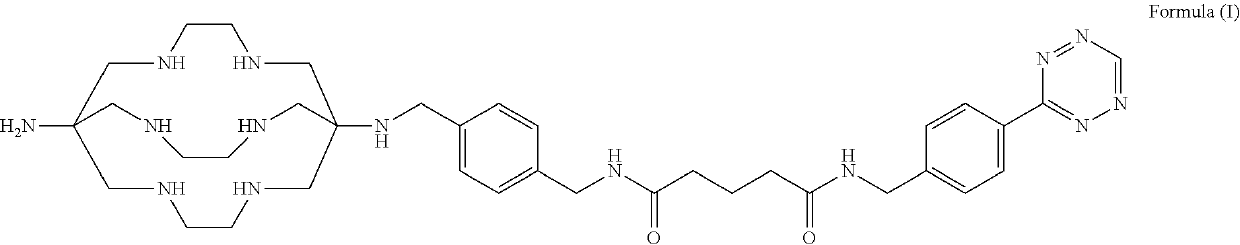
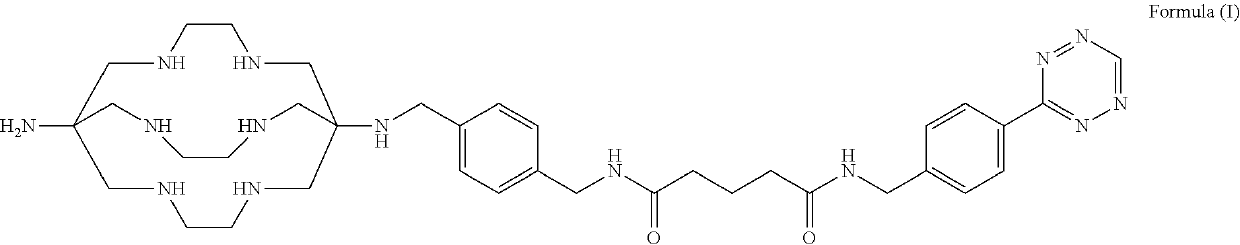
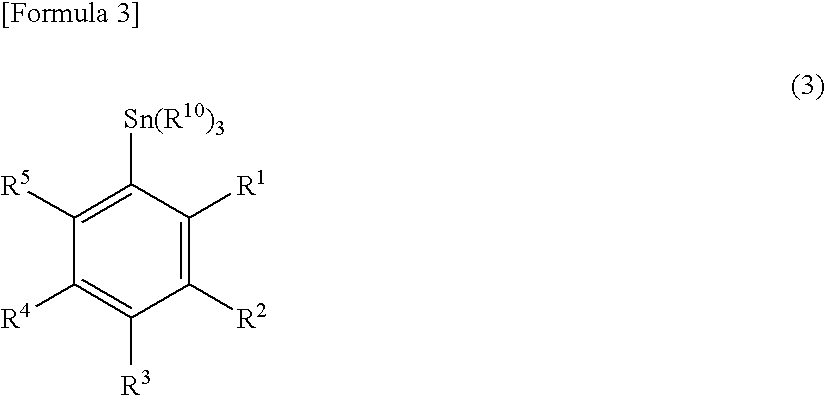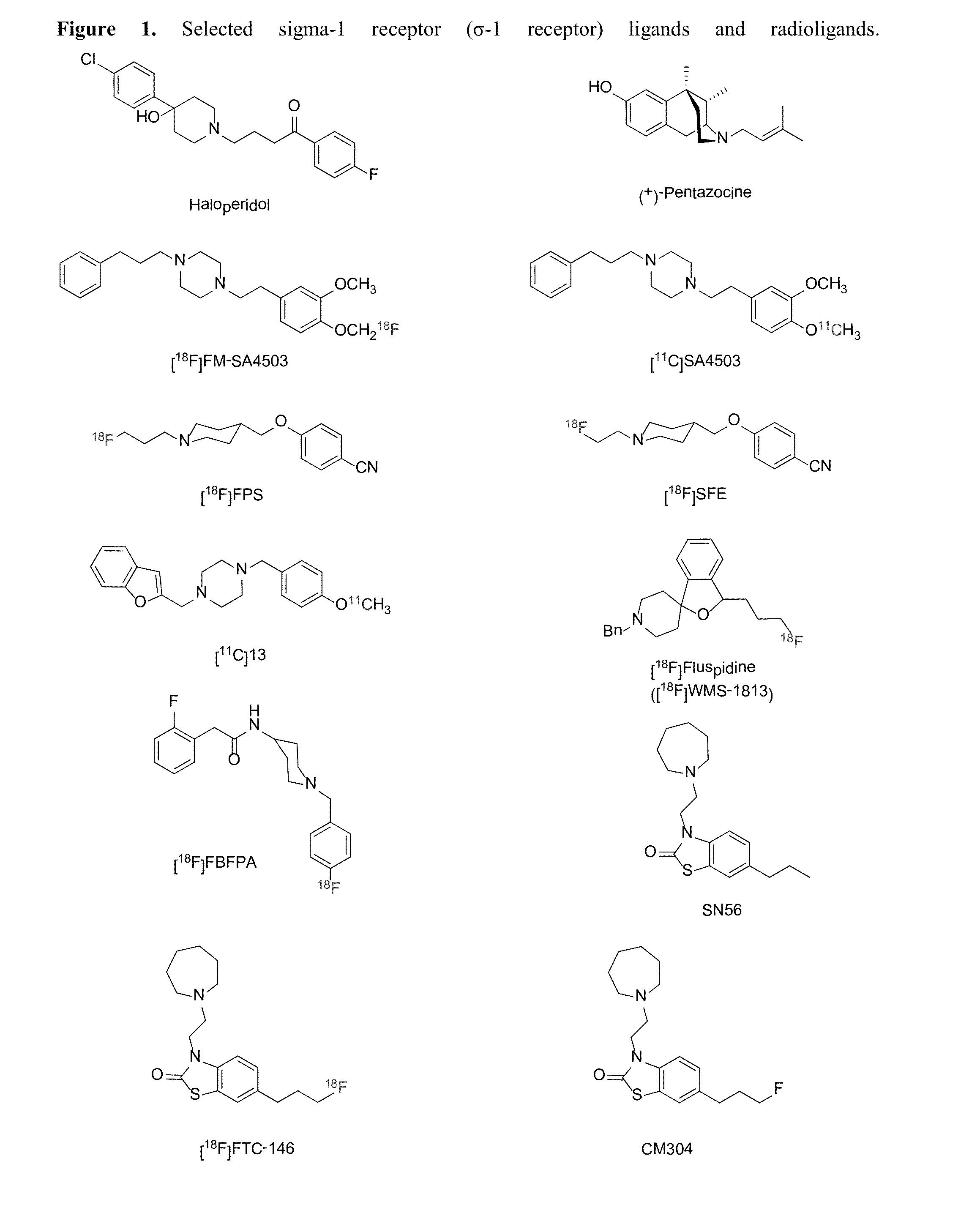Patents
Literature
46 results about "Radioligand" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A radioligand is a radioactive biochemical substance (in particular, a ligand that is radiolabeled) that is used for diagnosis or for research-oriented study of the receptor systems of the body. In a neuroimaging application the radioligand is injected into the pertinent tissue, or infused into the bloodstream. It binds to its receptor. When the radioactive isotope in the ligand decays it can be measured by positron emission tomography (PET) or single photon emission computed tomography (SPECT). In in vivo systems it is often used to quantify the binding of a test molecule to the binding site of radioligand. The higher the affinity of the molecule the more radioligand is displaced from the binding site and the increasing radioactive decay can be measured by scintillography. This assay is commonly used to calculate binding constant of molecules to receptors.
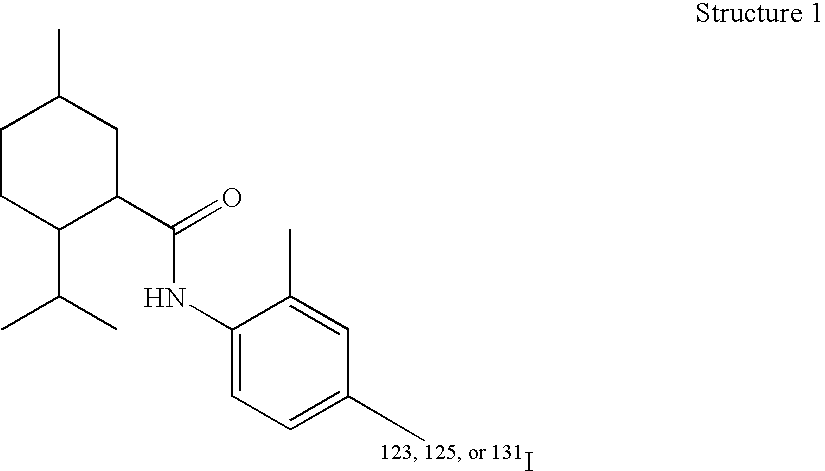
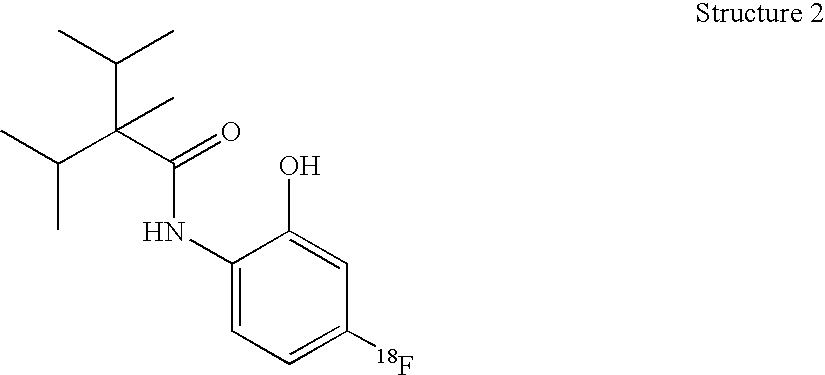
Radioligands for the TRP-M8 receptor and methods therewith
InactiveUS20050084447A1Radioactive preparation carriersGroup 3/13 element organic compoundsProstate cancer cellSensory potential
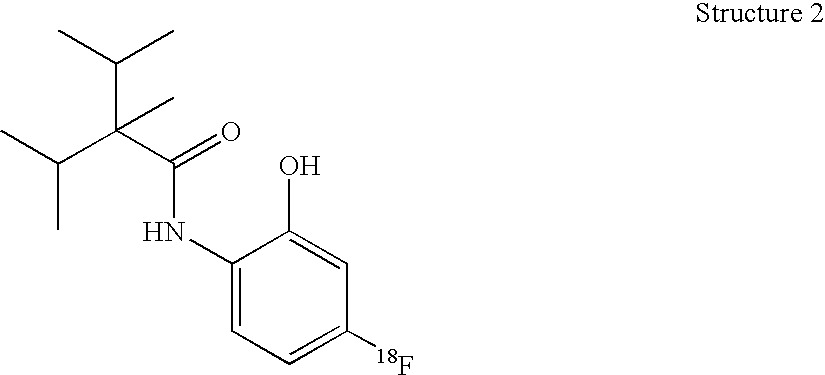
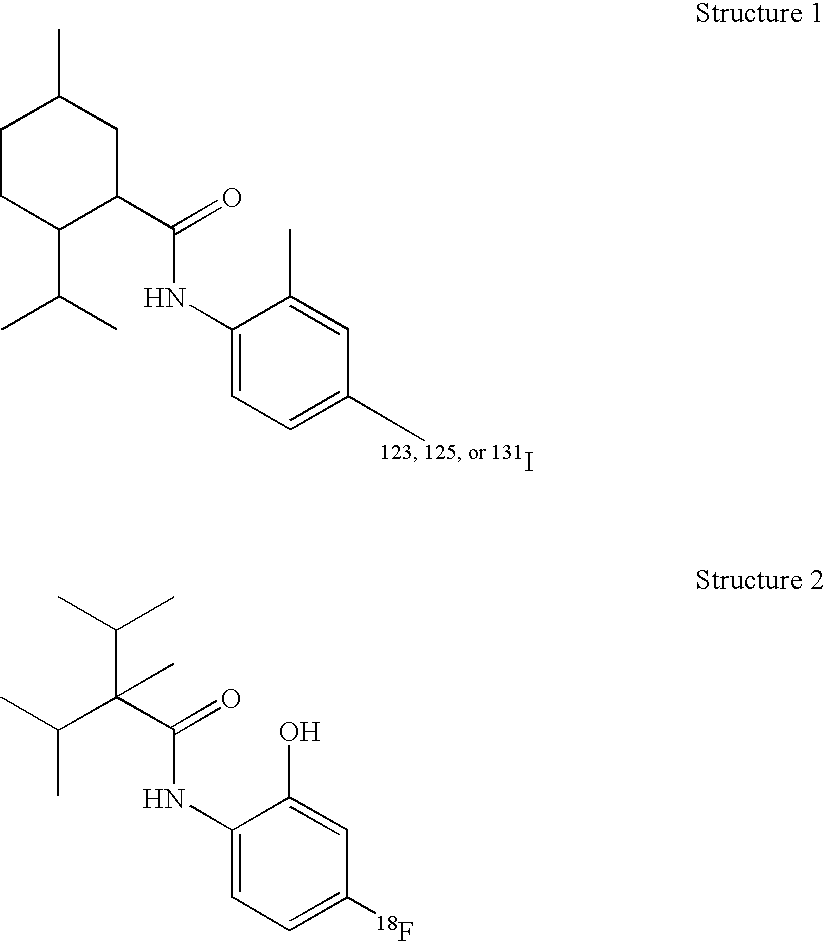
One embodiment of the invention is a composition that comprises a radioactive [18F], [123I], [125I], or [131I]—N-radiohaloaryl-alkylcarboxamide molecule. The composition binds to the transient receptor potential-M8 (TRP-M8) receptor of cells. The TRP-M8 receptor is selectively expressed in sensory neurons and in malignant tissues such as prostate cancer cells. The [18F], [123I], [125I], or [131I]—N-radiohaloaryl-alkylcarboxamide ligand may be used for radioreceptor binding studies, for diagnostic studies, and for radiotherapy of cancerous tissues. Affinity of the [125I] or [131I]—N-radiohaloaryl-alkylcarboxamide ligand for the TRP-M8 receptor confers selectivity and specificity in delivering lethal radiation to the diseased cells.
