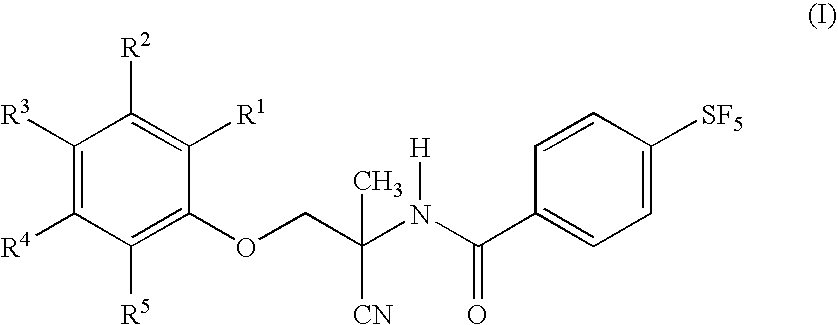
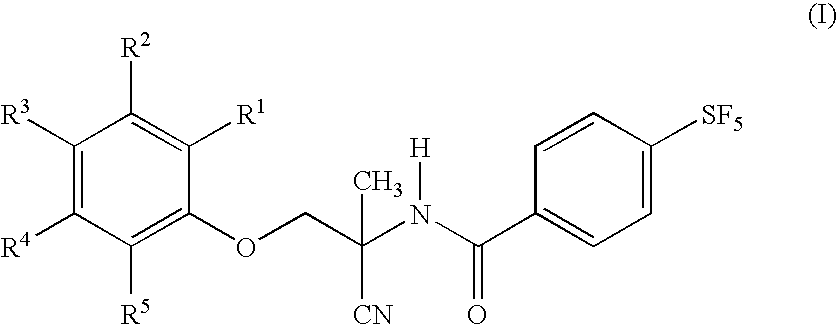
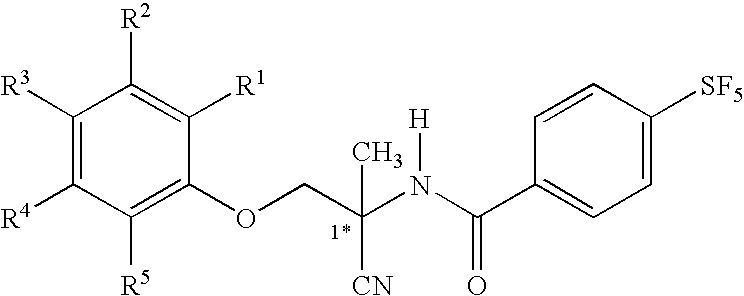
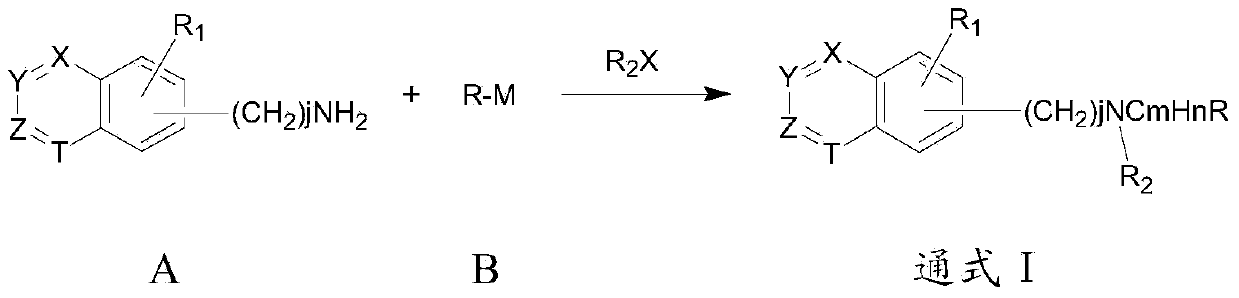
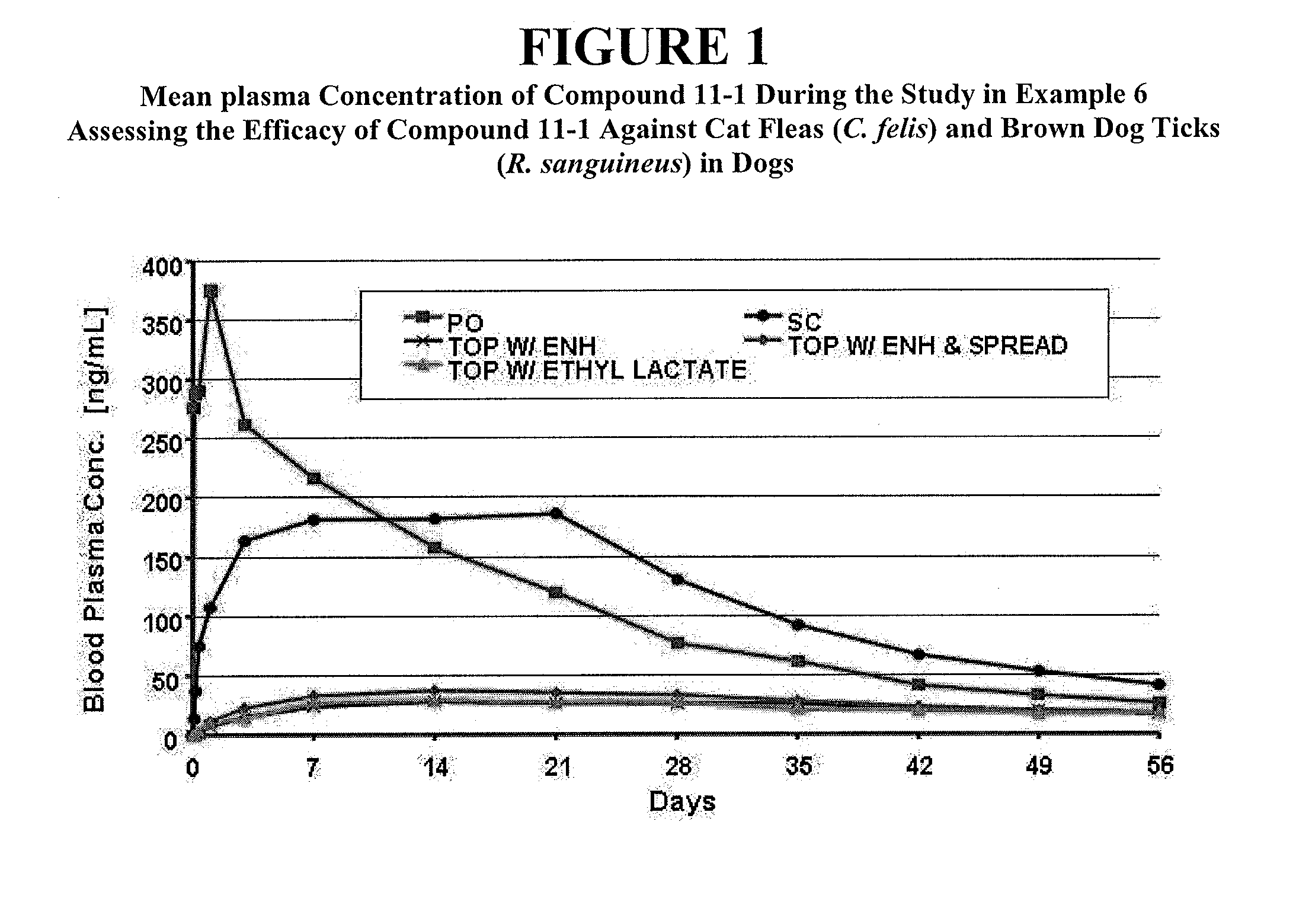
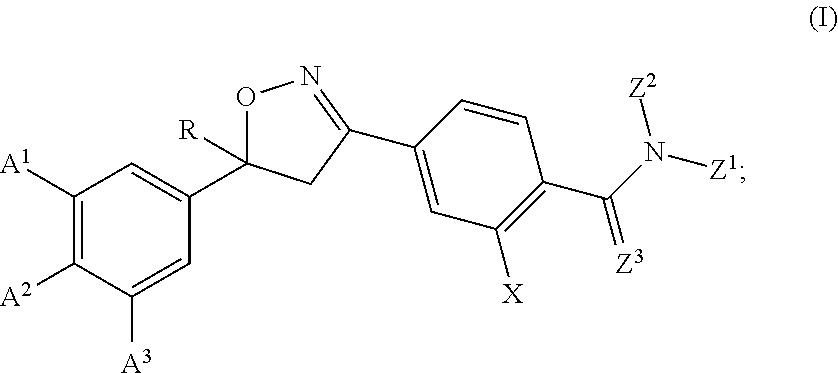
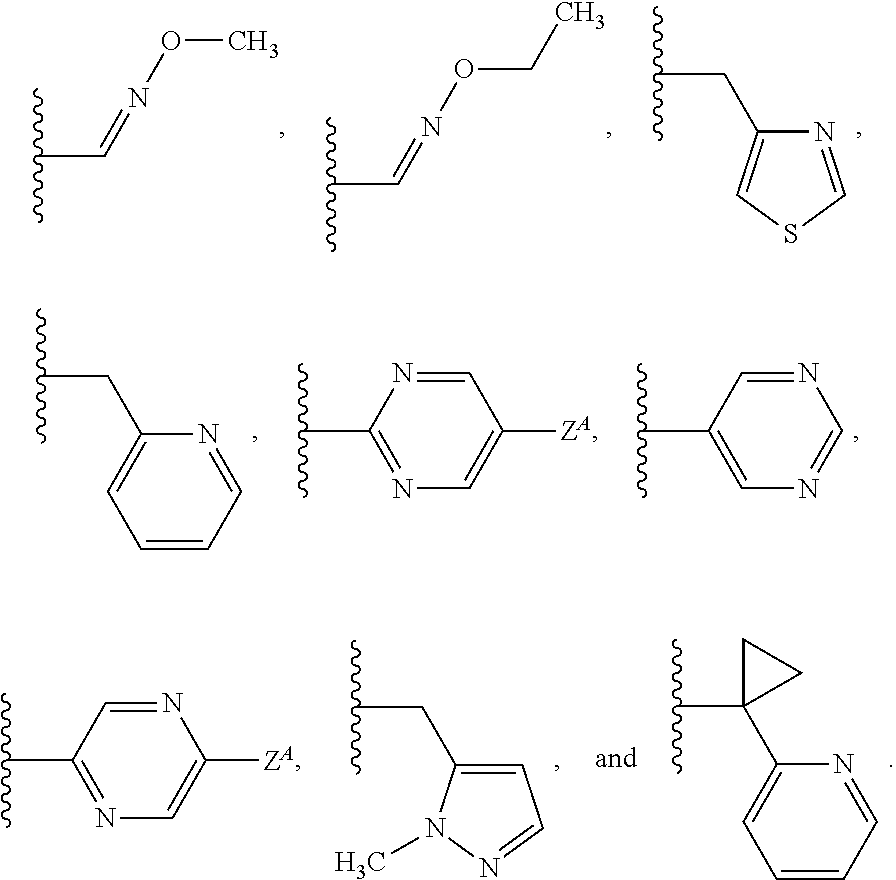
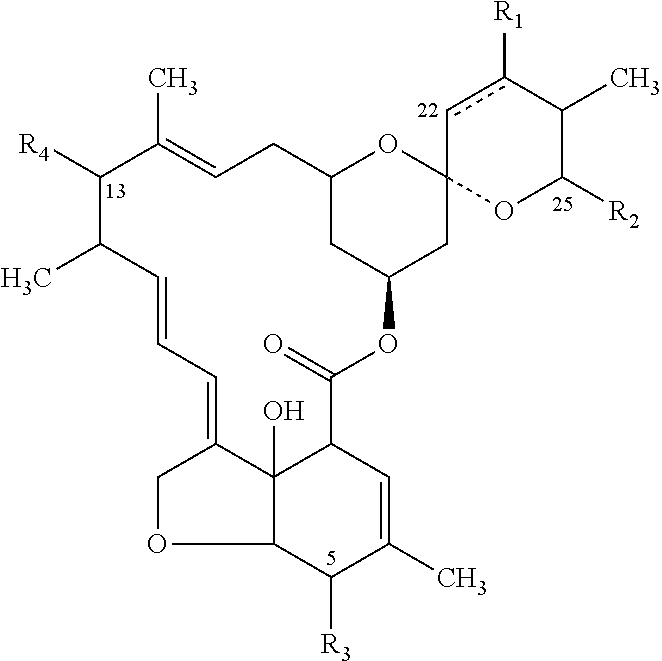
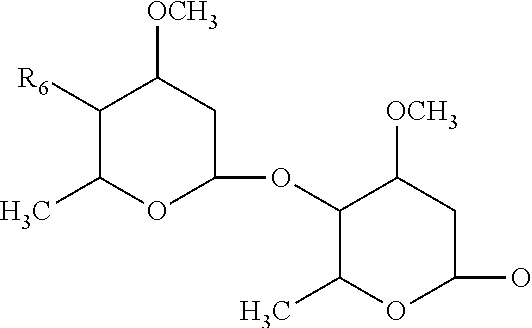
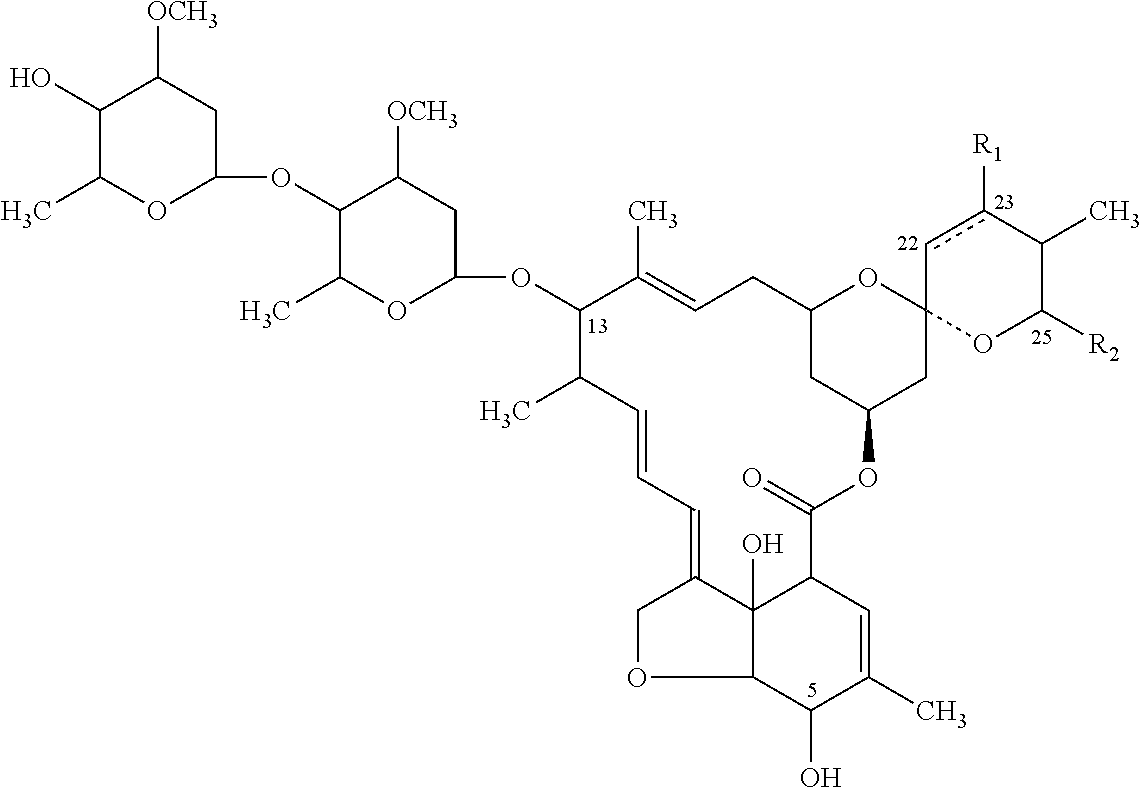
Patents
Literature
126 results about "Antiparasitic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antiparasitics are a class of medications which are indicated for the treatment of parasitic diseases, such as those caused by helminths, amoeba, ectoparasites, parasitic fungi, and protozoa, among others. Antiparasitics target the parasitic agents of the infections by destroying them or inhibiting their growth; they are usually effective against a limited number of parasites within a particular class. Antiparasitics are one of the antimicrobial drugs which include antibiotics that target bacteria, and antifungals that target fungi. They may be administered orally, intravenously or topically.
Spot-on formulations for combating parasites
InactiveUS6998131B2Effective and lasting destructionProphylaxis of parasite infestationsBiocideDead animal preservationAntiparasiticMammal
