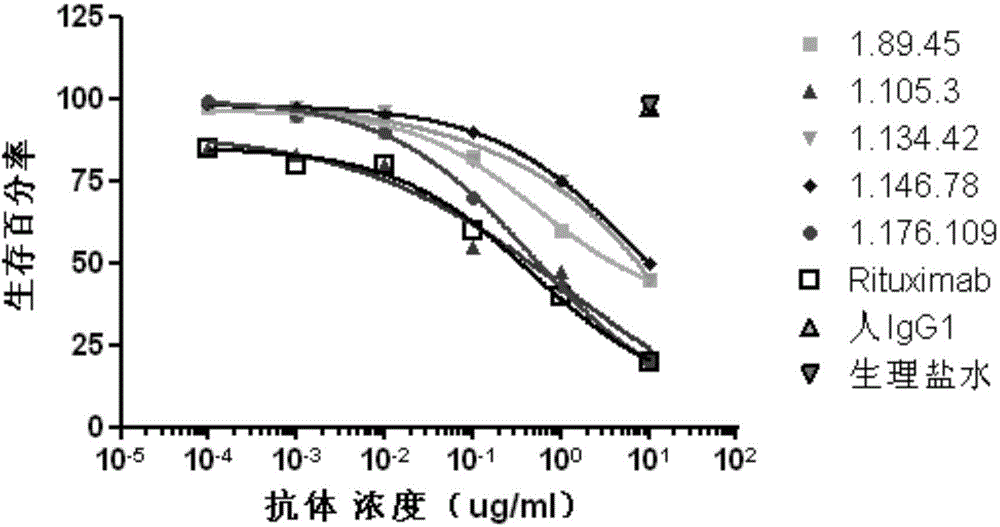
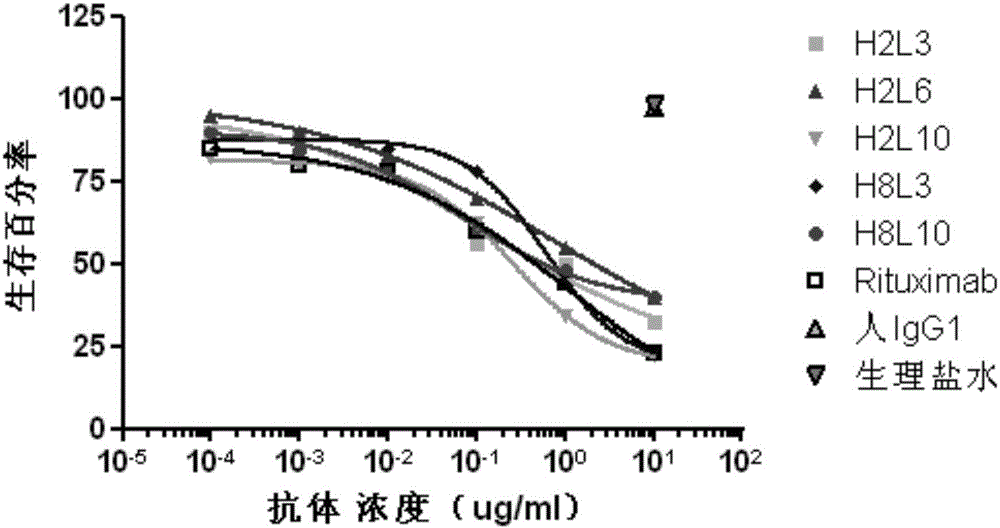
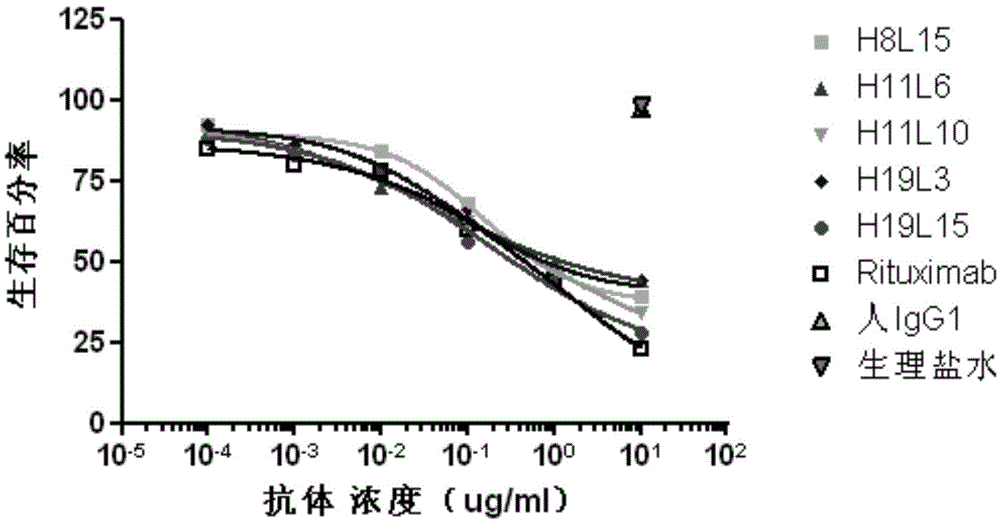
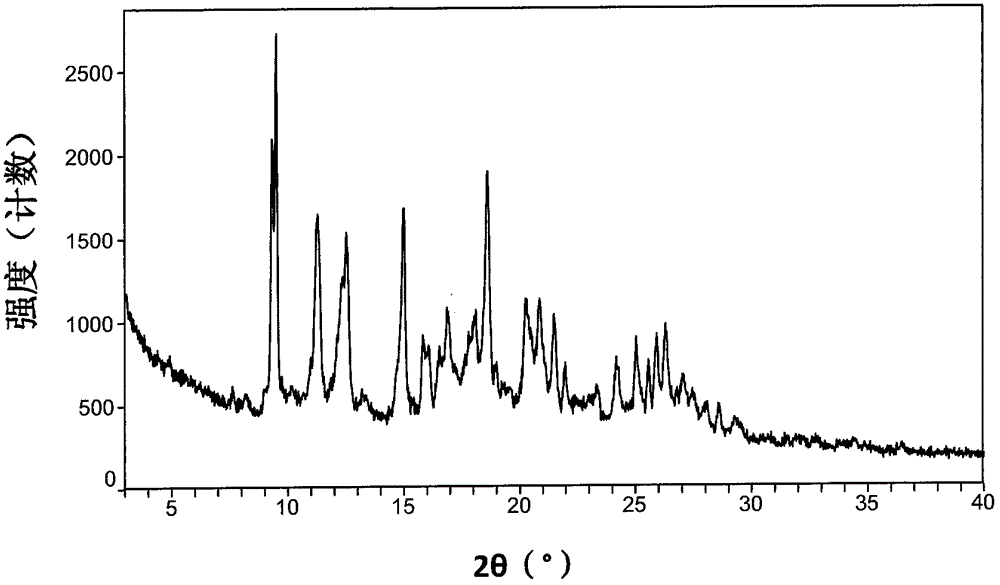
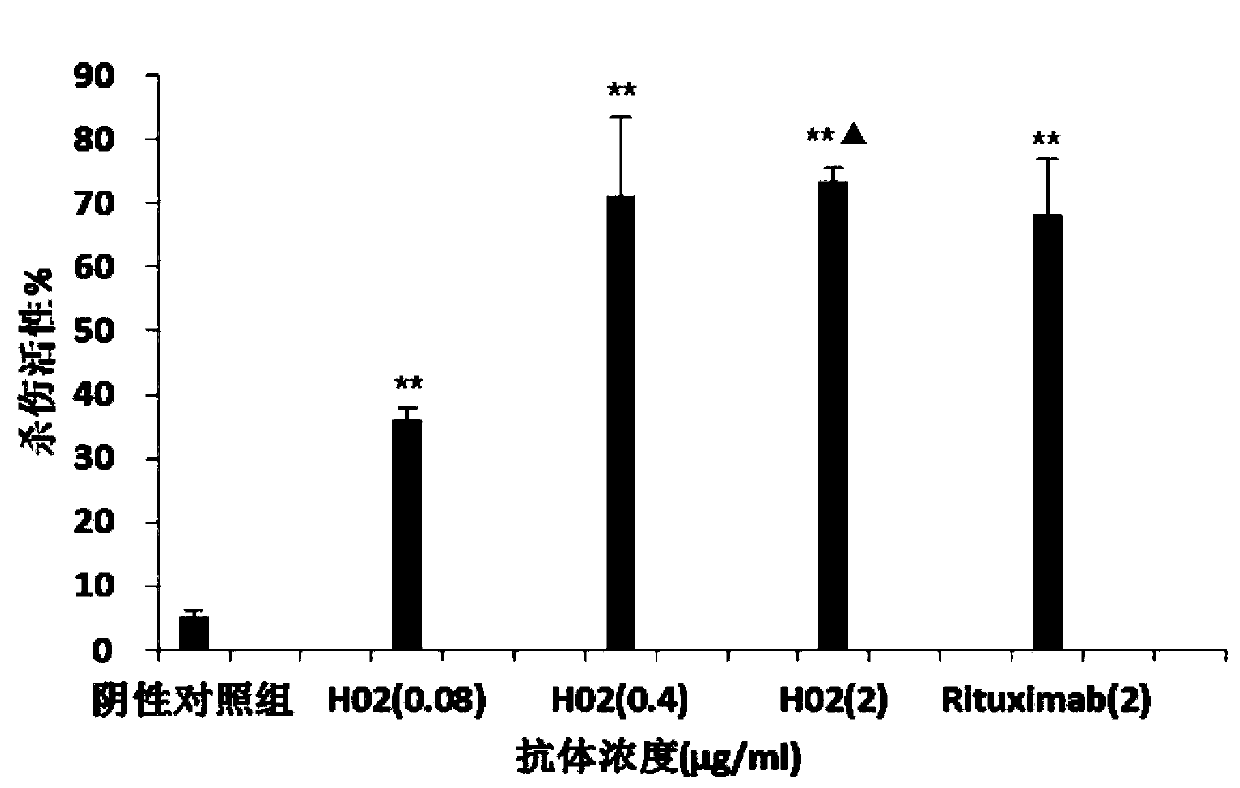
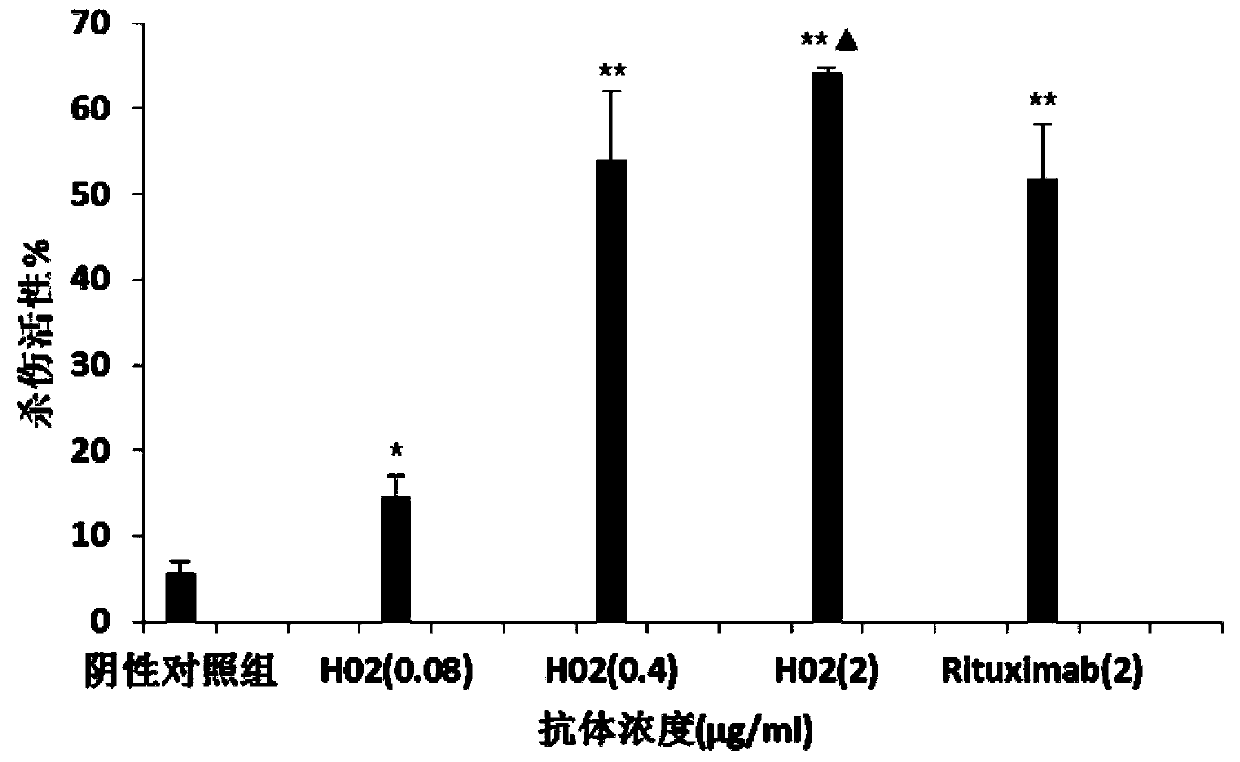
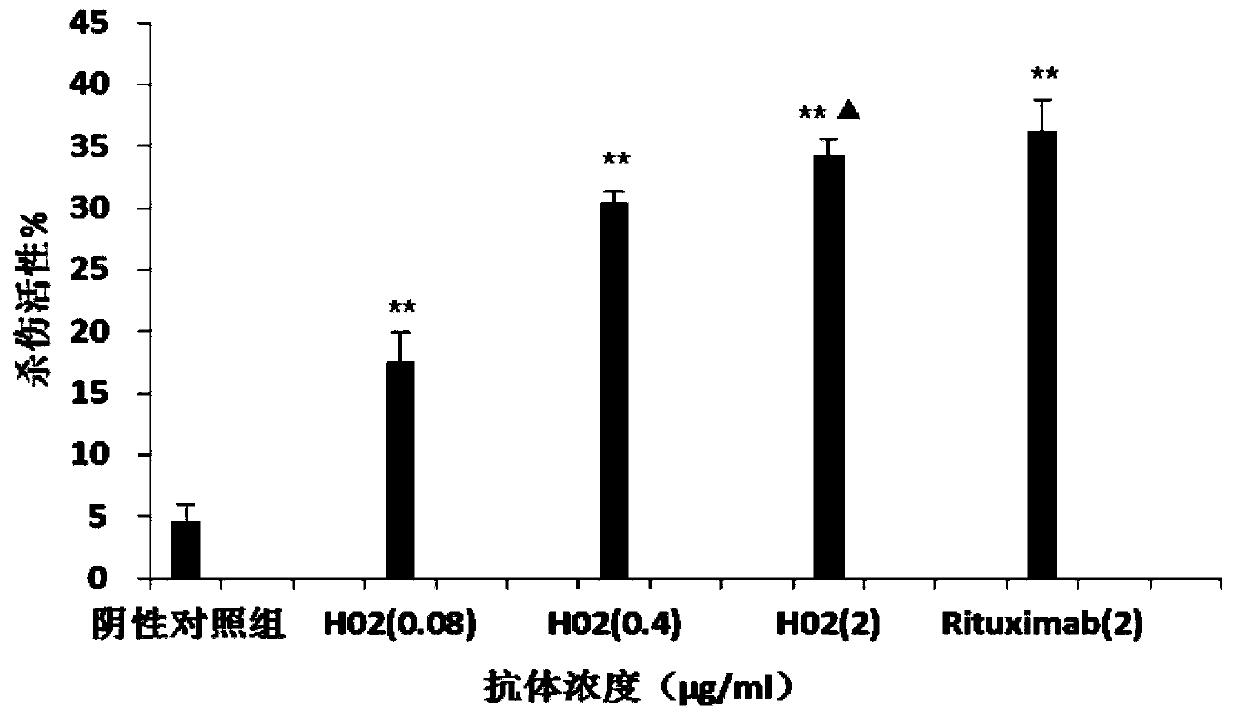
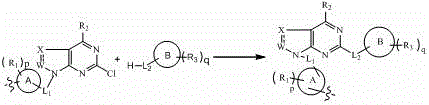Patents
Literature
47 results about "NH lymphoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-Hodgkin lymphoma (NHL) is a group of blood cancers that includes all types of lymphoma except Hodgkin's lymphomas. Symptoms include enlarged lymph nodes, fever, night sweats, weight loss and tiredness. Other symptoms may include bone pain, chest pain or itchiness.