Owner:WEI EDWARD T
Regularization of images
ActiveUS20170039706A1Suppress artifactsImage enhancementReconstruction from projectionMedicineReconstruction method
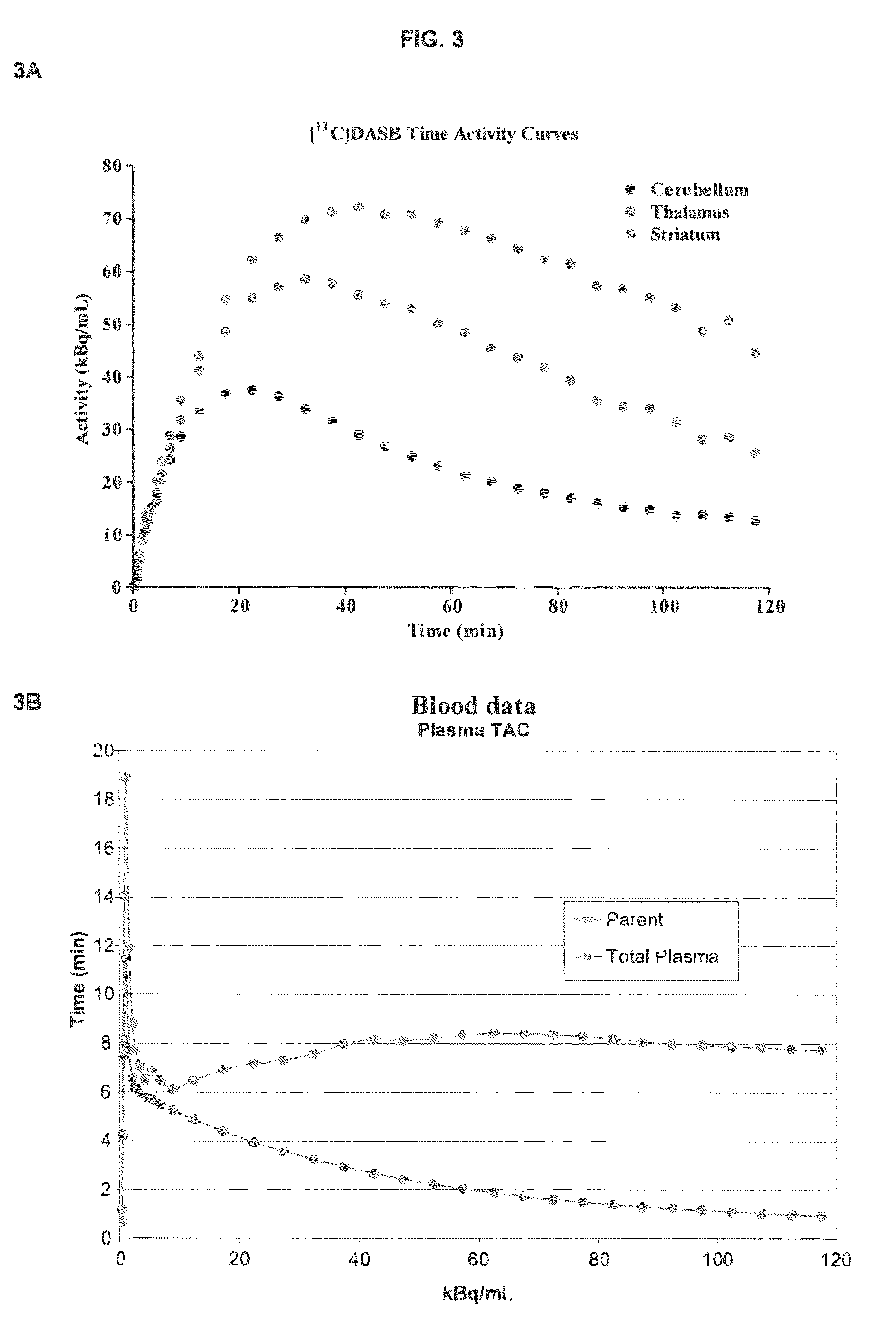
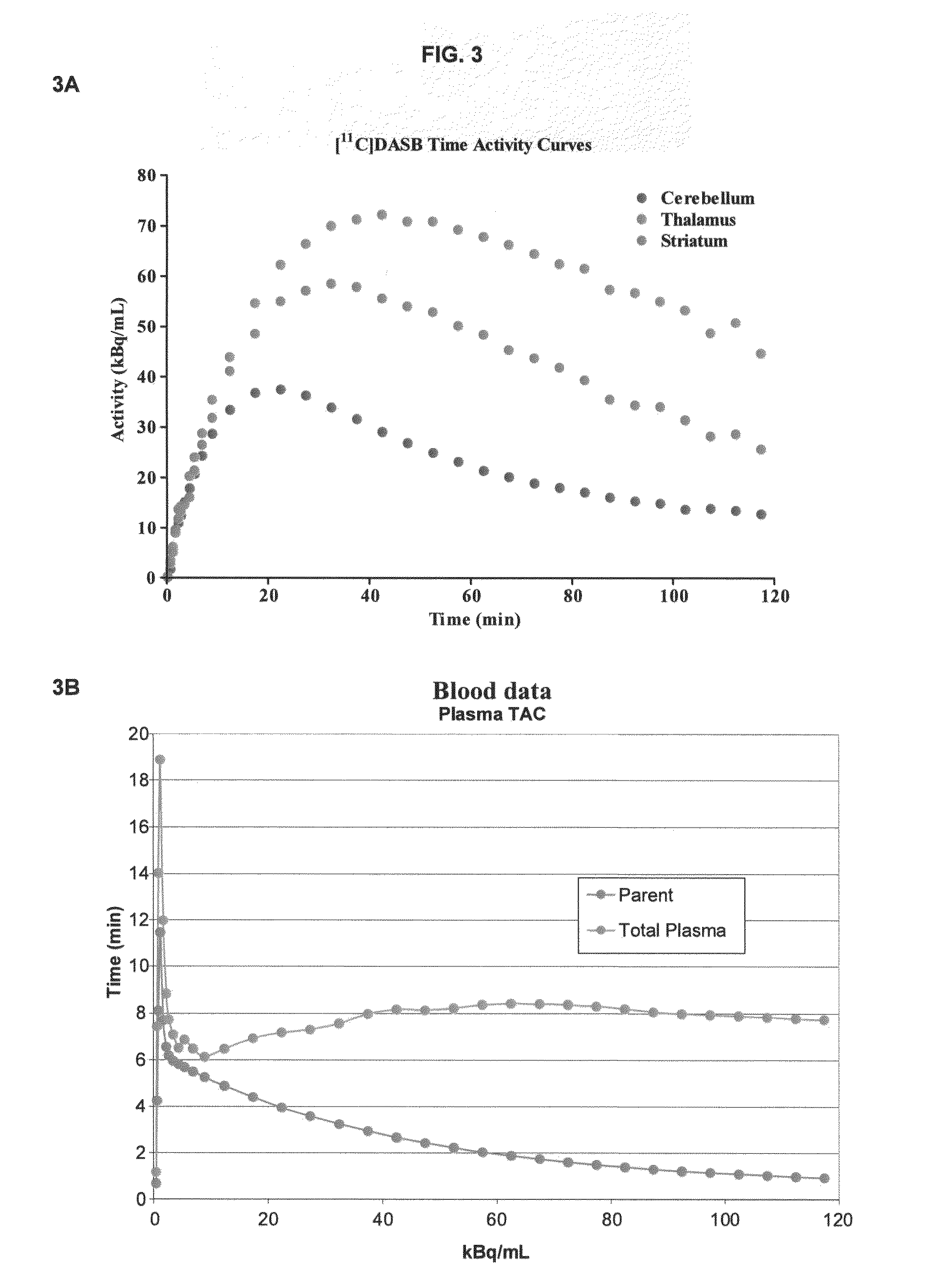
The present disclosure is directed to iterative regularized reconstruction methods. In certain embodiments, the methods incorporate locally-weighted total variation denoising to suppress artifacts induced by PSF modeling. In certain embodiments, the methods are useful for suppressing ringing artifacts while contrast recovery is maintained. In certain embodiments, the weighting scheme can be extended to noisy measures introducing a noise-independent weighting scheme. The present disclosure is also directed to a method for quantifying radioligand binding in a subject without collecting arterial blood. In certain embodiments, the methods incorporate using imaging data and electronic health records to predict one or more anchors, which are used to generate an aterial input function (AIF) for the radioligand.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
SSTR1-selective analogs
InactiveUS7019109B2High affinityEasy to markPeptide sourcesRadioactive preparation carriersNervous systemScreening method
Analogs of SRIF which are selective for SSTR1 in contrast to the other cloned SRIF receptors. These analogs are useful in determining the tissue and cellular expression of the receptor SSTR1 and its biological role in the endocrine, exocrine and nervous system, as well as in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,5[D-Trp8, NαMeIAmp9, Tyr11]-SRIF and counterparts incorporating Cbm at the N-terminus and / or NαSer13, inhibit the binding of a universal SRIF radioligand to the cloned human receptor SSTR1, but they do not bind with significant affinity to human SSTR2, SSTR3, SSTR4 or SSTR5. By incorporating an iodinated tyrosine in position-2 or in position-11 in these SSTR1-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. The N-terminus accommodates bulky moieties without loss of selectivity, and a carbamoyl moiety or a conjugating agent that will accept a radioactive nuclide or will link to a cytotoxin may be present at the N-terminus.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Click chemistry method for synthesizing molecular imaging probes
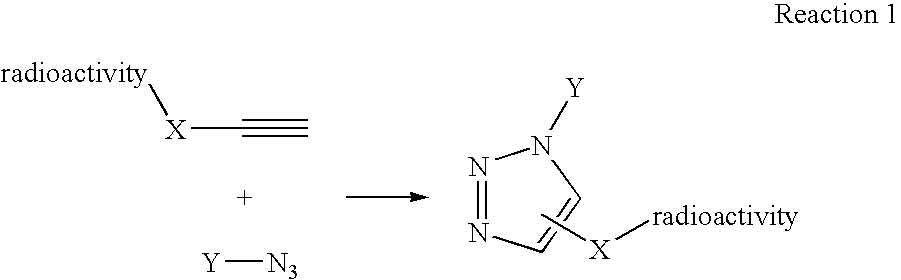
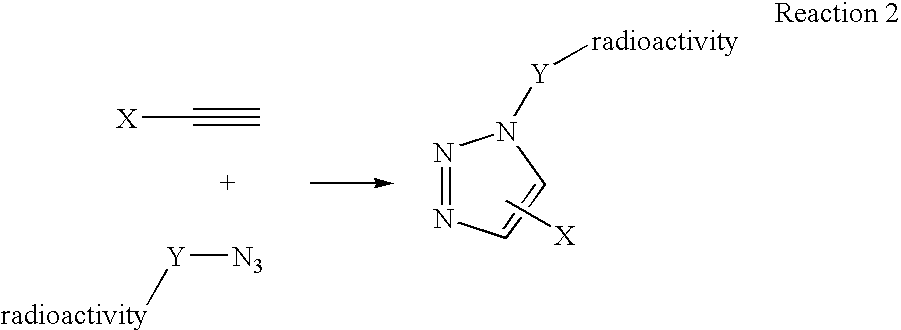
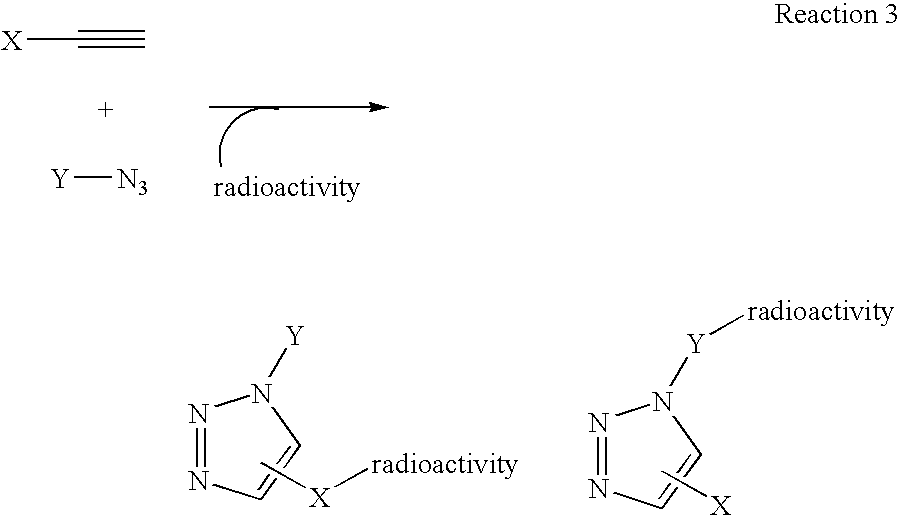
InactiveUS20060263293A1High reaction specificityEfficiently labeledSaccharide with heterocyclic radicalsIn-vivo radioactive preparationsLeaving groupChemical reaction
The present disclosure provides a method for preparing a radioactive ligand or radioactive substrate having affinity for a target biomacromolecule, the method comprising: (a) reacting a first compound comprising a first functional group capable of participating in a click chemistry reaction, with a radioactive reagent under conditions sufficient to displace the leaving group with a radioactive component of the radioactive reagent to form a first radioactive compound; (b) providing a second compound comprising a second complementary functional group capable of participating in a click chemistry reaction with the first functional group; (c) reacting the first functional group of the first radioactive compound with the complementary functional group of the second compound via a click chemistry reaction to form the radioactive ligand or substrate; and (d) isolating the radioactive ligand or substrate.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Use of small molecule radioligands to discover inhibitors of amyloid-beta peptide production and for diagnostic imaging
This invention relates to a novel method of screening for inhibitors of beta-amyloid production, and thereby identifying such inhibitors as therapeutics for neurological and other disorders involving APP processing and beta-amyloid production. This invention also relates to identifying macromolecules involved in APP processing and beta-amyloid production. Furthermore, inhibitors identified by the screening method of the present invention are useful in the treatment of neurological disorders, such as Alzheimer's disease, which involve elevated levels of Aβ peptides.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
Use of small molecule radioligands to discover inhibitors of amyloid-beta peptide production and for diagnostic imaging
InactiveUS20050129612A1Radioactive preparation carriersRadiation therapyRadioactive drugPharmaceutical drug
This invention relates to a method of using radiolabelled and / or radiopharmaceutical small molecule inhibitors of beta-amyloid peptide production for the diagnosis of neurological and other disorders involving APP processing and beta-amyloid production. Furthermore, radiolabelled small molecule inhibitors identified by the methods of the present invention would be useful in the diagnosis of neurological disorders, such as Alzheimer's disease, which involve elevated levels of Aβ peptides.