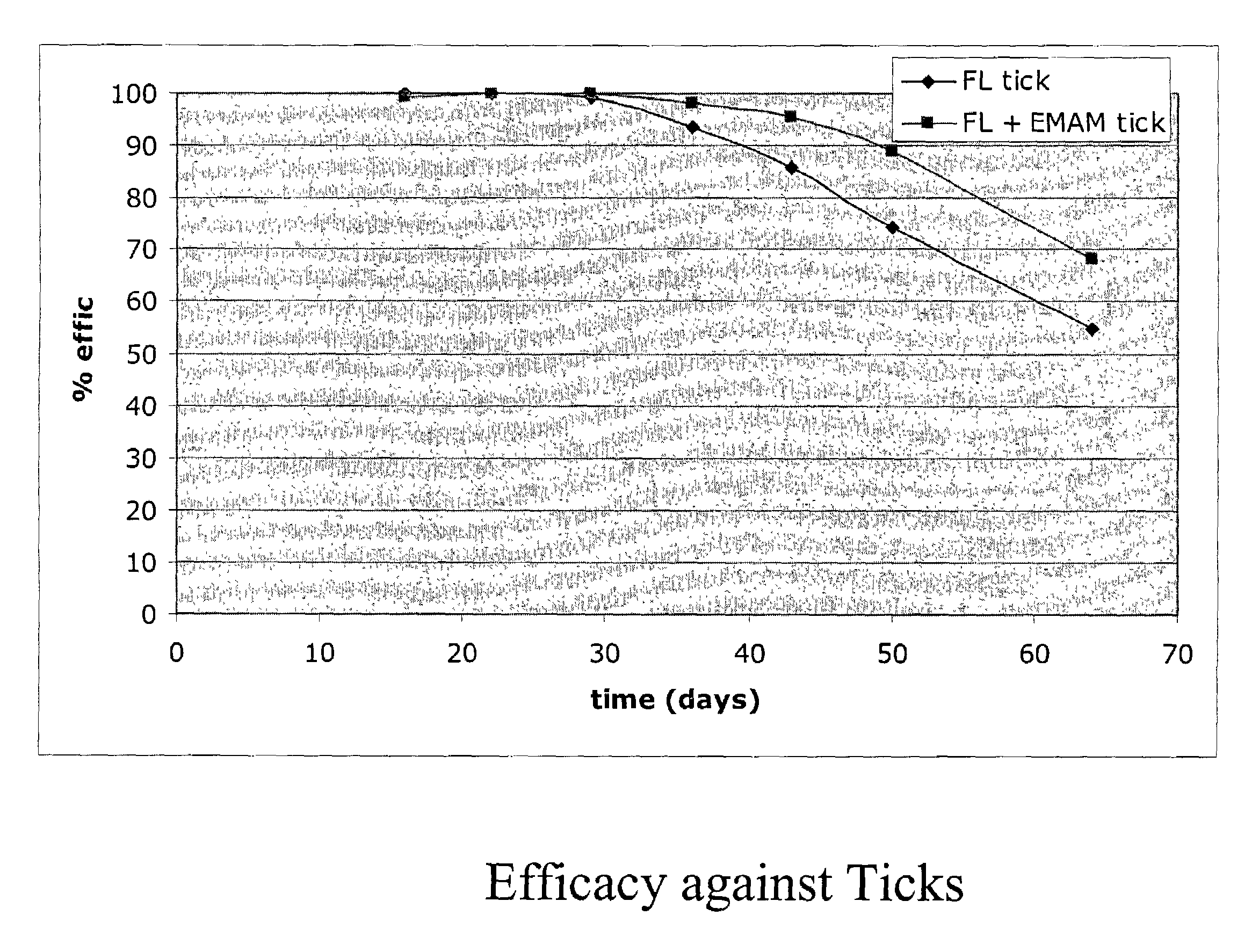
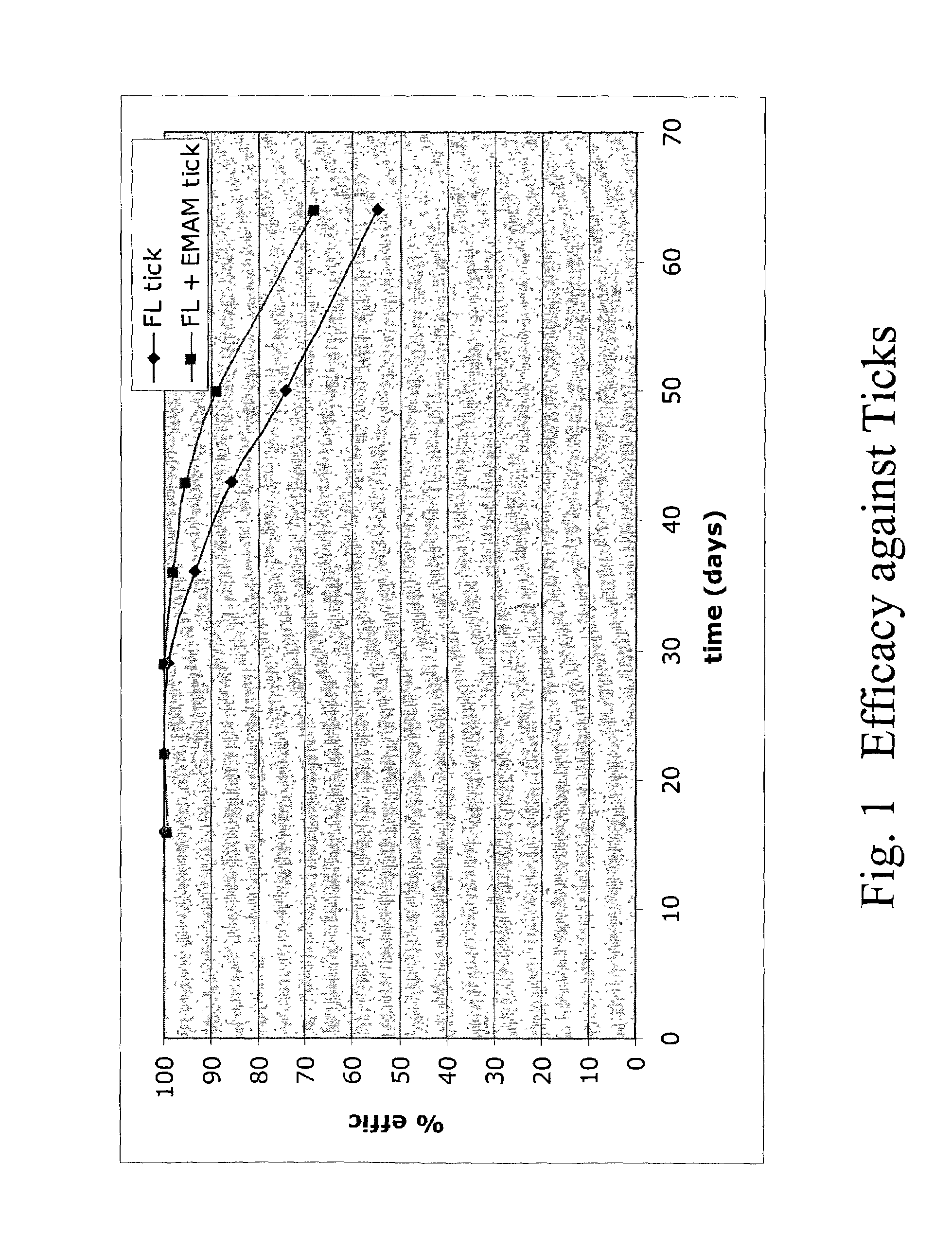
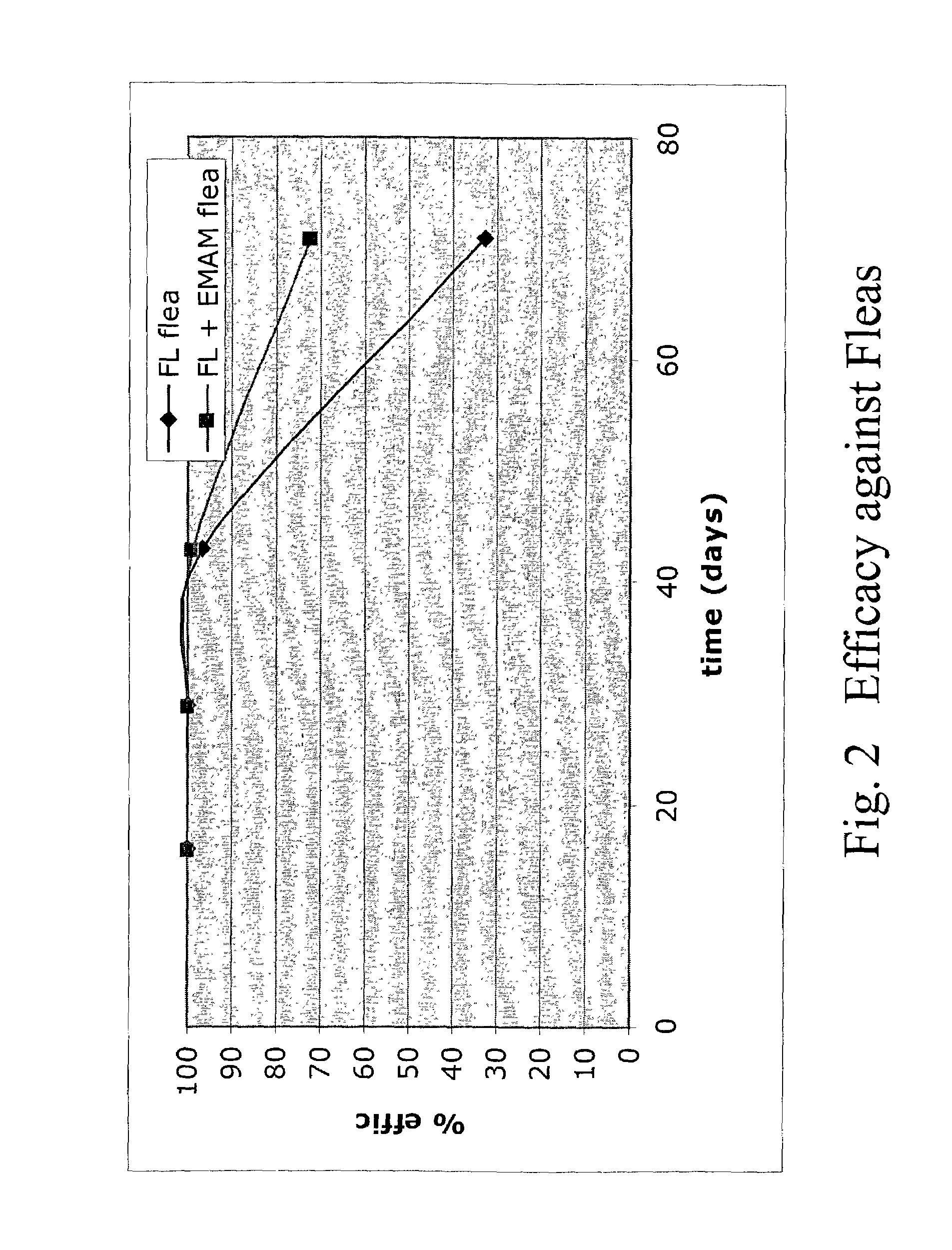
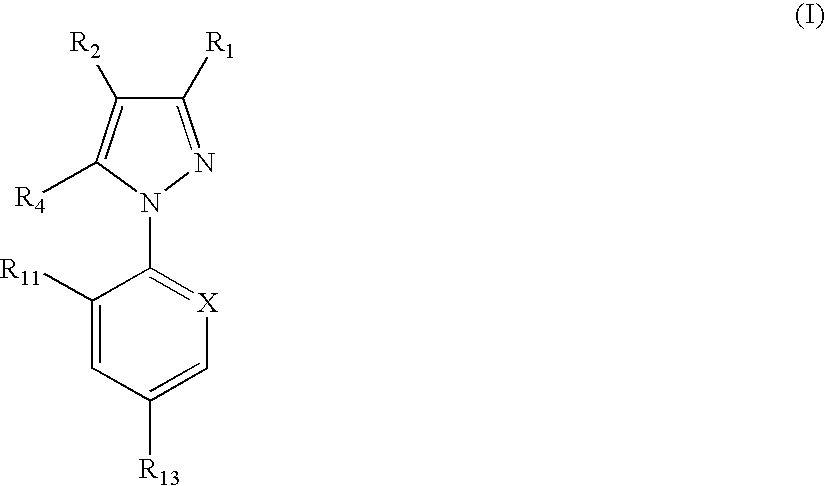
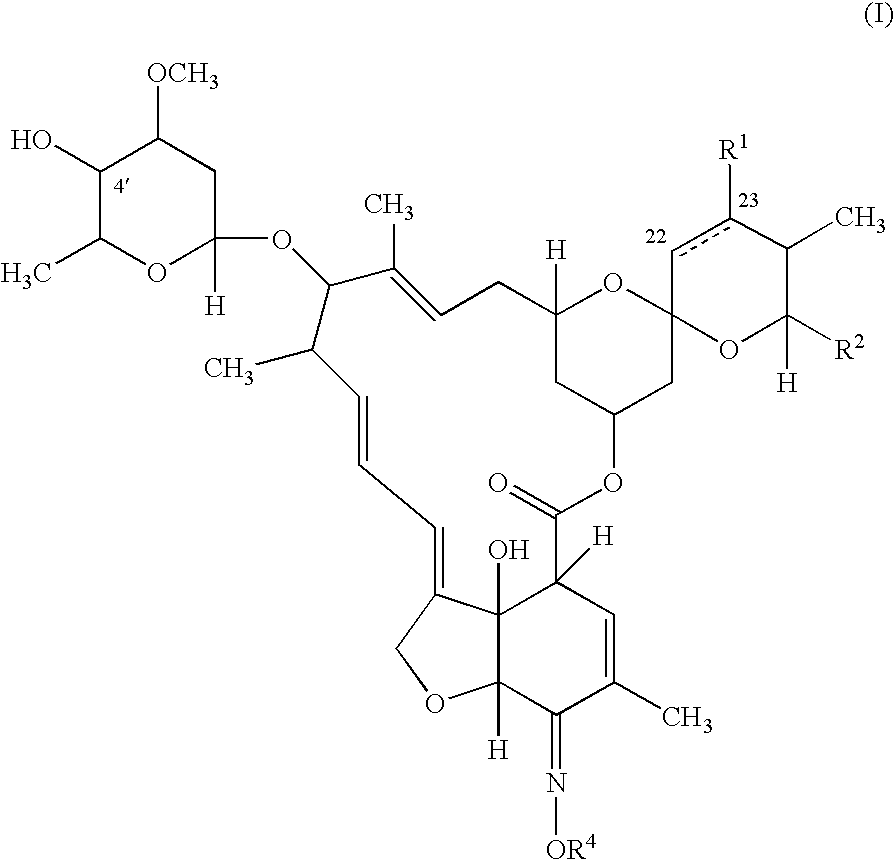
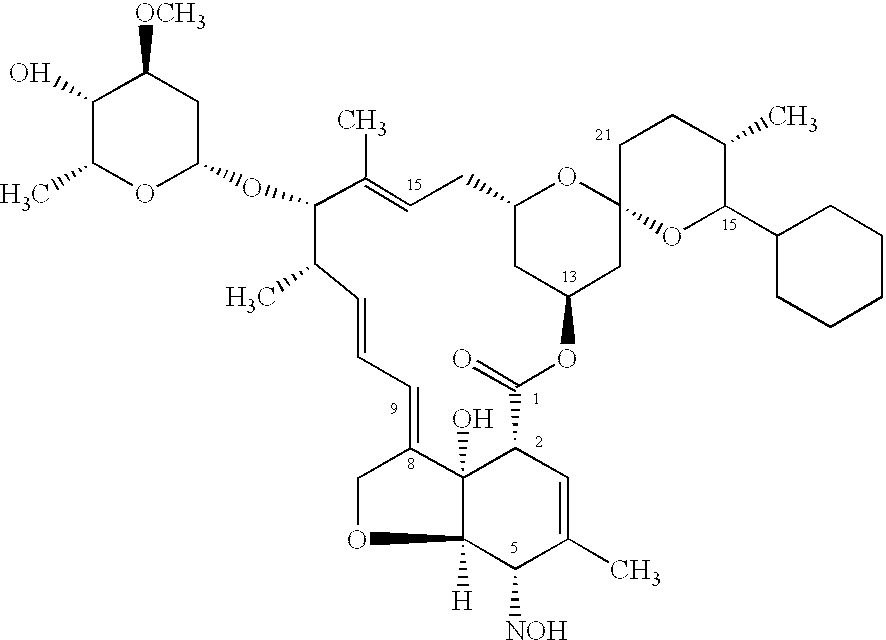
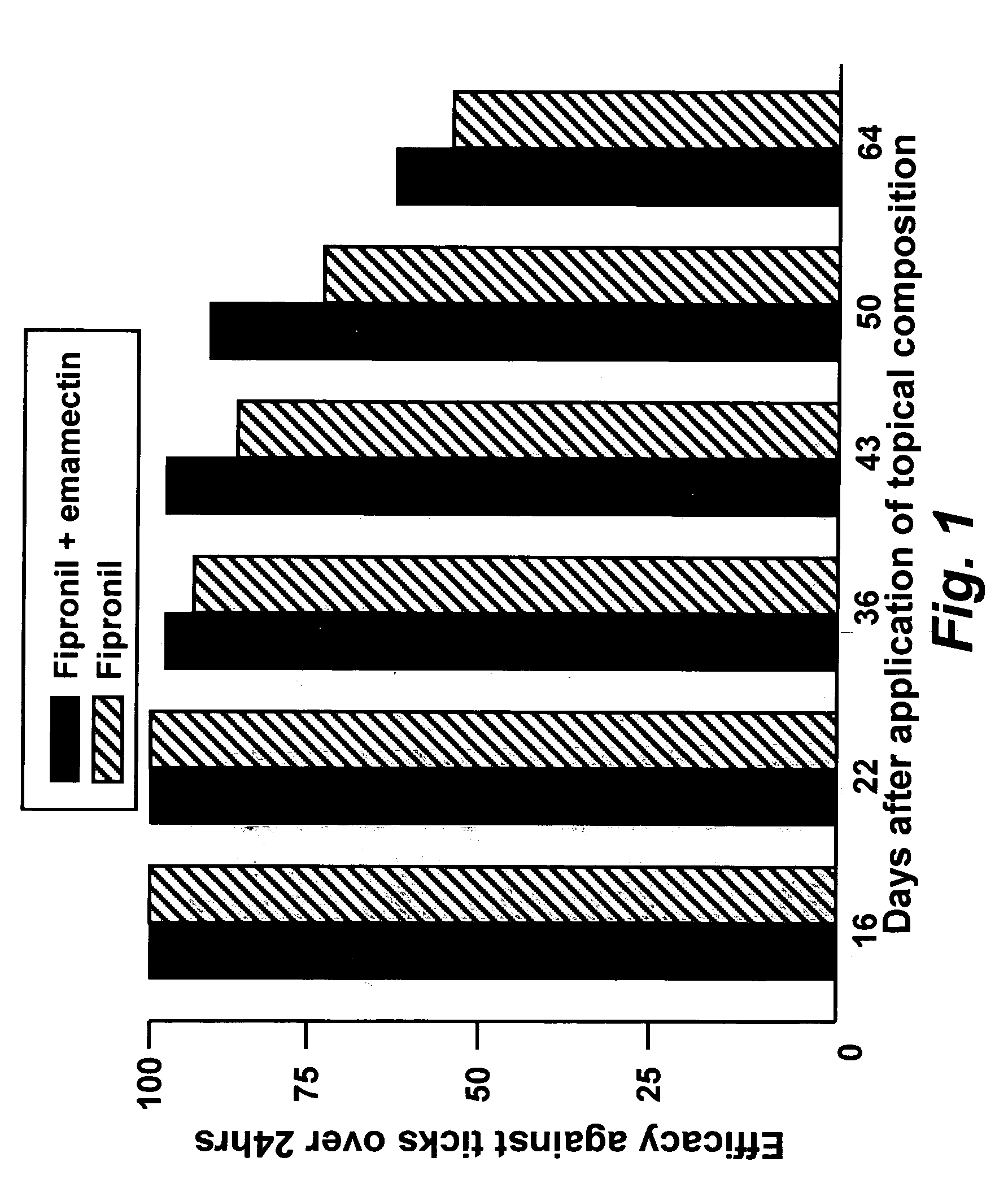
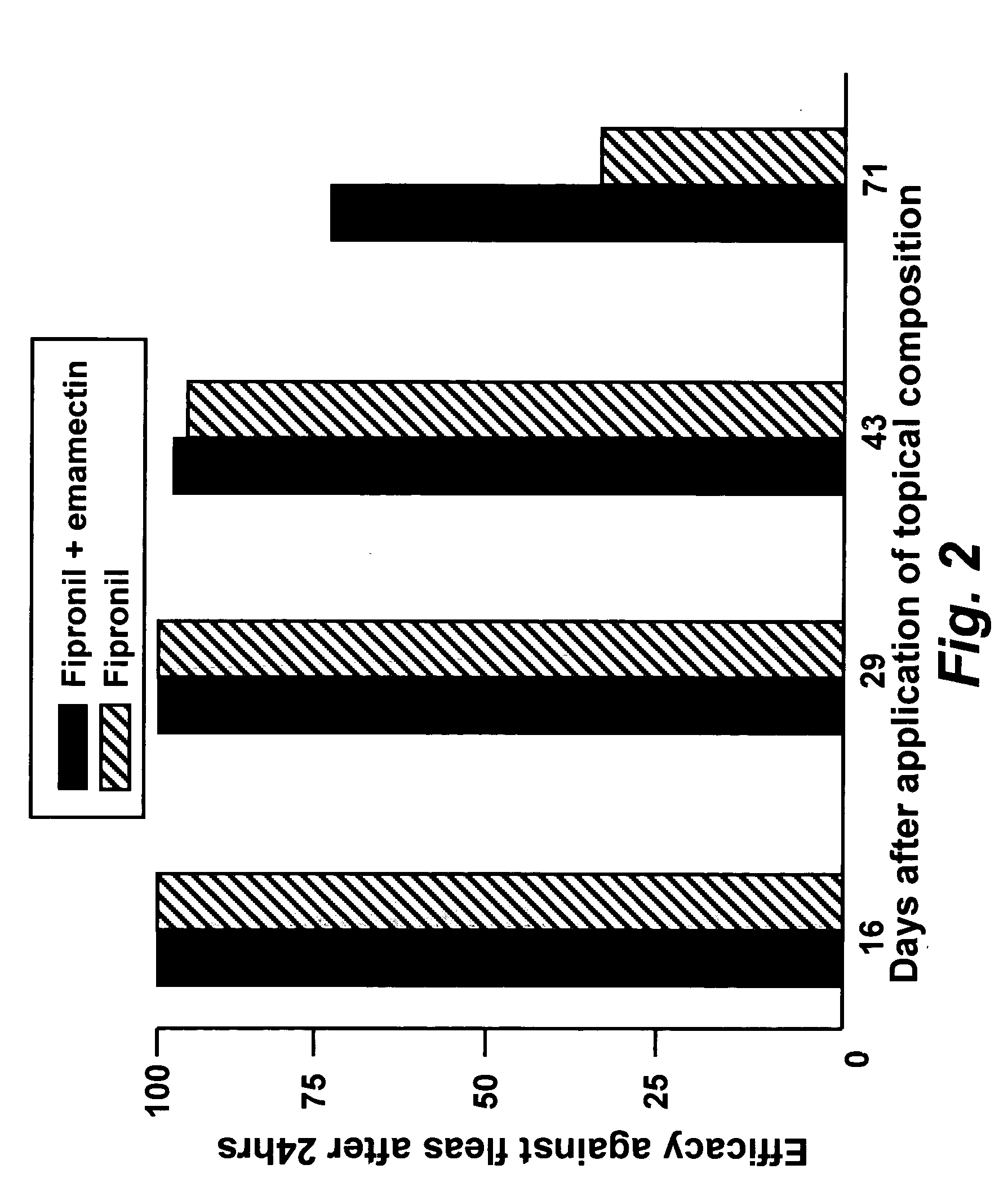
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and(B) an effective amount of emamectin;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
Spot-on formulations for combating parasites
InactiveUS6962713B2Toxic effectsEffective and lasting destructionBiocideDead animal preservationAntiparasiticAntiparasite agent
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and / or(B) an effective amount of a macrocyclic lactone antihelmintic or antiparasitic agent;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL SAS
Spot-on formulations for combating parasites
InactiveUS20050192319A1Effective and lasting destructionProphylaxis of parasite infestationsBiocidePharmaceutical delivery mechanismAntiparasiticAnimal science
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise: (1) a composition comprising (A) an effective amount of a 1-phenylpyrazole derivative; and (B) an effective amount of emamectin, latidectin or lepimectin; (2) an acceptable liquid carrier vehicle; and (3) optionally, a crystallization inhibitor. The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
