Alpha helical antibacterial peptide RL as well as preparation method thereof and application thereof
An antimicrobial peptide and α-helix technology, applied in the field of α-helical antibacterial peptide RL and its preparation, can solve the problems of high cytotoxicity, limited natural production, and poor antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
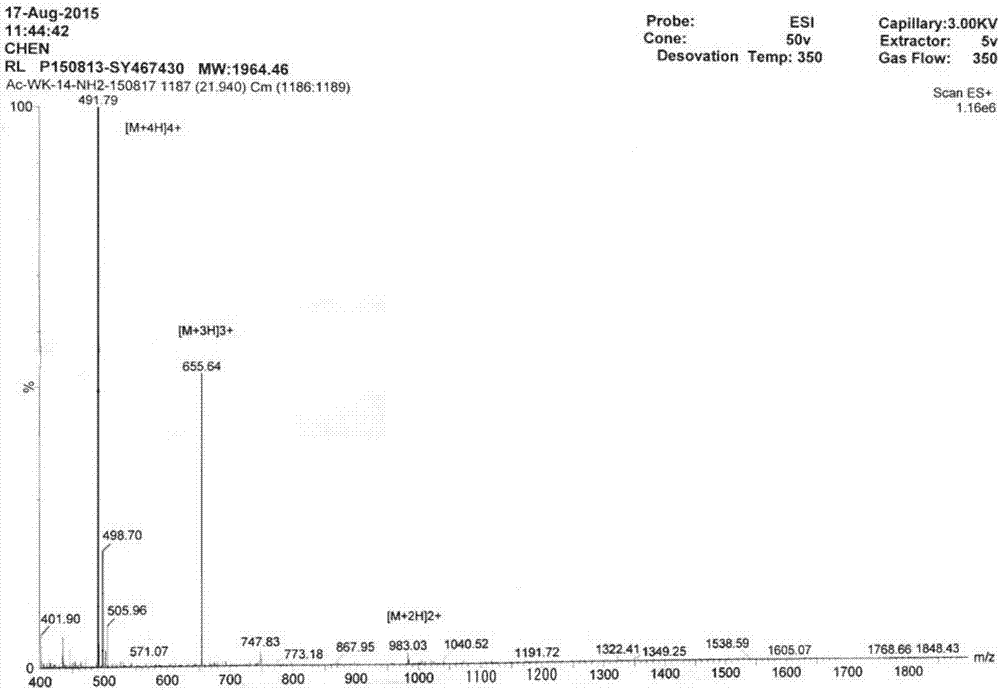
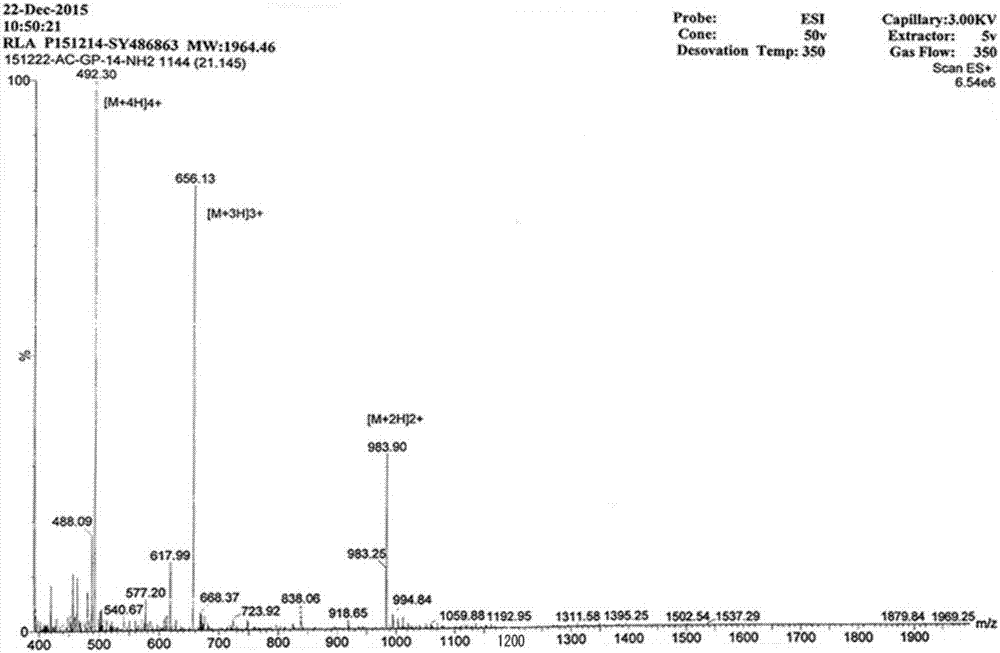
Image
Examples
Embodiment 1
[0017] Design of Antimicrobial Peptides
[0018] The amino acid sequence of the antimicrobial peptide RL is:
[0019]
[0020] Based on the folding principle of imperfect amphipathic α-helical peptides, an imperfect amphipathic α-helical peptide template Ac-WXKYWXZZYKXWYK-NH with a turn unit was designed 2 , X is a positively charged amino acid, Y is a hydrophobic amino acid, ZZ is a corner unit, when X=R, Y=L, ZZ= D When PG, the antimicrobial peptide is named RL; when D When the PG turn is disrupted, the antimicrobial peptide is named RLα. The sequences of the antimicrobial peptides are shown in Table 1.
[0021] Table 1 Amino Acid Sequence of Derived Peptides
[0022]
[0023] The charges of RL and RLα are both +7, and the hydrophobic value is -0.469. The C-termini of the two peptides were amidated to increase a positive charge, and the N-termini were acetylated to increase the stability of the peptides. The method enables the two peptides to have high antibacter...
Embodiment 2
[0025] Synthesis of Two Antimicrobial Peptides RL and RLα by Solid Phase Chemical Synthesis
[0026] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0027] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evapora...
Embodiment 3
[0031] Determination of antimicrobial activity of antimicrobial peptides
[0032] 1. Determination of antibacterial activity: The minimum inhibitory concentration of several antibacterial peptides was determined by the micro broth dilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 per mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Cultivate at a constant temperature of 37°C for 14-18h, measure the light absorption value at 492nm (OD492nm) with a microplate reader, and determine the minimum inhibitory concentration. The test results are shown in Table 2.
[0033] Ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com