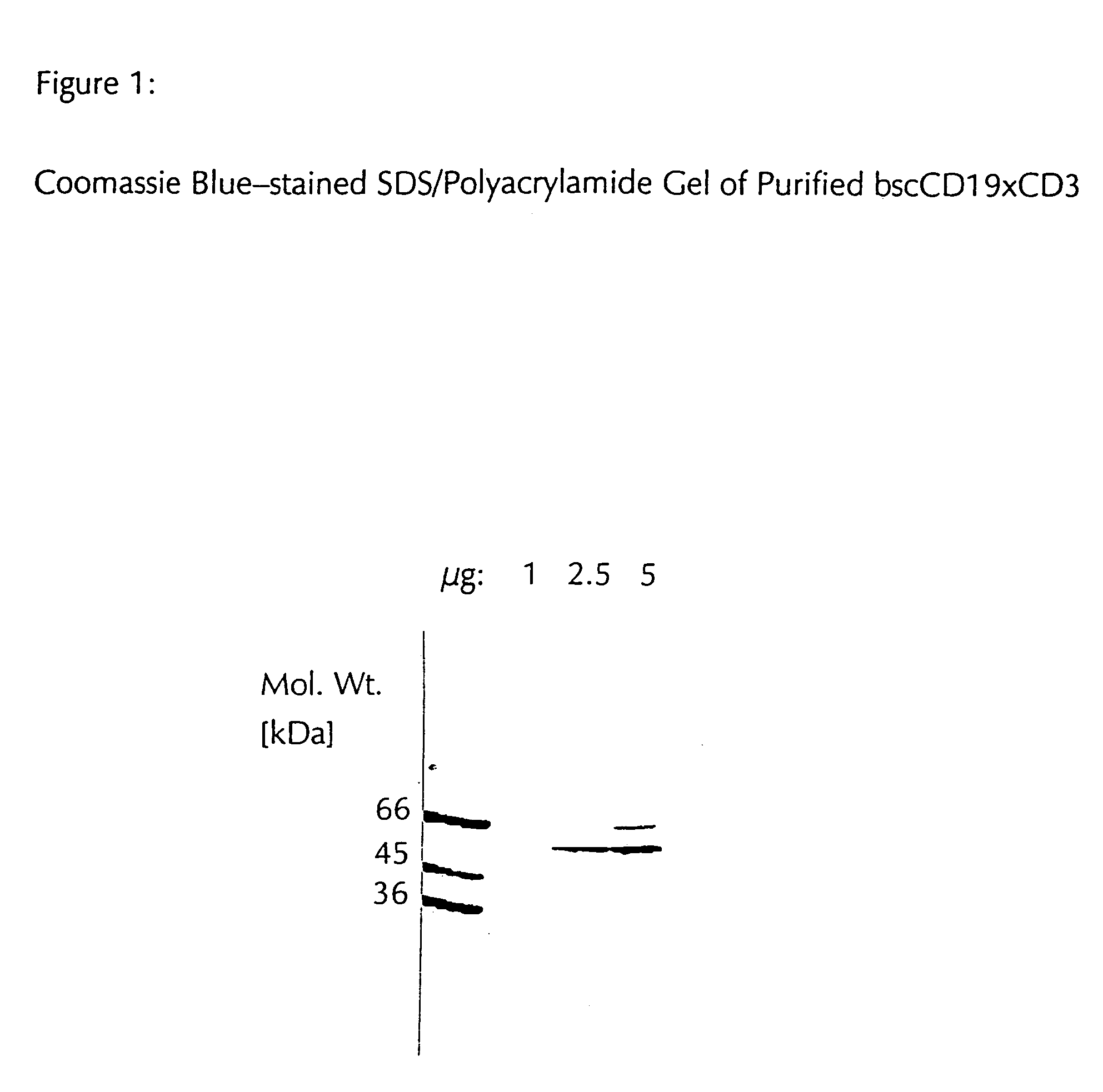
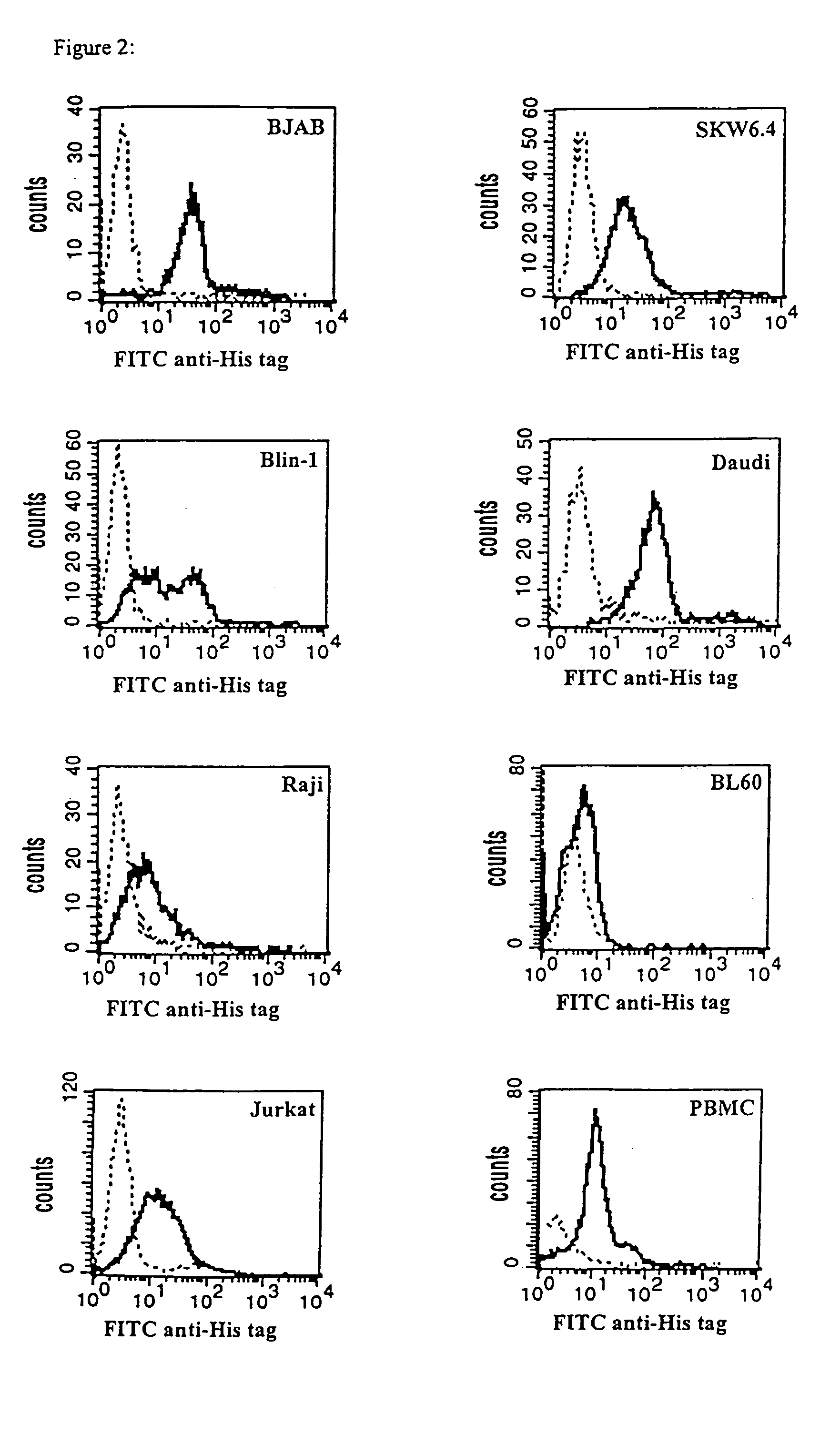
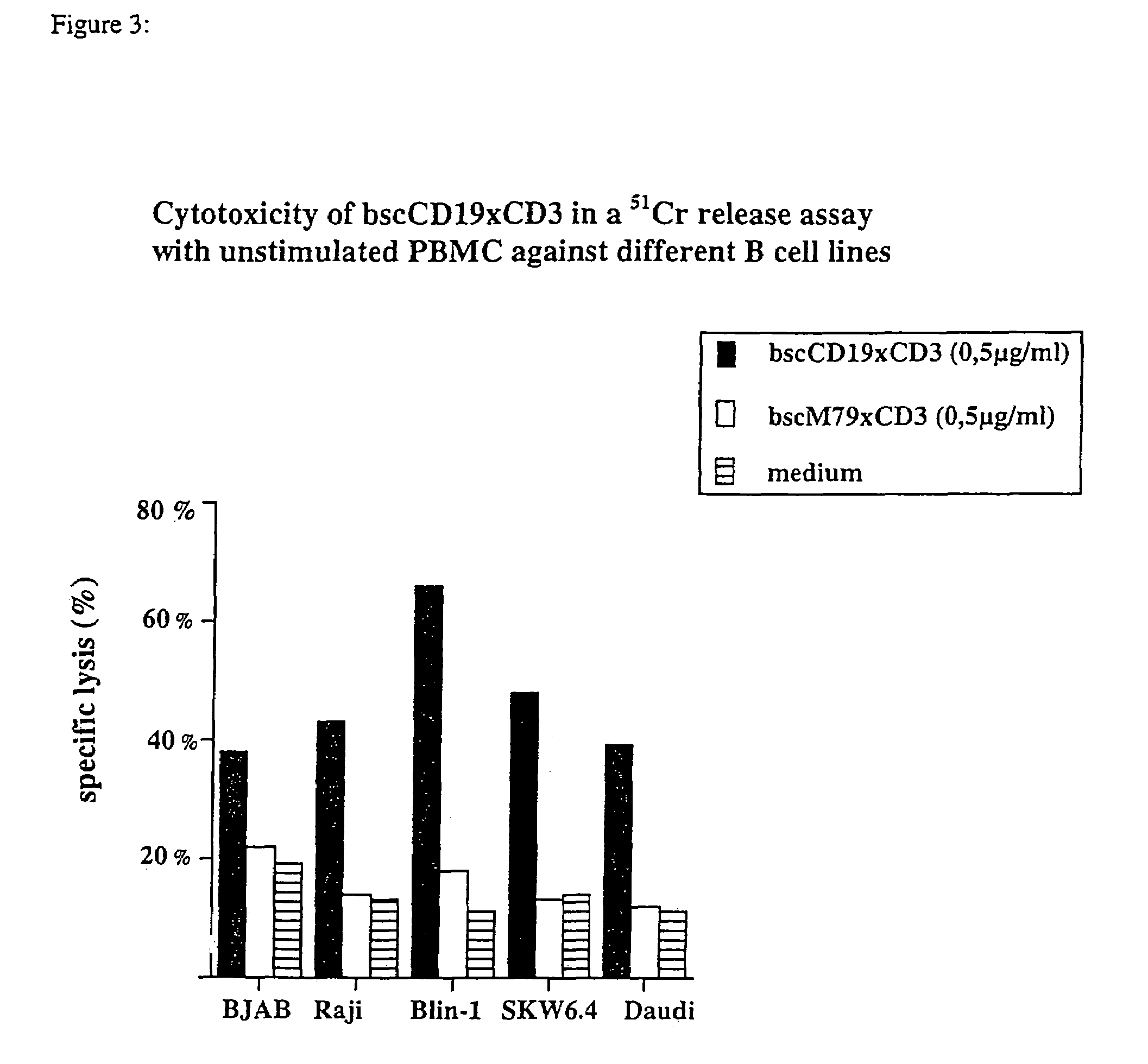
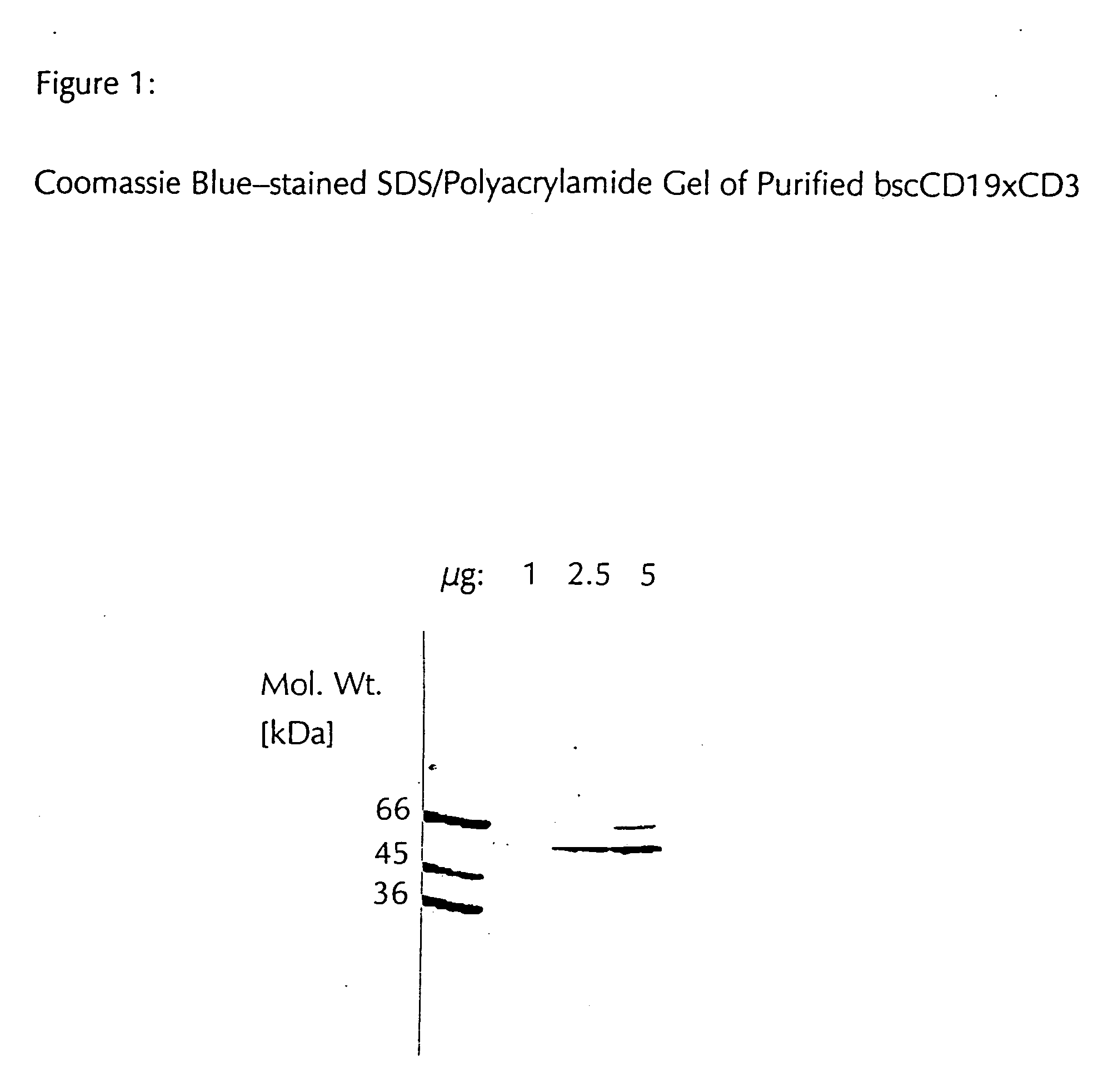
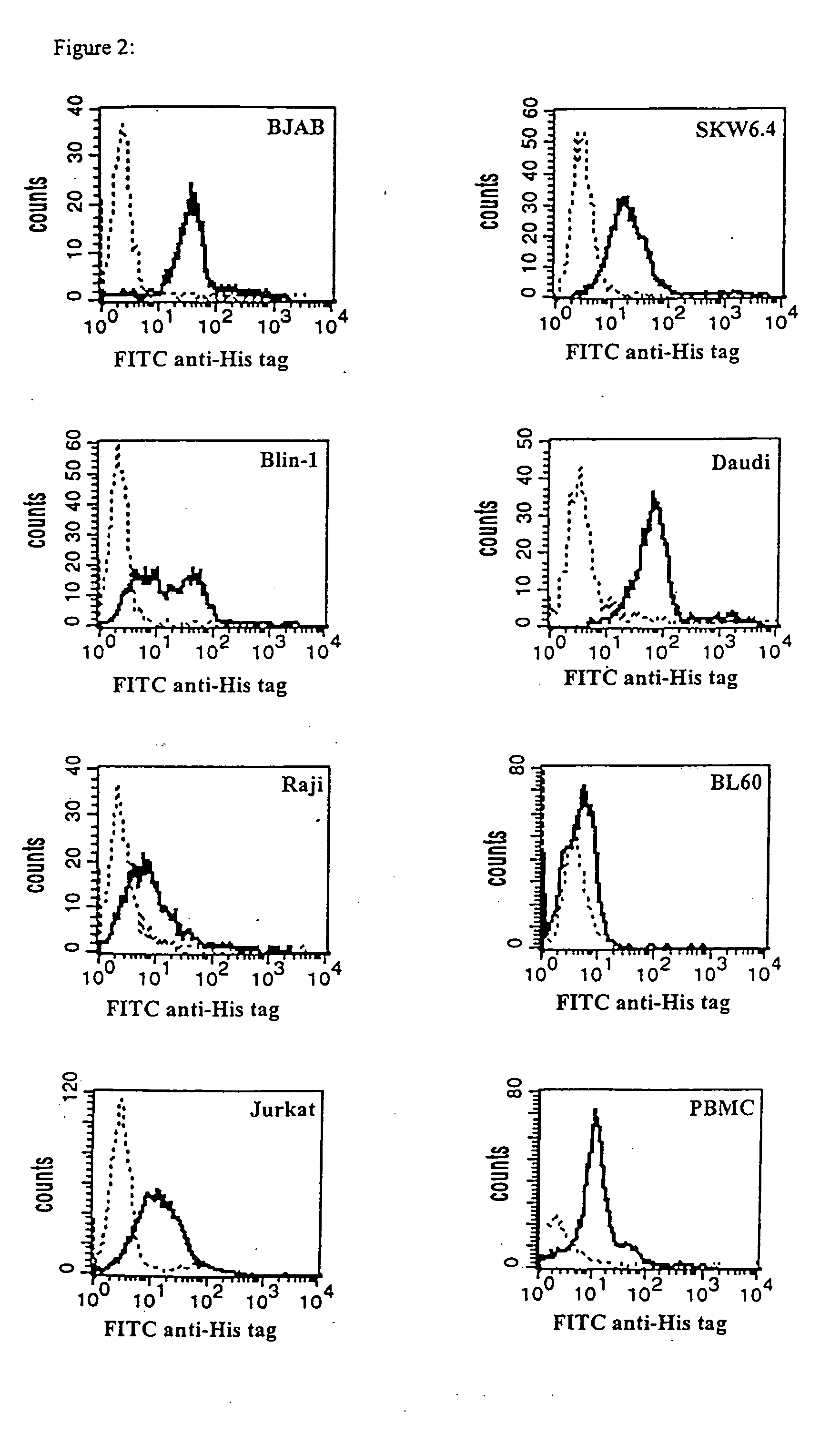
CD19xCD3 specific polypeptides and uses thereof
Described are novel single-chain multifunctional polypeptides comprising at least two binding sites specific for the CD19 and CD3 antigen, respectively. Further provided are polypeptides, wherein the above-described polypeptide comprises at least one further domain, preferably of pre-determined function. Furthermore, polynucleotides encoding said polypeptides as well as to vectors comprising said polynucleotides and host cells transformed therewith and their use in the production of said polypeptides are described. In addition, compositions, preferably pharmaceutical and diagnostic compositions are provided comprising any of the afore-described polypeptides, polynucleotides or vectors. Described is also the use of the afore-mentioned polypeptides, polynucleotides and vectors for the preparation of pharmaceutical compositions for immunotherapy, preferably against B-cell malignancies such as non-Hodgkin lymphoma.
Owner:AMGEN RES (MUNICH) GMBH
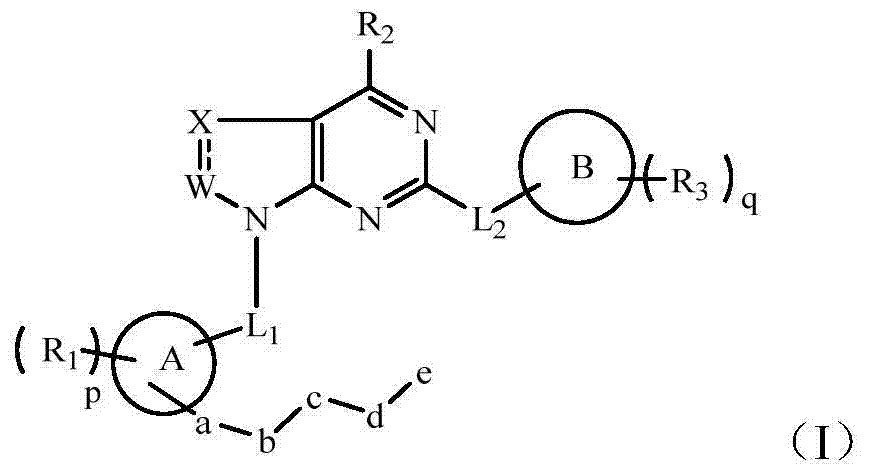
Hetero-aromatic ring and derivative type tyrosine kinase inhibitor thereof
InactiveCN103664878ABTK kinase inhibition is goodLittle side effectsOrganic active ingredientsOrganic chemistryImmunologic disordersDisease
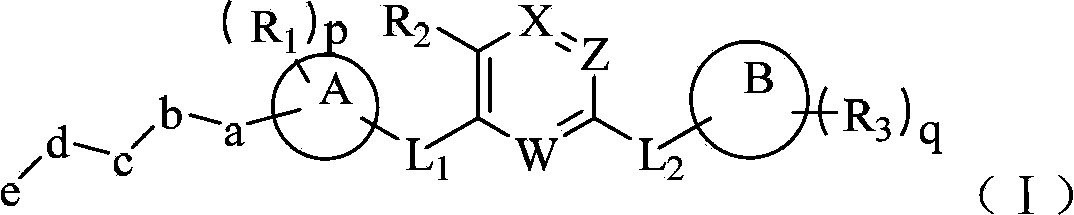
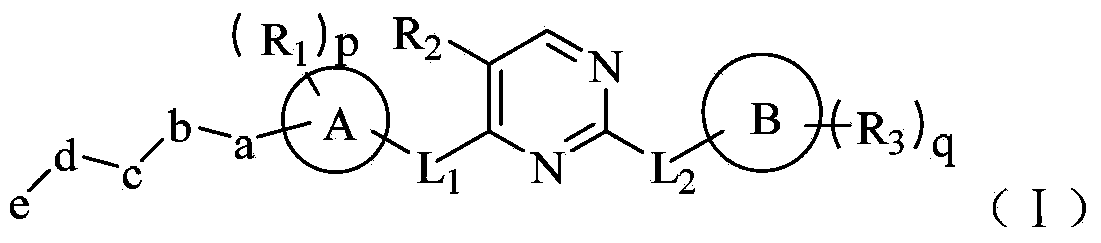
The invention belongs to the technical field of medicaments, and in particular relates to a hetero-aromatic ring shown by a general formula (I) as well as a derivative type tyrosine kinase inhibitor, a pharmaceutically acceptable salt or a stereoisomer thereof, wherein X, Z, W, R1, R2, R3, L1, L2, a, b, c, d, e, p, q, A and B are as defined in the specification. The invention also relates to preparation methods of these compounds, a pharmaceutical preparation containing these compounds, and important functions of these compounds in preparation of medicaments for treating B cell related leukemia (such as B cell chronic lymphocytic carcinoma and non-hodgkin lymphoma) and autoimmune diseases (such as rheumatoid arthritis, systemic lupus erythematosus and the like).
