Patents
Literature
107 results about "Antiparasitic agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug or agent used to treat or prevent parasitic infections.
Spot-on formulations for combating parasites
InactiveUS6962713B2Toxic effectsEffective and lasting destructionBiocideDead animal preservationAntiparasiticAntiparasite agent
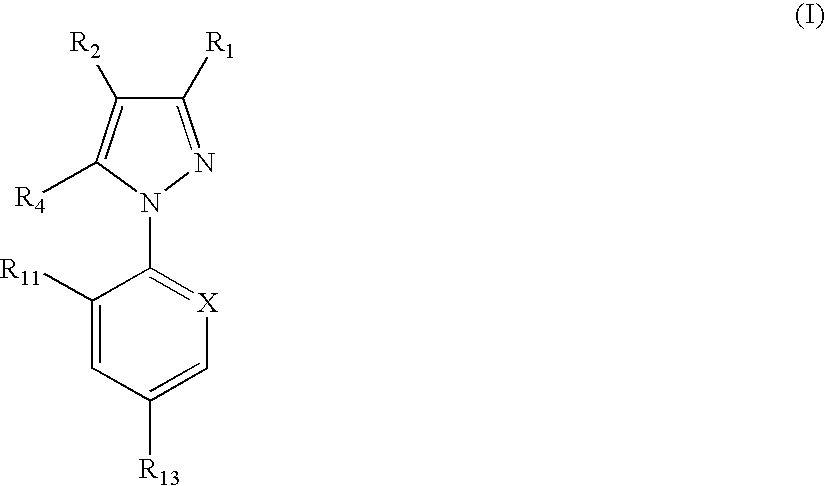
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and / or(B) an effective amount of a macrocyclic lactone antihelmintic or antiparasitic agent;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL SAS
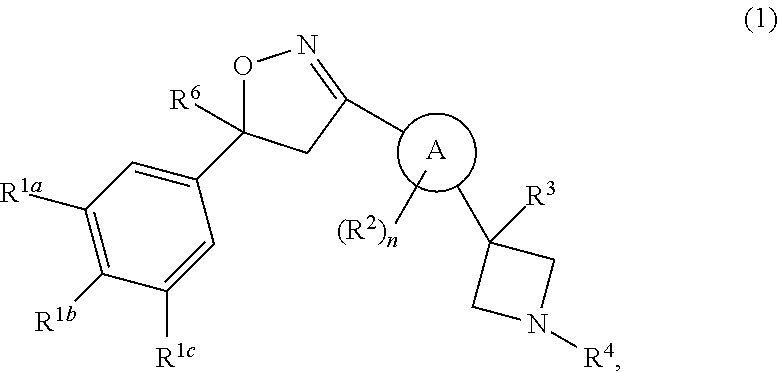
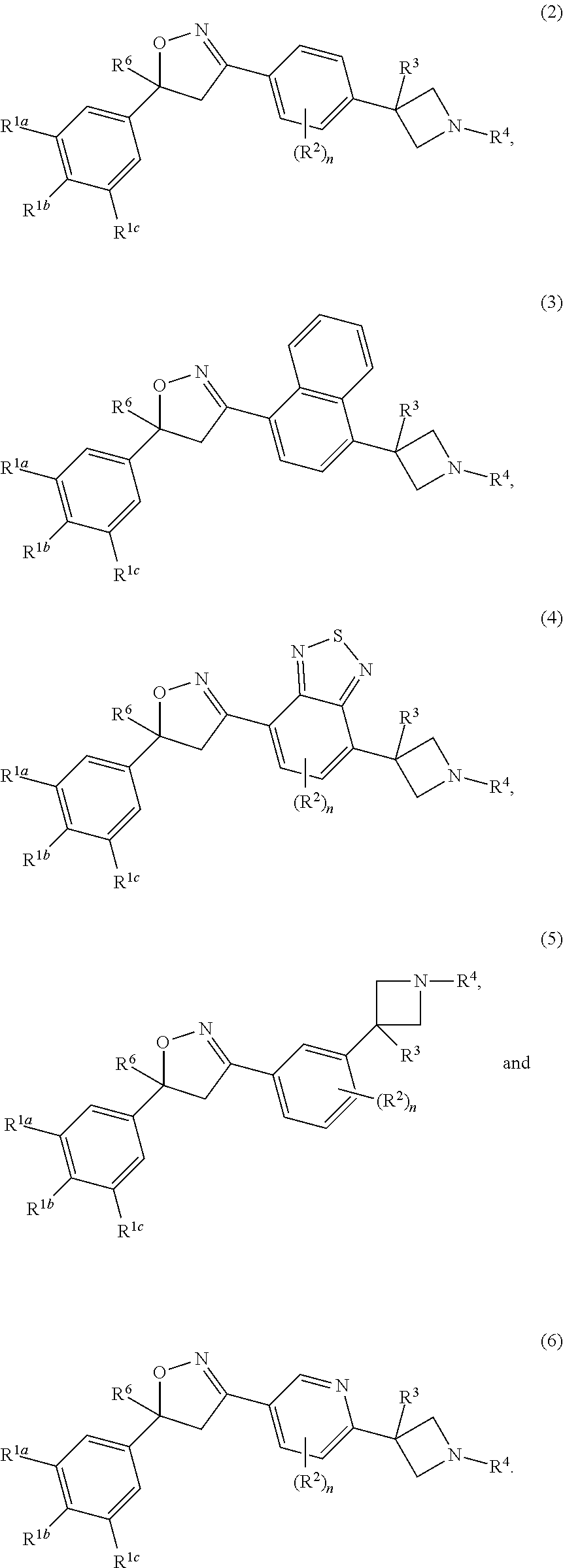
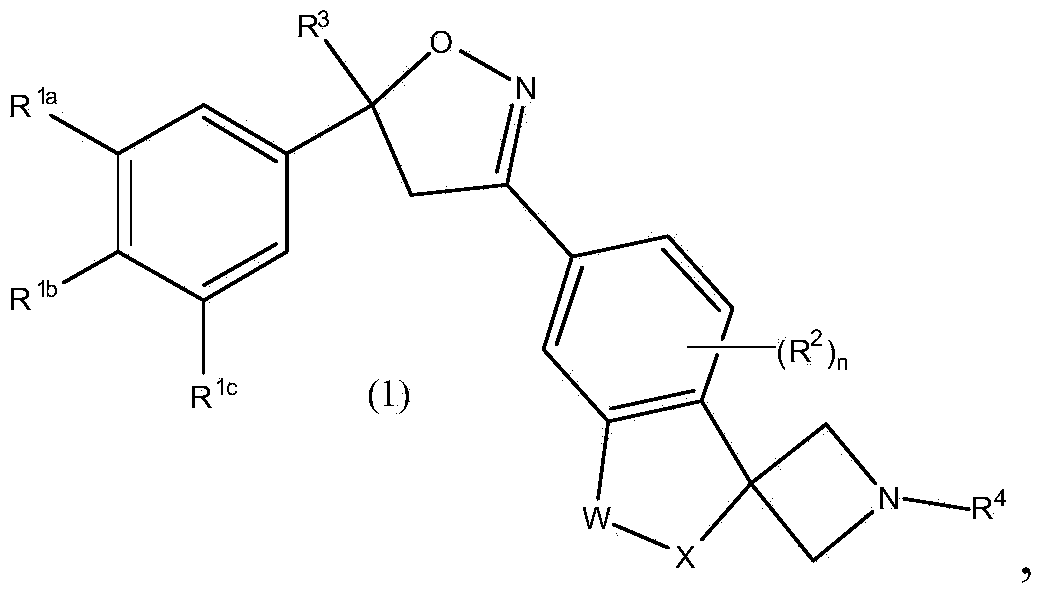
Isoxazoline derivatives as antiparasitic agents
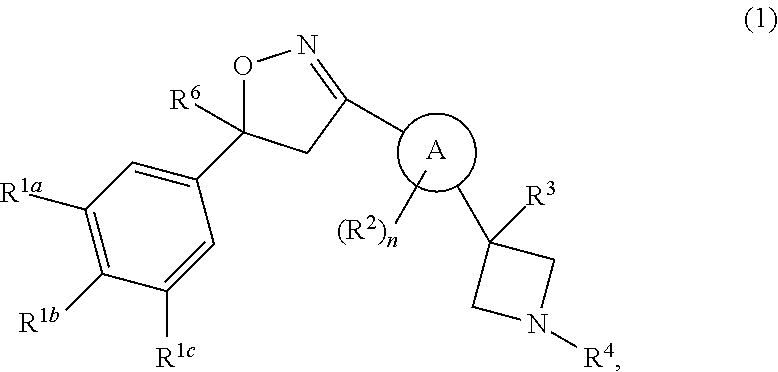
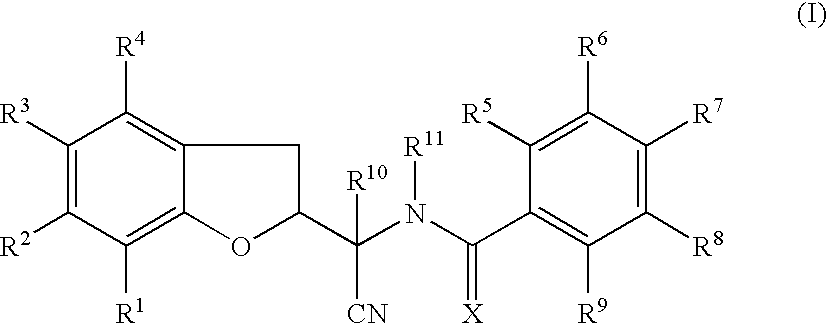
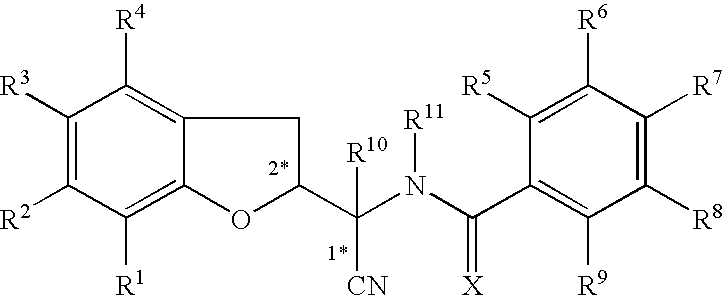
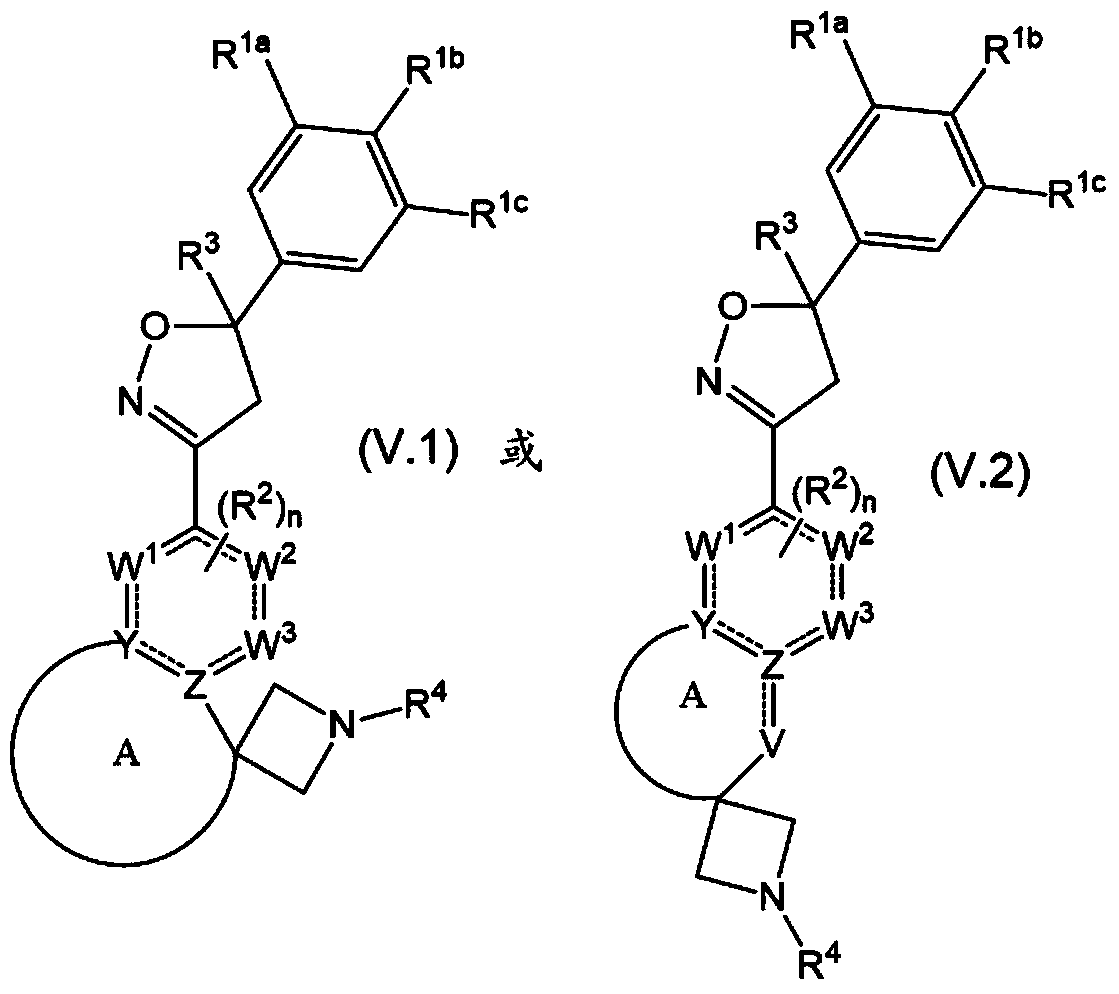
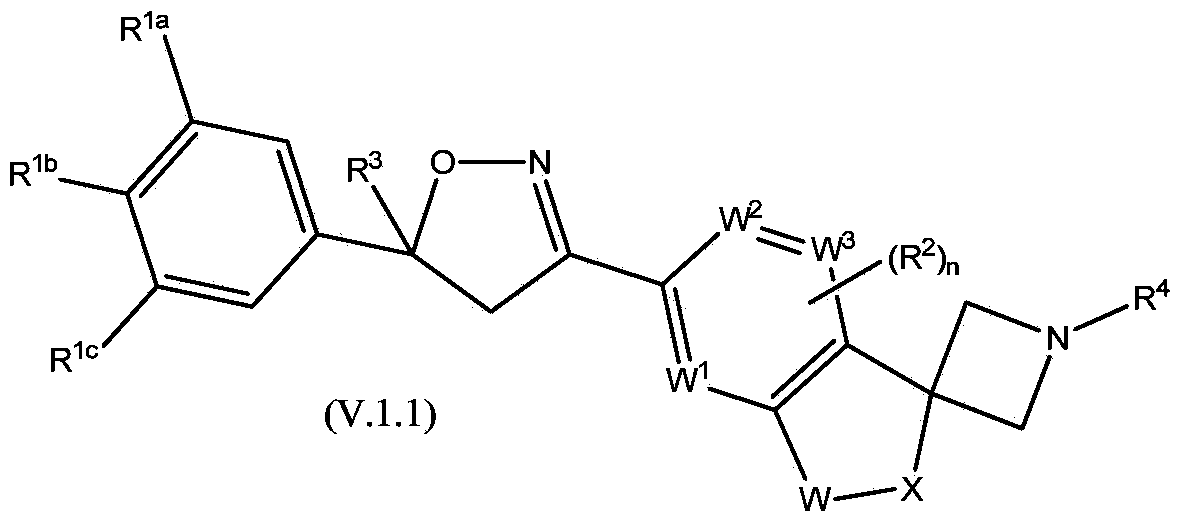
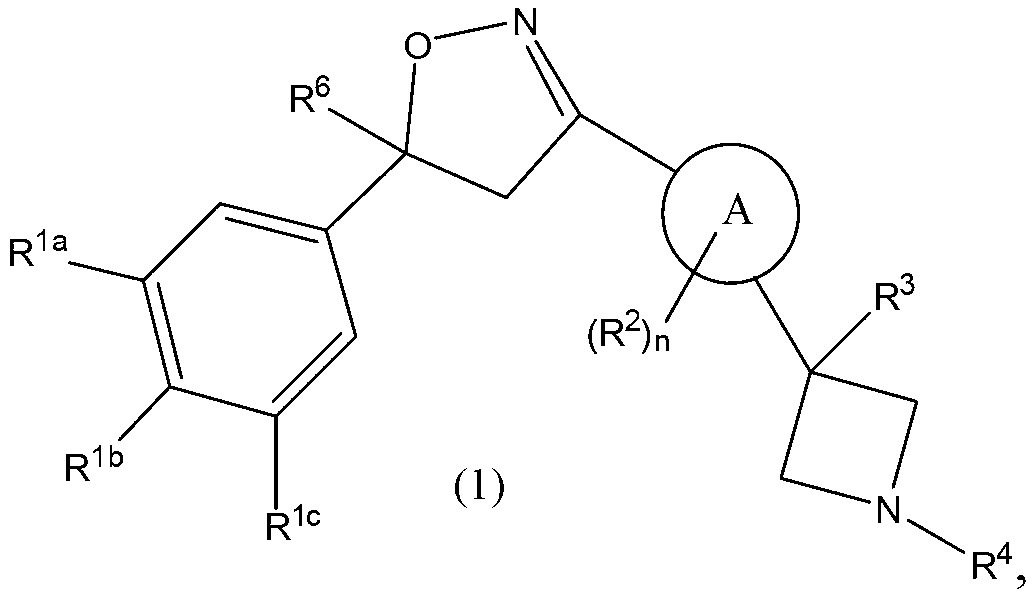
