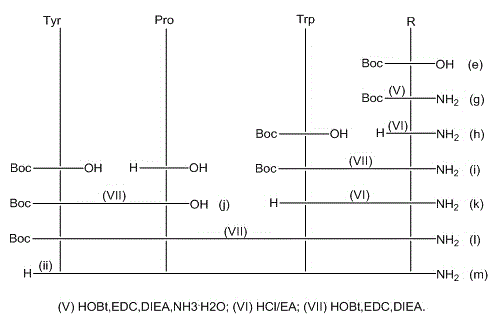
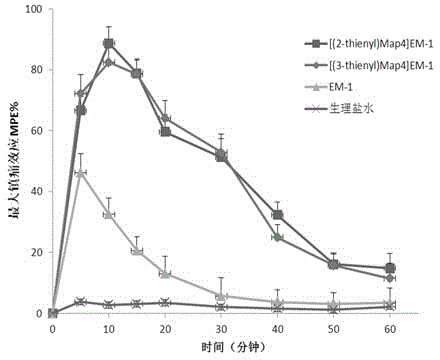
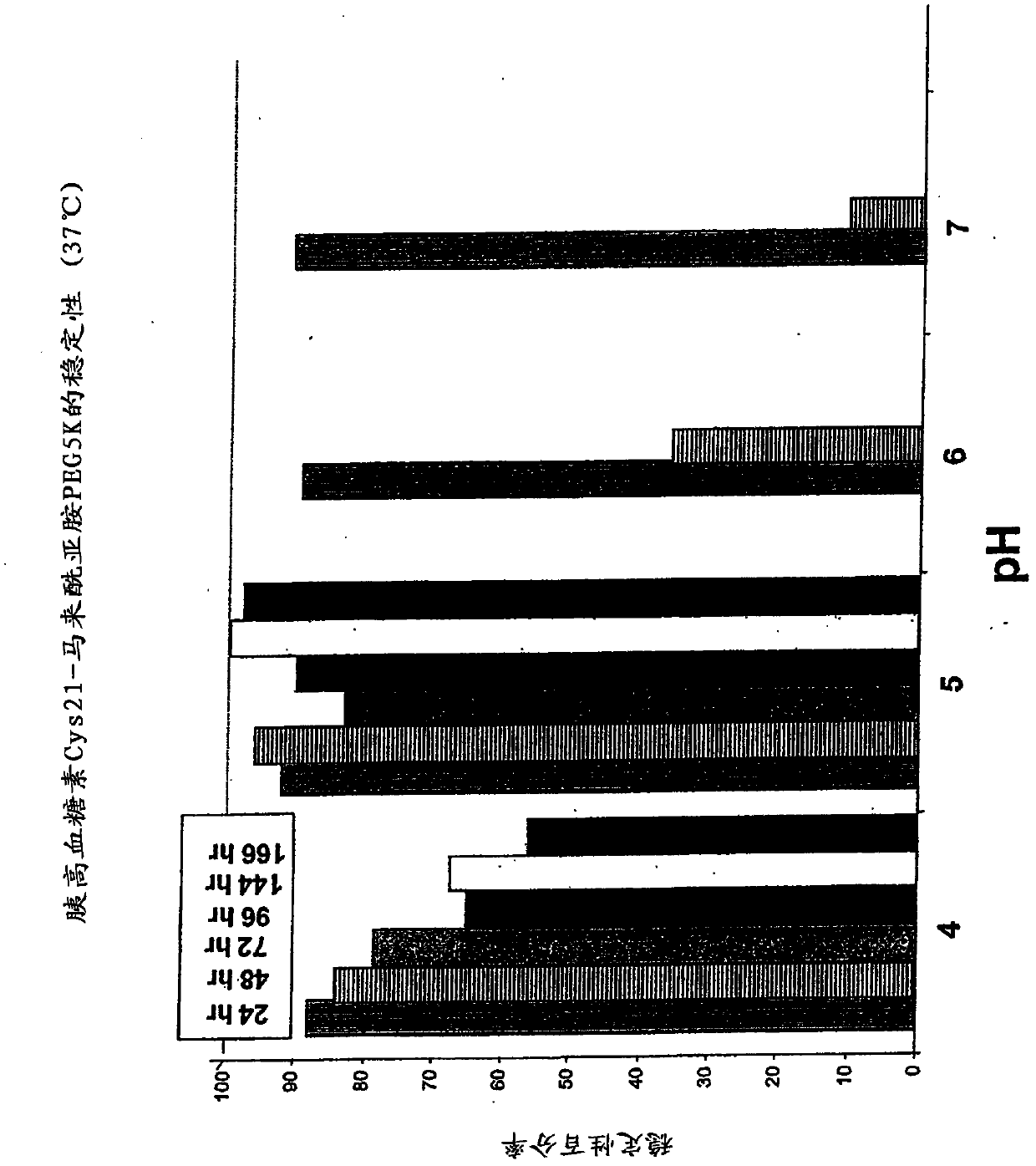
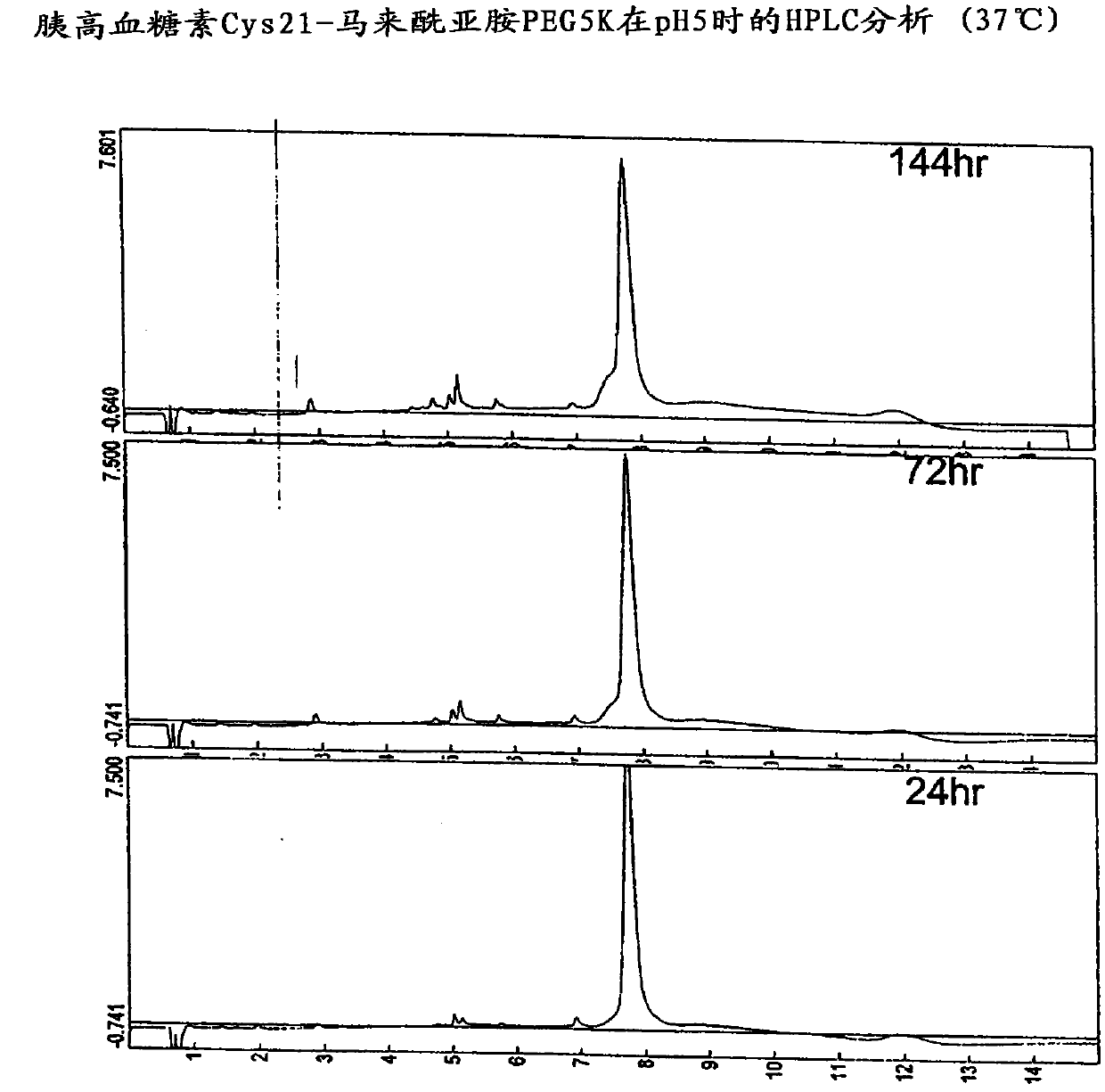
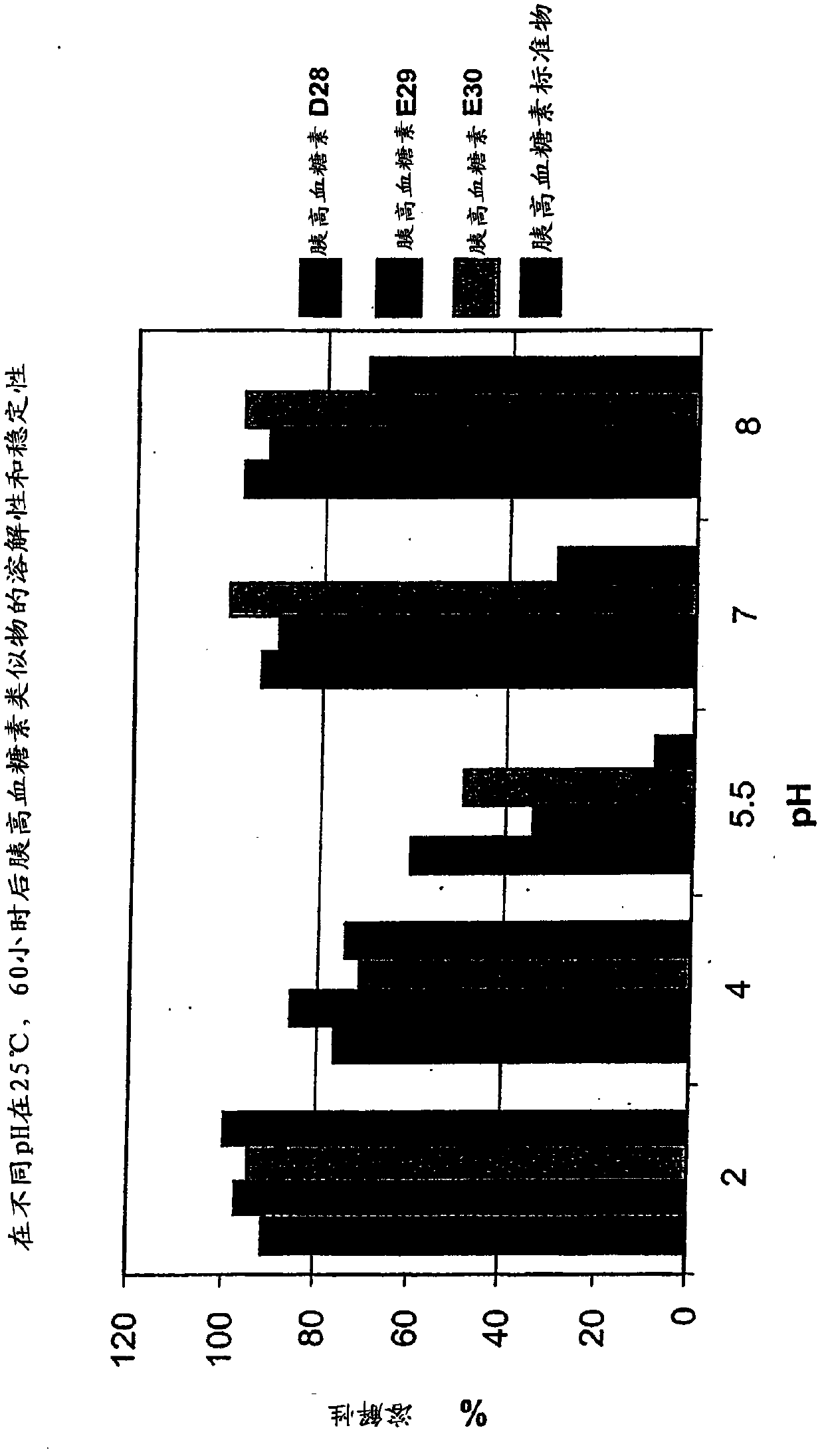
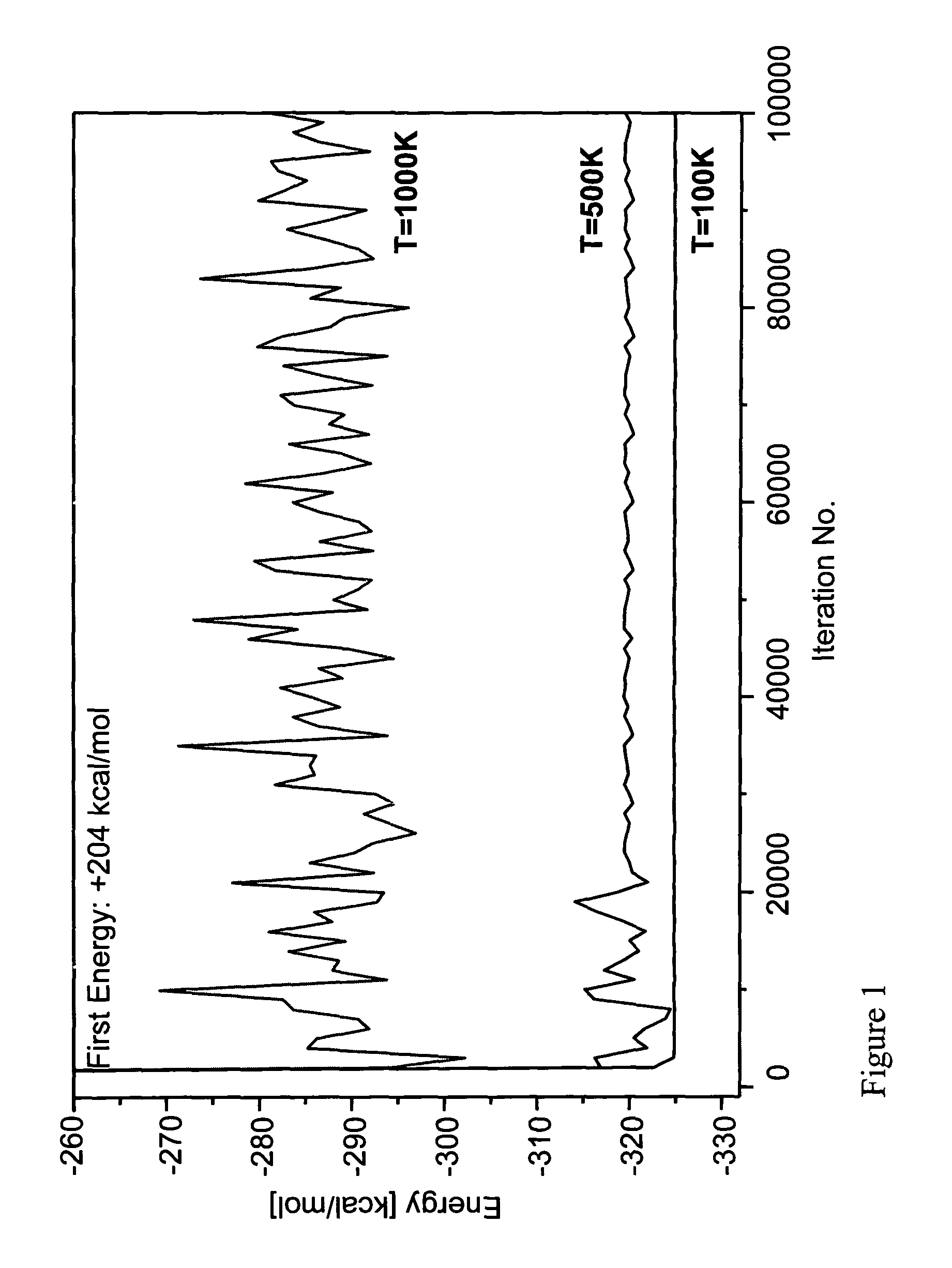
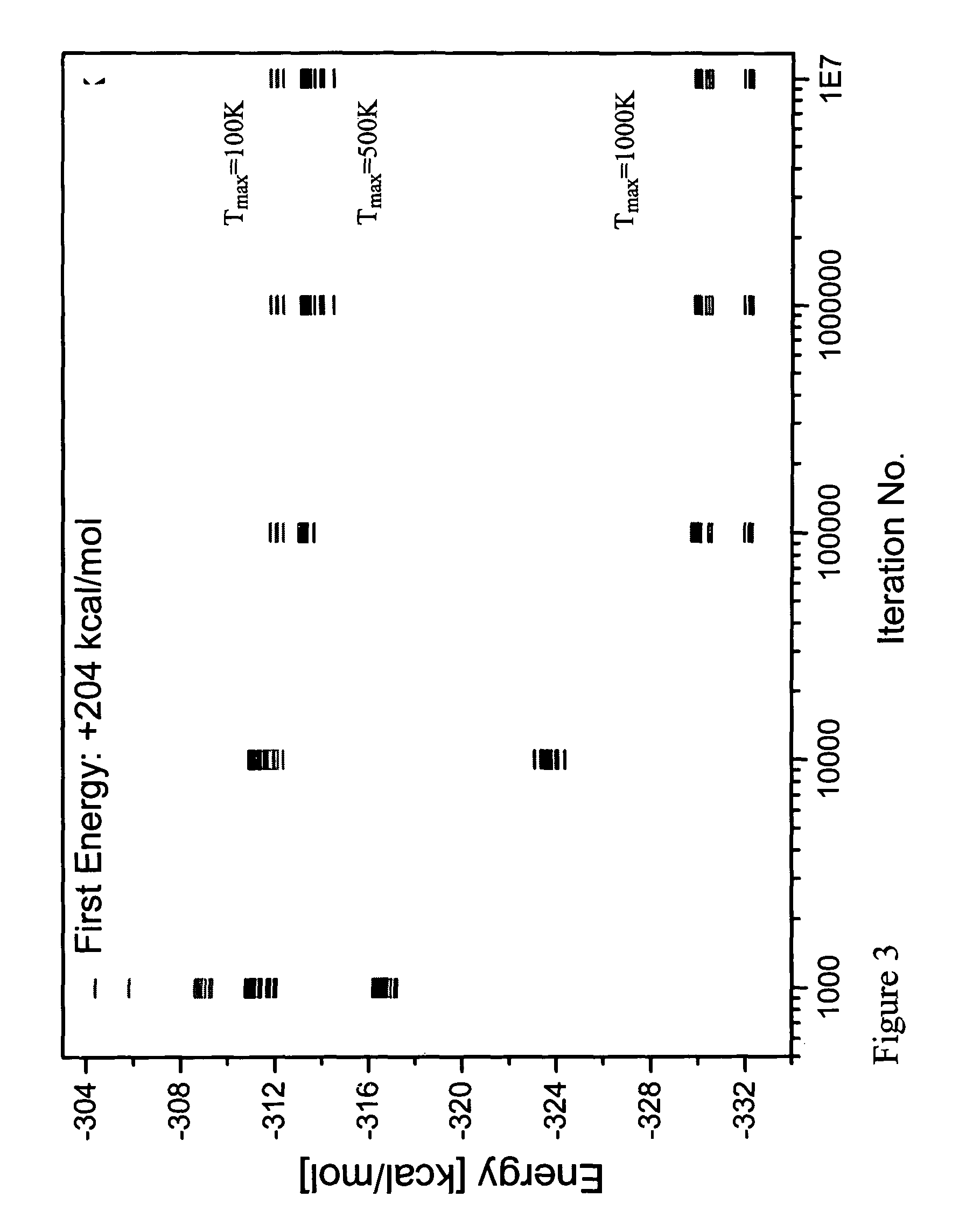
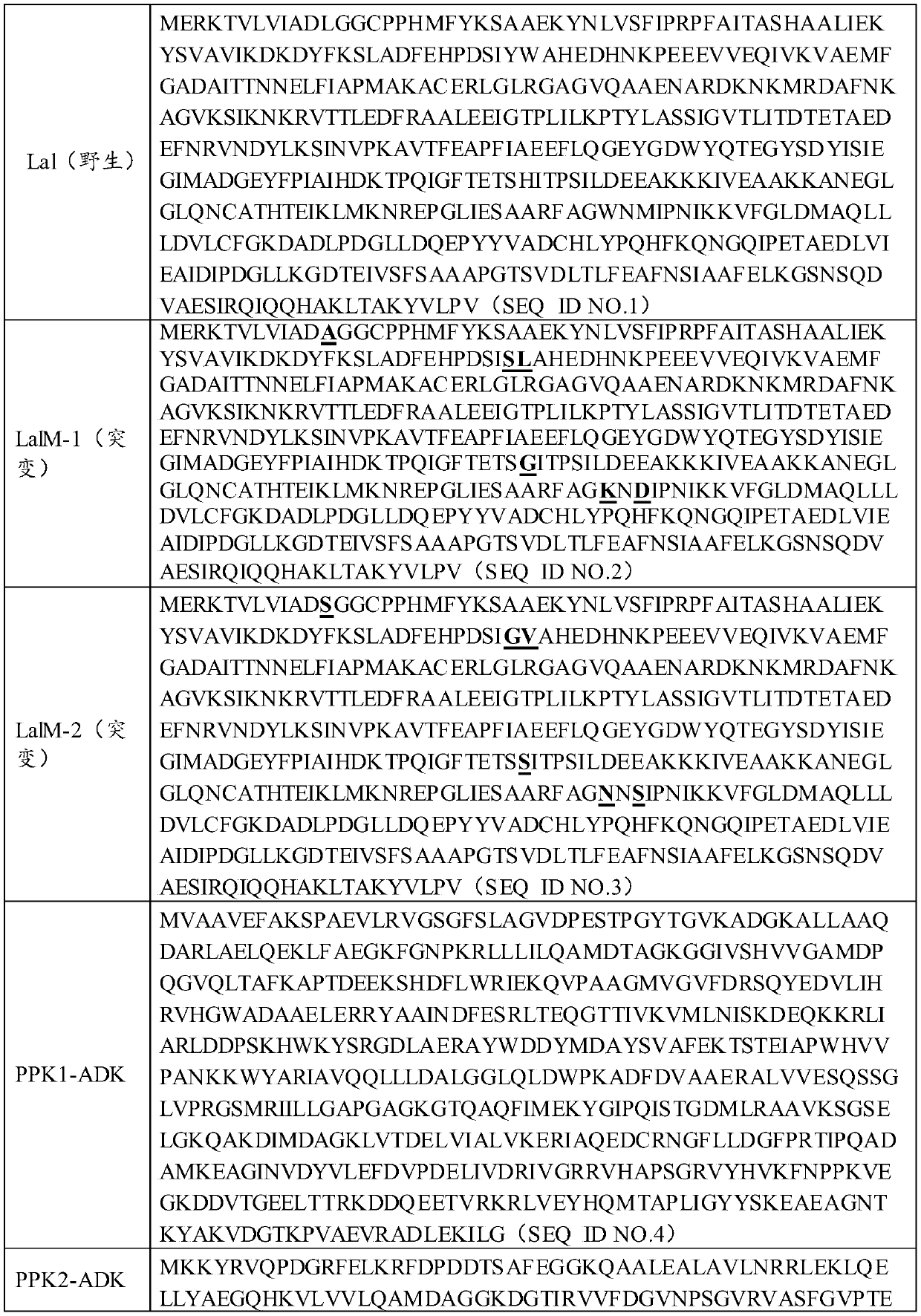
Patents
Literature
131 results about "Amino acid replacement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amino acid replacement is a change from one amino acid to a different amino acid in a protein due to point mutation in the corresponding DNA sequence. It is caused by nonsynonymous missense mutation which changes the codon sequence to code other amino acid instead of the original.
Humanized antibody specific for human 4-1BB and pharmaceutical compsn comprising same
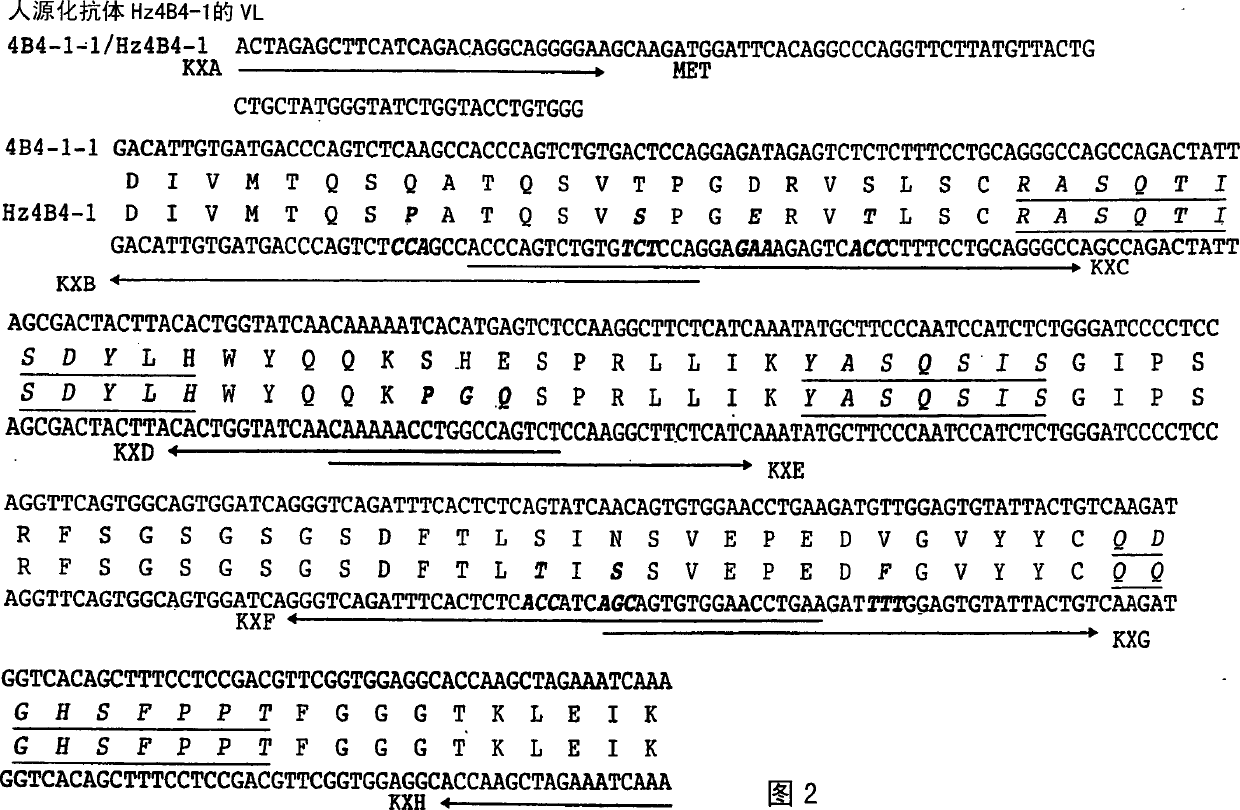