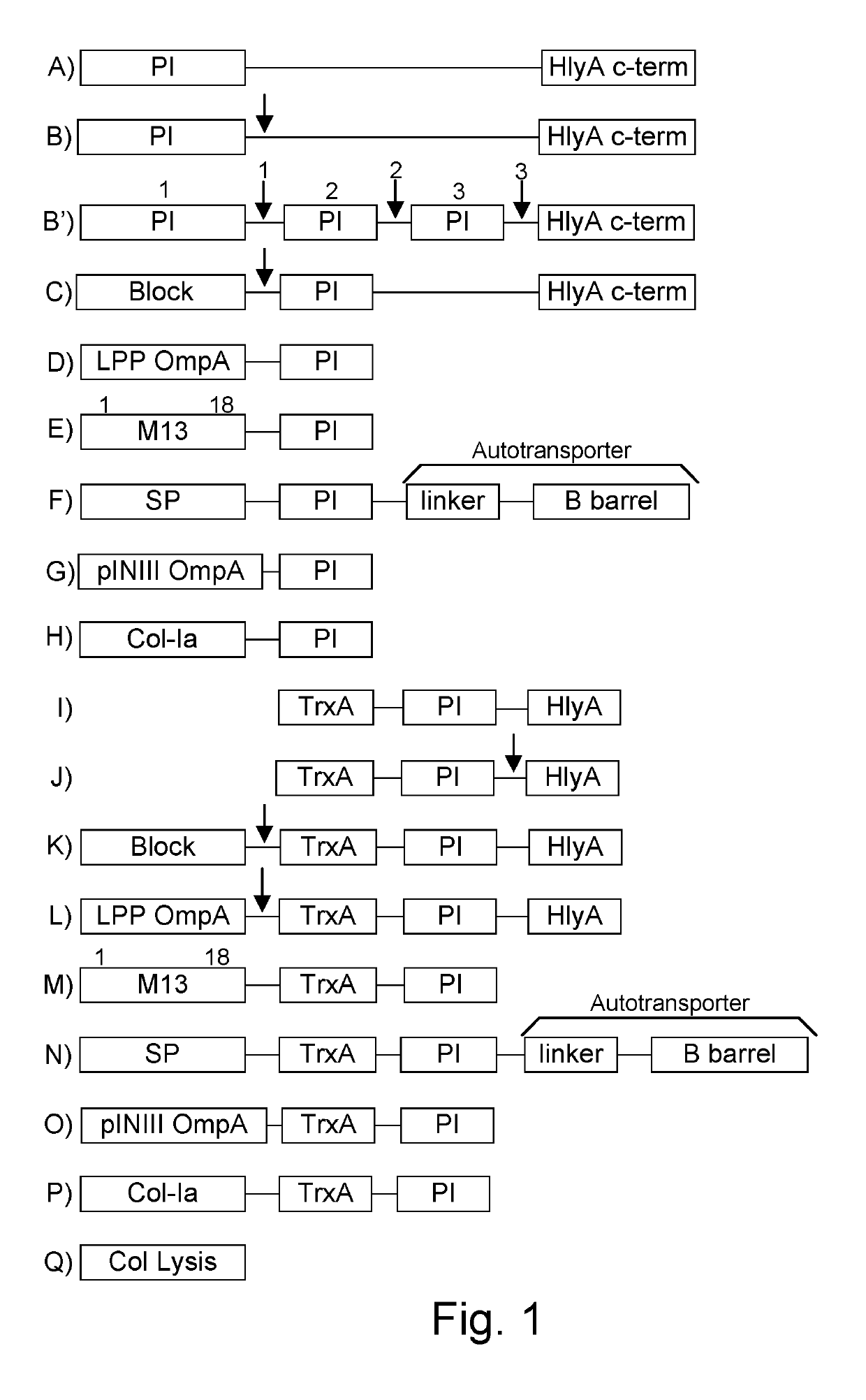
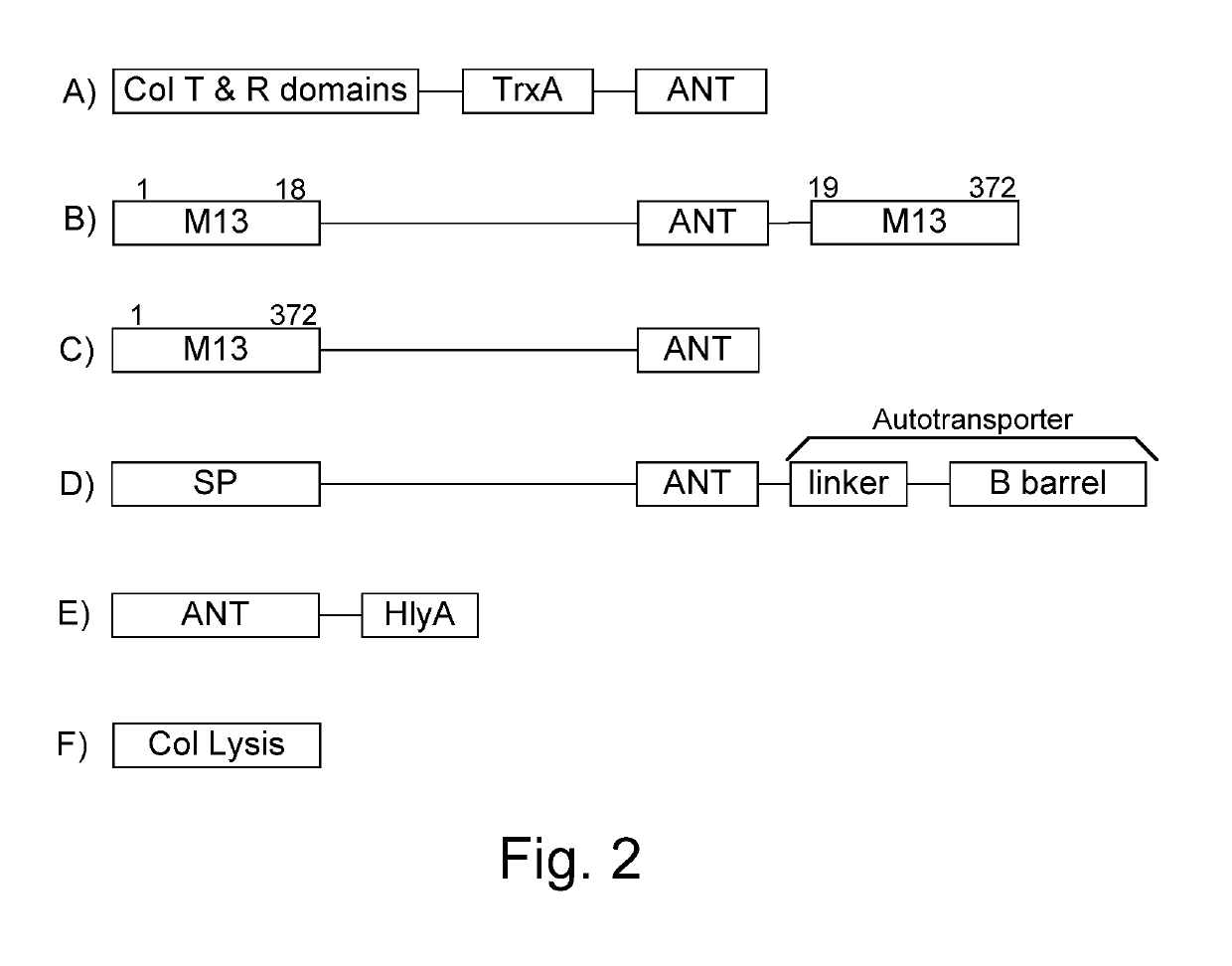
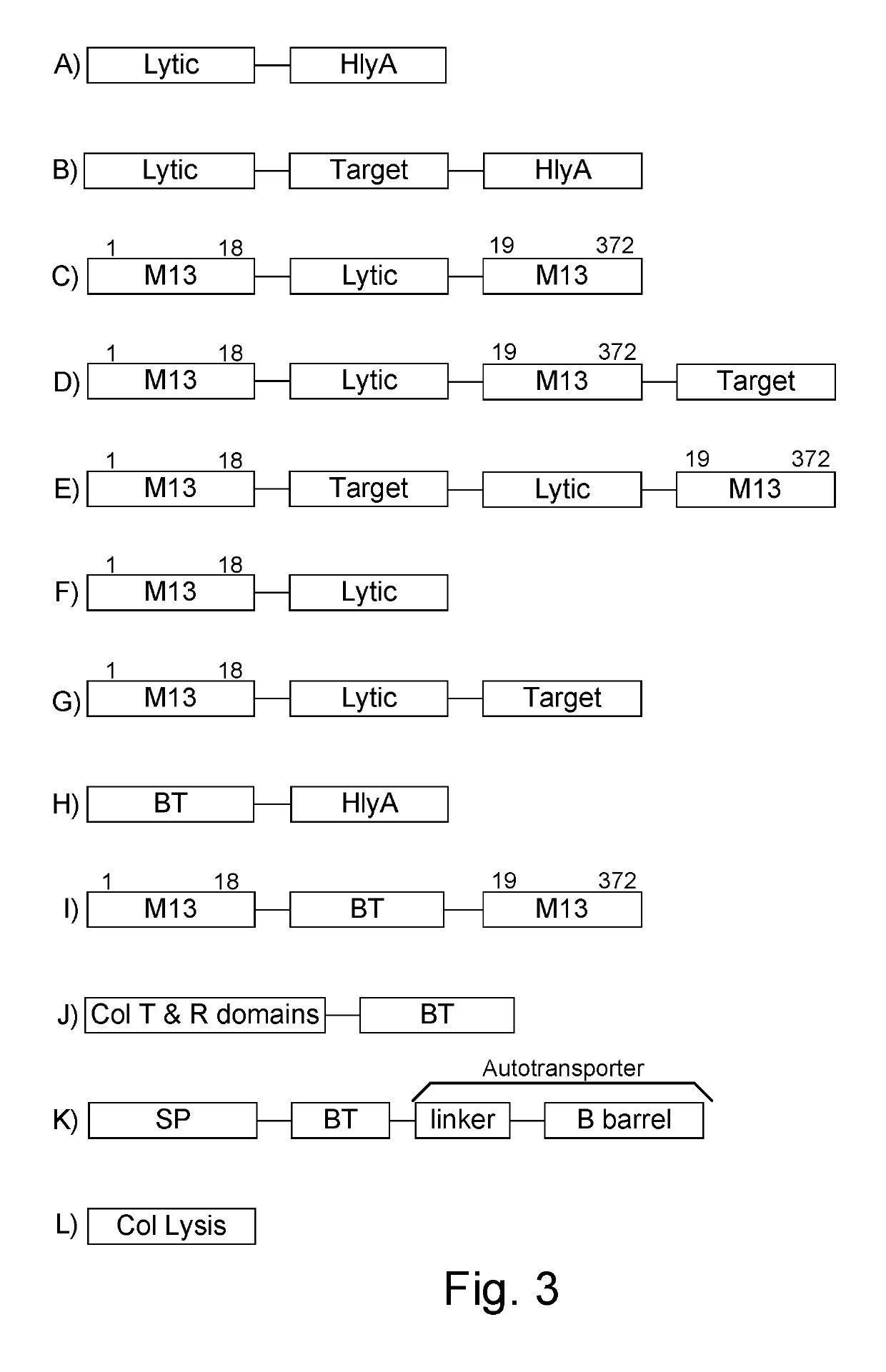
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS8771669B1Reducing eliminatingReducing or eliminating the targeted parasite, infectious diseaseVirusesBacteriaLytic peptideHuntingtons chorea
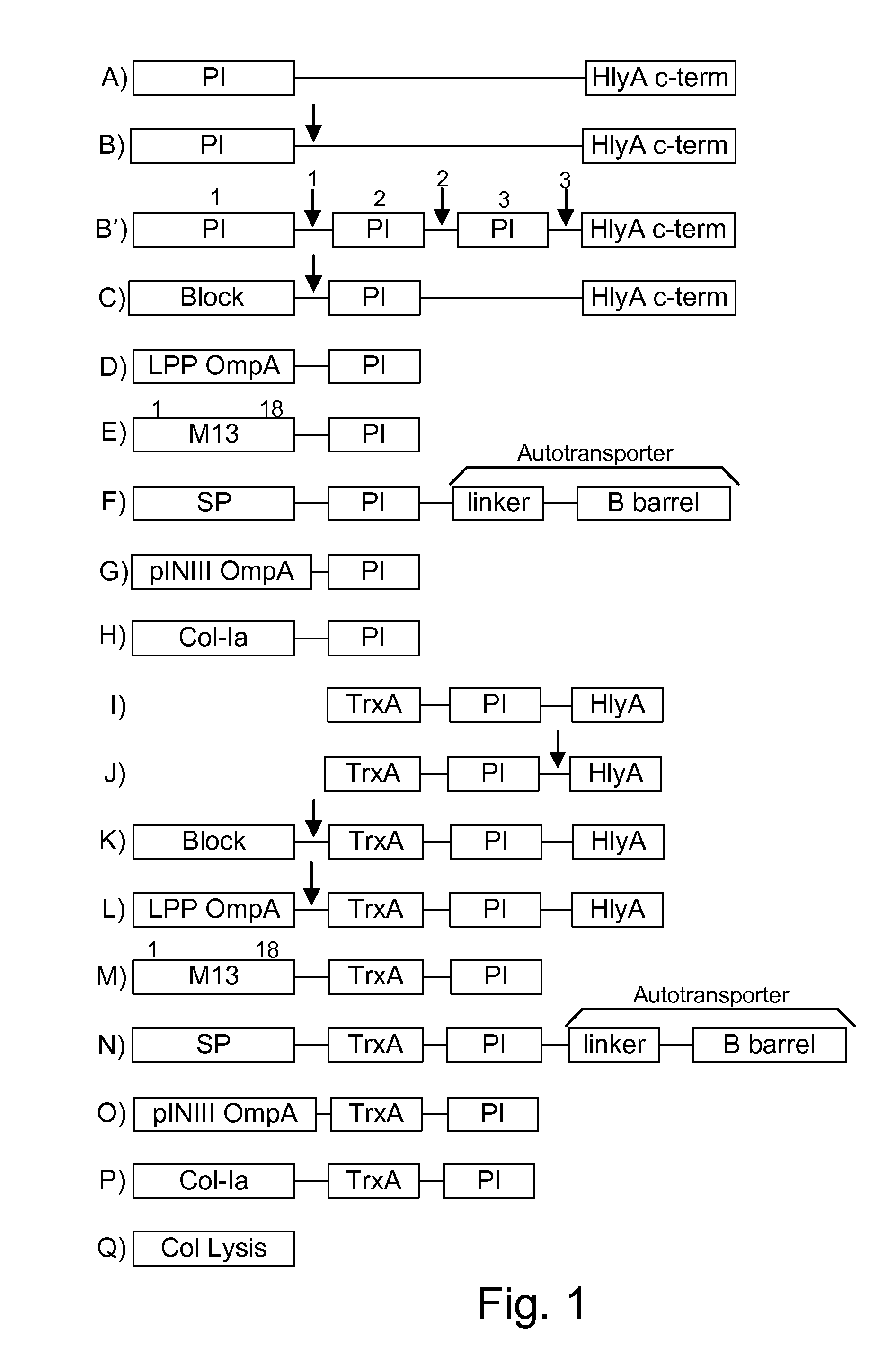
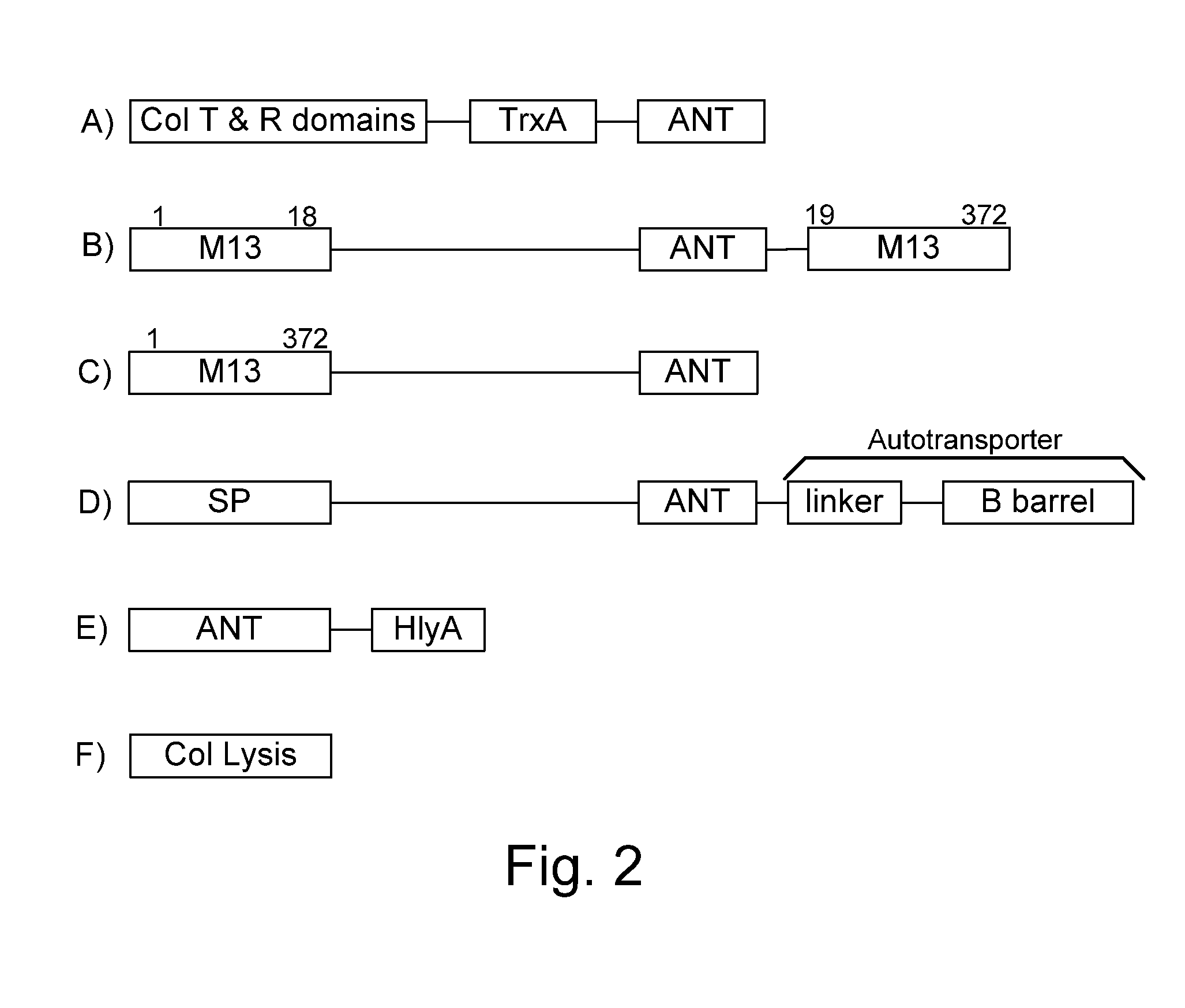
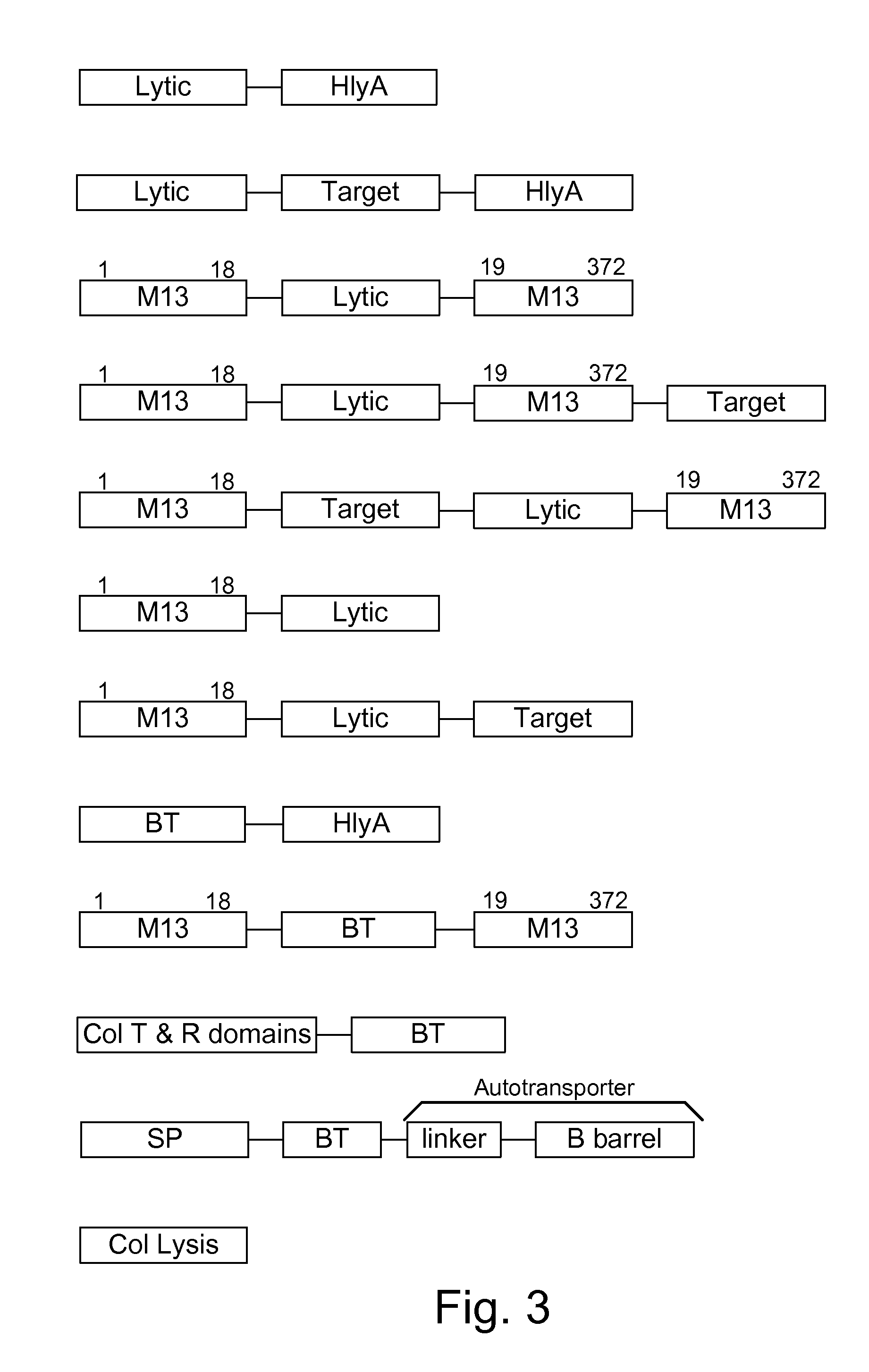
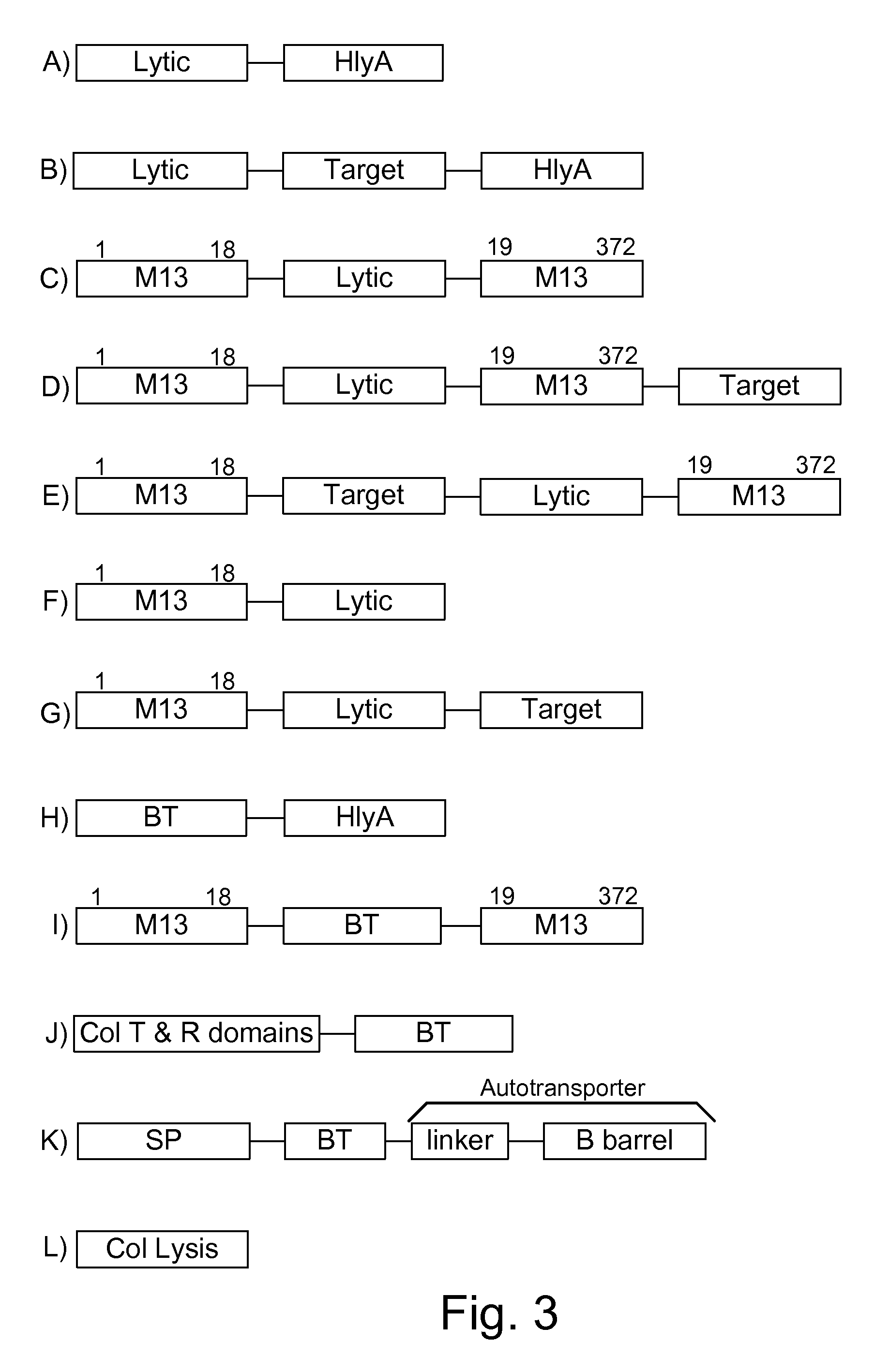
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID G DR
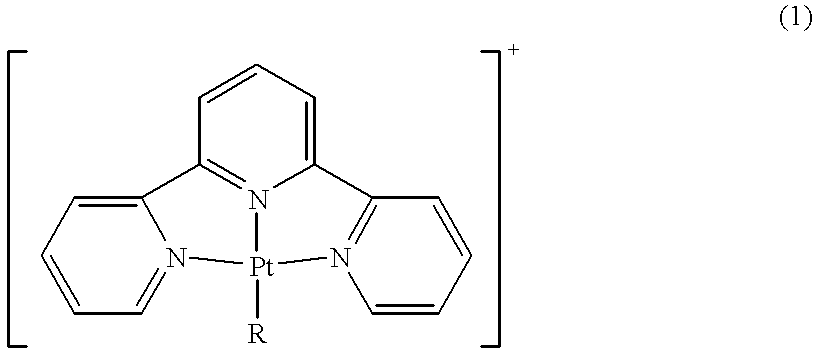
Terpyridine-platinum(II) complexes
InactiveUS20020013306A1Fast displacementImprove purification effectBiocideHeavy metal active ingredientsAntiparasiticChemical compound
A new class of 2,2':6',2''-terpyridine-platinum (II) and substituted 2,2':6',2''-terpyridine-platinum (II) complexes in which an N- or O- or halo nucleophile is the fourth ligand to platinum. The compounds are potent intercalators of DNA. Some have antitumour activity. Some have anti-parasitic activity. A new method of preparing the complexes involves reacting a Pt complex of 1,5-cyclooctadiene with a 2,2':6',2''-terpyridine.
