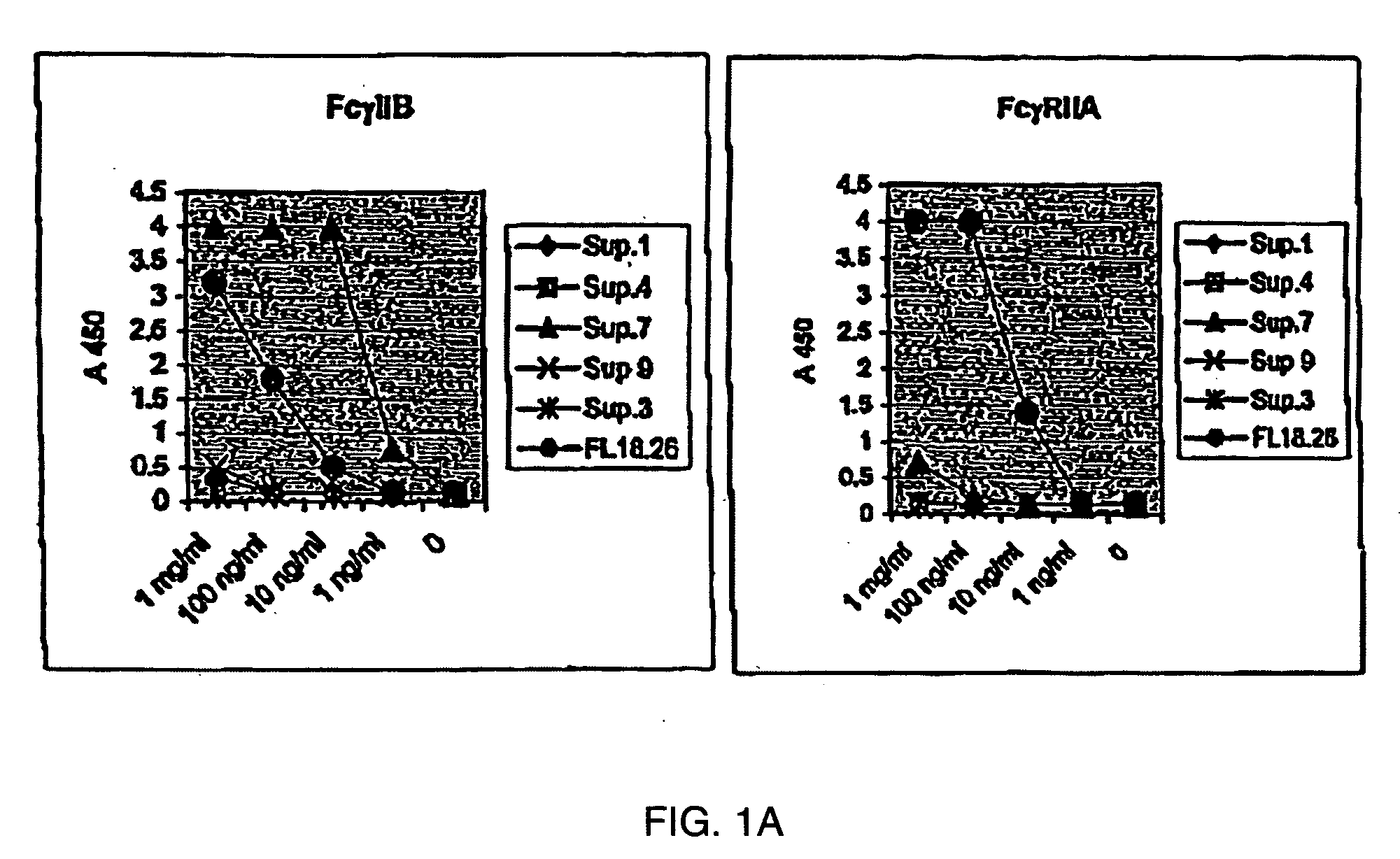
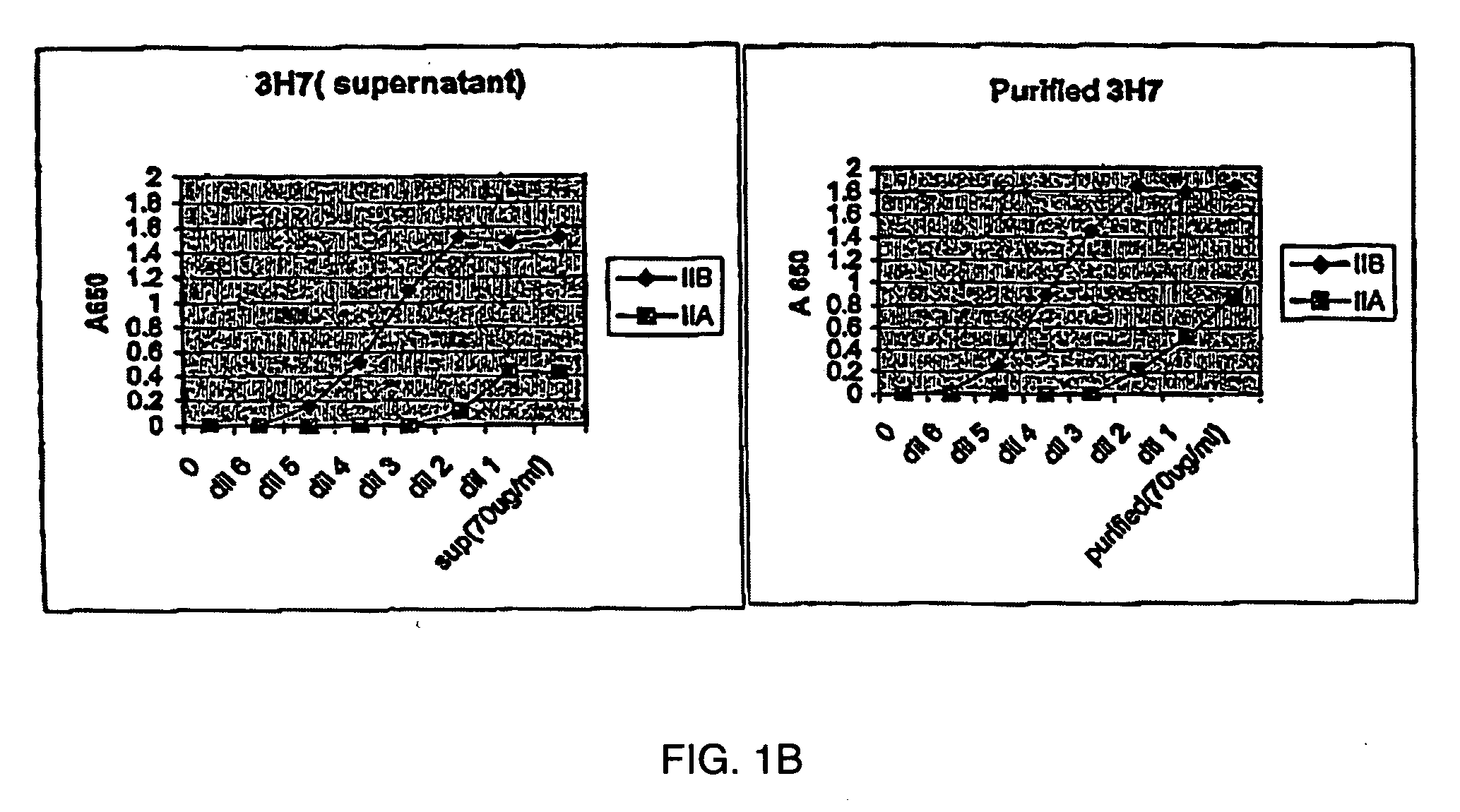
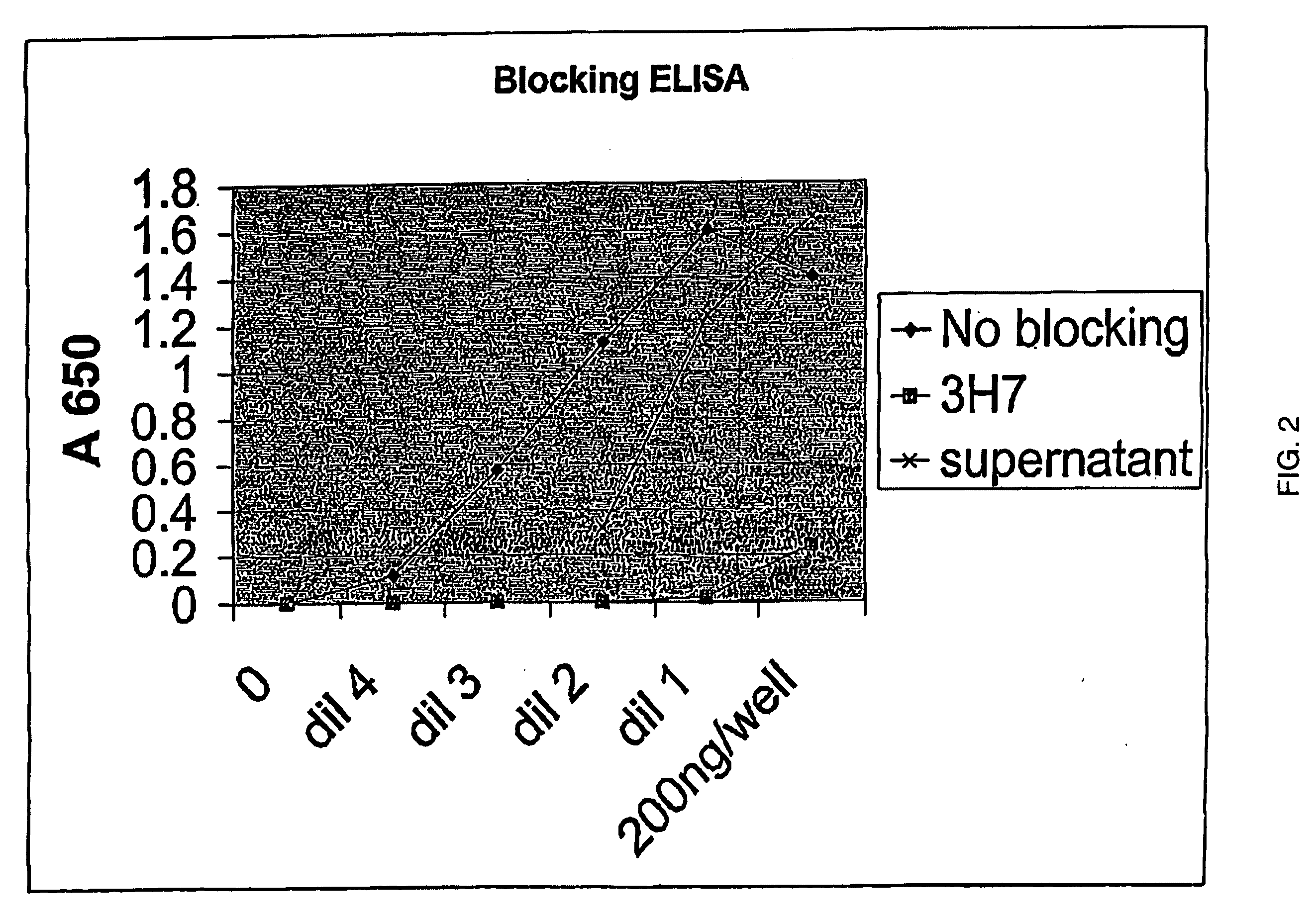
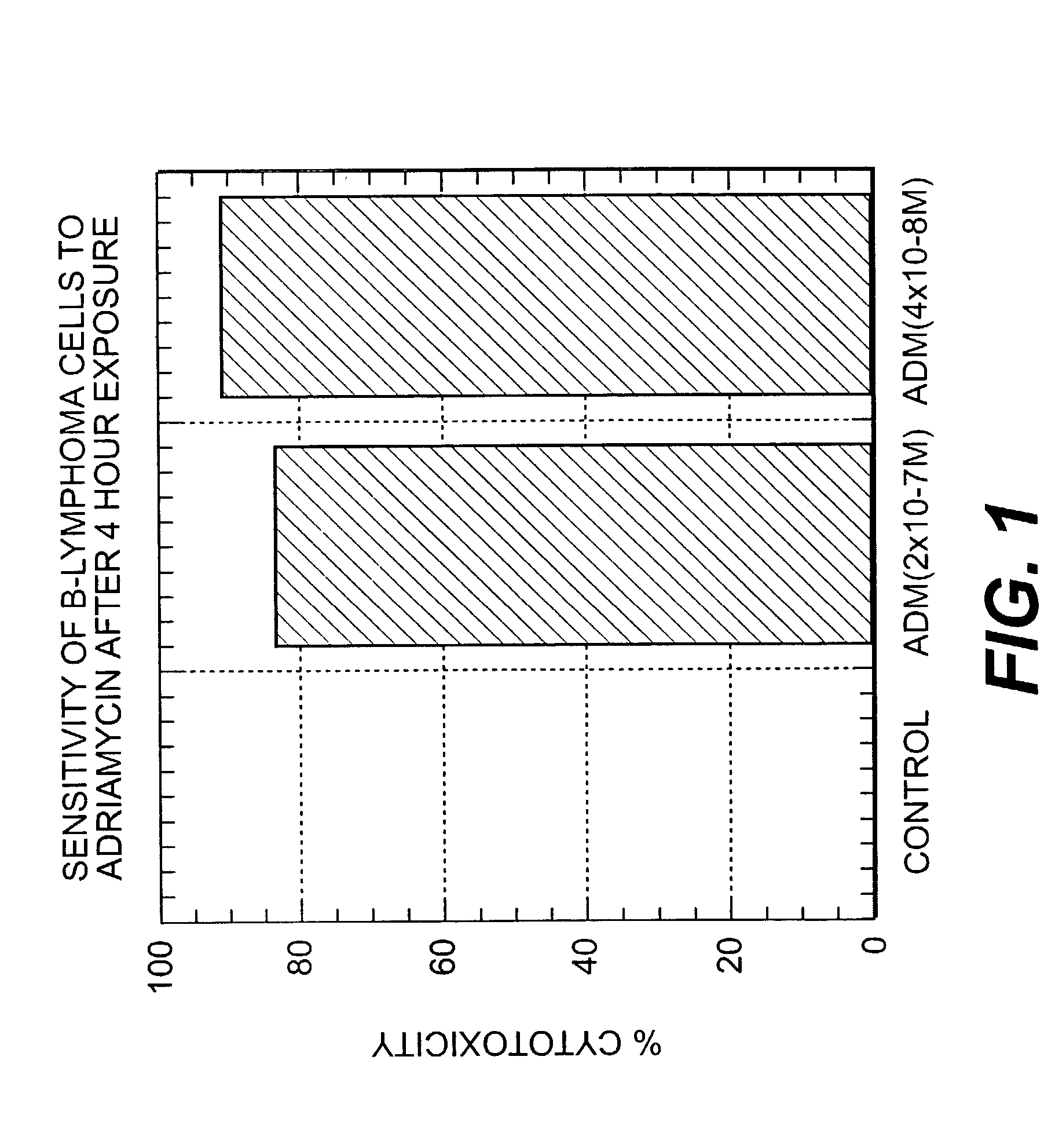
