Patents
Literature
1087 results about "Antigen receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term antigen originally described a structural molecule that binds specifically to an antibody. It was expanded to refer to any molecule or a linear molecular fragment that can be recognized by highly variable antigen receptors (B-cell receptor or T-cell receptor) of the adaptive immune system.
Method and compositions using a chimeric antigen receptor for enhanced anti-tumor effector functioning of T cells
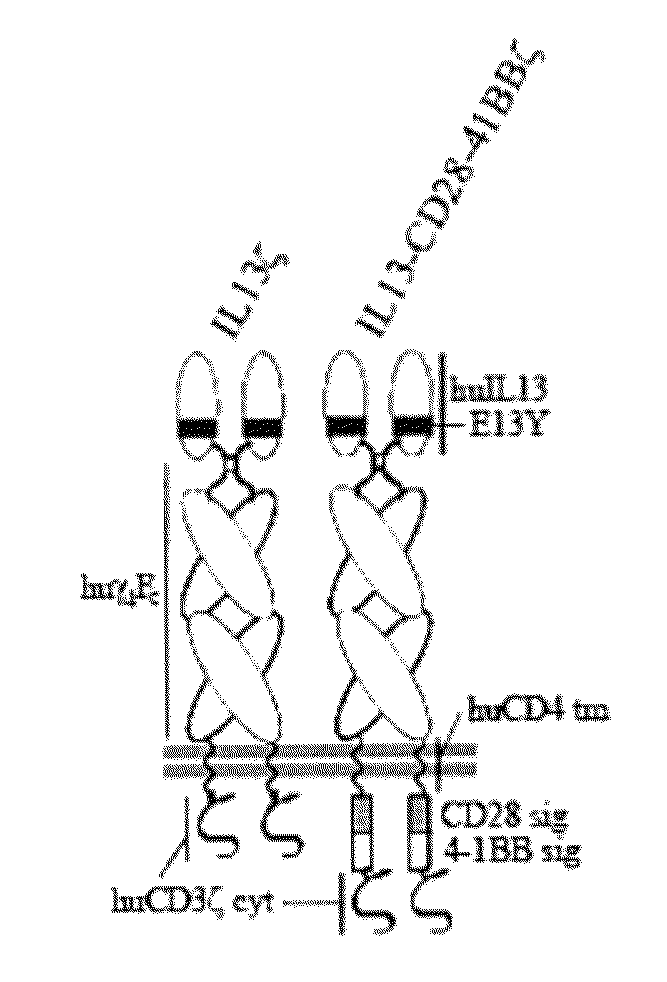
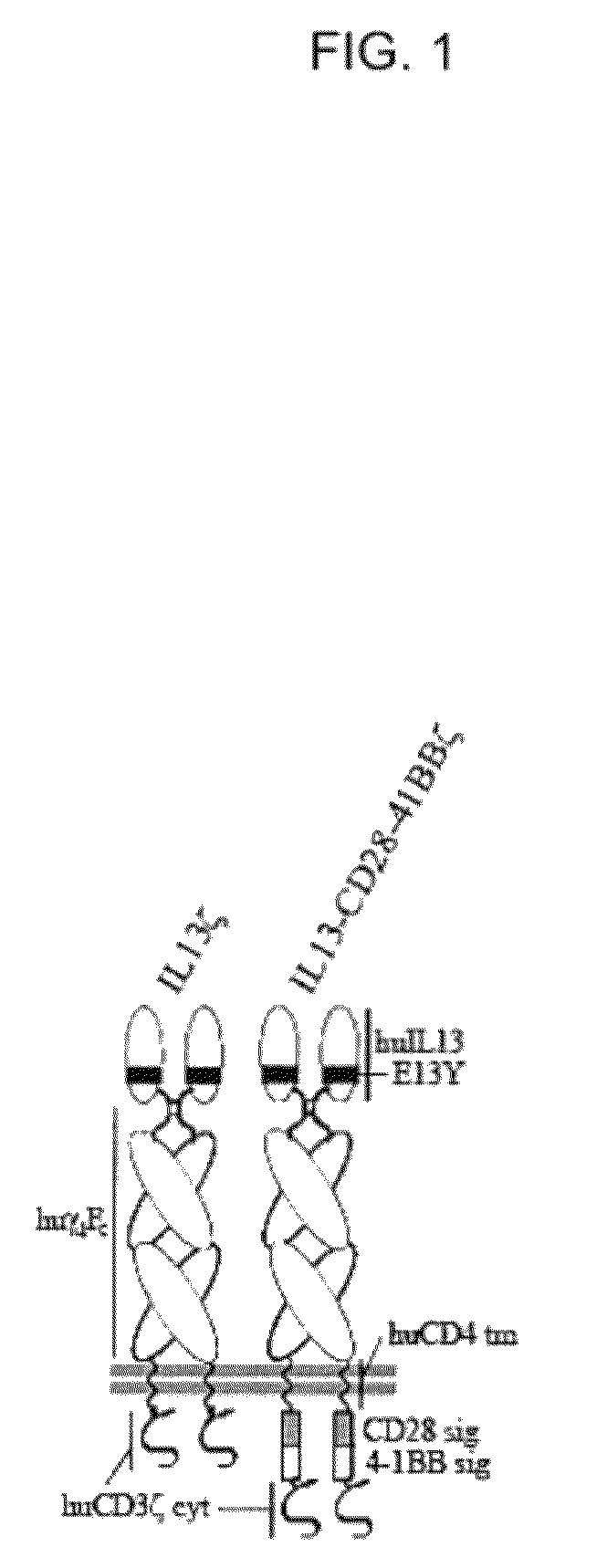
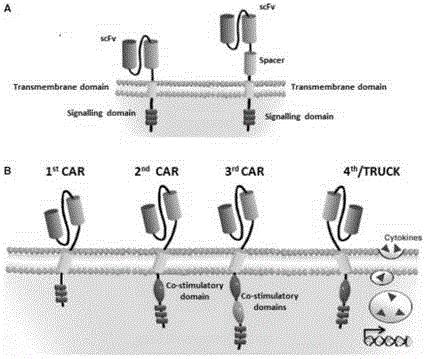
Integration of costimulatory signaling domains within a tumor targeting chimeric antigen receptor (CAR), such as the IL13Rα2 specific IL13-zetakine (IL13ζ), enhances T cell-mediated responses against tumors even in the absence of expressed ligands for costimulatory receptors.
Owner:CITY OF HOPE
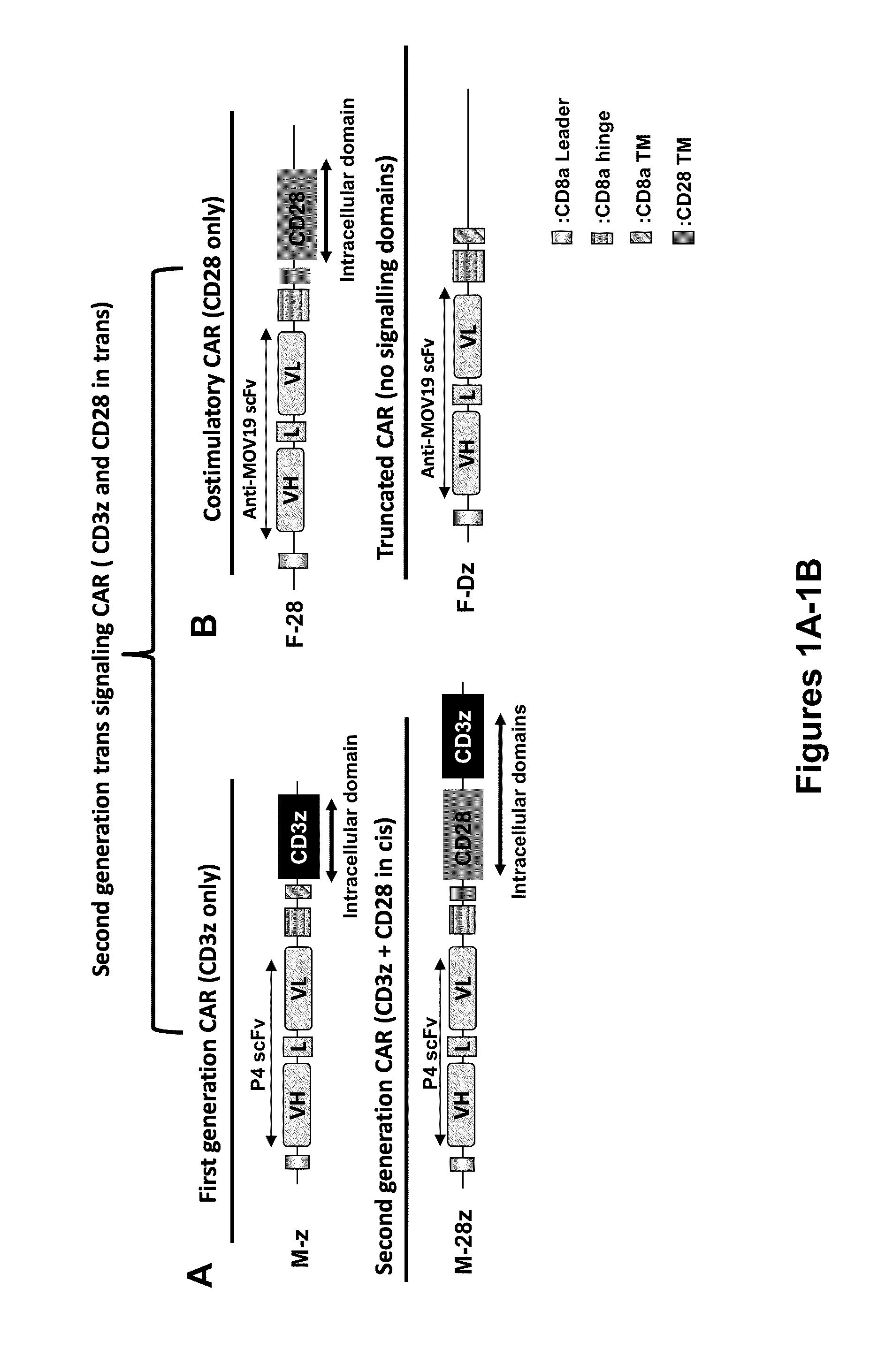
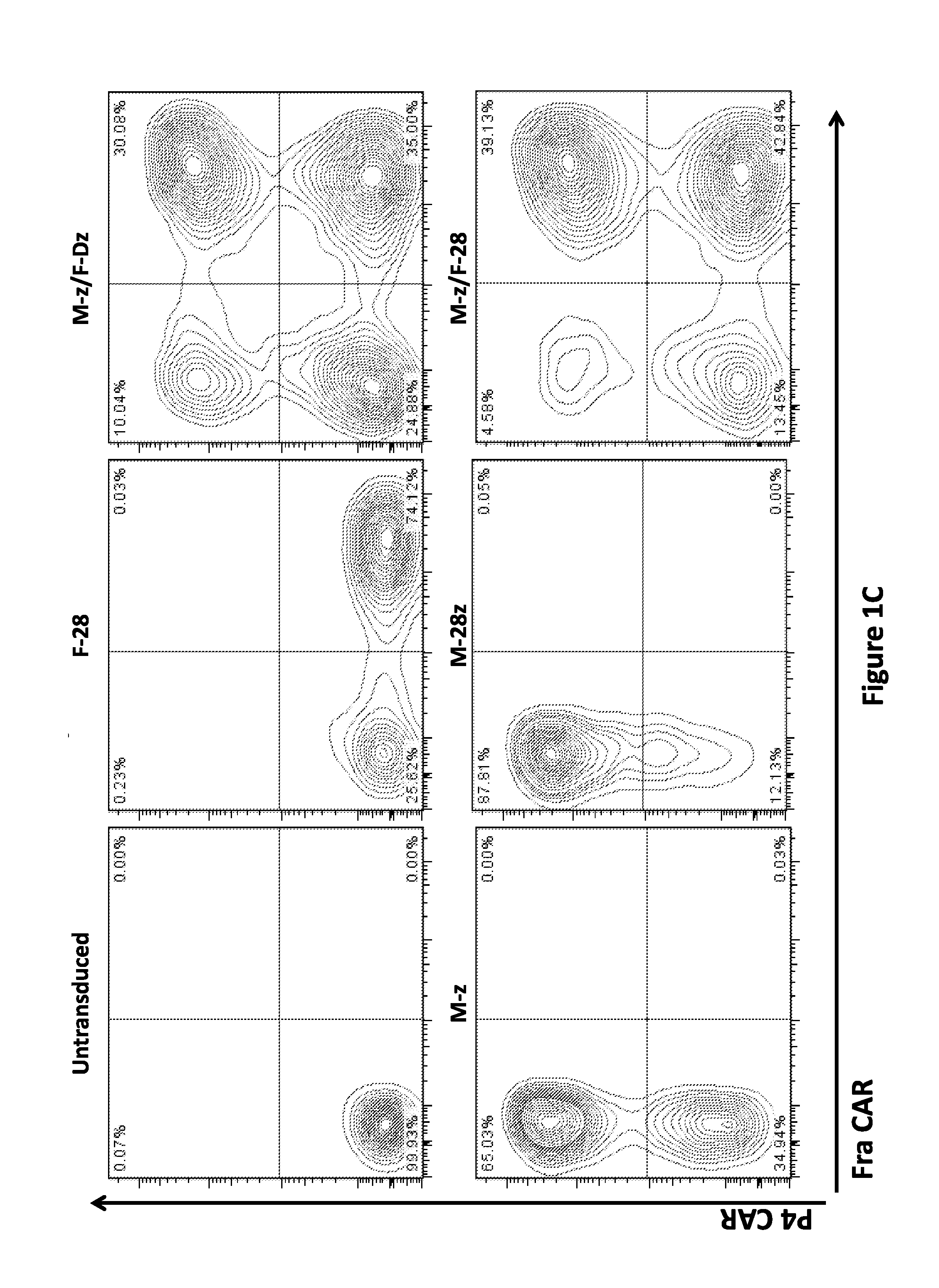
Use of a Trans-Signaling Approach in Chimeric Antigen Receptors
ActiveUS20140099309A1Heightened tumor specificitySugar derivativesAntibody mimetics/scaffoldsTrans signalingActivation cells
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Generation and application of universal T cells for B-ALL
InactiveUS20070036773A1Hinder recognitionEnhanced siRNA effectBiocideGenetic material ingredientsAntigenNatural Killer Cell Inhibitory Receptors
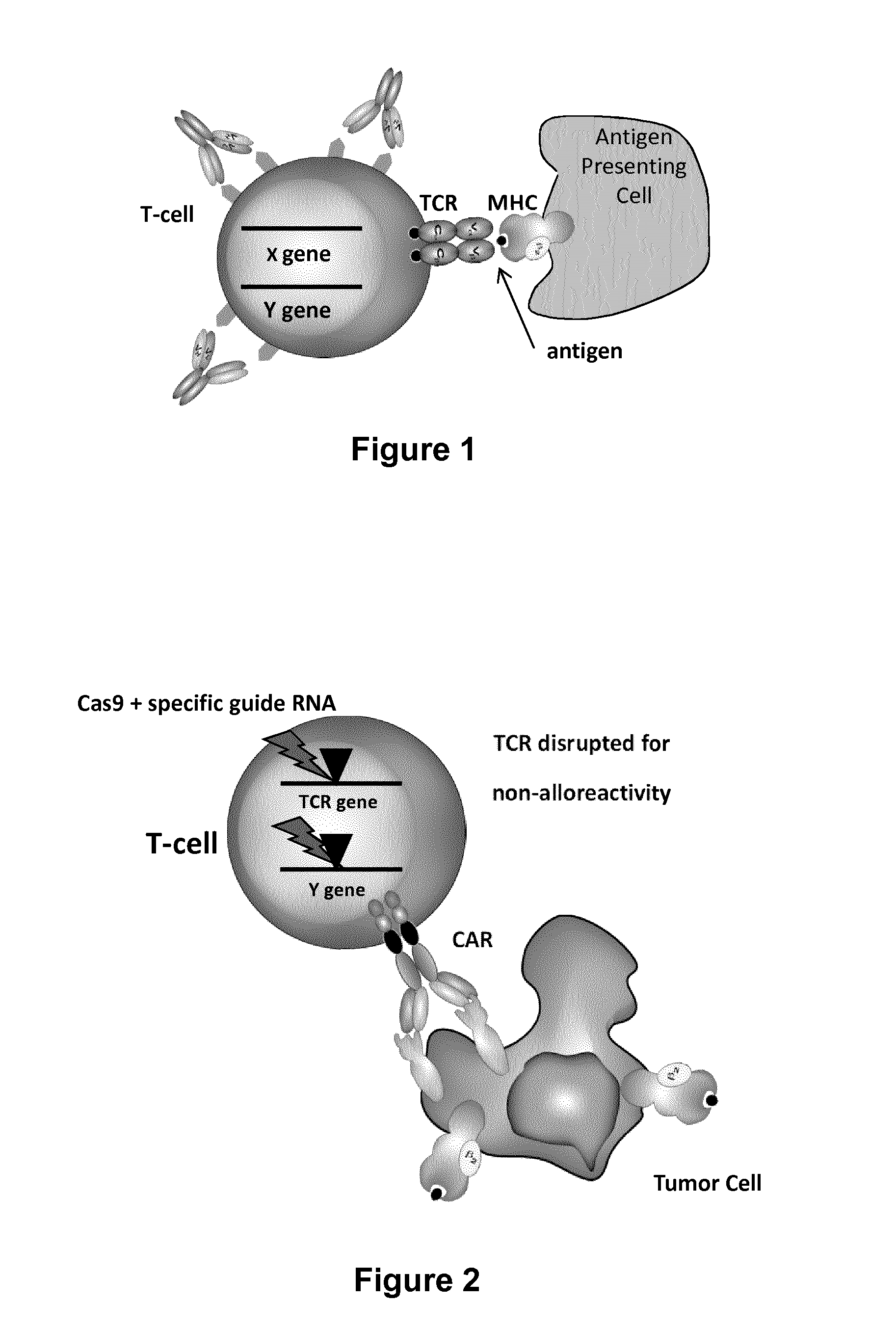
The present invention is directed to universal T cells and their use in treating diseases and other physiological conditions. More specifically, the present invention is directed to universal T cells and their use in treating treating B-lineage acute lymphoblastic leukemia (B-ALL) in particular and malignancy in general. The universal T cells contain (i) nucleic acid encoding a chimeric antigen receptor (CAR) to redirect their antigen specificity and effector function and (ii) nucleic acids encoding shRNA and / or siRNA molecules to down-regulate cell-surface expression of T cell classical HLA class I and / or II genes to avoid recognition by recipient T cells. The universal T cells may also contain a nucleic acid encoding a non-classical HLA gene, such as an HLA E gene to enforce expression of HLA E genes and / or an HLA G gene to enforce expression of HLA G genes, to avoid recognition by recipient NK cells. The universal T cells may further contain a nucleic acid encoding a selection-suicide gene.
