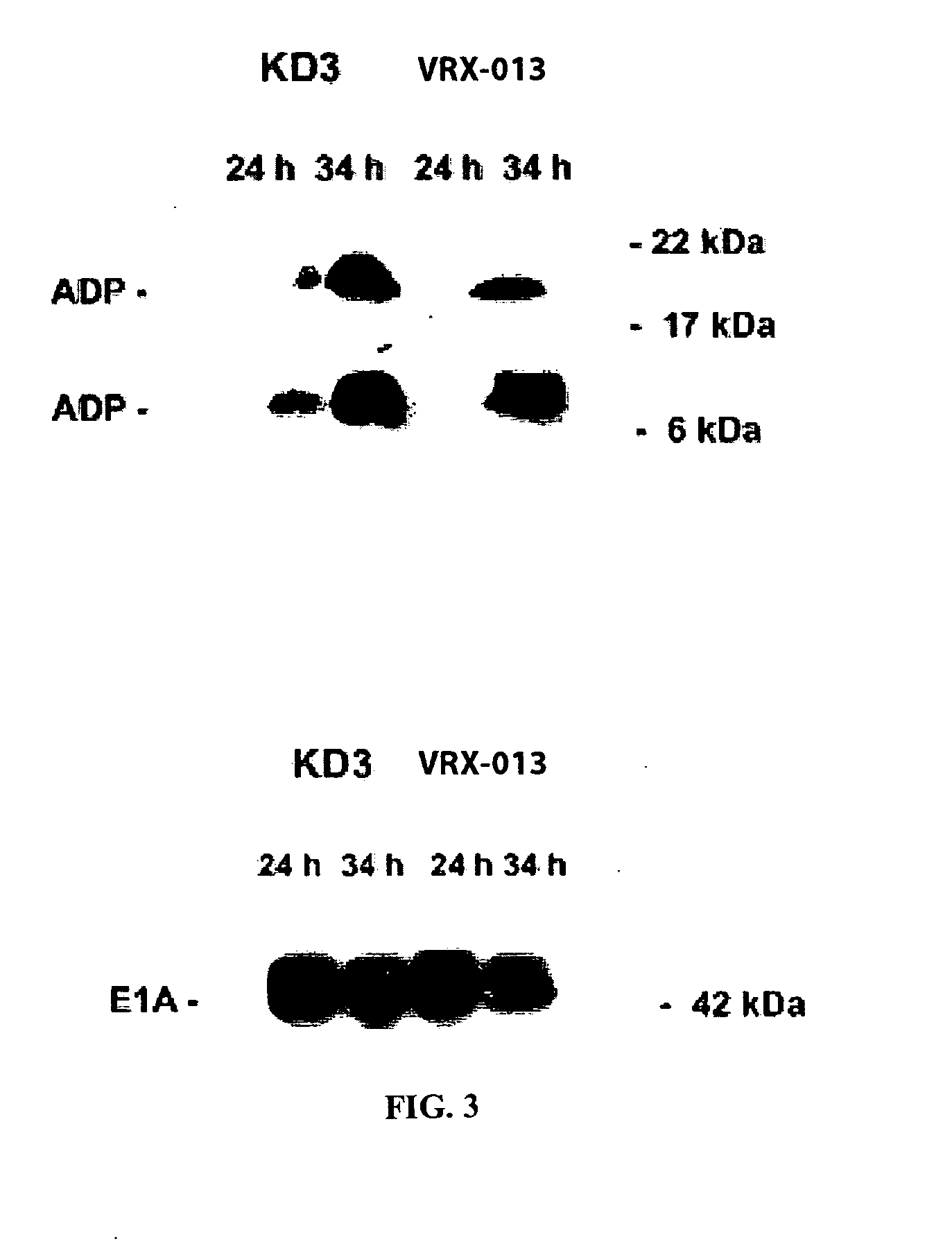Patents
Literature
584results about "NGF/TNF-superfamily" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
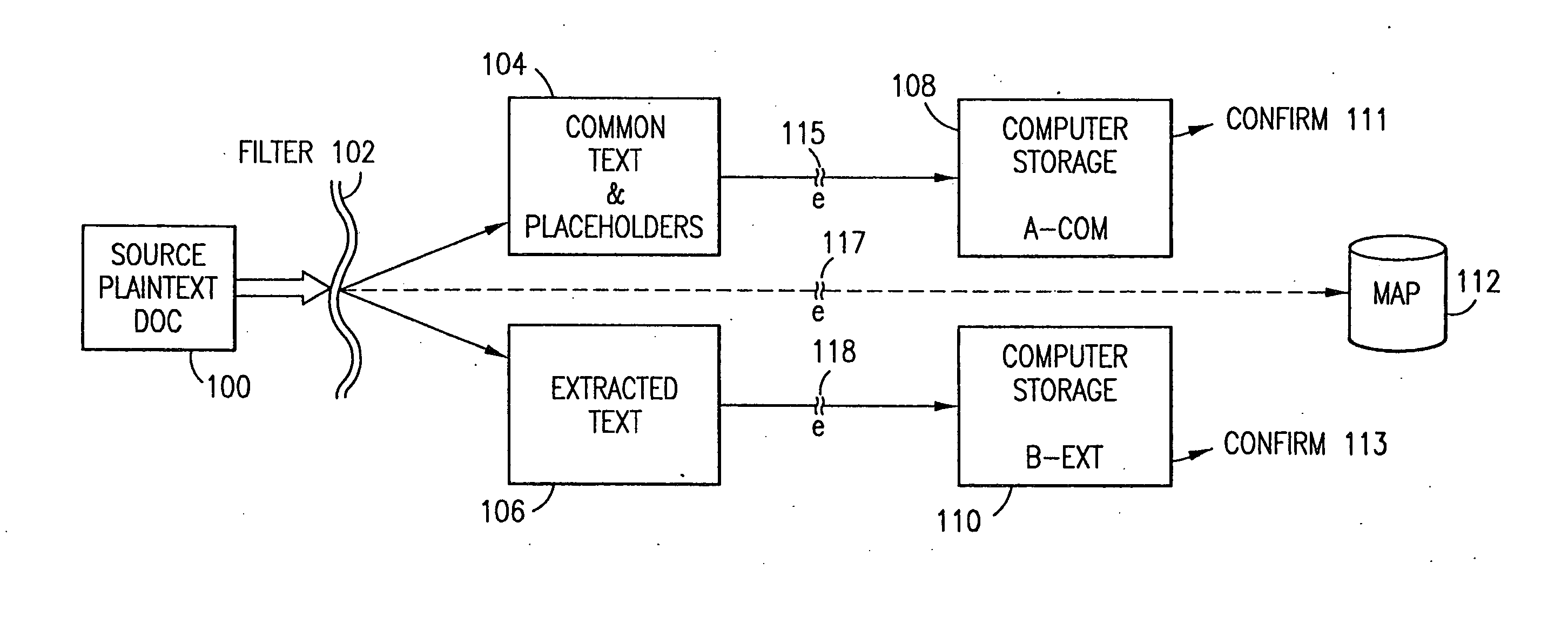
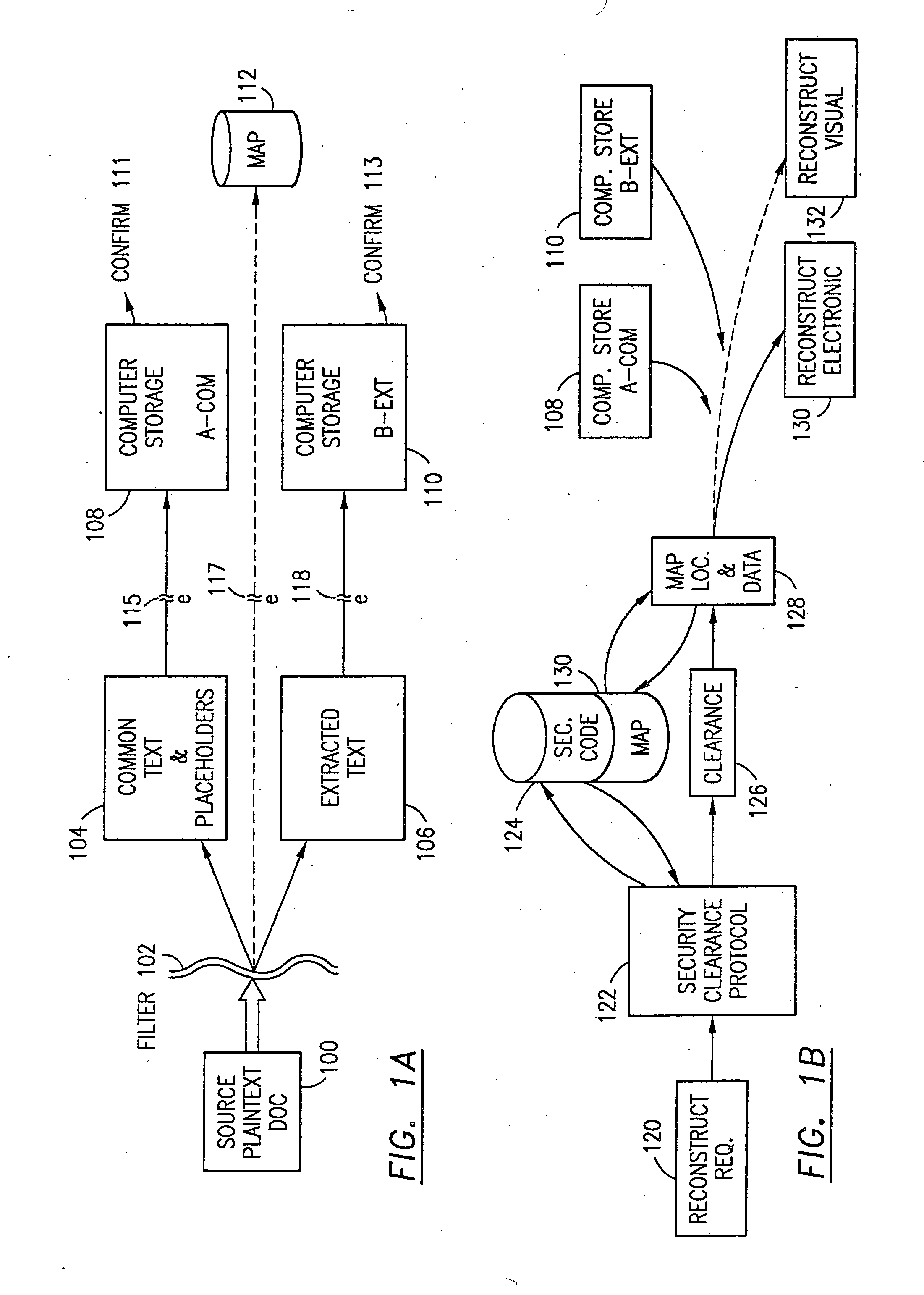
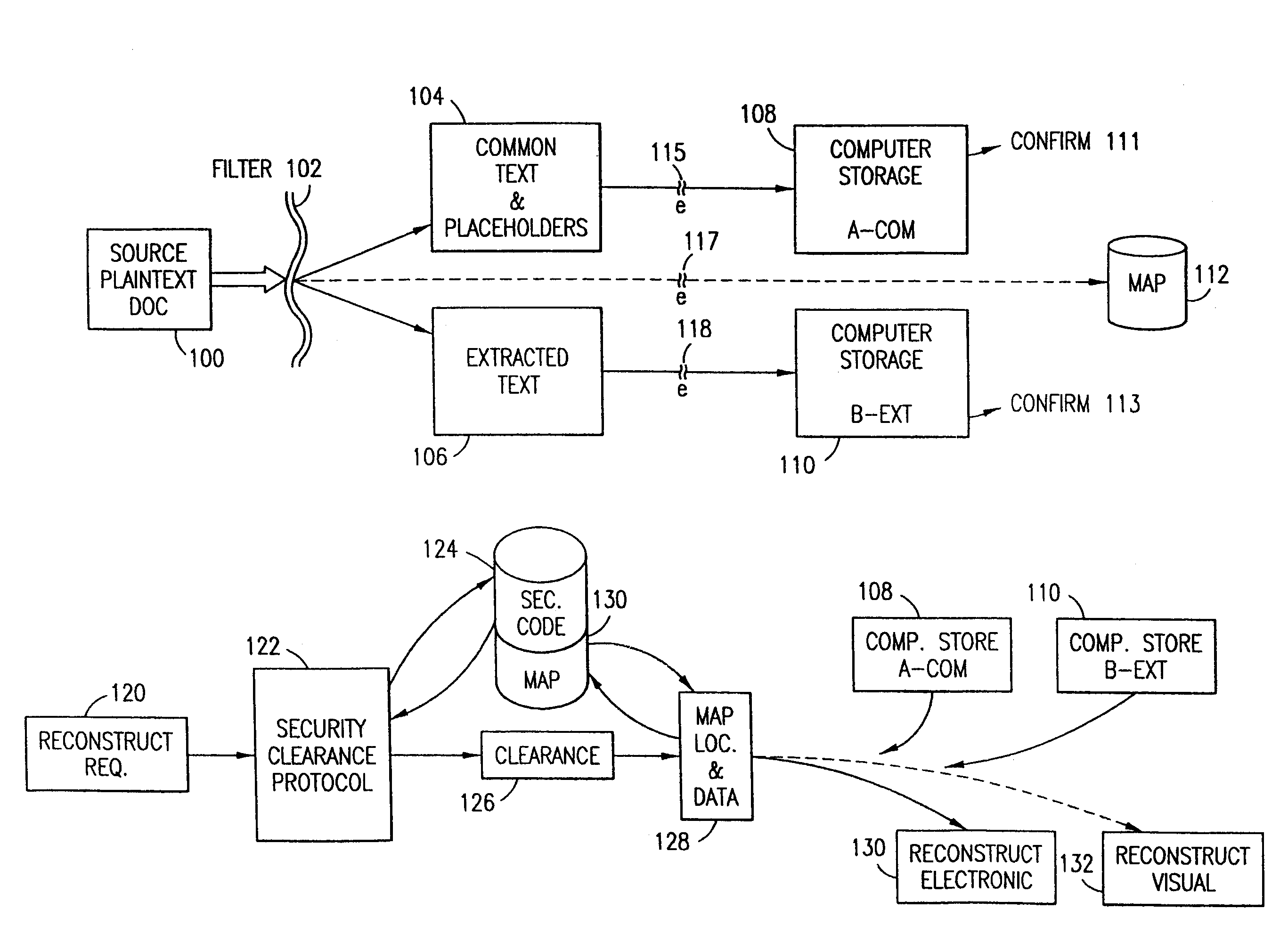
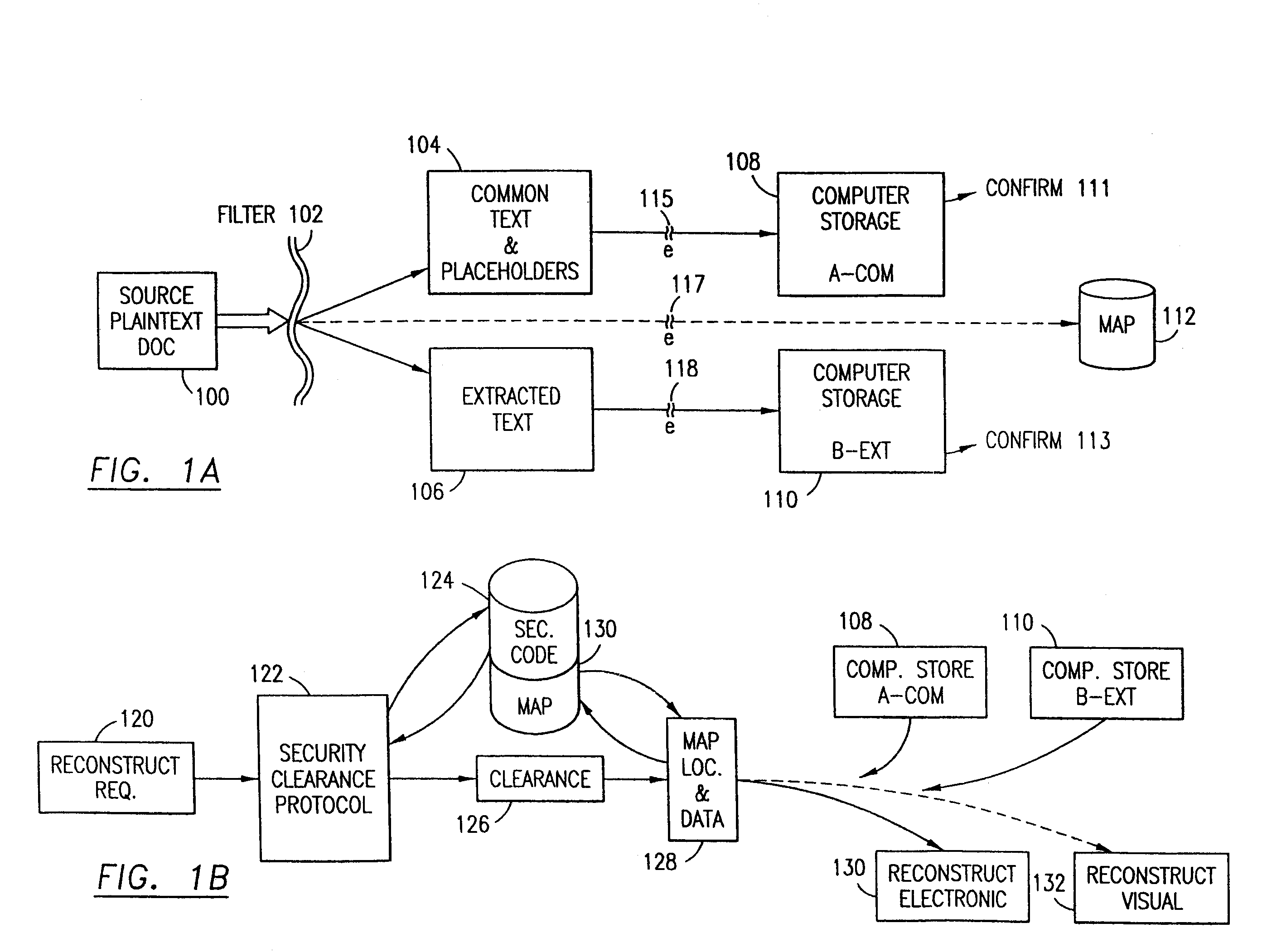
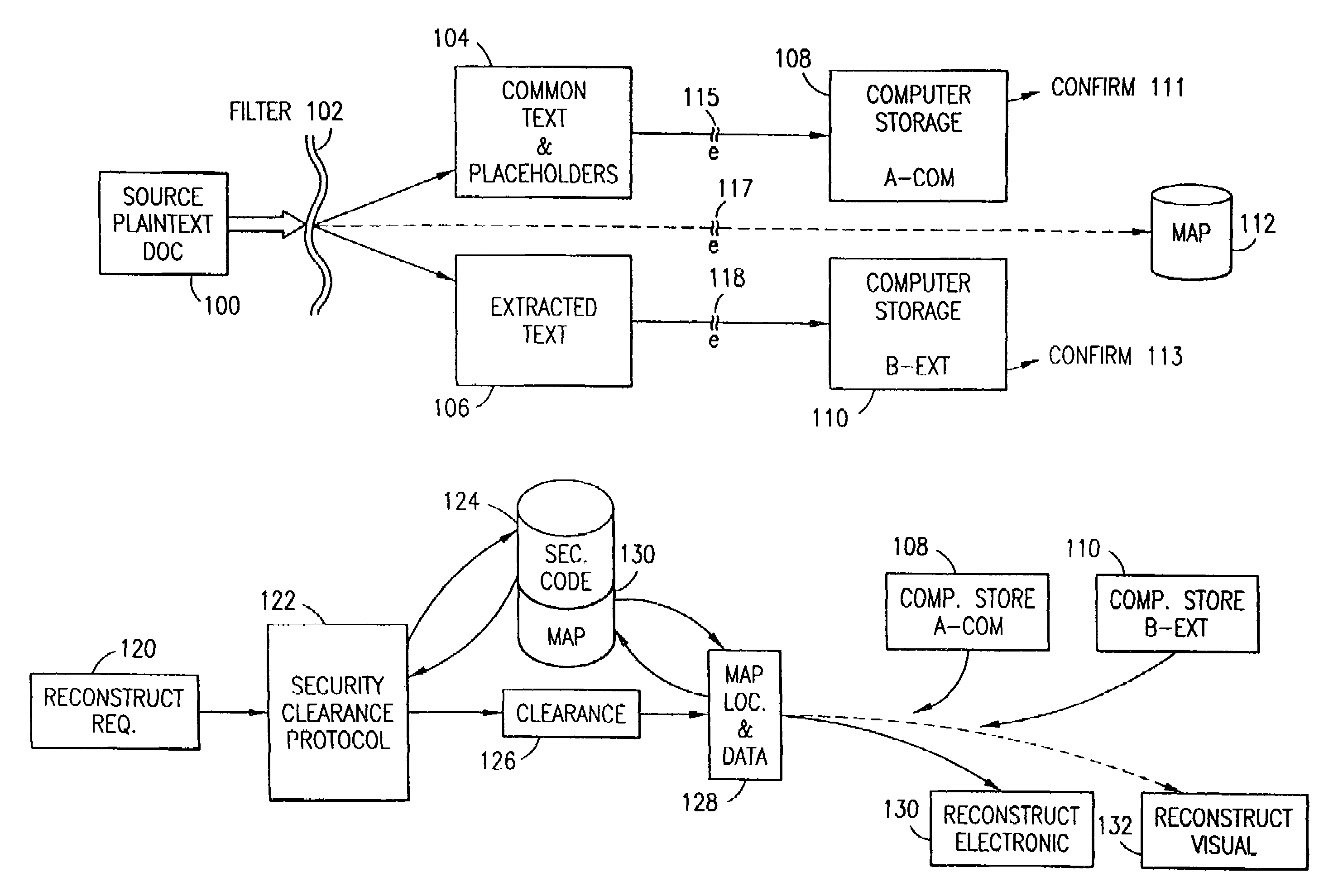
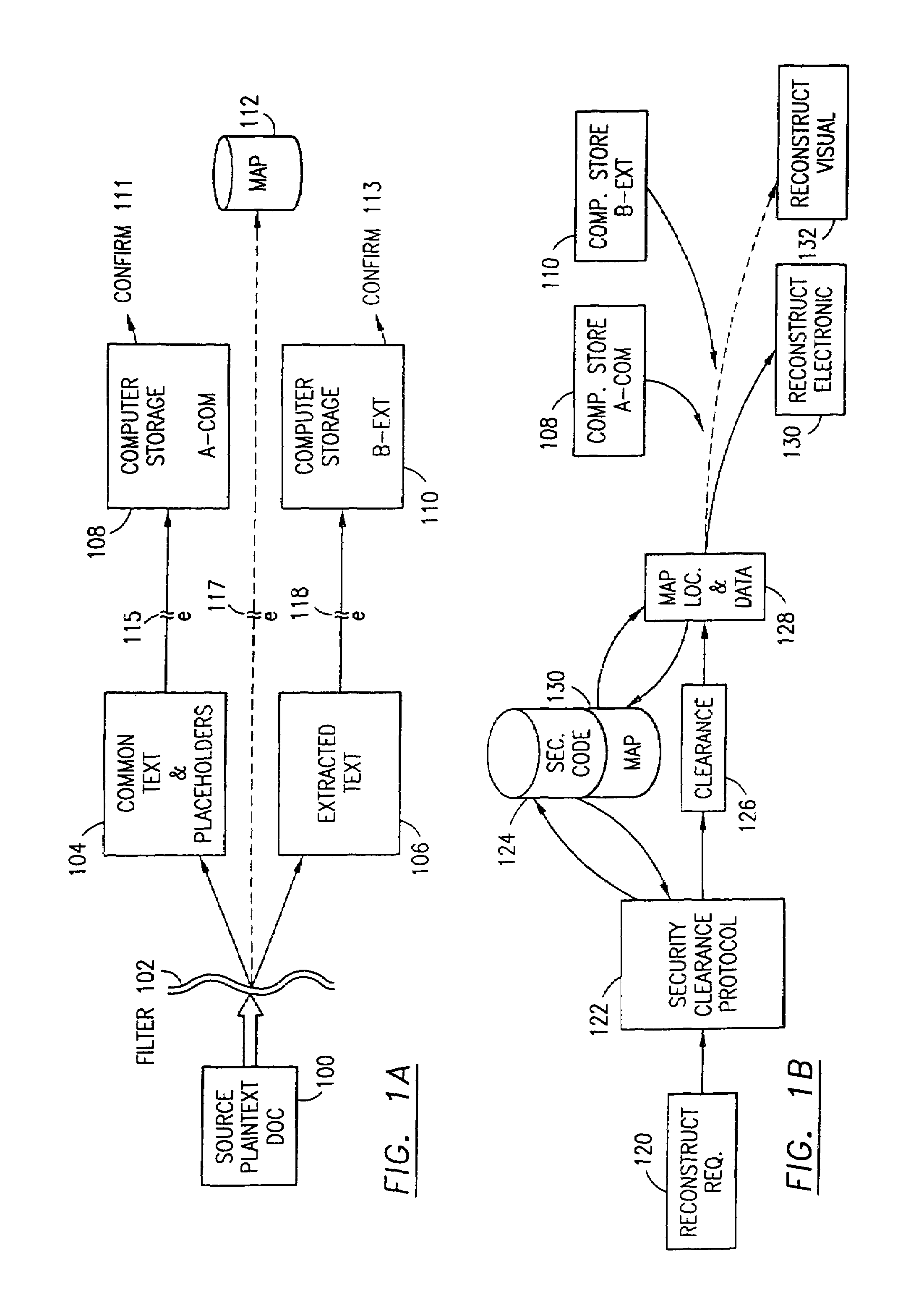
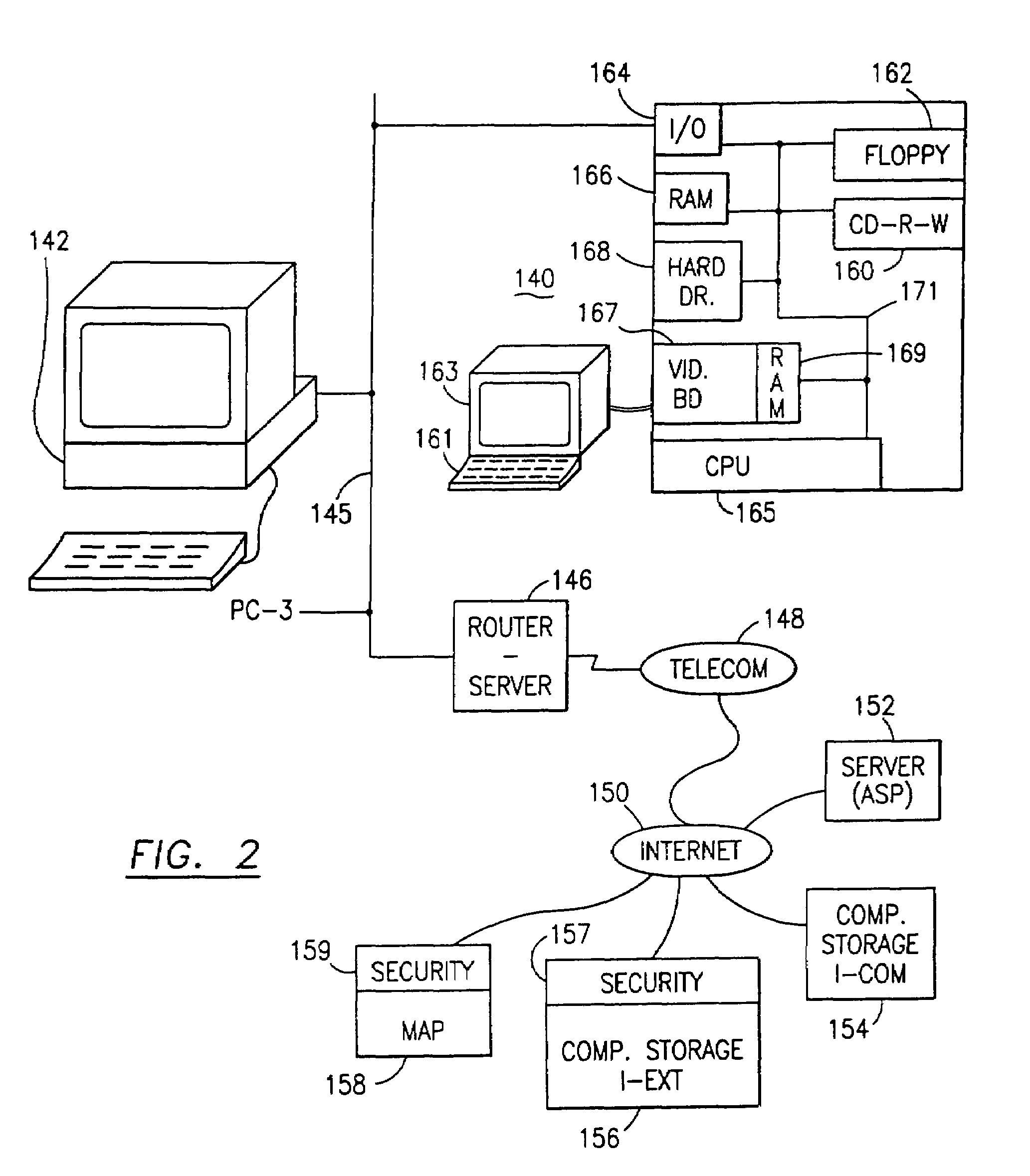
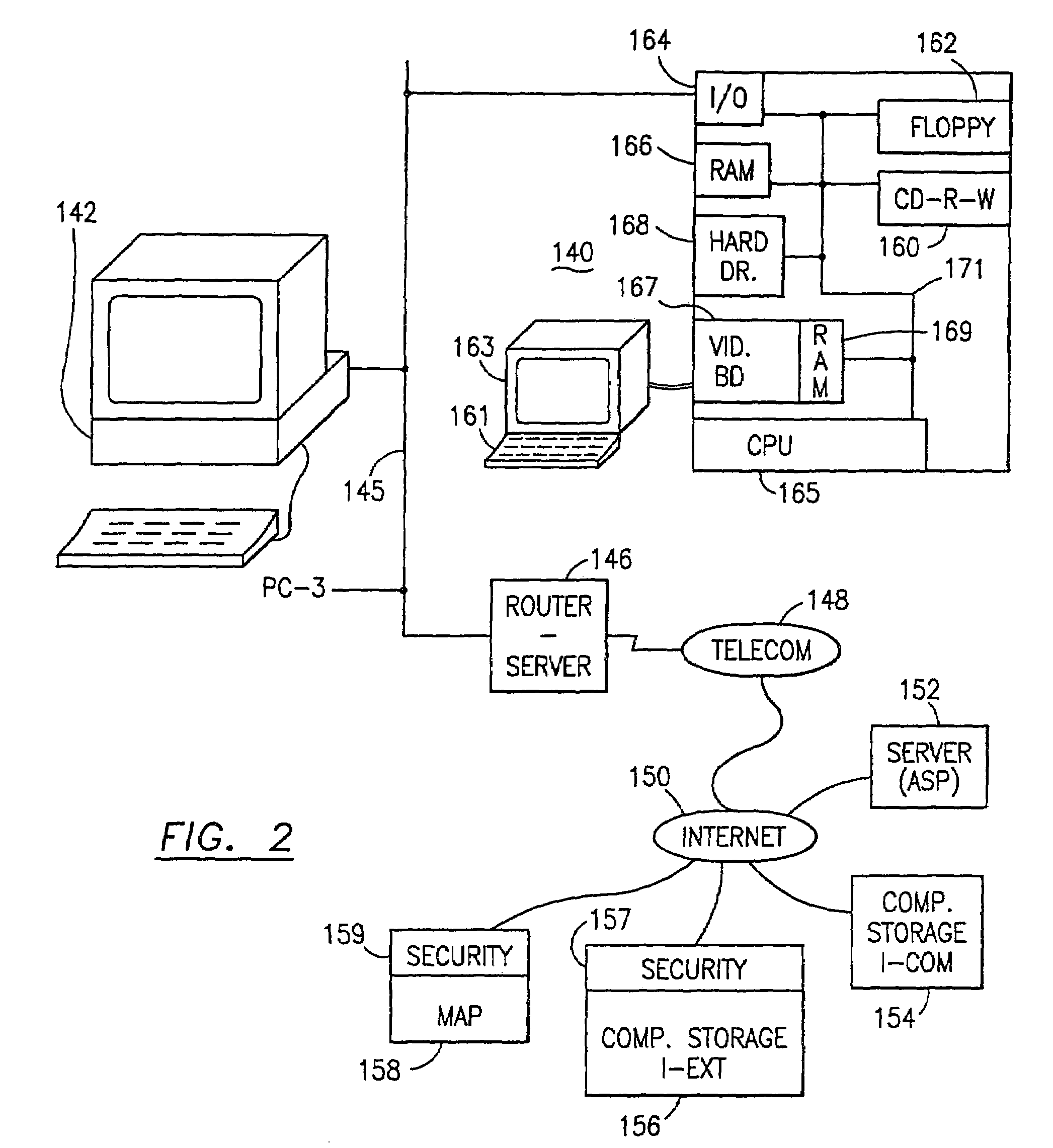
Data security system and method with multiple independent levels of security
InactiveUS20050138110A1Ease overhead performanceHigh overhead performancePeptide/protein ingredientsNGF/TNF-superfamilyInformation processingWorkstation
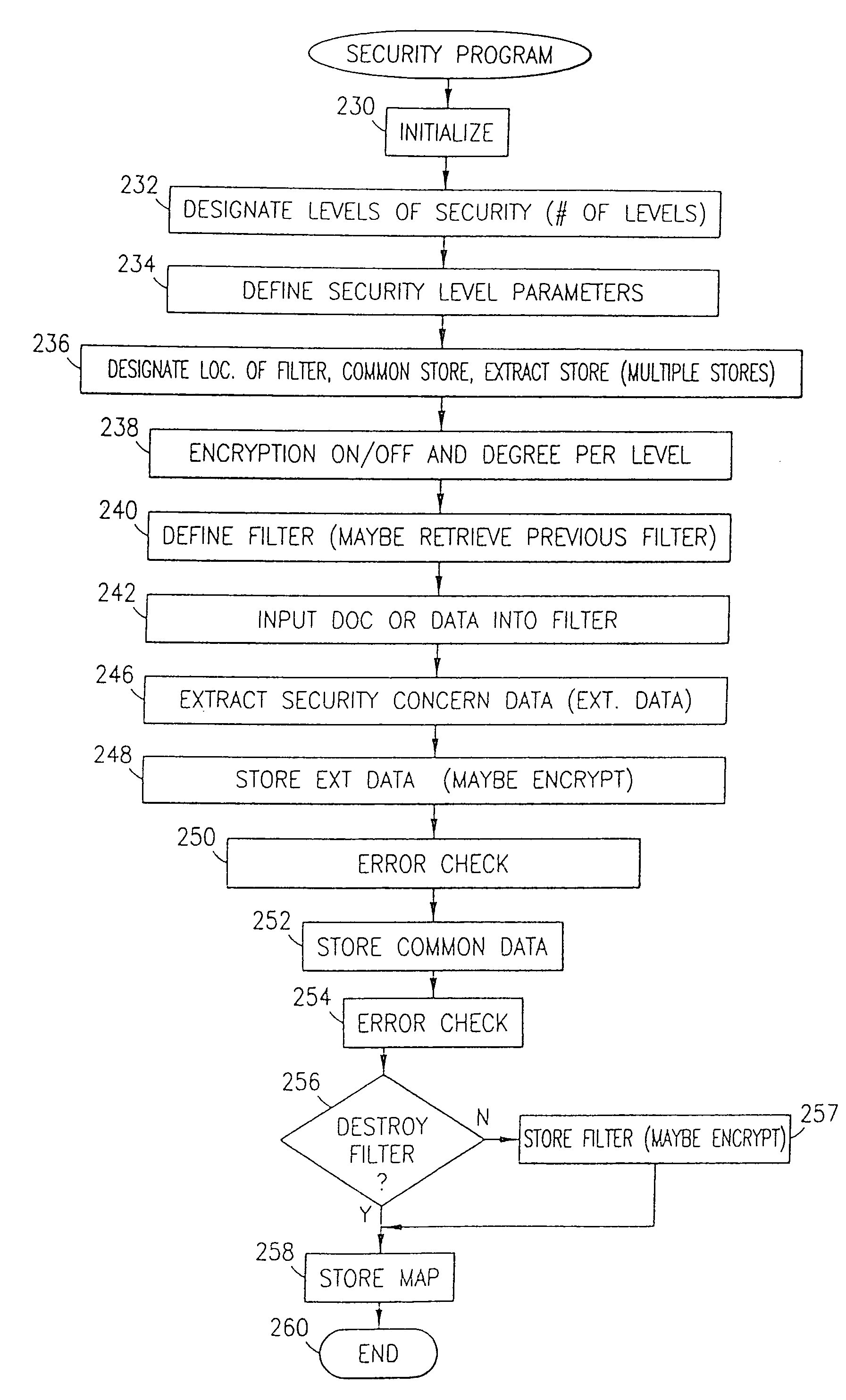
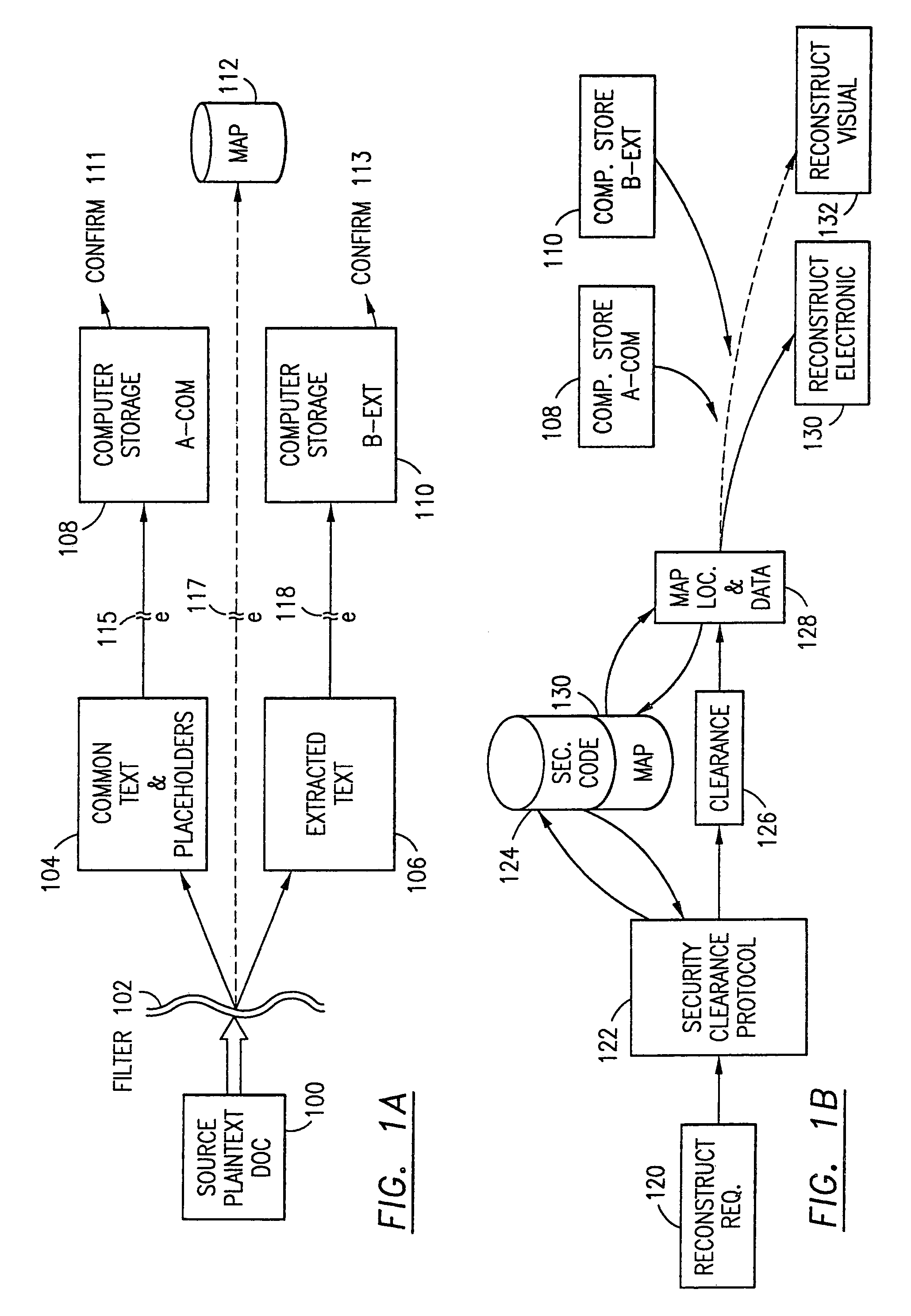
The method, program and information processing system secures data, and particularly security sensitive words, characters or data objects in the data, in a computer system with multiple independent levels of security (MILS). Each level of MILS has a computer sub-network with networked workstations. The MILS sub-networks are connected together via security guard computer(s) and each guard computer has separate memories for each level (TS, S, C, UC(or remainder)). The method extracts the security sensitive words / data (a granular action), from the source document for each MILS level, stores the extracted data in a corresponding extract store for each level and permits reconstruction / reassembly of the dispersed data via said extracted data at each said level of said multiple security levels and remainder data only in the presence of a predetermined security clearance commensurate with each MILS level.
