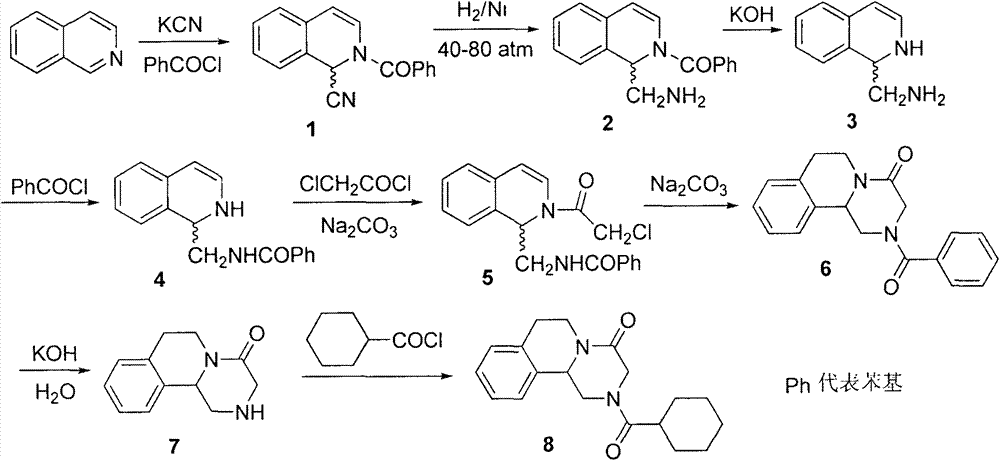
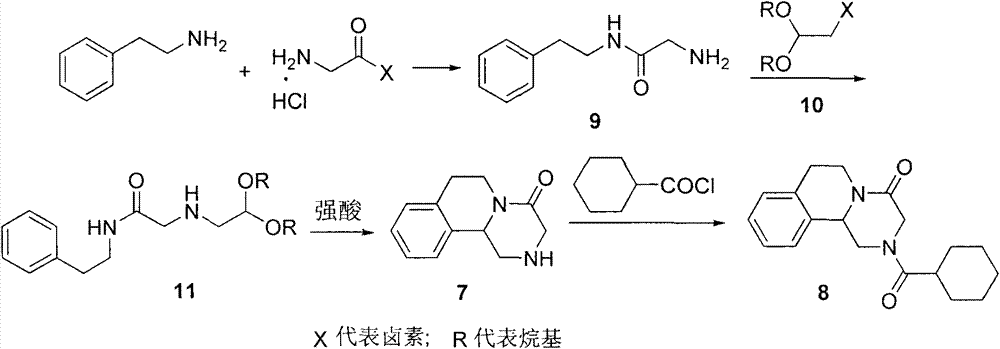
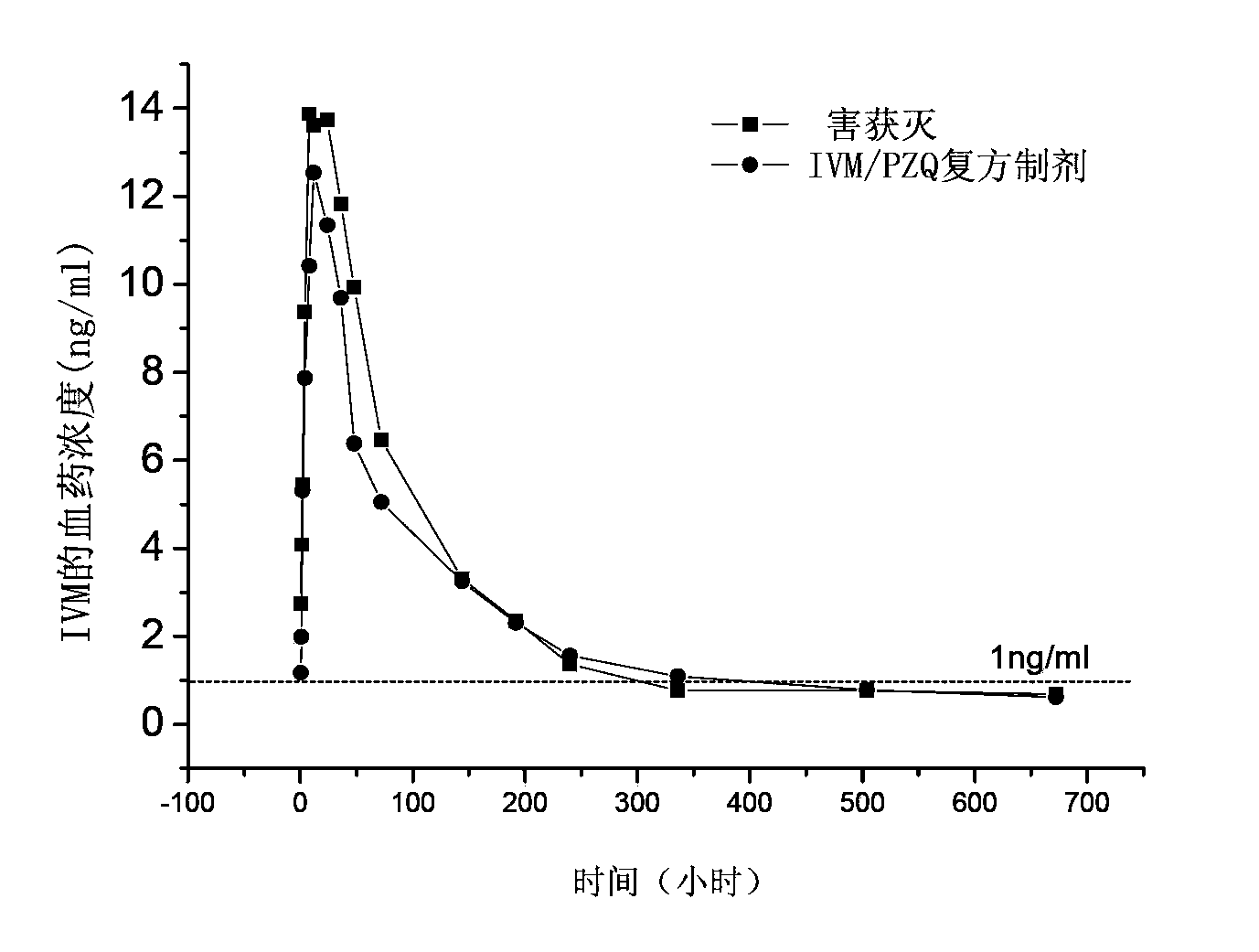
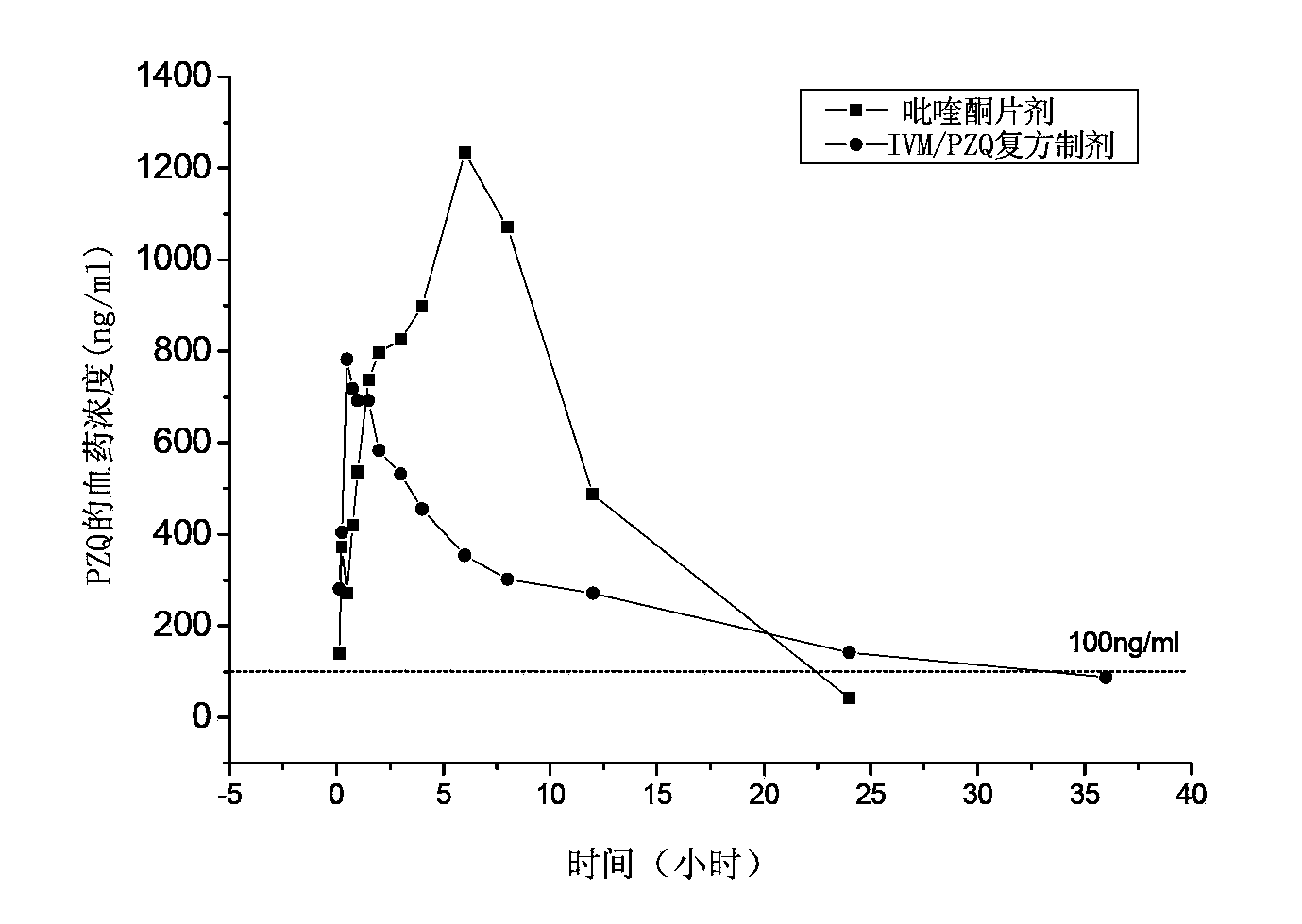
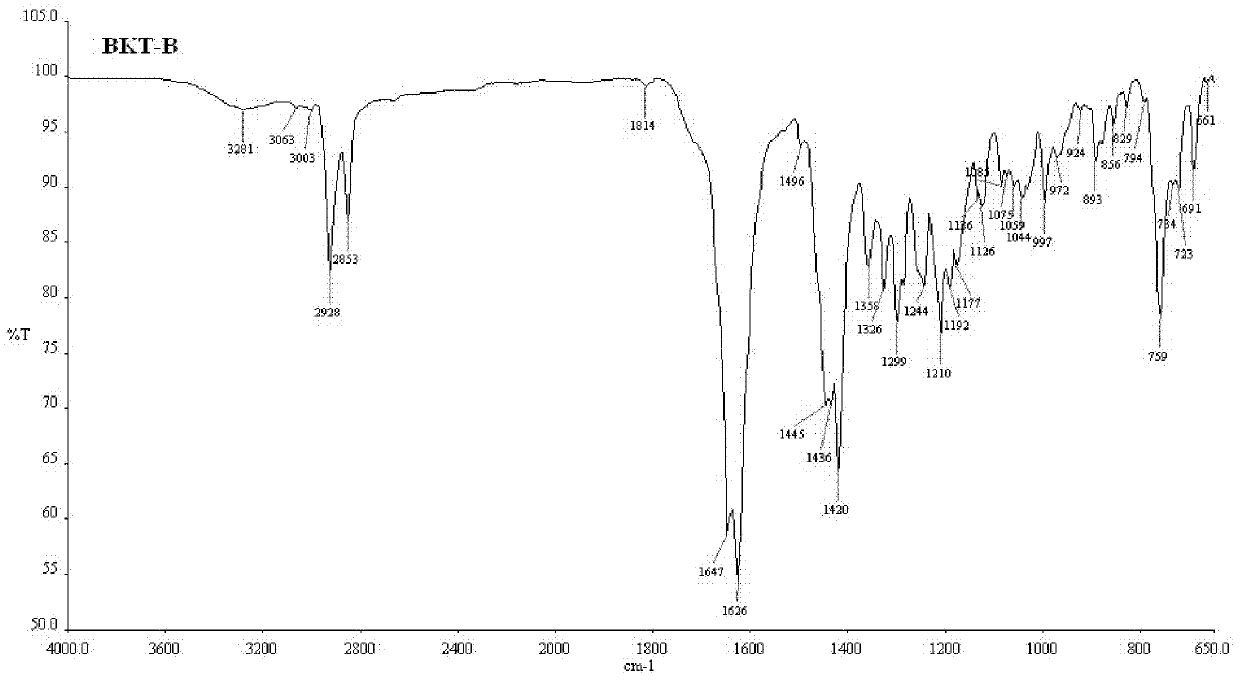
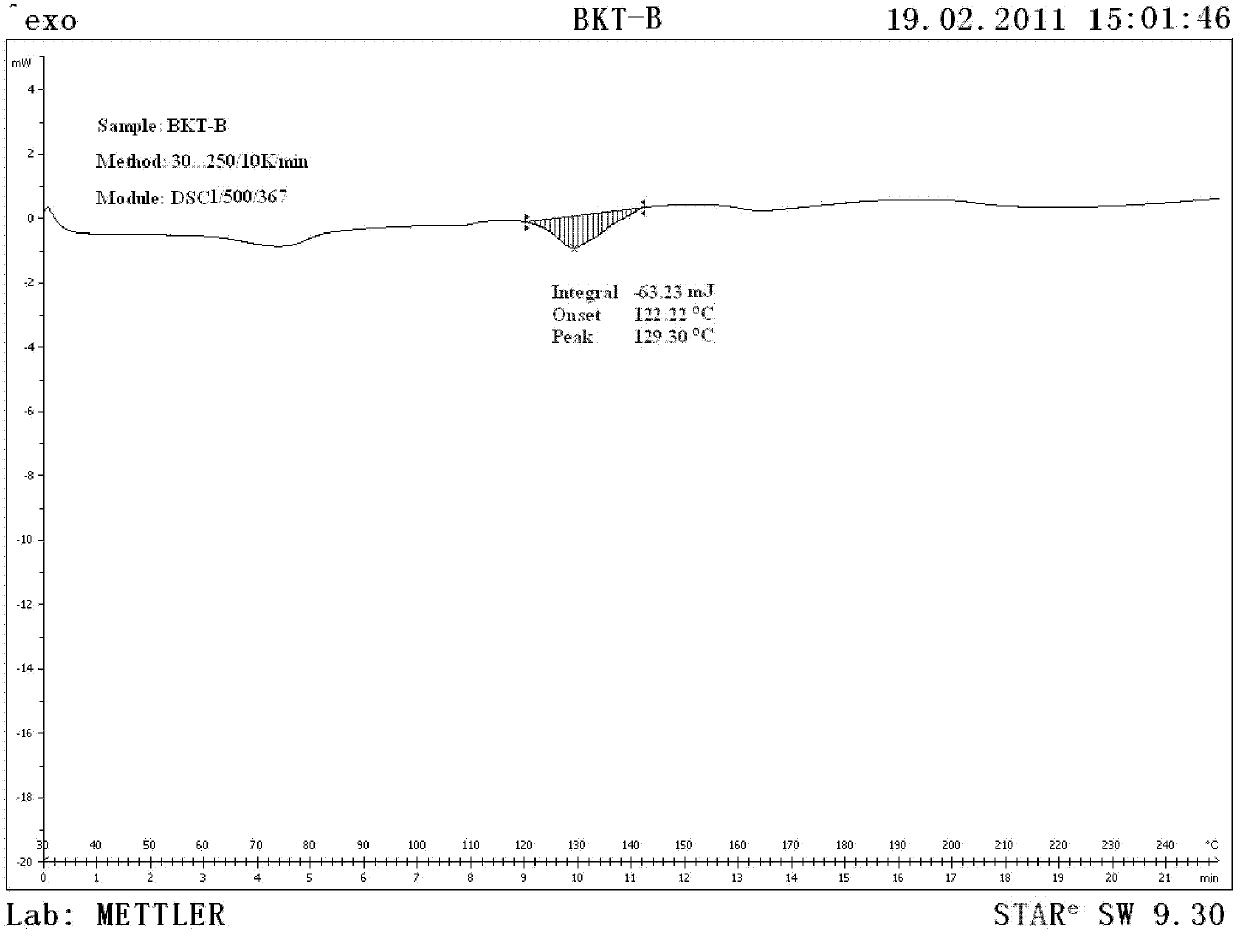
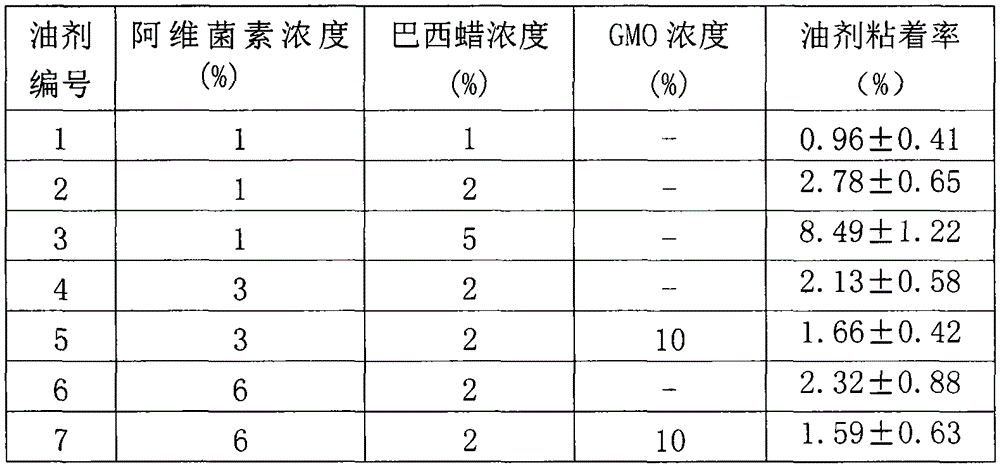
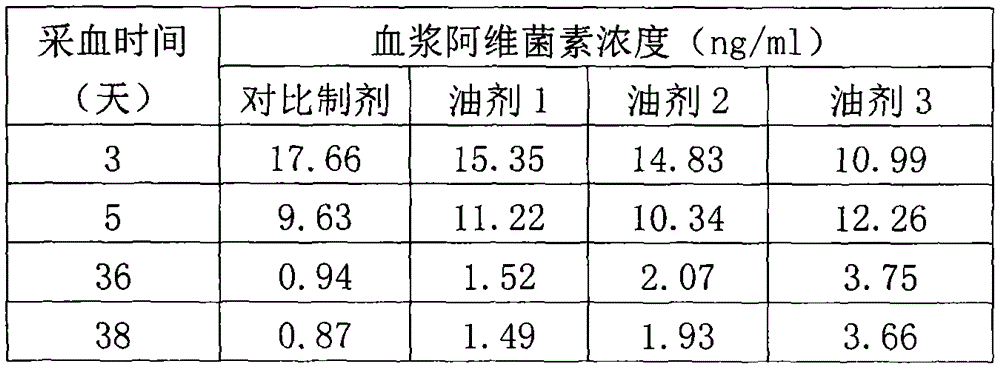
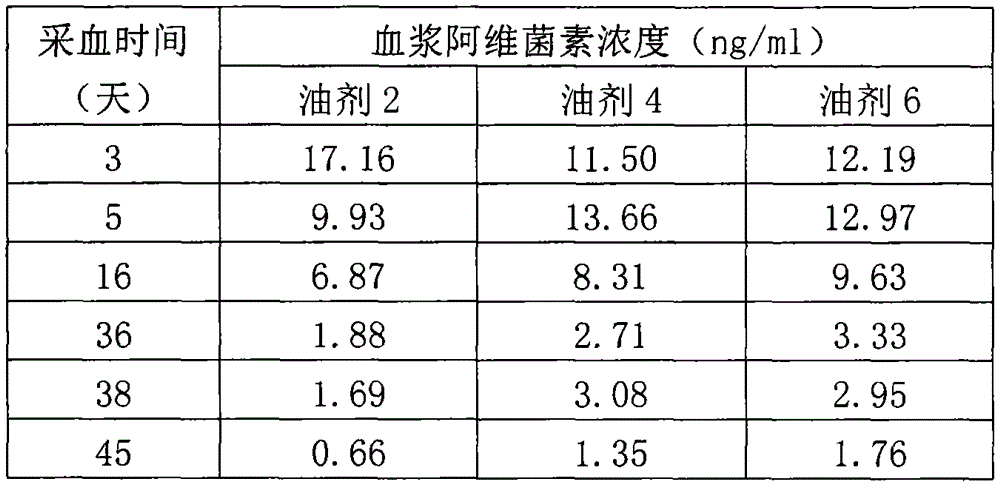
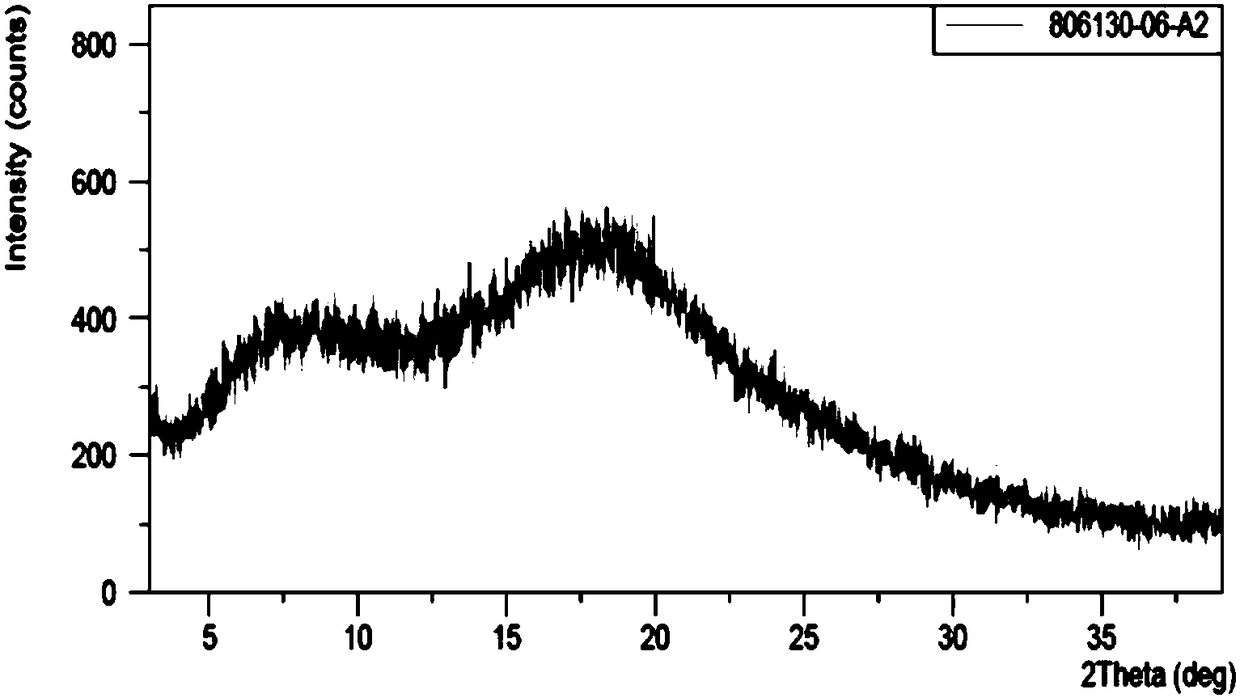
