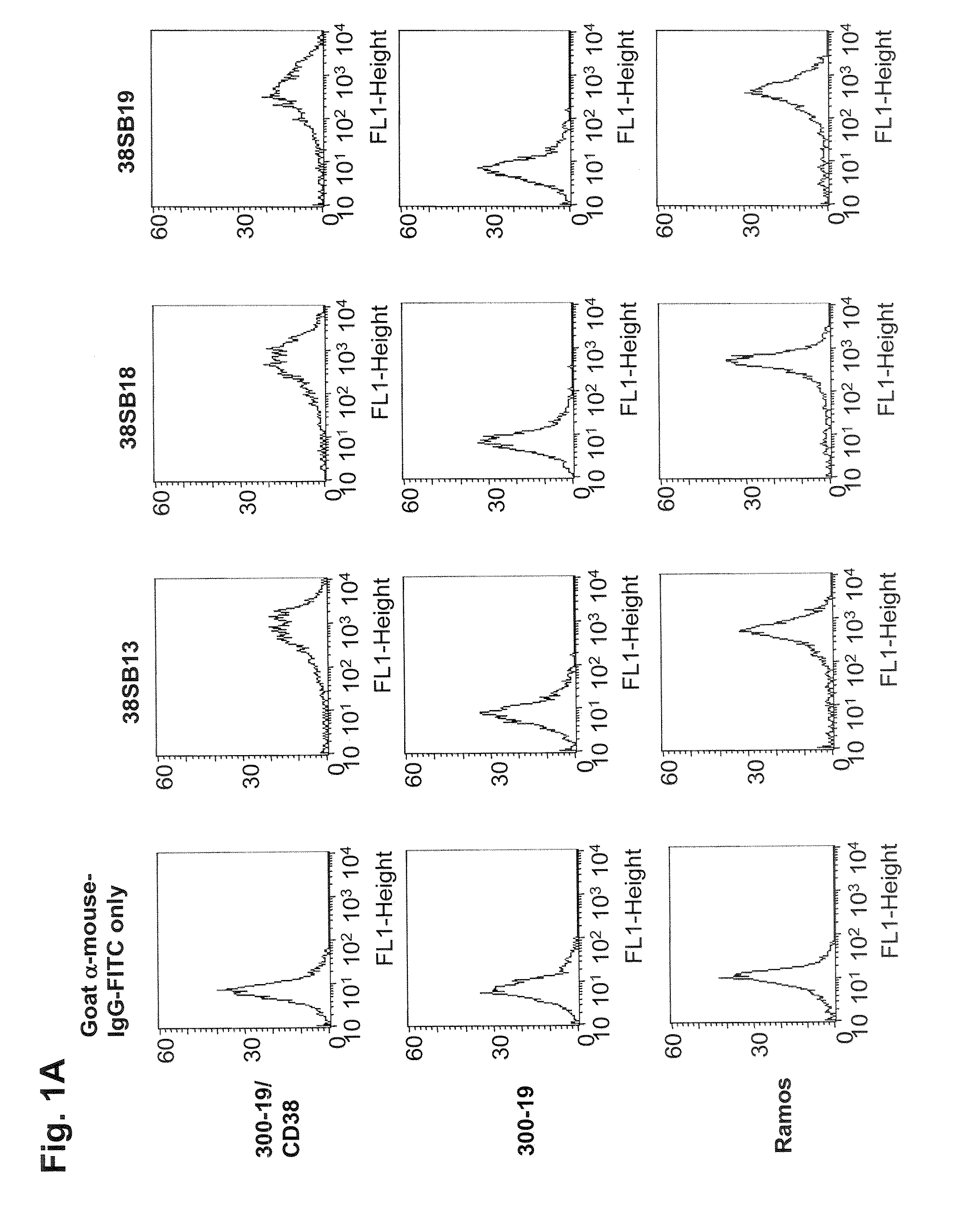
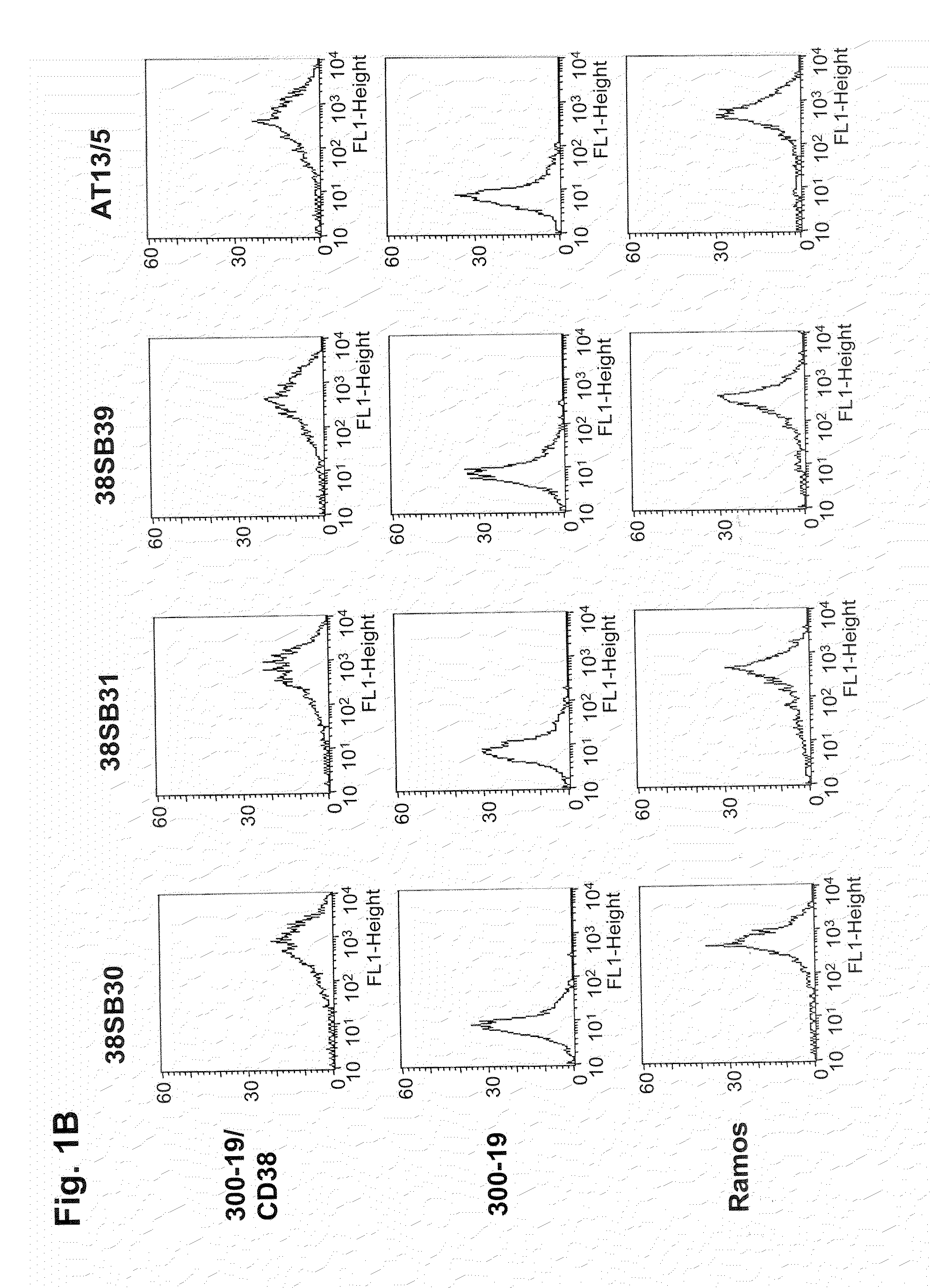
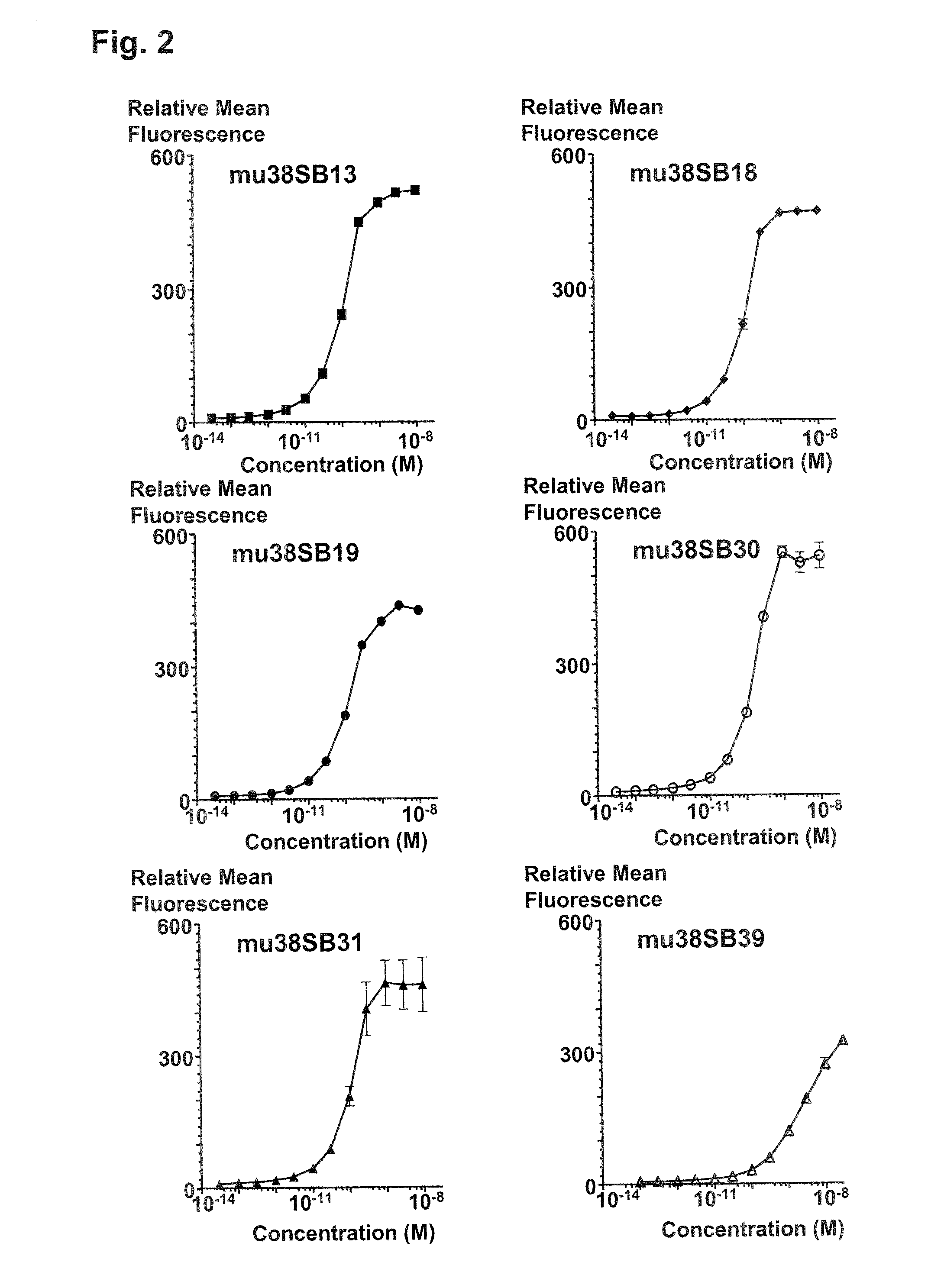
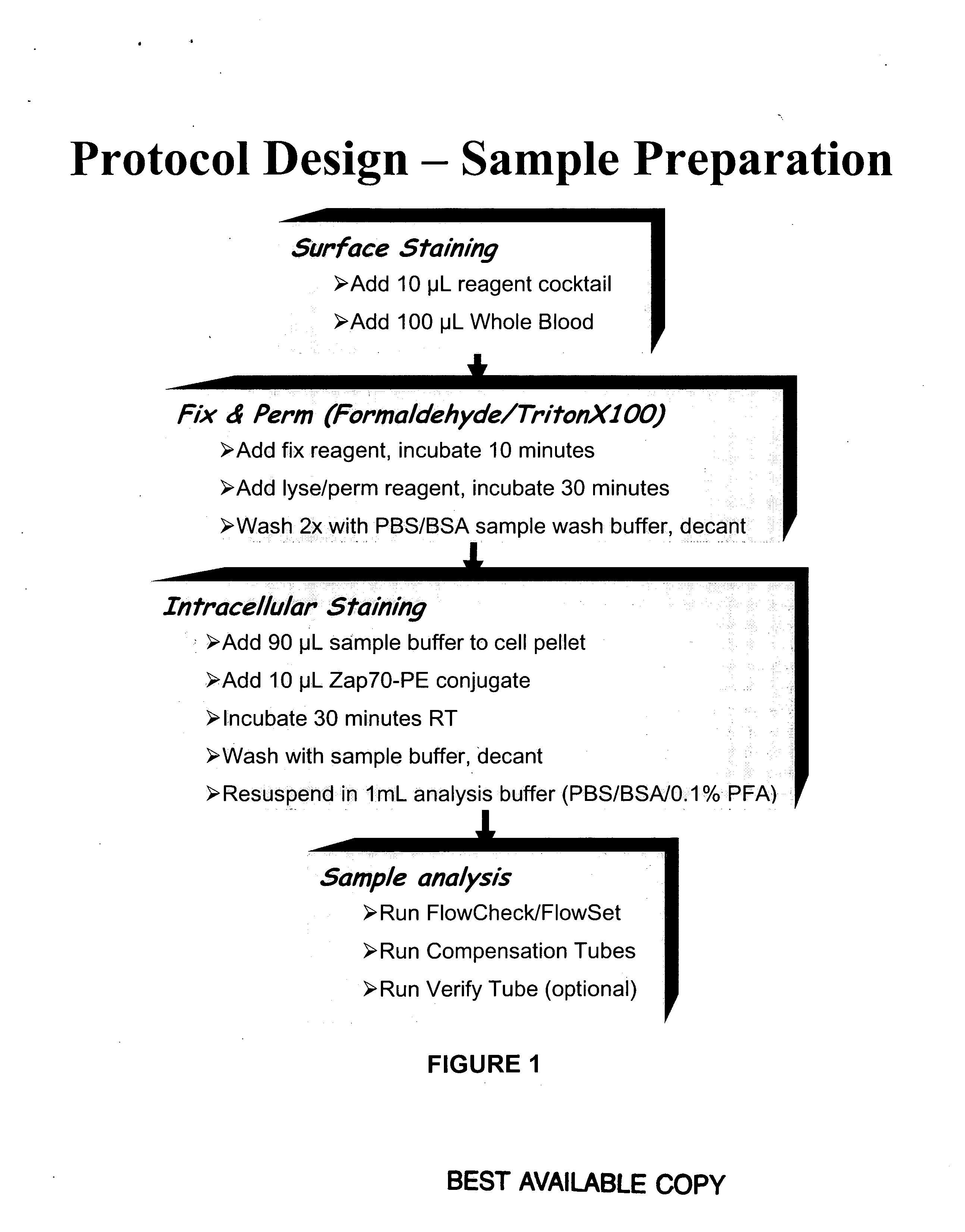
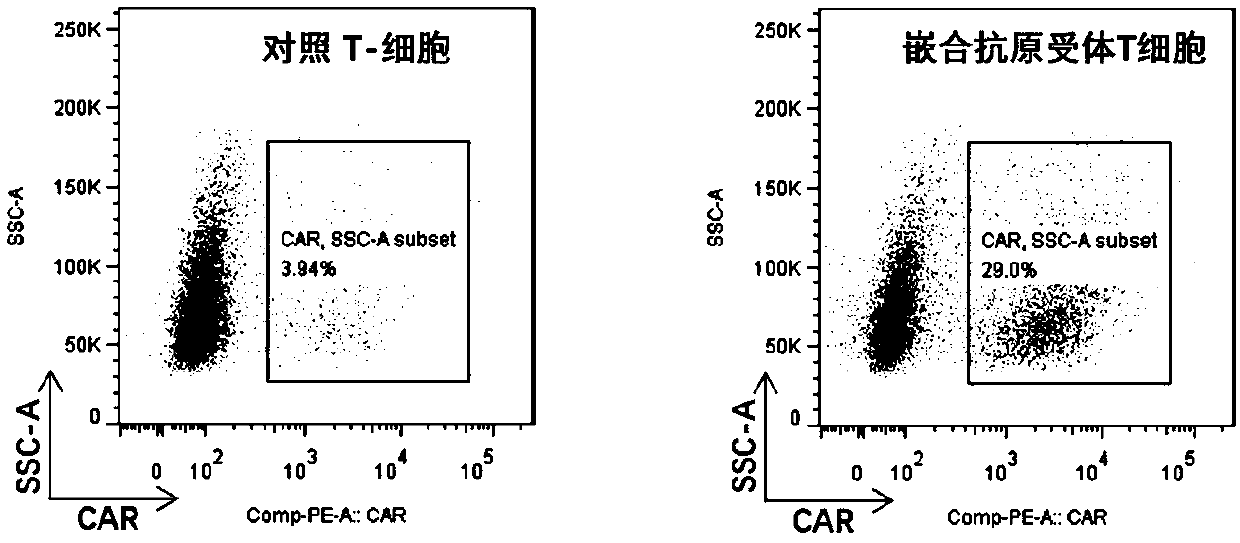
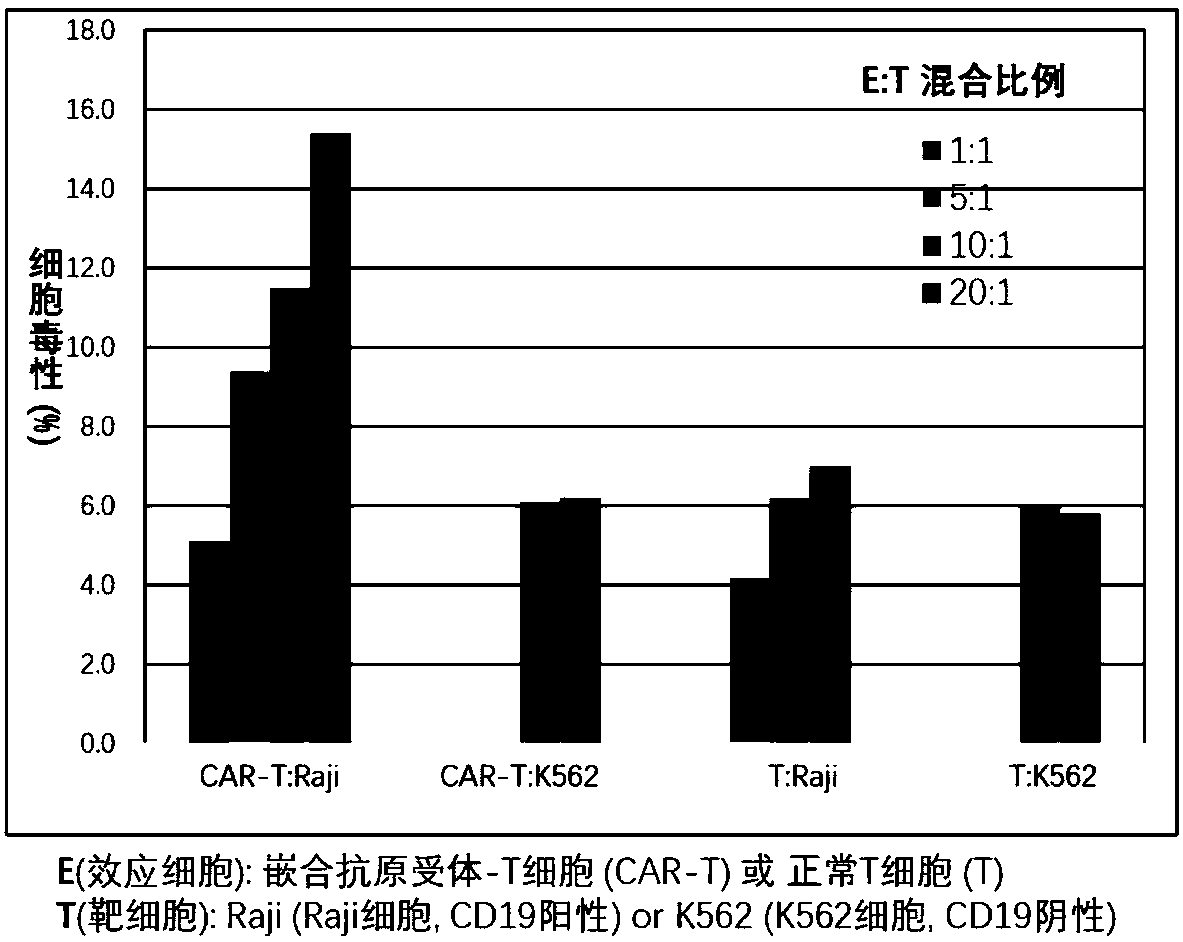
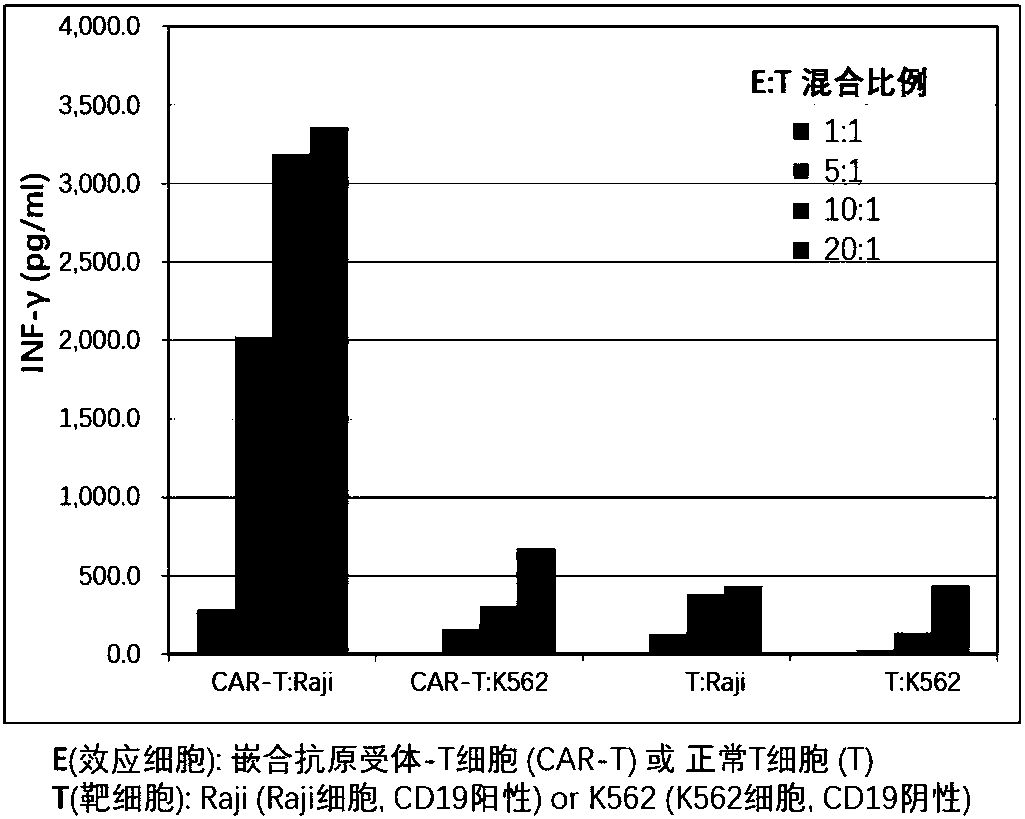
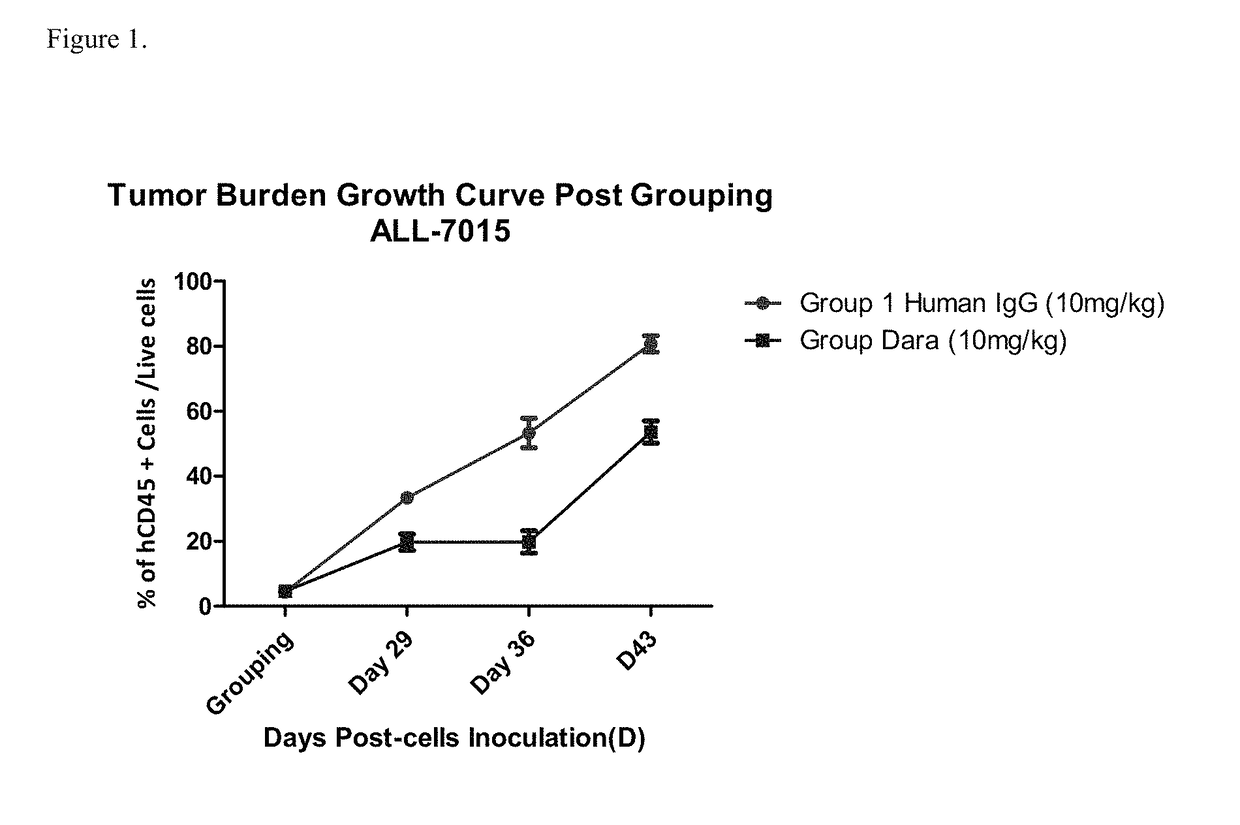
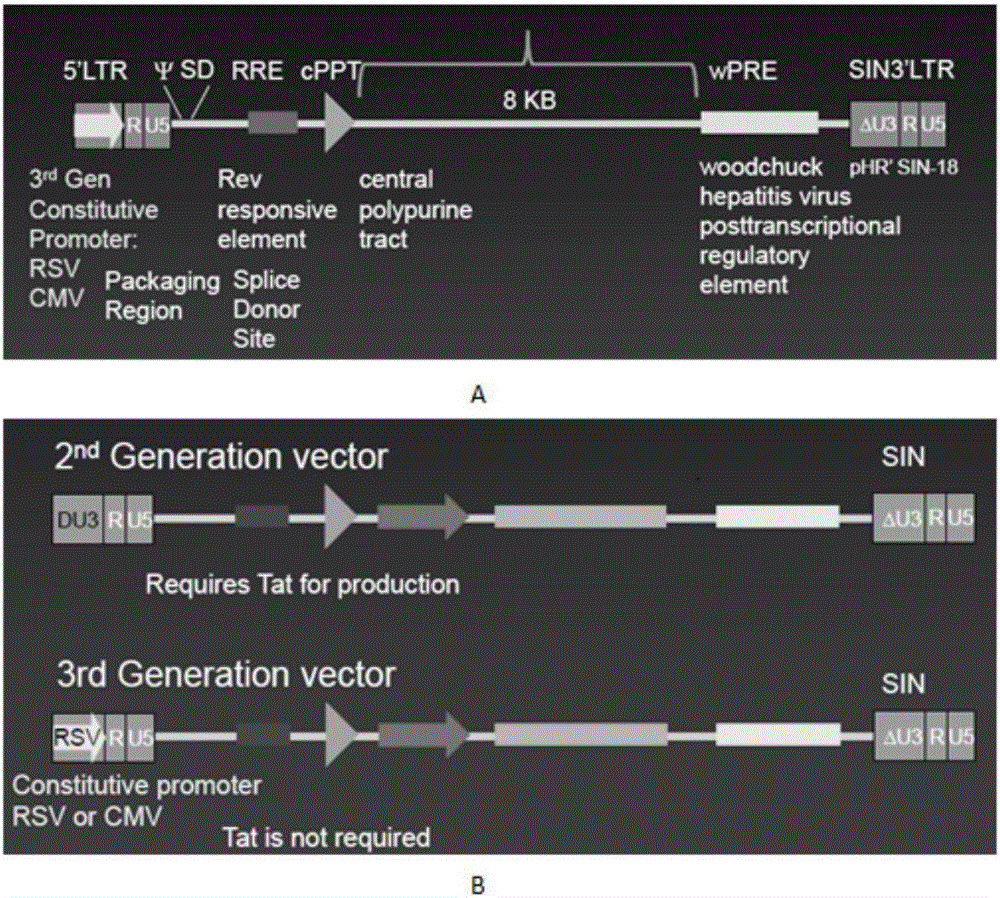
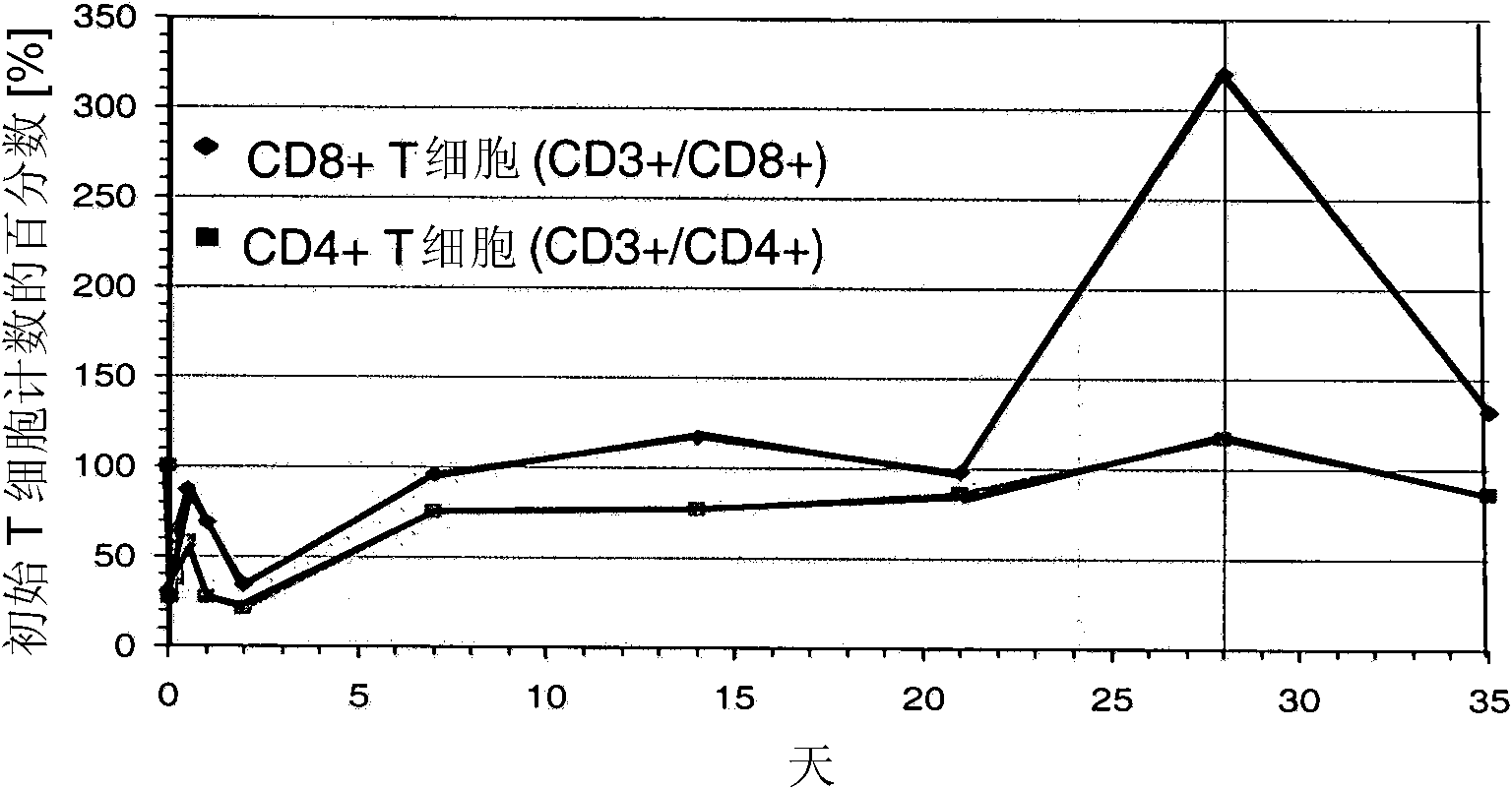
Patents
Literature
165 results about "Acute lymphocytic leukemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A type of blood and bone marrow cancer which mainly affects the white blood cells.
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Generation and application of universal T cells for B-ALL
InactiveUS20070036773A1Hinder recognitionEnhanced siRNA effectBiocideGenetic material ingredientsAntigenNatural Killer Cell Inhibitory Receptors
The present invention is directed to universal T cells and their use in treating diseases and other physiological conditions. More specifically, the present invention is directed to universal T cells and their use in treating treating B-lineage acute lymphoblastic leukemia (B-ALL) in particular and malignancy in general. The universal T cells contain (i) nucleic acid encoding a chimeric antigen receptor (CAR) to redirect their antigen specificity and effector function and (ii) nucleic acids encoding shRNA and / or siRNA molecules to down-regulate cell-surface expression of T cell classical HLA class I and / or II genes to avoid recognition by recipient T cells. The universal T cells may also contain a nucleic acid encoding a non-classical HLA gene, such as an HLA E gene to enforce expression of HLA E genes and / or an HLA G gene to enforce expression of HLA G genes, to avoid recognition by recipient NK cells. The universal T cells may further contain a nucleic acid encoding a selection-suicide gene.
