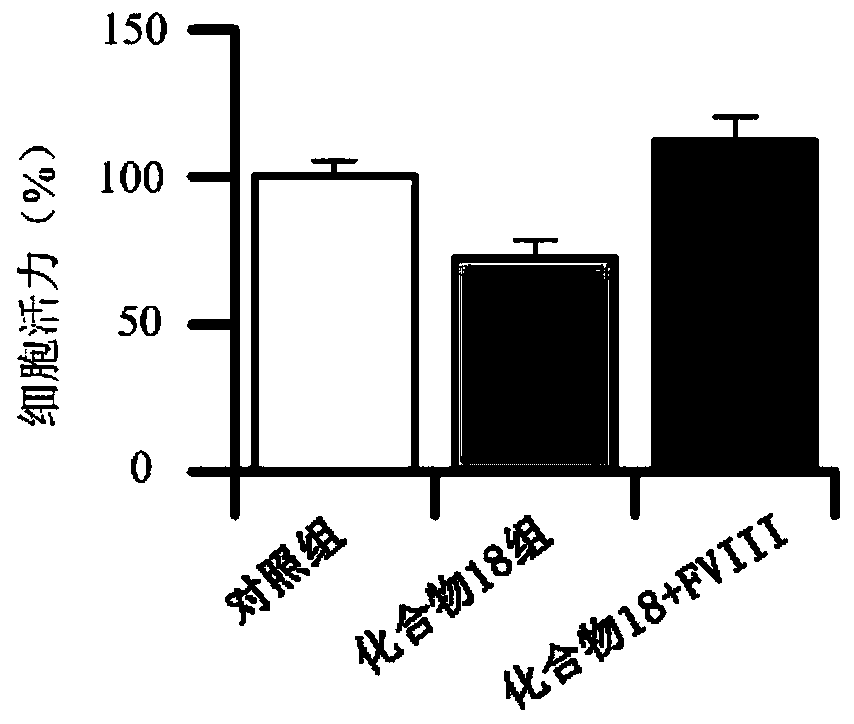
Cyclopentanopolyhydrophenanthrene skeleton compound capable of regulating and controlling blood coagulation factor VIII level to play anti-tumor role and application thereof
A skeleton compound, anti-tumor effect technology, applied in anti-tumor drugs, steroids, organic chemistry, etc., to achieve the effect of reducing production costs, high economy, and inhibiting lung metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this example, 3-acetylfurostane ester compounds were synthesized, specifically compounds 4-7 were synthesized.
[0043] 1. Synthesis of compound 4: 3-acetyl-aspirin furostanyl ester
[0044] Compound 3 (459mg, 1mmol), aspirin (216mg, 1.2mmol) and 4-dimethylaminopyridine (DMAP, 24mg, 0.2mmol) were dissolved in anhydrous dichloromethane (30mL) and stirred for 10min to form solution A. 1-Ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl, 383mg, 2mmol) was dissolved in 8mL of anhydrous dichloromethane to form solution B, at room temperature Solution B was slowly added dropwise to solution A, and the temperature was gradually raised to 35-40°C for about 8 hours. The reaction progress was detected by TCL, and the reaction was basically complete. The resulting reaction solution was washed successively with 5% sodium chloride solution, saturated sodium bicarbonate solution and 5% sodium chloride solution, the organic phase was separated and dried with anhydr...
Embodiment 2
[0056] In this example, furostane diester compounds were synthesized, specifically including compounds 9-12.
[0057] 1. Synthesis of compound 9: aspirin furostane diester
[0058] Compound 8 (416mg, 1mmol), aspirin (432mg, 2.4mmol) and DMAP (48mg, 0.4mmol) were dissolved in anhydrous dichloromethane (30mL) and stirred for 10min to form solution A, EDC.HCl (766mg, 4mmol ) was dissolved in 8 mL of anhydrous dichloromethane to form solution B, and solution A was slowly added dropwise to solution B at room temperature, and the temperature was gradually raised to 35-40°C for about 8 hours. The reaction progress was detected by TCL, and the reaction was basically complete. The resulting reaction solution was washed successively with 5% sodium chloride solution, saturated sodium bicarbonate solution and 5% sodium chloride solution, the organic phase was separated and dried with anhydrous sodium sulfate, and then washed with petroleum ether:ethyl acetate=10:1( v / v) As an eluent, it ...
Embodiment 3
[0070] In this example, spirostanoid ester compounds were synthesized, specifically including compounds 13-16.
[0071] 1. Synthesis of compound 13: aspirin spirostanyl ester
[0072] Compound 1 (414mg, 1mmol), aspirin (216mg, 1.2mmol) and DMAP (24mg, 0.2mmol) were dissolved in anhydrous dichloromethane (30mL) and stirred for 10min to form solution A, EDC.HCl (383mg, 2mmol ) was dissolved in 5 mL of anhydrous dichloromethane to form solution B, and solution B was slowly added dropwise to solution A at room temperature, and the temperature was gradually raised to 35-40°C for about 8 hours. The reaction progress was detected by TCL, and the reaction was basically complete. The resulting reaction solution was washed successively with 5% sodium chloride solution, saturated sodium bicarbonate solution, and 5% sodium chloride solution, and the organic phase was separated and dried with anhydrous sodium sulfate, and then washed with petroleum ether:ethyl acetate=10:1( v / v) used as e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com