Owner:CITY OF HOPE
Car+ t cells genetically modified to eliminate expression of t-cell receptor and/or HLA
ActiveUS20140349402A1Suitable for storageReduce/eliminate T cellsGenetically modified cellsMammal material medical ingredientsAntigen receptorsAutoimmunity
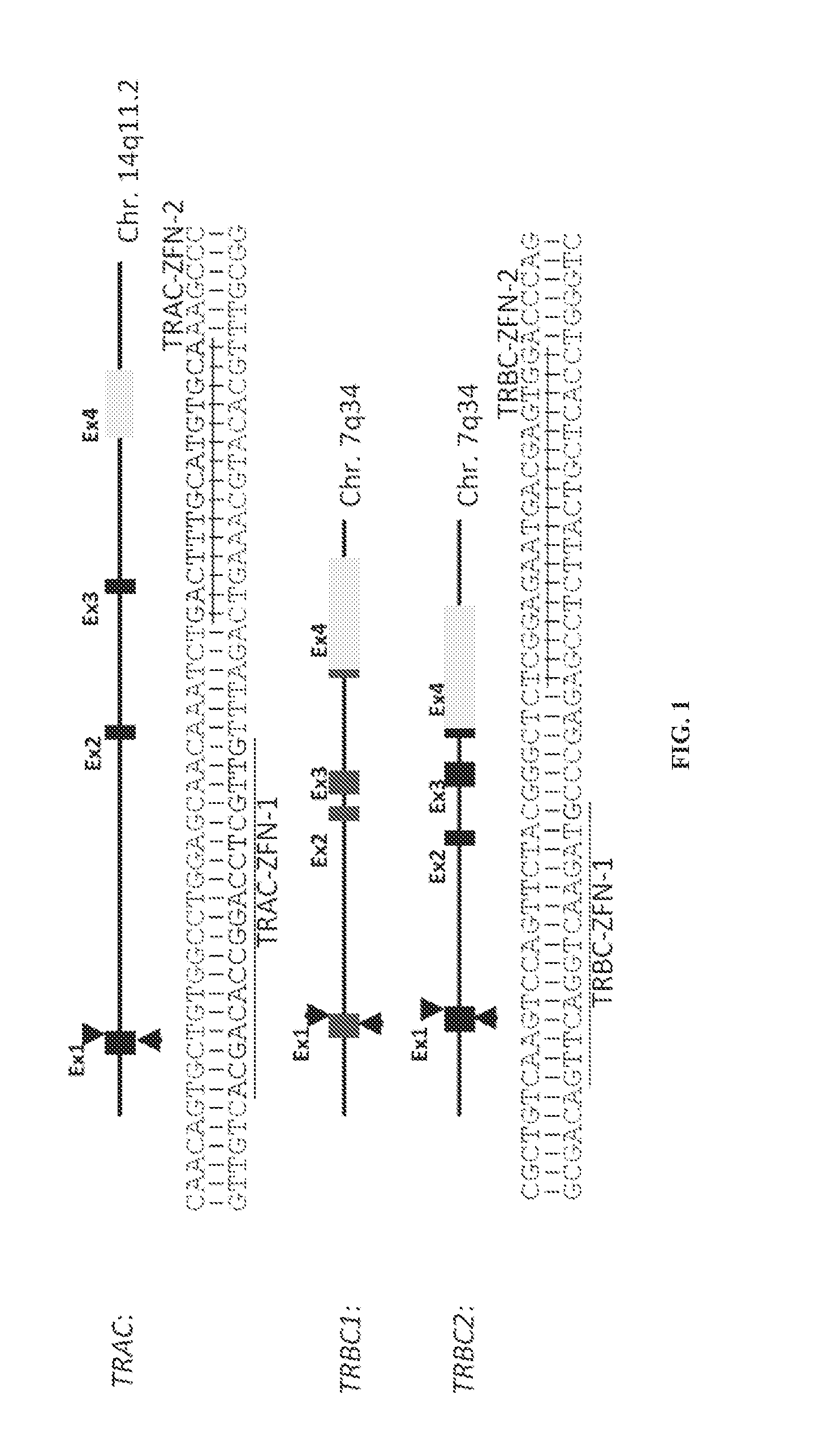
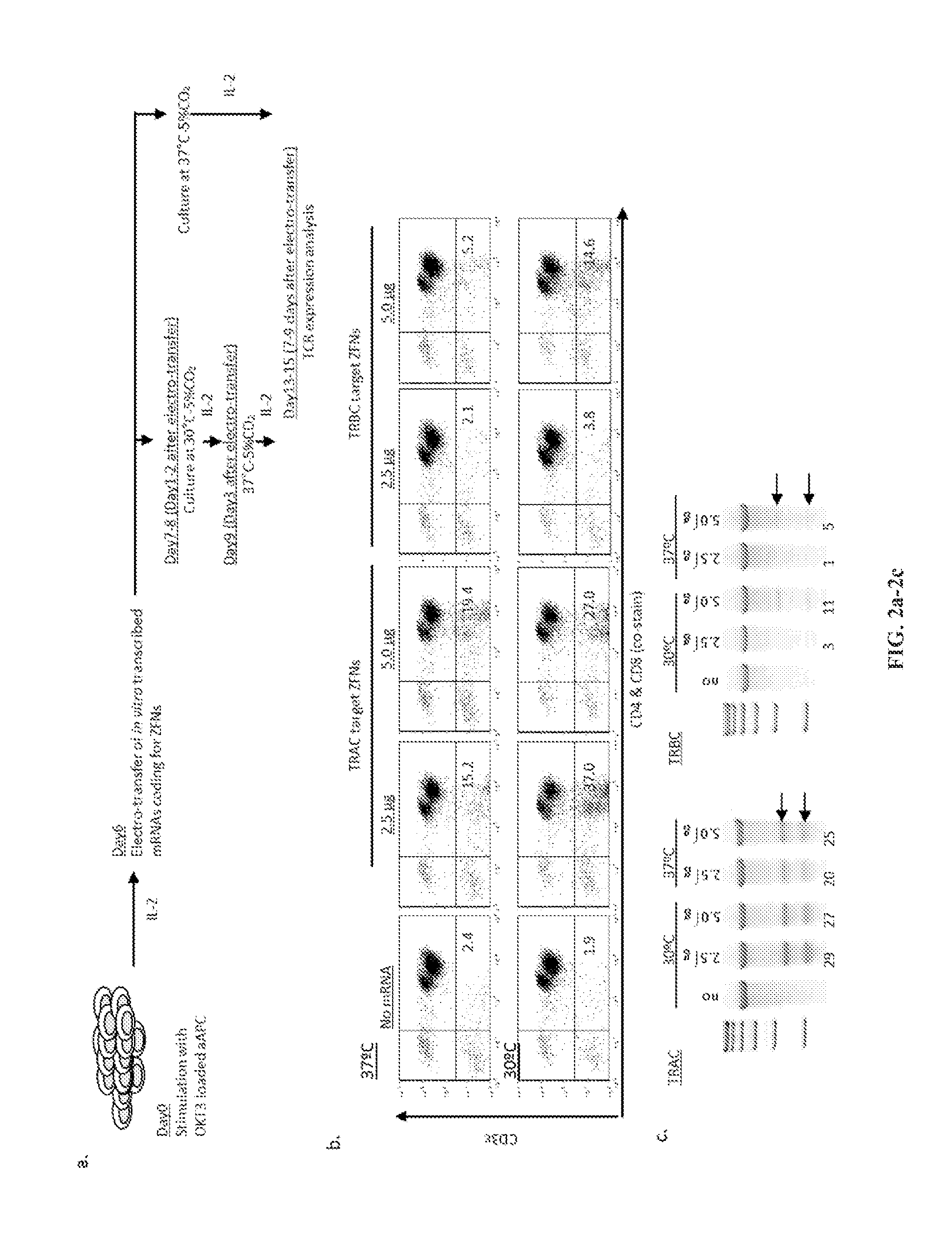
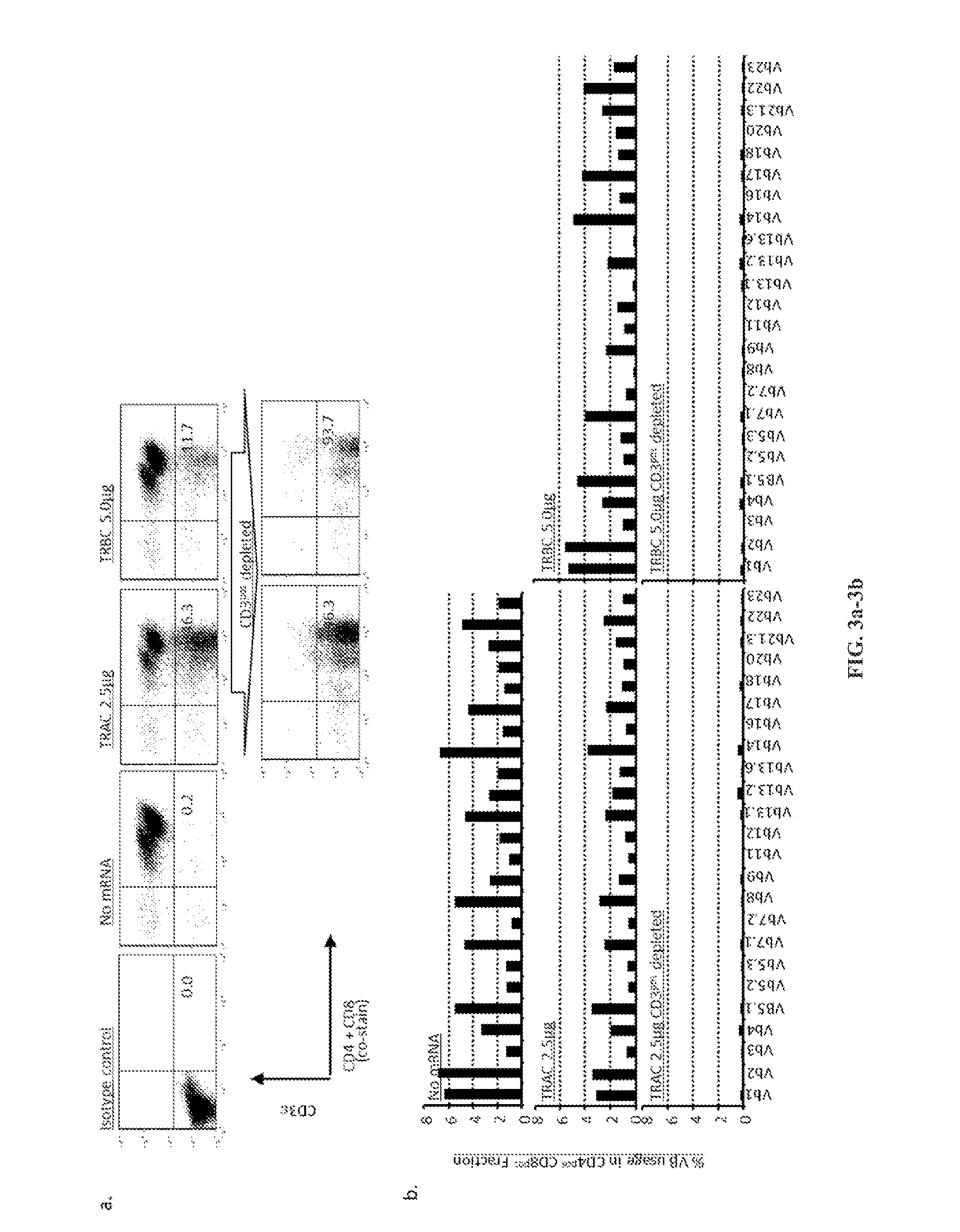
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising disrupted T cell receptor and / or HLA and comprising a chimeric antigen receptor. In certain embodiments, the compositions are employed allogeneically as universal reagents for “off-the-shelf treatment of medical conditions such as cancer, autoimmunity, and infection. In particular embodiments, the T cell receptor-negative and / or HLA-negative T cells are generated using zinc finger nucleases, for example.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Universal anti-tag chimeric antigen receptor-expressing T cells and methods of treating cancer
ActiveUS9233125B2Efficient productionPromote efficient proliferationBiocideAntibody mimetics/scaffoldsHuman cancerCancer cell
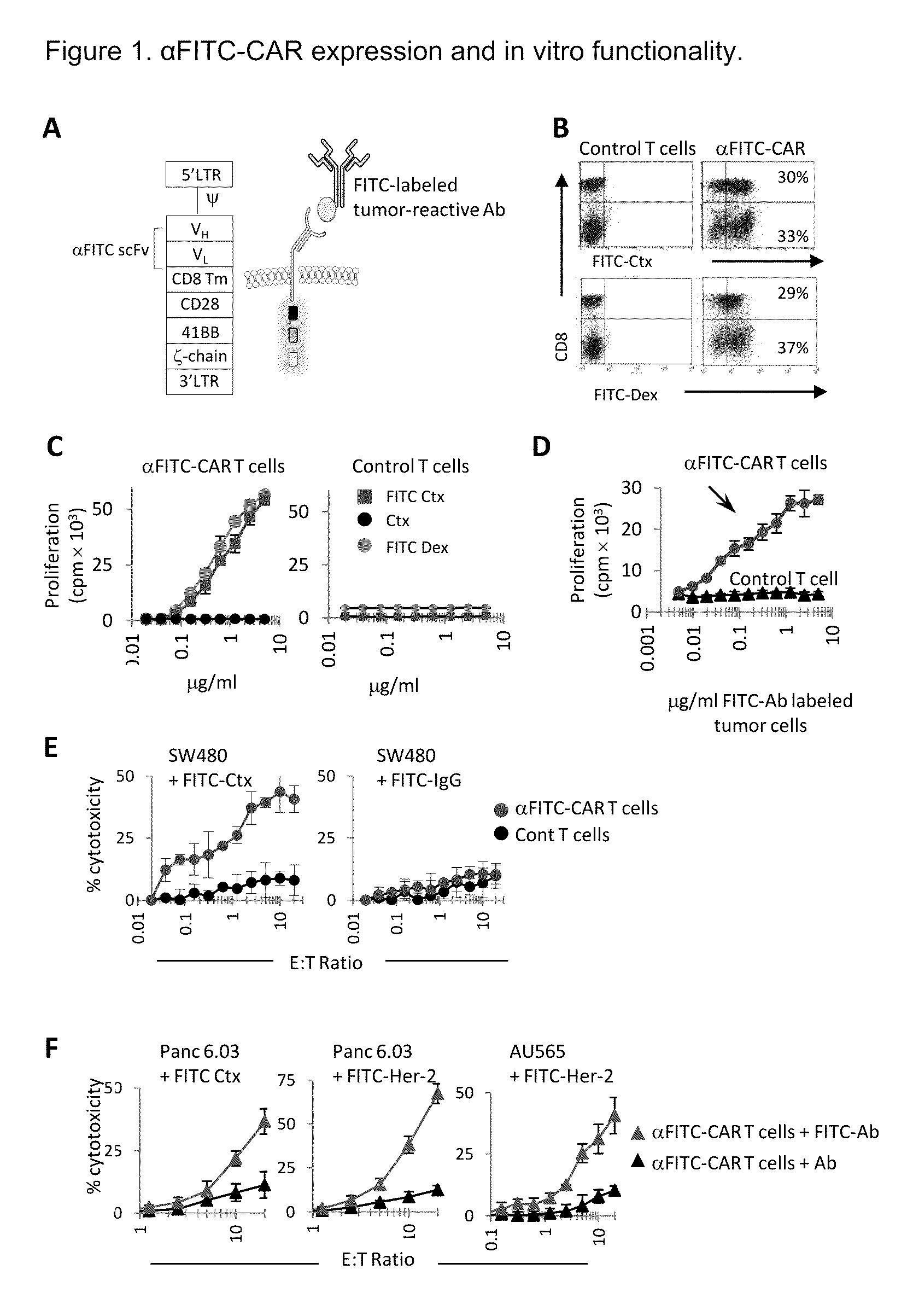
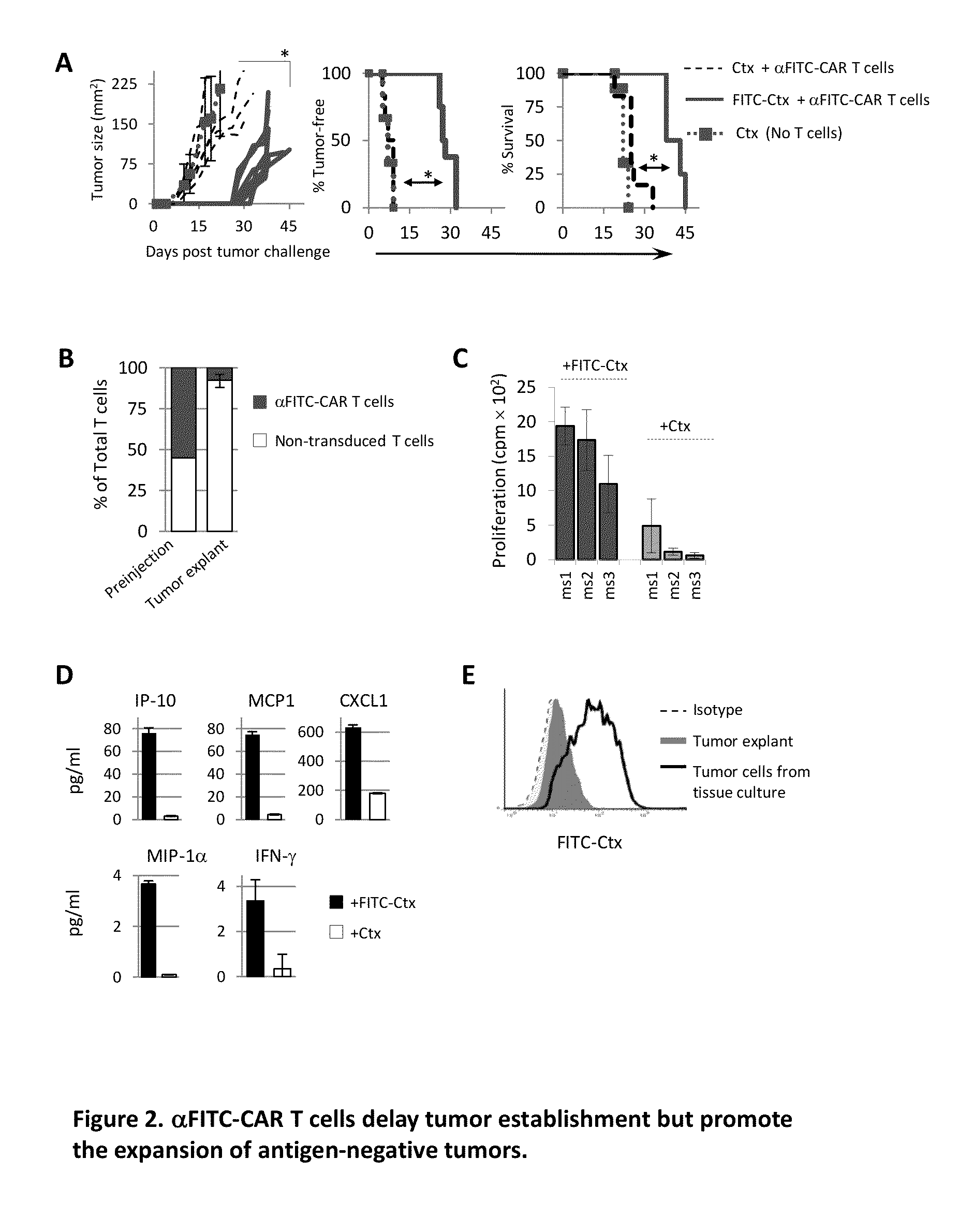
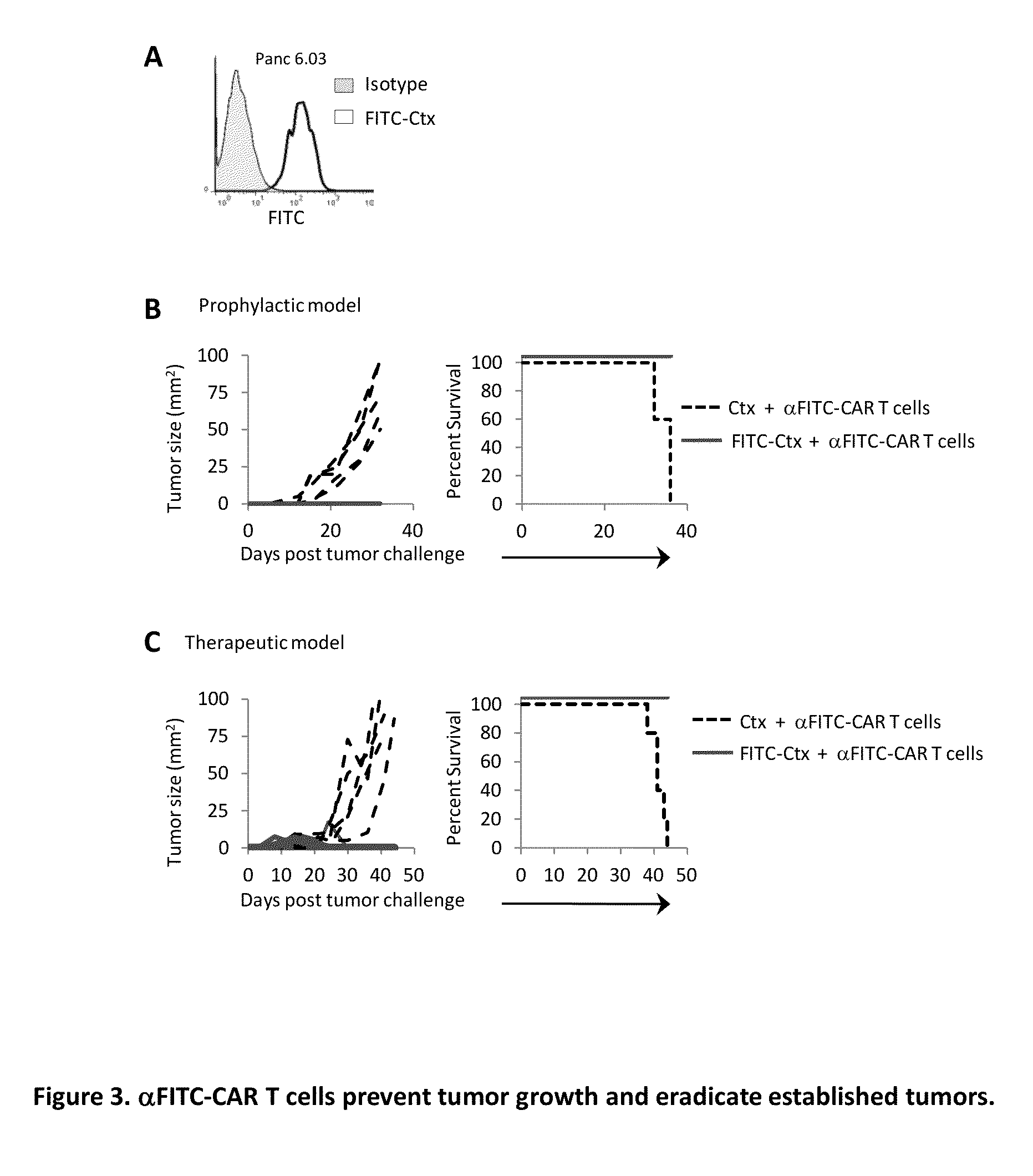
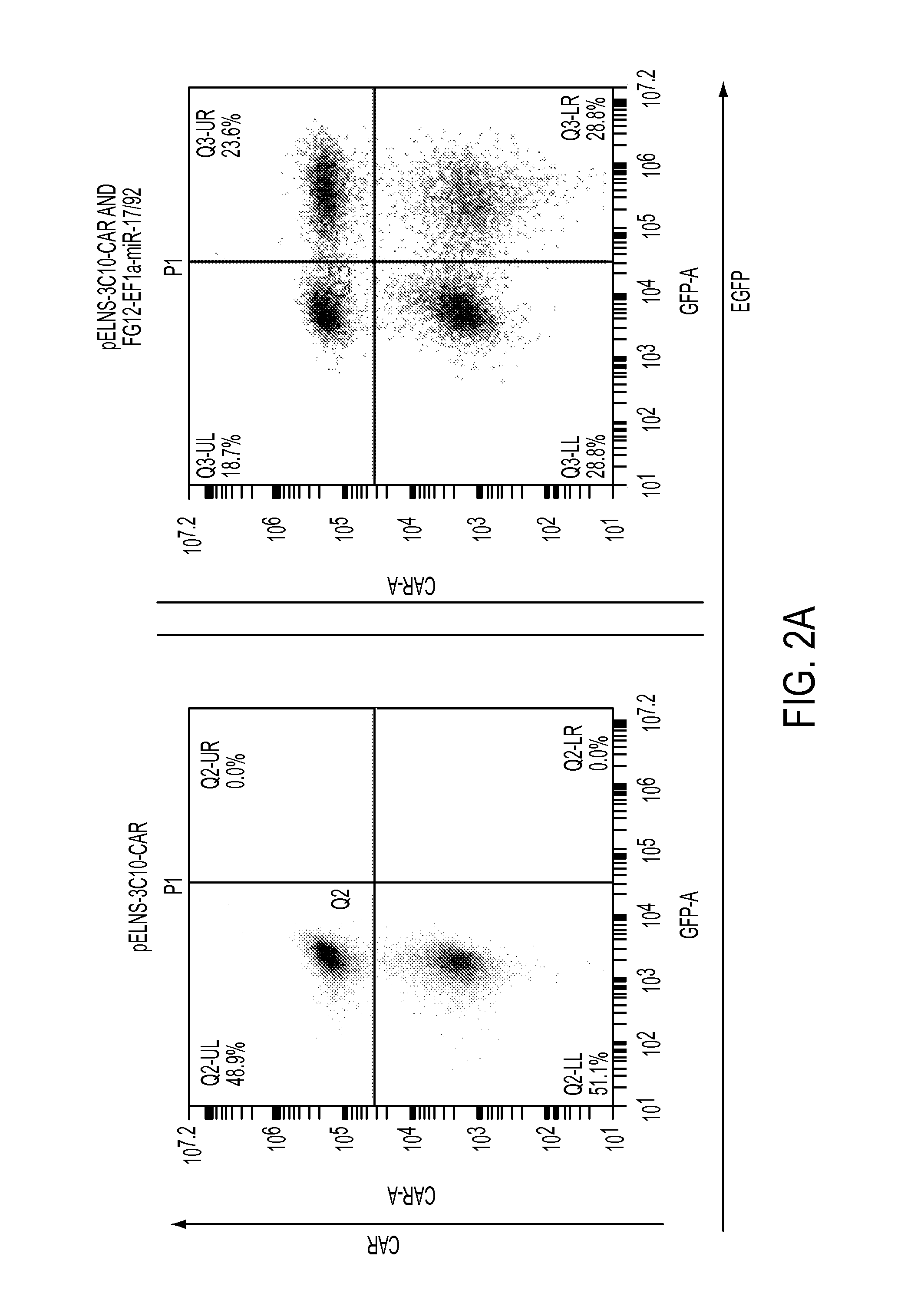
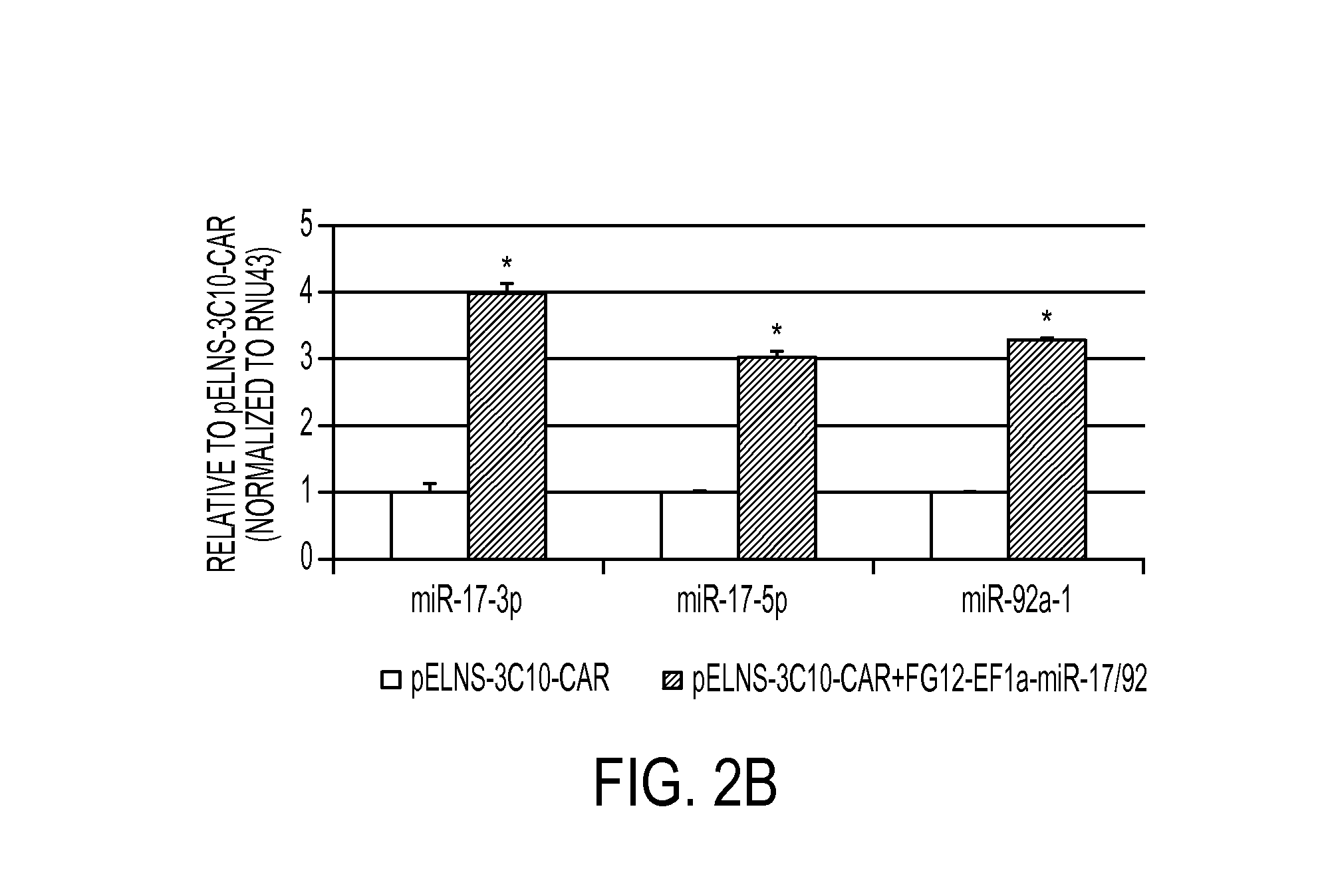
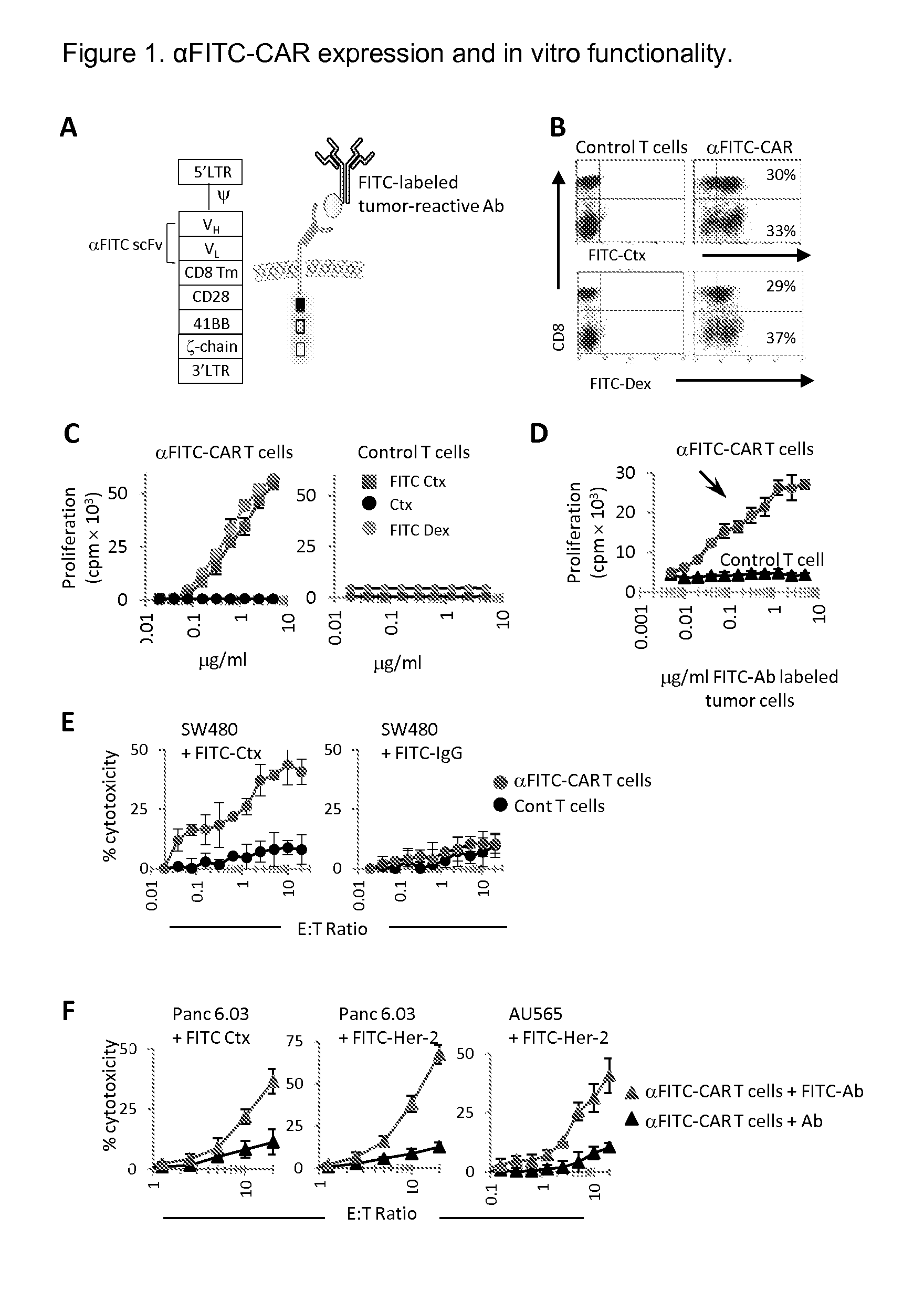
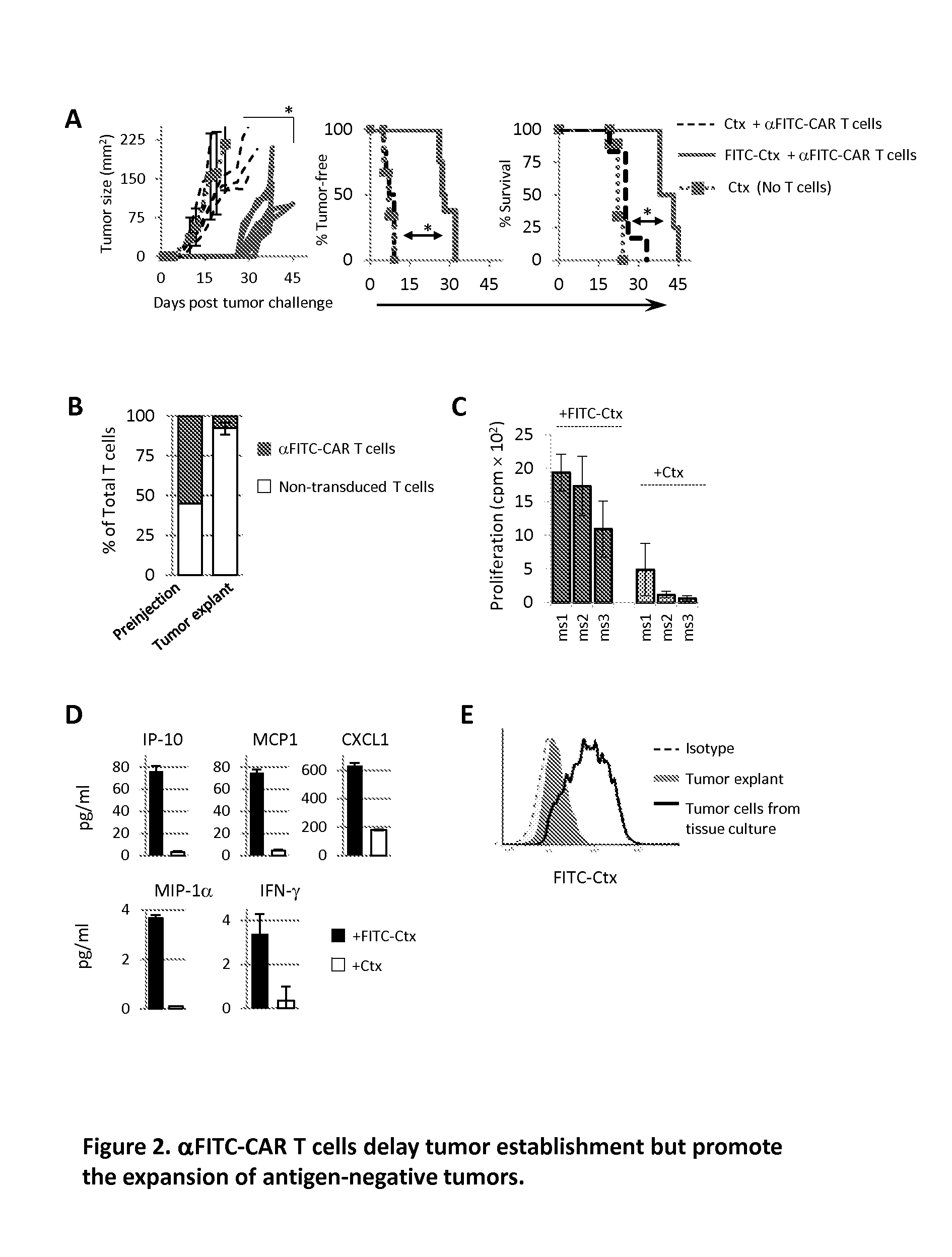
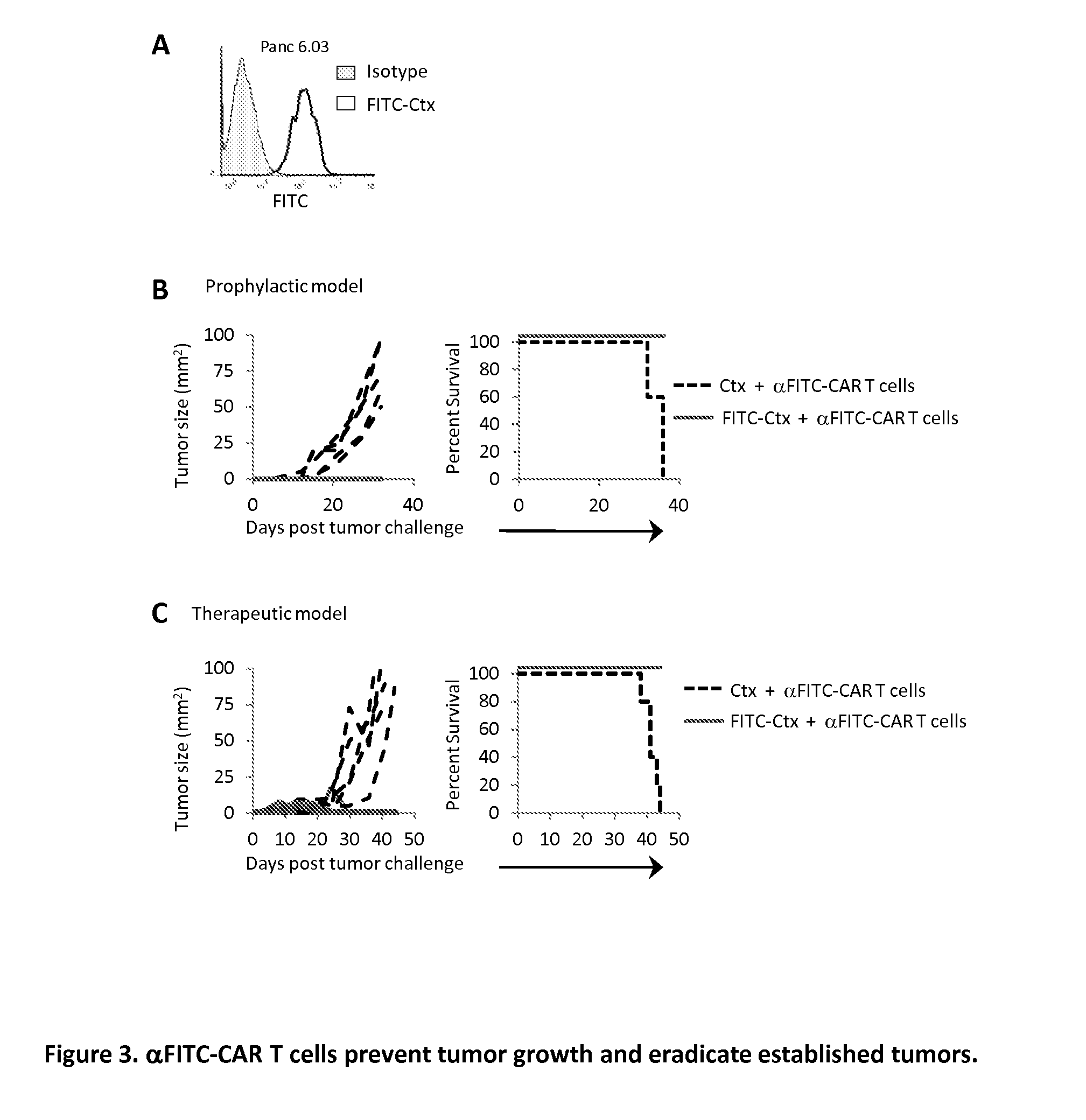
The present invention provides a universal, yet adaptable, anti-tag chimeric antigen receptor (AT-CAR) system which provides T cells with the ability and specificity to recognize and kill target cells, such as tumor cells, that have been marked by tagged antibodies. As an example, αFITC-CAR-expressing T cells have been developed that specifically recognize various human cancer cells when those cells are bound by cancer-reactive FITC-labeled antibodies. The activation of αFITC-CAR-expressing T cells is shown to induce efficient target lysis, T cell proliferation, and cytokine / chemokine production. The system can be used to treating subjects having cancer.
