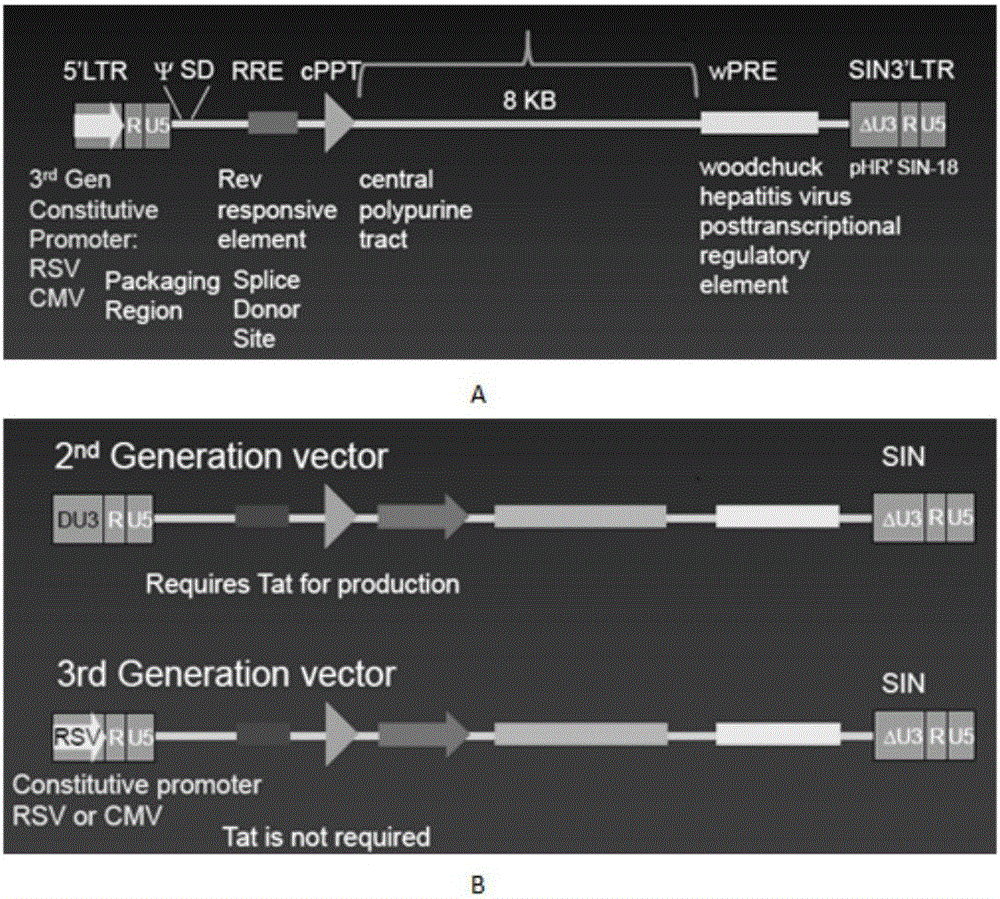
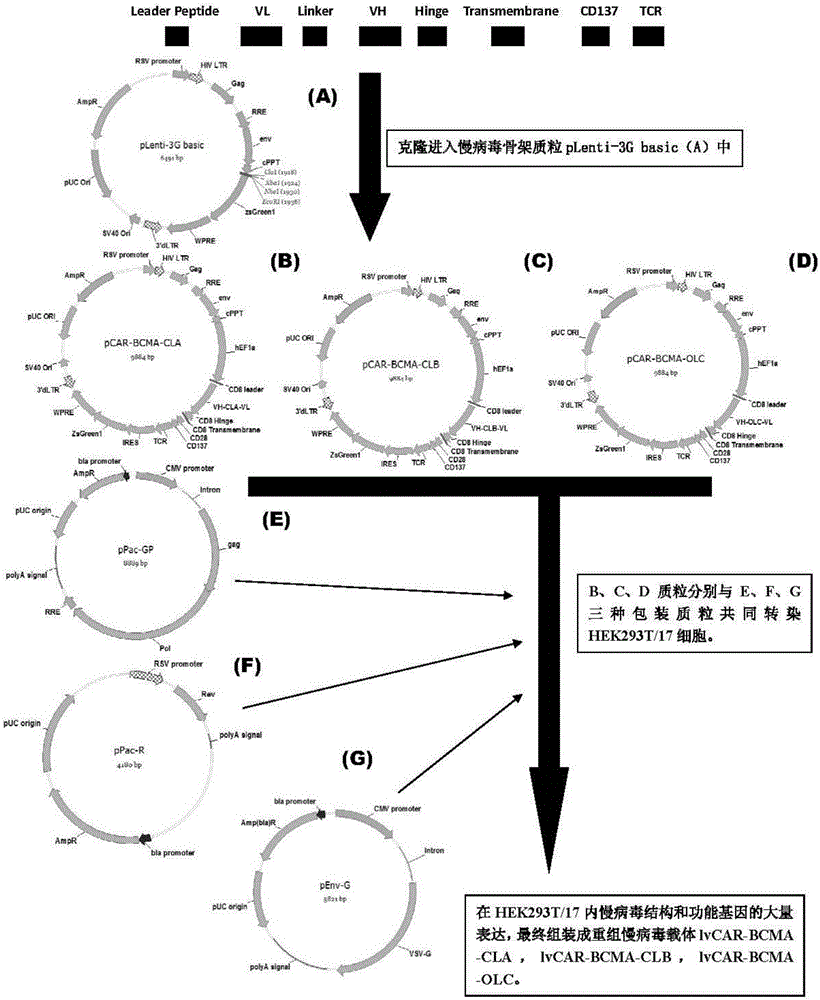
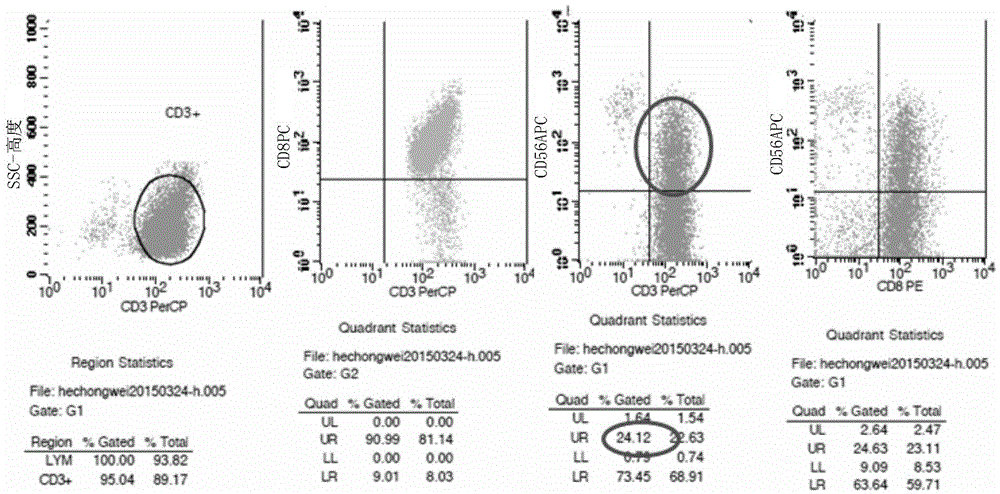
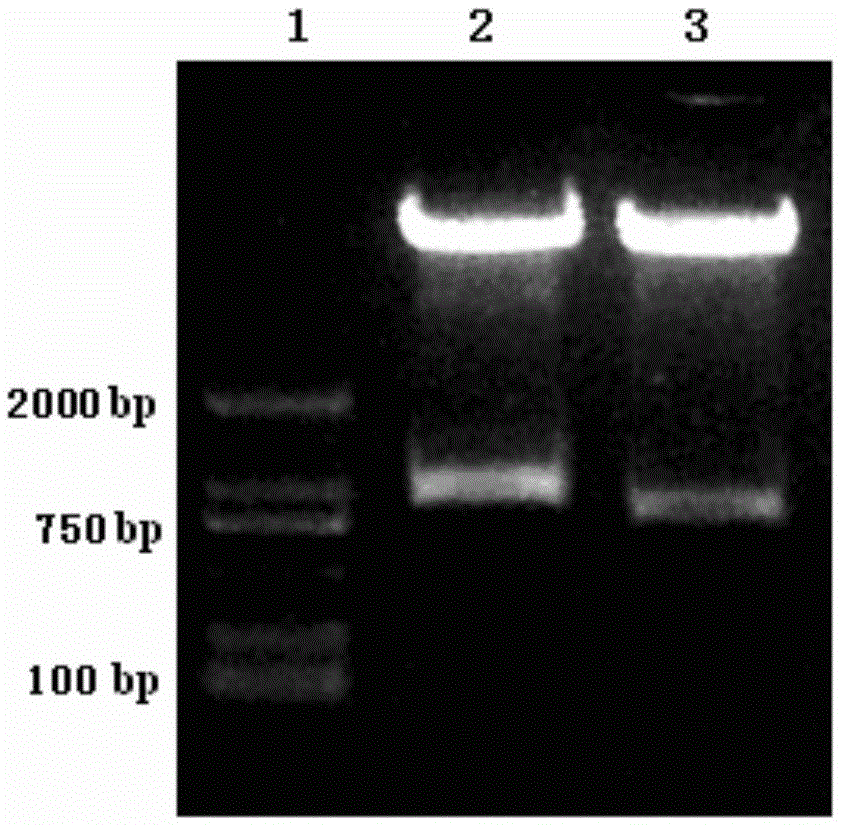
Patents
Literature
1072 results about "CAR - Chimeric antigen receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chimeric antigen receptors (CARs, also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors) are engineered receptors that combine a new specificity with an immune cell to target cancer cells.
Method and compositions using a chimeric antigen receptor for enhanced anti-tumor effector functioning of T cells
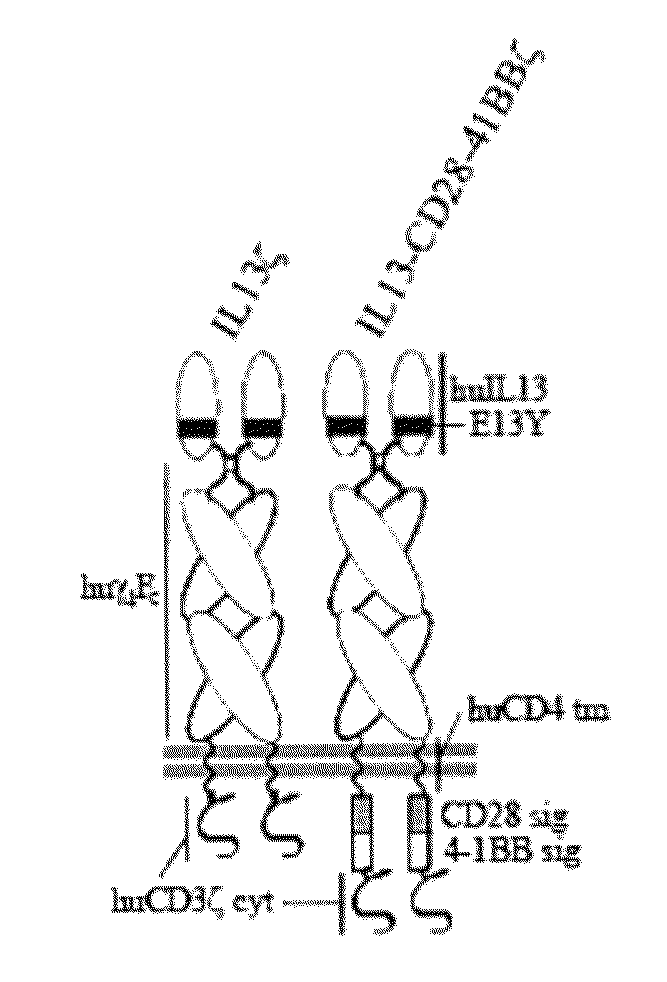
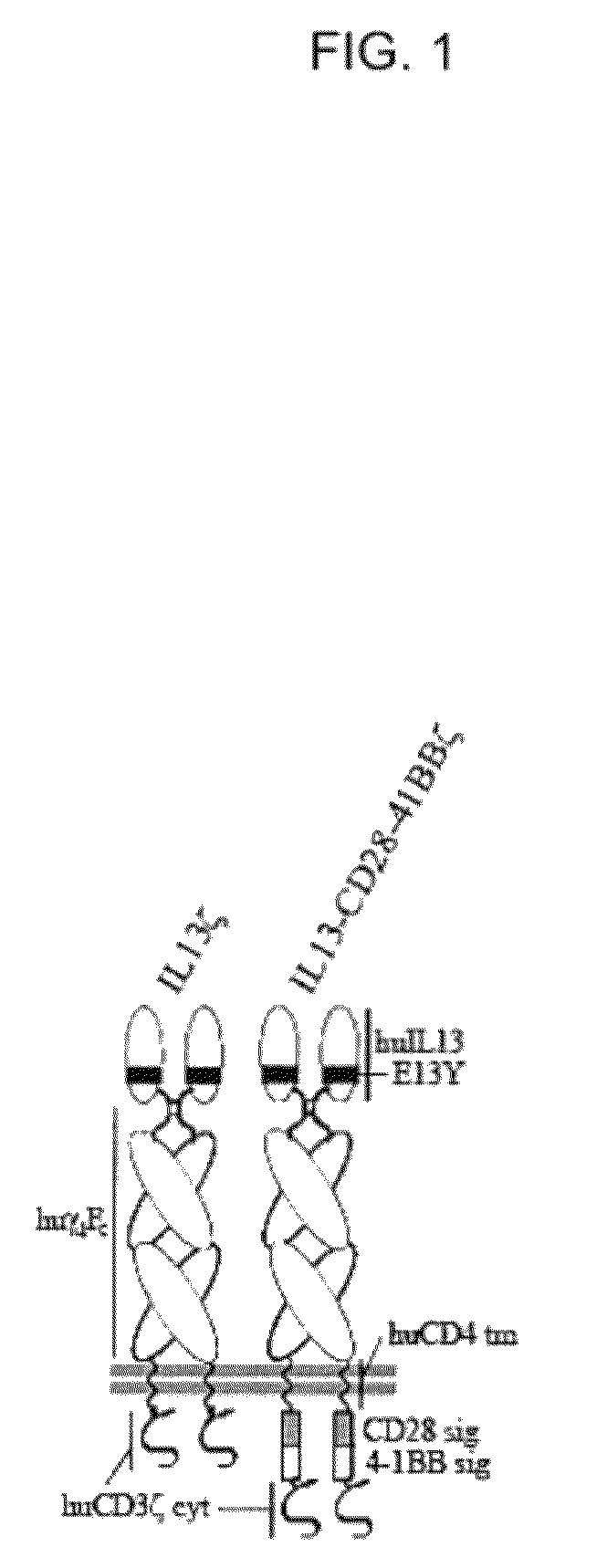
Integration of costimulatory signaling domains within a tumor targeting chimeric antigen receptor (CAR), such as the IL13Rα2 specific IL13-zetakine (IL13ζ), enhances T cell-mediated responses against tumors even in the absence of expressed ligands for costimulatory receptors.
Owner:CITY OF HOPE