Owner:KBP BIOSCIENCES CO LTD
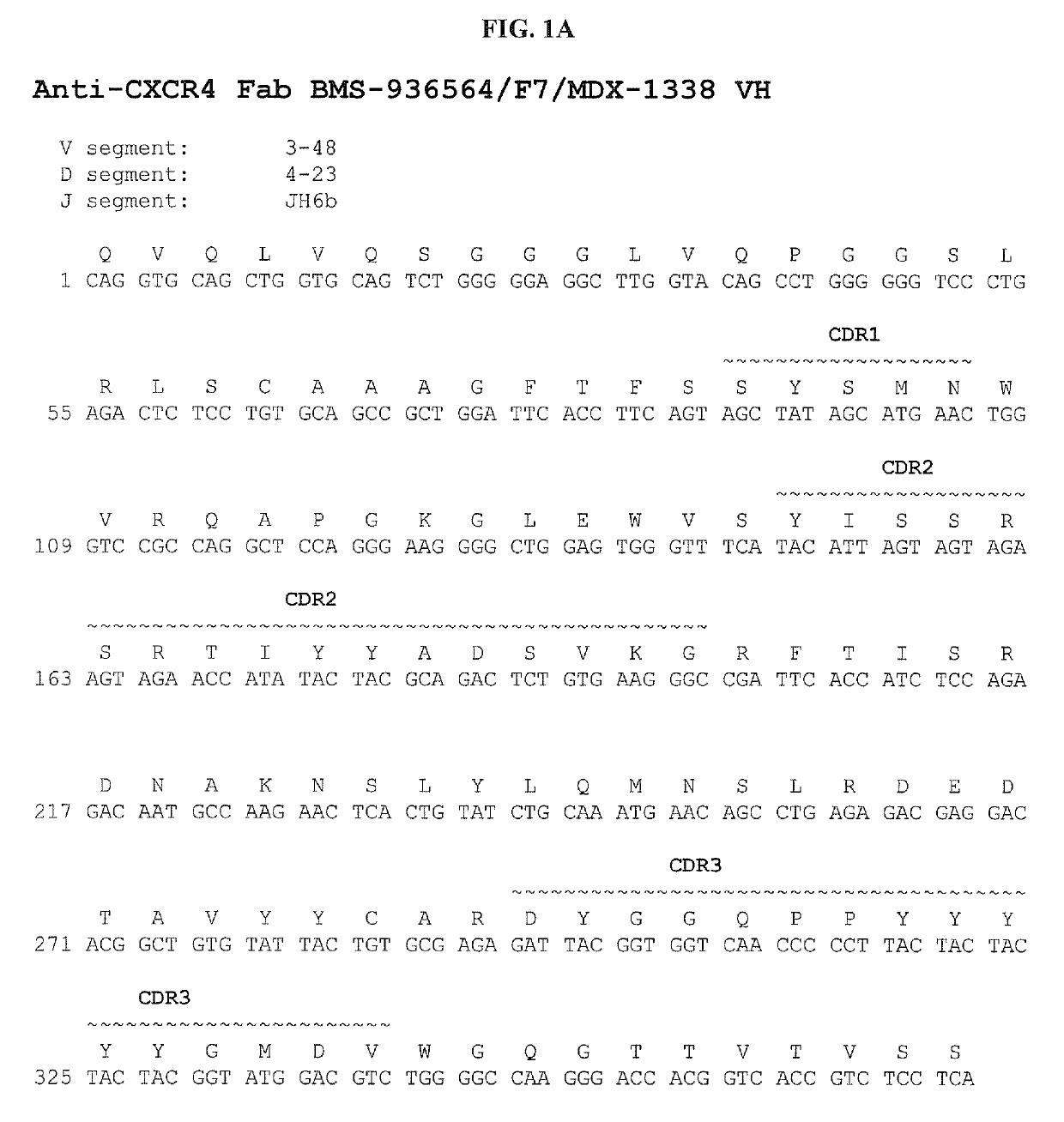
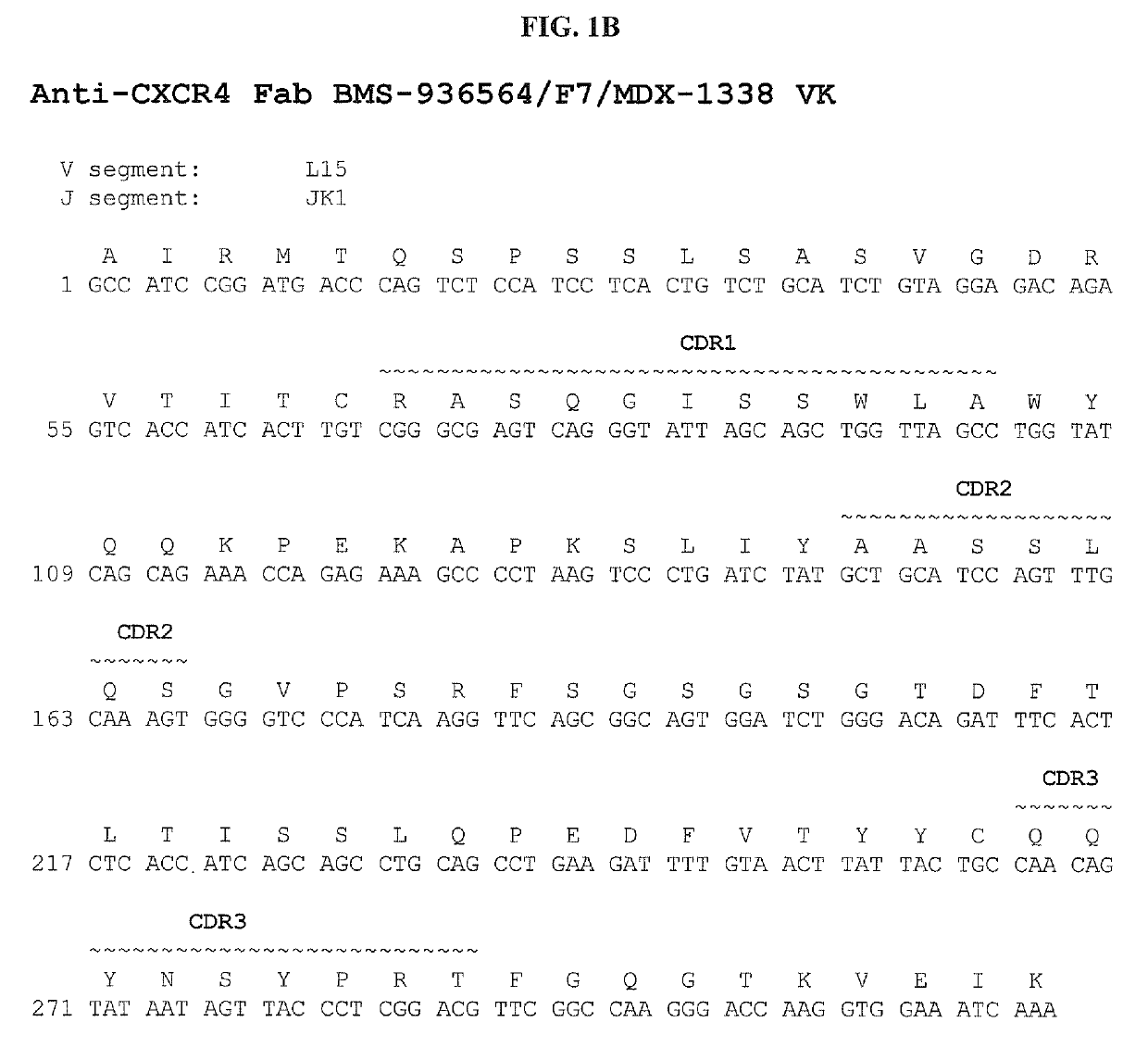
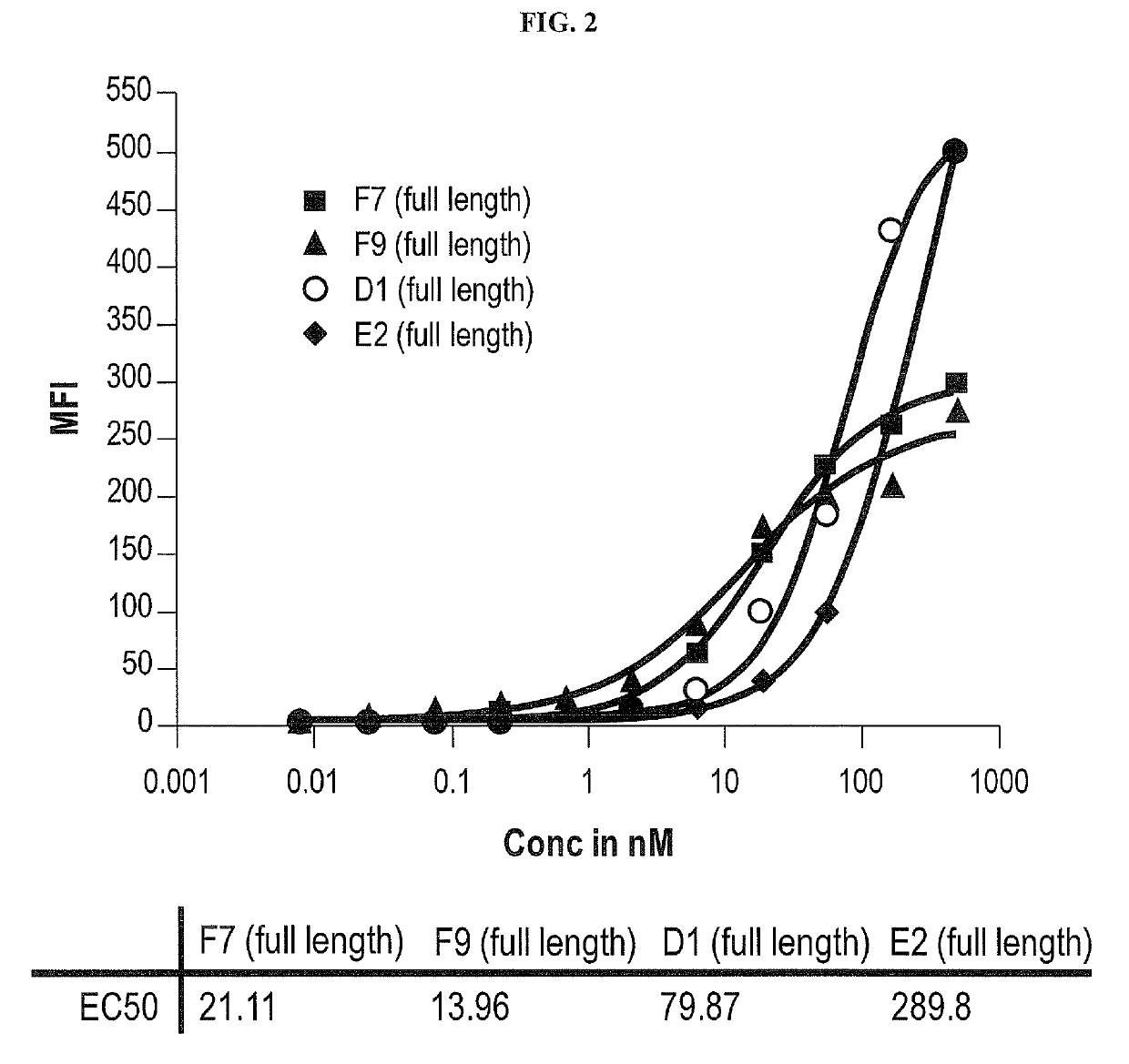
Treatment of hematologic malignancies with an Anti-cxcr4 antibody
ActiveUS20140322208A1Inhibit capillary tube formationBoron compound active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Monoclonal antibody
The present disclosure provides human monoclonal antibodies that bind specifically to CXCR4 with high affinity. This disclosure also provides a method for treating a subject afflicted with a CXCR4-expressing cancer, in particular a hematological malignancy such as multiple myeloma, acute myeloid leukemia, or non-Hodgkin's lymphoma, comprising administering to the subject a therapeutically effective amount of a pharmaceutical composition comprising an anti-CXCR4 antibody of the disclosure. The disclosure further provides a kit for treating a cancer in a subject comprising a dose of an anti-CXCR4 antibody and instructions for using the anti-CXCR4 antibody in the therapeutic methods of the disclosure.
Owner:BRISTOL MYERS SQUIBB CO
Novel CD19xCD3 specific polypeptides and uses thereof
Described are novel single-chain multifunctional polypeptides comprising at least two binding sites specific for the CD19 and CD3 antigen, respectively. Further provided are polypeptides, wherein the above-described polypeptide comprises at least one further domain, preferably of pre-determined function. Furthermore, polynucleotides encoding said polypeptides as well as to vectors comprising said polynucleotides and host cells transformed therewith and their use in the production of said polypeptides are described. In addition, compositions, preferably pharmaceutical and diagnostic compositions are provided comprising any of the afore-described polypeptides, polynucleotides or vectors. Described is also the use of the afore-mentioned polypeptides, polynucleotides and vectors for the preparation of pharmaceutical compositions for immunotherapy, preferably against B-cell malignancies such as non-Hodgkin lymphoma.
Owner:AMGEN RES (MUNICH) GMBH
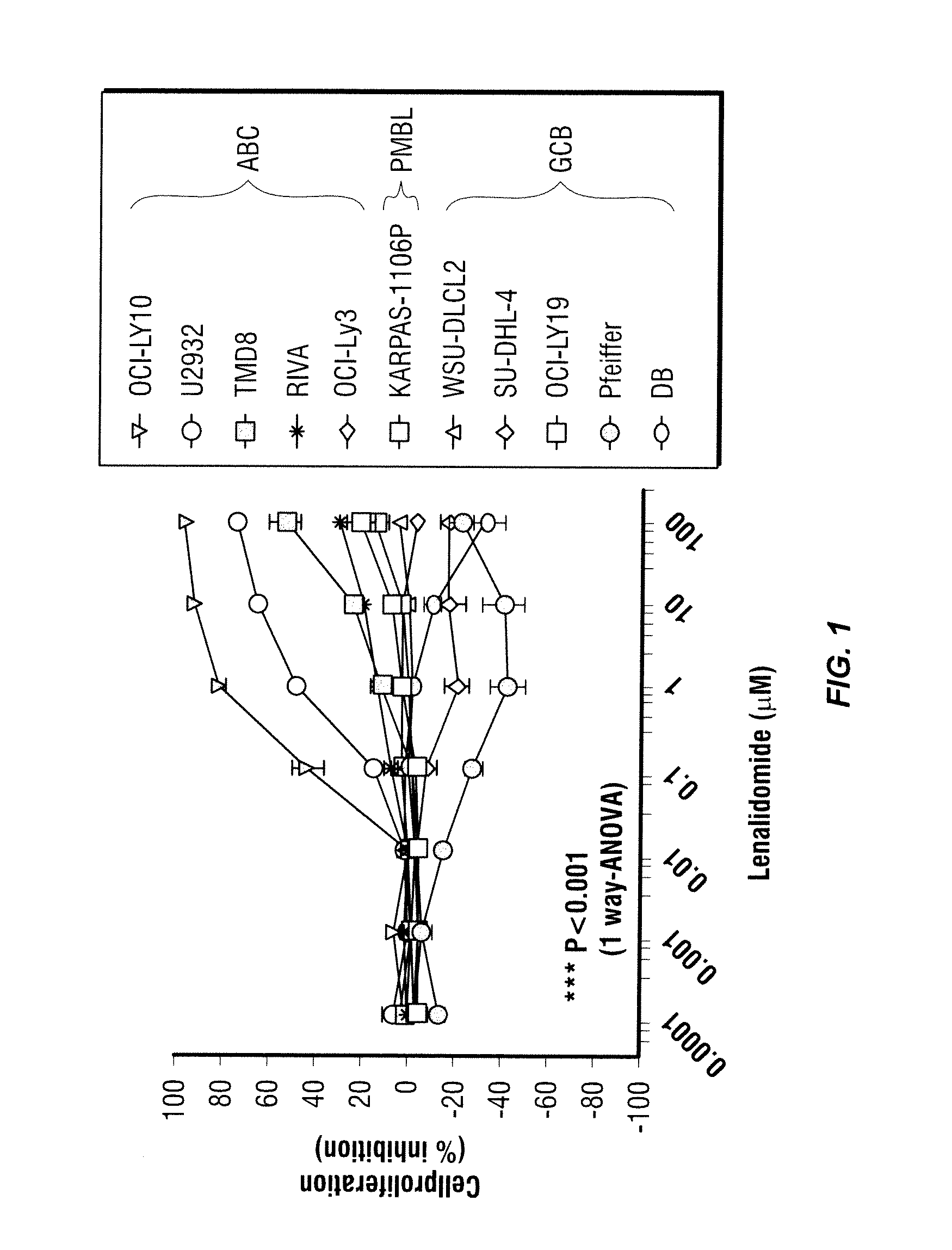
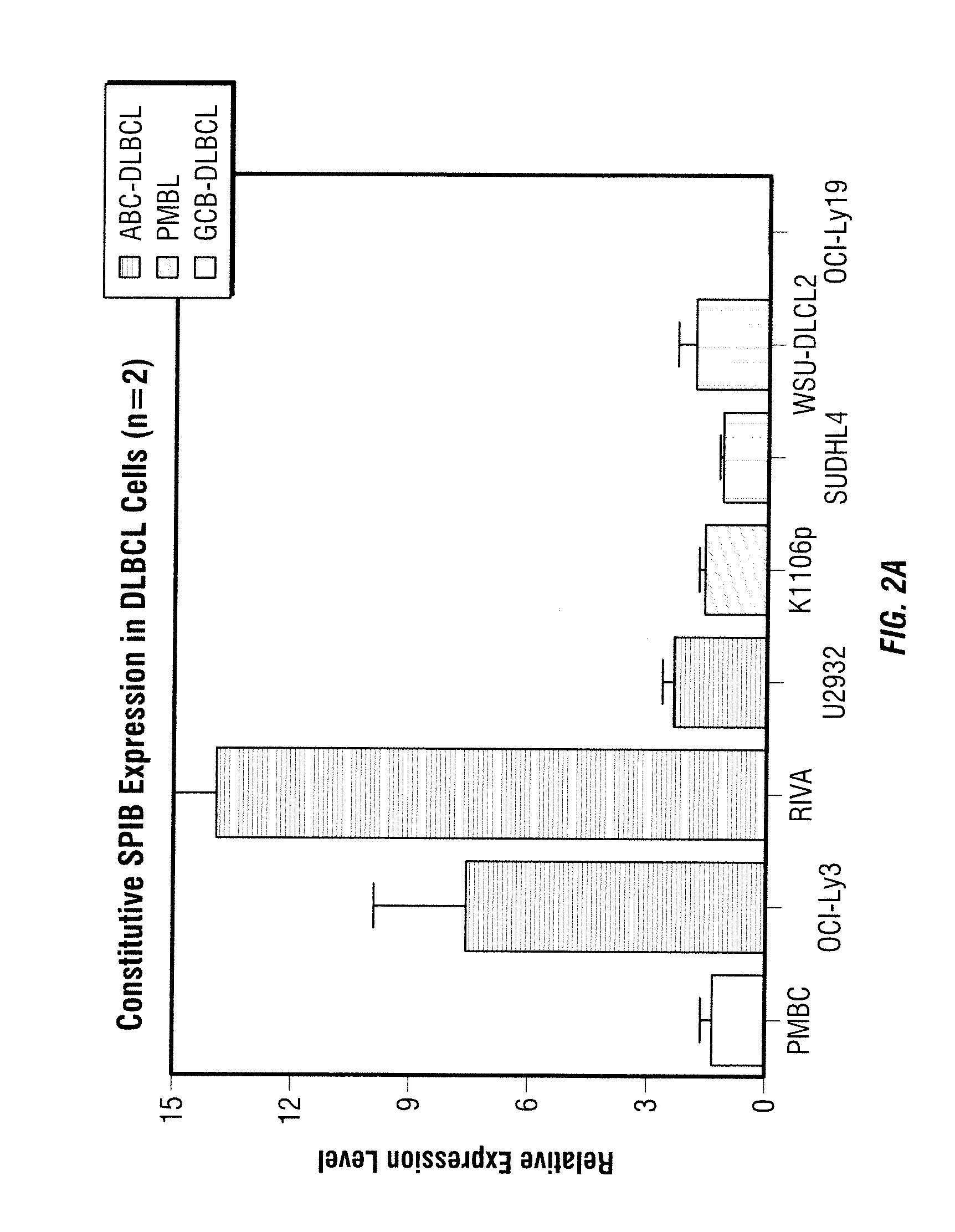
Methods for the treatment of non-hodgkin's lymphomas using lenalidomide, and gene and protein biomarkers as a predictor
InactiveUS20110223157A1Monitoring effectiveness of treatmentBiocideMicrobiological testing/measurementADAMTS ProteinsFhit gene
Methods of treating or managing specific cancers, including non-Hodgkin's lymphoma, by the administration of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are disclosed. Methods of using gene and protein biomarkers as a predictor of non-Hodgkin's lymphoma response to treatment with 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are also disclosed.