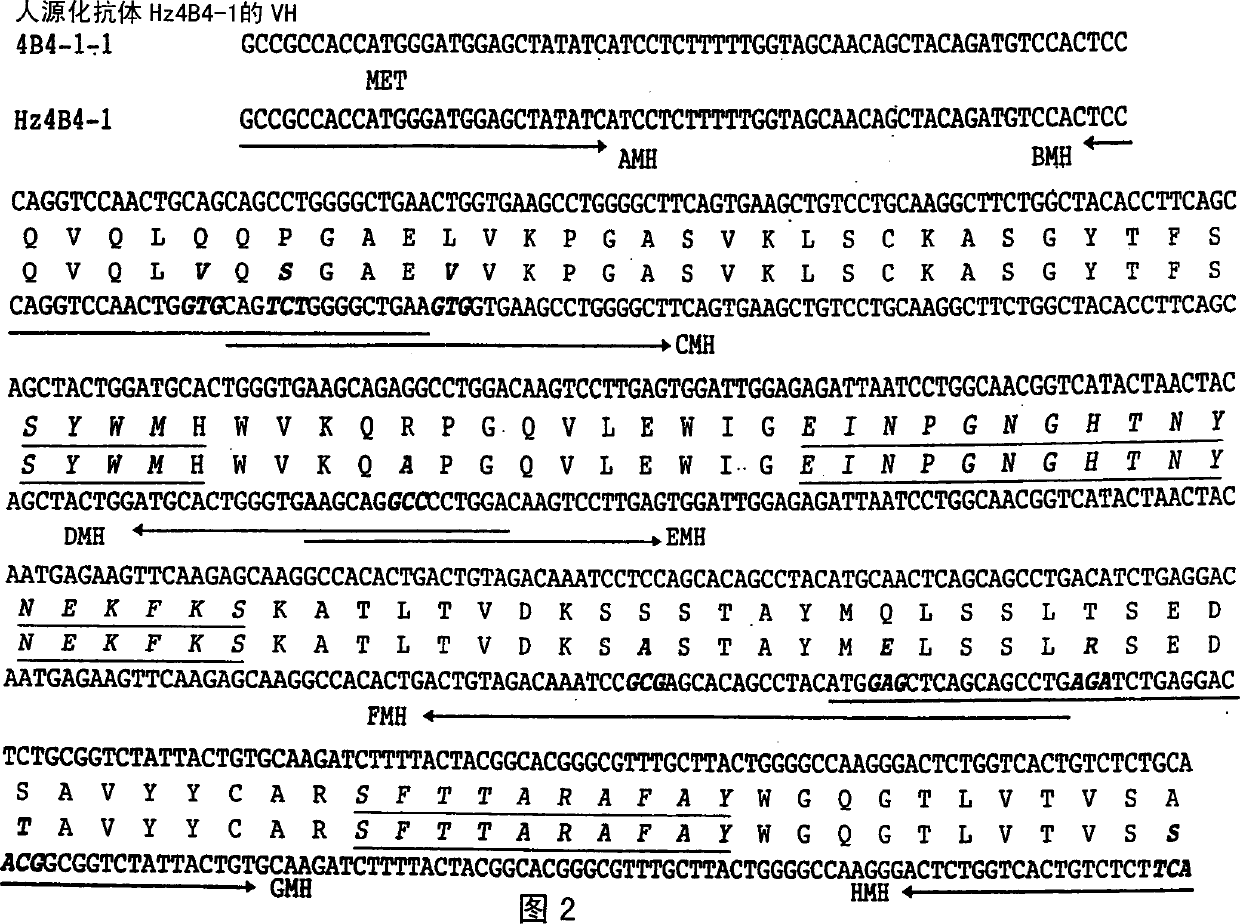
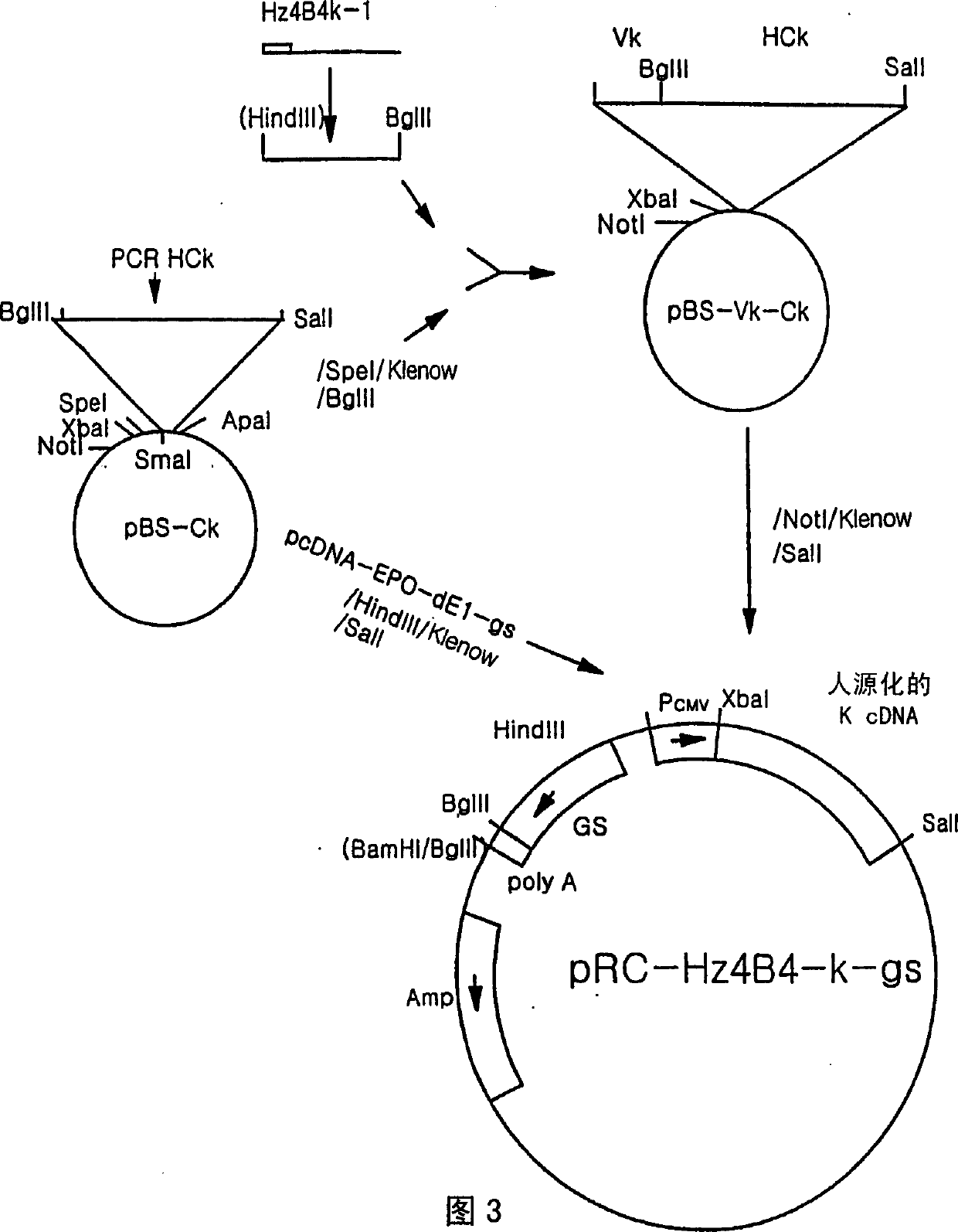
The present invention is directed to humanized antibodies that specifically bind the protein 4-1BB. The antibodies can be made by grafting of the complementarity determining regions (CDR's) of mouse monoclonal antibody to human 4-1BB to the remaining portions of a human antibody and by making further amino acid replacements. In addition, a pharmaceutical composition that includes the humanized antibody can be made and can be used to treat autoimmune diseases to suppress an immune response. The humanized antibody of the invention has high affinity for human 4-1BB, and exhibits sequence similarity to human antibody. As a result, the pharmaceutical composition of the present invention can be used to treat autoimmune disease and act as an immunosuppressant in humans without much side-effect.