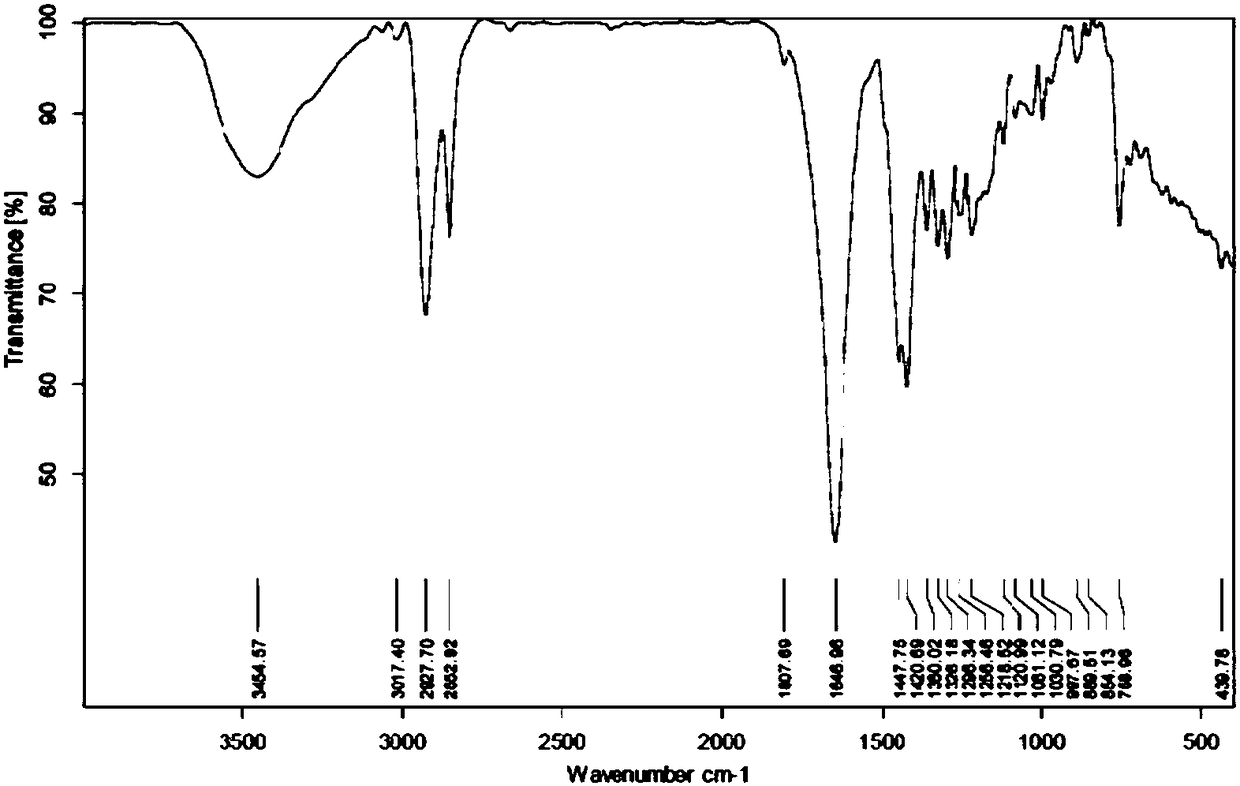
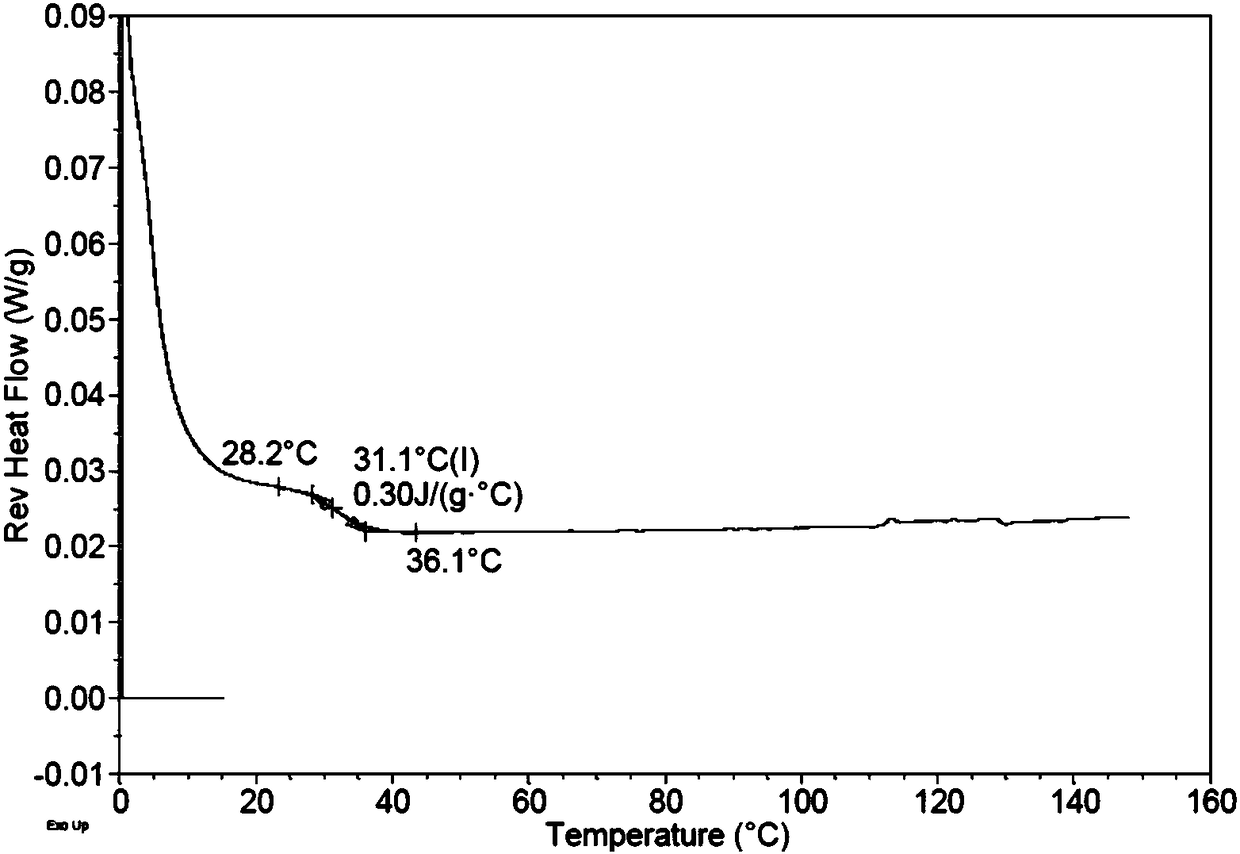
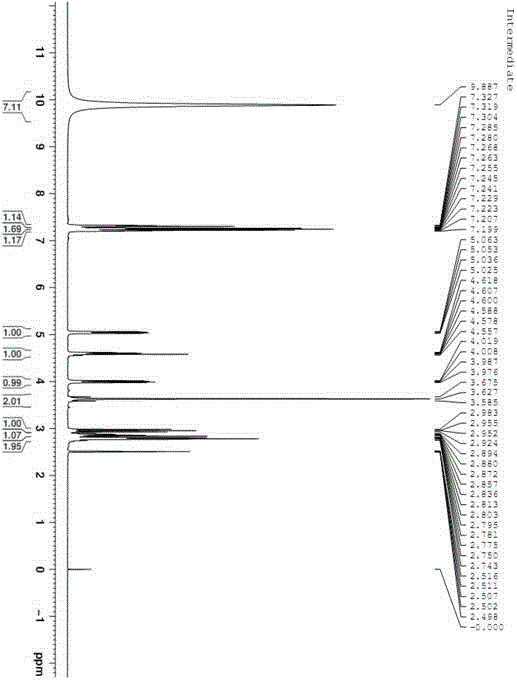
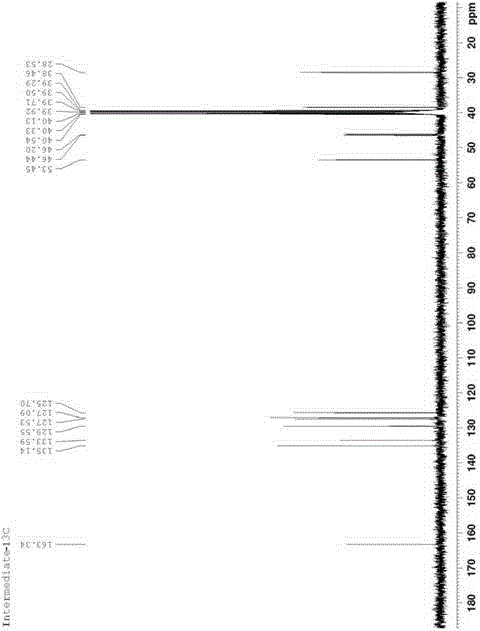
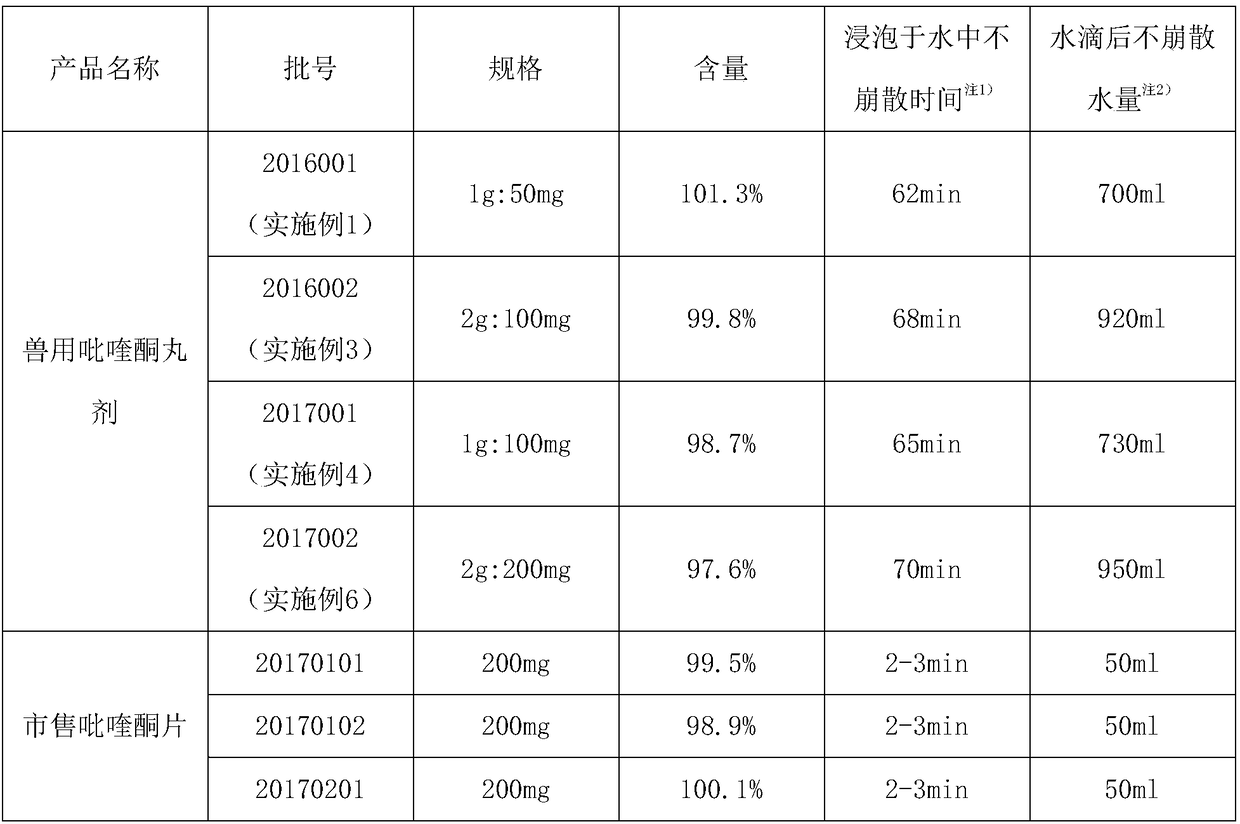
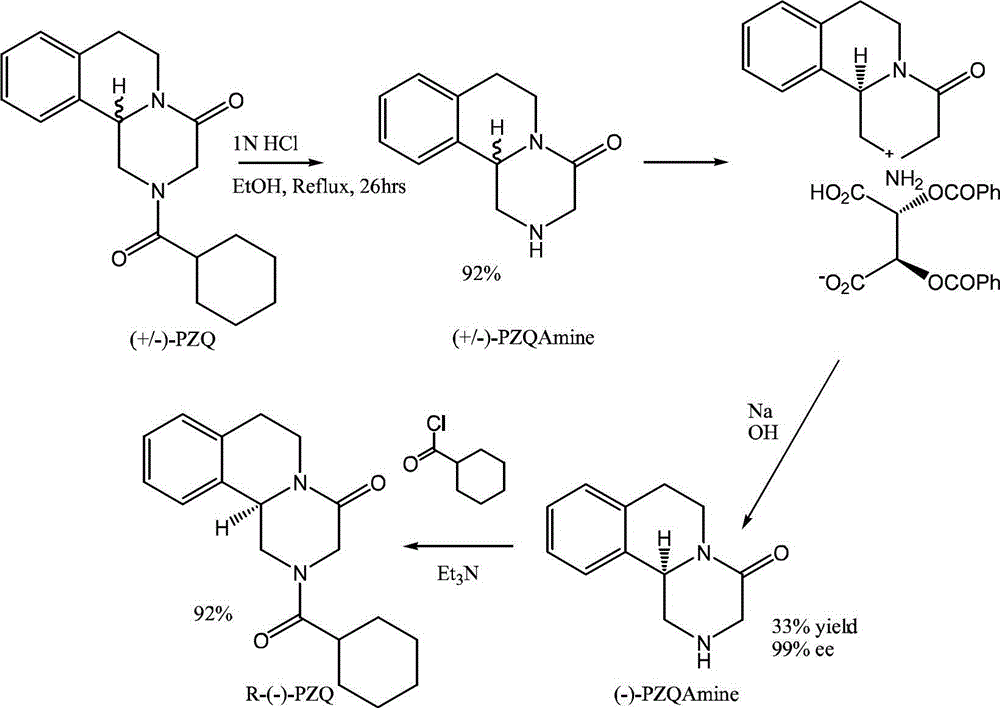
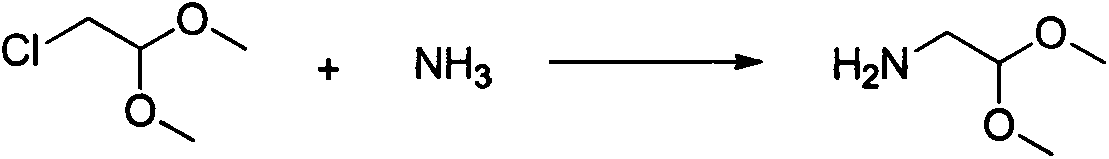Patents
Literature
171 results about "Praziquantel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat infections of certain parasites (e.g., Schistosoma and liver flukes).
Efficacious composition of a benzimidazole, an avermectin and praziquantel and related methods of use
Embodiments of the present invention generally comprise compositions comprising benzimidazole, avermectin and praziquantel for the control of various parasites and for the prevention of heartworm disease in dogs.