Owner:SCHAFER PETER H +3
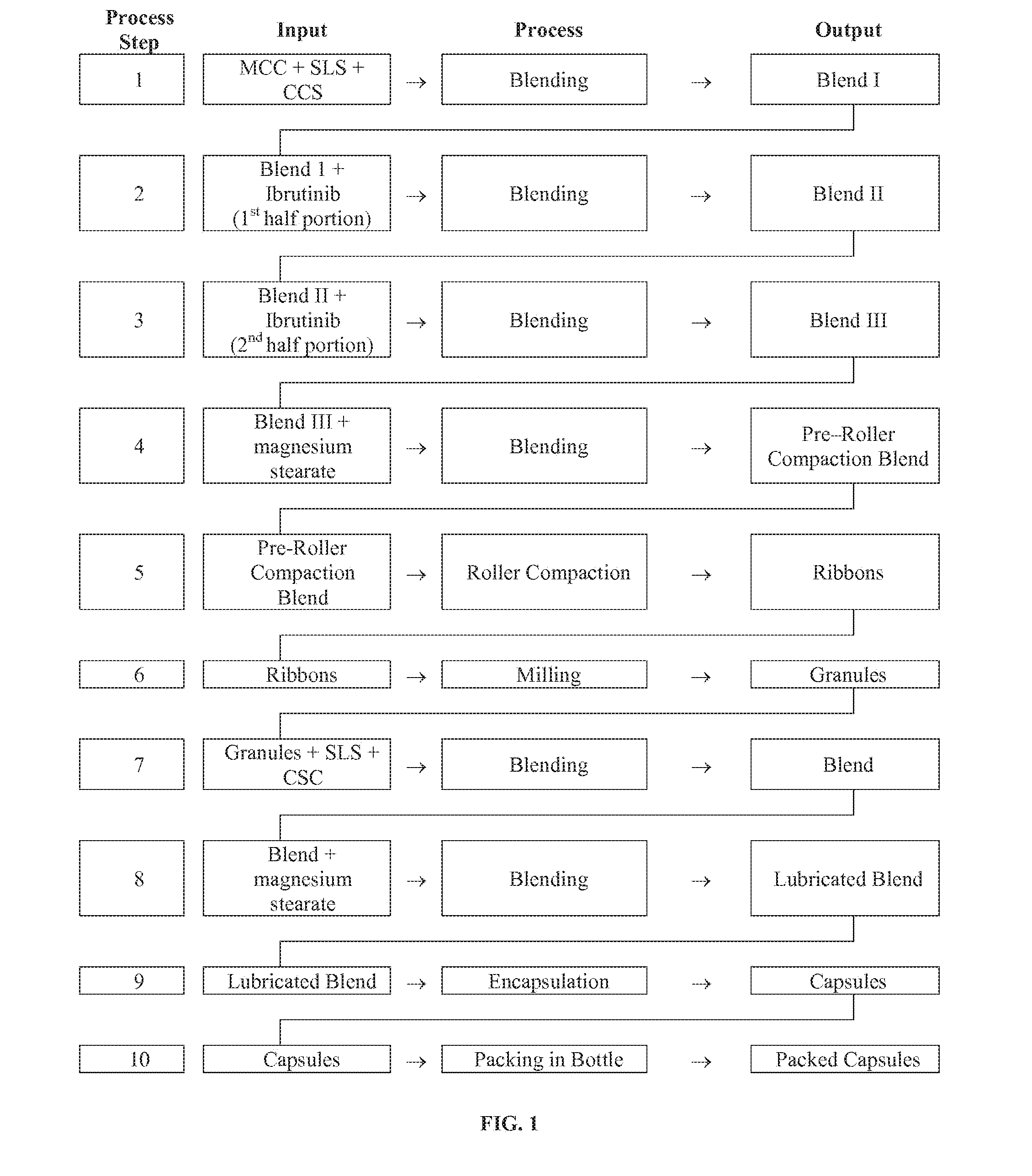
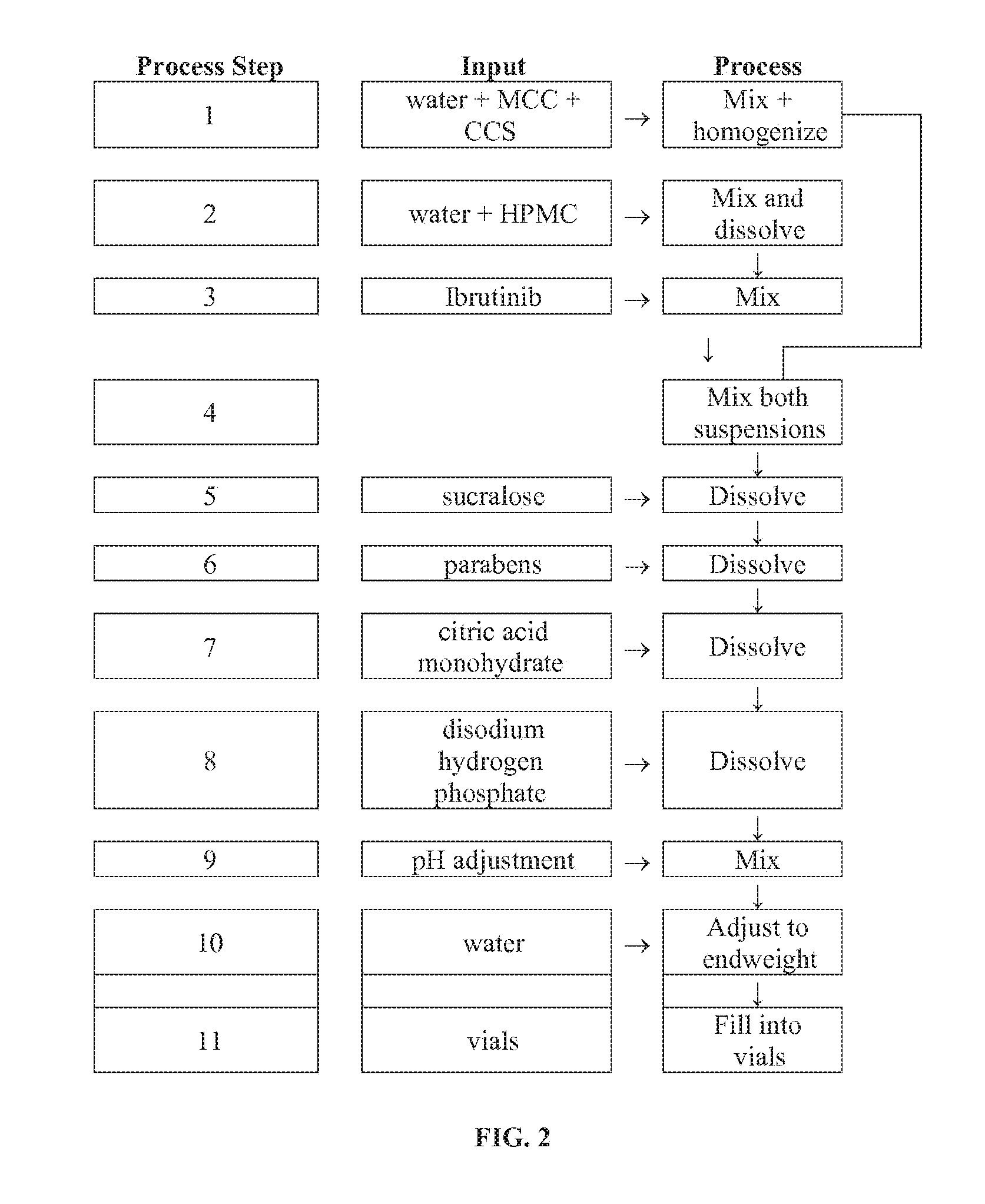
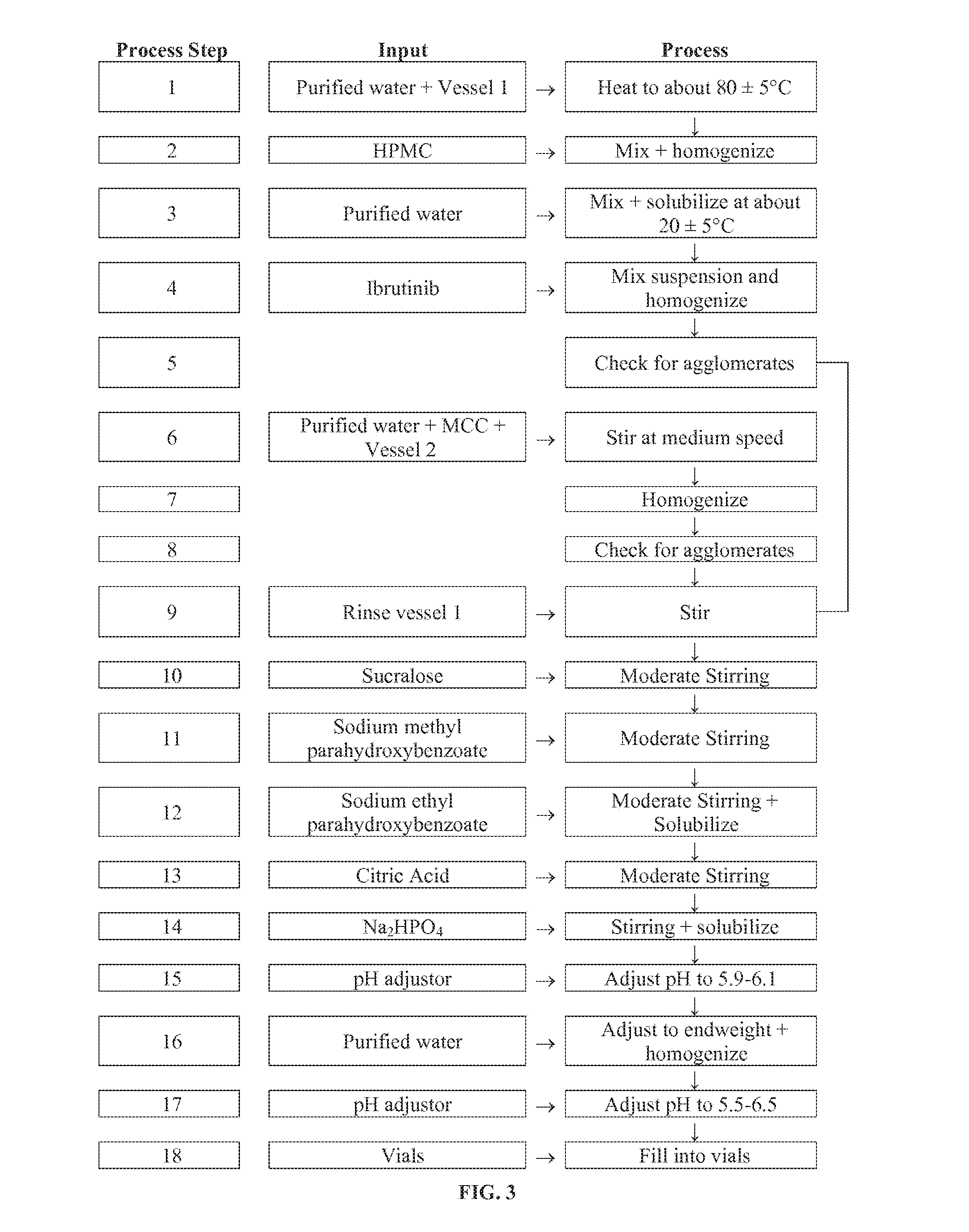
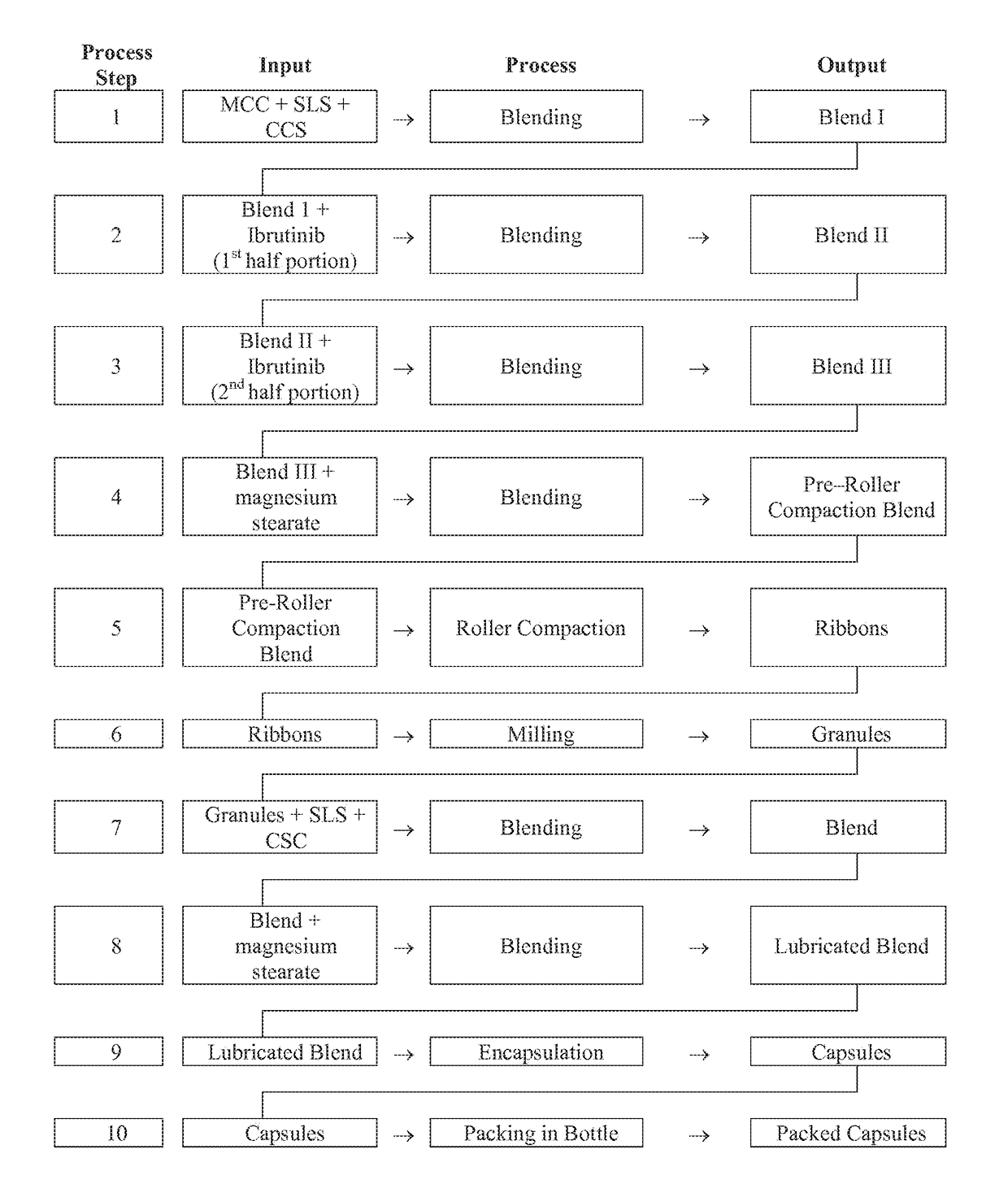
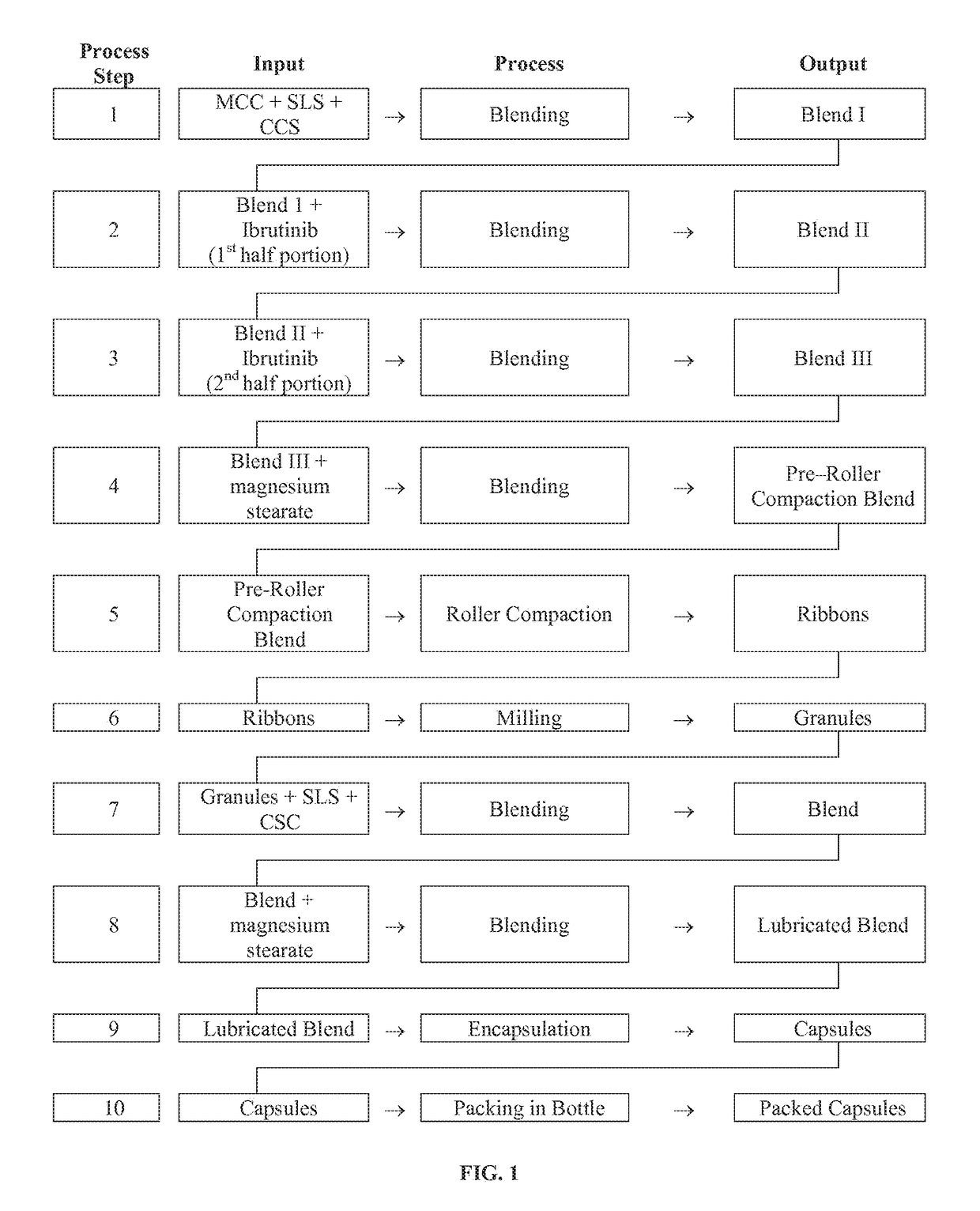
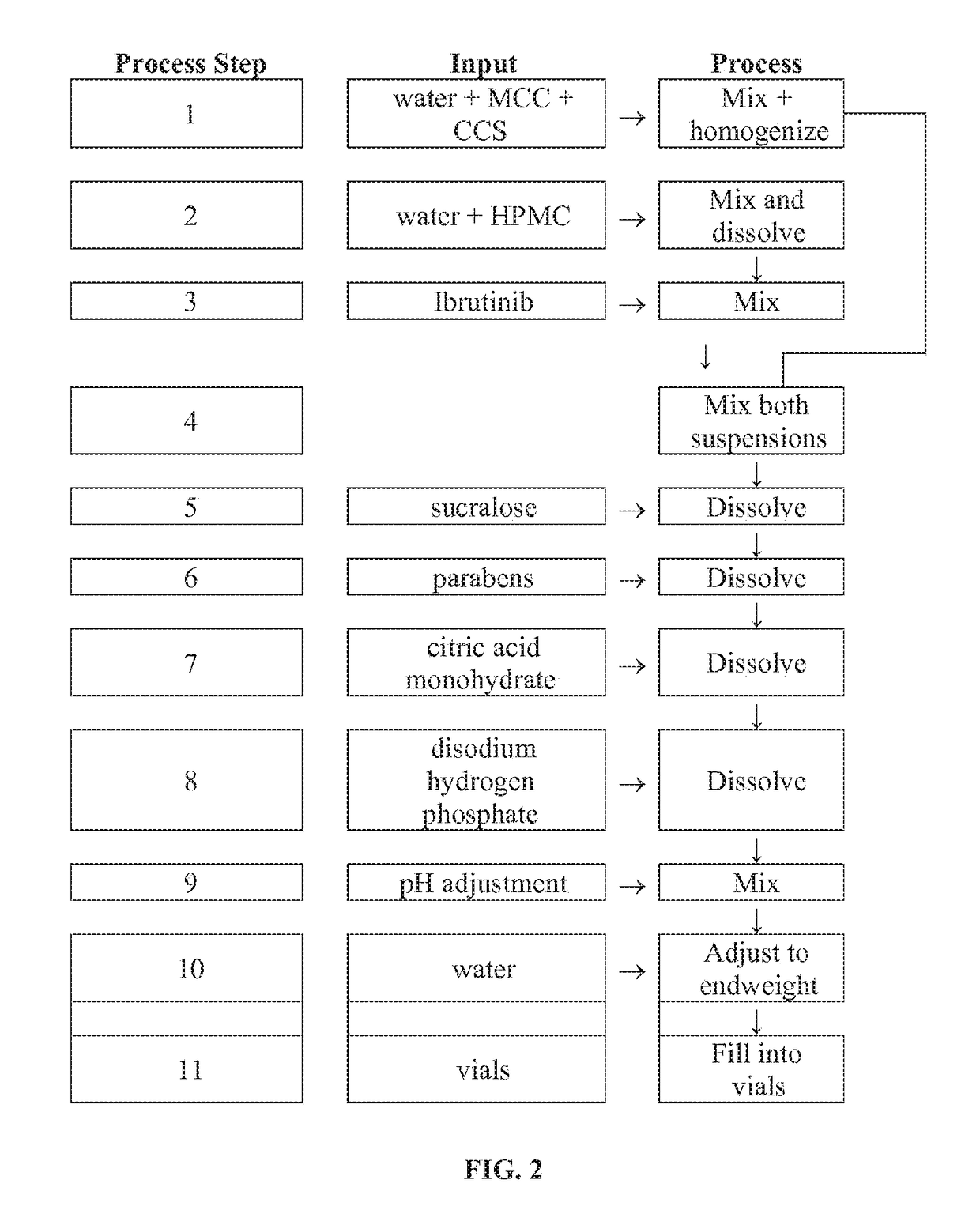
Compositions Containing Ibrutinib
InactiveUS20160287594A1Easy to sprinkleOrganic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Heterocyclic nitrogen compound acting as tyrosine kinase inhibitor
ActiveCN103864792ABTK kinase inhibition is goodGood biological stabilityNervous disorderOrganic chemistryImmunologic disordersDisease
The invention belongs to the technical field of medicine, particularly relates to a heterocyclic nitrogen compound which acts as a tyrosine kinase inhibitor and is represented by a general formula (I) as well as a deuterated material, pharmaceutically acceptable salt or stereisomer thereof. In the general formula (I), X, W, R1, R2, R3, L1, L2, a, b, c, d, e, p, q, a ring A and a ring B are defined in specification. The invention further relates to a preparation method of the compounds, a pharmaceutic preparation containing the compounds, and important actions of the compounds in treatment of leukemia (such as B cell chronic lymphocytic carcinoma and non-hodgkin lymphoma) related to B cell, and autoimmune diseases (such as rheumatoid arthritis and systemic lupus erythematosus).