Owner:LG CHEM LTD
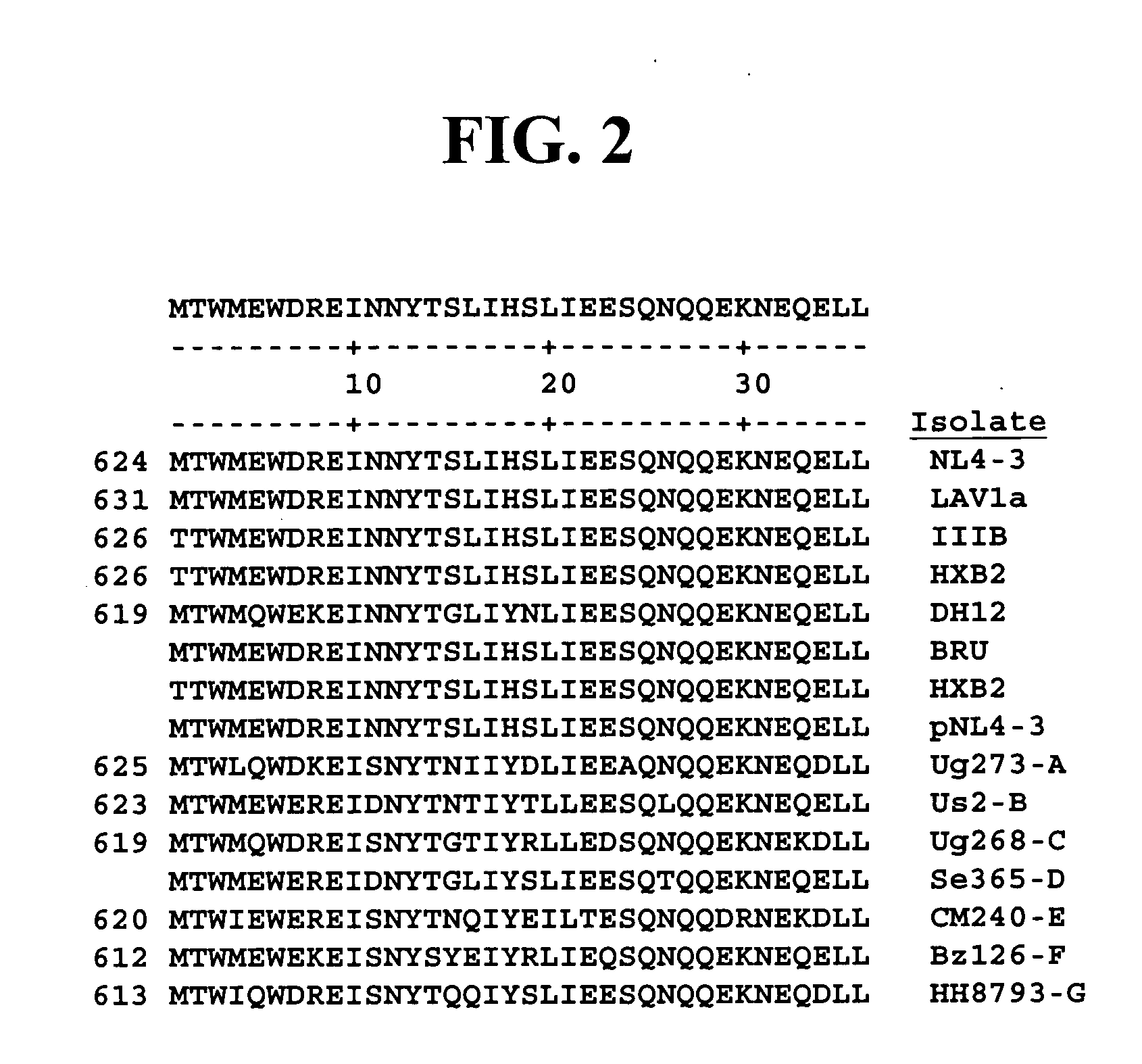
HIV gp41 HR2-derived synthetic peptides, and their use in therapy to inhibit transmission of human immunodeficiency virus
InactiveUS20060247416A1Improve biological activityStrong antiviral activityPolypeptide with localisation/targeting motifSugar derivativesImmunodeficiency virusAmino acid substitution
Provided are synthetic peptides based on a native sequence of HIV gp41 HR2 except that the synthetic peptides have a plurality of amino acid replacements comprising (a) a helix-promoting amino acid, or (b) a combination of helix-promoting amino acids, and charged amino acids introduced to form ion pairs in the synthetic peptide; wherein the synthetic peptides demonstrate an unexpected, improved biological activity, as compared to a peptide having an amino acid sequence without the plurality of amino acid substitutions. Also provided are polynucleotides encoding synthetic peptide, and methods of using these synthetic peptides in inhibition of, or as compositions to inhibit, transmission of HIV to a target cell.
Owner:TRIMERIS
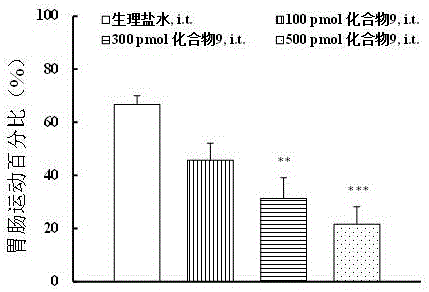
Multi-target peptide molecules of opium and neuropeptide FF receptors, and preparation and application thereof
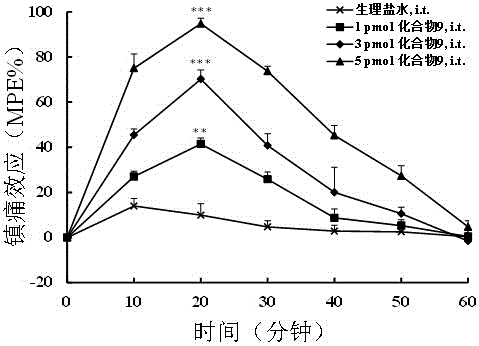
ActiveCN106084001AStrong central analgesic activityNo significant regulationNervous disorderPeptide/protein ingredientsAnalgesics drugsSide effect
The invention discloses multi-target polypeptides of opium and neuropeptide FF receptors; amino acid substitution is performed based on an opium peptide Biphalin and an NPFF chimeric peptide BN-9, and a series of multi-target polypeptides which can activate various receptors of an opium and NPFF system at the same time are obtained. By in-vitro cAMP function identification and identification of body analgesic activity and central side effects and other pharmacological activities, the multi-target polypeptides are indicated to activate the opium and NPFF receptors at the same time, have high-efficiency analgesic activity and cannot generate the analgesic tolerance phenomenon, and moreover, have the advantages of showing low side effects on body temperature, gastrointestinal motility, cardiovascular activity and the like. Therefore, the multi-target polypeptides have high application value in preparation of clinical analgesic drugs.
Owner:SHANGHAI TIANCI LIFE SCI DEV CO LTD
Superluminescent luciferase variant with prolonged bioluminescence
InactiveUS20120034672A1High activityHigh luminous intensityFungiSugar derivativesOptical propertyAmino acid replacement
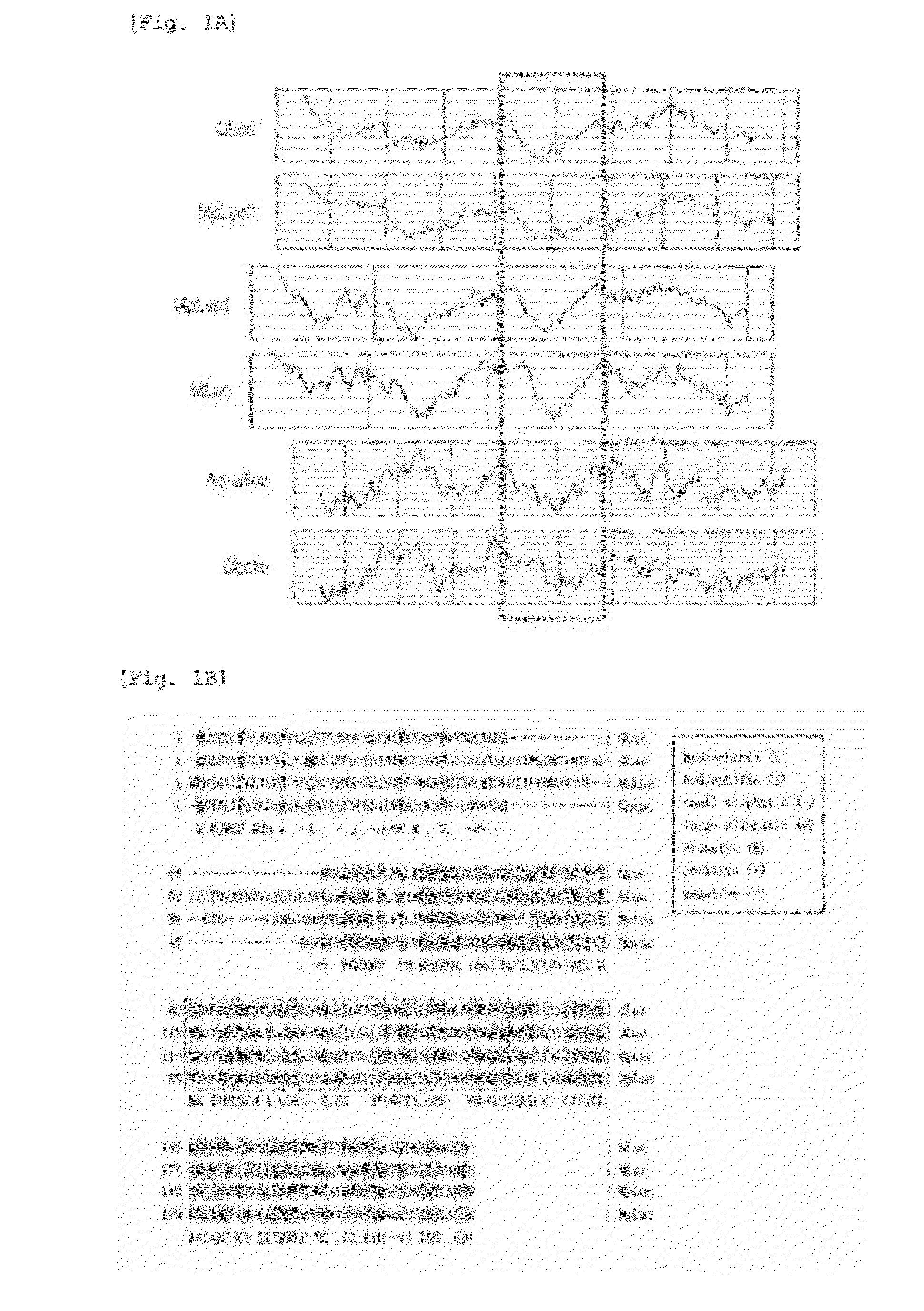
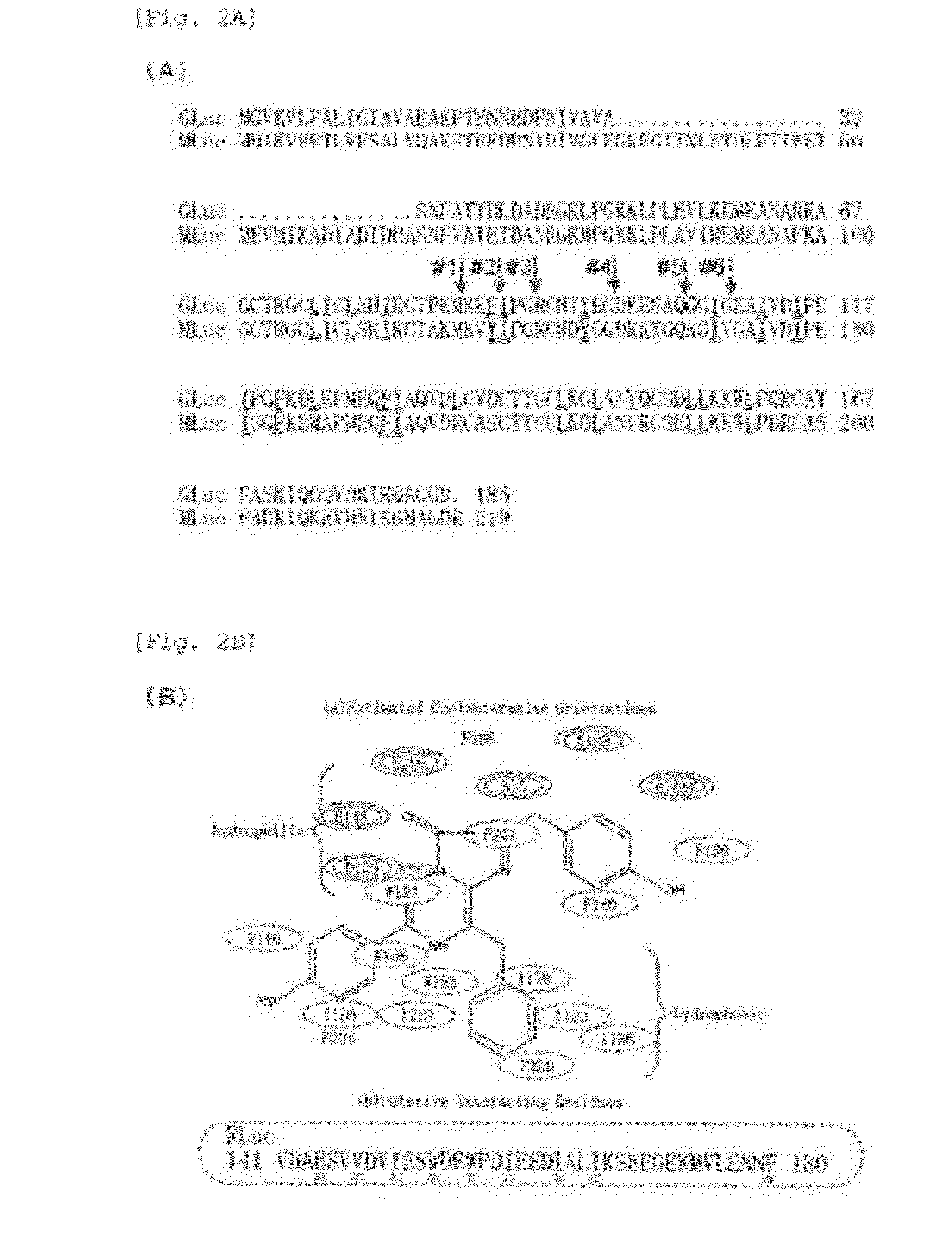
This invention provides a genetically modified marine luciferase such as Gaussia luciferase, which has high bioluminescence intensity, and has high bioluminescence stability and / or red-shifted wavelength. Specifically disclosed is a luciferase variant with improved optical property obtained by replacing at least one amino acid residue among the amino acid sequence of a marine luciferase at positions corresponding to positions 89 to 118 in the amino acid sequence of Gaussia luciferase (GLuc), wherein an amino acid residue at a position corresponding to at least one selected from positions 89, 90, 95, 97, 100, 108, 112, 115, and 118 in the amino acid sequence of GLuc is replaced by way of conservative amino acid replacement. The above-mentioned replacement in a marine luciferase improves enzymatic activity of the luciferase. Also disclosed is a bioluminescent probe having an improved optical property, which is produced using the luciferase variant of the present invention.