Owner:CITY OF HOPE
Methods and compositions for the treatment of myeloproliferative diseases and other proliferative diseases
Compounds of the present invention, alone and in combination with other active agents, find utility in the treatment of hyperproliferative diseases, mammalian cancers and especially human cancers including but not limited to for example malignant melanomas, myeloproliferative diseases, chronic myelogenous leukemia, acute lymphocytic leukemia, a disease caused by c-ABL kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Bispecific Anti-CD20/Anti-CD3 Antibodies to Treat Acute Lymphoblastic Leukemia
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of acute lymphoblastic leukemia. The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
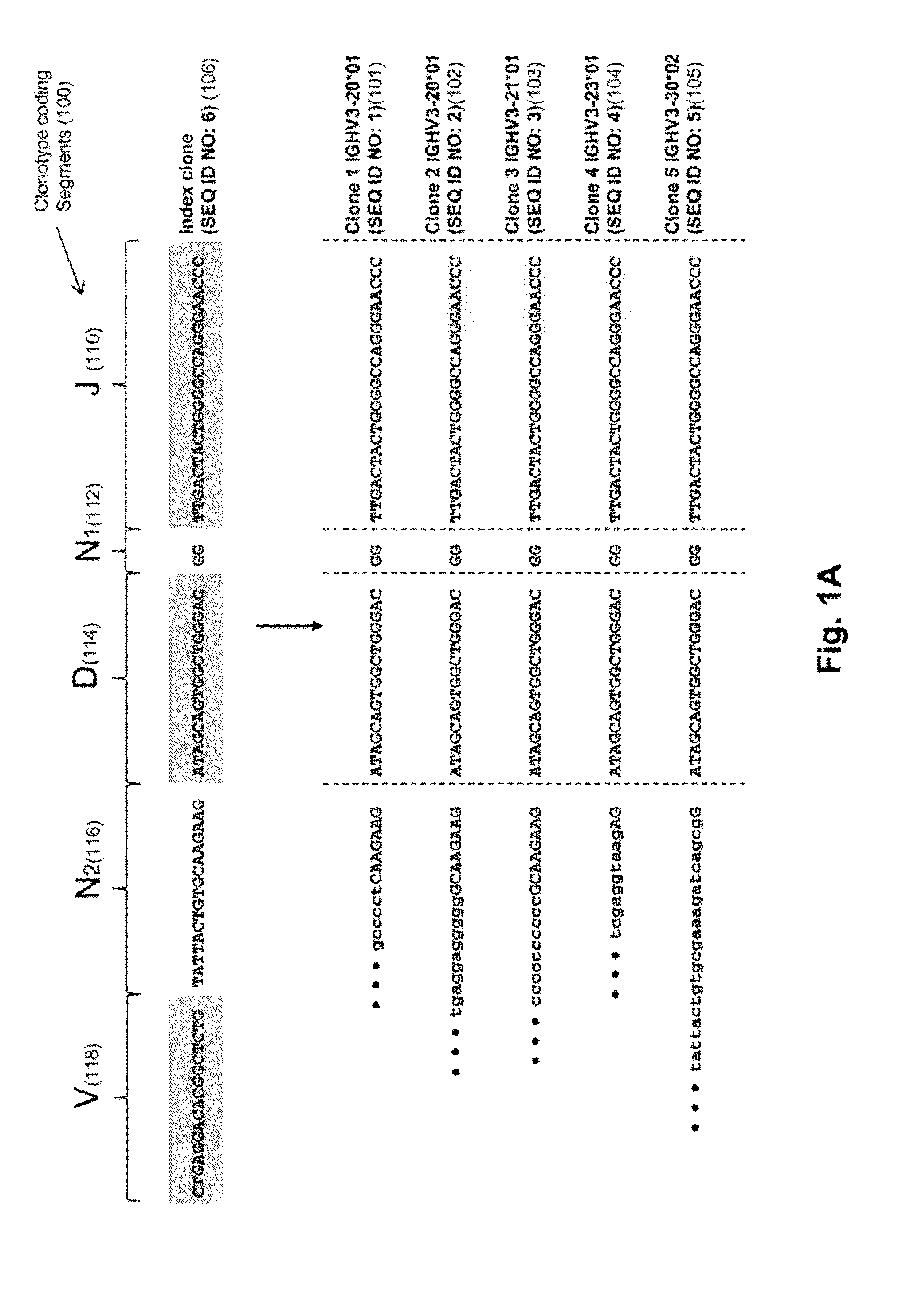
Monitoring immunoglobulin heavy chain evolution in b-cell acute lymphoblastic leukemia
ActiveUS20130202718A1Reduce the possibilityOvercome deficienciesHeavy metal active ingredientsMicrobiological testing/measurementImmunoglobulin heavy chainDisease
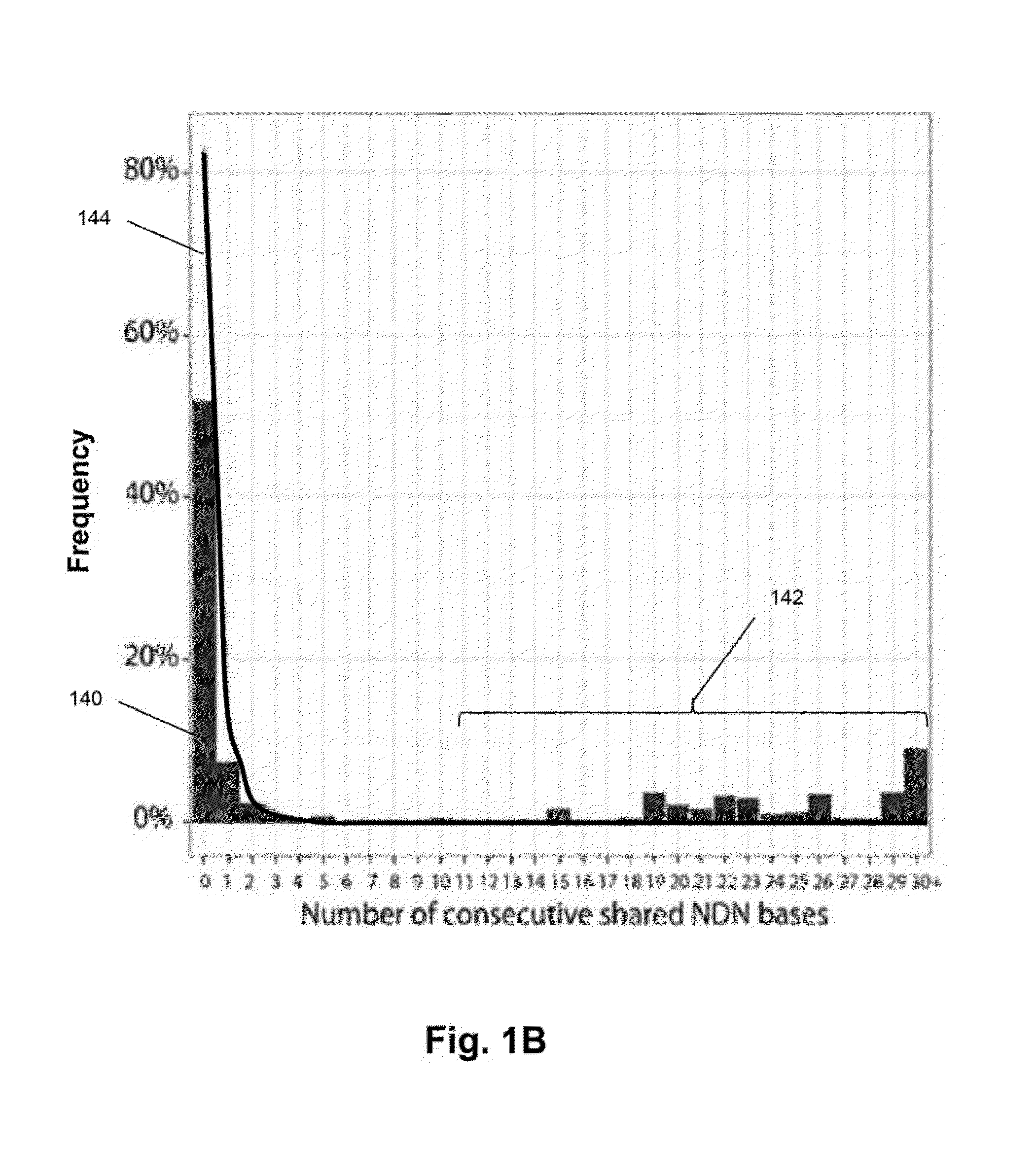
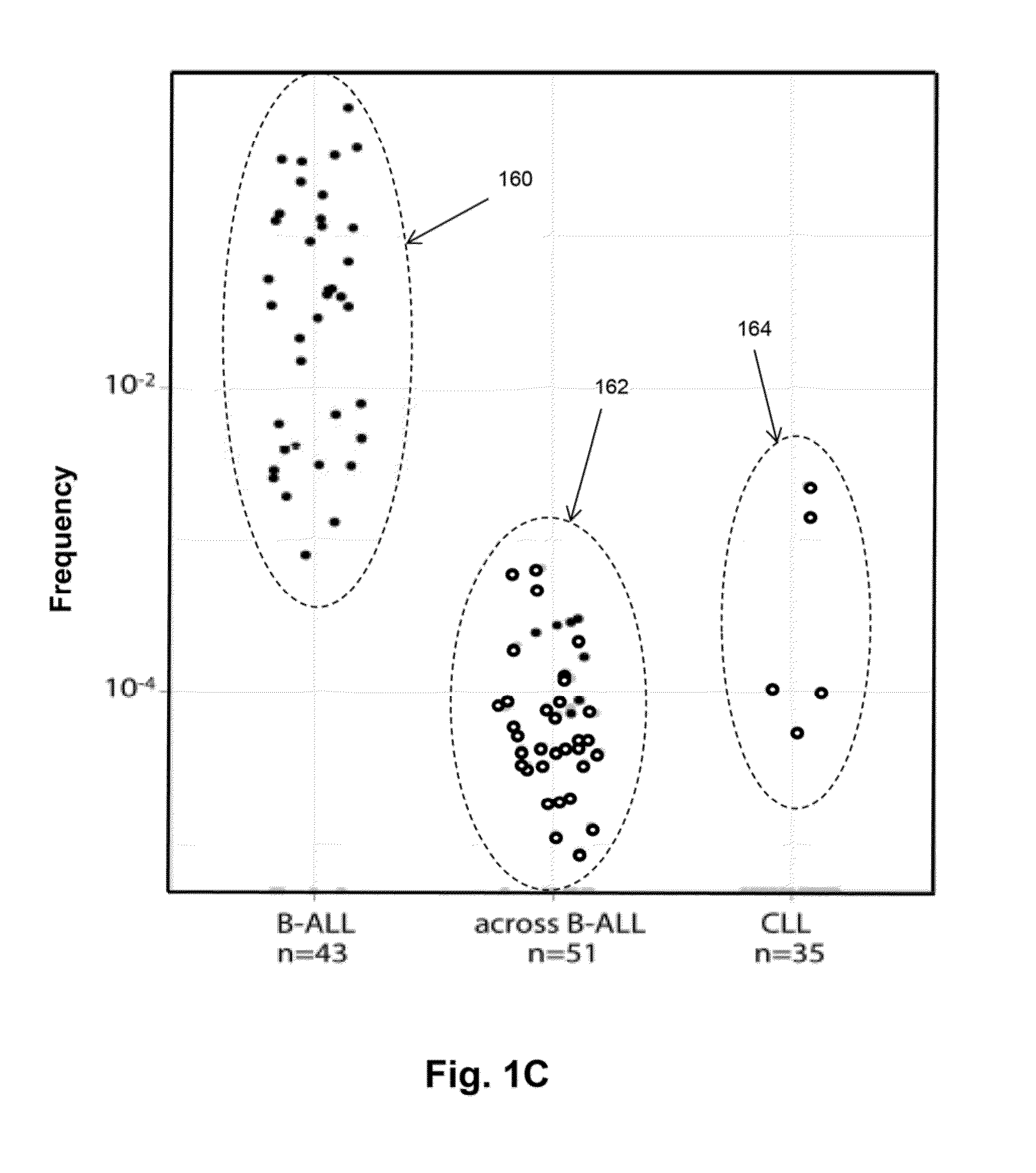
The invention is directed to methods of monitoring B-cell lymphoid proliferative disorders, such as B-cell acute lymphoblastic leukemias, by measuring the presence, absence and / or levels of correlating, or index, clonotypes and related clonotypes that have evolved therefrom, for example, as part of the disease condition. In one aspect, such methods are implemented by generating sequencing-based clonotype profiles and determining frequencies of correlating, or index, clonotypes present, including new clonotypes that have evolved therefrom, particularly, in the case of B-cell ALL, by VH substitution. The invention also includes use of such monitoring information to modify treatment status of a patient.