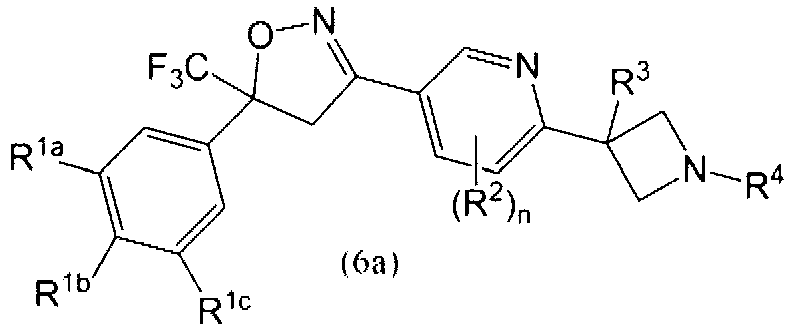
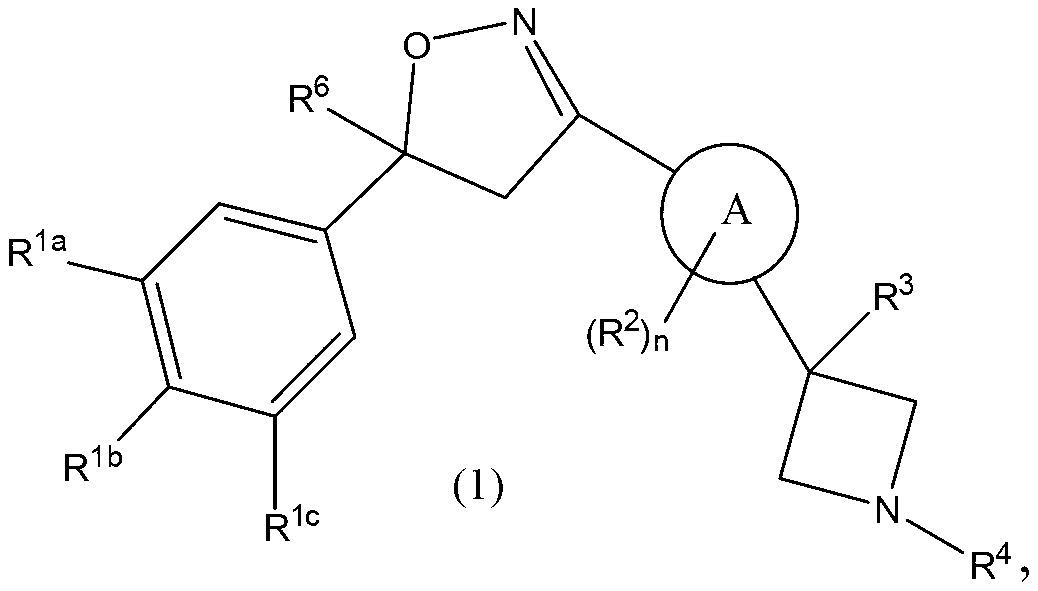
This invention recites isoxazoline substituted azetidine derivatives of Formula (1)stereoisomers thereof, veterinarily acceptable salts thereof, compositions thereof, and their use as a parasiticide in mammals and birds. R1a, R1b, R1c, R2, R3, R4, R6, and n are as described herein.
Owner:ZOETIS SERVICE LLC
Dimethicone-containing sustained release injection formulation
InactiveUS20070053943A1Prevention and treatment of problemBiocidePharmaceutical delivery mechanismDrugAnalgesic agents
A sustained release formulation by using dimeticone as the dispersion medium, which includes active ingredient (e.g., drugs against parasites, insecticides, NSAIDs, antibiotics, sex hormone like agents or oily soluble vitamins) and dimeticone as the medium. Suitable stabilizer, antioxidant, local analgesics and material for sustained release may be added. The formulation is bio-compatible, stable and injectable.