Owner:KBP BIOSCIENCES CO LTD
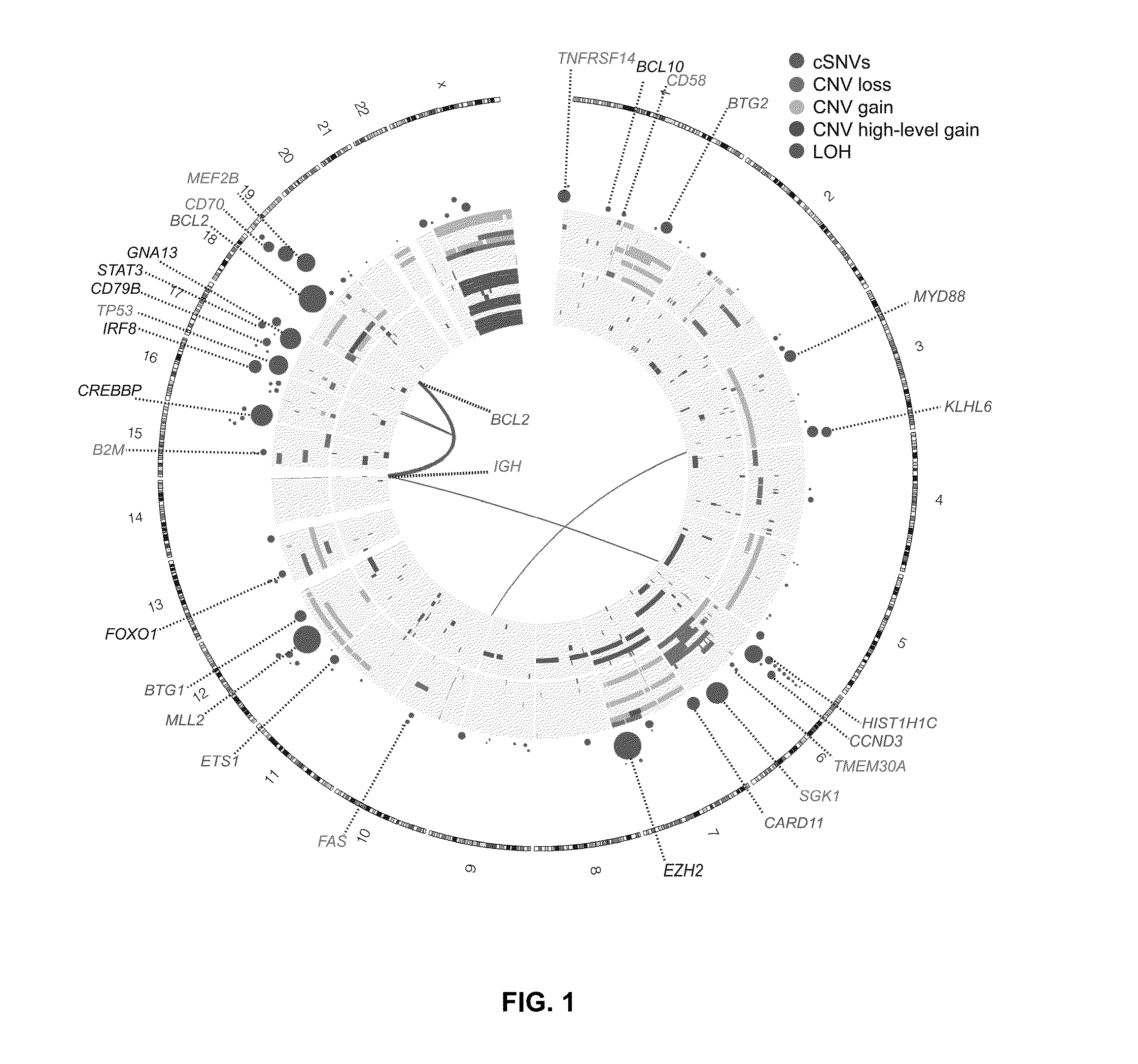
Biomarkers for Non-Hodgkin Lymphomas and Uses Thereof
InactiveUS20130195843A1Reduce molecular weightSmall sizeSugar derivativesMicrobiological testing/measurementBiologyB-Cell Non-Hodgkin Lymphoma
The disclosure provides a method of identifying a subject as having B-cell non-Hodgkin lymphoma (NHL) such as testing a sample from a subject for a mutation in one or more biomarkers. Also described are methods for classifying or monitoring a subject having, or suspected of having, B-cell non-Hodgkin lymphoma comprising testing the sample for a mutation in one or more biomarkers.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
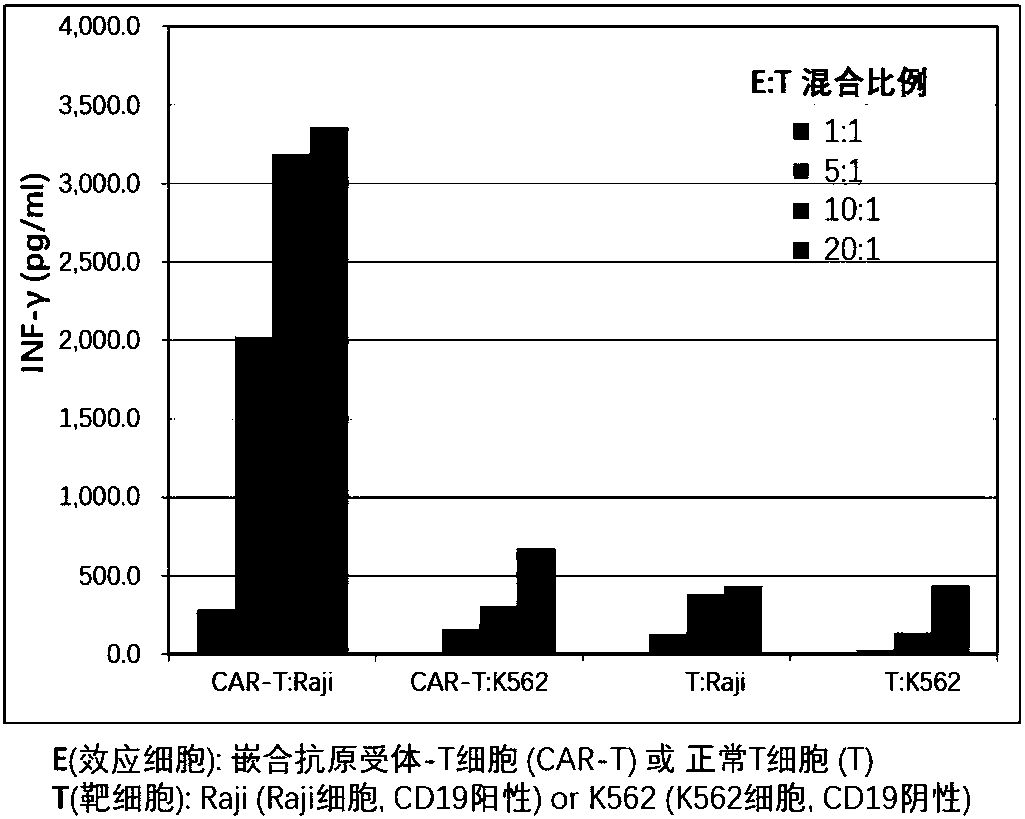
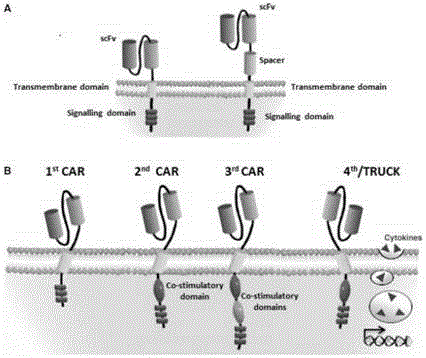
CAR-T (chimeric antigen receptor T cell) for targeting CD19 and application of CAR-T
ActiveCN107827991ARestrict growthPrevent proliferationMammal material medical ingredientsImmunoglobulinsSequence signalSingle-Chain Antibodies
The invention discloses a CAR-T (chimeric antigen receptor T cell) for targeting CD19 and an application of the CAR-T. A CAR for preparing the CAR-T comprises interleukin 2 signal peptides, an anti-CD19 single-chain antibody, a CD8 protein molecule hinge region, a transmembrane region, an intracellular signal structure region and a CD3 zeta protein molecule intracellular signal conduction structure region which are connected in series sequentially and has the amino acid sequence shown in SEQ ID NO:9. The CAR-T is applied to preparation of a medicine or a preparation for treating hematologicalmalignancy, wherein the hematological malignancy comprises CD19-positive B-cell acute lymphocytic leukemia, diffuse large B cell lymphoma and non-hodgkin's lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
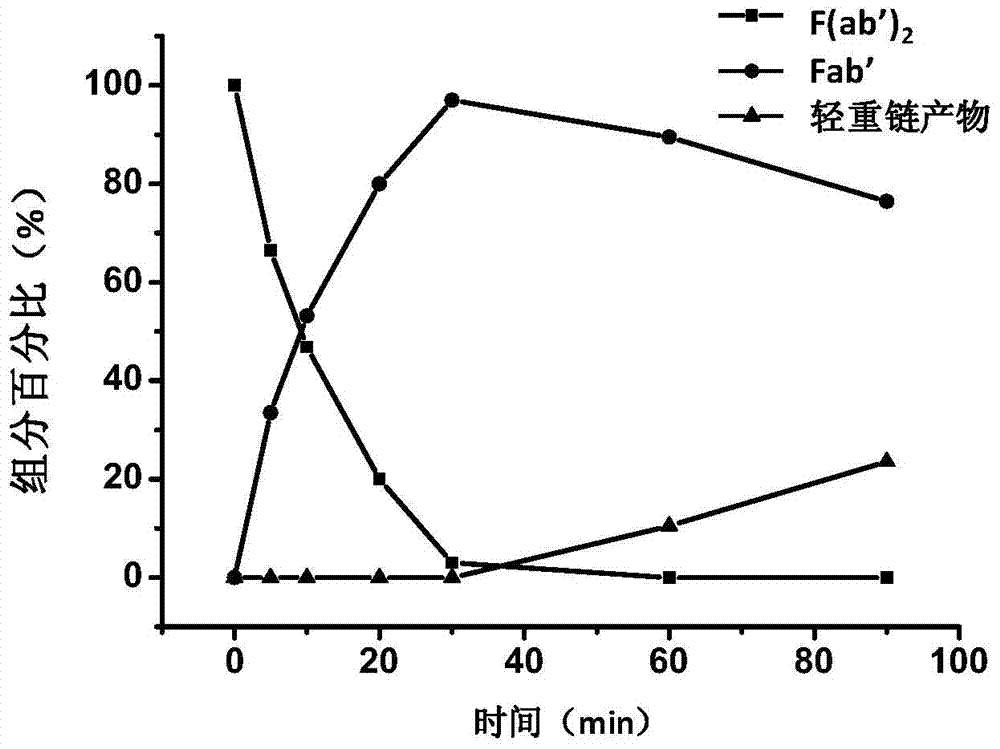
Antibody-coupled drug as well as preparation and application thereof
ActiveCN104758946AThe structure of the drug loading site is clearRetain on-target activityPharmaceutical non-active ingredientsAntineoplastic agentsBifunctionalCytotoxic drug
The invention discloses an antibody-coupled drug as well as a preparation and the application thereof. The antibody-coupled drug is obtained from a heresy-base bifunctional-group polyethylene glycol covalent coupled drug molecule and a Fab' fragment, wherein the structure of the antibody-coupled drug is shown in formula I: Fab'-heresy base bifunctional group polyethylene glycol-drug molecule (1), wherein the Fab' is a fragment of an anti-CD20 antibody and the drug molecule is a cytotoxic drug molecule. According to the antibody-coupled drug, the F(ab')2 fragment degraded from the anti-CD20 antibody is reduced to a Fab' fragment of which the hinge area comprises two free thiols; and the Fab' fragment is then covalently coupled with the drug molecule by the heresy-base bifunctional-group polyethylene glycol, so that the antibody-coupled drug is obtained. The antibody-coupled drug prepared by the invention has the characteristics of fixed drug loading ratio and clear drug loading site structure; the antibody-coupled drug also retains the binding capacity of the anti-CD20 antibody to CD20 proteins, so that the antibody-coupled drug has targeting ability to the CD20-positive cells and is applicable to treatment of non-Hodgkin lymphomas.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
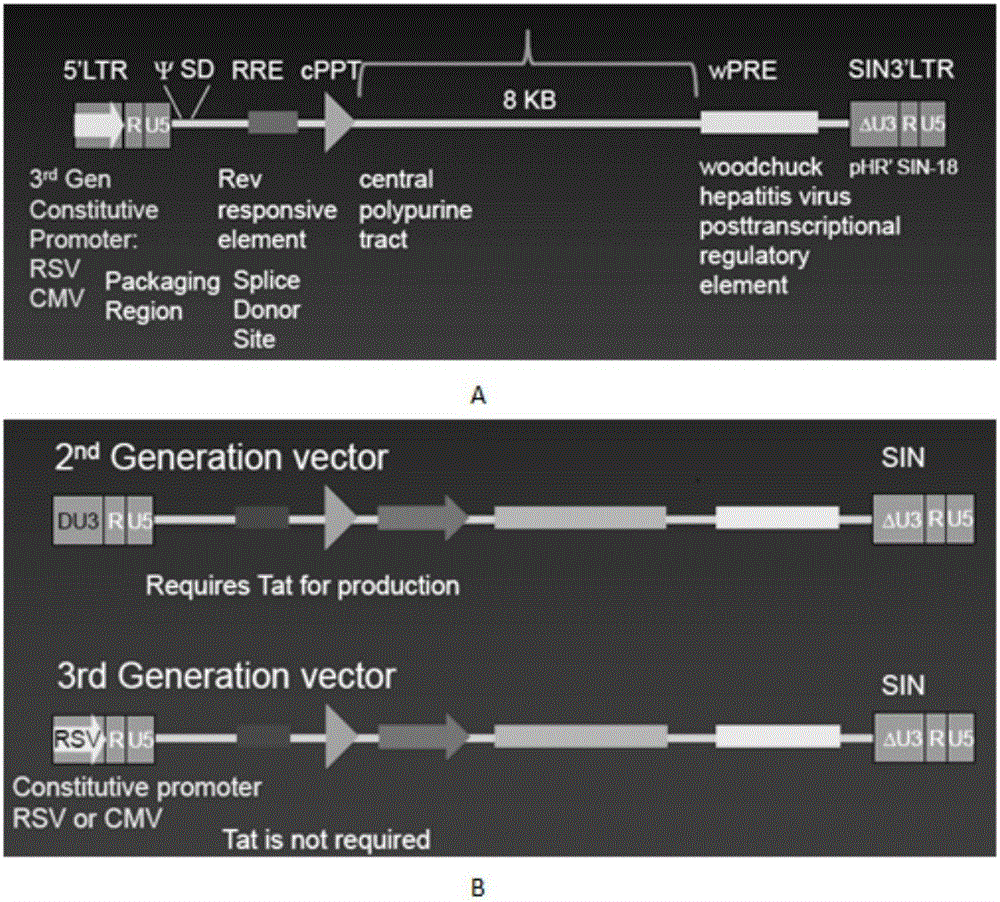
CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector as well as construction method and applications thereof
ActiveCN105950663APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorAntigenAmpicillin
The invention discloses a CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector. The CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector comprises a prokaryotic replicor pUC Ori sequence used for plasmid duplication; an ampicillin resistance gene AmpR sequence used for amplification of a large number of target strains; a virus replicor SV40 Ori sequence used for enhancing the replication in eukaryocytes; a lentivirus packaging cis element used for lentivirus packaging; ZsGreen 1 green fluorescent protein used for green fluorescence expression of the eukaryocytes; an IRES ribosome binding sequence used for co-transcription and co-expression of protein; a human EF1 alpha promoter used for eukaryotic transcription of genes of a chimeric antigen acceptor; the chimeric antigen acceptor used for forming second-generation CAR or third-generation CAR integrating recognition, delivery and promoting; and an eWPRE element used for enhancing the expression efficiency of transgenes. In addition, the invention further discloses a construction method and applications of the vector. With the vector, the secretion of the cell factors and the in-vitro lethal effect of the CAR-T cells can be obviously improved, and the effect in treating hodgkin lymphoma or non-hodgkin lymphoma clinically is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
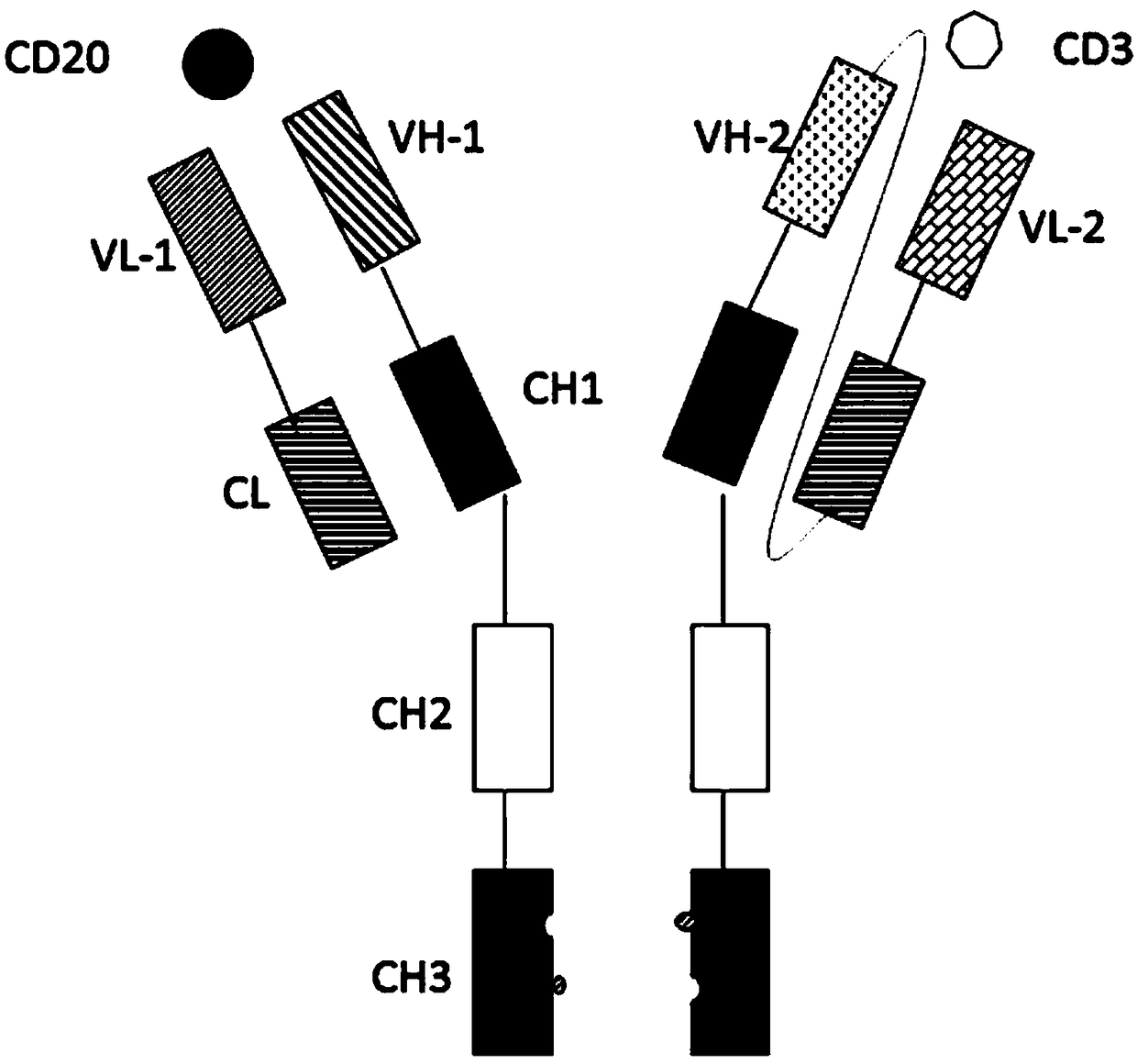
Bispecific antibody for CD20 and CD3
ActiveCN108059680AImprove stabilityOvercome stabilityHybrid immunoglobulinsAntibody ingredientsAntigenCD20
The invention relates to the technical field of biological pharmacy and particularly discloses a bispecific antibody for CD20 and CD3. The bispecific antibody comprises univalent unit, a single-chainunit and a linker, wherein the univalent unit is a light chain-heavy chain pair with a specific binding capability for an antigen CD20 on the surface of a tumor cell; the single-chain unit can be usedfor specifically binding a CD3 antigen on the surface of an immune cell; the end N of a heavy chain and the end C of a light chain of the single-chain unit are connected by the linker to form a Fab-Fc form; the univalent unit is connected with the single-chain unit through a disulfide bond of a hinge region and a pestle mortar structure of a CH3 structural domain. According to the bispecific antibody, CD20 and CD3 can be specifically bound and the immune effector cell can be targeted to the tumor cell, so that the killing effect of the immune effector cell on the tumor cell is improved; the bispecific antibody can be used for treating CD20 antigen positive B-cell neoplasms, comprising non-Hodgkin lymphoma, chronic lymphocytic leukemia and the like, and has a broad application prospect inthe field of immunotherapy of tumors.