Owner:DIGITAL DOORS
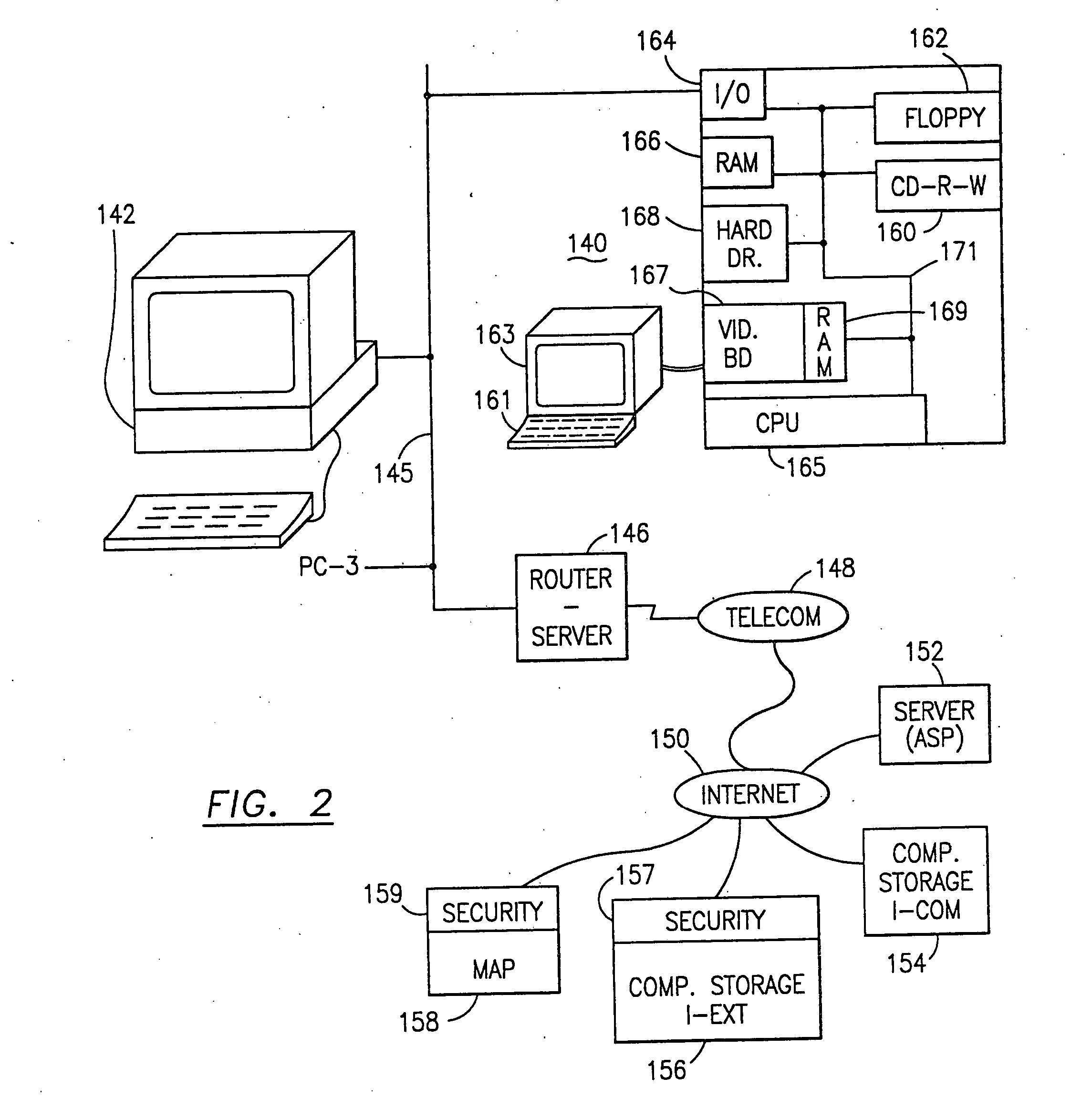
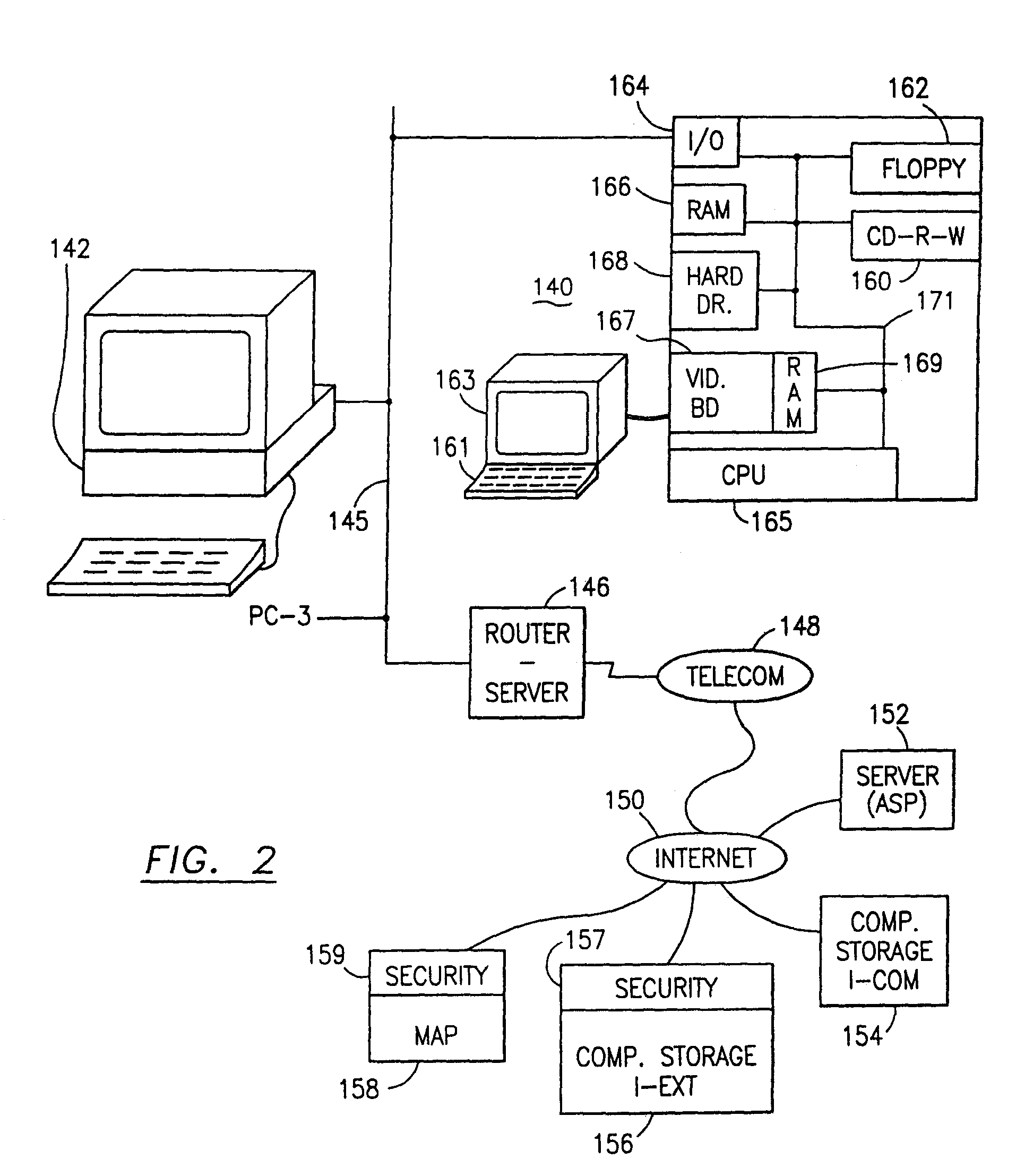
Data security system and method for portable device
ActiveUS7313825B2Ease overhead performanceHigh overhead performancePeptide/protein ingredientsDigital data processing detailsInformation processingEvent trigger
The method, used with a portable computing device, secures security sensitive words, icons, etc. by determining device location within or without a predetermined region and then extracting the security data from the file, text, data object or whatever. The extracted data is separated from the remainder data and stored either on media in a local drive or remotely, typically via wireless network, to a remote store. Encryption is used to further enhance security levels. Extraction may be automatic, when the portable device is beyond a predetermined territory, or triggered by an event, such a “save document” or a time-out routine. Reconstruction of the data is permitted only in the presence of a predetermined security clearance and within certain geographic territories. A computer readable medium containing programming instructions carrying out the methodology for securing data is also described herein. An information processing system for securing data is also described.
