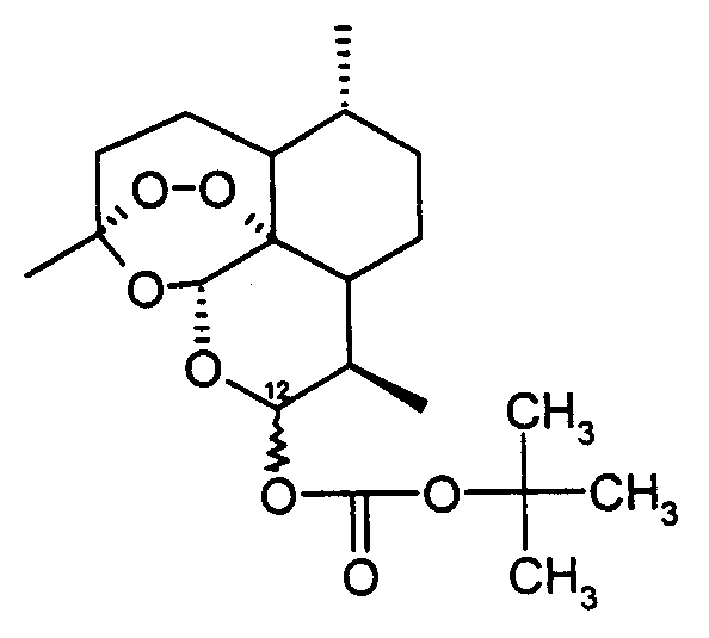
Tert-butoxy carbonyl dihydro artemisinin, preparation method and drug composition thereof
A technology of tert-butoxycarbonyl dihydroartemisinin and dihydroartemisinin, which is applied in the field of terpenoids, and can solve the problems that the solubility is not as good as artemether, and there is no in-depth research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0041] The specific implementation of the present invention will be further elaborated below in conjunction with examples, but these examples are by no means any limitation to the present invention.
Synthetic example 1
[0043] First dihydroartemisinin (2.84 grams, 10 millimoles) was dissolved in 40 milliliters of dichloromethane, bis-tert-butyl dicarbonate (2.84 grams, 12.8 millimoles) was added, and then the acylation catalyst dimethylamino Pyridine (20 mg), stirred at room temperature. After the reaction was basically completed, the reaction solution was washed with water and dried with magnesium sulfate. The solvent was evaporated, and the obtained crude product was recrystallized with ethyl acetate-petroleum ether to obtain 3.00 g (yield 78%) of tert-butoxycarbonyl dihydroartemisinin (12-alpha body) and tert-butoxycarbonyl dihydroartemisinin Artemisinin (12-β body) 0.19 g (yield 5%).
Synthetic example 2
[0045] First, dihydroartemisinin (2.84 g, 10 mg) was dissolved in 50 ml of anhydrous acetonitrile, bis-tert-butyl dicarbonate (2.5 g, 11.3 mg) was added, and heated to reflux for reaction. After the reaction was basically completed, the solvent was evaporated from the reaction solution under reduced pressure, and the resulting crude product was separated by column chromatography (silica gel, 6% ethyl acetate / petroleum ether as eluent) to obtain tert-butoxycarbonyldihydroartemisinin ( 12-α body) 1.46 g (yield 38%) and tert-butoxycarbonyldihydroartemisinin (12-β body) 1.54 g (yield 40%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com