Owner:WANG YUWAN +2
Combination
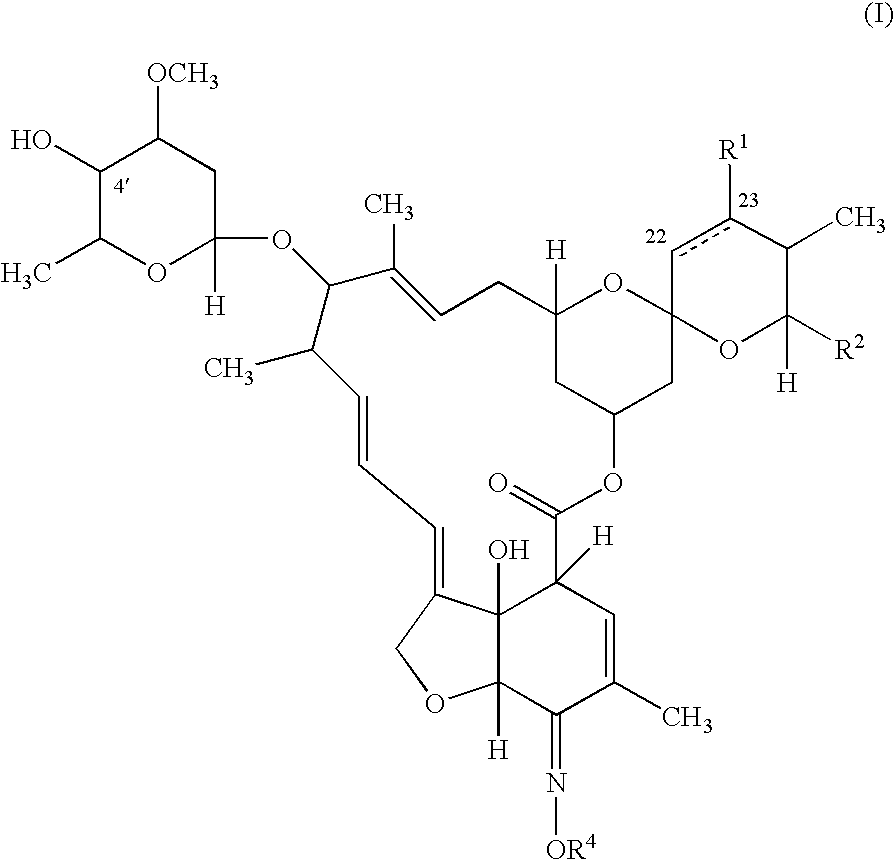
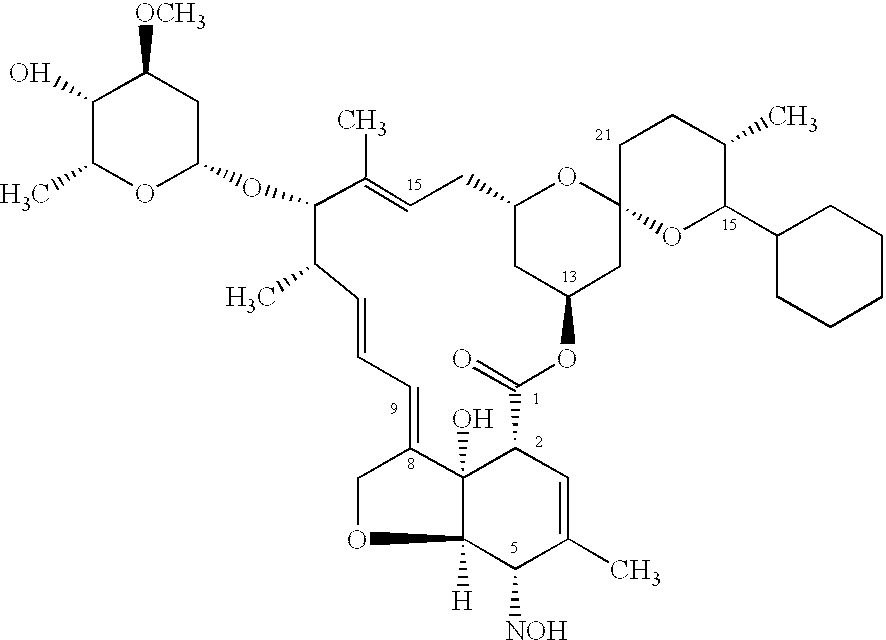
Compounds of formula (I) are used in combination with a second antiparasitic agent for the treatment of parasitic infestations in a host animal.
Owner:PFIZER LTD +1
Combination
Compounds of formula (I) are used in combination with a second antiparasitic agent for the treatment of parasitic infestations in a host animal.
Owner:PFIZER LTD
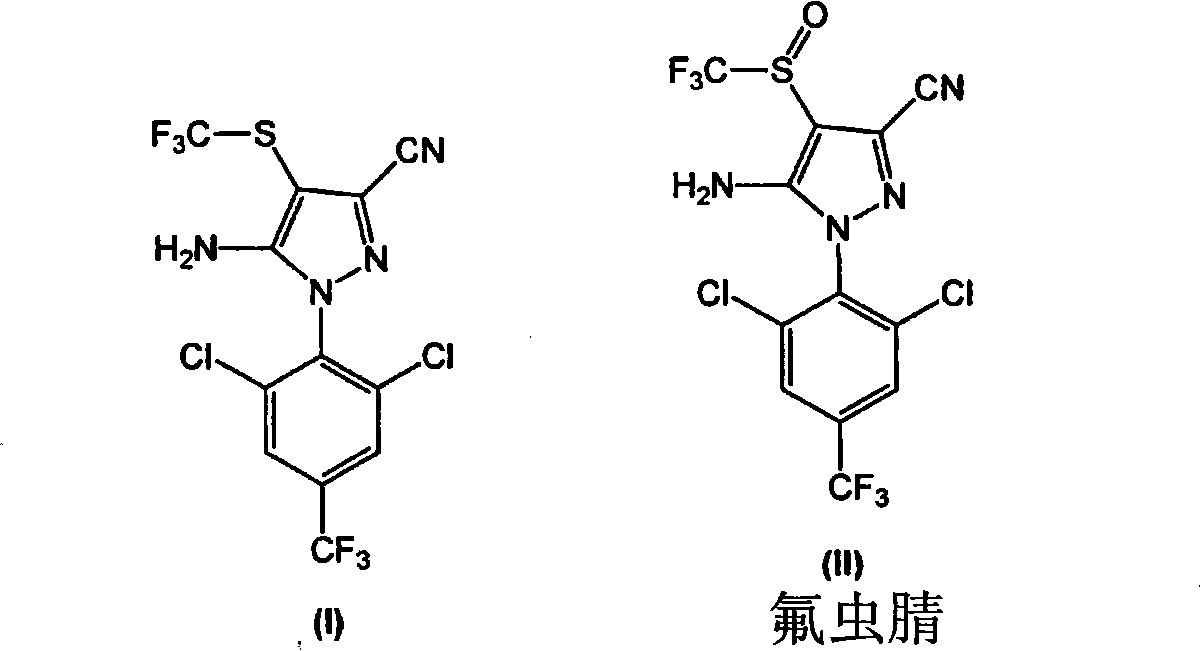
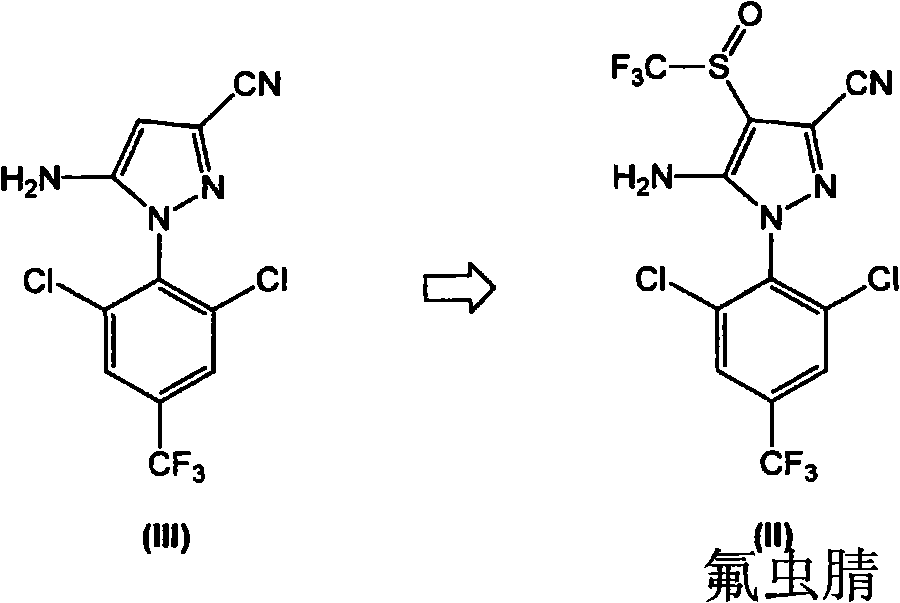
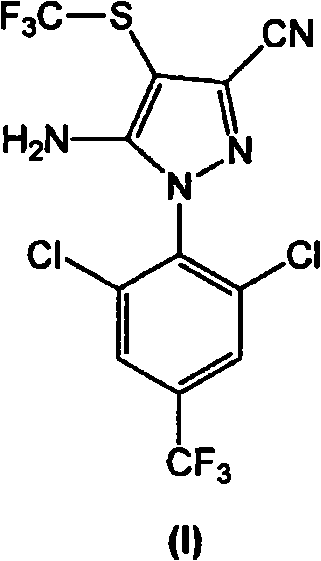
Process for the preparation of fipronil and analogues thereof
The present invention relates to a new and efficient process for preparing 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylthio)-IH-pyrazole-3-carbonitrile (hereinafter referred to as compound of formula I), which is useful as an intermediate for the antiparasitic agent fipronil, and a process for preparing 5-amino-3-cyano-l-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethyl sulfinylpyrazole (hereinafter referred to as compound of formula II or fipronil). In one aspect, there is provided a process for preparing fipronil comprising: a) a step of reacting CF3S(=O)ONa with the compound of formula (III) in the presence of a reducing / halogenating agent; and b) a step of oxidizing the compound of formula (I) obtained in step a) in the presence of a selective oxidizing agent, under suitable conditions, wherein the selective oxidizing agent selectively effects oxidation of (I) to the corresponding sulfoxide, Fipronil. In certain exemplary embodiments, the selective oxidizing agent is MHSO5, wherein M is an alkaline metal cation.