Owner:ADAPTIVE BIOTECH
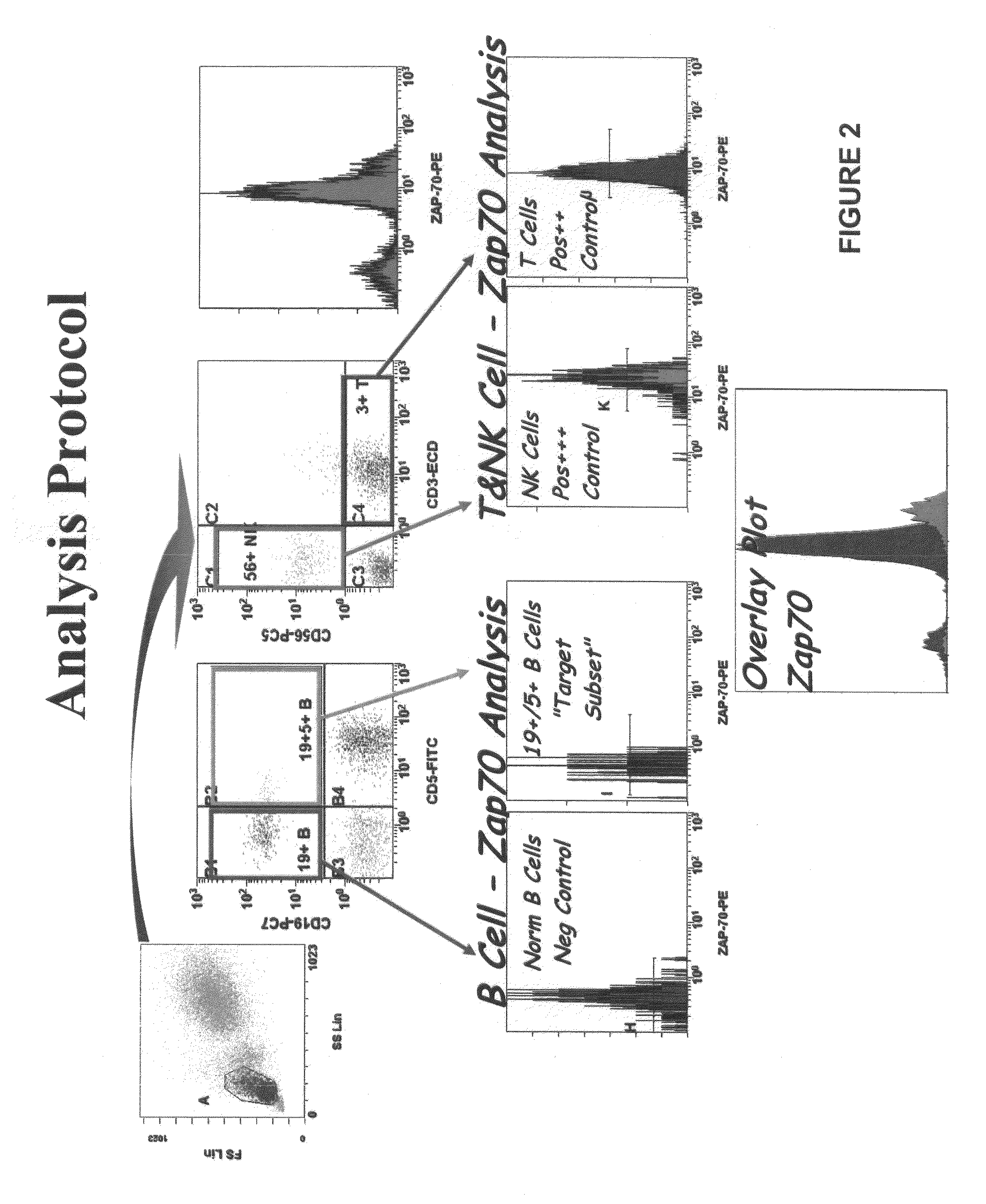
Composite Profiles of Cell Antigens and Target Signal Transduction Proteins for Analysis and Clinical Management of Hematologic Cancers
InactiveUS20100261204A1Increased riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
Owner:BECKMAN COULTER INC
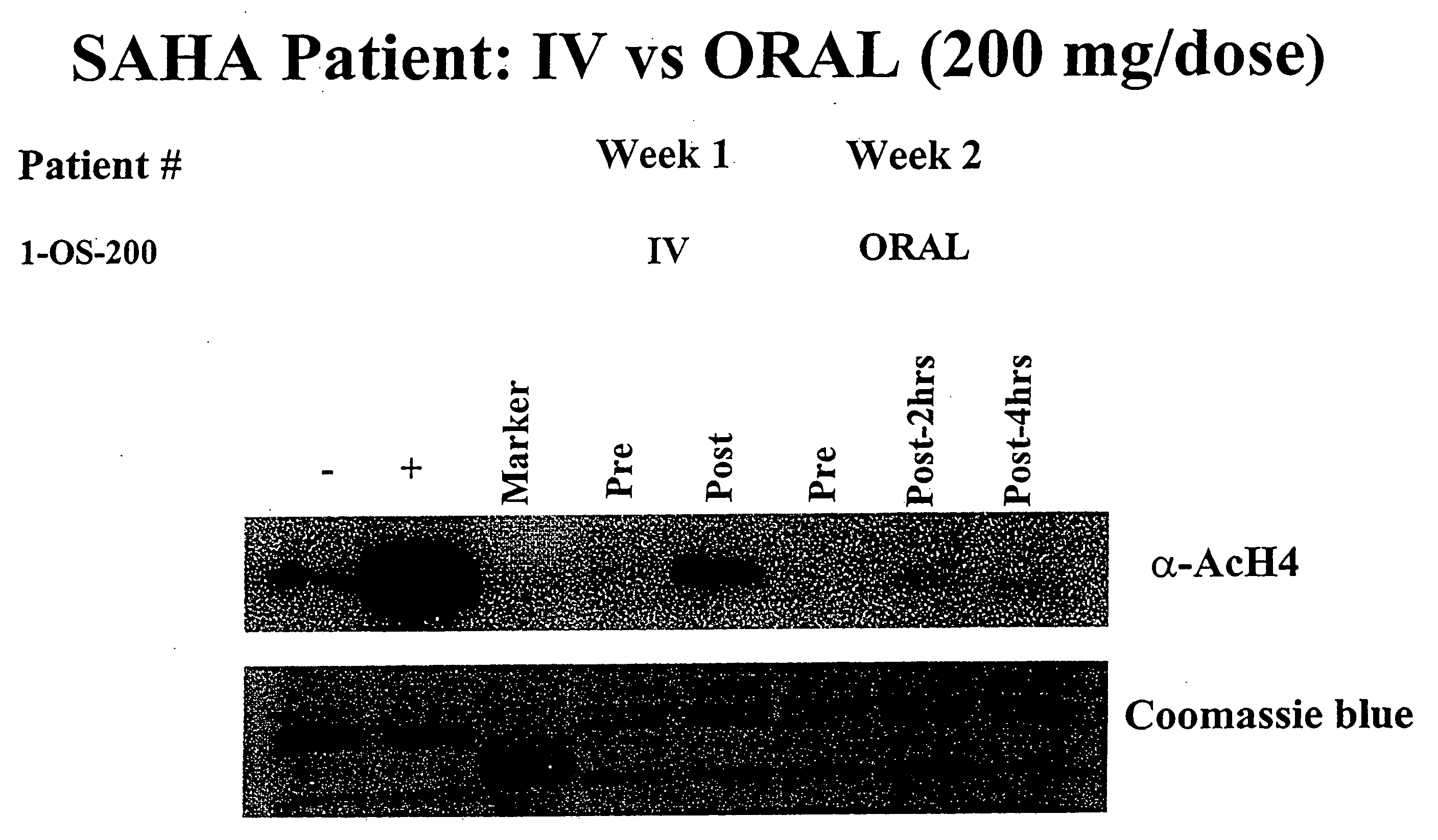
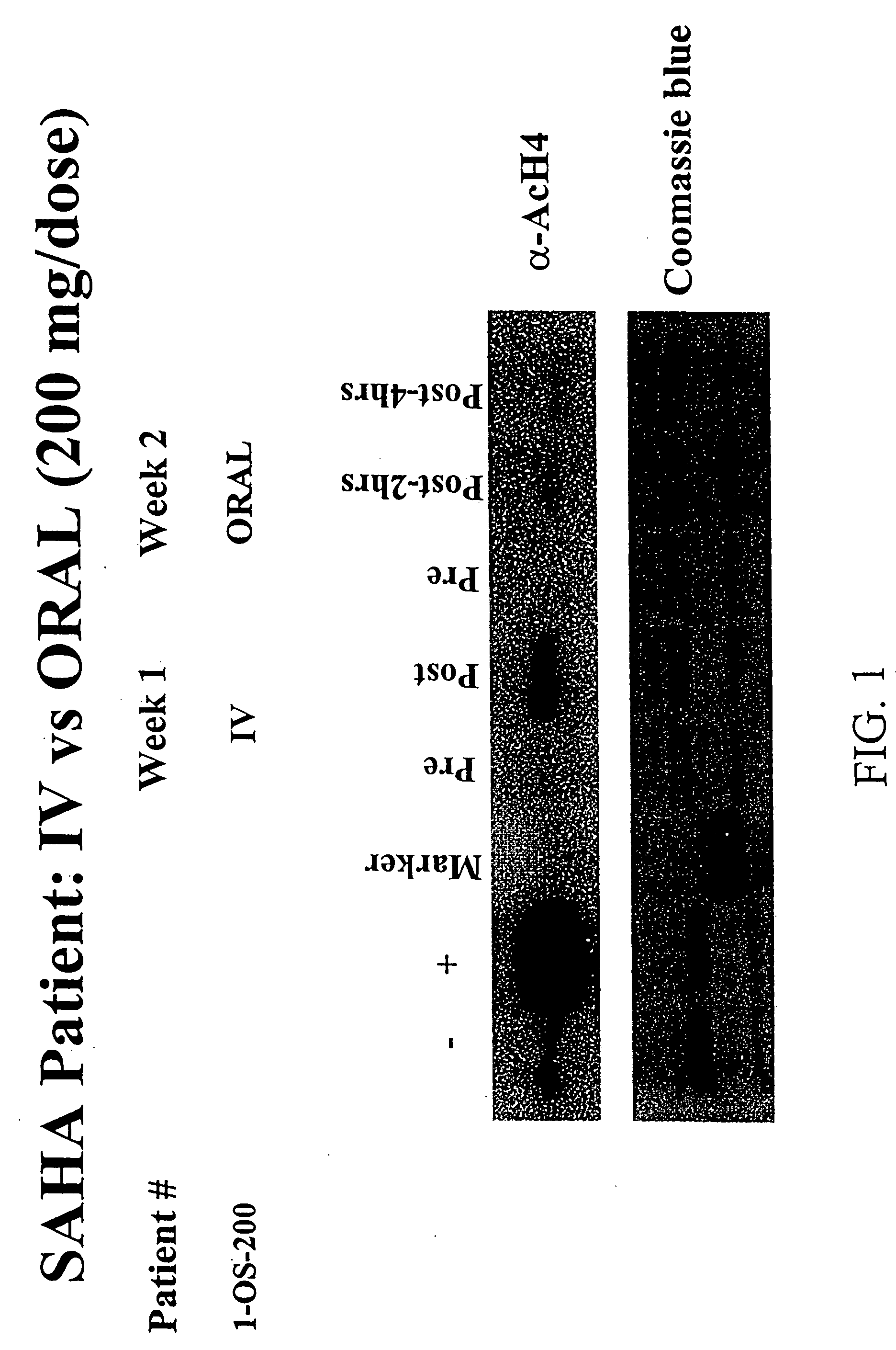
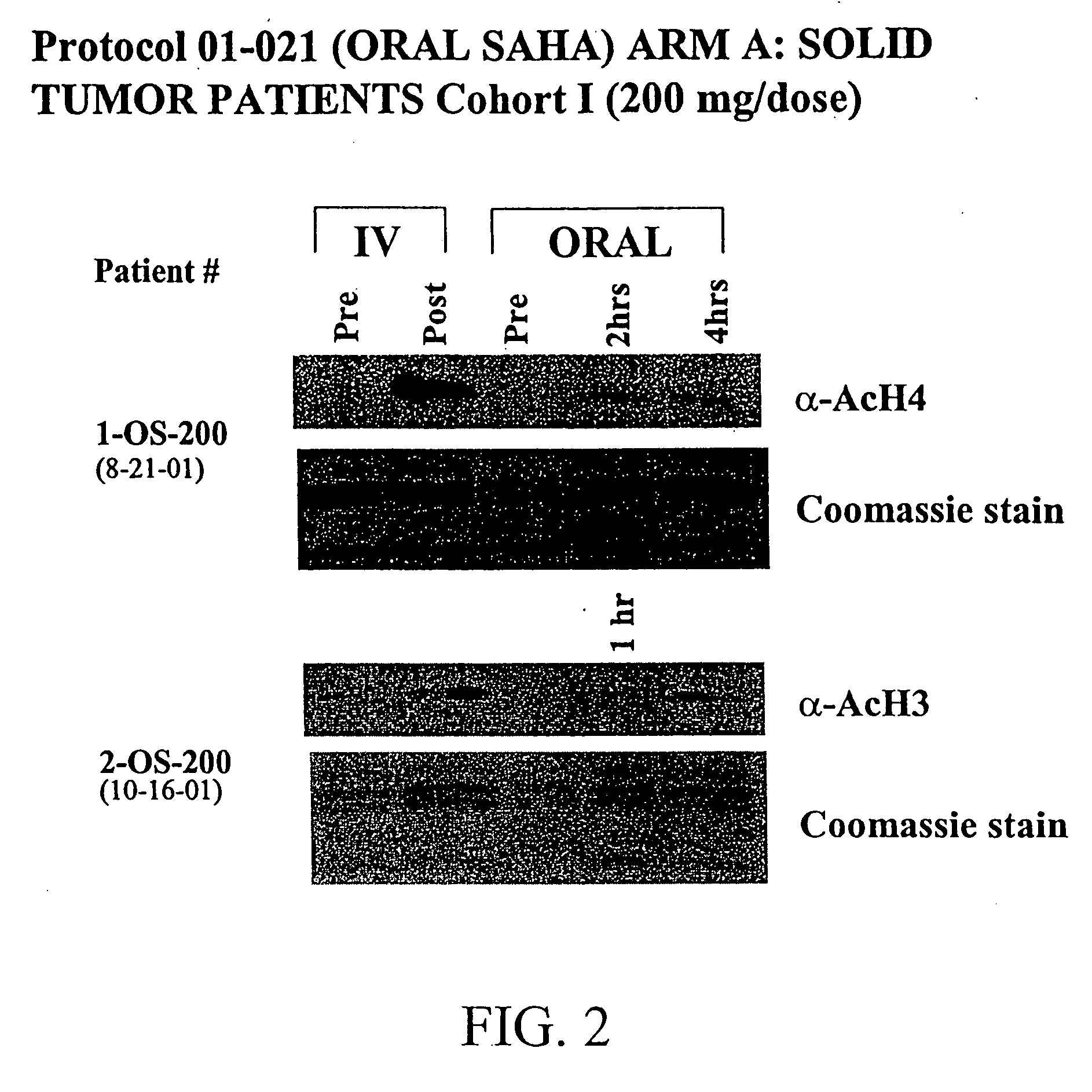
Methods of treating cancer with HDAC inhibitors
InactiveUS20080249179A1Better pharmacokinetic profileImprove bioavailabilityBiocideOrganic chemistryDosing regimenIn vivo
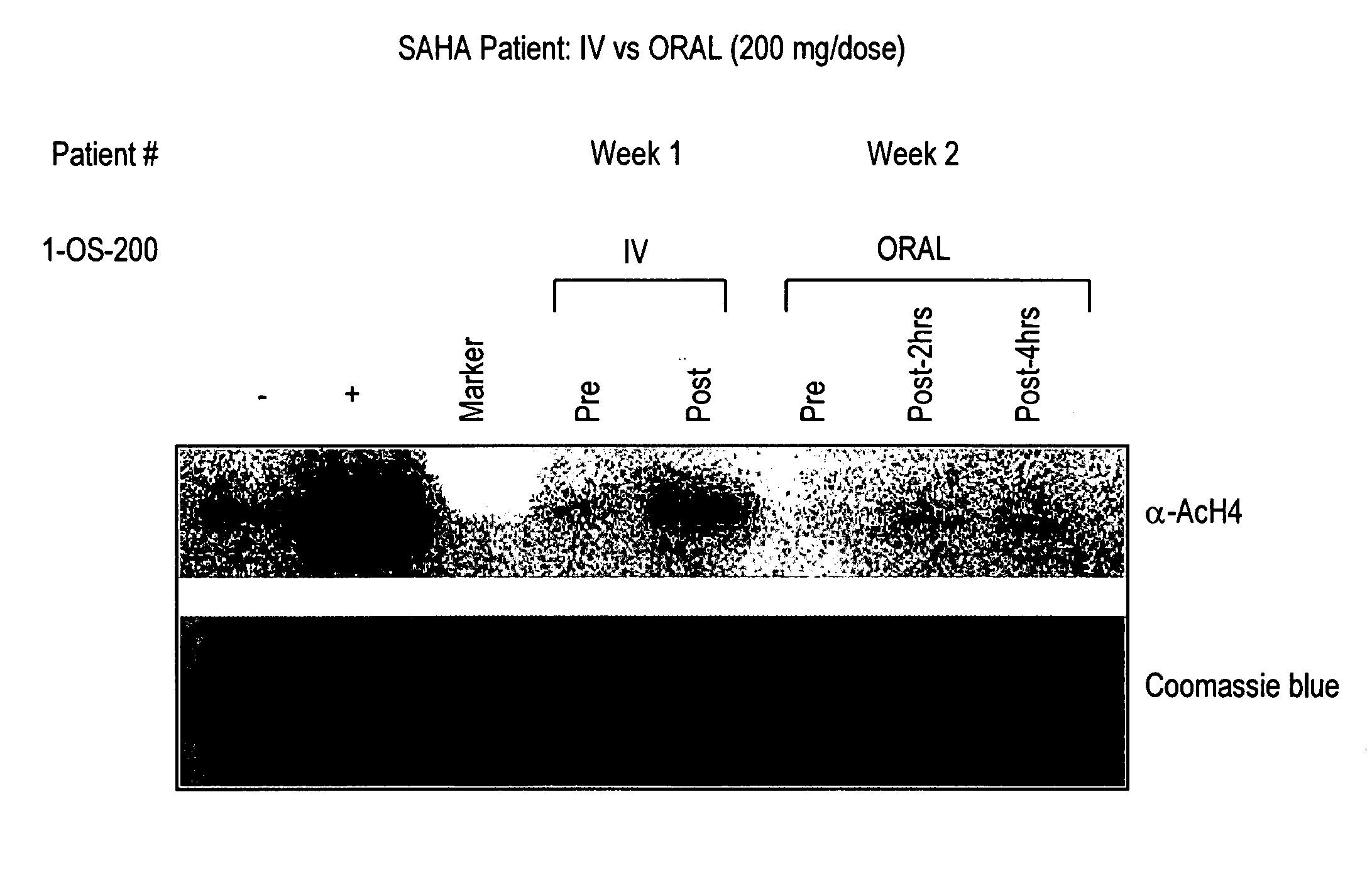
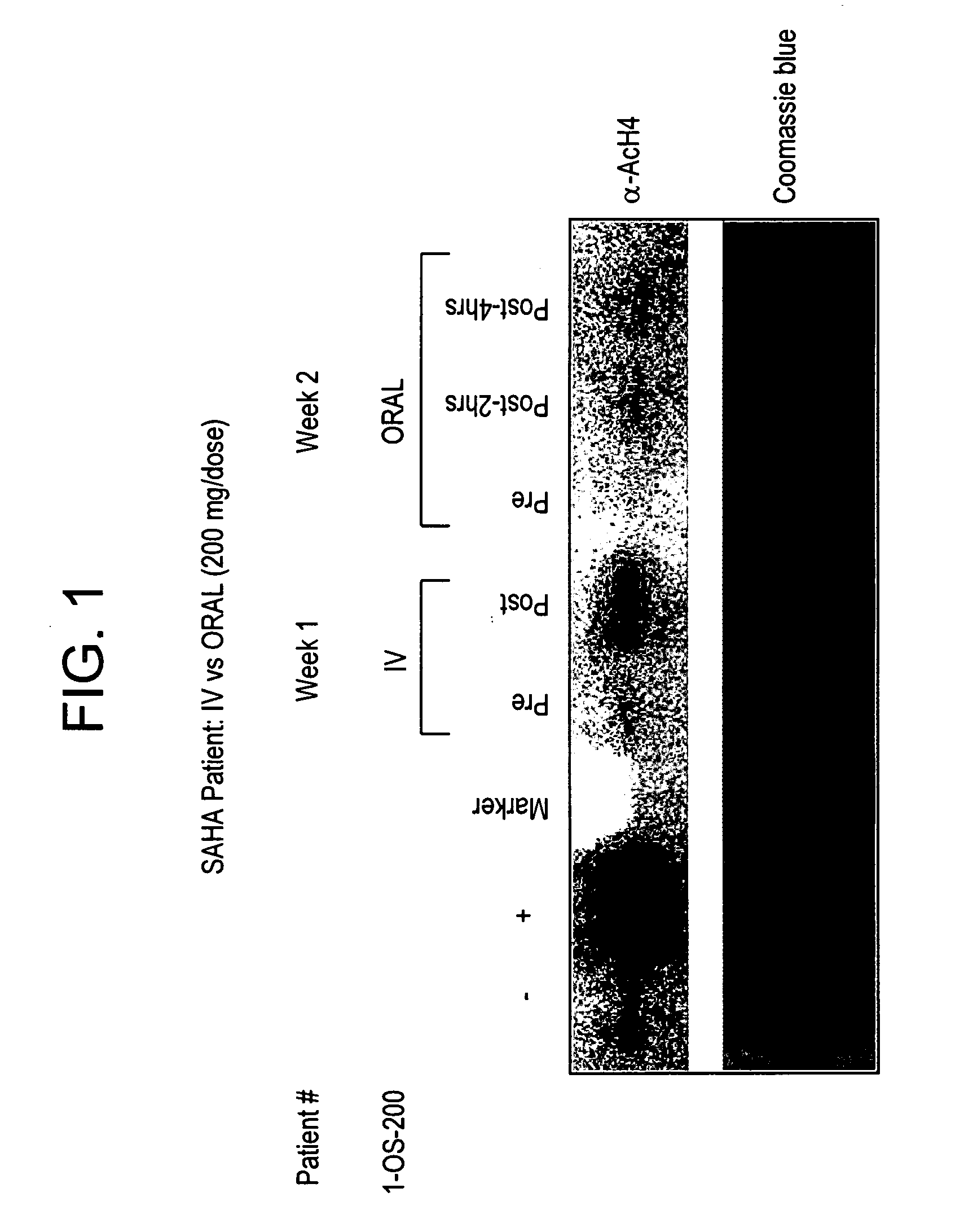
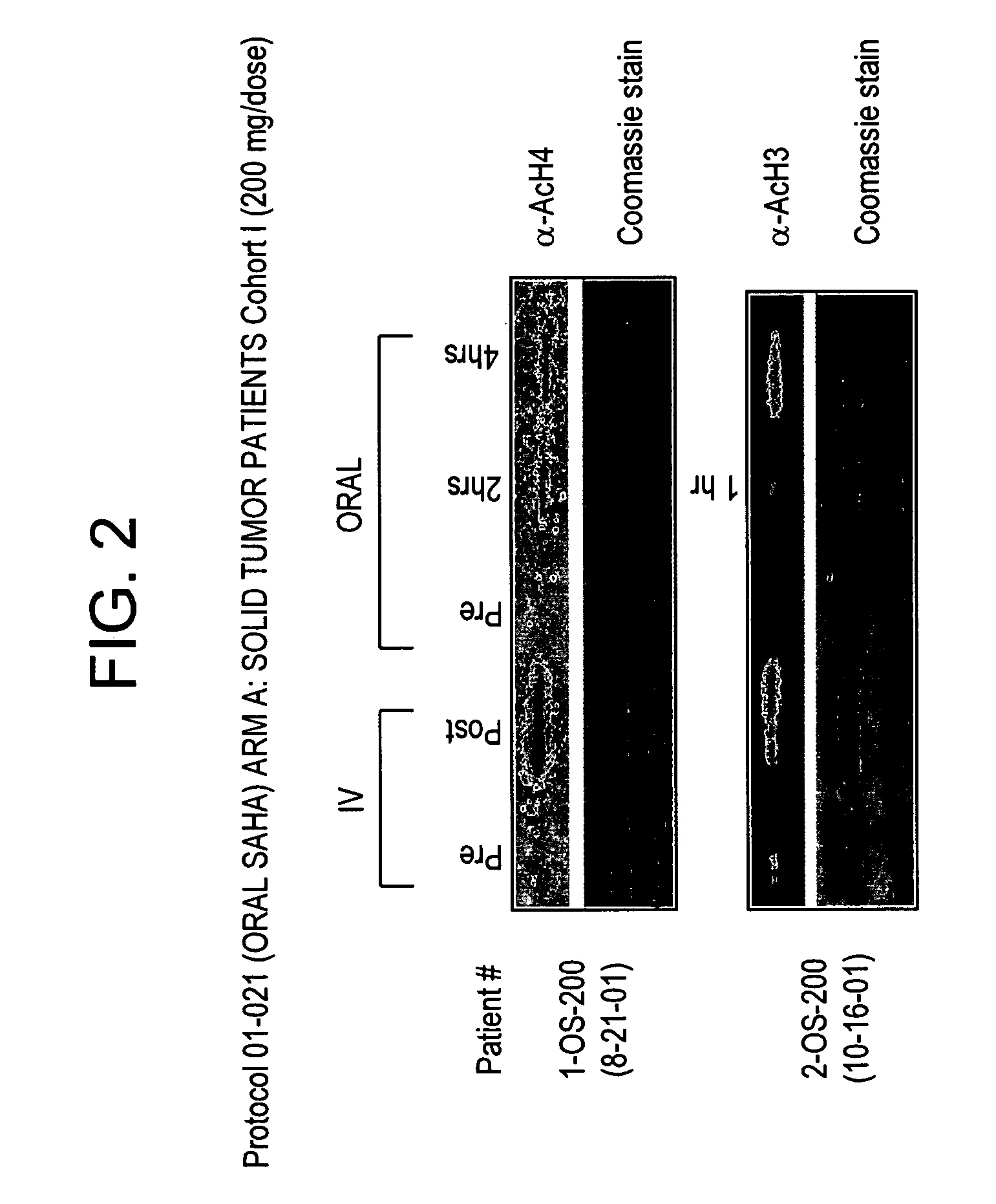
The present invention relates to methods of treating cancers, e.g., leukemia. More specifically, the present invention relates to methods of treating acute and chronic leukemias including Acute Lymphocytic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic leukemia (CLL), Chronic myeloid leukemia (CML) and Hairy Cell Leukemia, by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
Novel Anti-cd38 antibodies for the treatment of cancer
InactiveUS20110262454A1Improved propertyLess immunogenicSenses disorderAntipyreticDiseaseComplement-dependent cytotoxicity
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI SA
Composite profiles of cell antigens and target signal transduction proteins for analysis and clinical management of hematologic cancers
InactiveUS20070105165A1Increased relapse riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
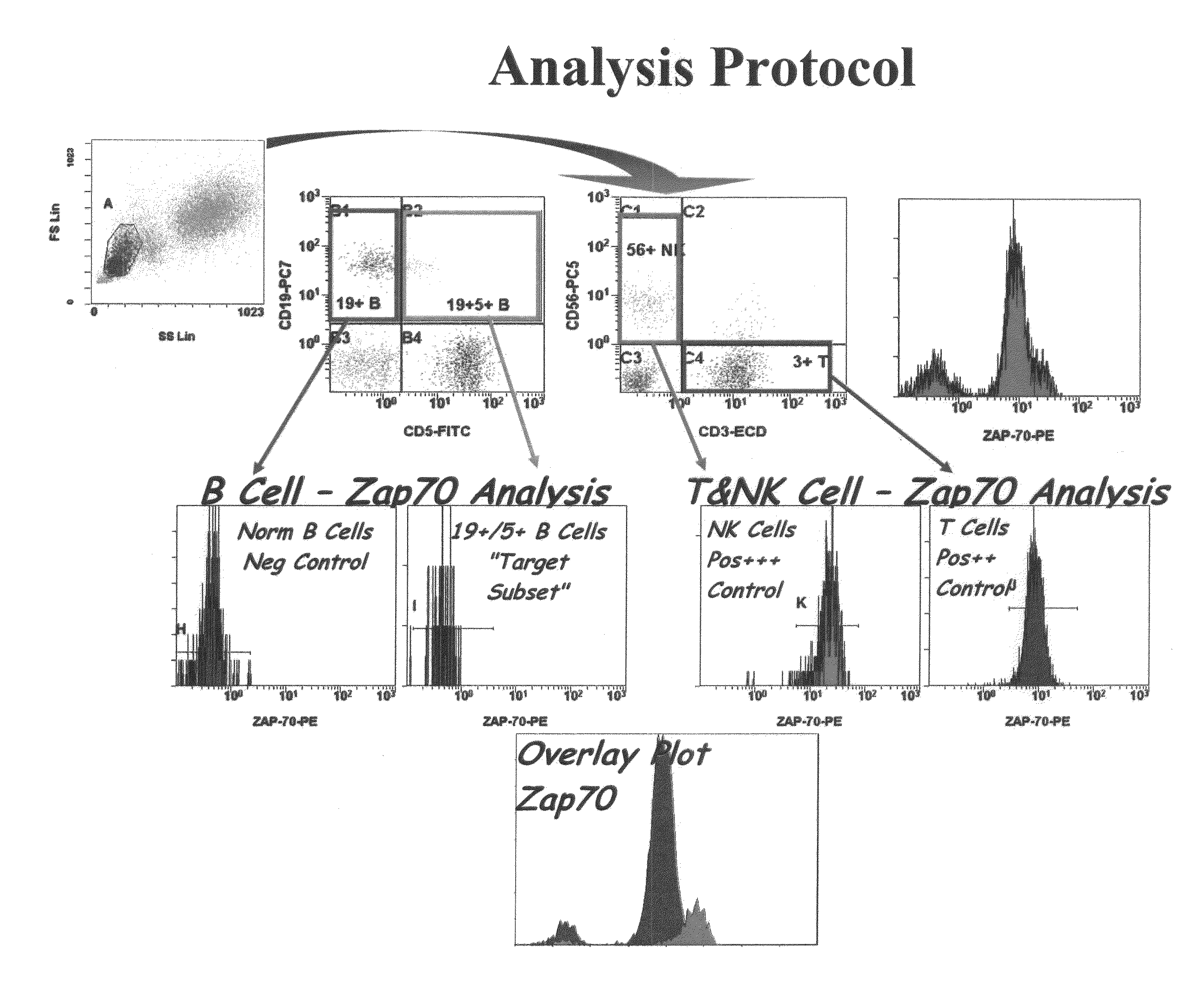