Owner:BEIJING DONGFANG BIOTECH
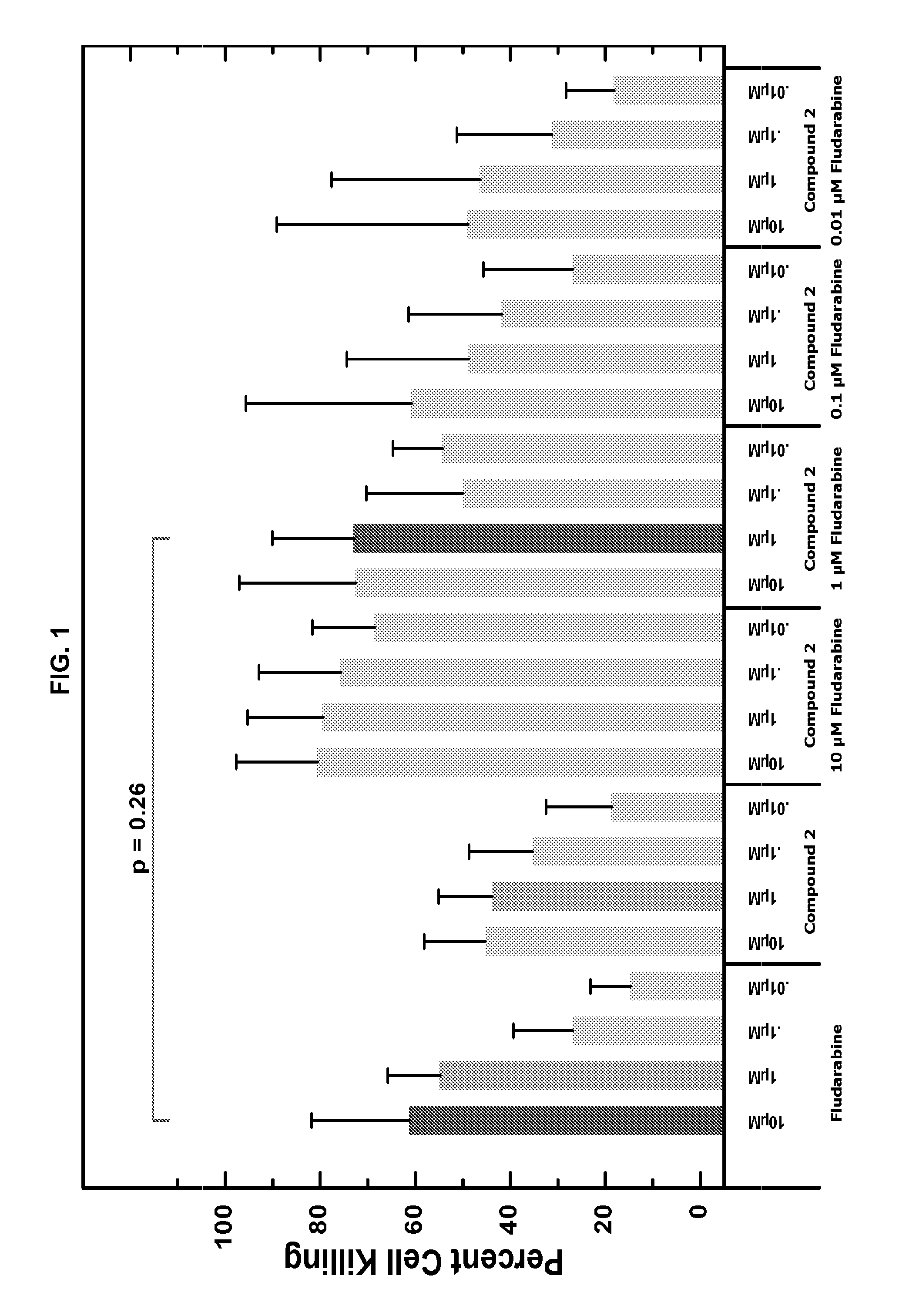
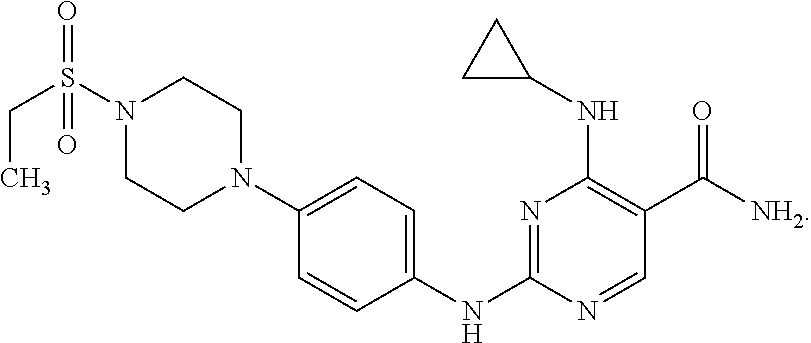
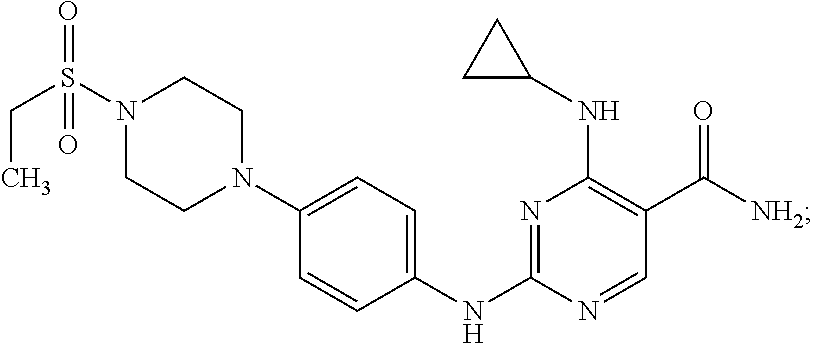
Combination therapy of 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide and fludarabine
InactiveUS20130237493A1Good effectGood treatment effectBiocideCarbohydrate active ingredientsMantle lymphomaCombination therapy
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide, or a pharmaceutically acceptable salt thereof, and fludarabine for the treatment of cell proliferative disorders, such as undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL); mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic Lymphoma (SLL) and multiple myeloma.
Owner:ALEXION PHARMA INC
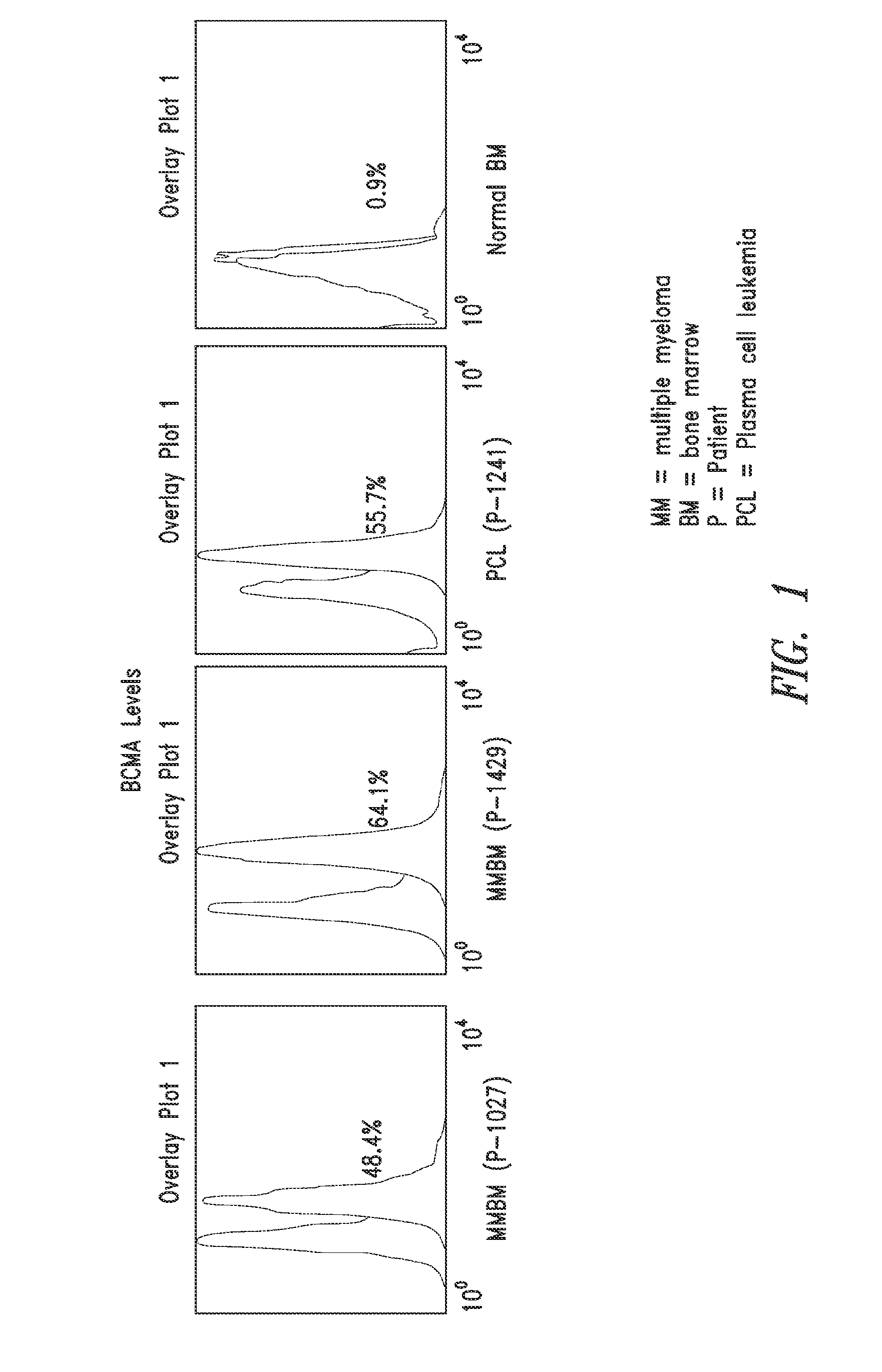
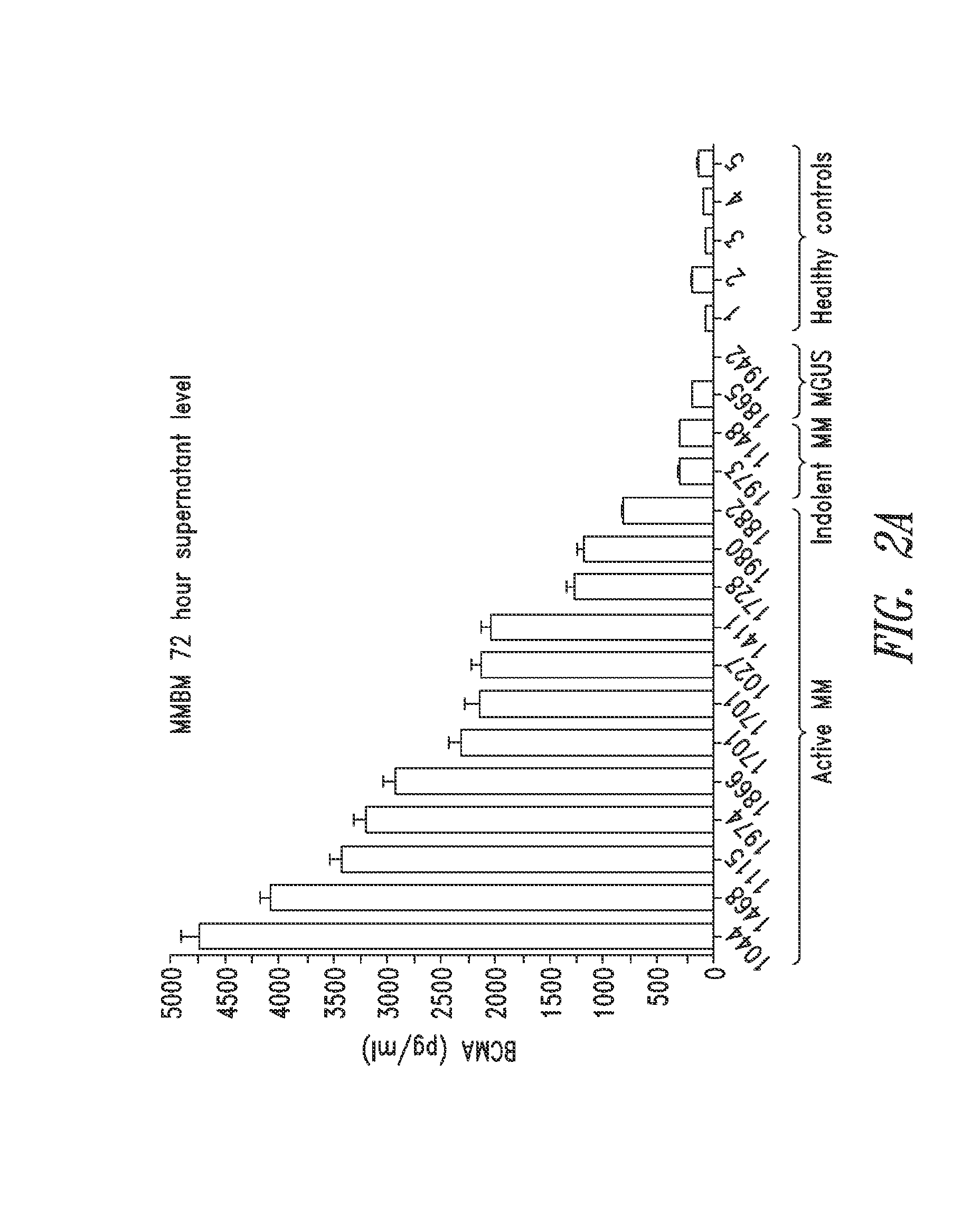
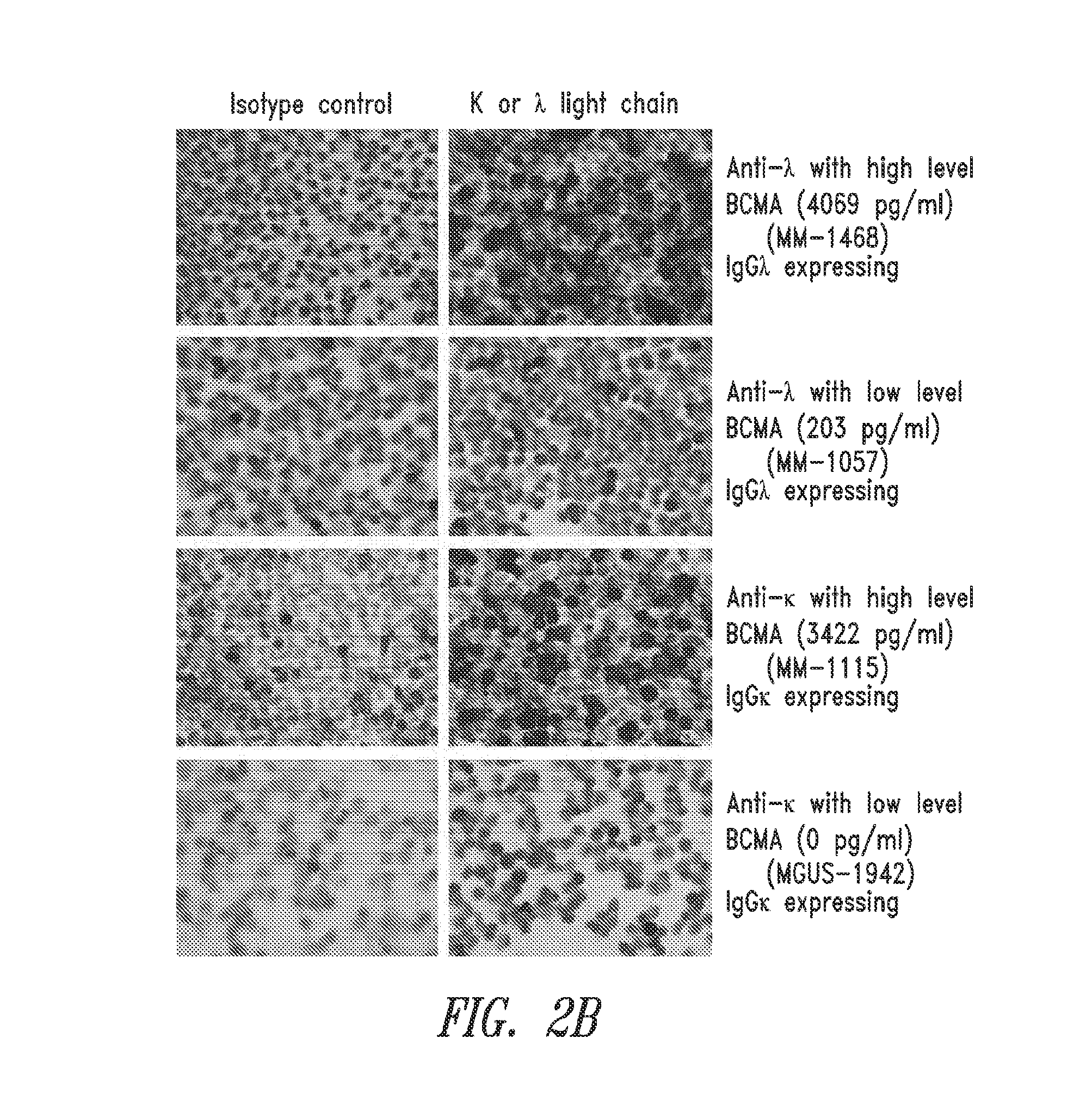
Diagnostic, prognostic, and monitoring methods for multiple myeloma, chronic lymphocytic leukemia, and b-cell non-hodgkin lymphoma
The invention generally provides improved compositions and methods for detecting, diagnosing, prognosing, and monitoring multiple myeloma, chronic lymphocytic leukemia, or B-cell non-Hodgkin lymphoma in a subject. In particular, the invention provides methods for detecting BCMA in subjects to reliably diagnose, predict survival, or monitor multiple myeloma, chronic lymphocytic leukemia, or B-cell non-Hodgkin lymphoma in the subject.
Owner:ONCOTRACKER INC
Combination therapy with 4-(3-(2h-1,2,3-triazol-2-yl)phenylamino)-2-((1r,2s)-2-aminocyclohexylamino)pyrimidine-5-carboxamide
InactiveUS20130244963A1Good treatment effectReduce the amount requiredBiocideCarbohydrate active ingredientsAnaphylaxisSystemic lupus erythematosus
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing a SYK inhibitor, or a pharmaceutically acceptable salt thereof, and a antineoplastic or antiinflammatory agent for the treatment of inflammatory, autoimmune and cell proliferative diseases, such as allergic reaction, transplant rejection, rheumatoid arthritis (RA), lupus, multiple sclerosis (MS) or psoriasis undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL) (including diffuse large B cell lymphoma (DLBCL)), mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic (SLL), Lymphoma, multiple myeloma, asthma, vasculitis, Idiopathic thrombocytopenic purpura (ITP), Heparin Induced Thrombocytopenia (HIT) and hemolytic anemia.