Owner:DIGITAL DOORS
Data security system and method associated with data mining
ActiveUS7322047B2Ease overhead performanceHigh overhead performancePeptide/protein ingredientsMultiple keys/algorithms usagePlaintextInternet privacy
The data security method, system and associated data mining enables multiple users, each having a respective security clearance level to access security sensitive words, data objects, characters or icons. The method extracts security sensitive words, data objects, characters or icons from plaintext or other source documents to obtain (a) subsets of extracted data and (b) remainder data. The extracted data is, in one embodiment, stored in a multilevel security system (MLS) which separates extract data of different security levels with MLS guards. Some or all of the original data is reconstructed via one or more of the subsets of extracted data and remainder data only in the presence of a predetermined security level. In this manner, an inquiring party, with the proper security clearance, can data mine the data in the MLS secured storage.
Owner:DIGITAL DOORS
Data security system and method with multiple independent levels of security
InactiveUS7669051B2Peptide/protein ingredientsNGF/TNF-superfamilyInformation processingSecurity guard
The method, program and information processing system secures data, and particularly security sensitive words, characters or data objects in the data, in a computer system with multiple independent levels of security (MILS). Each level of MILS has a computer sub-network with networked workstations. The MILS sub-networks are connected together via security guard computer(s) and each guard computer has separate memories for each level (TS, S, C, UC (or remainder)). The method extracts the security sensitive words / data (a granular action), from the source document for each MILS level, stores the extracted data in a corresponding extract store for each level and permits reconstruction / reassembly of the dispersed data via said extracted data at each said level of said multiple security levels and remainder data only in the presence of a predetermined security clearance commensurate with each MILS level.
Owner:DIGITAL DOORS
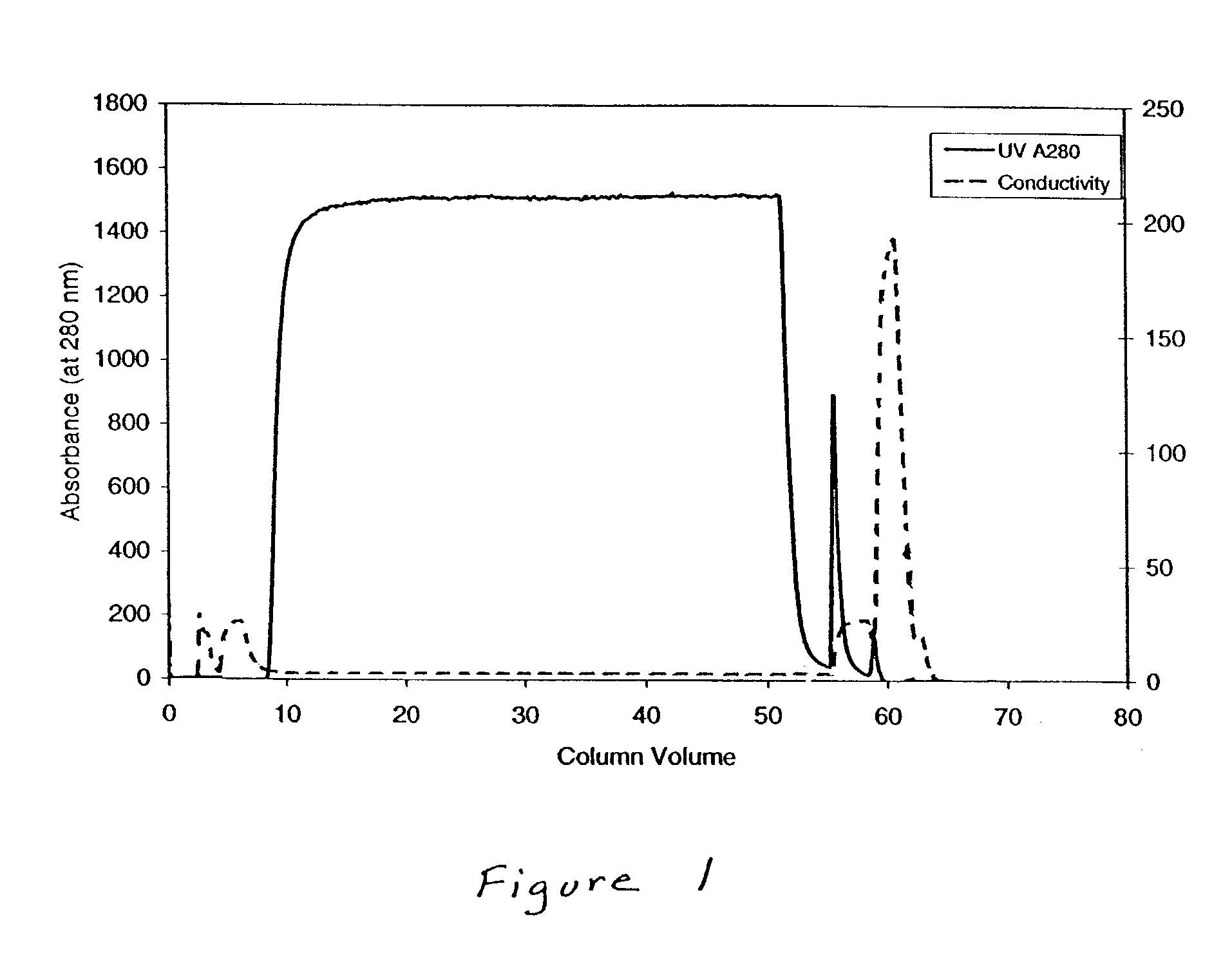
Methods for purifying protein
ActiveUS7122641B2Antibody mimetics/scaffoldsNGF/TNF-superfamilyAntibody Affinity ChromatographyImmunoglobulin domain
A method is provided for separating a protein from one or more other proteins using hydroxyapatite chromatography in which the protein does not bind to hydroxyapatite but the other protein(s) does. In some embodiments, a second protein affixed to a solid support has been used previously to purify the protein by affinity chromatography, and small amounts of the second protein are introduced in the sample during this process. The protein being purified can comprise at least one constant antibody immunoglobulin domain. The second protein can bind to proteins comprising such a domain.
Owner:IMMUNEX CORP
Methods and compositions for use in treatment of patients with autoantibody positive disease
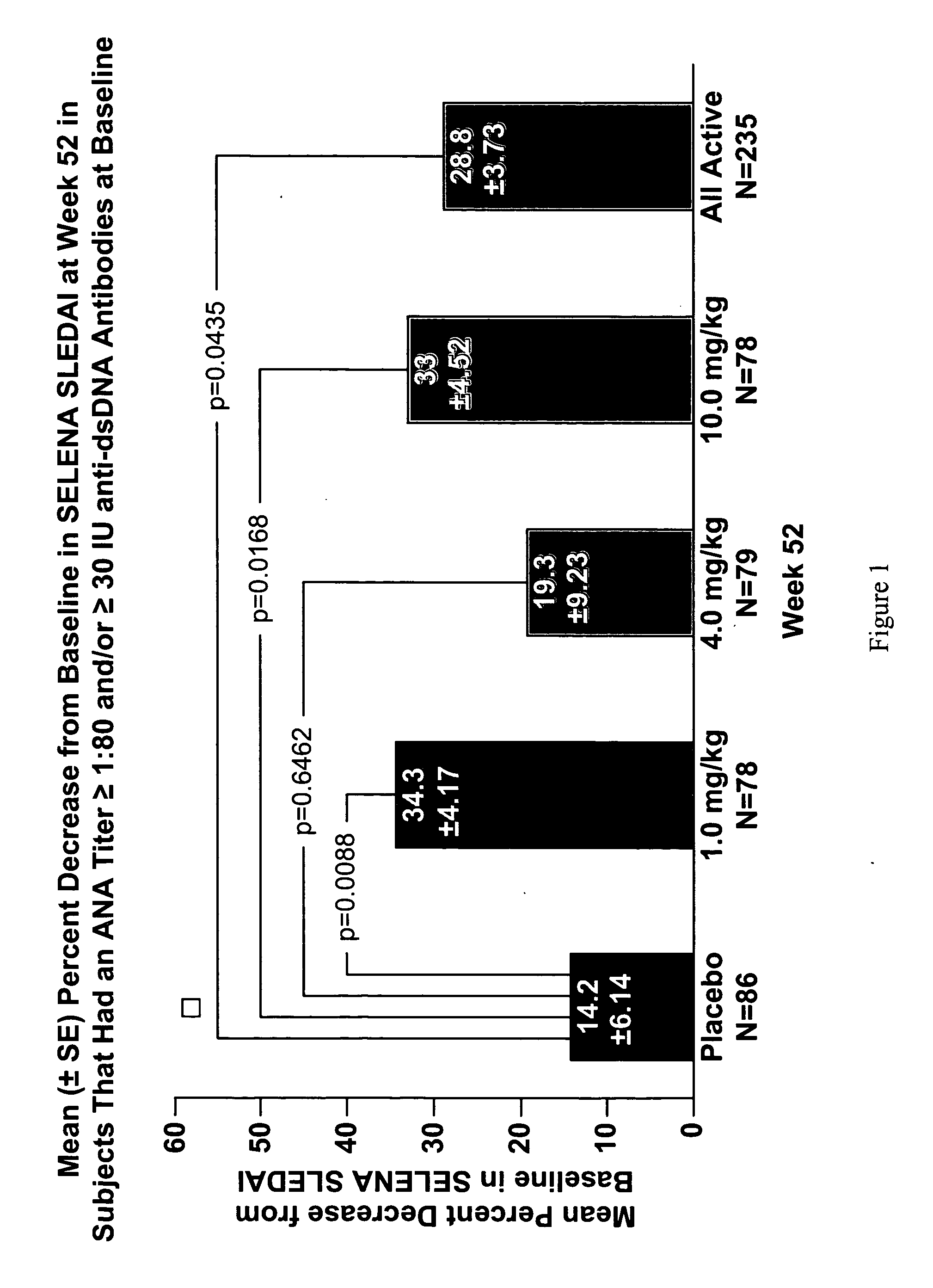
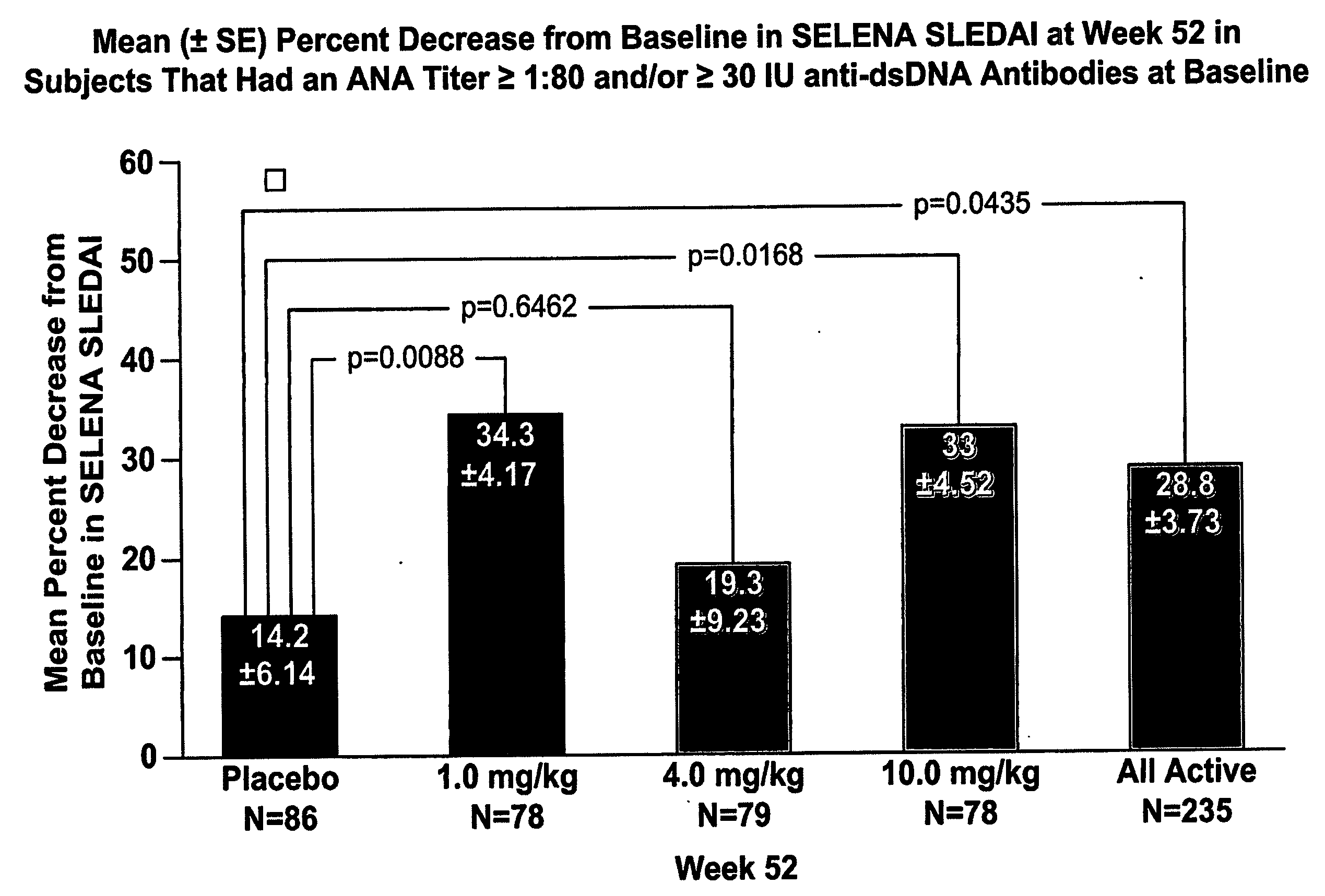
InactiveUS20070086979A1Reduce frequencyReduce in quantityPeptide/protein ingredientsAntipyreticAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