Owner:INTERVET INT BV
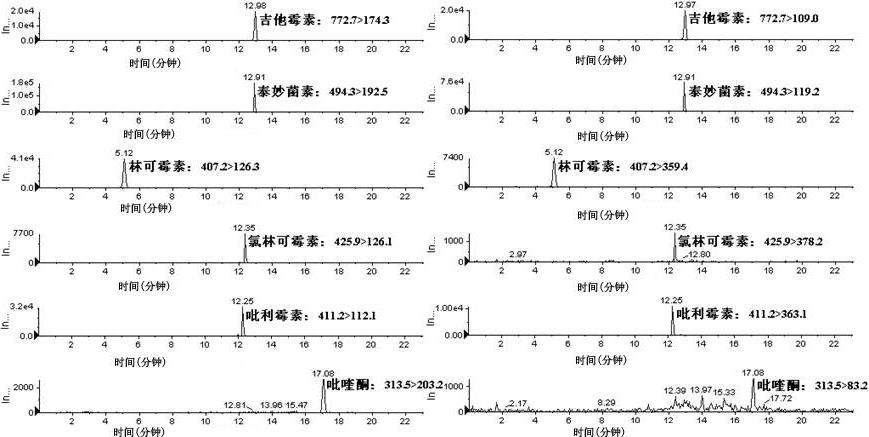
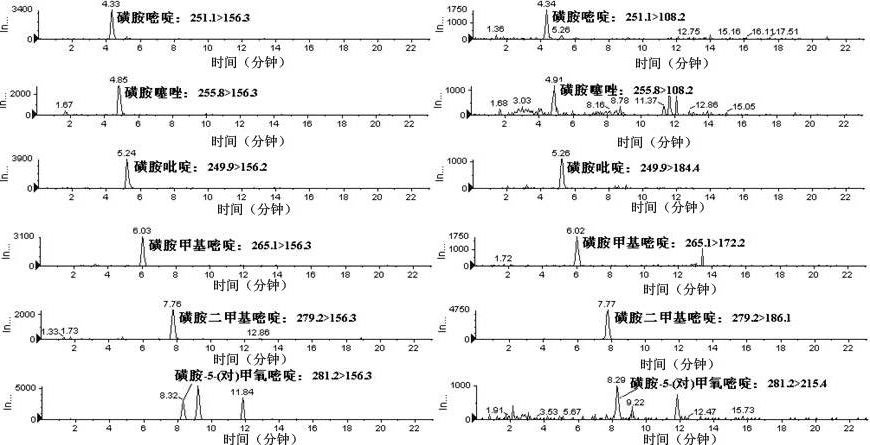
Method for simultaneously measuring various drug residues in honey by utilizing liquid chromatogram tandem mass spectrum isotope dilution method
The invention relates to a method for detecting various drug residues in honey, in particular relating to a method for simultaneously measuring various drug residues in honey by utilizing a liquid chromatogram tandem mass spectrum isotope dilution method. The method provided by the invention comprises the following steps: directly diluting by virtue of a phosphate buffer solution the pH value of which is equal to 8; carrying out HLB (Hydrophile Lipophile Balance) extraction and purification; carrying out measurement by utilizing the liquid chromatogram tandem mass spectrum isotope dilution method (LC-MS / MS); and quantifying by utilizing the internal standard method and external standard method of isotope internal standard dilution; and measuring low-limit sulfanilamide drugs to be 1.0 mu g / kg, nitromidazoles drugs to be 1.0 mu g / kg, carbostyril drugs to be 2.0 mu g / kg, macrolide drugs to be 3.0 mu g / kg, lincosamides drugs to be 2.0 mu g / kg and praziquantel to be 0.3 mu g / kg. The method provided by the invention is simple, convenient and rapid and has the advantages of small resource consumption and low detection cost; the front processing procedure, drug variety and instruments for measurement can be better complementary with the existing method; and the method provided by the invention is suitable for the simultaneous measurement requirements of various drugs in the honey and can provide a powerful technical guarantee for maintaining the food safety and guaranteeing that Chinese honey is successfully exported.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Taste masked veterinary formulation
ActiveUS7348027B2Quantity minimizationMinimal numberBiocidePharmaceutical delivery mechanismYeastActive component
A method of producing a self-take anthelmintic that includes active components that are undesirable to at least one sense of a target animal. The active ingredients including praziquantel are mixed with artificial beef and yeast components and subjected to a first compression. The resulting rough tablet is then ground to increase the density of the material by approximately 100% of the original density. Thereafter, the material is subjected to a second compression to form a final self-take tablet.
Owner:BAYER HEALTHCARE LLC
Anthelmintic formulations
The present invention provides a method for preparing a pharmaceutical formulation containing ivermectin and a method and composition that can contain ivermectin plus hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles or febantel. Examples of hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles include praziquantel, pyrantel pamoate and fenbendazole, respectively. A pharmaceutical formulation is provided for use in the treatment of helminthiasis of mammals, and particularly tapeworm, hookworm, roundworm, whipworm and heartworm of domestic animals and farm animals. The present invention also provides a method of treating helminthiasis in mammals, which method comprises administering to the mammal in need thereof an anthelmintically effective amount of a pharmaceutical formulation of the invention.
Owner:VIRB AC SA
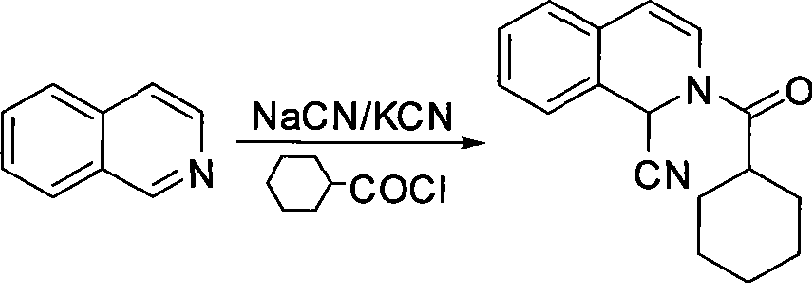
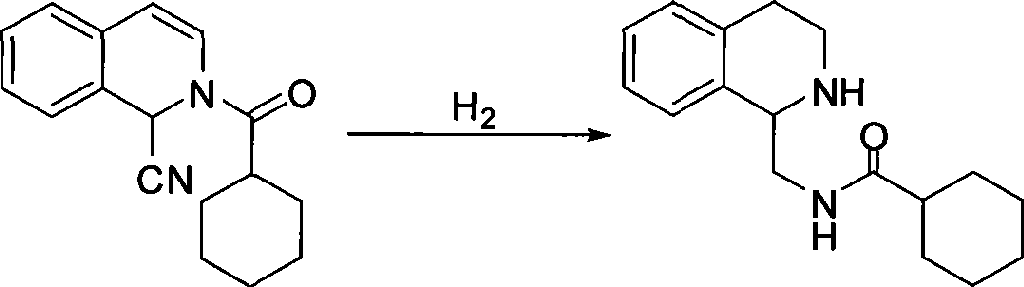
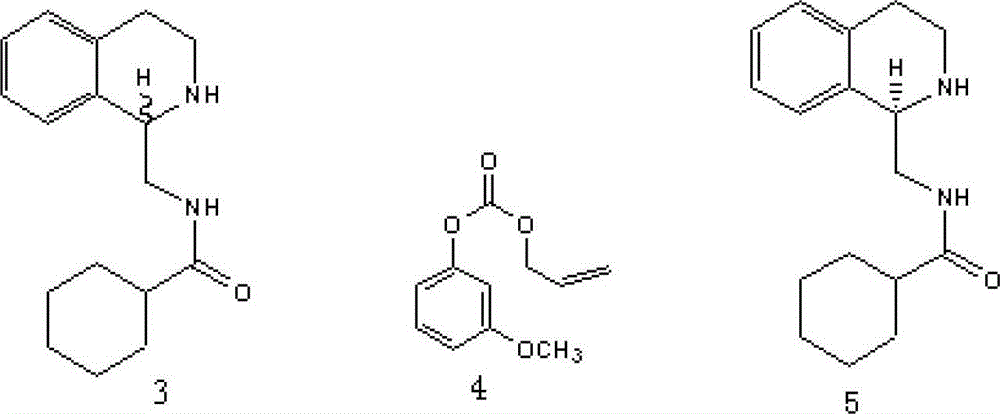
Method for preparing praziquantel
InactiveCN101445507AShort synthetic stepsMild reaction conditionsOrganic chemistryHigh pressureSodium cyanide
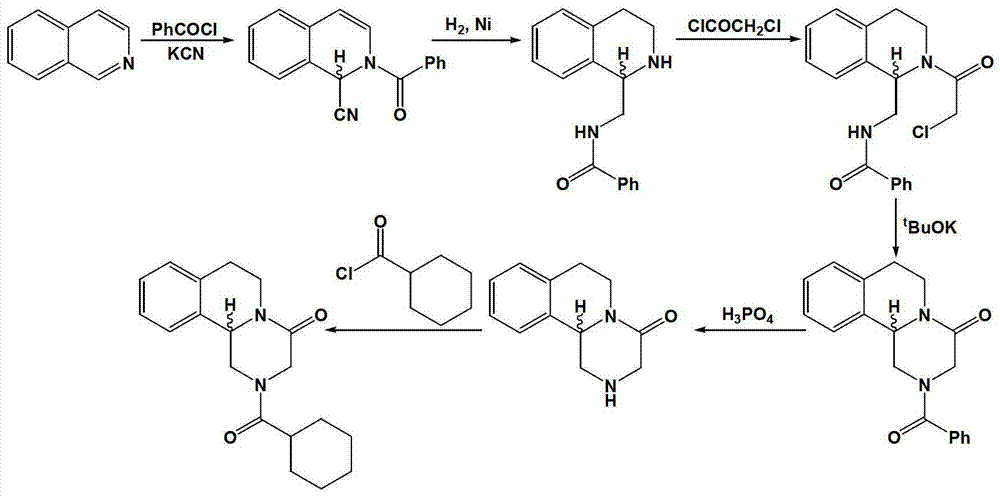
The invention relates to a method for preparing praziquantel, which is characterized by comprising the steps: (1) isoquinolin, sodium cyanide or potassuim cyanide and triethylamine or pyridine are added into a reactor to react, and yellow solid which is a first step product is obtained; (2) the first step product is added into a high-pressure hydrogenation kettle and filled with hydrogen to react under the action of catalyst, so that a second step product is obtained; (3) after being stirred and dissolved, the second step product and ethyl acetate is added with anhydrous potassium carbonate or anhydrous sodium carbonate, and then is stirred at the room temperature and dripped with chloracetyl chloride to be stirred at the room temperature to react, so that the solid obtained in the reaction is crude product of the praziquantel, and fine product is obtained by recrystallization of absolute methanol. Compared with the prior art, the method has the advantages of shortening the reaction process, reducing the energy consumption, improving the overall yield, etc.
Owner:SHANGHAI WANXIANG INDAL
Anthelmintic formulations
The present invention provides a method for preparing a pharmaceutical formulation containing ivermectin and a method and composition that can contain ivermectin plus hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles or febantel. Examples of hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles include praziquantel, pyrantel pamoate and fenbendazole, respectively. A pharmaceutical formulation is provided for use in the treatment of helminthiasis of mammals, and particularly tapeworm, hookworm, roundworm, whipworm and heartworm of domestic animals and farm animals. The present invention also provides a method of treating helminthiasis in mammals, which method comprises administering to the mammal in need thereof an anthelmintically effective amount of a pharmaceutical formulation of the invention.
Owner:VIRB AC SA
Method for preparing praziquantel
InactiveCN103739601AThe synthesis process is simpleImproved post-treatment processOrganic chemistryDimethyl acetalCyclohexanecarboxylic acid
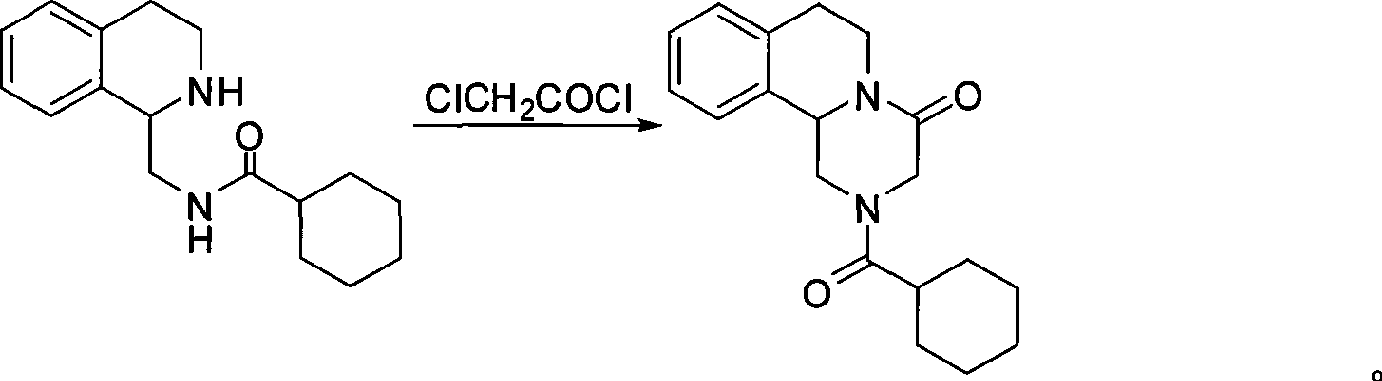
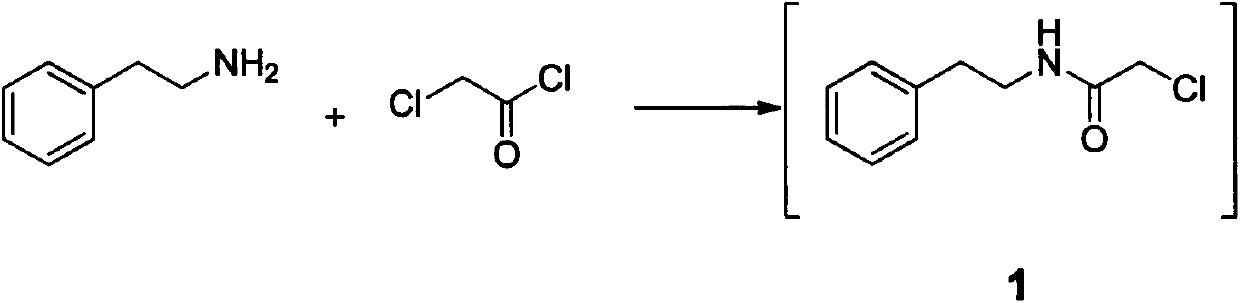
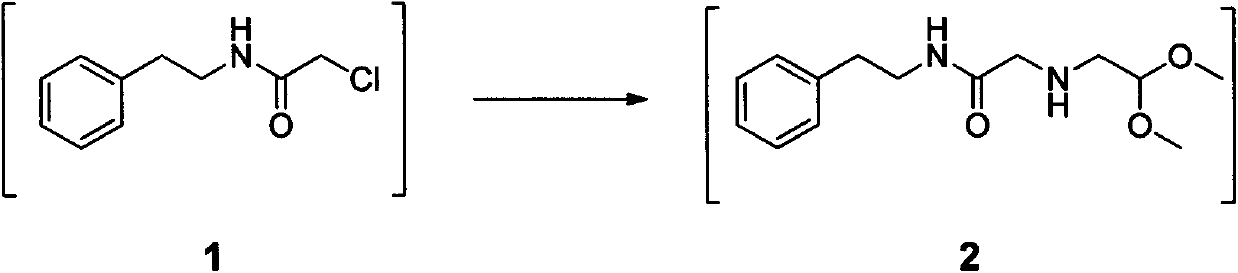
The invention relates to a method for preparing praziquantel, which is a one-pot method and comprises the following steps: performing an ammonolysis reaction of chloroacetaldehyde dimethyl acetal and an ammonia aqueous solution to generate aminoacetaldehyde dimethyl acetal; performing a condensation reaction of beta-phenylethylamine and chloroacetyl chloride in an organic solvent in alkaline environment to generate an intermediate 1; performing a condensation reaction of the intermediate 1 and the aminoacetaldehyde dimethyl acetal in an organic solvent to generate an intermediate 2; performing cyclization of the intermediate 2 in the presence of an acidic catalyst to generate an intermediate 3; performing a reaction of the intermediate 3 and cyclohexanecarboxylic acid chloride in an organic solvent in alkaline environment, and performing solvent crystallization to obtain the target product of praziquantel.
