Non-natural amino acid modified endomorphin-1 analogue as well as synthesis method and application thereof
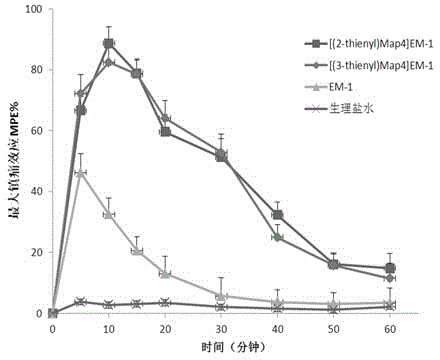
An unnatural amino acid, endomorphin technology, applied in the field of biomedicine, can solve the problems of easy enzymolysis and short action time, and achieve the effect of prolonging the effect, improving the affinity and the stability of enzymolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
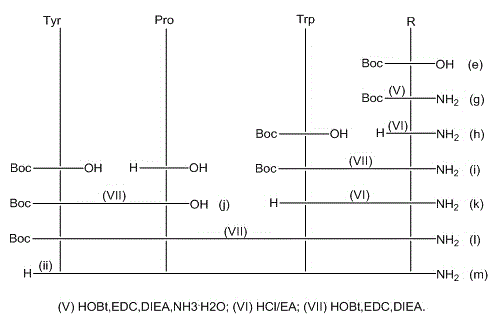
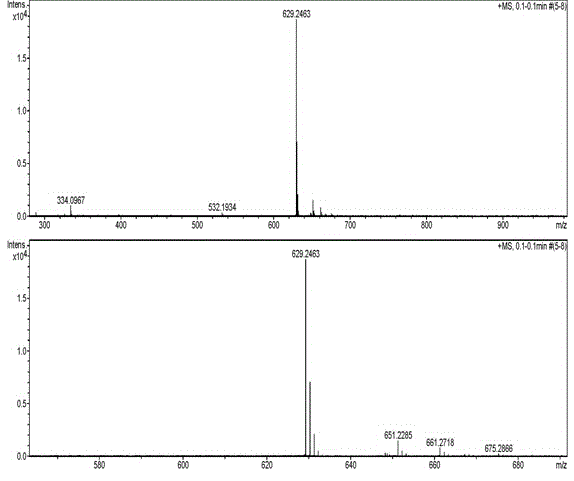
[0070] Example 1: Synthesis of [(2-thienyl)Map4]EM-1:
[0071] (1) Amide synthesis of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(2-thiophene)propionic acid
[0072] Dissolve N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(2-thiophene)propionic acid in anhydrous dichloromethane, and add N-tert-butoxy Carbonyl-3-amino-2-methenyl-3-(2-thiophene)propionic acid 4~5 times the amount of N,N-diisopropylethylamine, 1.45~1.5 times the amount of 1-hydroxyl Benzotriazole, 1.5~1.6 times the amount of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, stir and dissolve fully, add N-tert-butoxycarbonyl-3-amino-2- Ammonia water with 1.2 to 1.5 times the molar weight of methenyl-3-(2-thiophene)propionic acid was reacted at room temperature for 10 to 12 hours; after the reaction was completed, it was washed, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain a white solid N-tert Amide of butoxycarbonyl-3-amino-2-methenyl-3-(2-thiophene)propionic acid.
[0073] (2) ...
Embodiment 2
[0087] Embodiment 2: Synthesis of [(3-thienyl)Map4]EM-1:
[0088] (1) Amide synthesis of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(3-thiophene)propionic acid
[0089] Dissolve N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(3-thiophene)propionic acid in anhydrous dichloromethane, add N-tert-butoxy Carbonyl-3-amino-2-methenyl-3-(3-thiophene)propionic acid 4~5 times the amount of N,N-diisopropylethylamine, 1.45~1.5 times the amount of 1-hydroxyl Benzotriazole, 1.5~1.6 times the amount of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, stir and dissolve fully, add N-tert-butoxycarbonyl-3-amino-2- Ammonia water with 1.2 to 1.5 times the molar weight of methenyl-3-(3-thiophene)propionic acid was reacted at room temperature for 10 to 12 hours; after the reaction was completed, it was washed, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain a white solid N-tert Amide of butoxycarbonyl-3-amino-2-methenyl-3-(3-thiophene)propionic acid;
[0090] (2) S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com