Compositions and Methods for Targeted Immunomodulatory Antibodies and Fusion Proteins
ActiveUS20130039911A1Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antobodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antobodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immuno-suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β)) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By conteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for targeted immunomodulatory antibodies and fusion proteins
ActiveUS8993524B2Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antibodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antibodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immune suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By counteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Chimeric proteins and methods for using the same
InactiveUS7569663B2Enhanced advantageImprove propertiesCompounds screening/testingPeptide/protein ingredientsTrans signalingDisease
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
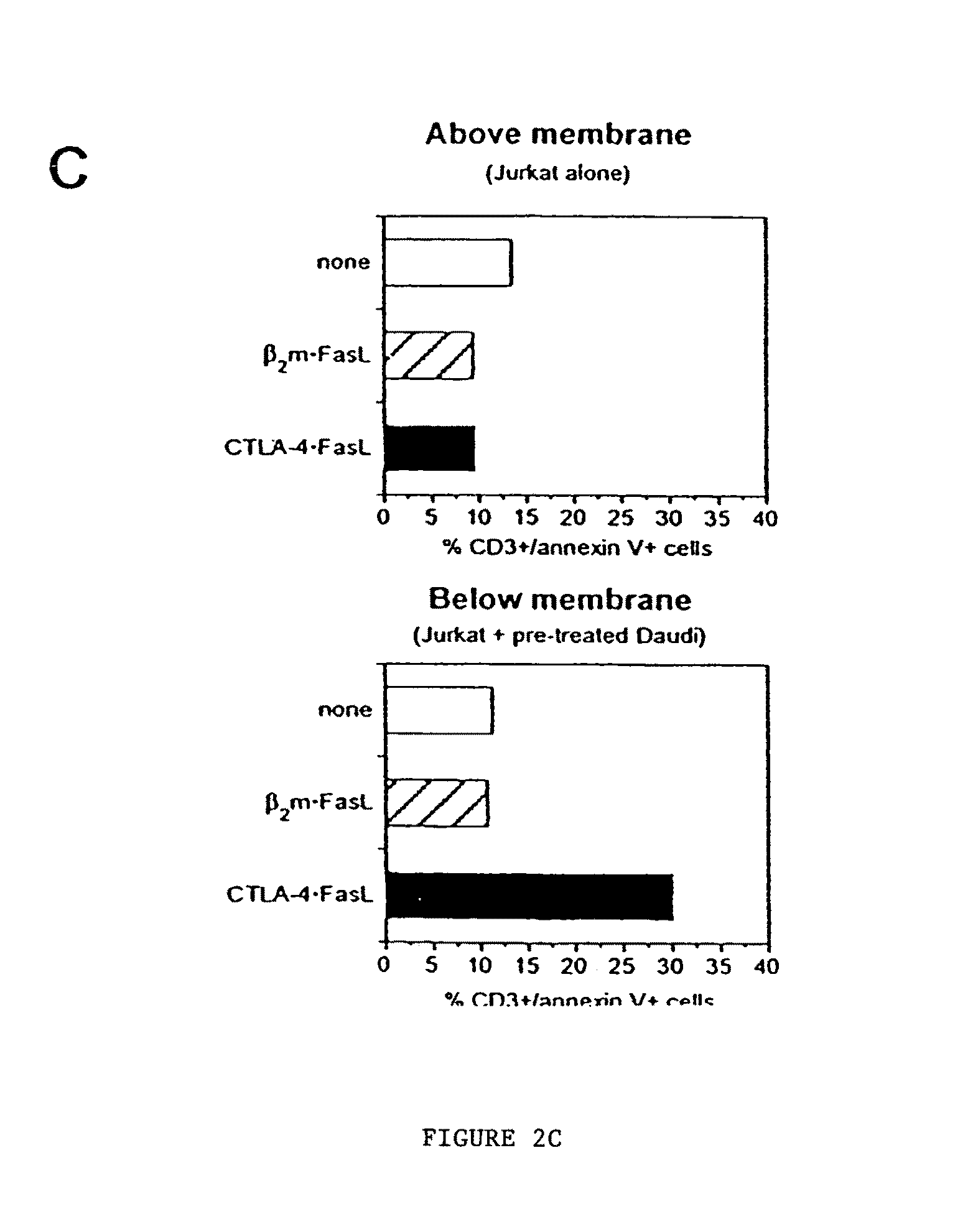
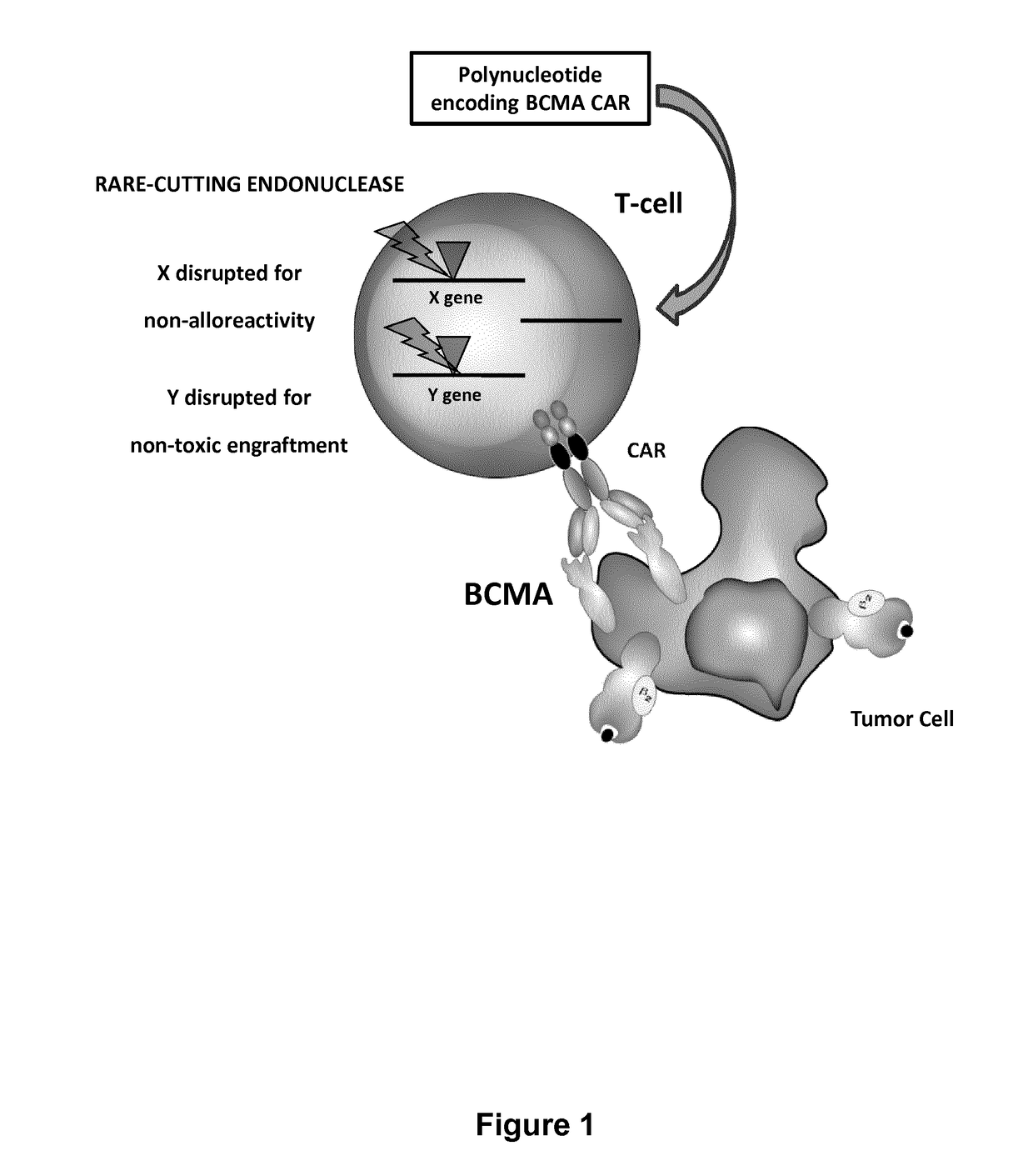
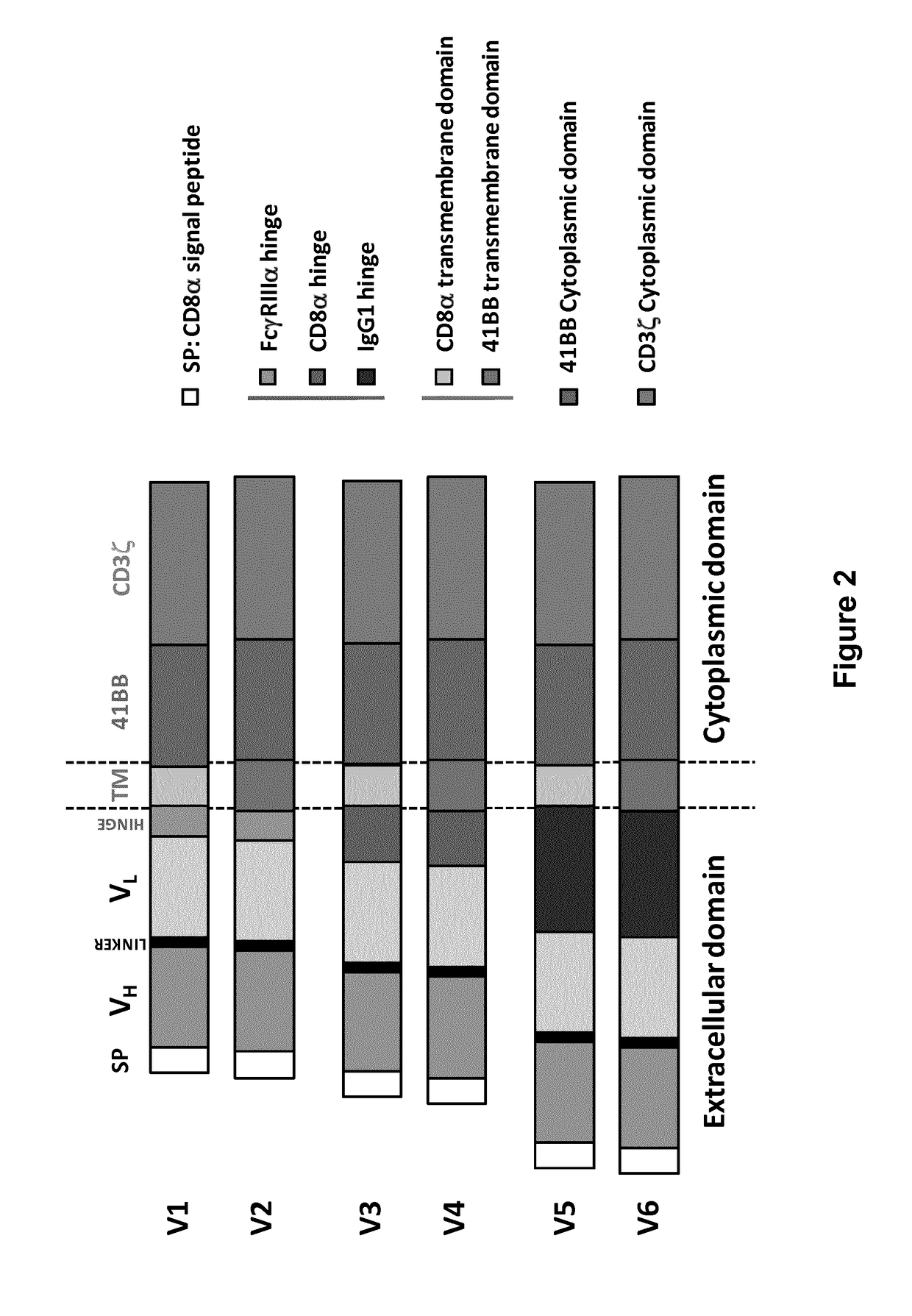
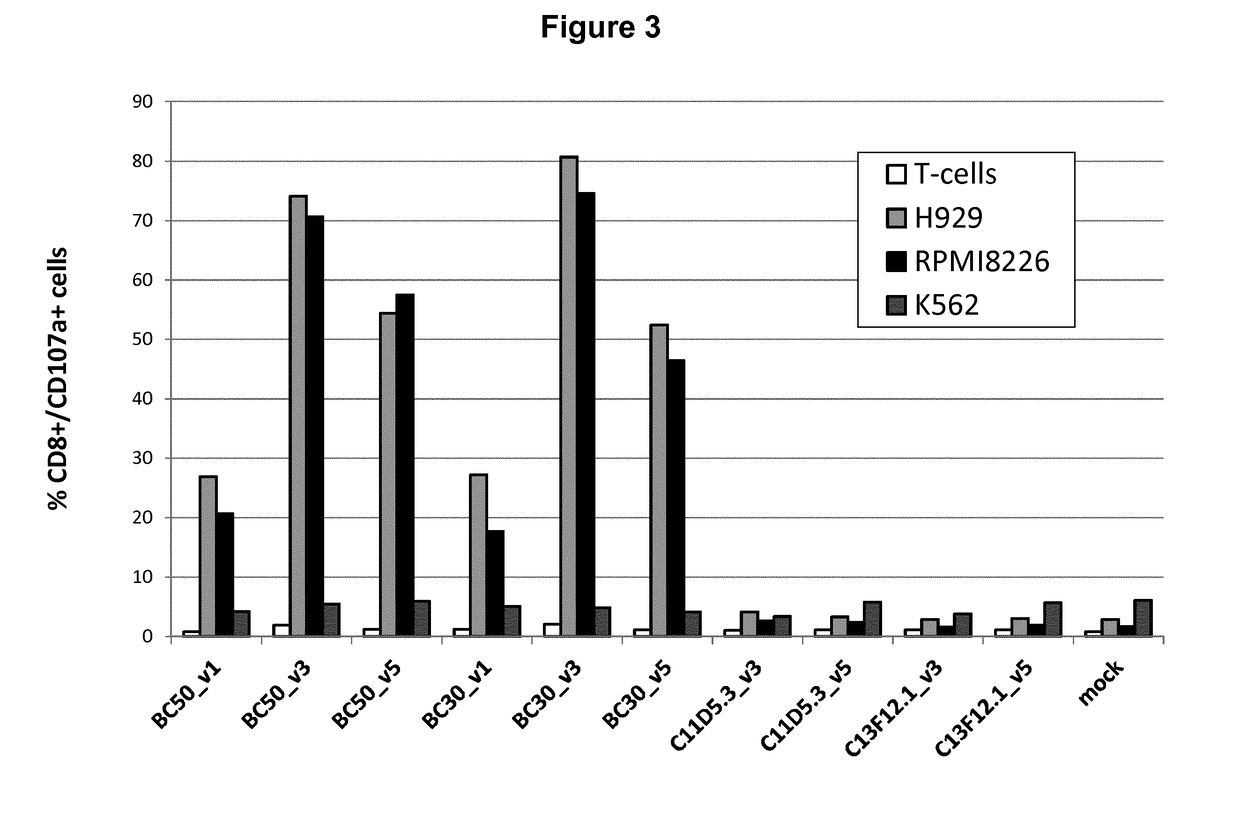
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
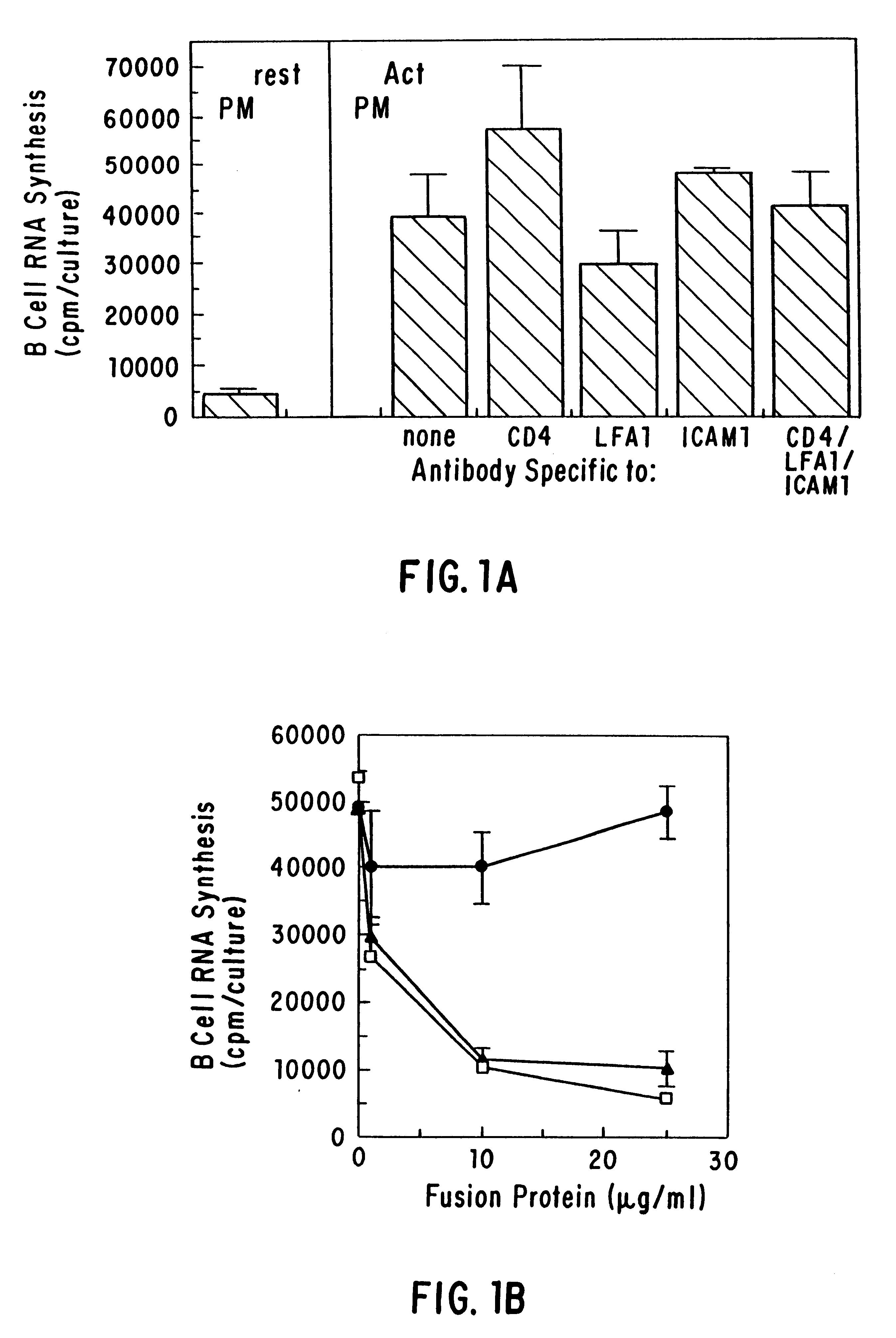
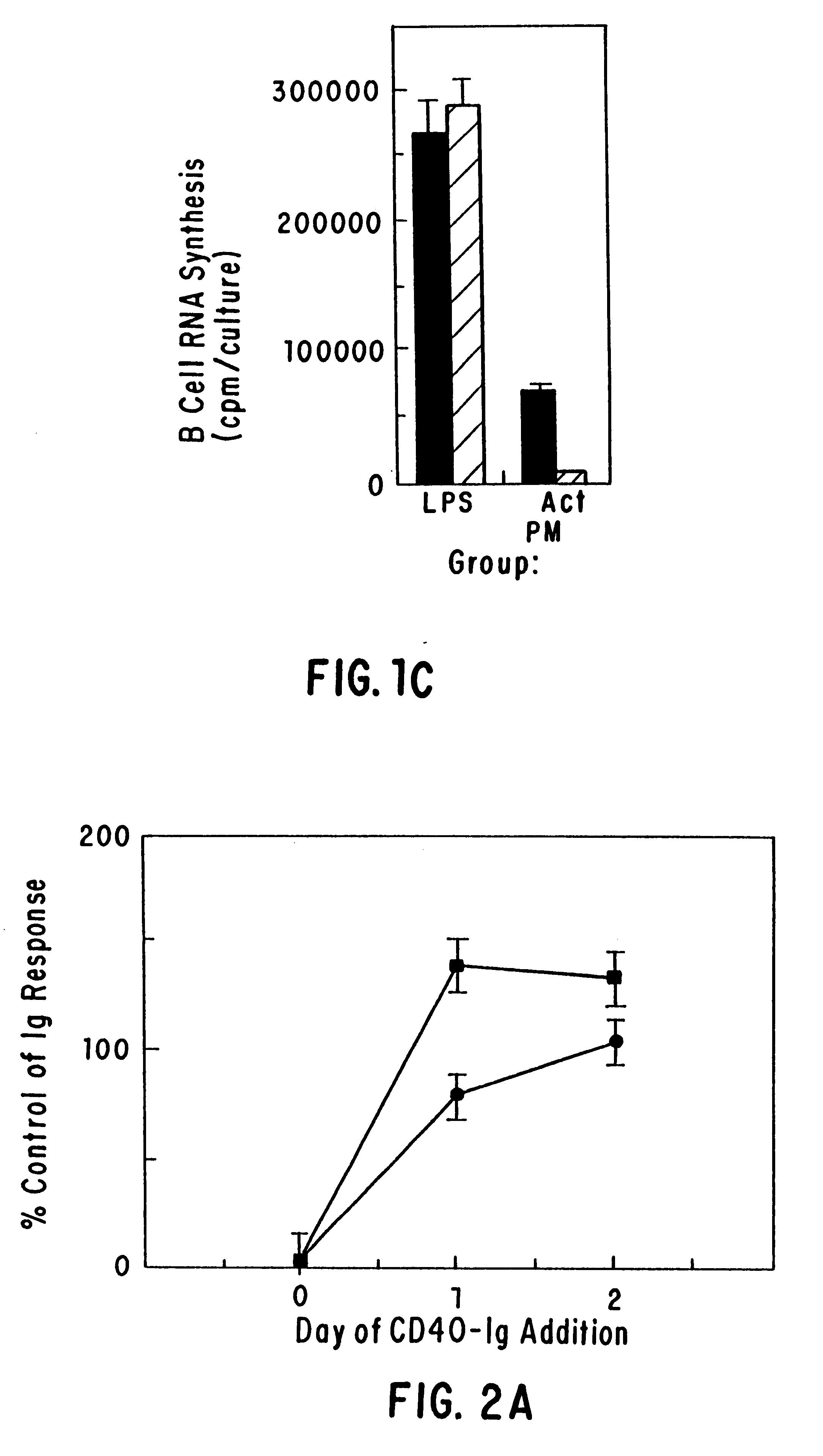
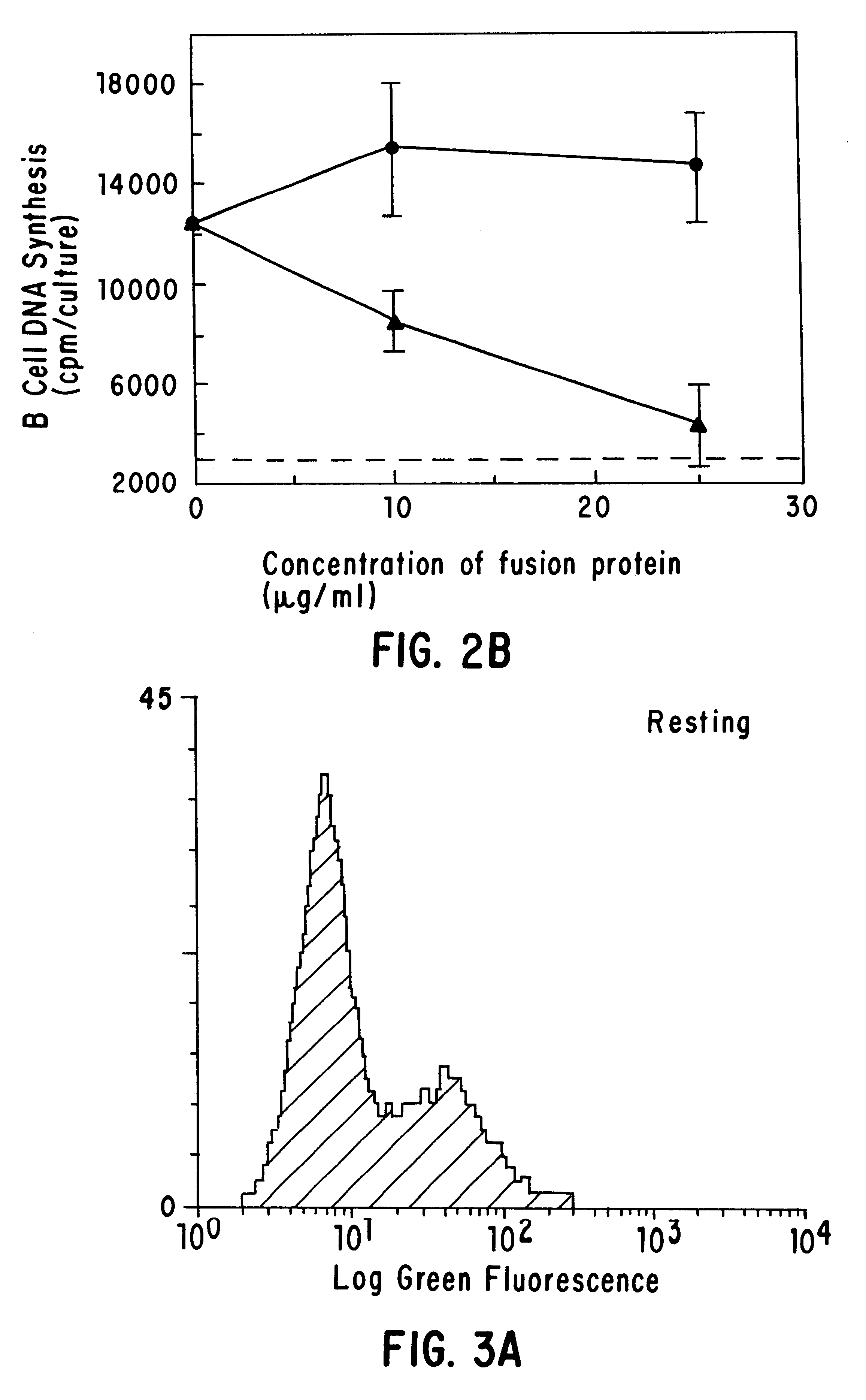
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
Inhibitor which is deactivatable by a reagent produced by a target cell
ActiveUS8809504B2Undesirable effectModulating responseNGF/TNF-superfamilyAntibody ingredientsActive agentWhole body
The invention relates to molecules inhibiting biologically active compounds and further comprising moieties specifically cleavable by a reagent produced by a target cell. The invention relates to inhibitors that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent. Inhibitors comprise at least one moiety that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent and at least one moiety that can be cleaved specifically by a reagent produced by target cells. The cleavage deactivates the inhibitor. Following cleavage, the active agent is liberated into the local environment. Administration of the inhibitor alone or together with the active agent suppress the compound's activity until it reaches the proximity of a target cell. Targeted specific release enables the agent concentration in specific site to reach levels that have desired therapeutic effects without systemic toxicity.
Owner:VYTACERA BIO LLC
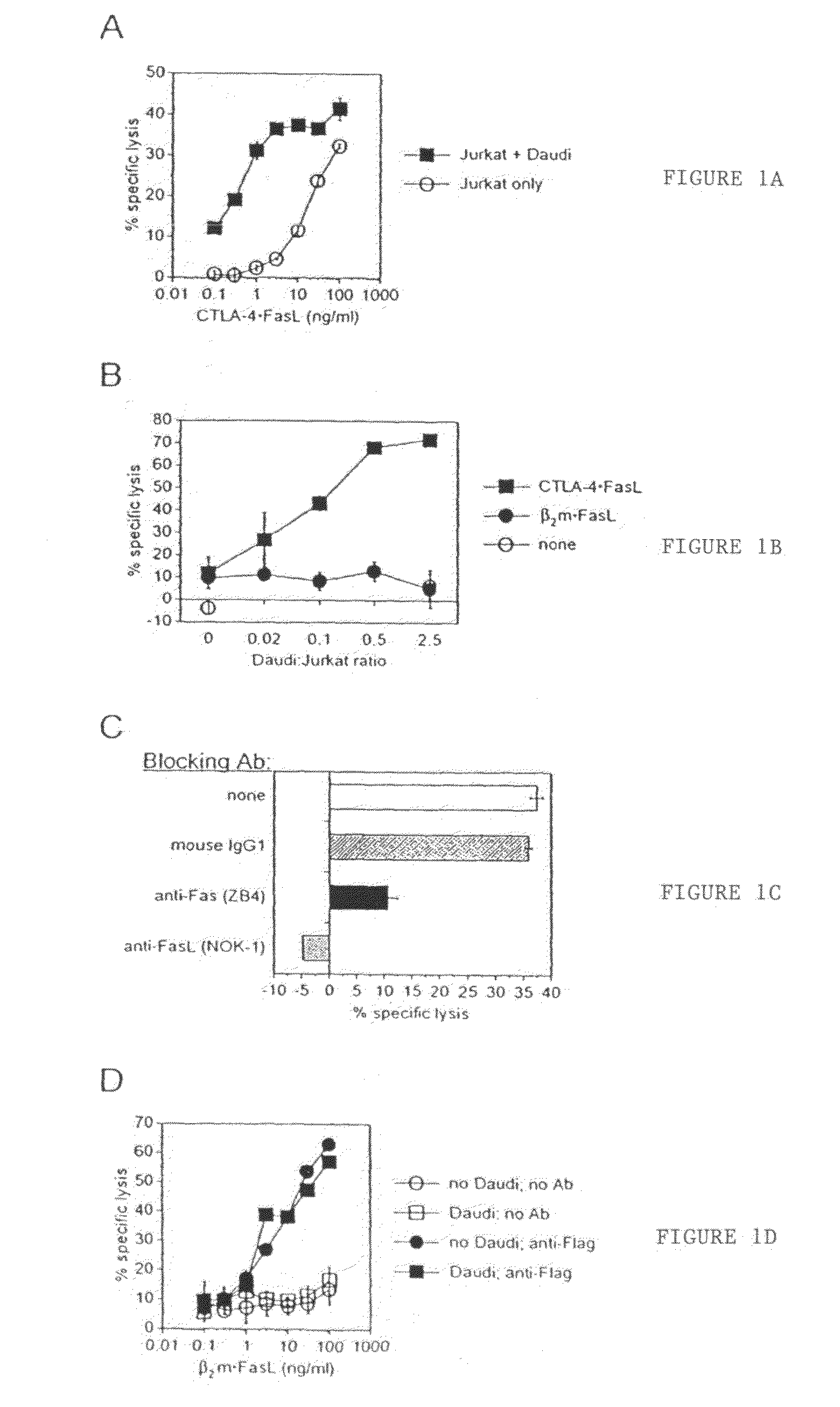
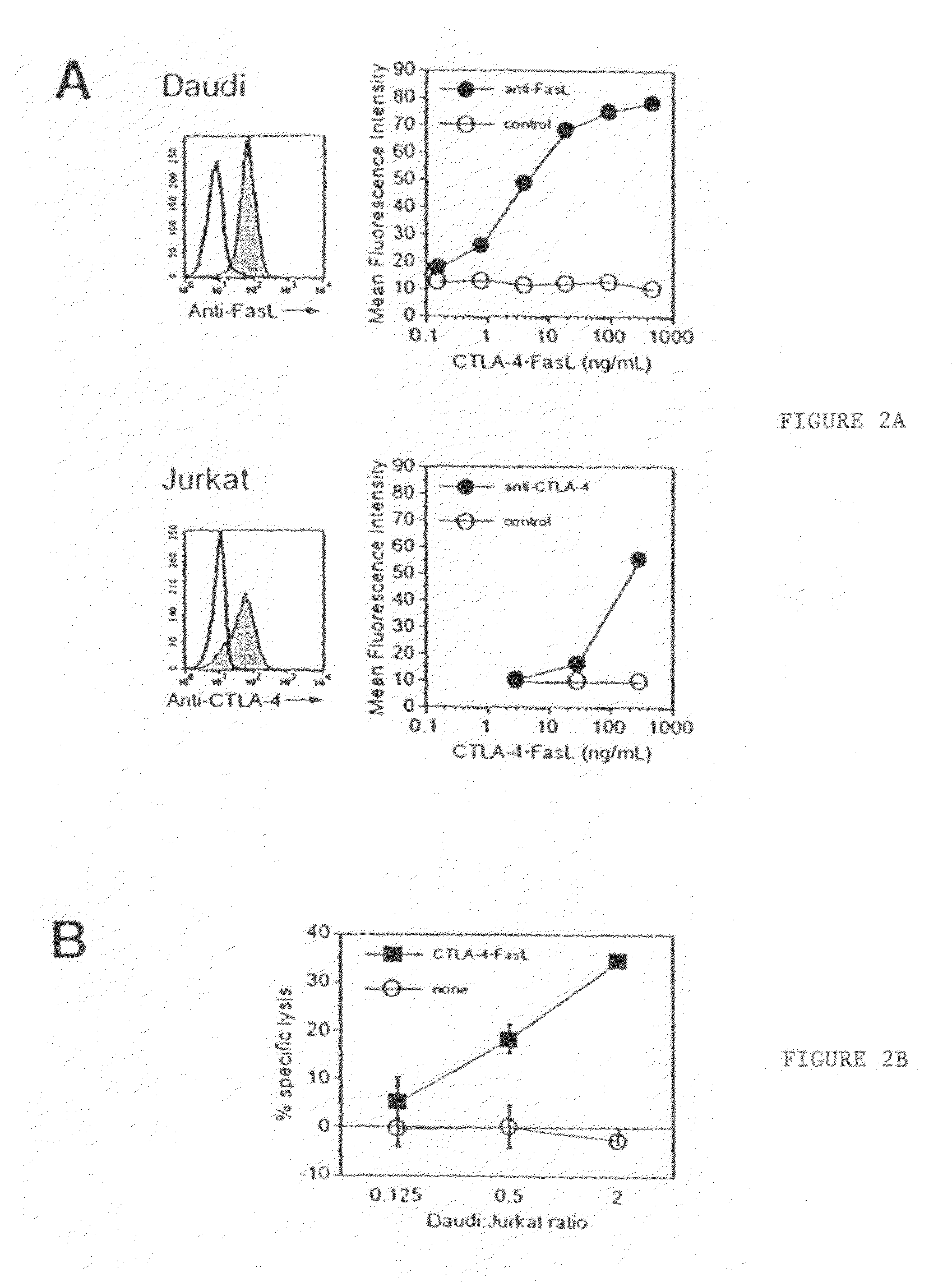
Apoptosis inducing molecule II and methods of use
The present invention relates to a novel member of the TNF-Ligand superfamily. More specifically, isolated nucleic acid molecules are provided encoding a human Apoptosis Inducing Molecule II (AIM II). AIM II polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of AIM II activity. Also provided are therapeutic methods for treating lymphadenopathy, aberrant bone development, autoimmune and other immune system diseases, graft versus host disease, rheumatoid arthritis, osteoarthritis and to inhibit neoplasia, such as tumor cell growth.