Owner:NAT INST OF ADVANCED IND SCI & TECH
NY-ESO-1 tumour antigen mimic epitope and use thereof
InactiveCN101381402AImproving immunogenicityStrong specificityPeptidesAntibody medical ingredientsCtl epitopePredictive methods
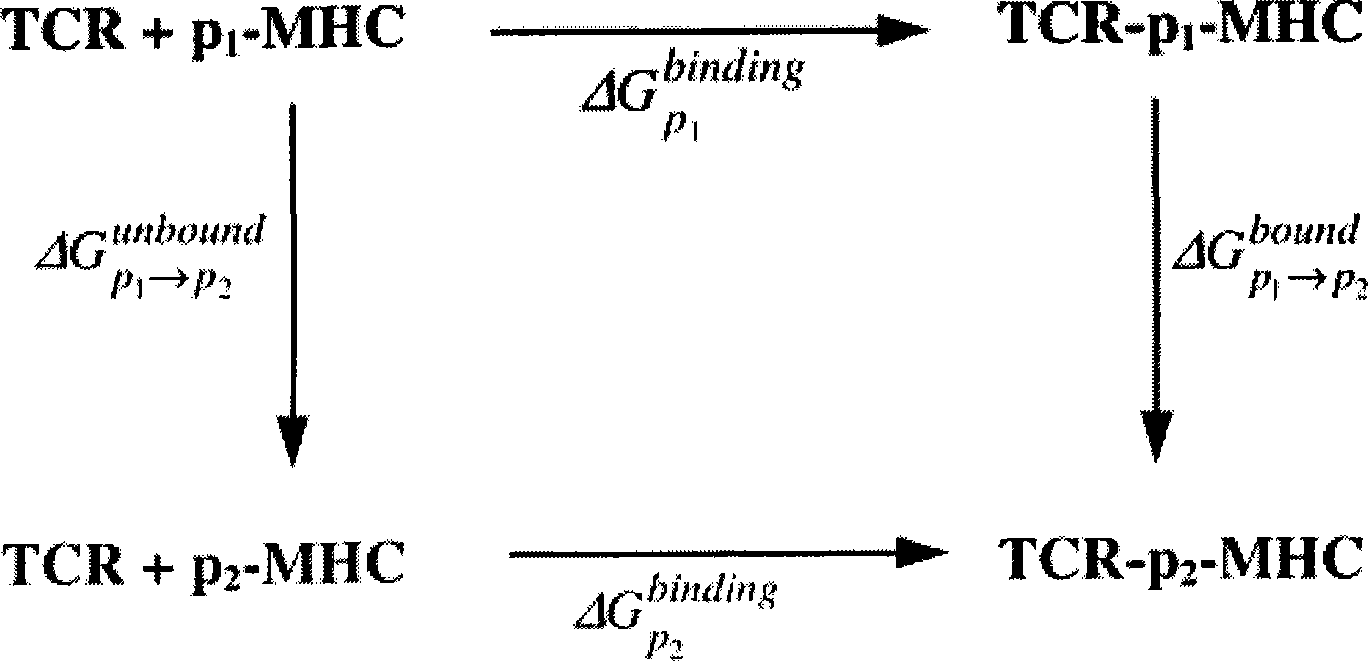
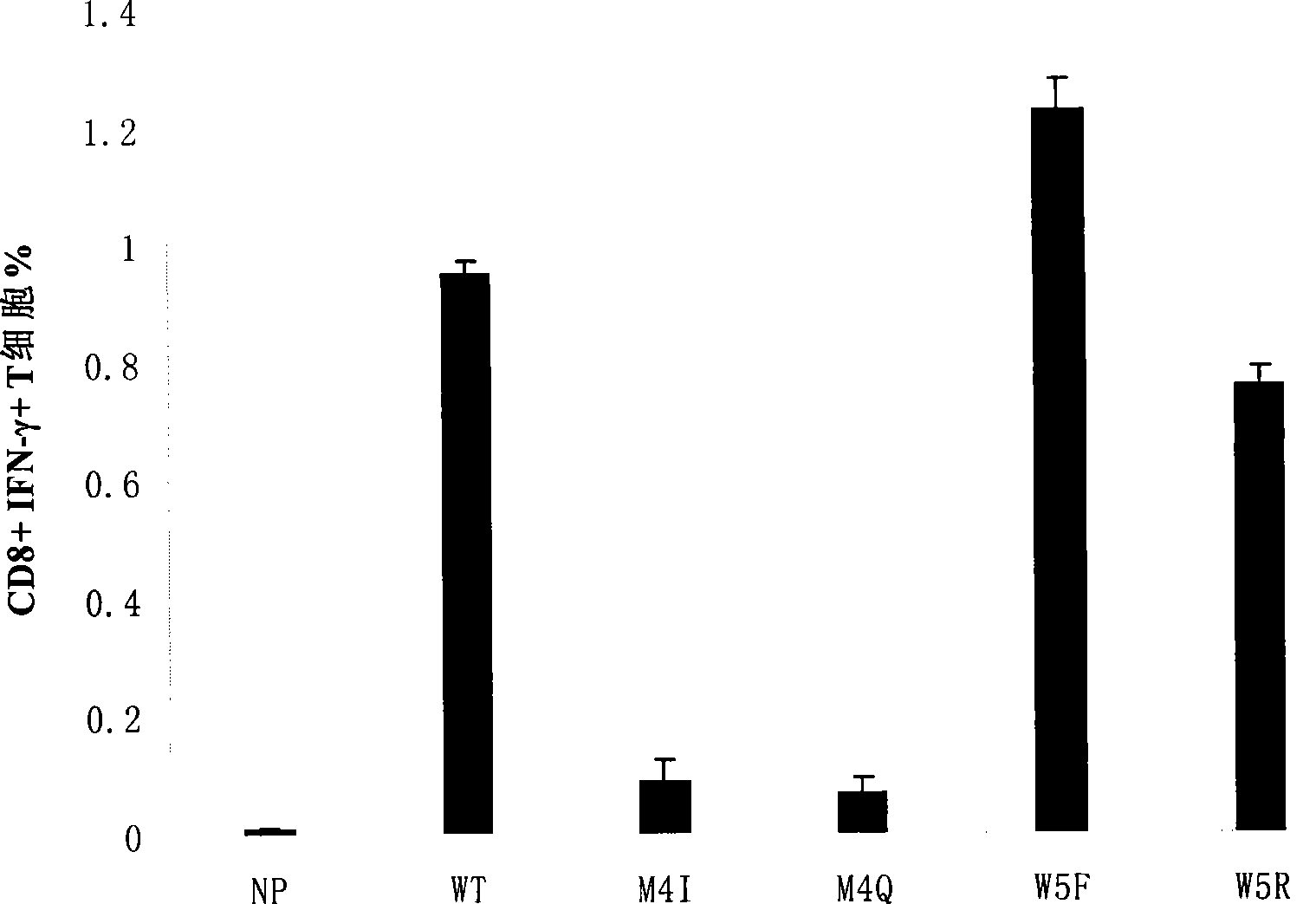
The invention discloses epitope for an NY-ESO-1 tumor antigen. The amino acid sequence of the mimic epitope is Ser-Leu-Leu- Met-Phe-Ile-Thr-Trp-Cys, namely SLLMFITWC; the mimic epitope can raise a CTL immunity response, can undergo a cross reaction with a natural epitope, has the characteristics of strong immunogenicity, immune tolerance breaking, strong specificity, safety, low cost, and easy synthesis and storage, and can be used to prepare tumor-therapeutic polypeptide vaccine. The invention also discloses a method for predicting the mimic epitope, which comprises steps of the establishment of a structural model of a TCR-pMHC complex, the analysis of TCR binding sites of the epitope, and the analysis of amino acid replacement of the TCR binding sites of the epitope. The method is also applicable to the computer-aided modification of CTL epitopes of other antigens, and can provide a useful tool for the design and research of therapeutic polypeptide vaccine.
Owner:ARMY MEDICAL UNIV
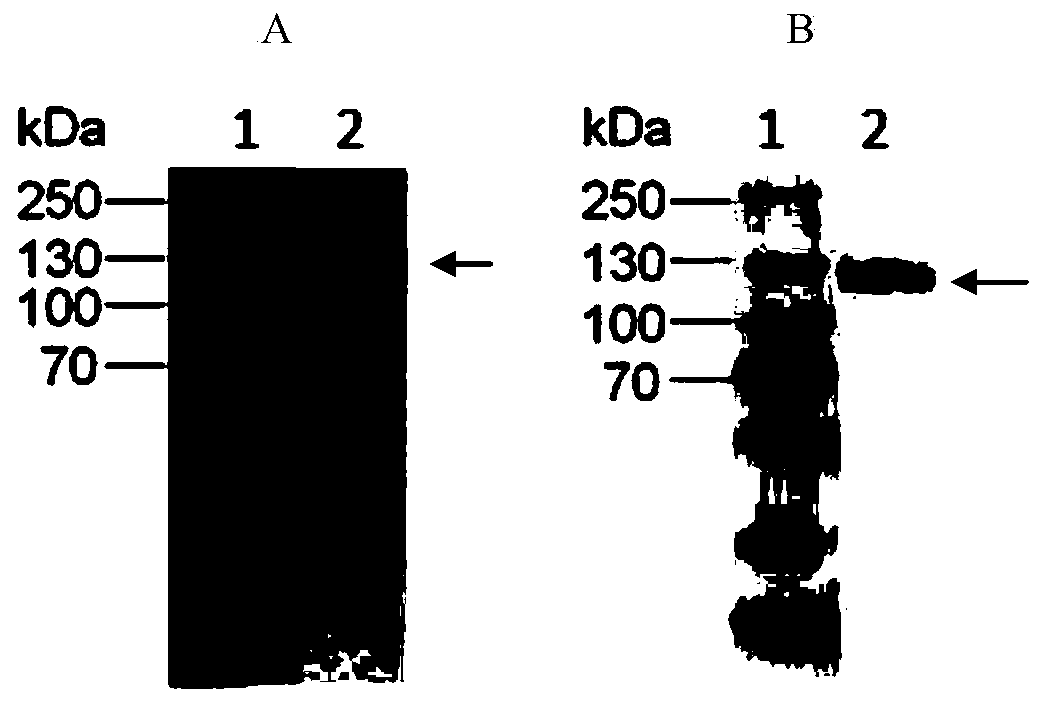
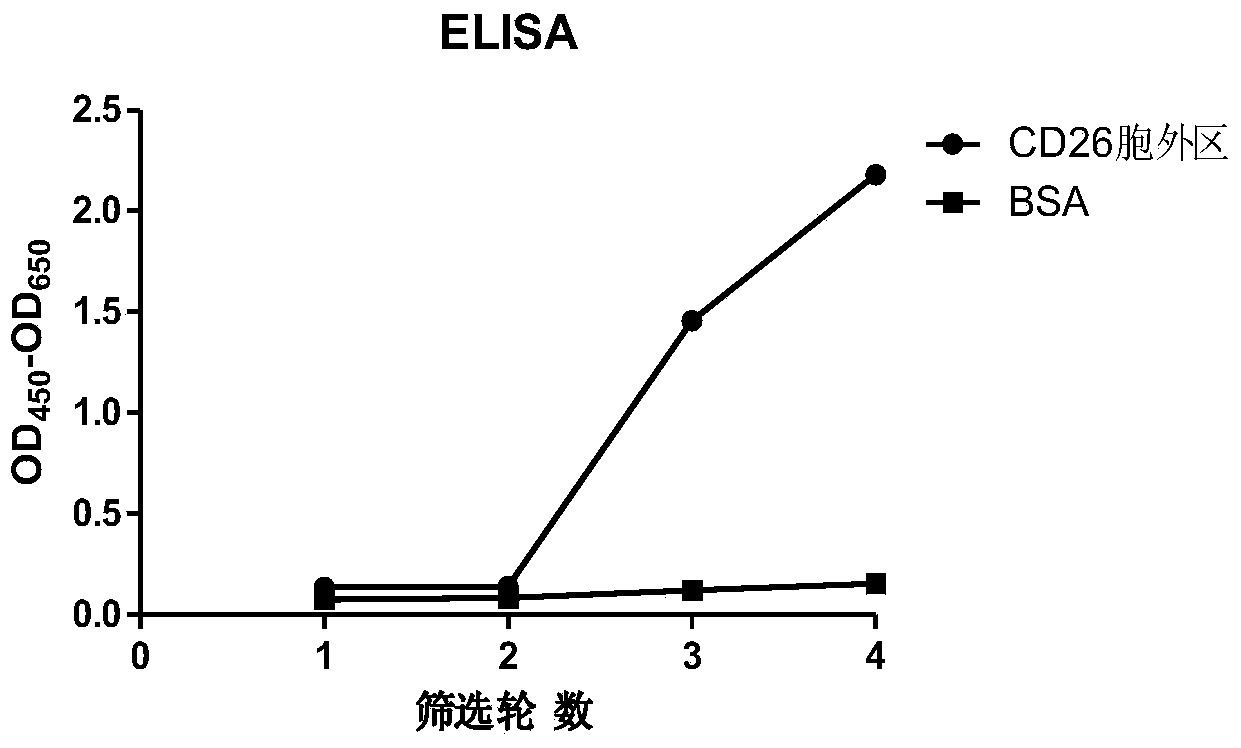
Humanized anti-CD26 antibody and application thereof
ActiveCN103724431APrevent proliferationPrevent invasionFungiBacteriaSingle-Chain AntibodiesComplementarity determining region
The invention provides a novel high-affinity completely humanized antibody which can specifically bind with CD26 as well as a preparation method and application thereof and belongs to the technical field of genetic engineering antibodies. The CD26 is a ubiquitous multifunctional II type transmembrane glycoprotein, has various biological functions and can be interacted with various proteins such as ADA, CD45, FAP-alpha and the like. The invention provides the humanized antibody or a fragment thereof, wherein the antibody or the fragment thereof is capable of specifically binding with the human CD26, preferably the CD26 extracellular domain; the amino acid sequence of the antibody or the fragment thereof comprises an amino acid sequence containing a monoclonal antibody or a fragment thereof or a conjugate of the fragment in any one of 6 complementary determining regions in one of SED ID NO: 2, SED ID NO: 3, SED ID NO: 4, SED ID NO: 6, SED ID NO: 7, and SED ID NO: 8, or an amino acid sequence obtained through amino acid replacement or modification. The obtained anti-CD26 single-chain antibody provided by the invention can highly specifically bind with the CD26 and is simultaneously capable of obviously inhibiting the proliferation, the invasion and the metastasis of tumor cells.
Owner:ZONHON BIOPHARMA INST
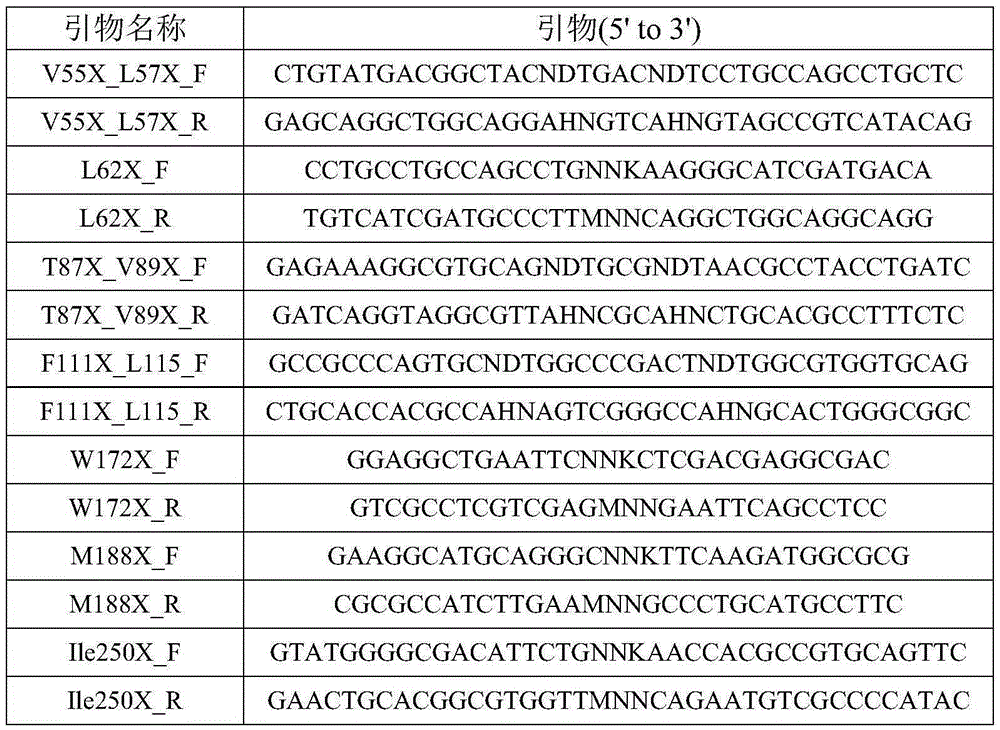
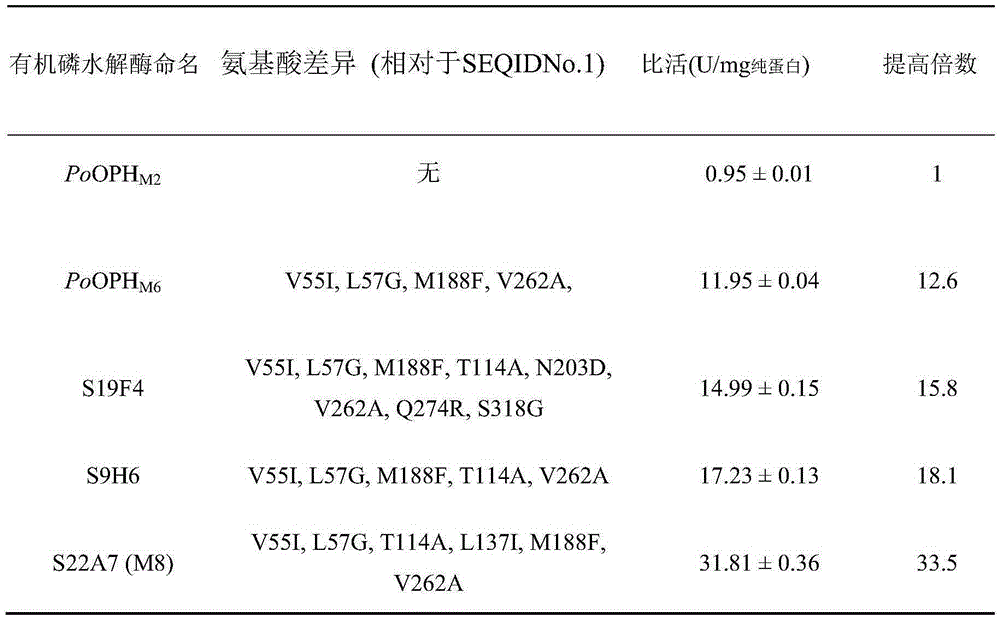
Engineering organophosphorus hydrolase, nucleic acid, mutant and application of engineering organophosphorus hydrolase and mutant
ActiveCN105255846AImprove activity stabilityImprove thermal stabilityHydrolasesContaminated soil reclamationHalf-lifeMalathion
The invention relates to engineering organophosphorus hydrolase, nucleic acid, a mutant and an application of the engineering organophosphorus hydrolase and the mutant and belongs to the technical field of bioengineering. The engineering organophosphorus hydrolase is an organophosphorus hydrolase mutant constituted by new amino acid sequences formed after amino acid substitution of one or more of amino acid residues on the 55th site, the 57th site, the 83th site, the 114th site, the 137th site, the 188th site or the 262th site of an amino acid sequence shown as SEQ ID No.1, and the activity of the engineering organophosphorus hydrolase is improved. Compared with the prior art, the organophosphorus hydrolase mutant has the advantages that the malathion catalytic activity is improved by 36.7 times, the mutant keeps excellent thermal stability of a female parent, and the half-life period at 50 DEG C reaches 65 h. The organophosphorus hydrolase mutant can efficiently degrade common organophosphorus pesticides such as malathion and the like, has excellent thermal stability and has very good application prospect in the field of degradation of the organophosphorus pesticides.
Owner:EAST CHINA UNIV OF SCI & TECH
Anti-CD26 antibody and application thereof
ActiveCN103641917AGrowth inhibitionPrevent proliferationFungiBacteriaSingle-Chain AntibodiesComplementarity determining region
The invention provides a novel full-humanized antibody which is high in affinity and can be specifically combined with CD26, and a preparation method and application thereof, and belongs to the technical field of a genetically engineered antibody. CD26 is a ubiquitous multifunctional II-type transmembrane glycoprotein, has a plurality of biological functions, and can interact with a plurality of proteins, such as ADA, CD45, FAP-alpha and the like. The invention provides an antibody from a human source or a segment thereof. The antibody or the segment thereof is specifically combined with human CD26, and preferably specifically combined with a CD26 extracellular region; an amino acid sequence of the antibody or the segment thereof comprises a monoclonal antibody which is selected from any region of six complementary determining regions containing a group of sequences of SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:6, SEQ ID NO:7 and SEQ ID NO:8, or a segment thereof or a conjugate of the segment thereof, or an amino acid sequence which is obtained by amino acid replacement or modification. The anti-CD26 single-chain antibody obtained by the method is highly specifically combined with the CD26, and meanwhile, multiplication of tumor cells can also be obviously inhibited.