Owner:UNIV OF MARYLAND BALTIMORE
Bispecific chimeric antigen receptors and therapeutic uses thereof
ActiveUS20150038684A1Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingAntigen receptors
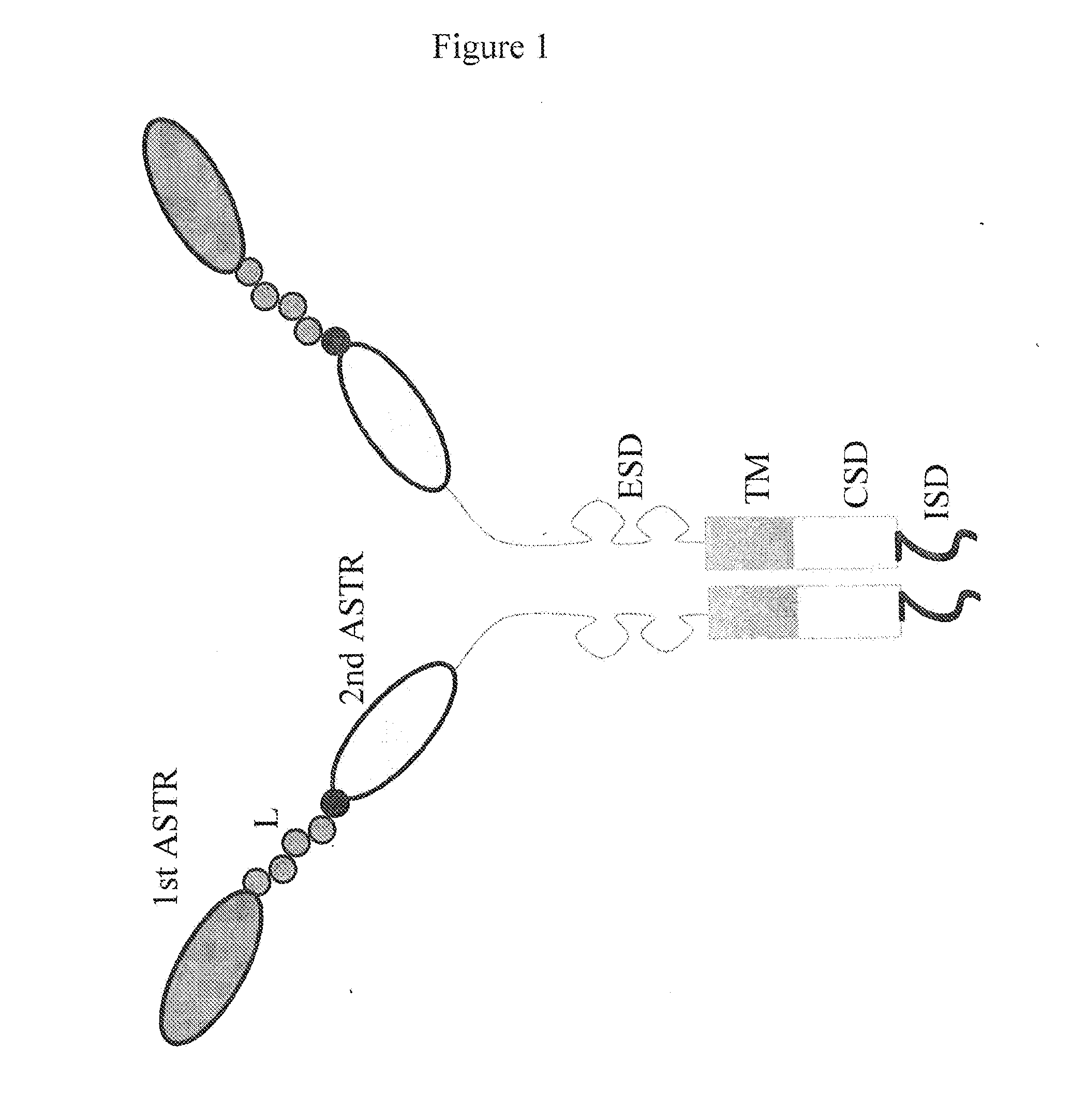
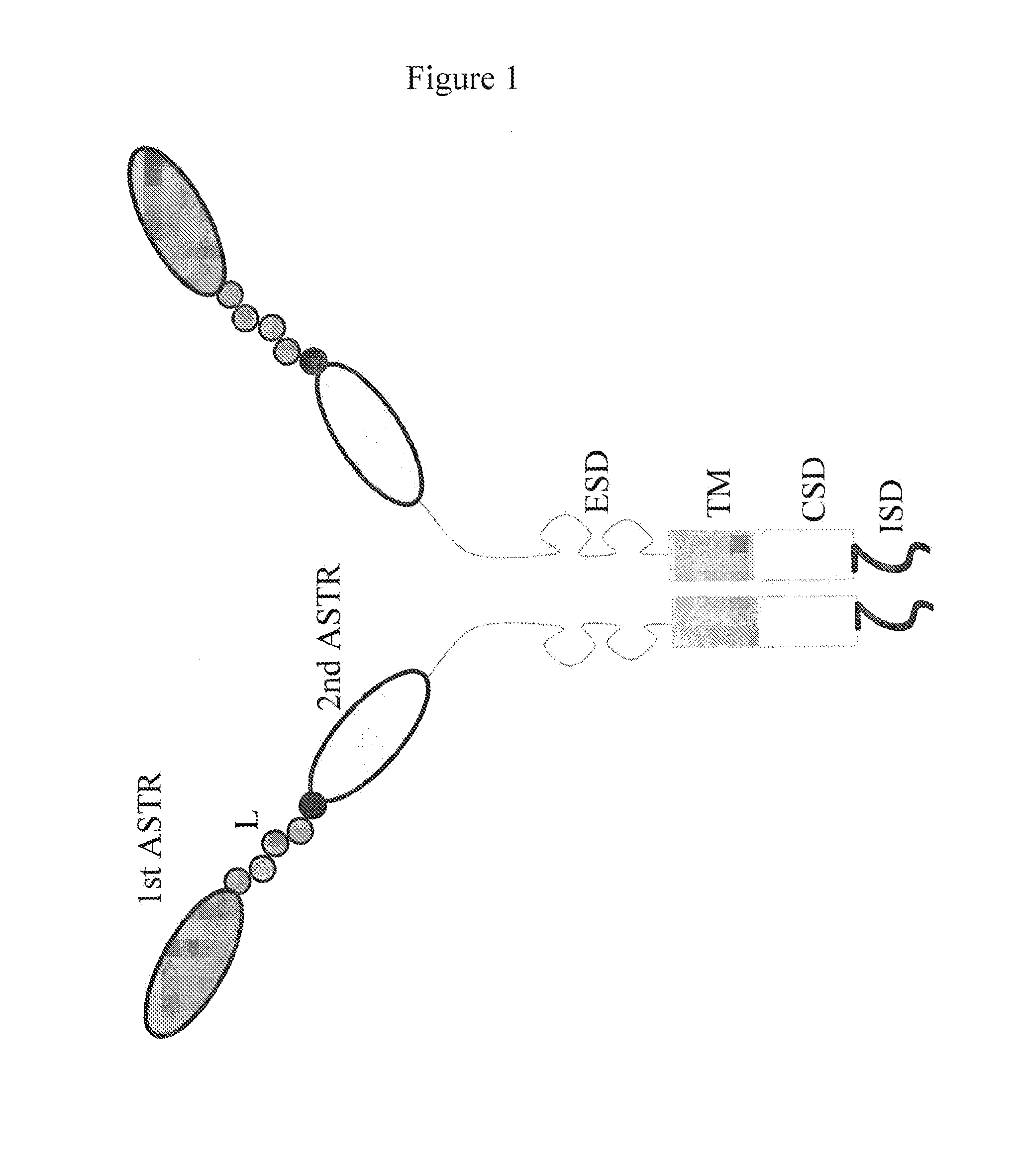
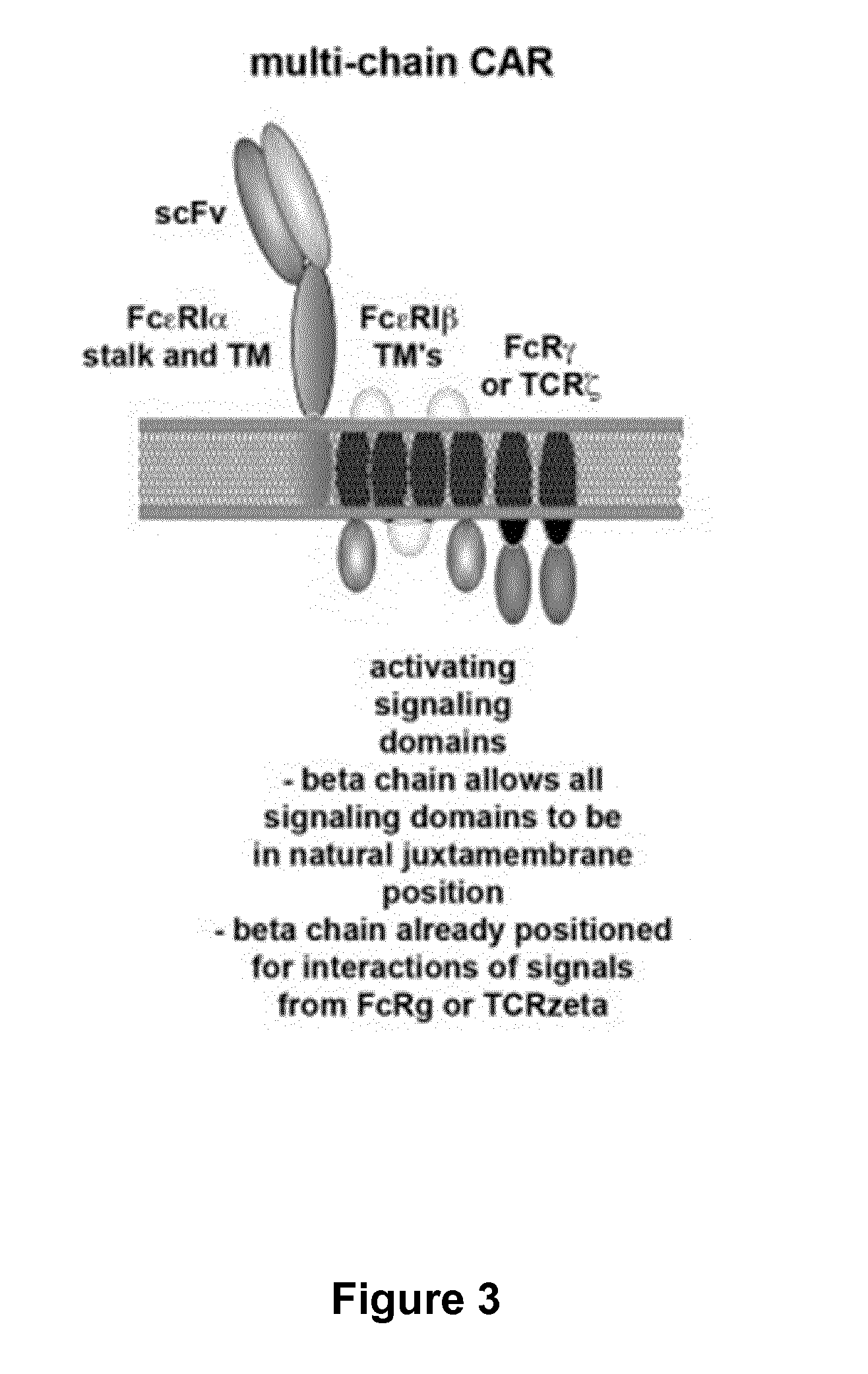
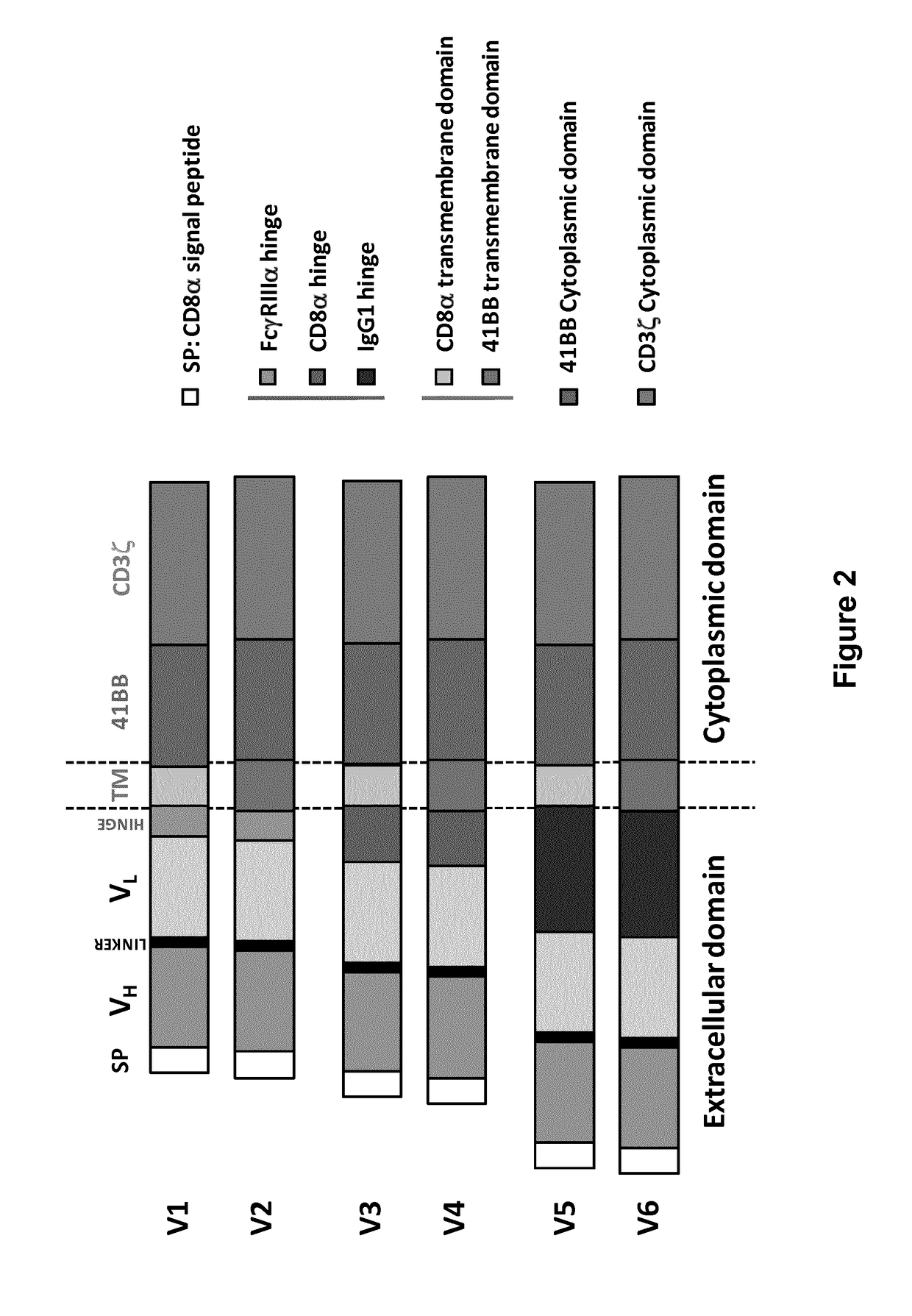
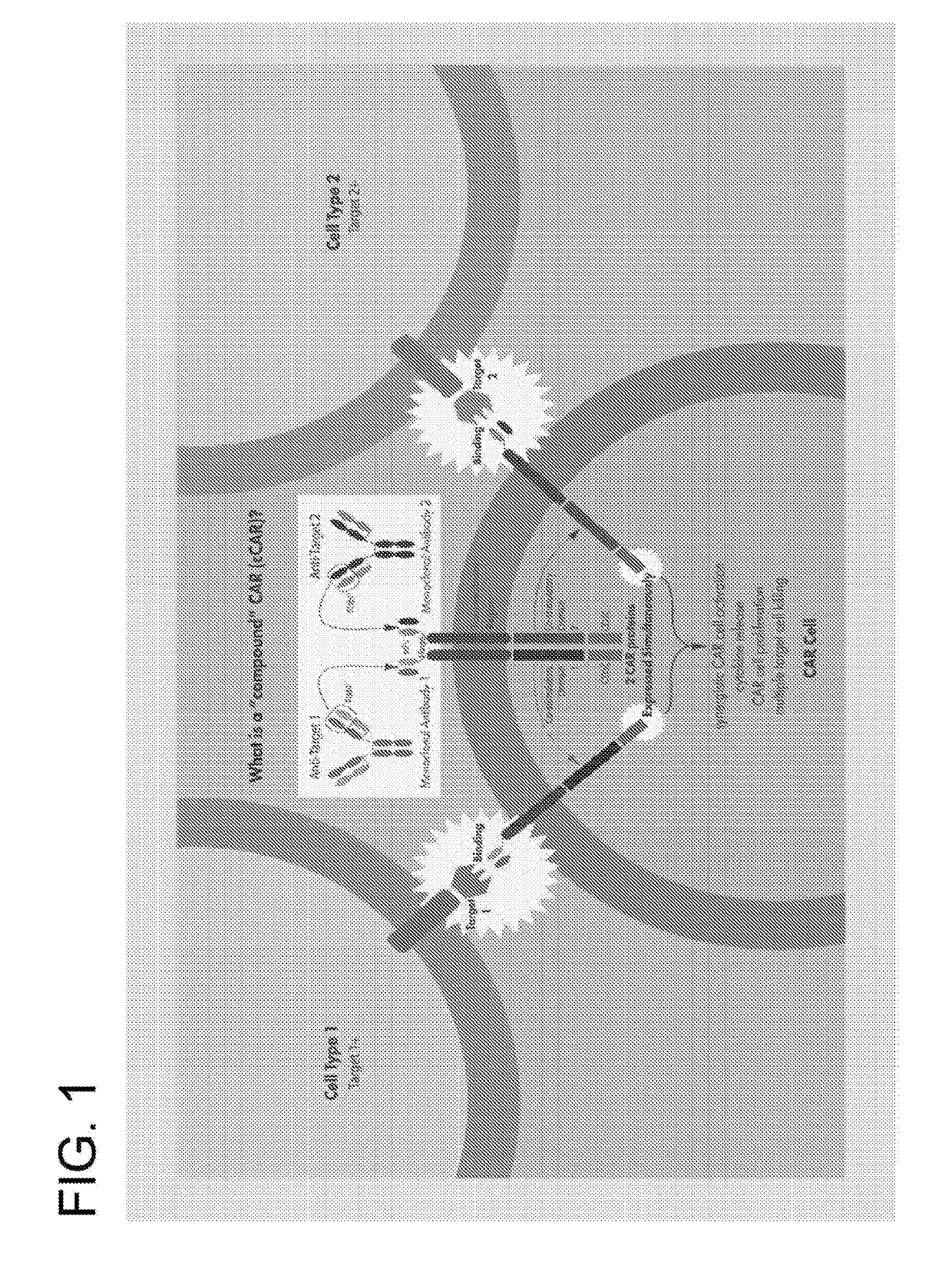
The invention is directed to a bispecific chimeric antigen receptor, comprising: (a) at least two antigen-specific targeting regions; (b) an extracellular spacer domain; (c) a transmembrane domain; (d) at least one co-stimulatory domain; and (e) an intracellular signaling domain, wherein each antigen-specific targeting region comprises an antigen-specific single chain Fv (scFv) fragment, and binds a different antigen, and wherein the bispecific chimeric antigen receptor is co-expressed with a therapeutic control. The invention also provides methods and uses of the bispecific chimeric antigen receptors.
