Isolated monoclonal antibodies that specifically bind to human Claudin 18.2
An antibody, monoclonal antibody technology, applied in the direction of antibodies, antibody medical components, medical preparations with non-active components, etc., can solve the problems of poor prognosis and low survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0133] Preparation of monoclonal antibody of the present invention
[0134] The monoclonal antibodies of the present invention can be prepared using the somatic cell hybridization (hybridoma) technique of Kohler and Milstein (1975) Nature 256:495. Other embodiments for producing monoclonal antibodies include viral or oncogenic transformation of B lymphocytes and phage display techniques. Chimeric or humanized antibodies are also well known in the art. See, eg, US Patents 4,816,567; 5,225,539; 5,530,101; 5,585,089; 5,693,762 and 6,180,370.
[0135] Generation of Transfectomas to Produce Monoclonal Antibodies of the Invention
[0136] Antibodies of the invention can also be produced in host cell transfectomas using, for example, recombinant DNA techniques combined with gene transfection methods (eg Morrison, S. (1985) Science 229:1202). In one embodiment, DNA encoding partial or full-length light and heavy chains obtained by standard molecular biology techniques is insert...
Embodiment 1
[0176] Example 1 Construction of HEK193A cell strain stably expressing human Claudin 18.1 or Claudin 18.2
[0177] The cDNA sequences (SEQ ID NOs: 69 and 71) encoding human Claudin 18.1 (SEQ ID NO: 70) and Claudin 18.2 (SEQ ID NO: 72) were synthesized, and the coding sequences of the above two genes were digested by EcoRI and BamHI. respectively cloned into the pLV-sfGFP(2A)puro plasmid. The resulting pLV-EGFP(2A)-Puro-Claudin 18.1 or pLV-EGFP(2A)-Puro-Claudin 18.2 was co-transfected with psPAX2 and pMD2.G into HEK-293T cells (Cobioer, China) to generate lentivirus. The resulting lentivirus was transfected into HEK-293A cells (Cobioer, China) to generate stable cell lines overexpressing human Claudin 18.1 or Claudin 18.2. The cell line was cultured in DMEM medium (Cat: SH30022.01, Gibco) supplemented with 10% FBS (Cat: FND500, Excell) and 0.2 μg / ml puromycin (Cat: A11138-03, Gibco). The time is more than 7 days.
[0178] The expressions of Claudin 18.1 and Claudin 18.2 we...
