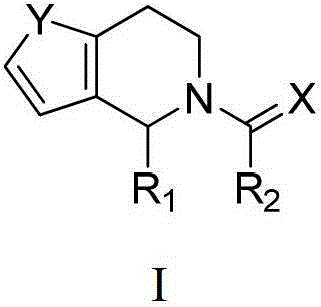
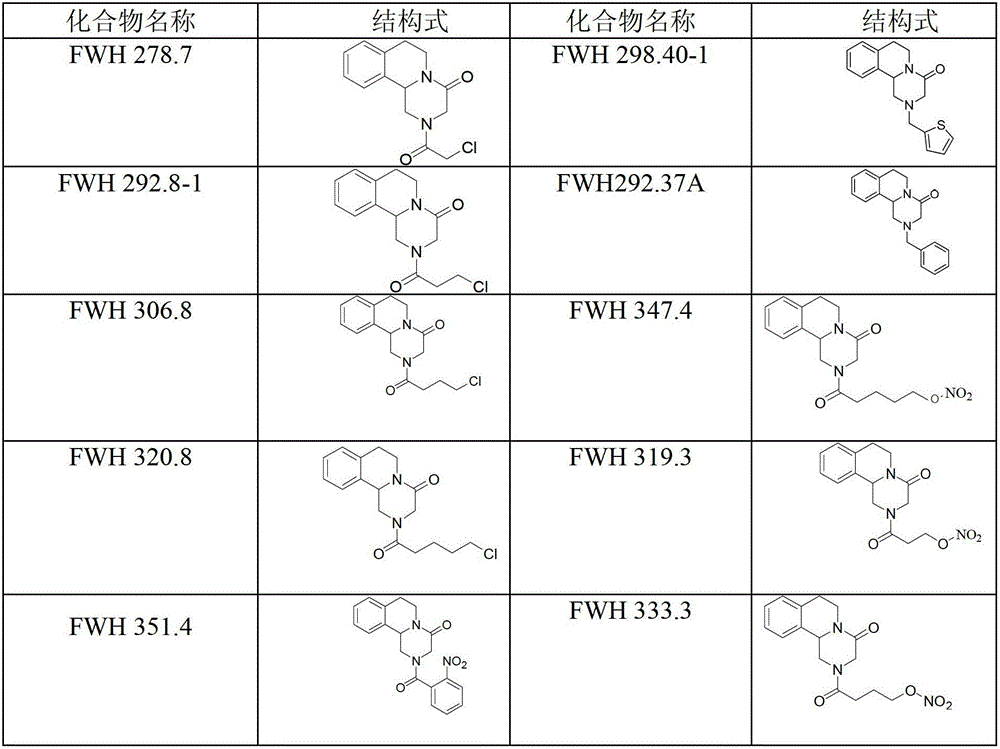
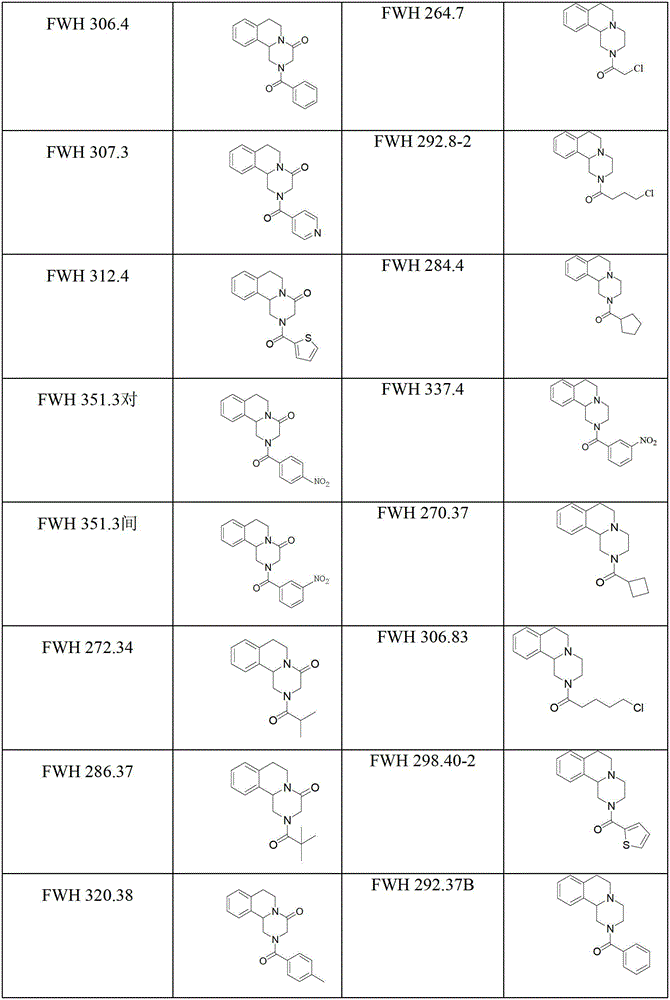
Praziquantel analogue, preparation method and application thereof
A scheme and compound technology, applied in the field of praziquantel analogs, can solve the problems such as the prevalence of praziquantel-resistant strains and the poor treatment effect of schistosomiasis adults
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation method of compound among the following preparation examples 1-11 mainly comprises following eight reaction operations:
[0073] React operation 1:
[0074]
[0075] Compound 1 was mixed with 2N HCl aqueous solution and heated to 110°C overnight. After the reaction was completed, neutralization, extraction, and concentration were performed to obtain reactant 2. Afterwards, the reactant 2 (FWH202.2) (1.0eq) was dissolved in dichloromethane, and the acid chloride (R 4 COCl) (1.5eq), triethylamine (TEA) (1.0eq), and TLC to track and determine the degree of completion of the reaction. After the reaction was completed, compound 3 was obtained by extraction, concentration of the reaction solution to remove the solvent, and column chromatography.
[0076] where R 4 is unsubstituted or fluorine or chlorine substituted C 2 -C 5 Alkyl; C 3 -C 6 Cycloalkyl; cyclohexenyl; unsubstituted or substituted phenyl, the substituents are selected from C 1 -C 3 1-3 ...
Embodiment 1
[0110]
[0111] Compound 1 (10.0g, 32mmol) was added to HCl aqueous solution (2N, 50mL), then heated to 110°C and refluxed overnight. After the reaction, the aqueous phase was extracted with ethyl acetate to remove impurities, and the aqueous phase was washed with sodium bicarbonate ( NaHCO 3 ) saturated