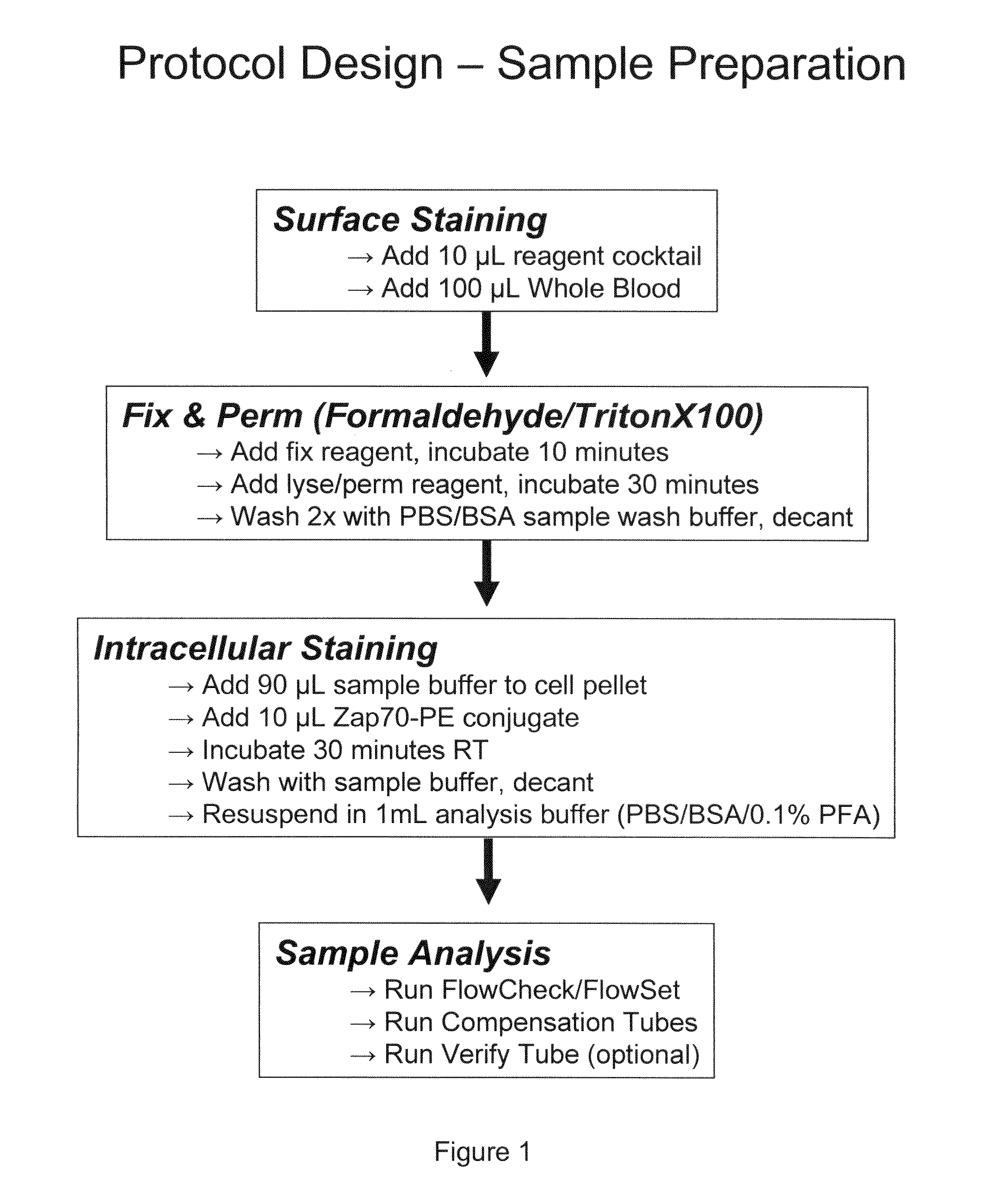
The present invention is directed to methods for establishing a composite marker profile for a sample derived from an individual suspected having a neoplastic condition. A composite marker profile of the invention allows for identification of prognostically and therapeutically relevant subgroups of neoplastic conditions and prediction of the clinical course of an individual. The methods of the invention provide tools useful in choosing a therapy for an individual afflicted with a neoplastic condition, including methods for assigning a risk group, methods of predicting an increased risk of relapse, methods of predicting an increased risk of developing secondary complications, methods of choosing a therapy for an individual, methods of determining the efficacy of a therapy in an individual, and methods of determining the prognosis for an individual. In particular, the method of the present invention discloses a method for establishing a composite marker profile that can serve as a prognostic indicator to predict whether the course of a neoplastic condition in a individual will be aggressive or indolent, thereby aiding the clinician in managing the patient and evaluating the modality of treatment to be used. In particular embodiments disclosed herein, the methods of the invention are directed to establishing a composite marker profile for a leukemia selected from the group consisting of Chronic Lymphocytic Leukemia (CLL), Acute Myelogenous Leukemia (AML), Chronic Myelogenous Leukemia (CML), and Acute Lymphocytic Leukemia (ALL).