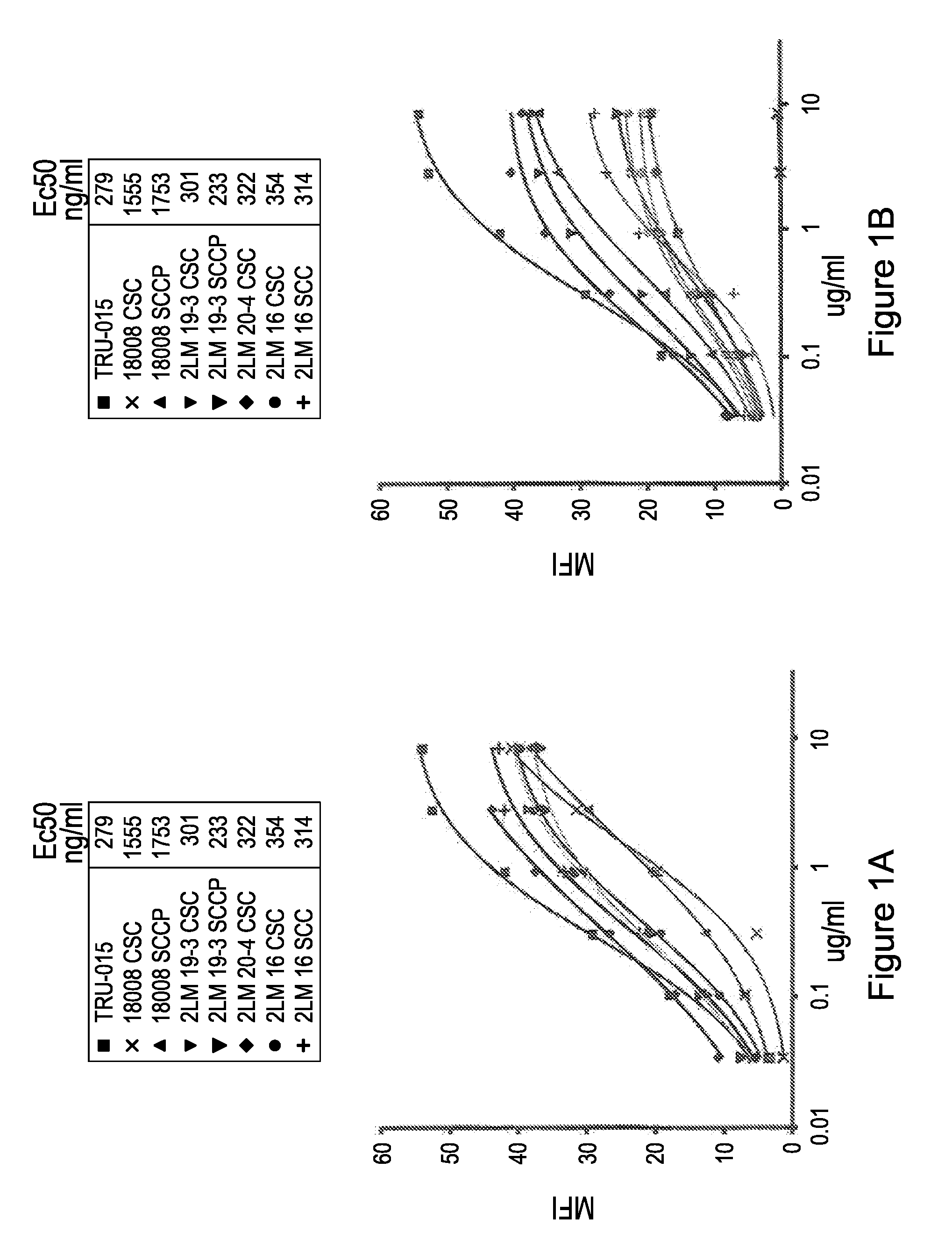
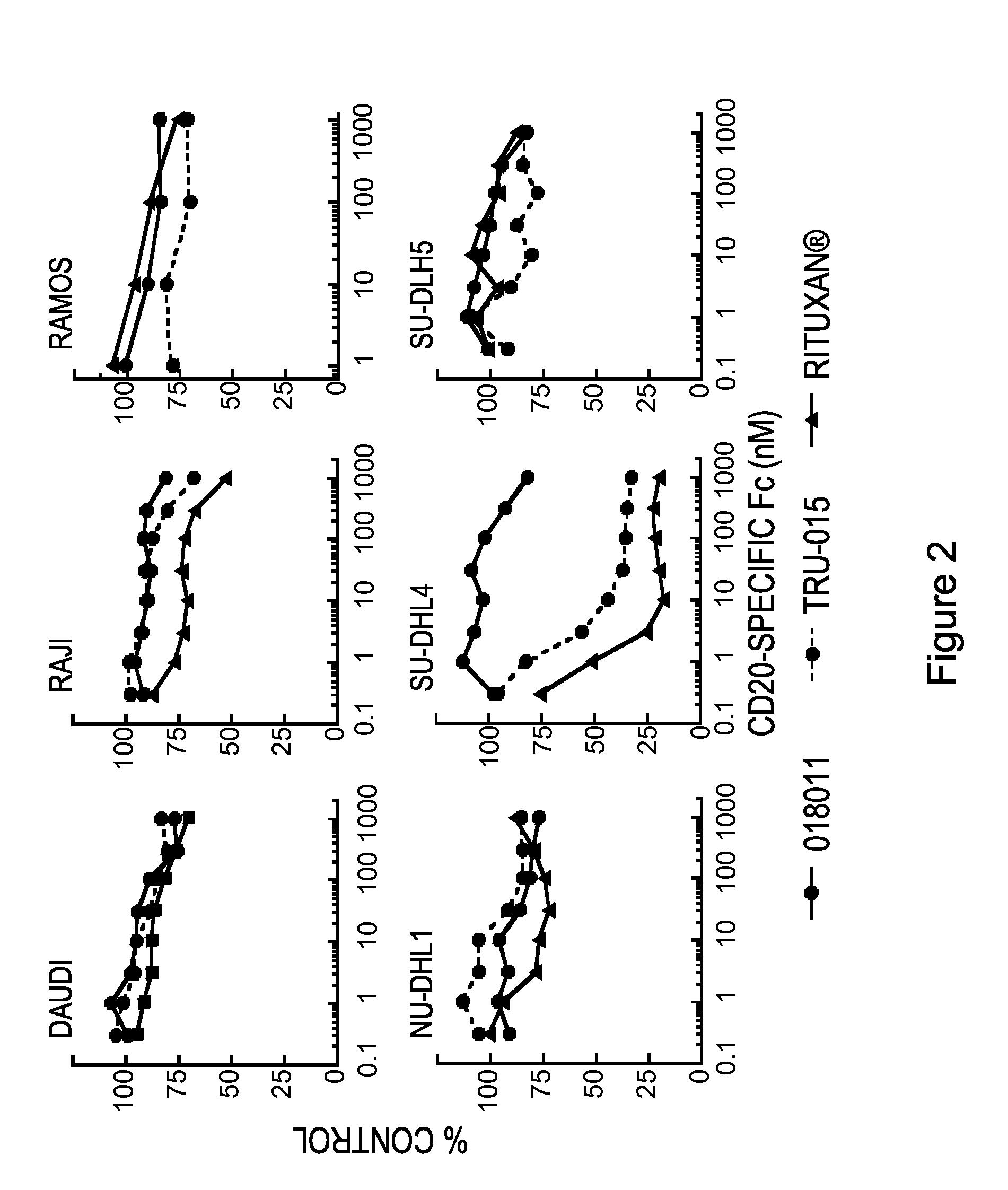
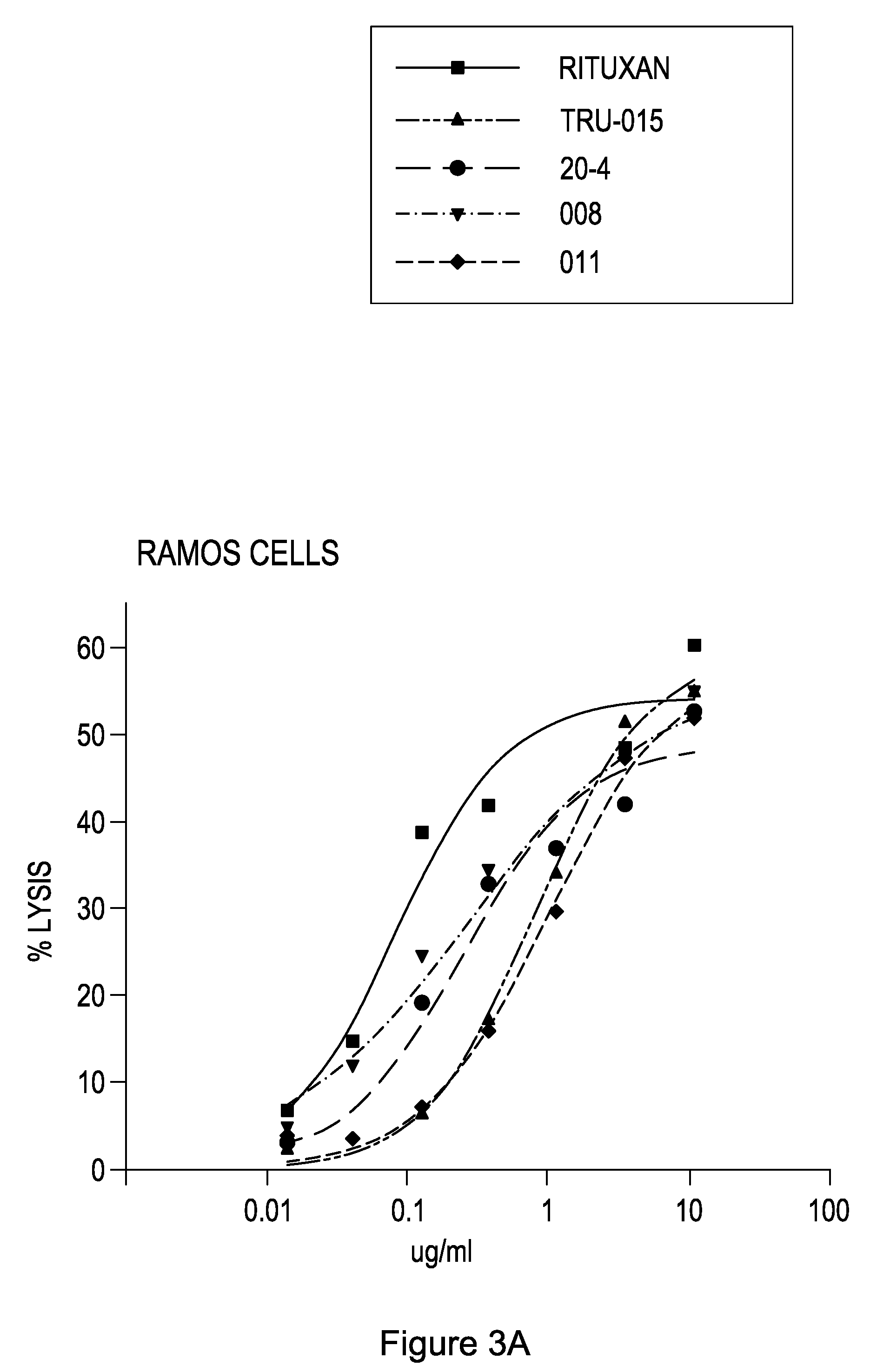
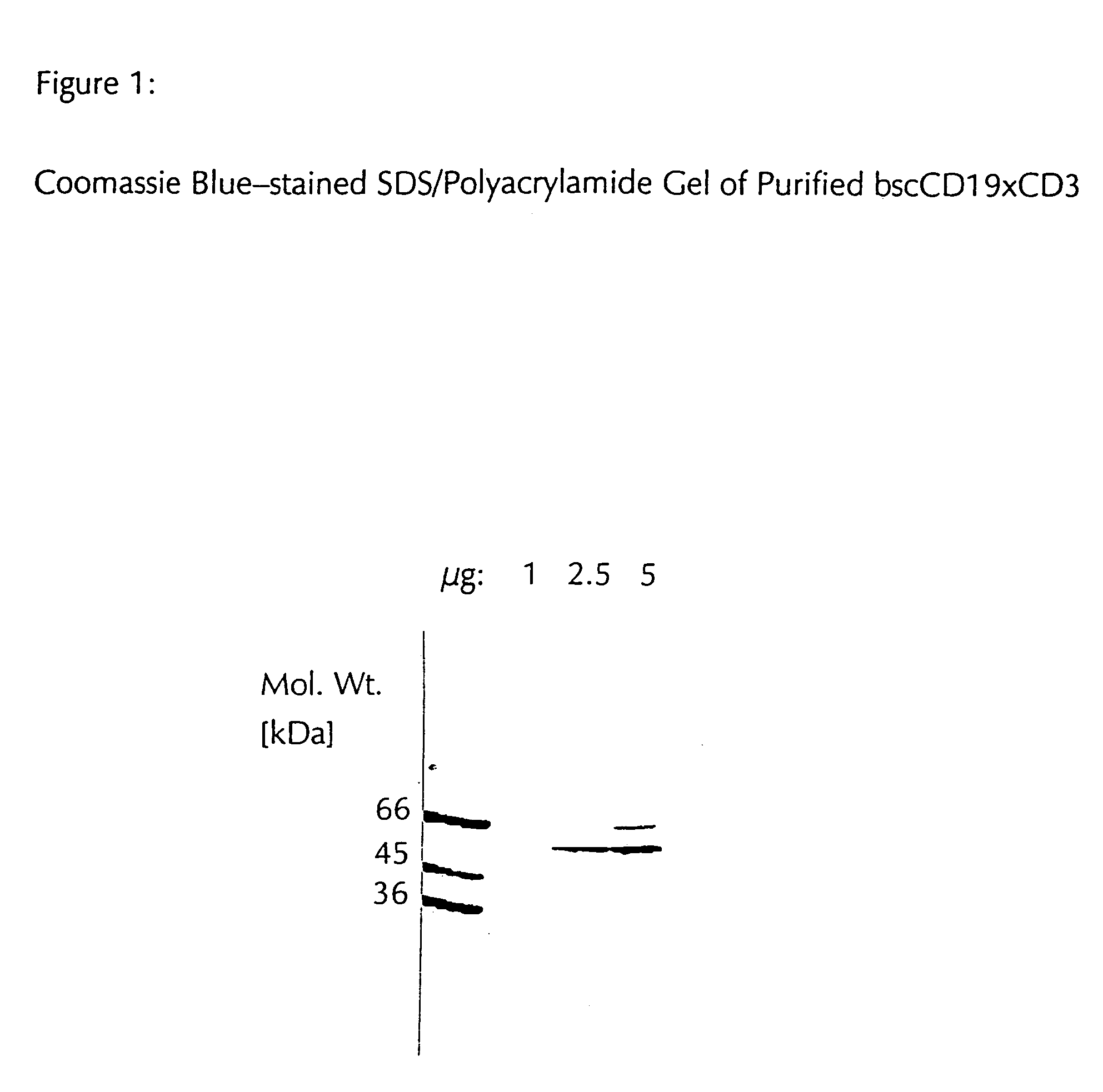