Owner:ZONHON BIOPHARMA INST
Engineered Cas12i nuclease as well as effect protein and application thereof
ActiveCN113151215AHigh activityExcellent gene editing efficiencyHydrolasesMicrobiological testing/measurementSimple aromatic ringSingle strand
The invention provides an engineered Cas12i nuclease, which comprises one, two, three or four of following mutations based on reference Cas12i nuclease: (1) replacing one or more amino acids which interact with PAM in the reference Cas12i nuclease with positively charged amino acids; and / or (2) replacing one or more amino acids which participat in opening DNA double chains in the reference Cas12i nuclease with amino acids with aromatic rings; and / or (3) replacing one or more amino acids which are located in the RuvC structural domain and interact with the single-stranded DNA substrate in the reference Cas12i nuclease with positively charged amino acids; and / or (4) replacing one or more amino acids which interact with the DNA-RNA double helix in the reference Cas12i nuclease with positively charged amino acids. Particularly, the reference Cas12i nuclease is natural Cas12i nuclease, such as natural Cas12i2 nuclease, and the amino acid sequence of the reference Cas12i nuclease is as defined in SEQ ID NO. 1.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI +1
DNA polymerase, nucleic acid test method and nucleic acid test kit
ActiveCN108588050ALow costShort detection timeMicrobiological testing/measurementTransferasesEscherichia coliPolymerase L
The invention provides a DNA polymerase with DNA or RNA as a template. The DNA polymerase is obtained through amino acid replacement, including G198W, V222I, E306K, Q354E, A381E and E582K, of klenow fragments of escherichia coli polymerases I. The invention also provides a primer with a stem loop structure and used for constant-temperature nucleic acid amplification. The invention further providesa nucleic acid test technique and a test kit both based on combination between rapid constant-temperature nucleic acid amplification and a Cas test system. The DNA polymerase, the nucleic acid test technique and the test kit can be used for testing nucleic acid targets in test samples at a constant temperature, have the advantages of low cost, short test time, simplicity and convenience in operation, high specificity, high sensitivity and the like, and are particularly applied to POCT (point-of-care testing).
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Soluble TNF acceptor mutant
ActiveCN101085813ASuppression of normal immune functionLow affinityPeptide/protein ingredientsTumor necrosis factorDiseaseSide effect
The invention discloses a soluble TNF acceptor mutant, relating to tumor necrosis factor TNF acceptor derivant and its medical application. The amnio acid at 89th position of mutant is replaced and the ability of neutralizing cell toxicity of TNF alpha is retained, while ability of neutralizing LT is reduced by more than 10 times. The mutant can be used to treat over expressing disease of TNF alpha, because the affinity to lymphotoxin is reduced, the side effect caused by lymphotoxin neutralisation can be reduced, and the inhibitive effect to normal immunity function from medicine is aslo reduced.
Owner:上海复旦张江生物医药股份有限公司
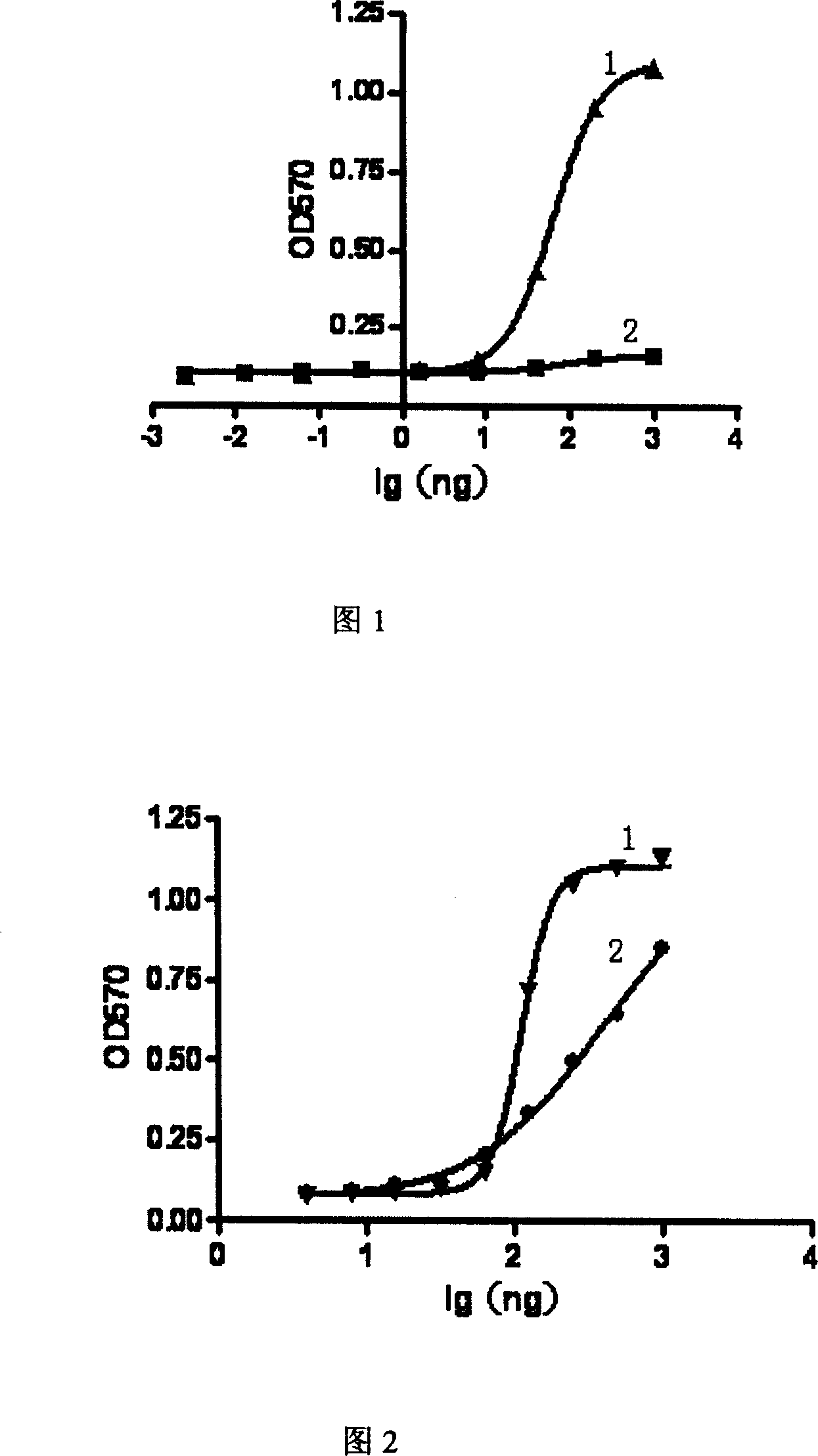
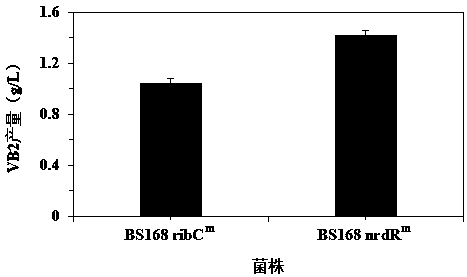
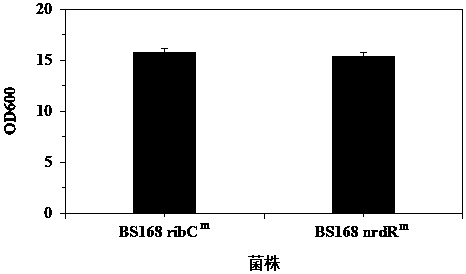
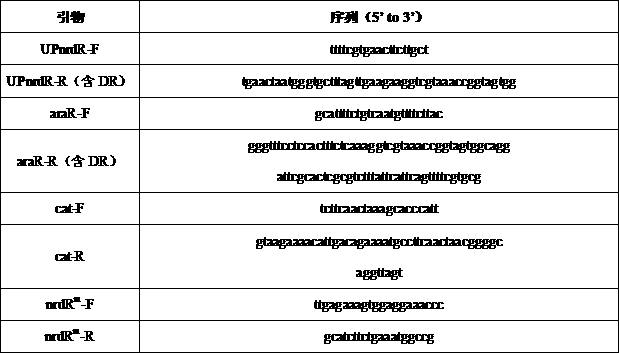
Ribonucleoside reductase transcription inhibition factor mutant, mutation gene and application of ribonucleoside reductase transcription inhibition factor mutant and mutation gene to preparation of vitamin B2
The invention discloses a ribonucleoside reductase transcription inhibition factor mutant, a mutation gene and an application of the ribonucleoside reductase transcription inhibition factor mutant andthe mutation gene to preparation of vitamin B2. The amino acid sequence of the mutant is subjected to the following mutation relative to the sequence as shown in SEQID No.3; and an amino acid at the26th site is replaced with H. For the nucleotide fixed point mutation that the amino acid at the 26th site, coded in the ribonucleoside reductase transcription inhibition factor gene on a bacillus subtilis chromosome is replaced with H, a genetic engineering bacterium is obtained, and the capacity for producing the vitamin B2 is greatly improved. The ribonucleoside reductase transcription inhibition factor mutant has great application and extension value.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
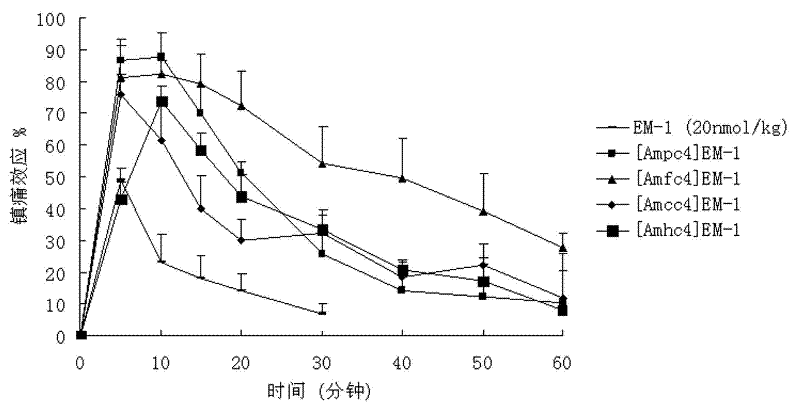
Non-natural amino acid modified endomorphin-1 analogue as well as synthesis method and application thereof
InactiveCN104877005AHigh affinityHigh enzymatic stabilityNervous disorderTetrapeptide ingredientsEnzymatic hydrolysisSynthesis methods
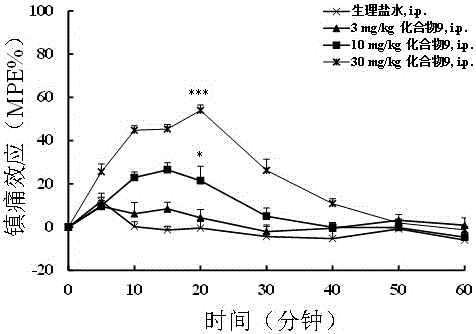
The invention discloses a non-natural amino acid modified endomorphin-1 analogue. A synthesis method comprises the following step: replacing amino acid phenylalanine at a fourth site from a terminal N to a terminal C of parent endomorphin-1 by respectively using 2-thienyl substituted alpha-alkenyl-beta-amino acid and 3-thienyl substituted alpha-alkenyl-beta-amino acid. The affinity and enzymatic hydrolysis stability of a mu opioid receptor of the non-natural amino acid modified endomorphin-1 analogue can be effectively improved, and thus the in-vivo analgesic effect of the non-natural amino acid modified endomorphin-1 analogue can be further improved and prolonged; and the non-natural amino acid modified endomorphin-1 analogue is subjected to pharmacological activity identification by virtue of radioligand receptor binding experiments, in-vitro organ biological assays and in-vitro enzymatic hydrolysis stability and warm bath tail-flick analgesic experiments, and results show that compared with parent endomorphin-1, the synthesized non-natural amino acid modified endomorphin-1 analogue disclosed by the invention has higher affinity, higher enzymatic hydrolysis stability and higher analgesic activity, and has potential application values of being taken as clinical polypeptide analgesic medicines.
Owner:LANZHOU UNIVERSITY
Glucagon analogs exhibiting enhanced solubility and stability physiological pH buffers
Modified glucagon peptides are disclosed having improved solubility and / or half-life while retaining glucagon agonist activity. The glycogen peptides have been modified by substitution of native amino acids with, and / or addition of, charged amino acids to the carboxy terminus of the peptide. The modified glucagon agonists can be further modified by pegylation, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 20, SEQ ID NO: 21, SEQ ID NO: 23, or both to further enhance the solubility of the glucagon agonist analogs.
Owner:INDIANA UNIV RES & TECH CORP
Method and system for predicting amino acid sequences compatible with a specified three dimensional structure
A method for predicting an amino acid sequence compatible with a three-dimensional (3D) structure of a protein. A reduced virtual representation of the 3D structure is constructed, and, for each position along the representation, its solvent accessibility is determined. For each position along the structure, an amino acid residue is randomly selected from a predefined group of amino acids having a solvent accessibility compatible with the solvent accessibility of the position. A Monte-Carlo simulation is performed on this devised protein in which an amino acid at a particular position is sequentially replaced with other amino acids having the same solvent accessibility, and an energy score is calculated for each rotamer. The lowest scoring rotamer for this position is then selected The Monte-Carlo simulation is repeated for each position along the sequence, to obtain an amino acid sequence with the lowest total energy score.
Owner:RAMOT AT TEL AVIV UNIV LTD
Less immunogenic binding molecules
InactiveCN1950399AAntibacterial agentsHybrid immunoglobulinsAmino acid substitutionAmino acid replacement
The present invention provides a bispecific binding molecule, wherein said molecule comprises or consists of at least two domains whereby one of said at least two domains specifically binds to / interacts with the human CD3 complex and said domain comprises an amino acid sequence of an antibody derived light chain, wherein said amino acid sequence is a particularly identified amino acid sequence comprising specific amino acid substitutions, and a second domain is or contains at least one further antigen-interaction-site and / or at least one further effector domain. The invention further provides nucleic acid molecules encoding the bispecific binding molecules of the invention, vectors comprising said nucleic acid molecules and host cells transformed or transfected with said vectors. Moreover, the invention concerns a method for the production of bispecific binding molecules of the invention and compositions comprising the bispecific binding molecules of the invention, the nucleic acid molecules of the invention or the host cells of the invention.
Owner:MICROMET AG
Multi-site combination modified endomorphin analogue, synthesis and application thereof
ActiveCN106967151AImprove pharmacological activityHigh medicinal valueNervous disorderTetrapeptide ingredientsMulti siteAnalgesics drugs
Belonging to the field of biomedicine, the invention provides an endomorphin analogue with the first position, the second position and fourth position respectively modified by tyrosine or 2, 6-dimethyltyrosine, N-methyl-D alanine and alpha-alkenyl-beta-amino acid and a synthesis method thereof. The endomorphin analogue is obtained by substituting the first position amino acid of endomorphin (EM1 and EM2) with tyrosine or 2, 6-dimethyltyrosine, substituting the second position amino acid with N-methyl-D alanine, and substituting the fourth position amino acid respectively with phenyl or 2-furyl replaced alpha-alkenyl-beta-amino acid, and is named as MEL-N16 series. Radioligand receptor binding experiment, isolated organ bioassay, in-vitro enzymolysis stability and warm bath tail flick analgesic experiment results show that the endomorphin analogue synthesized by the method provided by the invention has higher affinity than an endomorphine matrix, high enzymolysis stability and high analgesic activity, and has very good clinical application value in preparation of analgesic drugs.
Owner:LANZHOU UNIVERSITY
3alpha-hydroxysteroid dehydrogenase mutant, coding nucleotide sequence and kit
ActiveCN109082419AHigh catalytic activityImprove detection efficiencyMicrobiological testing/measurementBiological material analysisCholic acidNucleotide
The invention provides a 3alpha-hydroxysteroid dehydrogenase mutant, nucleotide sequence for coding the 3alpha-hydroxysteroid dehydrogenase mutant and a kit. The 3alpha-HSD mutant is obtained by subjecting amino acid sequence as shown in SEQ ID No. 2 to point mutation, wherein the point mutation is the amino acid substitution of at least one locus selected from the 25th-37th sites, the 100th-120thsites and the 145th-166th sites. By the point mutation, 3alpha-hydroxysteroid dehydrogenase mutant protease with high catalytic activity is obtained, enzyme catalytic activity can be evidently increased by more than 60% as compared with 3alpha-HSD, and the enzyme catalytic activity can reach up to twice of the original enzyme catalytic activity; by using the mutant protease as the tool enzyme, the total bile acid content and enzymatic method detection efficiency of the kit are improved greatly, production cost is lowered, and the market competiveness of corresponding products is increased.
Owner:SHENZHEN AMTECH BIOENGINEERING LTD INC
Endomorphin-1 analogue modified by alpha-alkenyl-beta-amino acid and composition and application thereof
ActiveCN102558297AEnhance receptor affinityImprove abilitiesNervous disorderOrganic compound preparationFuranAssay
The invention provides endomorphin-1 modified by unnatural alpha-alkenyl-beta-amino acid, which is characterized in that fourth amino acid of the endomorphin-1 is respectively formed by replacing the alpha-alkenyl-beta-amino acid replaced by phenyl, 2-furan, 3-chlorphenyl, 1, 3-dioxophenyl. Pharmacological activity identity is conducted on the endomorphin-1 modified by the unnatural alpha-alkenyl-beta-amino acid by radiation ligand receptor combination experiments, isolated organ biological assays, cAMP accumulation experiments, separation enzymolysis stability and analgesic tests with water baths. As the results show, the novel analogue has the advantages of high affinity, high enzymolysis stability and high analgesic activity compared with endomorphin-1, thereby having high application value in preparation of polypeptide analgesic medicine.