Owner:BECKMAN COULTER INC +4
P53 modulator and cancer target
Methods of screening for modulators of TRIM24 (also known as TIF1-ALPHA) expression and / or biological activity are described. In particular, methods of screening of screening for modulators of TRIM24 E3 ligase activity, and specifically an E3 ligase activity directed at p53 as the target polypeptide are also described. Modulators of TRIM24 expression and activity are provided and their use in treatment of cancer, particularly in breast, colon, prostate, renal cancers and in acute lymphoblastic leukaemia. Suitable modulators of TRIM24 expression include siRNA and shRNA and can be used in the treatment of cancer and for targeting cancer stem cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and compositions for identifying minimal residual disease in acute lymphoblastic leukemia
This invention provides methods and kits for diagnosing, ascertaining the clinical course of minimal residual disease associated with acute lymphoblastic leukemia (ALL). Specifically the invention provides methods and kits useful in the diagnosis and determination of clinical parameters associated with diseases associated with ALL based on patterns of surface marker expression unique to ALL.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
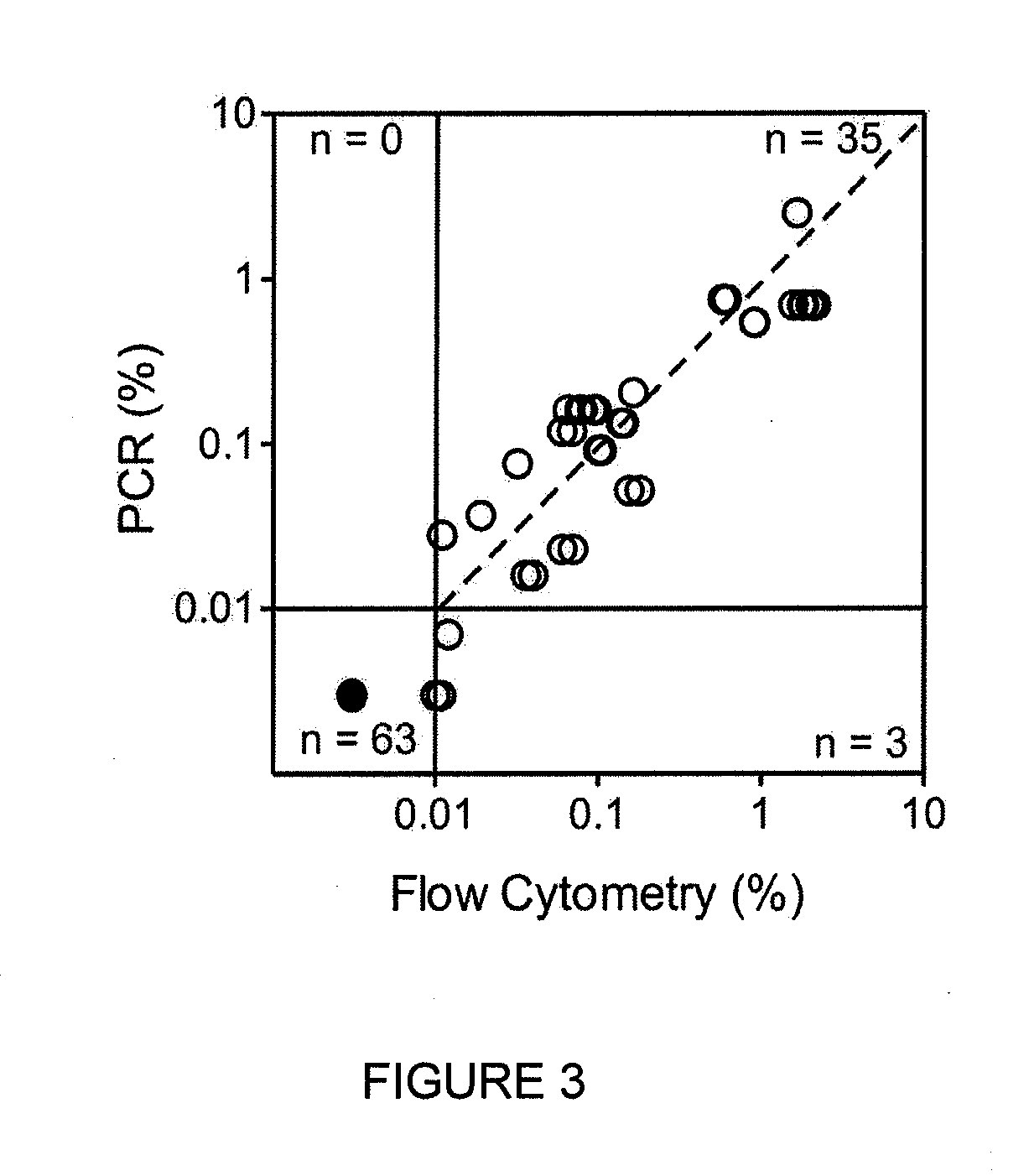
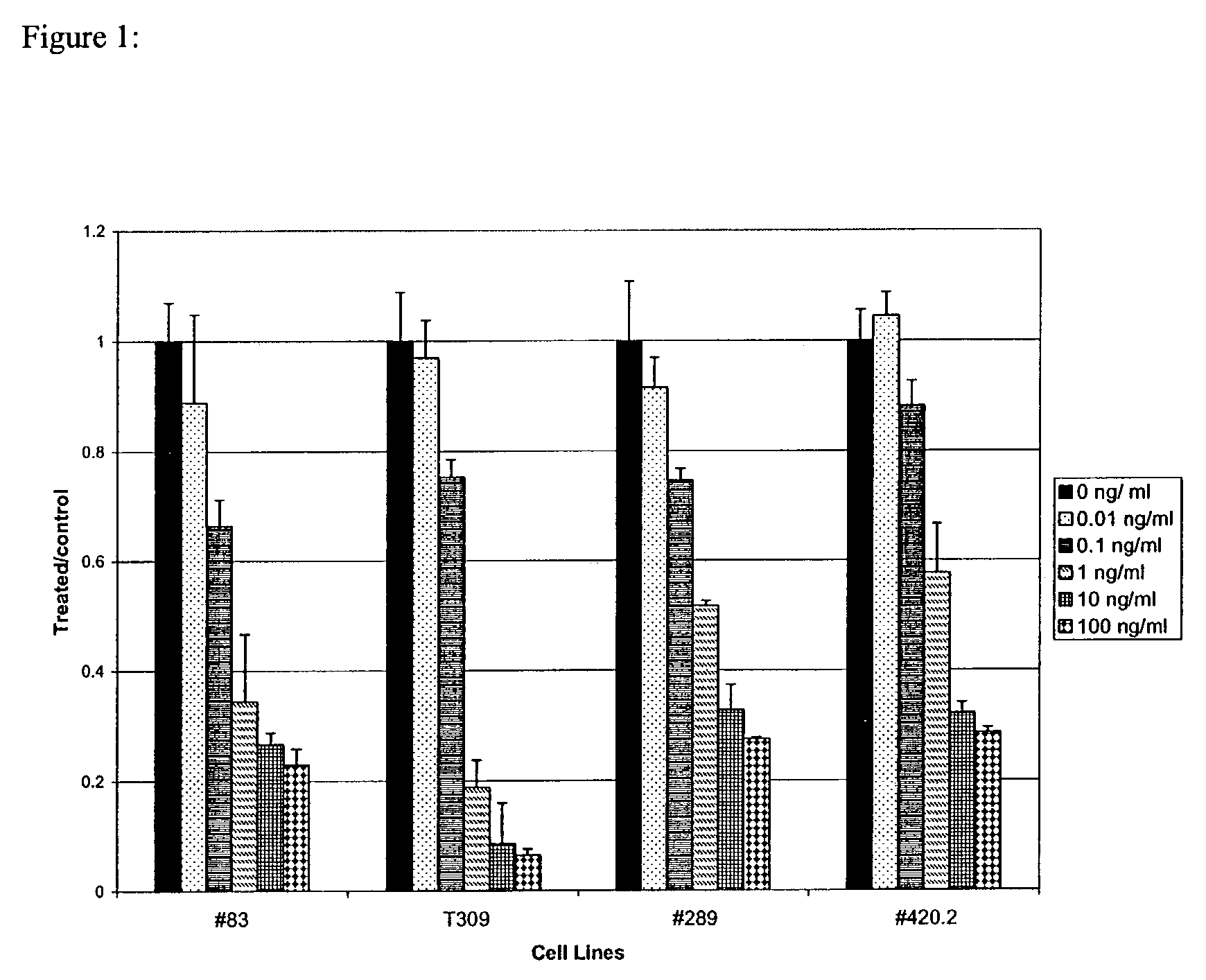
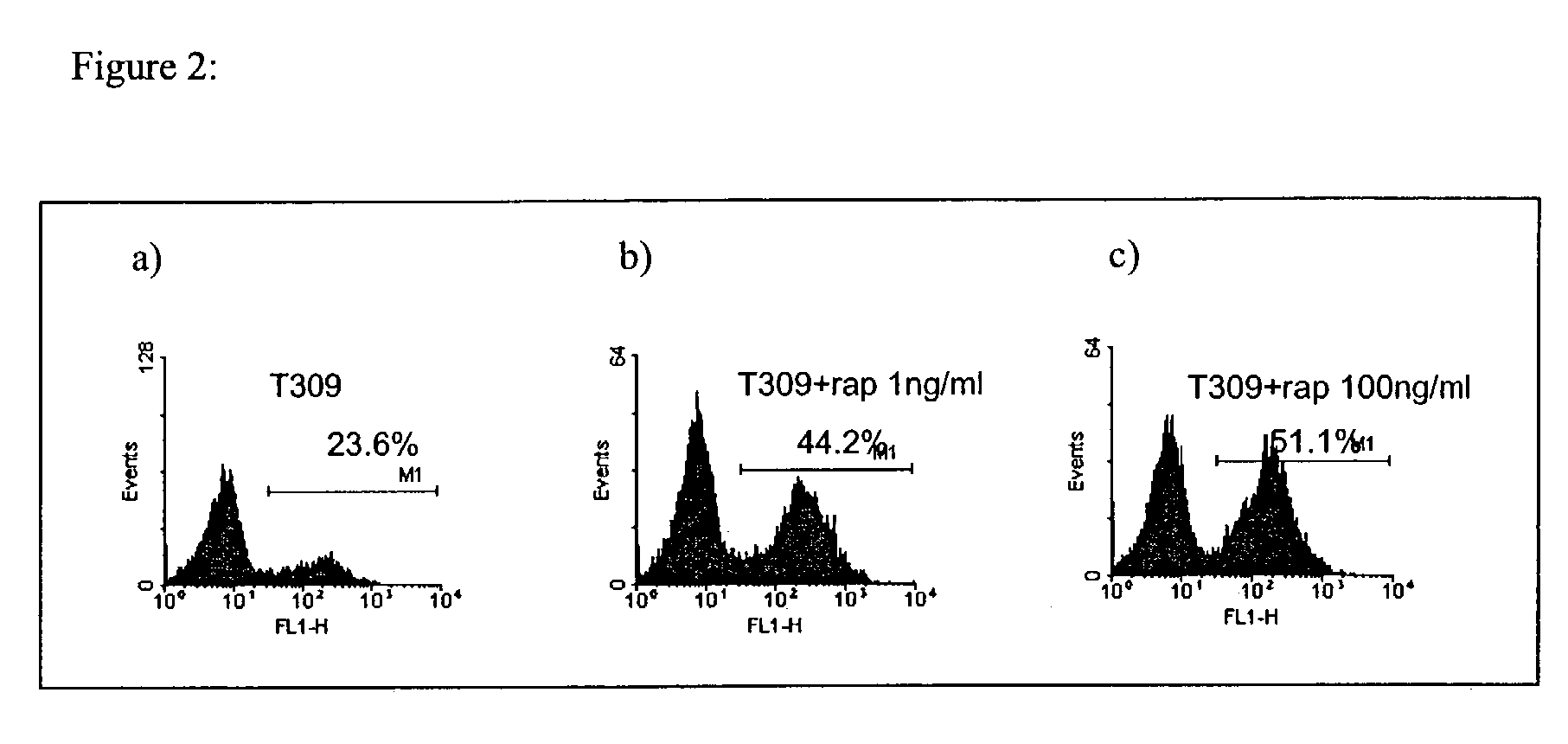
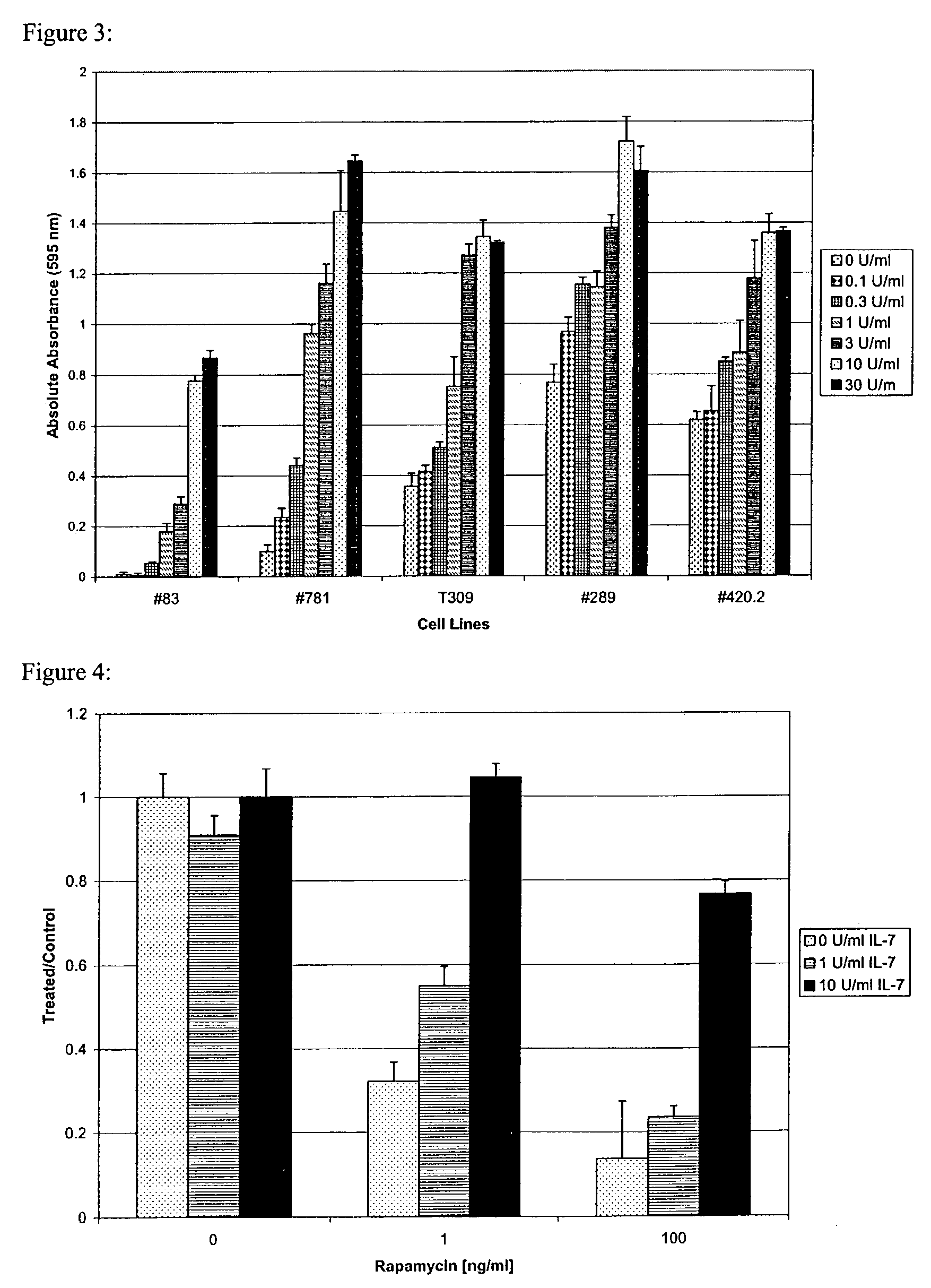
Methods for treatment of acute lymphocytic leukemia
InactiveUS7026330B2Improved prognosisAvoid seizuresBiocideAntibody ingredientsChronic lymphocytic leukemiaAcute lymphocytic leukemia
Methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof are provided. Also provided are methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof in combination with an IL-7 inhibitor. Finally methods for preventing GVHD in ALL patients following a bone marrow transplant are disclosed.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
BCR-ABL gene fluorescence quantitative RT-PCR primer and probe and reagent kit
InactiveCN1995386AEasy to detectImprove featuresMicrobiological testing/measurementReference genesAgricultural science
The invention discloses a quantitative RT-PCR primer and probe and agent box of BCR-ABL fusing gene mRNA fluorescence with BCR-ABL fusing gene primer and probe sequence as SEQ ID NO1-4 and internal reference gene primer and probe sequence as SEQ ID NO5-7, wherein the agent box contains cell cracking liquid, water, RT-PCR reacting liquid, internal reference TBP RT-PCR reacting liquid, BCR-ABL fusing gene detecting probe, TBP internal reference gene testing probe, composite enzyme, standard material and comparing material; the box can test the expressive level of mRNA of P210BCR / ABL and P190BCR / ABL in the specimen, which provides the reference to diagnose, recurrent and treat chronic granulocytic leukemia and acute lymphocyte leukemia.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Chimeric antigen receptor T cells targeting CD19, and application of chimeric antigen receptor T cells
InactiveCN108276497AInhibit tumor cell proliferationActivate the killing mechanismMammal material medical ingredientsImmunoglobulinsNH lymphomaInterleukin 2
The invention discloses chimeric antigen receptor T cells targeting CD19, and application of the chimeric antigen receptor T cells. A chimeric antigen receptor for preparing the chimeric antigen receptor T cells comprises interleukin 2 signal peptide, an anti-CD19 single chain antibody, a CD8 protein molecular hinge region, a transmembrane region, an intracellular signal structural domain, and anintracellular signal transduction structural domain of CD3 zeta protein molecules which are sequentially connected in series. The chimeric antigen receptor T cells are used for preparing medicines orpreparations for treating hematological malignancies, wherein the hematological malignancies comprise CD19-positive acute B-lymphocytic leukemia, diffuse large B-cell lymphoma and non-Hodgkin lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Methods of treating cancer with HDAC inhibitors
InactiveUS20080227862A1Better pharmacokinetic profileImprove bioavailabilityBiocideOrganic chemistryDosing regimenIn vivo
The present invention relates to methods of treating cancers, e.g., leukemia. More specifically, the present invention relates to methods of treating acute and chronic leukemias including Acute Lymphocytic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic leukemia (CLL), Chronic myeloid leukemia (CML) and Hairy Cell Leukemia, by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC
CAR-T (chimeric antigen receptor T cell) for targeting CD19 and application of CAR-T
ActiveCN107827991ARestrict growthPrevent proliferationMammal material medical ingredientsImmunoglobulinsSequence signalSingle-Chain Antibodies
The invention discloses a CAR-T (chimeric antigen receptor T cell) for targeting CD19 and an application of the CAR-T. A CAR for preparing the CAR-T comprises interleukin 2 signal peptides, an anti-CD19 single-chain antibody, a CD8 protein molecule hinge region, a transmembrane region, an intracellular signal structure region and a CD3 zeta protein molecule intracellular signal conduction structure region which are connected in series sequentially and has the amino acid sequence shown in SEQ ID NO:9. The CAR-T is applied to preparation of a medicine or a preparation for treating hematologicalmalignancy, wherein the hematological malignancy comprises CD19-positive B-cell acute lymphocytic leukemia, diffuse large B cell lymphoma and non-hodgkin's lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
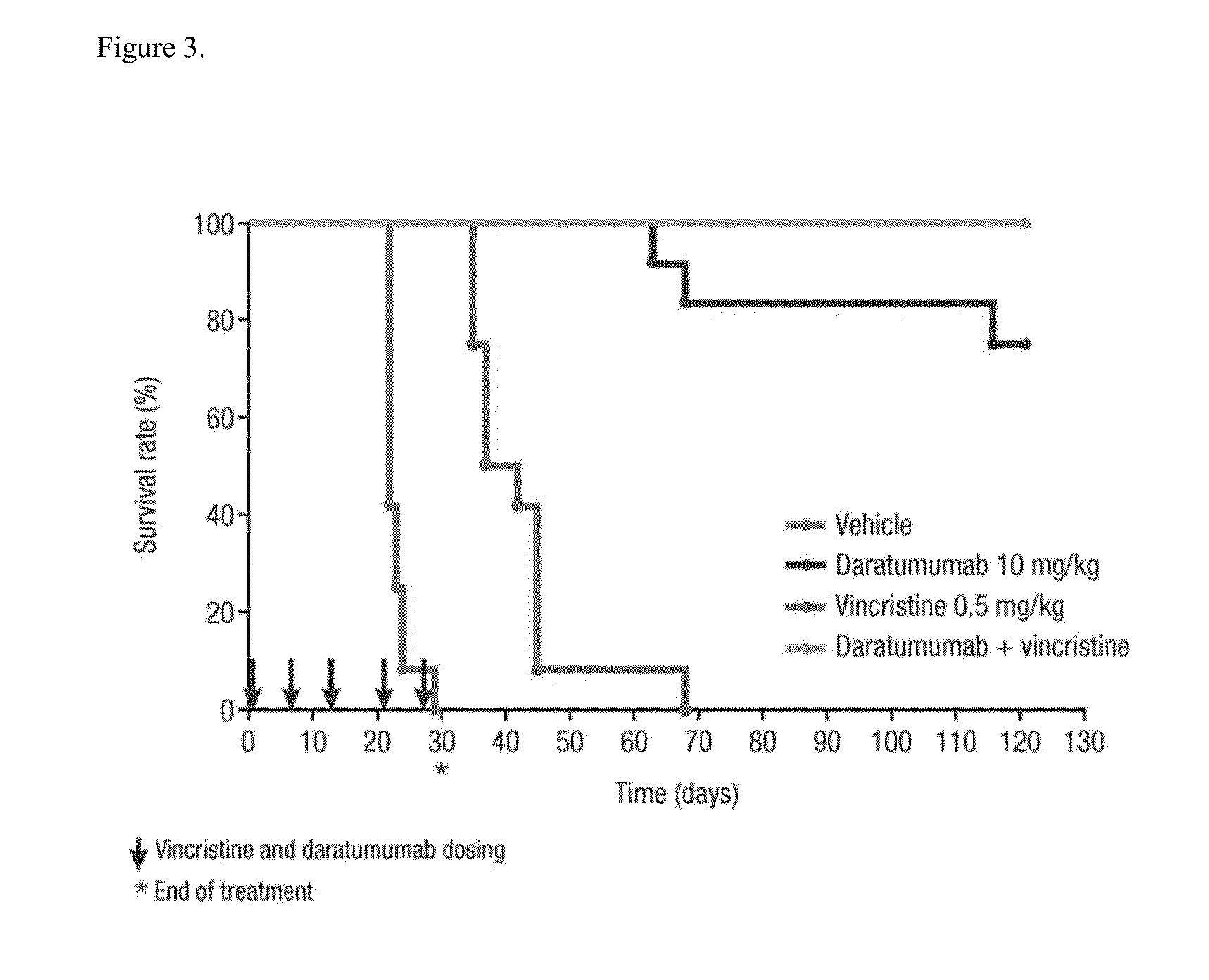
Anti-CD38 antibodies for treatment of acute lymphoblastic leukemia
ActiveUS9732154B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAcute lymphocytic leukemiaCombination therapy
Owner:JANSSEN BIOTECH INC
SiRNA of humanized interleukin 6, recombination expression carrier CAR-T and construction method and application of recombination expression carrier CAR-T
ActiveCN106636090ARelieve painReduce the risk of CRSOrganic active ingredientsGenetic material ingredientsAbnormal tissue growthInterleukin 6
