Method for making monoclonal antibodies and cross-reactive antibodies obtainable by the method
a monoclonal antibody and antibody technology, applied in the field of making monoclonal antibodies, can solve the problems of limited human use of therapeutic agents, and achieve the effect of reducing the number of animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Immunogens
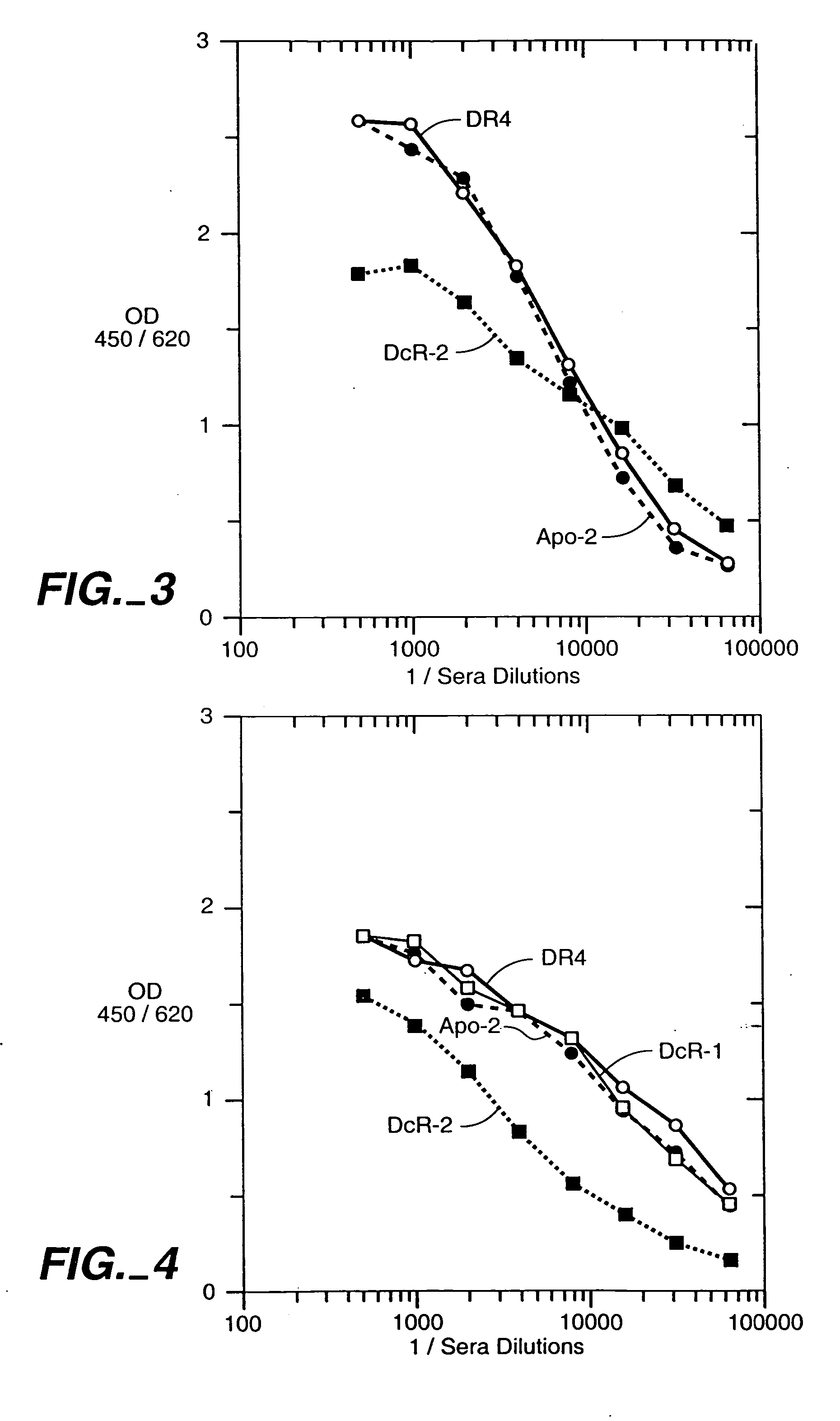
[0172] The receptor antigens in Examples 2 and 3 below were all receptors for Apo-2 ligand [Pitti et al., J. Biol. Chem., 271:12687-12690 (1996); and WO97 / 25428]. The Apo-2L receptors were: DR4 [Pan et al., Science, 276:111-113 (1997)]; Apo-2 [called “DR5” in Sheridan et al., Science 277:818-821 (1997)]; DcR1 [Sheridan et al., Science 277:818-821 (1997)]; and DcR2 [Marsters et al., Curr. Biol., 7:1003-1006 (1997)].