Owner:VETOQUINOL SA
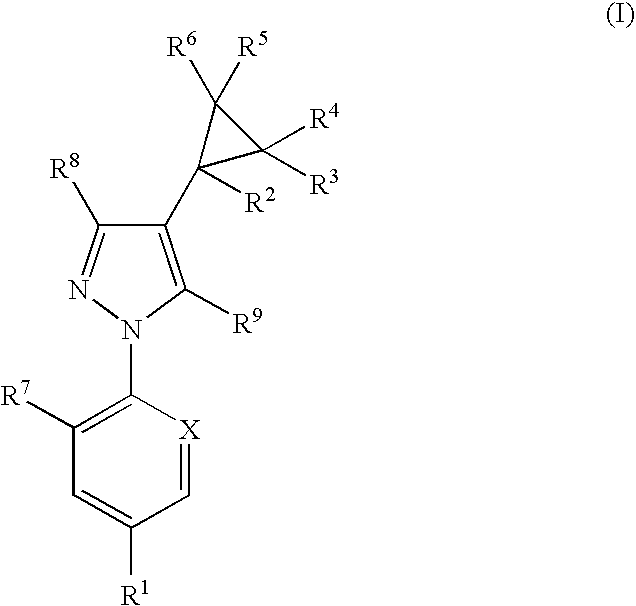
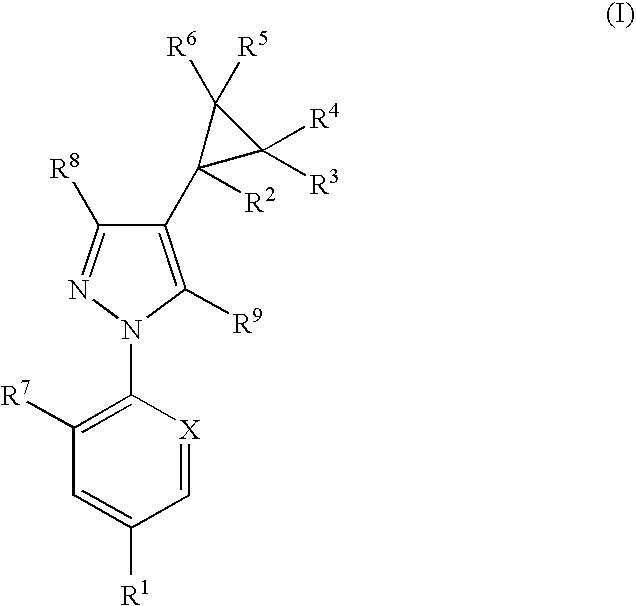
Antiparasitic agents
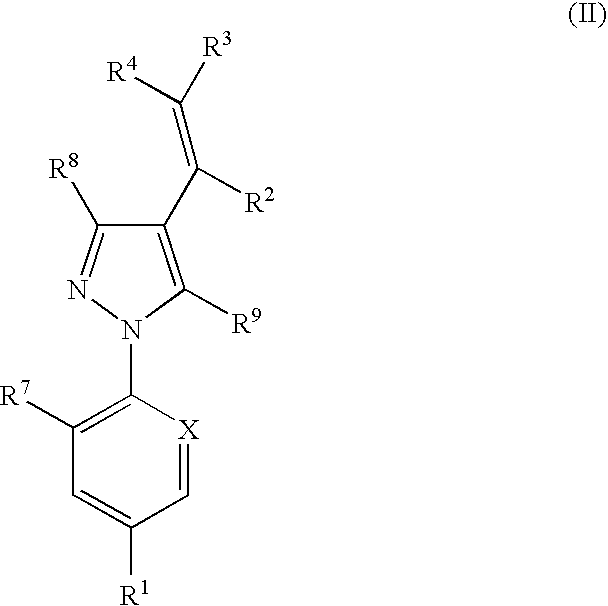
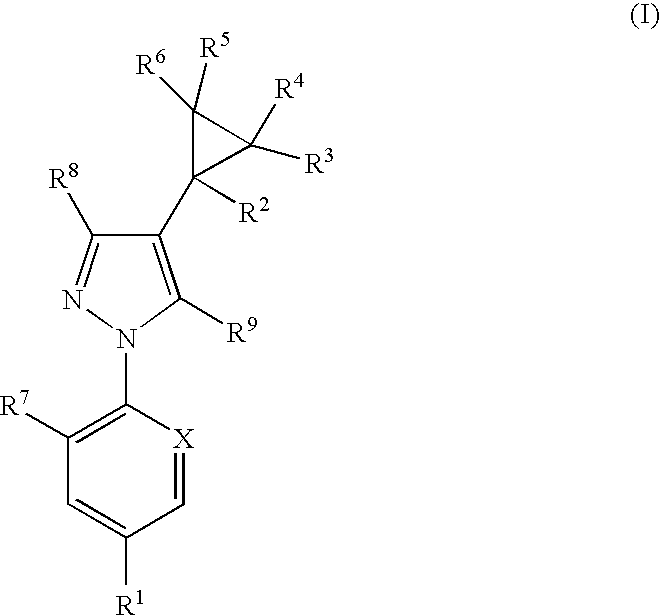
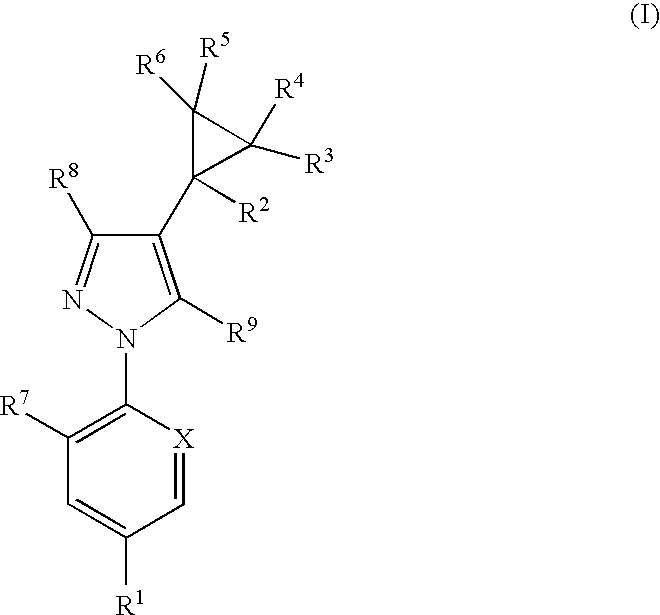
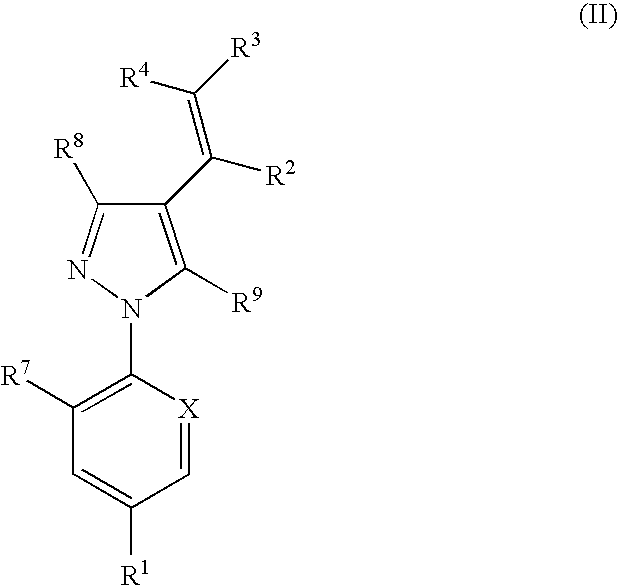
The present invention relates to compounds of the formula (I)and pharmaceutically acceptable salts thereof, compositions containing such compounds and the uses of such compounds as antiparasitic agents.
Owner:PFIZER ANIMAL HEALTH UK 1 LIMITED
Spirocyclic isoxazoline derivatives as antiparasitic agents
ActiveCN103517907AFight and control infectionResist and Control InvasionOrganic active ingredientsBiocideAntiparasitic agentStereochemistry
Owner:ZOETIS SERVICE LLC
Multi-purpose skin balm including skin balm for psoriasis
The invention is a user friendly, multi-purpose ointment whose simple basic formula is a combination of karaya gum powder and a vehicle for dispersing, such as petrolatum. It can be designed to treat a variety of skin problems by adding some non-essential ingredients such as Vitamin A&D Ointment, Vitamin E cream or Lotion at the discretion of the user, in addition to various pharmaceuticals based on the cause of the problem such as an antibacterial, an antifungal, an anti-inflammatory agent, an antiviral, an anti-parasitic, or an enzymatic agent at the discretion of the health care provider or user.
Owner:SMITH SADIE N
Water-soluble trioxanes as potent and safe antimalarial agents
InactiveUS6136847AGood water solubilityImprove efficacyBiocideOrganic chemistryArylAntiparasite agent
Biologically-active, water soluble, 3-substituted trioxanes of the formula wherein R represents a COOH- substituted aryl group, a substituted or unsubstituted heteroaryl group or an alkyl group, and C12-(p-carboxy)benzyloxy trioxanes of formula wherein R represents a substituted or unsubstituted alkyl, alkenyl, aryl or heteroaryl group and methods for their use as antiparasitic agents, particularly for the treatment of malaria.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pharmaceutical composition containing an anti parasitic agent and an active ingredient selected from carveol, thymol, eugenol, borneol, carvacrol, alpha-ionone, or beta-ionone
InactiveCN101175532ALow costMerit treatmentHydroxy compound active ingredientsAntiviralsEugenolBULK ACTIVE INGREDIENT
The present invention relates to a pharmaceutical composition. The pharmaceutical composition comprises: at least one first therapeutically-active substance which is selected from carveol, thymol, eugenol, borneol, carvacrol and the isomers, derivatives and mixtures thereof; and at least one second therapeutically-active substance which is selected from an antiviral agent, an antitumour agent and an antiparasitic agent.
Owner:ADVANCED SCIENTIFICS INC
Suspension containing albendazole, and preparation method thereof
The invention belongs to the technical fields of antiparasitic drugs for animals, and preparation methods thereof, and concretely relates to a suspension containing albendazole, and a preparation method thereof. The above preparation comprises an albendazole bulk drug, a suspending aid, a wetting agent, an antiseptic and a flocculating agent. The suspension containing albendazole is prepared through a dispersion process, and is an off-white sticky water suspension. The suspension containing albendazole developed in the invention has the advantages of good stability, good palatability, long elimination half life, and high bioavailability, and can be used by animals as an oral preparation.