Owner:ISIS INNOVATION LTD
Pesticidal and antiparasitic compositions
This invention relates to pesticide and antiparasitic compositions for the control of pests, diseases and parasites attacking plants and animals. The compositions include, at least one chitinolytic agent or a chitinolytic activity-inducing agent, and sulfide or a sulfide-producing agent from microorganisms or chemical compounds, wherein the chitinolytic agent or the chitinolytic activity-inducing agent and sulfur or a sulfur-producing agent obtaining from microorganisms or chemical compounds are concurrently applied at a range significantly lower than any of the above-mentioned compounds, when they are individually to attain effective control.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Geldanamycin derivatives and method of use thereof
The present invention relates to novel geldanamycin derivatives which have antitumor and antiparasitic properties. The geldanamycin derivatives disclosed herein have antitumor properties in humans due to their interaction with human heat shock protein 90 (hsp90). The human parasites Plasmodium falciparum, Trypanosoma Cruzi, and Leishmania donovani are lethally susceptible to exposure to geldanamycin via complexation of geldanamycin with their homologs (Pfhsp90, hsp83, and hsp90, respectively) of the human hsp90. The geldanamycin derivatives disclosed herein also interact with these parasitic hsp90 homologs so as to have antiparasitic properties.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS9486513B1Reducing or eliminating the targeted parasite, infectious diseaseSsRNA viruses negative-sensePeptide/protein ingredientsHuntingtons choreaAntiparasitic
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID GORDON
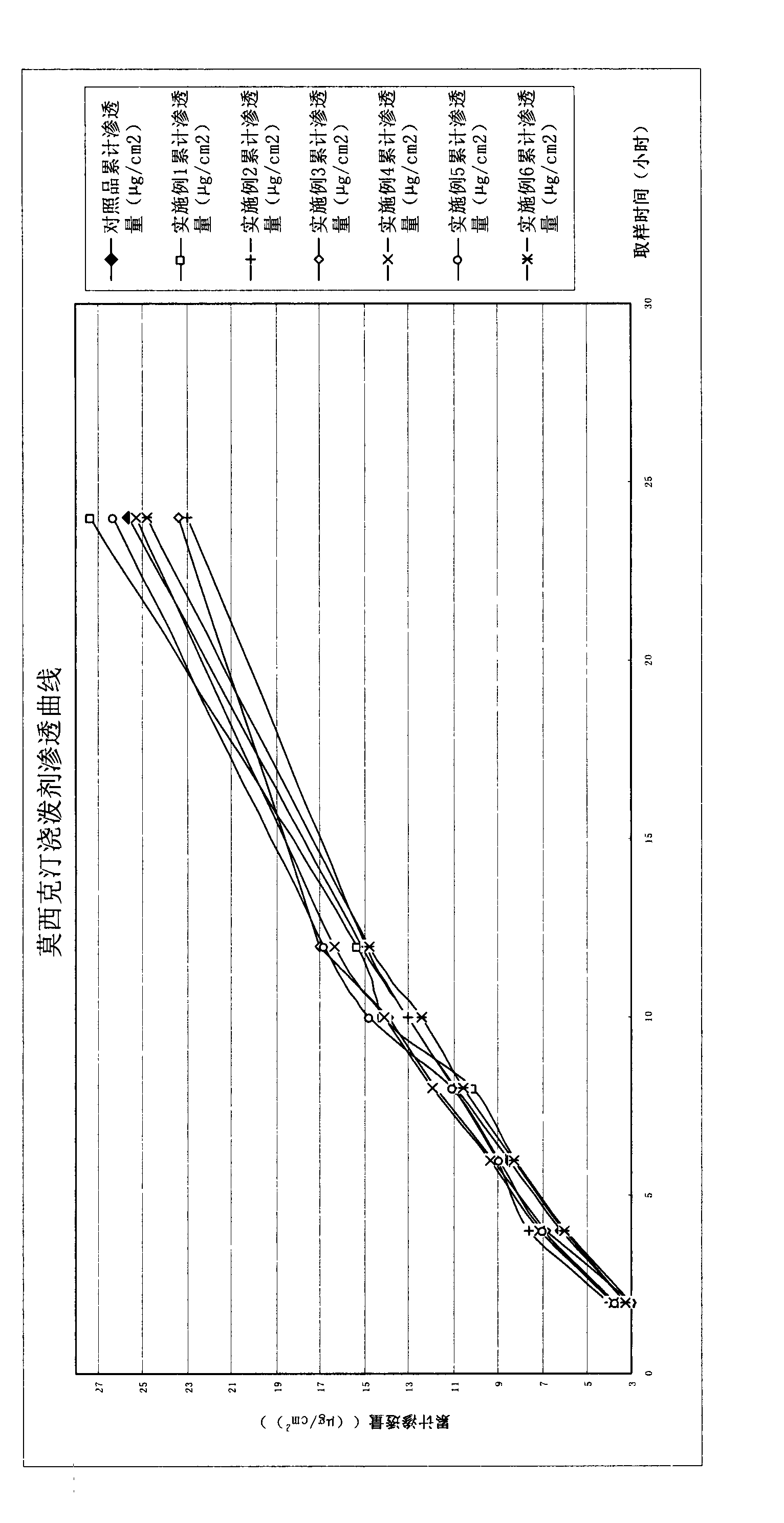
Anti-parasitic ivermectin transdermal solution used for livestock and preparation method thereof
InactiveCN101579309AFully reflect the advantages of preparationsPromote percutaneous absorptionOrganic active ingredientsPharmaceutical delivery mechanismSide effectThird generation
The invention relates to an anti-parasitic ivermectin transdermal solution used for livestock and a preparation method thereof. The anti-parasitic ivermectin transdermal solution comprises the following components respectively according to parts by volume: 5 to 15 parts of ethyl acetate, 20 to 30 parts of dimethyl sulfoxide, 2 to 6 parts of azone, 1 to 3 parts of isopropanol and 50 to 70 parts of propanediol; and the content of ivermectin is 0.3g to 1g in a solution of 100ml, and the finished product is prepared by the processes of dissolving, mixing, filtering and filling. The azone related in the prescription of the invention is a novel skin penetration enhancer, can increase the transdermal absorption of the skin to various medicines of different types, effectively avoids the pass effect of hepar, avoids peak and valley phenomena in the absorption process of the medicine, reduces the toxic or side effect caused by the correlative dosage in the medicine, prolongs the effective effect time, changes the administration area, effectively regulates the administration dosage and reduces differences among individuals. The invention has the characteristic of simple, convenient and fast use, and is more suitable for the anti-parasitic prevention and treatment of a pasturing area compared with other preparation forms.
Owner:TIANJIN BIJIA PHARMA CO LTD
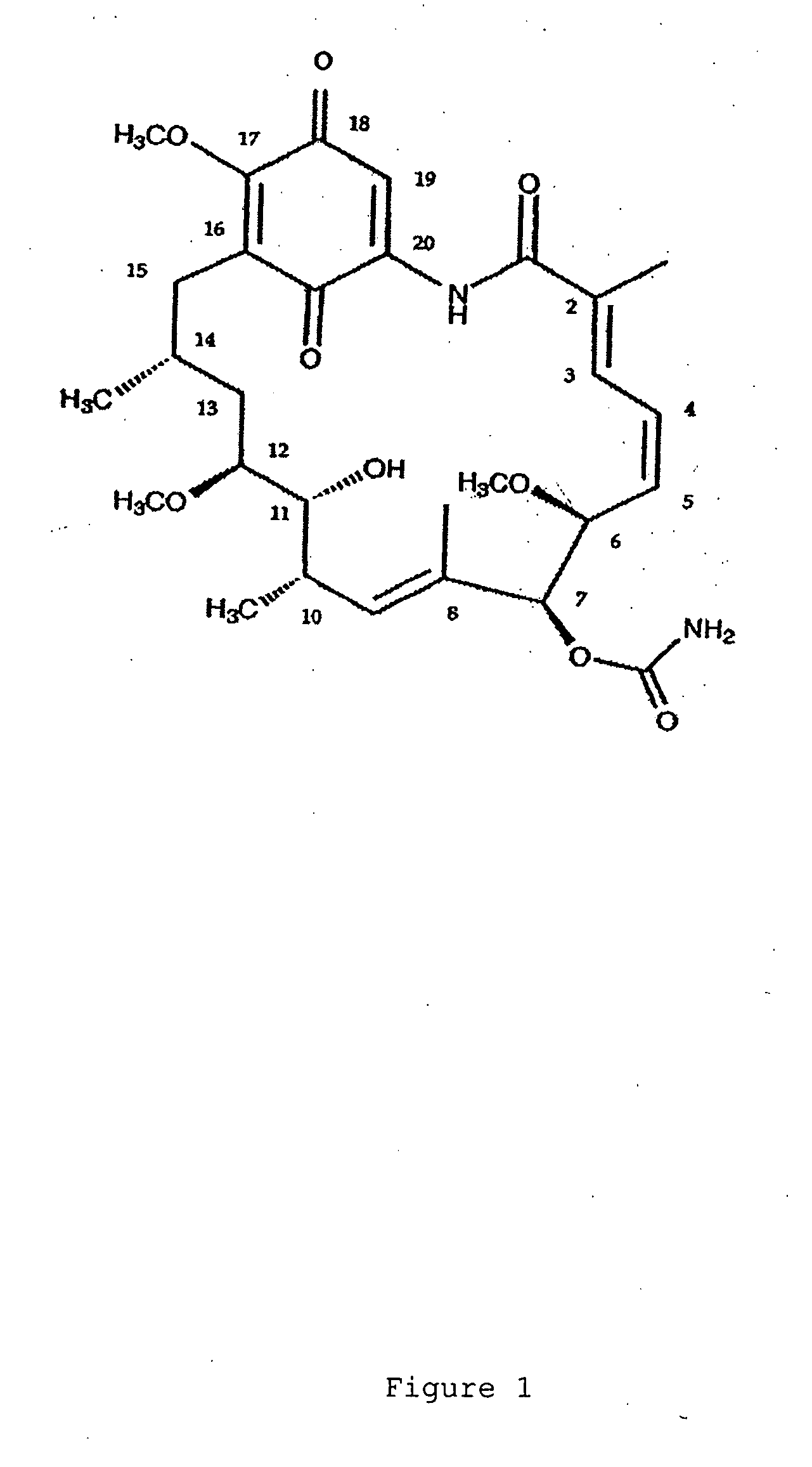
Optically pure alpha-ketoacyl harringtonine and preparing and purifying method thereof
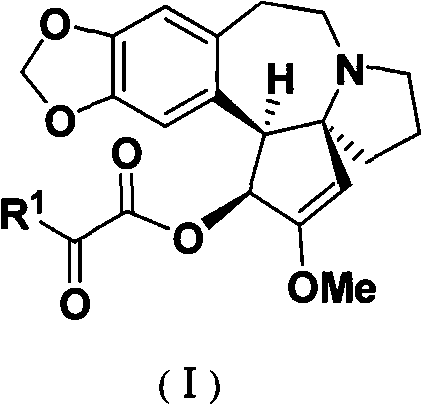
