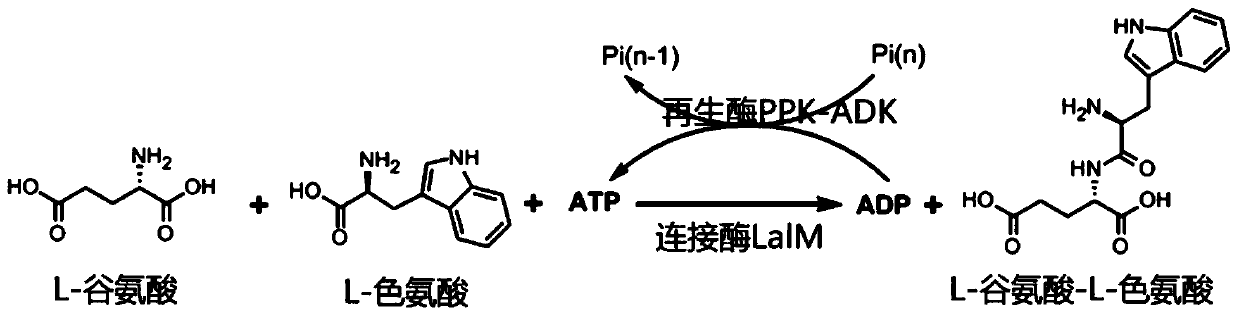
Mutated L-amino acid ligase and process for preparing L-glutamic acid-L-trp-trp by adopting enzyme catalysis method
A tryptophan dipeptide and amino acid technology, applied in the field of biochemistry, can solve the problems of not finding ligases, etc., and achieve the effects of reducing production costs, improving yield and production efficiency, and fast connection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of L-glutamic acid-L-tryptophan dipeptide (LalM-1&PPK1-ADK) catalyzed by liquid enzyme
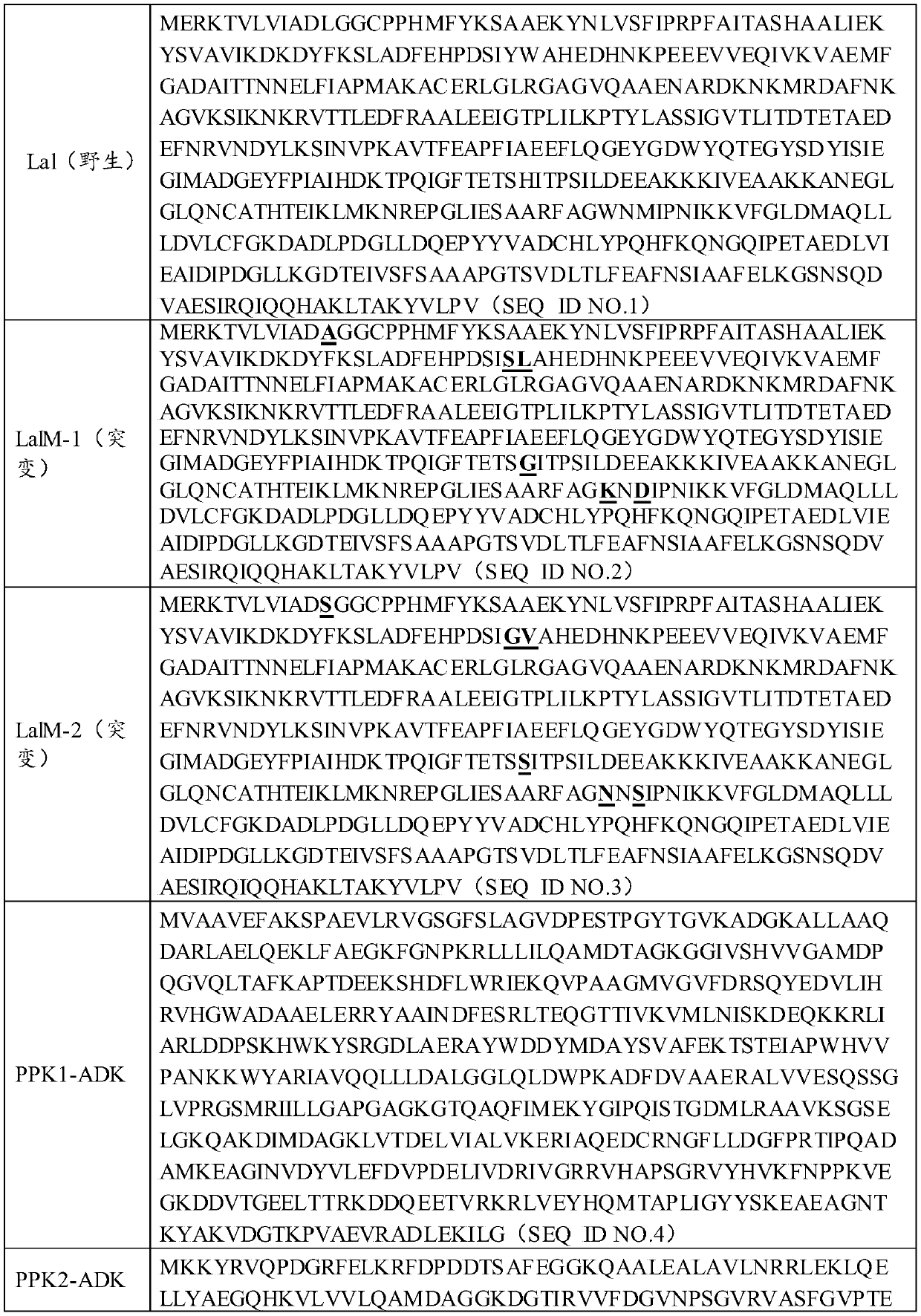
[0044] Obtain wet cells containing amino acid ligase LalM-1 (H276G, W332K, M334D, L12A, Y75S and W76L) or ATP regeneration enzyme PPK1-ADK according to the method in the specific embodiment; mix in 500ml 50mM Tris pH 8.0 buffer (Buffer A), after stirring evenly, the cells are broken by high pressure, and the cell wall is removed by high-speed centrifugation (16000rpm, 45min), and the collected supernatant and crude enzyme solution are directly subjected to the following catalytic reactions:
[0045] Add 11.1 grams of L-glutamic acid (75mM), 14.3 grams of L-tryptophan (70mM), and 2.8 grams of adenosine triphosphate disodium in 1000ml of trishydrochloride (Tris.HCl) solution containing 100mM pH 8.0 Salt ATP (5mM), 10.3 grams of polyphosphoric acid (Sigma, 25 poly, 100mM monophosphoric acid), 0.9 grams of magnesium chloride (10mM), 1.5 grams of potassium chloride...
Embodiment 2
[0049] Example 2: Preparation of L-glutamic acid-L-tryptophan dipeptide (LalM-2&PPK2-ADK) catalyzed by liquid enzyme