Owner:HUMAN GENOME SCI INC
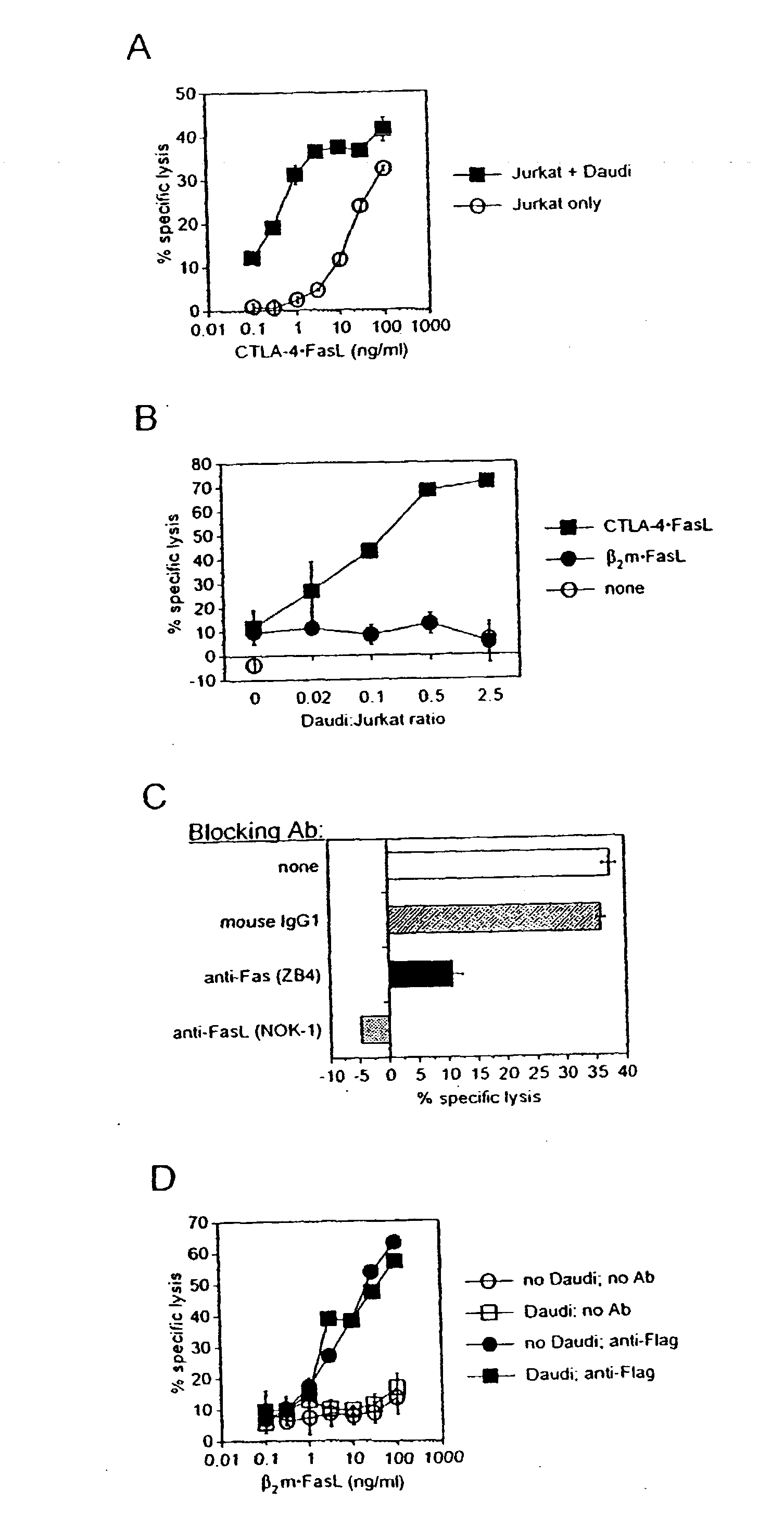
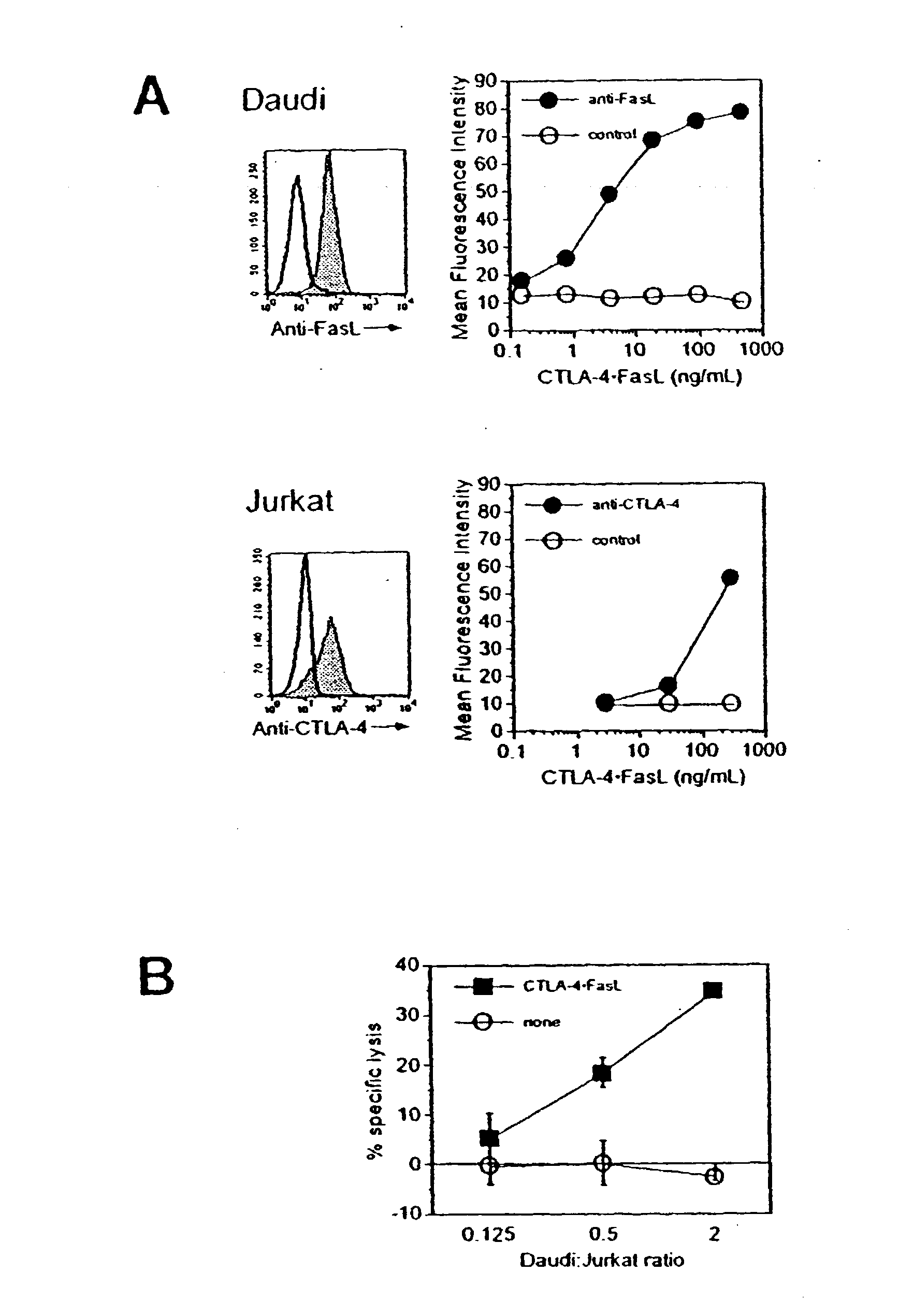
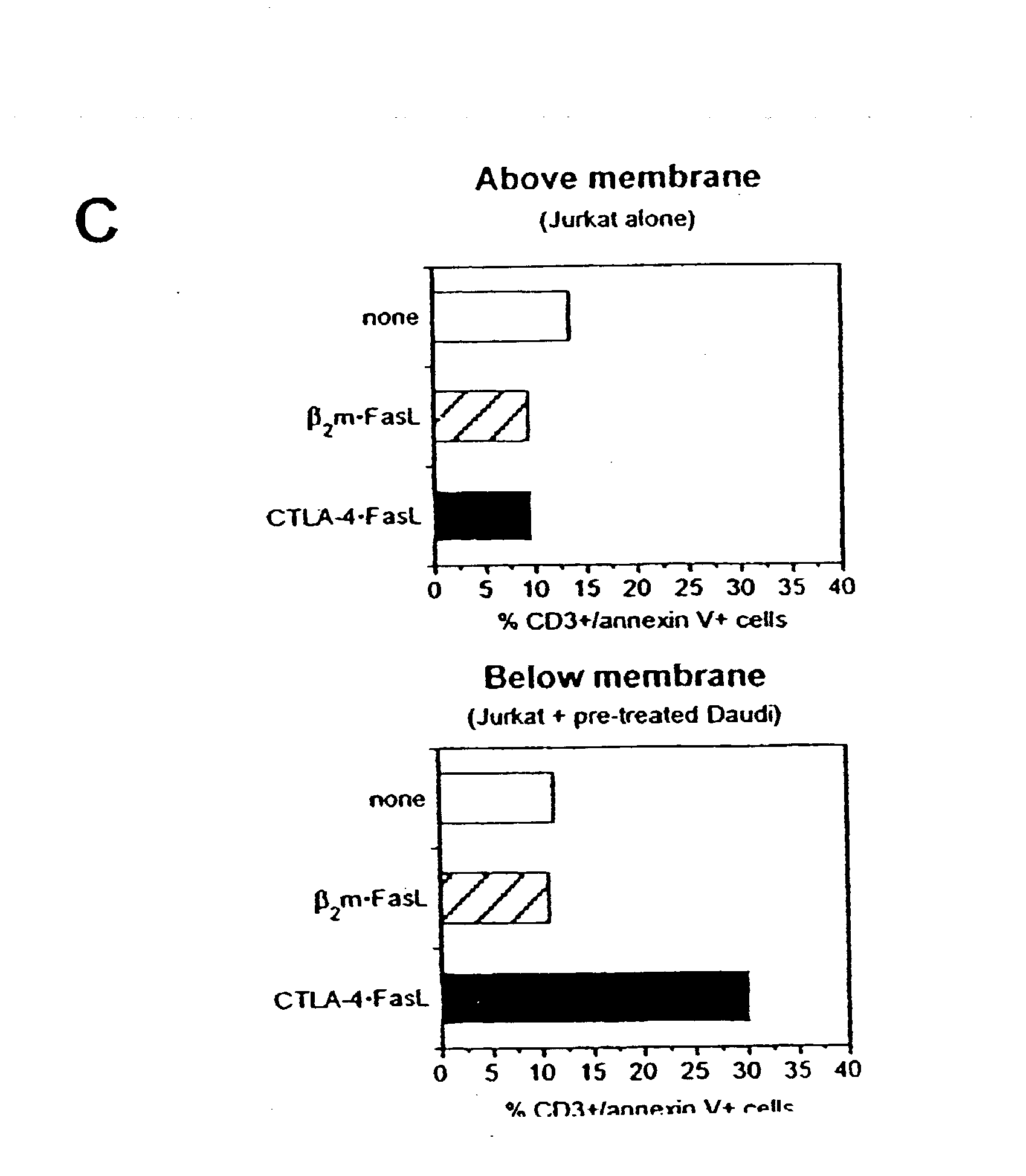
Anti-Fas antibodies
Anti-Fas antibodies which are cross-reactive with mouse and human Fas and are useful in the treatment of conditions attributable to abnormalities in the Fas / Fas ligand system.
Owner:SANKYO CO LTD
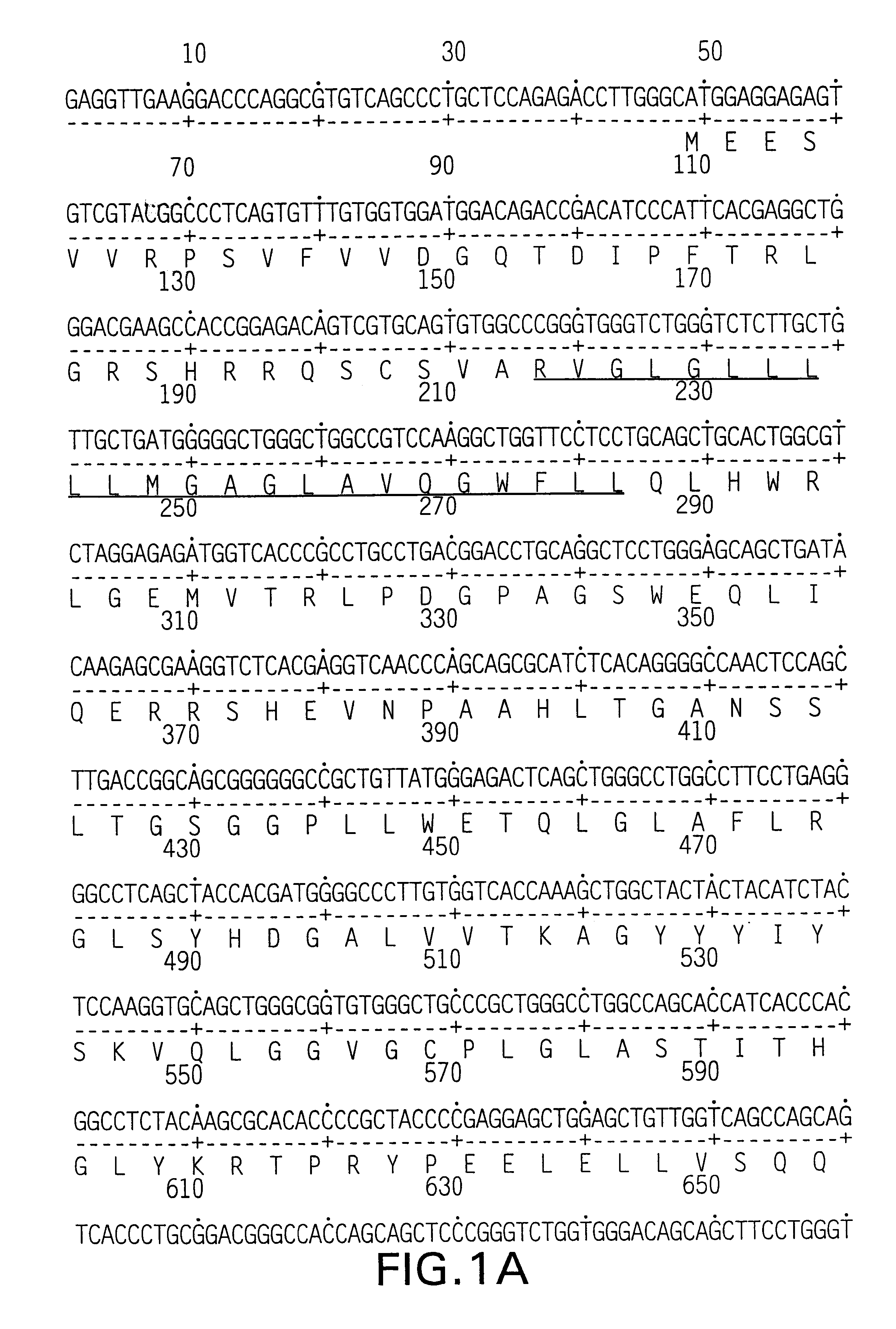
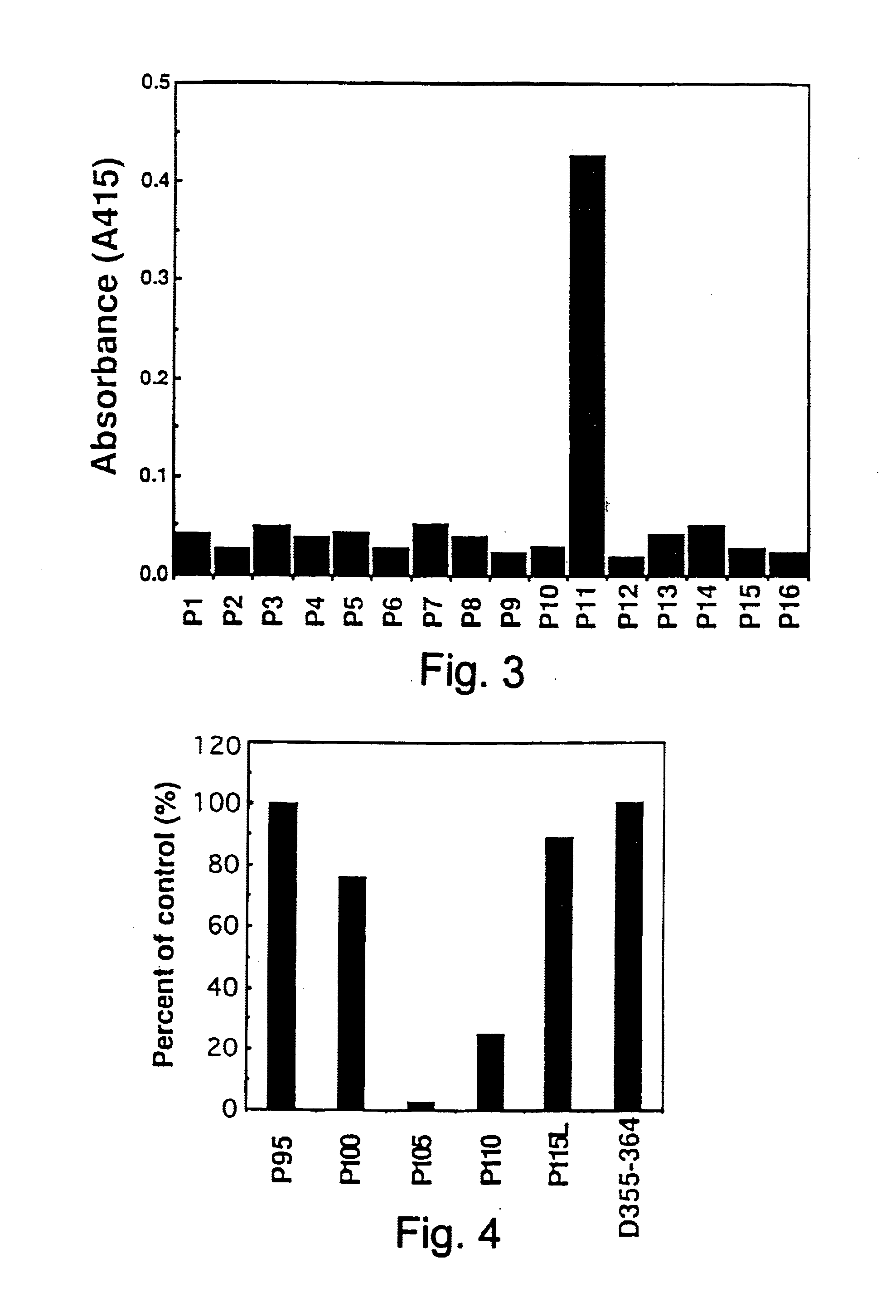
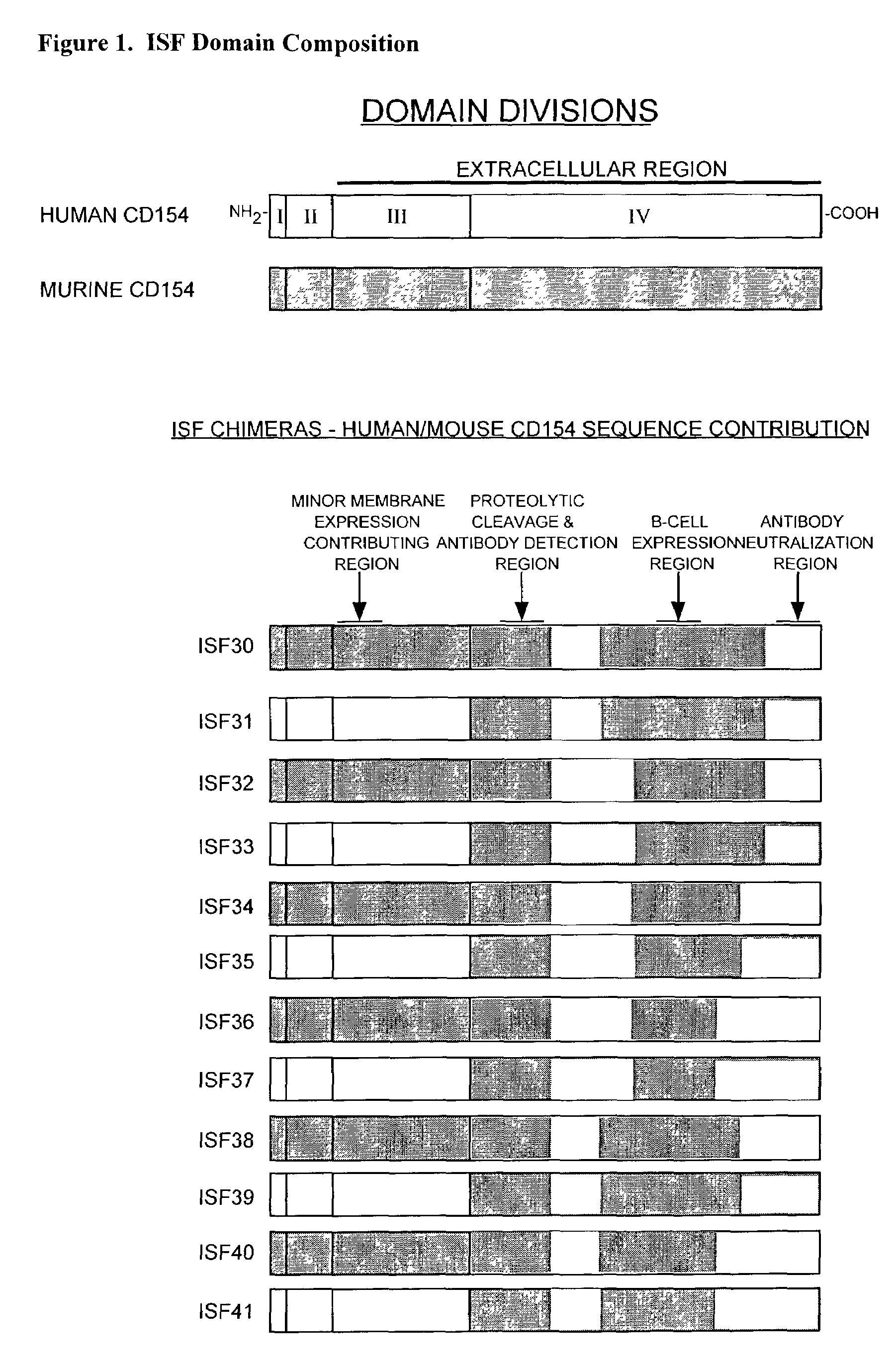
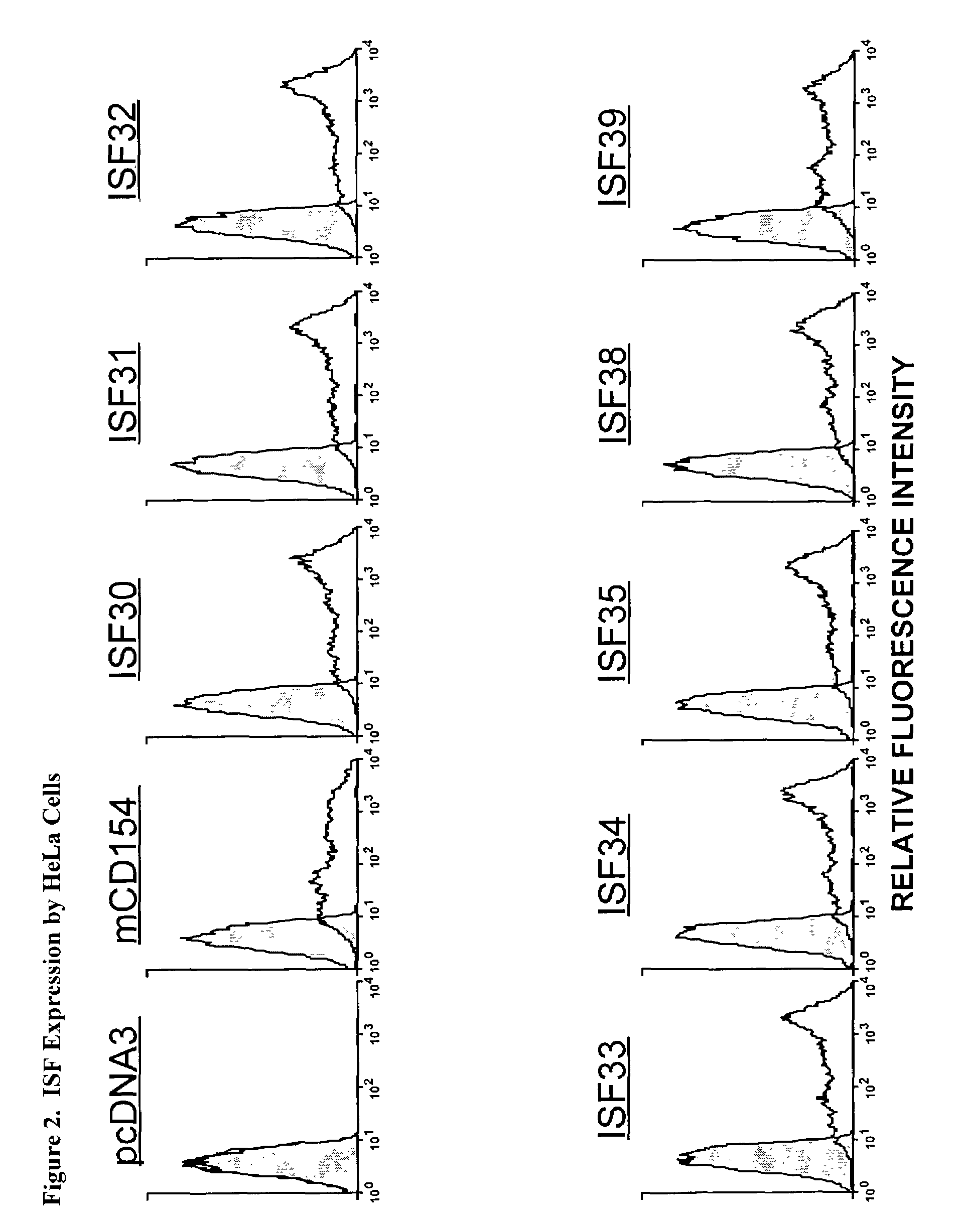
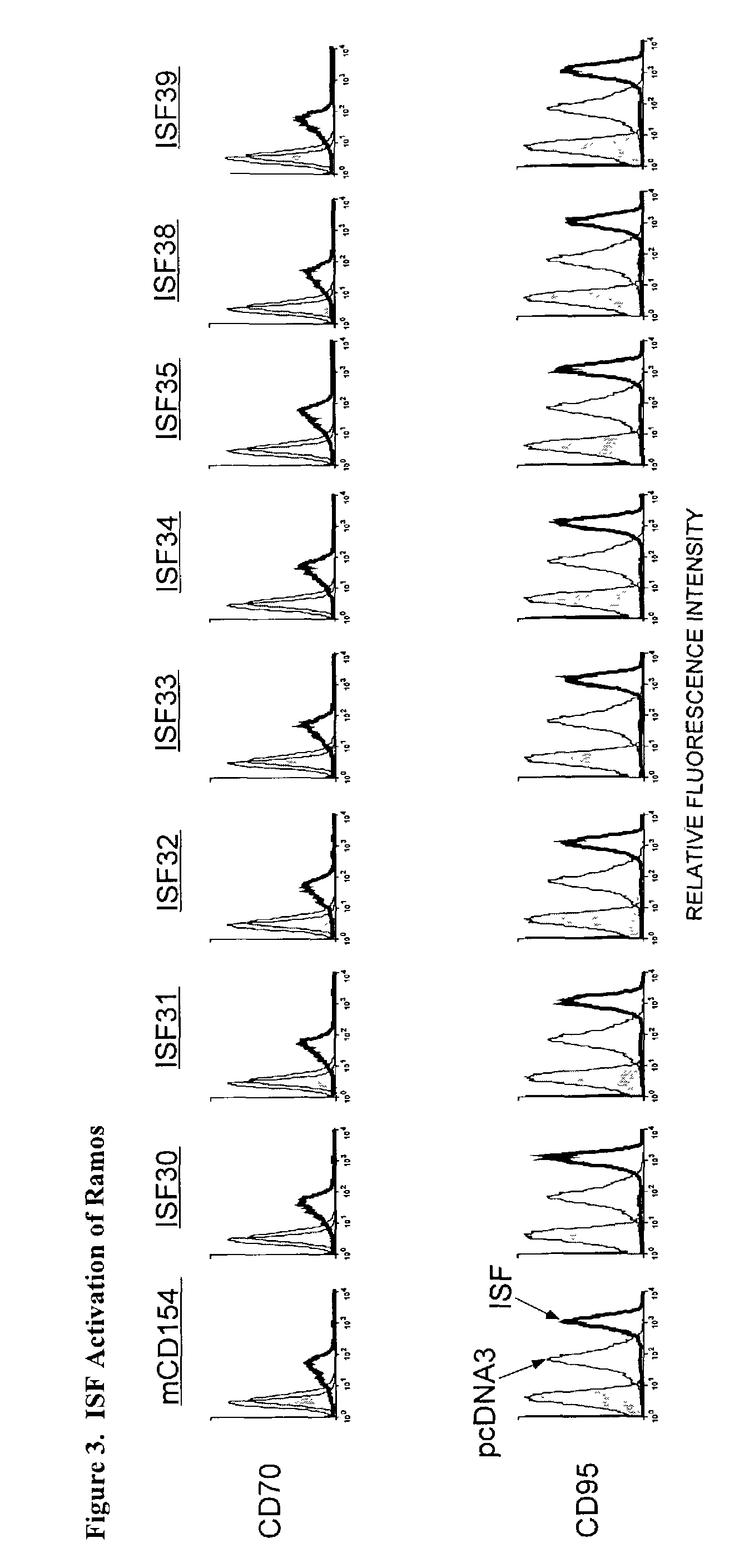
Nucleic acids encoding chimeric CD154 polypeptides
InactiveUS7495090B2Safe and effective for disease therapyLower resistanceBacteriaPeptide/protein ingredientsNucleotideNucleotide sequencing
The present invention provides for an isolated polynucleotide sequence encoding a chimeric CD154, comprising a first nucleotide sequence encoding an extracellular subdomain of non-human CD154, preferably murine CD154, that replaces a cleavage site of human CD154, and a second nucleotide sequence encoding an extracellular subdomain of human CD154 that binds to a human CD154 receptor. The present invention also provides for the chimeric CD154 that is encoded by the above-described polynucleotide sequence, an expression vector and a genetic vector comprising the polynucleotide sequence, a host cell comprising the expression vector or the genetic vector, a process for producing the chimeric CD154, and methods for utilizing the expression vectors and genetic constructs containing the chimeric CD154 polynucleotide sequences.
Owner:RGT UNIV OF CALIFORNIA
Combination therapy for the prevention or treatment of cancer, inflammatory disorders or infectious diseases in a subject
InactiveUS20030035790A1Good treatment effectReduce dosageVirusesPeptide/protein ingredientsDendritic cellT cell
The present invention relates to compositions comprising compounds which augment activated immune cells, such as T-cells, dendritic cells and natural killer ("NK") cells, and methods for the treatment or prevention of diseases and disorders, including cancer, inflammatory disorders, and infectious diseases, in a subject comprising the administration of said compositions to said subject. In particular, the present invention relates to