Owner:SEATTLE CHILDRENS HOSPITAL
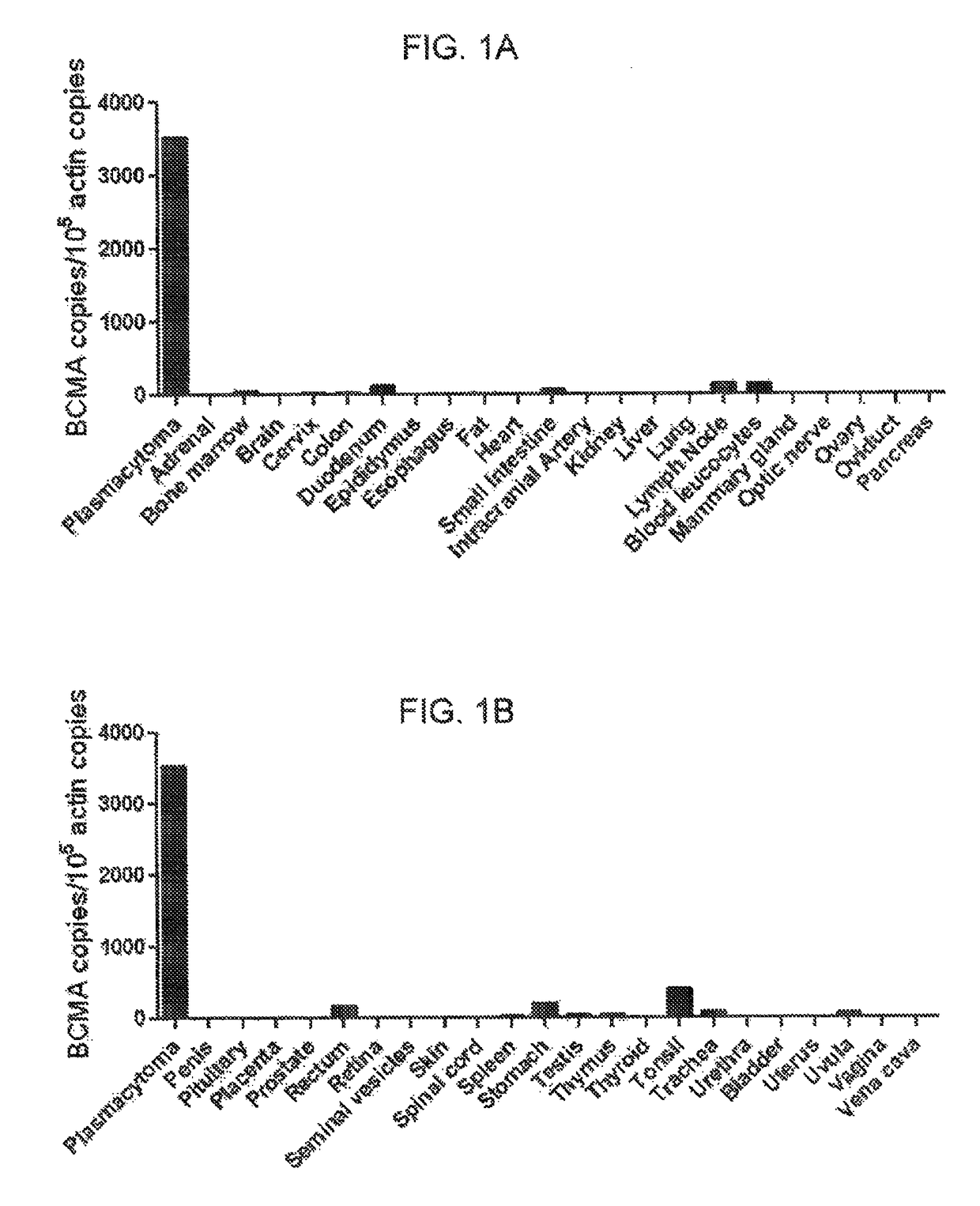
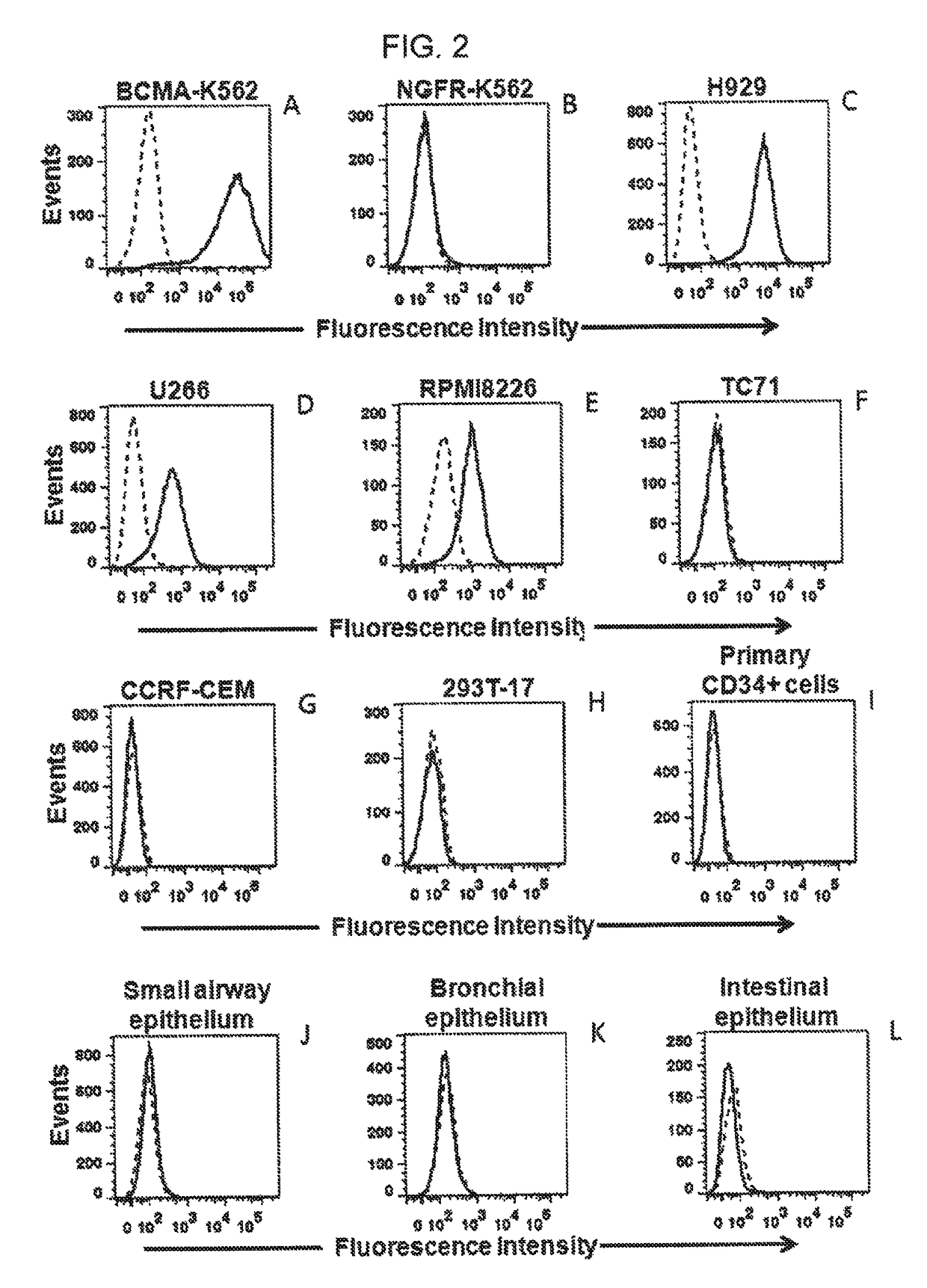
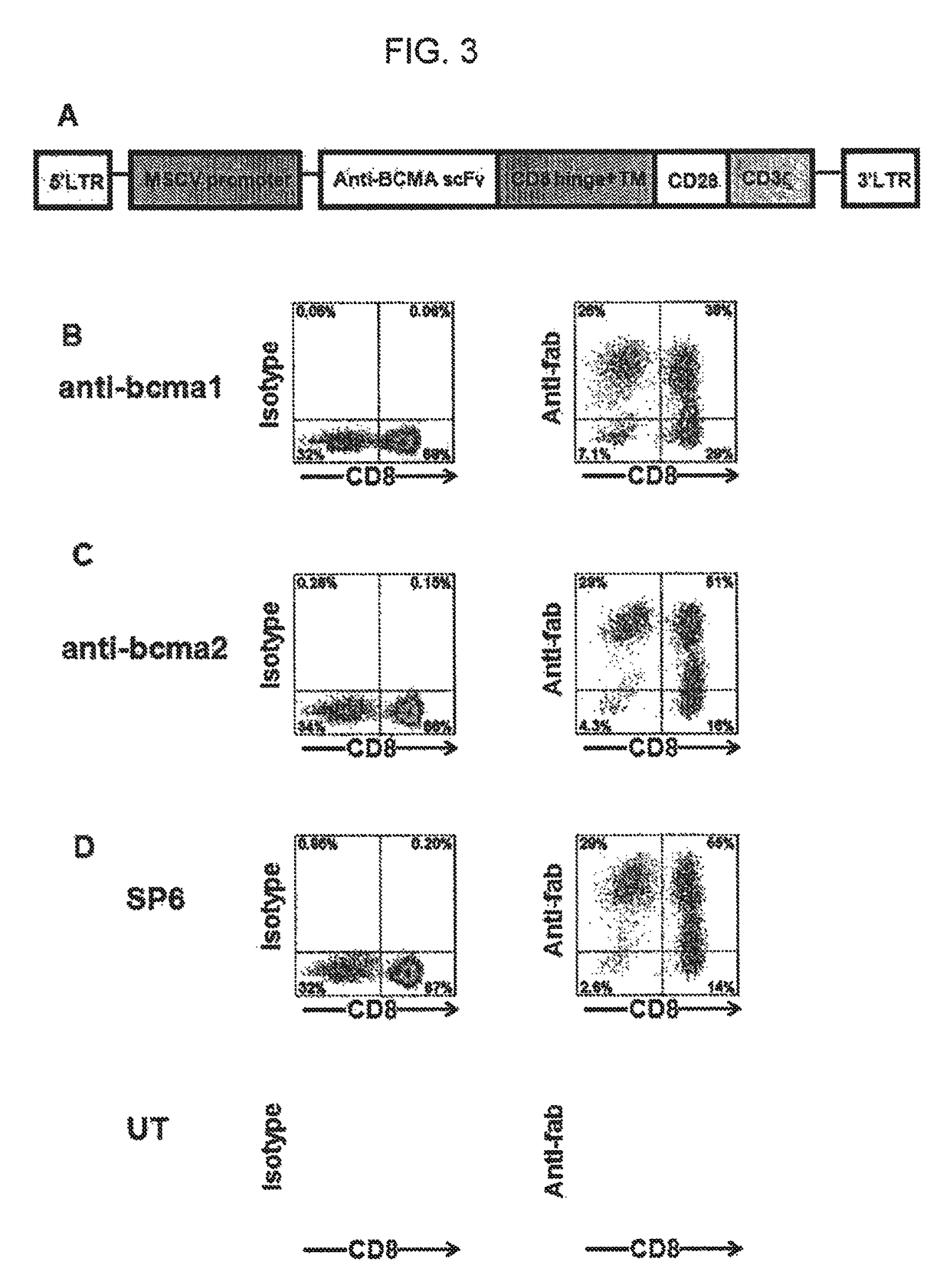
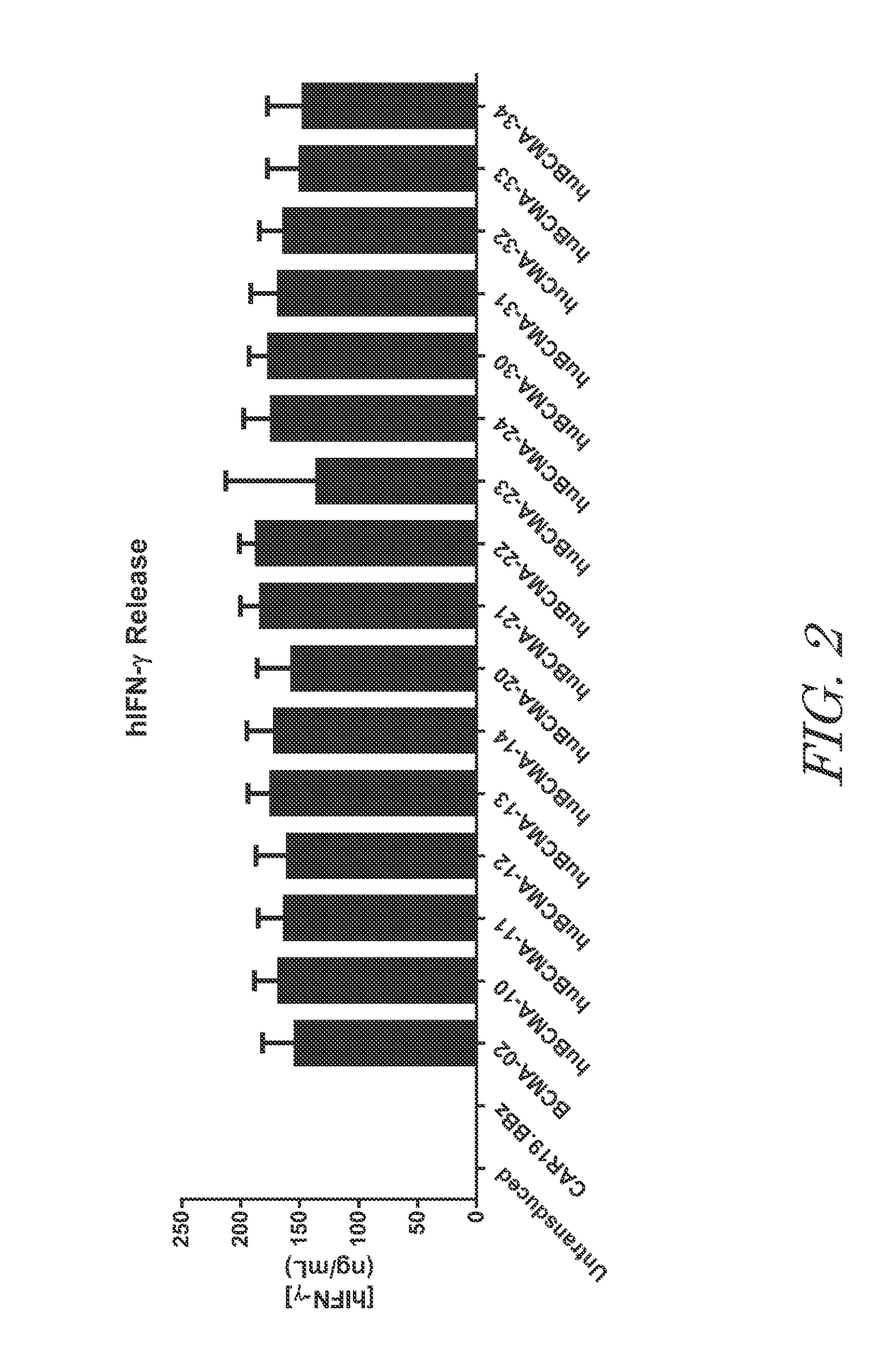
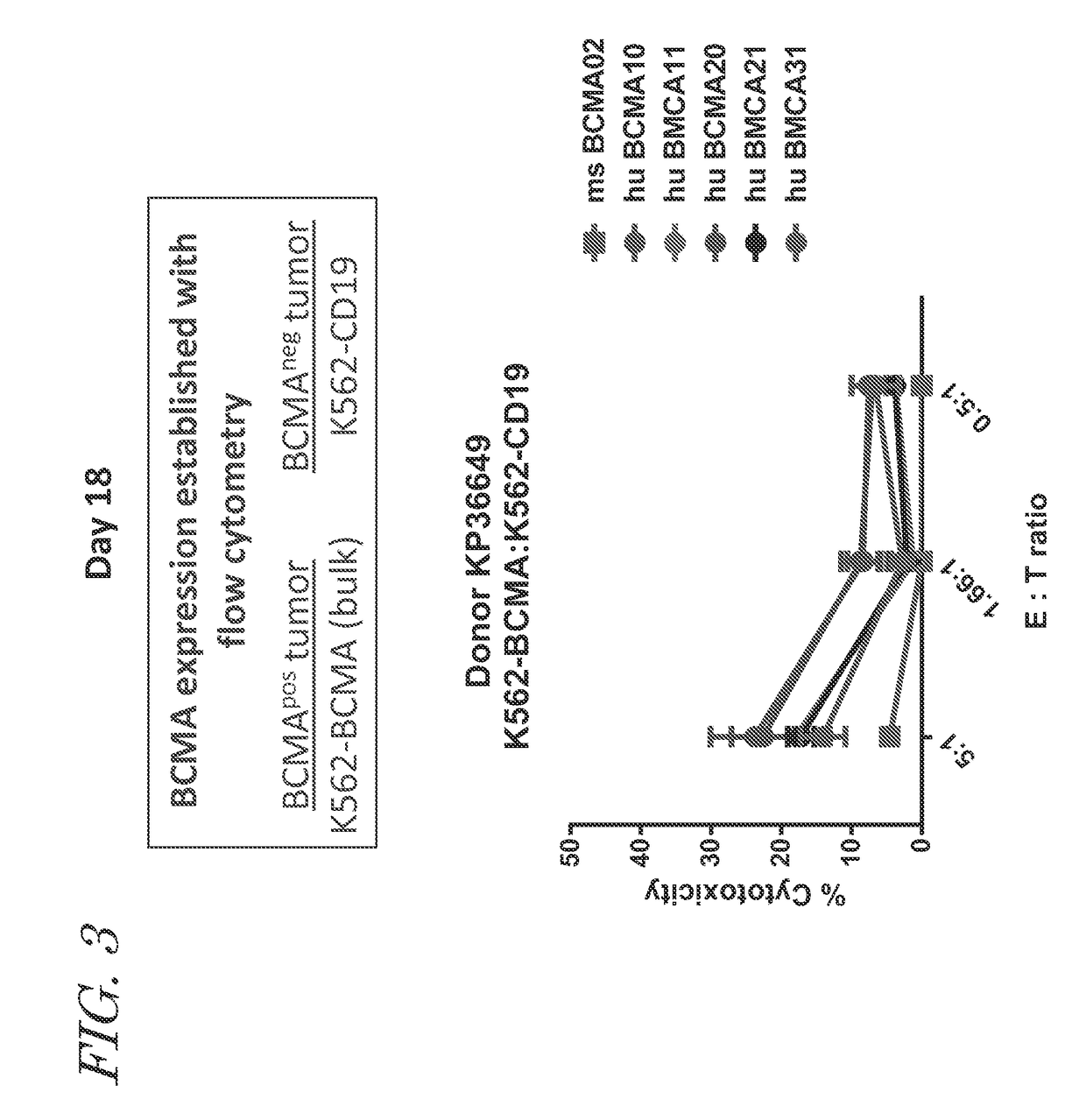
Chimeric antigen receptors targeting b-cell maturation antigen
ActiveUS20150051266A1Antibody mimetics/scaffoldsGenetic material ingredientsAntigenAntigen receptors
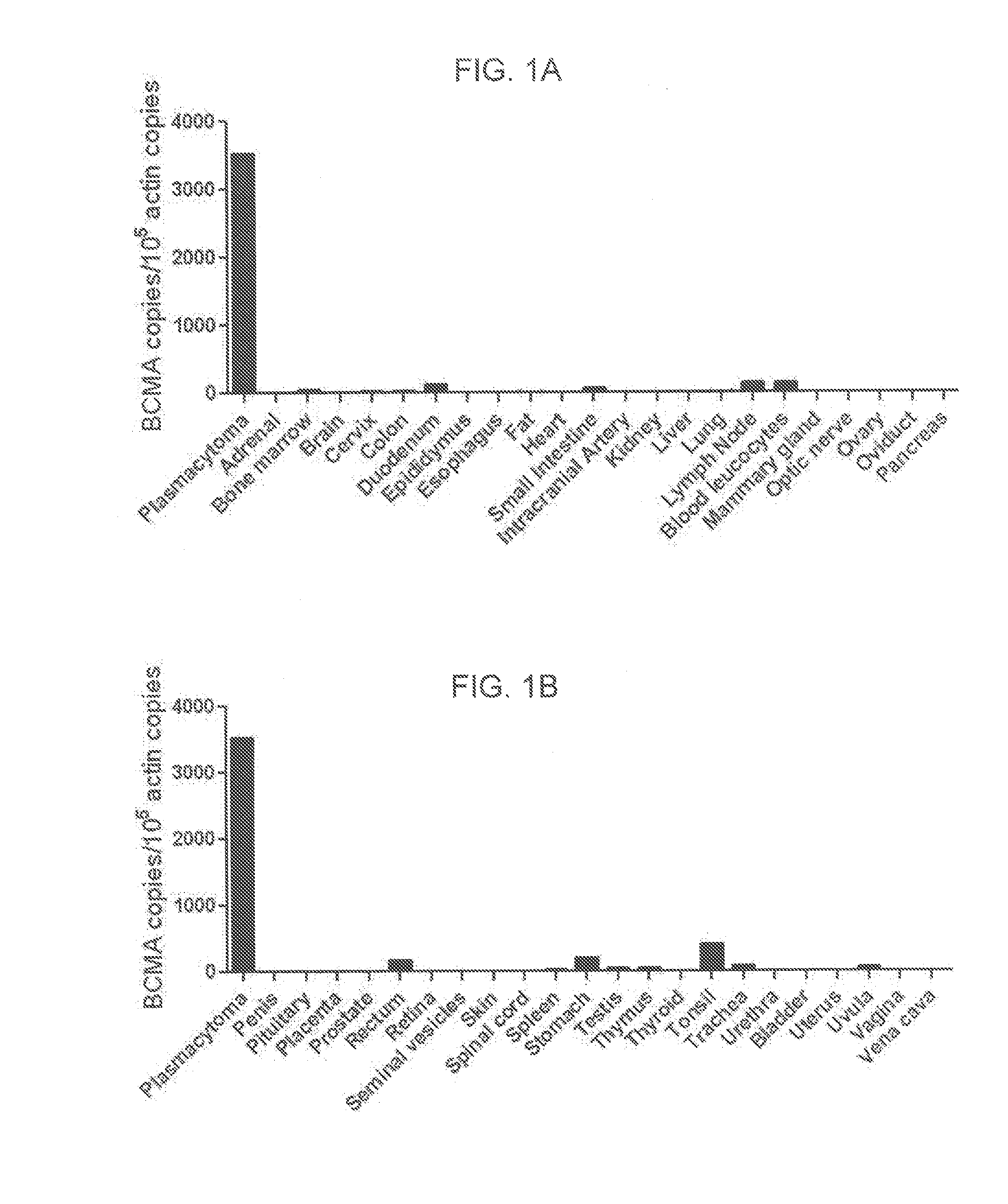
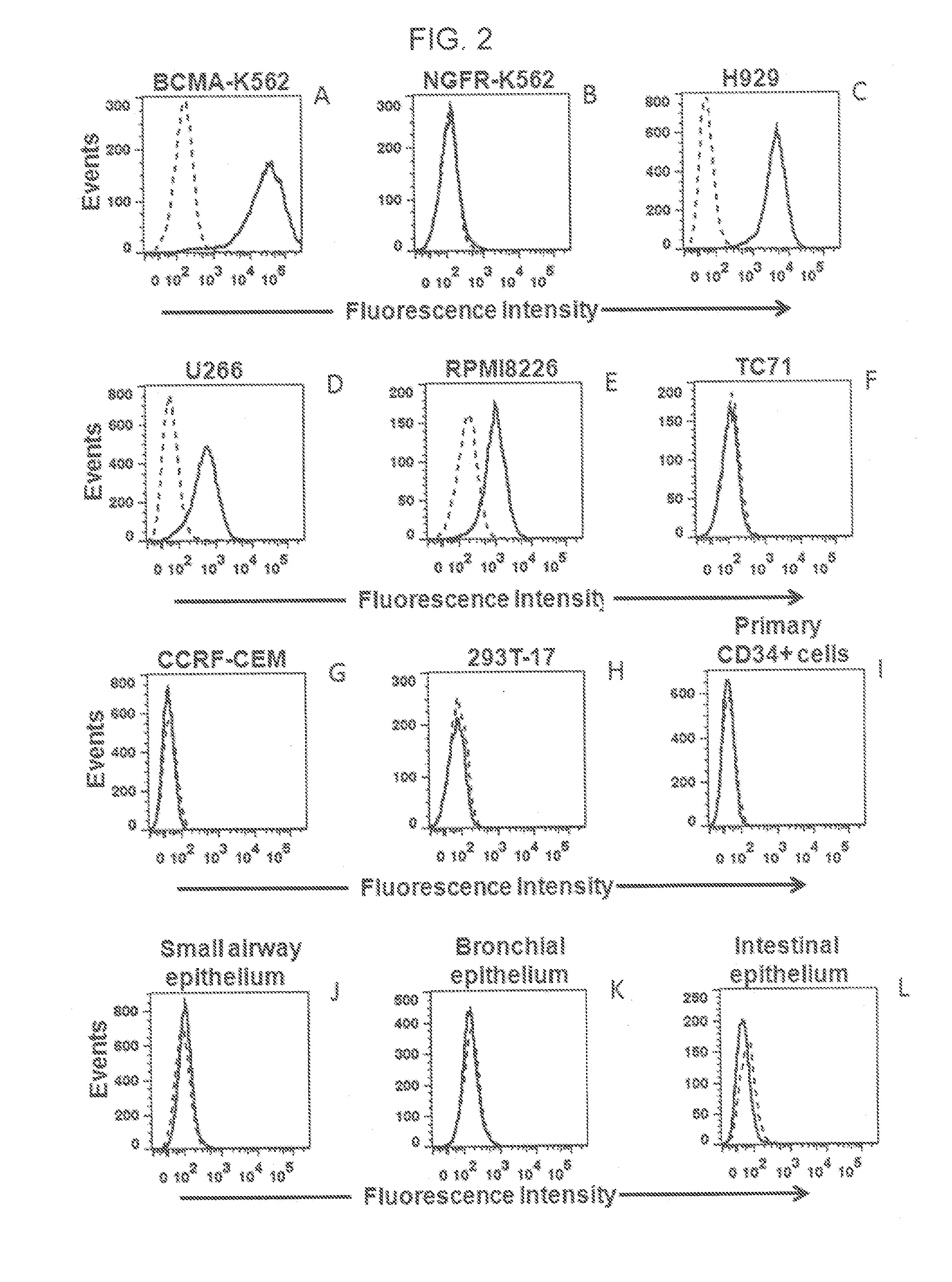
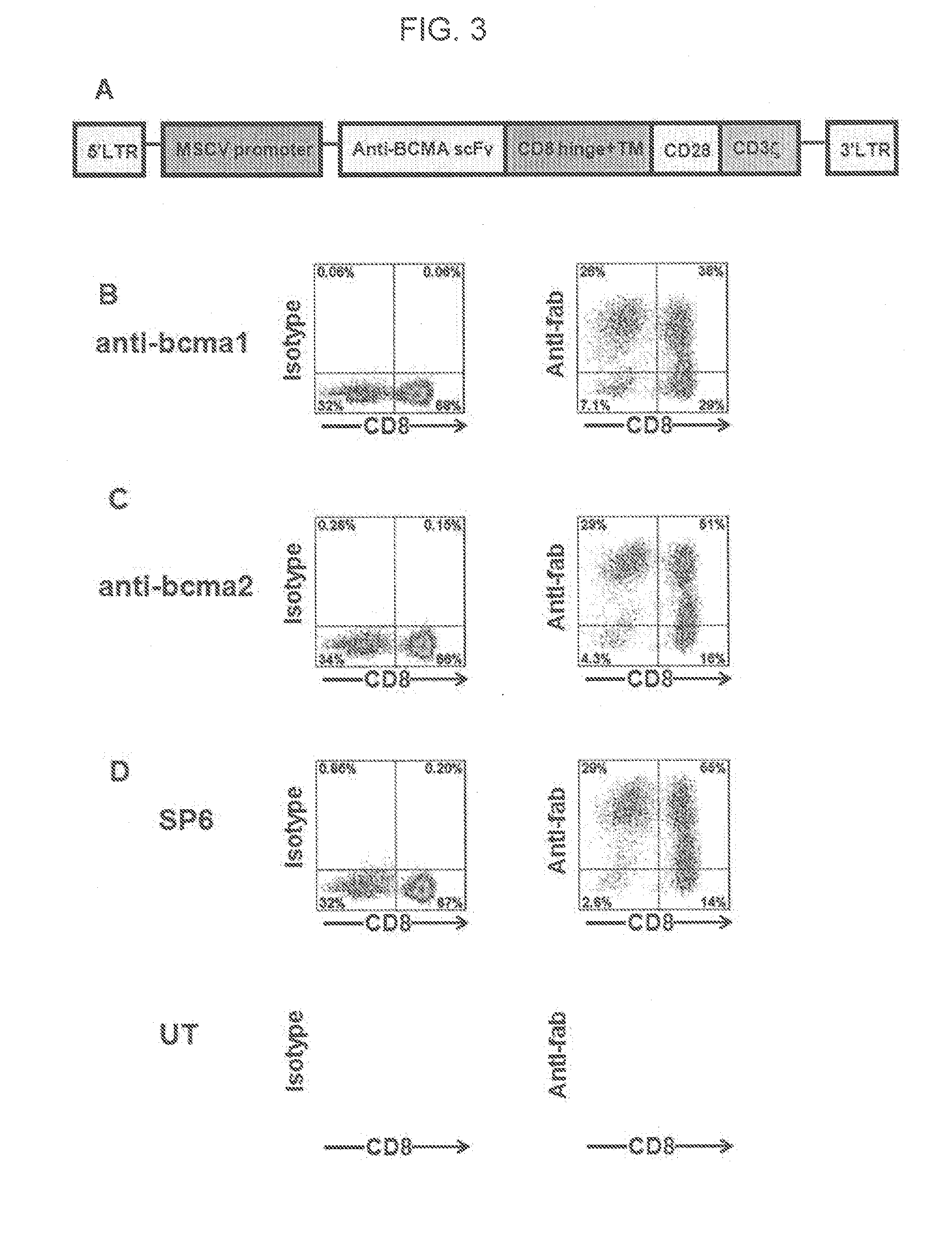
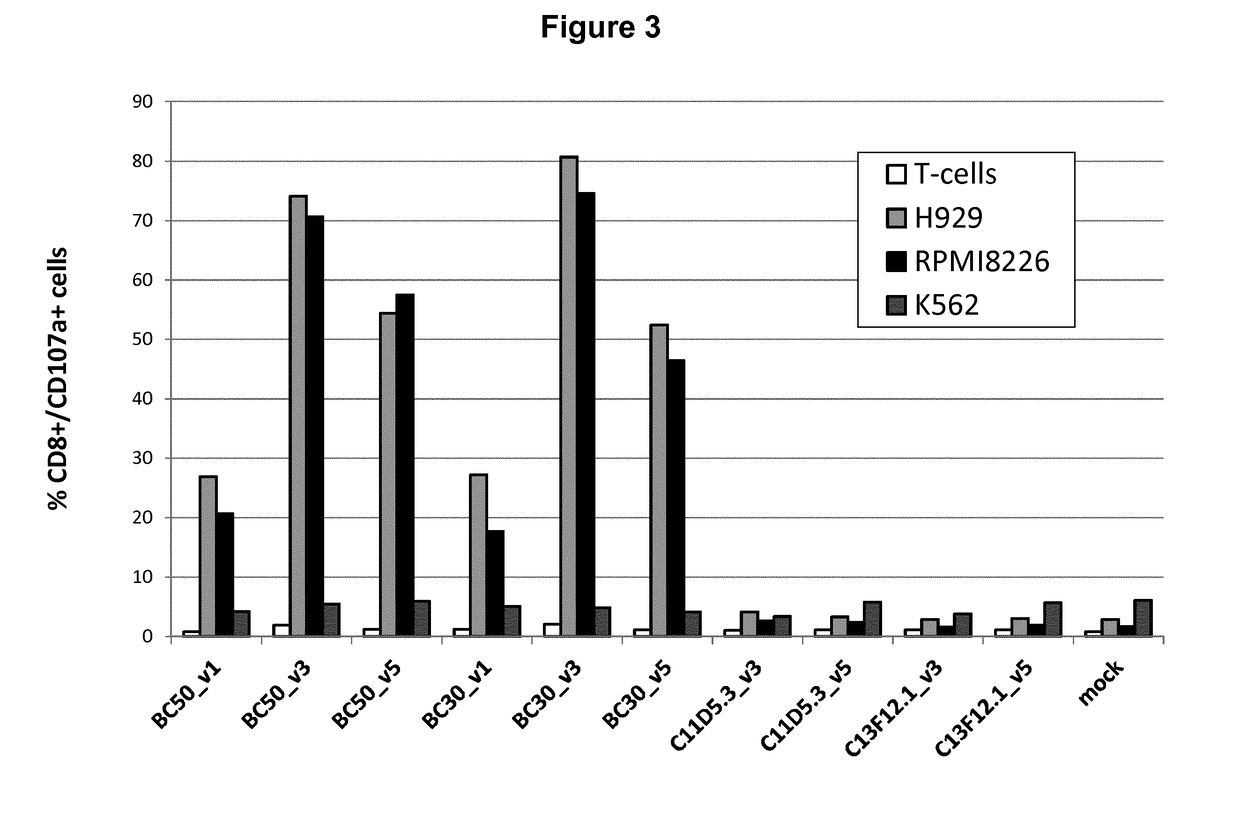
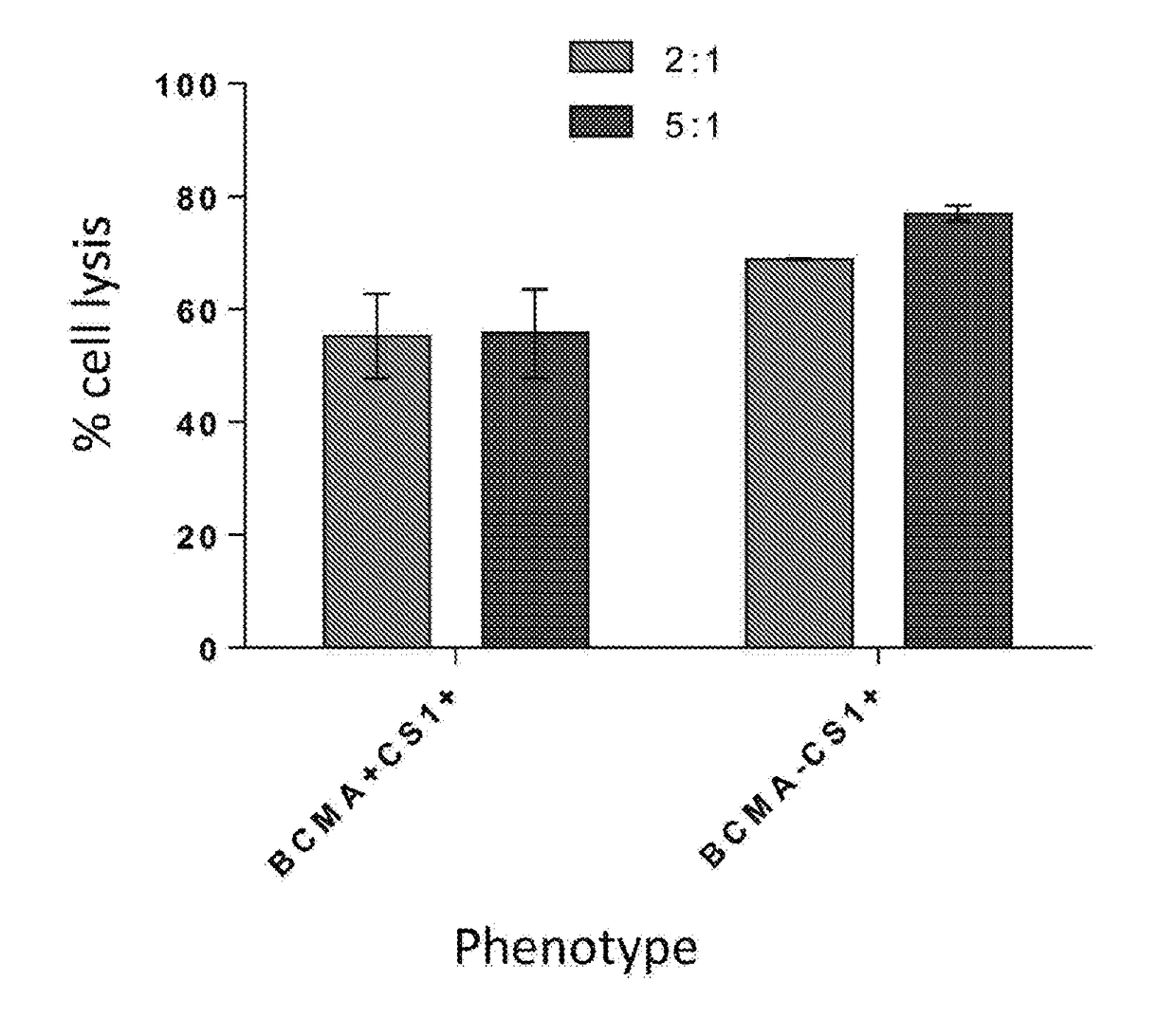
The invention provides an isolated and purified nucleic acid sequence encoding a chimeric antigen receptor (CAR) directed against B-cell Maturation Antigen (BCMA). The invention also provides host cells, such as T-cells or natural killer (NK) cells, expressing the CAR and methods for destroying multiple myeloma cells.