Owner:JIANGSU CHENGXIN PHARMA
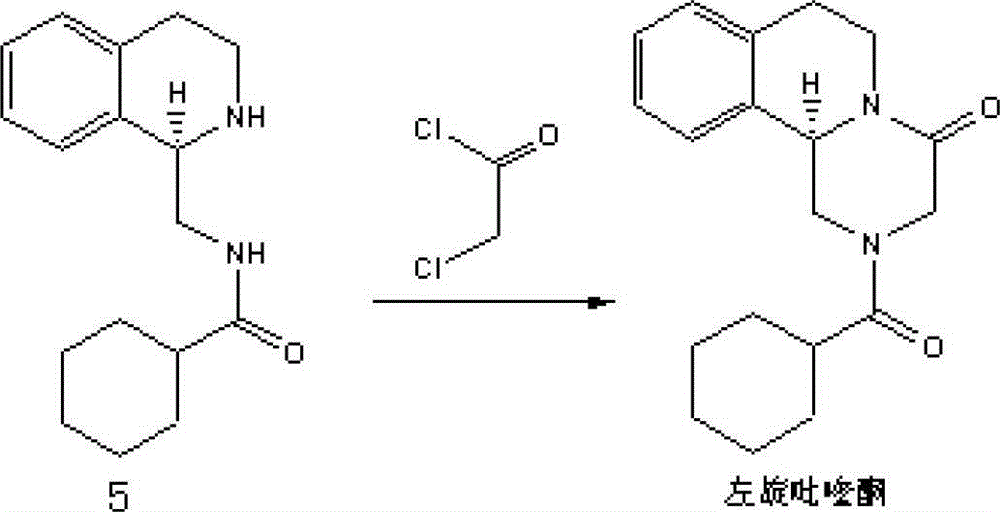
Method of synthetizing levo-praziquantel
ActiveCN103160562ASuitable for large-scale industrial productionMature technologyOrganic chemistryFermentationChemical reactionEnantiomer
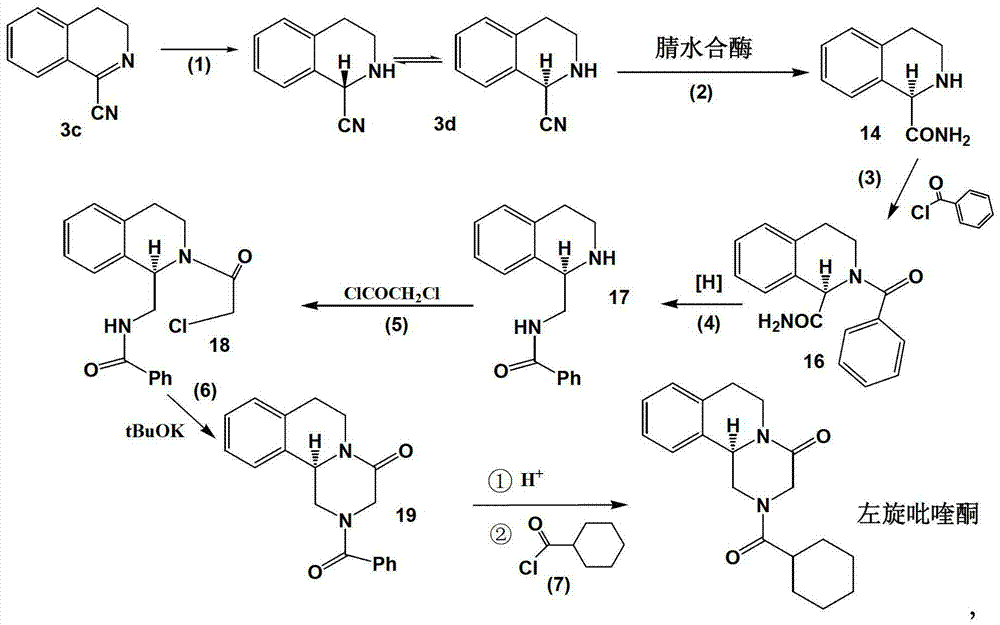
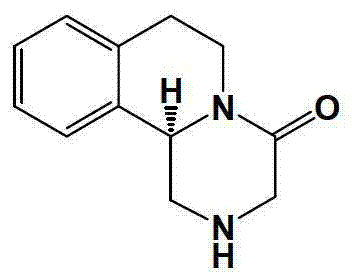
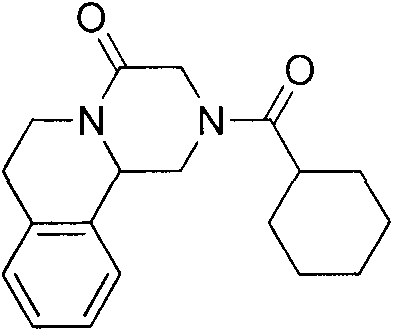
The invention relates to a novel method of synthetizing levo-praziquantel. High solid, locus and regioselectivity of enzyme are utilized to catalyze chemosynthesis racemic modification or a certain enantiomer of a derivative to conduct dynamic kinetic splitting and producing an optical homochiral levo-praziquantel midbody, and further the levo-praziquantel is obtained through various mature and high-yield conventional organic chemical reactions. The method is mature in process and low in cost, can be used for producing levo-praziquantel in mass, improves quality standard, establishes foundations for creating quality raw material medicine and preparation, and solves the industrial difficulty of purifying praziquantel which is suspended and unsolved for nearly 30 years. Raw materials can be easily obtained, and product purity can be higher than 98%.
Owner:TONGLI BIOMEDICAL
Preparation method of praziquantel
The invention belongs to the field of medicine, and relates to a preparation method of a veterinary drug praziquantel, in particular to a novel method for preparing praziquantel. Raw materials such as chloracetyl chloride and glycin used in the method are low in cost and easy to obtain; the reaction is conventional; the operation is simple; the method has no special requirements on equipment; one step that a key intermediate 14 is subjected to intramolecular cyclization to form a compound 15 is designed in a reaction route; the design is brand-new and is not reported previously; the reaction yield is higher in the whole route; the total yield can reach 50%; most solvents used in a reaction process can be recovered, so that the cost is saved, and the environmental pressure is reduced; the cost is lowered extremely; and the route has a very good industrial application prospect.
Owner:迪嘉药业集团股份有限公司
Veterinary compound suspension injection containing ivermectin and praziquantel and preparation method thereof
InactiveCN103417559AExpand the scope of insect resistanceHigh drug loadingOrganic active ingredientsSolution deliveryAntioxidantSuspending Agents
The present invention discloses a veterinary compound suspension injection containing ivermectin and praziquantel and a preparation method thereof, wherein effectively components of the veterinary compound suspension injection are ivermectin and praziquantel. The preparation method comprises the following steps: a) taking an auxiliary suspending agent, an antioxidant and a preservative, and dissolving or dispersing into 3-20% of a hot dispersion medium to obtain a solution A; 2) taking the dispersion medium and pouring into a colloid mill, opening the colloid mill, slowly adding the solution A, and adding a wetting agent while stirring after the solution A is completely added, wherein the amount of the dispersion is 30-90% of the formula amount; 3) after all the auxiliary materials are completely added, sequentially adding ivermectin and praziquantel, and adopting a cyclic grinding and acyclic grinding alternating manner to grind; and 4) checking a particle fineness, stopping grinding after meeting requirements, adding the dispersion medium until the amount achieves the formula amount, mixing, filling, sealing, and sterilizing to obtain the veterinary compound suspension injection containing ivermectin and praziquantel. The veterinary compound suspension injection containing ivermectin and praziquantel is mainly used for prevention and treatment of mixing infections caused by livestock flukes, tapeworms, threadworms and ectoparasites.
Owner:北京中农大动物保健品技术研究院
Praziquantel slow-released Implants for animals and preparation method thereof
InactiveCN101491494AAchieve hybridAchieve moldingOrganic active ingredientsPharmaceutical delivery mechanismOrganic solventMedicine
The invention relates to a veterinary praziquantel slow-release implant in the field of veterinary drug and a method for preparing the same. The slow-release implant comprises the following compositions in percentage by weight: 10 to 70 percent of praziquantel, 30 to 90 percent of a biocompatible material and 0 to 60 percent of a hydrophilic release regulator; the method for preparing the slow-release implant comprises the following steps: the praziquantel, the biocompatible material and the hydrophilic release regulator are weighed according to weight mixture ratio, thrown into an extruder, stirred and extruded out by a neck ring mold; a mixture of the extruded drug and material is cooled and solidified; and the fully cooled and solidified mixture of the drug and material is cut into drug bars so as to obtain the praziquantel slow-release implant. The method avoids the use of an organic solvent in the process of the preparation production and ensures the precision of the content of the preparation drug and the repeatability of preparation production.
Owner:SHANGHAI JIAO TONG UNIV
Praziquantel preparation process
ActiveCN103570710AReduced post-processingReduce processingOrganic compound preparationCarboxylic acid amides preparationAlkaneLower risk
The present invention discloses a praziquantel preparation process, which comprises the following one-pot reaction, wherein R1 and R2 in the reaction formula are respectively and independently selected from C1-C4 alkane. According to the present invention, the existing multi-step reaction is combined into the one-pot reaction without any separation and purification operations so as to reduce the material feeding ratio during the reaction process, save the raw materials, reduce the cost, substantially simplify the operations and reduce the three-waste treatment, the high purity product can be obtained with the simple post-treatment, the yield can be up to more than 95%, and the important significance is provided for large-scale praziquantel preparation; and with the process technology, synthesis of the high purity praziquantel by using inexpensive and easily available raw materials, simple operations and mild reaction conditions in the low toxicity, low risk and low cost manner can be achieved, and the industrial production requirements are met.
Owner:SHANGHAI DESANO CHEM PHARMA
Compound praziquantel parasite repellent tablet for pets and preparation method thereof
InactiveCN103083344ASmooth appearanceDisintegrates quicklyOrganic active ingredientsPill deliverySlurryCompanion animal
The invention relates to a compound praziquantel parasite repellent tablet for pets. The tablet is characterized by comprising the following components by mass percent: 68-80 of praziquantel, 3-5 of ivermectin, 13-20 of starch, 1-10 of 10% starch slurry for soft material preparation, 3-6 of sodium carboxymethyl starch, and 0.5-1 of magnesium stearate. The preparation method consists of: weighing the praziquantel and the ivermectin, adding the starch, mixing them, conducting crushing and sieving to obtain a mixture; and adding the starch slurry to make a soft material, carrying out granulation with a sieve, performing drying at 50DEG C-70DEG C, subjecting the obtained dry granules to grain straightening through the sieve, adding the magnesium stearate and the sodium carboxymethyl starch, mixing them uniformly, and conducting tabletting, thus obtaining the product. With a smooth and clean appearance, the tablet provided in the invention can disintegrate rapidly, has all indicators optimized, and can achieve a good parasite repellent effect after oral administration. And the preparation method has a simple and feasible process and a low cost, thus being suitable for industrial production.
Owner:QINGDAO VLAND BIOTECH INC
Taste masked veterinary formulation
ActiveUS20060228399A1Quantity minimizationMinimal numberBiocideAnimal feeding stuffYeastActive component
A method of producing a self-take anthelmintic that includes active components that are undesirable to at least one sense of a target animal. The active ingredients including praziquantel are mixed with artificial beef and yeast components and subjected to a first compression. The resulting rough tablet is then ground to increase the density of the material by approximately 100% of the original density. Thereafter, the material is subjected to a second compression to form a final self-take tablet.