methods for the treatment or prevention of diseases and disorders, including cancer, inflammatory disorders, and infectious diseases, in a subject comprising administrating to said subject one or more compounds that activate one or more cytokine receptors and one or more compounds that activate one or more co-stimulatory molecules expressed by activated immune cells. The present invention also relates to compositions and kits comprising a compound that activates one or more cytokine receptors and a compound that activates one or more co-stimulatory molecules expressed by activated immune cells.
Owner:MT SINAI SCHOOL OF MEDICINE
Novel chimeric proteins and methods for using the same
InactiveUS20030216546A1Compounds screening/testingPeptide/protein ingredientsDiseaseProtein insertion
Novel chimeric proteins are disclosed. The proteins comprise at least two portions. The first portion binds to a first cell and decreases the cell's ability to send a trans signal to a second cell; the second portion sends its own trans signal to the second cell. Methods for making and using these proteins in the treatment of cancer, viral infections, autoimmune and alloimmune diseases are also disclosed, as are pharmaceutical formulations comprising the novel chimeric proteins and genes. Either the proteins themselves or a genetic sequence encoding the protein can be administered. Other methods are also disclosed in which two molecular components result in decrement of a first trans signal from a first cell and the conferring of a second trans signal to a second cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
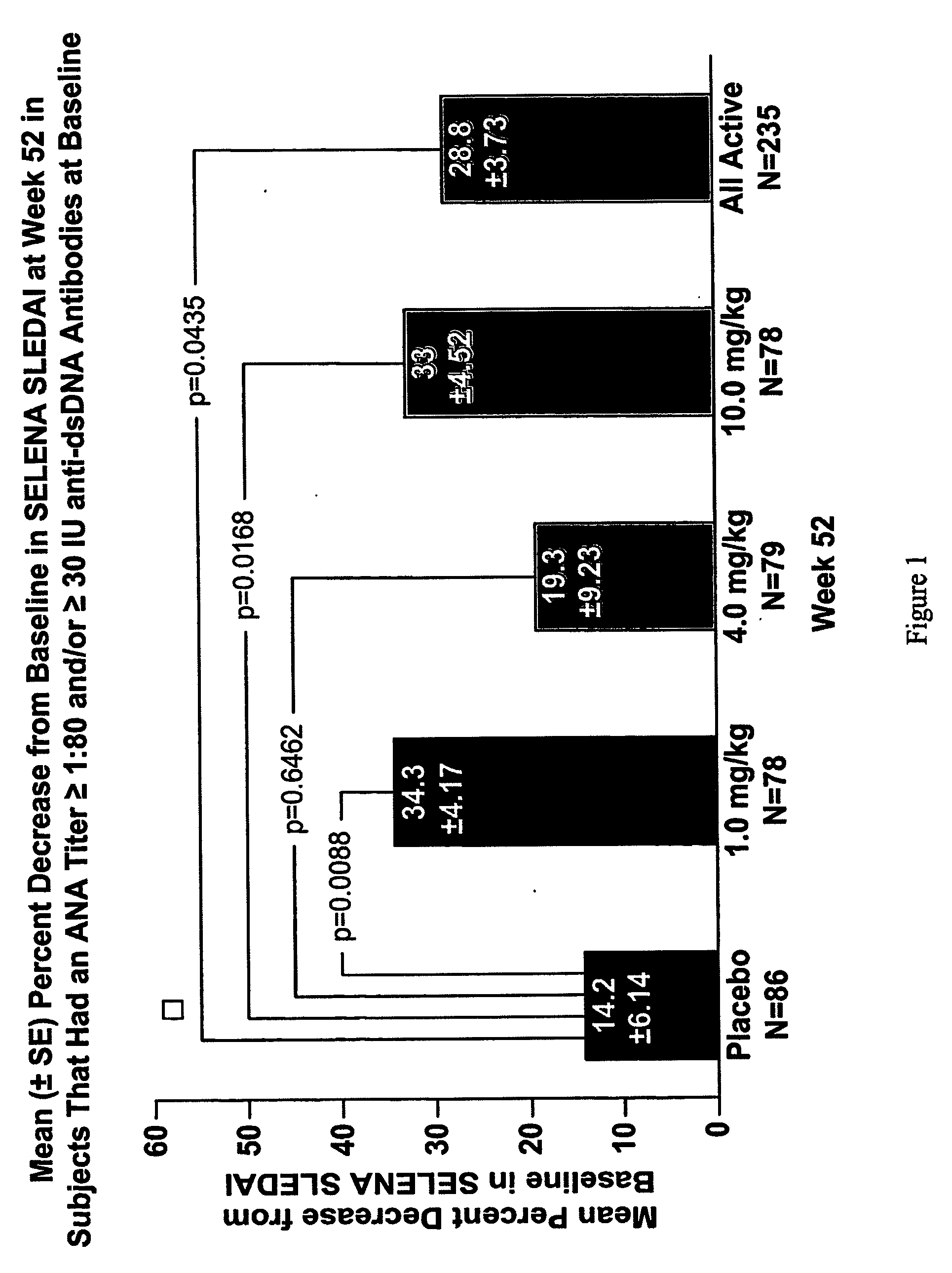
Methods and compositions for use in treatment of patients with autoantibody positive disease
ActiveUS20090148462A1Reduce frequencyReduce in quantityNervous disorderPeptide/protein ingredientsAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
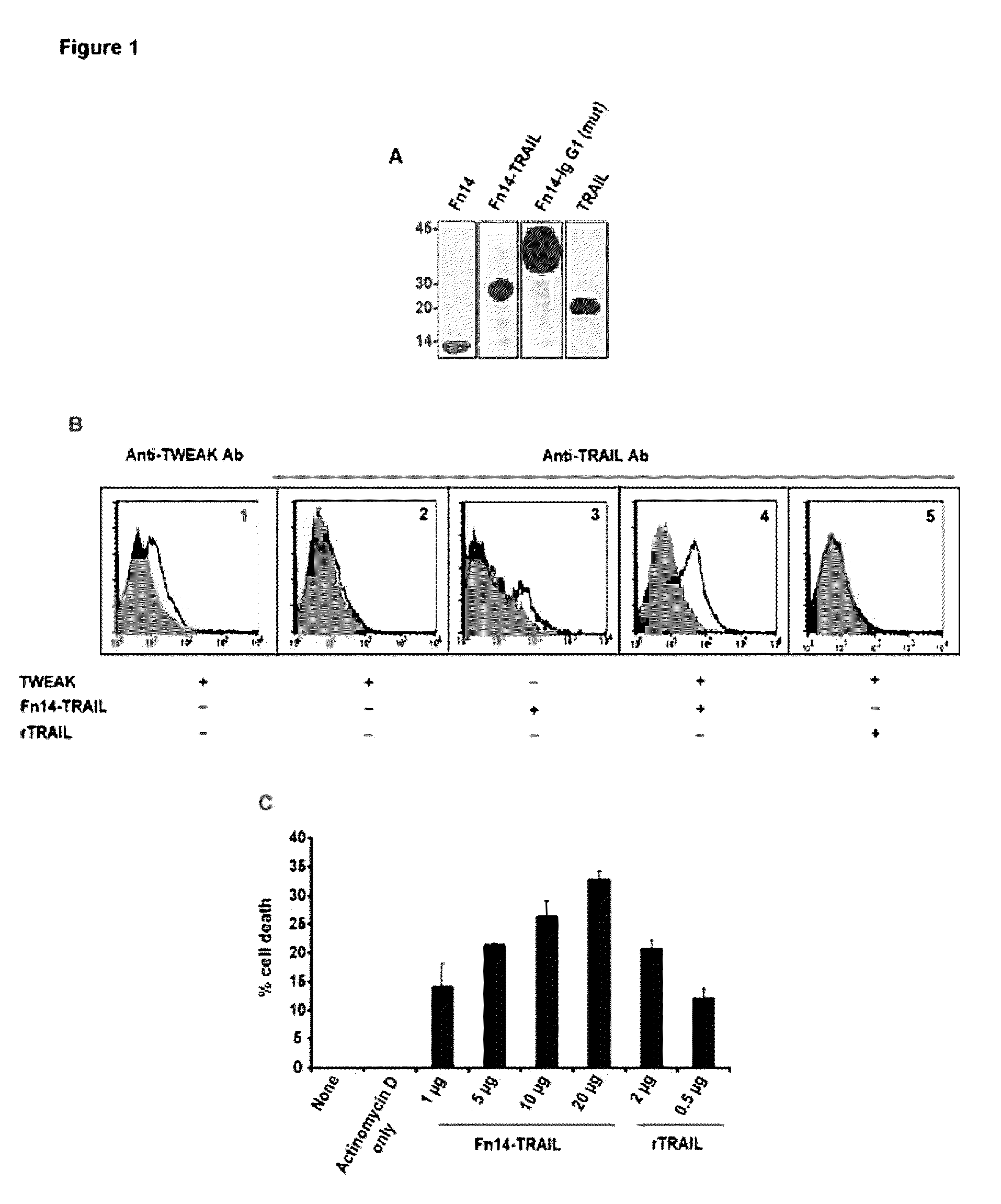
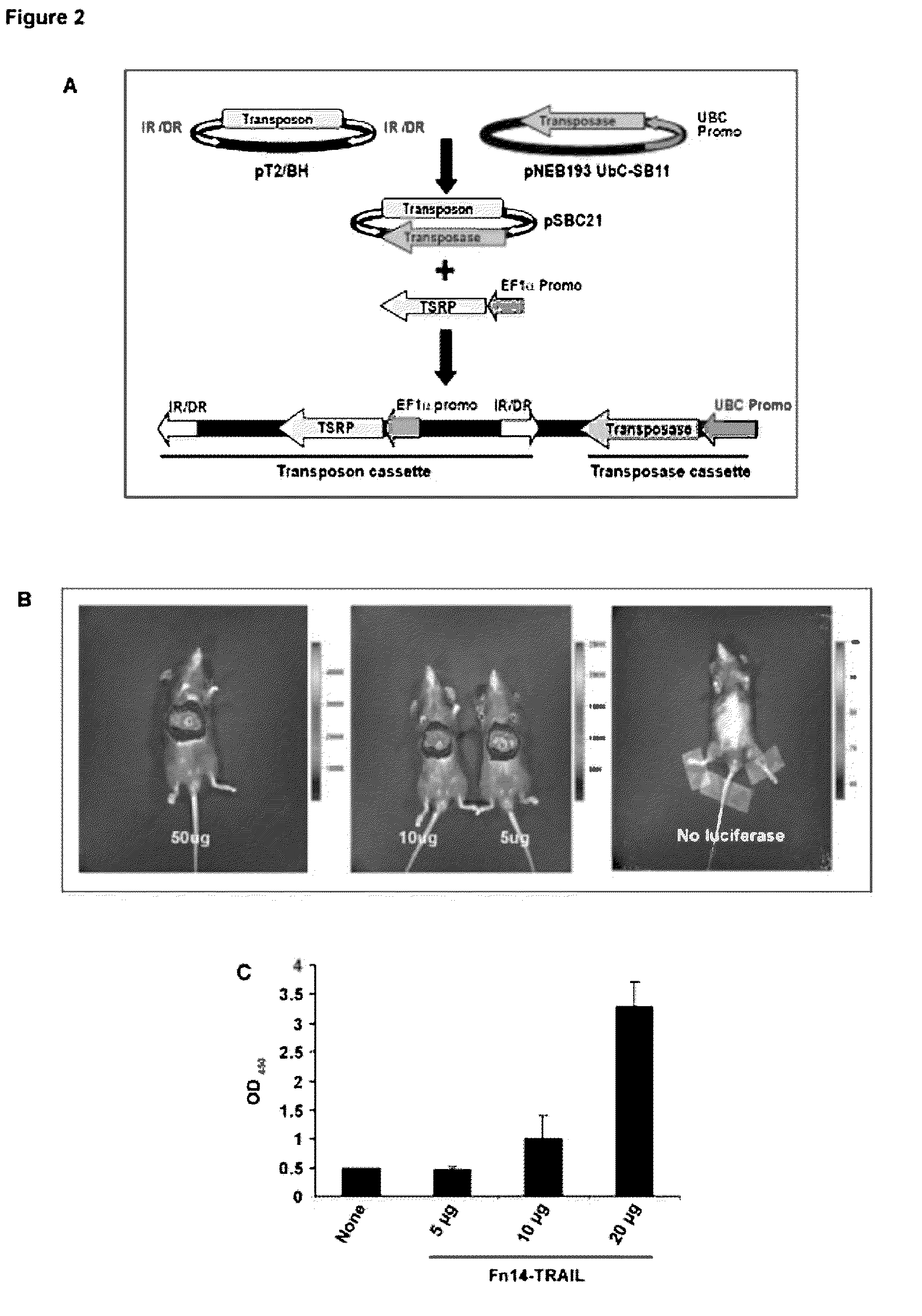
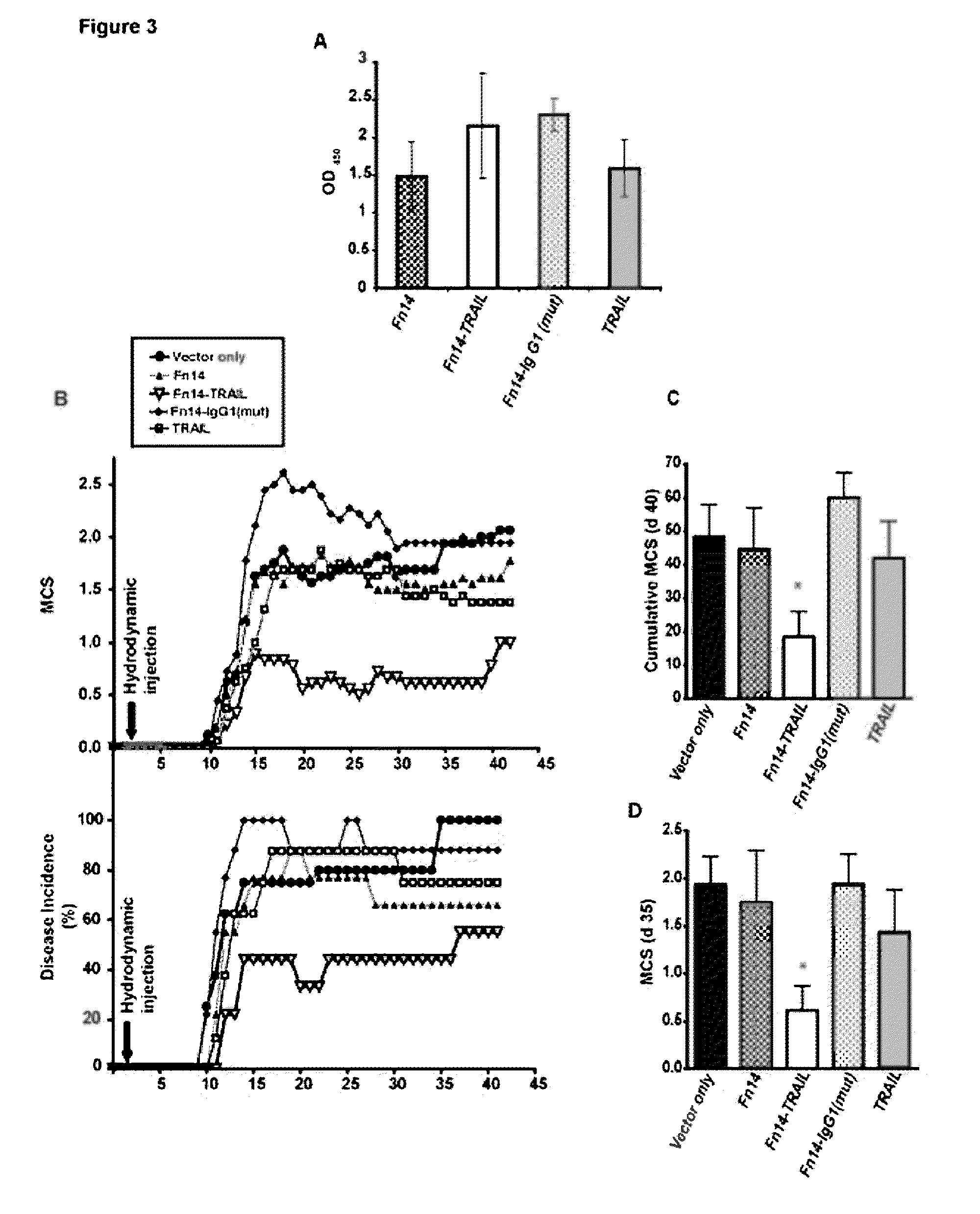
Fn14/TRAIL fusion proteins
Fusion proteins which act on the TWEAK and TRAIL signaling axes are provided. The proteins are useful in the treatment or amelioration of autoimmune diseases, particularly multiple sclerosis, as well as other diseases such as alloimmune diseases and cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Production of proteins in glutamine-free cell culture media