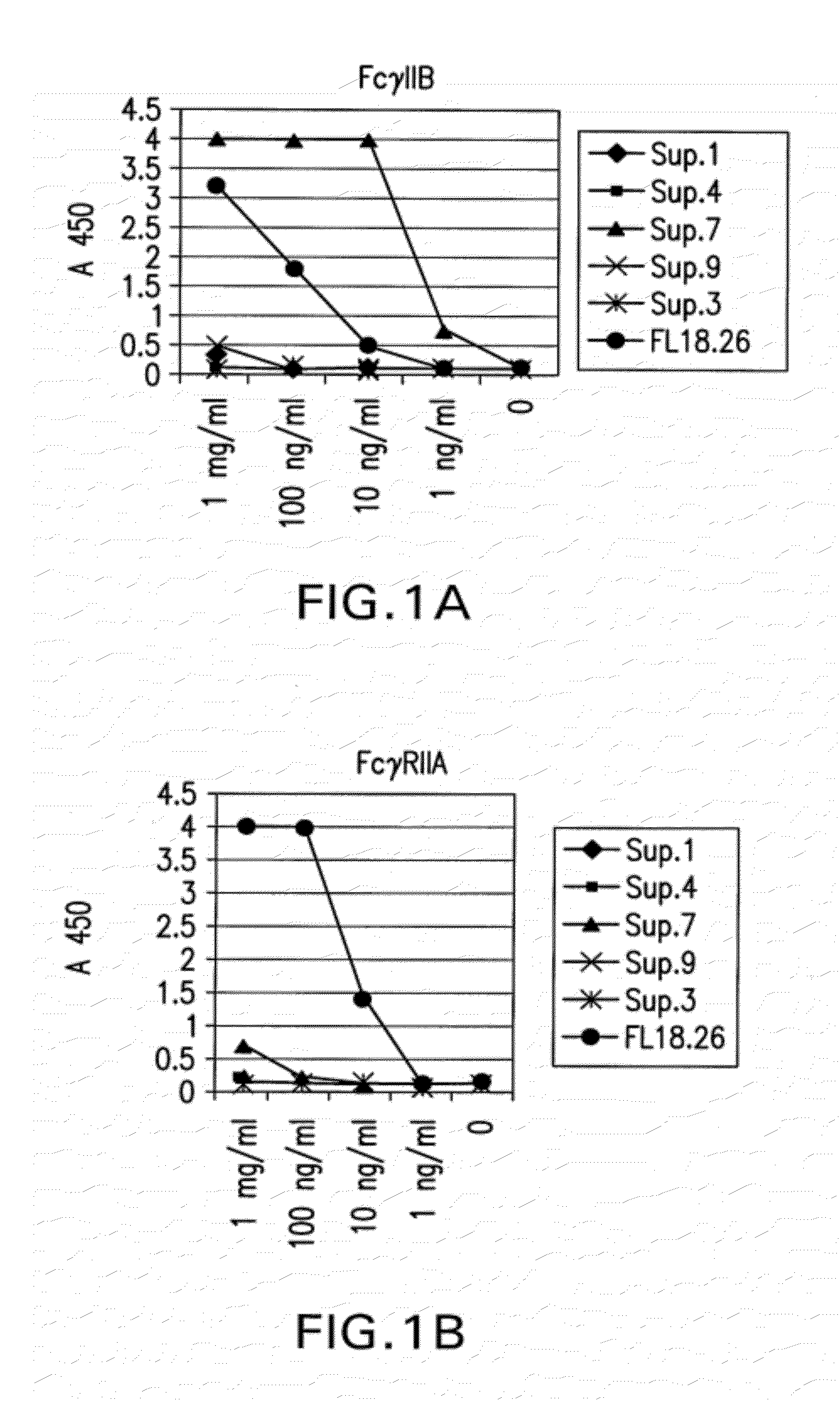
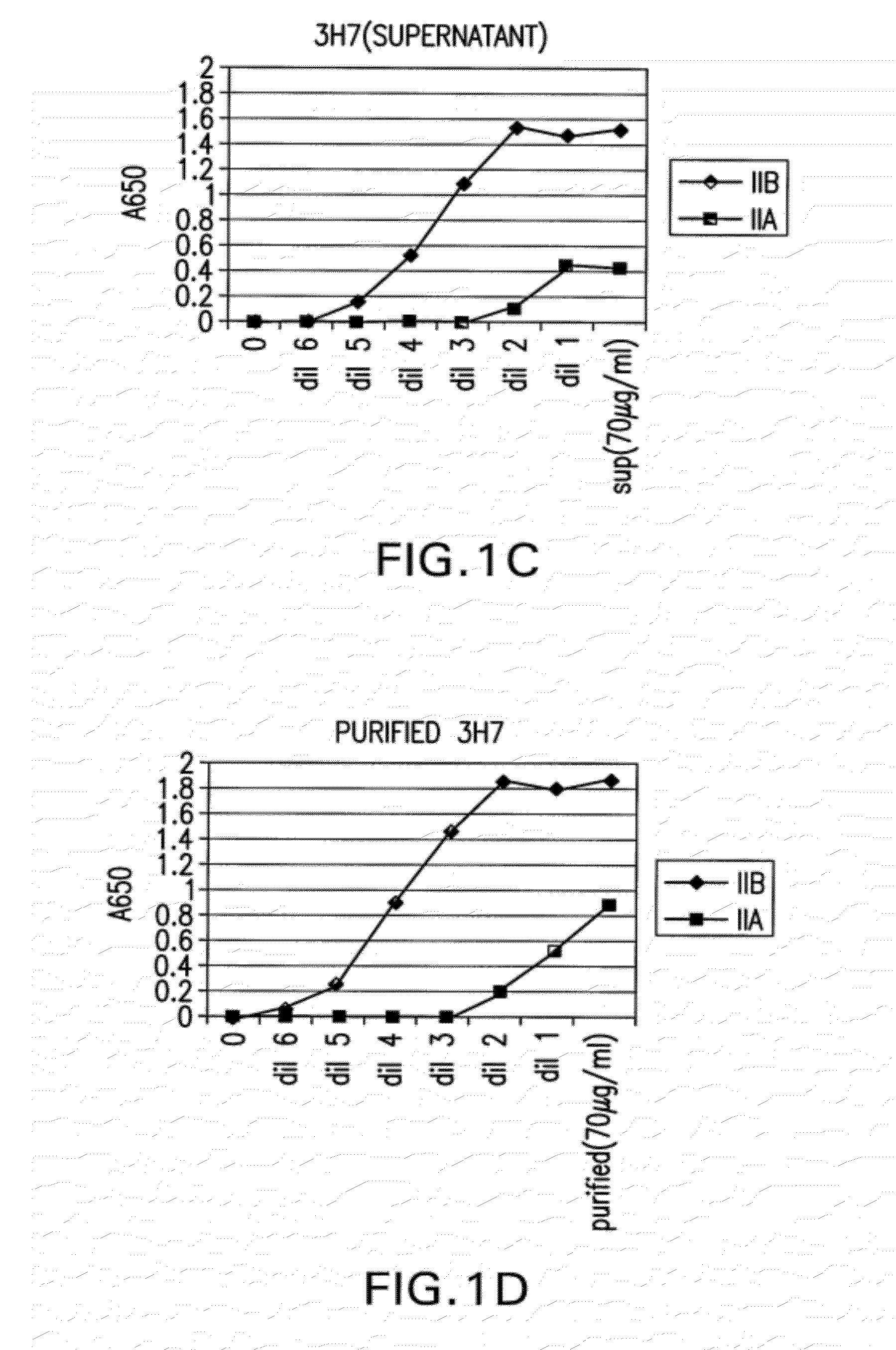
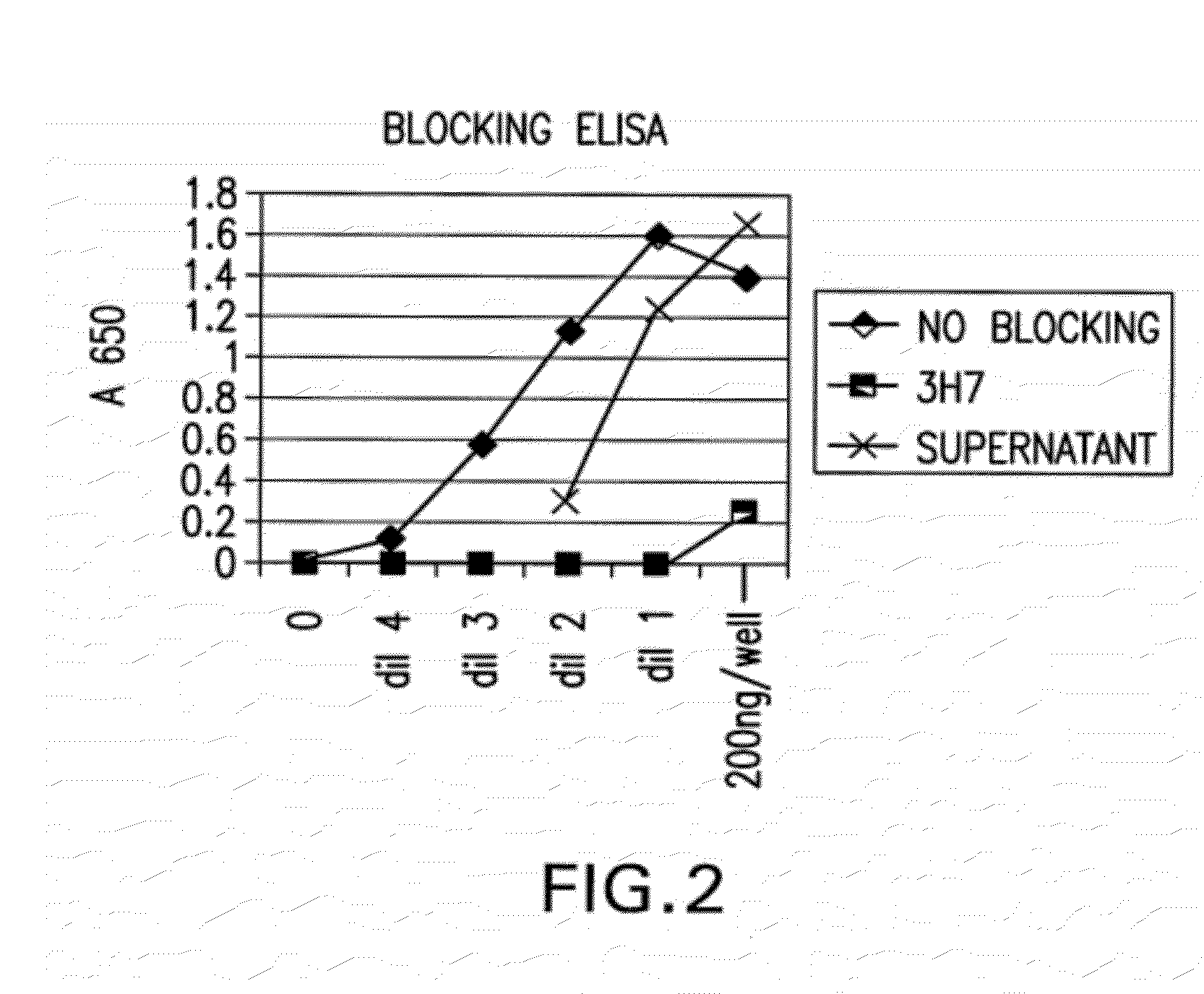
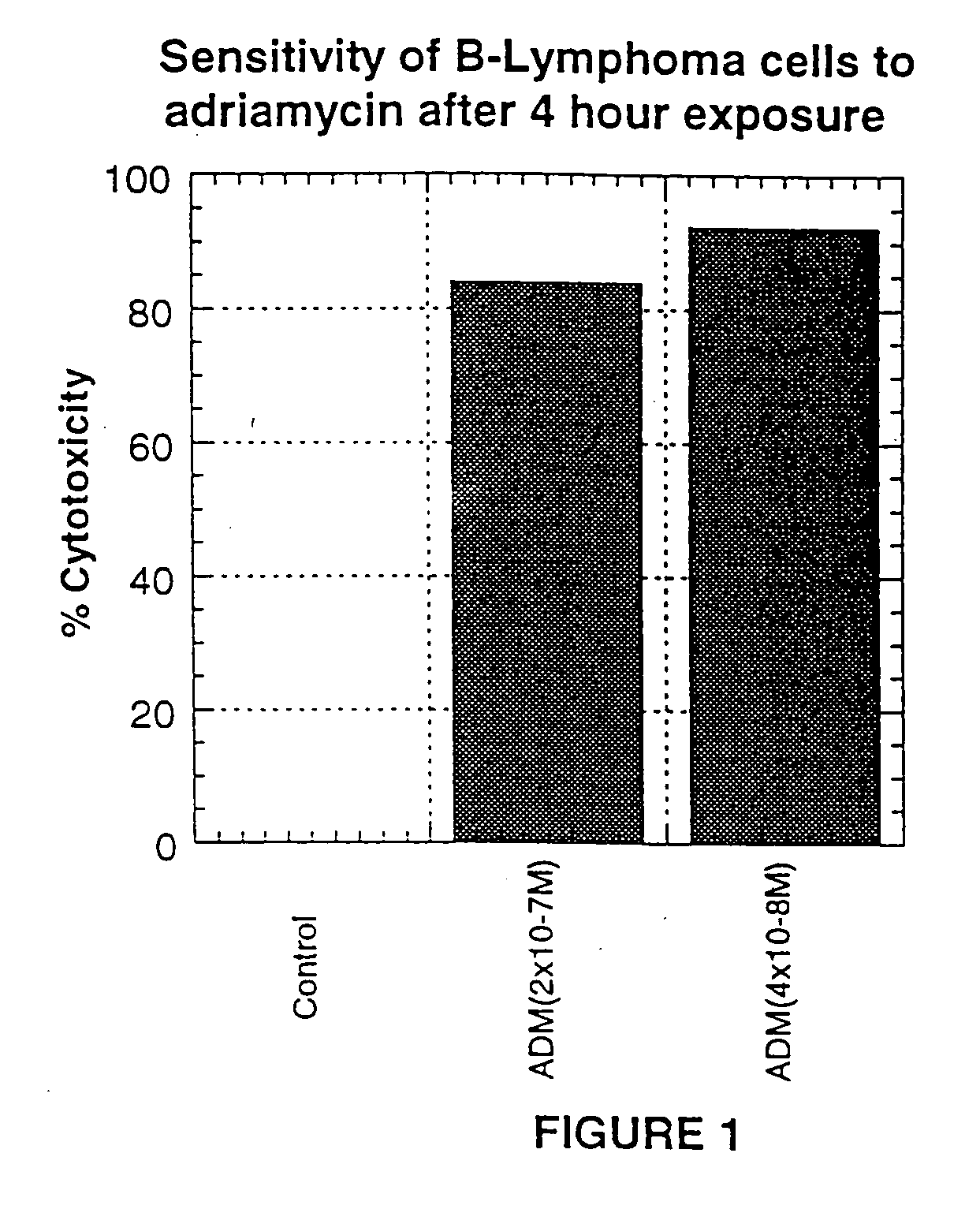