Owner:UNITED STATES OF AMERICA
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
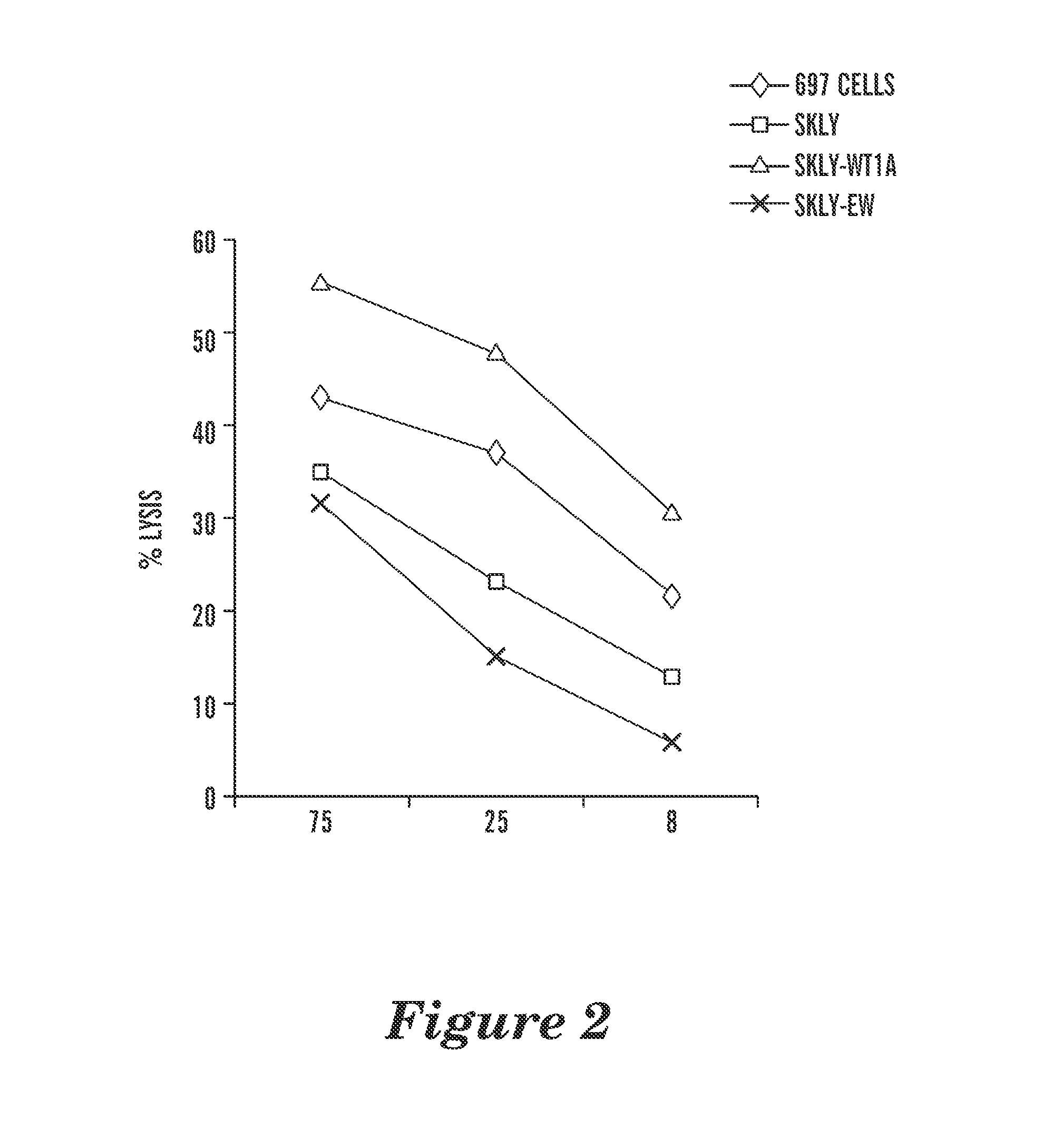
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
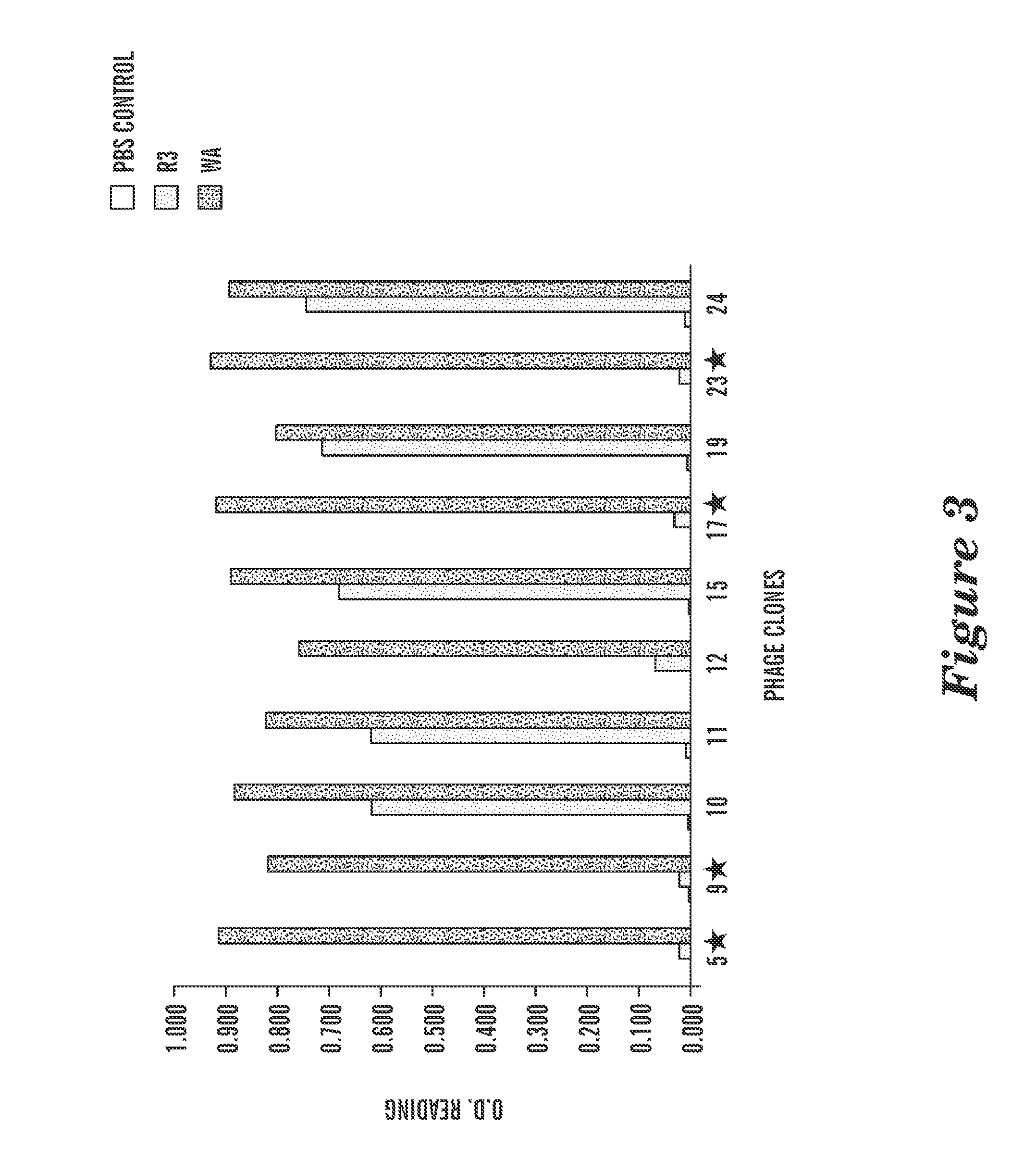
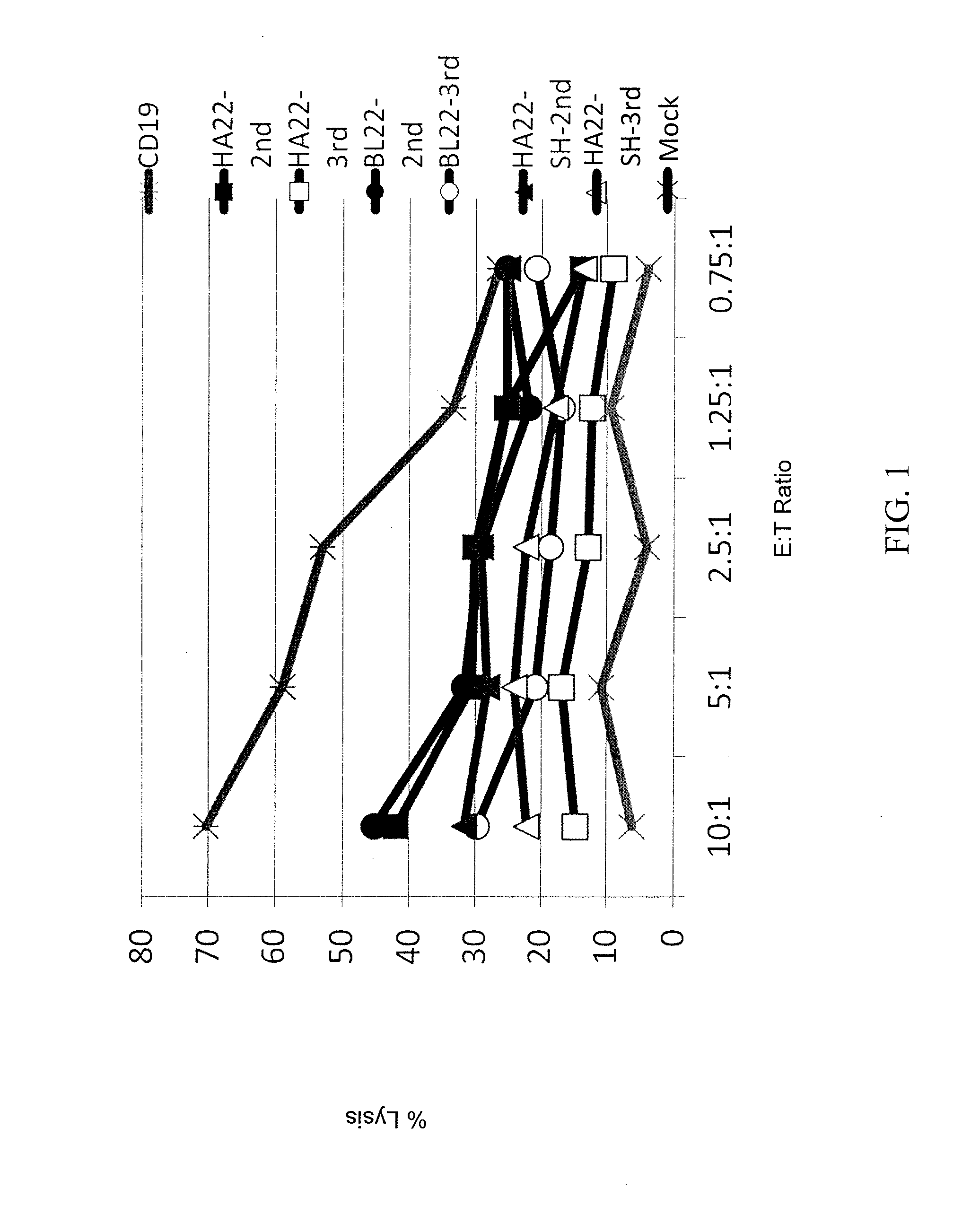
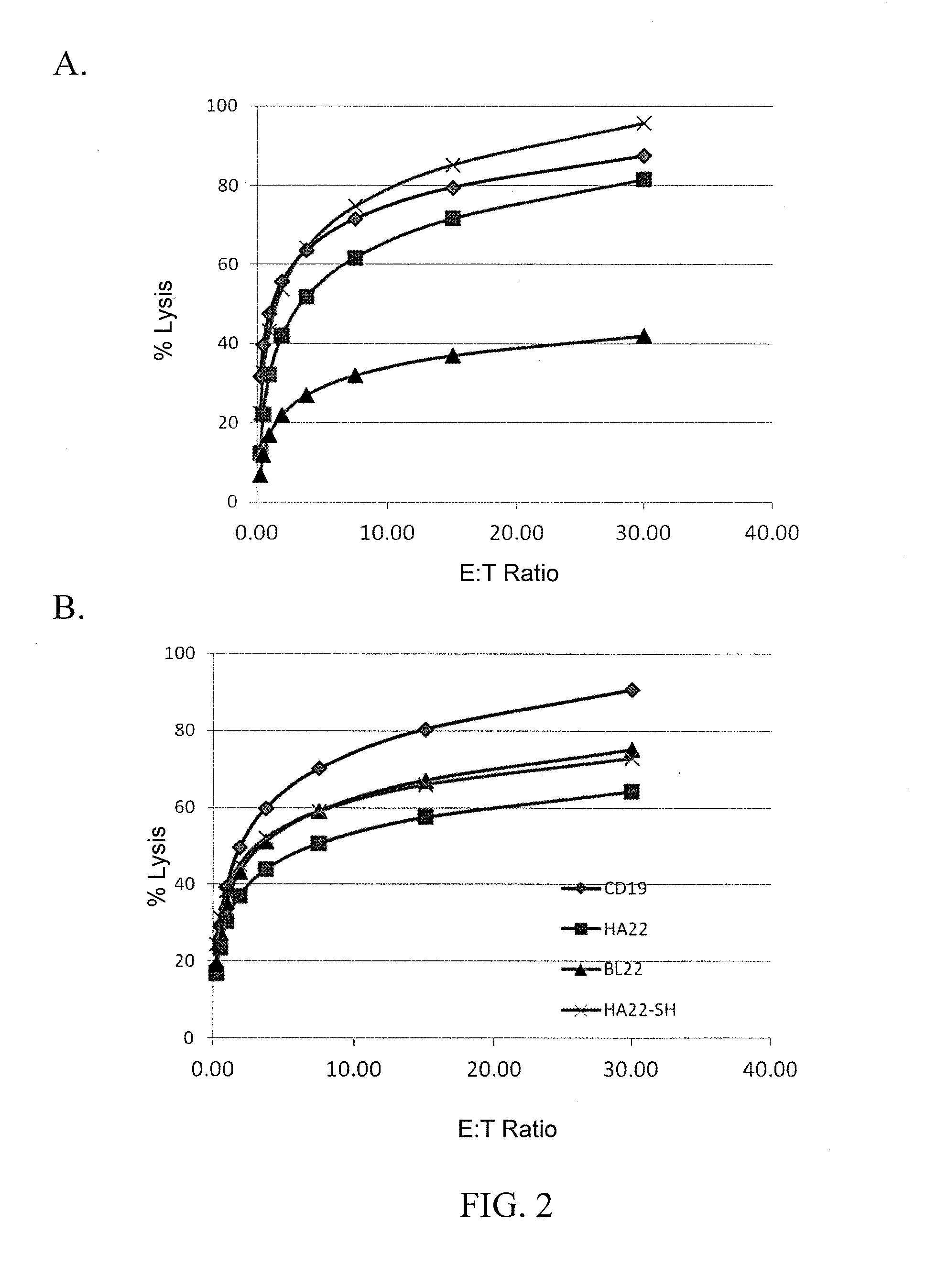
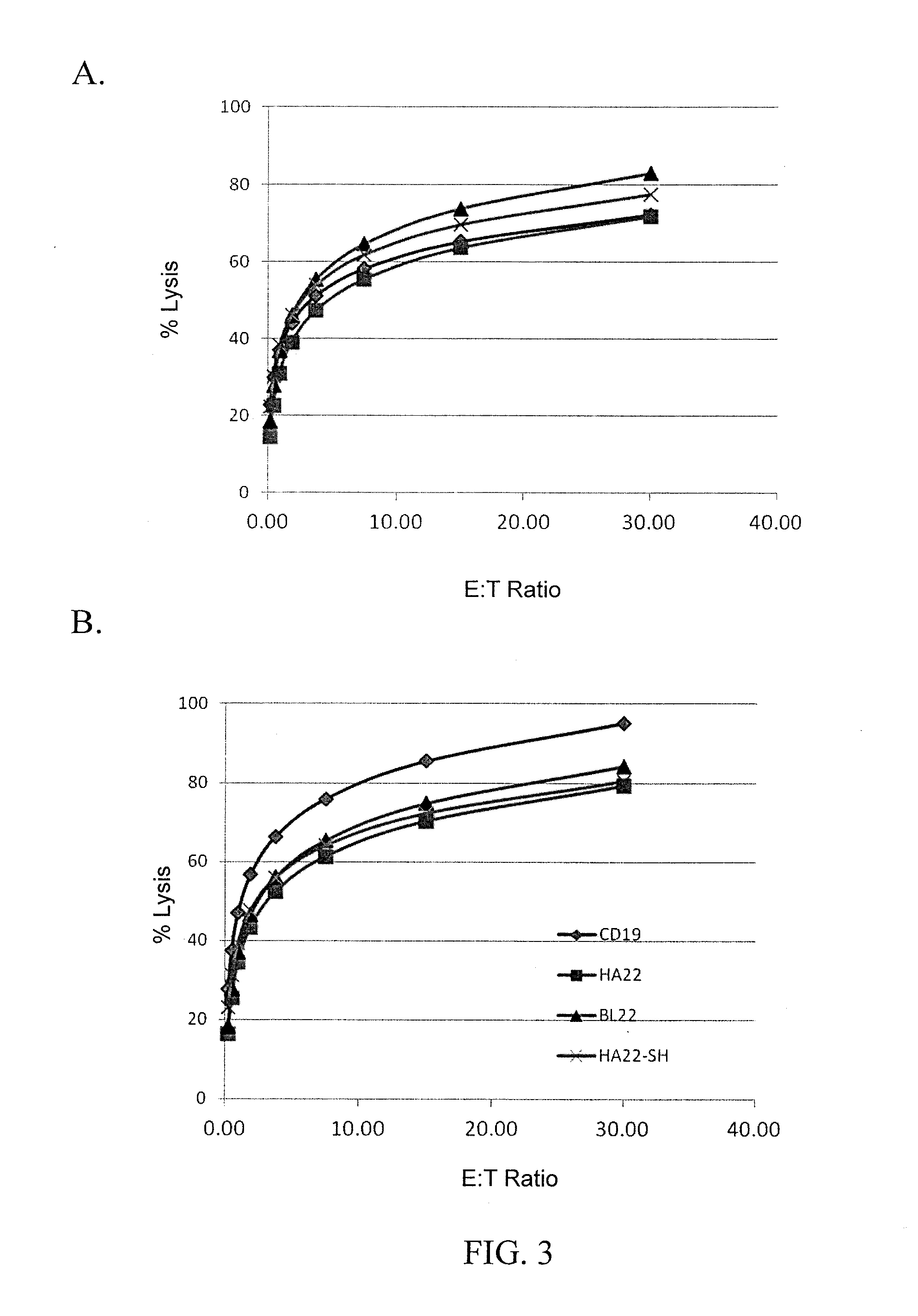
Anti-cd22 chimeric antigen receptors
The disclosure provides a chimeric antigen receptor (CAR) comprising a) an antigen binding domain of HA22, a transmembrane domain, and an intracellular T cell signaling domain; or b) an antigen binding domain of BL22, a transmembrane domain, and an intracellular T cell signaling domain comprising CD28 and / or CD137. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Disease therapy with chimeric antigen receptor (CAR) constructs and t cells (car-t) or nk cells (car-nk) expressing car constructs
InactiveUS20160361360A1Maintain self-toleranceModulate durationAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionDebulking Procedure
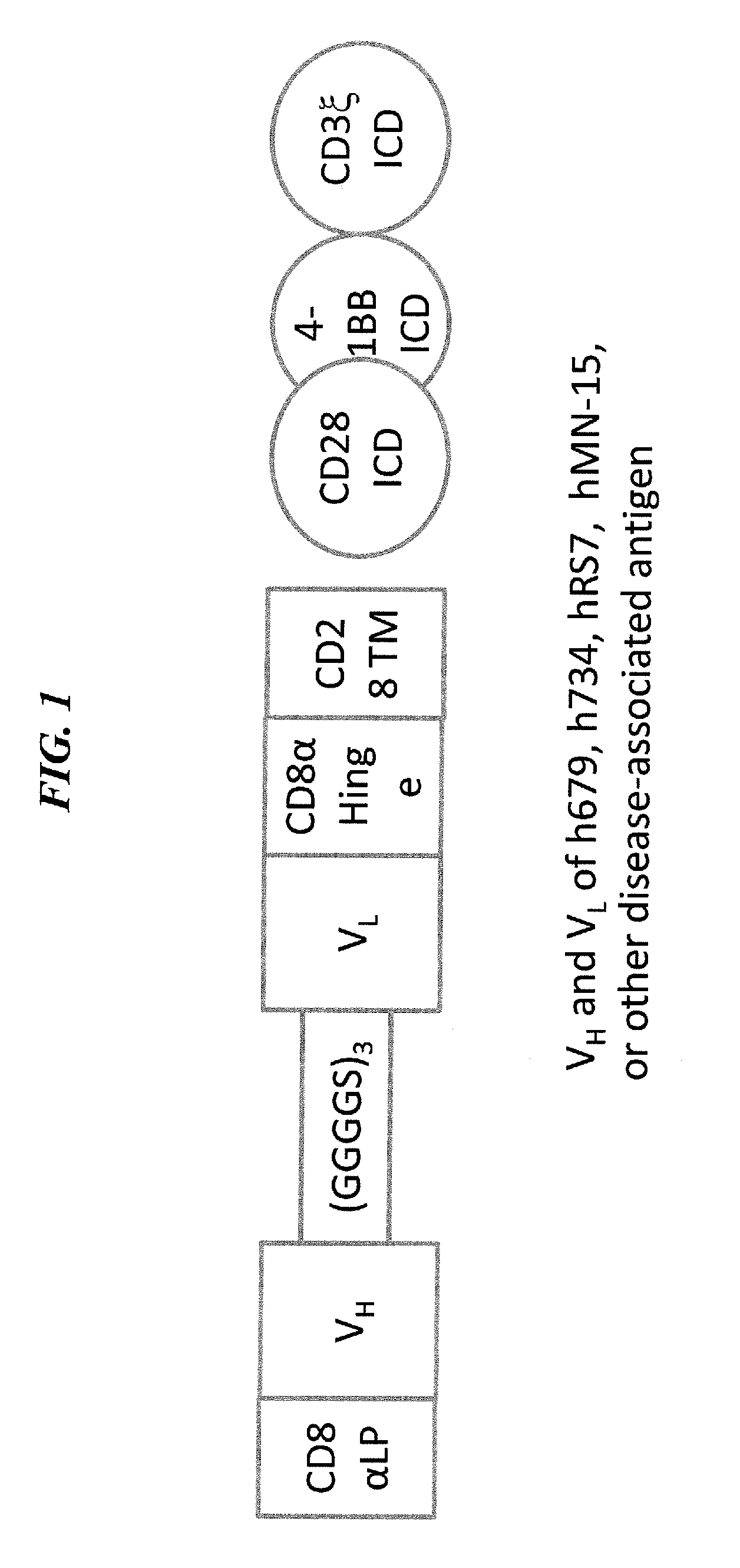
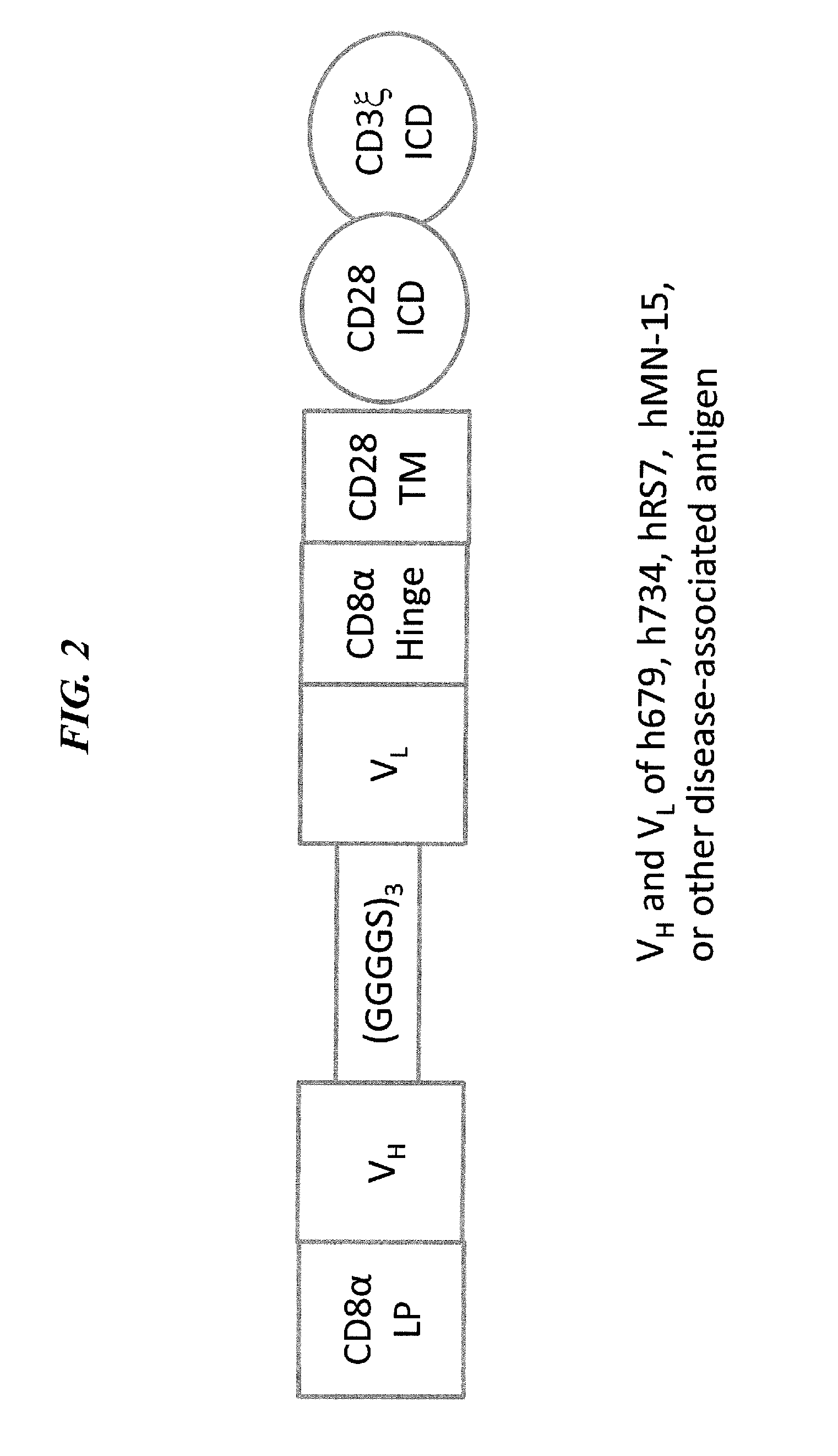
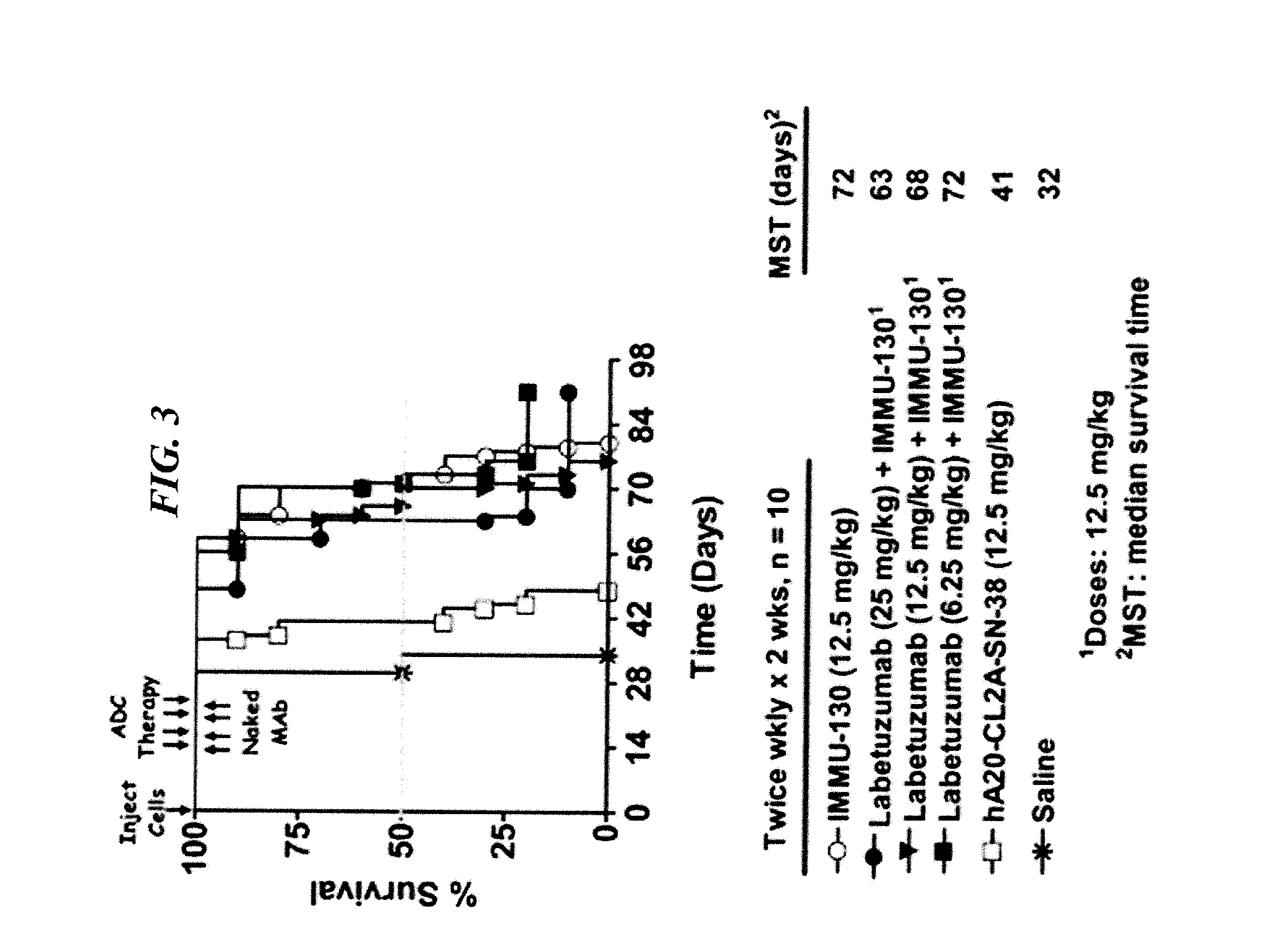
The present invention concerns CAR, CAR-T and CAR-NK constructs, preferably comprising a scFv antibody fragment against a disease-associated antigen or a hapten. More preferably, the antigen is a TAA, such as Trop-2. The constructs may be administered to a subject with a disease, such as cancer, autoimmune disease, or immune dysfunction disease, to induce an immune response against disease-associated cells. Where the constructs bind to a hapten, the subject is first treated with a hapten-conjugated antibody that binds to a disease associated antigen. Therapy may be supplemented by other treatments, such as debulking procedures (e.g., surgery, chemotherapy, radiation therapy) or coadministration of other agents. More preferably, administration of the construct is preceded by predosing with an unconjugated antibody that binds to the same disease-associated antigen. Most preferably, an antibody against CD74 or HLA-DR is administered to reduce systemic immunotoxicity induced by the constructs.
Owner:IMMUNOMEDICS INC
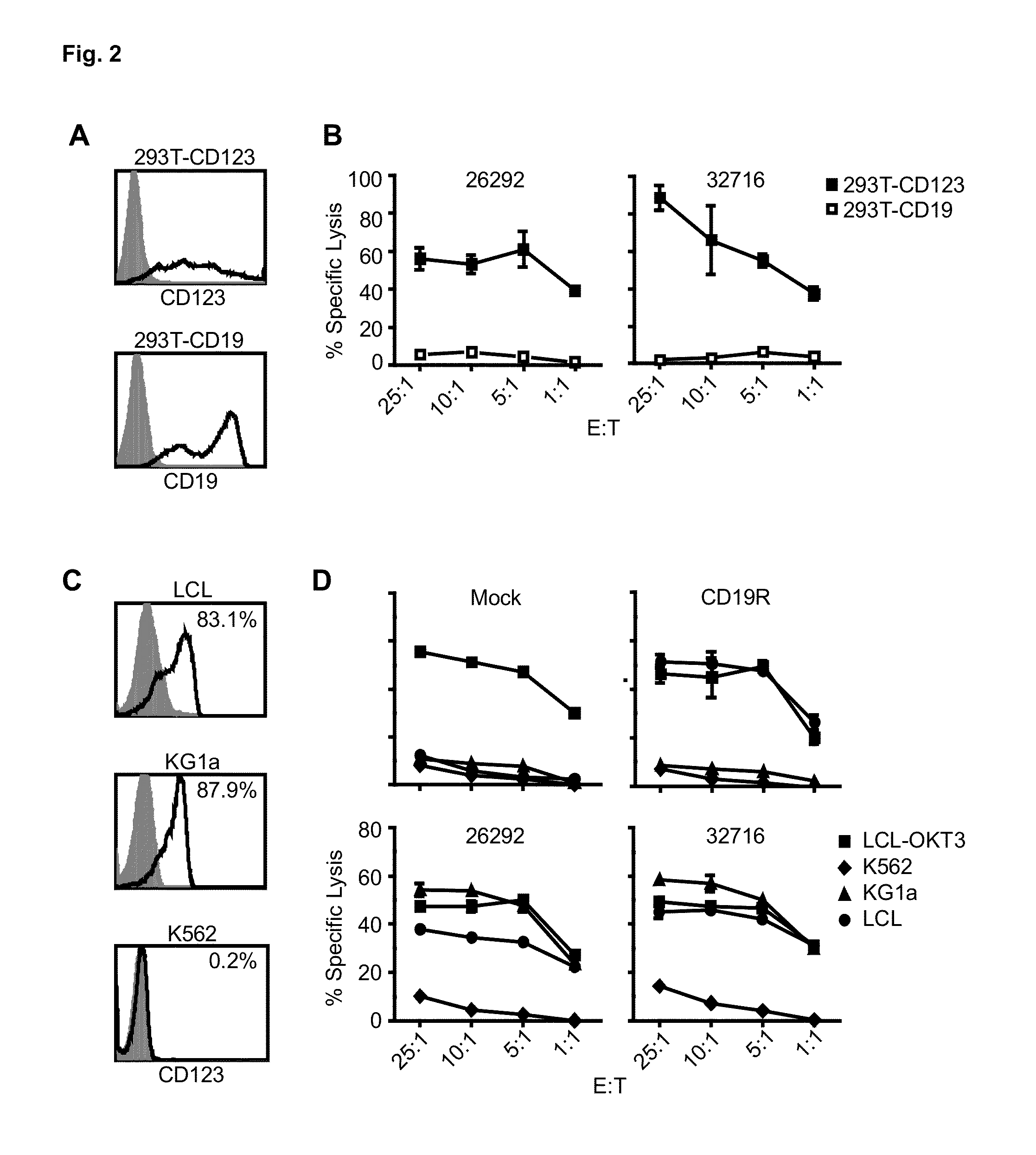
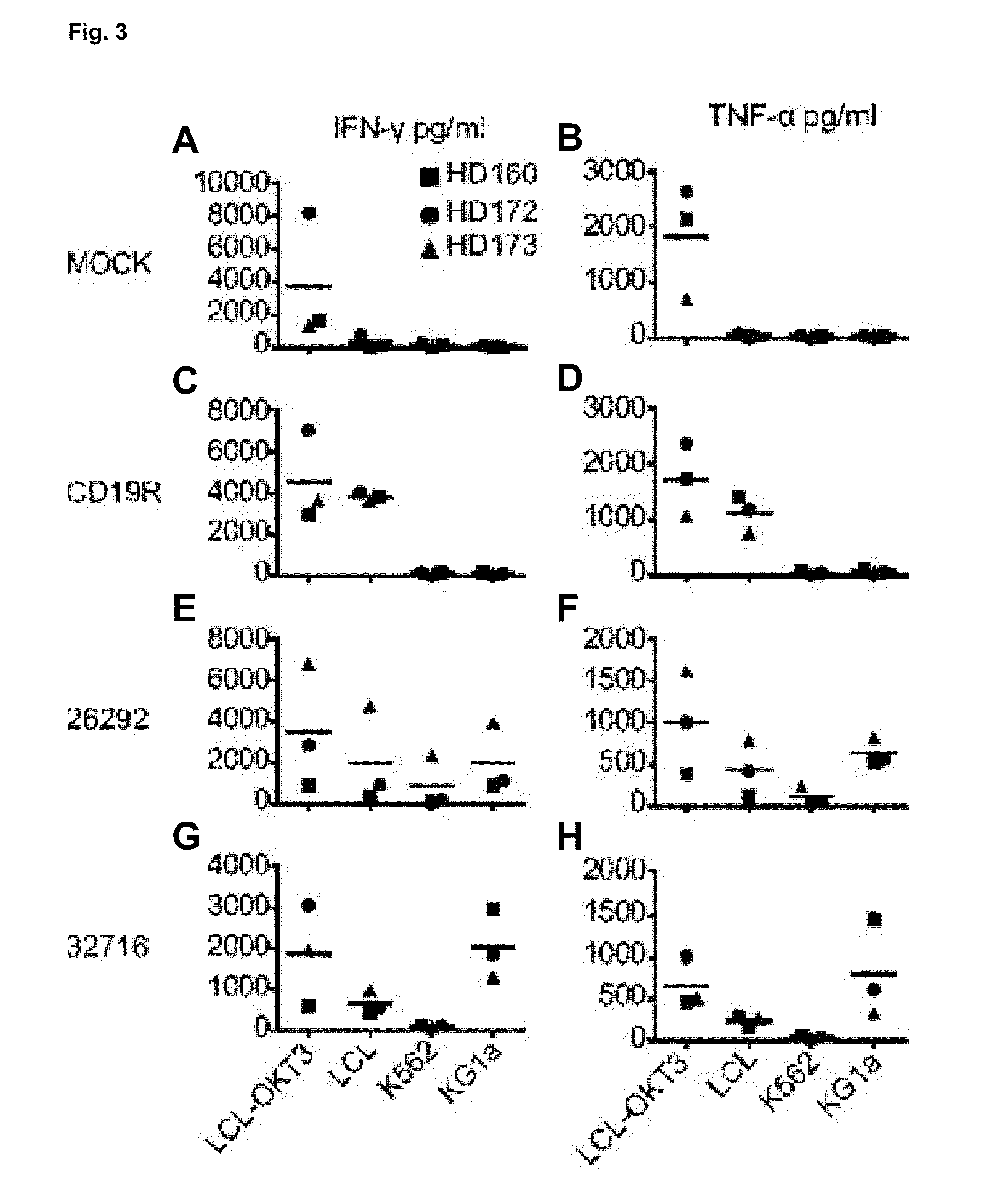
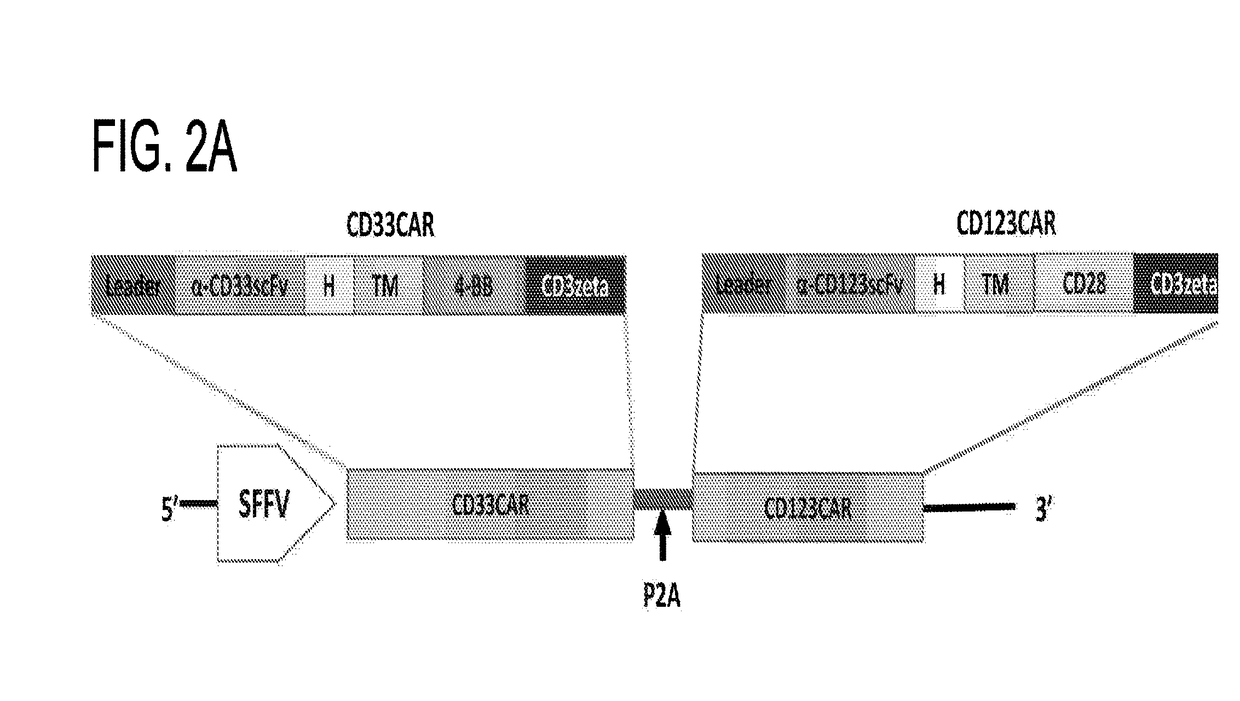
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
Chimeric antigen receptors targeting B-cell maturation antigen
ActiveUS9765342B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenAntigen receptors
The invention provides an isolated and purified nucleic acid sequence encoding a chimeric antigen receptor (CAR) directed against B-cell Maturation Antigen (BCMA). The invention also provides host cells, such as T-cells or natural killer (NK) cells, expressing the CAR and methods for destroying multiple myeloma cells.