ActiveUS20110091936A1Enhance cell viabilityIncrease culture longevityHydrolasesAntibody mimetics/scaffoldsCell culture mediaMammalian cell
Owner:F HOFFMANN LA ROCHE & CO AG
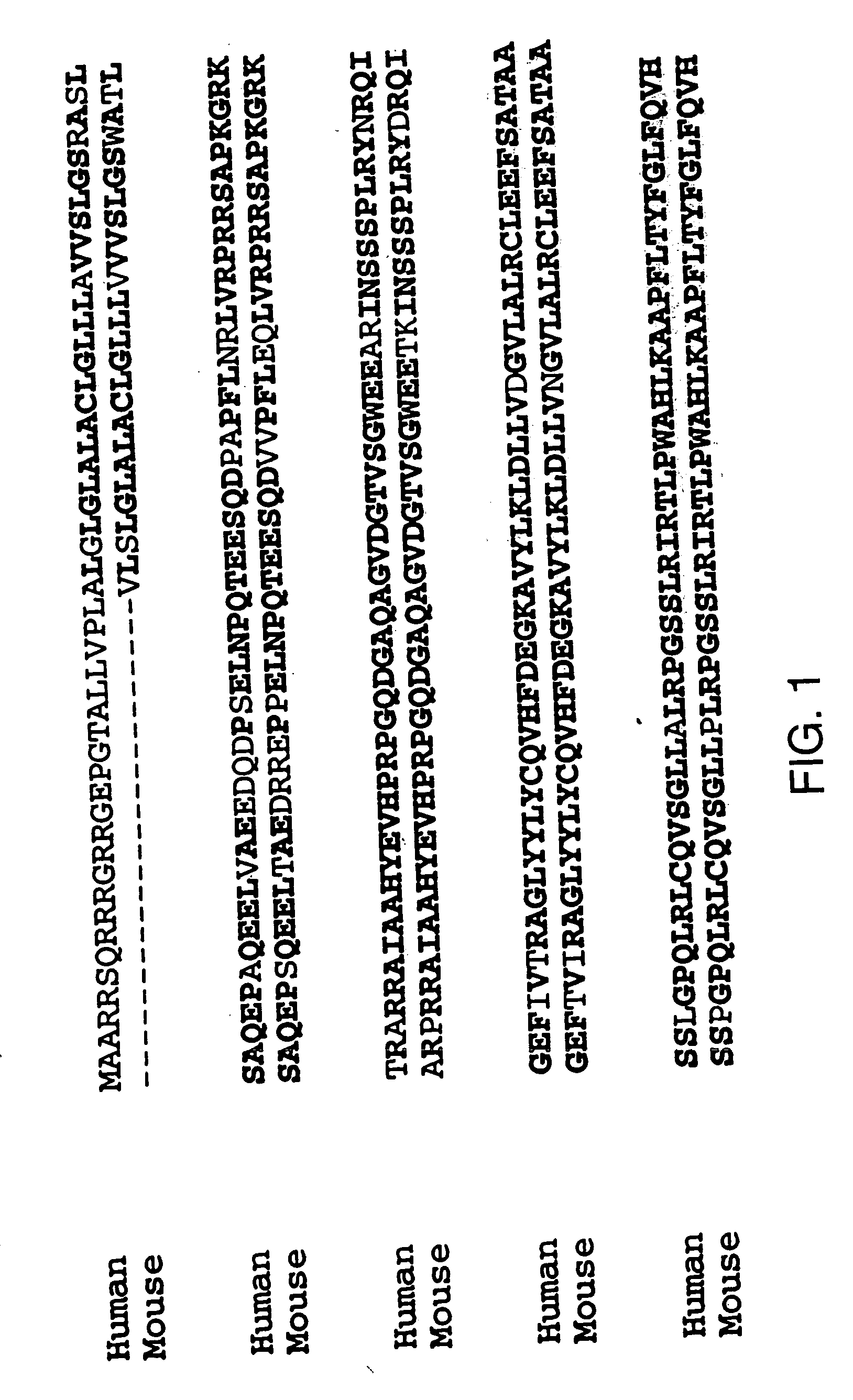
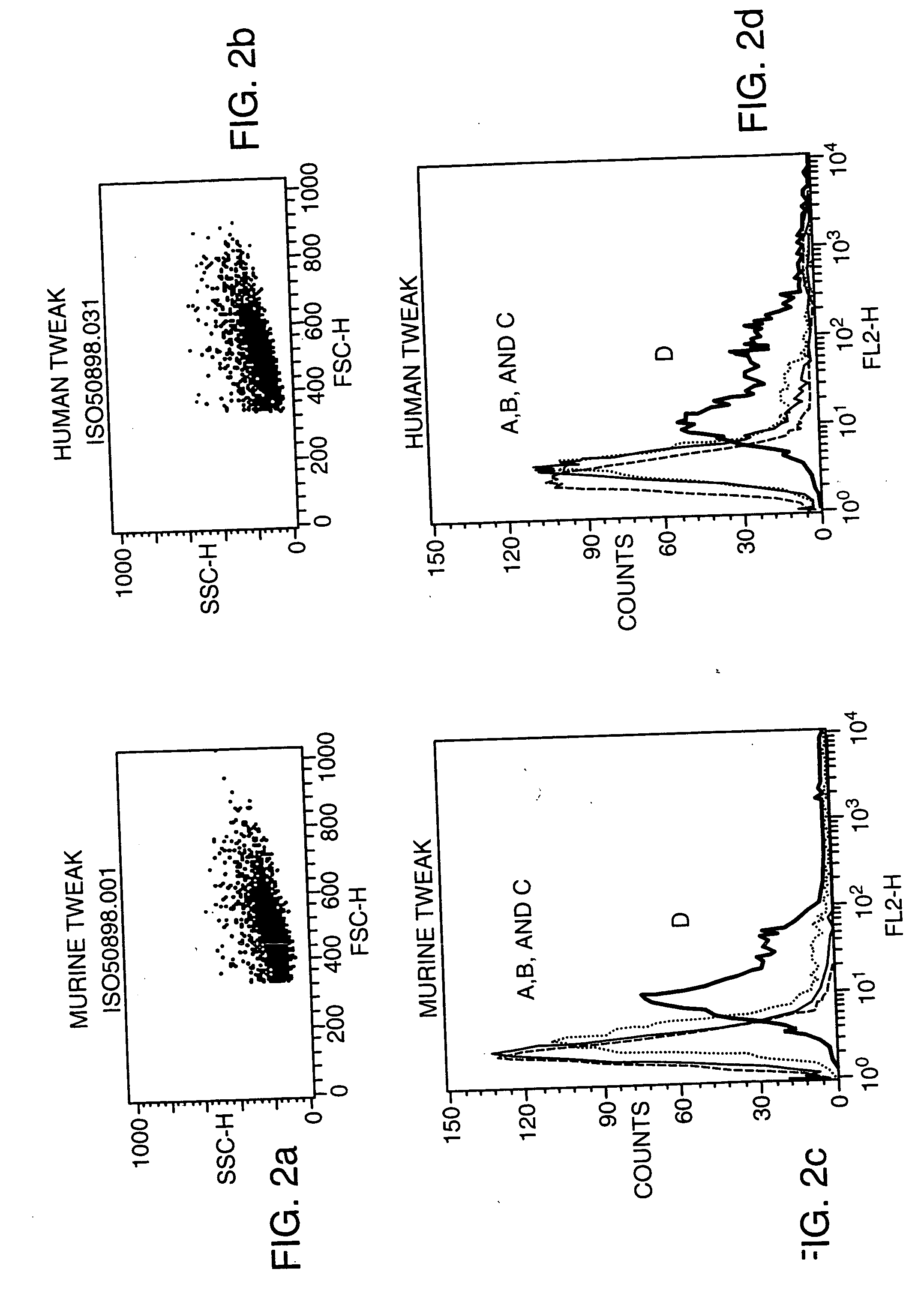
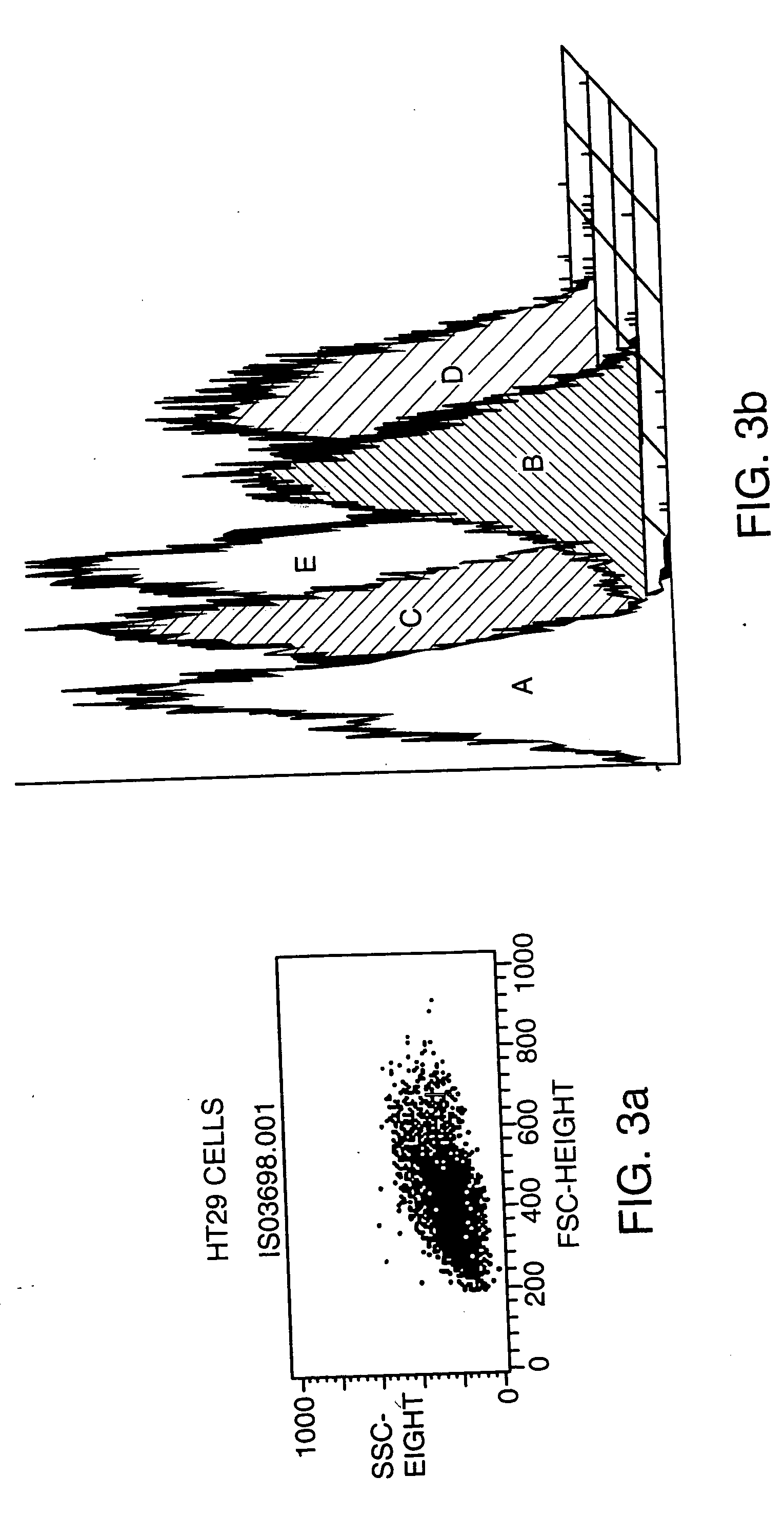
Antagonists of tweak and of tweak receptor and their use to treat immunological disorders
Owner:BIOGEN MA INC
Tumor necrosis factor-gamma
InactiveUS7597886B2Increase blockingInduce inflammatory activityOrganic active ingredientsIn-vivo radioactive preparationsAbnormal tissue growthDisease
Human TNF-gamma-alpha and TNF-gamma-beta polypeptides and DNA (RNA) encoding such polypeptides and a procedure for producing such polypeptides by recombinant techniques are disclosed. Also disclosed are methods for utilizing such polypeptides to inhibit cellular growth, for example in a tumor or cancer, for facilitating wound-healing, to provide resistance against infection, induce inflammatory activities, and stimulating the growth of certain cell types to treat diseases, for example restenosis. Also disclosed are diagnostic methods for detecting a mutation in the TNF-gamma-alpha and TNF-gamma-beta nucleic acid sequences or overexpression of the TNF-gamma-alpha and / or TNF-gamma-beta polypeptides. Antagonists against such polypeptides and their use as a therapeutic to treat cachexia, septic shock, cerebral malaria, inflammation, arthritis and graft-rejection are also disclosed.
Owner:HUMAN GENOME SCI INC
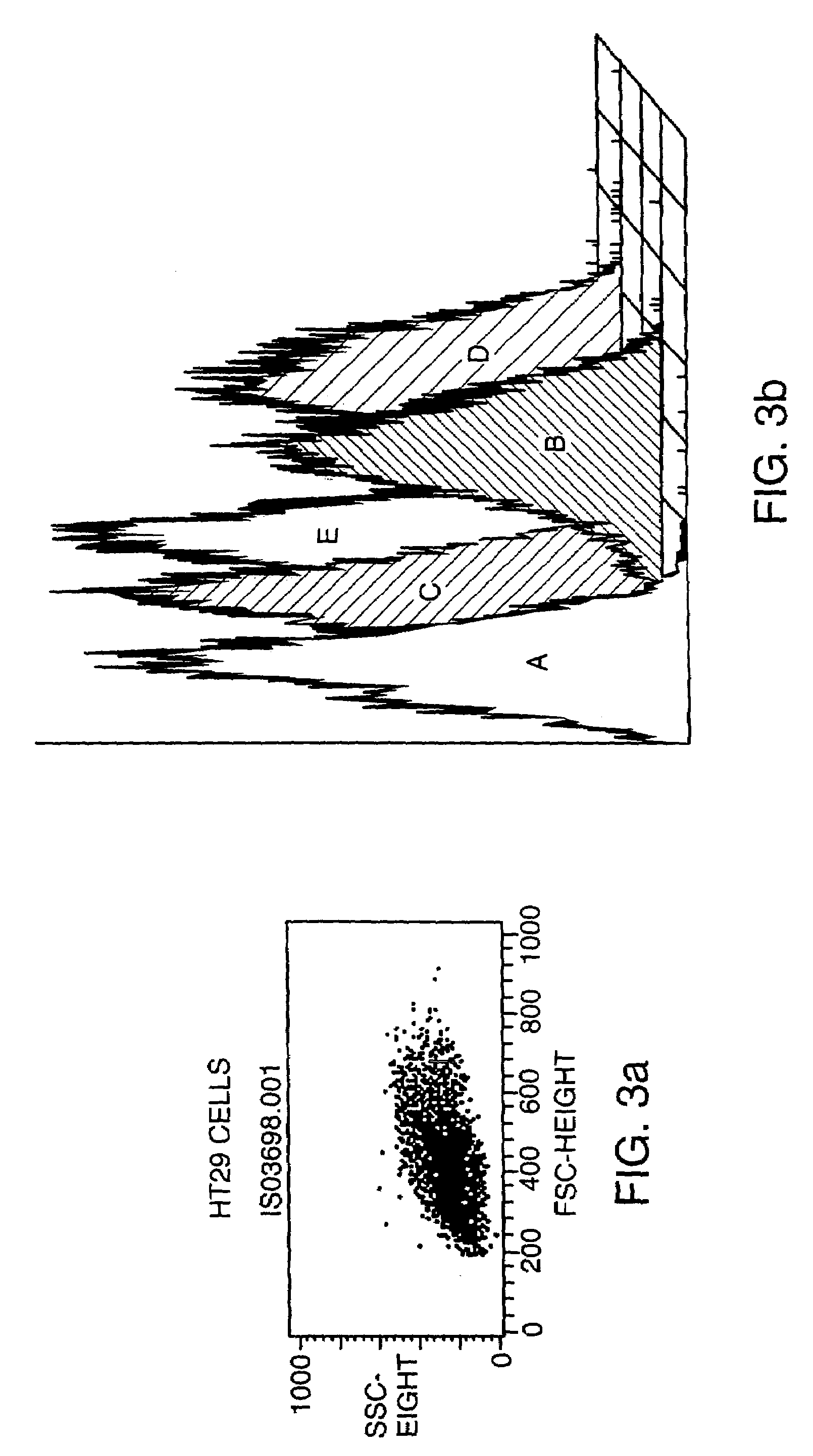
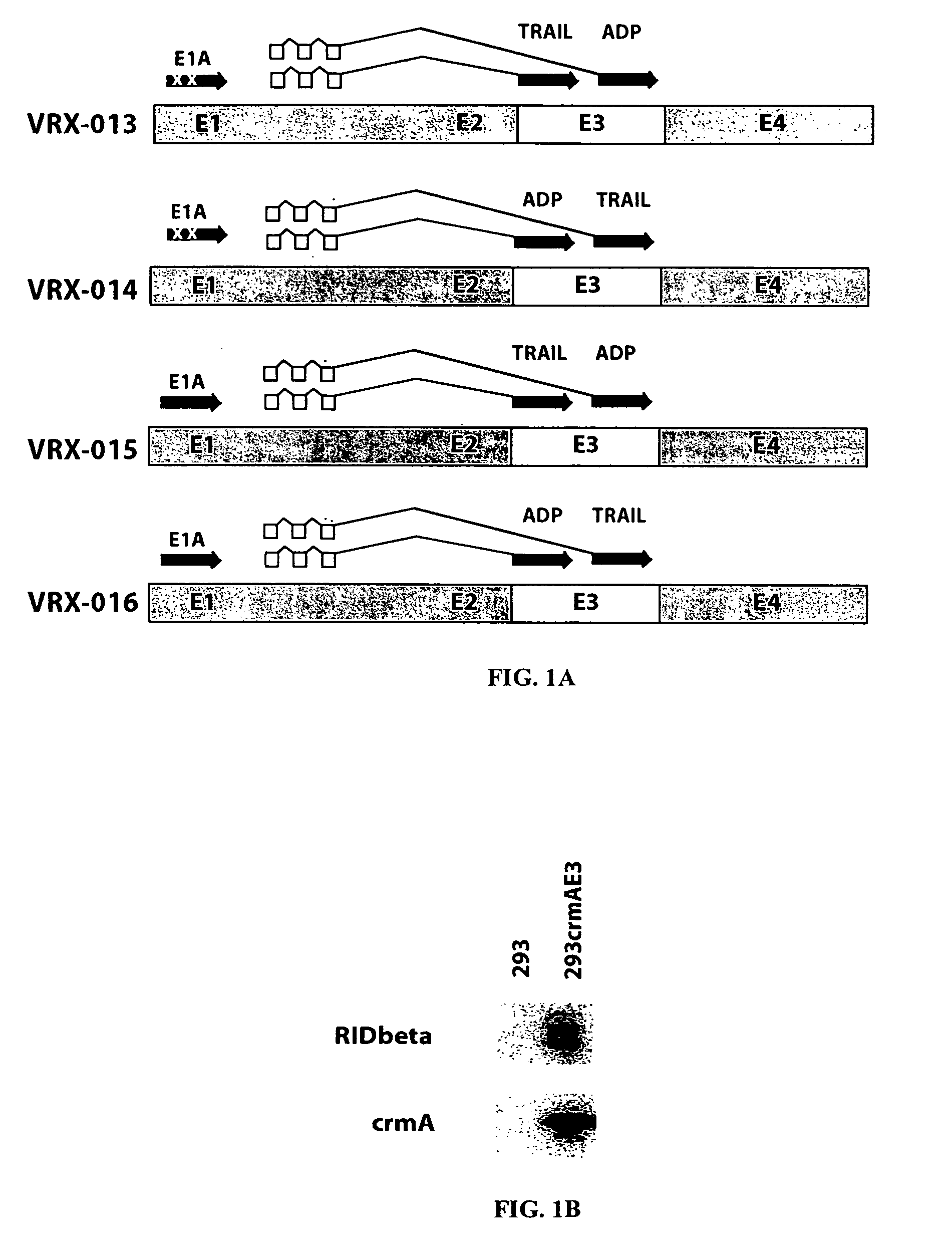
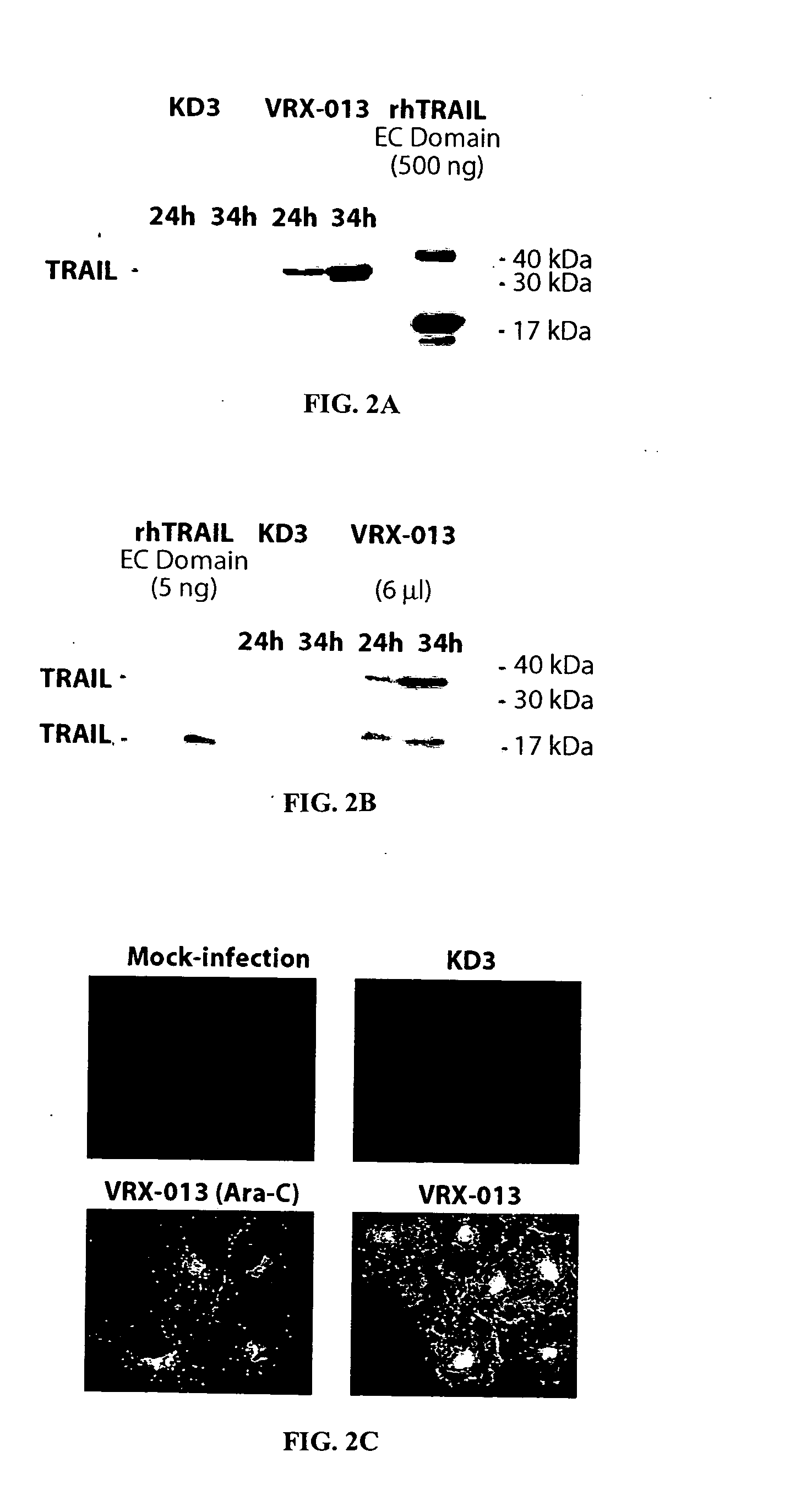
Adenovirus replication-competent vectors expressing trail
InactiveUS20040213764A1Improve the level ofMore replicationBiocidePeptide/protein ingredientsDiseaseCancer research
The present invention provides replication-competent Adenoviral vectors that express TRAIL and ADP. In a particular aspect, TRAIL and / or ADP are highly expressed using the Adenovirus major late promoter or other promoter. Methods of treating hyperproliferative disorders using such vectors, alone or in combination with secondary therapies, also are described.