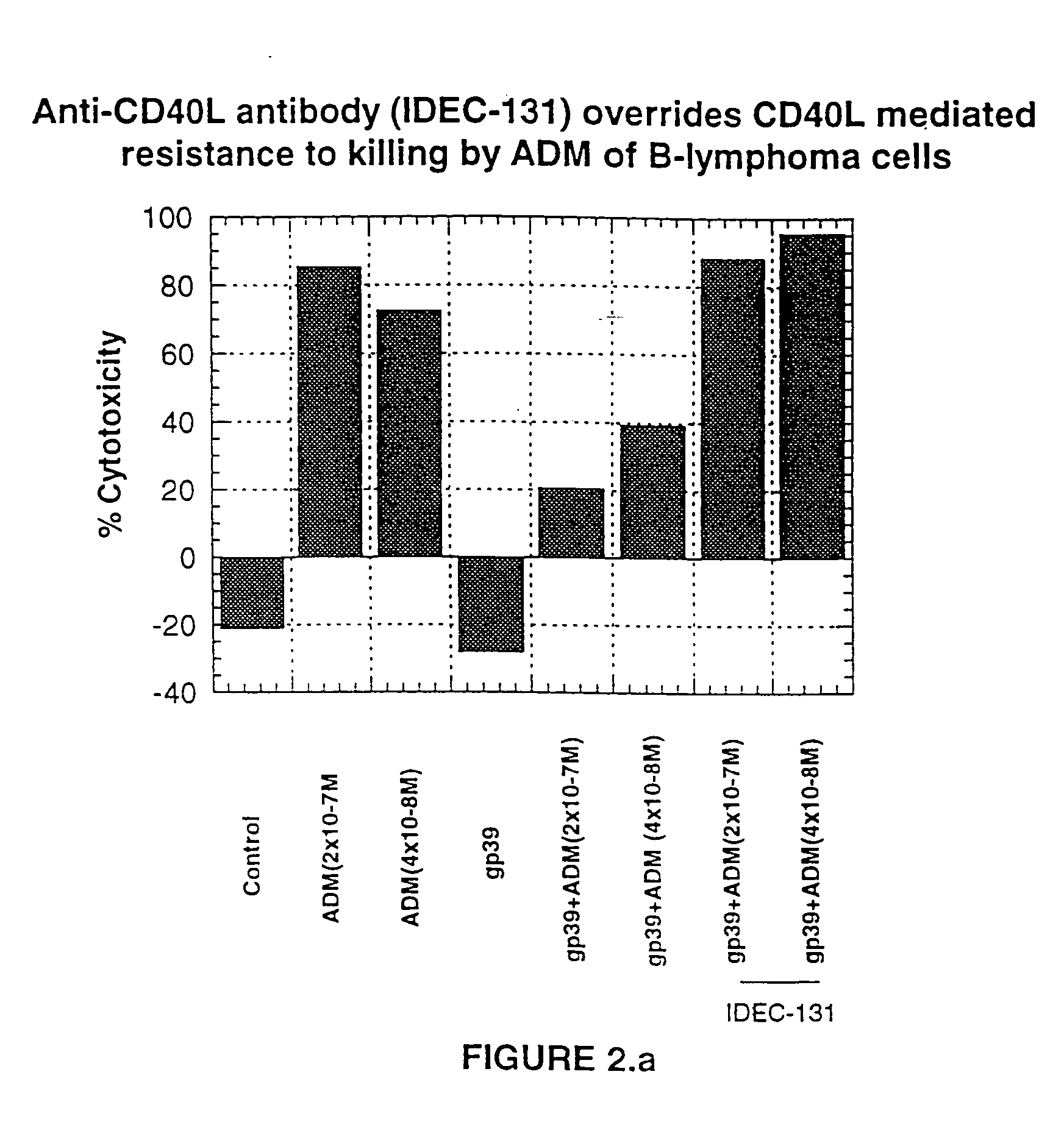
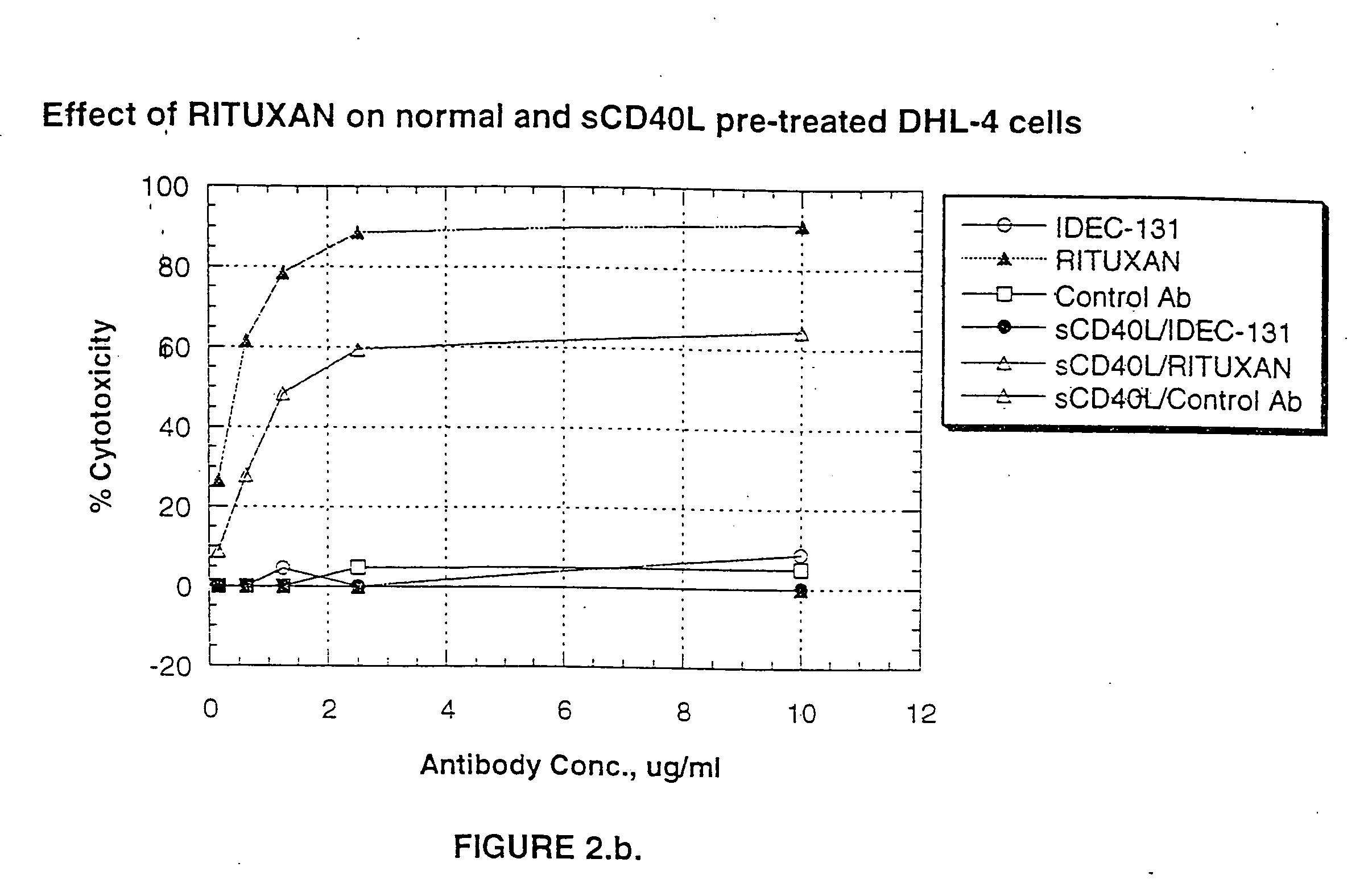
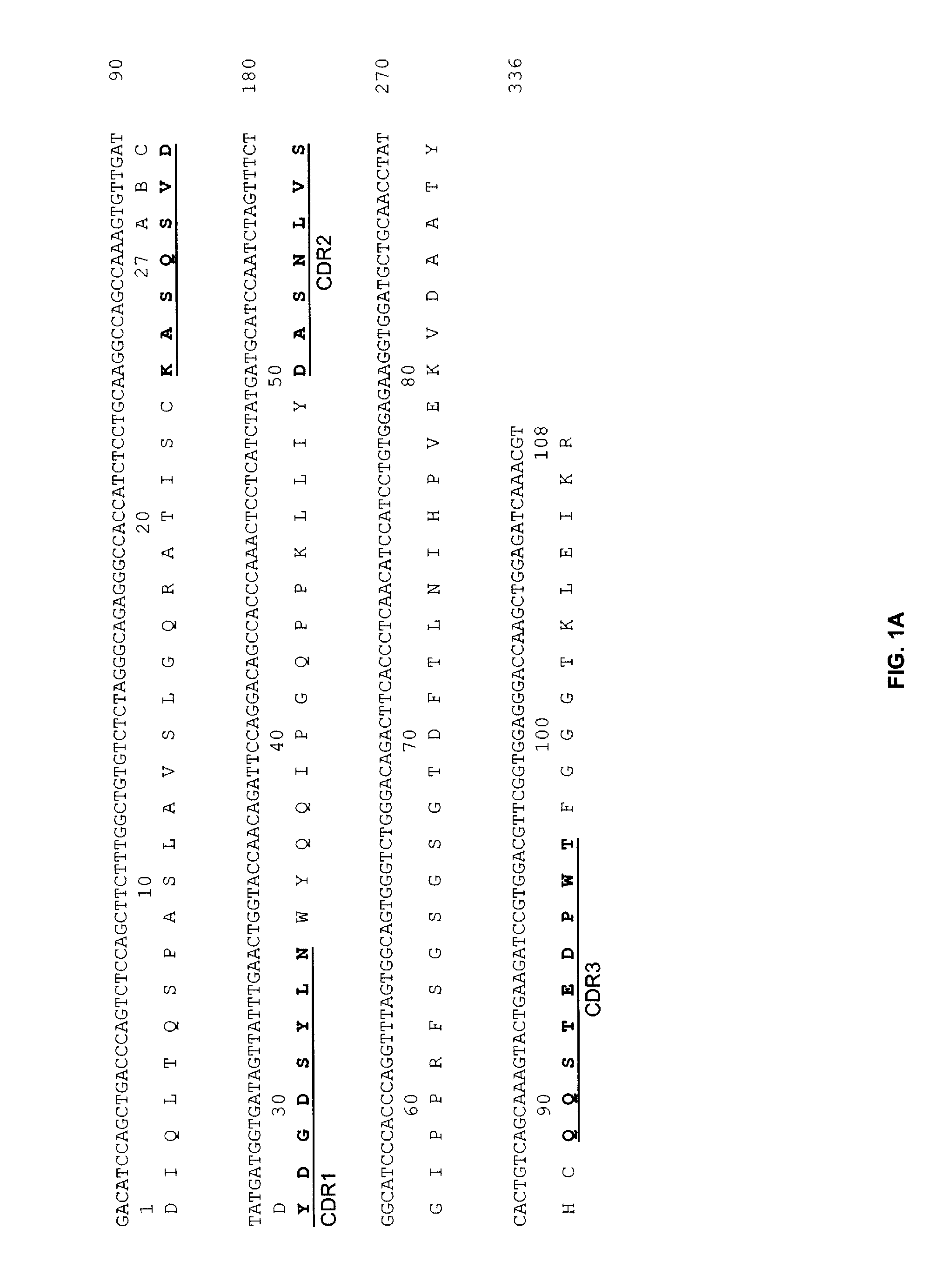
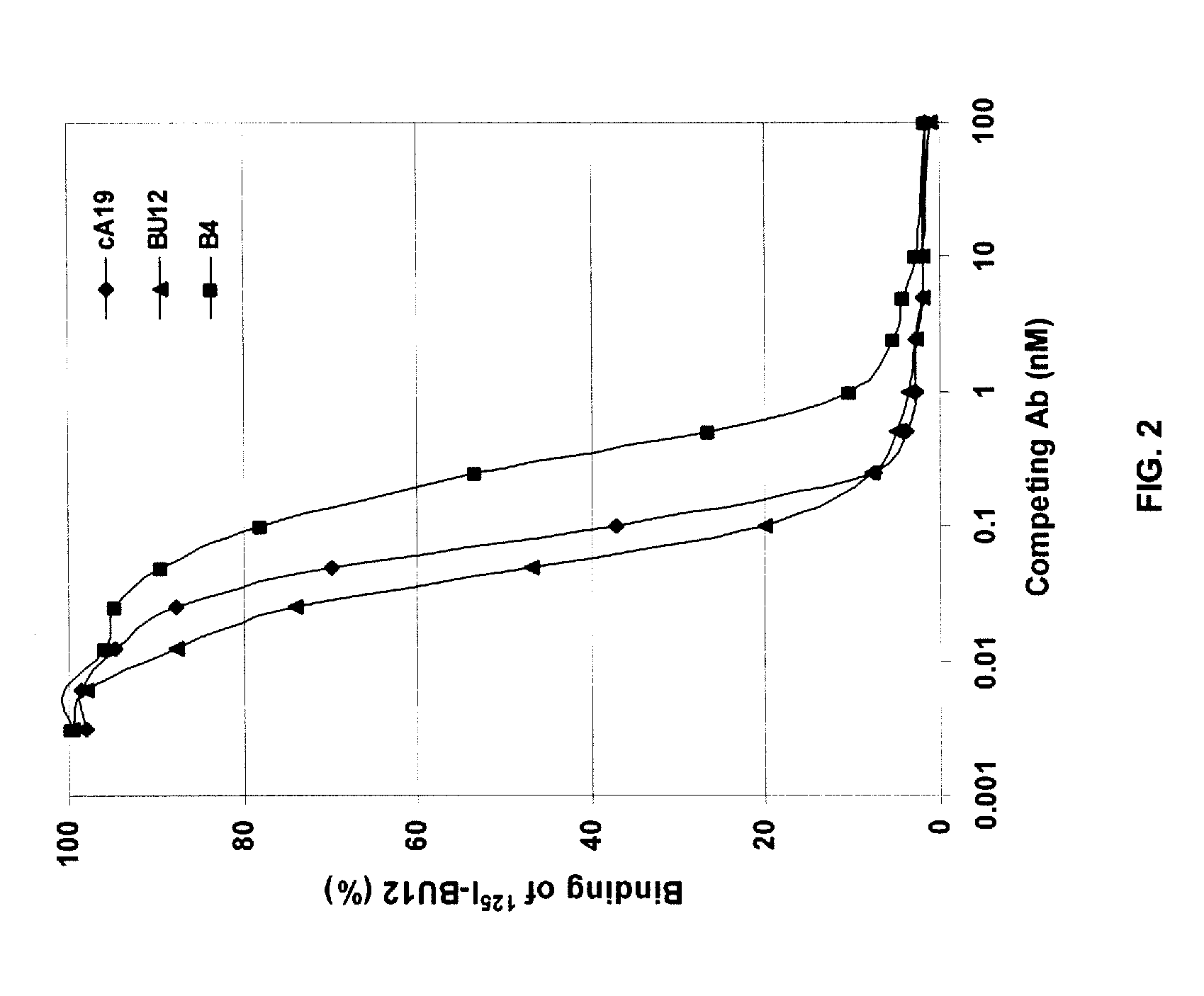
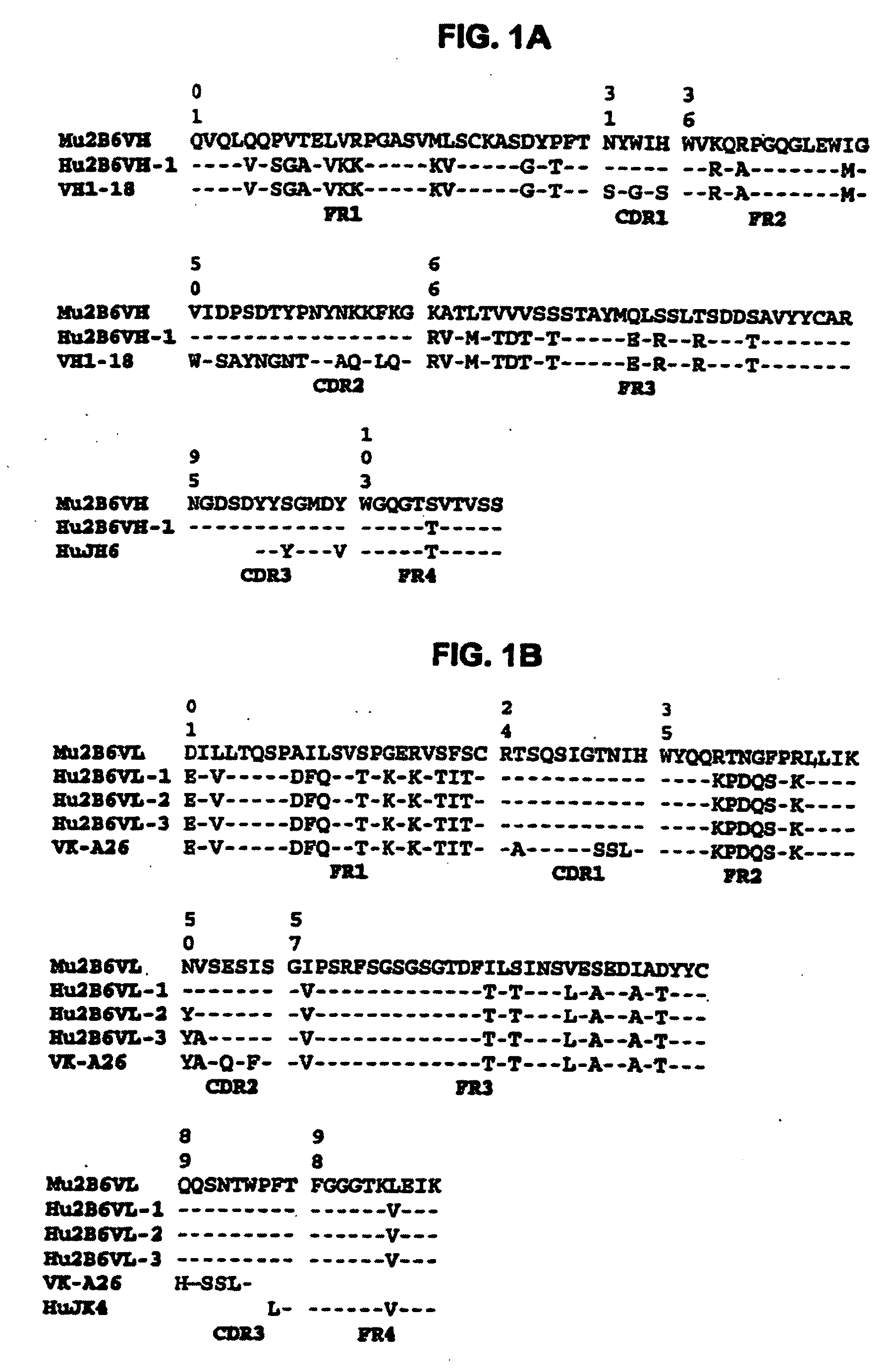