The invention discloses an siRNA of humanized interleukin 6, a recombination expression carrier CAR-T and a construction method and application of the recombination expression carrier CAR-T. An IL-6 knock-down siRNA expression cassette and an siRNA expression product not only can be applied to eliminating or alleviating of CRS symptoms in treatment of B lineage acute lymphoblastic leukemia (ALL) through CAR19-T, but also can be applied to alleviating of CRS symptoms caused in treatment of all types of tumors like B lymphoma, pancreatic cancer, brain glioma and myeloma through CAR-T, and is even applied to alleviating of CRS caused by other types of treatment.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Gene probe composition and kit for acute lymphocytic leukemia detection
ActiveCN102978279AEasy to detectImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceIn situ hybridisationFluorescence
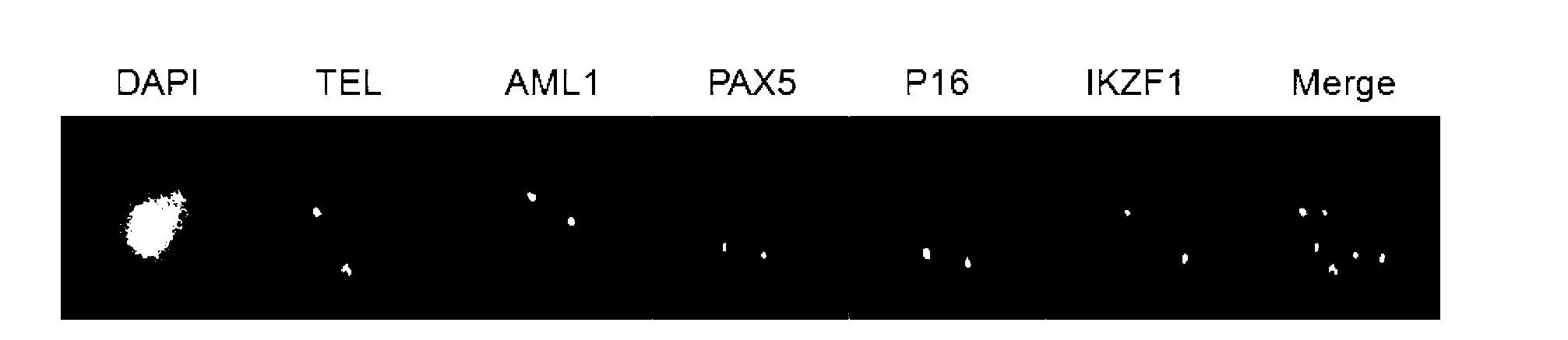
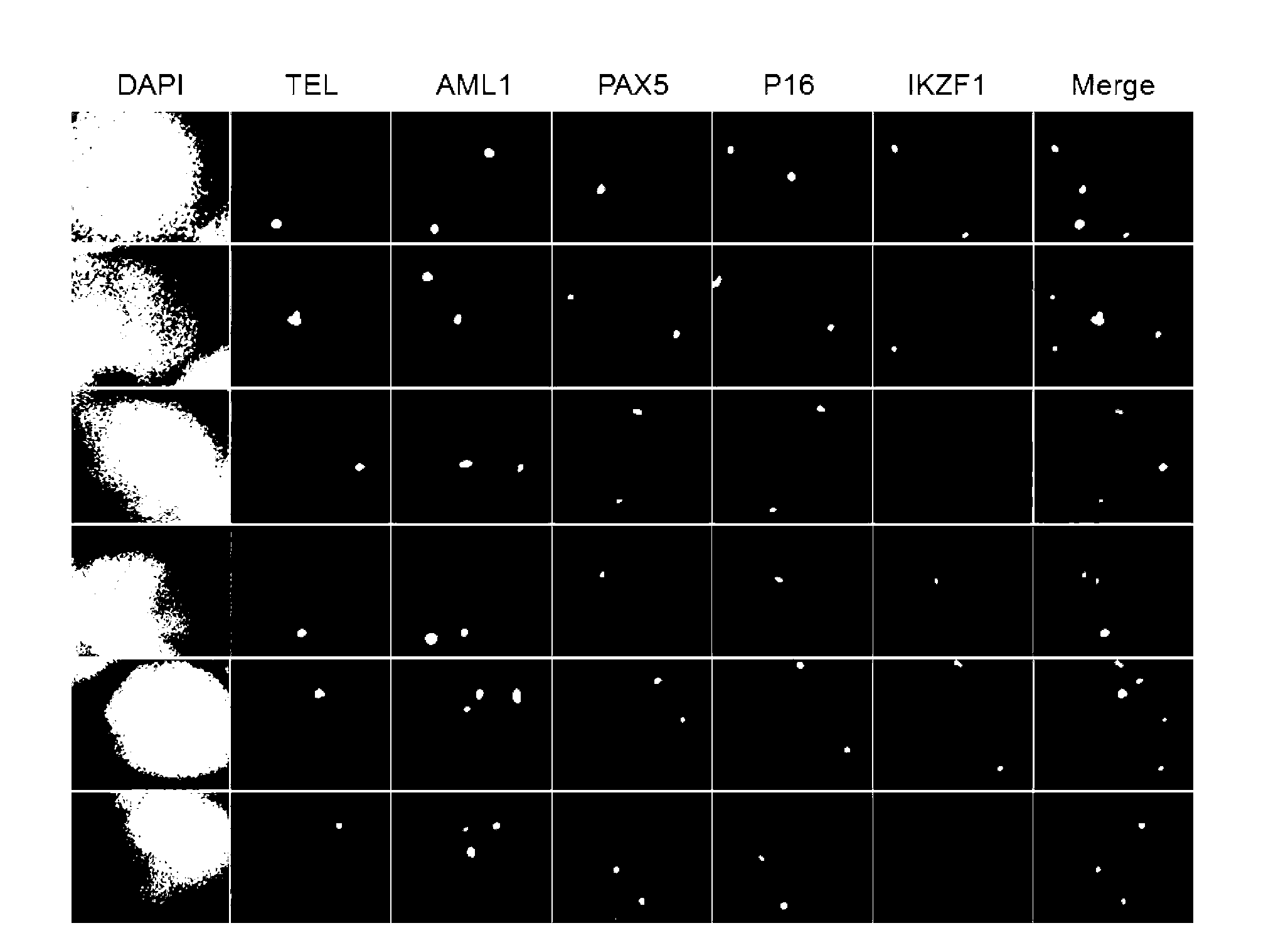
The present invention relates to a gene probe composition and a kit for acute lymphocytic leukemia detection. The gene probe composition comprises a TEL gene probe, an AML1 gene probe, a PAX5 gene probe, a P16 gene probe and an IKZF1 gene probe. The present invention further provides an acute lymphocytic leukemia fluorescence in situ hybridization detection kit, wherein the kit comprises the gene probe composition. With the kit, concurrent five gene detection can be performed on the same sample even the single leukemia cell, and detection capability and efficiency are significantly improved.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Children acute lymphoblastic leukaemia genotyping diagnosis chip
InactiveCN101525667AReduce complicationsHigh cure rateNucleotide librariesMicrobiological testing/measurementLife qualityA-DNA
The invention discloses a children acute lymphoblastic leukaemia genotyping diagnosis chip. The children acute lymphoblastic leukaemia genotyping diagnosis chip is a DNA chip which is fixed with 62 DNA fragment arrays on the surface of a carrier. .The nucleotide sequences of the 62 DNA fragment arrays are respectively the sequence 1 to the sequence 62 of the sequence list. The invention further discloses a children acute lymphoblastic leukaemia genotyping method. The genetic chip of the invention can provide precise typing so as to help correctly choose a chemo-treatment plan of appropriate strength, thereby reducing complicating disease and improving curative ratio and life quality. The genetic chip of the invention can be used to carry out genotyping on childhood ALL. Therefore, the genetic chip not only saves diagnosis cost, but also has significance in protecting labour resource and promoting family and society harmony.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV +1
Anti-CD38 Antibodies for Treatment of Acute Lymphoblastic Leukemia
ActiveUS20150246975A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAcute lymphocytic leukemiaCombination therapy
Owner:JANSSEN BIOTECH INC
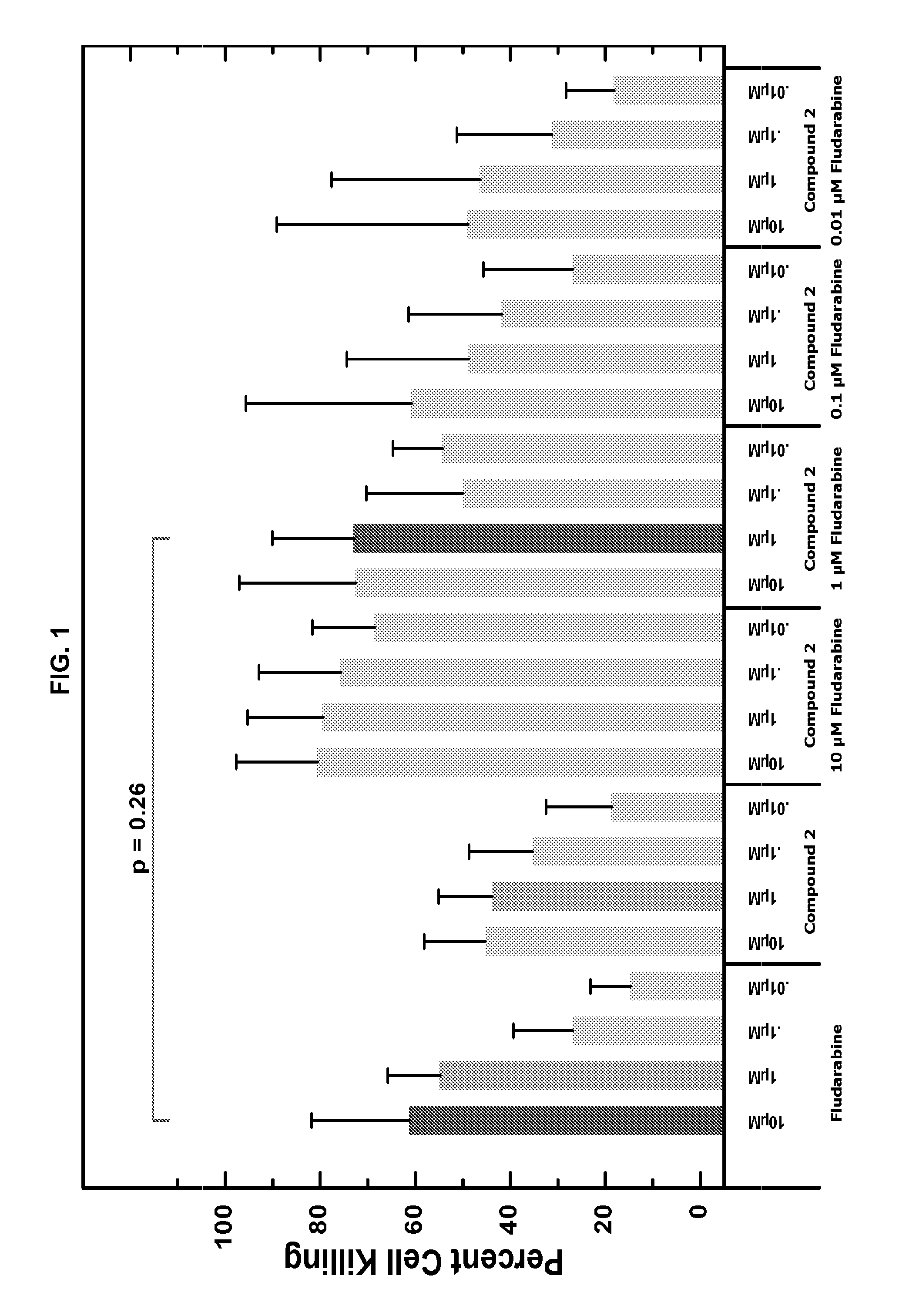
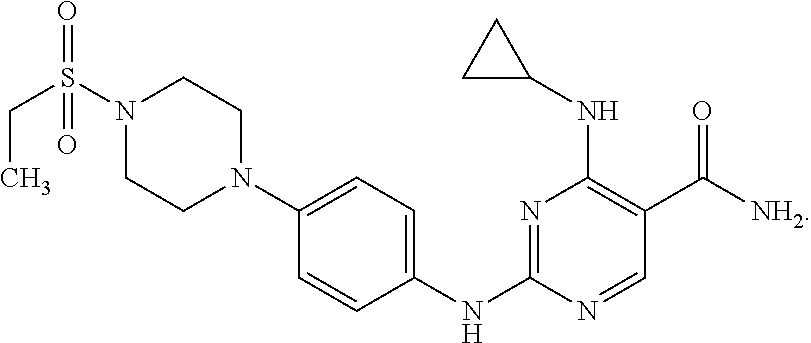
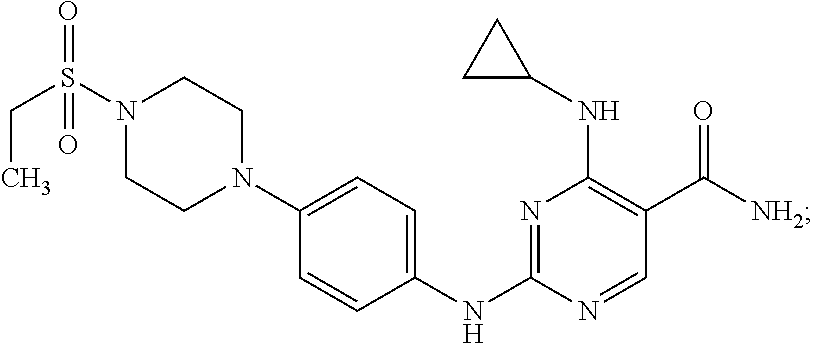
Combination therapy of 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide and fludarabine
InactiveUS20130237493A1Good effectGood treatment effectBiocideCarbohydrate active ingredientsMantle lymphomaCombination therapy
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide, or a pharmaceutically acceptable salt thereof, and fludarabine for the treatment of cell proliferative disorders, such as undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL); mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic Lymphoma (SLL) and multiple myeloma.
Owner:ALEXION PHARMA INC
Human acute B lymphocyte leukemia cell line and application thereof
The invention provides a human acute B lymphocyte leukemia cell line HXEX-ALL1. The human acute B lymphocyte leukemia cell line is preserved in China Center for Type Culture Collection (CCTCC) on October 25, 2017, and the preservation number is CCTCC NO: C2017232. The cell line is a primary line-established human acute B lymphocyte leukemia cell line and is a human acute B lymphocyte leukemia cellline with high tumorigenicity. The cell line provides a novel cell model for researches of relapse and refractory human acute lymphocyte leukemia and researches and development of biomedicines; the cell line can be provided for establishing a humanized acute lymphocyte leukemia animal model, an acute lymphocyte leukemia generation mechanism and relapse and refractory mechanism research platform,an acute lymphocyte leukemia novel drug research and development and screening platform, an acute lymphocyte leukemia immunological and microbiological research platform and the like. The human acuteB lymphocyte leukemia cell line HXEX-ALL1 provided by the invention has a wide prospect and relatively great application value for exploring a human acute lymphocyte leukemia pathogenesis, a relapse and refractory mechanism and drug research, development and application.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN
Method for promoting CD4+T proliferation and activation and suppressing Jurkat T cell by using red cassia tree lectin
InactiveCN102226170APromote proliferation and activationPrevent proliferationBlood/immune system cellsGrowth retardantAnti virus
The invention discloses a method for promoting cell proliferation and activation of peripheral blood CD4+T lymphocyte and suppressing cell proliferation and migration of acute lymphocytic leukemia (ALL) Jurkat T cells by using red cassia tree lectin. The separation and purification of red cassia tree lectin from red cassia tree seeds can be carried out at the temperature of 1-5 DEG C during the entire process, the proliferation and activation of the peripheral blood T lymphocyte CD4 subgroup can be promoted by the red cassia tree lectin through the verifications of an MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide) experiment, a DNA (deoxyribonucleic acid) analysis and an FCM (flow cytometry), and the red cassia tree lectin is CD4+T lymphocyte mitogen more efficient than PHA (Phytohaemagglutinin) and provides a new and effective way for anti-virus infection and vaccine intensifier development; through the verifications of CCK-8 (Cell Counting Kit-8) experiment, DNA analysis, cell apoptosis analysis, ELISA (enzyme-linked immuno sorbent assay) experiments and migration assay, the red cassia tree lectin can obviously suppress the proliferation and migration of the Jurkat T lymphocyte, is a growth inhibitor more efficient for the ALL cells and provides a new and effective supplementary means for treating the hematological malignancies.