Owner:QINGDAO KDN BIOTECH
Stable veterinary combination formulations of macrocyclic lactones and imidazothiazoles
ActiveUS20160051524A1Improve stabilityImprove solubilityBiocidePharmaceutical delivery mechanismAntiparasite agentControl parasites
The present invention is directed to stabilized compositions comprising at least one macrocyclic lactone, or derivative thereof, in combination with levamisole, and an amino sugar stabilizing agent, optionally an additional antiparasitic agent, and a method for treating or controlling a parasitic infection or infestation in an animal by administering said composition.
Owner:ZOETIS SERVICE LLC
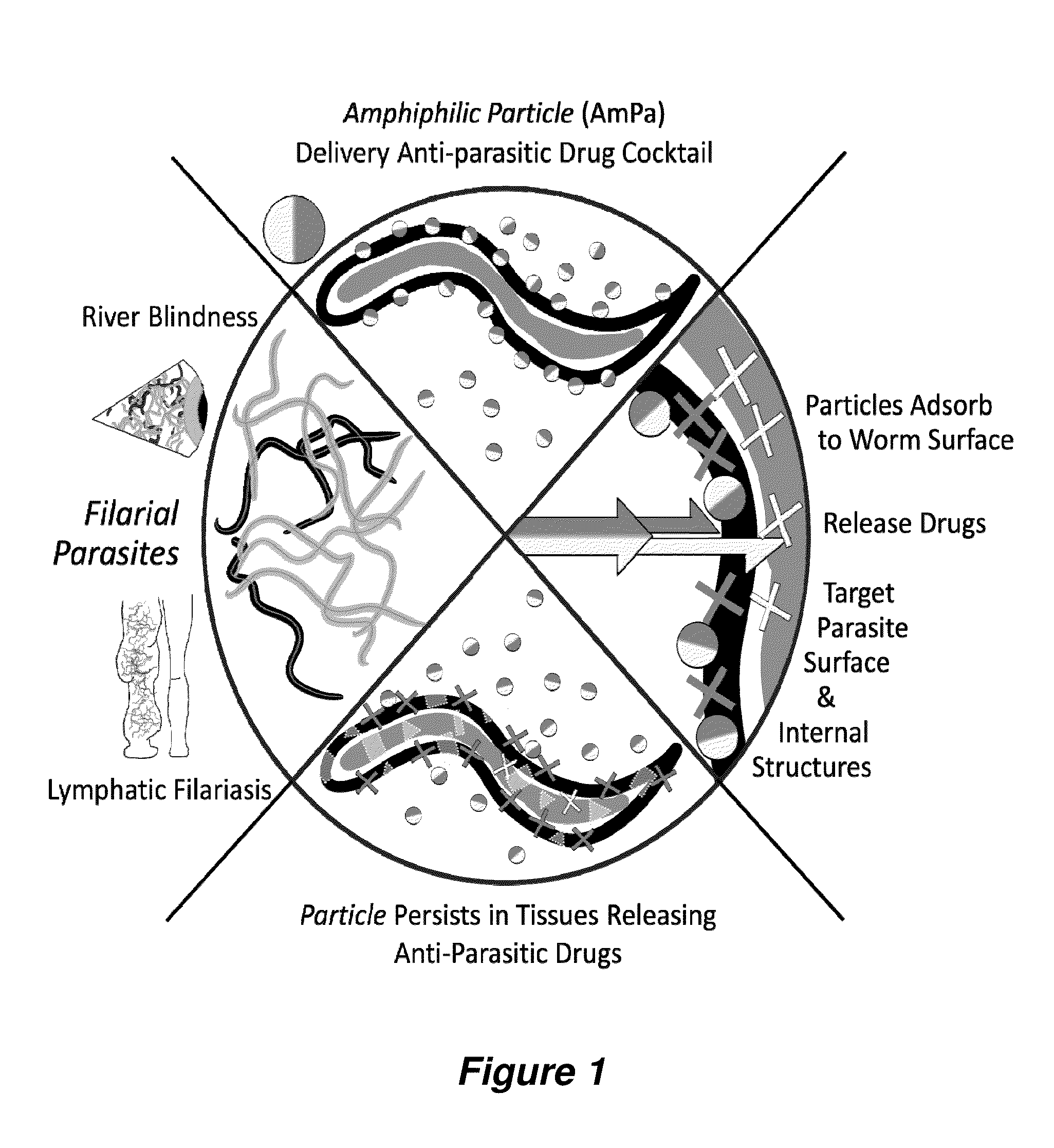
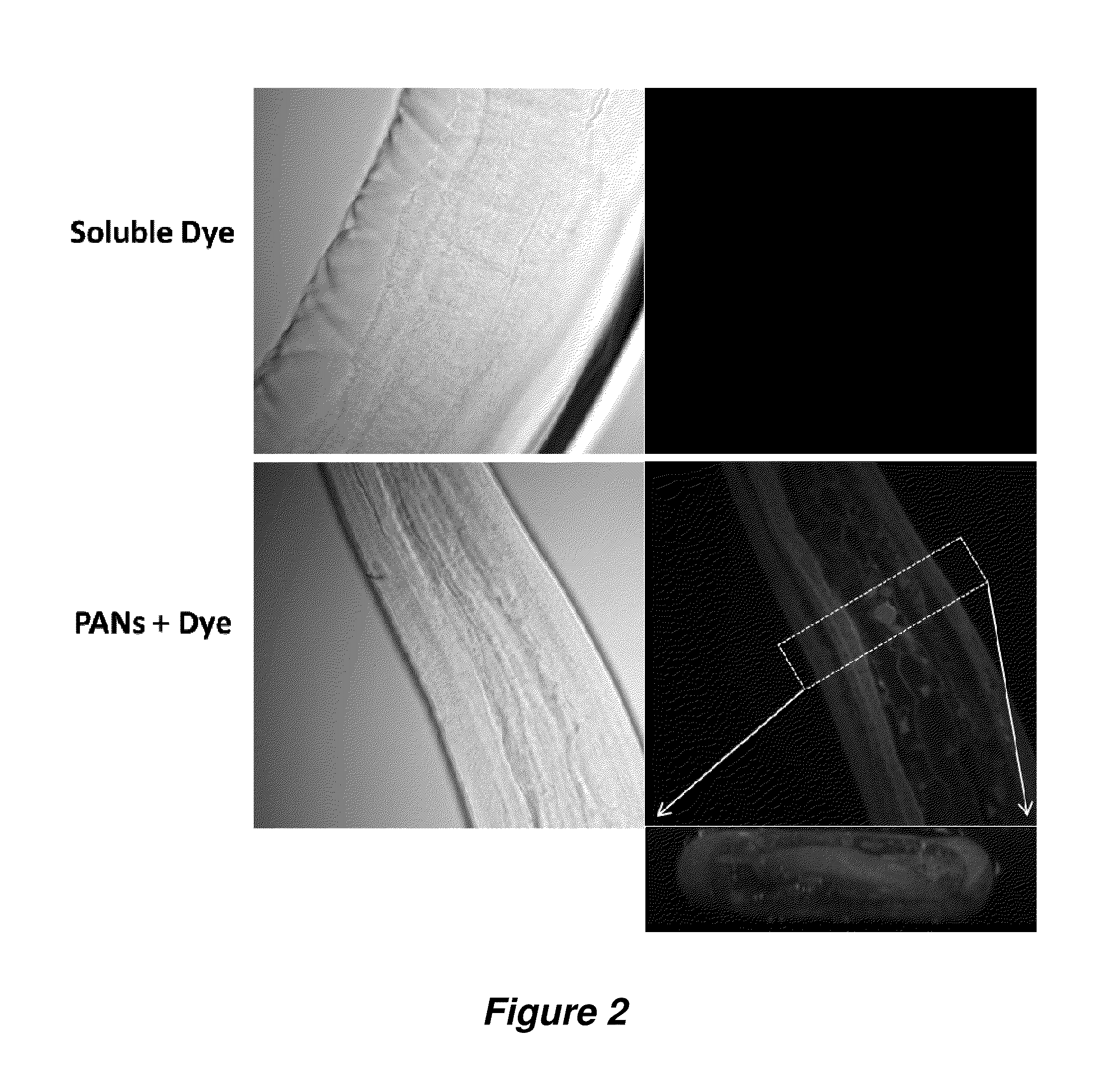
Antiparasitic polyanhydride nanoparticles
InactiveUS20150216888A1Good curative effectReducing microfilaria loadBiocideDispersion deliveryAntiparasiticAntiparasite agent
Filarial parasites Brugia, Wuchereria, Loa Loa and Onchocerca cause over 20 million infections worldwide and pose a significant social and economic burden in endemic areas. The invention provides compositions and methods to treat parasitic infections in animals and plants, and to kill and inhibit the replication of parasites in infected hosts. The methods can include administering to a host in need of treatment an effective antiparasitic amount of a composition comprising biodegradable polyanhydride microparticles or nanoparticles that encapsulate antiparasitic agents, optionally in combination with antibacterial agents. Through co-encapsulation of antiparasitic and antibacterial agents into the particles, the invention provides the ability to effectively kill parasitic helminthes, worms, and flukes, with up to a 40-fold reduction in the amount of drug used. The results described herein demonstrate the effectiveness of the drug carriers to reduce both the course of treatment and the amount of drug needed to treat parasitic infections.
Owner:IOWA STATE UNIV RES FOUND
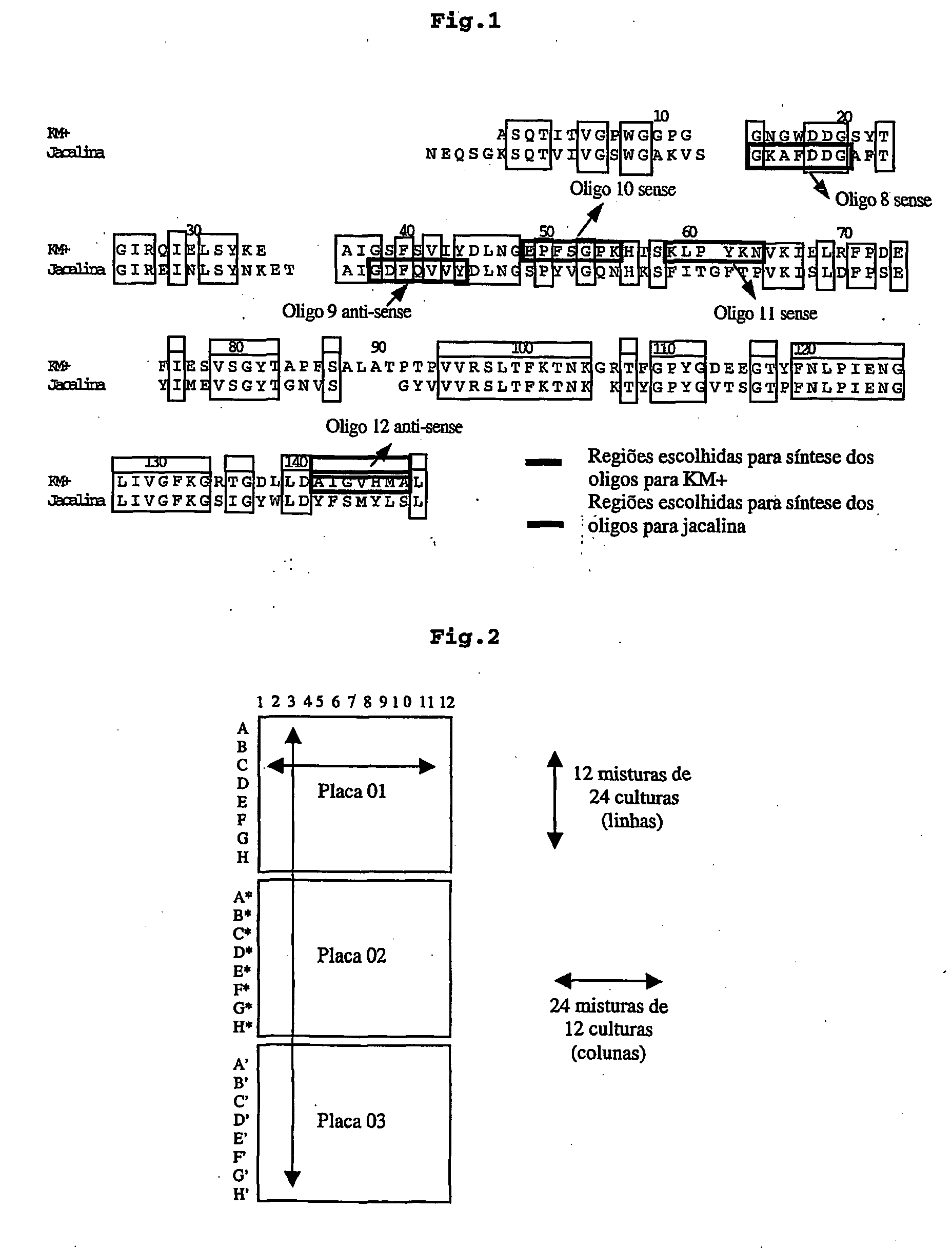
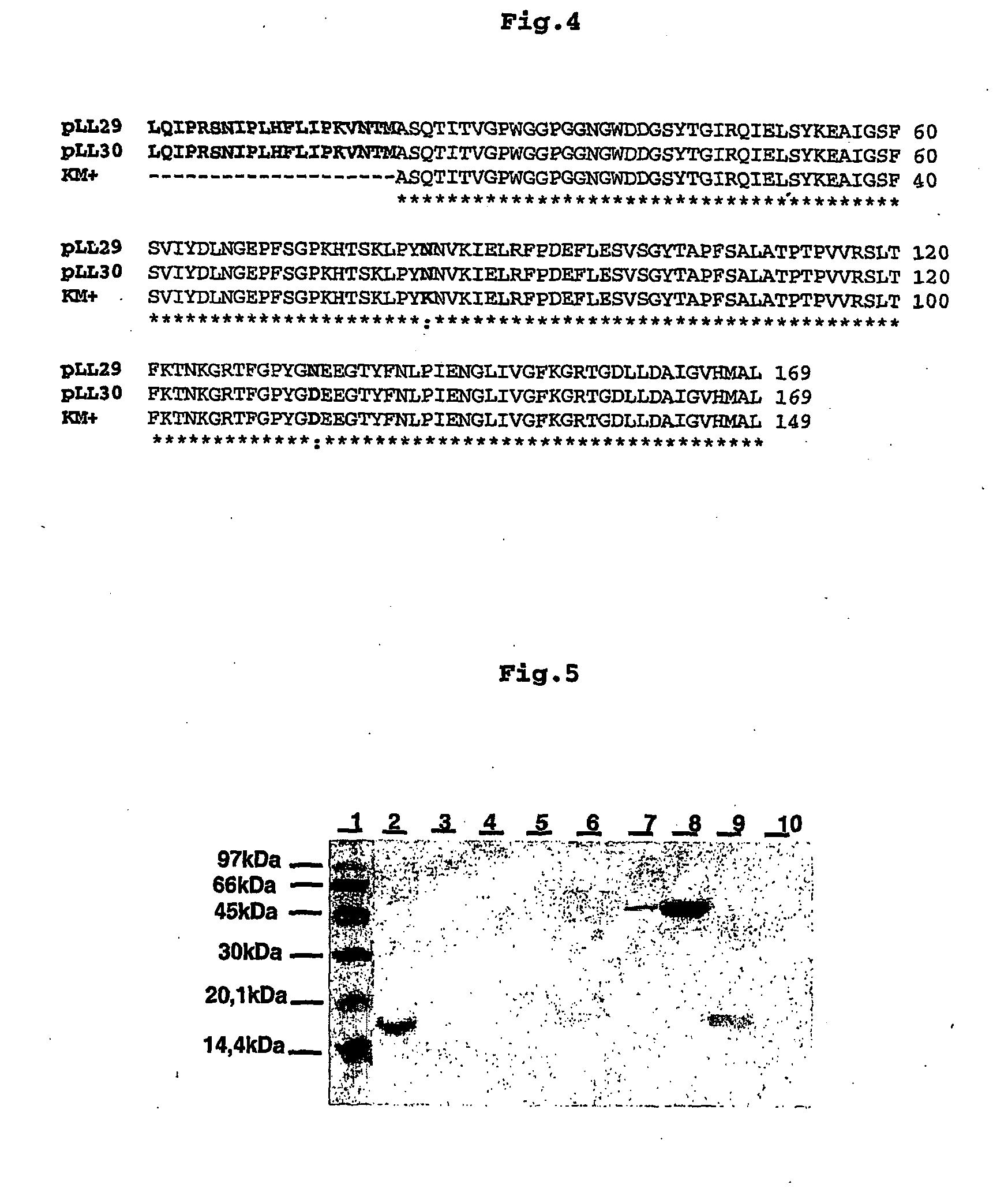
Pharmaceutical composition to prevent and treat epithelial wounds, immunomodulating pharmaceutical composition, pharmaceutical composition, insecticide, insecticide composition insecticide use of lectin KM+ to treat cicatrizations, use of lectin KM+ to prepare immunomodulating medicament, use of lectin KM+ to prepare anti-bacterial medicament, use lectin KM+ to prpare anti-viral medicament, use of lectin KM+ to prepare anti-parasite medicament, use of lectin KM+ to prepare anti-fungal medicament, use of lectin KM+ to prepare anti-parasite medicament expression method, DNA vector, recombinant
The invention deals with a pharmaceutical composition comprising lectin KM+ to prevent and heal epithelial wounds. The invention also comprises the use of lectin KM+, obtained from the plant (Artocarpus integrifolia) or recombinant (expression heterologue) to prepare medicaments.