Owner:ZACZEK ROBERT +3
SSTR1-selective analogs
InactiveUS20060155107A1Improve bindingHigh selectivityPeptide/protein ingredientsSomatostatinsNervous systemScreening method
Analogs of SRIF which are selective for SSTR1 in contrast to the other cloned SRIF receptors. These analogs are useful in determining the tissue and cellular expression of the receptor SSTR1 and its biological role in the endocrine, exocrine and nervous system, as well as in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,5[D-Trp8, IAmp9, Tyr11]-SRIF and counterparts incorporating Cbm at the N-terminus, as well as radioiodinated versions thereof, inhibit the binding of a universal SRIF radioligand to the cloned human receptor SSTR1, but they do not bind with significant affinity to human SSTR2, SSTR3, SSTR4 or SSTR5. By incorporating an iodinated tyrosine in position-2 or in position-11 in these SSTR1-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. The N-terminus accommodates bulky moieties without loss of selectivity, and a carbamoyl moiety or a conjugating agent that will accept a radioactive nuclide or will link to a cytotoxin may be present at the N-terminus.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Method for synthesizing radioactive ligand having 18f-labeled fluorobenzene ring
InactiveUS20090069592A1High yieldShort timeBiological testingIsotope introduction to acyclic/carbocyclic compoundsTosylic acidFluorobenzene
A phenyl tin compound is synthesized by using a derivative having various functional groups and a bromo- or iodo-benzene ring as a labeling material of a radioactive ligand. On the other hand, a novel hydroxytosyl iodobenzene compound having an electron-donating group is obtained by oxidizing iodobenzene having one or more electron-donating groups and reacting it with tosylic acid. Then, a diphenyliodonium salt which is a labeling precursor is synthesized by reacting the resulting compound with various phenyl tin compounds. Finally, a 18F-labeled ligand having various functional groups and a [18F] fluorobenzene ring is synthesized by reacting the resulting diphenyliodonium salt with [18F]F−.
Owner:NAT INST FOR QUANTUM & RADIOLOGICAL SCI & TECH
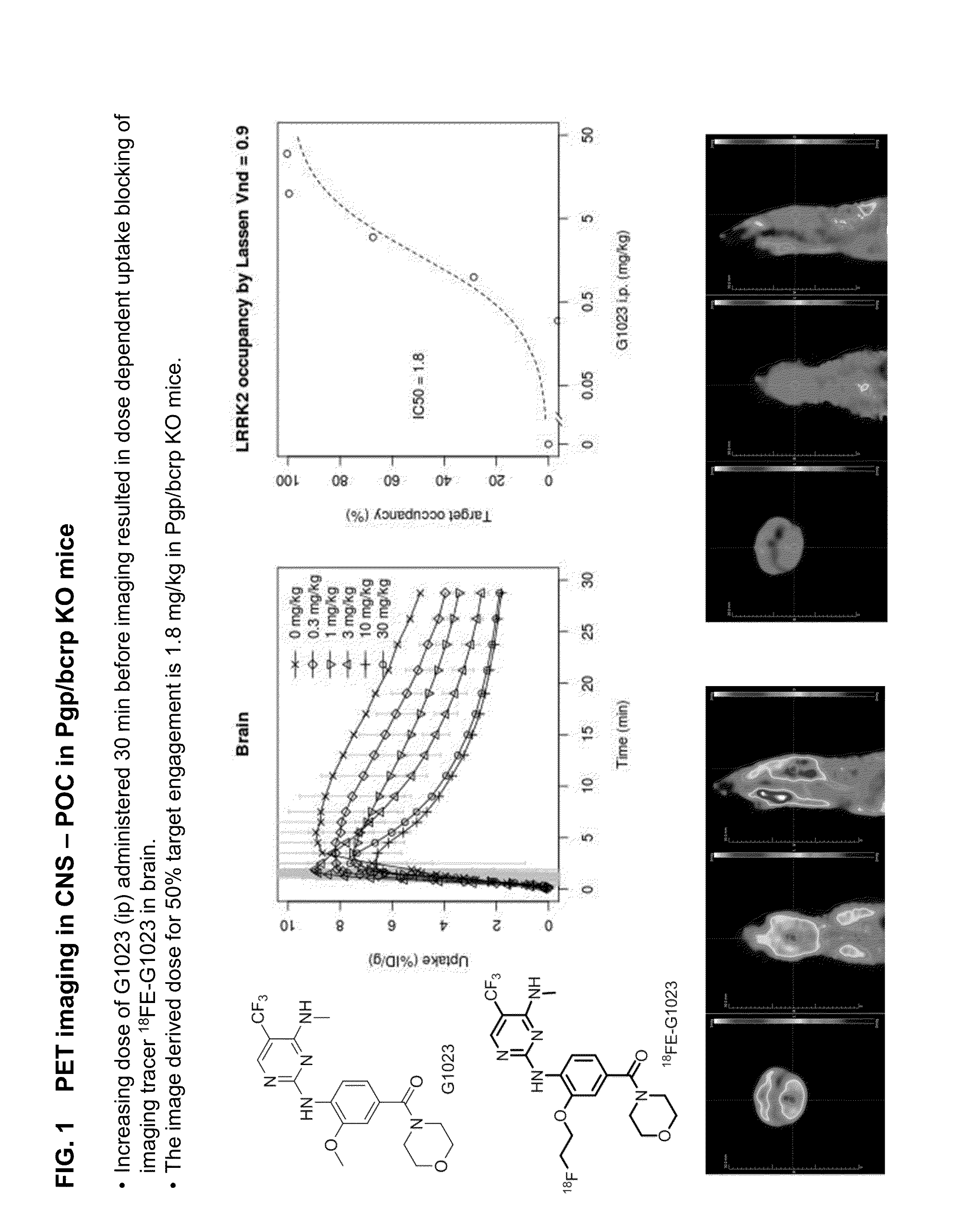
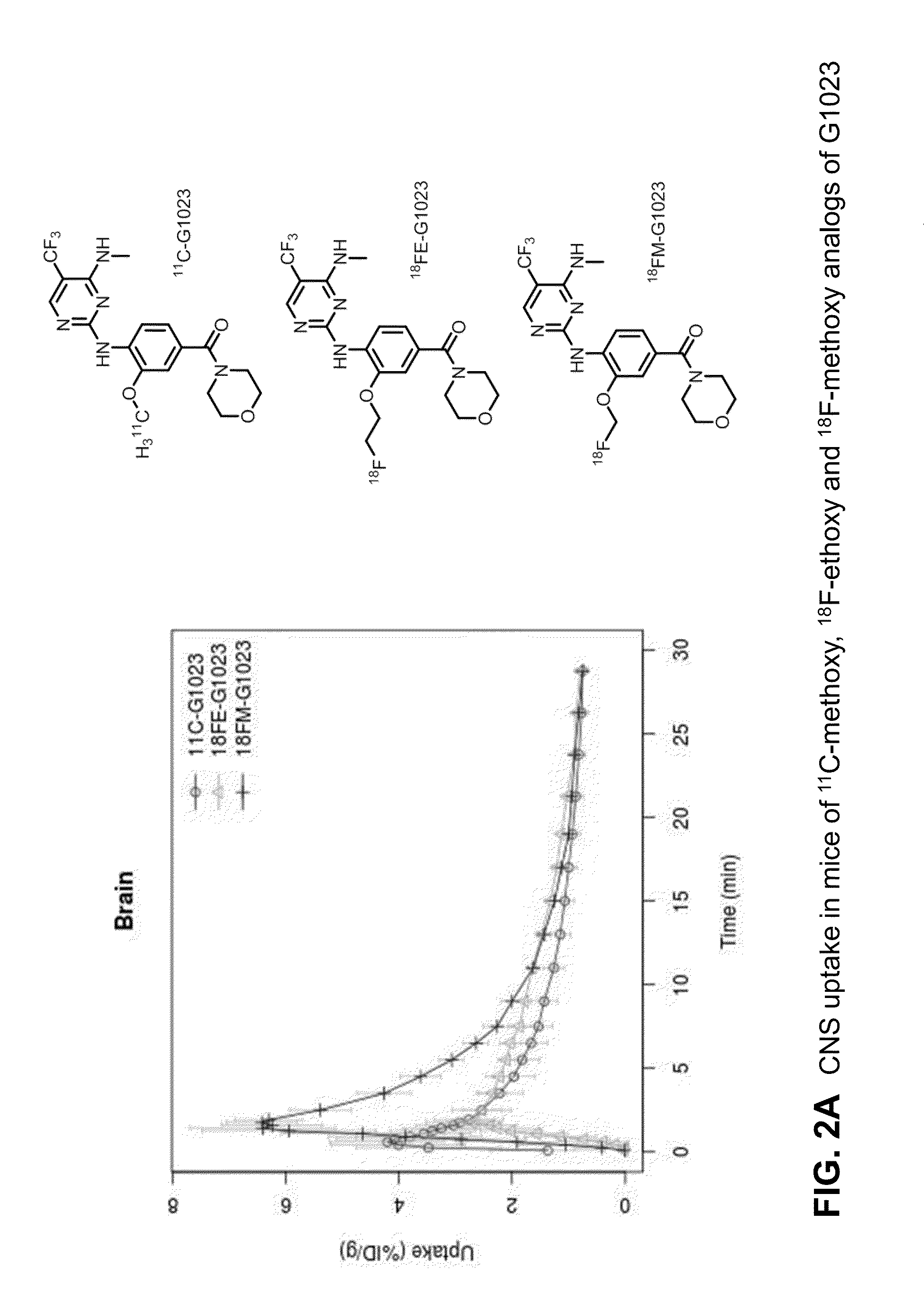
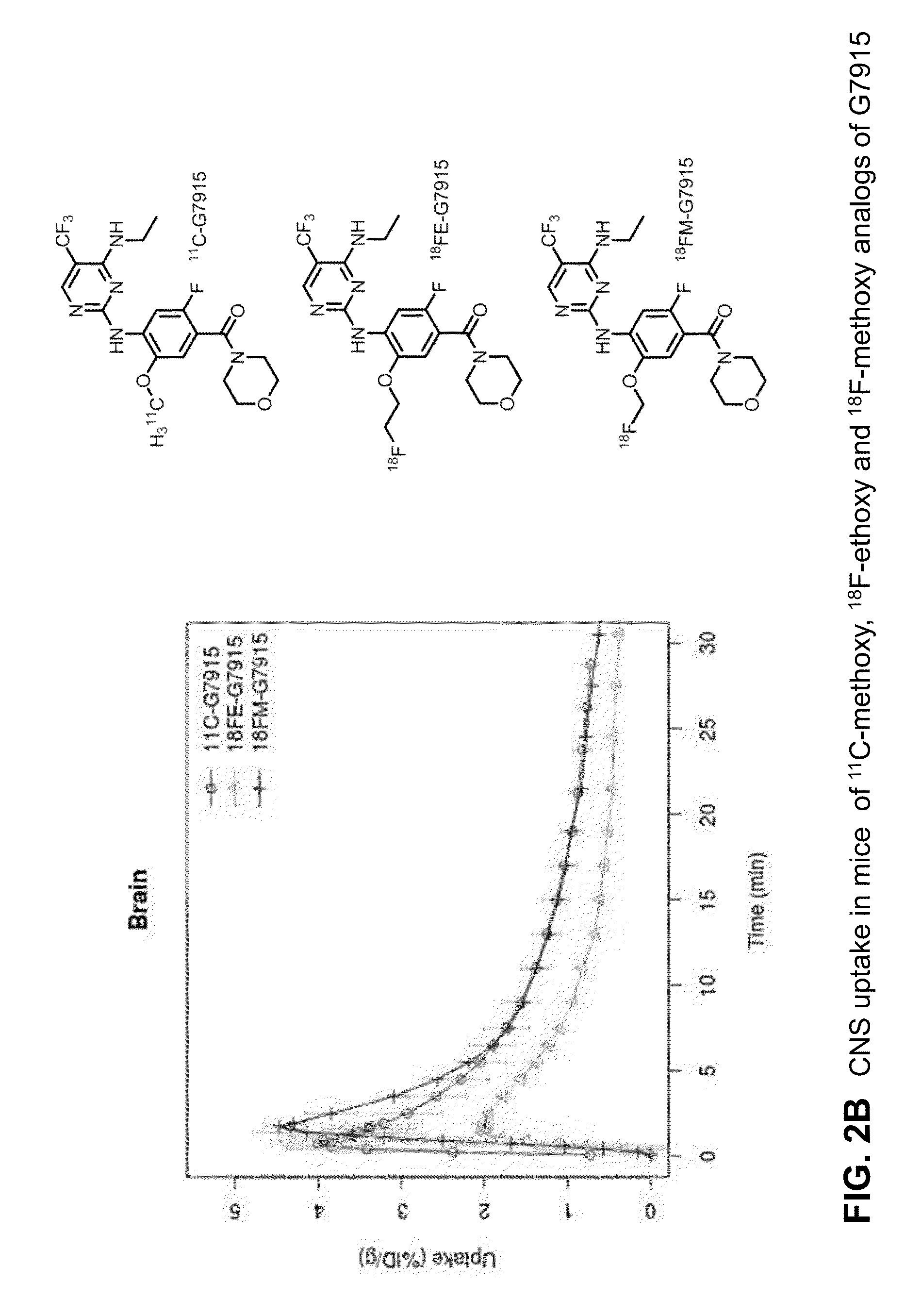
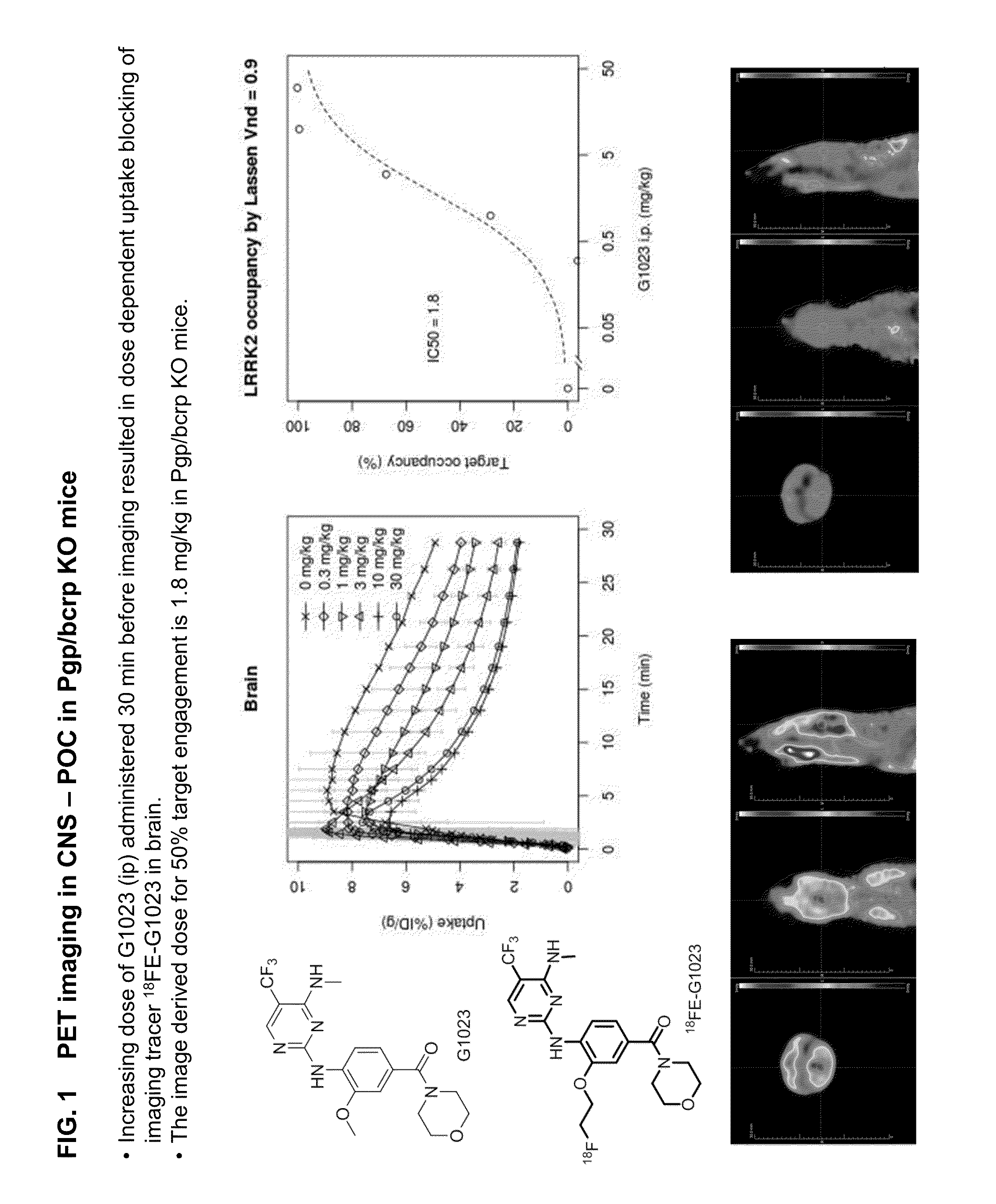
Fluorine-18 and carbon-11 labeled radioligands for positron emission tomography (PET) imaging for lrrk2
A method for positron emission tomography (PET) imaging of LRRK2 in tissue of a subject, the method comprising: administering a compound of formula I, formula II or formula III, or a pharmaceutically acceptable salt thereof to the subject, wherein the compound includes at least one C11 or F18 label thereon; allowing the compound to penetrate into the tissue of the subject; and collecting a PET image of the CNS or brain tissue of the subject