Owner:BAYER HEALTHCARE LLC
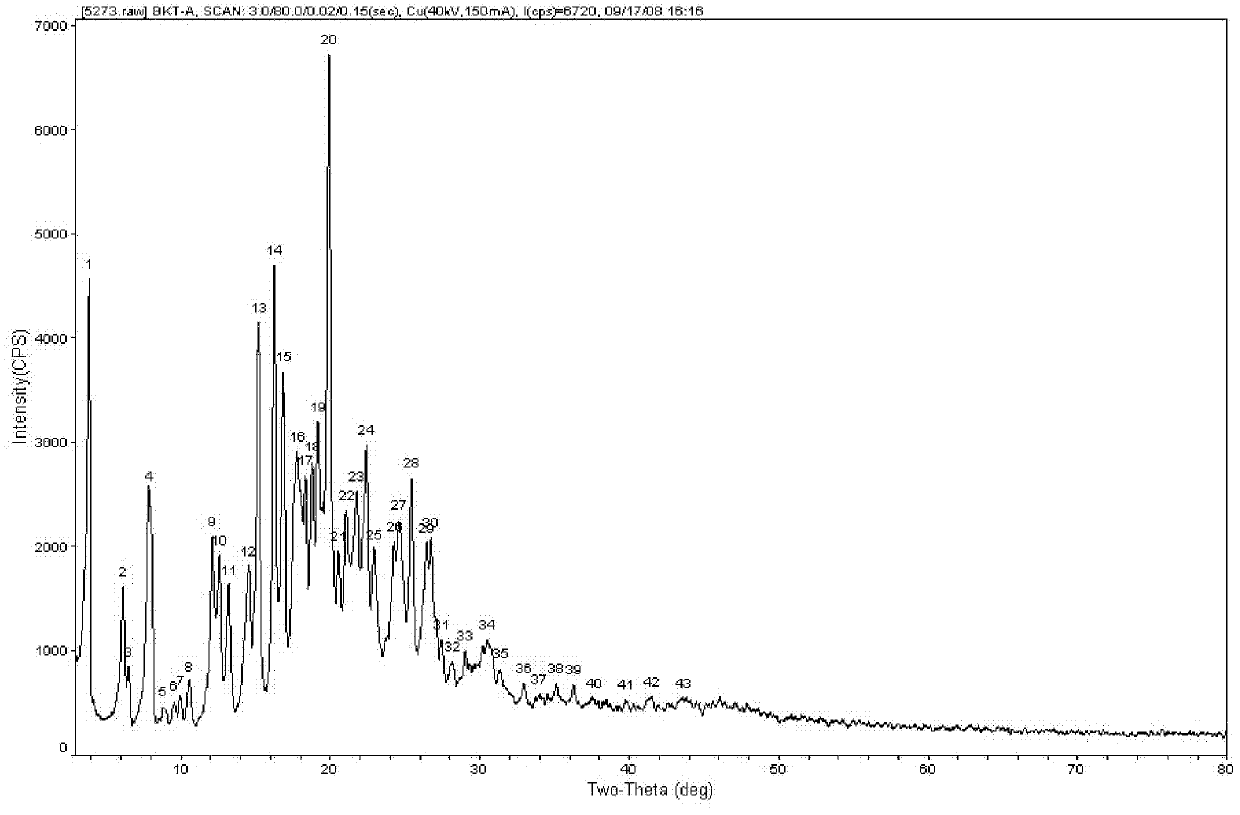
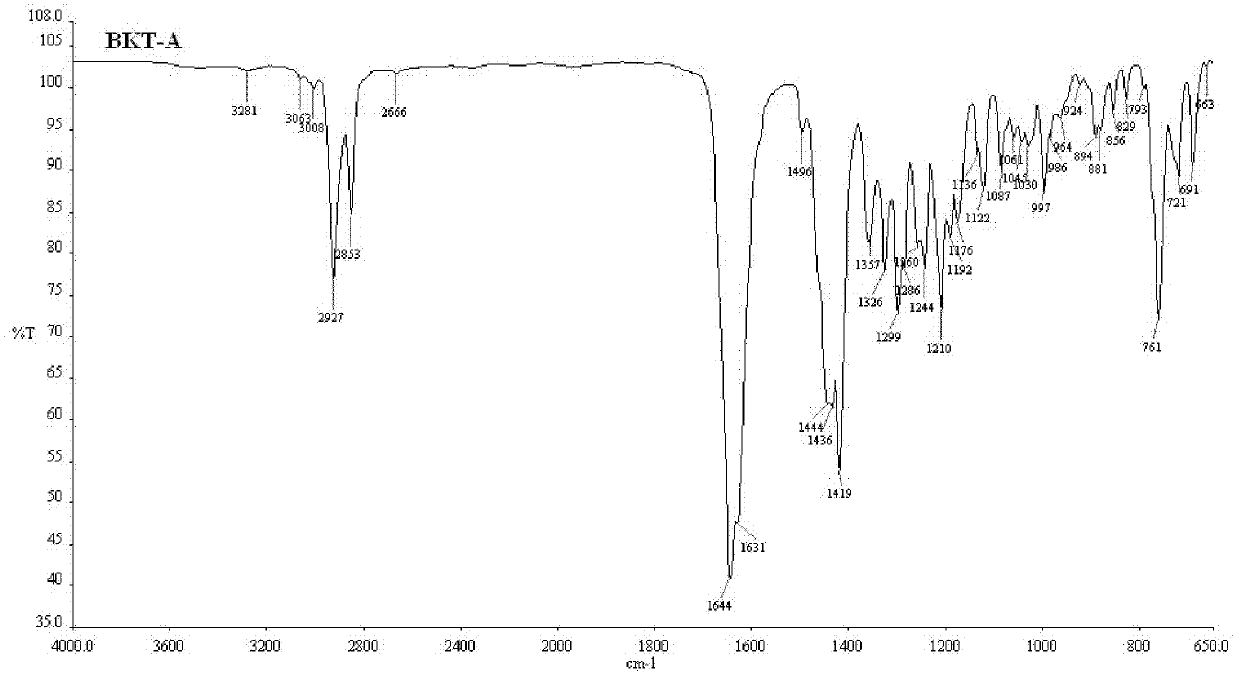
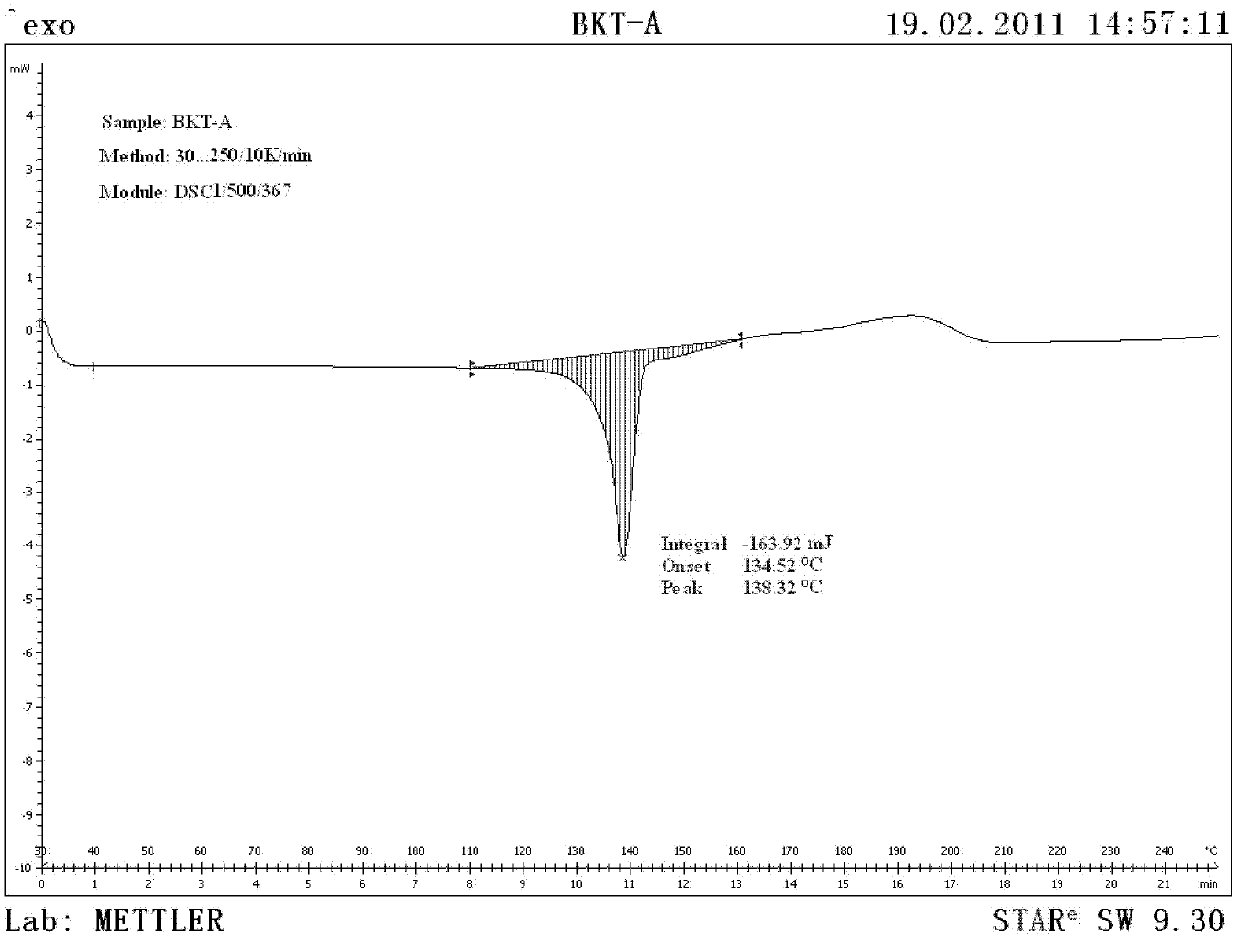
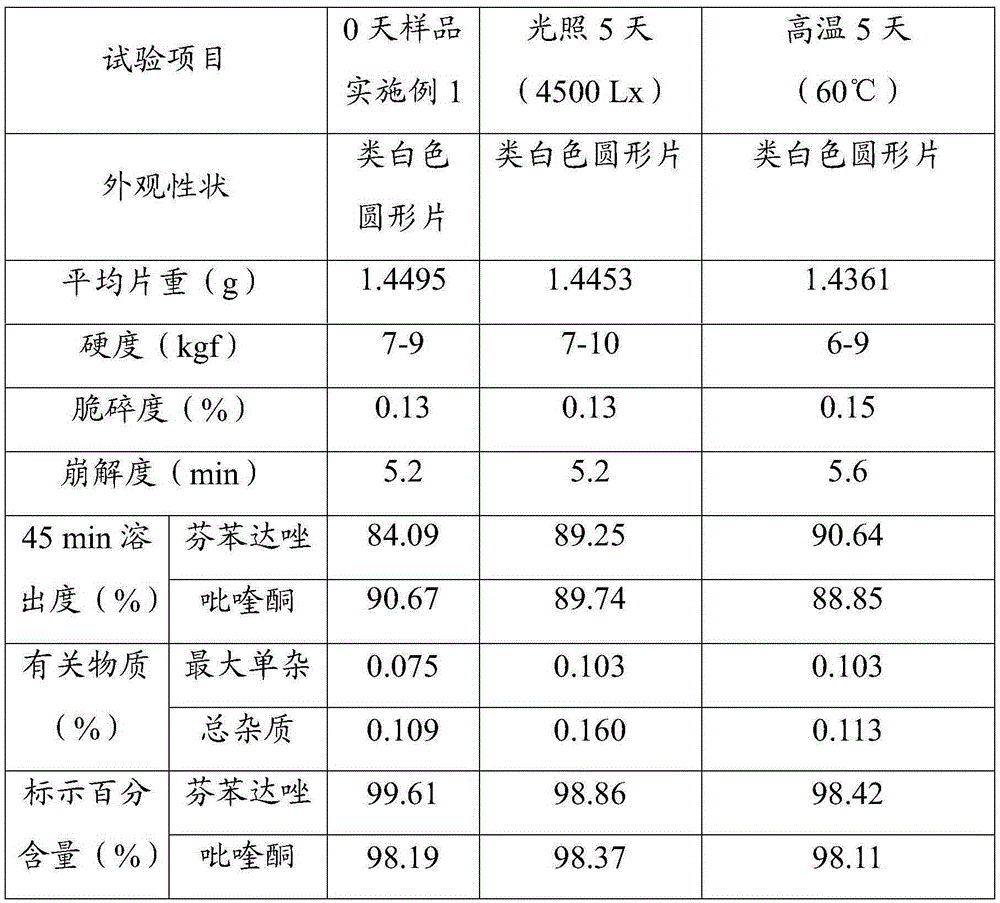
Praziquantel crystal A substance, its preparation method and its applications in medicines and healthcare products
The invention discloses a praziquantel crystal A substance with the structure represented by formula (I), a preparation method of a praziquantel crystal A sample, products prepared through treating the praziquantel crystal A substance as an active component, and applications of the praziquantel crystal A in the disease control and the healthcare, wherein the products comprise medicines and healthcare products.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Antiviral compound preparation for fish
InactiveCN103372211AReduce dosageExpand insecticidal spectrumOrganic active ingredientsAntiparasitic agentsSulfur drugMicrosphere
The invention provides an antiviral compound preparation for fish. The compound preparation composition comprises microparticles containing macrolide anthelmintic, as well as two or more than two of bithionol, praziquantel, diflubenzuron, triflumuron, pyriproxyfen, sulfur powder, salicylanilide, organic phosphorus, benzimidazole, pyrethroid, triazine, tetramisole anthelmintic, sulfonamides, anticoccidial drugs and Chinese herbal medicine anthelmintic, wherein the microparticles containing macrolide anthelmintic are microspheres or microcapsules composed of biodegradable or non-biodegradable carrier materials and macrolide anthelmintic, and the content of the macrolide anthelmintic in the microparticles is 0.1-50%. The compound preparation provided by the invention can not only expand the insecticidal spectrum, but also effectively improve the anthelmintic activity and reduce toxicity so as to ensure safer use.