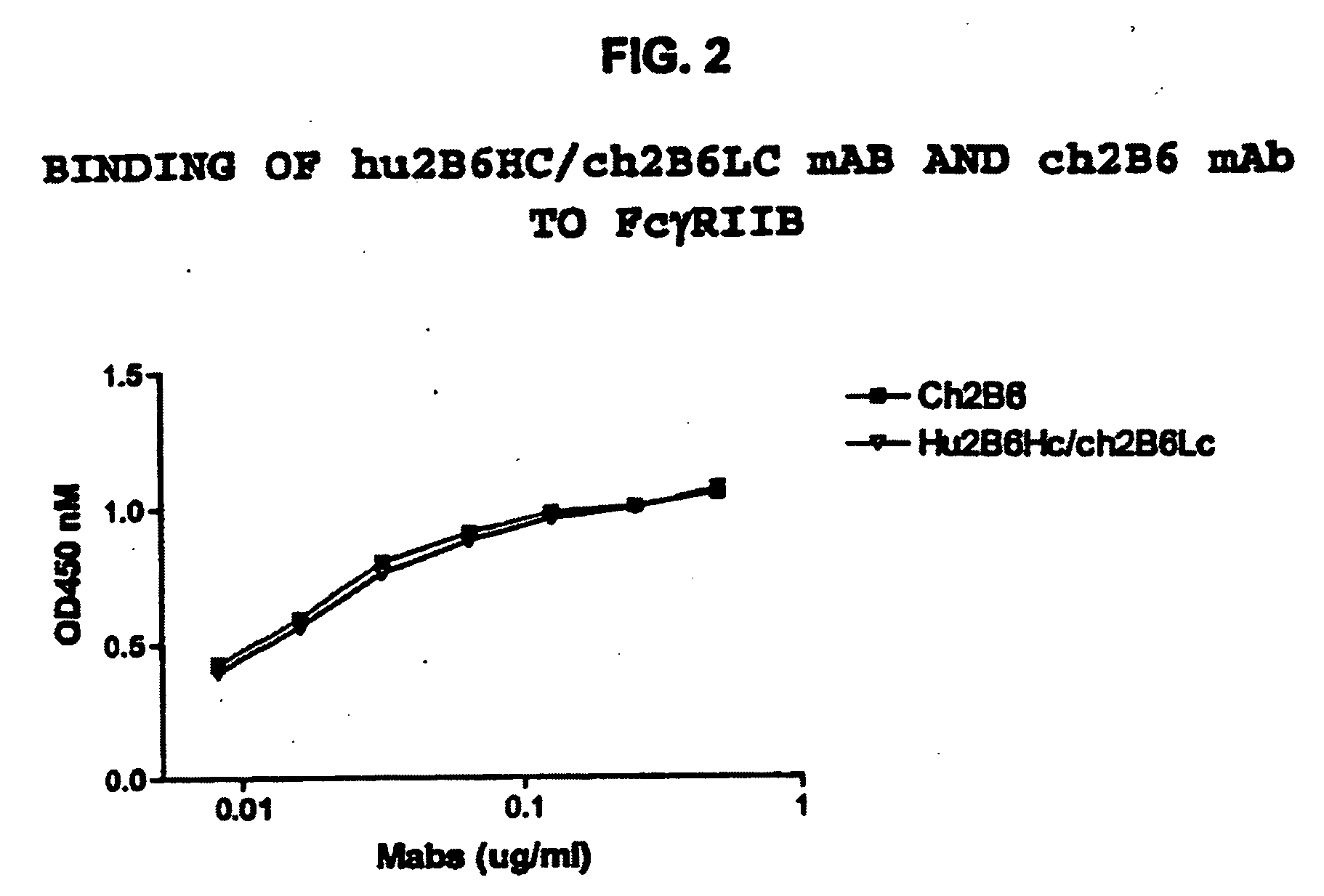
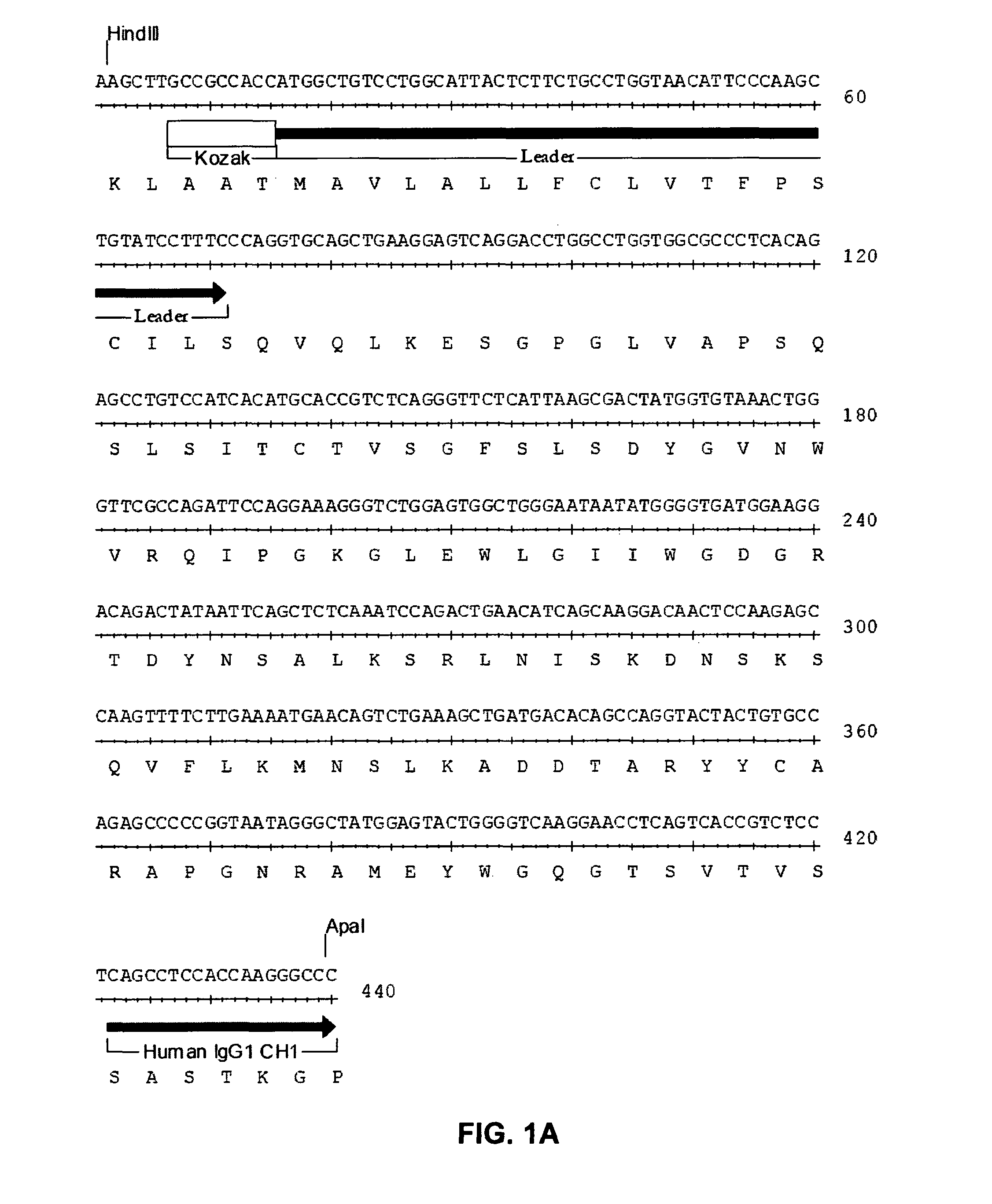
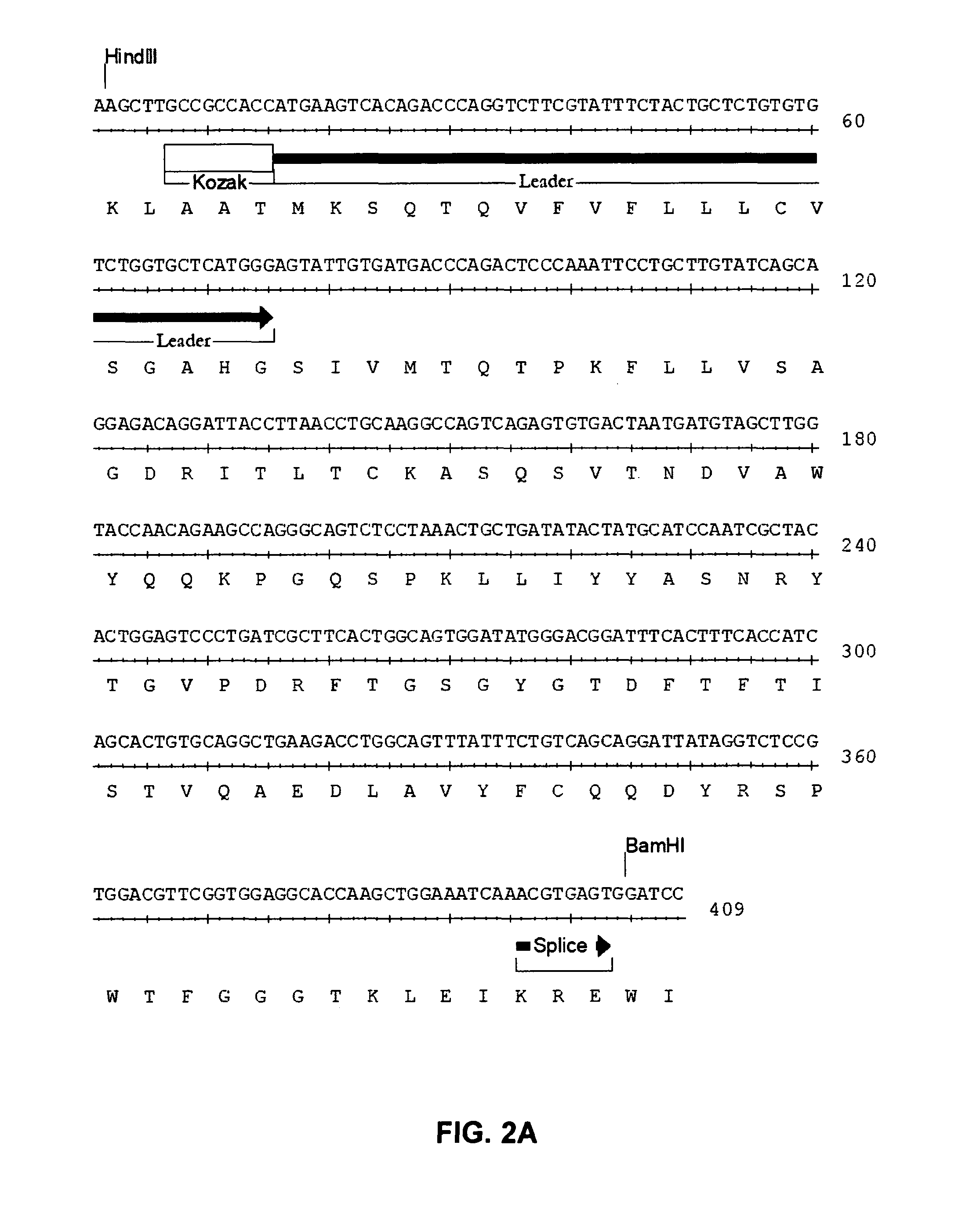
Patents
Literature