Owner:GENENTECH INC
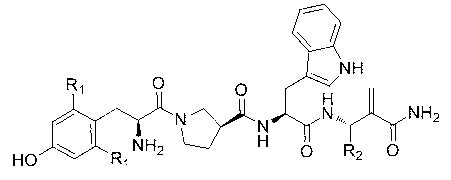
C-end phenylalanine p-amino modified endomorphine-1 analog and preparation and application thereof
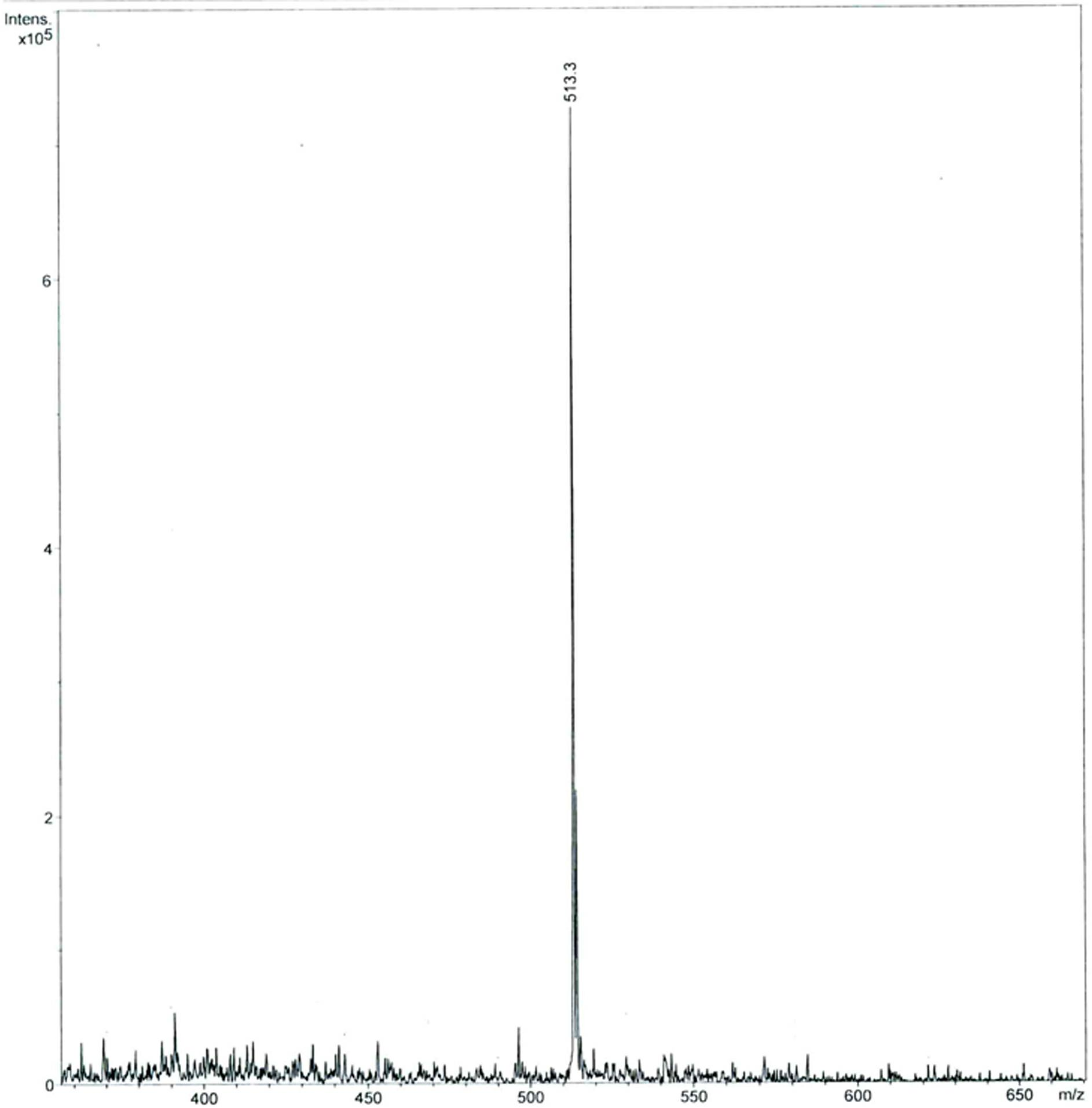
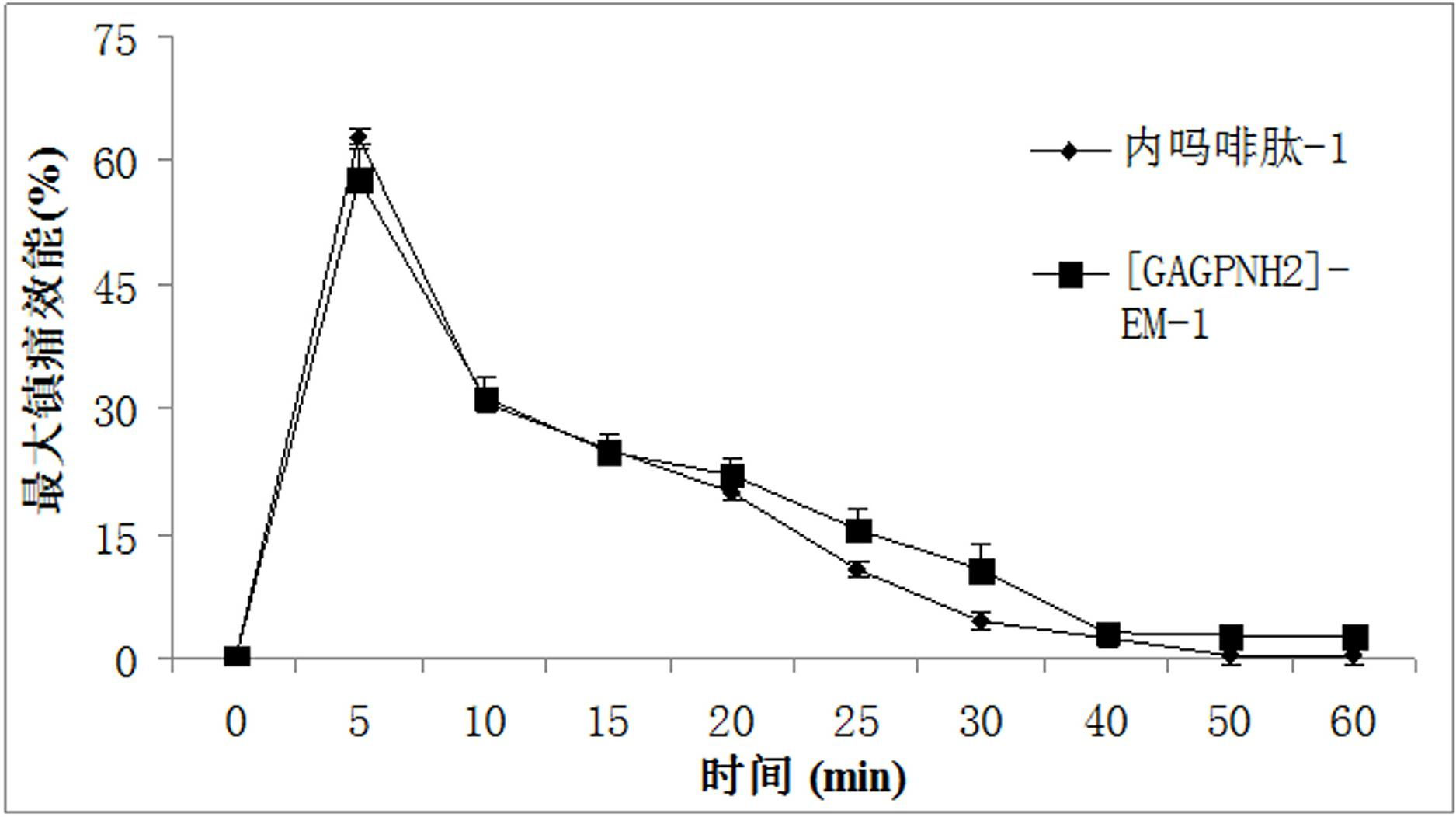
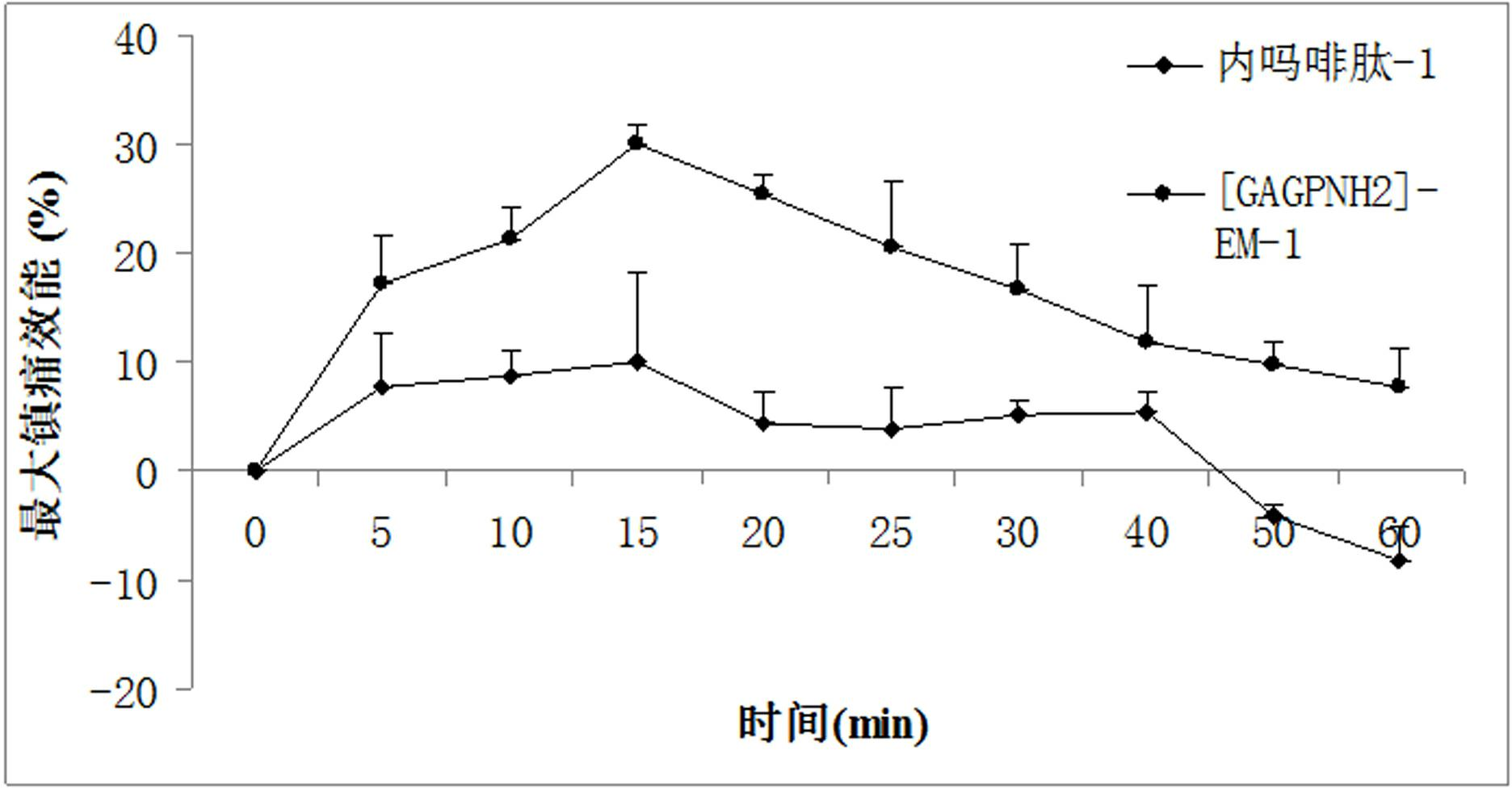
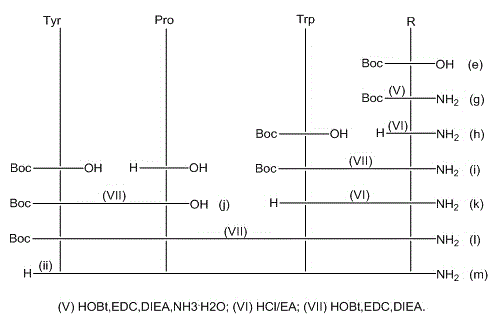
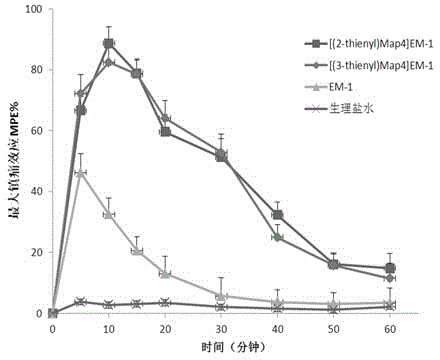
The invention provides a C-end phenylalanine p-amino modified endomorphine-1 analog. The N end of endomorphine-1 is modified by using guanidyl, Pro-Typ at the 2nd and 3rd positions of the endomorphine-1 is substituted by D-Ala-Gly, the benzene ring para-position at the 4th position is modified by using amino, and the novel analog [GAGPNH2]-EM-1 of the endomorphine-1 is synthesized by a liquid phase synthesis method. A series of physiological and pharmacological activity identification is performed on the analog through radioligand receptor binding experiment, in vitro enzymolysis stability experiment and warm bath drift analgesia experiment, and the results show that the analog has high affinity, high enzymolysis stability, high blood-brain barrier permeating capability and low side effect compared with parents of the analog. Therefore, the analog which is used as an active substance and used for preparing clinical analgesia medicaments has a good application prospect.
Owner:LANZHOU UNIVERSITY
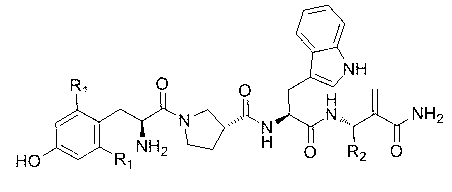
Non-natural amino acid modified endomorphin-1 analogue as well as synthesis method and application thereof
InactiveCN104877005AHigh affinityHigh enzymatic stabilityNervous disorderTetrapeptide ingredientsEnzymatic hydrolysisSynthesis methods
The invention discloses a non-natural amino acid modified endomorphin-1 analogue. A synthesis method comprises the following step: replacing amino acid phenylalanine at a fourth site from a terminal N to a terminal C of parent endomorphin-1 by respectively using 2-thienyl substituted alpha-alkenyl-beta-amino acid and 3-thienyl substituted alpha-alkenyl-beta-amino acid. The affinity and enzymatic hydrolysis stability of a mu opioid receptor of the non-natural amino acid modified endomorphin-1 analogue can be effectively improved, and thus the in-vivo analgesic effect of the non-natural amino acid modified endomorphin-1 analogue can be further improved and prolonged; and the non-natural amino acid modified endomorphin-1 analogue is subjected to pharmacological activity identification by virtue of radioligand receptor binding experiments, in-vitro organ biological assays and in-vitro enzymatic hydrolysis stability and warm bath tail-flick analgesic experiments, and results show that compared with parent endomorphin-1, the synthesized non-natural amino acid modified endomorphin-1 analogue disclosed by the invention has higher affinity, higher enzymatic hydrolysis stability and higher analgesic activity, and has potential application values of being taken as clinical polypeptide analgesic medicines.