Embodiment 2
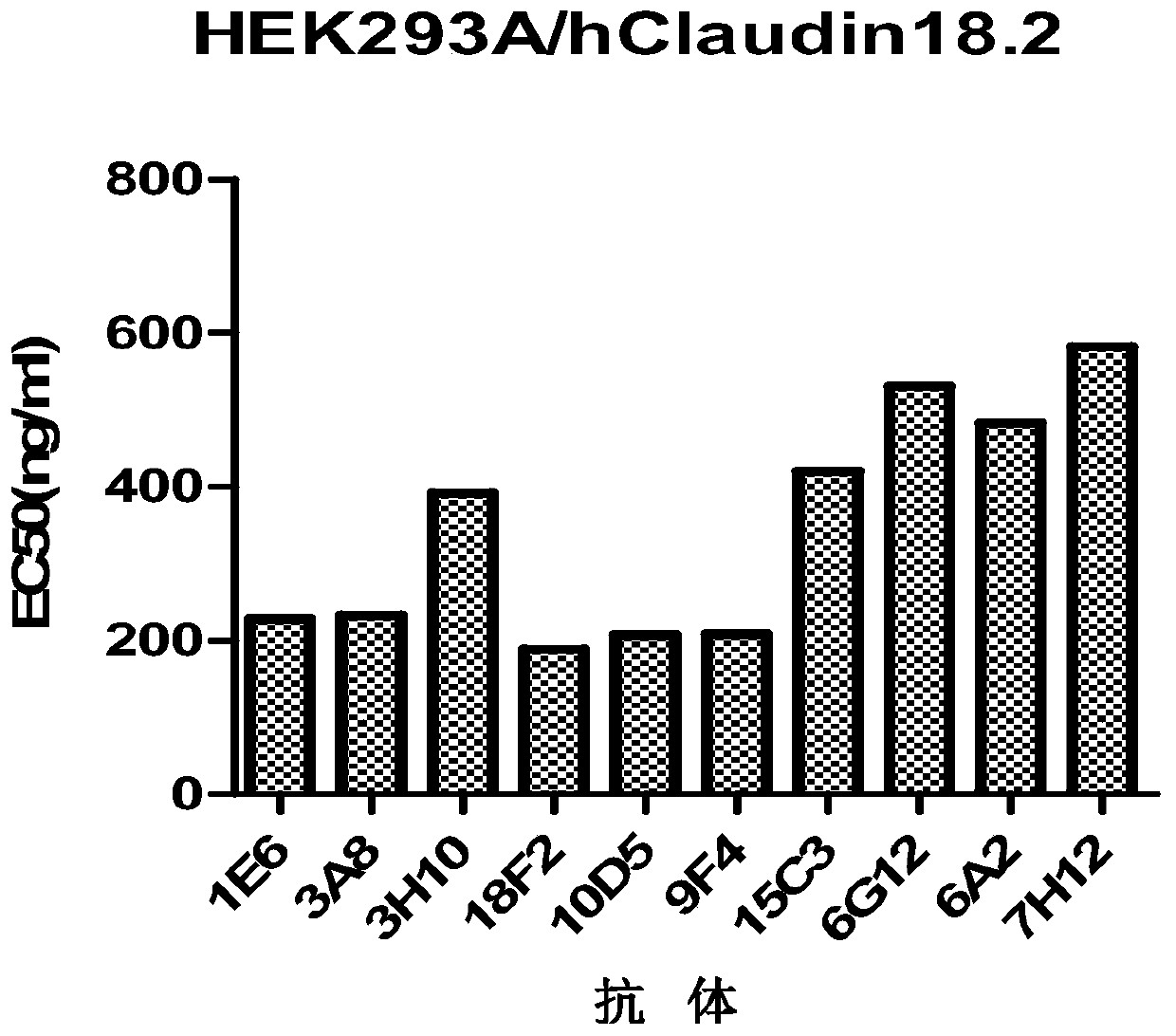
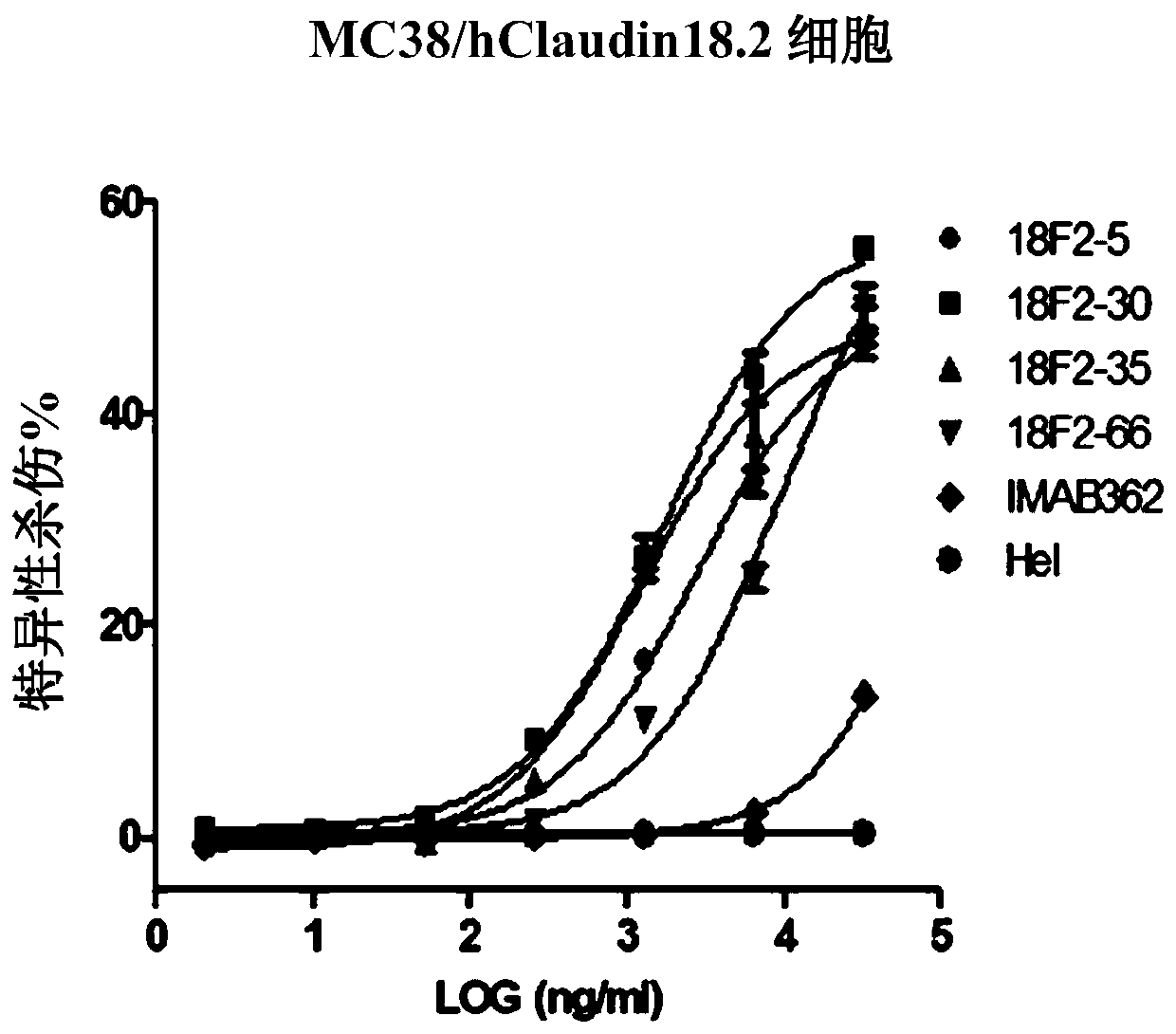
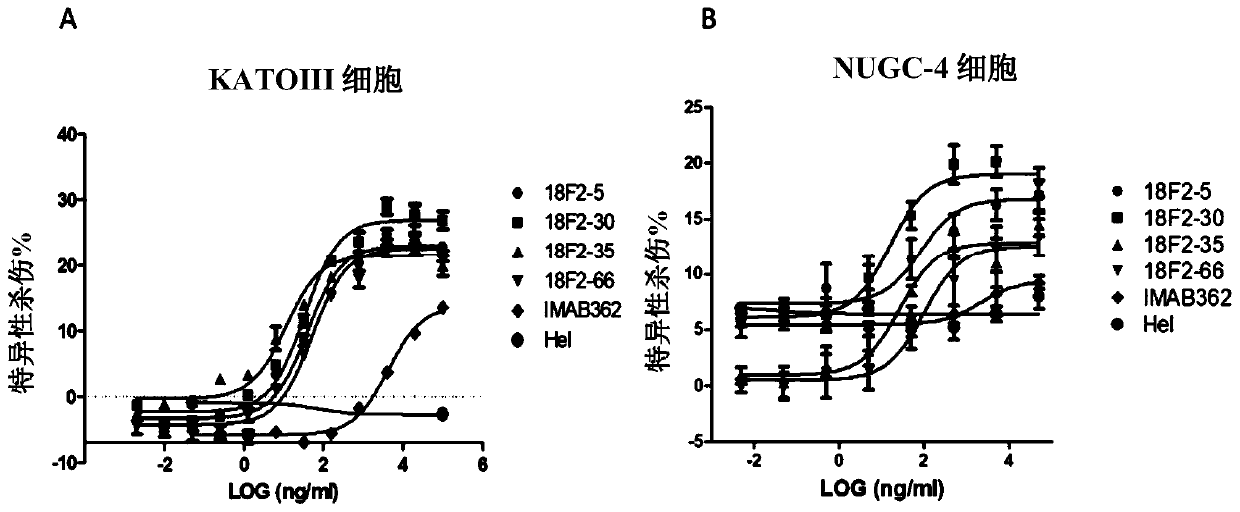
[0179] Example 2 Generation and Screening of Claudin 18.2 Monoclonal Antibody
[0180] Using HEK293A cells stably expressing human Claudin 18.2 in Example 1 to immunize 6-week-old BALB / c mice, the cells were injected subcutaneously, and each mouse was injected with 2×10 7 cells. After the primary immunization, booster immunizations were given every four weeks for a total of three times. Antibody titers in blood samples were determined by FACS using HEK293A cells expressing human Claudin 18.2. When the animal reached the appropriate antibody titer, with 5 × 10 in PBS 7 / mL cells for the last immune boost. Three days later, mice were sacrificed, bled, and spleens were collected for mRNA extraction and phage display library construction.
[0181] To construct the scFv phage display library, mouse spleen total RNA was extracted with Trizol kit (Invitrogen), and cDNA was synthesized using reverse transcription kit (Invitrogen). Using the above cDNA as a template, gene amplif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com