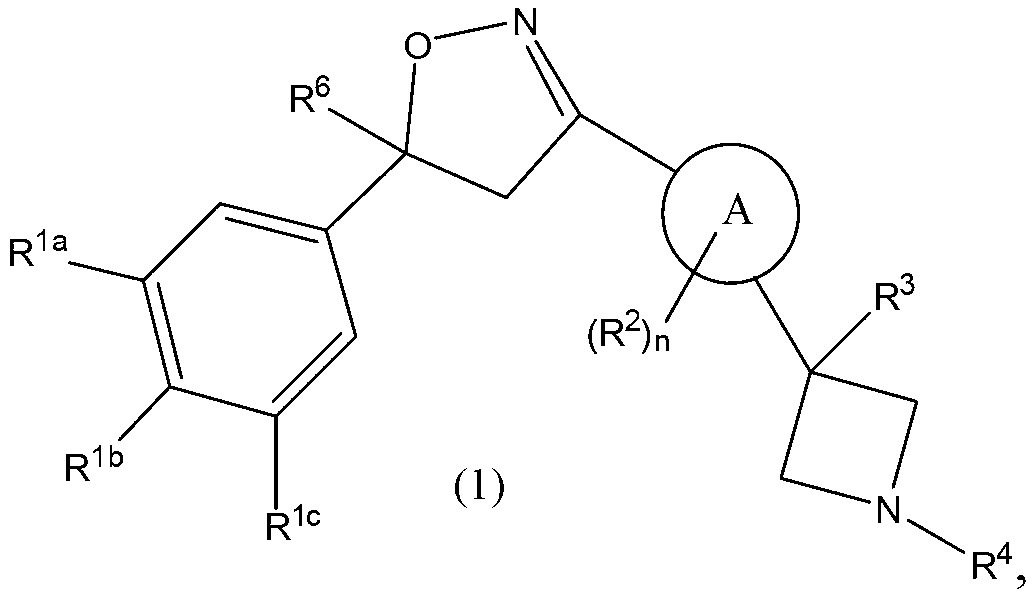
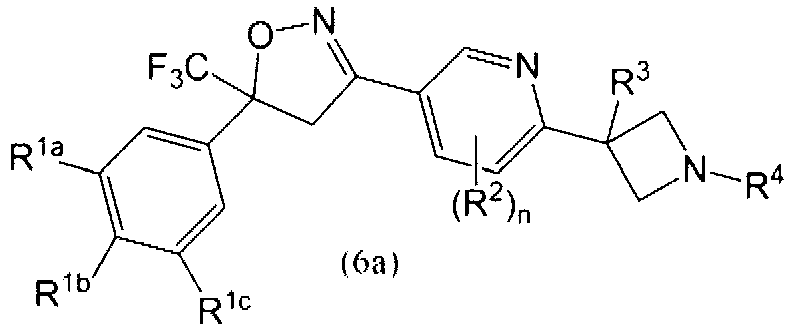
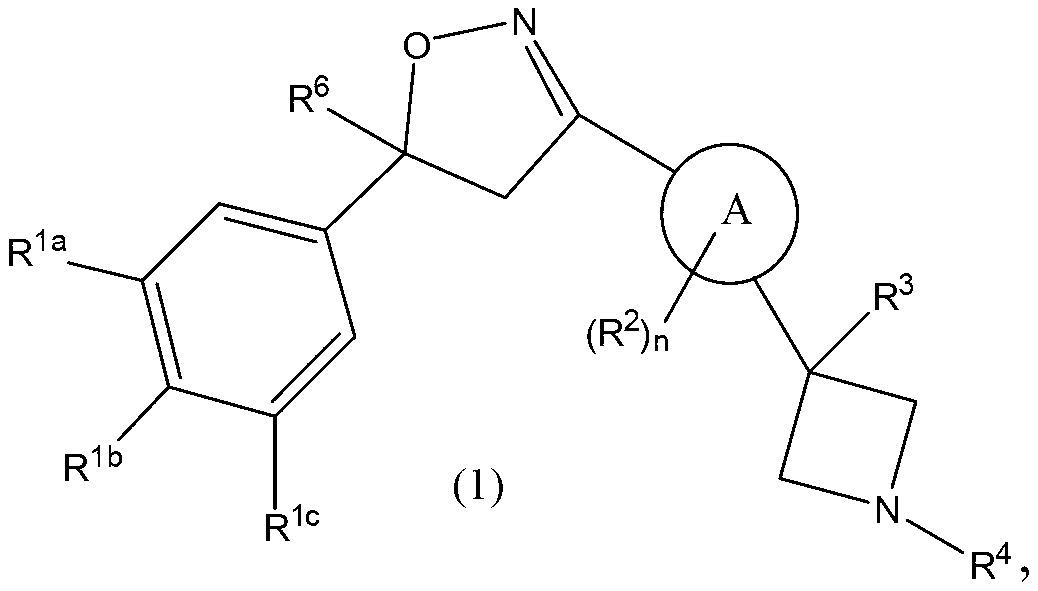
Isoxazoline derivatives as antiparasitic agents
A compound, C1-C6 technology, used in biocides, animal repellents, plant growth regulators, etc., can solve problems such as no compound antiparasitic species lineage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0653] Urea analogs can be prepared as shown in Scheme 16. Reaction of azetidine 70 with isocyanates or preformed carbamoyl chlorides in the presence of tertiary amine bases provides ureas.