Owner:UNITED STATES OF AMERICA
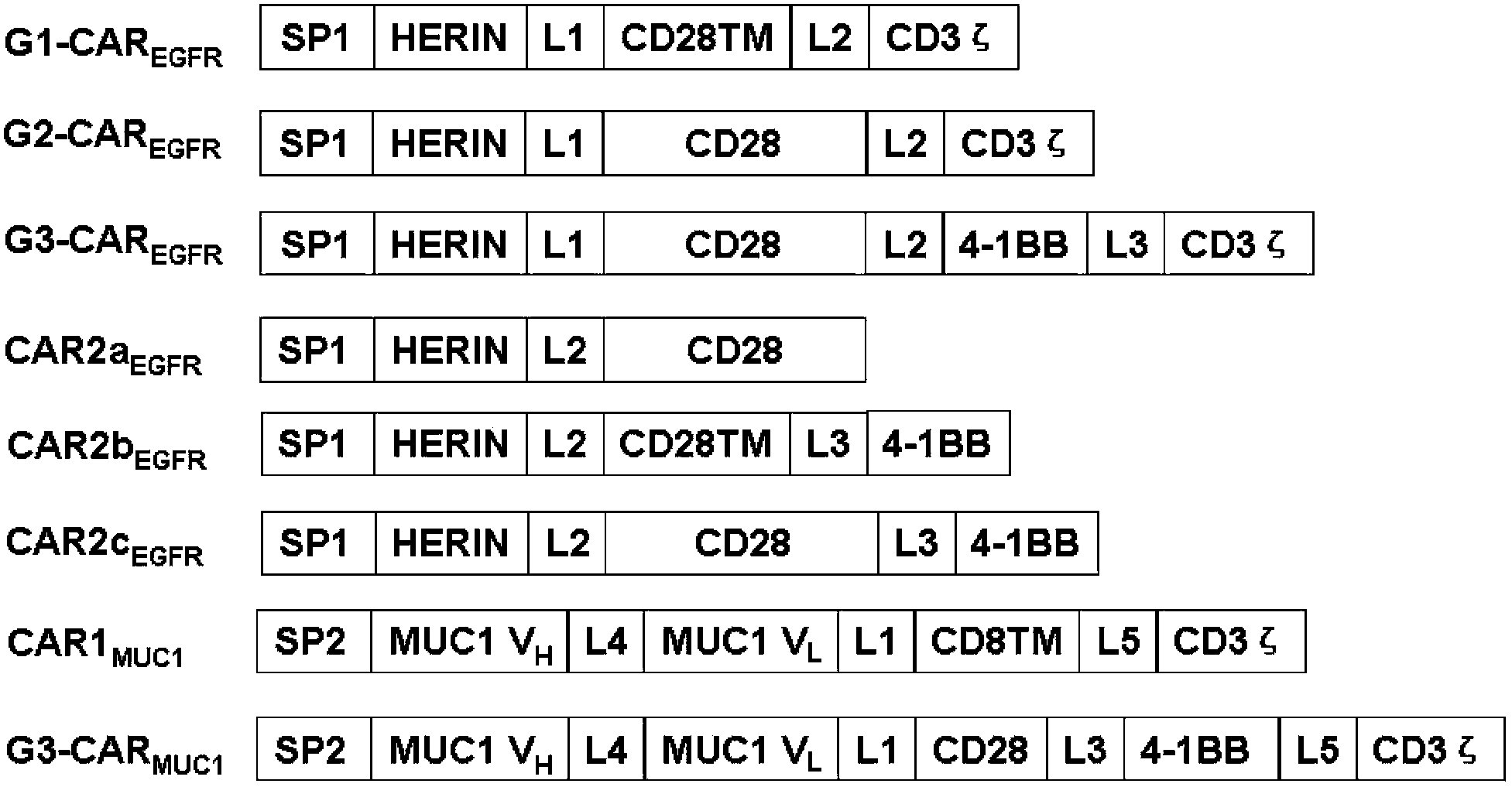
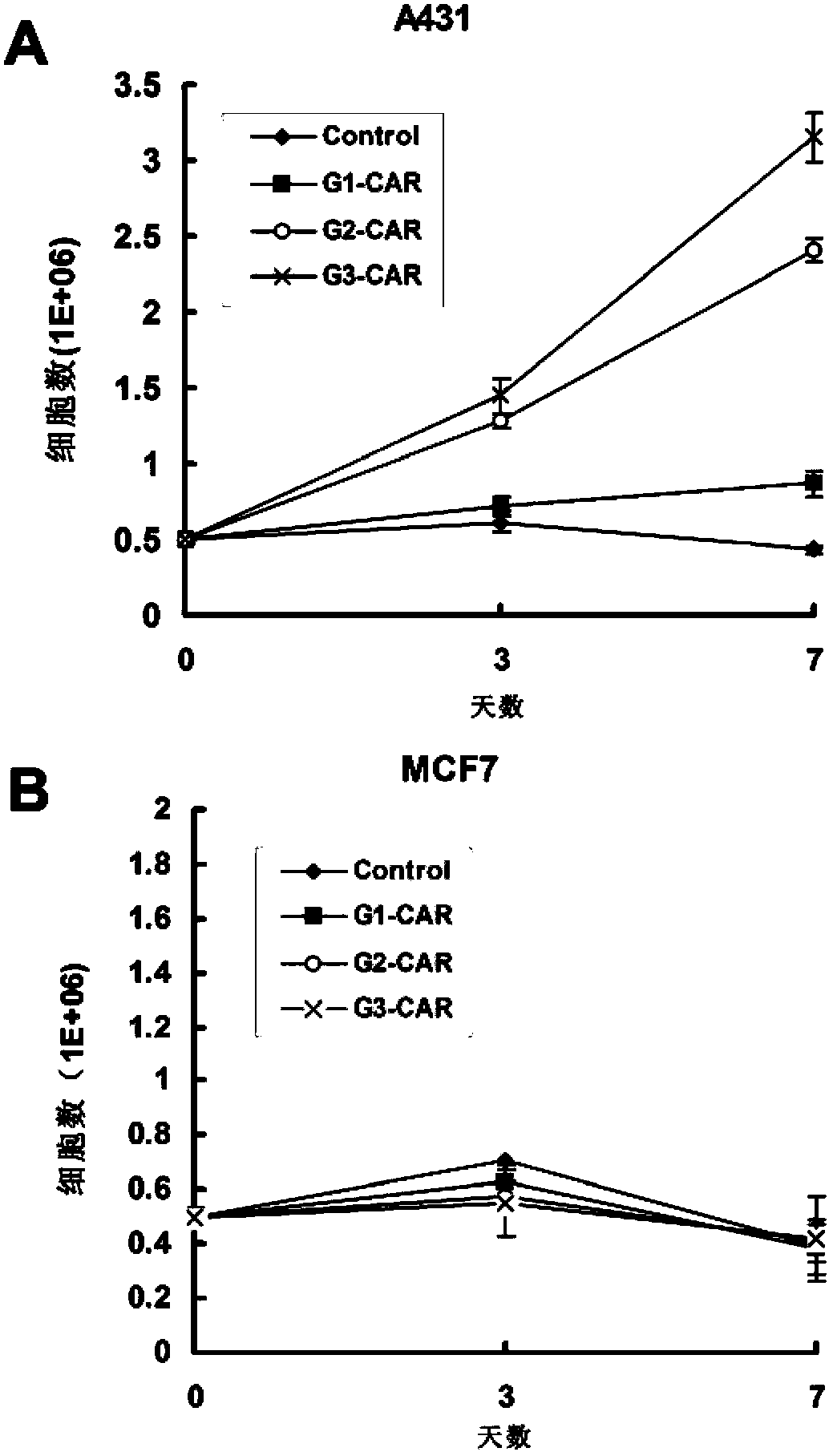
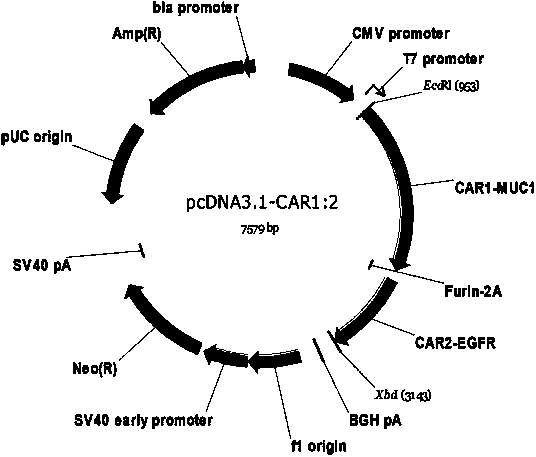
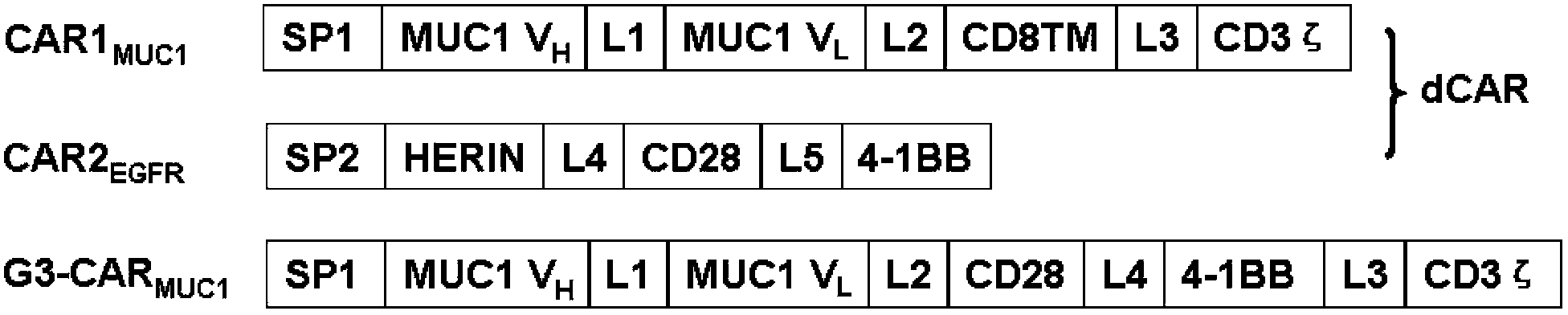
Chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and composition and uses thereof
ActiveCN103483453AFix security issuesImprove proliferative abilityPeptide/protein ingredientsGenetic material ingredientsEpidermal Growth Factor Receptor KinaseAntigen
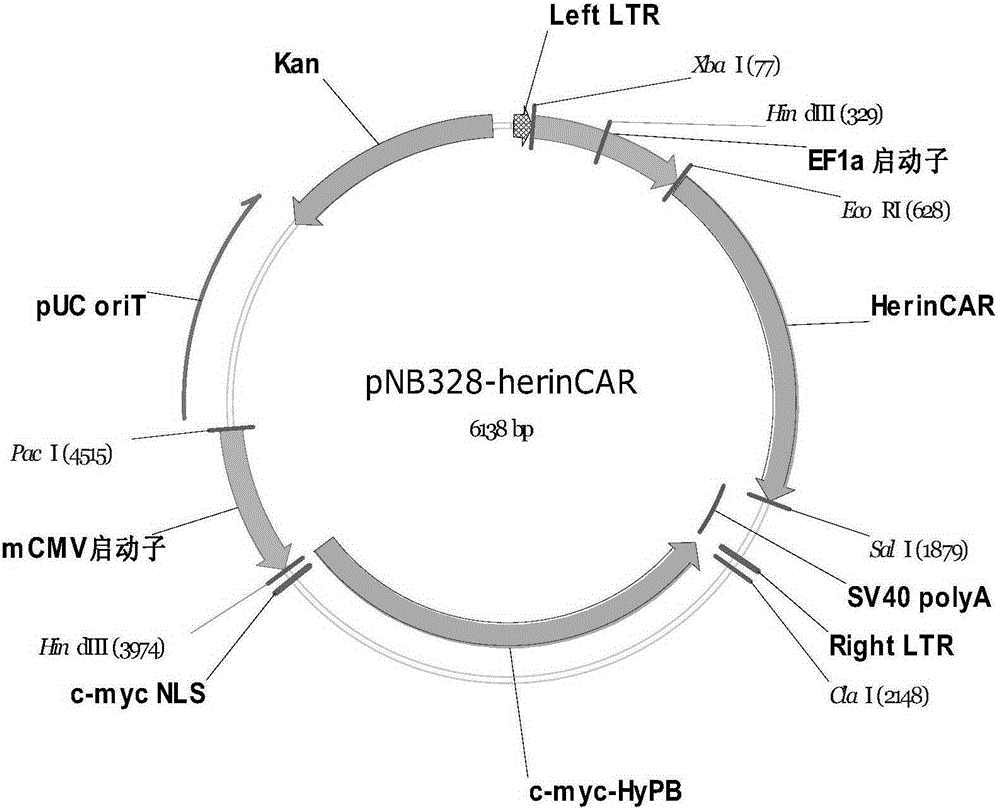
The present invention belongs to the fields of molecular biology and immunology and relates to a chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and a composition and uses thereof The present invention particularly relates to the chimeric antigen receptor comprising polypeptides efficiently combining the EGFR family proteins and transmembrane domains, wherein the polypeptides efficiently combining EGFR family proteins comprise HERIN. The chimeric antigen receptor can promote the proliferation ability and killing effect of T cell at the premise of maintaining the killing specificity of the T cell.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Antigen Receptor Variable Region Typing
A method of typing a variable region of a specific variant of an antigen receptor chain is disclosed. The method comprises: (a) exposing a probe set to a sense or antisense strand of a polynucleotide encoding at least a portion of the variable region of the specific variant of the antigen receptor chain, wherein the probe set includes a plurality of probe molecules, wherein each probe molecule of the plurality of probe molecules is substantially complementary to a sense or antisense strand of a nucleic acid sequence region of a specific polynucleotide encoding a variant of the antigen receptor chain, the nucleic acid sequence region encoding a specific combination of at least two variable region segments of the antigen receptor chain; and (b) measuring a hybridization of each probe molecule of the plurality of probe molecules with the sense or antisense strand of the nucleic acid sequence region of the polynucleotide encoding at least a portion of the variable region of the specific variant of the antigen receptor chain, thereby typing the variable region of the specific variant of the antigen receptor chain.
Owner:YEDA RES & DEV CO LTD
Bispecific chimeric antigen receptors and encoding polynucleotides thereof
ActiveUS9447194B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellularAntigen receptors
The invention is directed to a bispecific chimeric antigen receptor, comprising: (a) at least two antigen-specific targeting regions; (b) an extracellular spacer domain; (c) a transmembrane domain; (d) at least one co-stimulatory domain; and (e) an intracellular signaling domain, wherein each antigen-specific targeting region comprises an antigen-specific single chain Fv (scFv) fragment, and binds a different antigen, and wherein the bispecific chimeric antigen receptor is co-expressed with a therapeutic control. The invention also provides methods and uses of the bispecific chimeric antigen receptors.
Owner:SEATTLE CHILDRENS HOSPITAL