Owner:江苏海辰科技集团有限公司
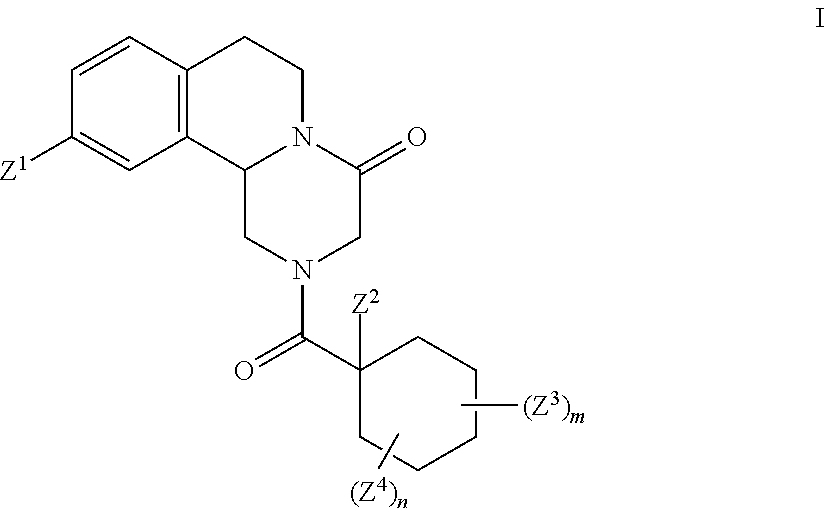
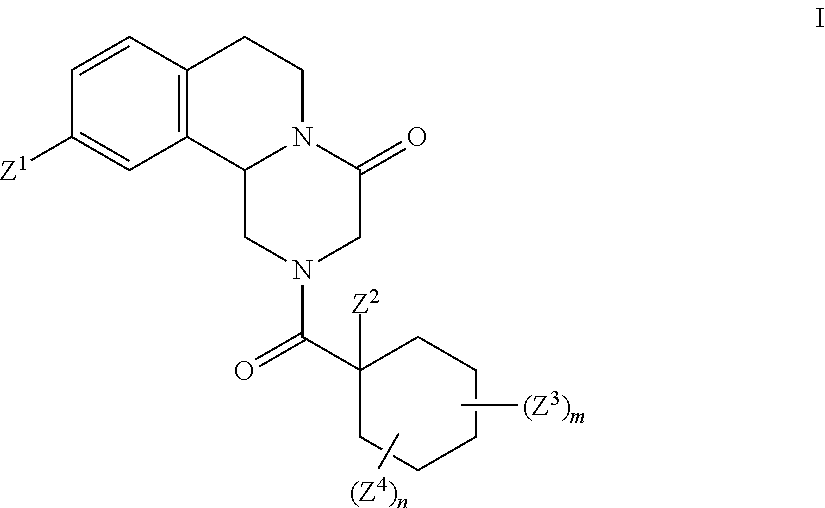
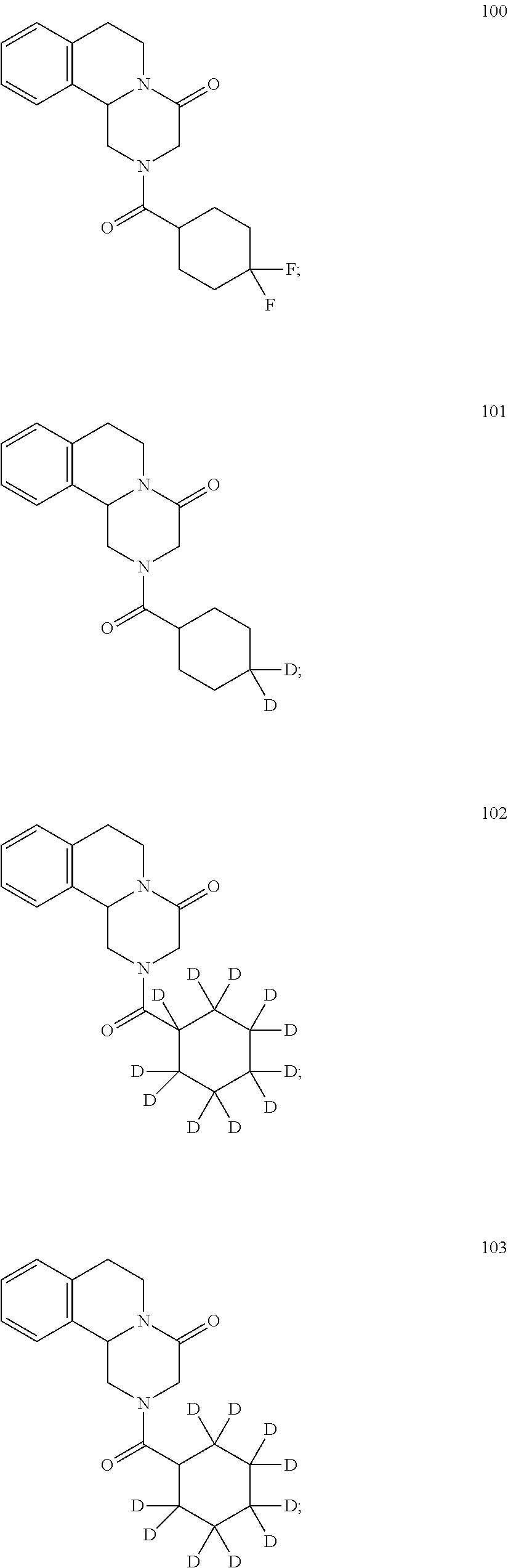
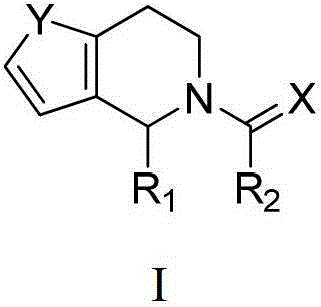
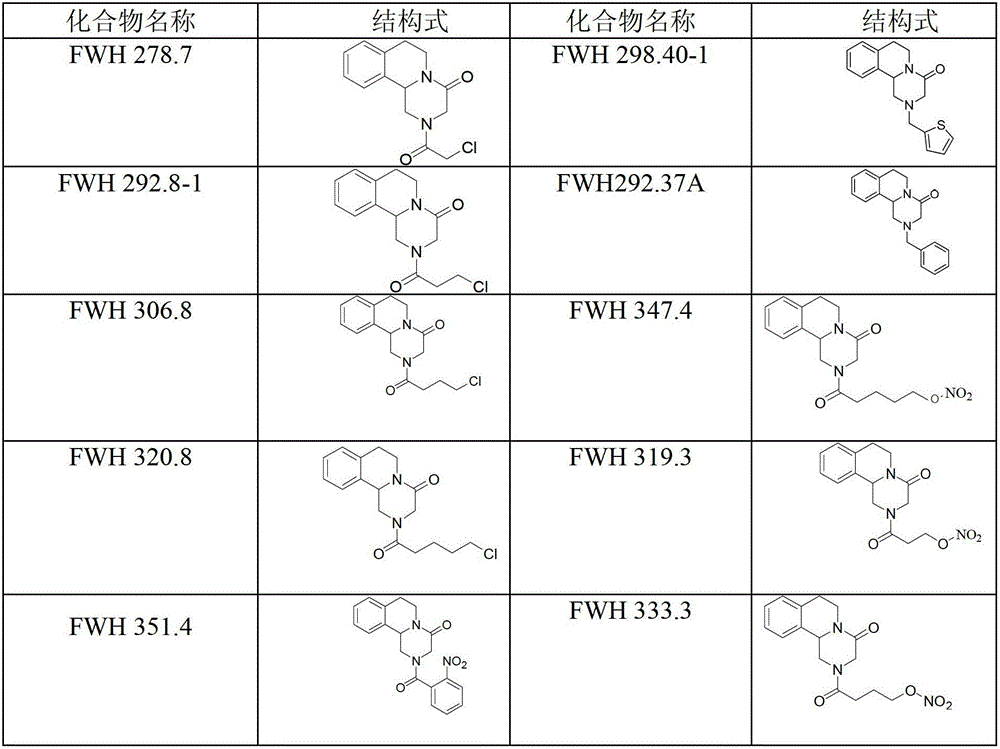
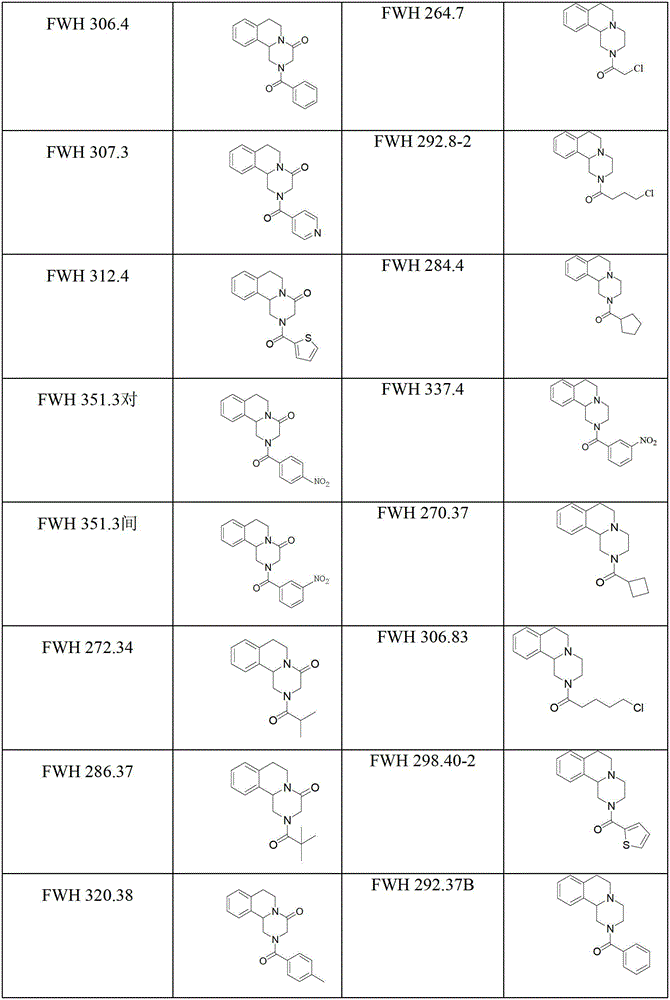
Deuterated pyrazinoisoquinoline compounds
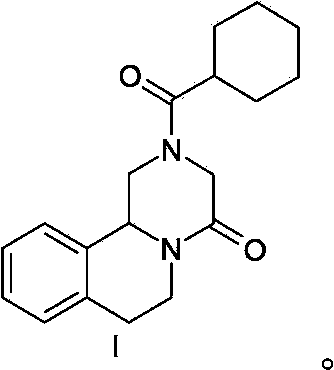
This invention relates to novel compounds that are pyrazinoisoquinoline derivatives, and pharmaceutically acceptable salts thereof. More specifically, this invention relates to novel pyrazinoisoquinoline derivatives that are derivatives of praziquantel, such as compounds of formula (I):or pharmaceutically acceptable salts thereof. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions that are beneficially treated by administering an antihelminthic agent, such as praziquantel.
Owner:SUN PHARMA IND INC
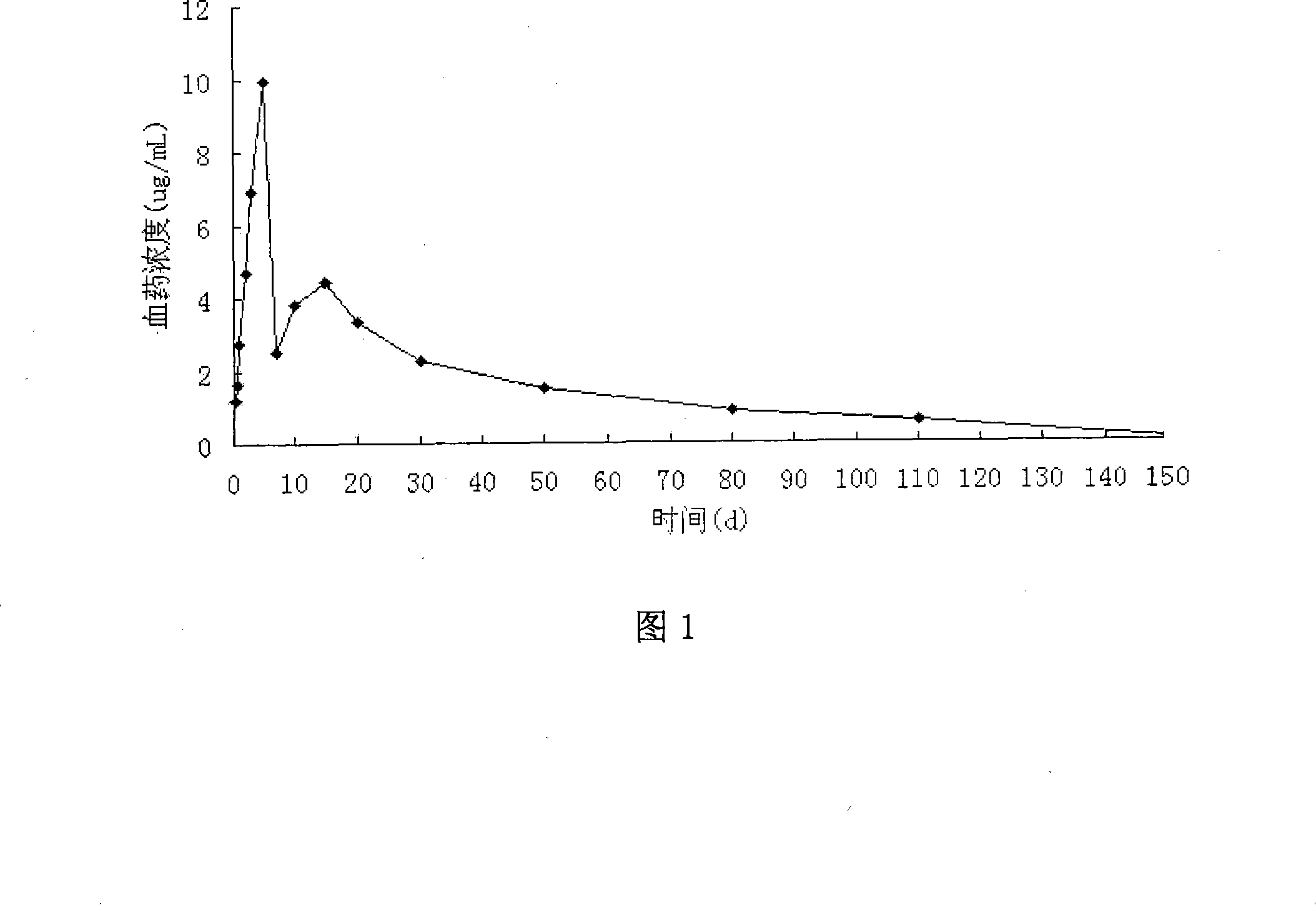
Praziquantel long-acting sustained release injection and preparing method thereof
InactiveCN101229123AStable in natureEasy to storeOrganic active ingredientsPharmaceutical delivery mechanismSolvent evaporationPraziquantel
The invention discloses a praziquantel long-acting sustained-release injection, following materials are formed with a certain mass fraction into 2 to 25 units of praziquantel, 20 to 250 units of ethanol, 0.5 to 2.5 units of aluminum monostearatenf, and 100 units of castor oil used for injection, the processing method is that first, oil glue of aluminum monostearatenf and castor oil is made, then, when the temperature is 50 to 60 DEG C, praziquantel is distributed into the oil glue of monostearatenf and castor oil through solvent evaporation, when the temperature is raised to 80 to 100 DEG C, light yellowish transparent thick liquid will be formed, after being cold and in split charging, the invention can be finished, the processing method of the invention is simple, and the storing process is stable, the invention can not only retain the wide anti-insect property and the strong activity of the traditional type of praziquantel but also be released slowly, stably in the body of animals for a long time, the control effect can be kept for three to four months after using the invention for once, and the invention adds a new dosage form, which solves the problems of traditional form such as the short sustained time and a plurality of times for medication.