120 results about "B cell malignancy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
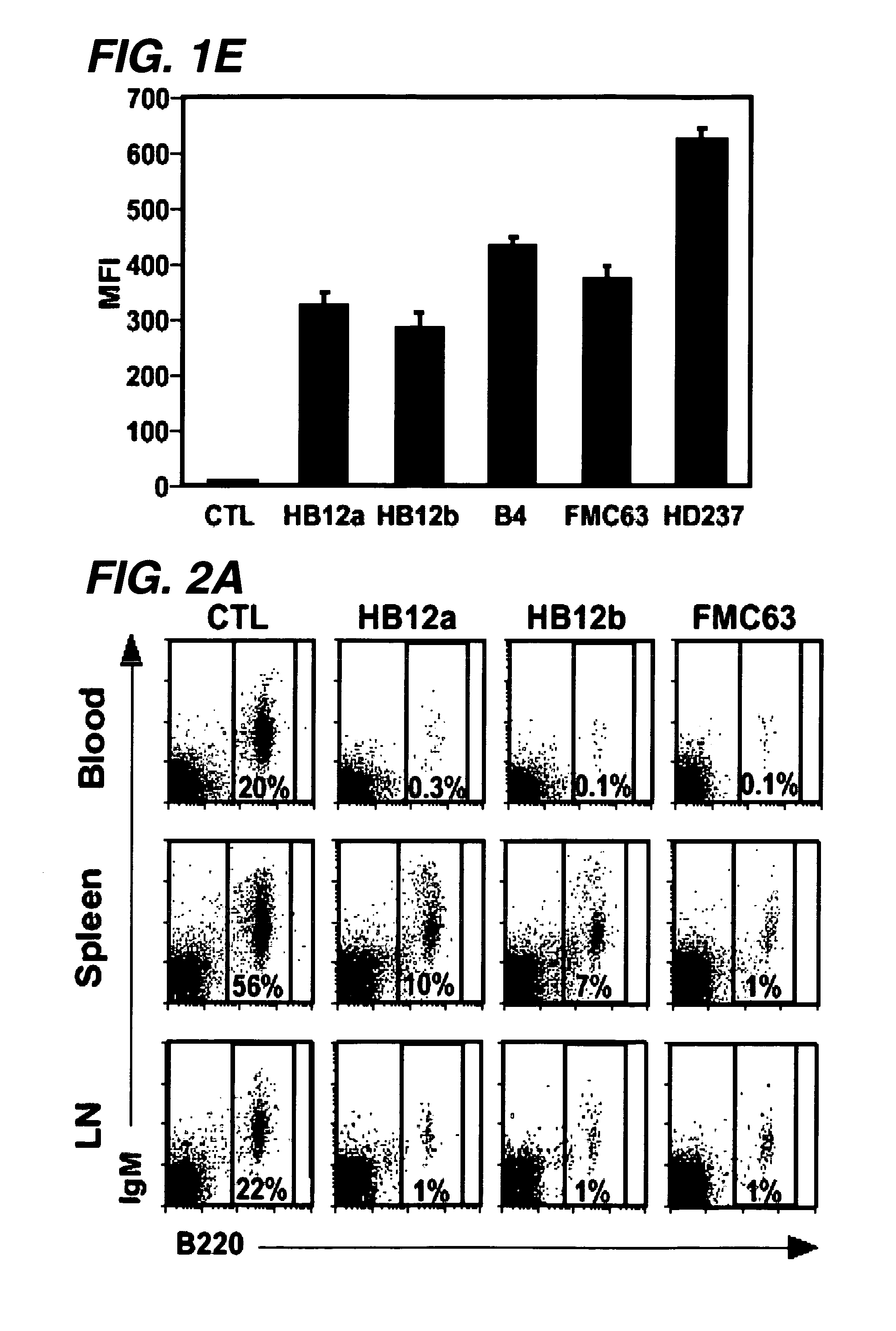
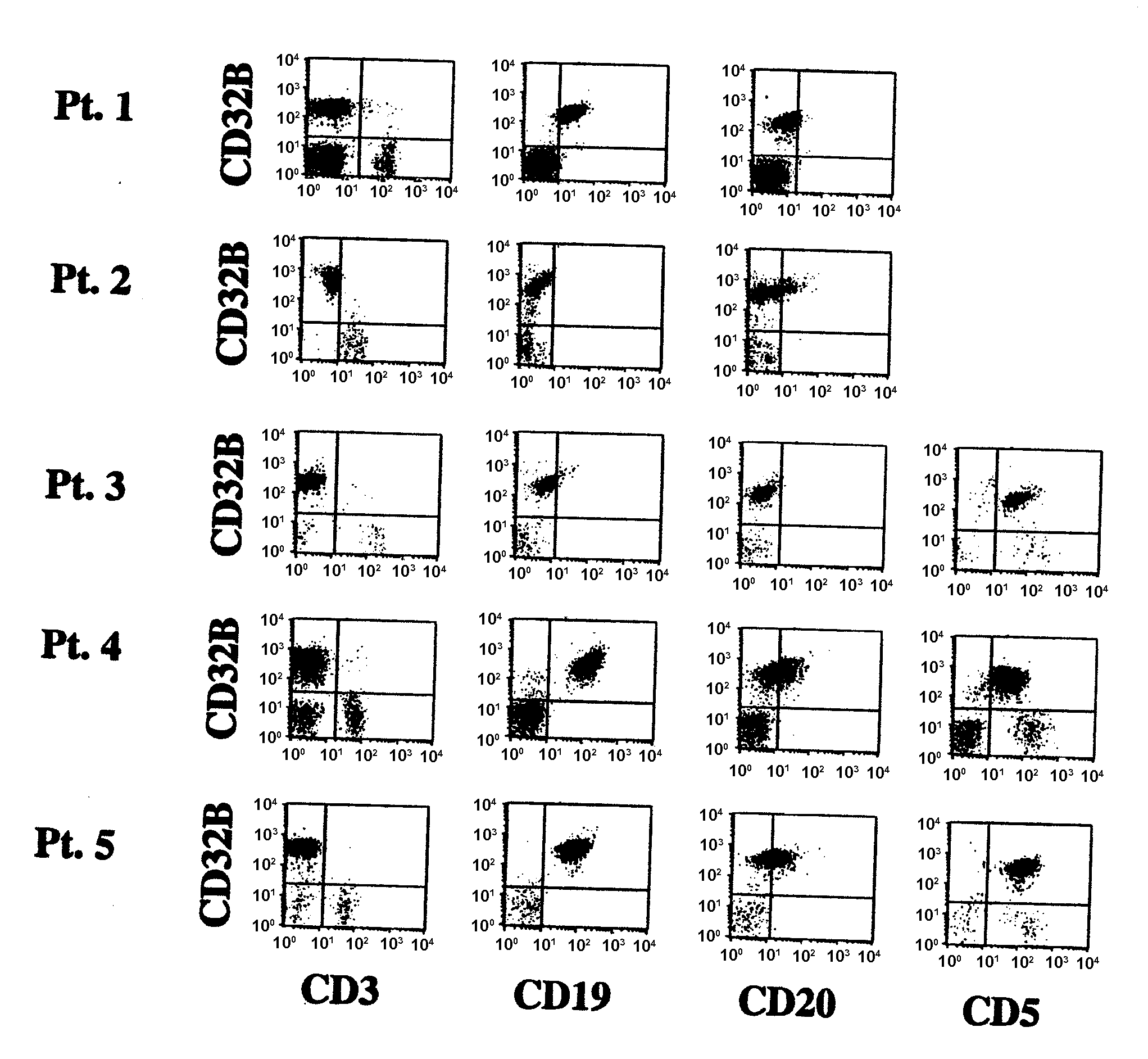
B-cell malignancies include non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL). NHLs are a heterogeneous group of more than 30 cancers of B lymphocytes and T lymphocytes.1.