Isolated monoclonal antibodies that specifically bind to human Claudin 18.2
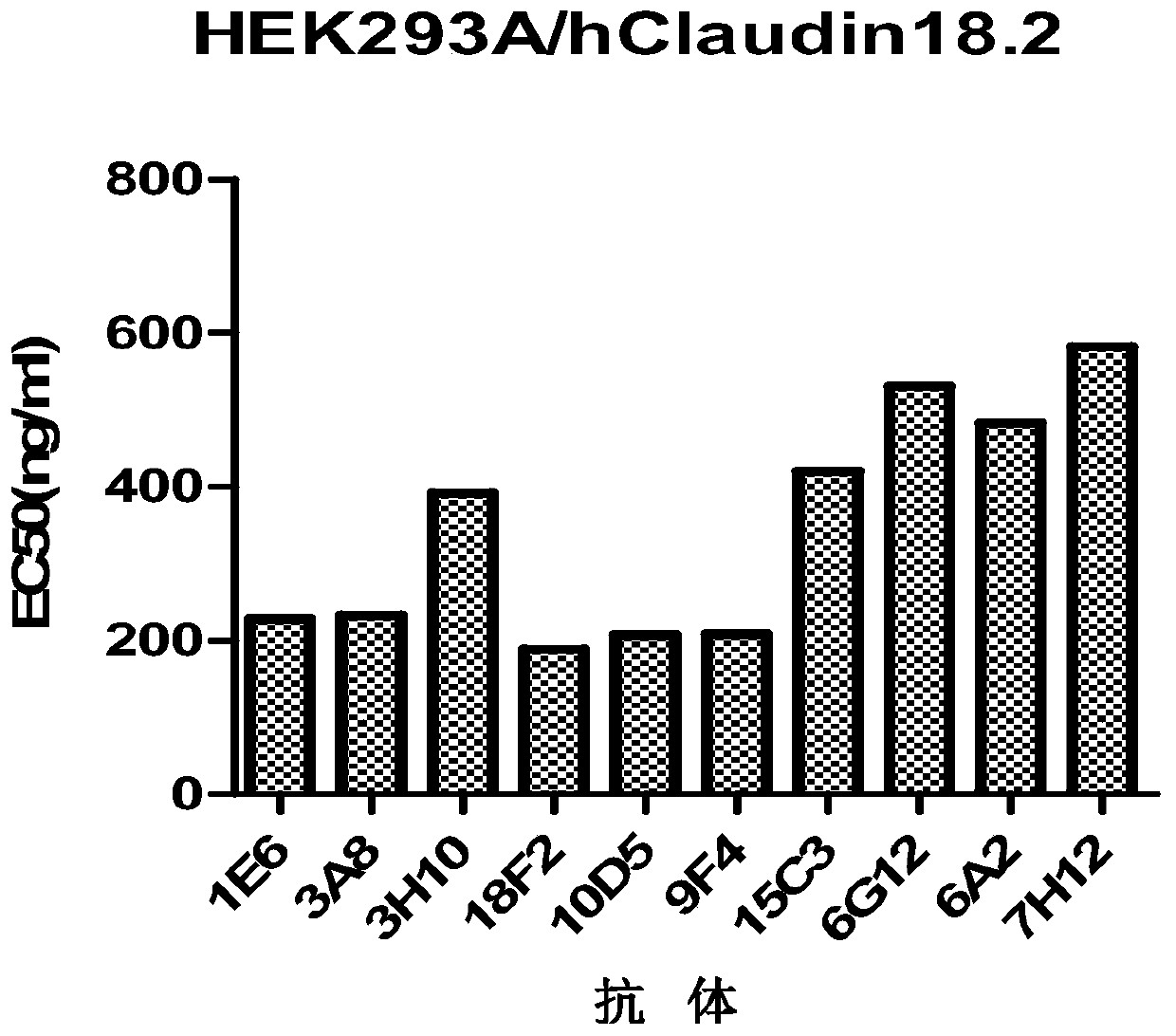
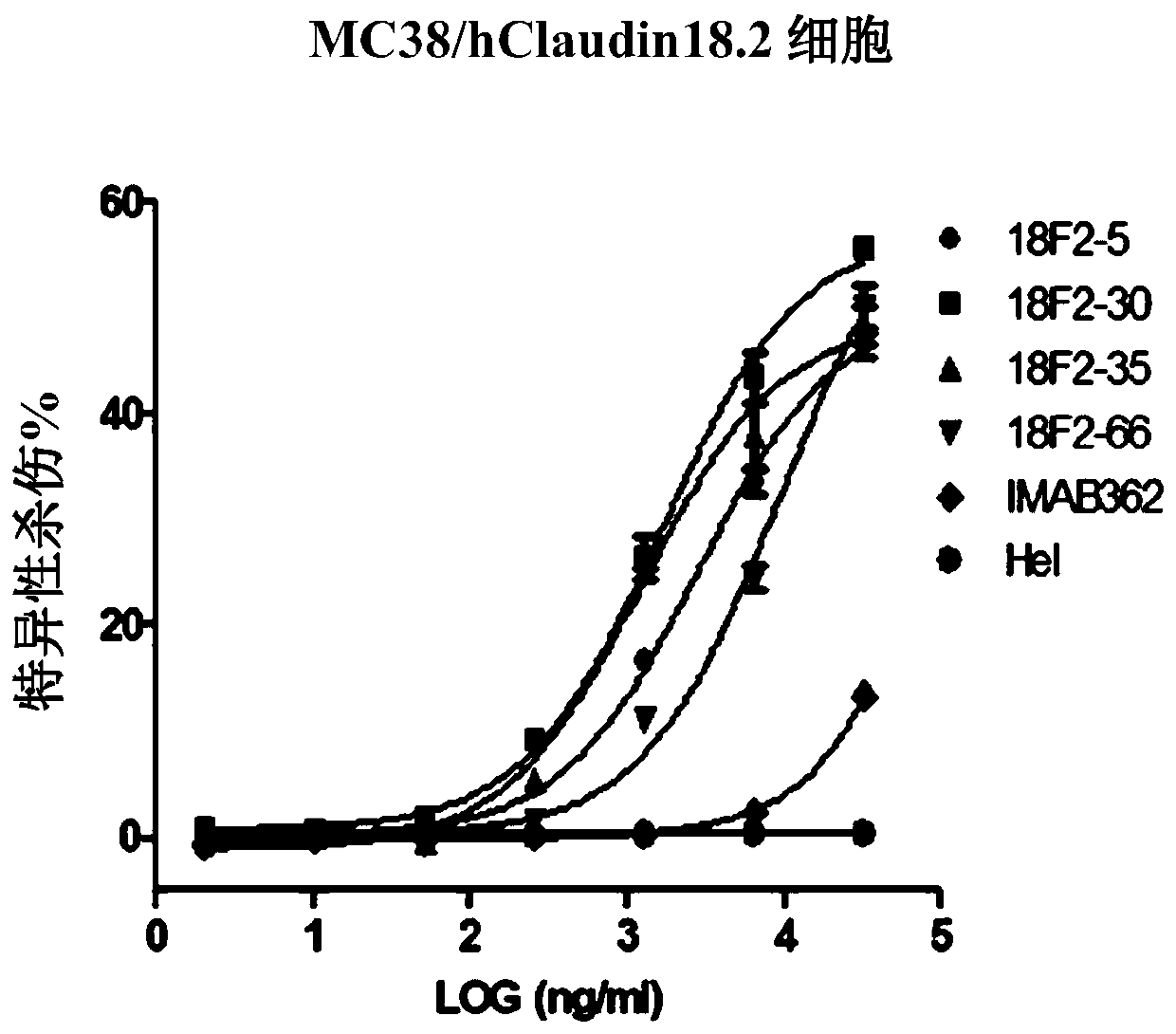
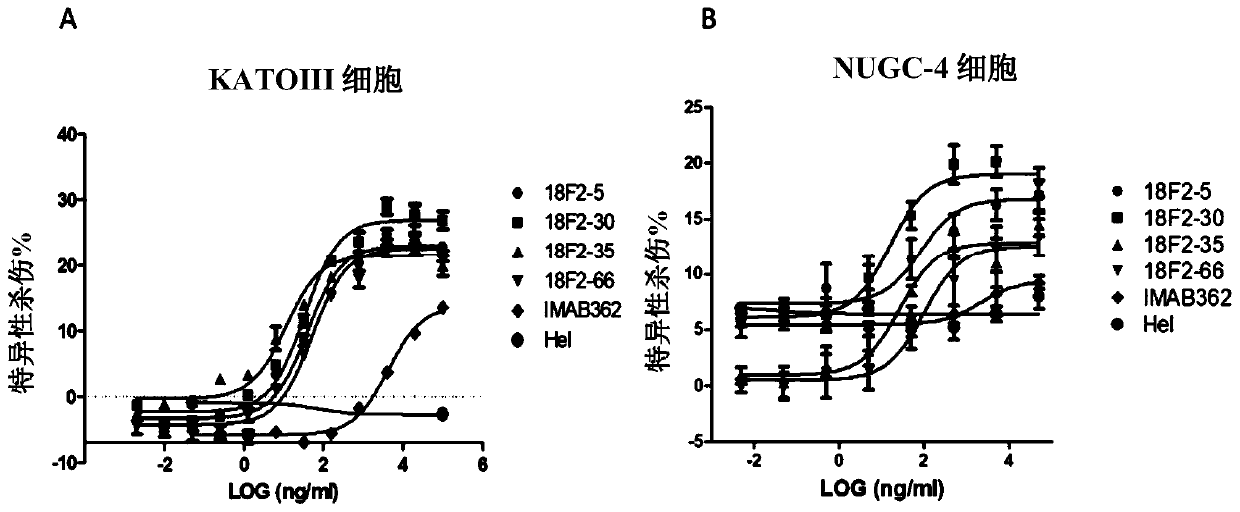
ActiveCN109762067AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonal antibody 14G2AAntigen receptors
Owner:BEIJING MABWORKS BIOTECH
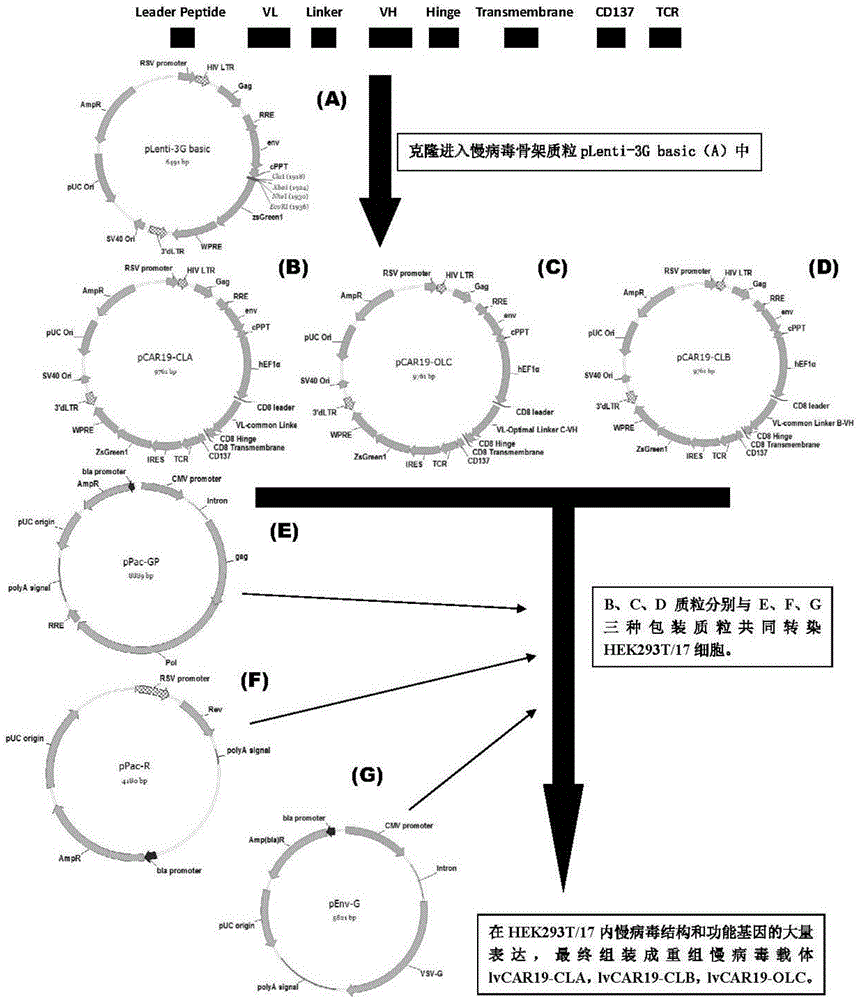
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
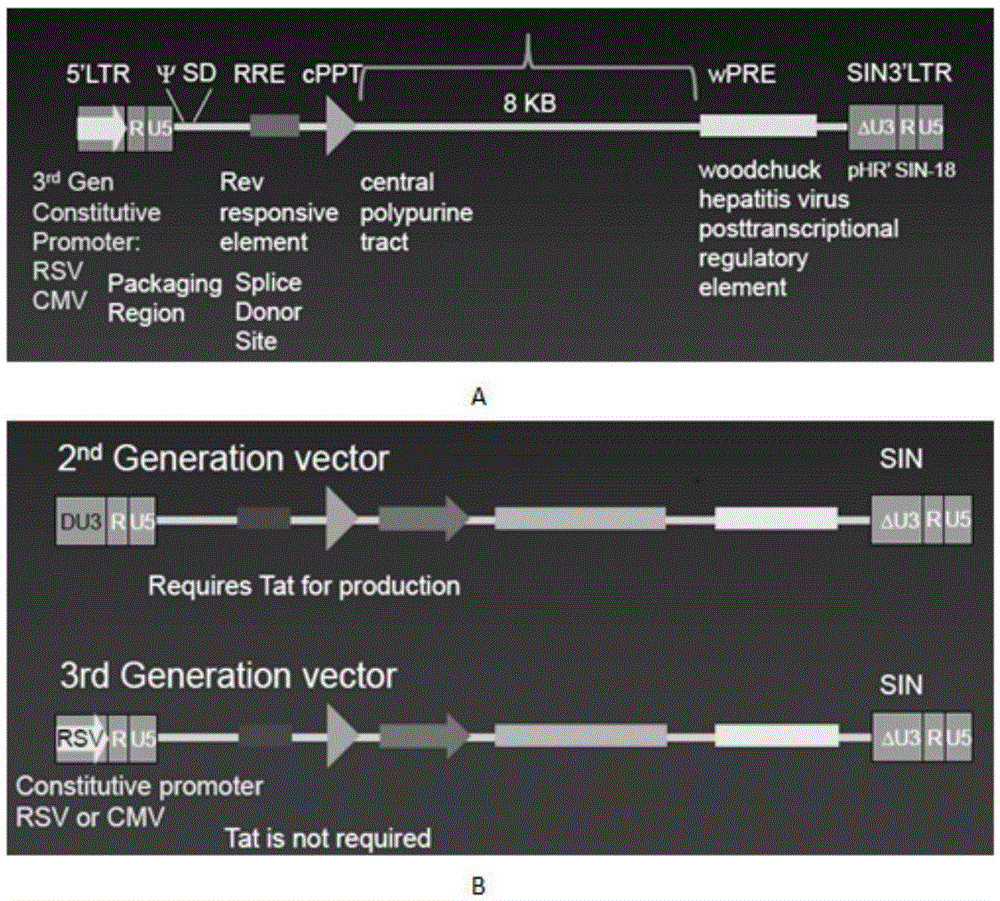
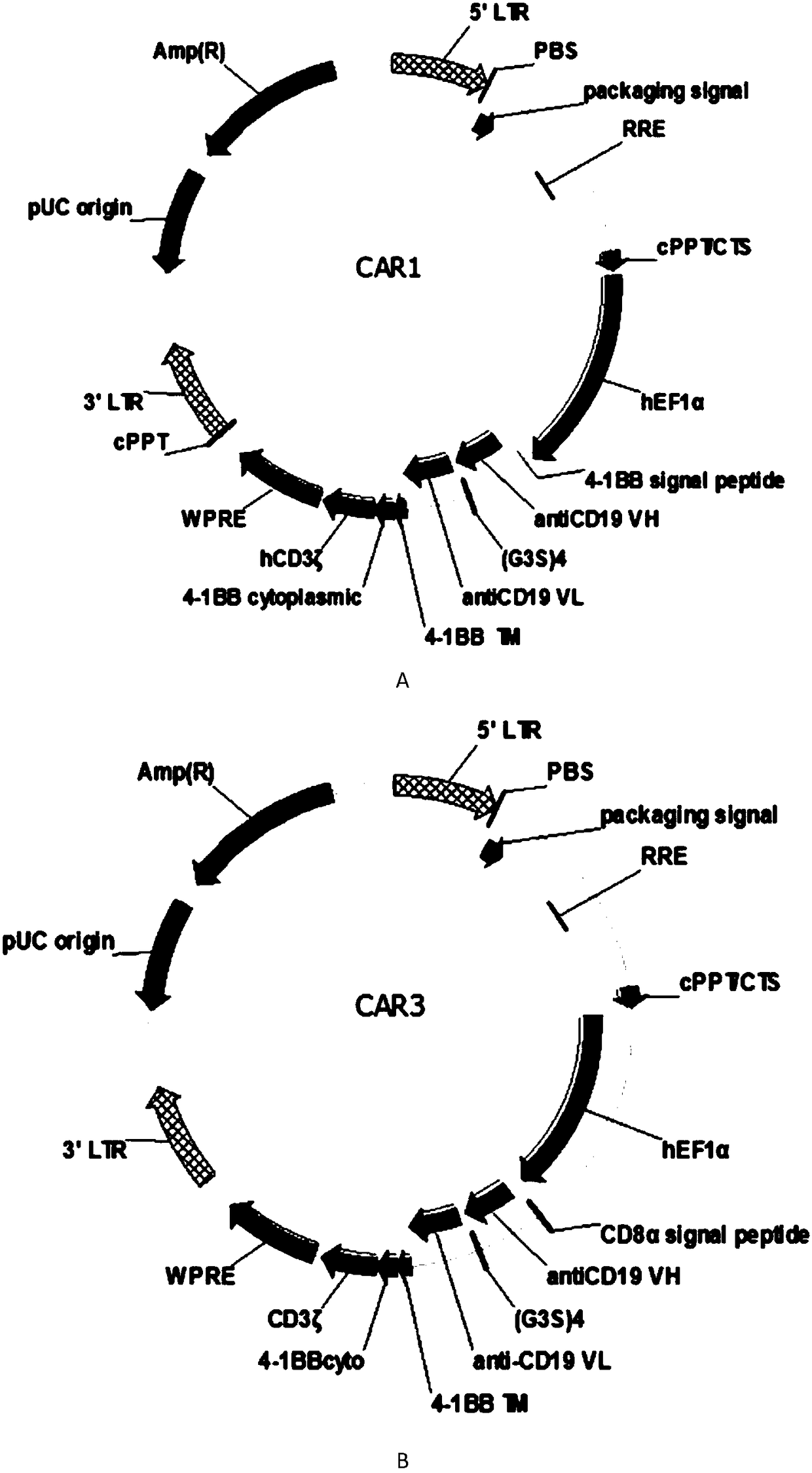
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
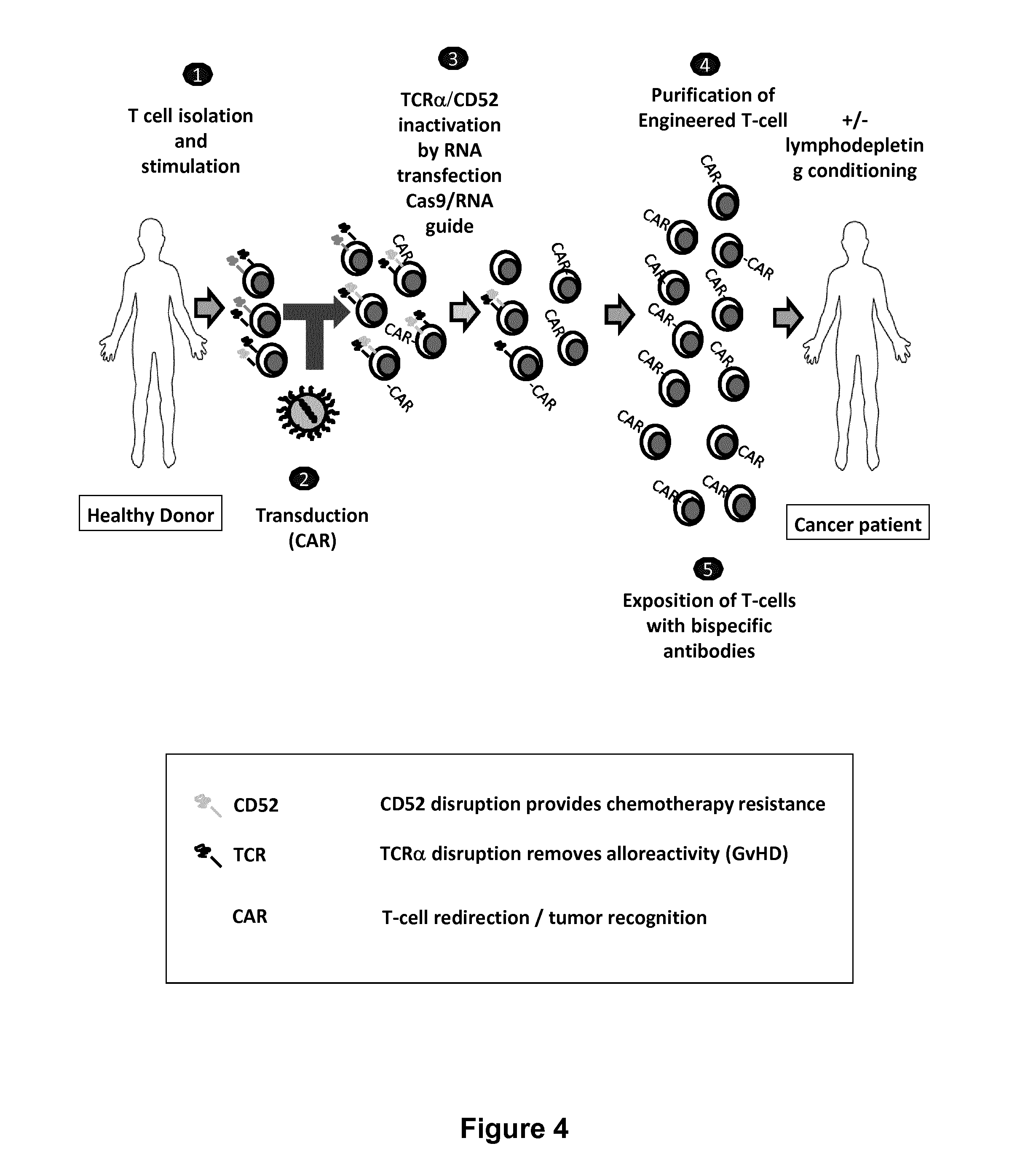
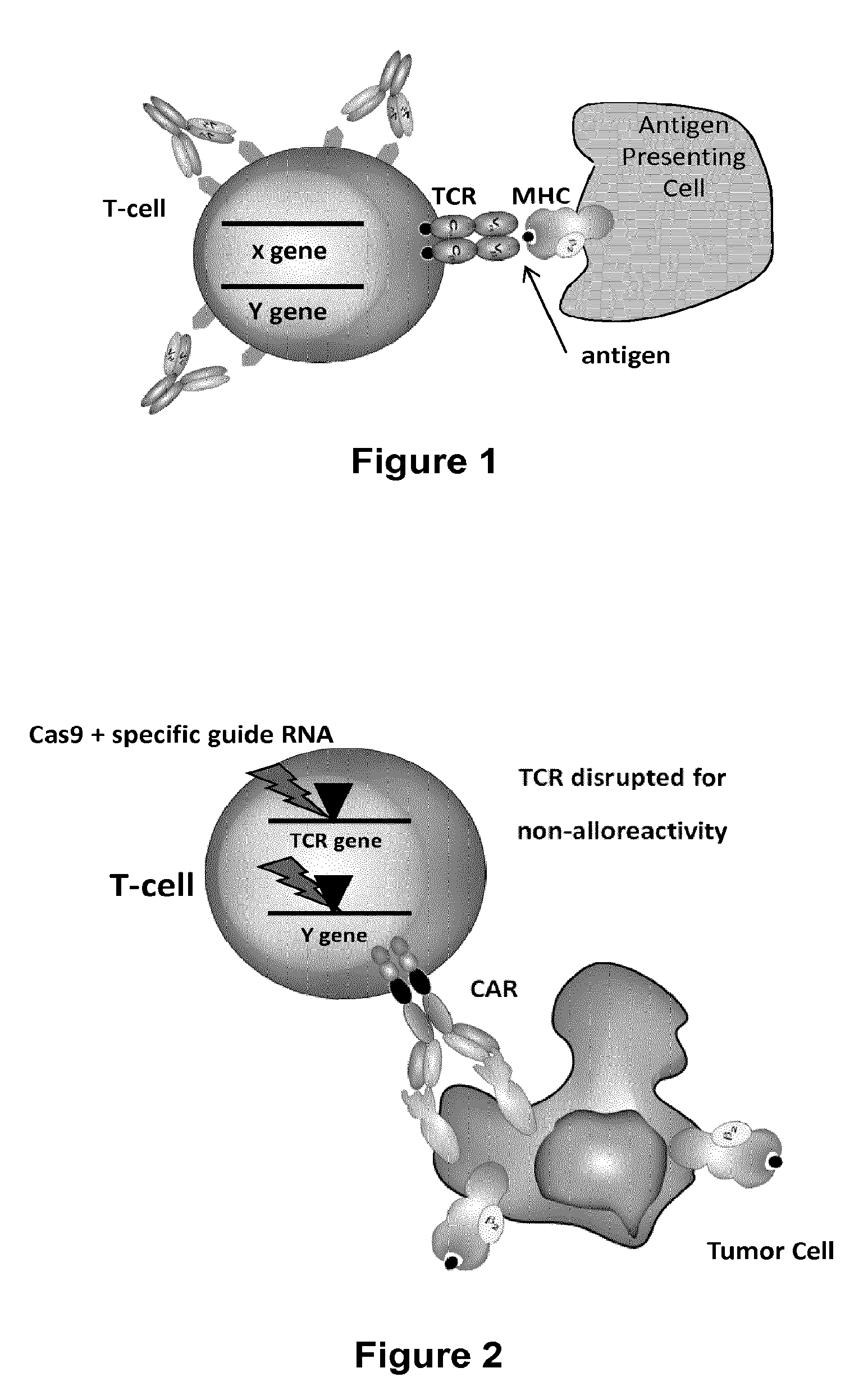
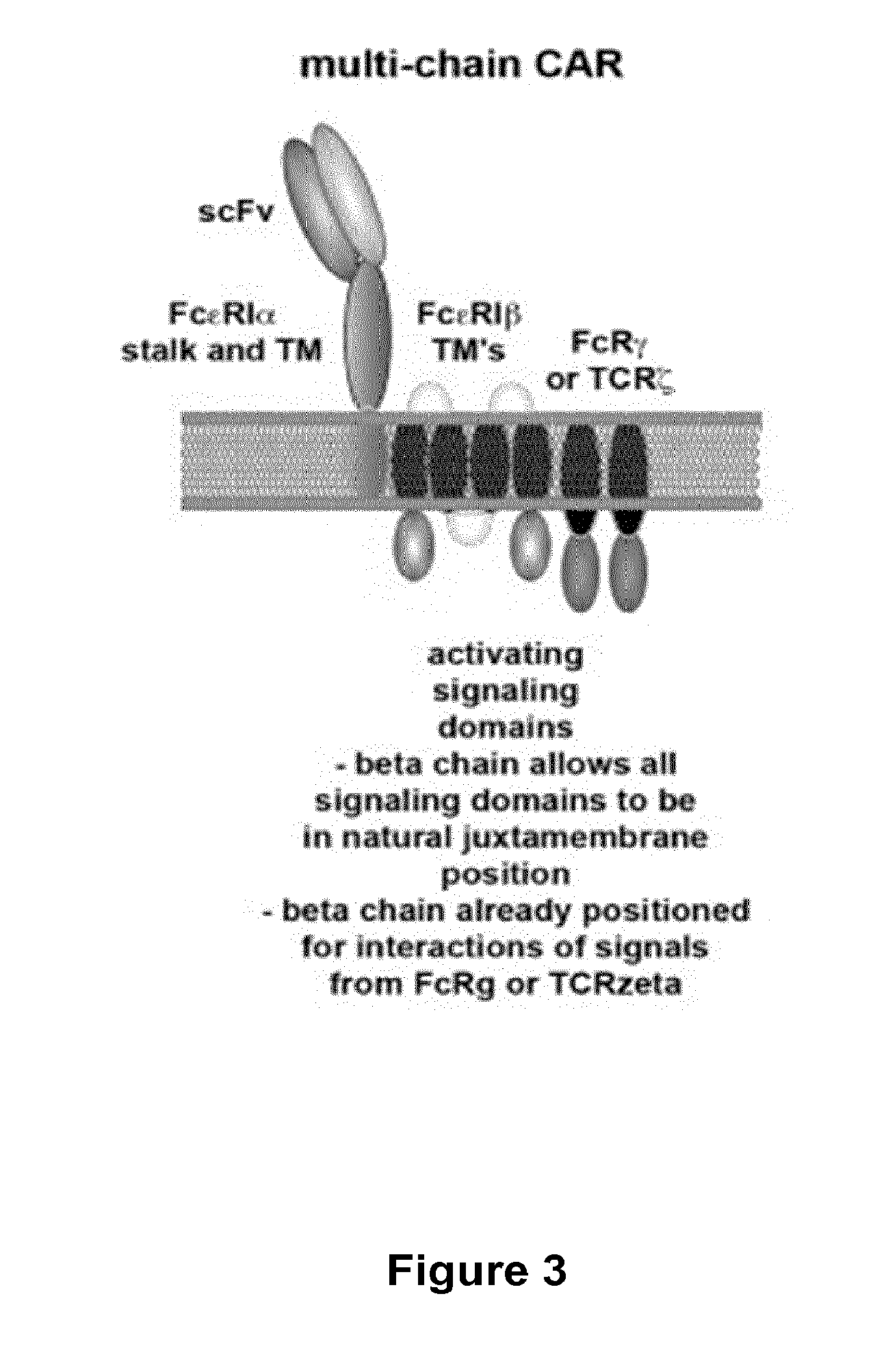
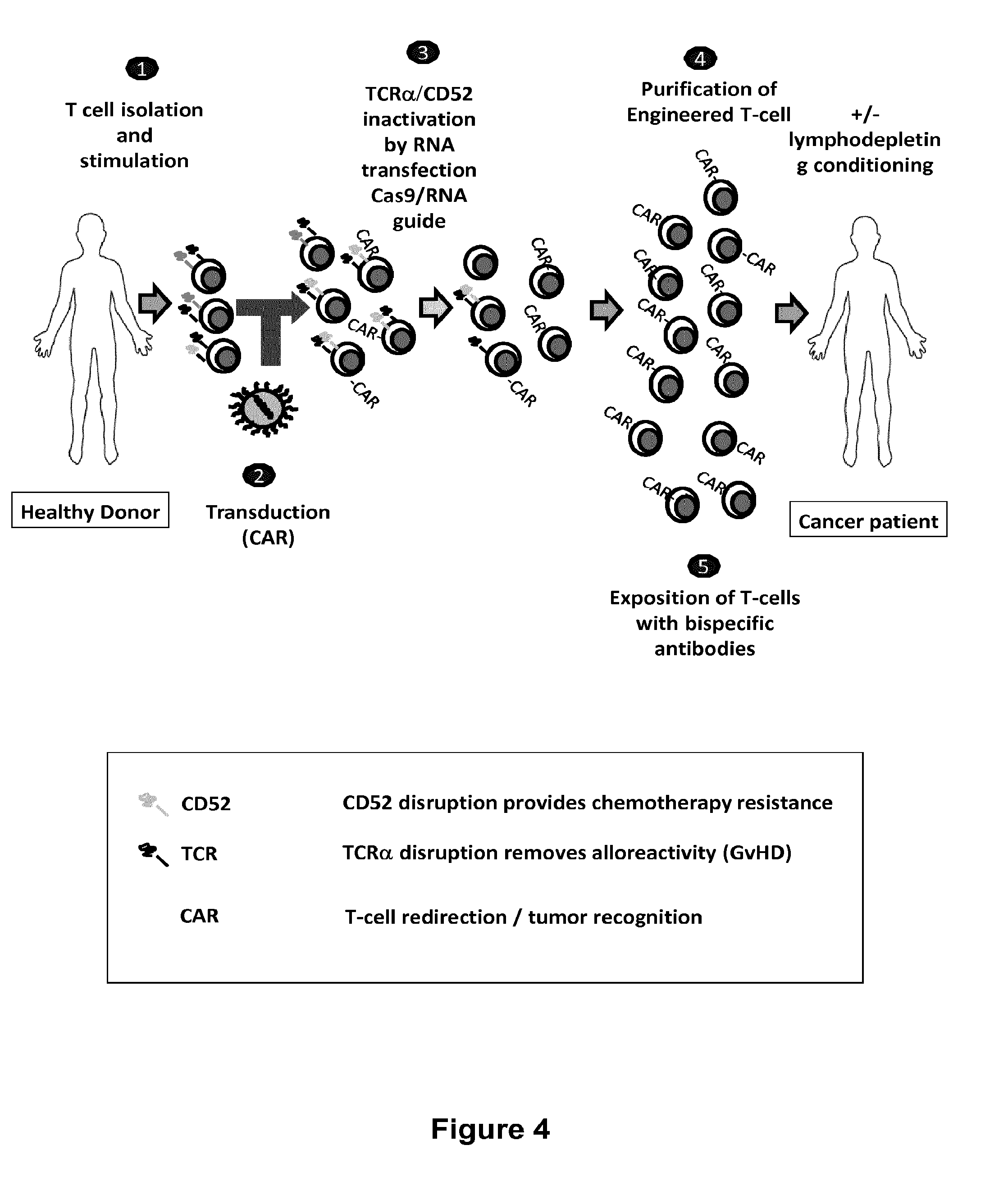
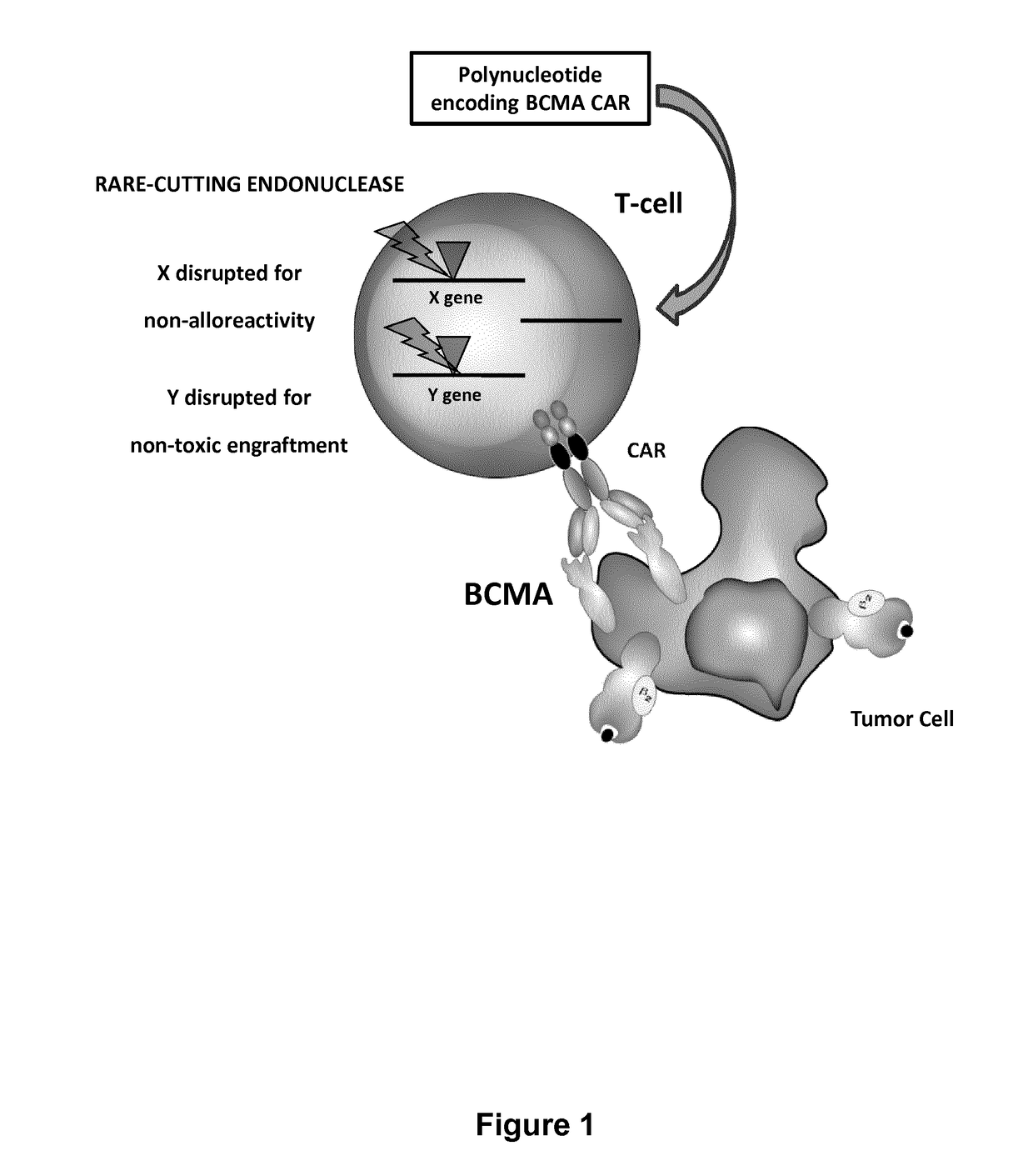
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160272999A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160184362A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
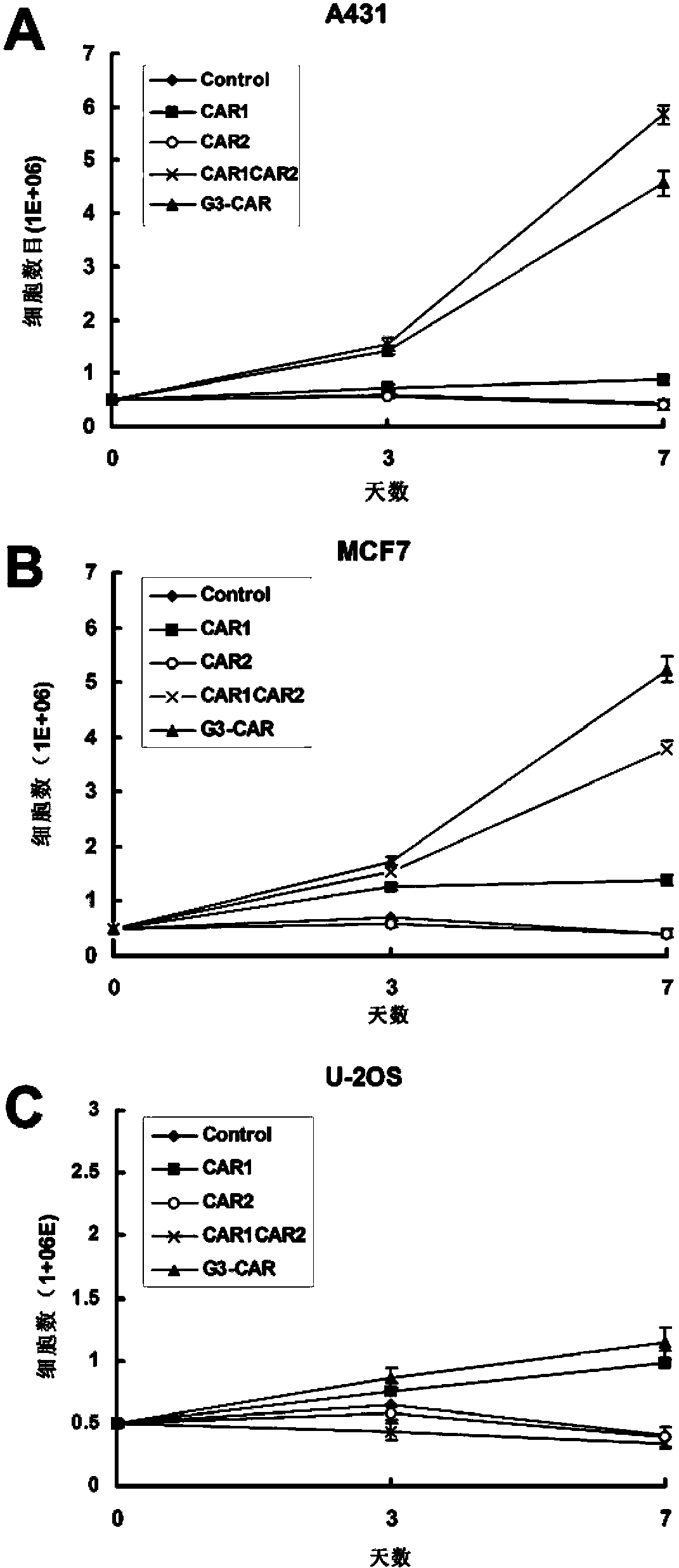
Dual-signal independent chimeric antigen receptors (dsCAR) and uses thereof
ActiveCN103483452APromote proliferationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsViral infectious disease
The invention relates to chimeric antigen receptors (CAR), particularly relates to dual-signal independent chimeric antigen receptors (dsCAR), and also relates to immune response cells of the dual-signal independent chimeric antigen receptors (dsCAR) and uses of the immune response cells in preparation of drugs for treatment of malignant tumor and virus infected diseases. In detail, the dual-signal independent chimeric antigen receptors (dsCAR) can respectively identify two different family antigens of tumor cells and can respectively transmit two T-cell-activation related signals. One of the CAR can transmit a first T-cell-activation related signal by combing a ligand of a tumor specific antigen or a tumor-associated antigen to decide T-cell killing specificity, and the other CAR can transmit a second T-cell-activation related signal by combing a ligand of a membrane receptor (such as EGFR (epidermal growth factor receptor) family protein) widely expressed by the tumor cells to promote T cell activation, proliferation and survival. The dual-signal independent chimeric antigen receptors (dsCAR) can avoid the potential safety problems on the basis of maintaining curative effects of second generation and third generation CAR.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
CHIMERIC ANTIGEN RECEPTORS (CARs), COMPOSITIONS AND METHODS OF USE THEREOF
ActiveUS20180187149A1Reduce in quantityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsCAR - Chimeric antigen receptor
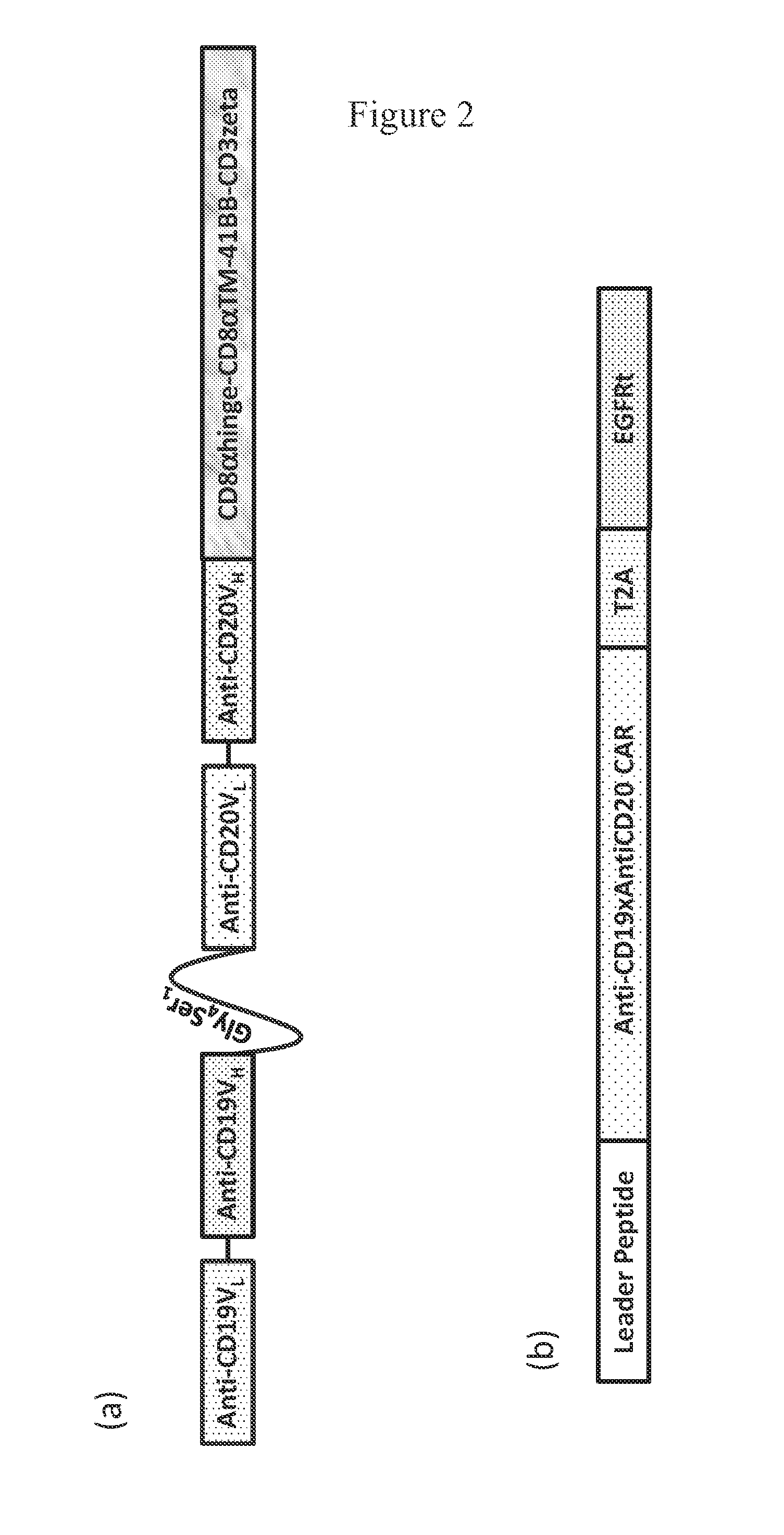
The present invention relates to compositions and methods relating to chimeric antigen receptor (CAR) polypeptides and methods relating thereto. In one embodiment, the present invention relates to engineered cells having chimeric antigen receptor polypeptides directed to at least two targets. In another embodiment, the present invention relates to engineered cells having chimeric antigen receptor polypeptides and an enhancer moiety.