Owner:LANZHOU UNIVERSITY
Multi-site combination modified endomorphin analogue, synthesis and application thereof
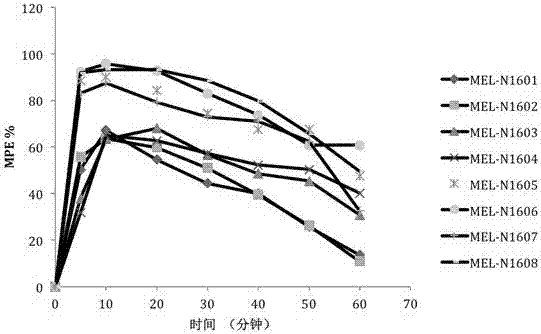
ActiveCN106967151AImprove pharmacological activityHigh medicinal valueNervous disorderTetrapeptide ingredientsMulti siteAnalgesics drugs
Belonging to the field of biomedicine, the invention provides an endomorphin analogue with the first position, the second position and fourth position respectively modified by tyrosine or 2, 6-dimethyltyrosine, N-methyl-D alanine and alpha-alkenyl-beta-amino acid and a synthesis method thereof. The endomorphin analogue is obtained by substituting the first position amino acid of endomorphin (EM1 and EM2) with tyrosine or 2, 6-dimethyltyrosine, substituting the second position amino acid with N-methyl-D alanine, and substituting the fourth position amino acid respectively with phenyl or 2-furyl replaced alpha-alkenyl-beta-amino acid, and is named as MEL-N16 series. Radioligand receptor binding experiment, isolated organ bioassay, in-vitro enzymolysis stability and warm bath tail flick analgesic experiment results show that the endomorphin analogue synthesized by the method provided by the invention has higher affinity than an endomorphine matrix, high enzymolysis stability and high analgesic activity, and has very good clinical application value in preparation of analgesic drugs.
Owner:LANZHOU UNIVERSITY
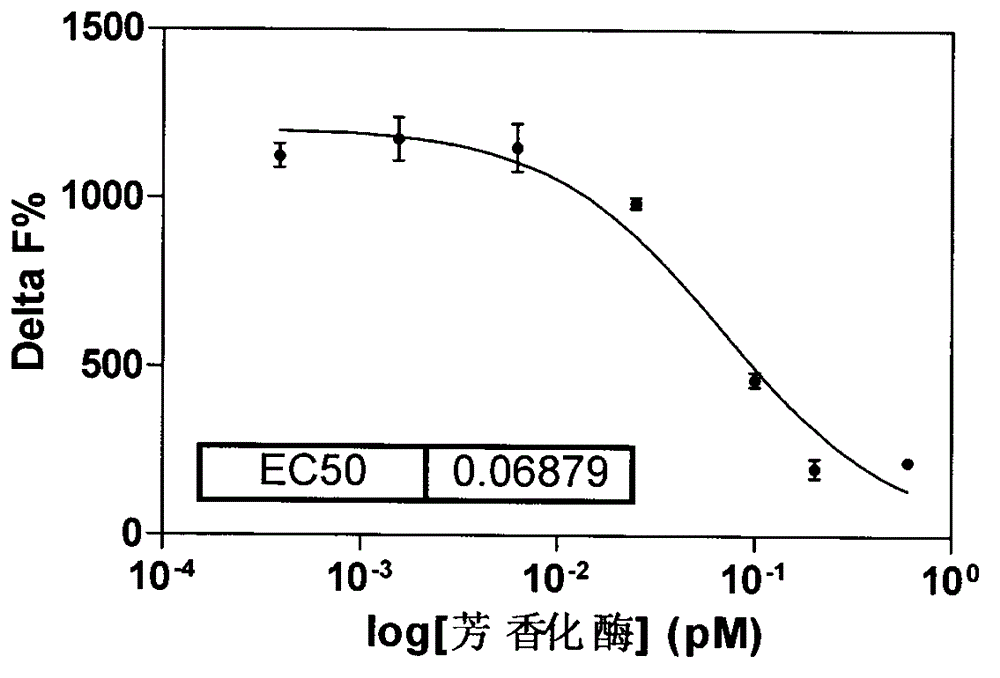
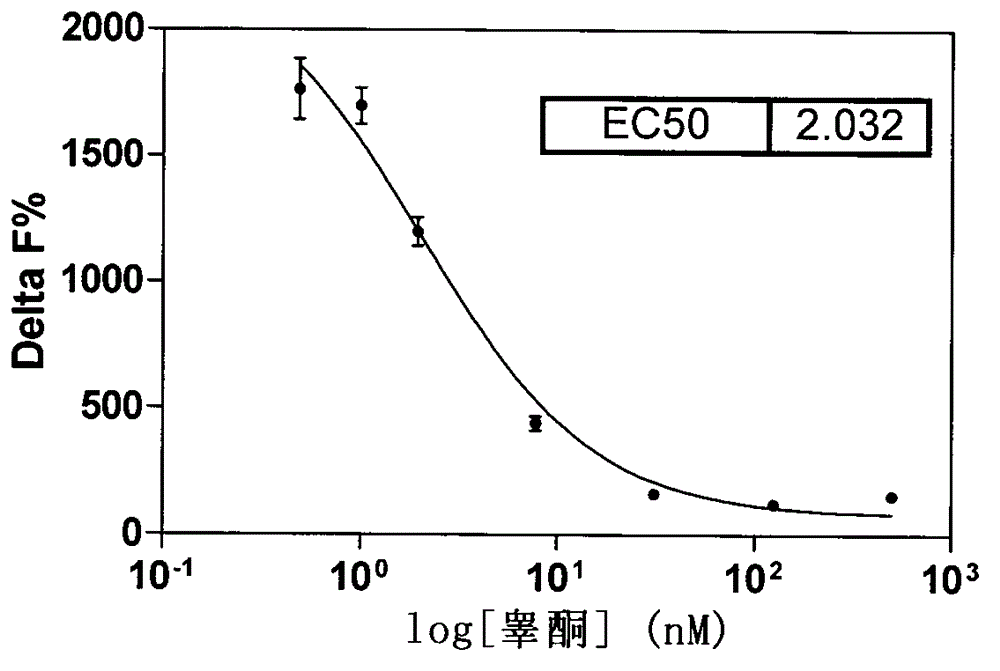
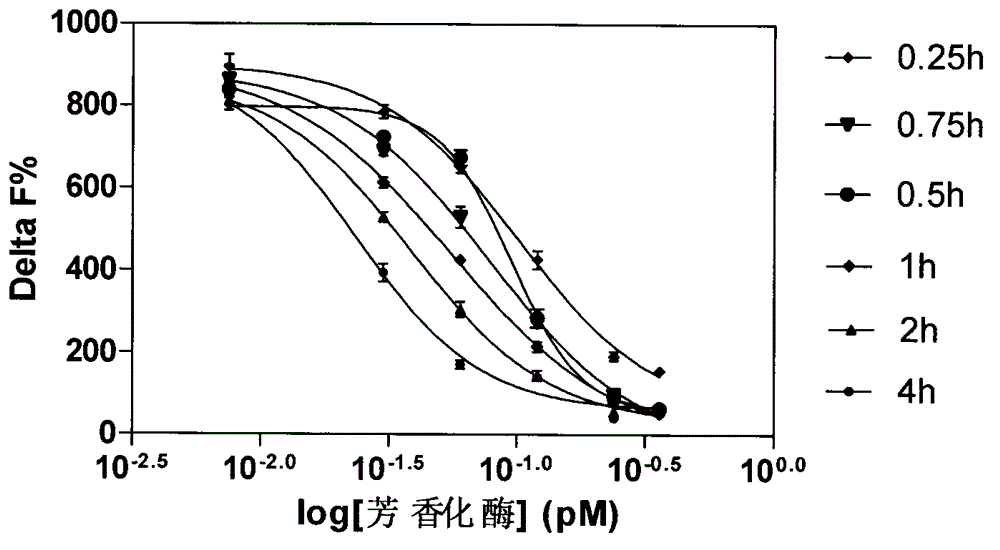
Aromatase inhibitor high-throughput screening model based on fluorescence detection principle
InactiveCN103149186AFluorescence/phosphorescenceHigh-Throughput Screening MethodsHigh-throughput screening
The invention relates to aromatase inhibitory activity high-throughput screening realized by means of a homogeneous time-resolved fluorescence technique. By detecting the fluorescence intensity ratio (665nm / 610nm) of an excitation light irradiated reaction system and performing formula conversion, the inhibitory activity of a to-be-detected sample on aromatase can be determined. By means of high-throughput screening new model, the harm of a radioligand binding principle-based experimental method to laboratory personnel and environment can be avoided. And the high-throughput screening model provided in the invention has the advantages of high efficiency, rapidity, accuracy, and inexpensiveness.