[0173] Receptor immunoadhesins (designated “DR4-IgG”, “Apo-2-IgG”, “DcR1-IgG” and “DcR2-IgG”) were prepared by fusing the extracellular domain sequence of each receptor to the hinge and Ec region of human immunoglobulin G1 heavy chain in pRK5 as described previously [Ashkenazi et al., Proc. Natl. Acad. Sci., 88:10535-10539 (1991)]. The immunoadhesin proteins were expressed by transient transfection into human 293 cells and purified from cell supernatants by protein A affinity chromatography, as described by Ashkenazi et al., supra. Purified immun...
example 2
Mixed Antigen Immunization
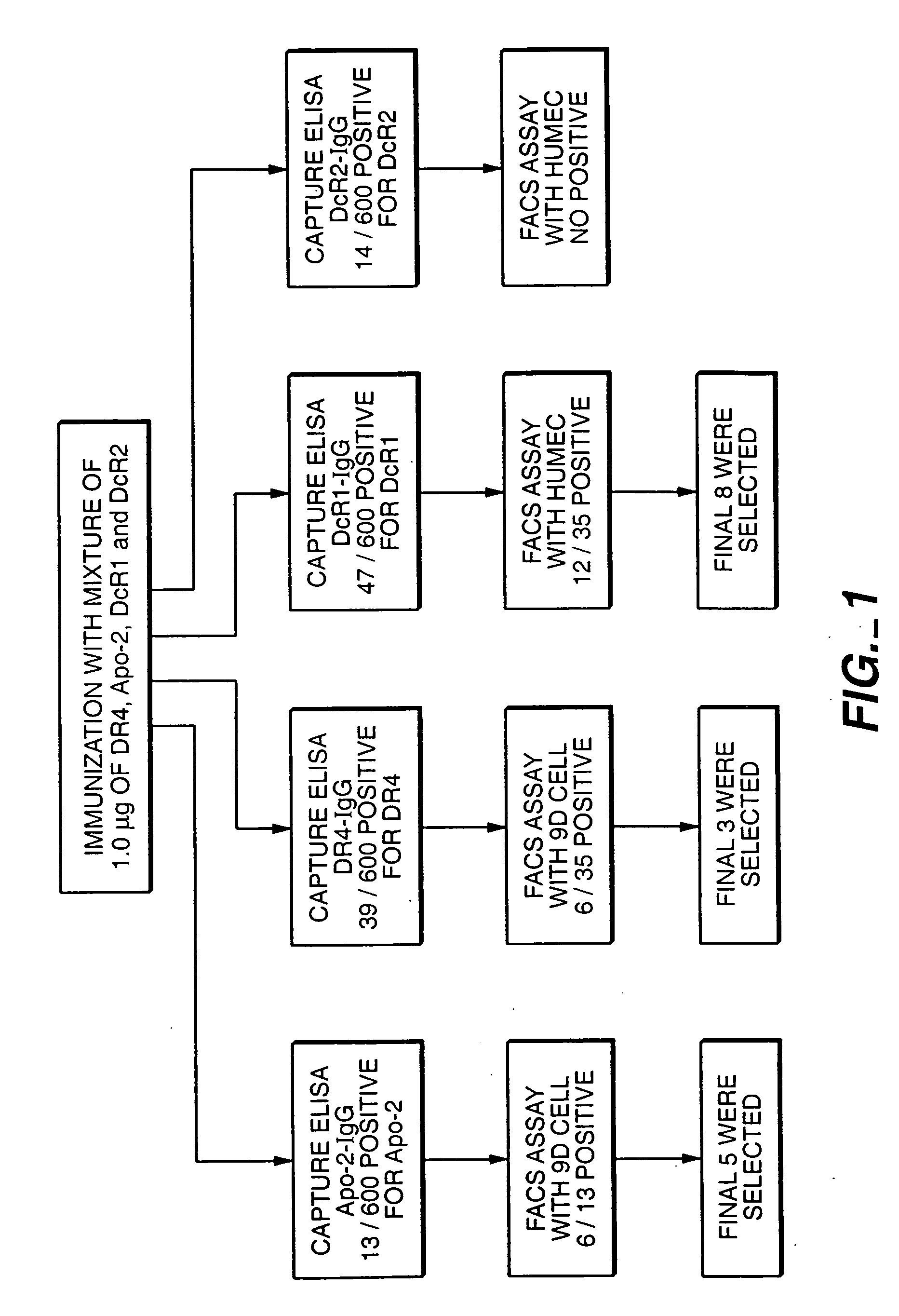
[0174] Animals in this example were immunized with the four receptor immunoadhesins of the preceding example. The mixed antigen immunization scheme used is shown in FIG. 1.
[0175] Balb / c mice (obtained from Charles River Laboratories) were immunized into each hind foot pad 14 times at 3-4 day intervals, with a mixture of DR4-IgG, Apo-2-IgG, DcR1-IgG and DcR2-IgG (1 μg each) suspended in monophosphoryl lipid A plus trehalose dicorynomycolate adjuvant (MPL-TDM; Ribi Immunochem. Research Inc., Hamilton, Mont.) at a 1:1 ratio of immunoadhesin:adjuvant (DcR2-IgG was only included in the mixture used for the final six immunizations).
