Process for the preparation of fipronil and analogues thereof
A technology for fipronil and a compound, which is applied in the field of preparing 5-amino-3-cyano-1--4-trifluoromethylsulfinylpyrazole, can solve the problem of difficult industrial application, difficult to scale up, and a Therapeutic use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 - Industrial Scale Purification of CF 3 SO 2 Na
[0136] In a 500L reactor, add 75.0kg of commercially available CF 3 SO 2 Na, followed by the addition of 210 kg of ethyl acetate. The resulting mixture was stirred at 25±5°C for 1 hour. Silica gel (10.7 kg) was added. The resulting mixture was stirred for 15 minutes, then centrifuged. The filter cake (residue) was placed in a 200 L reactor and 76.3 kg of ethyl acetate was added. The resulting mixture was stirred at 25±5°C for 1 hour, and then subjected to centrifugation. The filter cake (residue) was put back into the reactor and the process (ethyl acetate and filtration) was repeated one more time using 76.3 kg of ethyl acetate. The washing process is repeated 2 to 3 times.
[0137] The filtrates were combined, and 106.6 kg of pure deionized water was added. The resulting mixture was heated to 50±5°C and stirred at this temperature for 30 minutes, then cooled to room temperature. The organic layer w...
Embodiment 2
[0138] Embodiment 2-industrial scale prepares catalyst PTSA-NHMe 2