Owner:GUANGXI MEDICAL UNIVERSITY
Highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality
The invention belongs to the field of microbial animal cell lines, and relates to a new human acute B lymphocytic leukemia cell strain CHH-1. The in vitro isolate of the mononuclear cell of the marrow of a patient with firstly-diagnosed acute B lymphocytic leukemia undergoes cell primary culture to obtain the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality, the preservation number of the cell strain is CGMCC No.11797, and the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality is named as human acute B lymphocytic leukemia cell strain CHH-1, has a same clone source with patient's leukemia cells, can be infinitely and stably passed in vitro, and has the characteristics of clonality add(11)(q23) genetic abnormality, high tumorigenicity and high aggressiveness. The new human acute B lymphocytic leukemia cell strain CHH-1 provides a new good cell model for researches of human acute lymphocytic leukemia with No.11 chromosome long arm structure abnormality and development of biomedicines, and has wide prospect and great practical values in revelation of the pathogenesis of the human acute lymphocytic leukemia and development of medicines.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
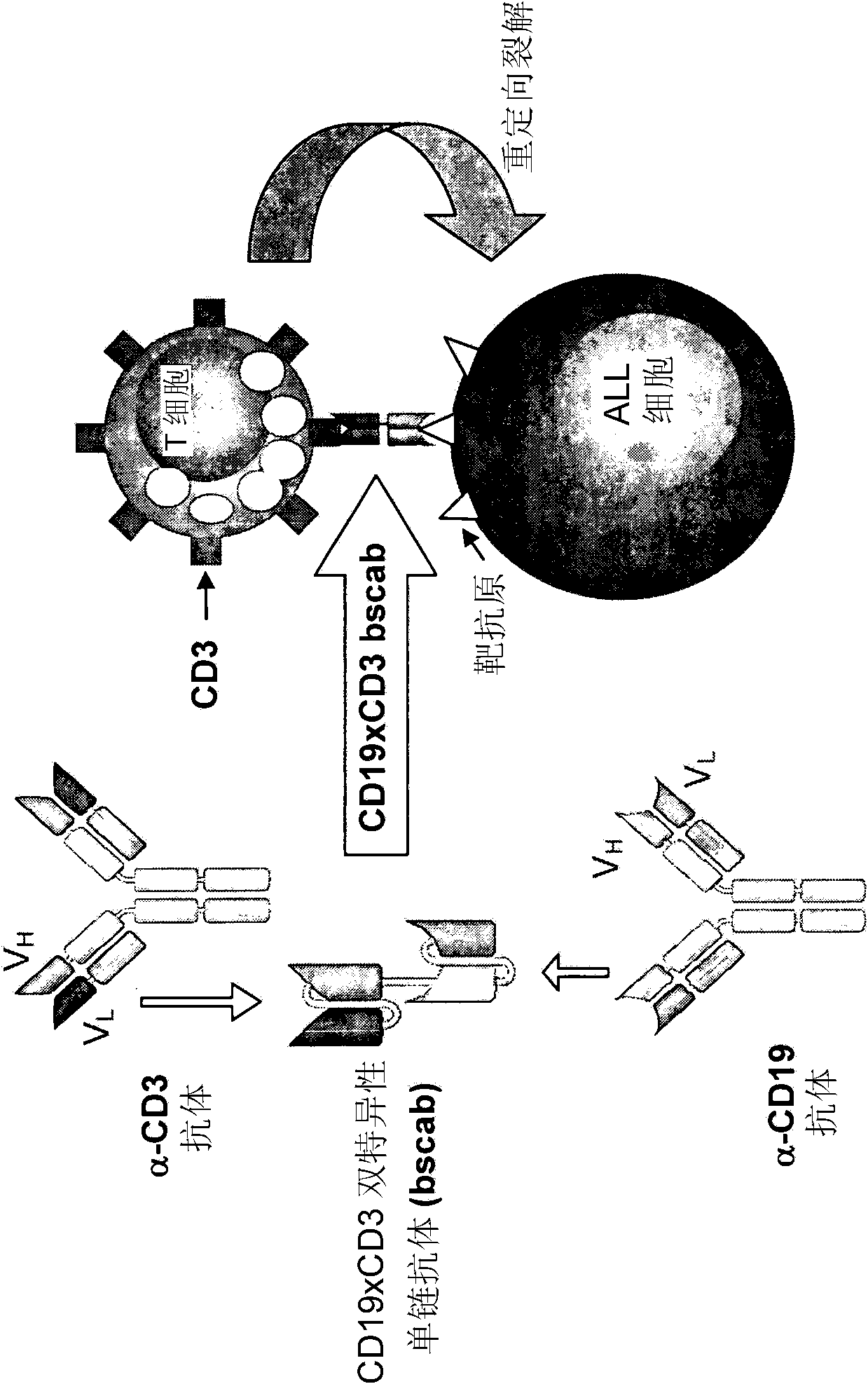
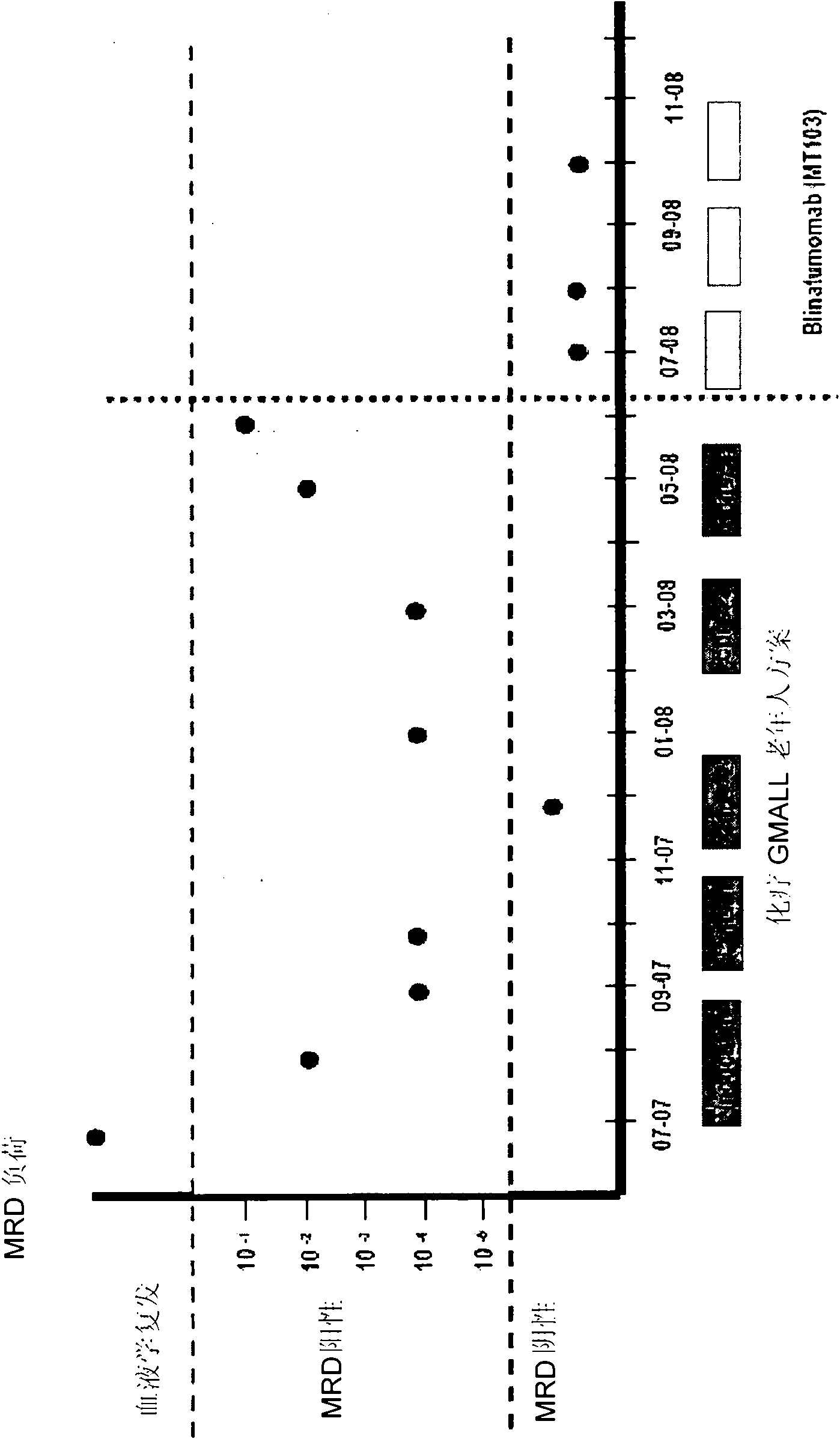
Treatment of acute lymphoblastic leukemia
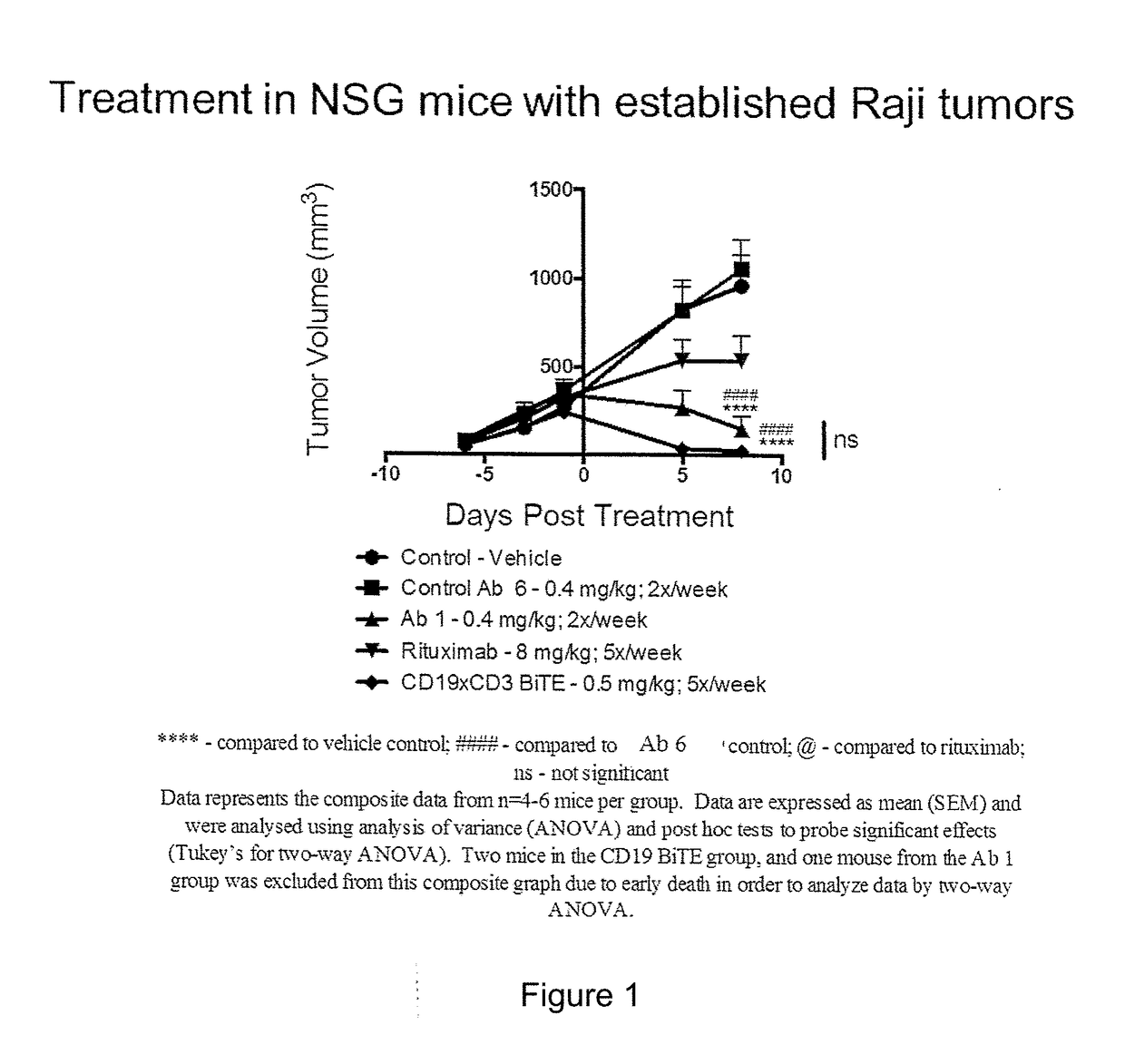
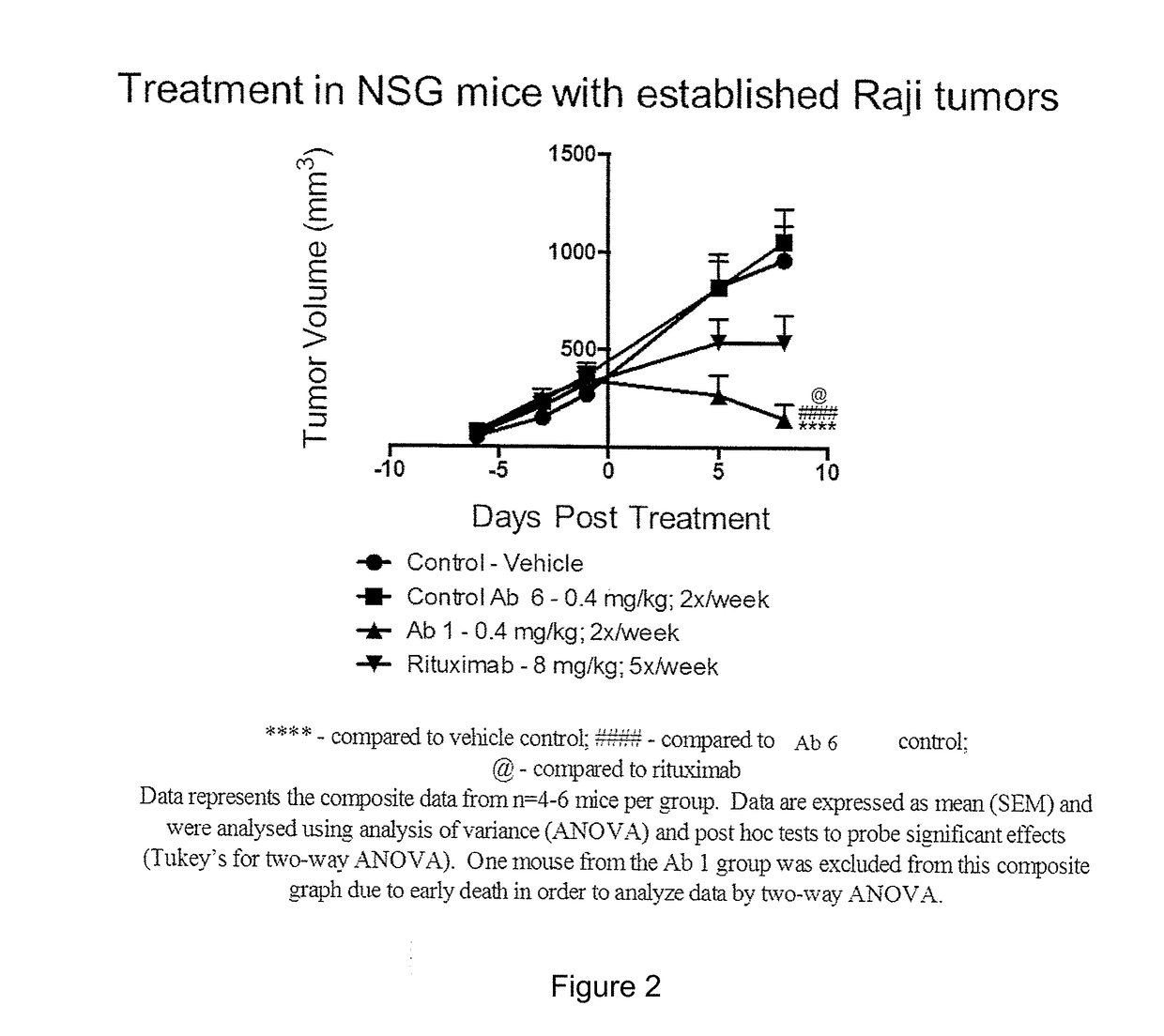
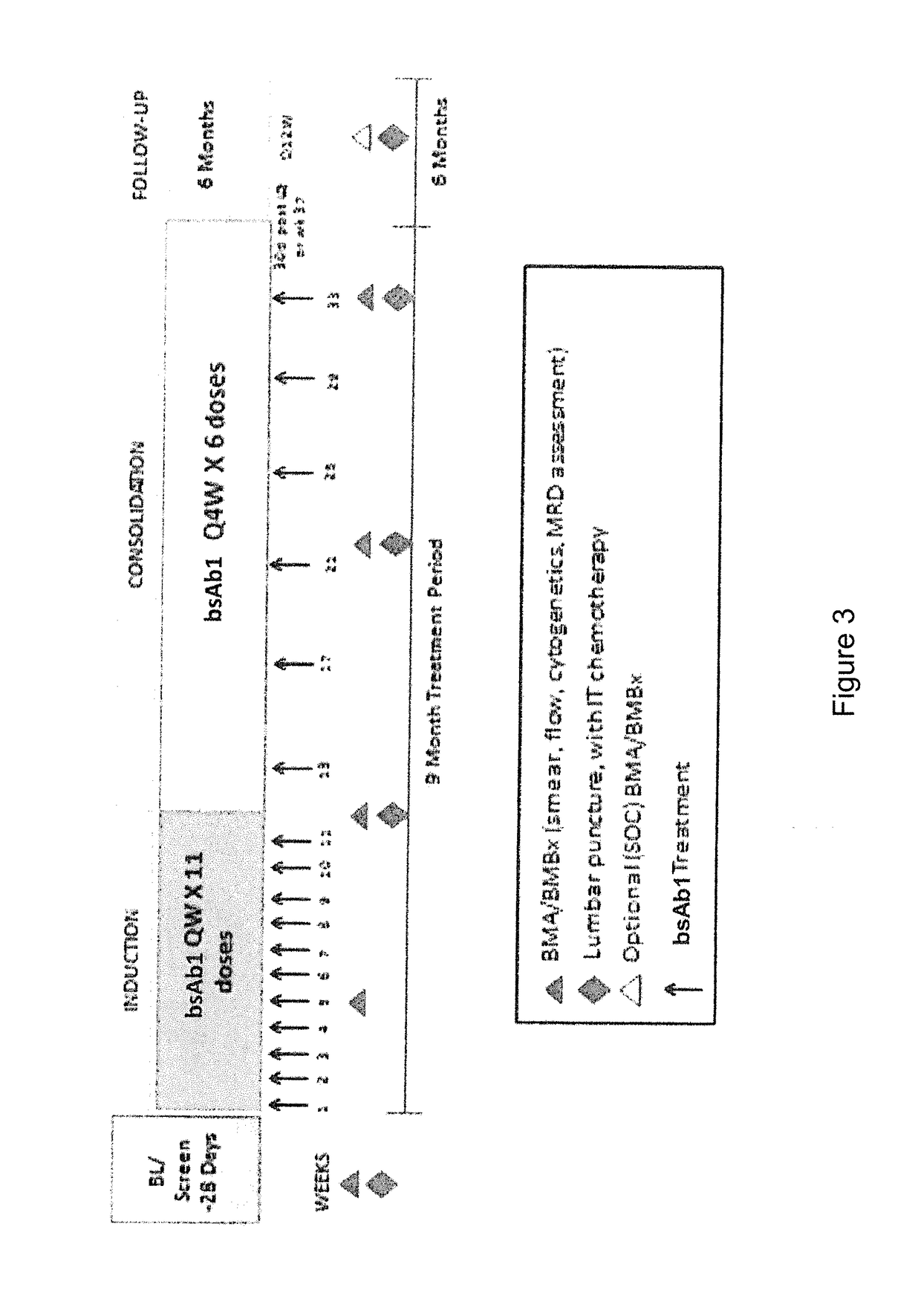
InactiveCN102209728AAmeliorating or palliative treatment pathwaysReduce or even eliminate the risk of recurrenceHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesAcute lymphocytic leukemia
The present invention relates to a method for the treatment, amelioration or elimination of acute lymphoblastic leukemia (ALL), the method comprising the administration of a pharmaceutical composition comprising a CD19xCD3 bispecific single chain antibody construct to an adult patient in the need thereof.
Owner:AMGEN RES (MUNICH) GMBH
Compositions and methods for treating cancers
ActiveUS20150099775A1Inhibit cancer cell proliferationPrevention and reduction of severityCompounds screening/testingBiocideAcute lymphocytic leukemiaOncology
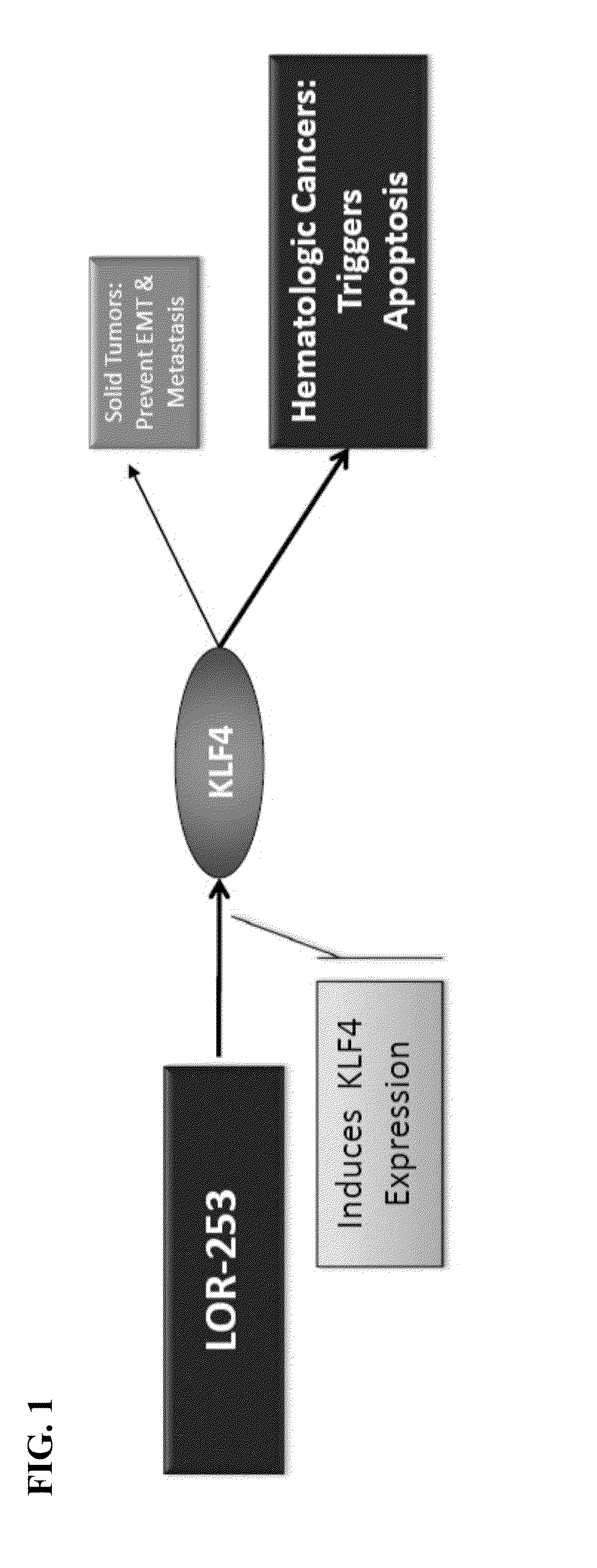
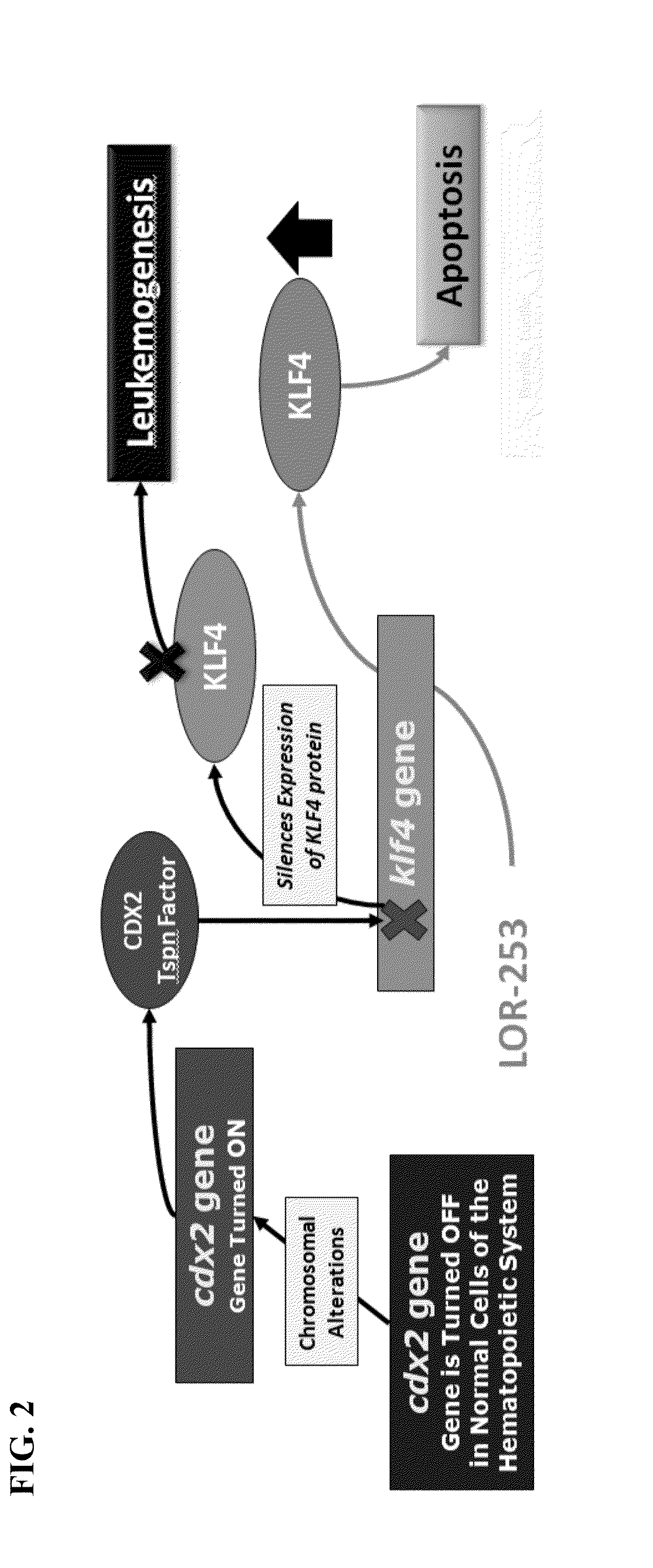
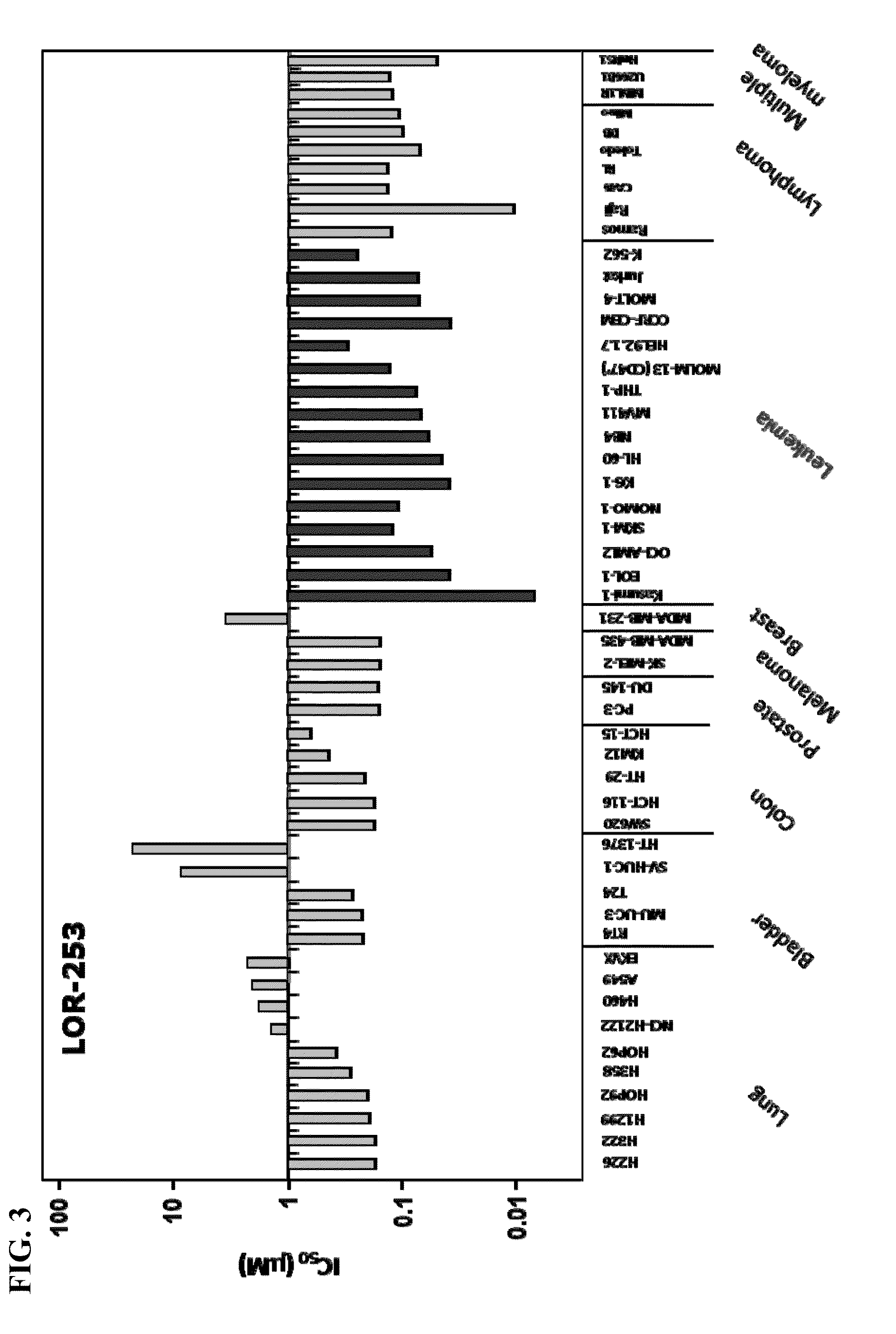
The present invention relates to compositions and methods for treating of cancer. In some embodiments, the invention relates to the use of agents that can modulate a component in the CDX2-KLF4 signaling pathway to treat myelodysplastic syndromes (MDS), acute myelogenous leukemia (AML), acute lymphocytic leukemia (ALL), adult T-cell leukaemia (ATLL), lymphoma, gastric cancer, multiple myeloma, or combinations thereof, or a condition associated with abnormal activity of the CDX2-KLF4 signaling pathway.