Owner:CHINA PHARM UNIV
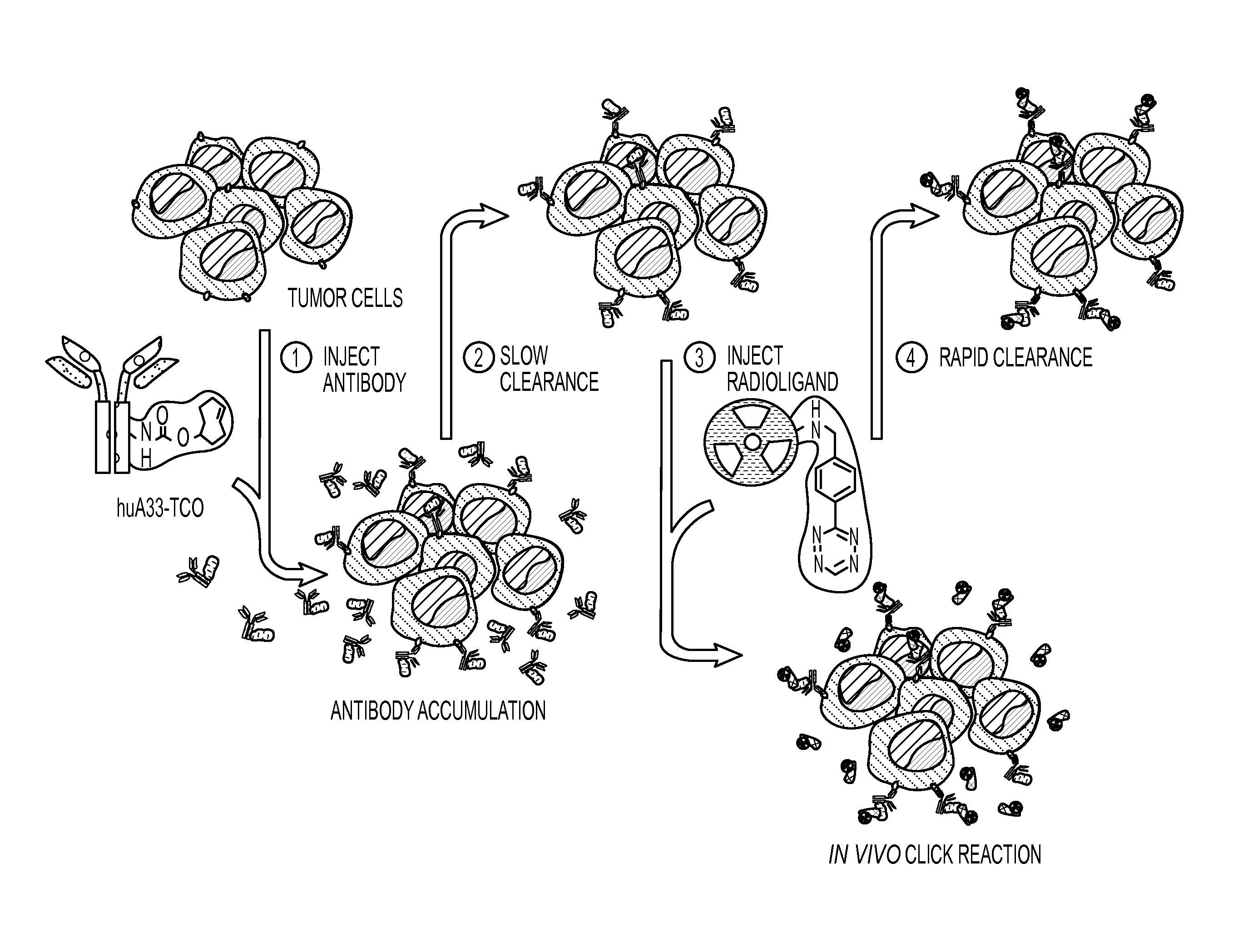
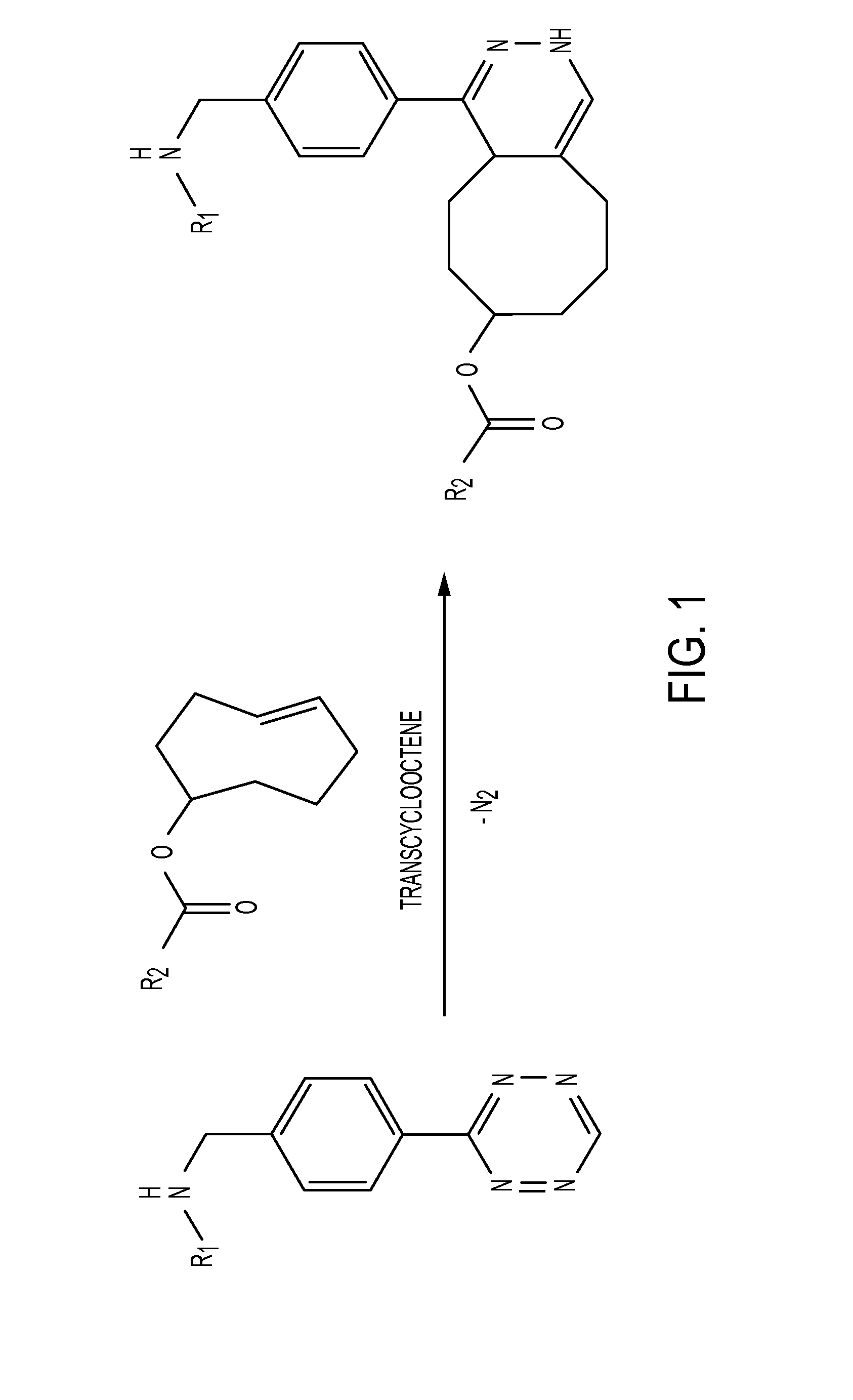
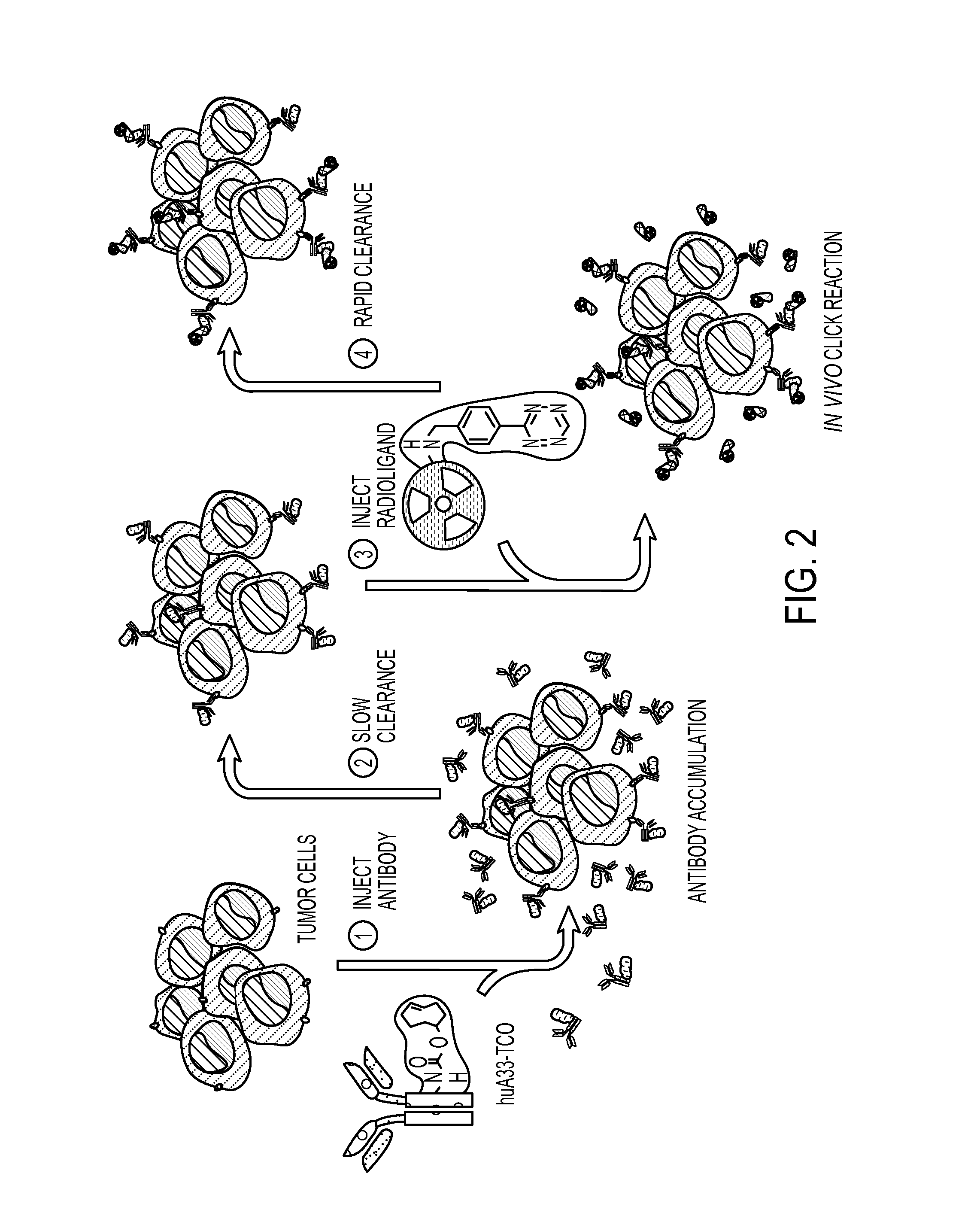
Radioligands for pretargeted pet imaging and methods of their therapeutic use
InactiveUS20160331852A1Fast excretionReduce radiation doseRadioactive preparation carriersPharmaceutical non-active ingredientsDecompositionRadioactive agent
Described herein are Tz / TCO-based pretargeting strategies using an Al[18F]-NOTA-labeled tetrazine radioligand. This imaging strategy enables delineation of cancer at earlier time points compared to other imaging strategies and further decreases the radiation dose to healthy tissues compared to directly labeled antibodies. Al-based 18F imaging of small molecules, such as tetrazine, has not been previously achieved due to the decomposition of tetrazine during radiofluorination. Radiofluorination is advantageous over other radiolabeling methods because, in addition to having a shorter half-life, 18F is more readily available to produce and therefore integrated into hospital workflows.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
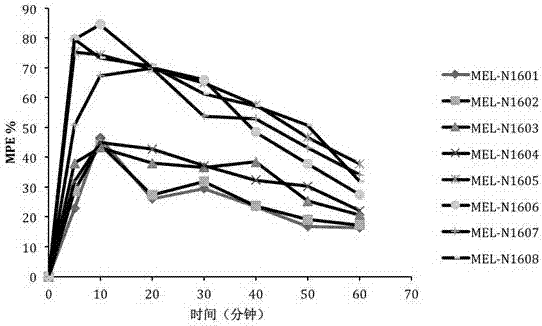
Synthesis and application of multi-site modified endomorphin-1 analogue
ActiveCN103214552AHigh affinityHigh enzymatic stabilityAntipyreticAnalgesicsAnalgesics drugsMethyltyrosines
The invention provides synthesis and application of multi-site modified endomorphin-1 analogues, belonging to a biological medicine field. The endomorphin-1 analogues are prepared by substituting a first amino acid of endomorphin-1 through tyrosine or 2,6-dimethyl tyrosine, substituting a second amino acid through proline or (S / R) beta-proline, and substituting a forth amino acid through phenyl, and 2-furyl substituted alpha-alkenyl-beta-amino acid respectively. By a radioligand receptor binding experiment, in vitro organ biological detection, a cAMP accumulation experiment, and an in vitro enzymolysis stability and warm water bath tail flick analgesic experiments, the multi-site modified endomorphin-1 analogues in the invention are performed pharmacological activity identification. A result shows that: compared with endomorphin-1, the novel multi-site modified endomorphin-1 analogues has advantages of high affinity, high enzymolysis stability and high analgesic activity, and has a high application value for designing and synthesizing polypeptide analgesic drugs.
