Tick cystatin Rhcyst-2, and gene and applications thereof
A cysteine protease, rhcyst-2 technology, applied in the field of bioengineering, can solve the problem that there is no research report on cysteine protease inhibitory molecules of R. falciparum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Gene cloning and sequence analysis of RHcyst-2, a cysteine protease inhibitor of Rhizocephalus falciparum
[0038] 1. Extraction of total RNA from female Rhizocephalus sylvestris half-saturated
[0039] Rhizocephalus falciparum was inoculated on rabbit ears at a ratio of 2:1 male to female, and half-full blooded female ticks were removed from rabbit ears 4 days later. Grind with liquid nitrogen on a sterilized mortar, then add 1ml of Trizol reagent to grind thoroughly and transfer to a nuclease-free EP tube. The total RNA of 4 Rhizocephalus falciparum half-saturated females was extracted by the classic Trizol method, the concentration was 11.3ug / ul, and the OD260 / OD280 value was 1.942, which could meet the requirements of subsequent experiments in terms of concentration and purity.
[0040] 2. Rapid amplification of the 3' end
[0041] 2.1 Primer design
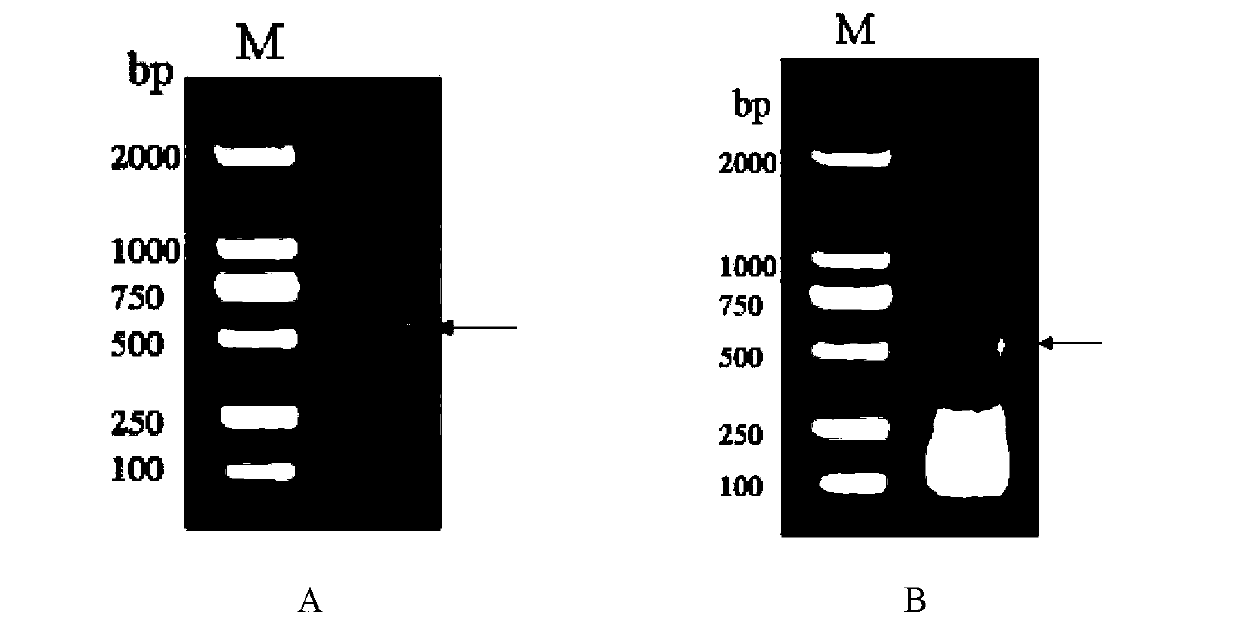
[0042] For the family 2 cystatin molecule of Rhizocephalus falciparum, based on the conserved QVVAGXN...
Embodiment 2
[0086] Example 2 Prokaryotic expression and purification of the cysteine protease inhibitor RHcyst-2 of Rhizocephalus falciparum
[0087] 1. Expression plasmid construction
[0088] According to the analysis results of the full-length sequence of RHcyst-2, gene-specific primers with appropriate enzyme cutting sites were designed at both ends of the ORF of the RHcyst-2 gene to remove the signal peptide. The primer sequences are as follows:
[0089] RHcyst-2express F: 5'-TA GAATTC CAACCGCTGGTTGGCGG-3' (SEQ ID NO. 9);
[0090] RHcyst-2express R:5'-TA CTCGAG CTAGGTGGATGCGCTGCT-3' (SEQ ID NO. 10).