Owner:ALEXION PHARMA INC
Methods of Prognosis for Non-Hodgkin Lymphoma
InactiveUS20120134986A1Predictive of survivalImprove the level ofBiocidePhosphorous compound active ingredientsBiologyTumor cells
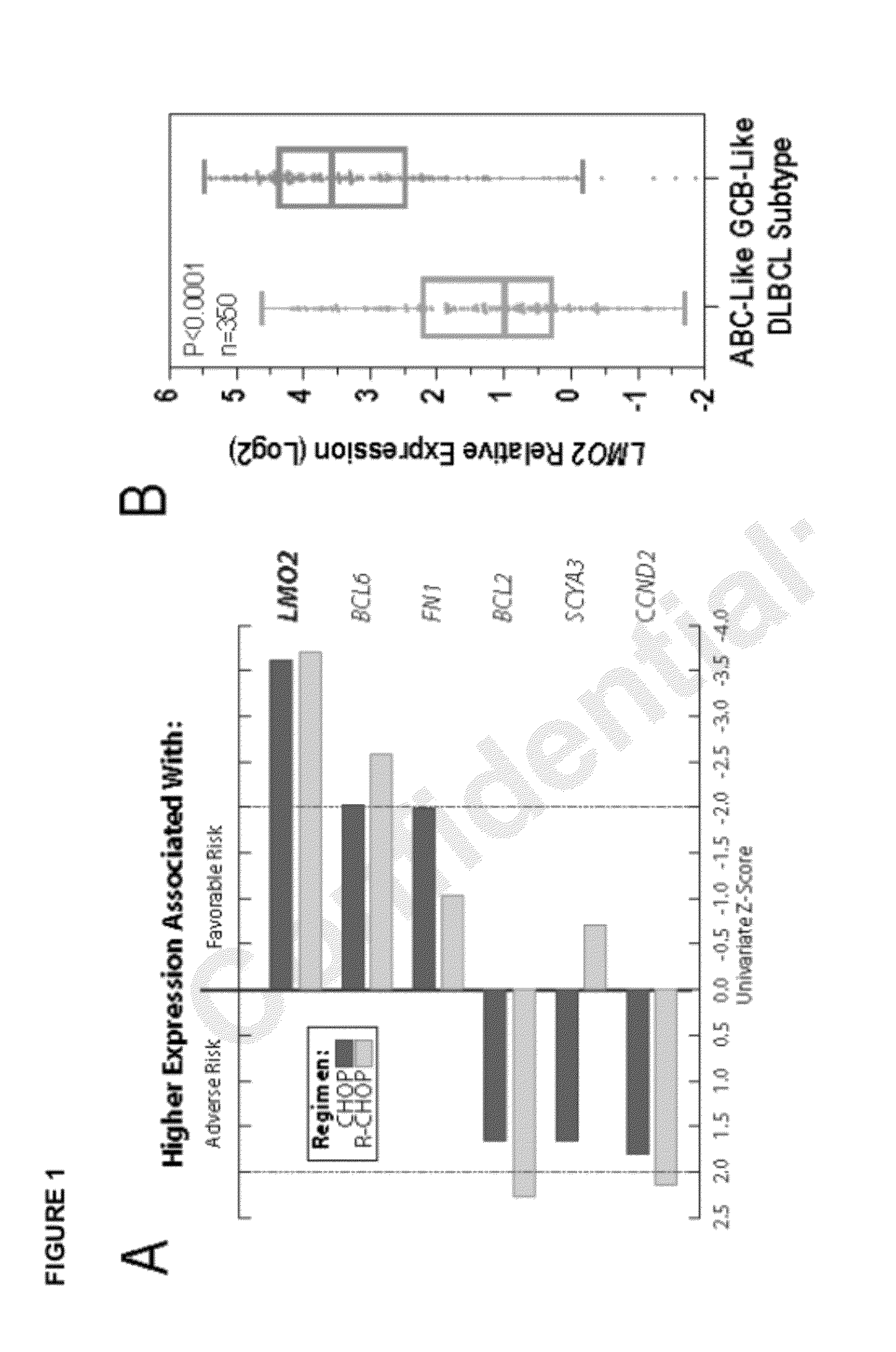
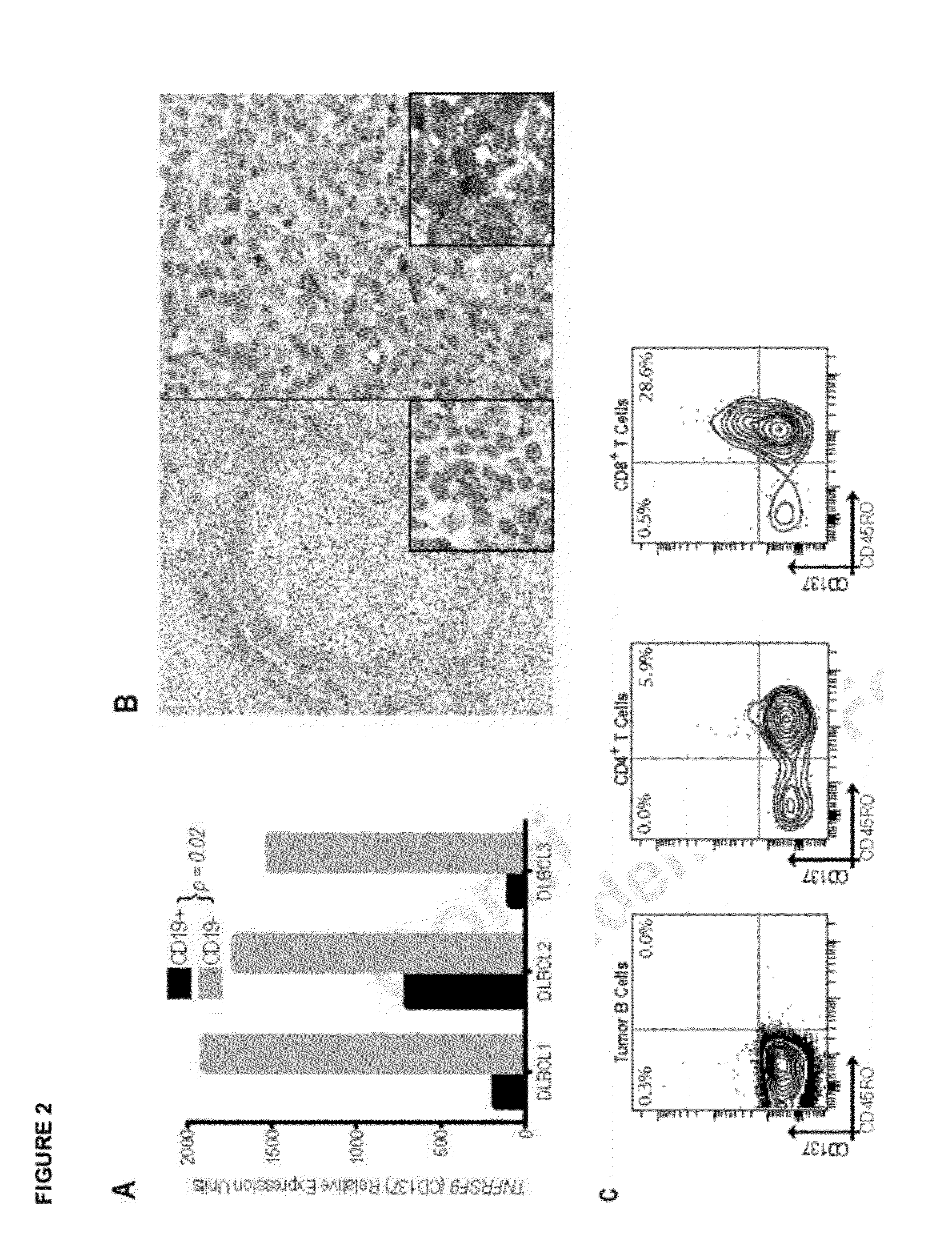
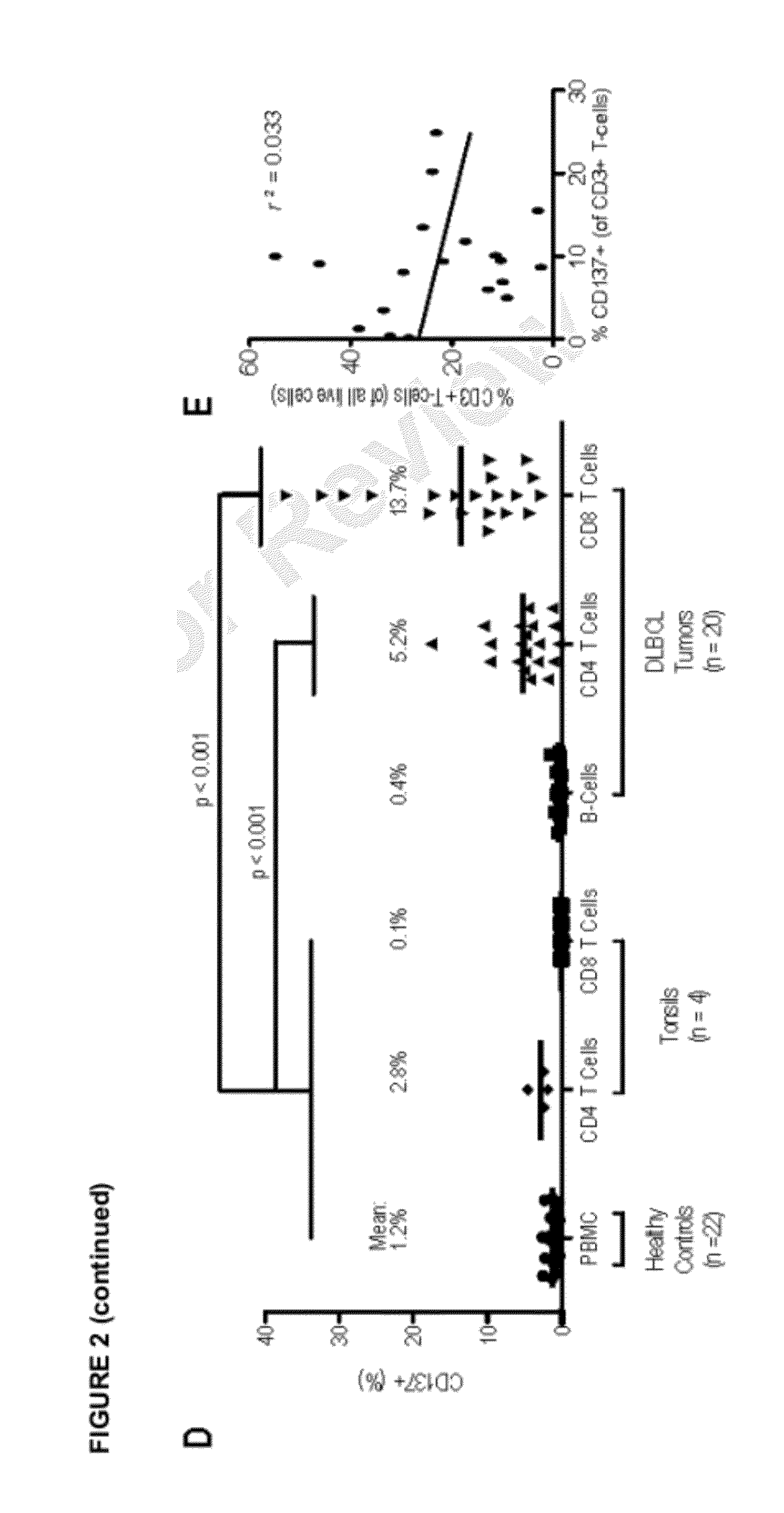
Measurement of a single gene expressed by tumor cells (LMO2) and a single gene expressed by the immune microenvironment (TNFRSF9), which determination may be referred to herein as a two gene score (TGS), powerfully predicts overall survival in patients with NHL, particularly overall survival in the context of anthracycline-based chemotherapy or co-treatment with anthracycline-based chemotherapy and anti-CD20 immunotherapy. It is shown herein that increased levels of LMO2 and TNFRSF9 correlate with a positive patient response and improved prognosis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Benzothiazine and benzothiadiazine compounds, preparation and application
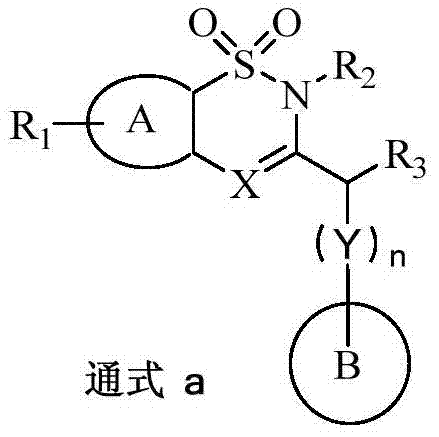
ActiveCN107033145AHas inhibitory effectEnhanced inhibitory effectOrganic active ingredientsSenses disorderBenzothiadiazinesDisease
The invention provides benzothiazine and benzothiadiazine compounds as well as analogues, pharmaceutically acceptable salts, stereoisomers and solvates of the compounds having a structure shown in a general formula a. Repeated experiments prove that the compounds have an inhibition action on PI3Kdelta, wherein most compounds selectively have a remarkableinhibition action on PI3Kdelta, the compounds can be applied to preparation of anti-inflammation and anti-tumor drugs, the range of involved tumor and inflammatory diseases is wide, and the compounds particularly have a better inhibition effect on FL (follicular B-cell non-Hodgkin lymphoma) relapsing in a treatment period, recurrent CLL (chronic lymphocytic leukemia) and recurrent SLL (small lymphocytic lymphoma). Prepared drugs can be combined with other anti-tumor drugs for use and have an obvious effect, and novel treatment drugs are provided for clinically resisting tumors and inflammation diseases.
Owner:ZHEJIANG UNIV
Tyrosine kinase inhibitor for nitrogen hetero-aromatic ring and derivatives thereof
InactiveCN103664884ABTK kinase inhibition is goodGood choiceOrganic active ingredientsOrganic chemistryDiseaseImmunologic disorders
The invention belongs to the technical field of medicines, and particularly relates to a tyrosine kinase inhibitor for nitrogen hetero-aromatic ring and derivatives thereof shown in the general formula (I), and pharmaceutically acceptable salts or stereoisomer thereof, wherein R1, R2, R3, L1, L2, a, b, c, d, e, p, q, A and B are defined in the specification. The invention further relates to a preparation method for the compounds, and pharmaceutical preparations containing the same, and the compounds play an important role in preparing drugs for preventing and / or treating blood cancers (such as B cell chronic lymphocytic leukemia, non-hodgkin lymphomas and the like) related to B cells, and autoimmune diseases (such as thrumatoid arthritis, systemic lupus erythematosus and the like).
Owner:KBP BIOSCIENCES CO LTD
Biomarkers for Non-Hodgkin Lymphomas and Uses Thereof
ActiveUS20140024099A1Convenient treatmentSugar derivativesMicrobiological testing/measurementBiomarker (petroleum)Hodgkin lymphoma
The disclosure provides a method of identifying a subject as having B-cell non-Hodgkin lymphoma (NHL) such as testing a sample from a subject for a mutation in one or more biomarkers. Also described are methods for classifying or monitoring a subject having, or suspected of having, B-cell non-Hodgkin lymphoma comprising testing the sample for a mutation in one or more biomarkers.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
Method for identifying, separating and culturing lymphoma stem cells from non-Hodgkin lymphoma cell line
InactiveCN107354135APrevent relapseStrong drug resistanceCell dissociation methodsCell culture active agentsSurface markerStudy methods
The invention provides a method for identifying, separating and culturing lymphoma stem cells from a non-Hodgkin lymphoma cell line and relates to the technical field of cells. The method comprises the steps that surface markers of initiating cells of amphicyte lymphoma are identified and separated, and the specificity is high; meanwhile, the initiating cells of the amphicyte lymphoma express higher drug resistance to clinical frequently-used chemotherapy drugs, the lymphoma stem cells which are identified and separated by using the surface markers of the initiating cells are subjected to specific treatment, and the method has very important significance for preventing lymphoma relapse and improving the survival rate of patients. The method for identifying, separating and culturing the lymphoma stem cells from the non-Hodgkin lymphoma cell line can provide a research method and technical support for the mechanism research of non-Hodgkin lymphoma generation and development and the development of therapeutic drugs.
Owner:QINGDAO UNIV
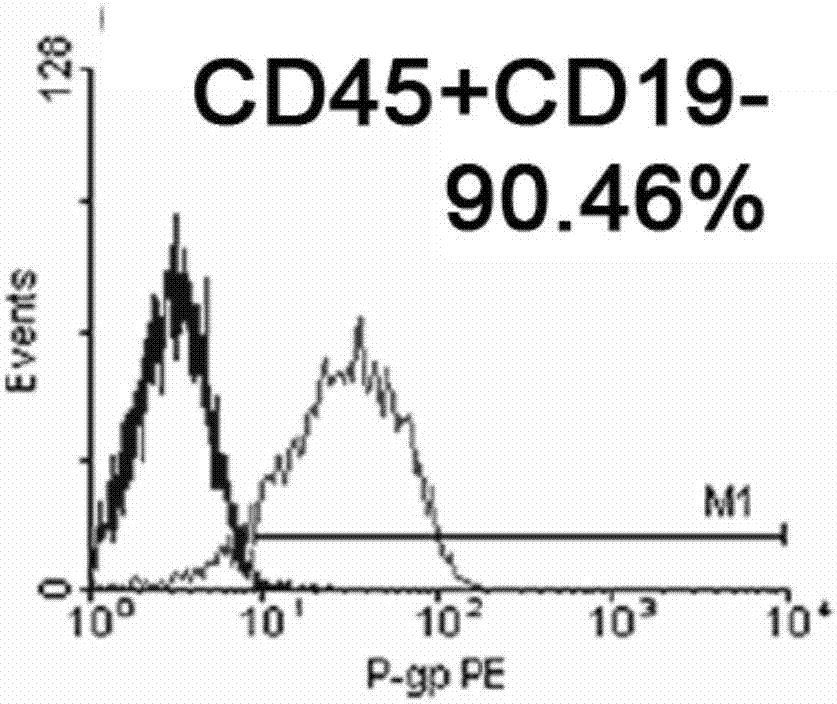
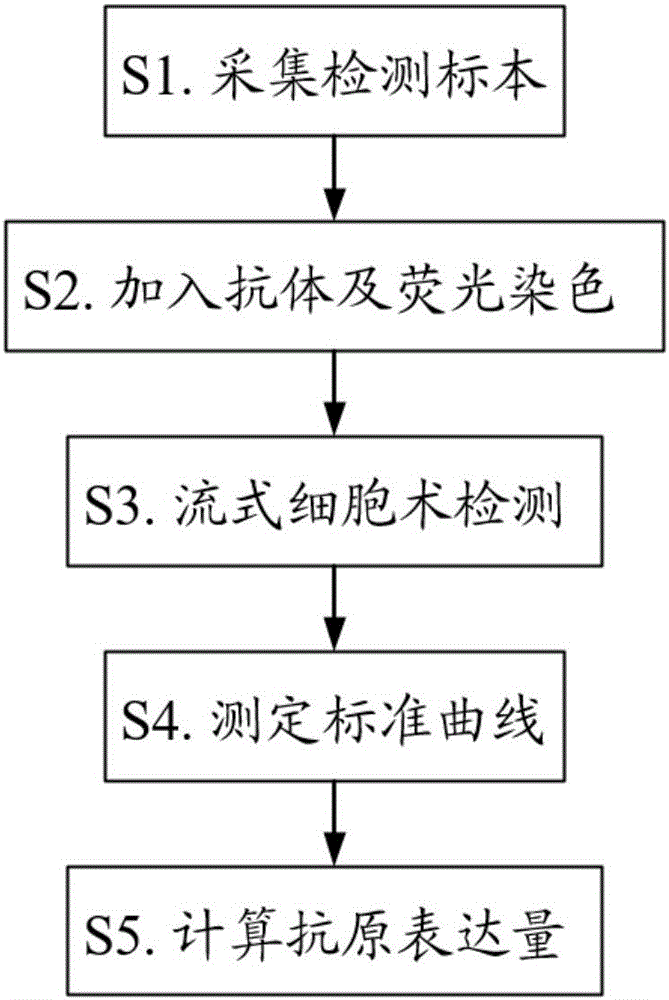
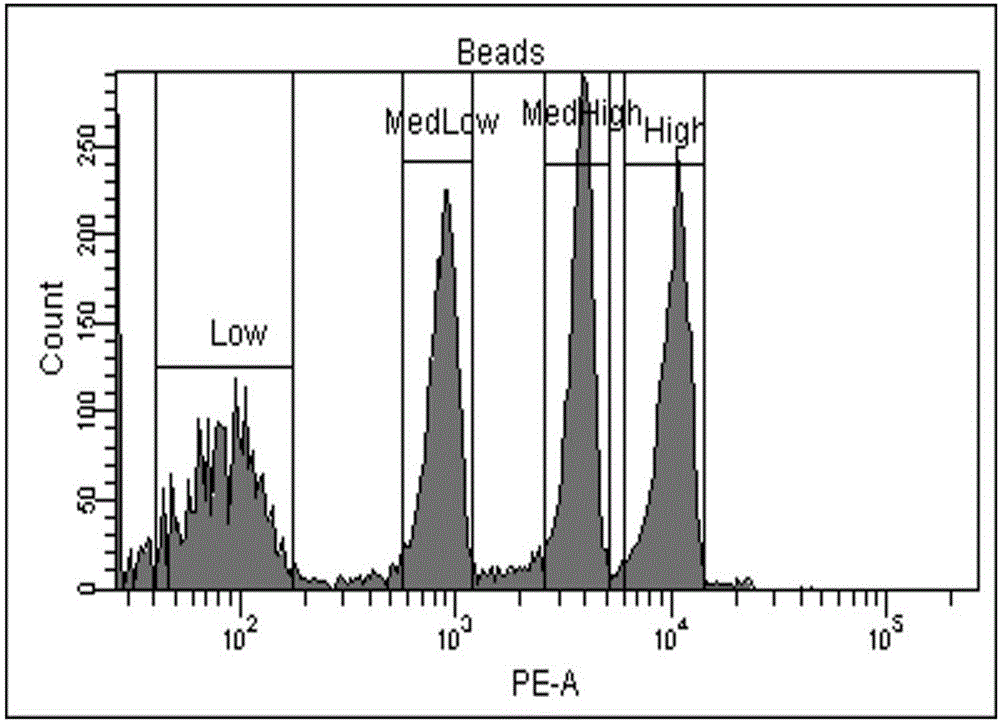
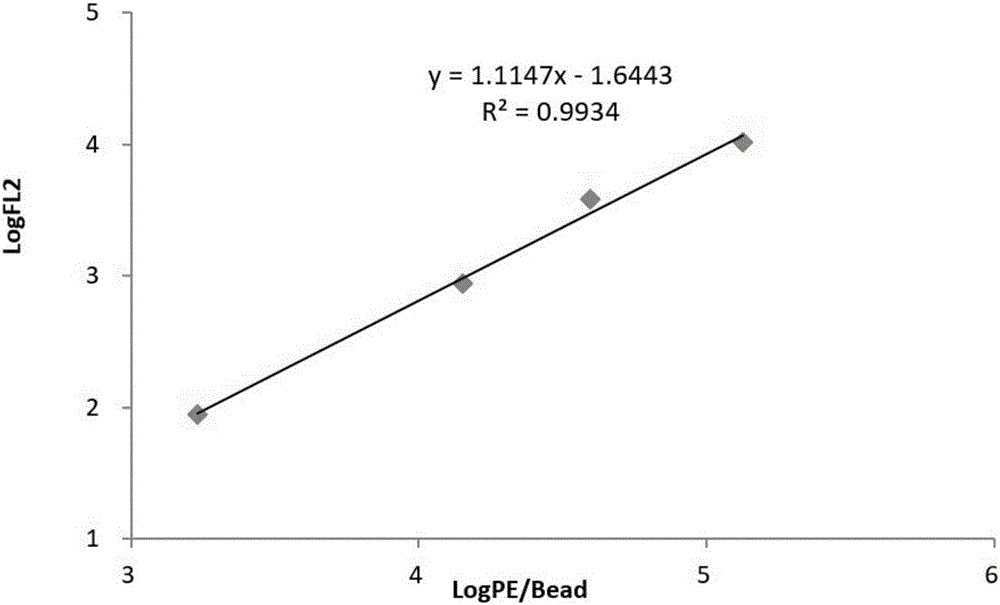
B cell malignant tumor-associated antigen expression quantity detection kit and detection method
The invention provides a B cell malignant tumor-associated antigen expression quantity detection kit and a detection method. Reagents of the kit include a Percp marked CD45 antibody, an APC marked CD19 antibody and a PE marked CD20 or CD22 antibody. Based on flow cytometry, the antigen expression quantity of peripheral blood and normal or abnormal B lymphocyte CD20 and CD22 in bone marrow is detected by a PE micro-sphere introduction method. Compared with existing other detection methods, the method has the advantages that quantitative detection can be realized, the antigen expression quantity of cell surfaces can be directly detected, characteristics of B lymphocyte non-Hodgkin lymphoma can be visually reflected, fewer specimens are used, operation is simple and rapid, and environmental pollution is avoided. Quantitative detection results can be used for instructing targeted therapy of B-NHL by clinical CD20 and CD22 monoclonal antibody-based drugs and judging treatment prognosis.
