Antibacterial peptide GLI23 derived from linear chicken beta-phylaxin4 (RL38) and preparation method thereof
A defensin and antimicrobial peptide technology, applied in the field of antimicrobial peptide GLI23 derived from linear chicken β-defensin 4 and its preparation, can solve the problem of few natural antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Design of antibacterial peptides
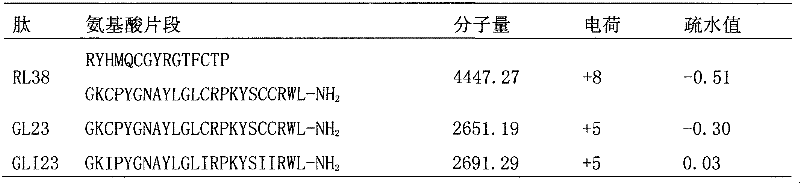
[0031] The amino acid sequence of linear chicken β-defensin 4 is:
[0032] Arg Tyr His Met Gln Cys Gly Tyr Arg Gly Thr Phe Cys Thr Pro
[0033] 1 5 10 15
[0034] Gly Lys Cys Pro Tyr Gly Asn Ala Tyr Leu Gly Leu Cys Arg Pro;
[0035] 20 25 30
[0036] Lys Tyr Ser Cys Cys Arg Trp Leu-NH 2
[0037] 35 38
[0038] By intercepting the 23 amino acids at the carboxyl terminal of linear chicken β-defensin 4 (RL38), two polypeptides with 4 positive charges and a hydrophobic value of -0.3 were obtained.
[0039] The obtained peptide GL23, its amino acid sequence is:
[0040] Gly Lys Cys Pro Tyr Gly Asn Ala Tyr Leu Gly Leu Cys Arg Pro
[0041] 1 6 10 15;
[0042] Lys Tyr Ser Cys Cys Arg Trp Leu--NH 2
[0043] 20 23
[0044] The first amino acid of GL23 is glycine to improve its stability.
[0045] Then replace the four cysteines in the GL23 peptide chain with isoleucine,
[0046] Obtain GLI23, whose amino acid se...
Embodiment 2
[0054] Synthesis and biological activity determination of antibacterial peptides
[0055] A peptide synthesizer is used to synthesize the above-mentioned antimicrobial peptides by a solid-phase synthesis method, and the biological activity of the antimicrobial peptides is identified and analyzed.
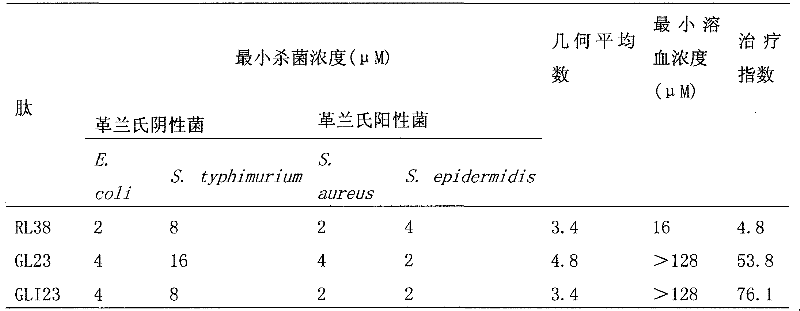
[0056] 2.1 Antibacterial activity
[0057] Prepare the peptide as a certain storage solution for use. The minimum bactericidal concentration of several antimicrobial peptides was determined by plate counting method. Using 10 mM sodium phosphate solution (pH 7.4) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially configured using the double dilution method. Take 100μl of the above solution and place it in a 96-well cell culture plate, and then add an equal volume of the test bacteria solution (10 6 Pcs / ml) in each well. Incubate at 37°C for 2h. Then take an appropriate amount of the above liquid diluted and spread it on an agar plate, incubate at 37°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com