Owner:SAINT LOUIS UNIVERSITY
Cd33 specific chimeric antigen receptors
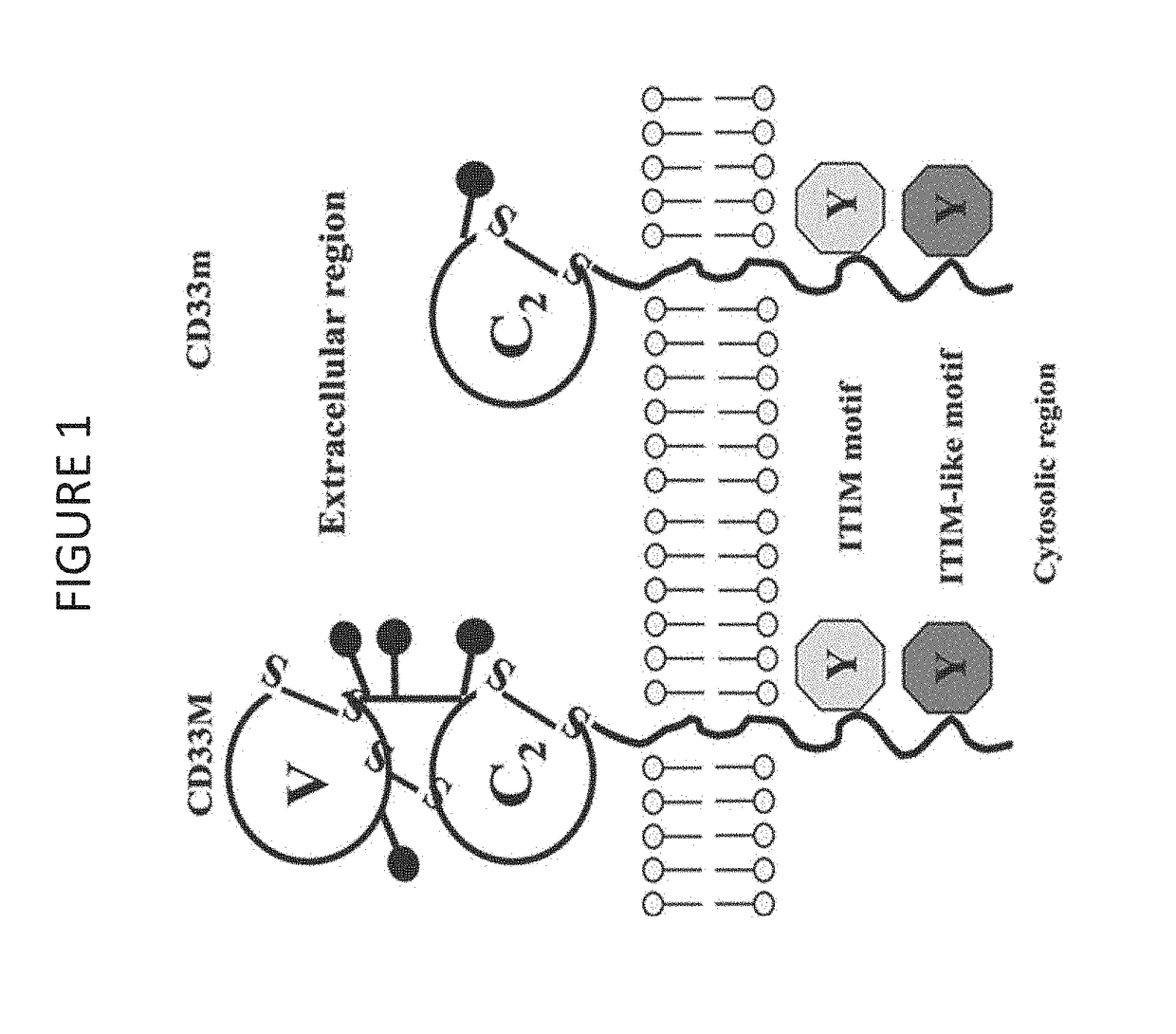
PendingUS20180002397A1Easy SurvivalEfficiently and specifically eliminatePolypeptide with localisation/targeting motifImmunoglobulin superfamilyMyeloid leukemiaCD33
Provided herein are chimeric antigen receptors (CARs) for cancer therapy, and more particularly, CARs containing a scFv from a CD33 monoclonal antibody. Provided are immune effector cells containing such CARs, and methods of treating proliferative disorders such as acute myeloid leukemia (AML), and relapsed or refractory AML.
Owner:PRECIGEN INC
Antagonists of tweak and of tweak receptor and their use to treat immunological disorders
Owner:BIOGEN MA INC
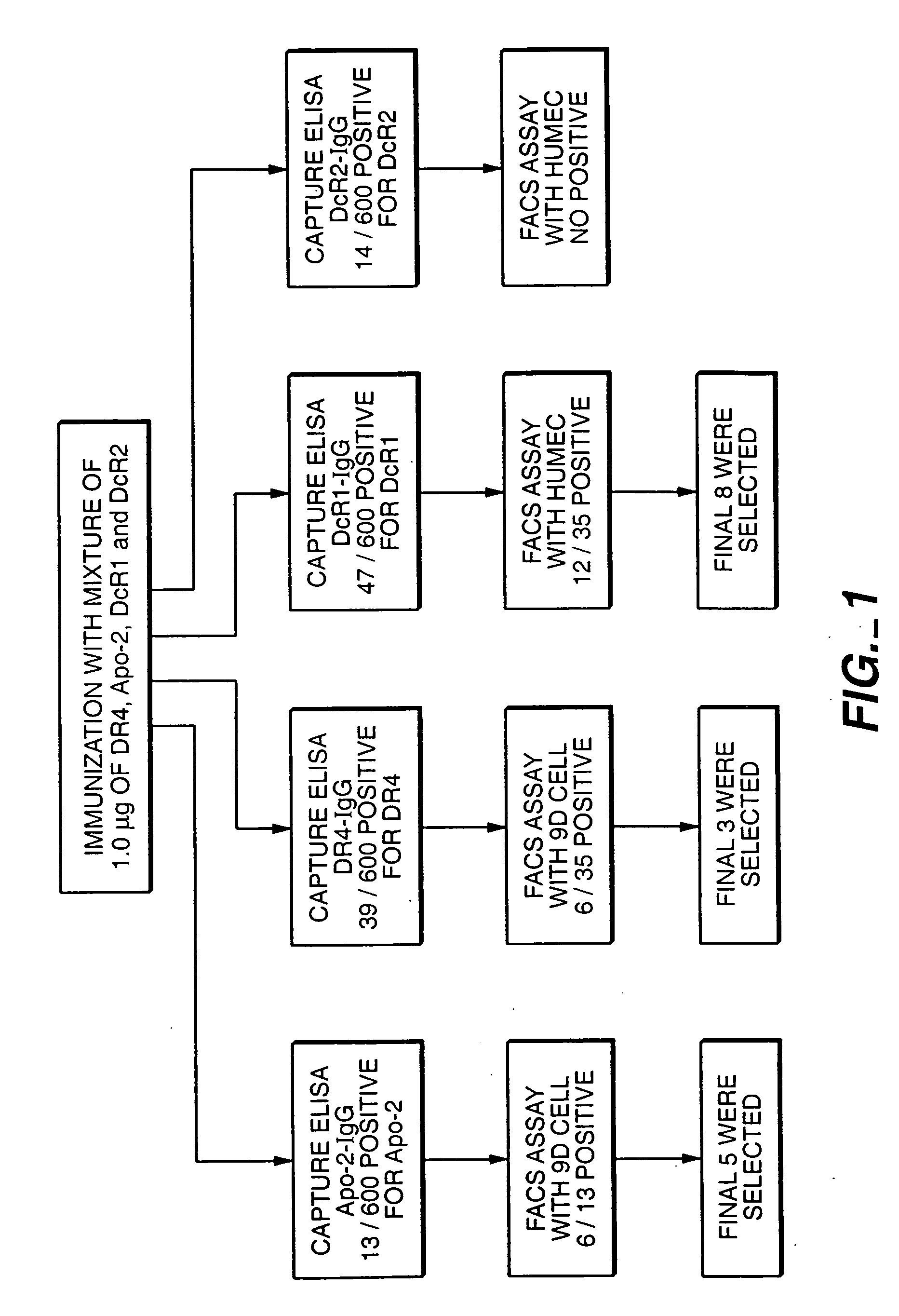
Method for making monoclonal antibodies and cross-reactive antibodies obtainable by the method
InactiveUS20050282230A1Reduce in quantityAntibody mimetics/scaffoldsNGF/TNF-superfamilyCross reactive antibodiesMonoclonal antibody
A method of making monoclonal antibodies according to a mixed antigen immunization protocol is described. In addition, antibodies obtainable by the method are disclosed which specifically cross-react with two or more different receptors to which Apo-2 ligand (Apo-2L) can bind.
Owner:GENENTECH INC
CD40 ligand fusion protein vaccine
ActiveUS20070128223A1Enhance immune responseAntibacterial agentsSsRNA viruses negative-senseAntigenInfectious agent
Provided are methods of generating an immune response to any of various antigens including foreign antigens such as infectious agent antigens. In general, the method comprises administering an expression vector encoding a transcription unit encoding a secretable fusion protein, the fusion protein containing the foreign antigen and CD40 ligand and also administering the encoded fusion protein. In another approach, an immune response to the foreign antigen is elicited using the encoded fusion protein without administering the vector. The invention methods may be used to immunize an individual against an infectious agent such as influenza virus. Methods of obtaining an immune response in older individuals also is described.
Owner:MICROVAX LLC
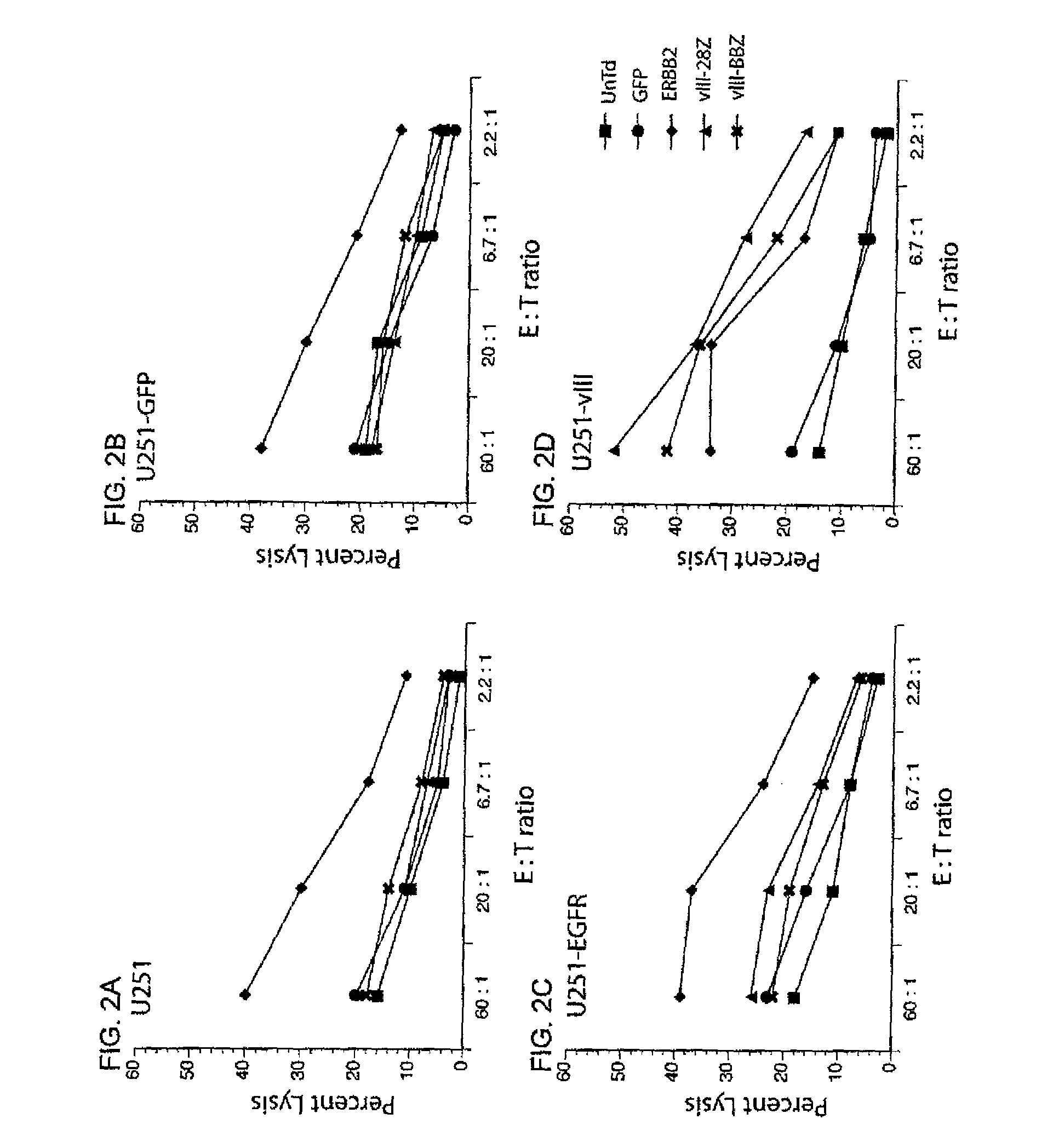
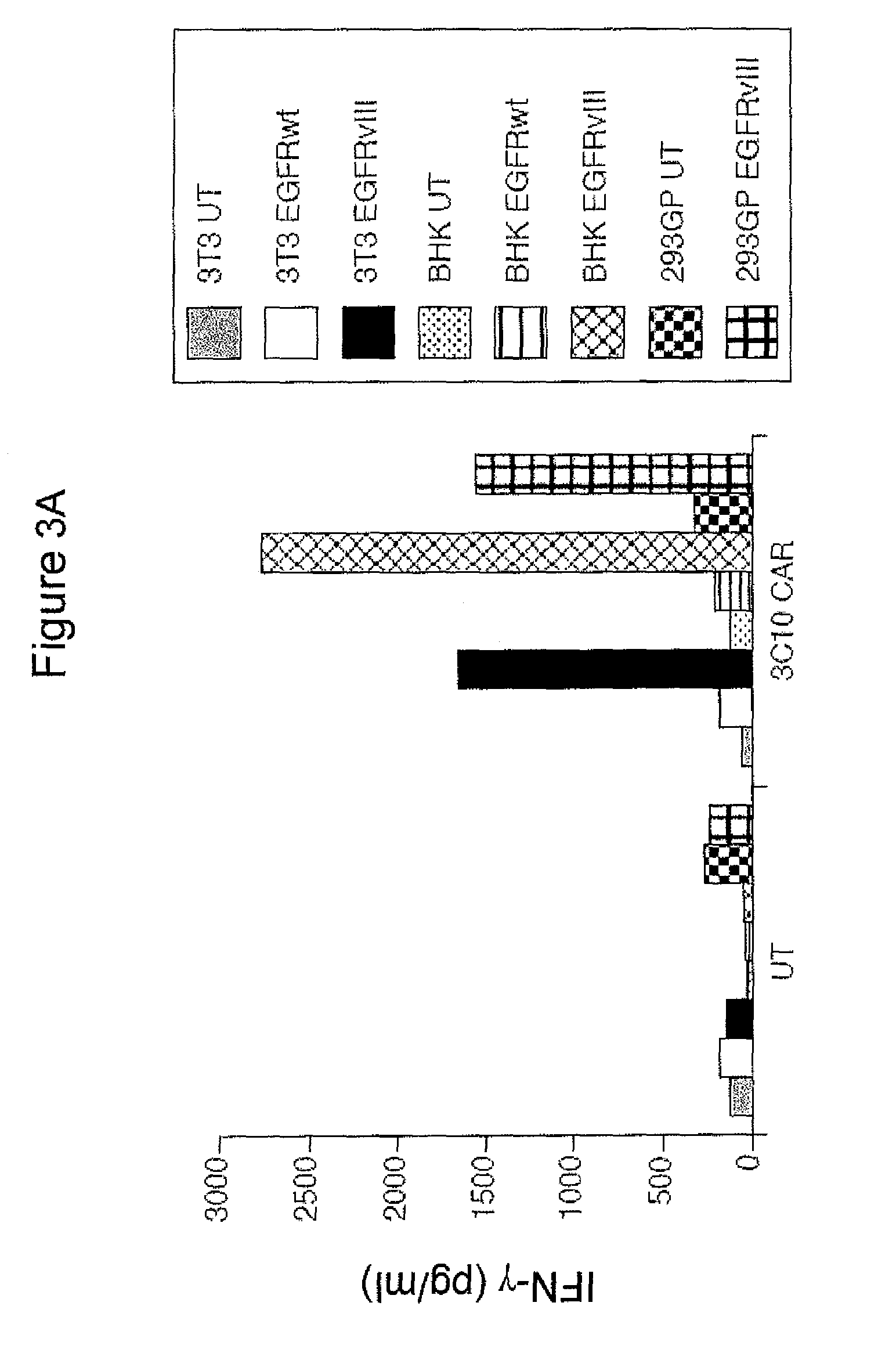
Anti-epidermal growth factor receptor variant III chimeric antigen receptors and use of same for the treatment of cancer
ActiveUS9266960B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsAntigen binding
The disclosure provides chimeric antigen receptors (CARs) comprising an antigen binding domain of human antibody 139, an extracellular hinge domain, a transmembrane domain, and an intracellular domain T cell receptor signaling domain. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a host and methods of treating or preventing cancer in a host are also disclosed.
Owner:UNITED STATES OF AMERICA
DNA cassette for the production of secretable recombinant trimeric TRAIL proteins, tetracycline/ doxycycline-inducible adeno-associated virus vector, their combination and use in gene therapy
InactiveUS20020128438A1Enhanced homotrimer-forming functionFunction increasePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseTwo-vector
The present invention relates to the construction of a TRAIL DNA cassette for the production of a secretable trimeric rTRAIL, the development of pCMVdw vectors and pAAVdw vectors harboring a feed-forward amplification loop type Tet-On system that can be packaged into AAV particles, the preparation of a recombinant vectors by the combination of the TRAIL DNA cassette and the two vectors, and the treatment of diseases including cancer using such vectors. The present invention provides a TRAIL DNA cassette comprising a secretion signal (SS) sequence, a trimer-forming domain (TFD) and a TRAIL(114-281) coding cDNA. The TRAIL cassette thus constructed can be cloned into an appropriate expression vector, and subsequently used in the mass production of a secretable recombinant trimeric TRAIL protein or administered to a patient for a gene therapy.
Owner:SEOL DAI WU
Auto-stimulating cells and methods for making and using the same
Methods for transferring one or more proteins to a cell are disclosed. The protein or proteins to be transferred are in the form of a fusion protein, and contain at least one domain encoding for a protein or peptide having trans signaling and / or adhesion function. The fusion protein is transferred to a cell by binding to a lipidated protein, which has been incorporated into the cell membrane. In an additional aspect of the invention, methods of making fusion proteins having cis signaling capabilities, as well as the ability to bind with receptors on the cell's own surface, are provided. Fusion proteins incorporating GPI or a homing element, and a costimulator or inhibitor domain can also be directly transferred to the cell surface. Methods for using cells which have undergone protein transfer according to the present methods are also disclosed. This includes use in a cancer vaccine, use for treatment of cancer or autoimmune disease, and use in determining costimulator threshold levels.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com