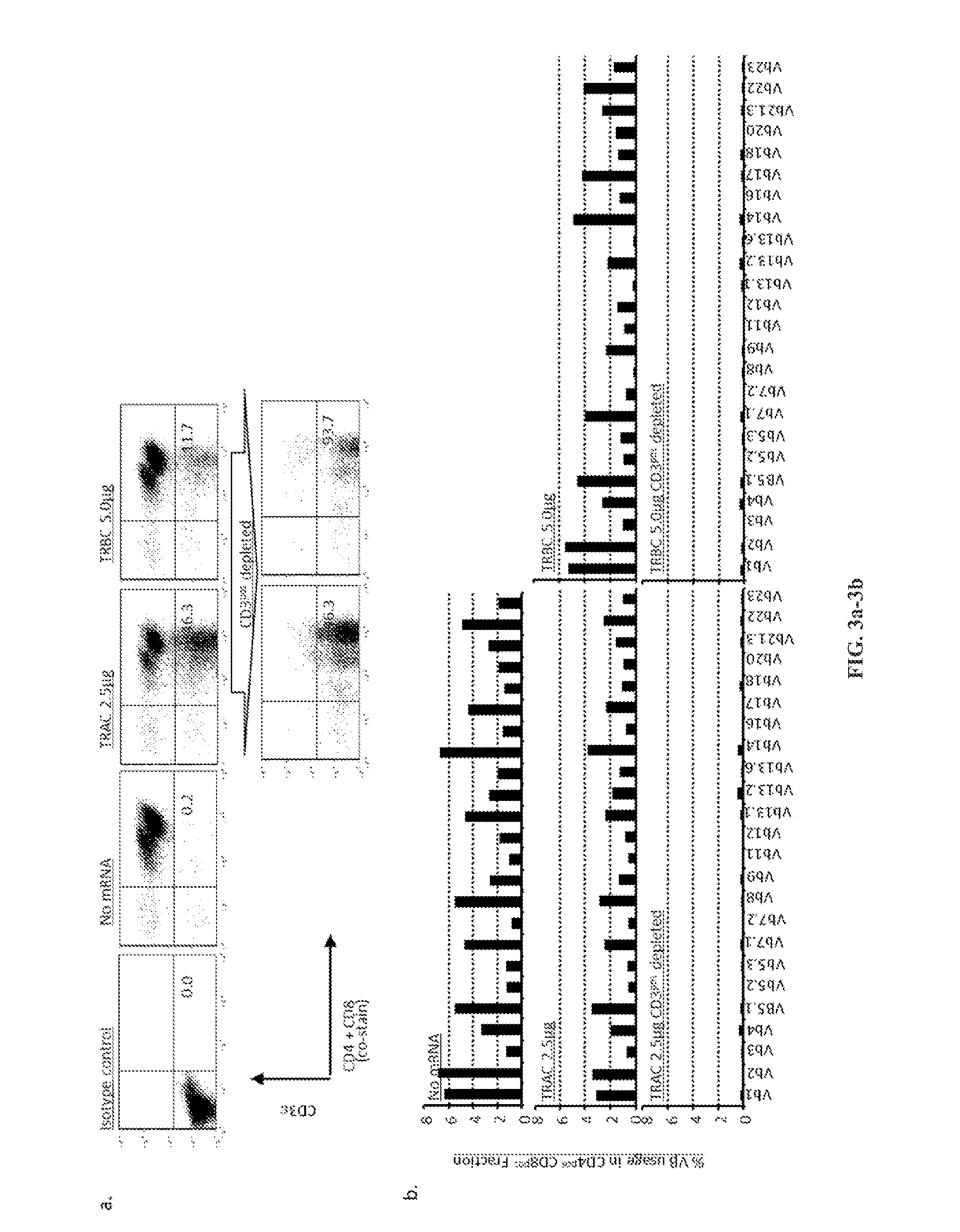
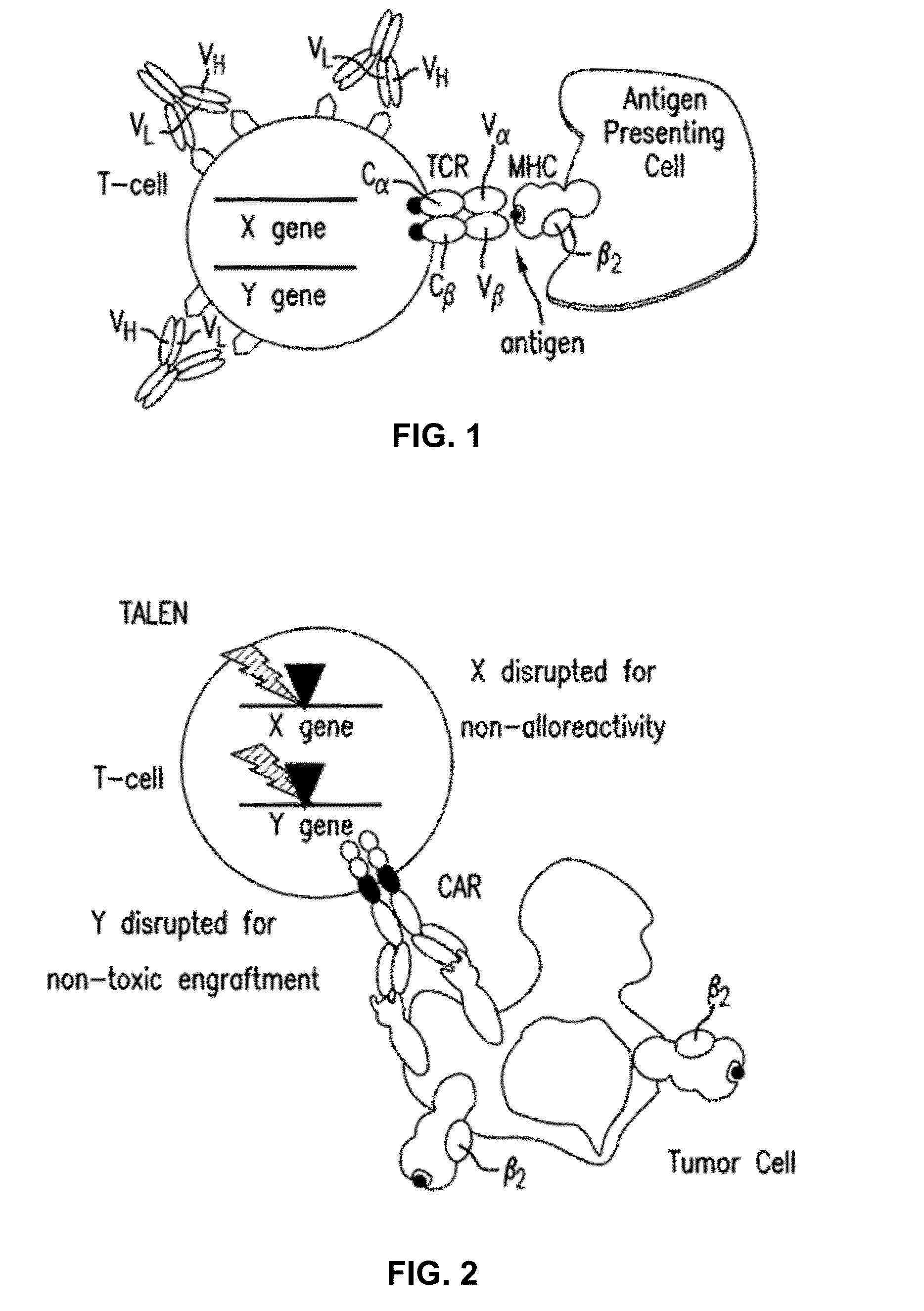
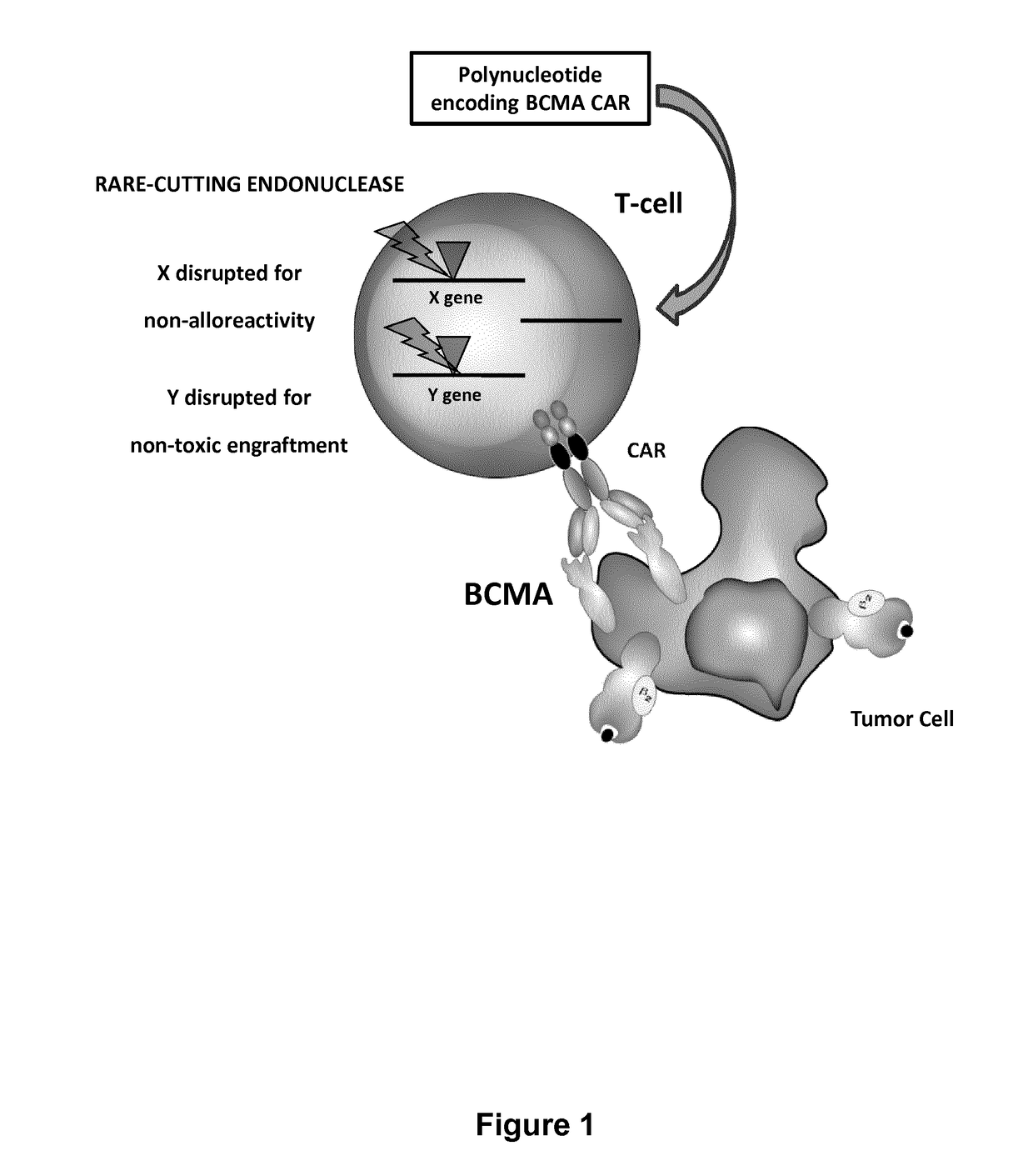
Car+ t cells genetically modified to eliminate expression of t-cell receptor and/or HLA
ActiveUS20140349402A1Suitable for storageReduce/eliminate T cellsGenetically modified cellsMammal material medical ingredientsAntigen receptorsAutoimmunity
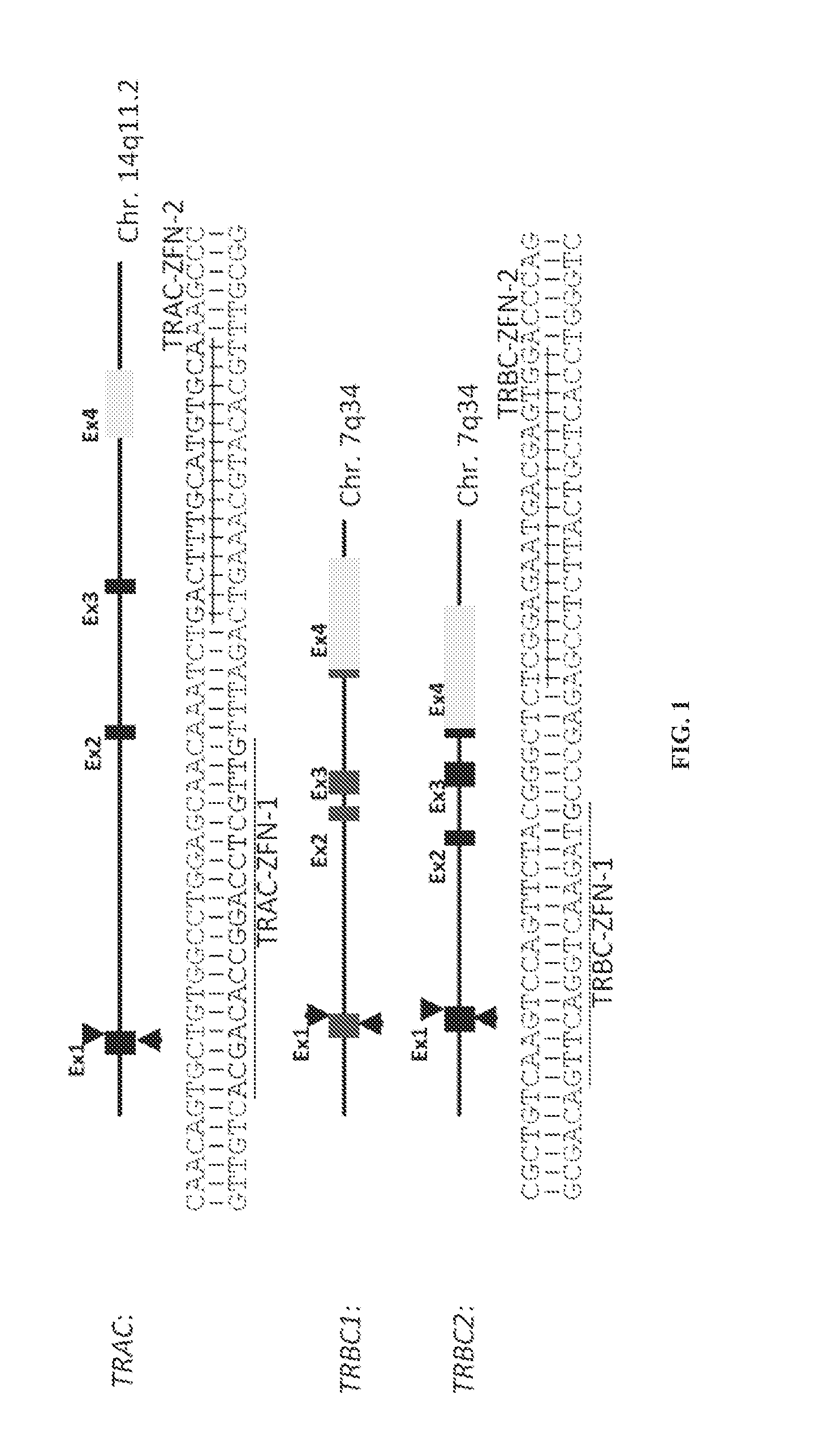
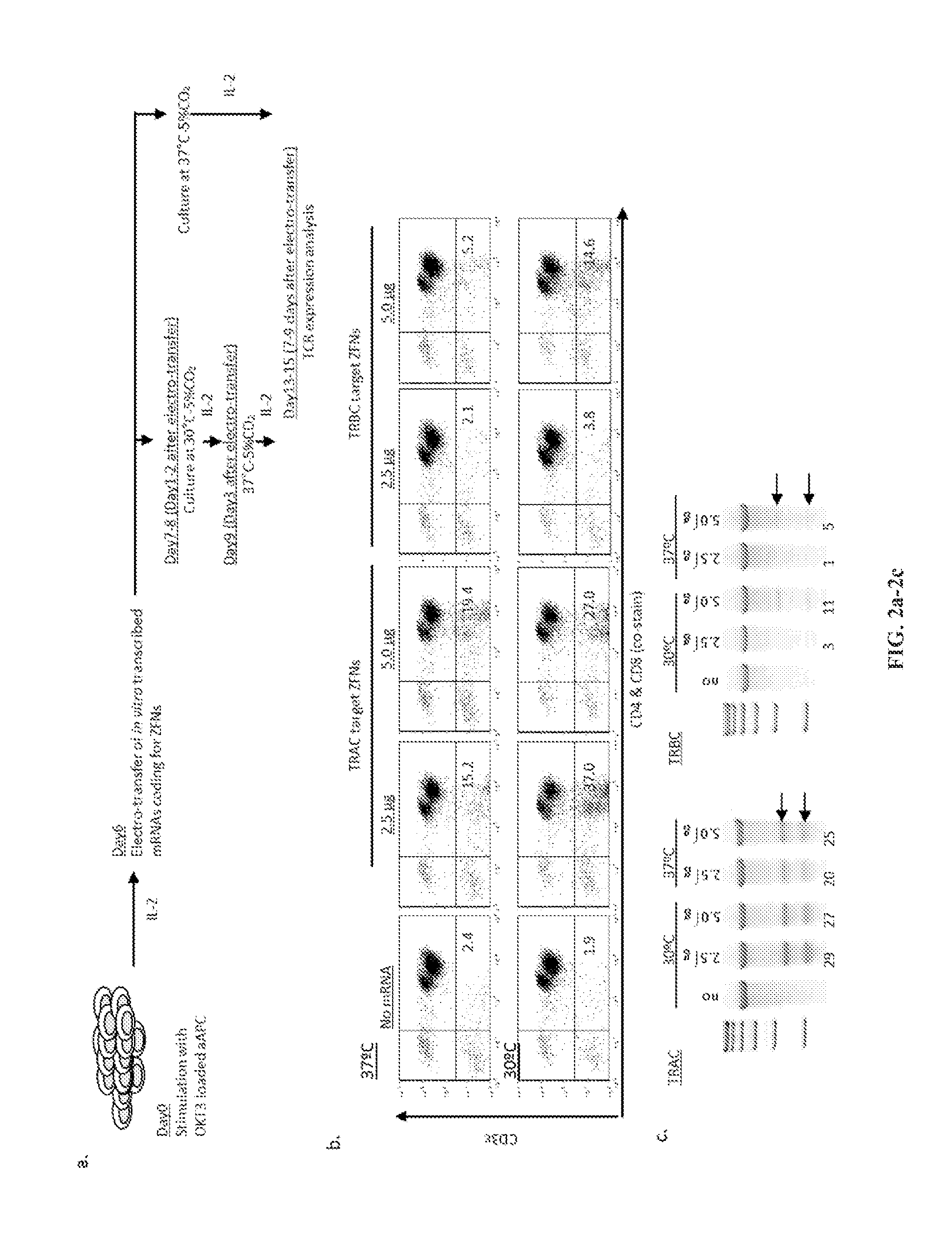
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising disrupted T cell receptor and / or HLA and comprising a chimeric antigen receptor. In certain embodiments, the compositions are employed allogeneically as universal reagents for “off-the-shelf treatment of medical conditions such as cancer, autoimmunity, and infection. In particular embodiments, the T cell receptor-negative and / or HLA-negative T cells are generated using zinc finger nucleases, for example.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
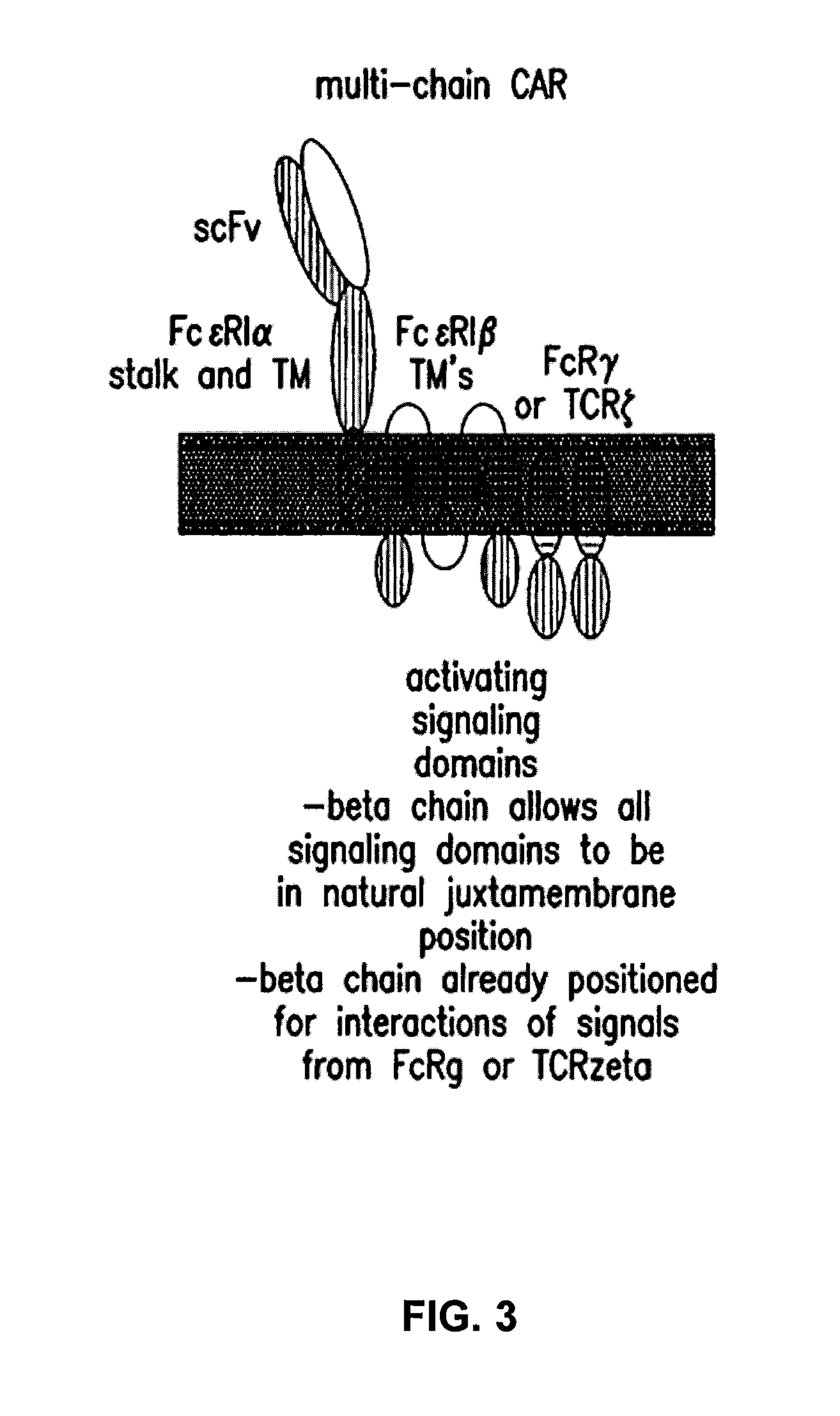