Owner:LANZHOU UNIVERSITY
Tumor vascular targeting peptide GX1
InactiveCN101531706AGood radiochemical purityGood specific activityIn-vivo radioactive preparationsPeptide preparation methodsTumor vesselDrug biological activity
The invention belongs to the technical field of biomedicine, and in particular provides tumor vascular targeting peptide GX1. The invention utilizes a radioactive nuclide <99>Tc<m>O4 <-> to successfully mark GX1 by a direct method. Quality identification proves that <99>Tc<m>-GX1 has good radiochemical purity, specific activity and in vivo / vitro stability. In vitro microcosmic receptor autoradiography shows that the <99>Tc<m>-GX1 can combine with human umbilical vein endothelial cells and has good biological activity. In vitro microcosmic autoradiography, receptor radioligand conjoint analysis and competitive inhibition analysis confirm that the combination of the <99>Tc<m>-GX1 and tumor vascular endothelial cells has good specificity and affinity. ECT imaging and biological distribution prove that the <99>Tc<m>-GX1 has good targeting property to in vivo tumor tissue and specific distribution characteristicto tumor tissue. The <99>Tc<m>-GX1 has certain theoretical significance and great potential application value in diagnosis of gastric cancer, study on vascular targeting therapy, and other aspects.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
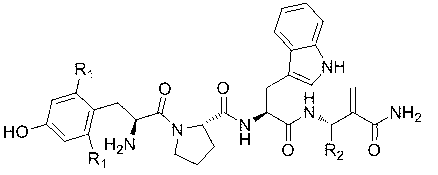
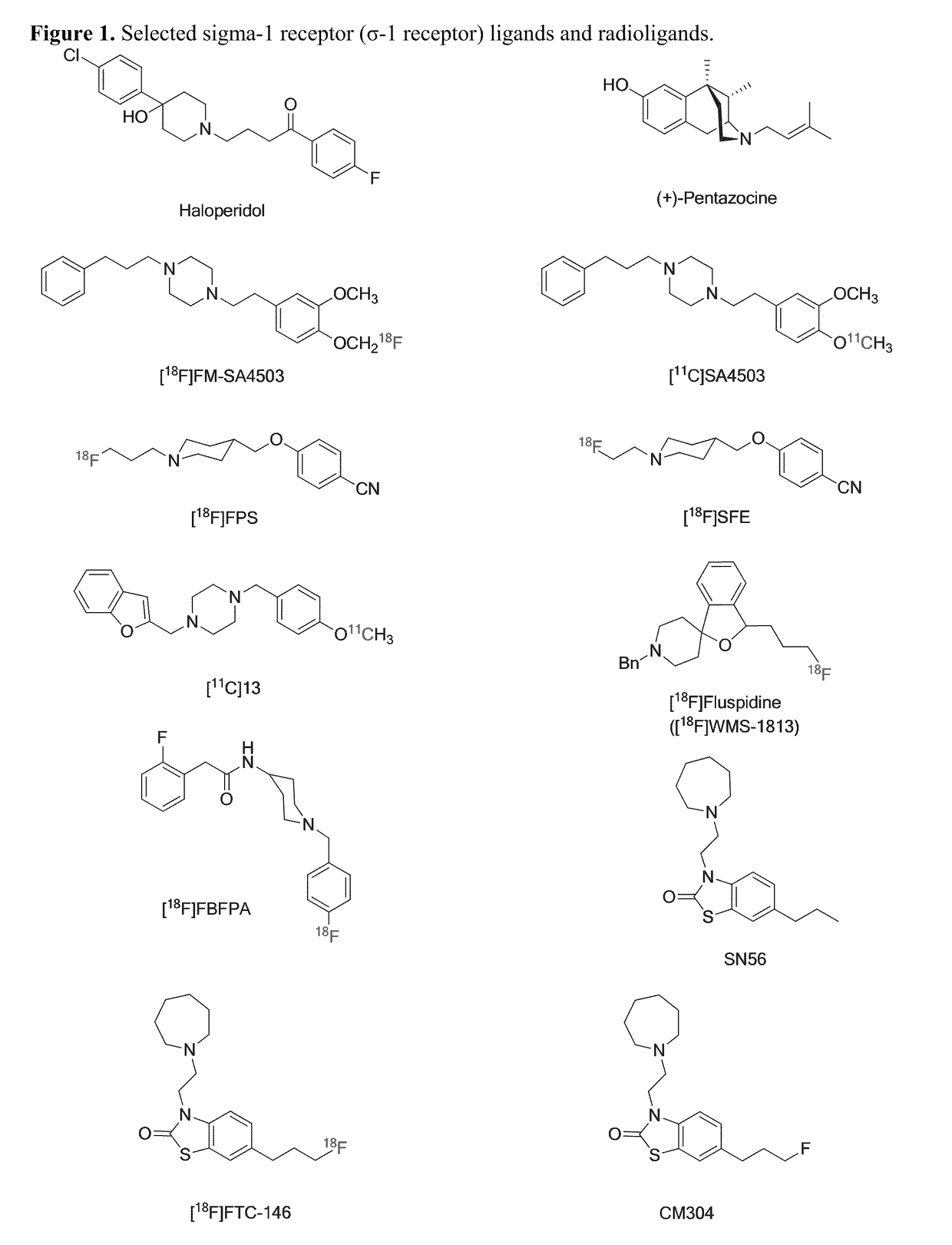
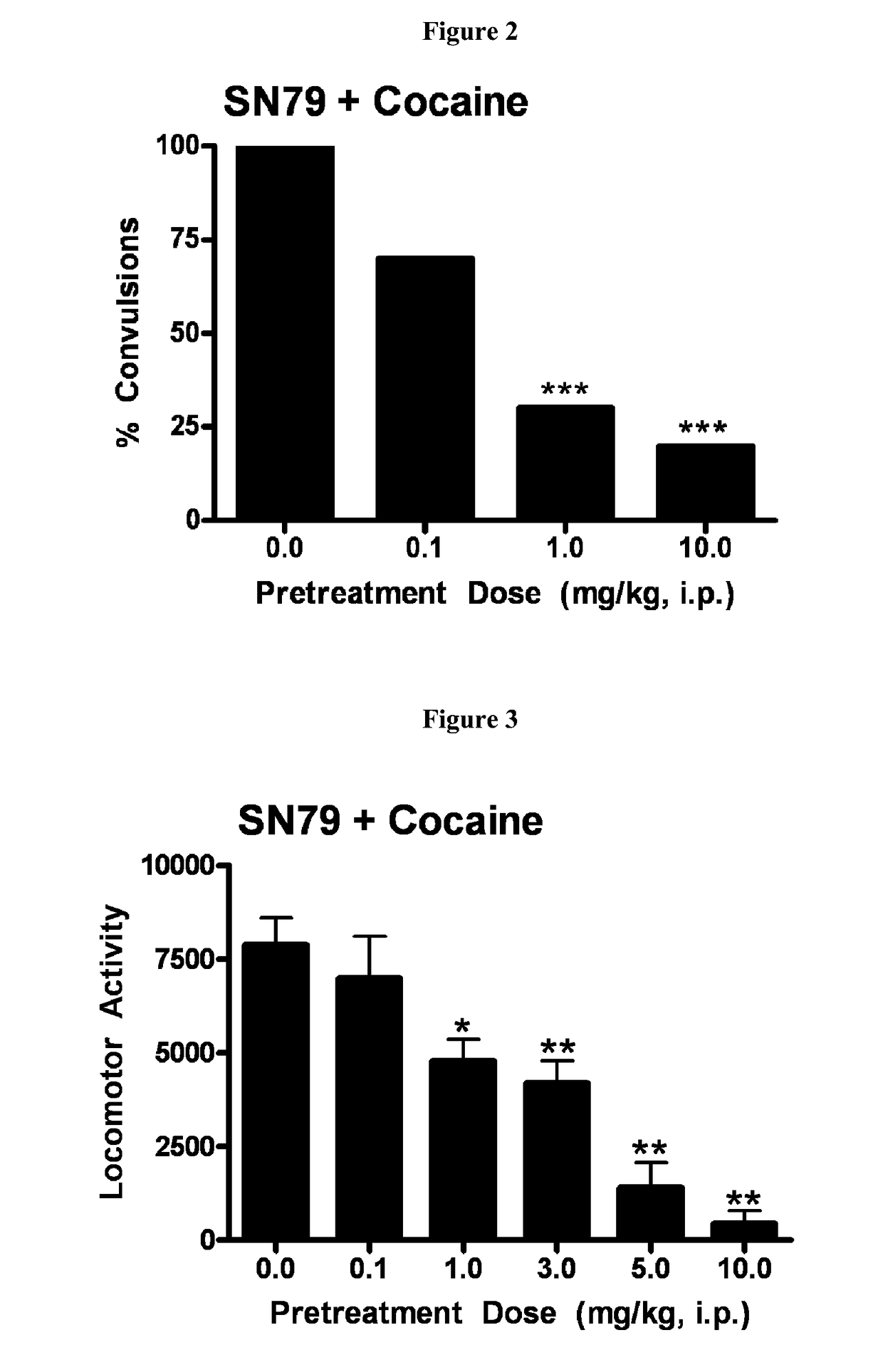
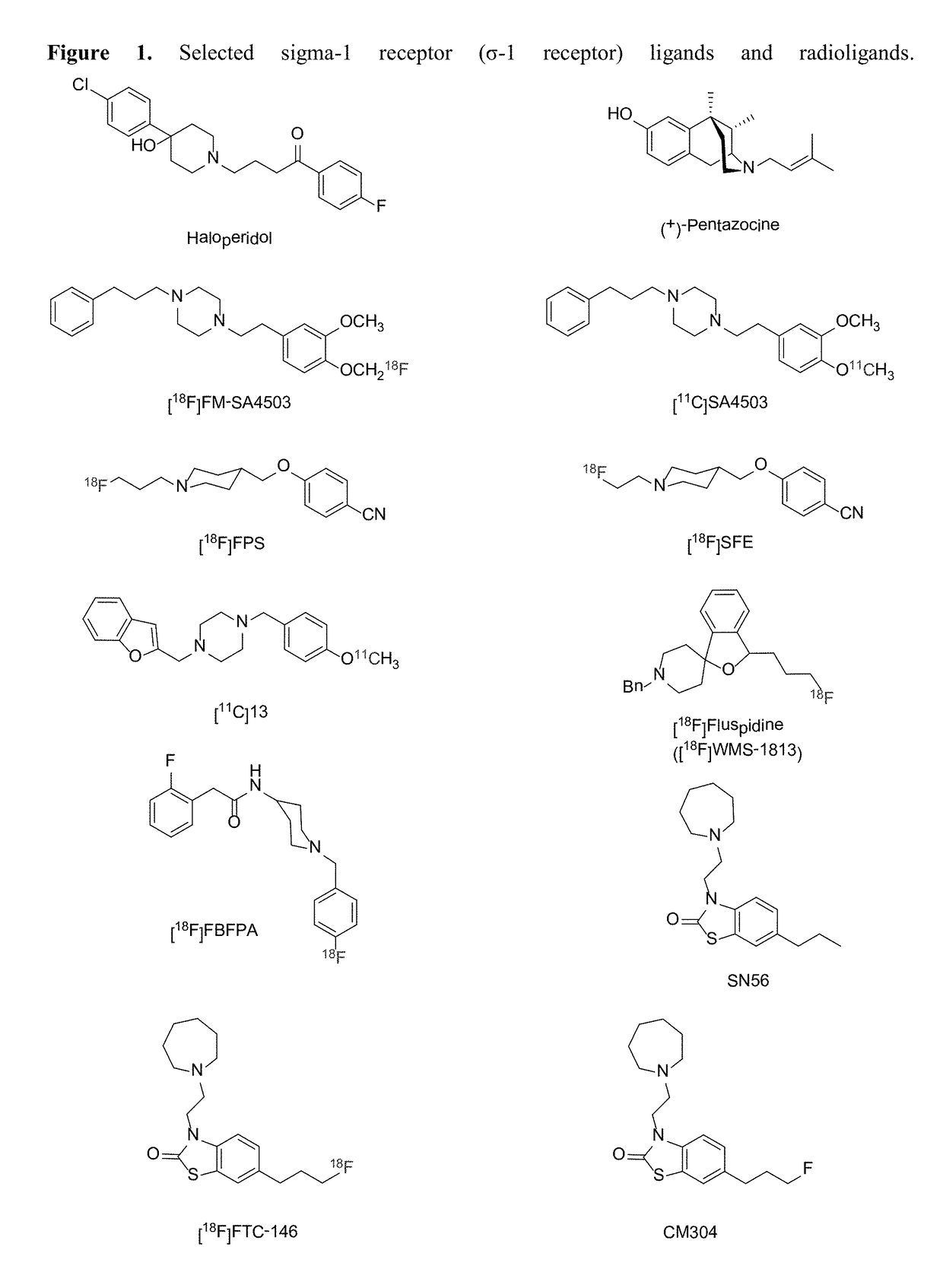
Highly selective sigma receptor ligands and radioligands as probes in nociceptive processing and the pathphysiological study of memory deficits and cognitive disorders
ActiveUS20140328755A1Simple methodUseful imageOrganic active ingredientsOrganic chemistryVolumetric Mass DensityPathology study
A method for localizing and quantifying S1R role in nociceptive processing; for providing a guide to providing an analgesic therapy; of using an S1R selective ligand as a biomarker for pathphysiological study of memory deficits and cognitive disorders; or of detecting increased S1R density at the site of nerve injury arising from neuropathic pain comprising using as a probe at least one SR1 selective compound or radioligand of the general formula III′, or IV′:
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
A high-throughput screening model for aromatase inhibitors based on the principle of fluorescence detection
InactiveCN103149186BFluorescence/phosphorescenceHigh-Throughput Screening MethodsHigh-throughput screening
The invention relates to aromatase inhibitory activity high-throughput screening realized by means of a homogeneous time-resolved fluorescence technique. By detecting the fluorescence intensity ratio (665nm / 610nm) of an excitation light irradiated reaction system and performing formula conversion, the inhibitory activity of a to-be-detected sample on aromatase can be determined. By means of high-throughput screening new model, the harm of a radioligand binding principle-based experimental method to laboratory personnel and environment can be avoided. And the high-throughput screening model provided in the invention has the advantages of high efficiency, rapidity, accuracy, and inexpensiveness.