The present invention relates to an optically pure alpha- ketoacyl harringtonine and a preparing and purifying method thereof. In the temperature of -80 DEG C to 50 DEG C, the alpha-ketoacyl chlorine which is prepared through reacting alpha-ketonic acid and oxalyl chloride reacts with the cephalotaxine in an inert organic solvent while the organic base is used as an acid-binding agent for obtaining the oily product represented by the formula (I). The purifying steps are as follows: dissolving the oily product with the inert organic solvent, adding the saturated NaHSO3 solution, mixing and separating the liquid; after washing the water phase with the organic solvent, adjusting the pH of the water phase with saturated NaHSO3 solution to 7-8, extracting with the organic solvent; washing the organic phase with the buffering solution with pH of 6.8 and the saturated saline solution, drying and filtering the organic phase, removing the solvent for obtaining the pale-yellow solid; and then recrystallizing with the organic dissolvent for obtaining the white solid or colorless crystal. The optically pure alpha- ketoacyl harringtonine is a key intermediate for synthesizing the medicine of harringtonine alkaloid, which is widely applied for anti-tumor (malignant tumor and benign tumor), antiparasitic, antifungal and antibacterial chemotherapy. The synthesizing method is suitable for purifying and preparing the large amount of optically pure compound represented by the structural formula of (I).
Owner:NANKAI UNIV
Antiparasitic agents
Owner:PFIZER ANIMAL HEALTH UK 1 LIMITED
Antileishmanial dinitroaniline sulfanomides with activity against parasite tubulin
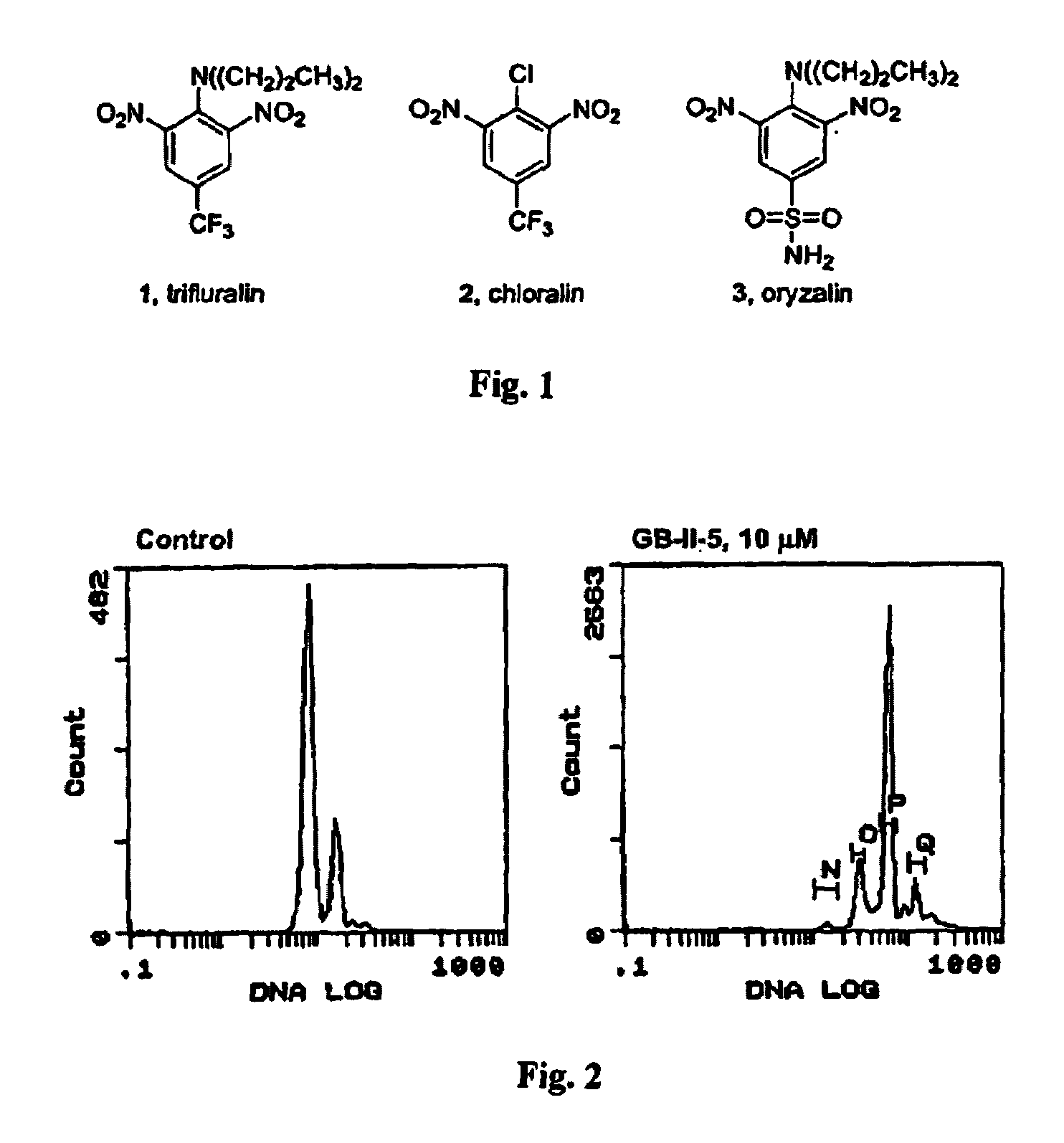
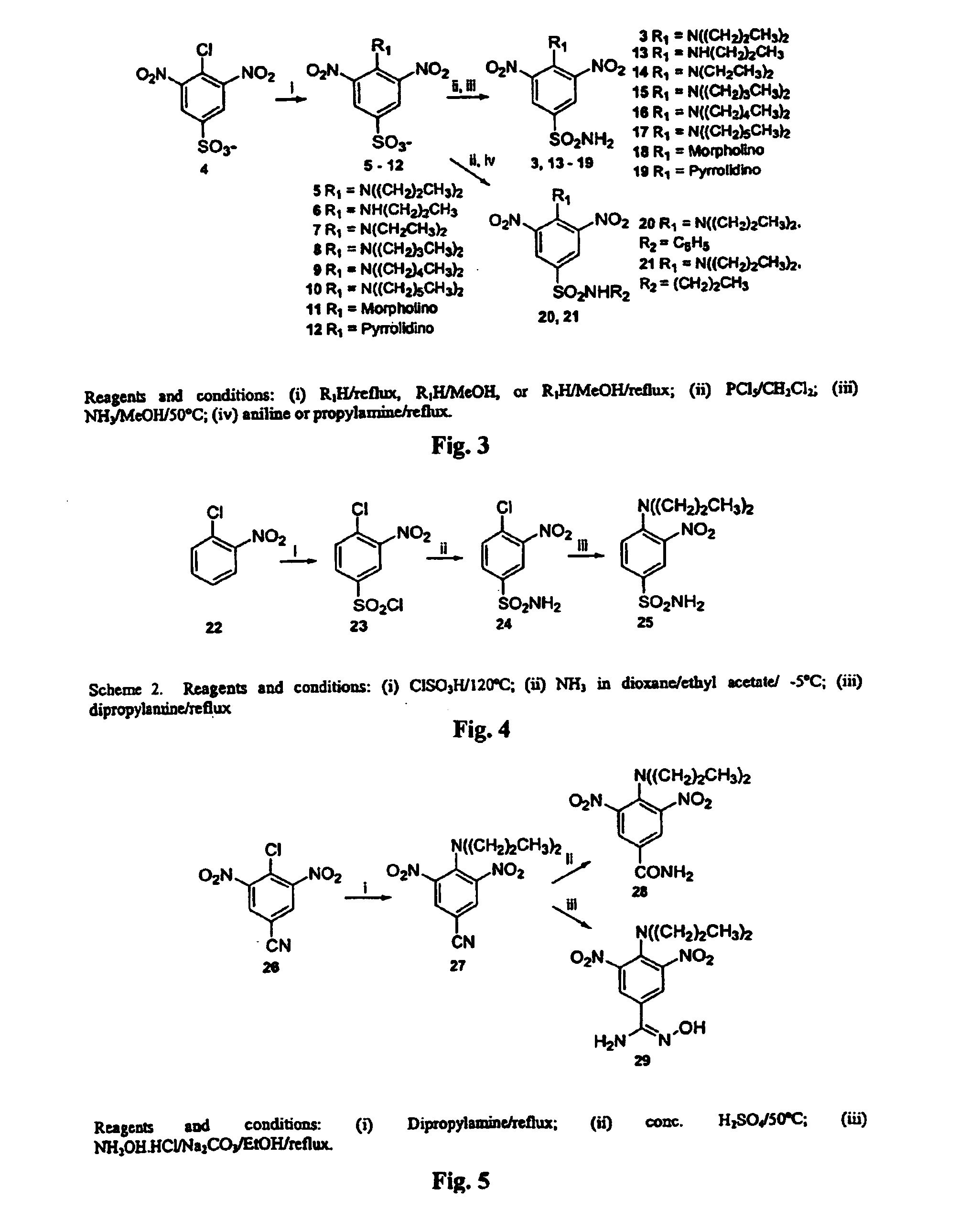
Dinitroaniline compounds useful for the treatment of diseases caused by parasitic protozoa in subjects in need of such treatment. The compounds are particularly useful in the treatment of leishmaniasis. The compounds are preferably less cytotoxic to normal cells than oryzalin. Also provided are methods of treating subjects having diseases caused by parasitic protozoa, preferably humans. The method comprising administering a therapeutically effective amount of a dinitroaniline compound of the present invention to a subject in need of such treatment
Owner:THE OHIO STATES UNIV +1
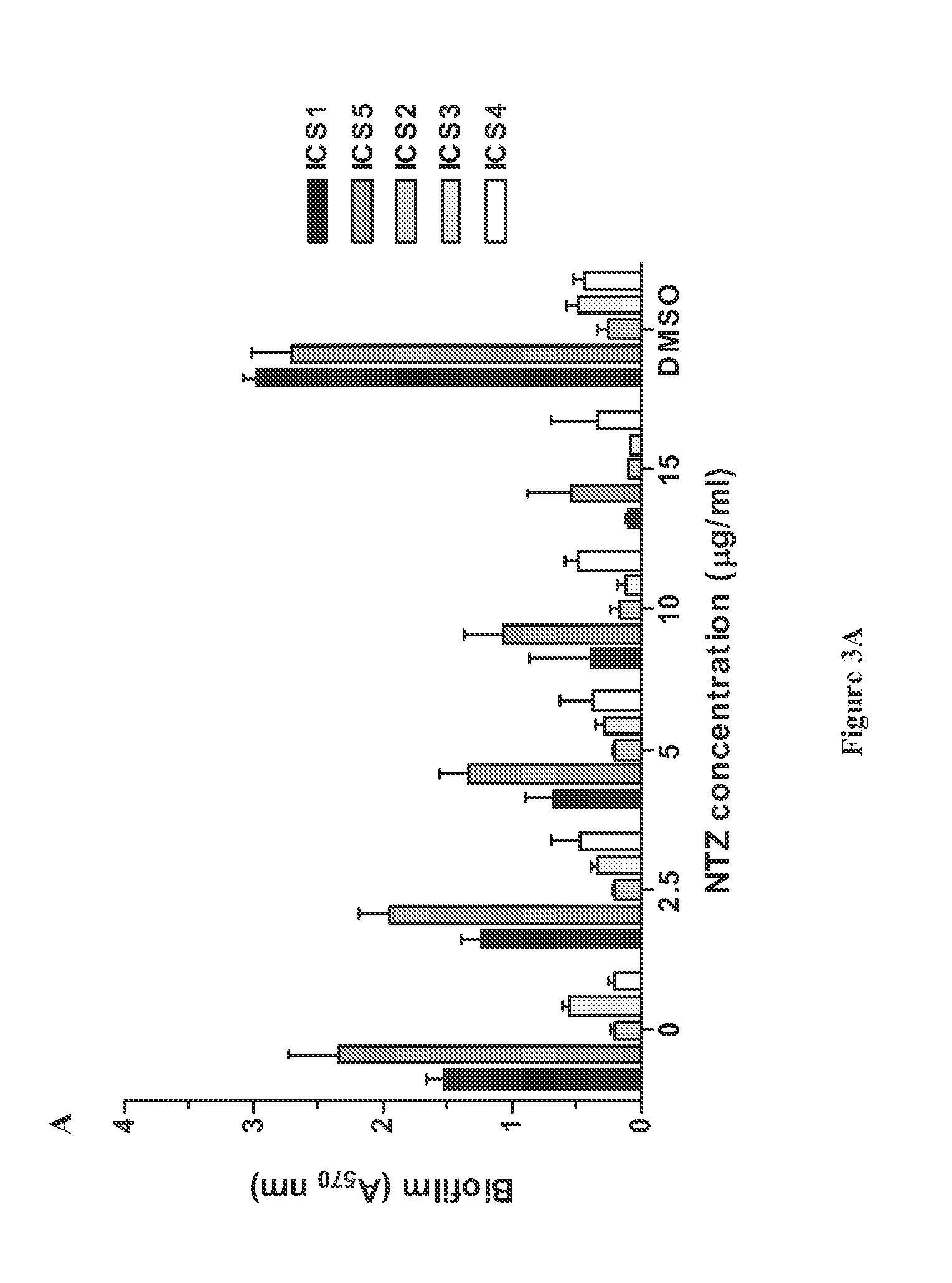
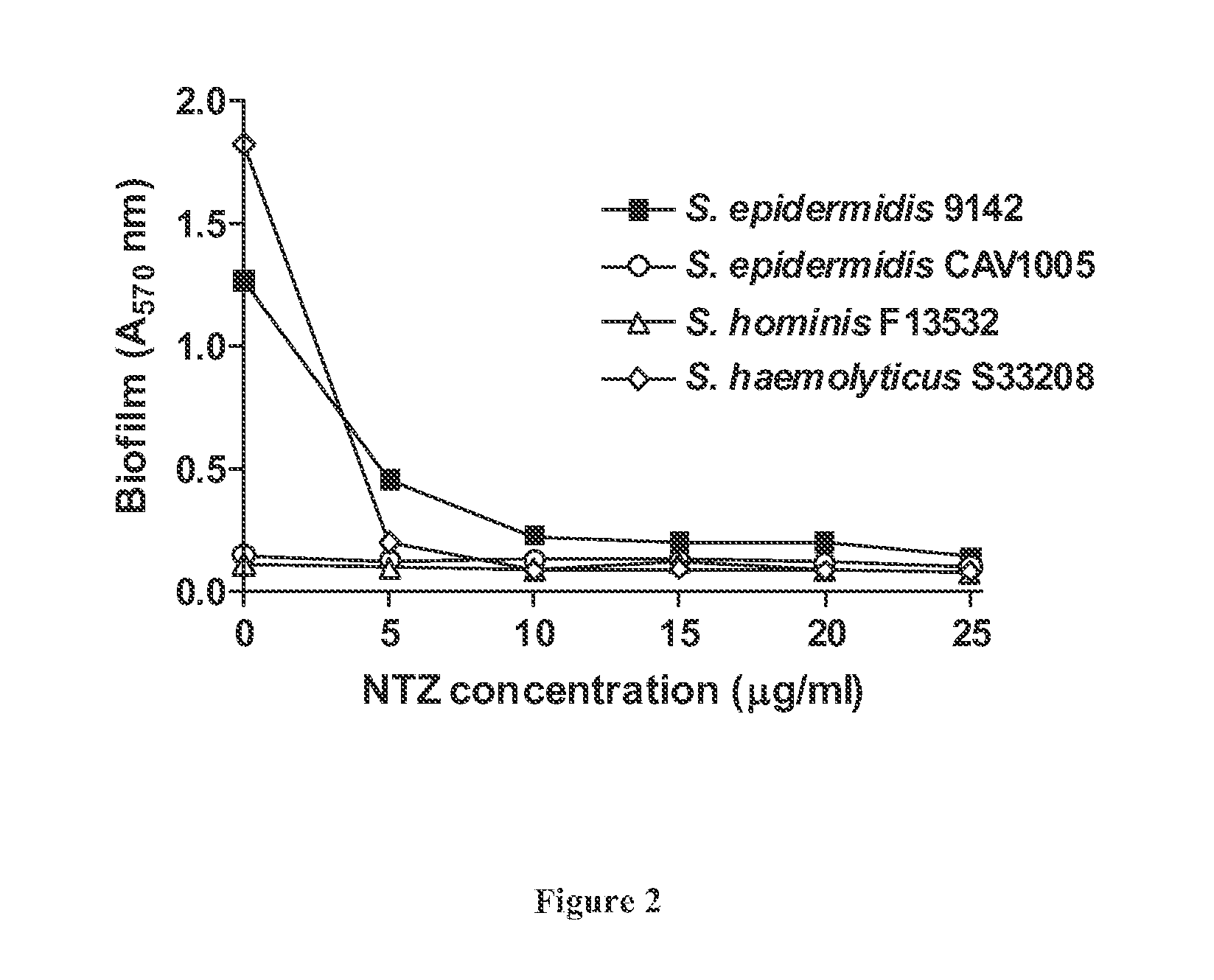
Broad spectrum benzothiophene-nitrothiazolide and other antimicrobials
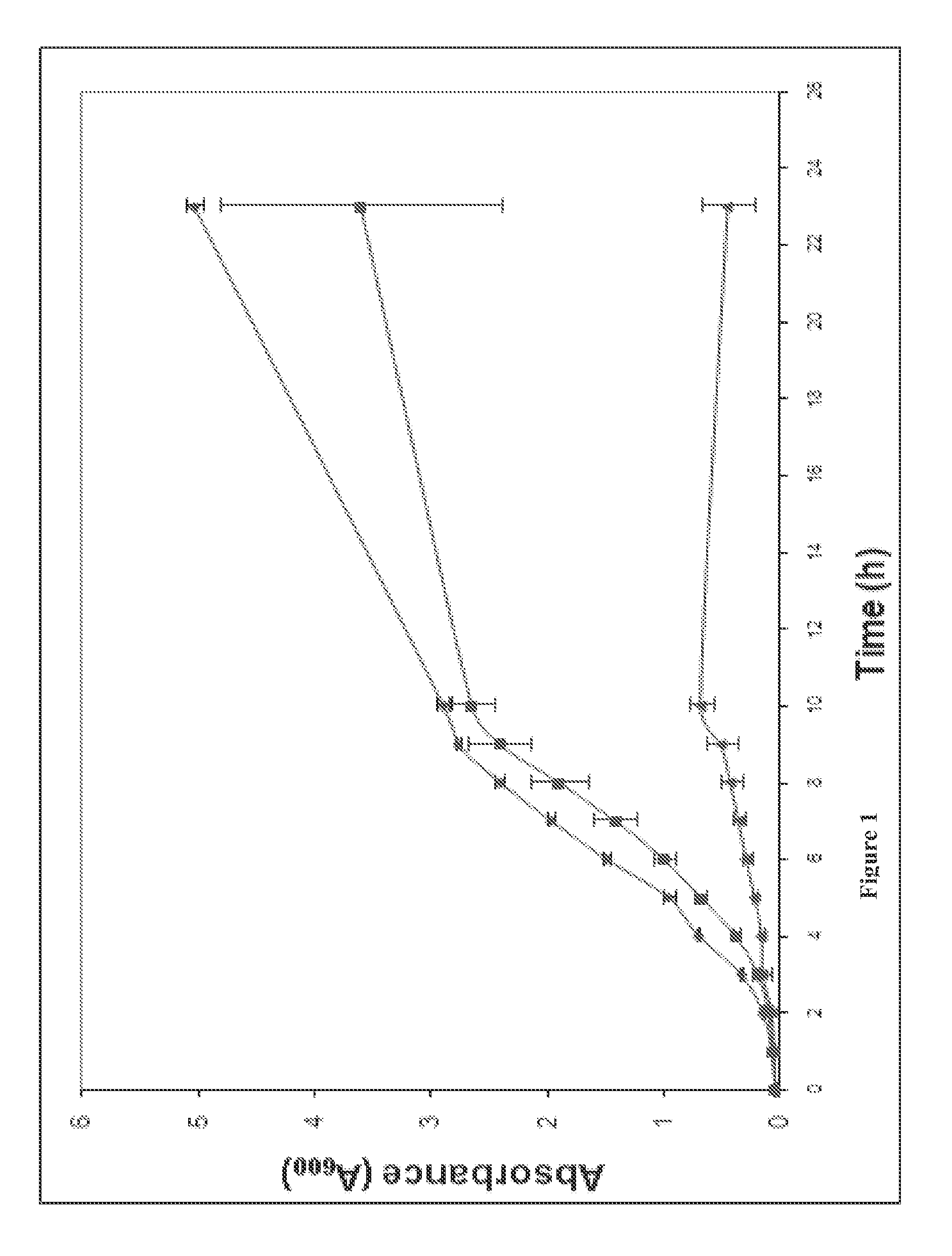
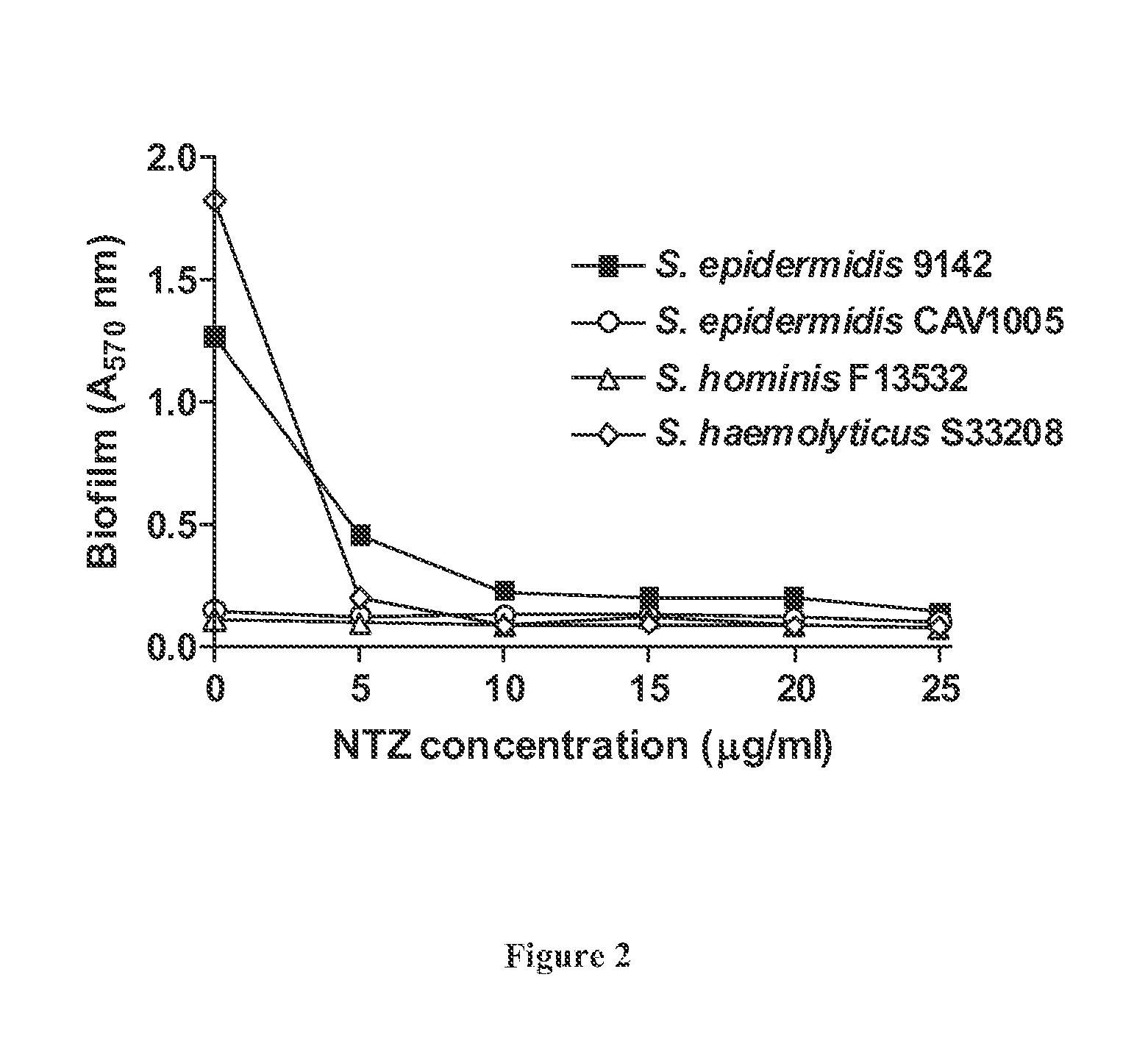
The invention provides FIG. 1 novel antimicrobial chemical entities based on a nitrothiazolide backbone that exhibit antibacterial and antiparasitic action against a wide range of human pathogens. The new classes of compounds show extended action against Gram positive bacteria including MRSA drug resistant pathogens. In the Gram-positive organisms, they specifically target and functionally inhibit microbial attachment to surfaces and biofilm formation. In Gram-negative bacteria, including enteroaggregative E. coli strains, these compounds function as pilicides by inhibiting the assembly of pilin subunits into adhesive filaments. Several of these compounds show potent antimicrobial action against Gram positive bacteria, perhaps involving novel targets. Many of the benzothiophene derivatives exhibit antimicrobial activity in the low micrograms per ml range and in blocking biofilm formation in the nanomolar range; ranges considered are well within the range of utility as therapeutics.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
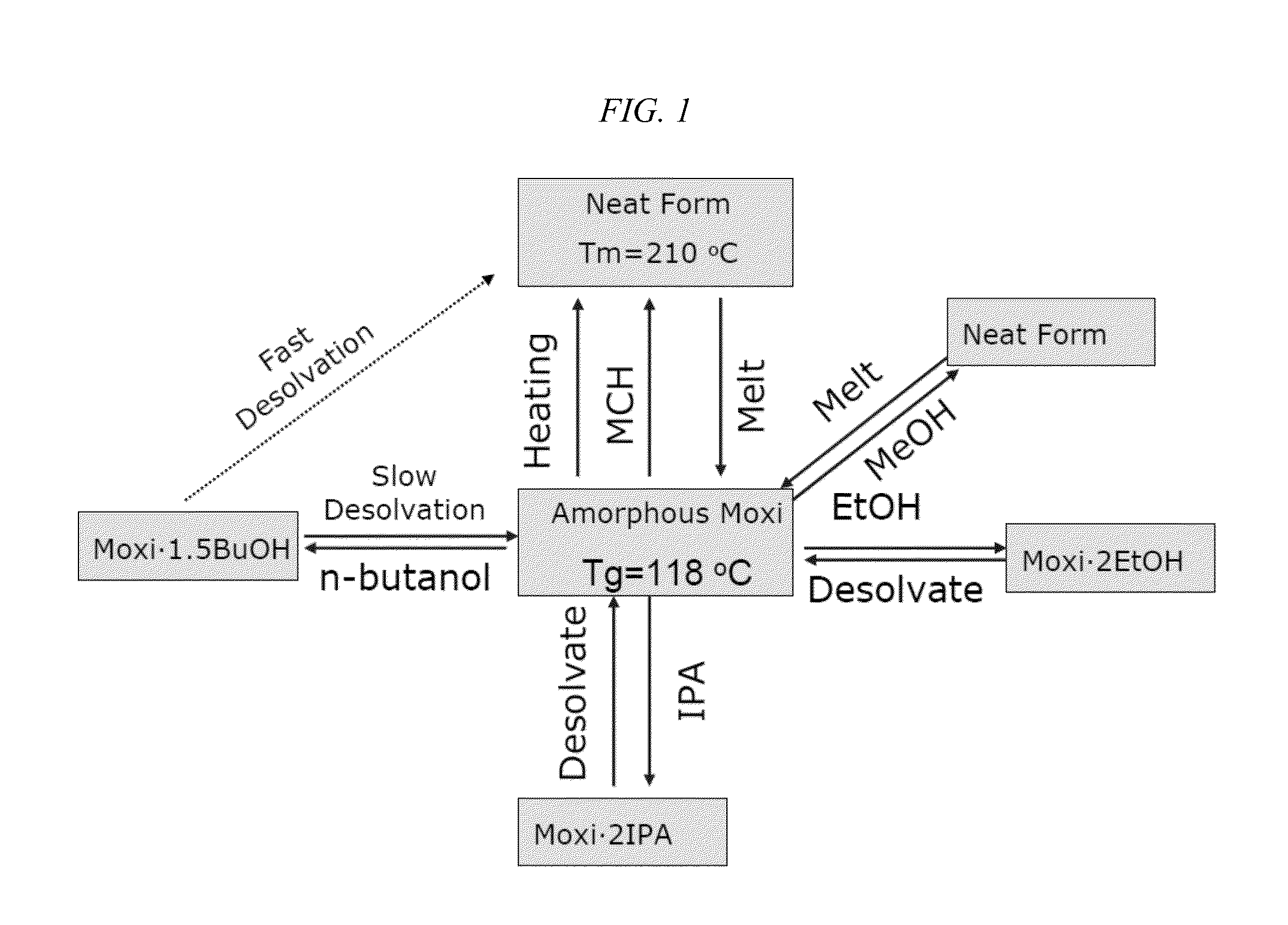
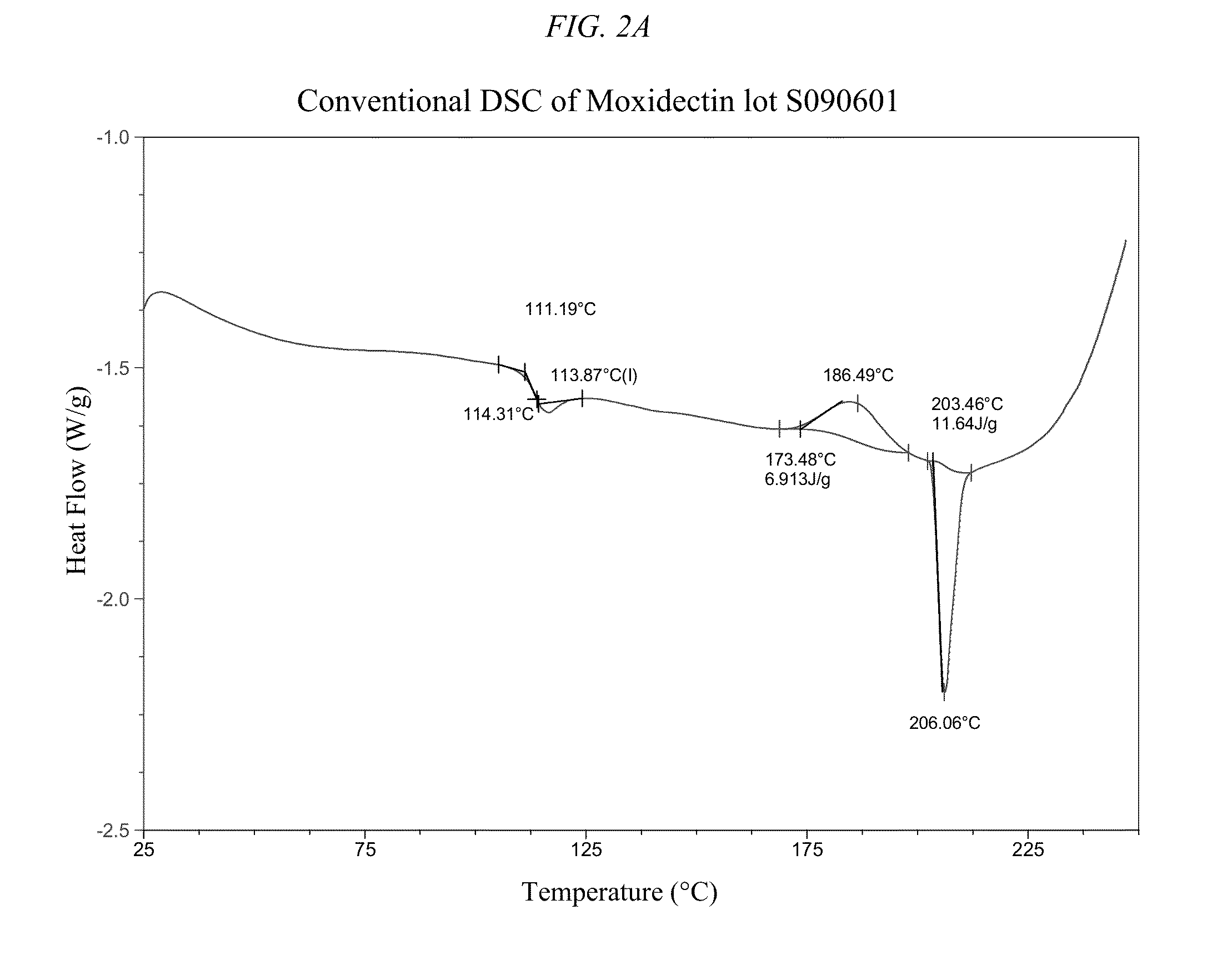
Long-acting injectable moxidectin formulations and novel moxidectin crystal forms
This invention provides for novel antiparasitic and pesticidal forms of moxidectin, including a long-acting polymeric implant. The resulting compounds may be used in veterinary compositions which are used in treating, controlling and preventing of endo- and ectoparasite infections in animals.