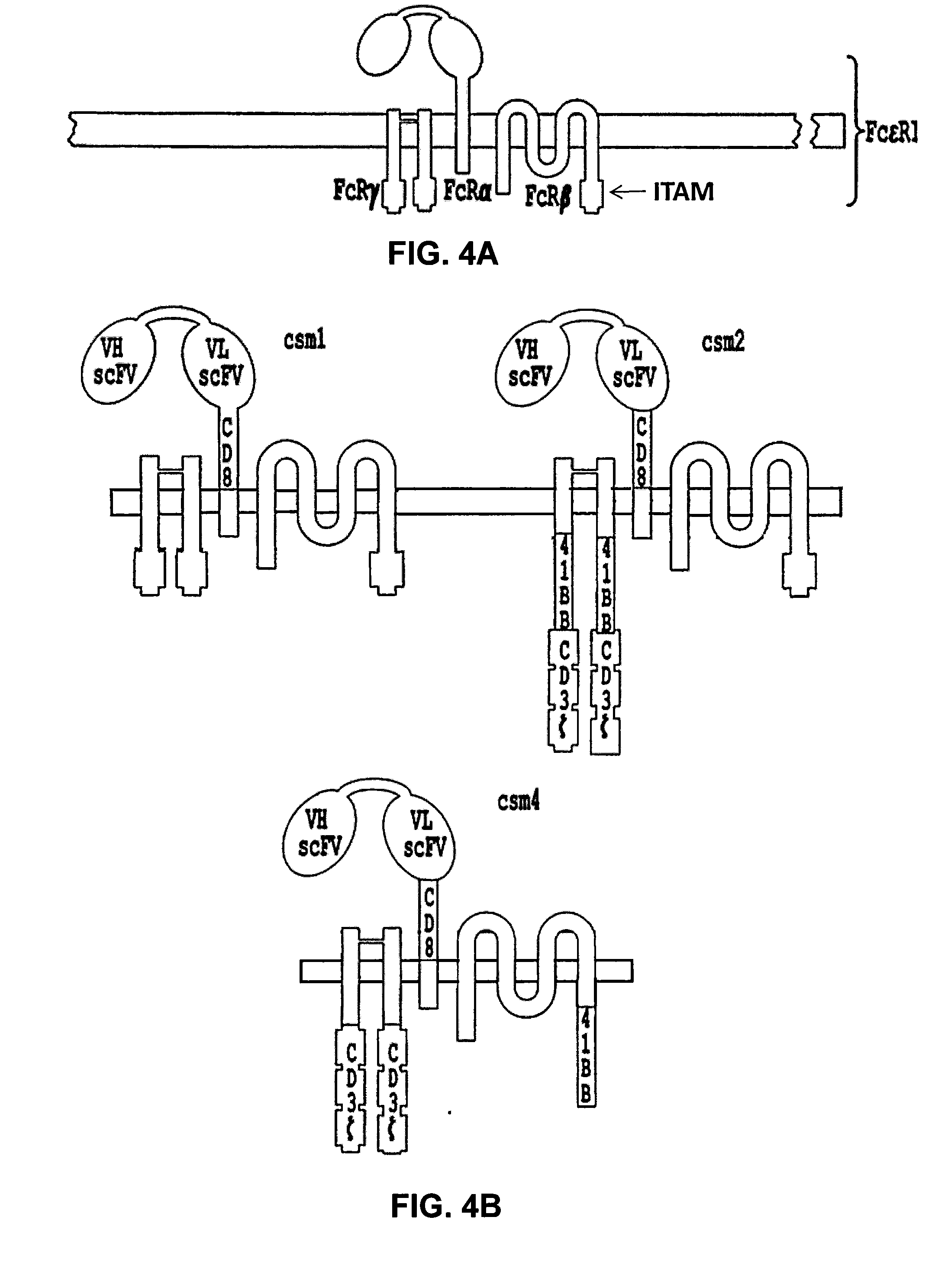
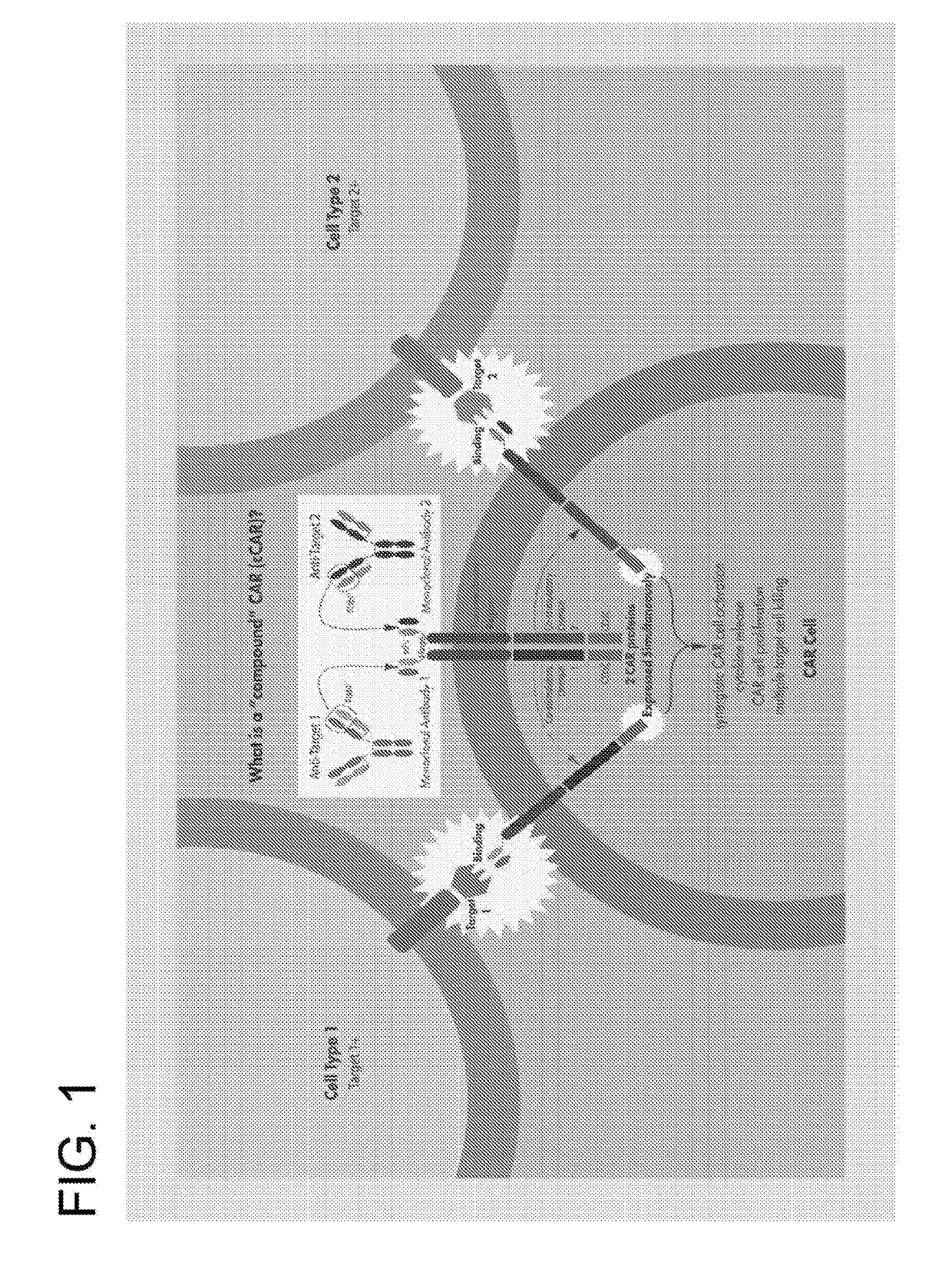
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
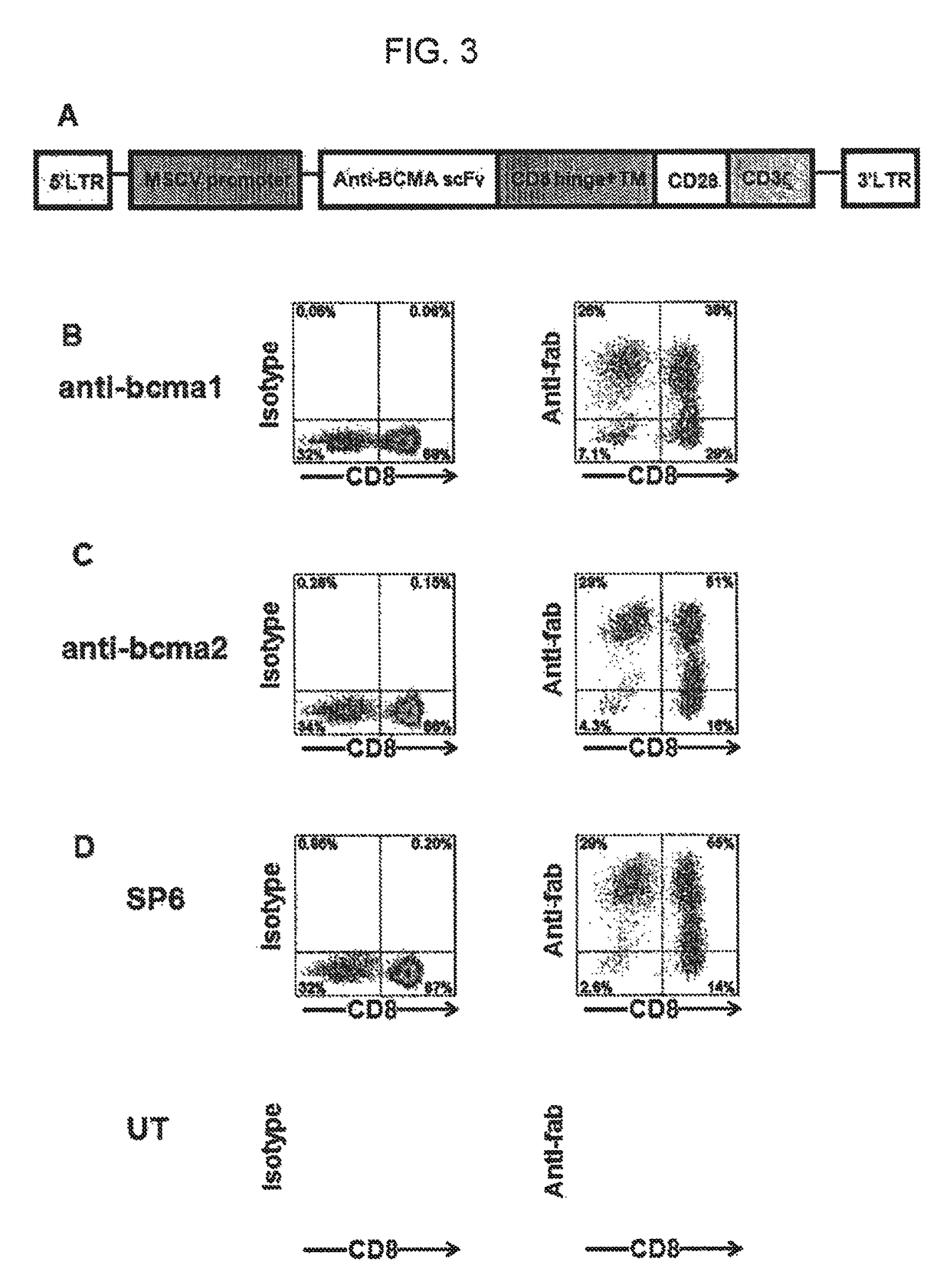
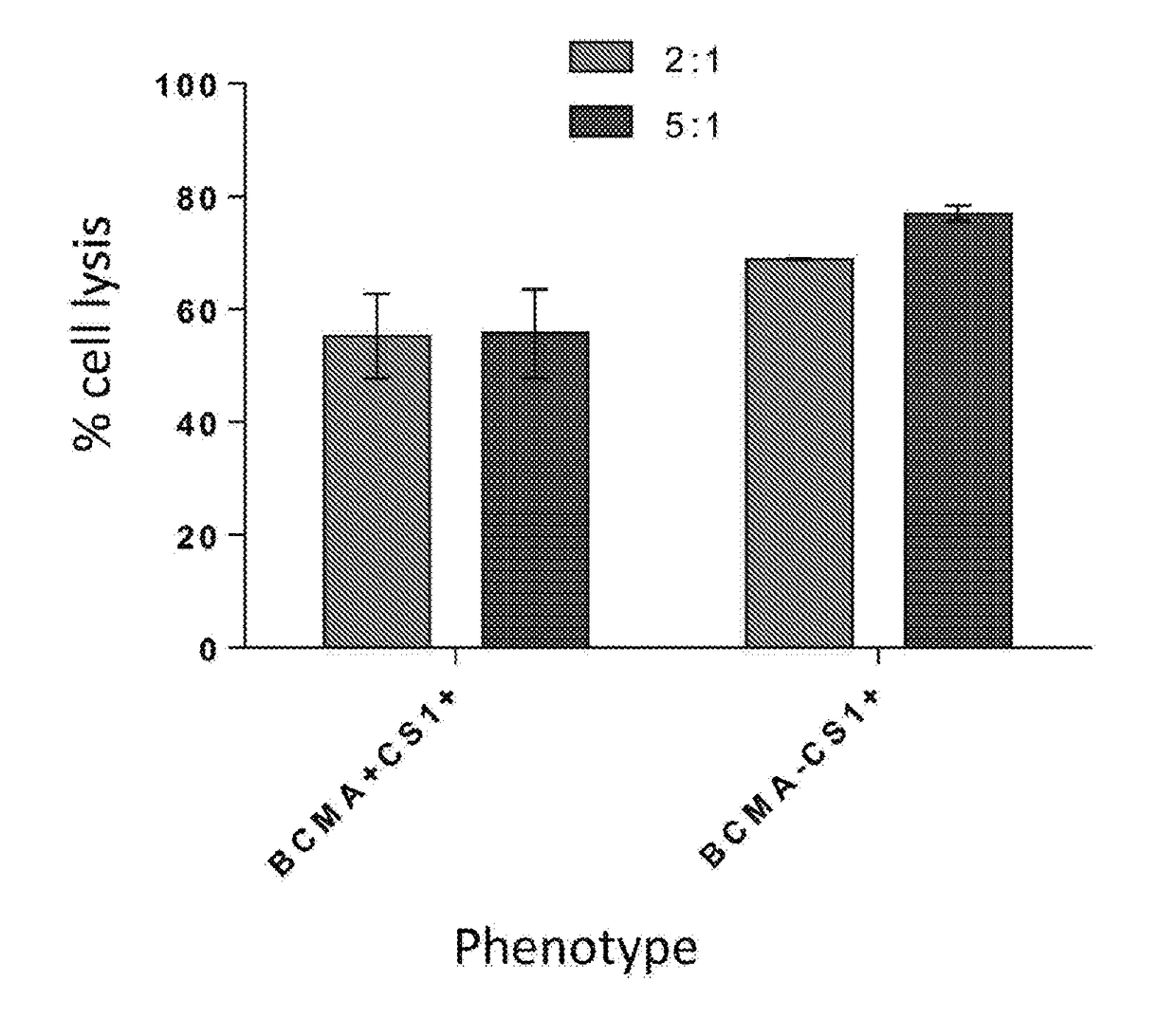
Chimeric antigen receptors targeting b-cell maturation antigen
ActiveUS20150051266A1Antibody mimetics/scaffoldsGenetic material ingredientsAntigenAntigen receptors
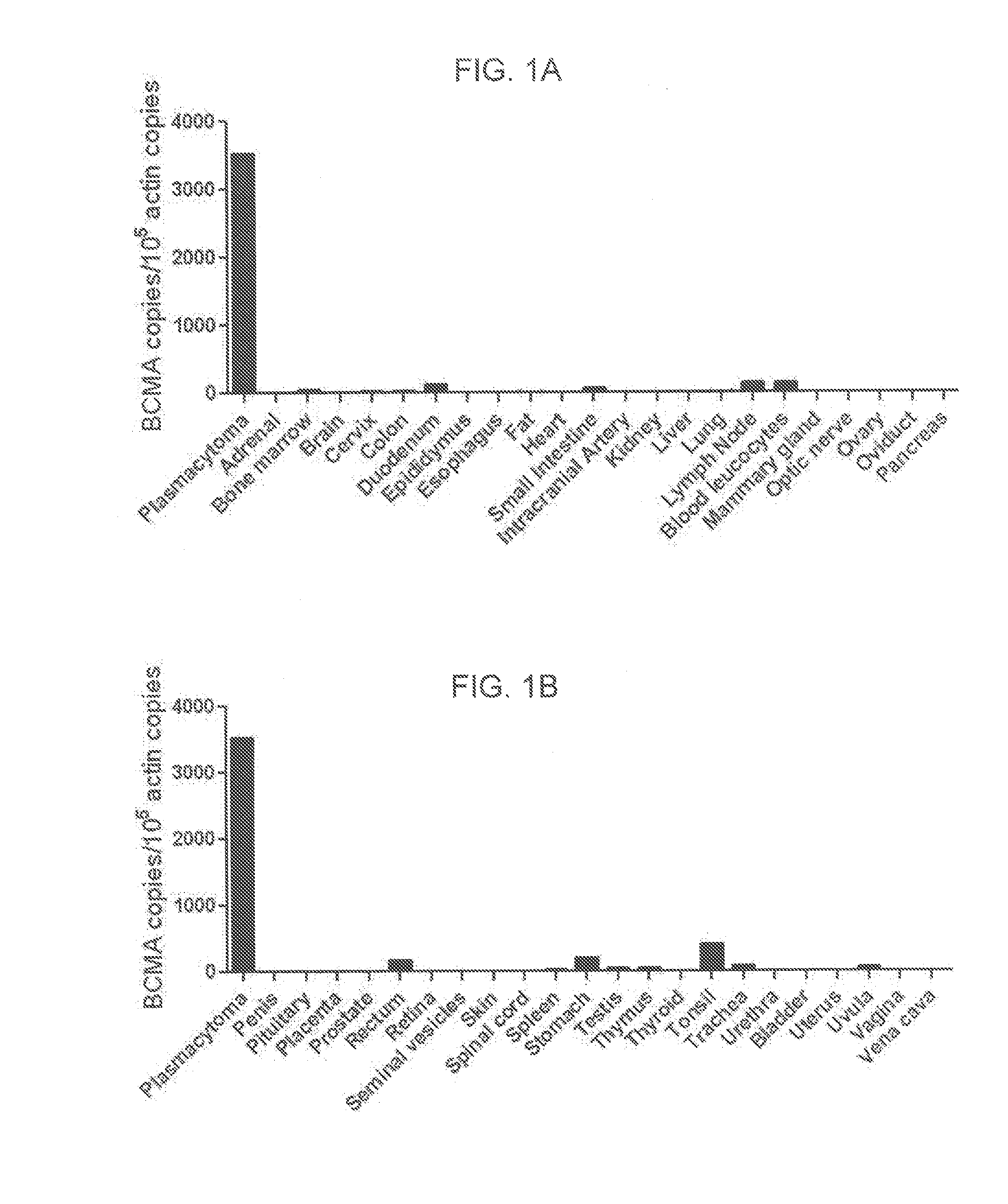
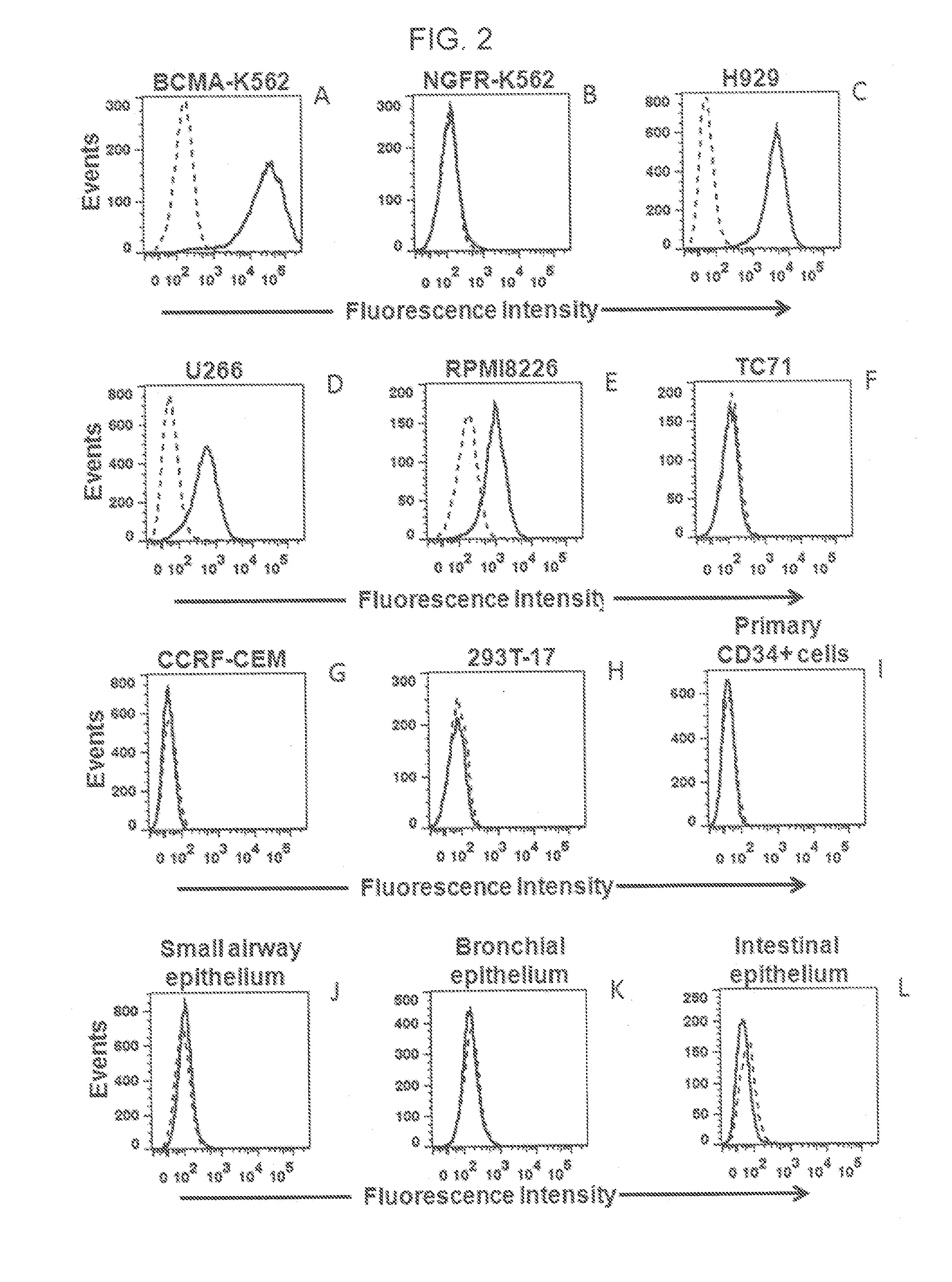
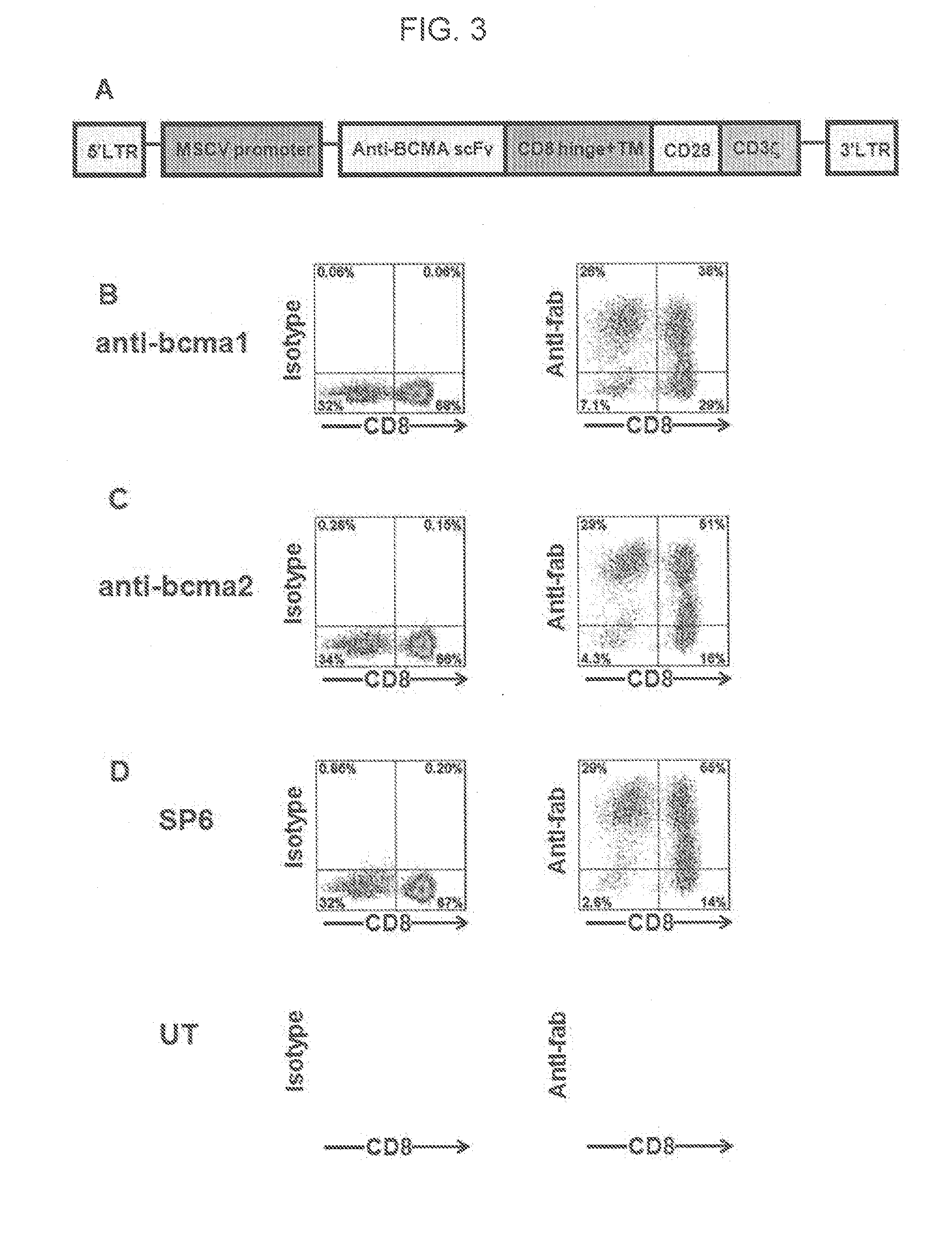
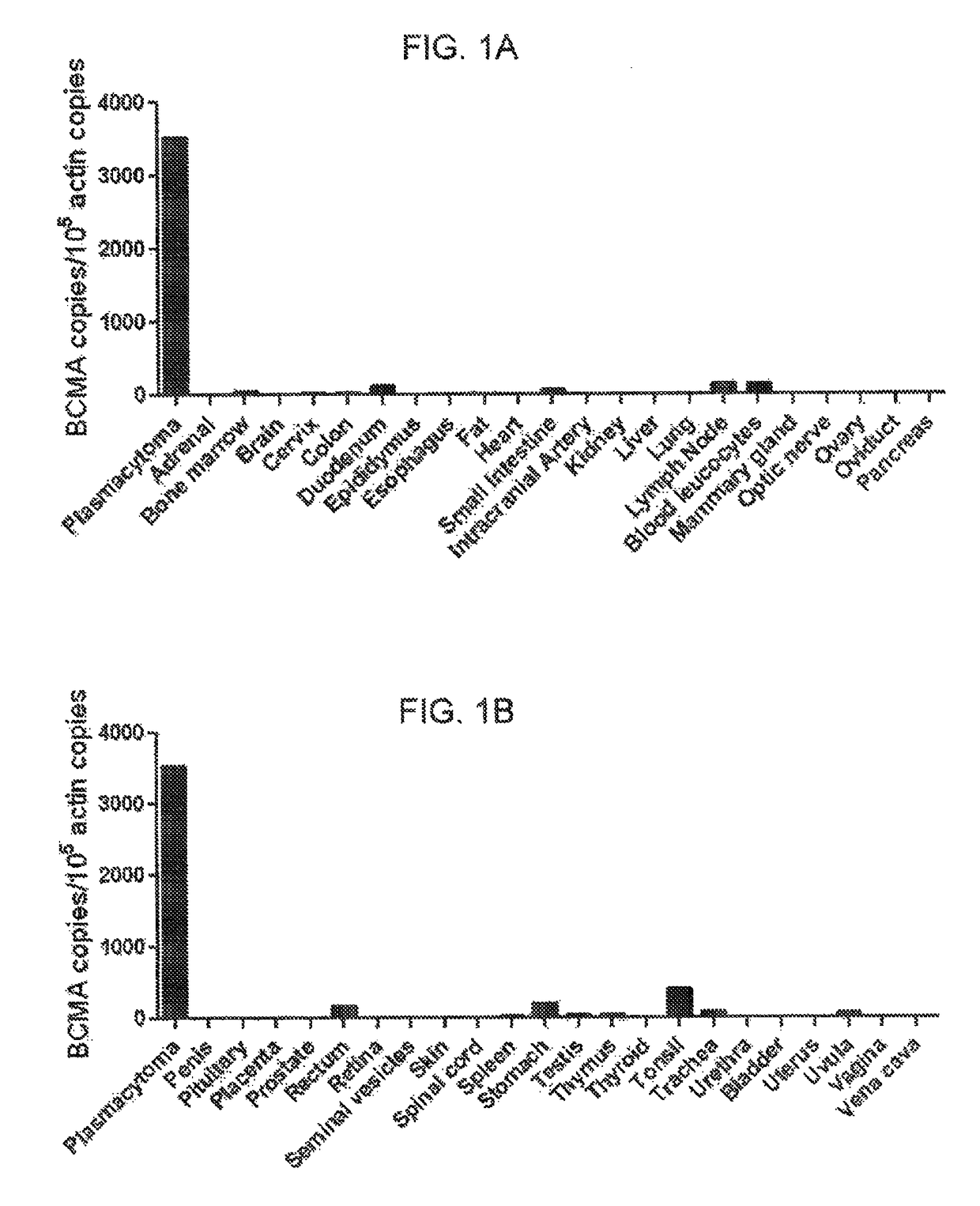
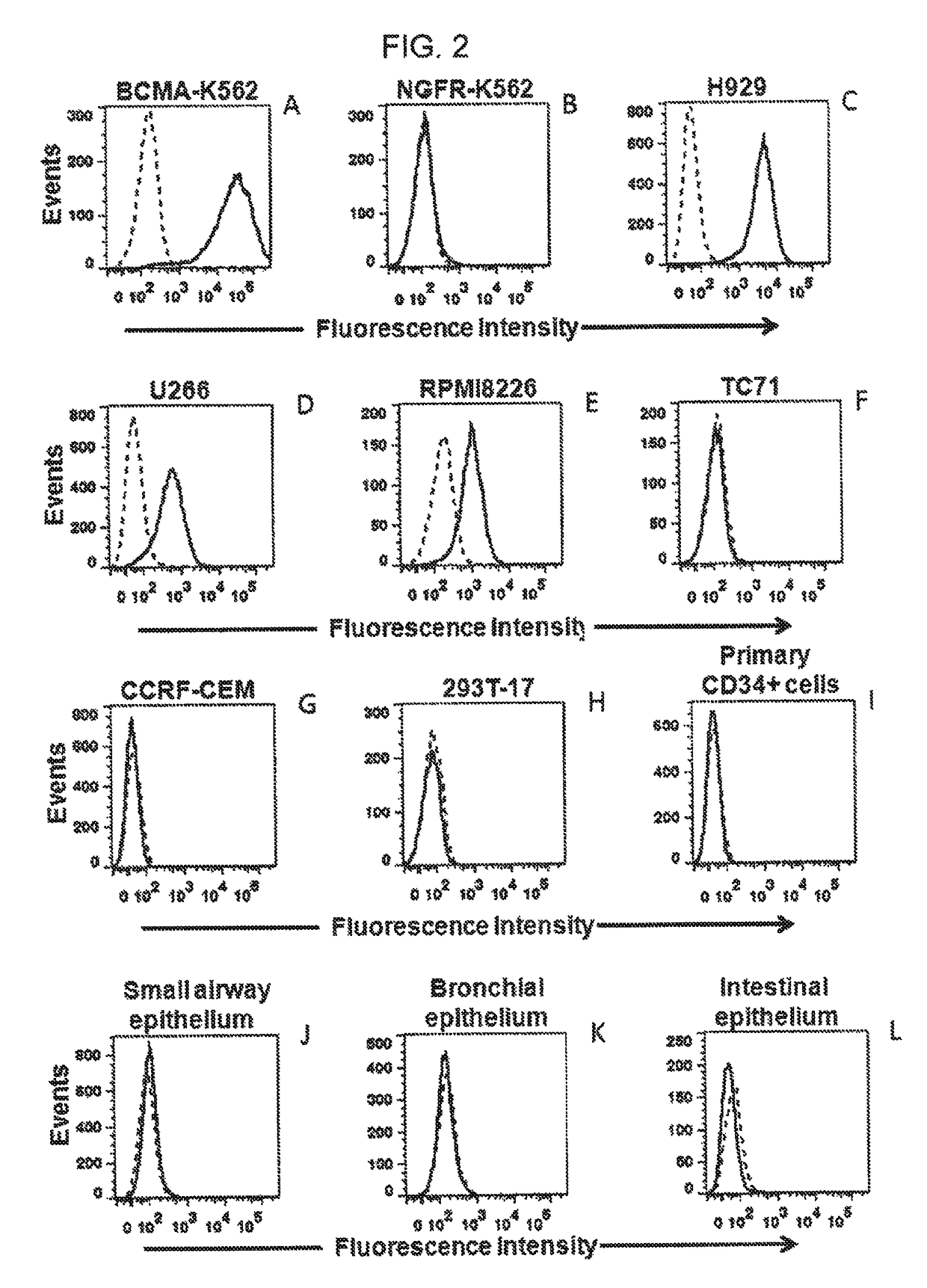
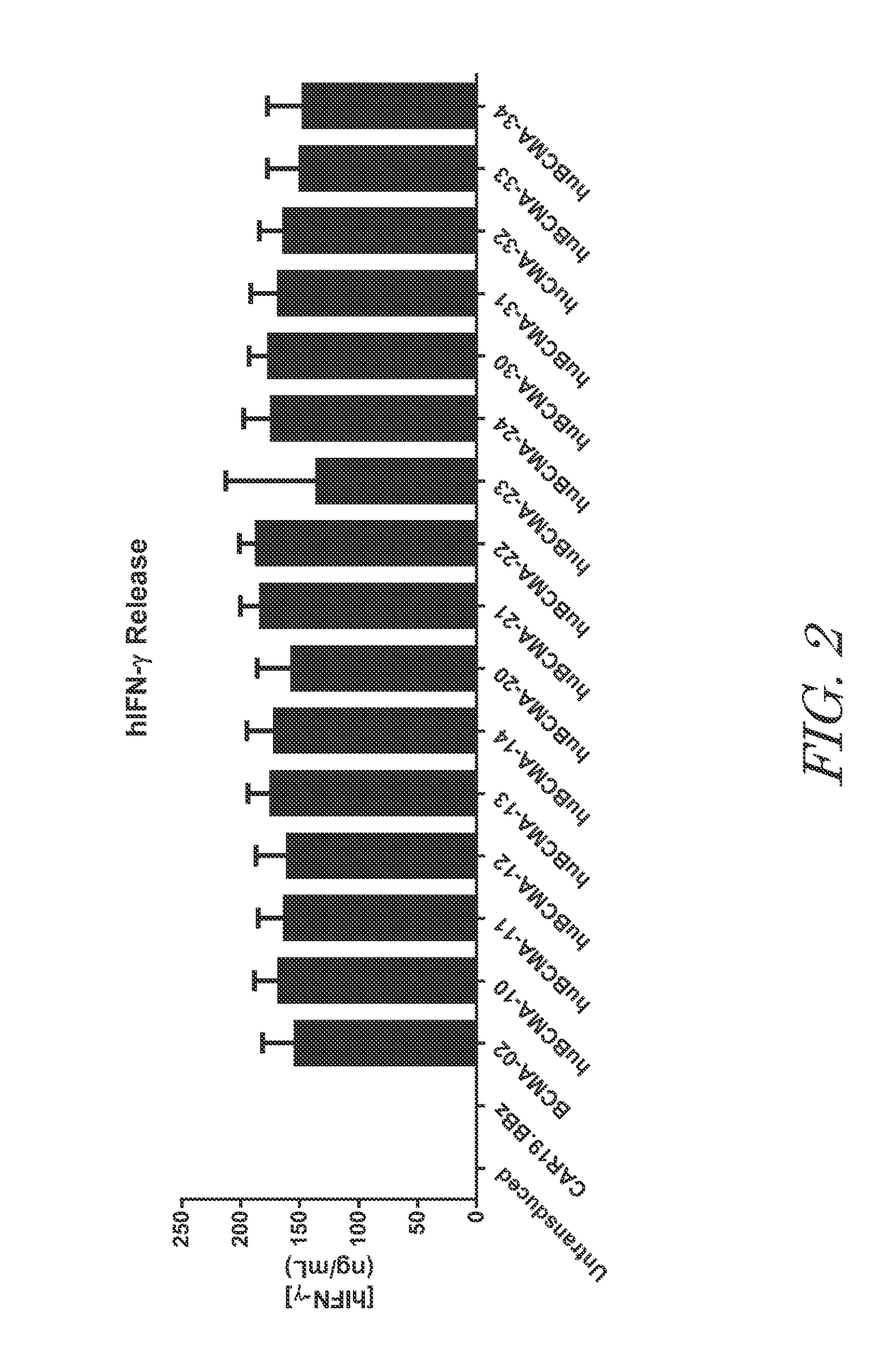
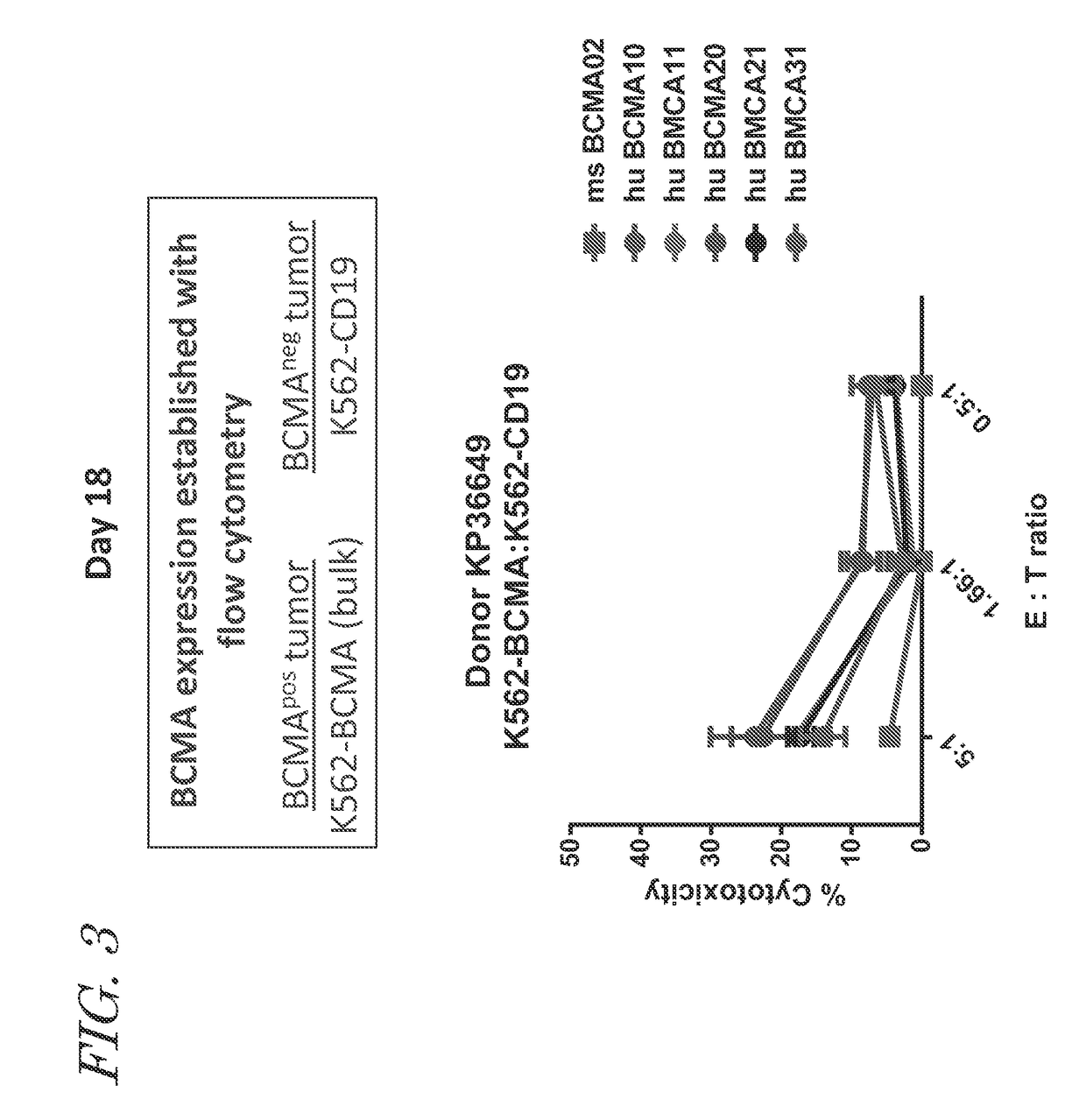
The invention provides an isolated and purified nucleic acid sequence encoding a chimeric antigen receptor (CAR) directed against B-cell Maturation Antigen (BCMA). The invention also provides host cells, such as T-cells or natural killer (NK) cells, expressing the CAR and methods for destroying multiple myeloma cells.