[0654] Scheme 17
[0655]
[0656] Thioamide 76 can be prepared by treating amide 71 with Lowe's reagent in refluxing toluene. Methyl triflate can be dissolved in a solvent such as CH 2 Cl 2 is added to thioamide 76 to form the thioimidate intermediate as a solution. Cyanamide and Hunig's base in THF can be added directly to the thioimidate solution in one portion to give cyanamide 77.
[0657] Those skilled in the art will appreciate that in some cases, following the addition of specified reagents depicted in the schemes, other routine synthetic steps not described in detail may have to be performed to complete the synthesis of compounds of formula (1) or formula (XX).
[0658] The present invention includes all veterinarily acceptable isotope-labeled formula (1) in which one o...
Embodiment 1
[0718] Example 1 : 1-(3-fluoro-3-(4-(5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazole-3- Base) phenyl) azetidin-1-yl) ethanone
[0719]
[0720] To 3-(4-(3-fluoroazetidin-3-yl)phenyl)-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5 - Dihydroisoxazole (Preparation 9, 94mg) in 2mL CH 2 Cl 2 To the solution in , pyridine (0.05 mL) was added, followed by acetyl chloride (31 mg). The reaction was stirred at room temperature for 1 hour. Water (3 mL) was added. Reaction with 3mL CH 2 Cl 2 Dilute, stir for 30 minutes, and pour through a phase extractor. Collect CH 2 Cl 2 layer and concentrate. The crude product from the reaction was adsorbed onto silica and chromatographed on a 12 g silica column eluting with a gradient of 50% EtOAc / hexanes to 100% EtOAc / hexanes to afford 90 mg of the desired compound as a white Foam. 1 H NMR (CDCl 3 )δ7.76(d,2H), 7.67(s,2H), 7.56(d,2H), 4.71-4.31(m,4H), 4.12(d,1H), 3.72(d,1H), 2.01(s ,3H). LC / MS dwell time = 3....
Embodiment 2
[0721] Example 2 : Cyclopropyl(3-fluoro-3-(4-(5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazole-3 -yl)phenyl)azetidin-1-yl)methanone
[0722]
[0723] To 3-(4-(3-fluoroazetidin-3-yl)phenyl)-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5 -Dihydroisoxazole (preparation 9,94mg) in 2mL CH 2 Cl 2 To the solution in , pyridine (0.05 mL) was added, followed by cyclopropanecarbonyl chloride (31 mg). The reaction was stirred at room temperature for 1 hour. Water (10 mL) was added. Reaction with additional 10mL CH 2 Cl 2 Dilute, stir at room temperature for 30 minutes, then pass through a phase separation tube. Collect CH 2 Cl 2 layer and concentrate. The crude material was adsorbed on silica and chromatographed on a 12 g silica column eluting with a gradient of 20% EtOAc / hexanes to 80% EtOAc / hexanes. Fractions containing the desired material were combined and concentrated. Will Et 2 O (~1 / 2 mL) was added to the resulting film. The flask was placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com