Owner:MERIAL INC
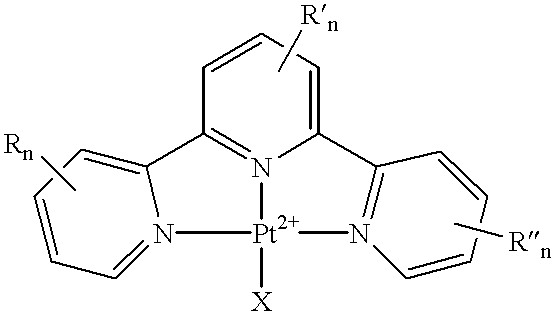
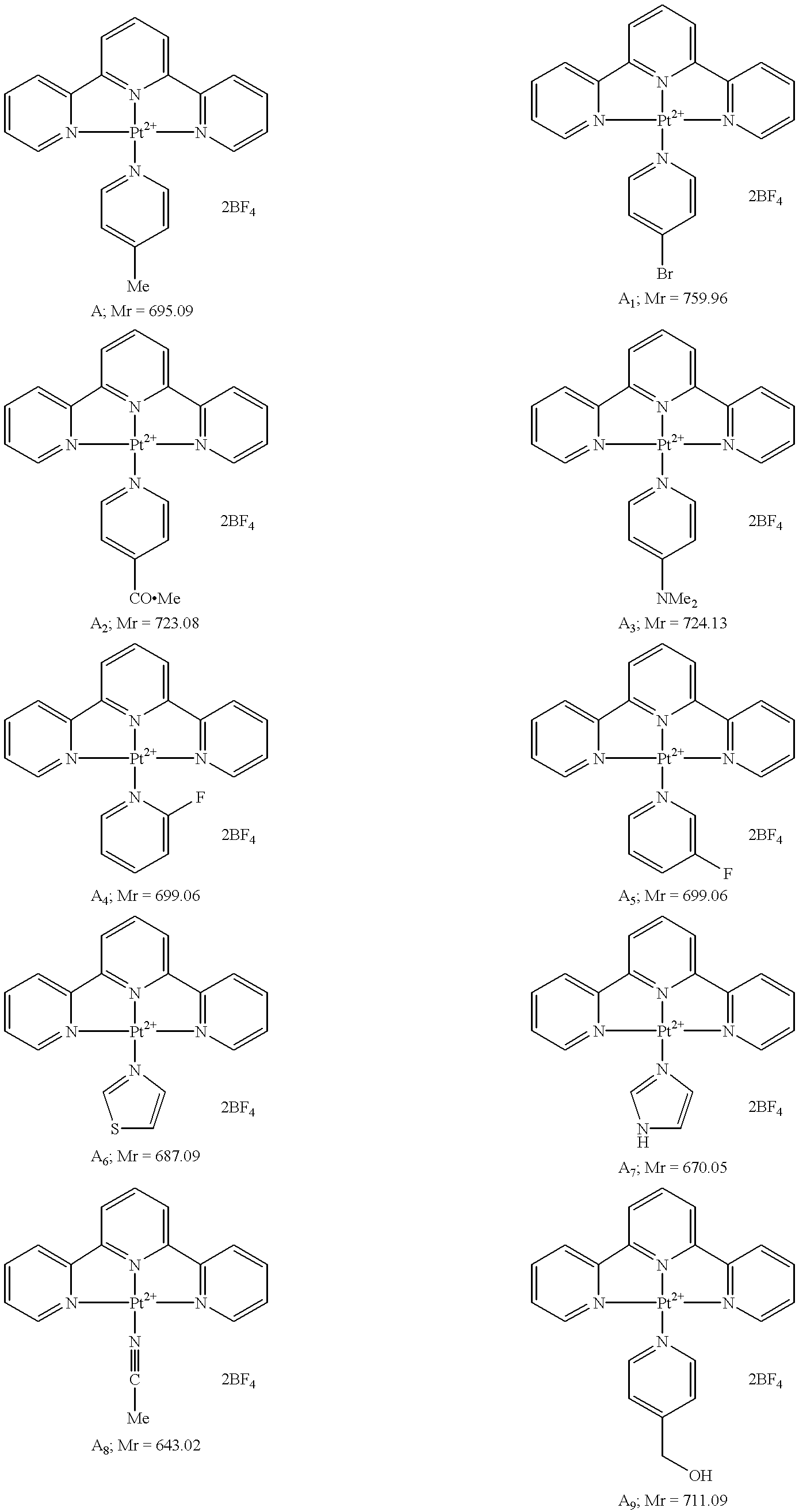
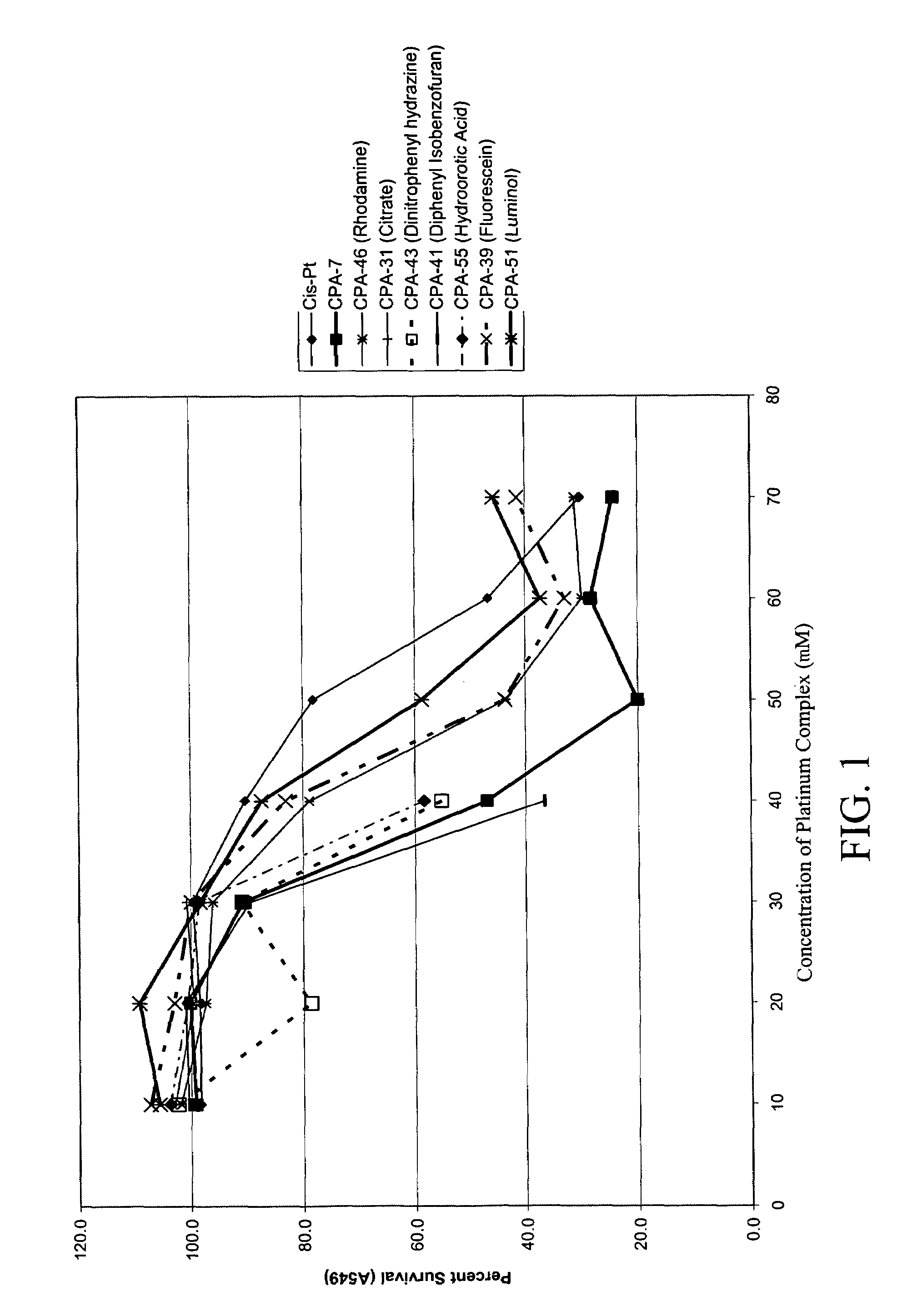
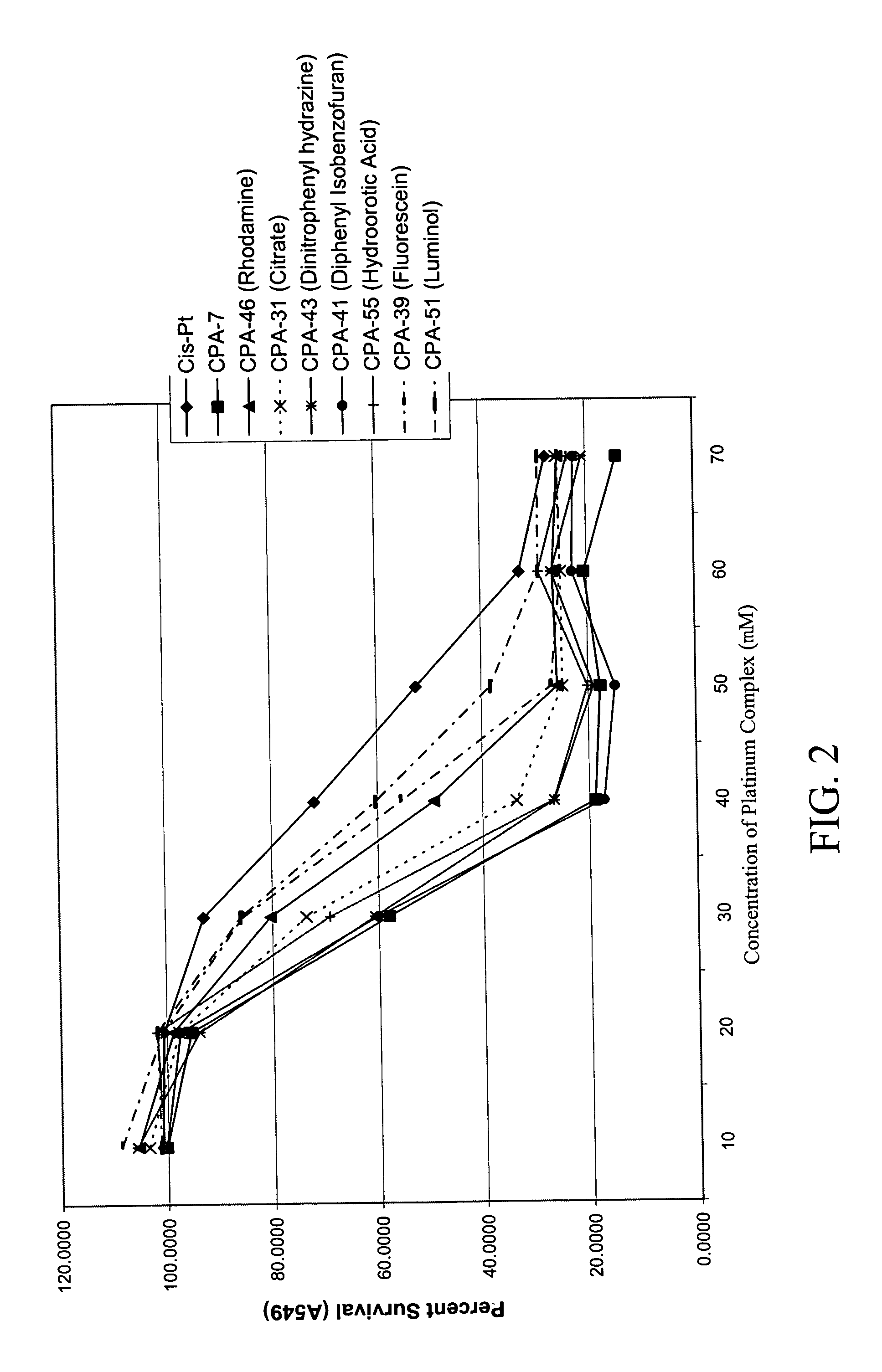
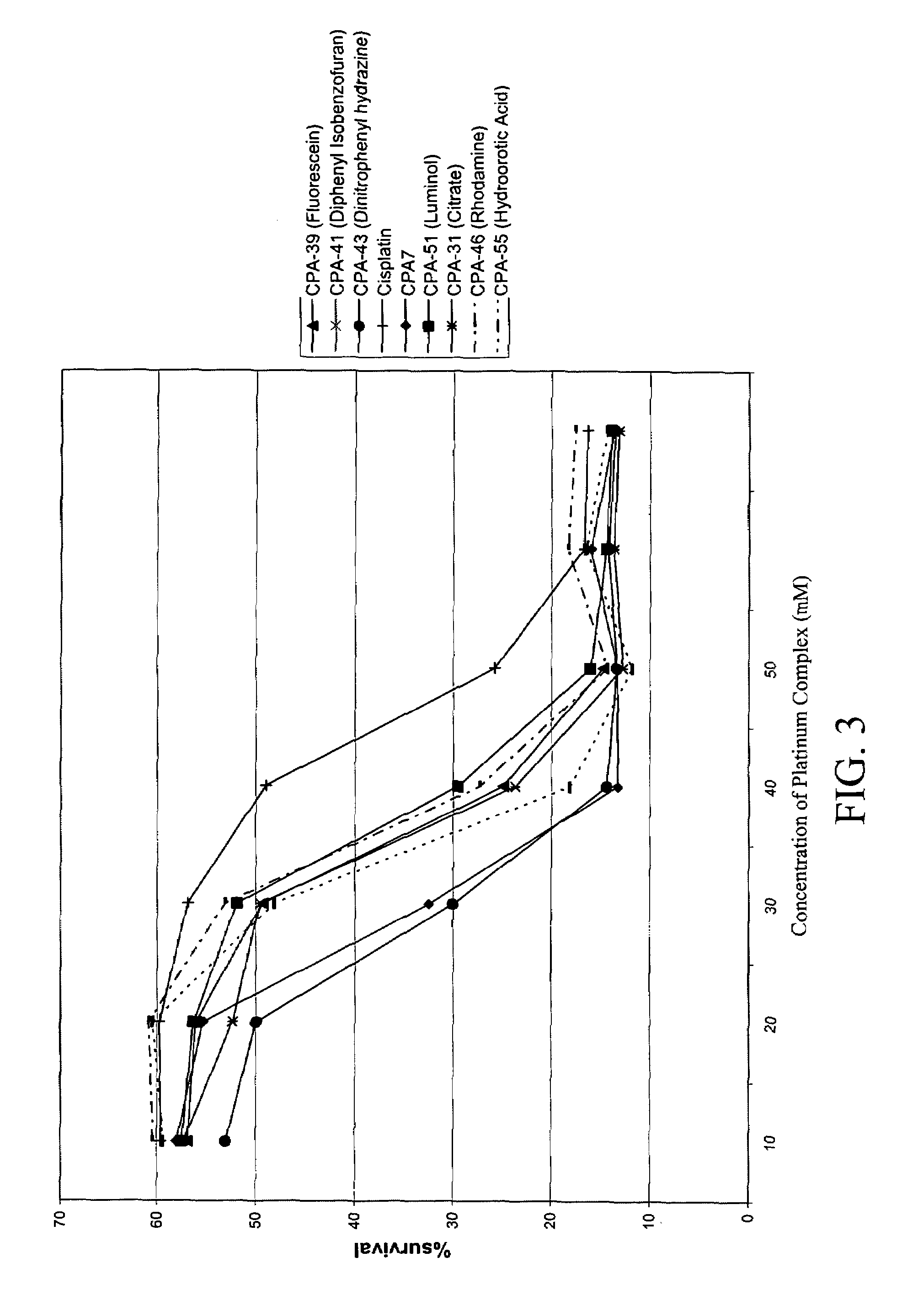
Platinum complexes and methods of use
The subject invention concerns platinum complexes that exhibit antitumor cell and / or antiparasitic activity. The subject invention also concerns the use of platinum complexes of the invention to treat oncological and inflammatory disorders. The platinum complexes of the invention can also be used to treat or prevent infection by a virus or a bacterial or parasitic organism in vivo or in vitro.
Owner:SOUTH FLORIDA UNIVESITY OF
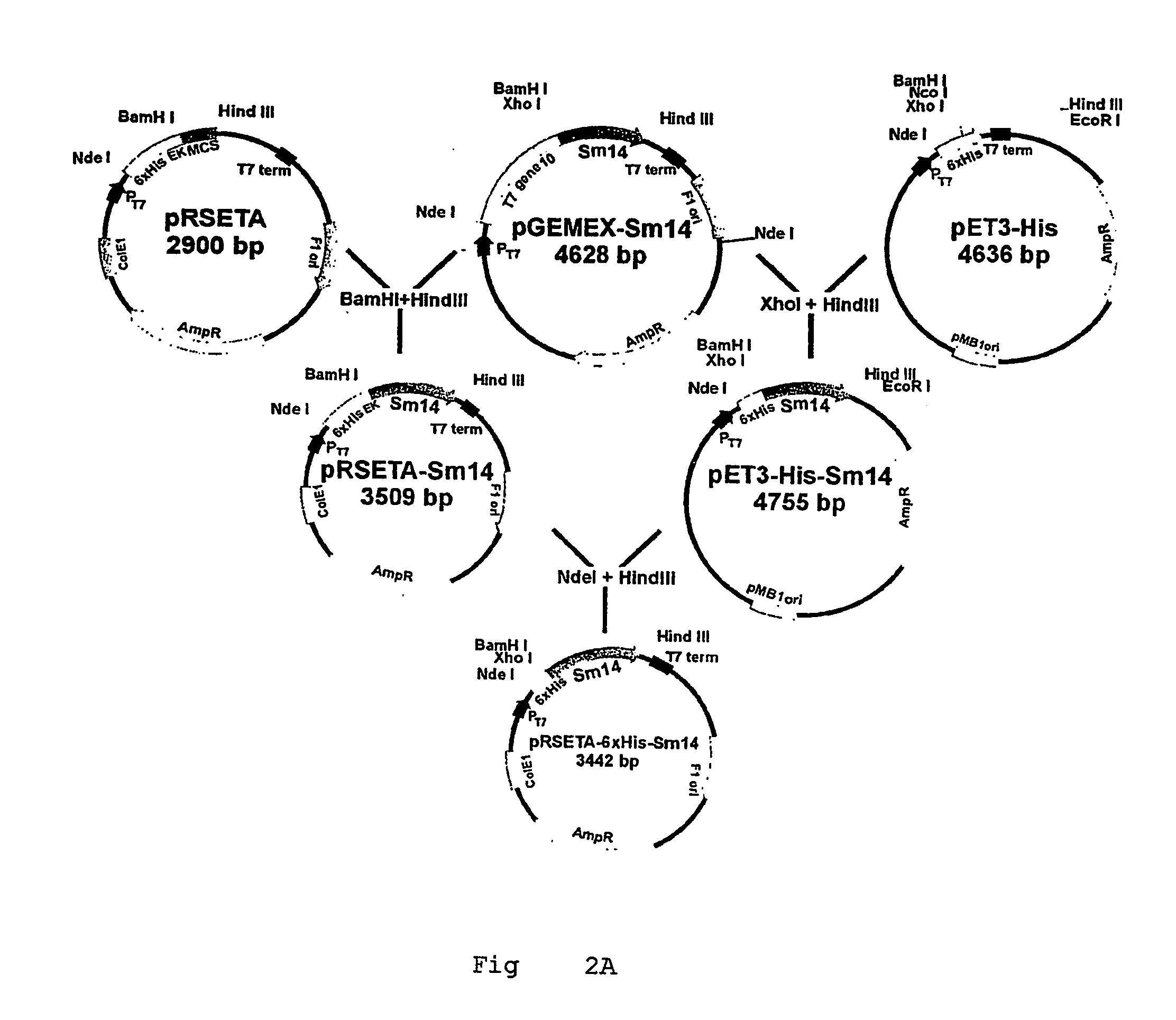
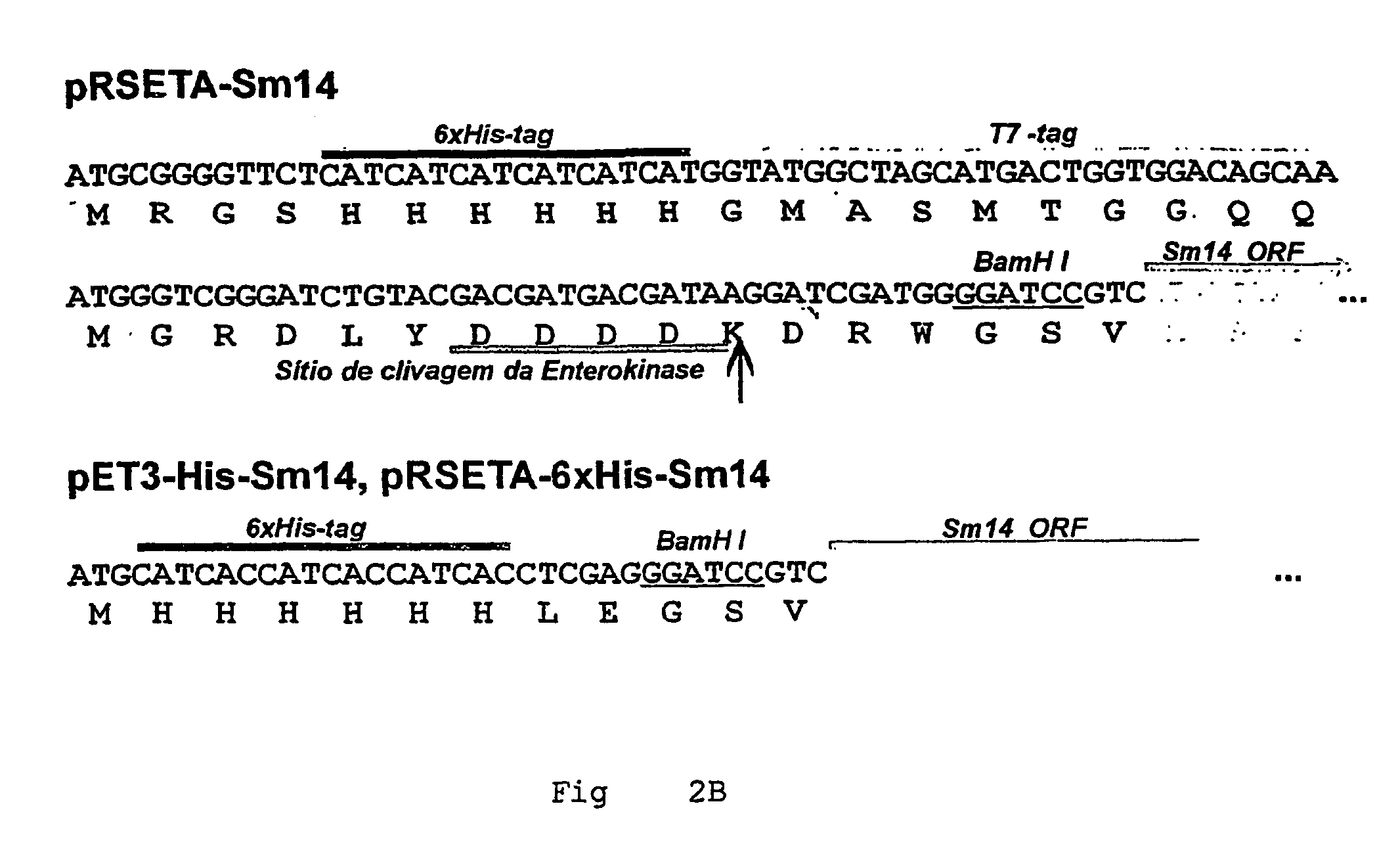
Helminth-derived antigens having capacity of providing protection against parasites
ActiveUS20070021332A1Stably formPeptide/protein ingredientsAntiparasitic agentsMutated proteinWild type
The primary objective of the present invention is the development of new mutant forms of the Sm14 protein, for producing a greater production volume. The recombinant proteins here obtained were capable of providing protection against schistosome and fasciola infection. The level of protection of Sm14 recombinant proteins obtained in the present invention was similar to that reached in the parasite saline extract. The mutant proteins of the present invention have reached approximately 100% of renaturation after the heating at 80° C., different from wild forms of the Sm14 protein. Moreover, after storage for 2 months at 4° C., mutant proteins have shown smaller β-structure loss than wild forms that have shown formation with random structure, as demonstrated by the circular dichroism analysis, indicating the success of mutations.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
Moxidectin pour sprinkling preparation and preparation method thereof
ActiveCN102133173ADoes not contain high toxicityLow flash pointOrganic active ingredientsPharmaceutical delivery mechanismMedicineAntioxidant
The invention provides a novel and environmental-friendly Moxidectin pour sprinkling preparation and an industrialized preparation method thereof. The pour sprinkling preparation developed in the invention is prepared by taking Moxidectin as an active ingredient for resisting parasites inside and out of animals and adding an appropriate amount of auxiliaries, such as an antioxidant, a trandermal absorbent, an adhesive, an oil solvent and the like; and the formulation of the preparation is the oily pour sprinkling preparation and belongs to the field of novel veterinary medicinal antiparasitic preparations. The pour sprinkling preparation does not contain an aromatic compound with a low flash point and the potential safety hazard in industrial production. In order to improve medicinal effect, the trandermal absorbent is selected through an in-vitro trandermal absorption test so as to improve the bioavailability of the Moxidectin pour sprinkling preparation in the animals. The environmental-friendly Moxidectin pour sprinkling preparation which has a long-term effect, and is safe and easy to industrially produce is prepared finally.