Owner:UNITED STATES OF AMERICA
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
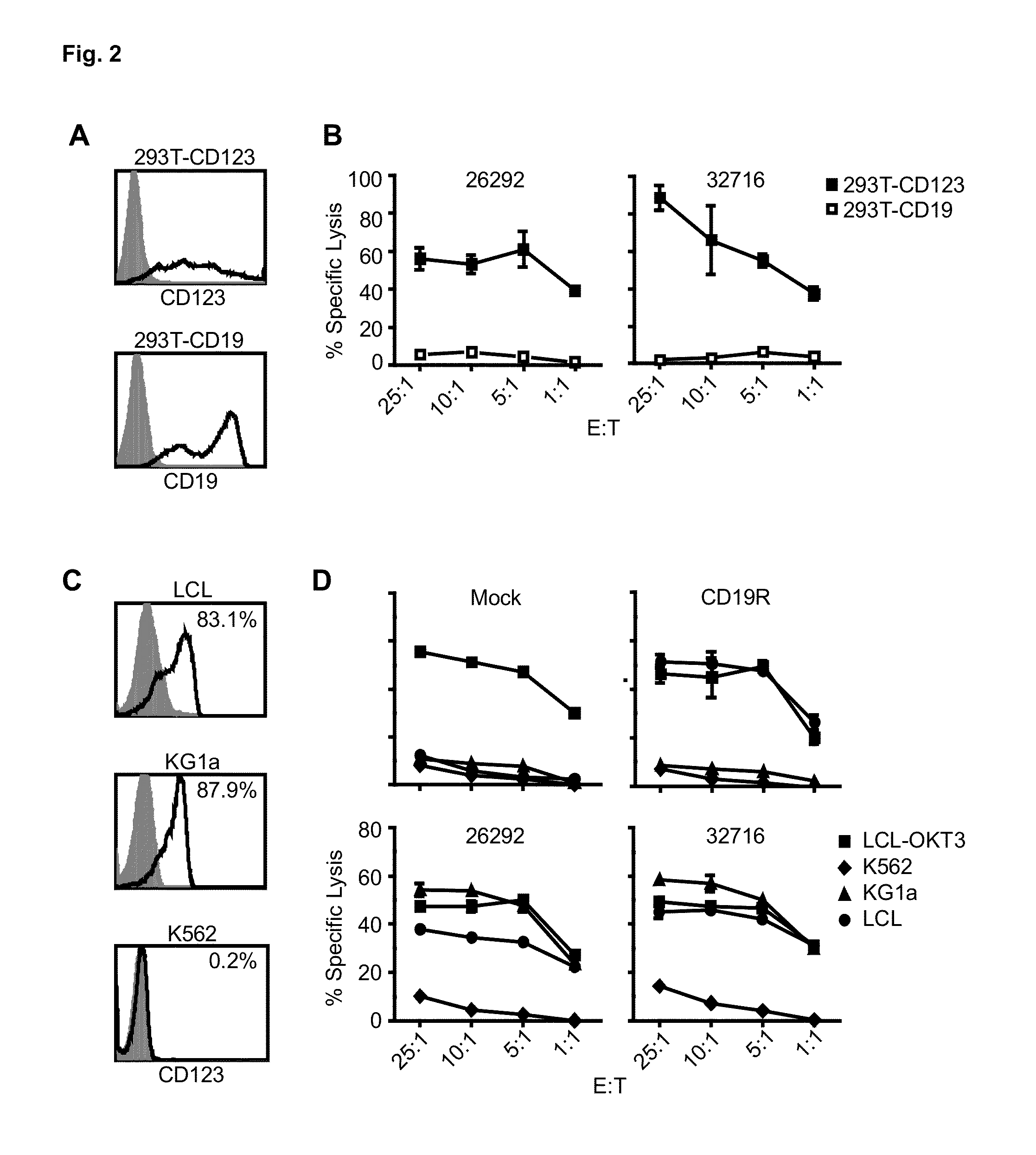
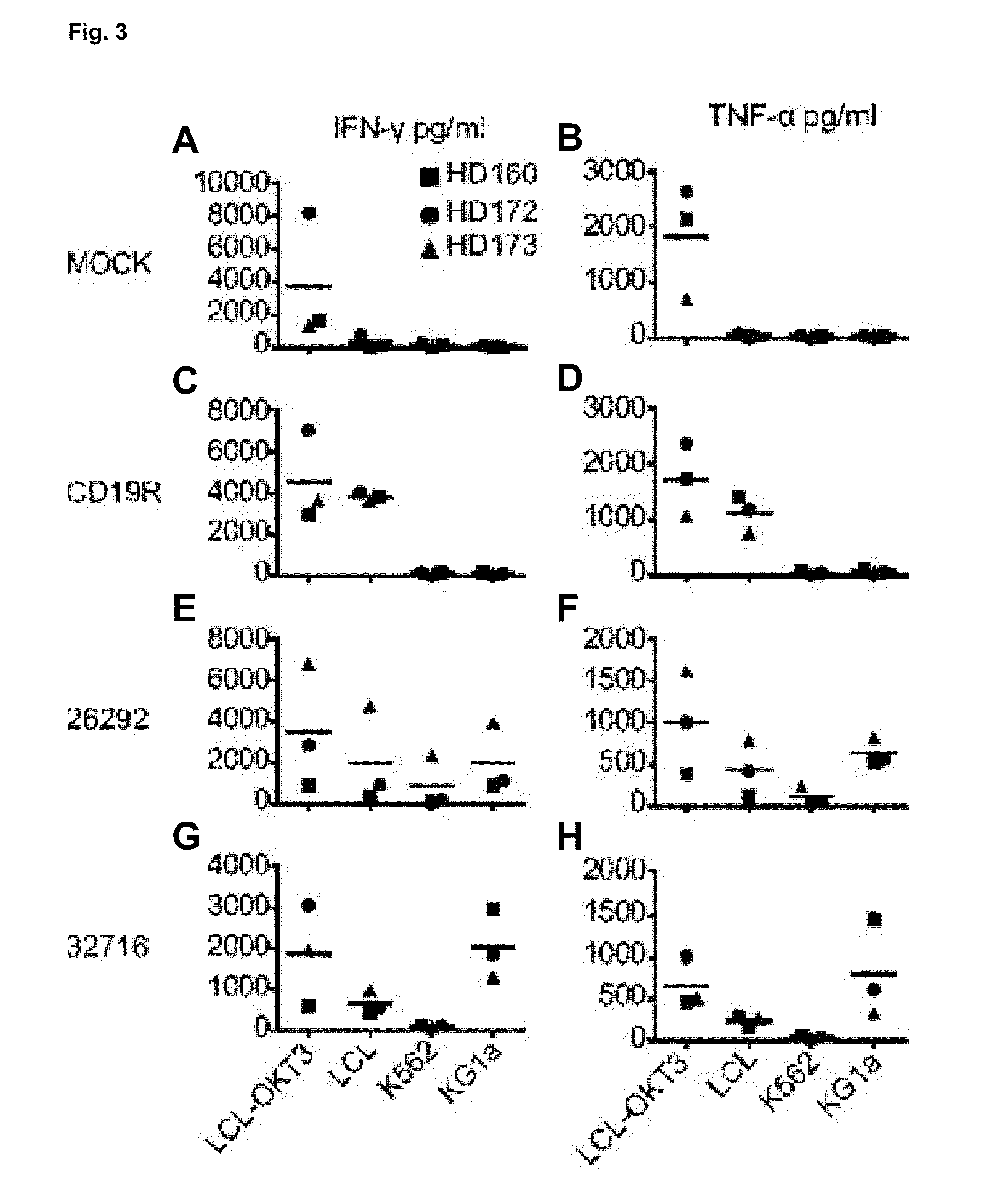
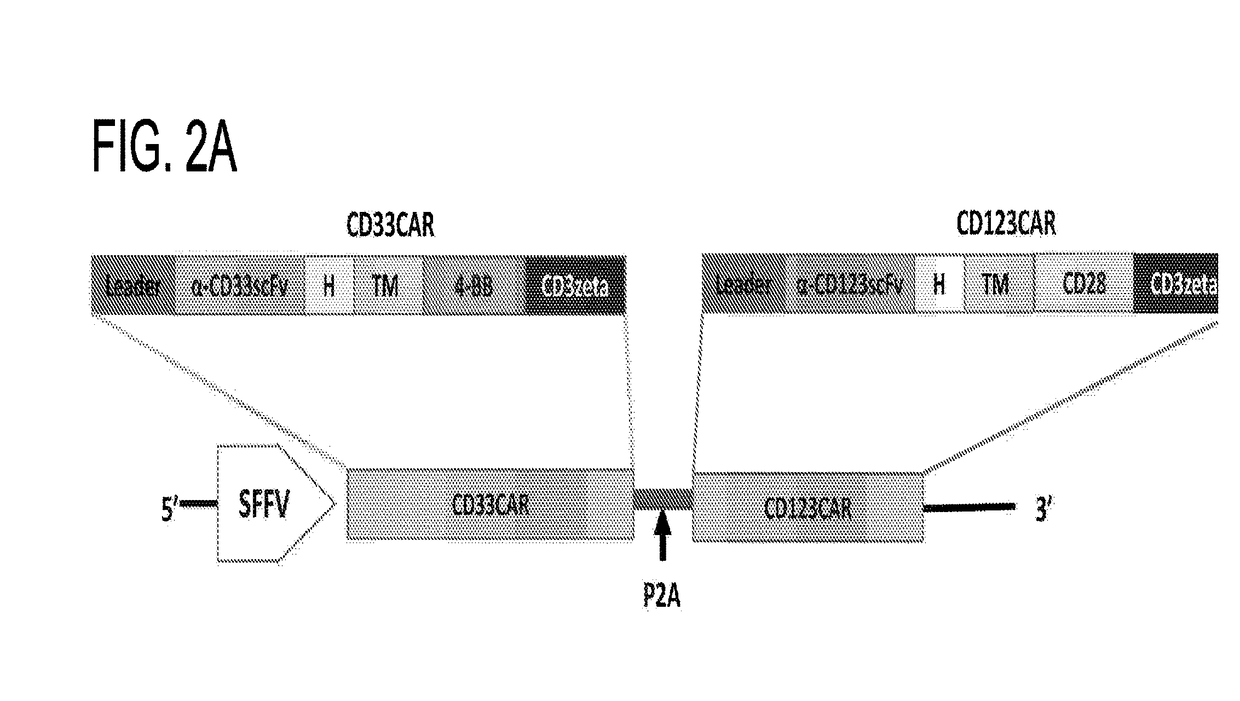
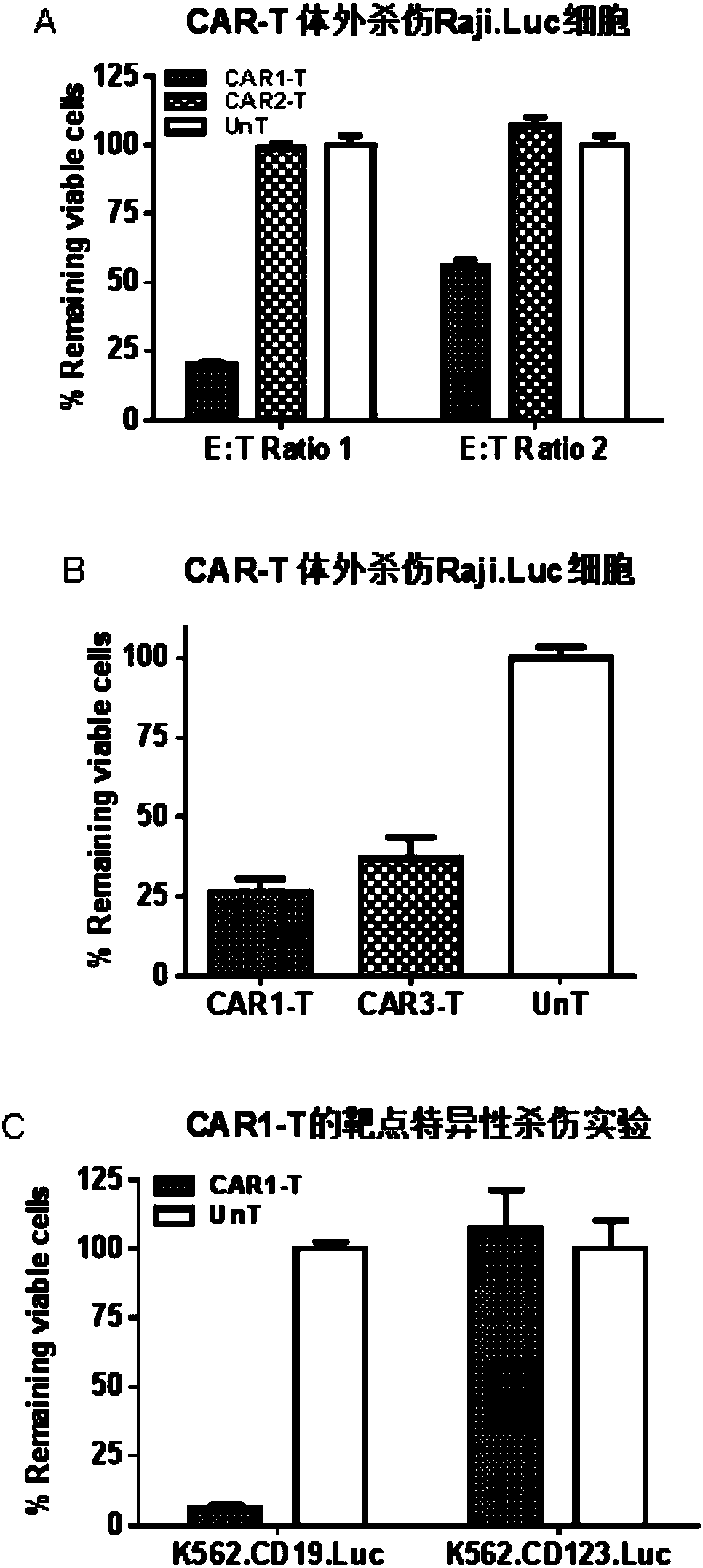
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
Chimeric antigen receptors targeting B-cell maturation antigen
ActiveUS9765342B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenAntigen receptors
The invention provides an isolated and purified nucleic acid sequence encoding a chimeric antigen receptor (CAR) directed against B-cell Maturation Antigen (BCMA). The invention also provides host cells, such as T-cells or natural killer (NK) cells, expressing the CAR and methods for destroying multiple myeloma cells.