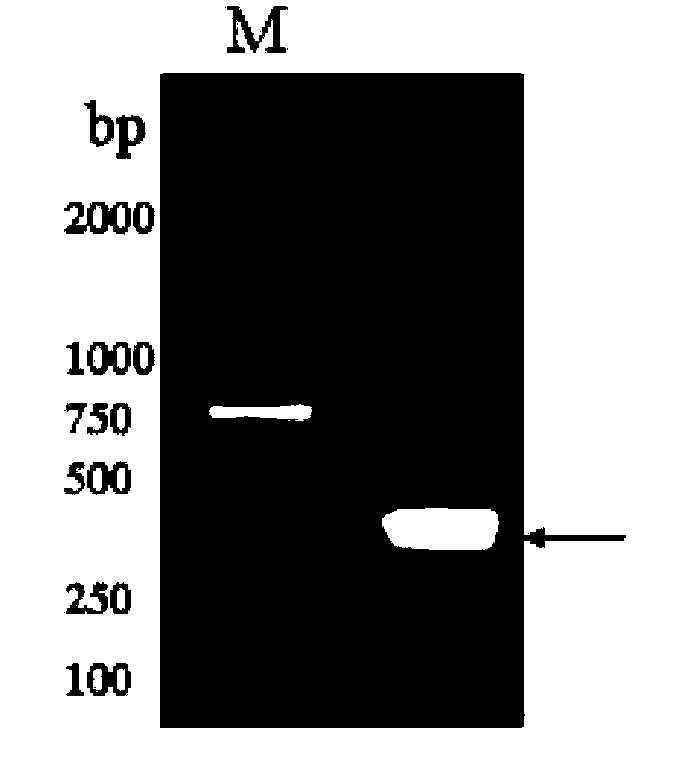
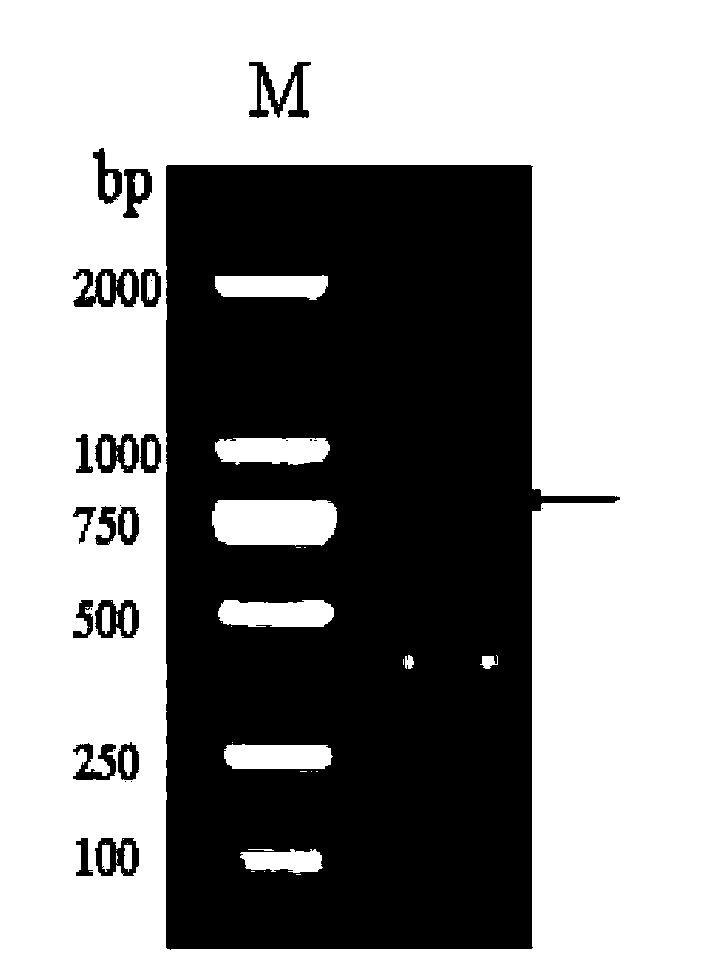
[0091] PCR amplified the coding region of the RHcyst-2 gene to remove the signal peptide, cloned it into the pGEX-4T-1 vector, constructed and expressed the recombinant plasmid RHcyst-2 / pGEX-4T-1, and subjected to double digestion (Xho I and EcoR I) and sequencing Identify the accurate insertion of the target fragment. Figure 5 It is the double enzyme digestion identification...
Embodiment 3
[0109] Example 3 Enzyme Activity Analysis Experiment of Recombinant Protein rRHcyst-2
[0110] 1. Method: The activity of rRHcyst-2 on papain-like cysteine protease was verified by measuring the residual activity of cathepsin L, B, C, H, S, papain and its corresponding fluorescent substrate after the action of rRHcyst-2. inhibitory activity. The specific method is as follows:
[0111] (1) The cysteine protease reaction solution was prepared according to the formula of 100mM NaAC, 100mM NaCl, 1mM EDTA, 1mg / ml cysteine and 0.005% TritonX-100, and the pH was adjusted to 5.5.
[0112] (2) Six cysteine proteases were prepared to 1.5uM with cysteine protease reaction solution, and their corresponding fluorescent substrates were prepared to 0.5mM.
[0113] (3) The purified RHcyst-2 recombinant protein was prepared into a concentration gradient of 12uM, 6uM, 3uM, 1.5uM, 0.75uM, 0.375uM and 0uM with PBS.
[0114] (4) Add 20ul of RHcyst-2 recombinant protein with seven conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com