Owner:ZHEJIANG HISUN ANIMAL HEALTH PROD CO LTD
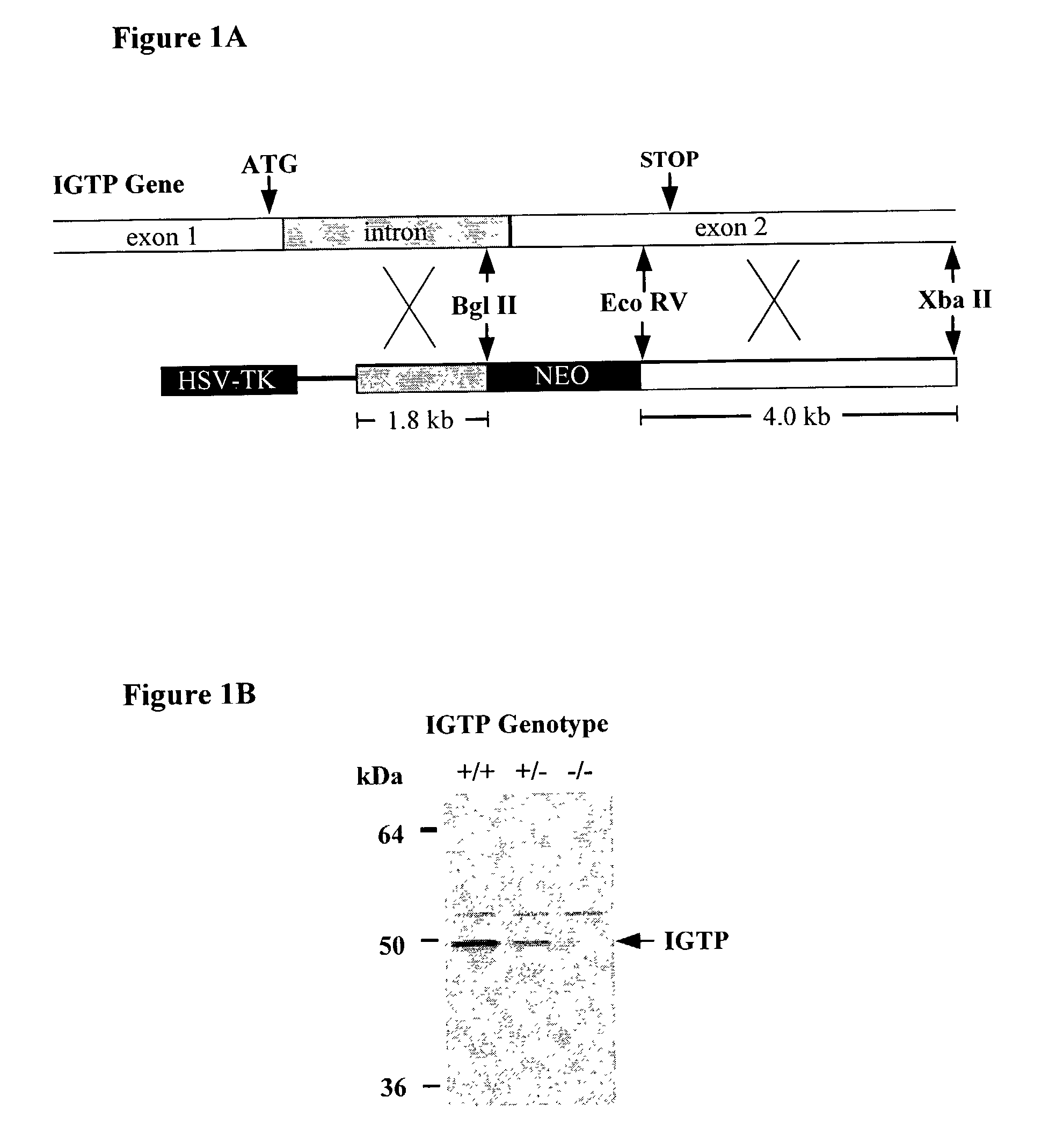
Molecules that influence pathogen resistance
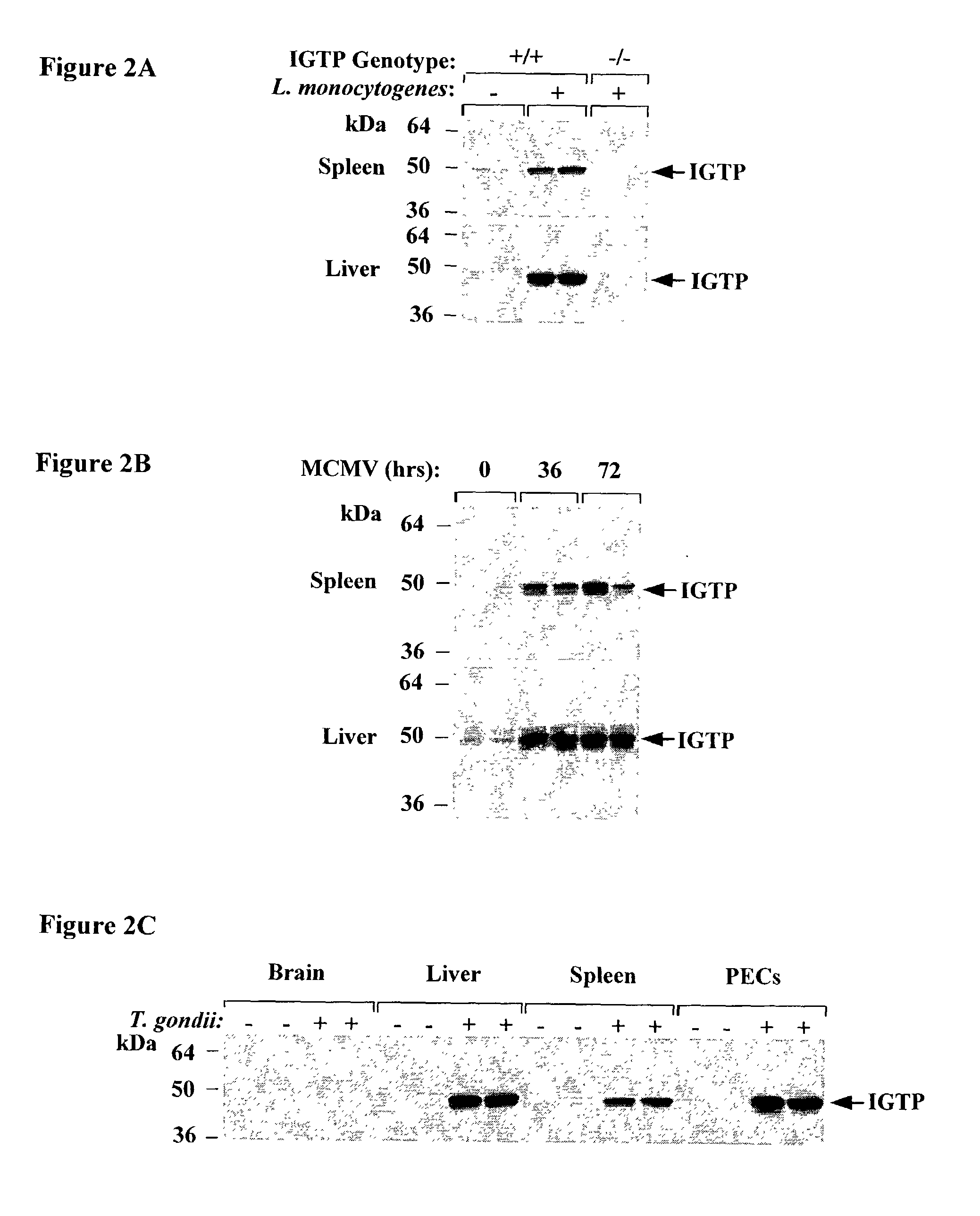
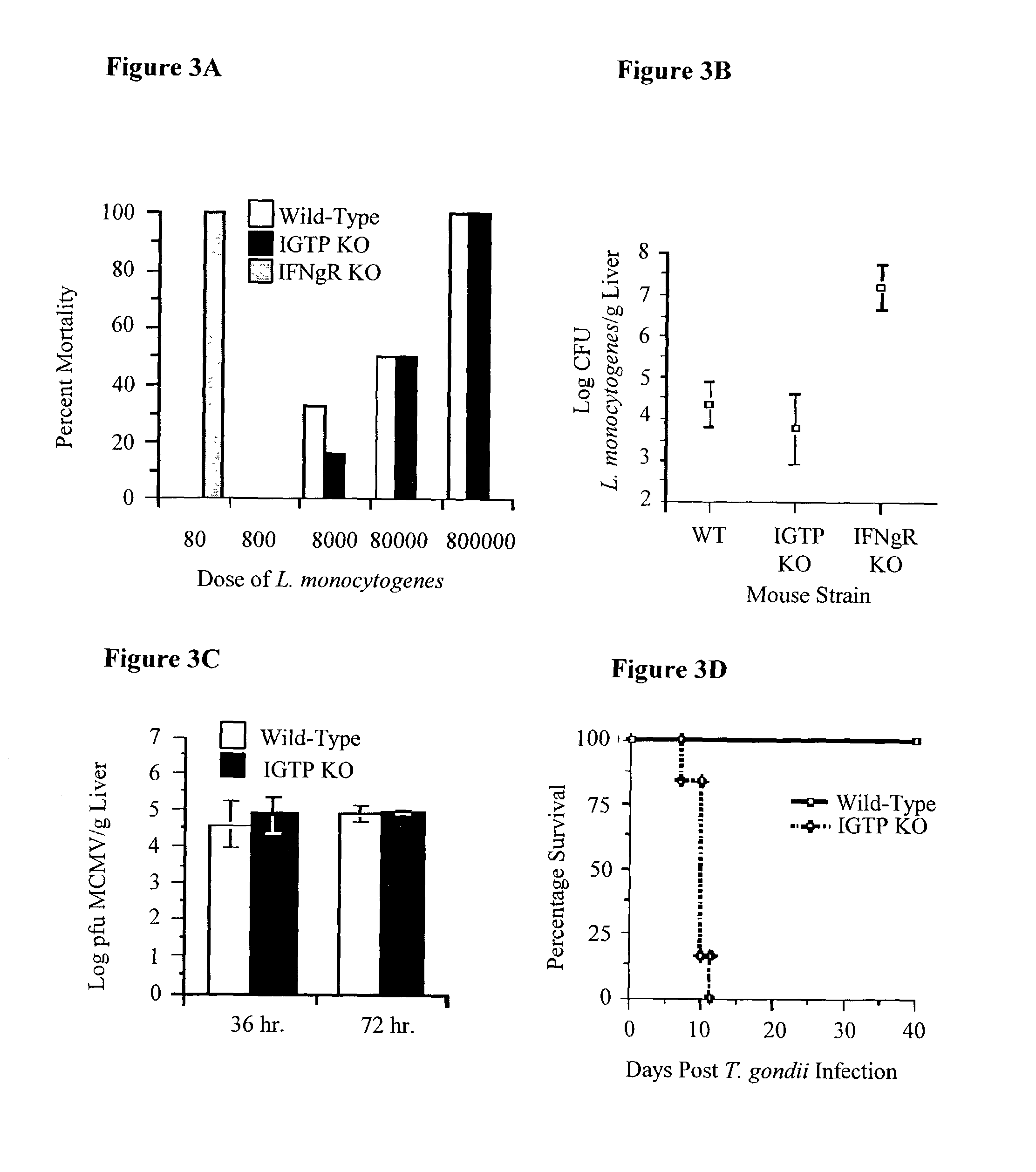
The functions of IFNgamma-induced GTPases of the IGTP-family as strong anti-infective agents, and more particularly a strong anti-parasite and / or anti-bacterial agents, are disclosed. These molecules (in both protein and nucleic acid forms) are effective to modify anti-microbial e.g., anti-bacterial and / or anti-parasitic) immune responses in a subject, to prevent or inhibit replication or infectivity of microbe, to treat microbial diseases, and to detect susceptibility of a subject to microbial infection. This invention also provides kits and compounds useful in such methods. Also provided are transgenic non-human animals in which IGTP-family member gene expression has been altered, and the use of such animals to screen for anti-microbial agents.
Owner:THE GOVERNMENT OF THE US ASREPRESENTED BY THE SEC OF THE DEPARMENT OF HEALTH & HUMAN SERVICES
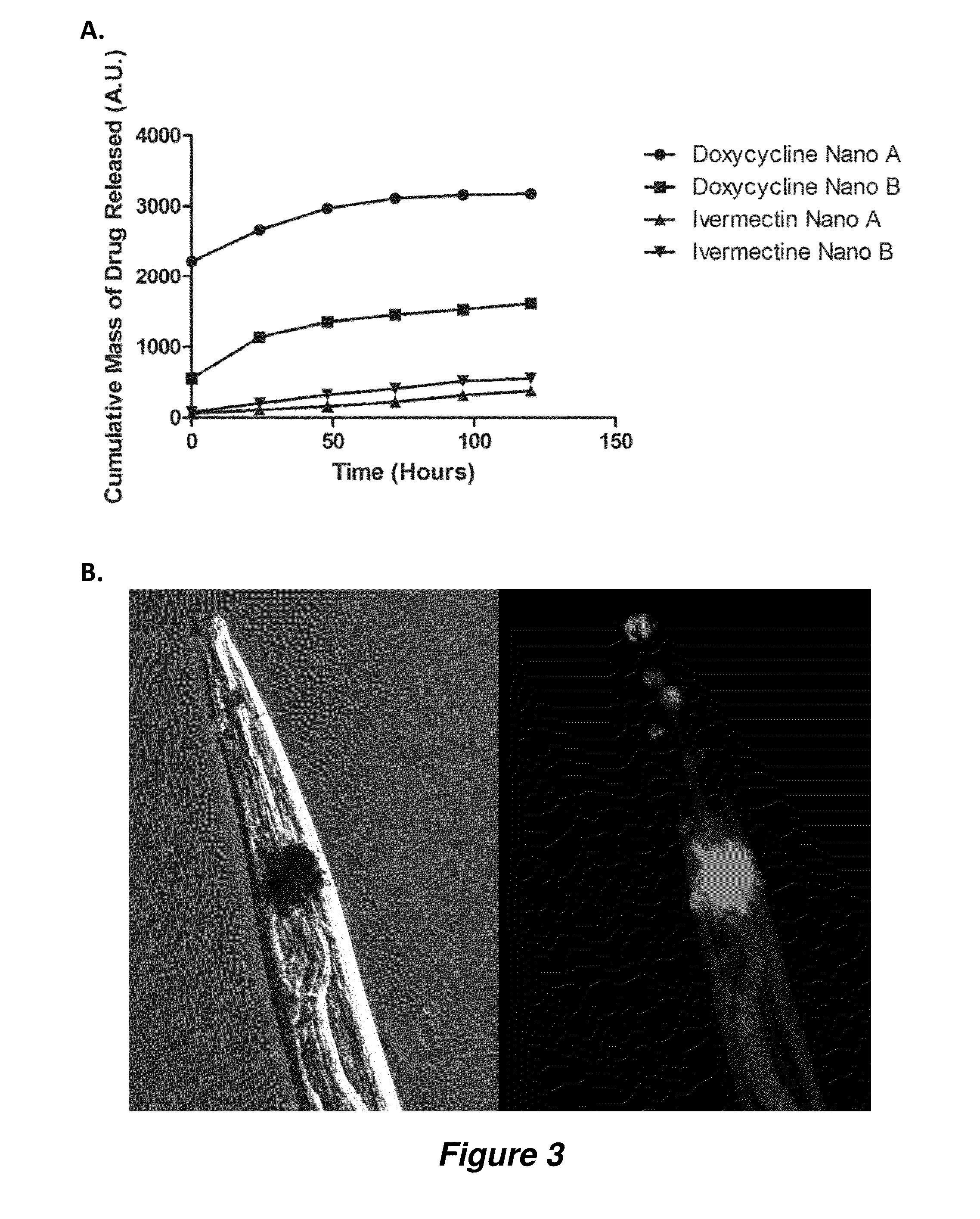
Antiparasitic polyanhydride nanoparticles
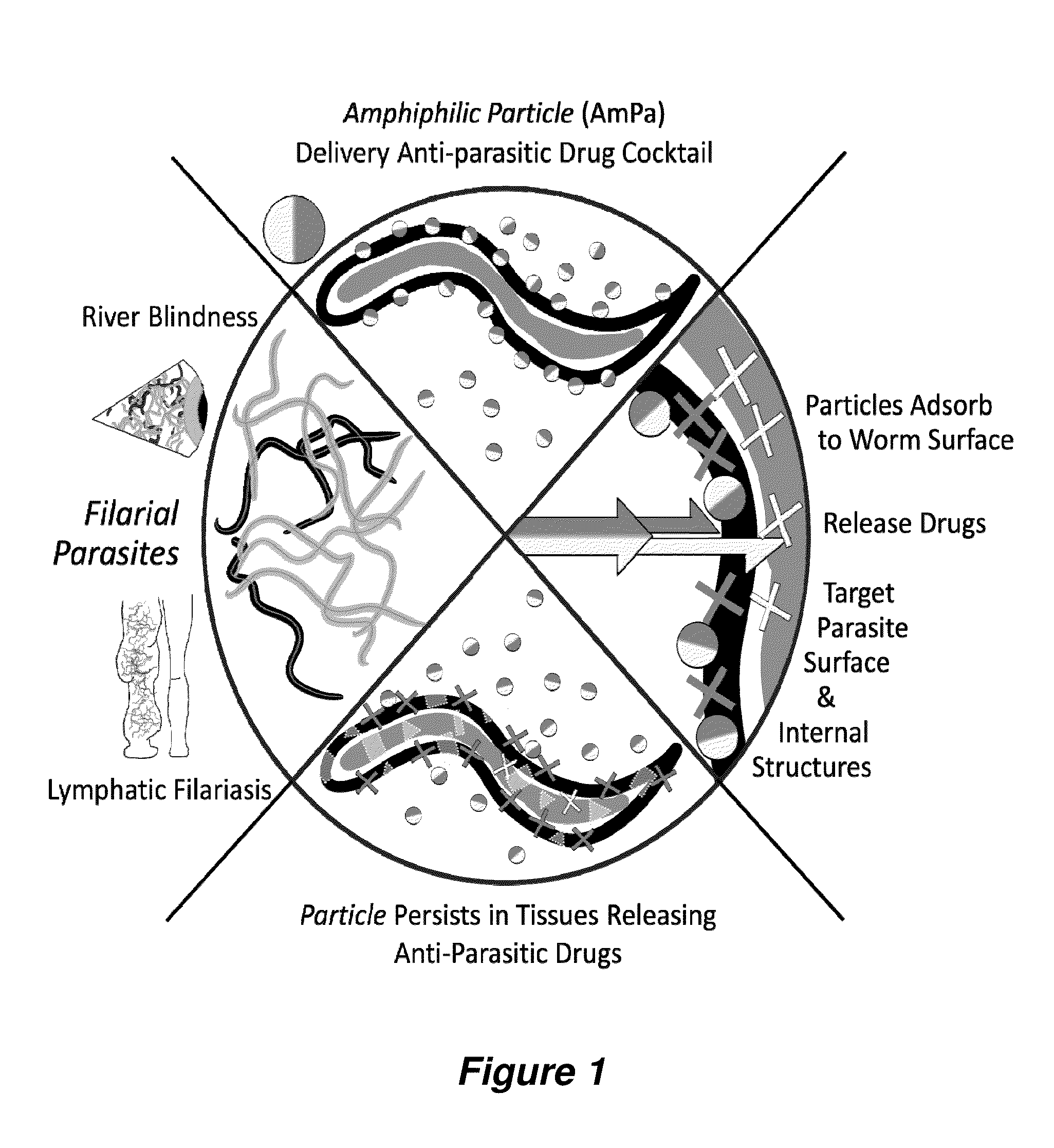
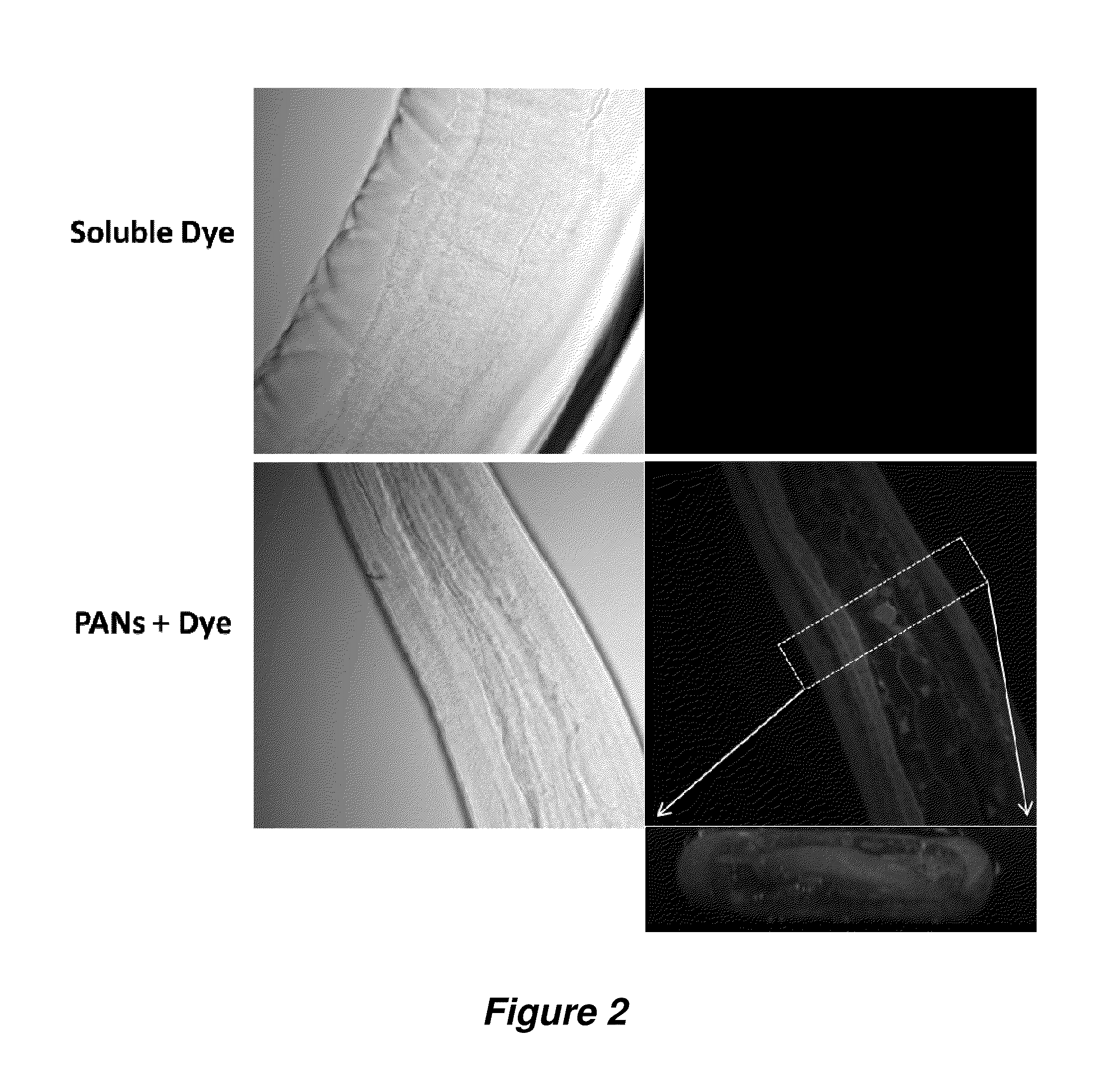
InactiveUS20150216888A1Good curative effectReducing microfilaria loadBiocideDispersion deliveryAntiparasiticAntiparasite agent
Filarial parasites Brugia, Wuchereria, Loa Loa and Onchocerca cause over 20 million infections worldwide and pose a significant social and economic burden in endemic areas. The invention provides compositions and methods to treat parasitic infections in animals and plants, and to kill and inhibit the replication of parasites in infected hosts. The methods can include administering to a host in need of treatment an effective antiparasitic amount of a composition comprising biodegradable polyanhydride microparticles or nanoparticles that encapsulate antiparasitic agents, optionally in combination with antibacterial agents. Through co-encapsulation of antiparasitic and antibacterial agents into the particles, the invention provides the ability to effectively kill parasitic helminthes, worms, and flukes, with up to a 40-fold reduction in the amount of drug used. The results described herein demonstrate the effectiveness of the drug carriers to reduce both the course of treatment and the amount of drug needed to treat parasitic infections.