[0050] Obtain wet cells containing amino acid ligase LalM-2 (H276S, W332N, M334S, L12S, Y75G and W76V) or ATP regeneration enzyme PPK2-ADK according to the method in the specific embodiment; mix in 500ml 50mM Tris pH8.0 buffer In (buffer solution A), after stirring evenly, the cells are broken by high pressure, and the cell wall is removed by high-speed centrifugation (16000rpm, 45min), and the collected supernatant and crude enzyme solution are directly subjected to the following catalytic reactions:
[0051] Similar to Example 1, but the reaction concentration is slightly lower, 7.4 grams of L-glutamic acid (50 mM), 13.4 grams of L-glutamic acid (13.4 grams of L- Tryptophan (47mM), 2.8g adenosine triphosphate disodium salt ATP (5mM), 6.7g polyphosphoric acid (Sigma, 25 poly, 65mM monophosphate), 0.9g magnesium chloride (10mM), 1.5g potassium chloride (20mM);...
Embodiment 3
[0053] Example 3: Preparation of L-glutamic acid-L-tryptophan dipeptide (LalM-3&PPK1-ADK) catalyzed by liquid enzyme
[0054] Obtain the wet cell containing amino acid ligase LalM-3 (N108H, L110A, L182F, G331D) or ATP regeneration enzyme PPK1-ADK with reference to the method in the specific embodiment; Mix in the damping fluid of 500ml 50mM Tris pH 8.0 (buffer A ), after stirring evenly, the cells are broken by high pressure, and the cell wall is removed by high-speed centrifugation (16000rpm, 45min), and the clear liquid crude enzyme solution collected directly carries out the following catalytic reactions:
[0055] Similar to Examples 1 and 2, but the amount of enzyme is more; 7.4 grams of L-glutamic acid (50 mM), 13.4 grams of L-glutamic acid (50 mM), and 13.4 grams of L-tryptophan (47mM), 2.8g adenosine triphosphate disodium salt ATP (5mM), 6.7g polyphosphoric acid (Sigma, 25 poly, 65mM monophosphate), 0.9g magnesium chloride (10mM), 1.5g potassium chloride (20mM ); after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com