Owner:CHINA PHARM UNIV
Fluorine-18 and carbon-11 labeled radioligands for positron emission tomography (PET) imaging for LRRK2
A method for positron emission tomography (PET) imaging of LRRK2 in tissue of a subject, the method comprising: administering a compound of formula I, formula II or formula III, or a pharmaceutically acceptable salt thereof to the subject, wherein the compound includes at least one C11 or F18 label thereon; allowing the compound to penetrate into the tissue of the subject; and collecting a PET image of the CNS or brain tissue of the subject.
Owner:GENENTECH INC
Methods for diagnosing diseases and evaluating treatments therefor using PET
InactiveUS8652440B2Compounds screening/testingIn-vivo radioactive preparationsDiabetes mellitusDisease
The present invention relates to methods for determining whether a mammal has a disease, such as diabetes, using PET data analysis techniques. These methods include administering to a mammal a PET-compatible tracer, such as a radioligand specific for a vesicular monoamine transporter 2 (VMAT2) receptor, and measuring total functional β-cell capacity (volume) of the mammal's pancreas using PET data analysis techniques. Methods for tracking the efficacy of a treatment for diabetes, for evaluating the regeneration of β-cells in a pancreas, and for monitoring a patient with a transplanted pancreas are also provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods for Diagnosing Diseases and Evaluating Treatments Therefor Using Pet
The present invention relates to methods for determining whether a mammal has a disease, such as diabetes, using PET data analysis techniques. These methods include administering to a mammal a PET-compatible tracer, such as a radioligand specific for a vesicular monoamine transporter 2 (VMAT2) receptor, and measuring total functional β-cell capacity (volume) of the mammal's pancreas using PET data analysis techniques. Methods for tracking the efficacy of a treatment for diabetes, for evaluating the regeneration of β-cells in a pancreas, and for monitoring a patient with a transplanted pancreas are also provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
PSMA targeted radiotherapy medicine and preparation method thereof
PendingUS20210121585A1Improve the quality of lifeTime can be spentRadioactive preparation carriersAntigenDisease
The invention features a novel precursor provided for radioisotope labeling with ligands for specific binding of prostate-specific membrane antigen (PSMA) for prostate cancer diagnosis and treatment, and the pharmacophore of a PSMA inhibitor composed of three molecules of glutamic acid, urea and lysine is provided with three variable linkers based on pharmacological activity of the PSMA inhibitor for labeling with radioactive nucleus Ga-67, Ga-68, In-111, Lu-177, Cu-64, or Y-90 through a chelating agent for imaging analysis of human tumor models of prostate cancer and serving as a PSMA-targeted radioligand therapy for prostate cancer diseases.
Owner:LI MING HSIN +7
Radioligands for the TRP-M8 receptor and methods therewith
InactiveUS7169377B2Radioactive preparation carriersGroup 3/13 element organic compoundsProstate cancer cellSensory potential
Owner:WEI EDWARD T
Chimeric gaba receptor
The present invention provides an isolated GABAB receptor protein comprising at least one GABABR1a subunit and at least one GABABR2a subunit, characterized in that said GABAB receptor has one high affinity agonist binding site and one low affinity agonist binding site. In particular the isolated recombinant GABAB receptor protein expressed by the hGABABR1a / GABABR2 CHO cell line deposited at the Belgian Coordinated Collections of Microorganisms (BCCM) as CHO-K1 h-GABA-b R1a / R2 clone on Aug. 22, 2003 with the accession number LMBP 6046CB. It is thus an object of the present invention to provide the hGABABR1a / GABABR2 CHO cell line deposited at the Belgian Coordinated Collections of Microorganisms (BCCM) as CHO-K1 h-GABA-b R1a / R2 clone on Aug. 22, 2003 with the accession number LMBP 6046CB. The invention also provides the use of the aforementioned cell line in a method to identify GABAB receptor agonists using a functional or a binding assay. In particular in a radioligand-binding assay comprising the use of radiolabeled agonists such as for example 3H-GABA or 3H-baclofen. In a particular embodiment the present invention provides the use of the aforementioned GABAB receptor in a method to identify a high affinity GABAB receptor agonist using a functional or a binding assay. In particular in a radioligand-binding assay comprising the use of radiolabeled agonists such as for example 3H-GABA or 3H-baclofen. Alternatively, the aforementioned binding assays are performed on cellular extracts, in particular cellular membrane preparations of the aforementioned cells.
Owner:JANSSEN PHARMA NV
Regularization of images
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Method for preventing human glands from radiation injury
PendingCN114746107AAvoid damagePrevent permanentIn-vivo radioactive preparationsPeptide/protein ingredientsAnticholinergic DrugsSide effect
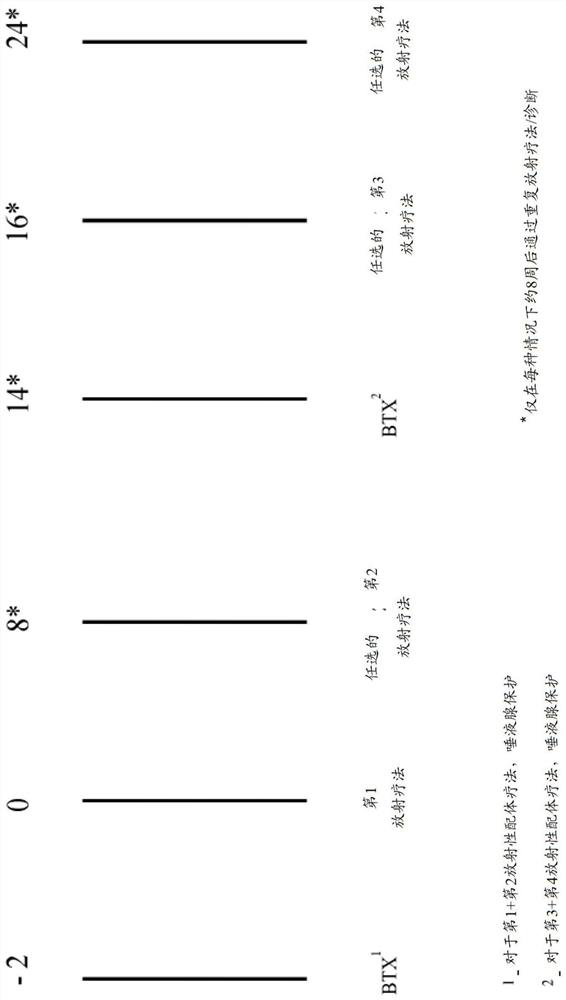
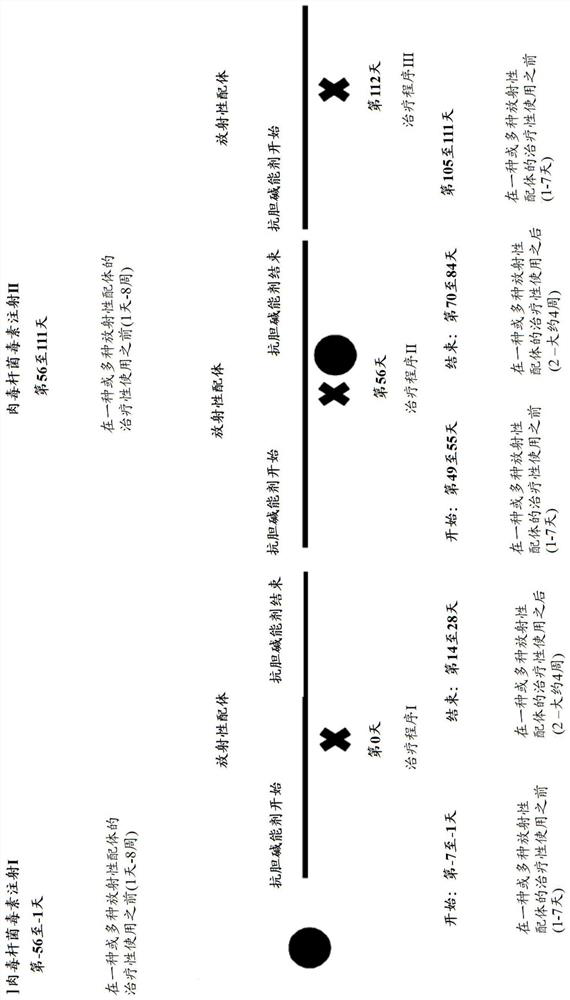
The invention relates to the field of radiooncology. The methods according to the invention comprise the use of botulinum toxins and anticholinergic agents to prevent glands from radiation damage and side effects due to radioactive ligands. The method according to the invention further comprises the use of botulinum toxins to prevent the glands from radiation damage, wherein the radiation damage is caused by radioactive ligands. By using the method according to the invention, the invention can provide effective protection of the glands to avoid radiation damage.
Owner:MERZ THERAPEUTICS GMBH
Highly selective sigma receptor radioligands
Compounds having the general formula III′, or IV′wherein R1 can be a radical of an optionally substituted C-4 to C-7 N-containing heterocycle or a radical of an optionally substituted cyclic or acyclic tertiary amine or isoindoline-1,3-dione: R2,4,5,6 can each independently be any one or combinations of the following moieties, cyano, nitro, acyl, alkyl, amido, azido, isothiocyanate, isocyanate, optionally substituted anilino, halogens, ethers, sulfonamides, thioacyl, nitro, aromatic, heterocyclic, olefinic, acetylene, deuterium, or tritium; Y is S; Z can be either H, O, S, S—R or NR where R groups can be either H, aryls, alkyls, or cycloalkyls; “n” can be 1 to 5 carbons in length and stereoisomers, functional analogs, and pharmaceutically acceptable salts thereof and wherein the moiety bridging R1 and N can be a substituted alkylene, optionally substituted alkenylene or optionally substituted alkynylene and where the alkylene group can include an inserted C3-C5 cycloalkyl group, aromatic, and heterocyclic group; and wherein X is C1-C4 radiohaloalkyl.
Owner:UNIVERSITY OF MISSISSIPPI +1
Sample preparation compositions, devices, systems, and methods
PendingCN114555204AEfficient separationShorten the timeOther chemical processesSolid sorbent liquid separationDownstream processingBiotin
The present specification relates to compositions, devices, apparatuses, methods, kits, and systems for sample preparation (e.g., isolation, reduction, or removal of small molecules from biomolecules in a sample). The molecular weight range lt of exemplary small molecules that can be separated, reduced or removed; 2,000 Da, and may include, but not limited to, dyes, biotins, affinity tags, cross-linkers, reducing agents, labels, nanoparticles, radioligands, mass tags, unreacted molecules, and combinations, intermediates, and derivatives of the foregoing. Exemplary biomolecules present in a sample include, but are not limited to, proteins, glycoproteins, antibodies, peptides, nucleic acids, polysaccharides, carbohydrates, and lipids. The methods, compositions, kits, devices, apparatuses, and systems of the present disclosure can advantageously provide excellent separation of small molecule contaminants and additionally can reduce the time and cost associated with separating small molecules from larger biomolecules in a sample. Isolated biomolecules as shown herein are suitable for better downstream processing.
Owner:PIERCE BIOTECHNOLOGY
Highly selective sigma receptor ligands and radioligands as probes in nociceptive processing and the pathphysiological study of memory deficits and cognitive disorders
A method for localizing and quantifying S1R role in nociceptive processing; for providing a guide to providing an analgesic therapy; of using an S1R selective ligand as a biomarker for pathphysiological study of memory deficits and cognitive disorders; or of detecting increased S1R density at the site of nerve injury arising from neuropathic pain comprising using as a probe at least one SR1 selective compound or radioligand of the general formula III′, or IV′:
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
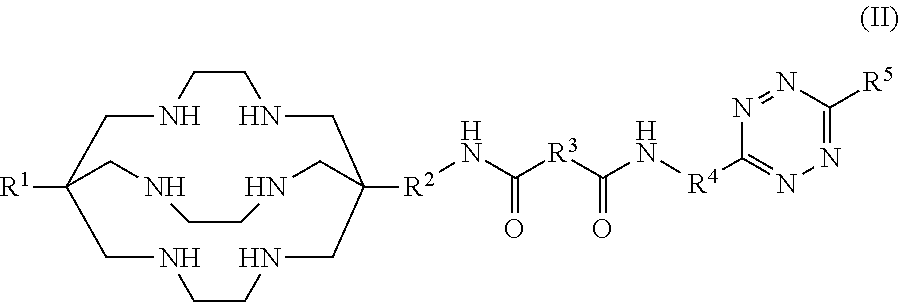
Formulations and kits for radiotherapy and diagnostic imaging
PendingUS20210015950A1Loss in overall efficacyGood effectOrganic active ingredientsPharmaceutical delivery mechanismRadio isotopesAntiendomysial antibodies
Aqueous formulations and kits of radiopharmaceutical compounds of general Formula (II) protected from radiolysis with stabilisers, such as L-methionine and gentisic acid, are disclosed, wherein the compounds are based on sarcophagine ligands coordinated to a radioisotope, such as 64-copper, and linked to a tetrazine group for reaction with tumour targeting antibodies having functional reactive groups such as trans-cyclooctene, processes of preparing said radioligand formulations, and uses thereof for radioimaging, diagnosing and treating cancer.
Owner:CLARITY PHARMA PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com