Owner:PINTO DA SILVA LUIS LAMBERTI +6
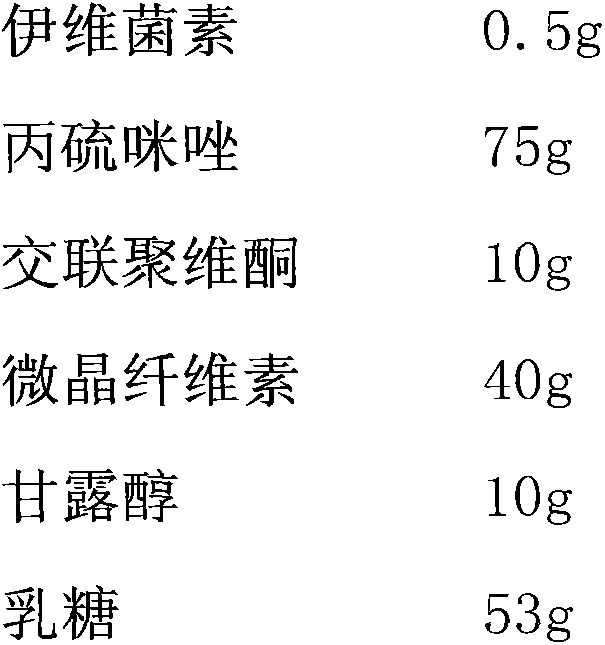
A kind of compound ivermectin injection and preparation method thereof
ActiveCN102258529AIncreased sensitivityDelay drug resistanceOrganic active ingredientsClimate change adaptationTherapeutic effectBENZYL ALCOHOL/WATER
The invention belongs to the technical field of veterinary anti-parasitic medicaments, particularly relates to a compound ivermectin injection and a preparation method thereof, and aims to provide an injection for treating parasitic infection of livestock, expanding an anti-parasitic spectrum and enhancing a therapeutic effect and a preparation method thereof. Every 100ml of compound ivermectin injection comprises 0.5-2g of ivermectin, 2.5-10g of albendazole sulfoxide, 5-15ml of polyethylene glycol 400 (PEG400), 1-10ml of acetic ether, 0.5-2ml of benzyl alcohol, 0.05-0.2ml of alpha-thioglycerol, 10-30ml of dimethyl sulfoxide and the balance of propylene glycol. In the injection, the ivermectin and the albendazole sulfoxide are used as anti-parasitic main components; the injection has double complementary advantages; the anti-parasitic spectrum is expanded; the drug resistance probability of parasites can be effectively reduced; and the sensitivity of the parasites is improved. The invention brings a completely new situation for safely, effectively and conveniently preventing and treating parasitic diseases in animal husbandry and aquaculture of China.
Owner:长沙施比龙动物药业有限公司
Sarah rhzomorph nano emulsion medicine composition and preparation method thereof
InactiveCN102949340AHigh thermodynamic stabilityGood storage stabilityOrganic active ingredientsAntiparasitic agentsAcute toxicity testingSide effect
The invention discloses a method for preparing a Sarah rhzomorph nano emulsion medicine composition and a formula. The preparation is an O / W (oil-in-water type) nano emulsion system which consists of ethylis oleas, Tween-80, n-butyl alcohol, ultrapure water and Sarah rhzomorph; the nano emulsion system has high medicine loading quantity (more than 11 percent); the medicine can be diluted by injection water or normal saline according to any rate in accordance with the clinical practical requirements; and the medicine is stably placed, so that the bioavailability of the medicine is greatly improved, the high dosage of the traditional transdermal medicine is effectively overcome; the insecticidal insect repellent function on the Sarah rhzomorph is greatly improved; and the clinical dosage of the Sarah rhzomorph is reduced to an extreme limit. The content of 1 percent of the preparation auxiliary materials (ethylis oleas, Tween-80, n-butyl alcohol and the like) is reduced to 4.1 percent on year-on-year basis; the content of 1 millesimal of the preparation auxiliary materials is reduced to 4.1 millesimal; the external properties are the same as those of true solution; compared with the transdermal preparation, the clinical dosage of the Sarah rhzomorph is reduced from 6mg / kg to 0.2mg / kg, and the curative effect is same; and therefore, at the present of deficiency of raw material medicines, the Sarah rhzomorph nano emulsion medicine is successfully researched, so that the dosage quantity is reduced, the dosage cost of a livestock master is reduced, the damage of the medicines to environment and public hygiene is greatly reduced; and in addition, according to an acute toxicity test and clinical pharmacodynamic test on mice, the nano emulsion medicine is free of toxic and side effects, so that the nano-level anti-parasitic medicine is safe, reliable and effective.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Anti-parasitical medicine in situ setting slow release injection and preparation method thereof
ActiveCN107811967AEasy to prepareImprove stabilityOrganic active ingredientsSolution deliveryAntiparasitic agentAnti parasitic
The invention discloses an anti-parasitical medicine in situ setting slow release injection and a preparation method thereof. The injection comprises the following components in ratio: 1-80g of an anti-parasitic disease medicine, 1-100g of a biodegradable high polymer material, 2-300mL of a dispersion medium and 0-50g of a solubilizer, wherein the content of the anti-parasitic disease medicine is1-800mg / mL. The injection can maintain long-term control effect when being medicated to a medicated object only once, the medicated object is protected from being infected by pathogens, propagation ofparasitic diseases can be economically and effectively controlled, material safety is high, prescription of a commercially available anti-parasitic disease medicine is simplified, compliance of the medicated object is improved, and control cost is reduced. Meanwhile, subcutaneous injection slow release injection is prepared by innovatively using a molluscicide, namely niclosamide, of the WHO, andthe effect of preventing schistosoma japonicum infection is achieved. The anti-parasitical medicine in situ setting slow release injection is simple in the preparation method, has good stability andis easy to use and popularize.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
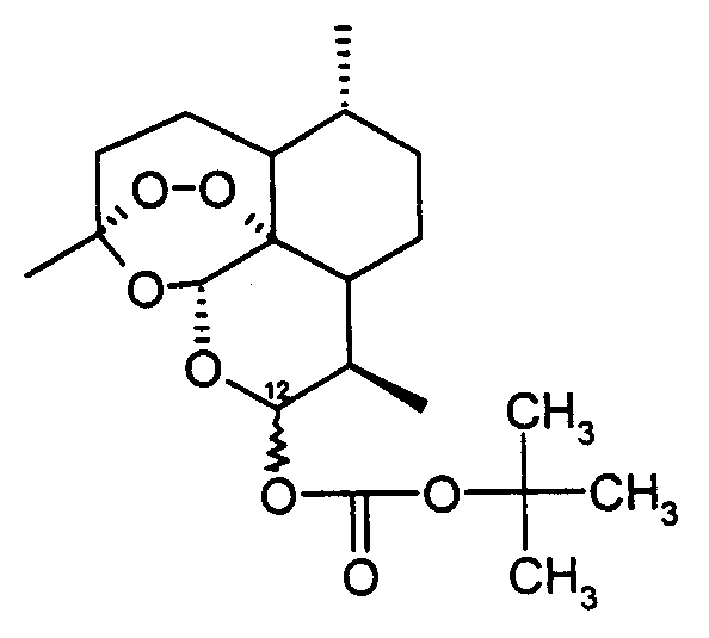
Tert-butoxy carbonyl dihydro artemisinin, preparation method and drug composition thereof
InactiveCN1405168AHigh activityLow toxicityOrganic active ingredientsOrganic chemistryDicarbonateTert-Butyloxycarbonyl protecting group
The invention provides a tert-butoxycarbonyl dihydroartemisine, which is characterized by that said invention provides its structural formula, and it is made up by using dihydroartemisine as initiation raw material, and making it and double tertiary butyl dicarbonate implement acidation reactino in organic solvent. The invented mecicine composition for resisting parasitic disease contains tert-butoxycarbonyl dihydroartemisine with therapeutic effective dose and pharmaceutically-acceptable carrier. Said invented product is high in therapeutical effect and low in toxicity, can be used for preventing and curing the parasitic diseases of schistosomiasis and malaria, etc.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Improved dimethicone-containing injection
ActiveCN103550150AGood compatibilityReduce stimulationSolution deliveryPharmaceutical non-active ingredientsALUMINUM STEARATESTissue Compatibility
The invention relates to a method for preparing an economical and efficient veterinary long-acting injection by adding a proper sustained-release material in an oil-based medium to improve the traditional oil-based injection. The method comprises the following specific steps: dissolving sucrose acetate isobutyrate and dimethicone with viscosity less than 20cs in isopropyl myristate or dissolving the dimethicone with viscosity less than 20cs and aluminum stearate in the isopropyl myristate to prepare the veterinary long-acting injection containing antibacterial agents or containing anti-parasitic drugs or containing non-steroidal anti-inflammatory drugs. The preparation is good in sustained release effect, stable in character, good in tissue compatibility and easy to prepare.