Owner:ICELL GENE THERAPEUTICS LLC
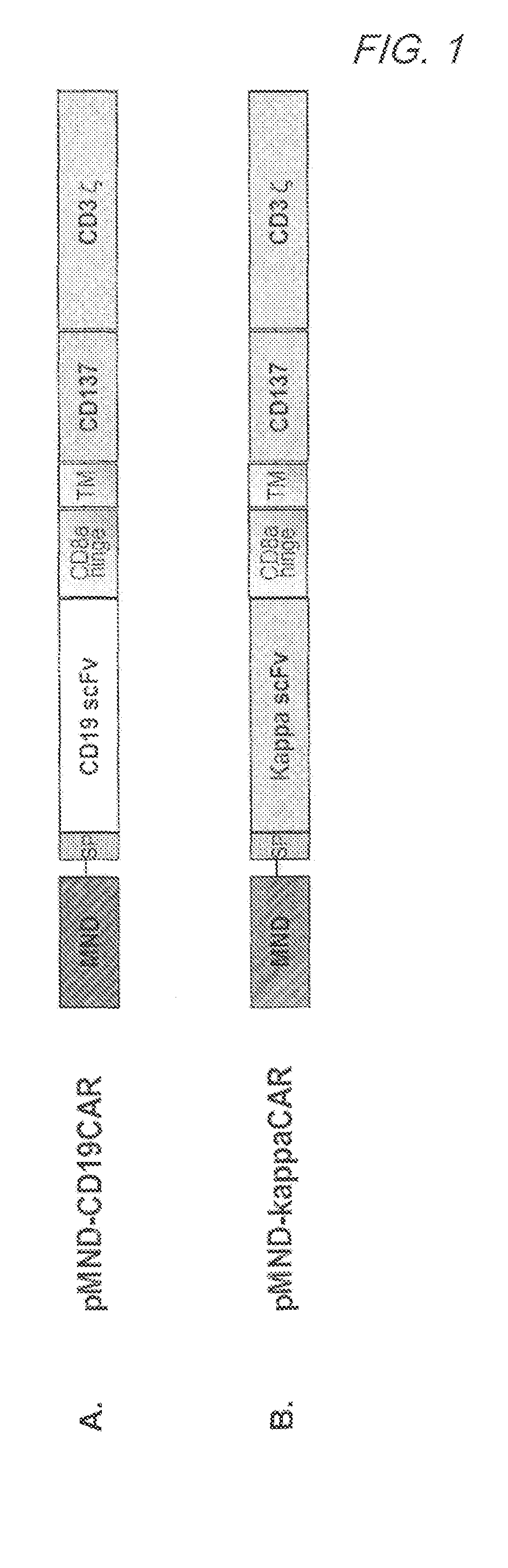
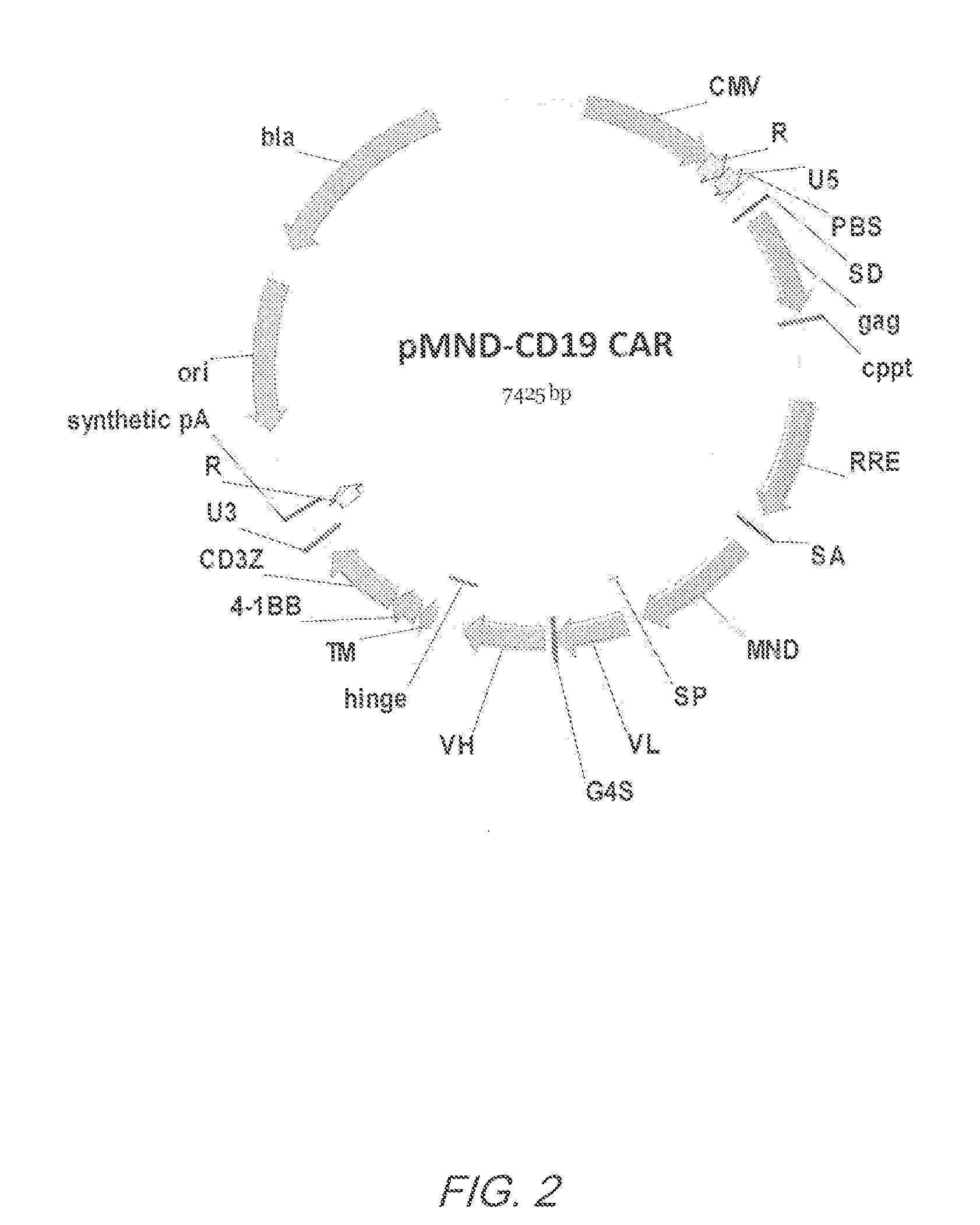
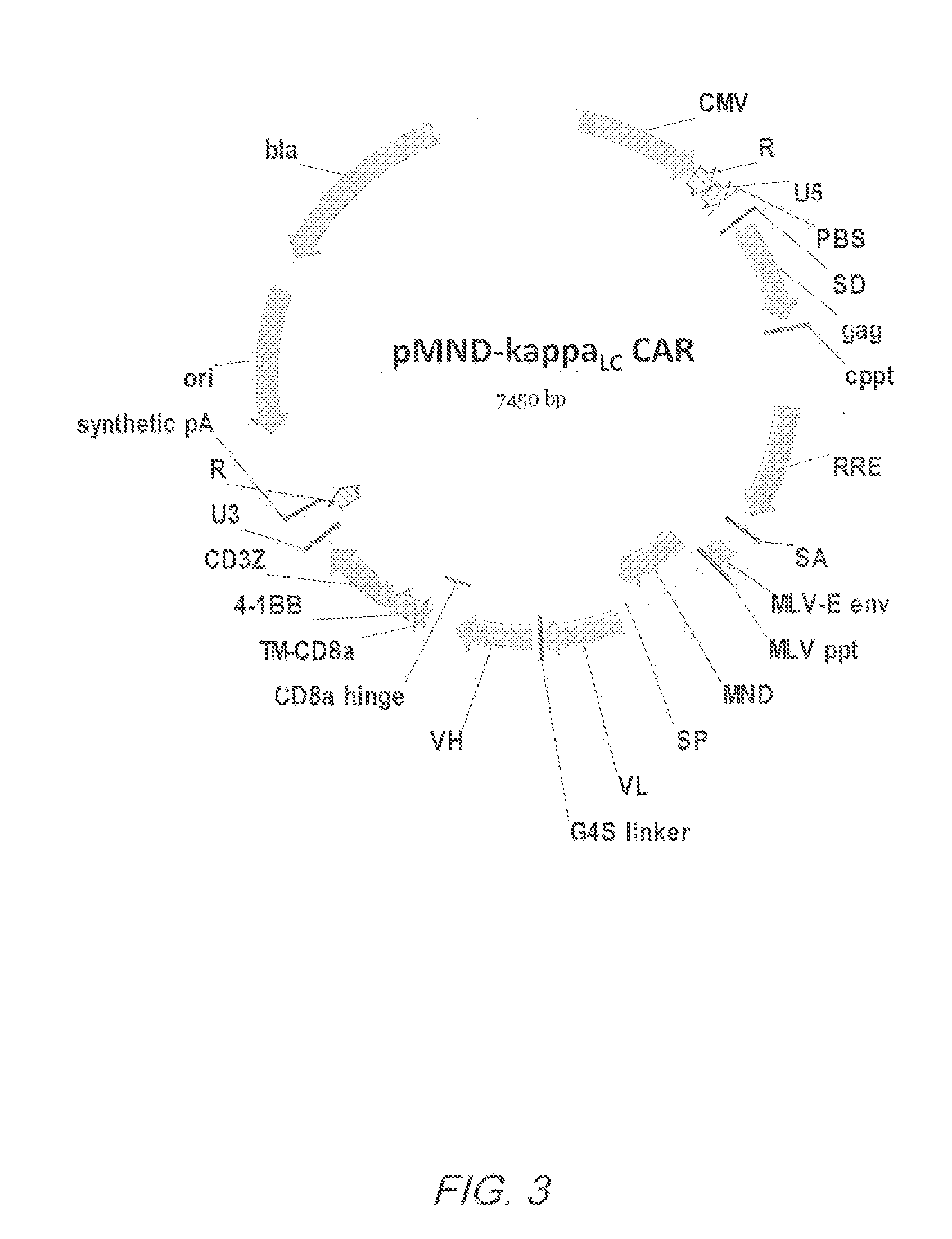
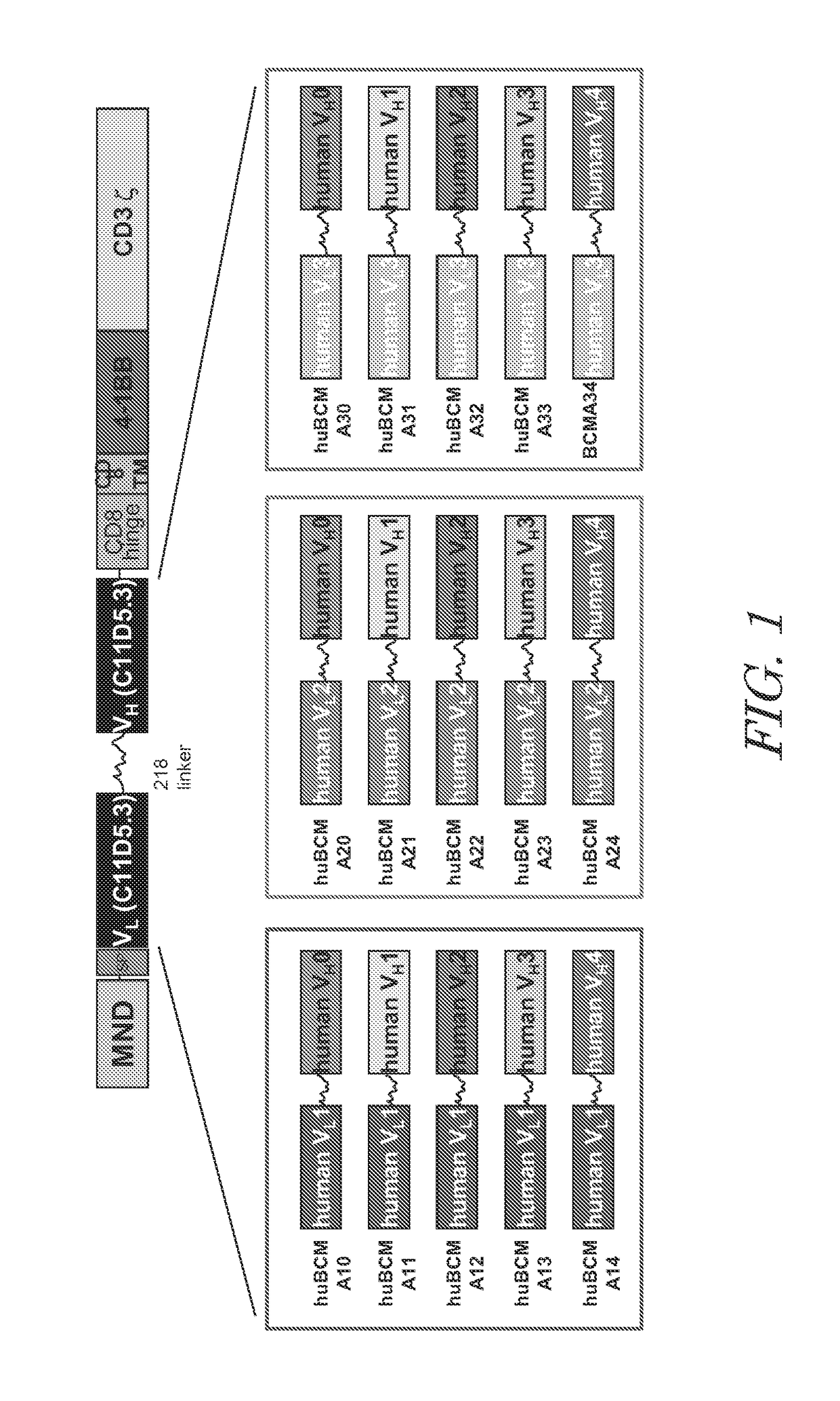
Mnd promoter chimeric antigen receptors
ActiveUS20170051308A1Antibody mimetics/scaffoldsGenetic material ingredientsBinding siteAntigen receptors
Vector compositions comprising a myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted (MND) promoter operably linked to a chimeric antigen receptor (CAR) are provided.
Owner:2SEVENTY BIO INC
Novel chimeric antigen receptor and applications thereof
PendingCN108276493AMild release responseHigh ability to target and recognize tumor antigensPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
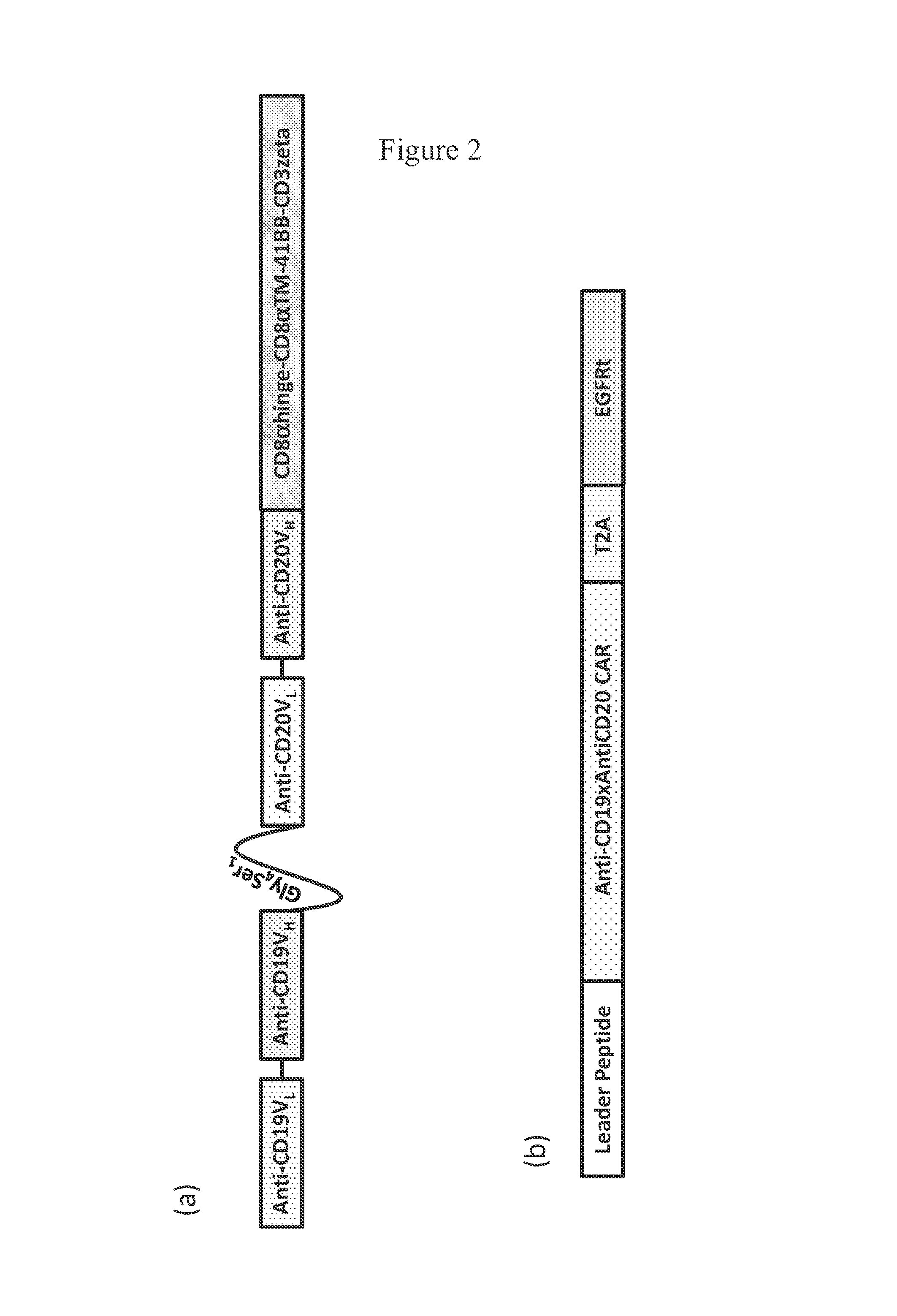
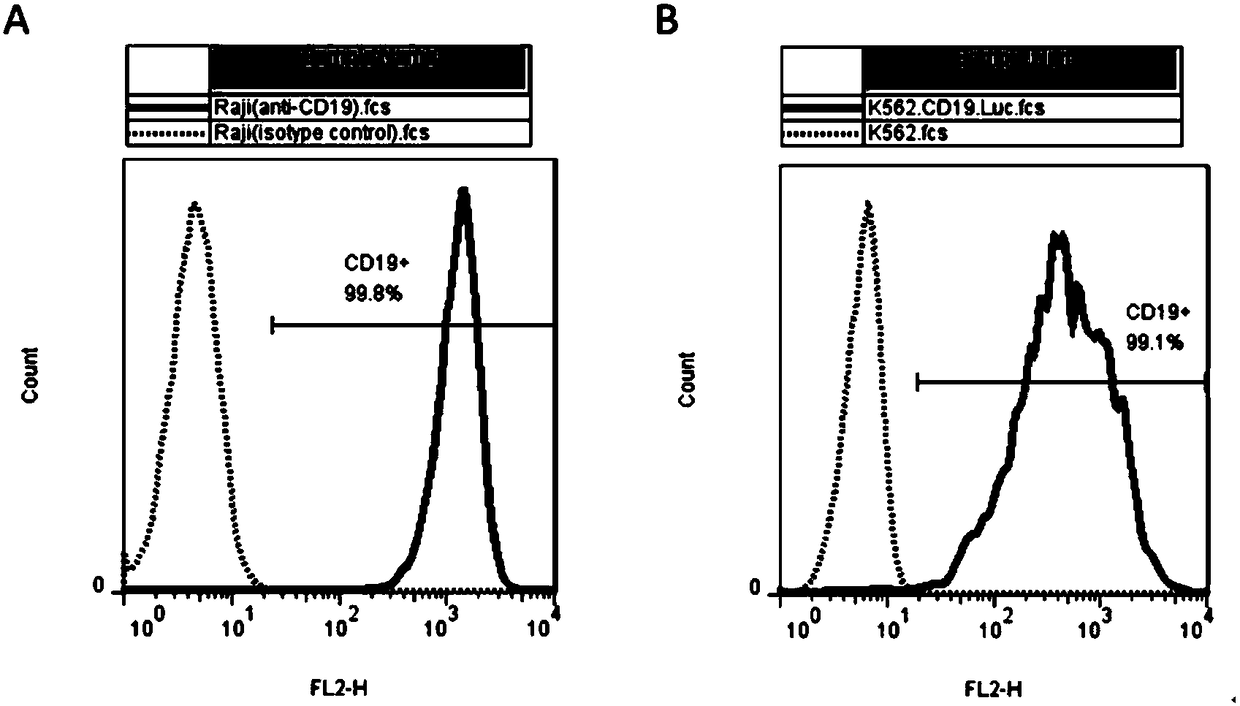
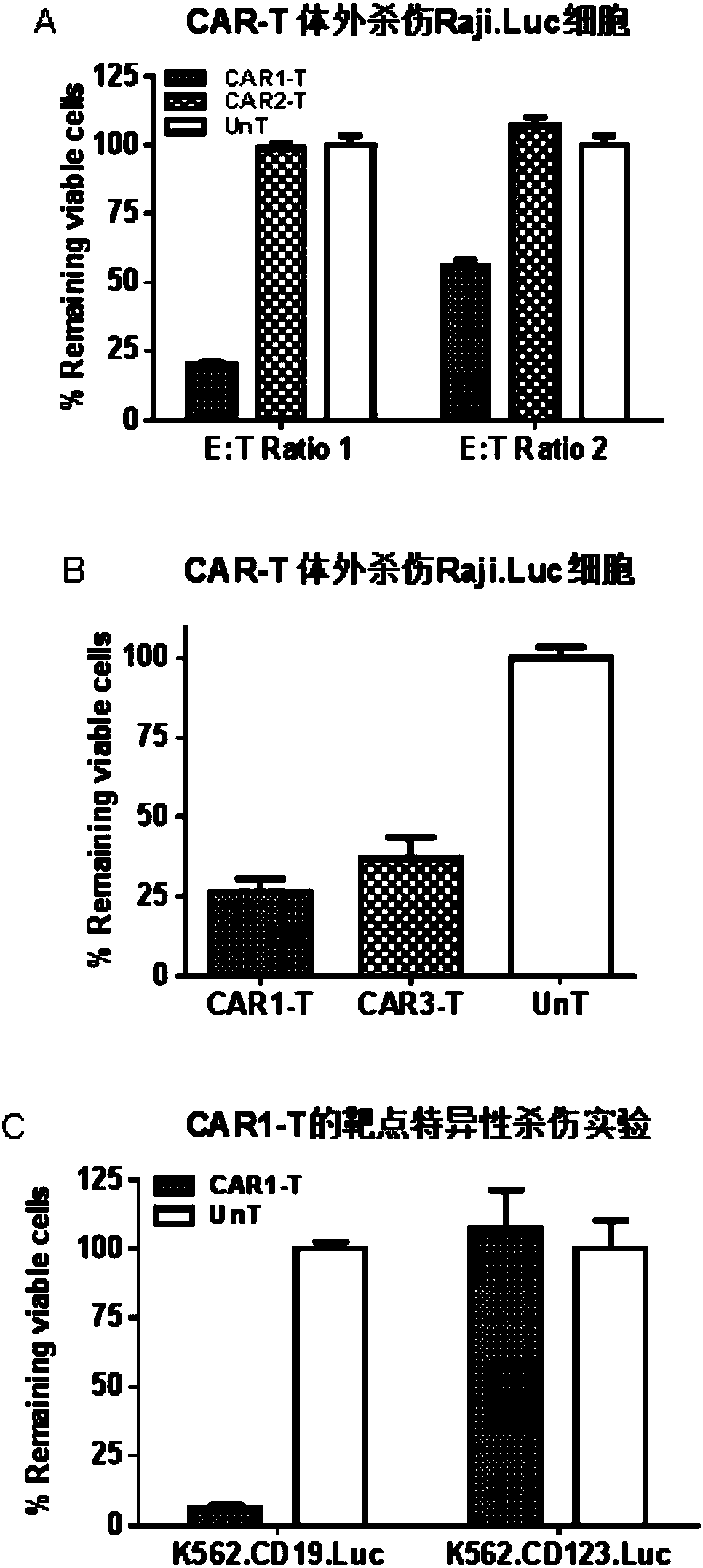
The present invention discloses a novel chimeric antigen receptor and applications thereof, wherein the novel chimeric antigen receptor comprises a signal peptide, an antigen binding domain, a transmembrane domain and an intracellular signal domain, and comprises a 4-1BB signal peptide and / or a 4-1BB molecular transmembrane domain. According to the present invention, a variety of chimeric antigenreceptor nucleic acid sequences are separated and purified, the chimeric antigen receptor specifically for CD19 malignant tumor antigens and the CAR-T cells are provided, and the blood cell line malignant tumor killing test results show that the tumor-cell-targeting ability of immune cells is significantly enhanced, and the tumor cell killing activity is enhanced.
Owner:NANJING LEGEND BIOTECH CO LTD
Treatment of cancer using humanized anti-EGFRvIII chimeric antigen receptor
The invention provides compositions and methods for treating diseases associated with expression of EGFRvIII. The invention also relates to chimeric antigen receptor (CAR) specific to EGFRvIII, vectors encoding the same, and recombinant T cells comprising the anti-EGFRvIII CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises an anti-EGFRvIII binding domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Universal Anti-tag chimeric antigen receptor-expressing t cells and methods of treating cancer
ActiveUS20130287752A1Efficient productionPromote efficient proliferationBiocideAntibody mimetics/scaffoldsHuman cancerCancer cell
The present invention provides a universal, yet adaptable, anti-tag chimeric antigen receptor (AT-CAR) system which provides T cells with the ability and specificity to recognize and kill target cells, such as tumor cells, that have been marked by tagged antibodies. As an example, αFITC-CAR-expressing T cells have been developed that specifically recognize various human cancer cells when those cells are bound by cancer-reactive FITC-labeled antibodies. The activation of αFITC-CAR-expressing T cells is shown to induce efficient target lysis, T cell proliferation, and cytokine / chemokine production. The system can be used to treating subjects having cancer.
Owner:UNIV OF MARYLAND BALTIMORE
Chimeric antigen receptor with stable antigen binding units, method for preparing chimeric antigen receptor and application thereof
ActiveCN104829733APeptide/protein ingredientsPharmaceutical non-active ingredientsTumor targetAntigen receptors
The invention relates to a chimeric antigen receptor with stable antigen binding units. The chimeric antigen receptor comprises the extracellular antigen binding units, trans-membrane domains and intracellular co-stimulation signal domains. The antigen binding units can be stably expressed as compared with specific tumor targets and comprise heavy chains and light chains which are connected with one another by hinges of SEQ ID NO.2 (sequence identity number.2) amino acid sequence codes. The invention further relates to cells for expressing the receptor and application of the receptor.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Bcma chimeric antigen receptors
InactiveUS20170226216A1Treating and preventing and ameliorating B cell related conditionVirusesPeptide/protein ingredientsAntigenAntigen receptors
Owner:2SEVENTY BIO INC
Tumor precision T cell containing efficient killing starting mechanism and application of tumor precision T cell
ActiveCN105331586AActivate proliferationActivate growthImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsAntigen receptorsT lymphocyte
The invention belongs to the fields of immunology and cell biology, and relates to a tumor precision T cell containing an efficient killing starting mechanism and an application of the tumor precision T cell, in particular to a CAR (chimeric antigen receptor) having moderate-affinity binding characteristic with a broad-spectrum expression membrane antigen on the tumor cell surface as well as a new-generation tumor precision T cell, namely, Baize T. T cell activation is started rapidly under the action of the CAR having the moderate-affinity binding characteristic, an activation signal of the CAR is superposed with a TCR (T cell receptor) signal with tumor antigen natural recognition capacity in a CTL (tumor-specific cytotoxic T lymphocyte), the CTL is activated to proliferate and grow in a tumor microenvironment, and a tumor cell is killed preciously by the tumor-antigen-specific TCR. The tumor precision T cell has broad anti-tumor application prospect.
Owner:SHANGHAI CELL THERAPY RES INST +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com