Owner:UNITED STATES OF AMERICA
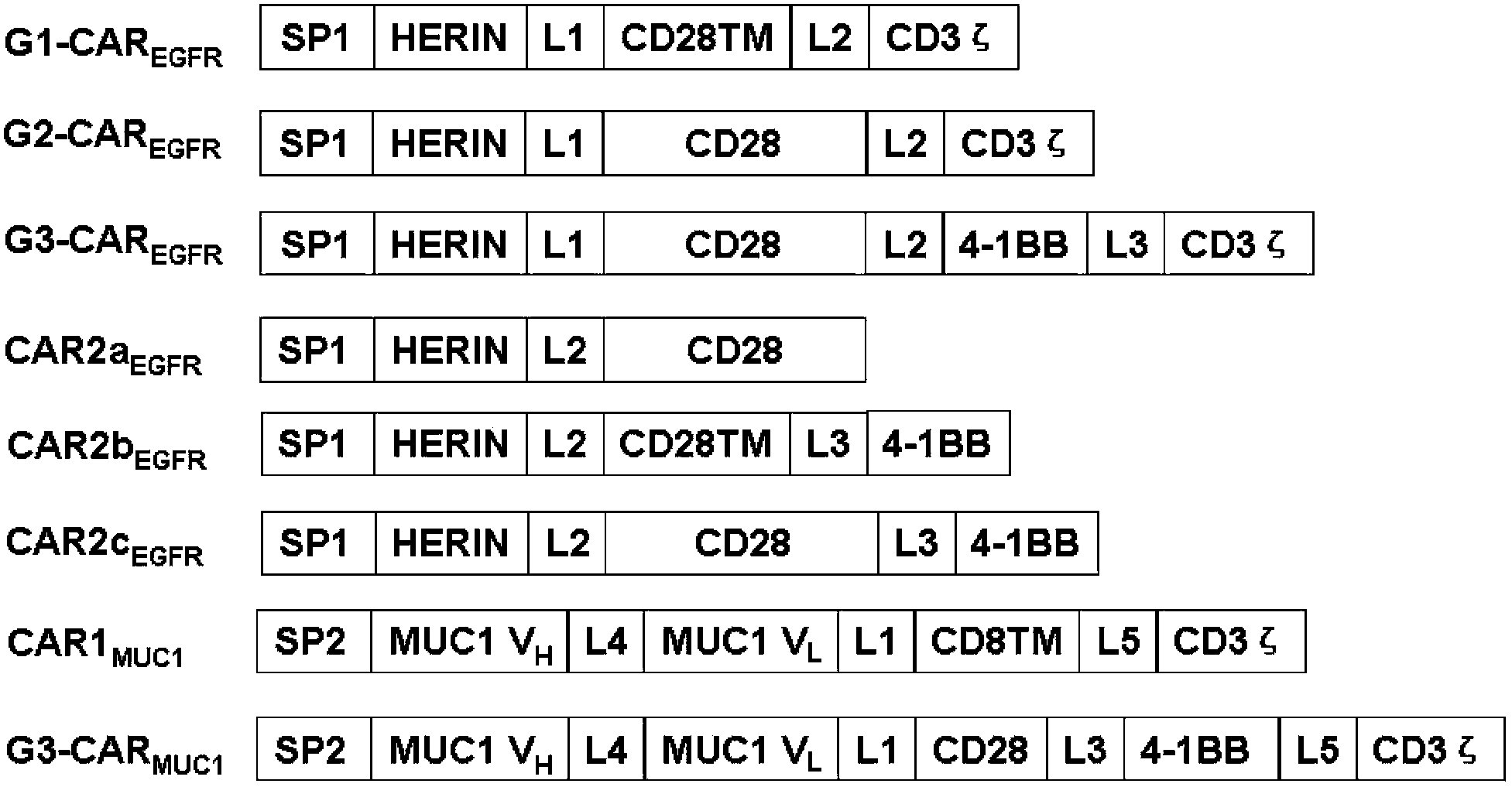
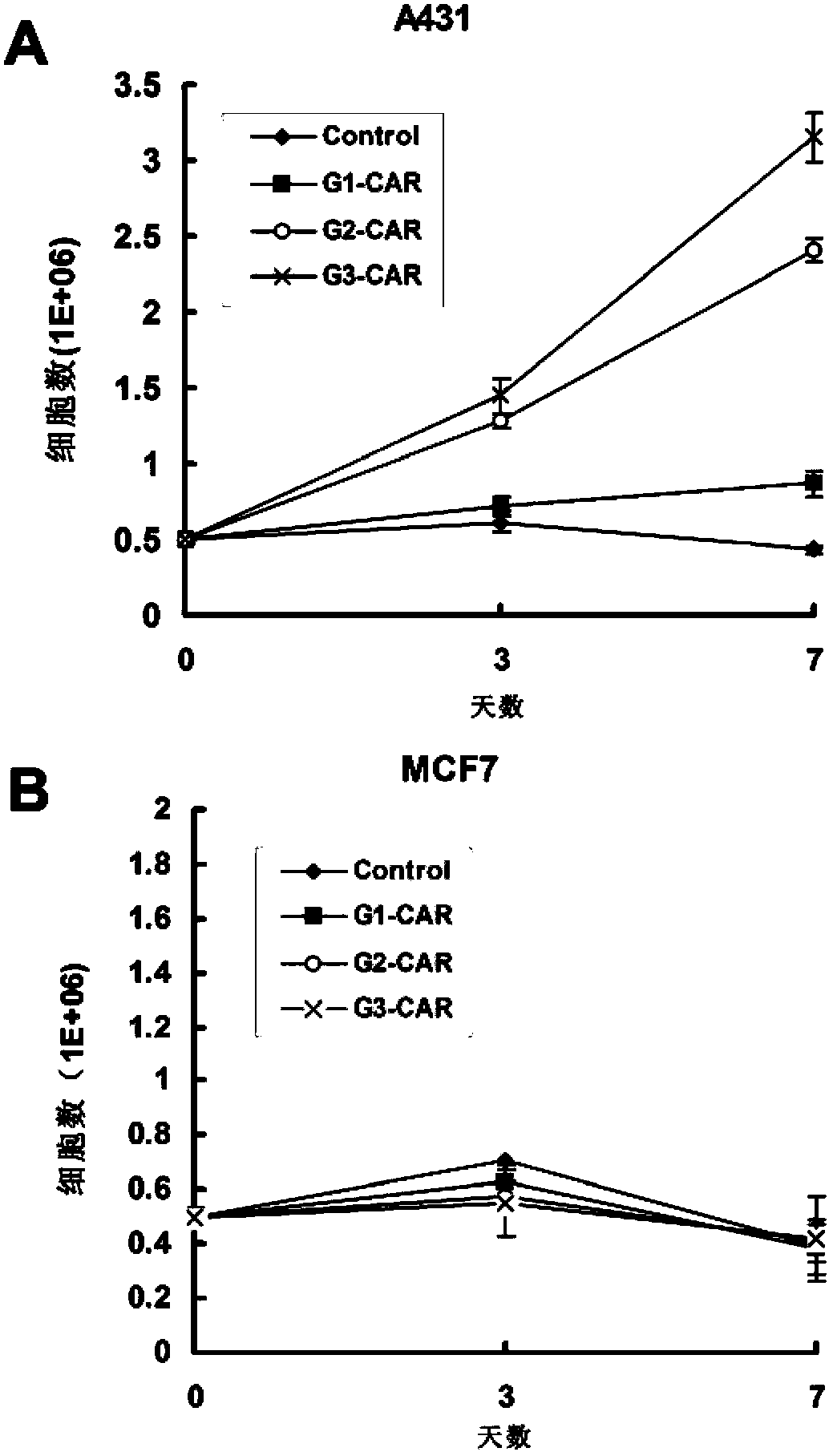
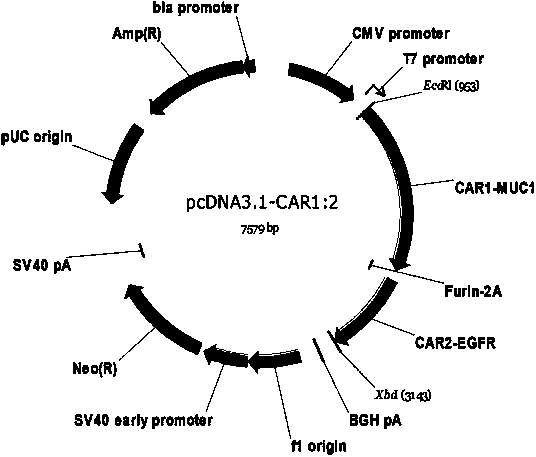
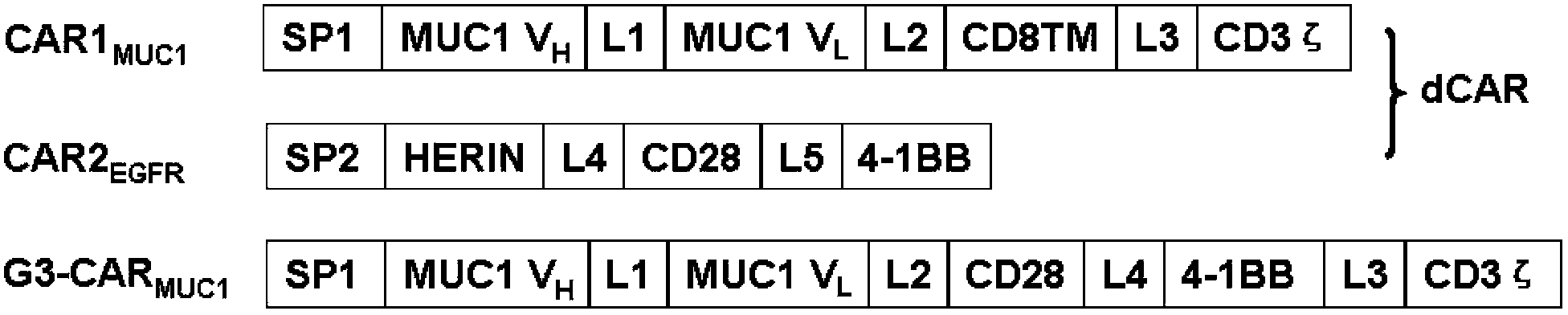
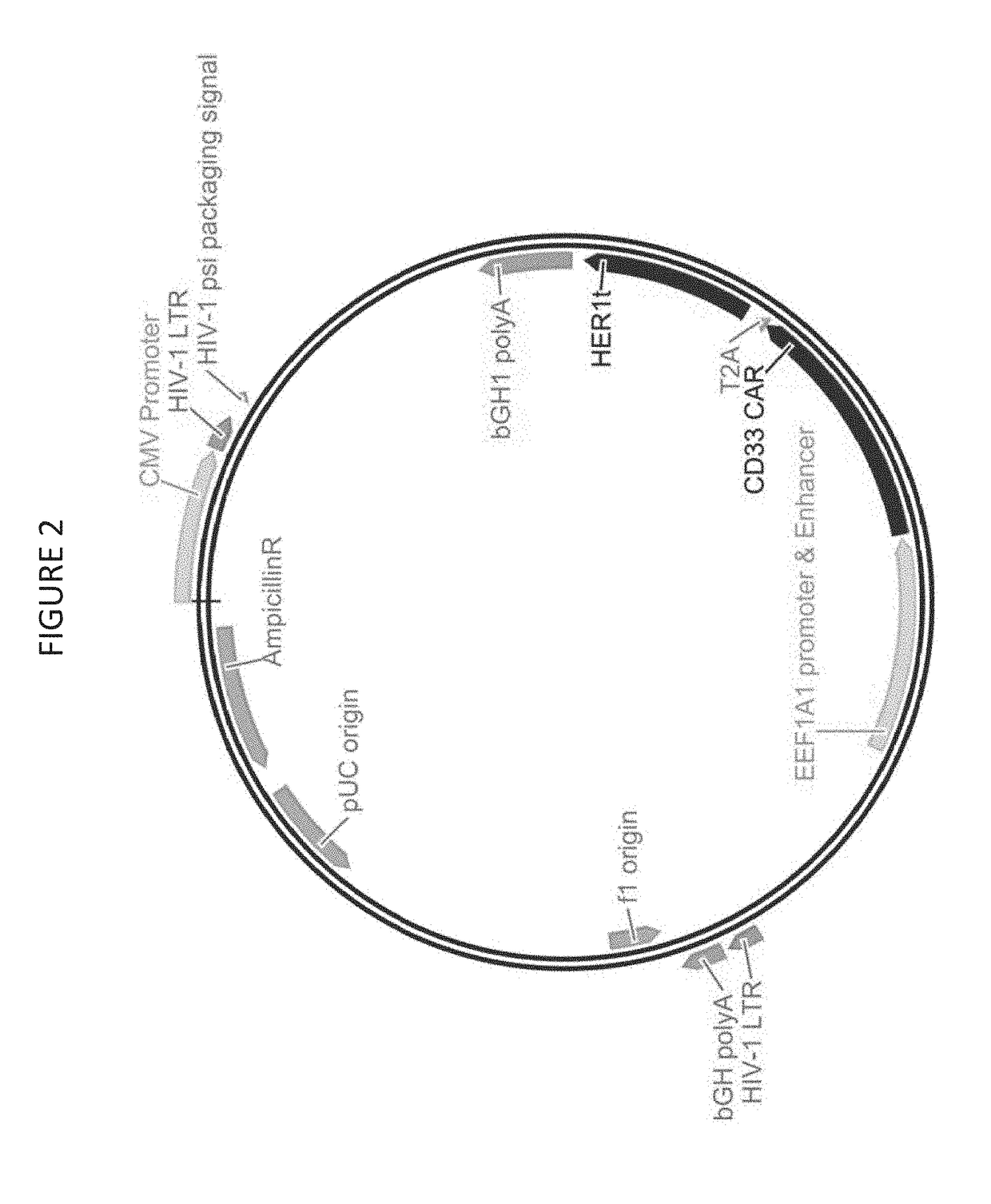
Chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and composition and uses thereof
ActiveCN103483453AFix security issuesImprove proliferative abilityPeptide/protein ingredientsGenetic material ingredientsEpidermal Growth Factor Receptor KinaseAntigen
The present invention belongs to the fields of molecular biology and immunology and relates to a chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and a composition and uses thereof The present invention particularly relates to the chimeric antigen receptor comprising polypeptides efficiently combining the EGFR family proteins and transmembrane domains, wherein the polypeptides efficiently combining EGFR family proteins comprise HERIN. The chimeric antigen receptor can promote the proliferation ability and killing effect of T cell at the premise of maintaining the killing specificity of the T cell.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Bispecific chimeric antigen receptors and encoding polynucleotides thereof
ActiveUS9447194B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellularAntigen receptors
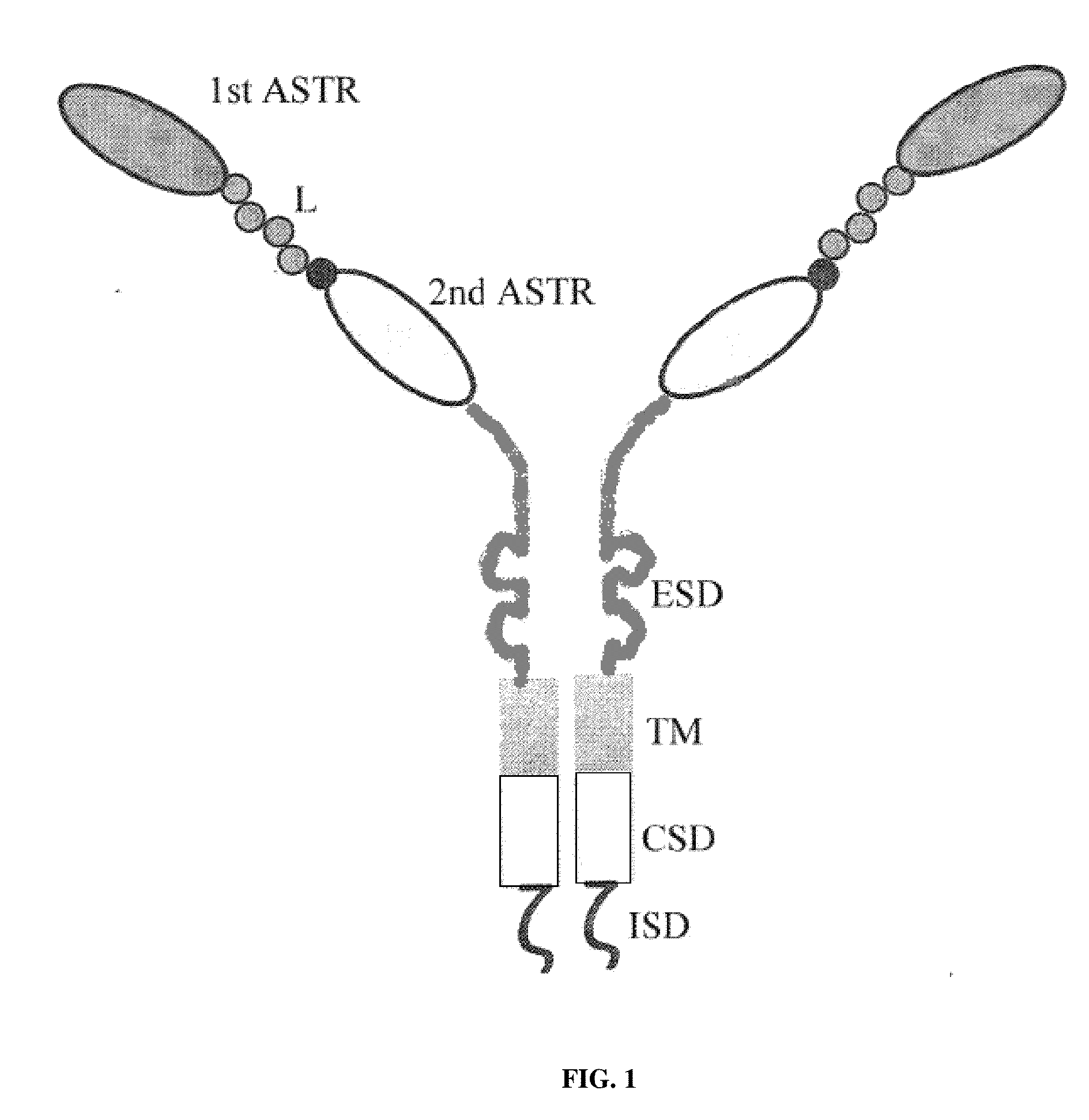
The invention is directed to a bispecific chimeric antigen receptor, comprising: (a) at least two antigen-specific targeting regions; (b) an extracellular spacer domain; (c) a transmembrane domain; (d) at least one co-stimulatory domain; and (e) an intracellular signaling domain, wherein each antigen-specific targeting region comprises an antigen-specific single chain Fv (scFv) fragment, and binds a different antigen, and wherein the bispecific chimeric antigen receptor is co-expressed with a therapeutic control. The invention also provides methods and uses of the bispecific chimeric antigen receptors.
Owner:SEATTLE CHILDRENS HOSPITAL
Isolated monoclonal antibodies that specifically bind to human Claudin 18.2
ActiveCN109762067AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonal antibody 14G2AAntigen receptors
Owner:BEIJING MABWORKS BIOTECH
Fully human, Anti-mesothelin specific chimeric immune receptor for redirected mesothelin-expressing cell targeting
The present invention relates to compositions and methods for treating diseases, disorders or conditions associated with dysregulated expression of mesothelin. In one embodiment, the invention relates to a fully human chimeric antigen receptor (CAR) wherein the CAR is able to target mesothelin.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
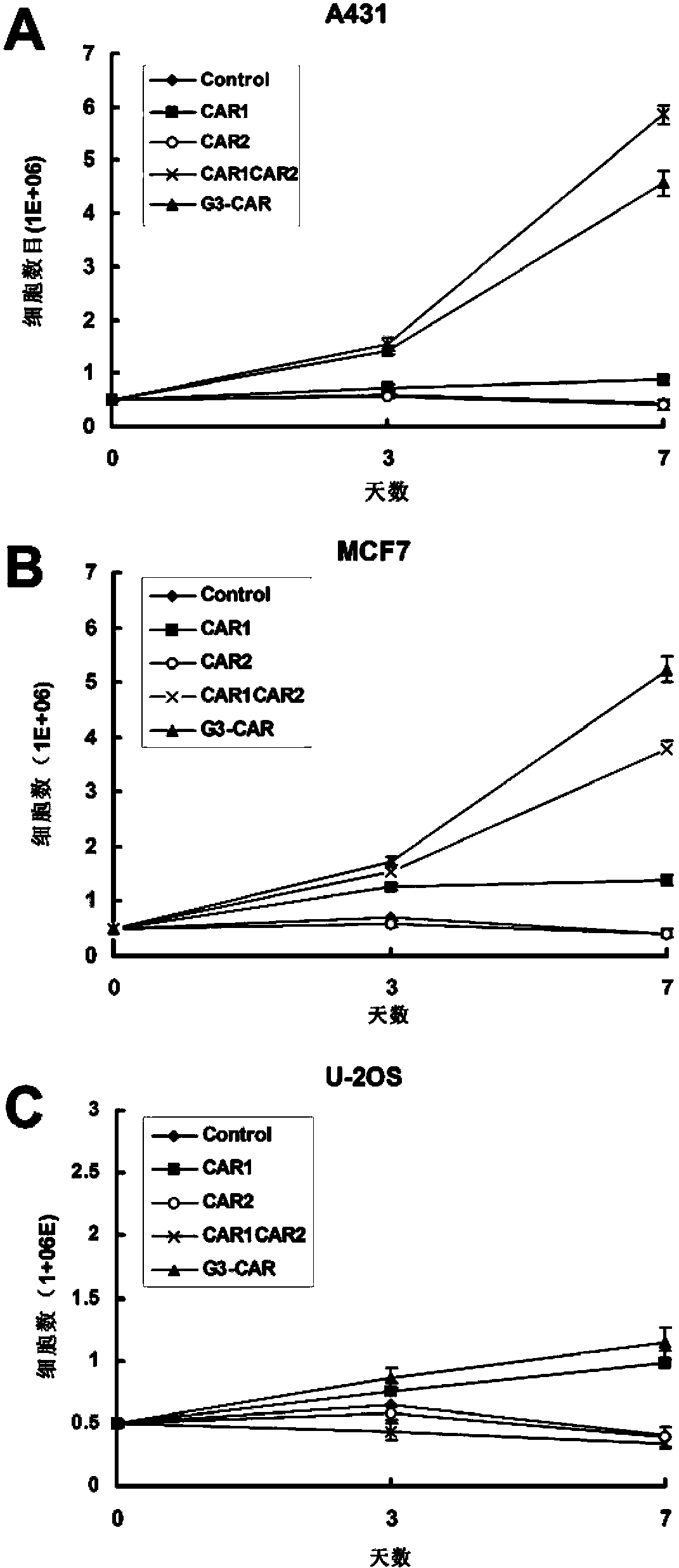
Dual-signal independent chimeric antigen receptors (dsCAR) and uses thereof
ActiveCN103483452APromote proliferationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsViral infectious disease
The invention relates to chimeric antigen receptors (CAR), particularly relates to dual-signal independent chimeric antigen receptors (dsCAR), and also relates to immune response cells of the dual-signal independent chimeric antigen receptors (dsCAR) and uses of the immune response cells in preparation of drugs for treatment of malignant tumor and virus infected diseases. In detail, the dual-signal independent chimeric antigen receptors (dsCAR) can respectively identify two different family antigens of tumor cells and can respectively transmit two T-cell-activation related signals. One of the CAR can transmit a first T-cell-activation related signal by combing a ligand of a tumor specific antigen or a tumor-associated antigen to decide T-cell killing specificity, and the other CAR can transmit a second T-cell-activation related signal by combing a ligand of a membrane receptor (such as EGFR (epidermal growth factor receptor) family protein) widely expressed by the tumor cells to promote T cell activation, proliferation and survival. The dual-signal independent chimeric antigen receptors (dsCAR) can avoid the potential safety problems on the basis of maintaining curative effects of second generation and third generation CAR.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
CHIMERIC ANTIGEN RECEPTORS (CARs), COMPOSITIONS AND METHODS OF USE THEREOF
ActiveUS20180187149A1Reduce in quantityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsCAR - Chimeric antigen receptor
The present invention relates to compositions and methods relating to chimeric antigen receptor (CAR) polypeptides and methods relating thereto. In one embodiment, the present invention relates to engineered cells having chimeric antigen receptor polypeptides directed to at least two targets. In another embodiment, the present invention relates to engineered cells having chimeric antigen receptor polypeptides and an enhancer moiety.
Owner:ICELL GENE THERAPEUTICS LLC
Novel chimeric antigen receptor and applications thereof
PendingCN108276493AMild release responseHigh ability to target and recognize tumor antigensPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
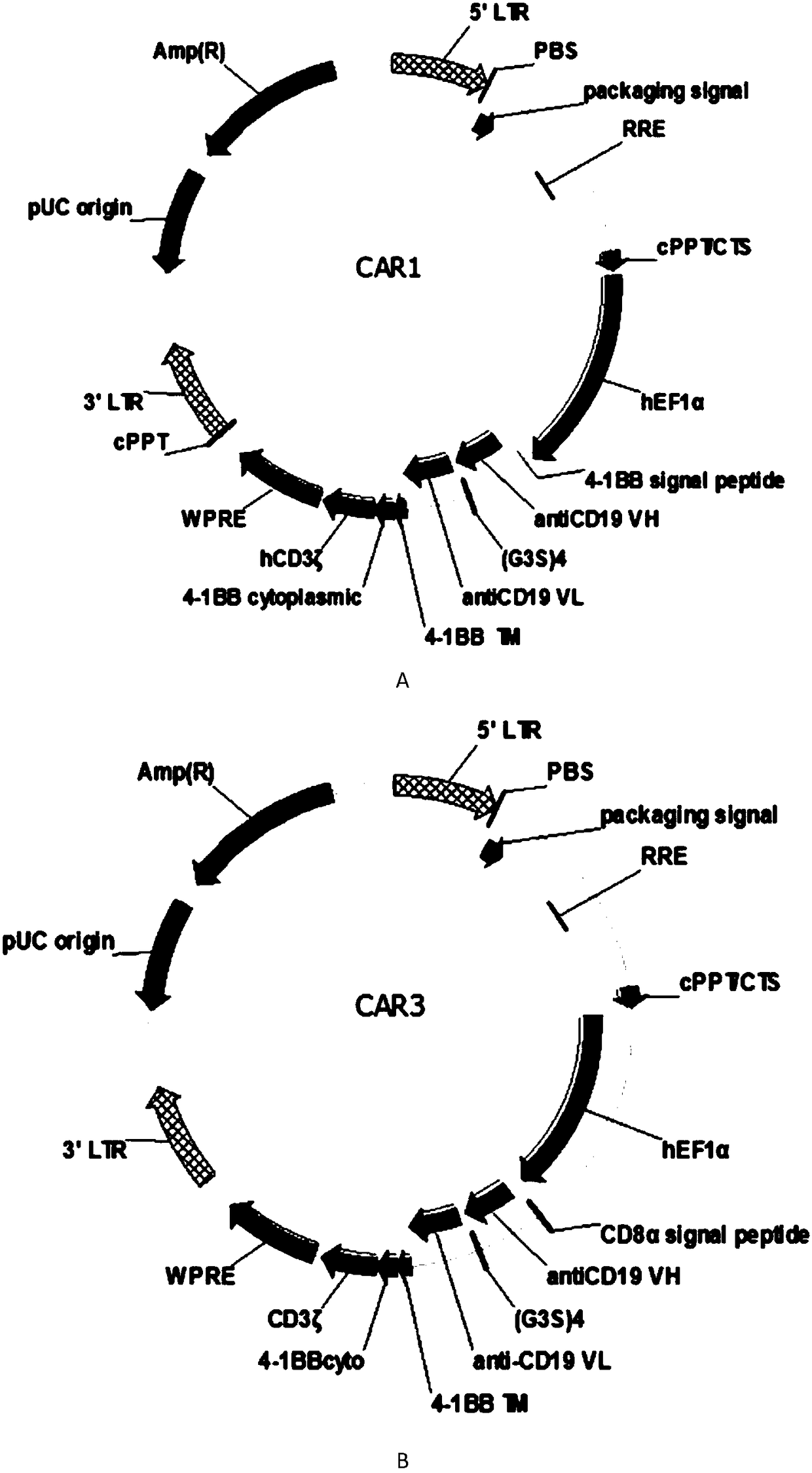
The present invention discloses a novel chimeric antigen receptor and applications thereof, wherein the novel chimeric antigen receptor comprises a signal peptide, an antigen binding domain, a transmembrane domain and an intracellular signal domain, and comprises a 4-1BB signal peptide and / or a 4-1BB molecular transmembrane domain. According to the present invention, a variety of chimeric antigenreceptor nucleic acid sequences are separated and purified, the chimeric antigen receptor specifically for CD19 malignant tumor antigens and the CAR-T cells are provided, and the blood cell line malignant tumor killing test results show that the tumor-cell-targeting ability of immune cells is significantly enhanced, and the tumor cell killing activity is enhanced.