Owner:中农华威制药股份有限公司
Veterinary anti-parasitic preparation containing carnauba wax
ActiveCN104666244AThe determination method is simpleOrganic active ingredientsSolution deliveryVegetable oilMethyl oleate
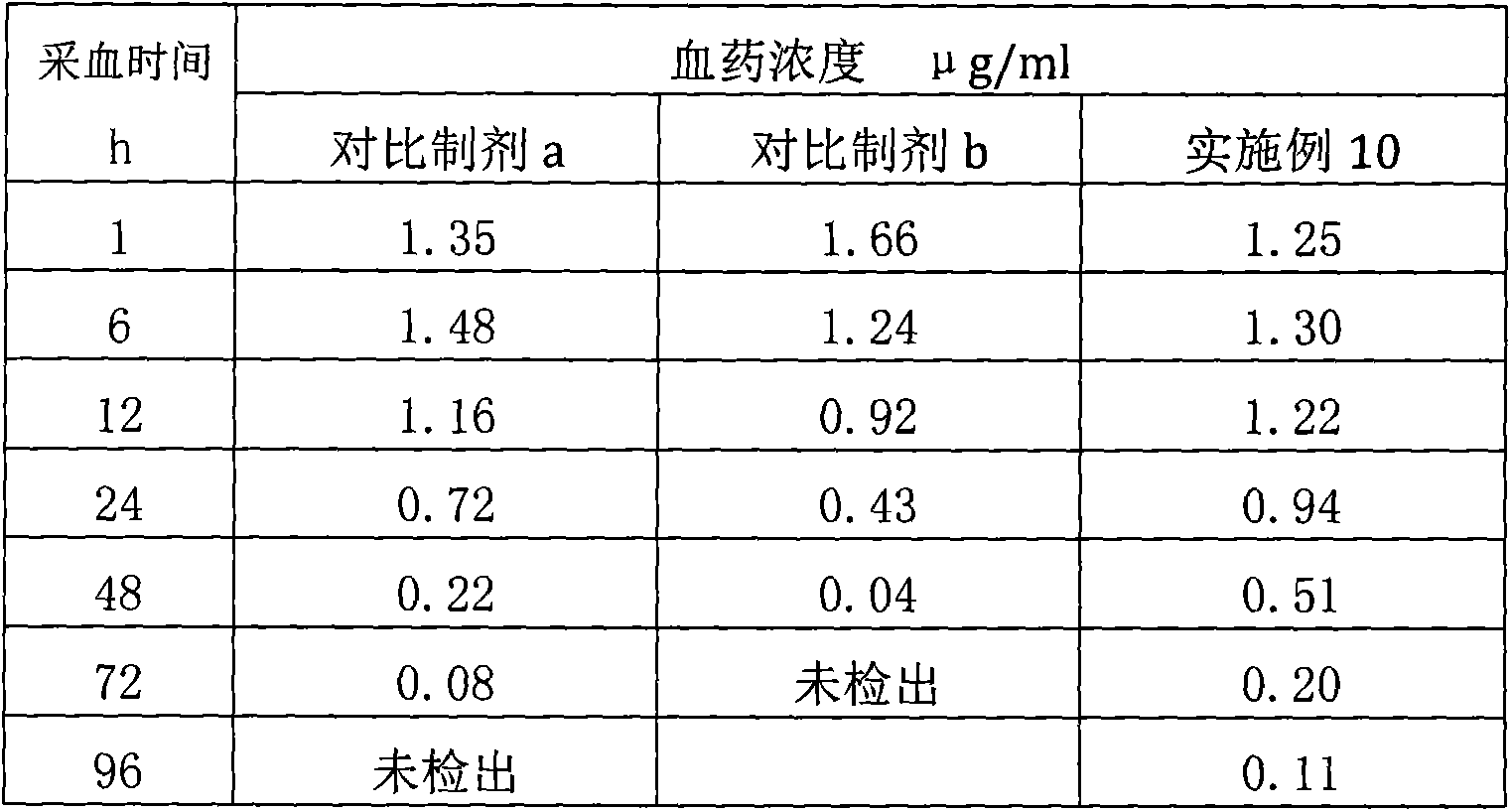
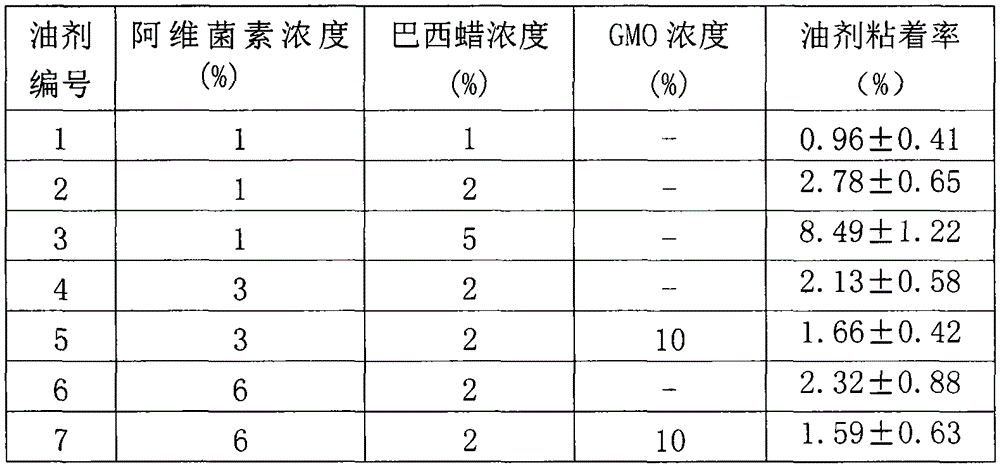
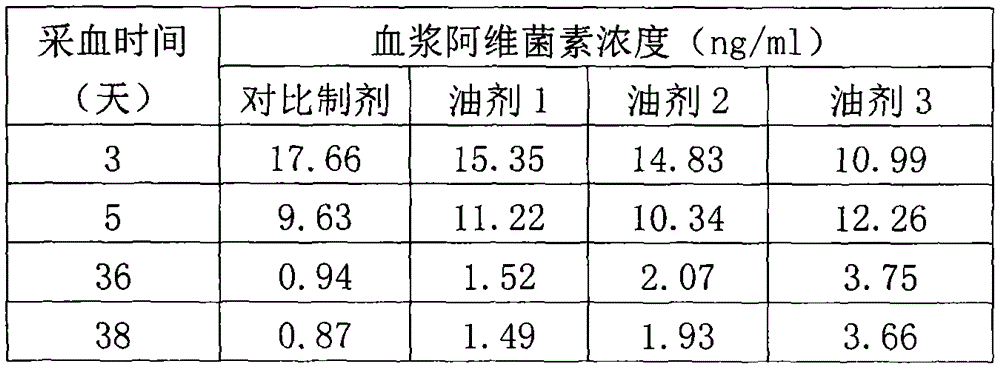
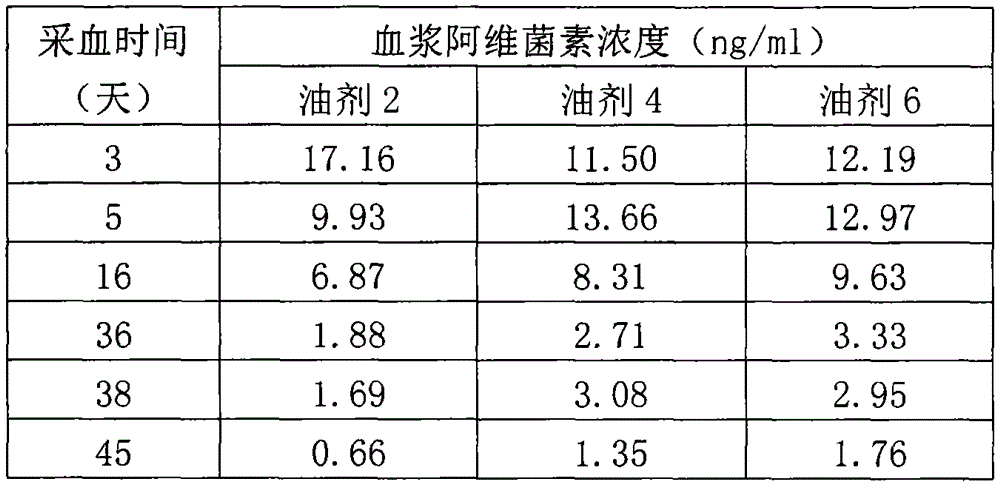
The invention relates to a veterinary anti-parasitic injection containing carnauba wax. The preparation is mainly prepared from anti-parasitic medicine, the carnauba wax and an oily medium, wherein the anti-parasitic medicine is avermectins, praziquantel and oxfendazole; the oily medium is vegetable oil, ethyl oleate or benzyl benzoate, and more than one of the vegetable oil, ethyl oleate or benzyl benzoate can be combined to use. Glycerol mono-oleate can be added in the preparation, the carnauba wax and the glycerol mono-oleate are compositely applied, and the slow-release effect of the preparation is better. The selected preparation is prepared from 36-80 g of abamectin anti-parasitic medicine, 10-70 g of the carnauba wax, 80-120 g of the glycerol mono-oleate, 0.1-0.3 g of an antioxidant, 10-20 g of benzyl alcohol and the balance of the oily medium (per 1000ml).
Owner:中农华威制药股份有限公司
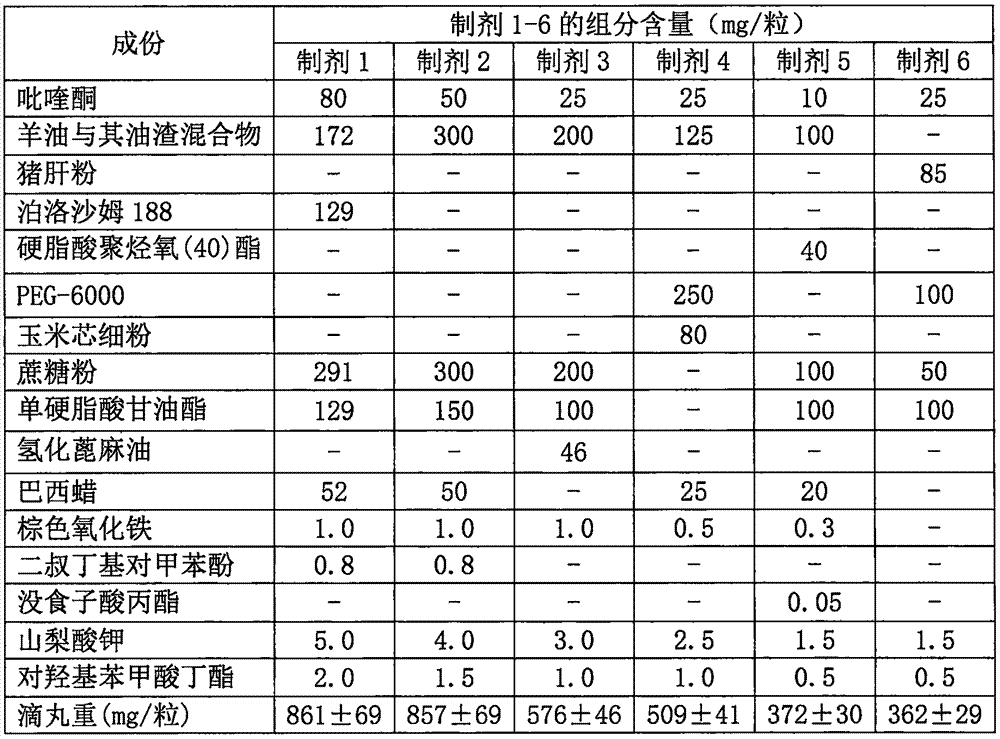
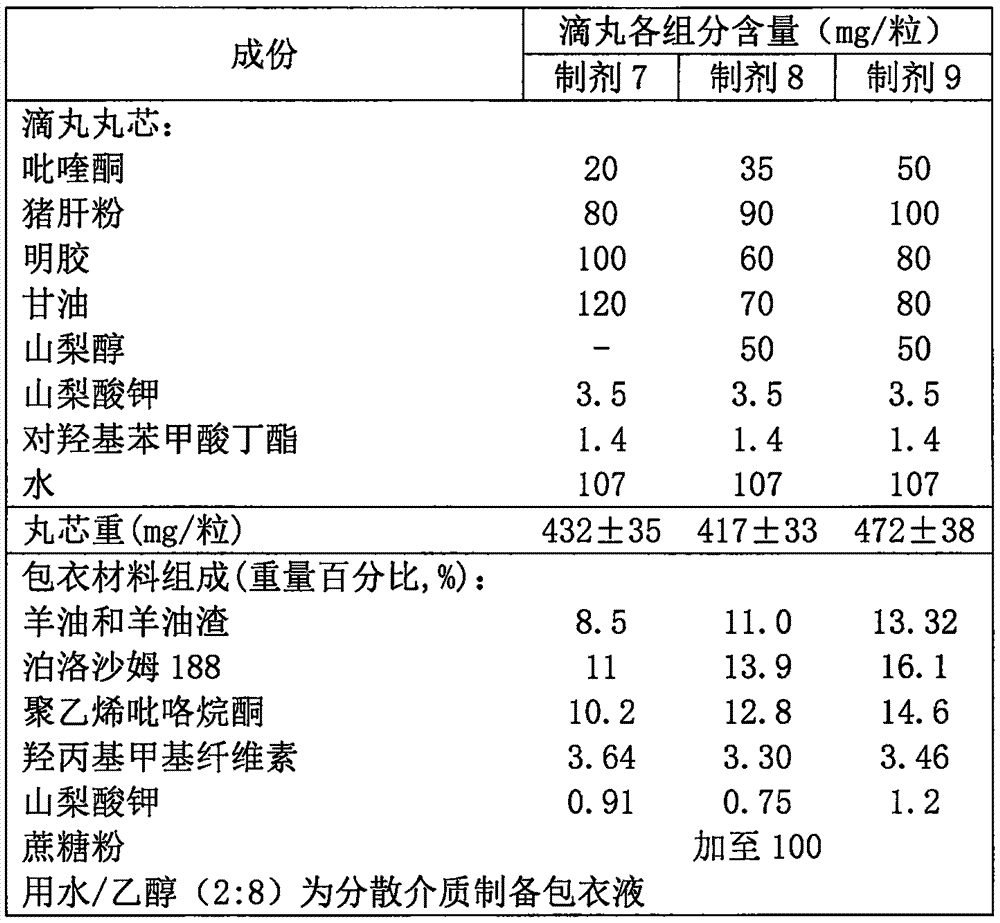
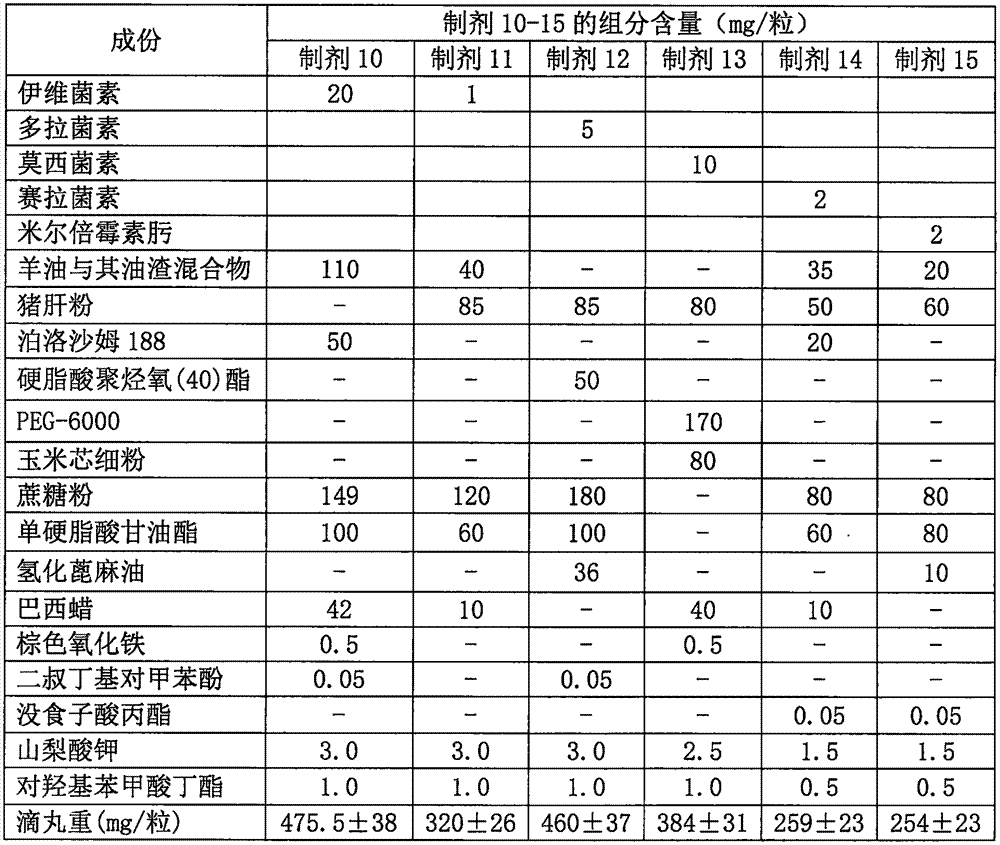
Anti-parasitic orally disintegrating tablet for dogs and cats and preparation method thereof
InactiveCN103751201AImprove bioavailabilityFast absorptionOrganic active ingredientsPill deliveryOrally disintegrating tabletAntiparasitic agent
The invention relates to an anti-parasitic orally disintegrating tablet for dogs and cats and a preparation method thereof and belongs to the field of veterinary preparations. The formula of the anti-parasitic orally disintegrating tablet provided by the invention comprises the following components in parts by weight: 0.1-2 parts of ivermectin, 30-60 parts of albendazole, 20-30 parts of disintegrant, 15-40 parts of filler, 1-2 parts of lubricant, 1-5 parts of glidant, 1-4 parts of surfactant and 1-4 parts of flavoring agent. The ivermectin and the albendazole are matched to prepare the orally disintegrating tablet, and the anti-parasitic orally disintegrating tablet has the advantages of simple production process, stable dosage and high production efficiency; and simultaneously, the flavoring agent is added into the orally disintegrating tablet, so that the taste of the medicament is improved, the medicament has the beef flavor, the palatability is increased, the compliance in medicament delivery of the dogs and the cats is further improved, and the anti-parasitic orally disintegrating tablet further has the advantages of more convenience in administration, fast disintegration after entering the mouth, higher bioavailability, good insect-dispelling effect in clinical use and broad application prospects.
Owner:LIAOCHENG UNIV
Dog and cat dropping pill preparation containing feed attractant
InactiveCN107510669AGood food attractantOvercome the disadvantages of drug administration difficultiesOrganic active ingredientsPharmaceutical non-active ingredientsAcrylic resinChicken Liver
The invention provides a dog and cat dropping pill preparation containing a feed attractant and treatment drugs (anti-parasite drugs, antibacterial drugs, non-steroidal anti-inflammatory drugs). The dropping pill preparation has excellent feeding attraction effect. In the dropping pill preparation, the feed attractant accounts for 5-55% of the weight of the dropping pill, the treatment drugs account for 0.1-35% of the weight of the dropping pill, and the matrix content at least accounts for 10% of the weight of the dropping pill. The treatment drugs contained in dropping pill can be combined with a capsule wall material and prepared into a microcapsule, and exist in the dropping pill in a microcapsule state. The capsule wall material for preparation of the microcapsule is selected from at least one of polyvinylpyrrolidone, acrylic resin, gelatin and Arabic gum. The feed attractant composing the dropping pill includes at least one of mutton tallow, mutton tallow residue, beef tallow, beef tallow residue, pork liver powder, chicken liver powder, fish meal, beef powder, chicken powder, natural bacon baking powder, and ham baking powder.
Owner:中农华威生物制药(湖北)有限公司
Solvent systems for pour-on formulations for combating parasites
ActiveUS20090163575A1Effective and lasting destructionTreatment or prophylaxisBiocideOrganic non-active ingredientsActive agentMacrolide resistance