Owner:LANZHOU UNIVERSITY
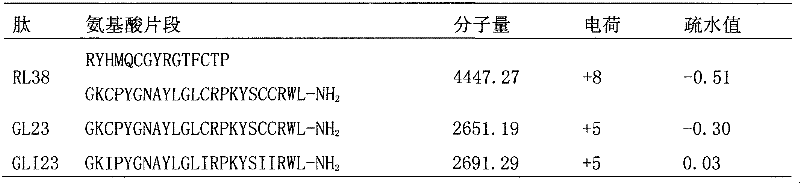
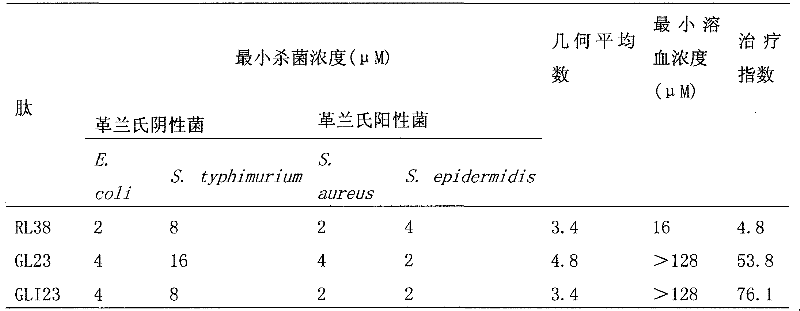
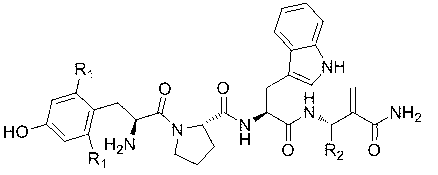
Antibacterial peptide GLI23 derived from linear chicken beta-phylaxin4 (RL38) and preparation method thereof
InactiveCN102382186ALow hemolytic activityHigh selectivityDepsipeptidesAmino acid replacementMammalian cell
The invention provides an antibacterial peptide GLI23 derived from linear chicken beta-phylaxin4 (RL38) and a preparation method thereof. According to the invention, methods of fixed-point amino acid segment interception and amino acid replacement are adopted so as to simplify the linear chicken beta-phylaxin4 and obtain two polypeptides; the antibacterial and the hemolytic activities the polypeptides are measured; and the therapeutic index of the polypeptides are calculated so as to evaluate the selectivity of the polypeptides to cells. Found by researches, the intercepted peptide GLI23 and the peptide GLI23 after being subject to amino acid replacement have obvious bactericidal activity and obviously-decreased hemolytic activity compared with the chicken beta-phylaxin4, especially the GLI23 of which the therapeutic index is as high as 76.1. The method provided by the invention has the advantages that: under the circumstance that the bactericidal activity of the antibacterial peptideis not decreased, the hemolytic activity of the antibacterial peptide is decreased; the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is improved; and the development potential of substituting for antibiotic is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Synthesis and application of multi-site modified endomorphin-1 analogue
ActiveCN103214552AHigh affinityHigh enzymatic stabilityAntipyreticAnalgesicsAnalgesics drugsMethyltyrosines
The invention provides synthesis and application of multi-site modified endomorphin-1 analogues, belonging to a biological medicine field. The endomorphin-1 analogues are prepared by substituting a first amino acid of endomorphin-1 through tyrosine or 2,6-dimethyl tyrosine, substituting a second amino acid through proline or (S / R) beta-proline, and substituting a forth amino acid through phenyl, and 2-furyl substituted alpha-alkenyl-beta-amino acid respectively. By a radioligand receptor binding experiment, in vitro organ biological detection, a cAMP accumulation experiment, and an in vitro enzymolysis stability and warm water bath tail flick analgesic experiments, the multi-site modified endomorphin-1 analogues in the invention are performed pharmacological activity identification. A result shows that: compared with endomorphin-1, the novel multi-site modified endomorphin-1 analogues has advantages of high affinity, high enzymolysis stability and high analgesic activity, and has a high application value for designing and synthesizing polypeptide analgesic drugs.
Owner:LANZHOU UNIVERSITY
Bst DNA polymerase recombinant mutant, coding DNA thereof and ultrafast magnetic bead LAMP detection method
ActiveCN113583996AHigh sensitivityImprove accuracyMicrobiological testing/measurementTransferasesMagnetic beadWild type
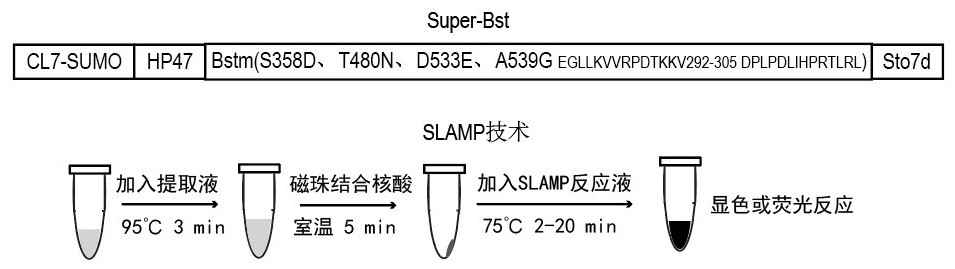
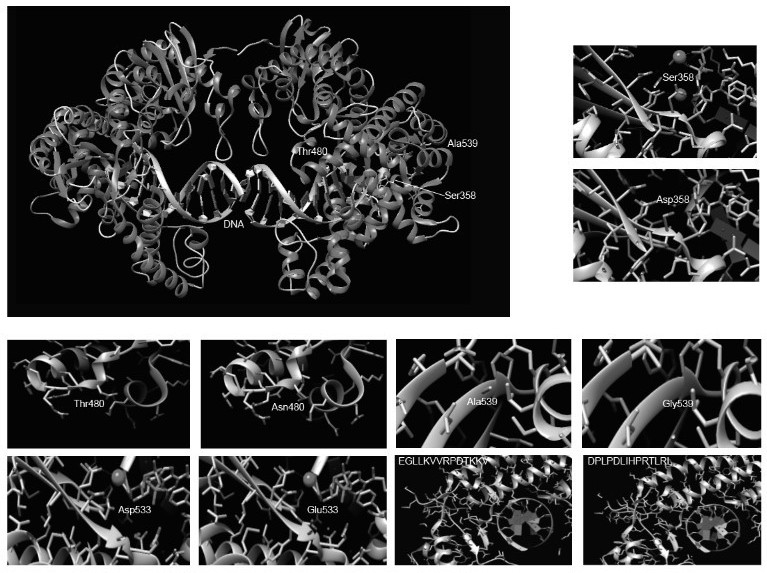
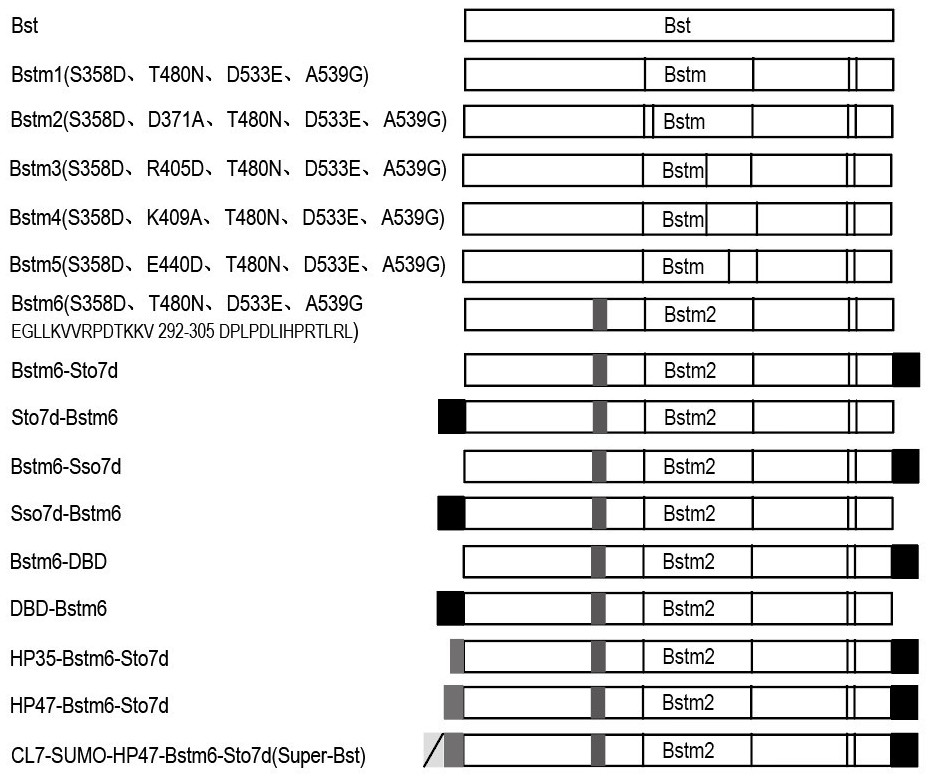
According to the invention, mutation of several sites of Ser358Asp, Thr480Asn, Asp533Glu and Ala539Gly is carried out on a wild type Bst DNA polymerase sequence, then 292-305 amino acid EGLLKVVRPDTKKV of the Bst DNA polymerase subjected to point mutation is replaced with DPLPDLIHPRTLRL, a DNA binding protein is fused at the C end of the mutated Bst DNA polymerase sequence, an HP47 polypeptide sequence (SEQ ID No.17) is fused at the N end of the mutated Bst DNA polymerase sequence, a CL7-SUMO-Tag is fused in front of the HP47 polypeptide sequence, and the recombinant mutant Super-Bst (SEQ ID No.16) of the Bst DNA polymerase with high activity and thermal stability is obtained. The thermal stability, specificity, strand displacement capacity, extension capacity and reverse transcriptase activity of Super-Bst are remarkably improved, and Super-Bst can tolerate high salt and various inhibitors and can be massively obtained through prokaryotic expression and affinity purification. The invention also discloses coding DNA of the gene and an ultrafast magnetic bead LAMP detection method.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
Mutated L-amino acid ligase and process for preparing L-glutamic acid-L-trp-trp by adopting enzyme catalysis method
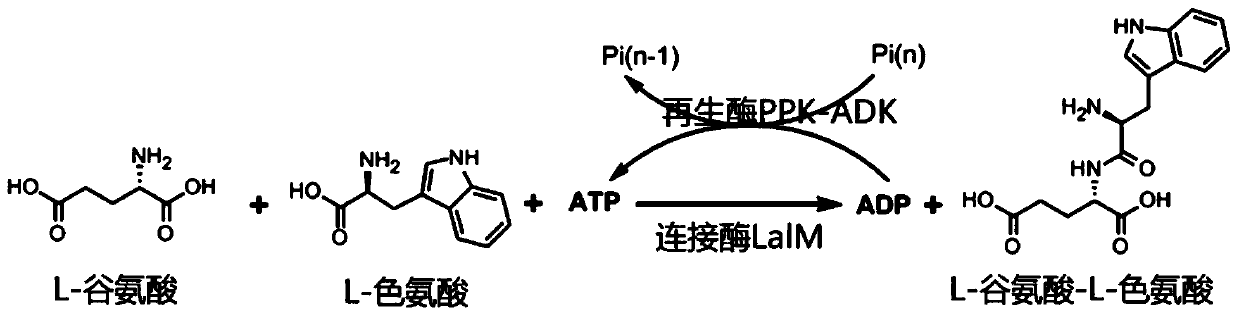
ActiveCN110777123AAchieve connectionHigh yieldMicroorganism based processesPeptidesDipeptideBinding site
The invention relates to the technical field of biochemistry, and discloses mutated L-amino acid ligase and a process for preparing L-glutamic acid-L-trp-trp by adopting an enzyme catalysis method. Based on wild type L-amino acid ligase, the mutated L-amino acid ligase has site mutation of H276G / S, W332K / N / Q / S, M334D / S, L12A / S, Y75S / G and W76L / V. According to the mutated L-amino acid ligase and the process for preparing the L-glutamic acid-L-trp-trp by adopting the enzyme catalysis method disclosed by the invention, based on a known-reported research on non-specific L-amino acid ligase, systematical amino acid replacement on binding sites of the activity of a substrate of the mutated L-amino acid ligase is carried out, and finally, quick connection of L-glutamic acid and L-tryptophan is realkzed; and meanwhile, through combination of cyclic regeneration of coenzyme ATP and catalysis of immobilized enzyme, on one hand, the production cost is reduced; and on the other hand, the quality of trp-trp products and the stability of production of the trp-trp products are improved preferably, and therefore, an application requirement is met.
Owner:SHENZHEN READLINE BIOTECH CO LTD
Polypeptide and application thereof to preparation of medicament for treatment and/or prevention of tumor
ActiveCN107474115ASignificant effectSmall toxicityPeptide/protein ingredientsPeptidesSide chainMedicine
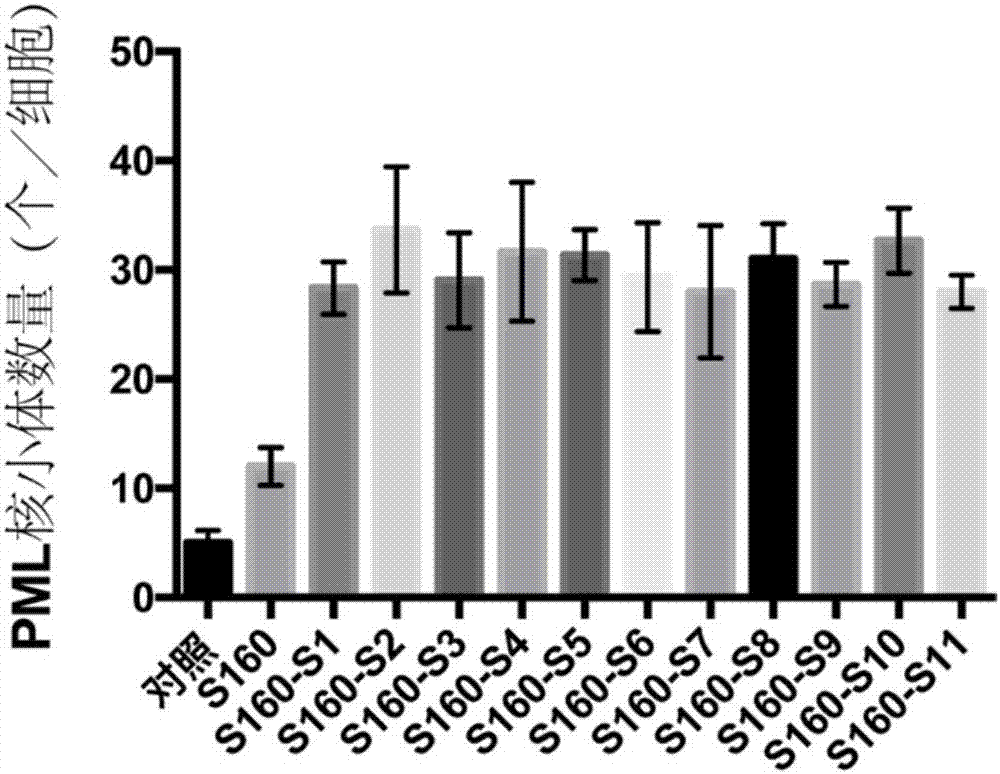
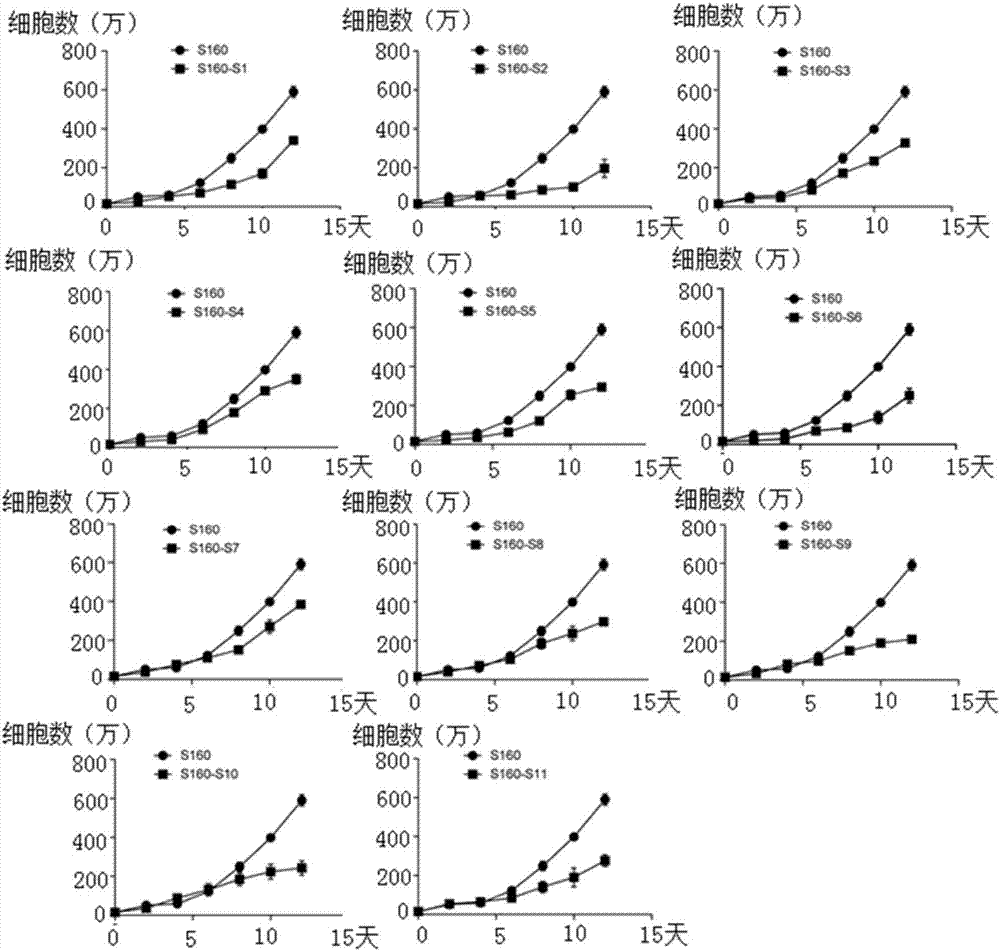
The invention provides a polypeptide and an application thereof to preparation of medicament for treatment and / or prevention of tumor. An amino acid sequence of the polypeptide is shown in a sequence table SEQ ID No.1, or shown in the sequence table SEQ ID No.1 amino acid sequence with two or more amino acids substituted by unnatural amino acids with connectable side chains, and a derivative comprises a chimeric peptide formed by connection of the polypeptide and a cell-penetrating peptide, a fusogenic peptide formed by the polypeptide and a virus, a methylated polypeptide, a glycosylated polypeptide, and a pegylated polypeptide. The polypeptide or the polypeptide derivative can targetedly increase number of PML nucleosomes, and can be applied to preparation of medicament for treatment and / or prevention of tumor.