Owner:NANJING LEGEND BIOTECH CO LTD
Subset-optimized chimeric antigen receptor-containing t-cells
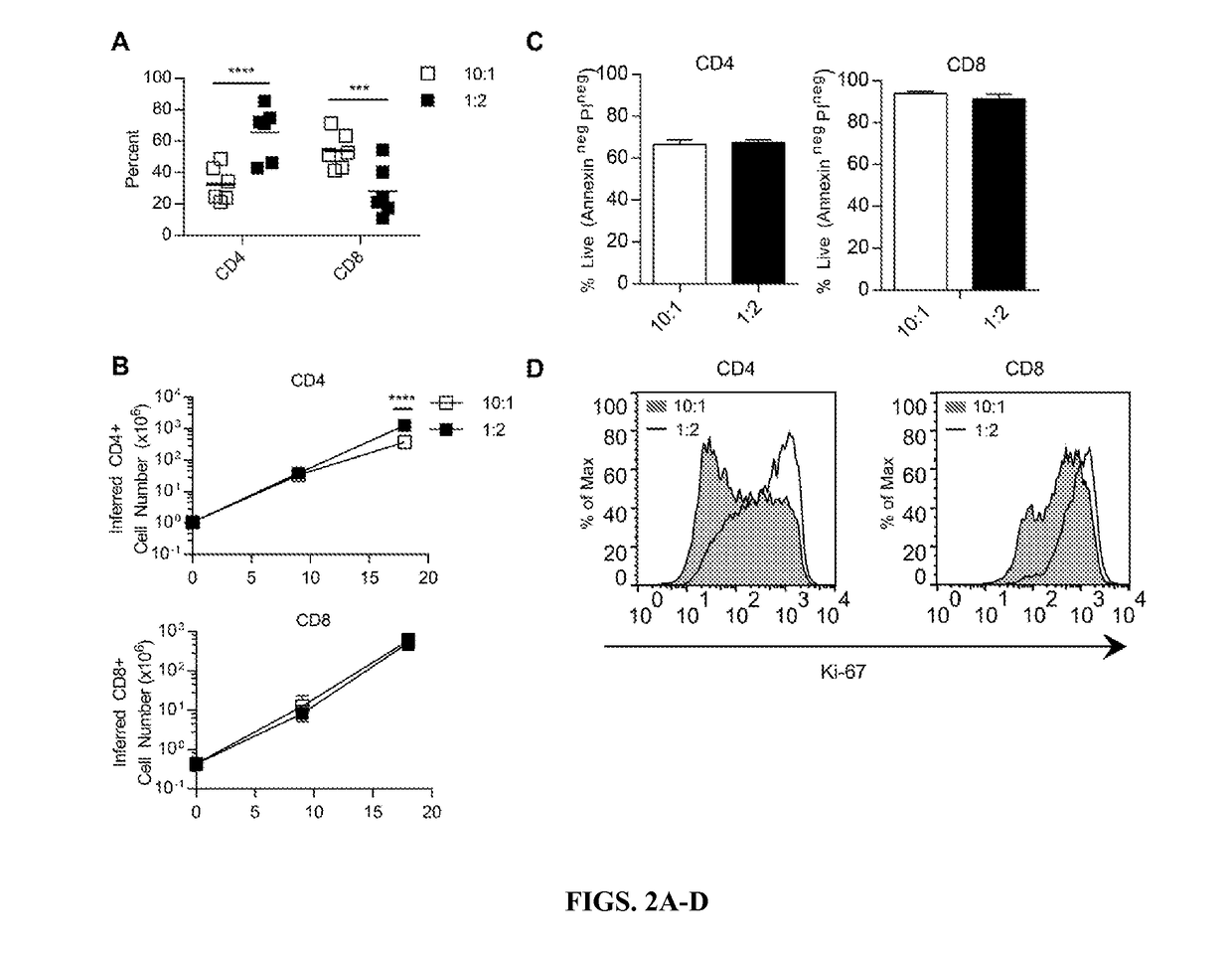
PendingUS20170209492A1Improve anti-tumor activityIncreased persistenceOrganic active ingredientsAntibody mimetics/scaffoldsDiseaseIntracellular signalling
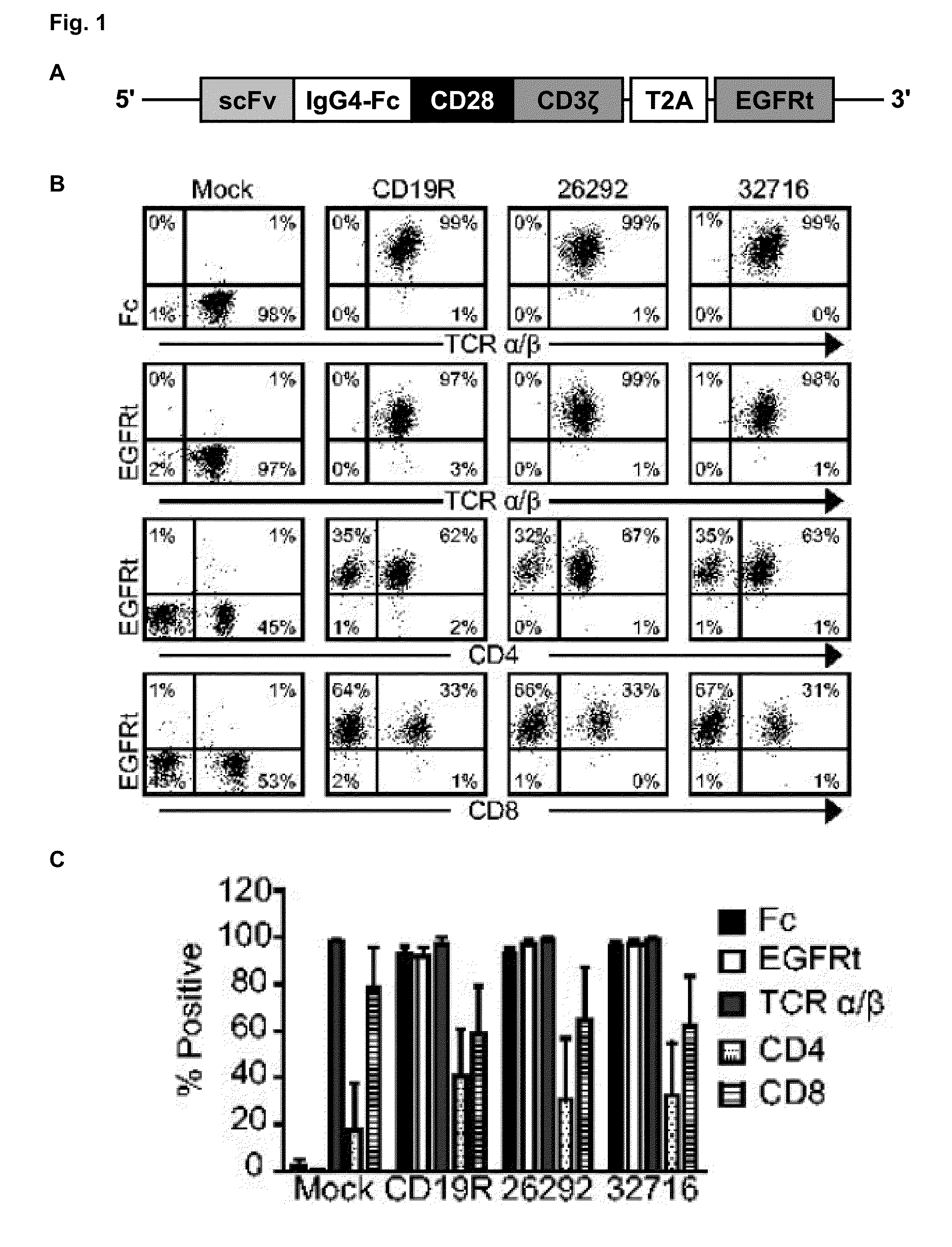
This disclosure provides, for instance subset-optimized CART cells and related methods. For instance, the disclosure describes methods and compositions of CD4′ and CD8′ T cells that express CARs containing specific combinations of intracellular signaling domains can be used to increase persistence and anti-tumor activity of the infused CAR-expressing T cells for treating a subject having a disease, e.g., a cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Bcma chimeric antigen receptors
InactiveUS20170226216A1Treating and preventing and ameliorating B cell related conditionVirusesPeptide/protein ingredientsAntigenAntigen receptors
Owner:2SEVENTY BIO INC
Chimeric antigen receptor and its use
InactiveCN103145849ASpeed up entryGood treatment effectGenetic material ingredientsAntiviralsLatent Membrane Protein-1Single-Chain Antibodies
The invention belongs to the biotechnical field of tumors, and discloses a preparation method of a chimeric antigen receptor and an application of the chimeric antigen receptor. The chimeric antigen receptor is formed through the structural series connection of a single chain antibody of a human anti-EB virus latent membrane protein 1, CH2CH3 of a human antibody IgG1, and intracellular signals of immune co-stimulating signal molecules CD28, CD134 and CD3zeta. The chimeric antigen receptor is used for modifying T lymphocytes, and the modified lymphocytes can be used for treating EB virus related tumors and preparing EB virus related tumor resisting medicines.
Owner:SINOBIOWAY CELL THERAPY CO LTD
Chimeric antigen receptor-targeting monoclonal antibodies
ActiveUS20160096902A1Easy to understandBacteriaImmunoglobulinsMonoclonal antibodyChimeric antigen receptor
Provided are monoclonal antibodies that detect CD 19 CAR-modified immune cells and CAR-modified immune cells irrespective of the tumor associated antigen they target. Methods of using these functional monoclonal antibodies include, but are not limited to, detection, quantification, activation, and selective propagation of CAR-modified immune cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chimeric antigen receptors (CAR) and methods for making and using the same
InactiveUS20170158749A1Facilitate cell targetingReduce off-target cytotoxicity of cellAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenCAR T-cell therapy
Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (e.g., cancer cells) with CAR T-cell therapies are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
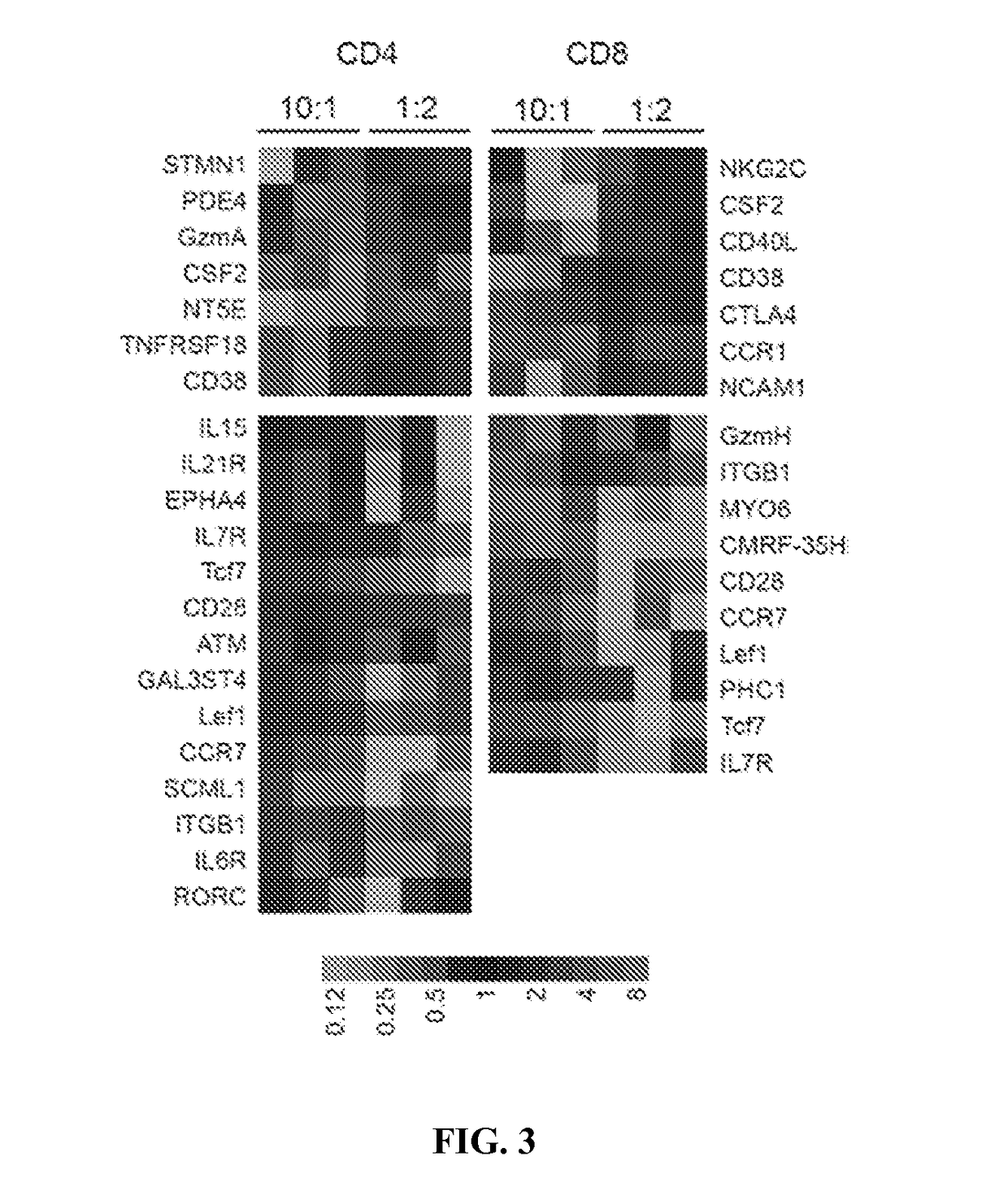
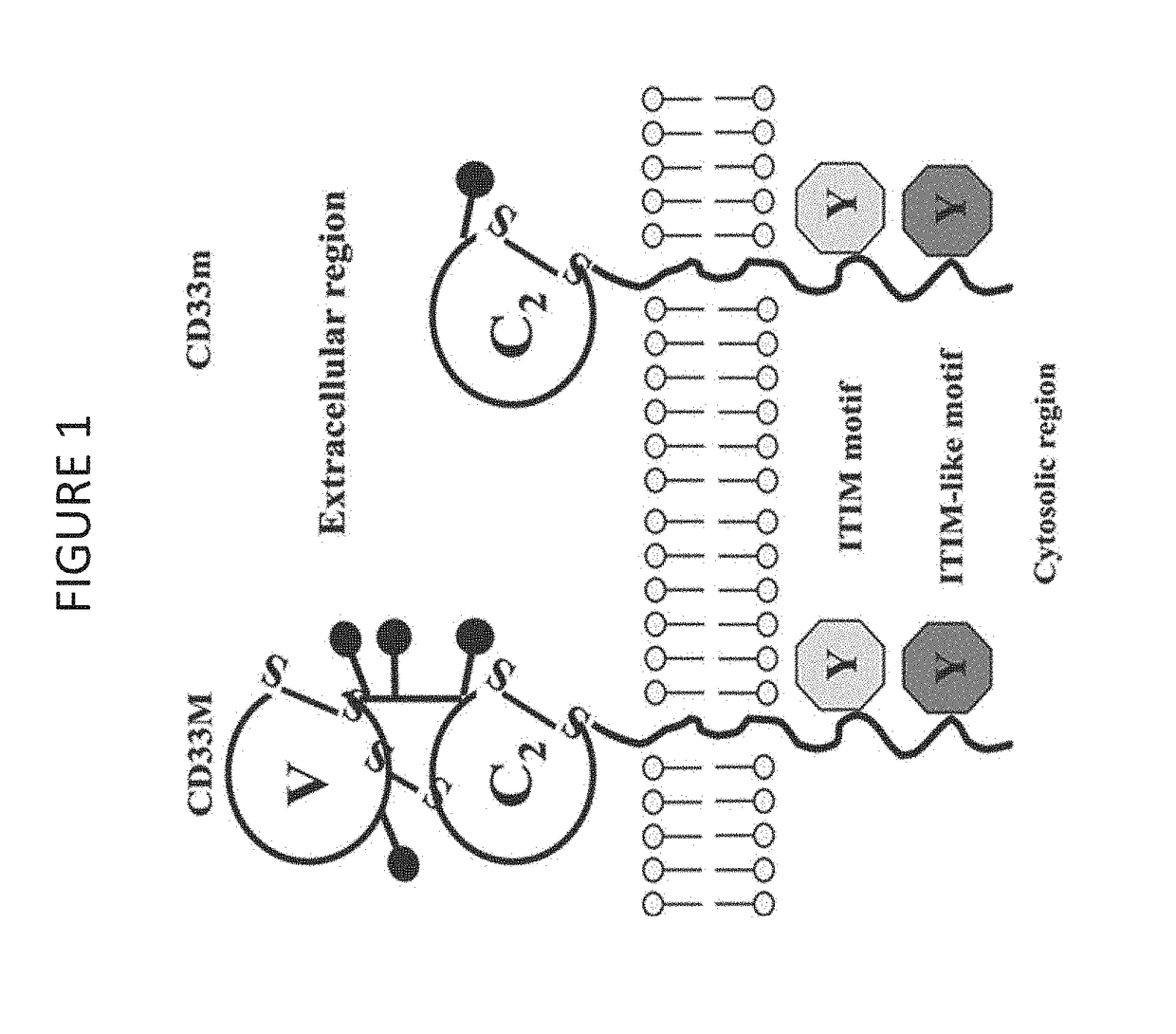
Cd33 specific chimeric antigen receptors
PendingUS20180002397A1Easy SurvivalEfficiently and specifically eliminatePolypeptide with localisation/targeting motifImmunoglobulin superfamilyMyeloid leukemiaCD33
Provided herein are chimeric antigen receptors (CARs) for cancer therapy, and more particularly, CARs containing a scFv from a CD33 monoclonal antibody. Provided are immune effector cells containing such CARs, and methods of treating proliferative disorders such as acute myeloid leukemia (AML), and relapsed or refractory AML.
Owner:PRECIGEN INC
Chimeric antigen receptors and immune cells targeting b cell malignancies
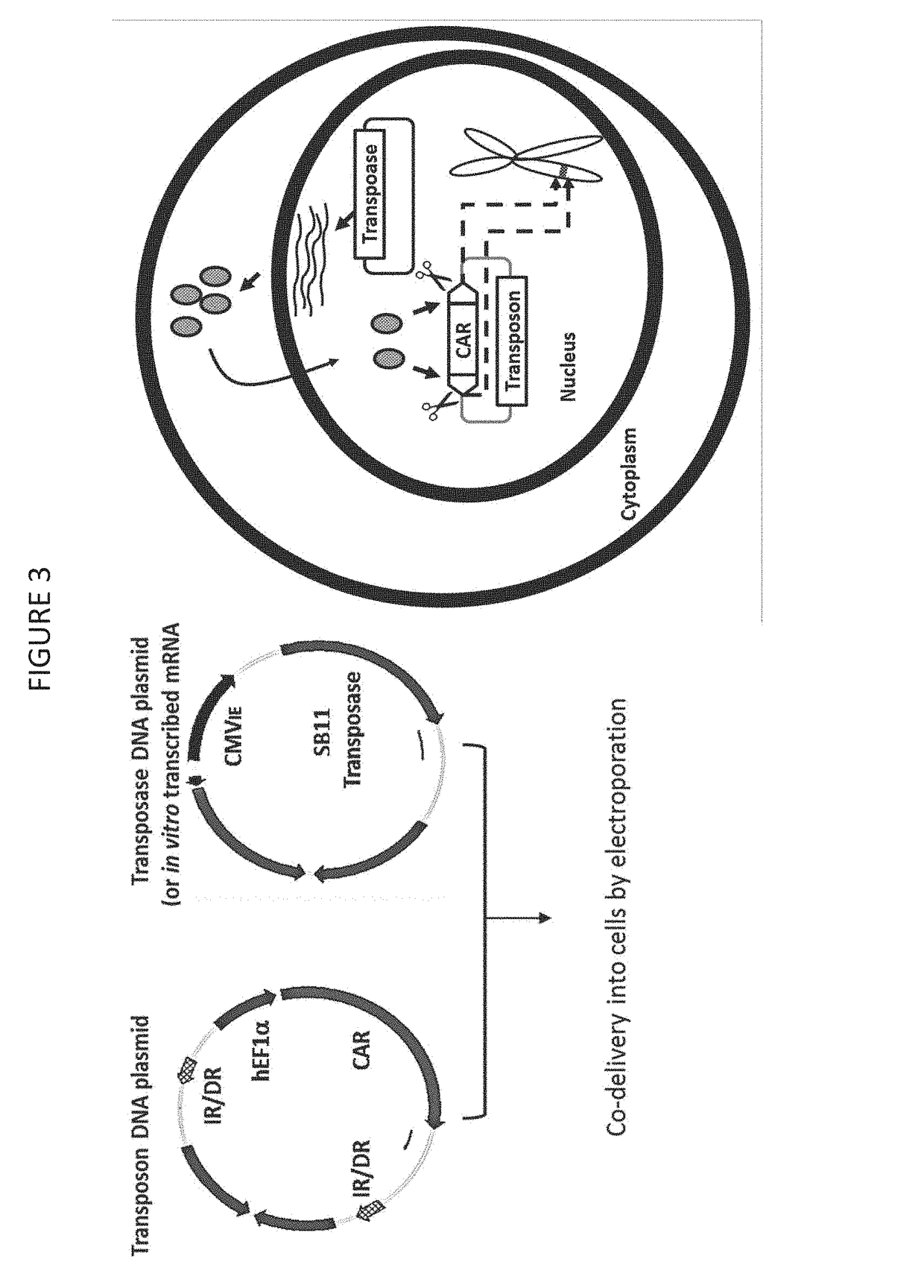
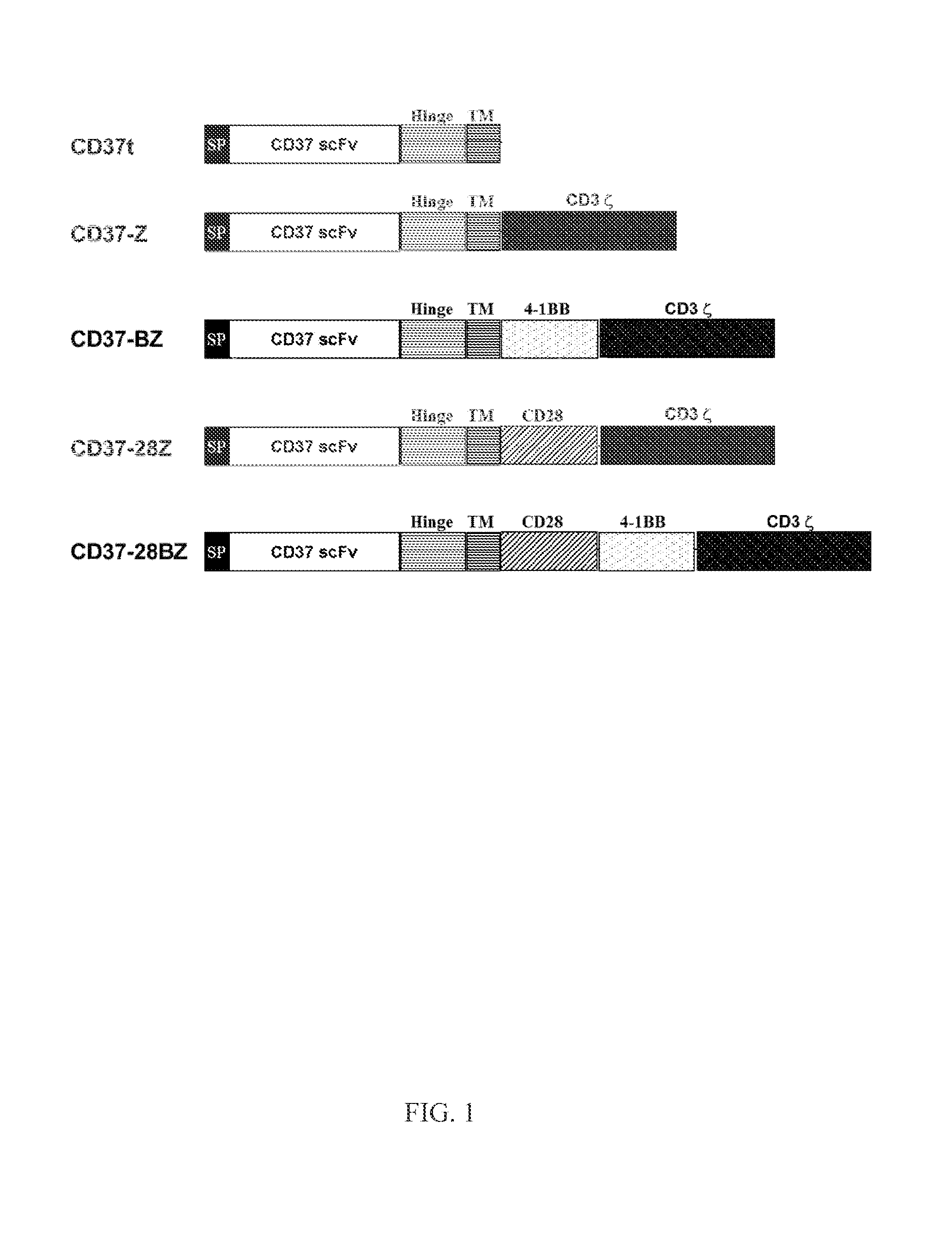
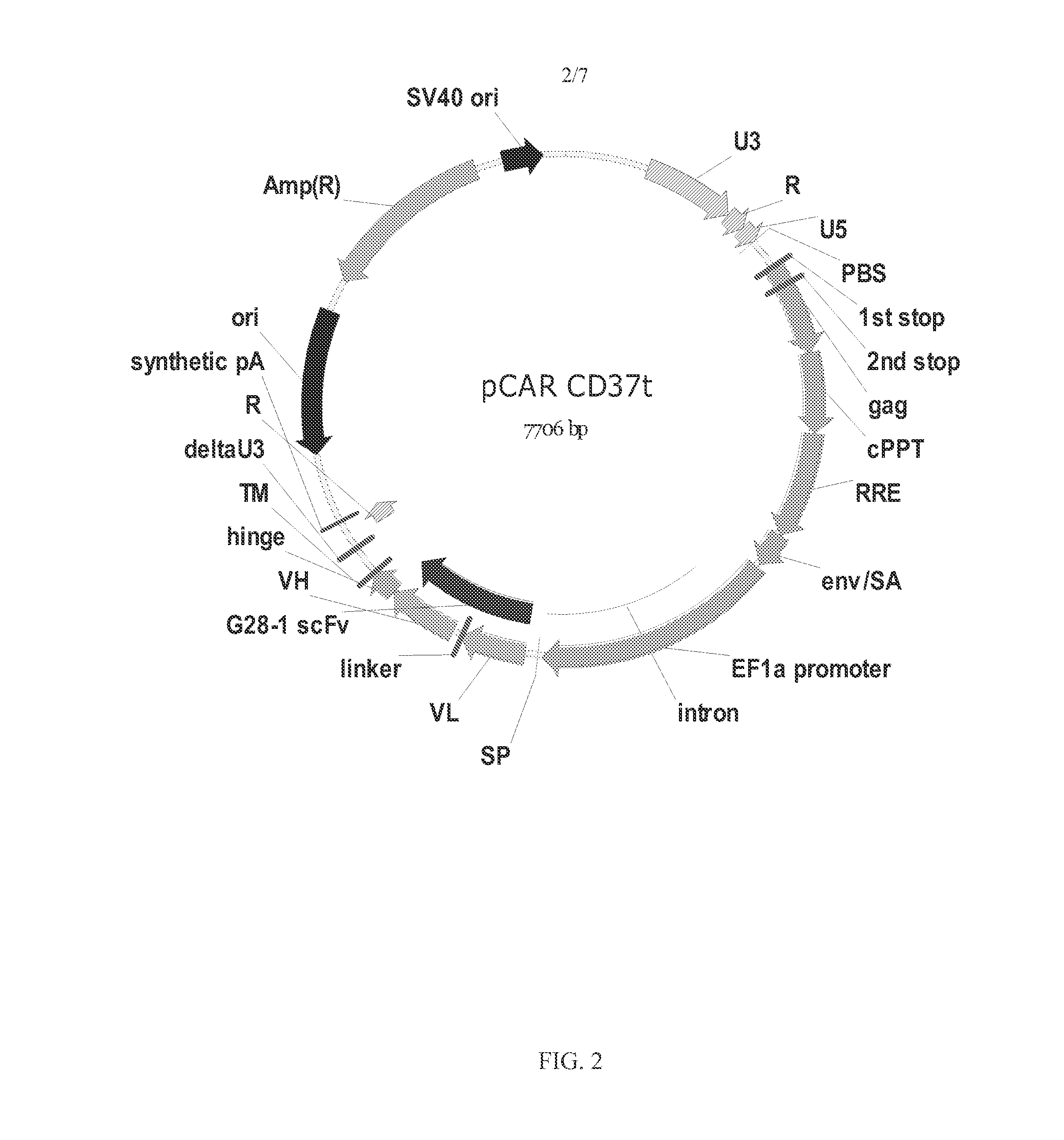
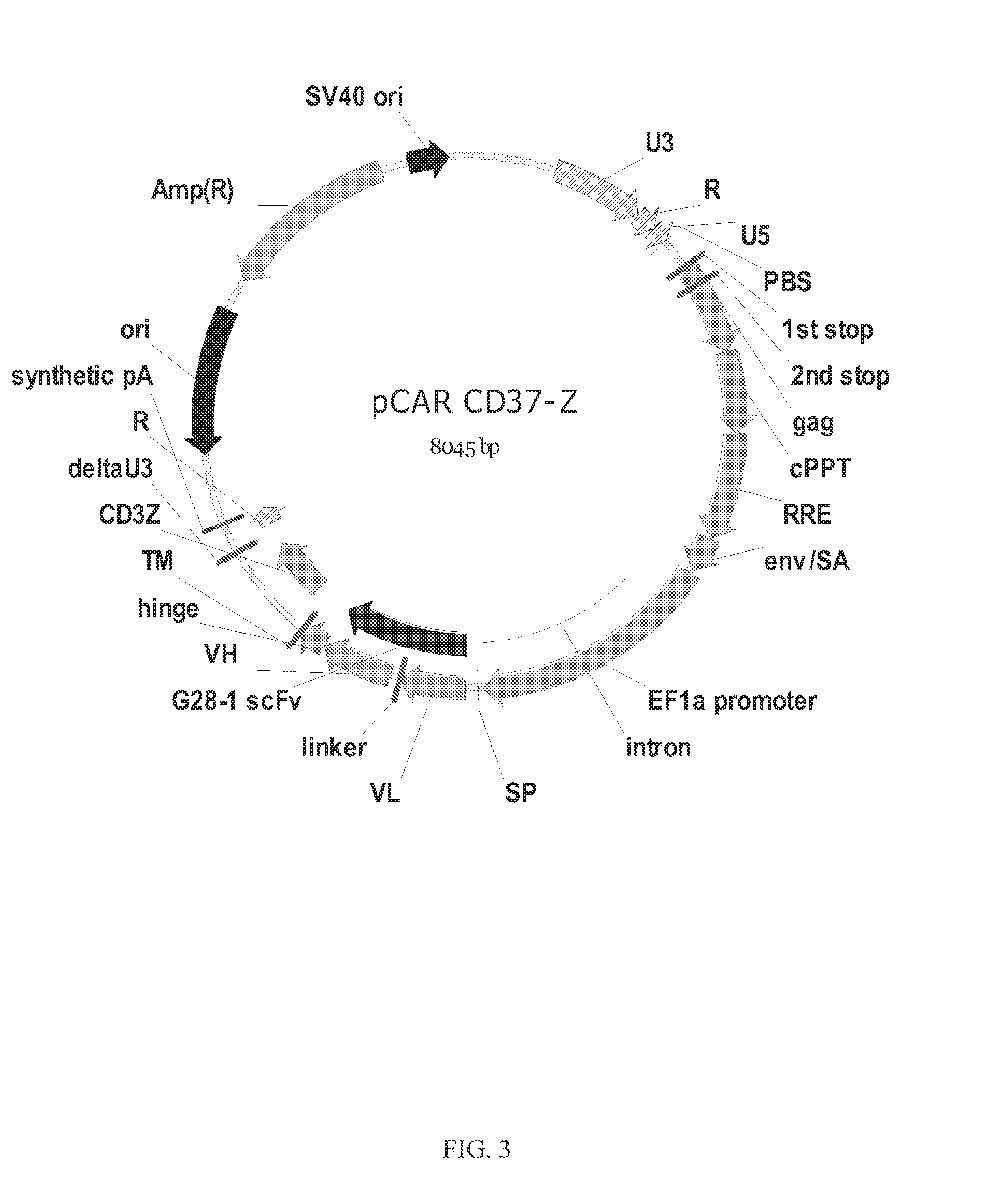
The disclosure describes genetically engineered CD37 specific redirected immune effector cells expressing a chimeric antigen receptor (CAR) protein comprising an antigen binding domain derived from an antibody, a single chain antibody or portion thereof that binds CD37; a hinge region; a transmembrane domain and an intracellular signaling domain derived from human CD3ζ or FcRγ; and optionally one or more co-stimulatory intracellular signaling domains The invention includes nucleic acids, vectors and immune effector cells associated with the production of the CAR protein, as well as methods of treating B cell malignancies in humans by cellular immunotherapy.