Owner:IOWA STATE UNIV RES FOUND
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS10364435B1Reducing or eliminating the targeted parasite, infectious diseaseSsRNA viruses negative-sensePeptide/protein ingredientsBacteroidesLytic peptide
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID GORDON
Long-acting spiro-isoxazoline antiparasitic compositions
InactiveUS20160081986A1Improved long-acting topical compositionOrganic active ingredientsBiocideAntiparasiticAntioxidant
The invention describes a long-acting composition comprising a spiro-azetidine isoxazoline of Formula (1) or (2) wherein R1a, R1b, R1c and R2 are as described herein, and stereoisomers thereof. The composition is a veterinary composition and also comprises a glycol ether and at least one veterinarily acceptable solvent, and optionally, at least one precipitation inhibitor, antioxidant and additional veterinary agent, and any mixture thereof. The invention also includes a method of treating an animal with a parasitic infestation by administering the long-acting composition to the animal in need thereof.
Owner:ZOETIS SERVICE LLC
Group of substituted benzoheterocycle amine derivatives and preparation method and related application thereof as IMPDH (inosine monophosphate dehydrogenase) inhibitor
ActiveCN103992310AOrganic active ingredientsOrganic chemistryInosine-5′-monophosphate dehydrogenaseScreening study
The invention discloses a group of substituted benzoheterocycle amine derivatives and a preparation method and related application thereof as an IMPDH (inosine monophosphate dehydrogenase) inhibitor. The IMPDH inhibitor has good application prospects in virus resistance, immunosuppression, tumor resistance, bacterium resistance, parasite resistance and the like. In the invention, a new-structure IMPDH inhibitor shown by the formula (I) is obtained through the design, synthesis and activity screening study on an active compound targeting IMPDH, and a foundation is laid for the development and application of the compounds as the medicines and medicinal compositions thereof with related effects such as virus resistance, tumor resistance, immunosuppression and the like.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Isoxazoline compositions and their use as antiparasitics
Owner:INTERVET INC
Anthelminthic formulations
The present invention relates to veterinary or pharmaceutical antiparasitic formulations which may comprise a macrocyclic lactone, one or more alcohol cosolvents and an oil wherein the crystallization of the macrocyclic lactone is minimalized. This invention also provides for, inter alia, antiparasitic formulations for the treating, controlling and preventing of endo- and ectoparasite infections in warm-blooded animals, such as livestock.
Owner:MERIAL LTD
Antiparasitic formulations
Fipronil co-formulations are provided herein. The formulations comprise an organic solvent, an alcohol co-solvent, and one or more antioxidants. The formulations provided herein are antiparasitic, and can be used, for example, to combat dog and cat parasites, such as, fleas and ticks.
Owner:FIDOPHARM
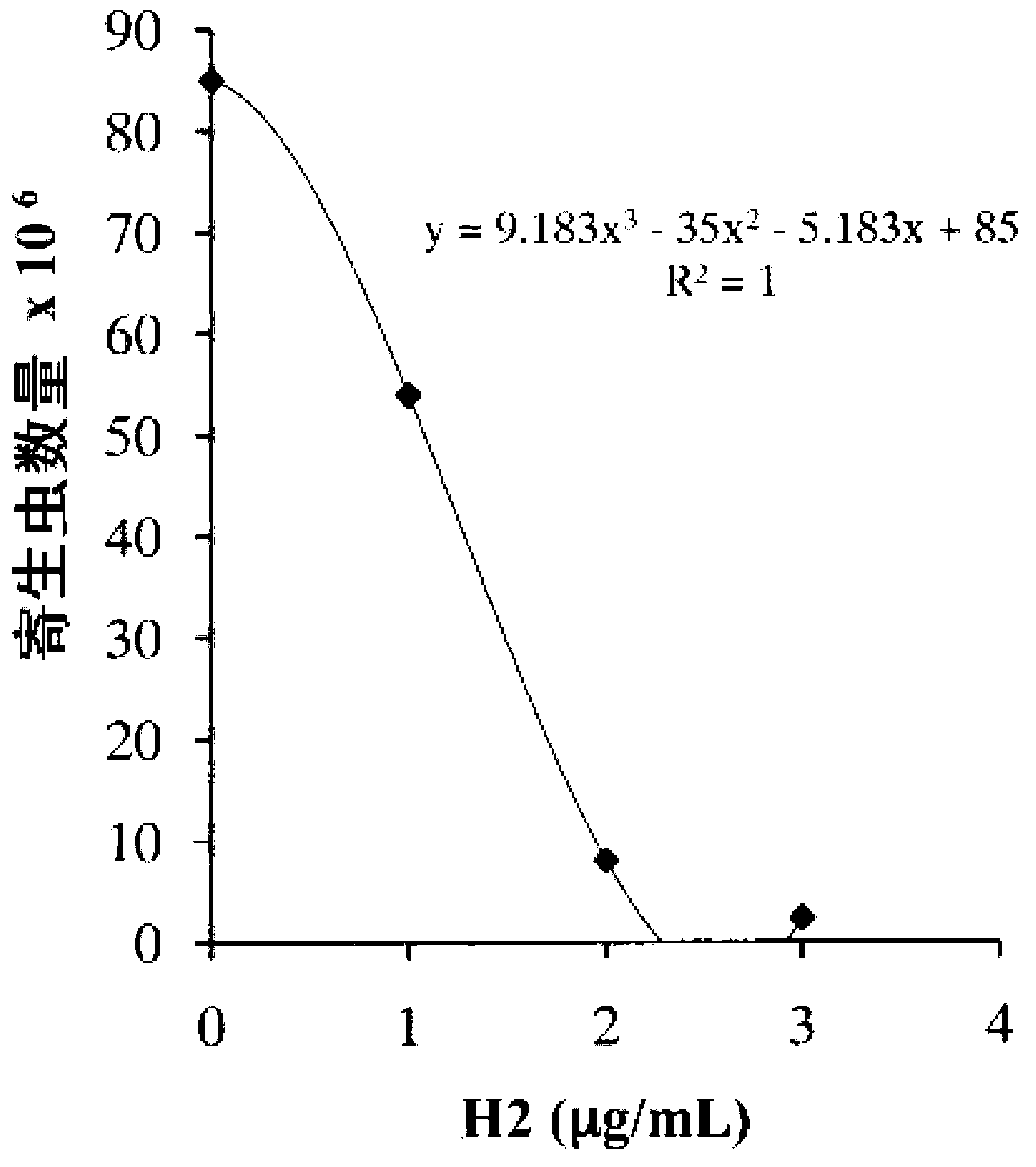
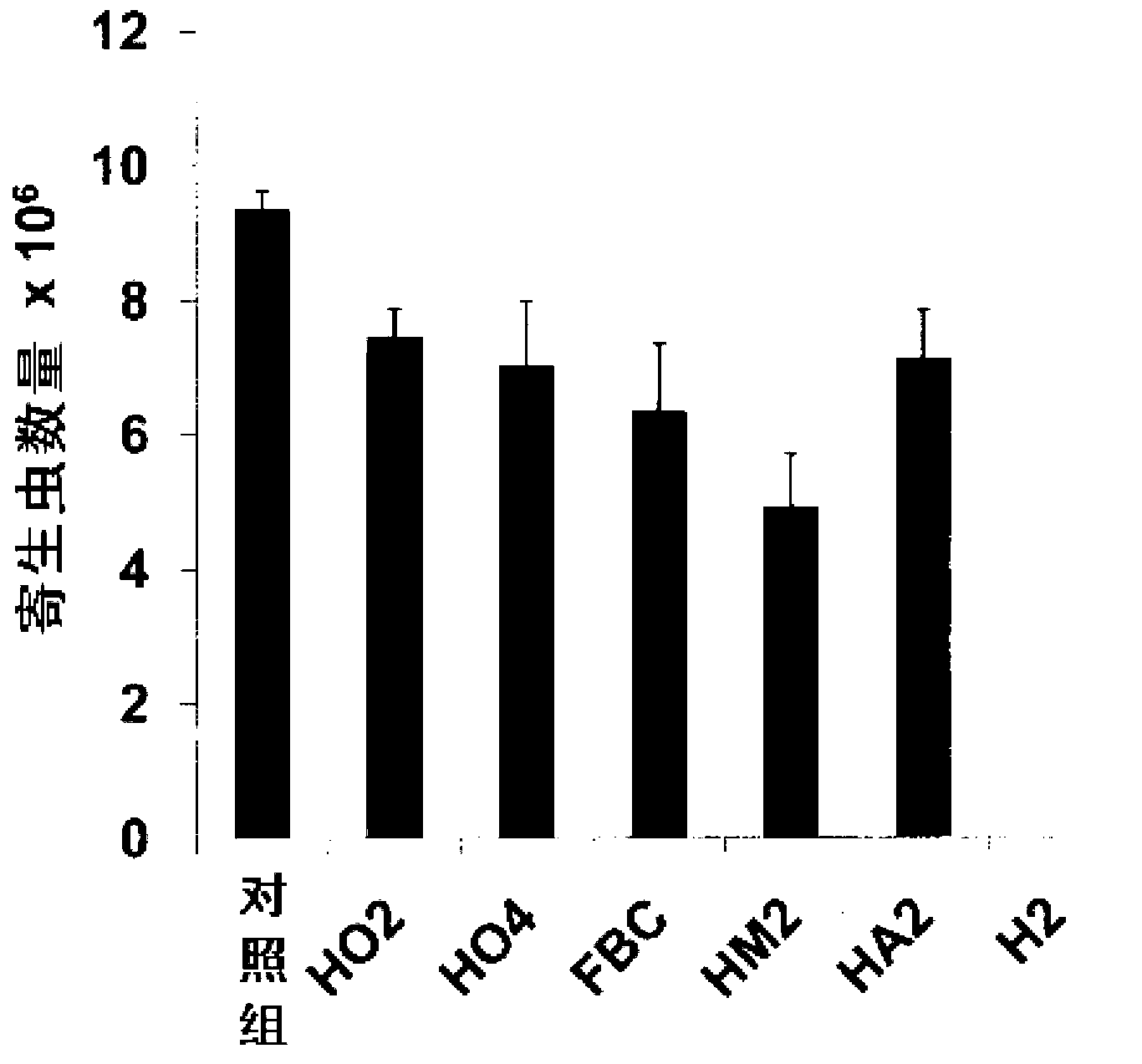
N6-(ferrocene methyl) quinazolin-2,4,6-triamine (h2) and the derivatives and prodrugs thereof as antimicrobial, antiparasitic, antiprotozoal and antileishmania agents
InactiveCN102918051AOrganic active ingredientsHeavy metal active ingredientsAntiparasiticVertebrate Animals
The invention relates to the use of compound N 6 -(ferrocenmethyl)quinazolin-2,4,6-triamin (H2) and the derivatives and prodrugs thereof having an antimicrobial (antibiotic, microbicidal), antiparasitic (parasiticidal), antiprotozoal (protozoacidal), antileishmanial (leishmanicidal) activity, and to the use thereof as a drug for (human and animal) vertebrates.
Owner:诺马德卡谟·加林多塞维利亚 +1
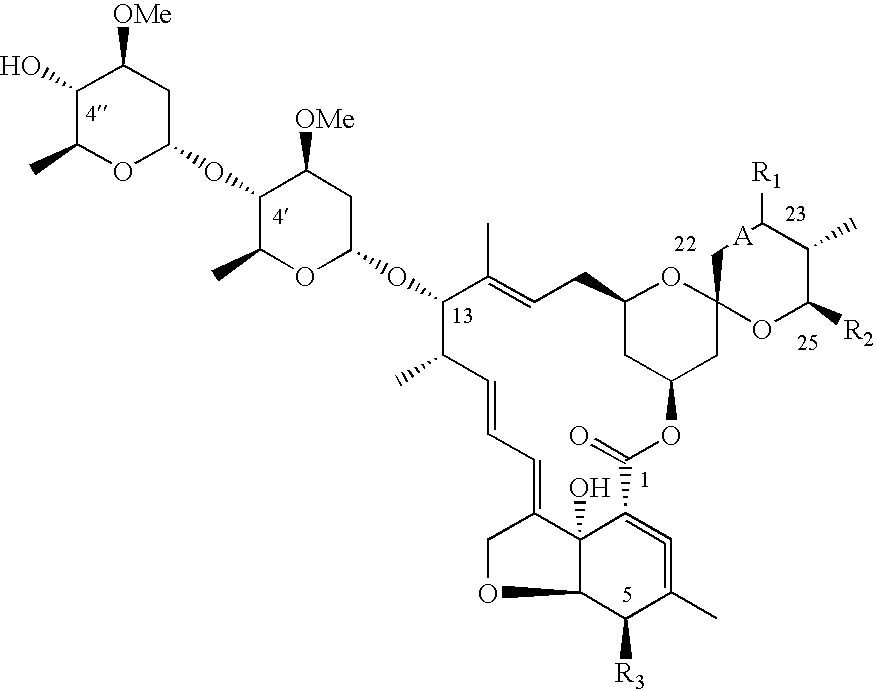
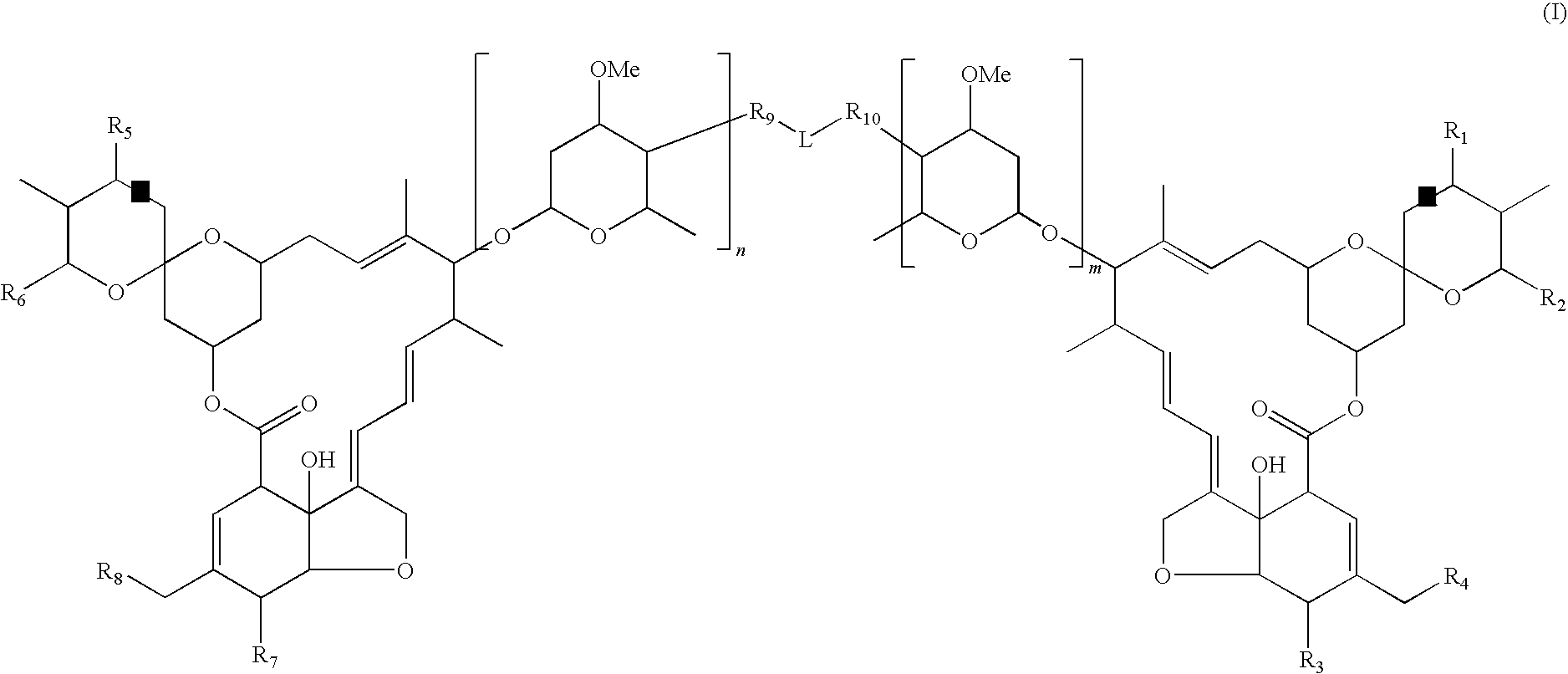
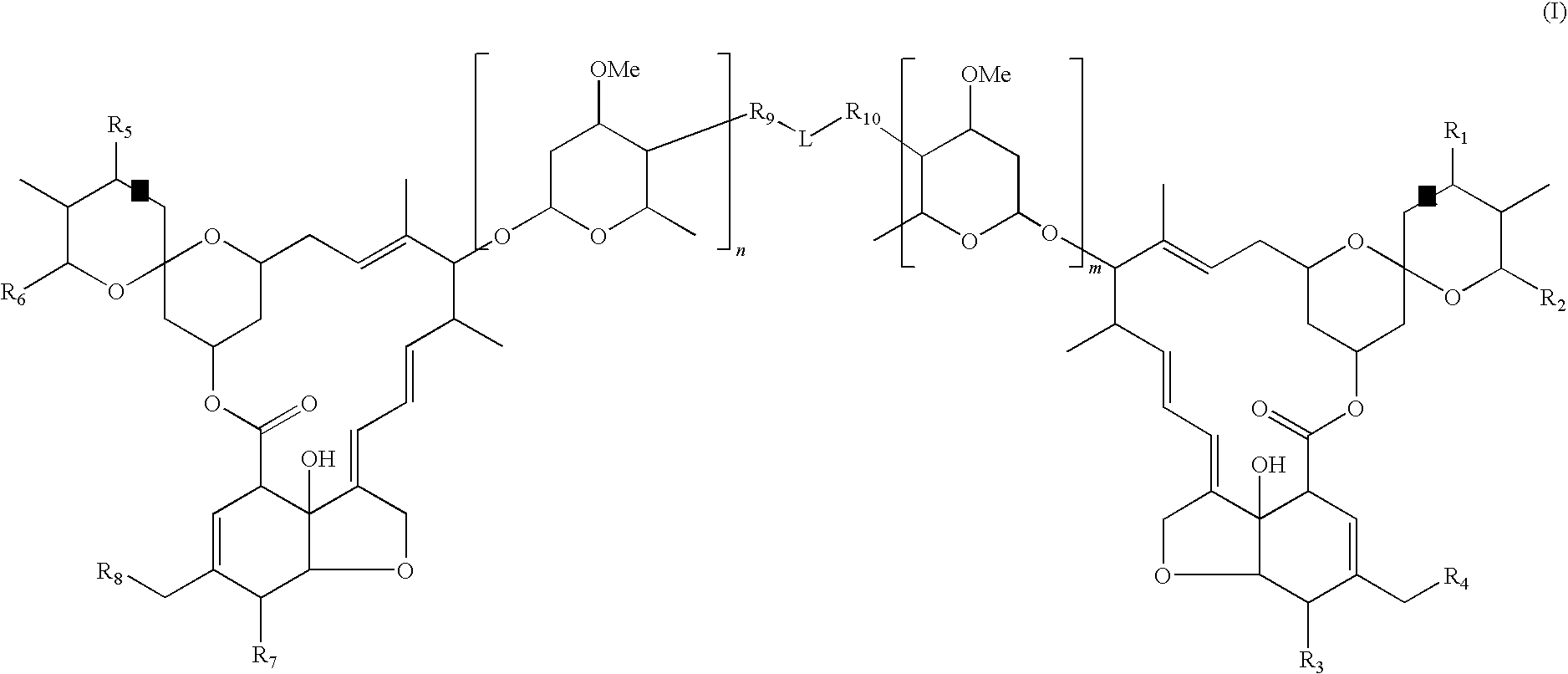
Dimeric avermectin and milbemycin derivatives
This invention provides for novel antiparasitic and pesticidal derivatives of avermectin and milbemycin compounds in which two avermectin or milbemycin members are linked together by a chemical linker. The resulting compounds may be used in veterinary compositions which are used in treating, controlling and preventing of endo- and ectoparasite infections and infestations in animals or for combating pests in plants or plant propagation material.
Owner:MERIAL INC
Anti-parasite compound preparation for pets
InactiveCN102107005AAvoid infectionEasy to useOrganic active ingredientsAntiparasitic agentsAntiparasiticNematode
The invention discloses a compound preparation for expelling and killing endobiotic eelworms epizoic fleas, lice and other parasites of cats and dogs. The compound preparation adopts the formula comprising pyrethrin having a specific effect for fleas and macrolide anthelmintics having specific effects for mites, ticks, lice and eelworms, and has the remarkable characteristics that the compound preparation is convenient for use and the infection from parasites such as fleas and lice of cats and dogs can be effectively prevented; and the medicament is safe for use and has no adverse reaction after being administrated by animals.
Owner:QINGDAO VLAND BIOTECH INC +1
Broad spectrum benzothiophene-nitrothiazolide and other antimicrobials
The invention provides novel antimicrobial chemical entities based on a nitrothiazolide backbone that exhibit antibacterial and antiparasitic action against a wide range of human pathogens. The new classes of compounds show extended action against Gram positive bacteria including MRSA drug resistant pathogens. In the Gram-positive organisms, they specifically target and functionally inhibit microbial attachment to surfaces and biofilm formation. In Gram-negative bacteria, including enteroaggregative E. coli strains, these compounds function as pilicides by inhibiting the assembly of pilin subunits into adhesive filaments. Several of these compounds show potent antimicrobial action against Gram positive bacteria, perhaps involving novel targets. Many of the benzothiophene derivatives exhibit antimicrobial activity in the low micrograms per ml range and in blocking biofilm formation in the nanomolar range; ranges considered are well within the range of utility as therapeutics.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Antiparasitic closantel sodium transdermal solution for ox and sheep and preparation method thereof
InactiveCN101693009AFully reflect the advantages of preparationsPromote percutaneous absorptionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAntiparasiticSide effect
The invention relates to an antiparasitic closantel sodium transdermal solution for ox and sheep and a preparation method thereof, the composing components of the solution and the parts by weight thereof are respectively that 5-15 parts of ethyl acetate, 20-30 parts of dimethyl sulfoxide, 2-6 parts of azone, 1-3 parts of isopropanol and 50-70 parts of propanediol or ethanol, the content of closantel sodium is 2.5-5 g per 100 ml, and the finished product is formed through dissolving, mixing, filtering and injecting. The azone related in the solution is a novel skin penetration accelerant, can effectively increase the absorption of the skin to various drugs through the skin, effectively prevents the pass effect of hepar, avoids the peak valley phenomenon during the absorbing process of the drugs, reduces the toxic side effect caused by about the drugs and the dosage, prolongs the effective acting time, changes the drug feeding area and effectively regulates the drug feeding dosage, reduces the difference between independent bodies, and has the characteristic of simple, convenient and rapid use. Compared with the other types of preparations, the solution is more suitable for antiparasitic preventive treatment and parasite killing in pasturing areas.
Owner:TIANJIN BIJIA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com