Owner:胡卓伟
Enzyme and application thereof
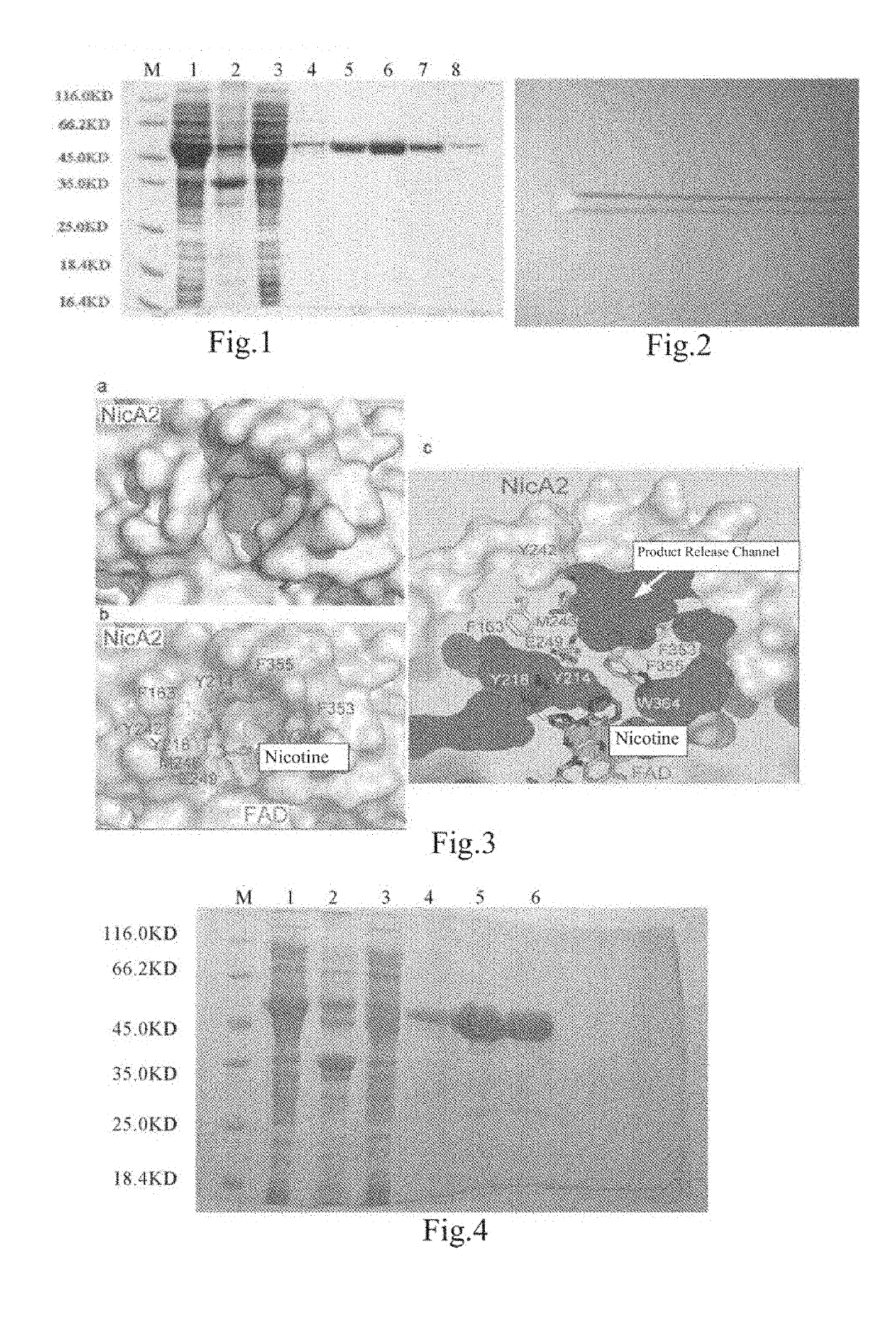
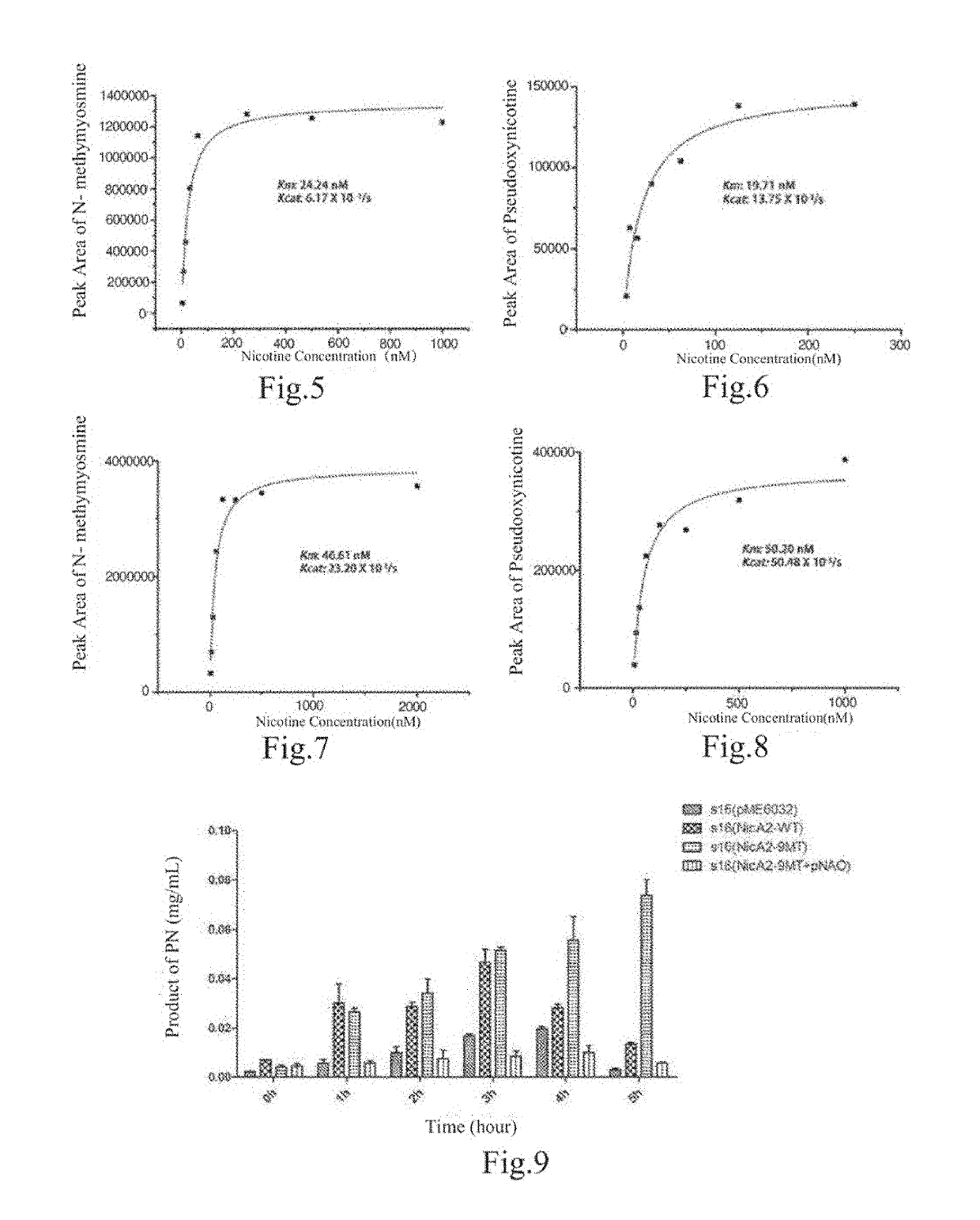
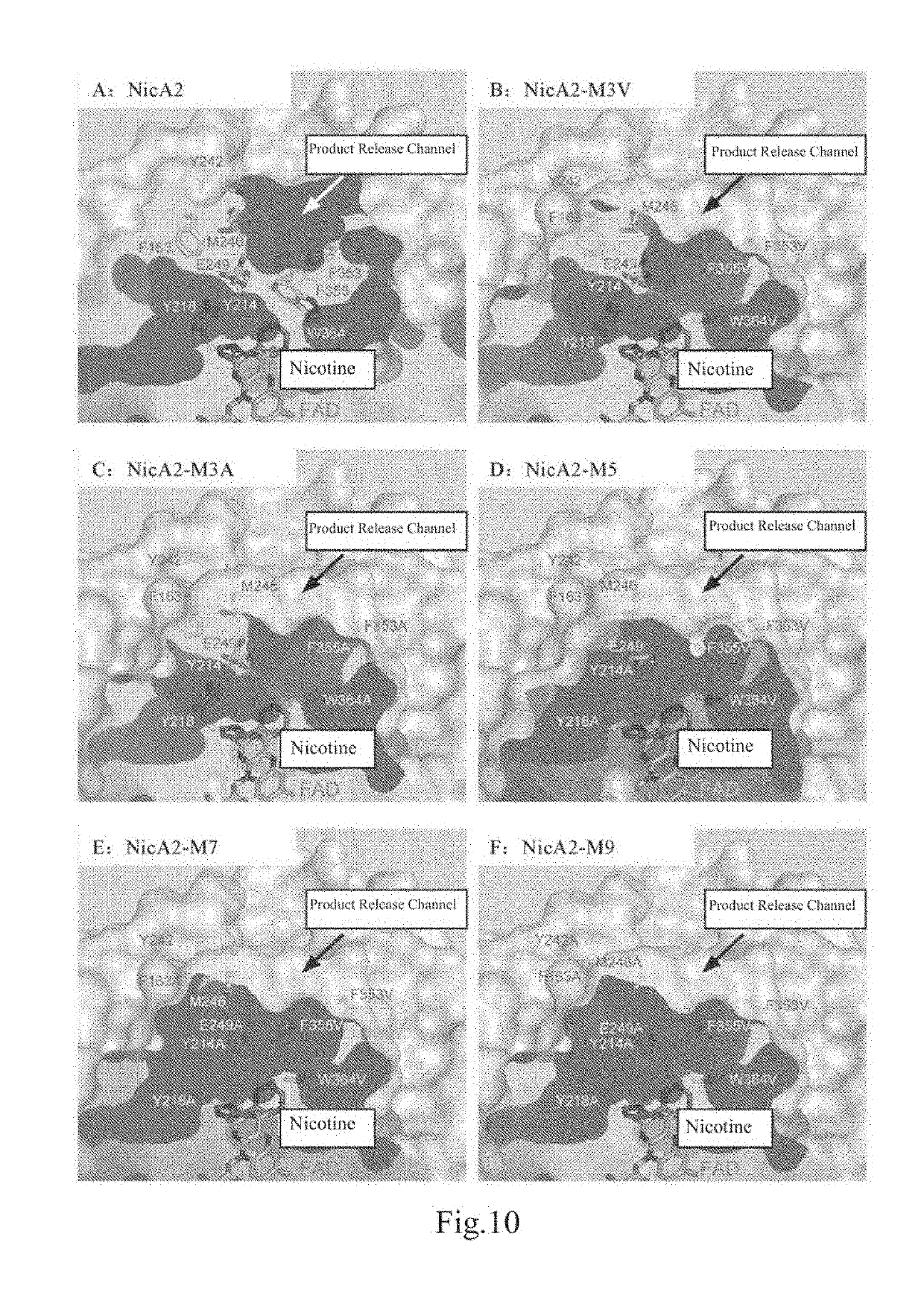
ActiveUS20190153403A1High catalytic efficiencyImprove nicotine transformation abilityNervous disorderPeptide/protein ingredientsArtificial enzymeSide chain
Provides an artificial enzyme obtained by improving upon a sequence of a natural nicotine dehydrogenase, wherein the improvement comprises replacing at least one amino acid hindering product release with an amino acid with smaller side chains, thereby improving a catalytic rate.
Owner:SHANGHAI JIAO TONG UNIV
Polypeptide and application thereof to preparation of medicine for treating and preventing tumors
ActiveCN107056887ASignificant effectSmall toxicityPeptide/protein ingredientsPeptidesDiseaseSide effect
The invention discloses a specific binding TRB3 polypeptide and application thereof to preparation of a medicine for treating and preventing tumors. The polypeptide has the amino acid sequence as shown in that two or more amino acids in an amino acid sequence shown in the sequence table SEQ ID No.8 are replaced with unnatural amino acids which enable other side chains to be connected. The polypeptide can be specifically bound with TRB3, so that mutual action between TRB3 and P62 protein is prevented, and accordingly, the polypeptide can be applied to preparation of the medicine for treating and preventing tumors. The prepared medicine has the advantages of remarkable curative effect, less toxic and side effect and use safety.