This invention relates to pharmaceutical and veterinary formulations providing enhanced solvency and stability for pharmaceutical and veterinary agents for administration to animals, especially ruminants. In addition, the invention relates to pour-on formulations for combating parasites in animals, such as cattle and sheep. In some embodiments, this invention provides glycol-ether-based pour-on formulations comprising a composition comprising a flukicide, such as, for example, clorsulon (4-amino-6-trichloroethenyl-1,3-benzene disulfonamide) and / or a macrolide anthelmintic or antiparasitic agent. In other embodiments, the invention provides pour-on formulations comprising at least one active agent, a glycol ether, and a stability enhancer. This invention also provides for methods for eradicating, controlling, and / or preventing parasite infestation in animals, such as cattle and sheep.
Owner:MERIAL INC
Tick cystatin Rhcyst-2, and gene and applications thereof
InactiveCN103965350AStrong inhibitory activityInfectiousBacteriaPeptide/protein ingredientsNucleotideNucleotide sequencing
The invention discloses a tick cystatin. The tick cystatin is a Rhipicephalus haemaphysaloide cystatin Rhcyst-2. The invention also discloses a Rhipicephalus haemaphysaloide cystatin Rhcyst-2 gene. The Rhipicephalus haemaphysaloide cystatin Rhcyst-1 gene includes a nucleotide sequence coding an amino acid sequence represented by SEQ ID NO.1. The Rhipicephalus haemaphysaloide cystatin Rhcyst-2 has substantial cysteine protease inhibition activity and toxoplasma infection resistance, and is suitable for preparing anti-parasitical medicines.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Broad-spectrum anti-parasitic compound ivermectin tablet for pets
InactiveCN103083345AImprove palatabilityImprove drug deliveryOrganic active ingredientsInorganic non-active ingredientsHepatic first pass effectCompanion animal
The invention relates to a broad-spectrum anti-parasitic compound ivermectin tablet for pets and its preparation method. The tablet is composed of the following components by mass: 0.5%-1% of ivermectin, 1%-3% of praziquantel, 40%-70% of lactose, 10%-20% of mannitol, 15%-30% of calcium carbonate, 10%-20% of dextrin 400, and 1%-5% of microcrystalline cellulose. The tablet is prepared by subjecting the main drugs to mixing, filtering and tabletting. Compared with traditional anti-parasitic drugs, the broad-spectrum and high efficiency anti-parasitic tablets provided in the invention can be absorbed quickly through oral administration, can mitigate the hepatic first-pass effect, and can rapidly reach a maximum plasma concentration, thus achieving better clinical effects.
Owner:QINGDAO VLAND BIOTECH INC
Isoxazoline derivatives as antiparasitic agents
This invention recites isoxazoline substituted azetidine derivatives of Formula (1), stereoisomers thereof, veterinarily acceptable salts thereof, compositions thereof, and their use as a parasiticide in mammals and birds. R1a, R1b, R1c, R2, R3, R4, R6, and n are as described herein.
Owner:ZOETIS SERVICE LLC
Pharmaceutical composition containing an N-phenylpyrazole derivative, and use thereof for preparing a topical veterinary for flea control
InactiveUS8501799B2Effectively prevent and treat flea infestationEasy to prepareBiocideOrganic active ingredientsOrganic solventVeterinary Drugs
The invention relates to a liquid pharmaceutical composition that contains N-phenylpyrazole derivative as an active principle, benzyl alcohol, and an appropriately selected organic solvent, and to the use of such a composition for preparing a topically applied antiparasitic veterinary drug for preventing and / or treating flea infestation in pets, in particular, in dogs and cats.
Owner:VIRB AC SA
Antiparasitic agents
The present invention relates to compounds of the formula (I)and pharmaceutically acceptable salts thereof, compositions containing such compounds and the uses of such compounds as antiparasitic agents.
Owner:PFIZER ANIMAL HEALTH UK 1 LIMITED
Ivermectin nanoemulsion antiparasitic medicine and preparation method thereof
InactiveCN104208023AHigh thermodynamic stabilityGood light transmissionOrganic active ingredientsAntiparasitic agentsSolubilityHigh absorption
The invention discloses an ivermectin nanoemulsion antiparasitic medicine and a preparation method thereof. A particle size range of the nanoemulsion is 1-100nm and the nanoemulsion comprises the following raw materials in percentage by mass: 0.10-1.00% of ivermectin, 1.00-20.00% of cosurfactant, 10.00-20.00% of surfactant, 1.00-5.00% of oil phase and the balance being distilled water. The nanoemulsion disclosed by the invention is a colorless, clear and transparent liquid in appearance, and has characteristics of low viscosity, good stability, strong dispersion, high absorption speed and targeted medicine release; in addition, the dissolubility and bioavailability of the medicines are improved and toxic and side effects are reduced. The nanoemulsion can take a good expelling and killing effect on in vitro and vivo parasites, particularly on nematode and arthropod, and can effectively prevent and treat parasitic diseases of animals within a wide spectrum.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com