Owner:BLUEBIRD BIO INC
Chimeric antigen receptors and methods of making
ActiveUS20170183407A1Improved therapeutic potentialConvenient treatmentAntibacterial agentsTumor rejection antigen precursorsAntigen receptorsBinding domain
Provided are methods of generating chimeric antigen receptors (CAR). In some embodiments, library screening of CAR is performed by generating a vector encoding the CAR from random attachment of vectors from libraries of vectors encoding antigen-binding domains (e.g., scFv regions), hinge regions, and endodomains. In some embodiments, the vectors contain a transposon.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
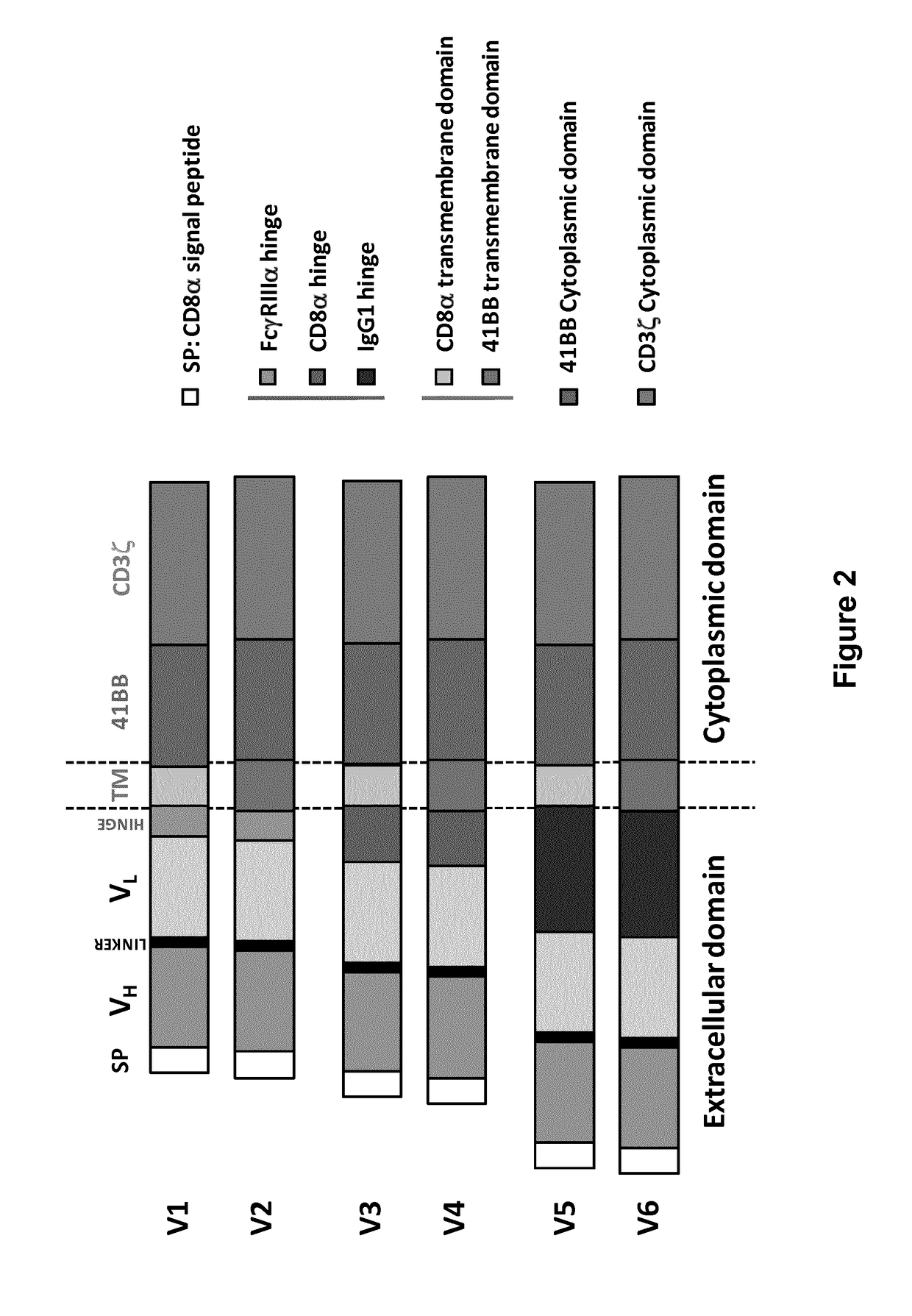
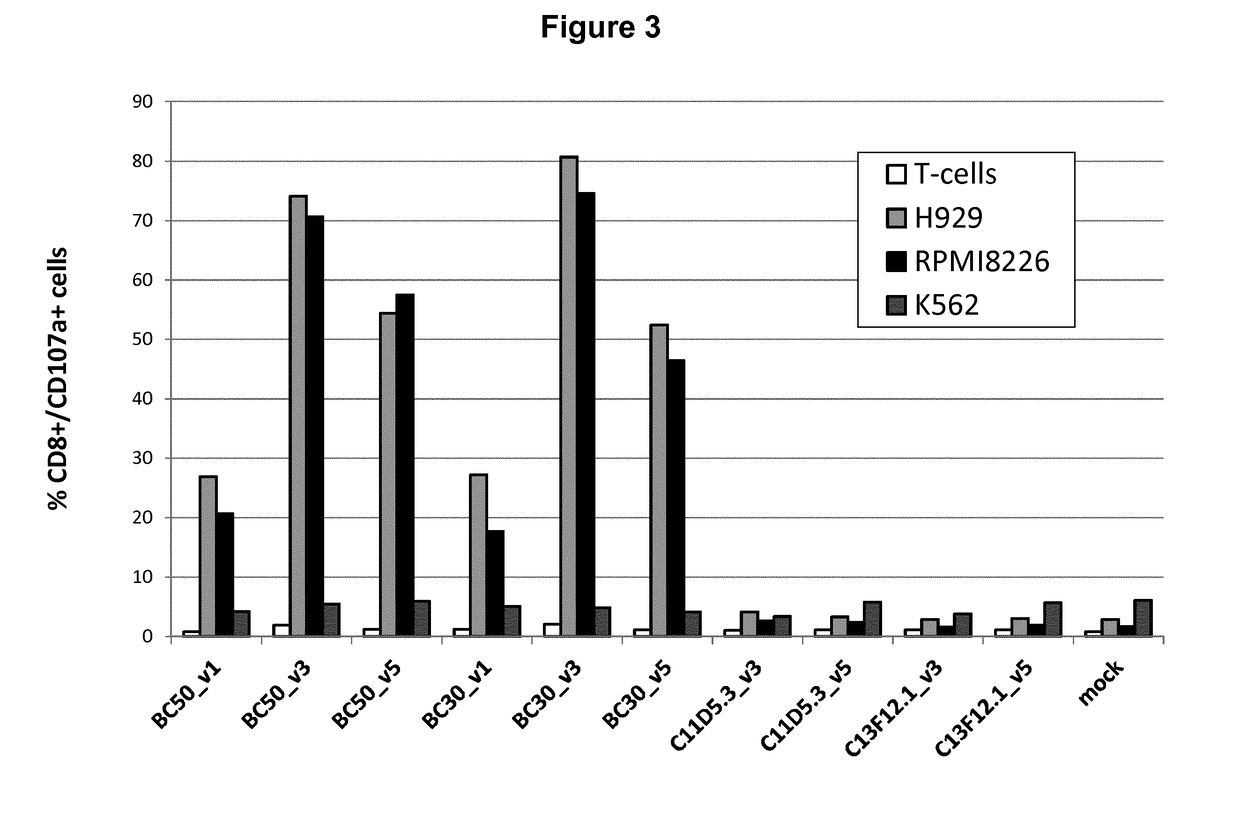
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer
InactiveUS20170151281A1High proliferation rateIncrease productionPolypeptide with localisation/targeting motifPeptide/protein ingredientsIntracellular signallingDendritic cell
The current invention provides monocytic cells transfected with chimeric antigen receptor (CAR) to selectively home to tumors and upon homing differentiate into dendritic cells capable of activating immunity which is inhibitory to said tumor. In one embodiment of the invention, monocytic cells are transfected with a construct encoding an antigen binding domain, a transcellular or structural domain, and an intracellular signaling domain. In one specific aspect of the invention, the antigen binding domain interacts with sufficient affinity to a tumor antigen, capable of triggering said intracellular domain to induce an activation signal to induce monocyte differentiation into DC.
Owner:MYELOID THERAPEUTICS INC
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
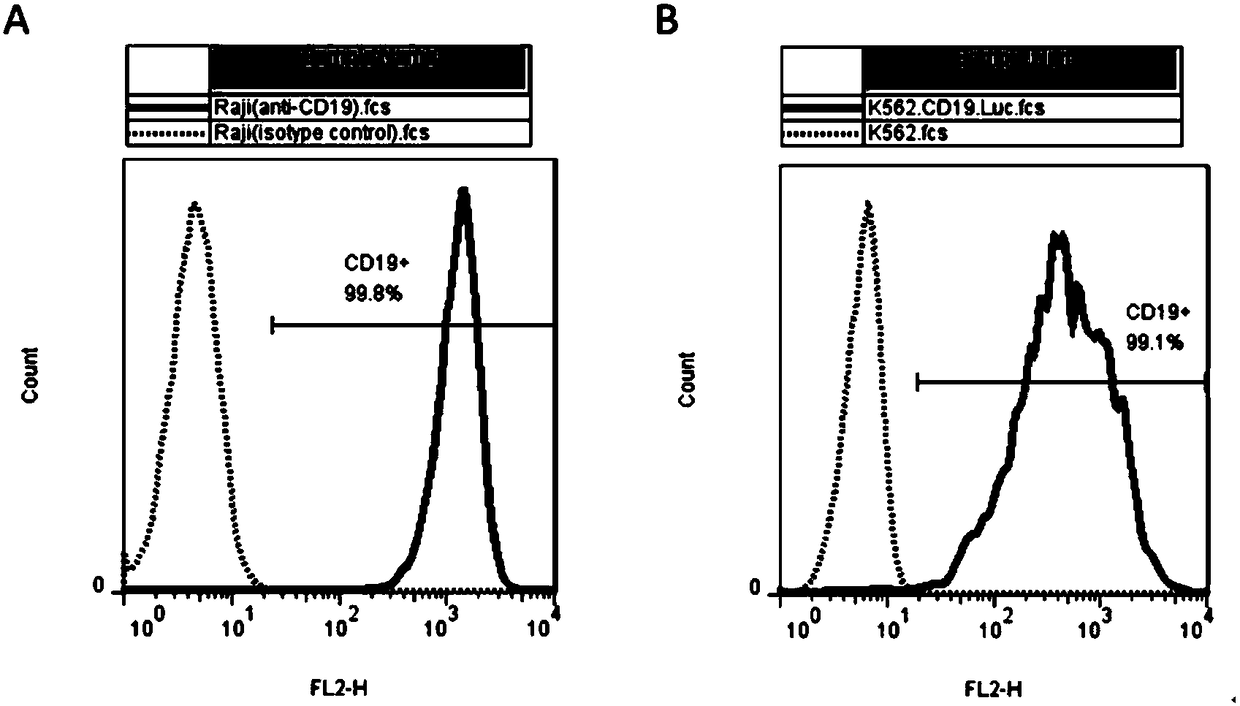
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
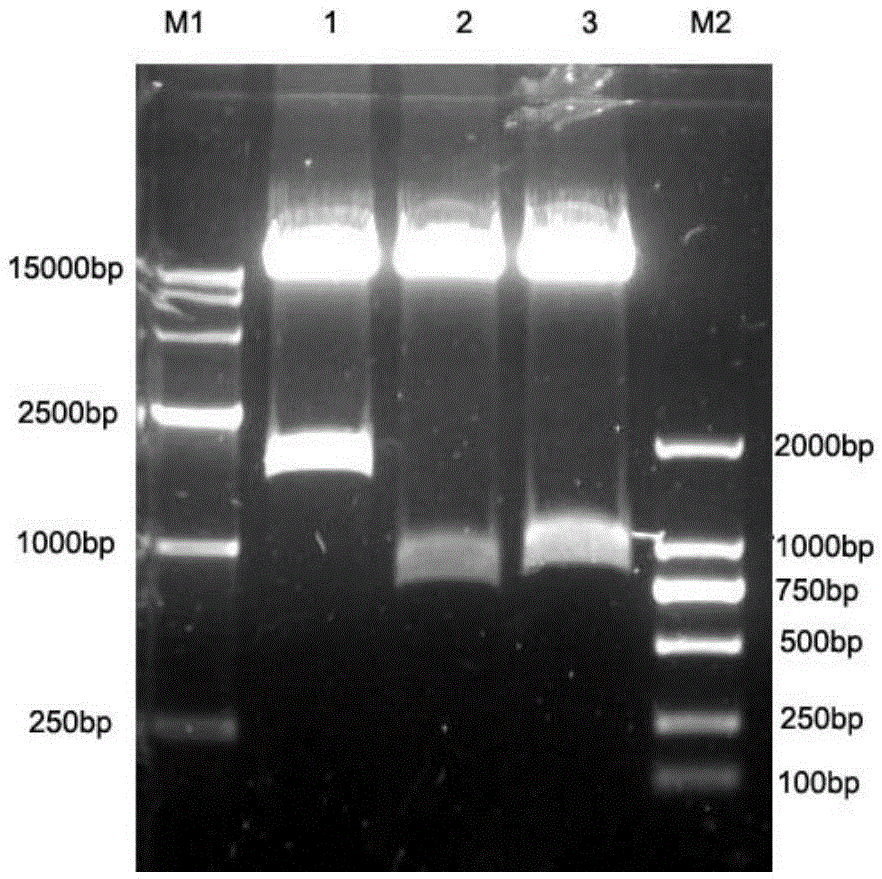
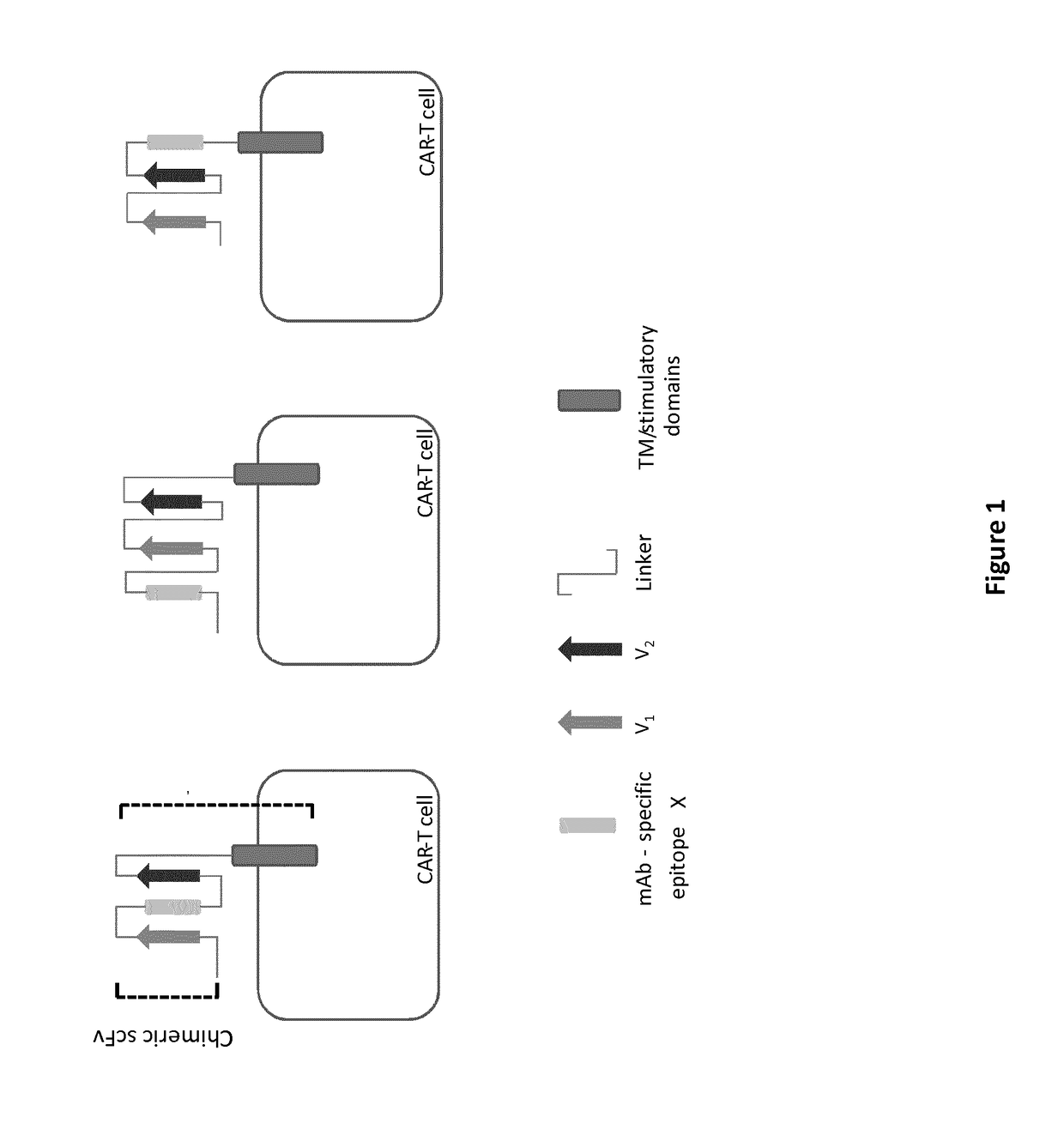
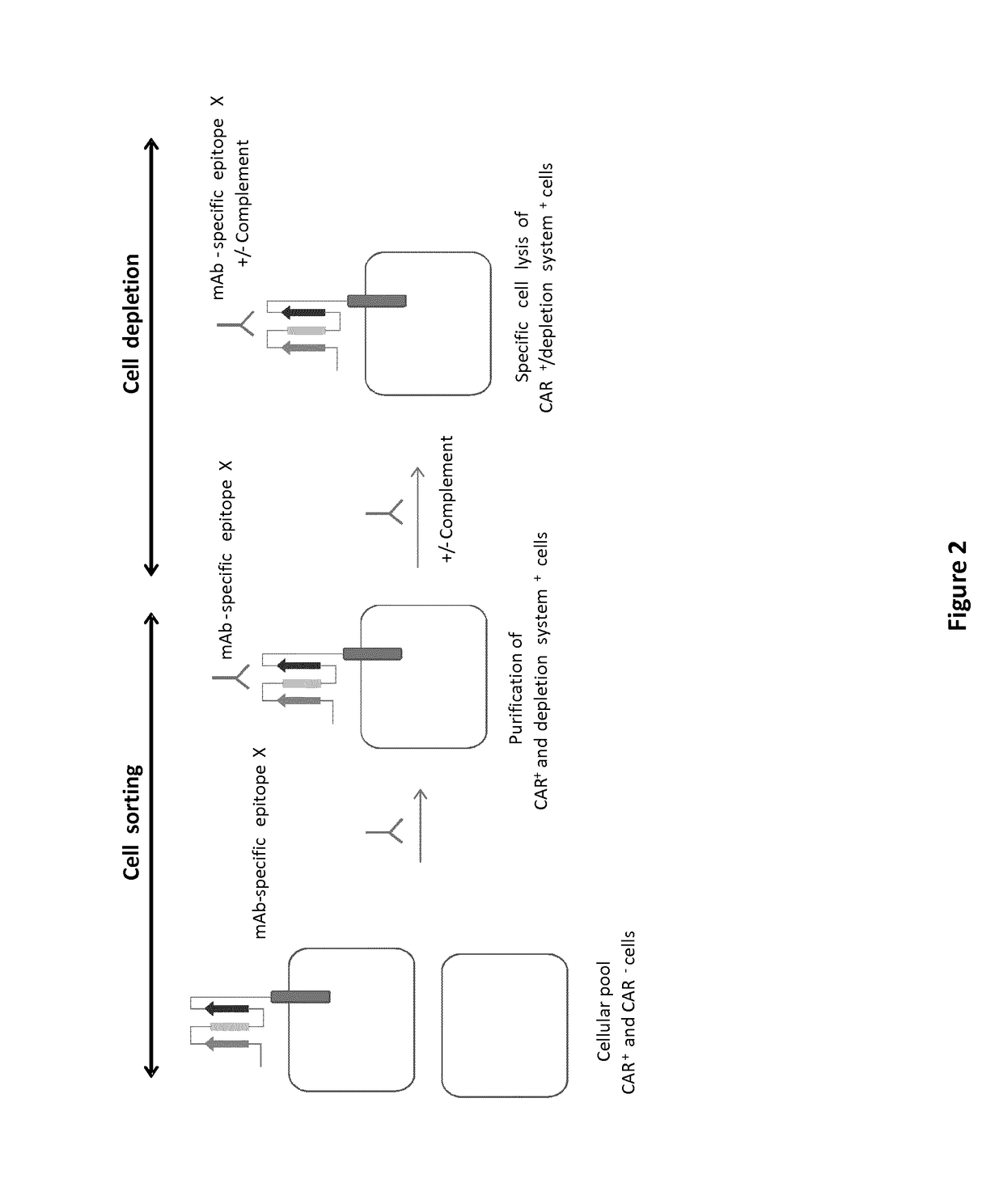
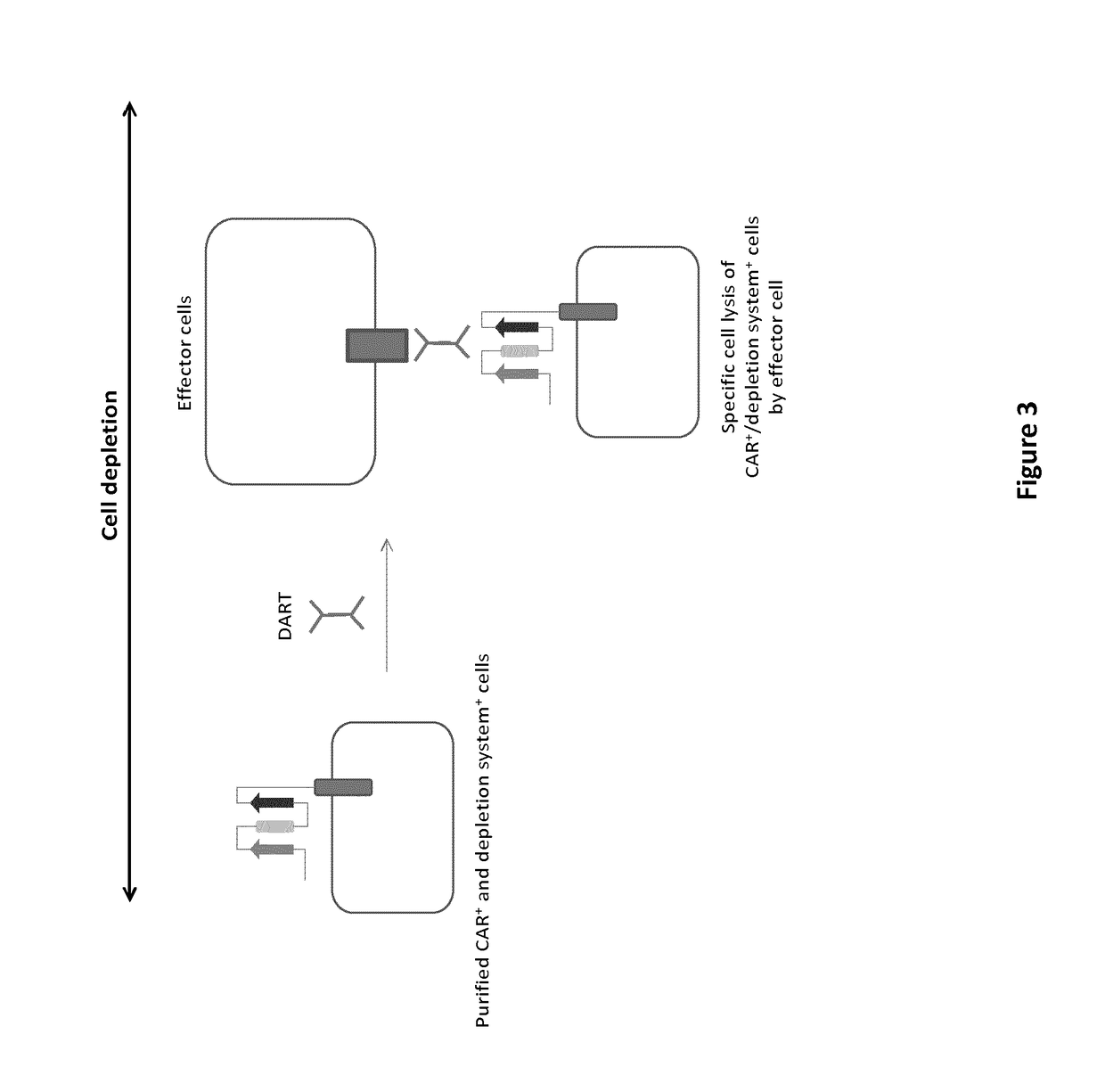
mAb-DRIVEN CHIMERIC ANTIGEN RECEPTOR SYSTEMS FOR SORTING/DEPLETING ENGINEERED IMMUNE CELLS
PendingUS20180002435A1Strong cytotoxicityPromote CDC cytotoxicityAnimal cellsAntibody mimetics/scaffoldsEpitopeBinding domain
A polypeptide encoding a chimeric antigen receptor (CAR) comprising at least one extracellular binding domain that comprises a scFv formed by at least a VH chain and a VL chain specific to an antigen, wherein said extracellular binding domain comprises at least one mAb-specific epitope.
Owner:CELLECTIS SA +1
Chimeric antigen receptor of anti-human CD19 antigen and application thereof
ActiveCN107226867ANo side effectsIncreased proliferationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer cellSide effect
The invention belongs to the field of gene engineering, and relates to chimeric antigen receptor of anti-human CD19 antigen and application thereof. The chimeric antigen receptor of anti-human CD19 antigen comprises polypeptide (scFv) for recognizing human CD19 antigen, a hinge region, a transmembrane region and an intracellular signal domain which are connected in sequence, wherein an amino acid sequence of the polypeptide (scFv) for recognizing human CD19 antigen is as shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3 or SEQ ID NO.4. The chimeric antigen receptor can be more stably expressed in T lymphocyte, has the capacity for properly removing cancer cell, can not only maintain the positive rate of CD19 targeted chimeric antigen receptor during the cell culture of a patient, but also enhance the capacity for CAR-T multiplication and tumor killing, does not have toxic and / or side effects on antigen-negative cell, and can be applied to the targeted therapy of tumor.