Owner:胡卓伟
Methods and compositions for modulating C5-a-mediated inflammatory responses
InactiveUS7763708B2Impair C5a anaphylatoxin bindingHigh activityPeptide/protein ingredientsImmunoglobulinsEnprofyllineIn vivo
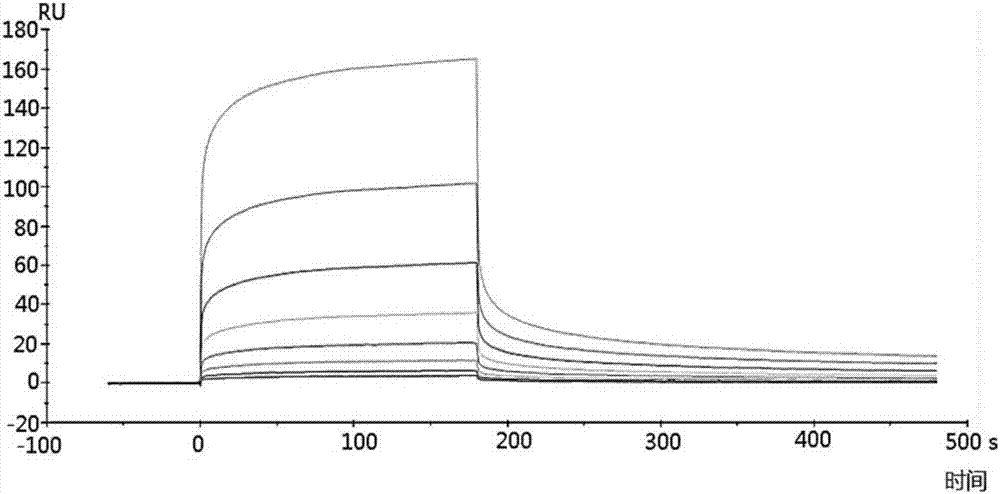
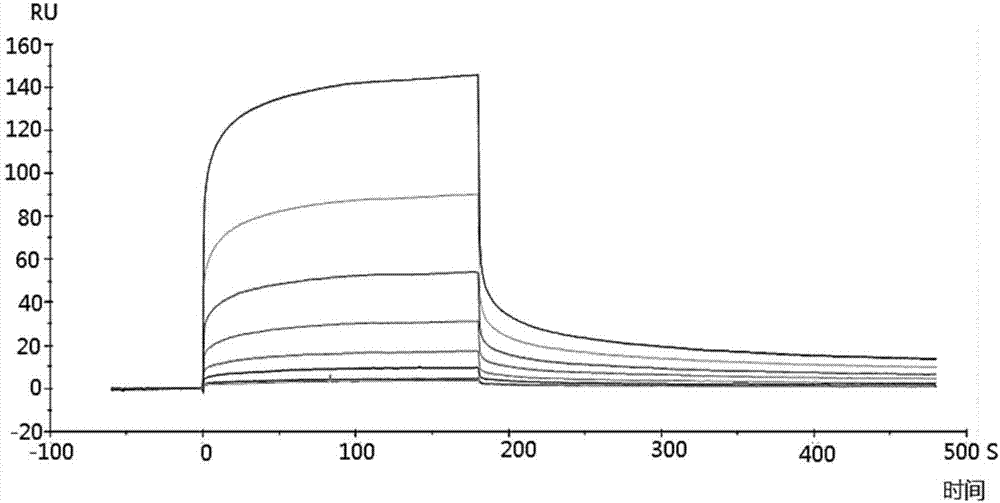
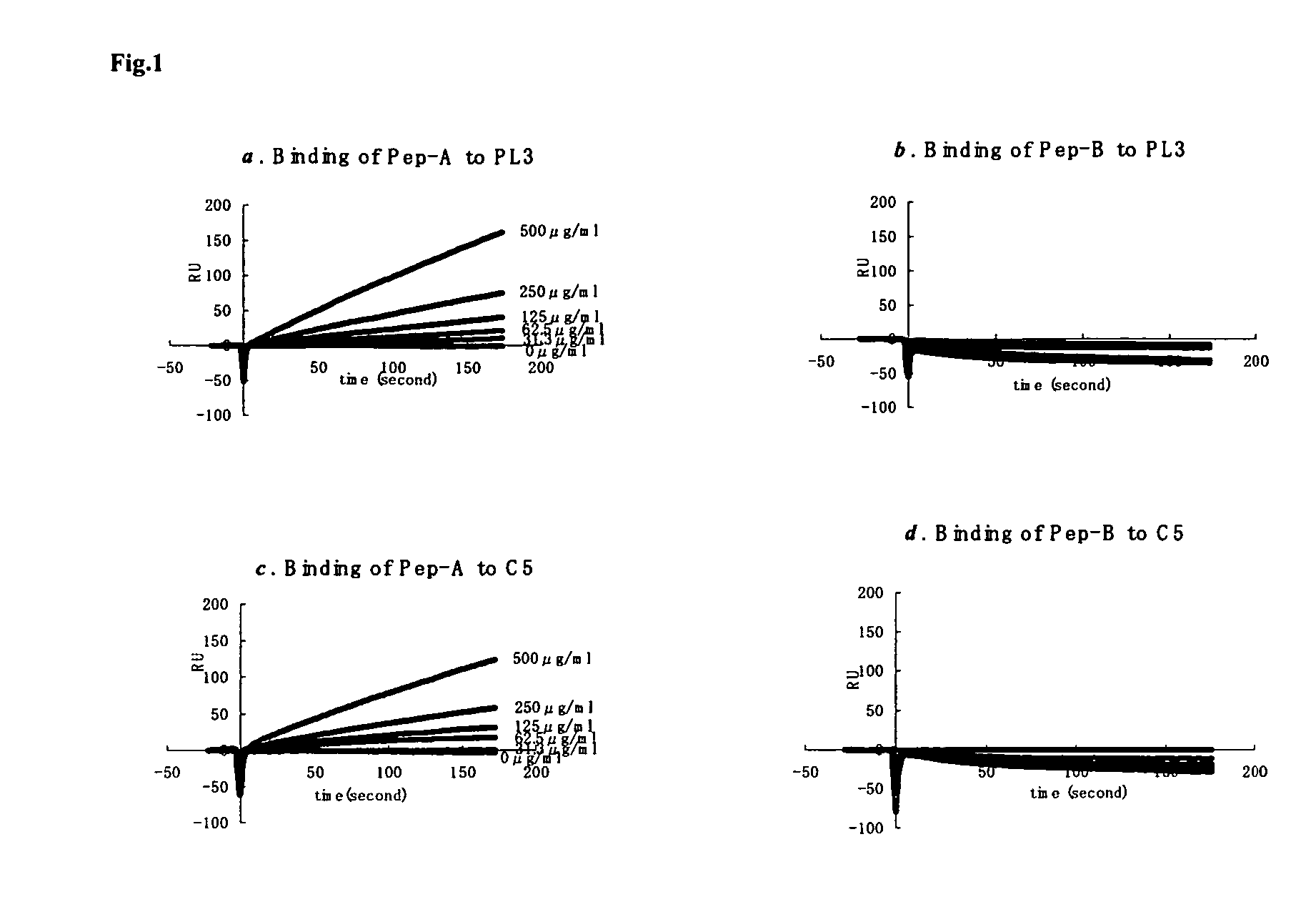
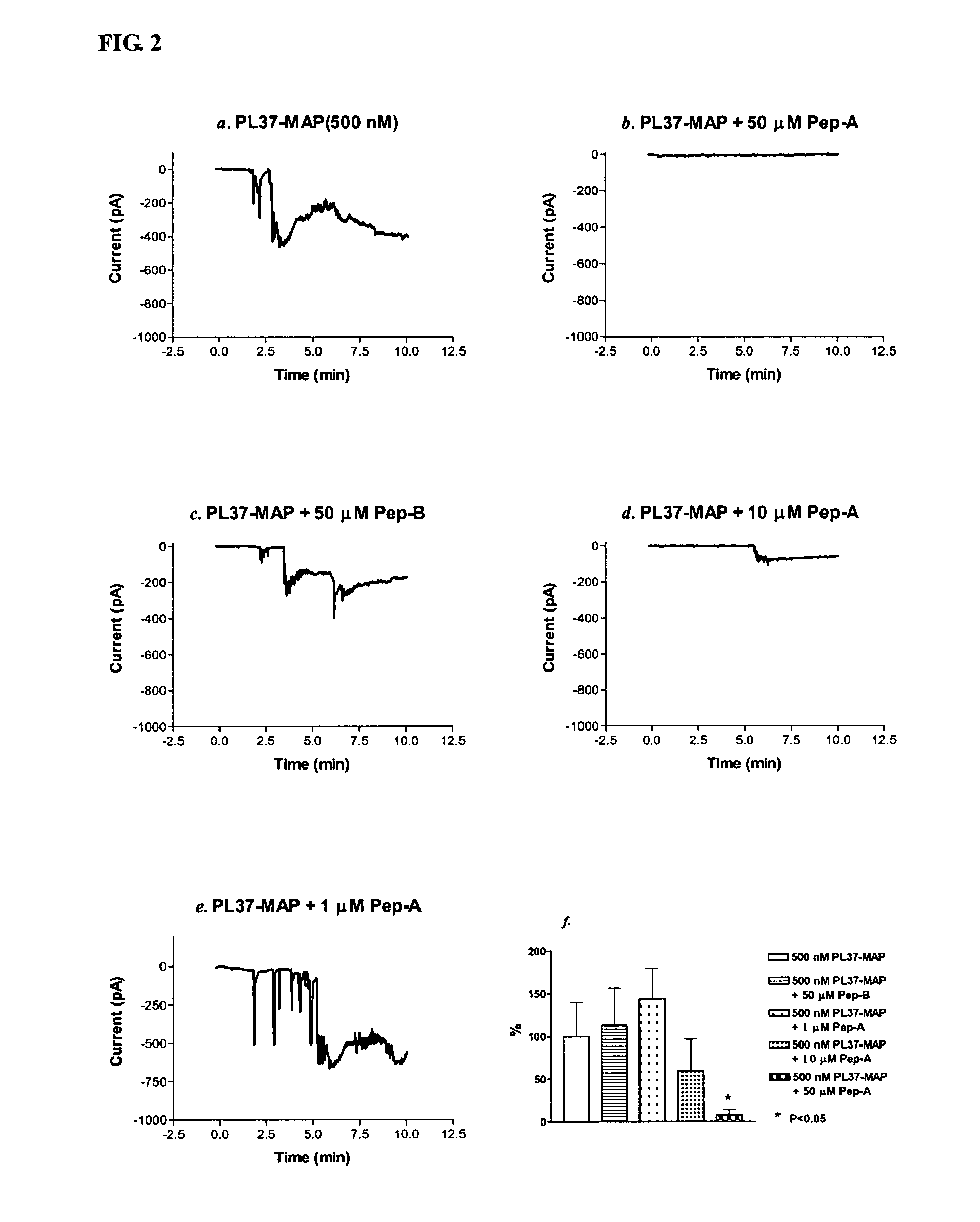
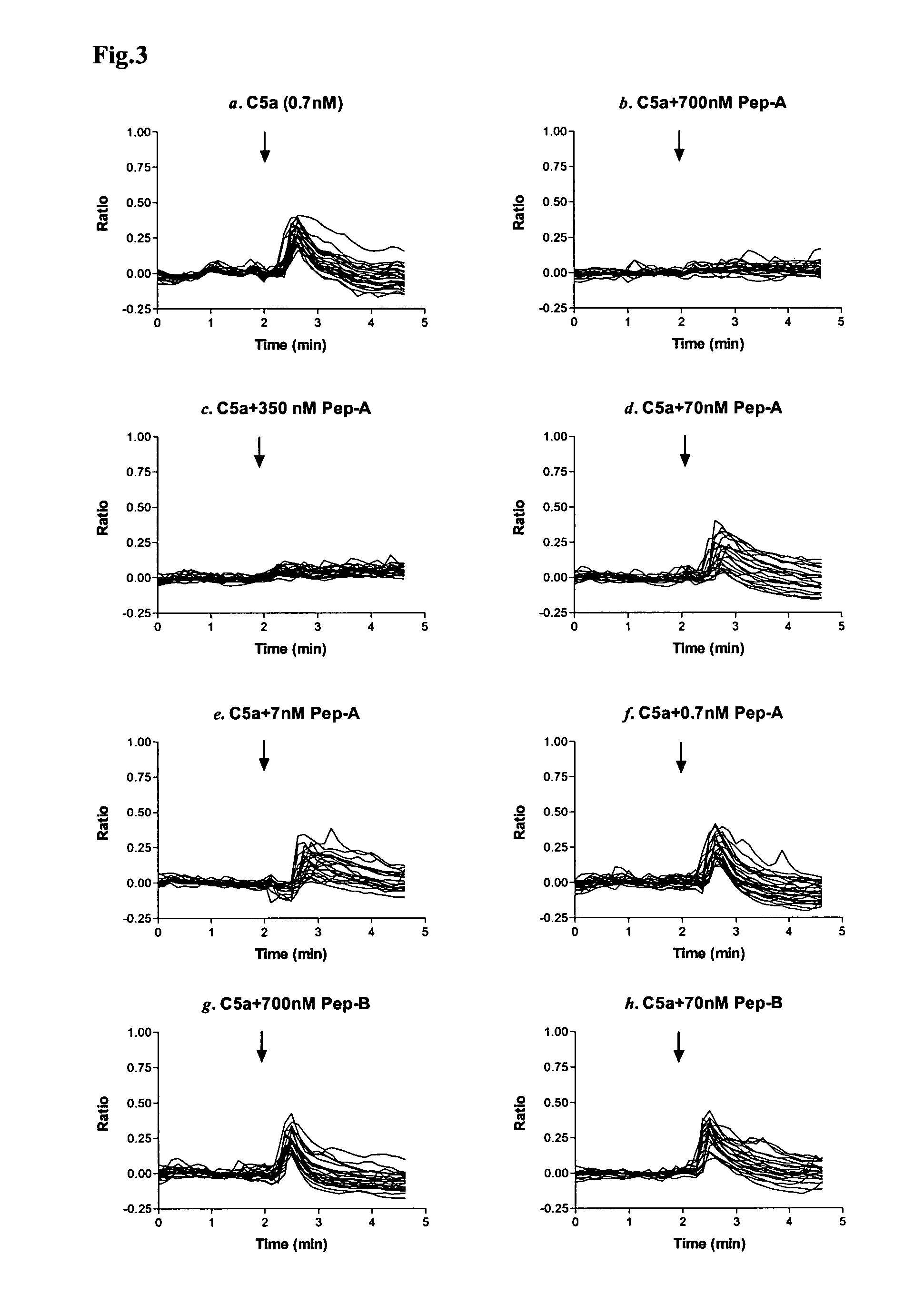
PL37 (RAARISLGPRCIKAFTE [SEQ ID NO: 2]) is an Antisense Homology Box peptide composed of amino acids 37 to 53 of C5a-anaphylatoxin. Complementary peptides, ASGAPAPGPAGPLRPMF (Pep-A [SEQ ID NO: 1]) and ASTAPARAGLPRLPKFF (Pep-B [SEQ ID NO: 3]) were designed and characterized. Pep-A bound to PL37 and to C5a with very slow dissociation, whereas Pep-B failed to bind at all. C5a was inactivated by 7 nM or more of Pep-A and this concentration of Pep-A inhibited induction of intracellular Ca++ influx in neutrophils. Patch clamp studies also showed the effectiveness of Pep-A in C5a-receptor-expressing neuroblastoma cells. Pep-A administration prevented rats from C5a-mediated rapid lethal shock. A-Pep-A (Pep-A acetylated with alanine at the amino-terminus) was more stable in vivo and showed stronger inhibition of inflammatory reactions in mice and rats. Chemical modification of Pep-A (e.g., acetylation, or single or multiple amino acid replacement, insertion, or deletion within the native Pep-A sequence) will yield effective inhibitors, and will often improve inhibitory function on C5a anaphylatoxin. In such modified constructs it will often be desired to conserve some or all 5 prolines found in Pep-A to preserve inhibitory function on C5a.
Owner:RES INST FOR PROTEIN SCI
Application of active peptide and mesenchymal stem cell exosome for improving skin physiological characteristics in drugs or cosmetics
ActiveCN113307851AHigh activityEasily damagedCosmetic preparationsPeptide-nucleic acidsRepair processesBiophysics
The invention belongs to the technical field of biological pharmacy, and particularly relates to application of active peptide and a mesenchymal stem cell exosome for improving skin physiological characteristics in drugs or cosmetics. Amino acid replacement is performed on the basis of original active peptide, and other conventional amino acids and unusual amino acids are introduced, so that the antioxidant capacity, collagen secretion promoting capacity and cell proliferation promoting capacity are improved. The effect of combined use between the amino acids and the human umbilical cord mesenchymal stem cell exosome is more obvious, skin injuries caused by ultraviolet rays can be effectively prevented and treated, and the repair process after the injuries is promoted.
Owner:珠海医美企业管理有限公司
Polypeptide or its derivatives and application thereof in preparation of tumor drugs
ActiveCN108570096APromote degradationInhibitory activityPolypeptide with localisation/targeting motifPeptide/protein ingredientsSide chainWilms' tumor
The invention discloses a polypeptide to specifically promote degradation of EGFR (epidermal growth factor receptor) protein, or its derivatives, and application of the polypeptide in the preparationof tumor drugs. An amino acid sequence of the polypeptide is shown as in sequence table SEQ ID No. 1; alternatively, two or more amino acids in the amino acid sequence shown as in the sequence table SEQ ID No. 1 are replaced with side-chain-connectable non-natural amino acids; the derivatives include chimeric peptides that are formed by connecting the polypeptide with cell penetrating peptides. The polypeptide or its derivatives can promote degradation of EGFR protein and inhibit EGFR signal pathway activity; therefore, the polypeptide or its derivatives are applicable to the preparation of tumor drugs. Drugs prepared herein are suitable for treating the tumors, such as lung cancer, intestinal cancer, pancreatic cancer, breast cancer, liver cancer, and glioma.
Owner:BEIJING WEIFENG YIMIN TECH
Immunogenic detoxified mutants of cholera toxin
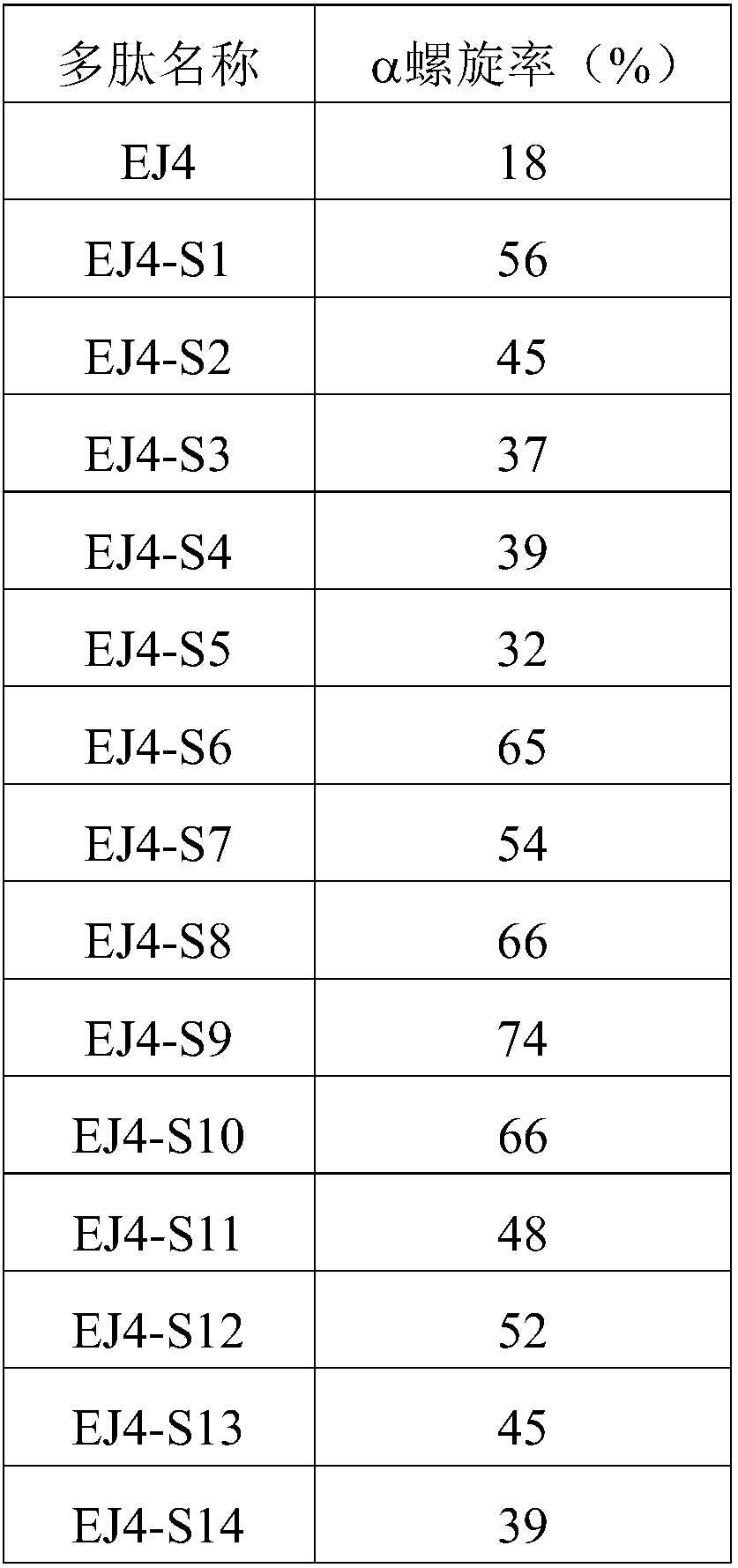
An immunogenic detoxified protein comprising the amino acid sequence of subunit A of a cholera toxin (CT-A) or a fragment thereof or the amino acid sequence of subunit A of an Escherichia coli heat labile toxin (LT-A) or a fragment thereof wherein the amino acids at, or in positions corresponding to Ser-63 and Arg-192 are replaced with another amino acid. The immunogenic detoxified protein is useful as vaccine for Vibrio cholerae or an enterotoxigenic strain of Escherichia coli and is produced by recombinant DNA means by site-directed mutagenesis.
Owner:CHIRON CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com