aqueous solution was neutralized to alkaline, extracted (DCM:MeOH=10:1), concentrated to remove the solvent to obtain compound 2 (4.0 g, yield 62.5%). Compound 2 (500mg, 2.5mmol) was dissolved in dichloromethane (DCM) (10mL), and benzoyl chloride (520mg, 3.75mmol), triethylamine (TEA) (250mg, 2.5mmol) were added at 0°C After the addition, stir at room temperature, TLC to track and measure the degree of completion of the reaction. After the reaction is completed, add a saturated aqueous solution of sodium bicarbonate, extract with ethyl acetate, concentrate to remove the solvent, and use petroleum ether / ethyl acetate (volume ratio 1:1 ) column chromatography to obtain compo...
Embodiment 2
[0119]
[0120] Dissolve compound 2 (300mg, 1.5mmol) in methanol (10mL), add cyclohexaneformaldehyde under ice bath, add glacial acetic acid (180mg, 3mmol) after 40min, raise the reaction temperature to room temperature, stir for 1h, then heat Stir at 60°C for 2h, then cool down to 0°C, add sodium borohydride (450mg, 12mmol) in batches, heat to 60°C overnight after addition. Then the reaction system was cooled to room temperature, quenched with water, the reaction solution was concentrated to remove the solvent, extracted, and separated by petroleum ether / ethyl acetate (volume ratio 2:1) column chromatography to obtain compound 4-1 (FWH298.42) (140mg , yield 32%).
[0121] 1 HNMR (400MHz, CDCl 3 )δ:1.58-1.83(m,6H),1.69-1.79(m,7H),2.75-2.99(m,5H),3.02-4.39(m,2H),4.79-4.90(m,2H),7.16- 7.28(m,4H).ESI-MS(m / s):299[M+1] +
[0122] Except replacing the cyclohexyl formaldehyde in embodiment 2 with following corresponding reaction compound, synthesize following compound with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com