Owner:NORTHWEST A & F UNIV
A process of synthesizing praziquantel
InactiveCN106866663AHigh yieldSimple and fast operationOrganic chemistryTemperature controlReaction temperature
A process of synthesizing praziquantel is provided. According to the process, beta-phenylethylamine and dichloromethane are added into a reactor and subjected to an acylation reaction; the mixture is stirred and cooled and the acylation reaction temperature is lowered to 0-35 DEG C or below; chloroacetyl chloride and liquid caustic soda are added dropwise under temperature control of the reaction solution; the pH value of the acylation reaction solution is controlled to be 6-12 during a dropwise adding procedure; and after the dropwise adding procedure is finished, the temperature is maintained, and the mixture is stirred and layered. A one-pot method is adopted by the process, the cost of the process is low, operation methods are simple and controllable, environment protection safety is good and product quality is stable.
Owner:JIANGSU CHENGXIN PHARMA
Compound fenbendazole tablet
ActiveCN105267230AThe killing effect is obviousNo side effectsOrganic active ingredientsPill deliveryReduction rateFenbendazole
The invention discloses a compound fenbendazole tablet. Specifically, each tablet is prepared from the following components by mass: 300-500mg of fenbendazole, 15-30mg of praziquantel, 0.01-0.05mg of ivermectin, 600-1000mg of a filler, 80-120mg of a disintegrating agent, 100-200mg of an adhesive, 10-20mg of a lubricant and 1-3mg of a flavoring agent. The compound fenbendazole tablet provided by the invention has significant repelling and killing effects on naturally infected worms of dogs, and single egg reduction rate reaches over 95%. The compound fenbendazole tablet can expel more species of parasites, can achieve better therapeutic effect, also is safe, has no toxic or side effect, good drug dissolution, high temperature resistance, sunlight resistance, good reproducibility, and stability.
Owner:QINGDAO AGRI UNIV
Compound preparation for expelling in-vivo and in-vitro parasites from dogs and cats and preparation method thereof
The invention relates to a compound preparation for expelling in-vivo and in-vitro parasites from dogs and cats and a preparation method thereof. The compound preparation mainly comprises milbemycin oxime and praziquantel, wherein the milbemycin oxime is a novel and specific 16-membered ring macrolide medicament for preventing and controlling the in-vivo and in-vitro parasites of the dogs and the cats, which can specially prevent and control heartworms and efficiently prevent and control in-vivo parasites such as hookworms, roundworms, whipworms, belly worms and the like and in-vitro parasites such as hair follicle mites, scabies, louses, fleas and the like; and the praziquantel is effective to nematodes, trematodes and tapeworms in animal bodies. The milbemycin oxime and the praziquantel expel the parasites complementarily. The two medicaments are combined, so that a parasite expelling range is expanded and one-step parasite expelling effect is improved. The invention also provides a method for preparing compound tablets. The tablets prepared by the method can be released rapidly and dispersed fully.
Owner:TIANJIN RINGPU BIO TECH
Praziquantel crystal B substance, its preparation method and its applications in medicines and healthcare products
The invention discloses a praziquantel crystal B substance with the structure represented by formula (I), a preparation method of a praziquantel crystal B sample, products prepared through treating the praziquantel crystal B substance as an active component, and applications of the praziquantel crystal B in the disease control and the healthcare, wherein the products comprise medicines and healthcare products.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Veterinary anti-parasitic preparation containing carnauba wax
ActiveCN104666244AThe determination method is simpleOrganic active ingredientsSolution deliveryVegetable oilMethyl oleate
The invention relates to a veterinary anti-parasitic injection containing carnauba wax. The preparation is mainly prepared from anti-parasitic medicine, the carnauba wax and an oily medium, wherein the anti-parasitic medicine is avermectins, praziquantel and oxfendazole; the oily medium is vegetable oil, ethyl oleate or benzyl benzoate, and more than one of the vegetable oil, ethyl oleate or benzyl benzoate can be combined to use. Glycerol mono-oleate can be added in the preparation, the carnauba wax and the glycerol mono-oleate are compositely applied, and the slow-release effect of the preparation is better. The selected preparation is prepared from 36-80 g of abamectin anti-parasitic medicine, 10-70 g of the carnauba wax, 80-120 g of the glycerol mono-oleate, 0.1-0.3 g of an antioxidant, 10-20 g of benzyl alcohol and the balance of the oily medium (per 1000ml).
Owner:中农华威制药股份有限公司
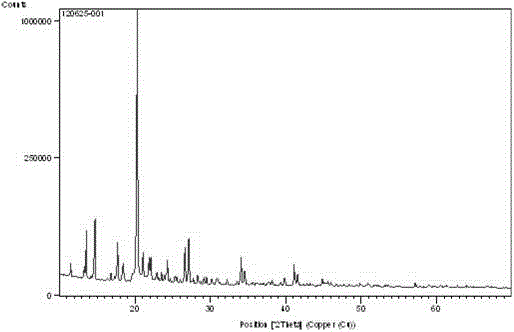
Amorphous levo-praziquantel solid and preparation method and application thereof
PendingCN108794466AAmorphous structure has high reactivitySimple methodOrganic active ingredientsOrganic chemistry methodsBULK ACTIVE INGREDIENTSolvent
The invention discloses an amorphous levo-praziquantel solid and a preparation method thereof. The solid has no characteristic absorption peaks in a powder X-ray diffraction pattern. The preparation method of the amorphous levo-praziquantel solid includes a step (a) and a step (b) as follows: (a) dissolving levo-praziquantel crystal form powder and / or oily levo-praziquantel in a mixed solvent ofa first organic solvent or a second organic solvent and water, and then placing the mixture at 50 DEG C to 100 DEG C for volatilization; and (b) dissolving levo-praziquantel crystal form powder and / or oily levo-praziquantel in a third organic solvent, and then adding a high molecular polymer to induct precipitation of the solid. The method combines multiple clinical advantages of the levo-praziquantel and the advantages that the reactivity of an amorphous structure is generally greater than that of the same substance, and paves the way for the listing of the amorphous levo-praziquantel solidand / or medicine which contains the amorphous levo-praziquantel solid as an active ingredient and treats schistosomiasis and a plurality of parasitic diseases.
Owner:TONGLI BIOMEDICAL
Praziquantel analogue, preparation method and application thereof
The invention provides a compound shown as the structural formula I, a preparation method and application thereof. The compound has polypide paralysis and insecticidal effects, can be used for preparing anti-schistosomiasis drugs, and overcomes poor curative effect or ineffective treatment phenomenon for the reason of drug resistance caused by long-term use of the anti-schistosomiasis drugs in the prior art. The compound has the advantages of simple structure and easy preparation.
Owner:JIANGNAN UNIV +1
Anthelmintic and preparation method thereof
InactiveCN103933045AEfficient killingGood effectOrganic active ingredientsPill deliveryFenbendazolePraziquantel
The invention discloses an anthelmintic for pets and a preparation method thereof. The anthelmintic disclosed by the invention is prepared from the following components: 83.3-87.2% of fenbendazole and 12.8-16.7% of praziquantel. The invention further provides the preparation method of the anthelmintic. The components are matched according to a specific proportion; the fenbendazole is wide in anthelmintic spectrum; the praziquantel has low toxicity to dogs, cats and the like; conversion products of the fenbendazole and the praziquantel can effectively interfere transportation of glucose by polypides in dogs and cats; the fenbendazole and the praziquantel are matched according to a specific proportion, so that the anthelmintic can take effect rapidly and is unnecessary to take on an empty stomach; in addition, the polypide-repelling degree is relatively thorough.
Owner:SHENZHEN REDRAY BIOTECHNOLOGY CORP LTD
Praziquantel preparation process
ActiveCN103059018AReduce generationEmission reductionOrganic chemistryChemical recyclingDistillationFormylation reaction
The invention relates to a praziquantel preparation process which comprises the following steps of: carrying out hydrolysis reaction on 1, 2, 3, 6, 7, 11beta-hexahydro-4H-pyrazino[2,1-alpha]isoquinolin-4-one and phosphoric acid, carrying out reduced-pressure distillation on a reaction solution to remove generated byproducts, adding deionized water to dilute the reaction solution, and crystallizing to obtain a phosphate compound intermediate; carrying out cyclohexaformylation on the obtained phosphate compound intermediate and cyclohexaformyl chloride in an organic solvent in the presence of an alkali compound to obtain a praziquantel crude product; and decoloring and filtrating the obtained praziquantel crude product, and recrystallizing a filtrate to obtain a refined praziquantel. Compared with the prior art, the praziquantel preparation process has the advantages of shortening the process flow, reducing the use of the solvent and improving the quality and yield of the product.
Owner:SHAOXING MINSHENG PHARMA
Praziquantel pill for veterinary use and preparation method of praziquantel pill
PendingCN108451912AEasy to feedStrong food attractantOrganic active ingredientsPill deliveryVegetable oilAntioxidant
The invention discloses a praziquantel pill for veterinary use and a preparation method of the praziquantel pill. The praziquantel pill is prepared from the following raw materials by weight percent:2.5 to 15 percent of praziquantel, 81.5 to 91.5 percent of basic nutrition powder, 1 to 5 percent of disintegrating agent, 0.05 to 1 percent of flavor enhancer, 0.02 to 0.2 percent of preservative, 0.02 to 0.2 percent of antioxidant, and 2 to 8 percent of medicinal starch or / and hydroxypropyl methylcellulose; and the basic nutrition powder is prepared from cereal, meat, bone powder, corn protein powder, vegetable oil, animal grease, table salt, vitamins and minerals. The praziquantel bill has a high attractant effect, and is unlikely to disintegrate, particularly suitable for being widely dispensed in the field or for manually feeding canidae host animals with an infection source at a fixed point, convenient for feeding the livestock, complete in basic nutrition, and capable of preventingand treating parasitic infection of the animal such as schistosomiasis, teniasis and cysticercosis.
Owner:南京制药厂有限公司 +1
Method for preparing levo-praziquantel
ActiveCN102911996ASolve industrial problemsLow costOrganic chemistryFermentationChemical synthesisRegioselectivity
The invention relates to a method for preparing levo-praziquantel. The method includes resolving certain antipode in raceme under actions of characteristics of high stereoscopic property, locus and regioselectivity of biological enzymes to obtain a mixture of optical isomers which are reacted or unreacted; and further separating and synthesizing the optical isomers to obtain the target product. The raceme is chemically synthesized. The method has the advantages that raw materials are easy to obtain, the cost is low, the purity of the product can be higher than 98%, quality standards are improved, a foundation is laid for manufacturing high-quality bulk drugs and preparations, and the difficult problem, which cannot be solved in the past 30 years, of purifying praziquantel is solved.
Owner:TONGLI BIOMEDICAL
Praziquantel chewable tablets for dog or cat
ActiveCN101190197ABreak through the shortcomings of poor palatabilityHigh cure rateOrganic active ingredientsPill deliveryOral glucoseDisease
The invention discloses a praziquantel chewable tablet used for dogs and cats. The invention overcomes the poor palatability defect of the existing tablet to promote the thorough chewing absorption by dogs and cats, so as to effectively guarantee the medicine-supply dose, to enhance the cure rate of diseases of dogs and cats and to reduce the medicine waste. The tablet of the invention comprises the following components represented by weight-percentage: 1 to 5 percent of aspartame; 20 to 30 percent of a mixture of oral dextrose and degreased milk powder; the ratio of 1:1 to 1:4 between the oral dextrose and degreased milk powder in the mixture; 40 to 60 percent of excipient; 0.5 to 1 percent of glidant and 30 to 50 percent of praziquantel.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com