[0176] Three days after the final boost, popliteal lymph node cells nodes were removed from the mice and a single cell suspension was prepared in DMEM media (obtained from Biowhitakker Corp.) supplemented with 1% penicillin-streptomycin. The lymph node cells were fused with murine myeloma cells P3X63AgU.1 (ATCC CRL 1597) using 35% p...
example 3
Single Antigen Immunization
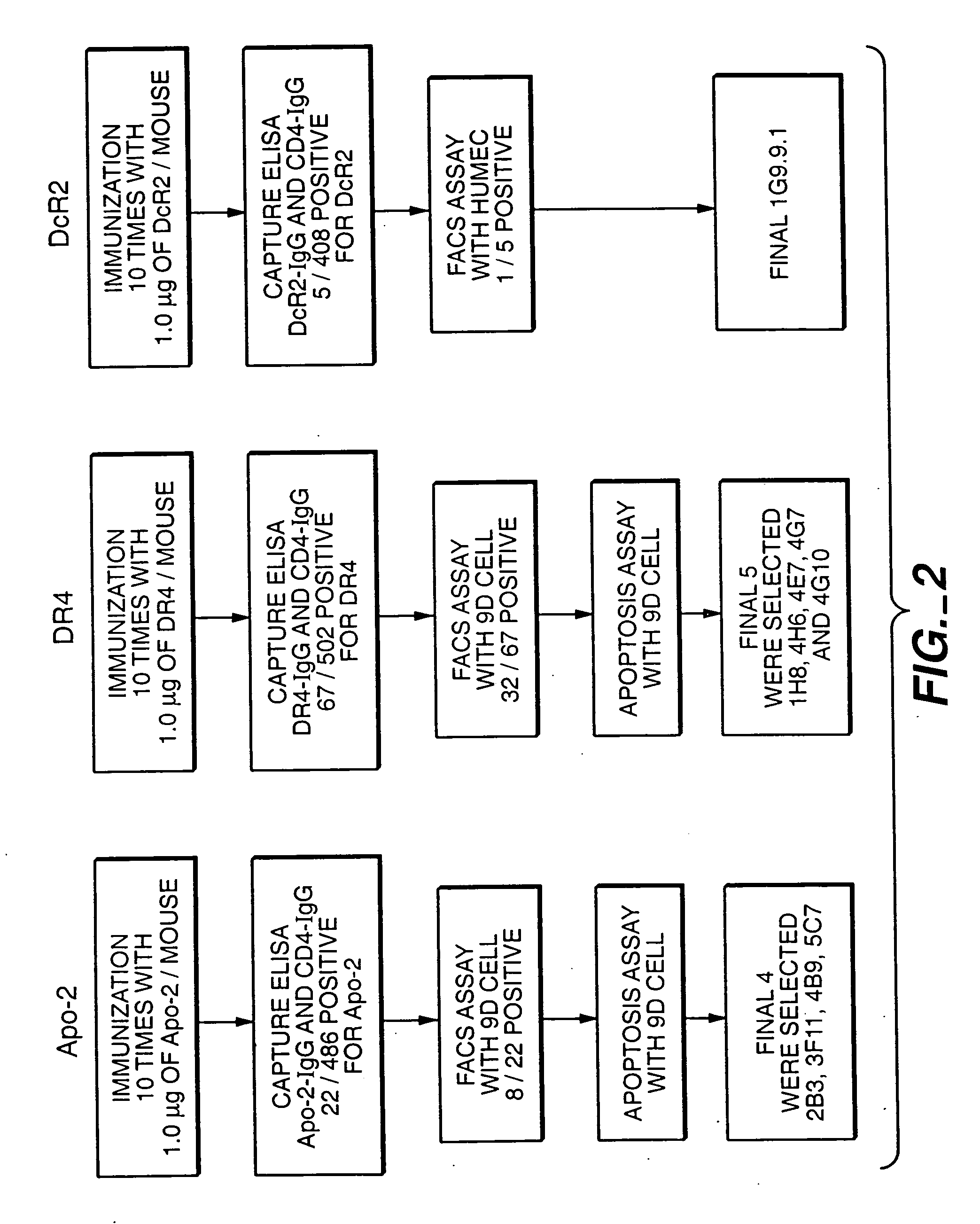
[0180] The single antigen immunization scheme is shown in FIG. 2. The general method was almost the same as the mixed antigen immunization protocol in Example 2 above, except that only a single antigen was used as the immunogen and during the screening of hybridomas supernatant (180 μl) was collected only once to screen for the presence of positive monoclonal antibodies to the particular antigen and control CD4-IgG.
[0181] Balb / c mice (obtained from Charles River Laboratories) were immunized by injecting 0.5 μg / 50 μl of immunoadhesin protein (diluted in MPL-TDM adjuvant purchased from Ribi Immunochemical Research Inc., Hamilton, Mont.) 10 times into each hind foot pad at 3-4 day intervals. Three days after the final boost, popliteal lymph nodes were removed from the mice and a single cell suspension was prepared in DMEM media (obtained from Biowhitakker Corp.) supplemented with 1% penicillin-streptomycin. The lymph node cells were then fused with murine m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com