Owner:北京海思特医学检验实验室有限公司
CD20-resisting all humanized monoclonal antibodies and application thereof
ActiveCN104987420AImprove tumor killing effectGood curative effectAntipyreticAnalgesicsDiseaseAntiendomysial antibodies
Owner:BEIJING ANBAOKANG BIO PHARMA CO LTD
A kind of crystal form of inhibitor and its preparation method and use
ActiveCN104736538BNot easy to absorb moistureHigh content of active ingredientsOrganic chemistry methodsAntineoplastic agentsIndolent Non-Hodgkin LymphomaMedicine
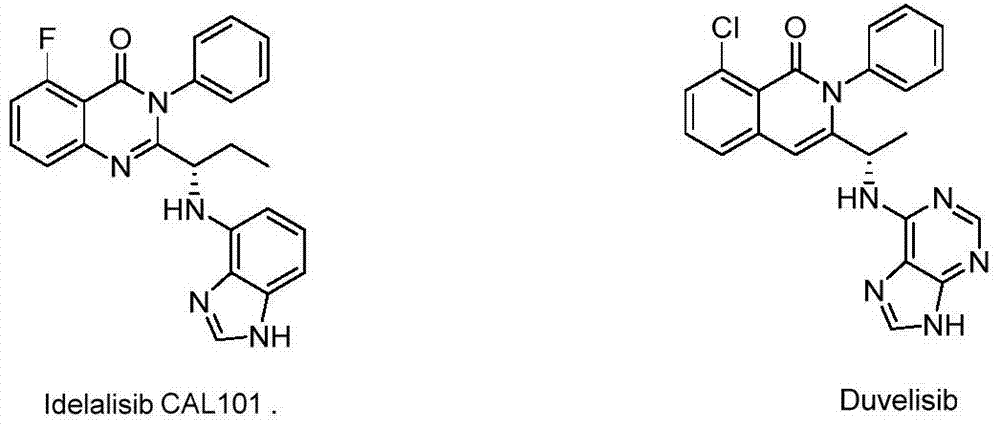
The present invention relates to a kind of inhibitor 5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one New crystalline forms having one or more improved properties compared to known crystalline forms. The present invention also relates to a new crystal form of 5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one The preparation method, its pharmaceutical composition and its use in the preparation of medicines for treating and / or preventing diseases such as chronic lymphocytic leukemia and inert non-Hodgkin's lymphoma.
Owner:SOLIPHARMA
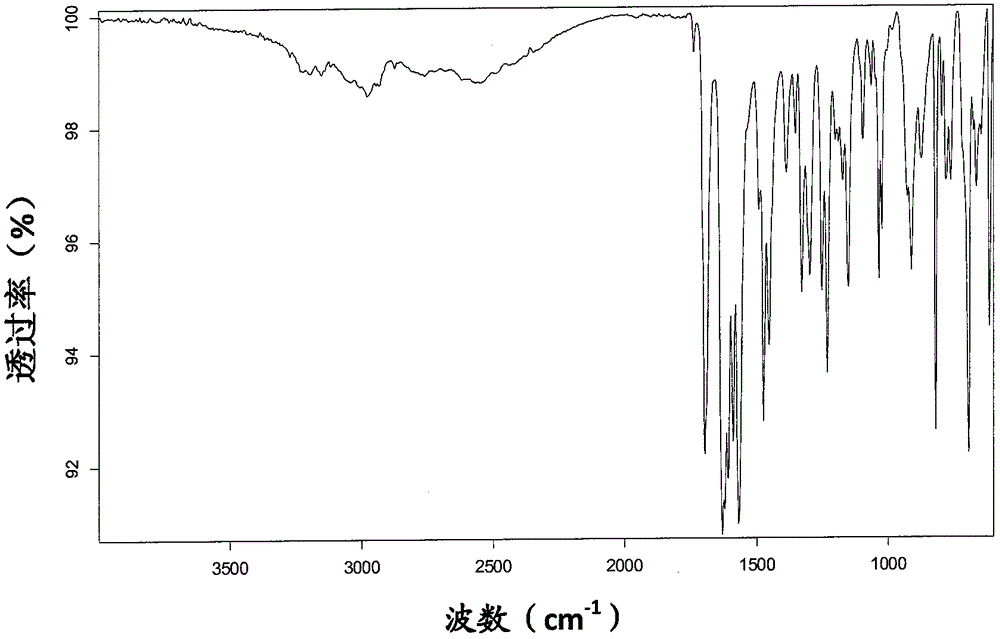
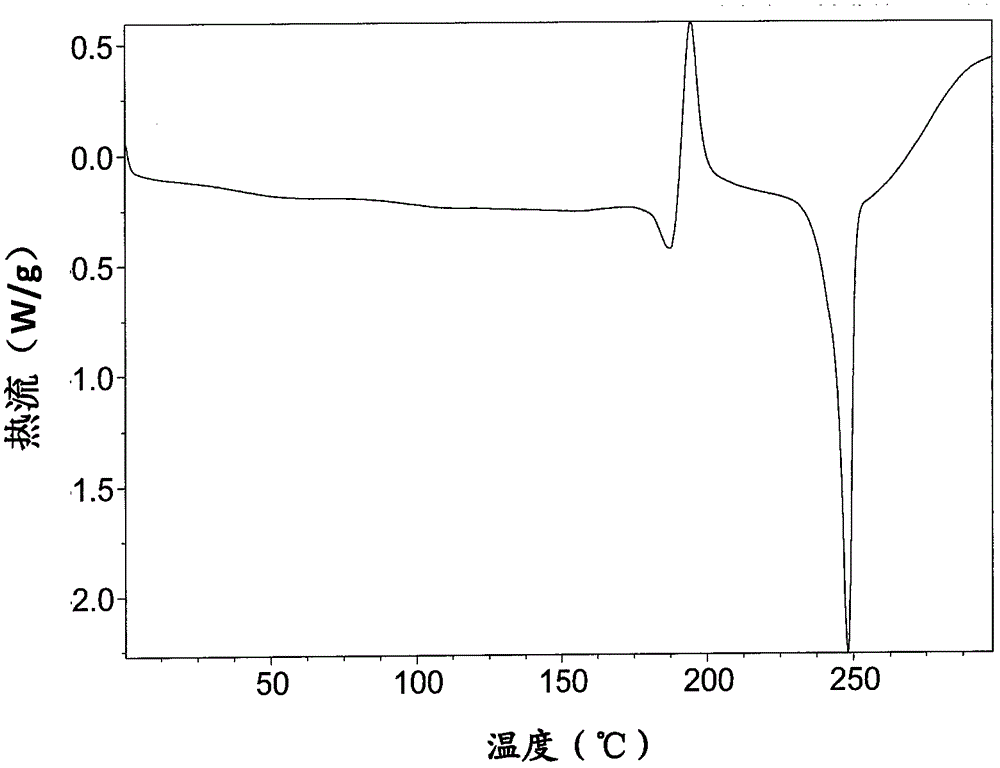
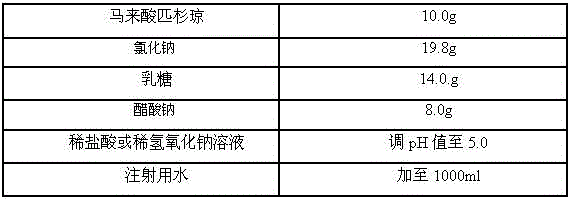
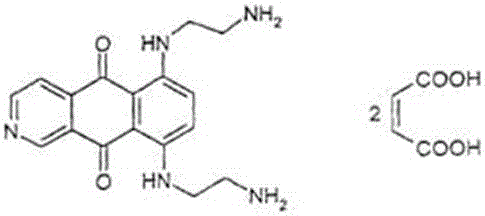
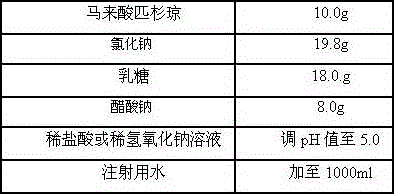
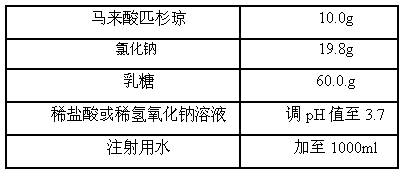
Freeze-dry composition for treating NHL (non-hodgkin lymphoma) and preparation method thereof
InactiveCN105769776AQuality improvementEasy to preparePowder deliveryOrganic active ingredientsFreeze-dryingBULK ACTIVE INGREDIENT
The invention belongs to the technical field of medicine, and particularly relates to a freeze-dry composition for treating NHL (non-hodgkin lymphoma) and a preparation method thereof. The freeze-dry composition for treating NHL mainly consists of active ingredients including maleic acid pixantrone, sodium chloride, lactose, natrium aceticum and pH regulators. The The freeze-dry composition for treating NHL has the advantages that the quality is stable; all indexes conform to the quality requirements; in the clinical use process, the preparation by medical personnel is convenient; the prepared medicine can be stored for a long time; meanwhile, the use safety of a patient is also improved.
Owner:QINGDAO TUMOR HOSPITAL
Compositions Containing Ibrutinib
InactiveUS20170252344A1Organic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Treatment of hematologic malignancies with an anti-CXCR4 antibody
ActiveUS10428151B2Boron compound active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Monoclonal antibody
The present disclosure provides human monoclonal antibodies that bind specifically to CXCR4 with high affinity. This disclosure also provides a method for treating a subject afflicted with a CXCR4-expressing cancer, in particular a hematological malignancy such as multiple myeloma, acute myeloid leukemia, or non-Hodgkin's lymphoma, comprising administering to the subject a therapeutically effective amount of a pharmaceutical composition comprising an anti-CXCR4 antibody of the disclosure. The disclosure further provides a kit for treating a cancer in a subject comprising a dose of an anti-CXCR4 antibody and instructions for using the anti-CXCR4 antibody in the therapeutic methods of the disclosure.
Owner:BRISTOL MYERS SQUIBB CO
Treatment of relapsed and/or refractory solid tumors and non-hodgkin's lymphomas
ActiveUS10328077B2Increase volumeImprove toleranceBiocideOrganic chemistrySolid tumorCancer research
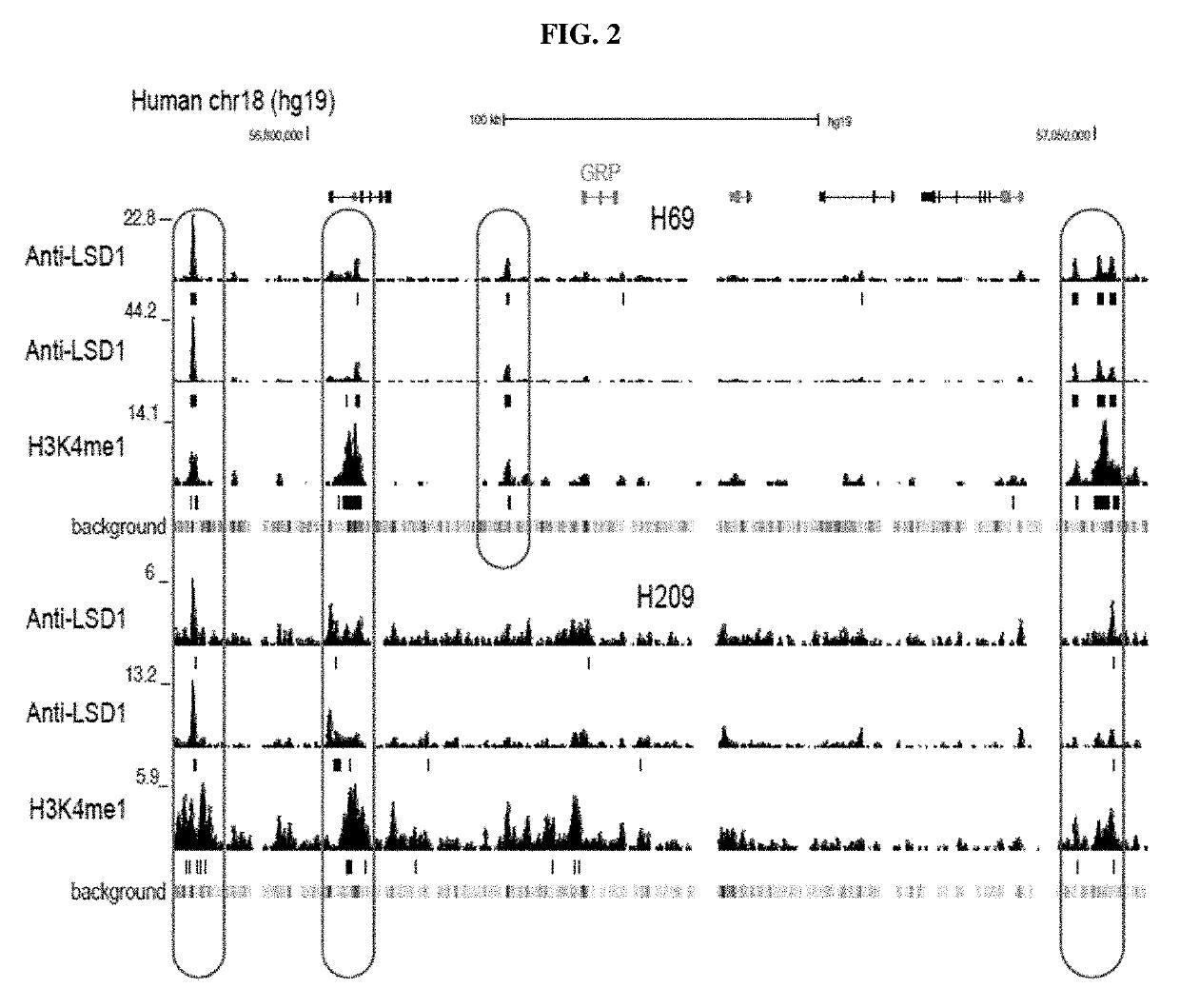
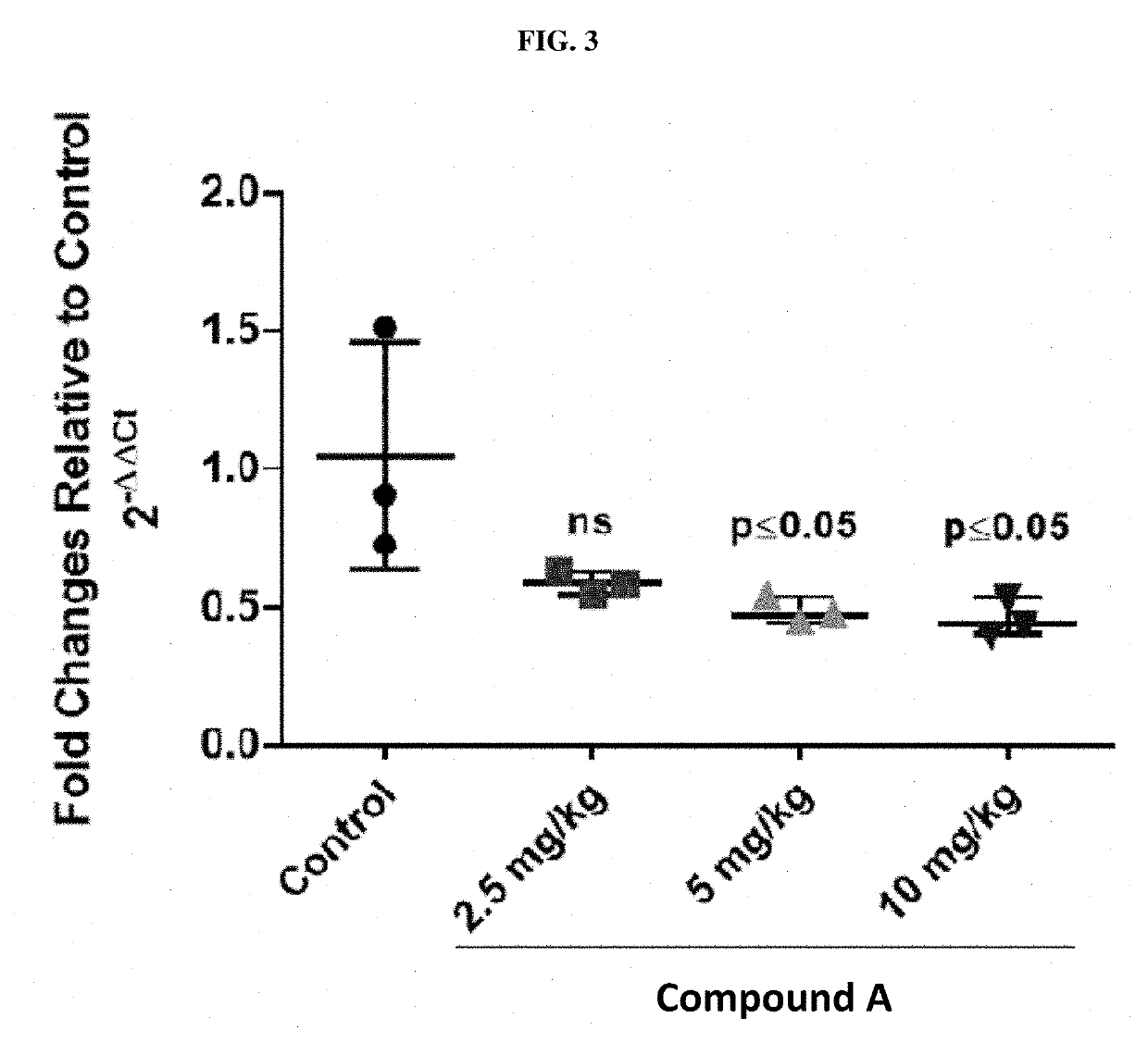
Methods are provided for the treatment of relapsed and / or refractory solid tumors (including neuroendocrine carcinomas (NEC)) and non-Hodgkin's lymphomas (NHLs) and the like, using substituted heterocyclic derivative compounds and pharmaceutical compositions comprising compounds useful for the inhibition of lysine specific demethylase-1 (LSD-1).
Owner:CELGENE QUANTICEL RES
Nitrogenous ring compounds as tyrosine kinase inhibitors
ActiveCN103864792BBTK kinase inhibition is goodGood biological stabilityNervous disorderOrganic chemistryImmunologic disordersDisease
The invention belongs to the technical field of medicine, particularly relates to a heterocyclic nitrogen compound which acts as a tyrosine kinase inhibitor and is represented by a general formula (I) as well as a deuterated material, pharmaceutically acceptable salt or stereisomer thereof. In the general formula (I), X, W, R1, R2, R3, L1, L2, a, b, c, d, e, p, q, a ring A and a ring B are defined in specification. The invention further relates to a preparation method of the compounds, a pharmaceutic preparation containing the compounds, and important actions of the compounds in treatment of leukemia (such as B cell chronic lymphocytic carcinoma and non-hodgkin lymphoma) related to B cell, and autoimmune diseases (such as rheumatoid arthritis and systemic lupus erythematosus).
Owner:KBP BIOSCIENCES CO LTD
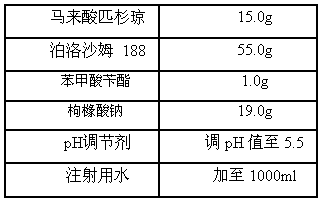
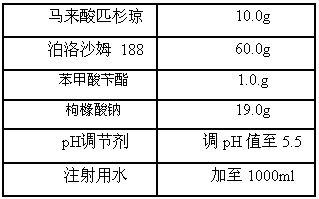
A kind of injection for treating non-Hodgkin's lymphoma and preparation method thereof
InactiveCN105769757BQuality improvementMeet quality requirementsOrganic active ingredientsPharmaceutical delivery mechanismBULK ACTIVE INGREDIENTActive ingredient
The invention belongs to the technical field of medicine, and particularly relates to an injection for treating NHL (non-hodgkin lymphoma) and a preparation method thereof. The injection for treating NHL is mainly prepared from active ingredients of maleic acid pixantrone, sodium chloride, dispersing agents, nzyl benzoate, sodium citrate, pH regulators and injection water. The injection for treating NHL has the advantages that the quality is stable; all indexes conform to the quality requirements; the process is simple; the operation is convenient.
Owner:QINGDAO TUMOR HOSPITAL
Anti-CD20 monoclonal antibody, preparation method and application thereof
InactiveCN103012590BPracticalSimple preparation processAntipyreticAnalgesicsAbnormal tissue growthImmunologic disorders
Owner:SHANGHAI HANKANG BIO PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com