Owner:CHONGQING PRECISION BIOTECH CO LTD
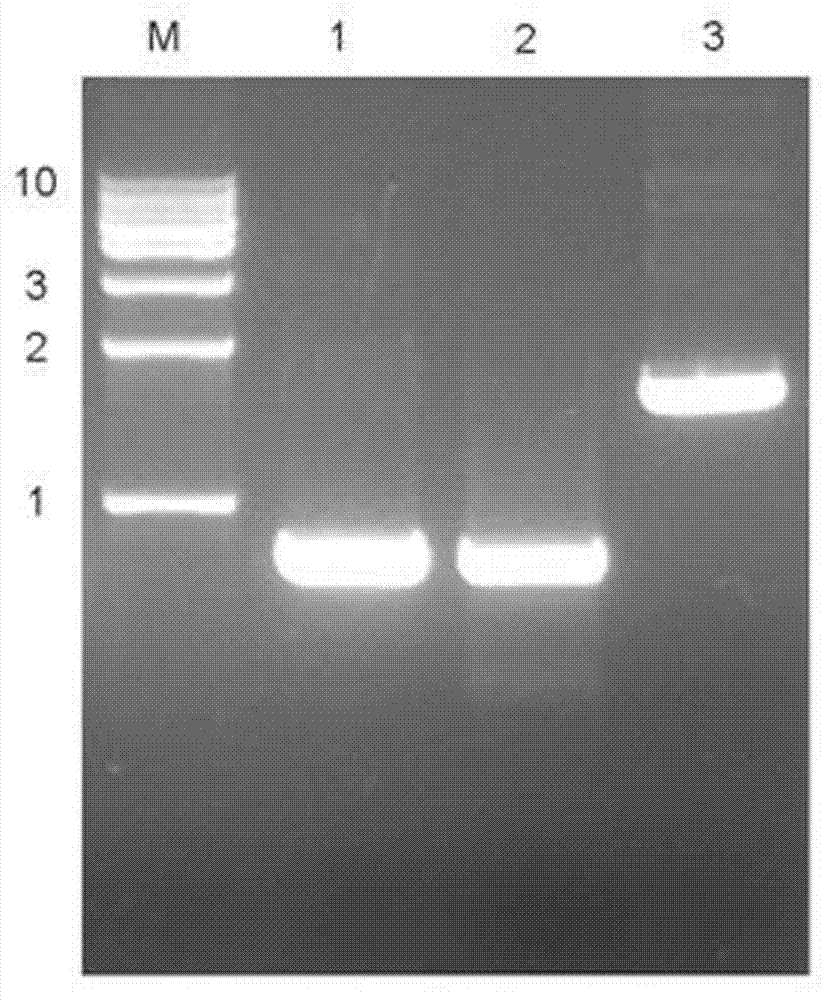
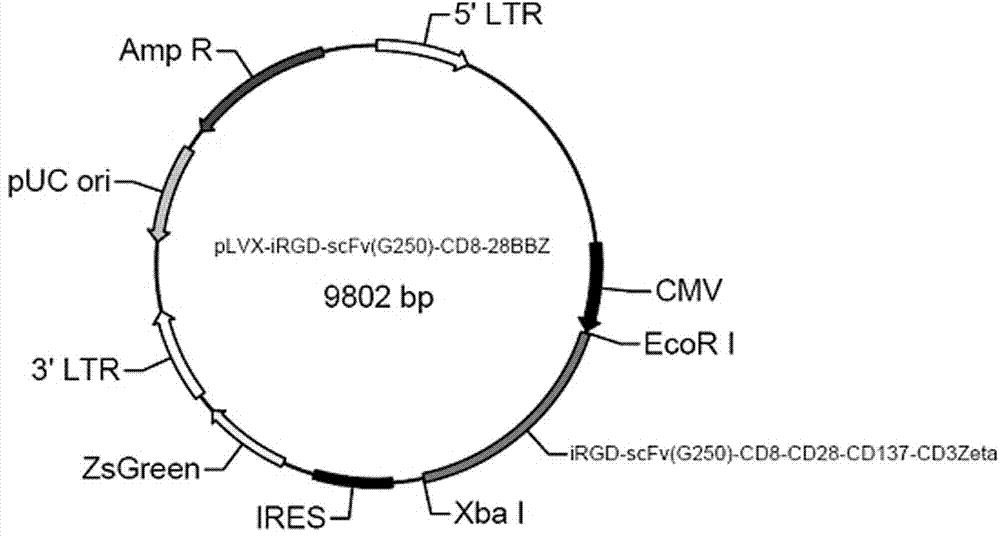
Chimeric antigen receptor iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof
InactiveCN102775500APeptide/protein ingredientsPharmaceutical non-active ingredientsAntigen receptorSingle-Chain Antibodies
The invention belongs to the technical field of biology and new medicines and discloses a chimeric antigen receptor (CAR) iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof. The chimeric antigen receptor is formed by series connection of tumor penetrating peptide iRGD, a single-chain antibody of a humanized anti-human renal carcinoma antigen G250, a hinge region and a transmembrane region of CD8, and intracellular signal structural domains of CD28, CD137 and CD3zeta. The chimeric antigen receptor can be used for modifying T lymphocytes, and the modified T lymphocytes can be used for renal carcinoma treatment.
Owner:郑骏年
Conditionally active chimeric antigen receptors for modified t-cells
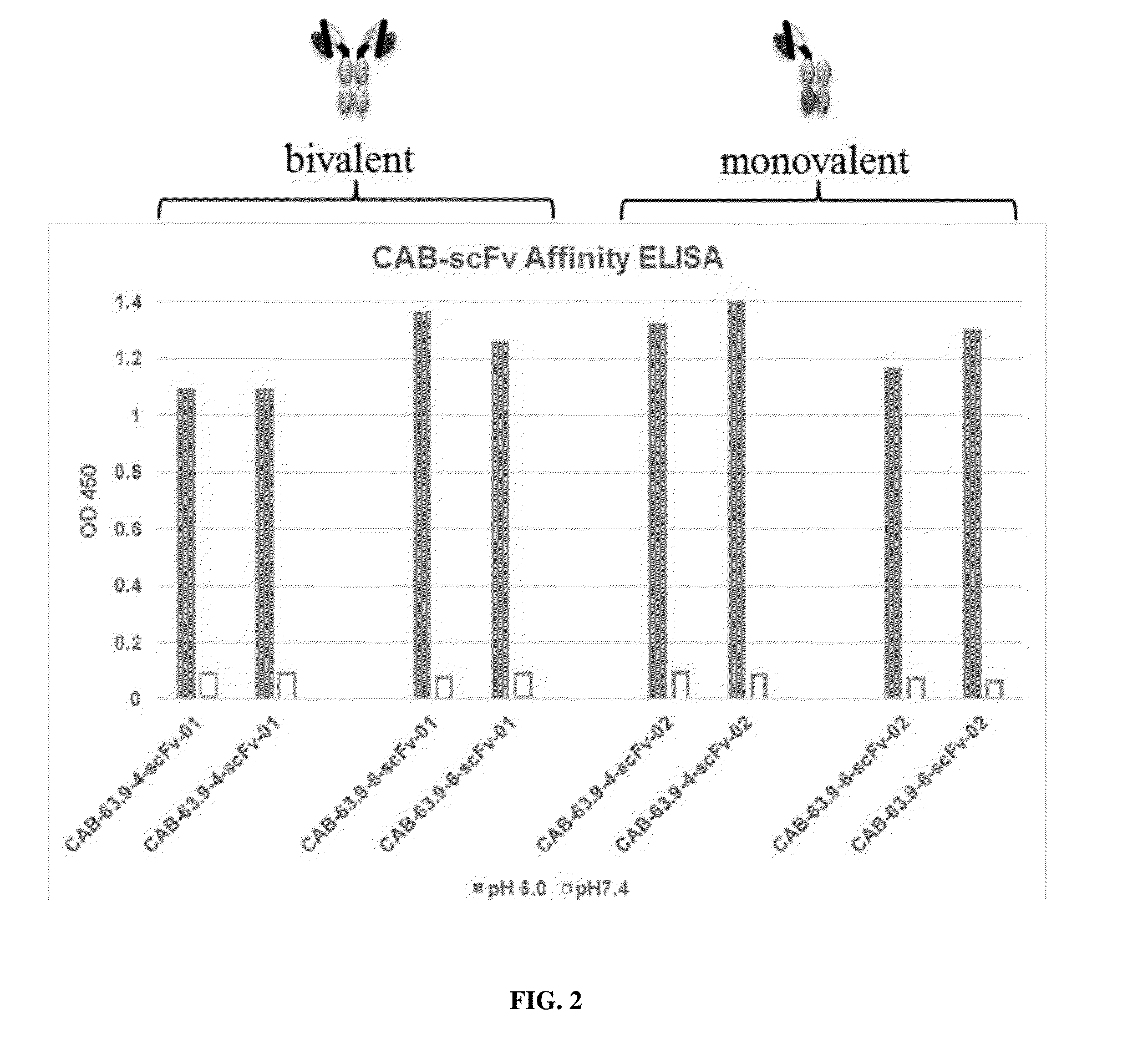
InactiveUS20160207989A1Reduced activityHigh activityBiocidePeptide/protein ingredientsAntigen receptorsWild type
This disclosure relates to a chimeric antigen receptor for binding with a target antigen. The chimeric antigen receptor comprises at least one antigen specific targeting region including a multispecific antibody evolved from a wild-type antibody or a fragment thereof and having at least one of: (a) a decrease in activity in the assay at the normal physiological condition compared to the wild-type antibody or the fragment thereof, and (b) an increase in activity in the assay under the aberrant condition compared to the wild-type antibody or the fragment thereof. A method for using the chimeric antigen receptor and cytotoxic cells for cancer treatment is also provided. A method for producing the chimeric antigen receptor is also provided.
Owner:BIOATLA LLC
Novel chimeric antigen receptor and application thereof
ActiveCN106279438AImprove securityAdd control switchFermentationHybrid peptidesAntigenAntigen receptors
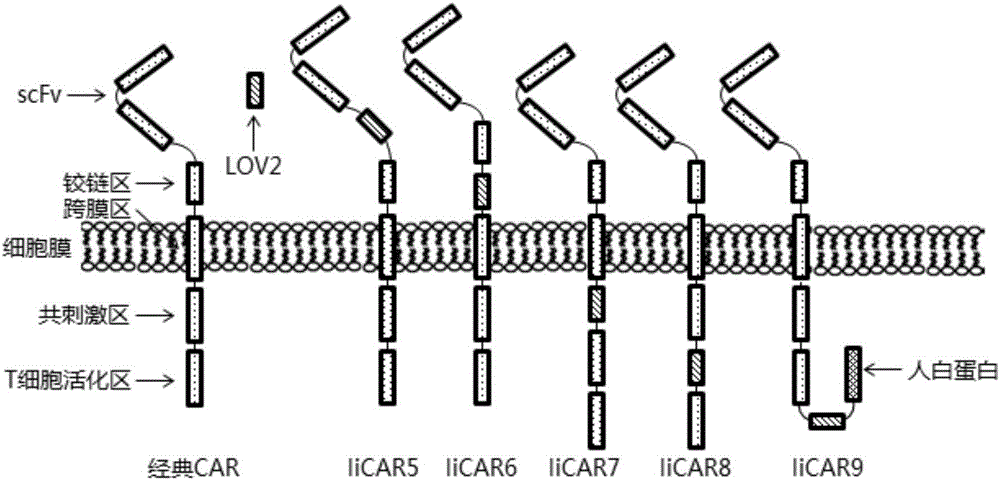
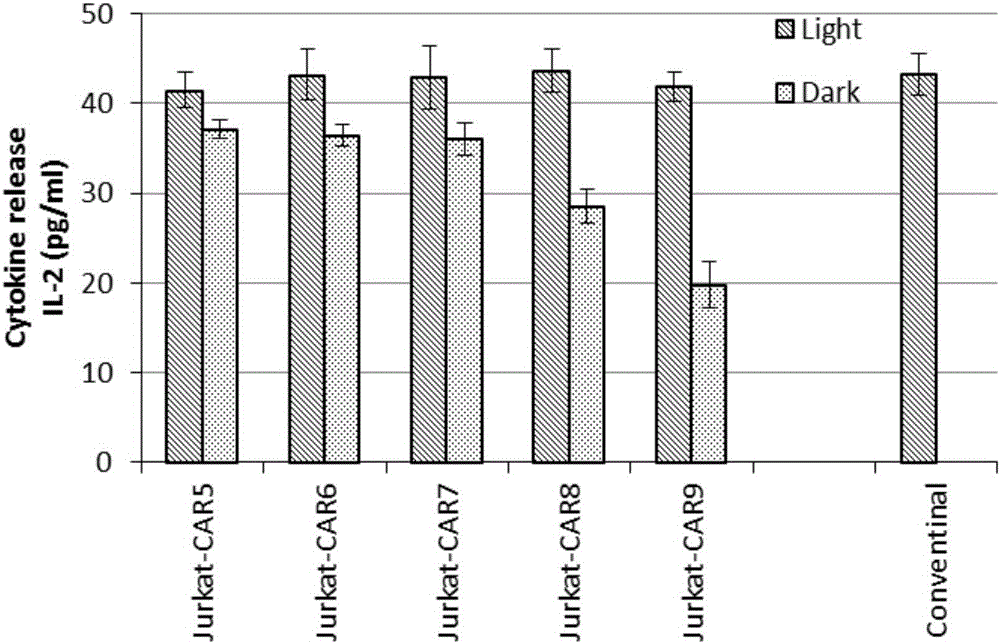
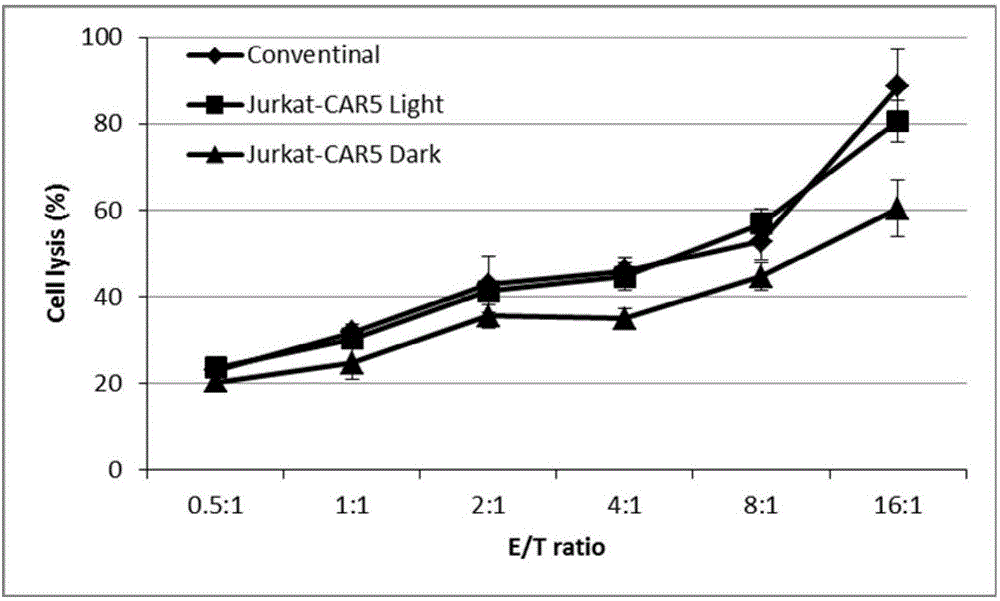
The invention discloses a recombined chimeric antigen receptor with a photoinduction element. The chimeric antigen receptor comprises an antigen combining area, a transmembrane structure area, a co-stimulatory signal transduction area and T cell signal transduction area functional structure domains, wherein the photoinduction element is inserted at the N terminal or C terminal of the recombined chimeric antigen receptor or between the functional structure domains. According to the chimeric antigen receptor, photoinduction element LOV2 genes are cloned into plasmids Lenti-EF1alpha-CD19CAR with a molecular cloning method to establish a co-expression vector of the recombined chimeric antigen receptor with the photoinduction element, 293T cells are used for packaging to obtain a lentiviral vector with photoinduction CAR molecules. After the recombined chimeric antigen receptor is expressed in T cells through the lentiviral vector, the damage toxicity of T cells is reversely controlled by LOV2, and an importable approach is provided for improving the safety of CAR-T in treating cancer clinically.
Owner:北京领柯生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com