Owner:APTOSE BIOSCIENCES INC
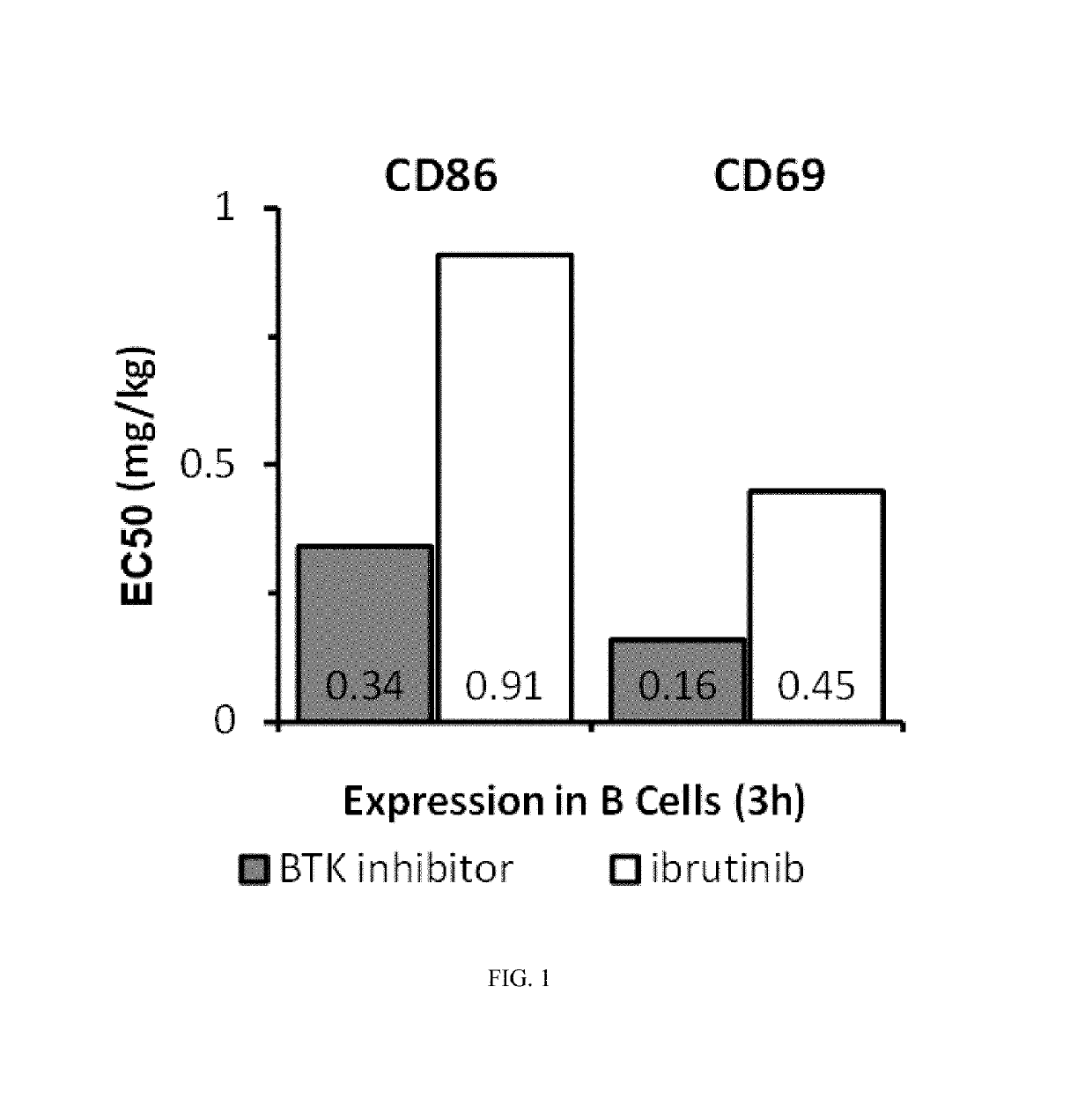
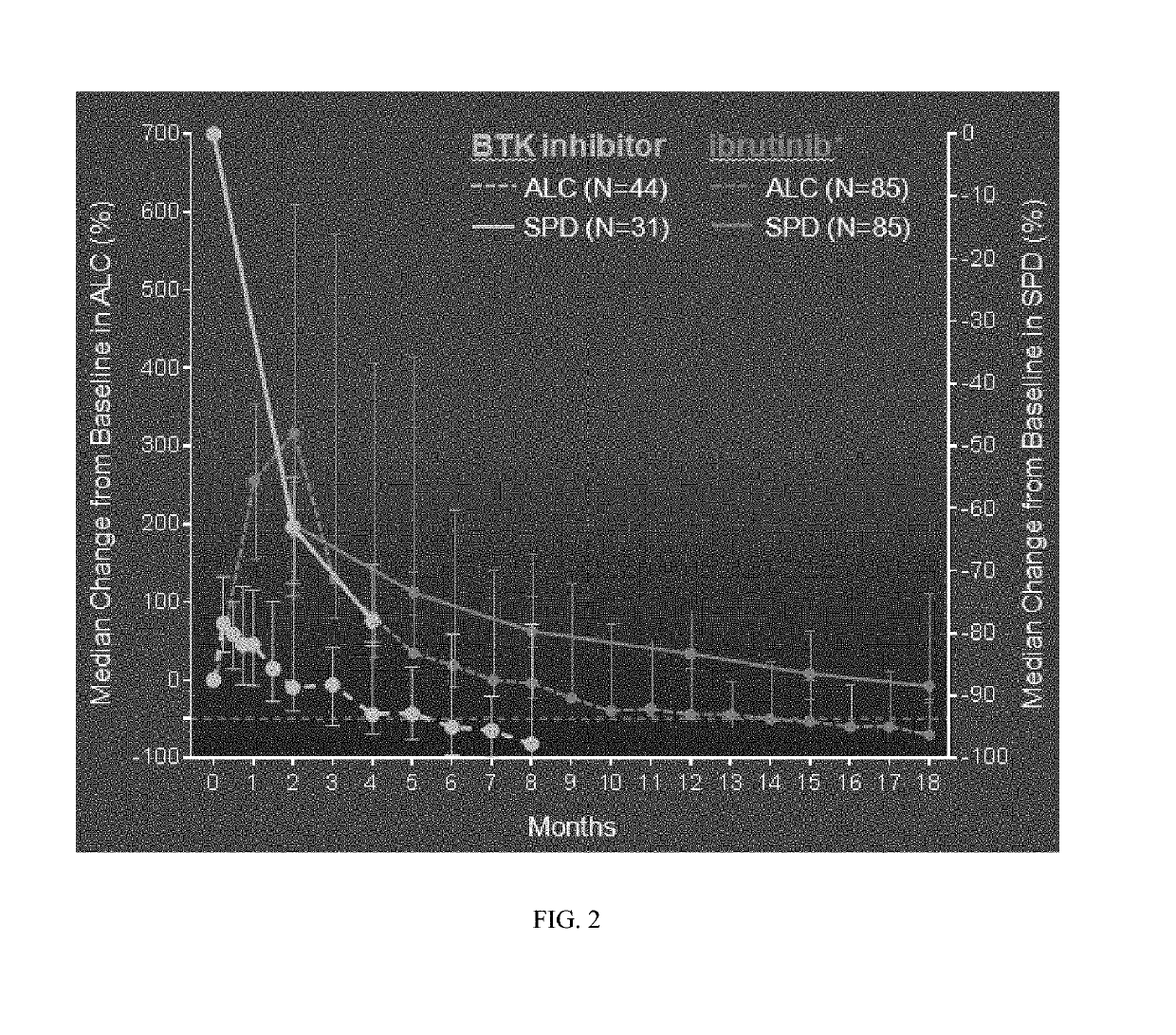
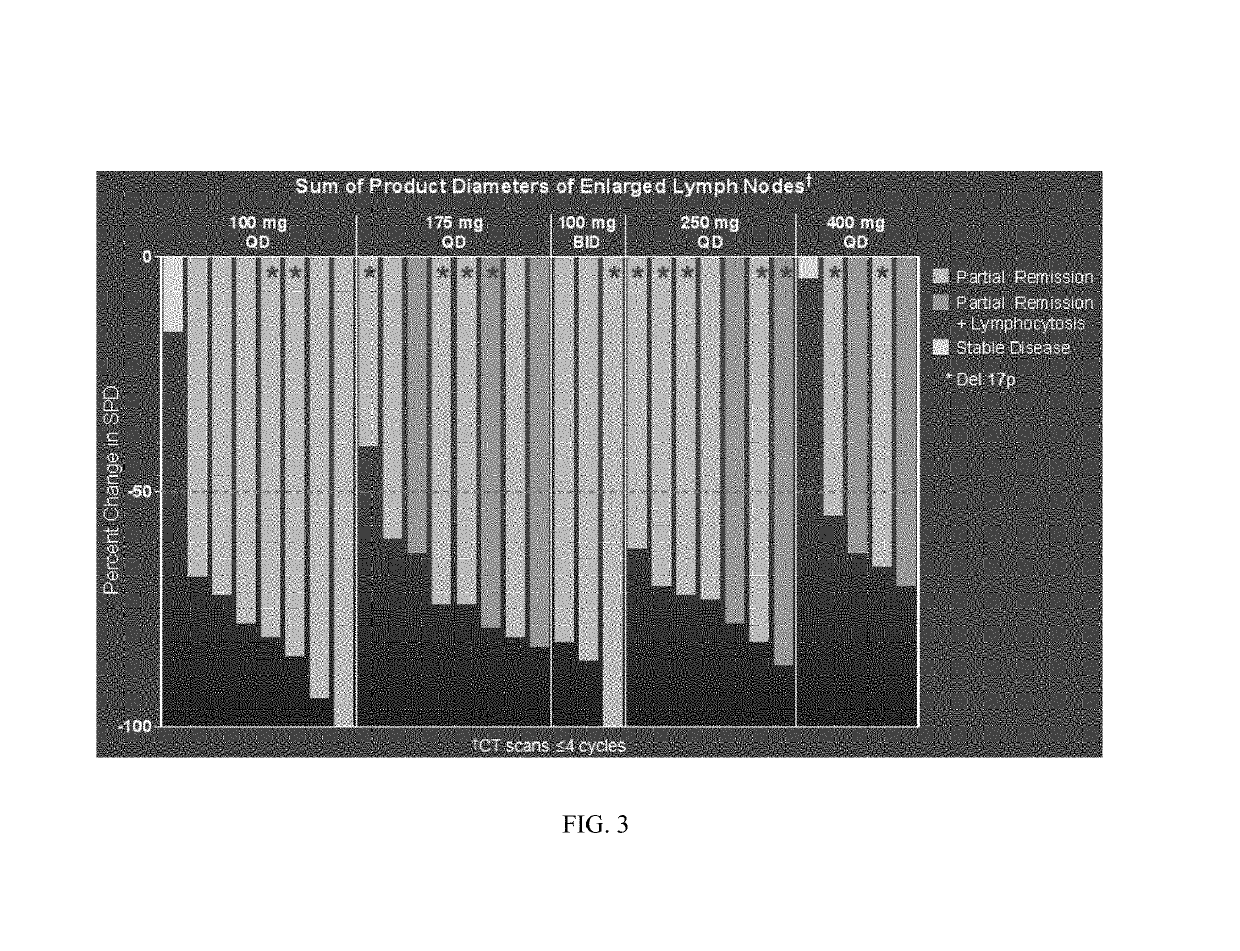
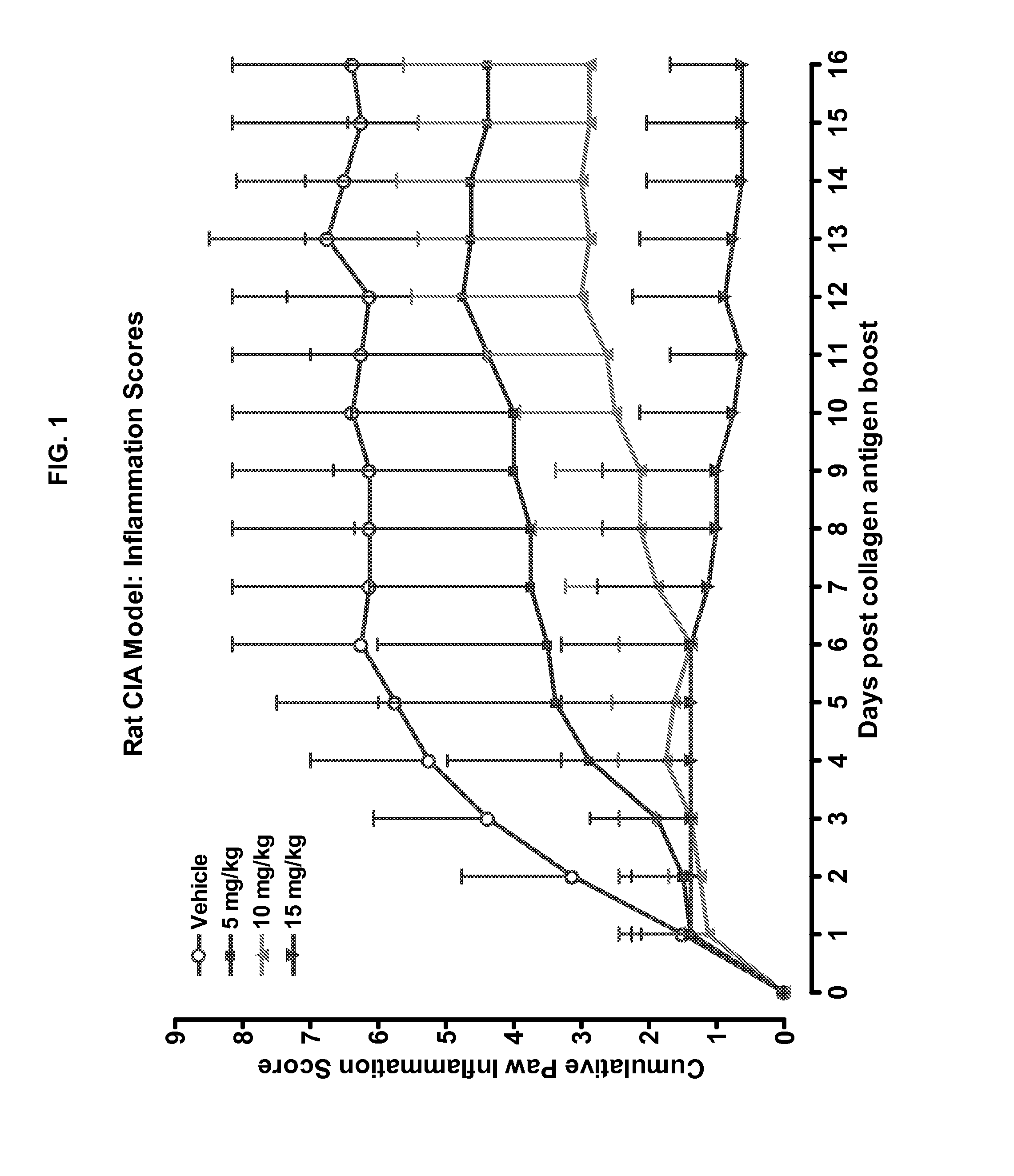
Methods of treating chronic lymphocytic leukemia and small lymphocytic leukemia using a BTK inhibitor
ActiveUS10272083B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsOncologyThrombosis
Owner:ACERTA PHARMA BV
Combination therapy with 4-(3-(2h-1,2,3-triazol-2-yl)phenylamino)-2-((1r,2s)-2-aminocyclohexylamino)pyrimidine-5-carboxamide
InactiveUS20130244963A1Good treatment effectReduce the amount requiredBiocideCarbohydrate active ingredientsAnaphylaxisSystemic lupus erythematosus
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing a SYK inhibitor, or a pharmaceutically acceptable salt thereof, and a antineoplastic or antiinflammatory agent for the treatment of inflammatory, autoimmune and cell proliferative diseases, such as allergic reaction, transplant rejection, rheumatoid arthritis (RA), lupus, multiple sclerosis (MS) or psoriasis undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL) (including diffuse large B cell lymphoma (DLBCL)), mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic (SLL), Lymphoma, multiple myeloma, asthma, vasculitis, Idiopathic thrombocytopenic purpura (ITP), Heparin Induced Thrombocytopenia (HIT) and hemolytic anemia.
Owner:ALEXION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com