[0139] In a 200 L reactor, 70.0 kg PTSA was added. Add Me dropwise at 25±5°C 2 NH (5805 g, 30% in water). The resulting solution was stirred at this temperature for 1 hour. The solution was then concentrated under vacuum at 70±5°C. Toluene (100.0 kg) was added to the residue; the remaining water was removed by azeotropic distillation under vacuum at 70±5°C. When water could no longer be separated, the mixture was cooled to 20±5°C and filtered through a 1.0 mm porous titanium alloy filter cartridge under a pressurized nitrogen purge. The filter cake was dried under vacuum at 70±5°C.
Embodiment 3
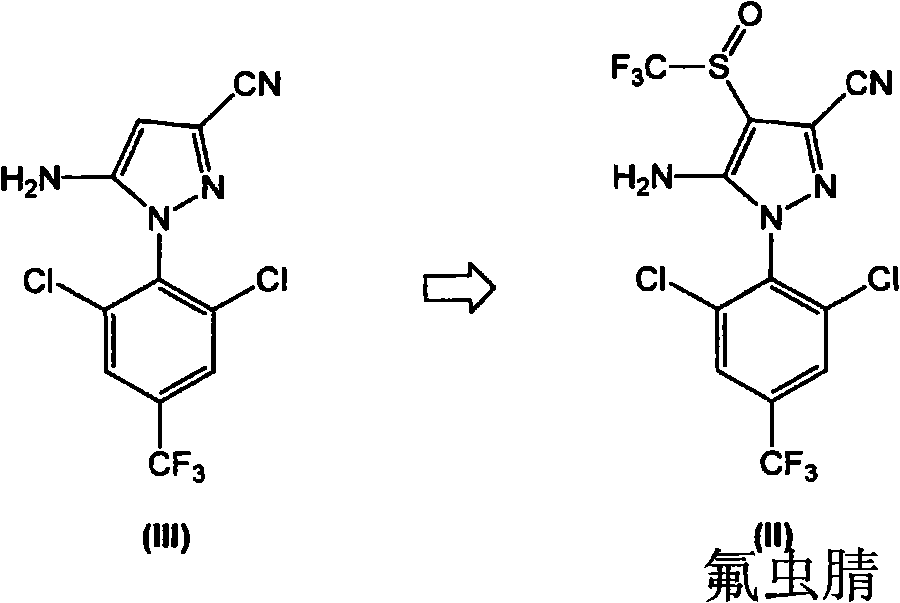
[0140] Embodiment 3-industrial scale preparation formula I compound
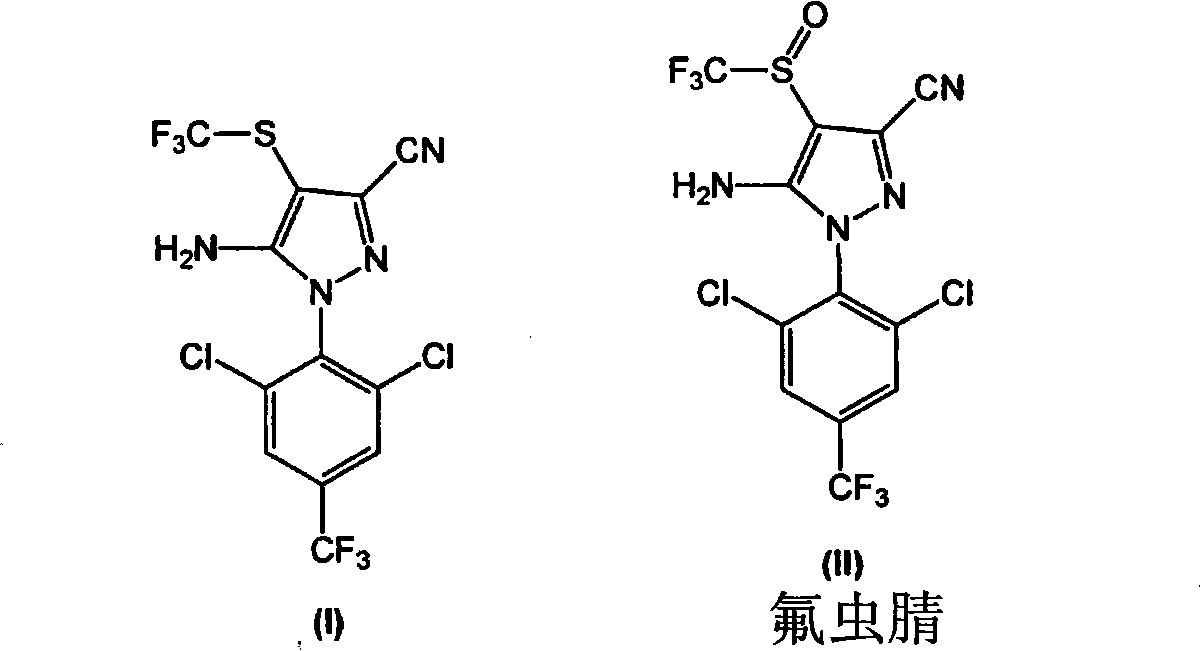
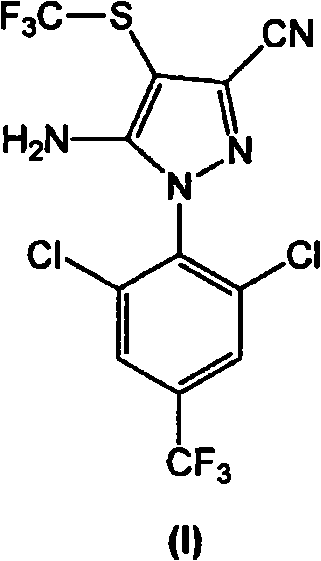
[0141] In a 200L reactor, add 12.0kg 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-1H-pyrazole-3-carbonitrile (compound of formula III), 11.7kg The CF obtained in Example 1 3 SO 2 The catalyst PTSA.NHMe that obtains in Na, 12.4kg embodiment 2 2 and 90.8kg toluene. The resulting mixture was stirred at room temperature (25±5° C.) for 15 minutes, then 0.11 kg of DMF was added. The resulting mixture was stirred at room temperature for 30 minutes. Allow the mixture to cool to 0±2 °C and add PCl dropwise at this temperature 3 (5.1g). The resulting mixture was stirred at 0±2°C for 1 hour. It was then allowed to warm to room temperature and stirred at 20±5°C for 1 hour. The mixture was then heated to about 65°C to 70°C and stirred at this temperature for 8 hours.
[0142] Water (48.0 kg) and 16.1 kg ethyl acetate were added. The resulting mixture was stirred for 30 minutes, cooled at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com