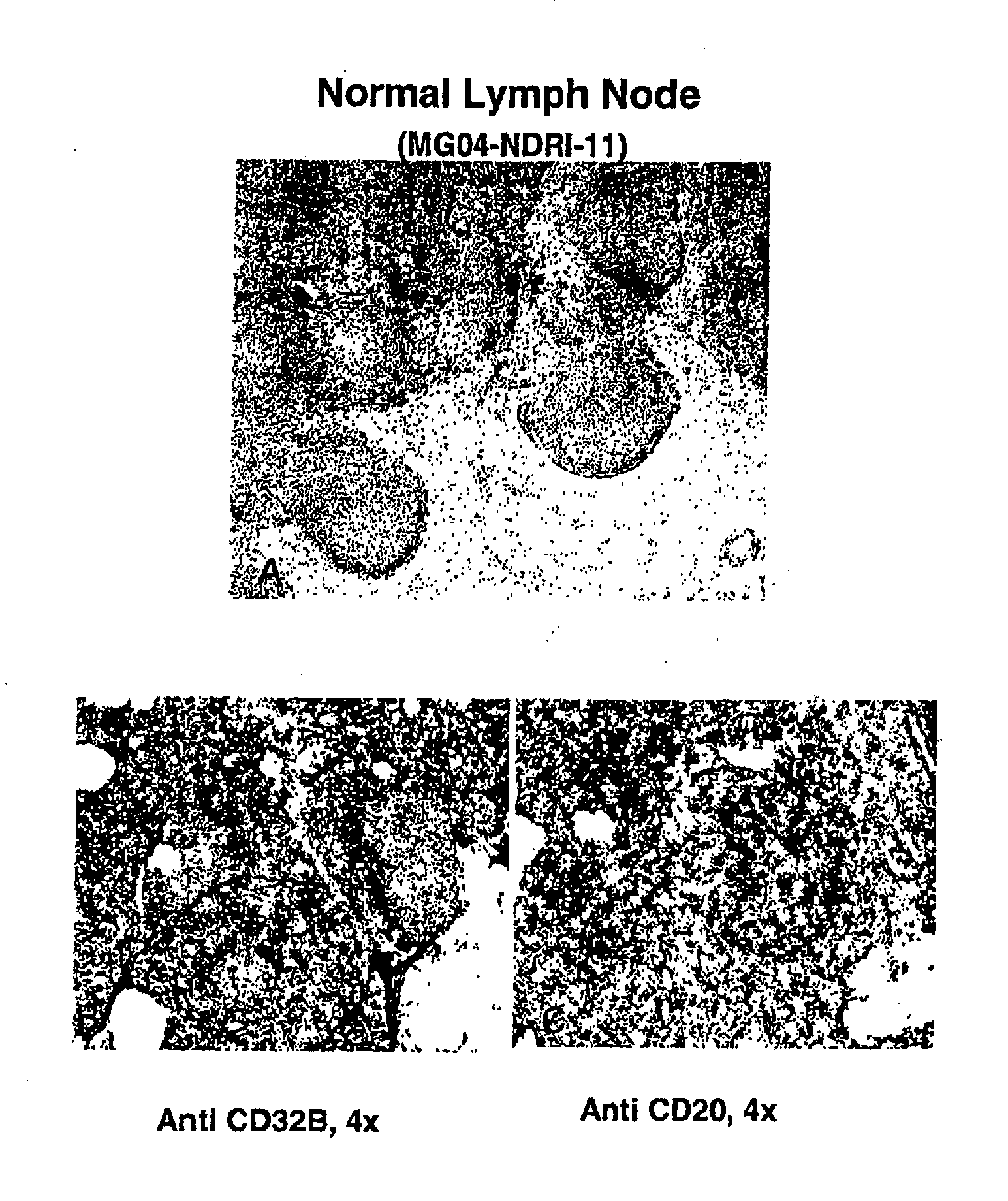
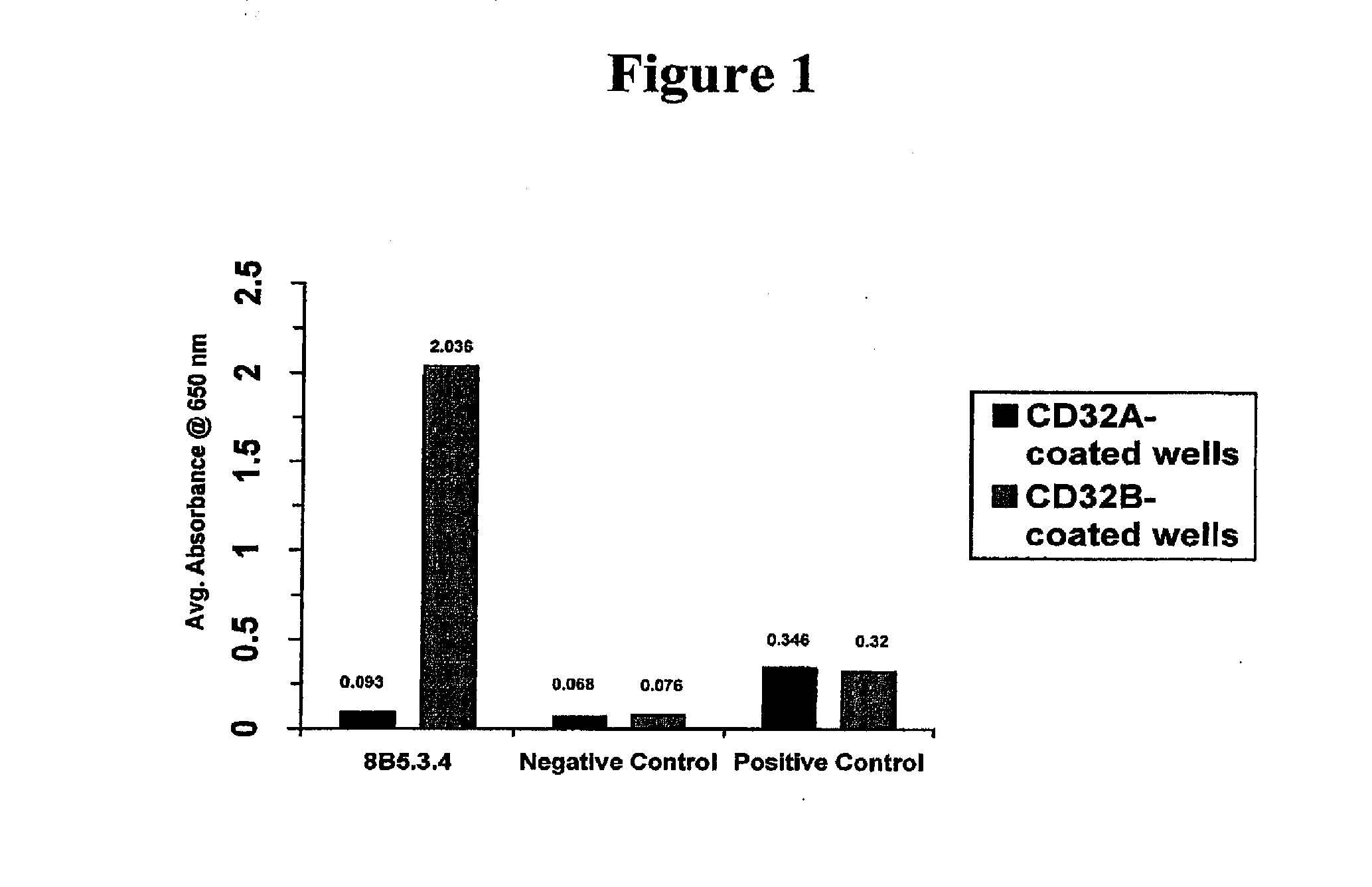
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
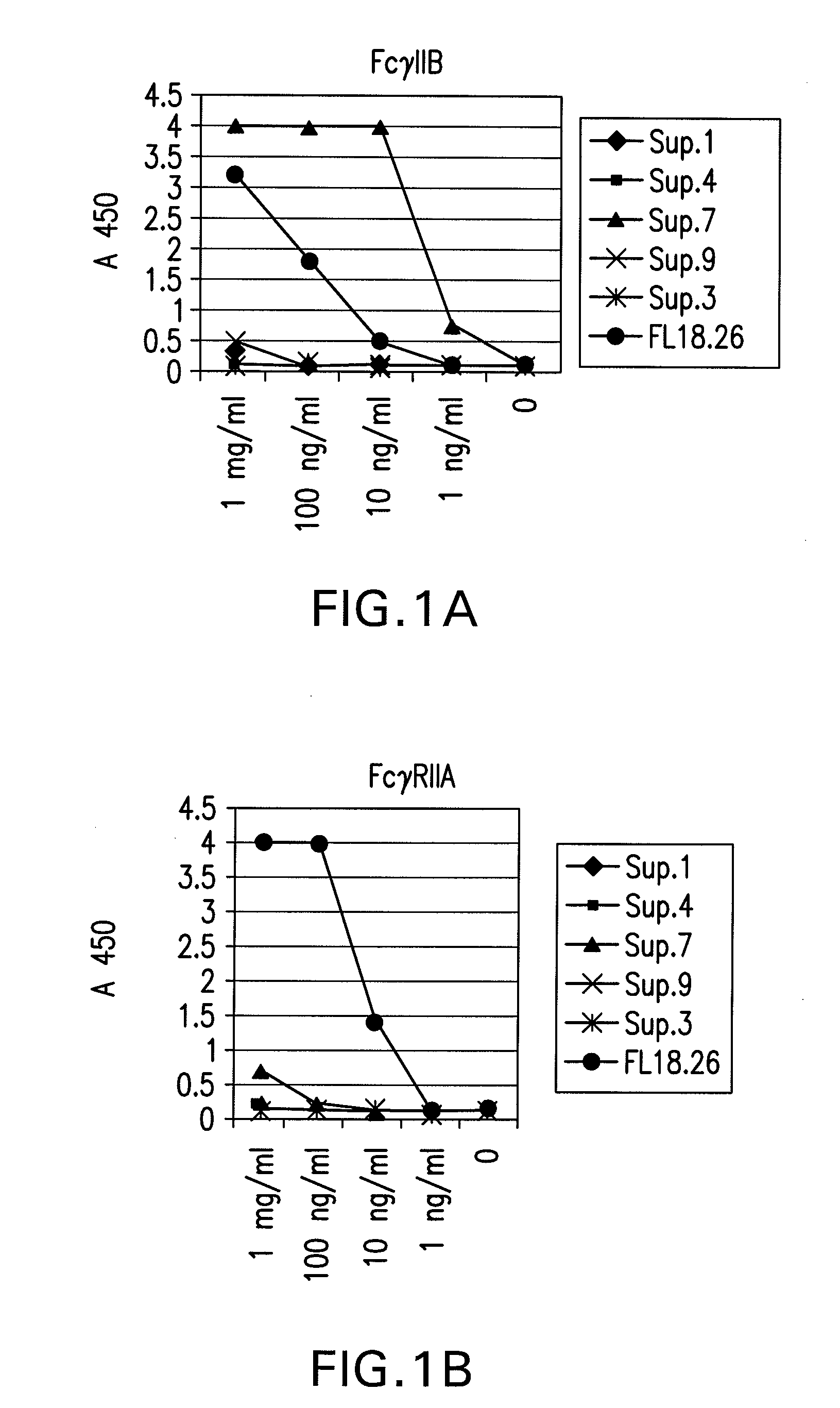
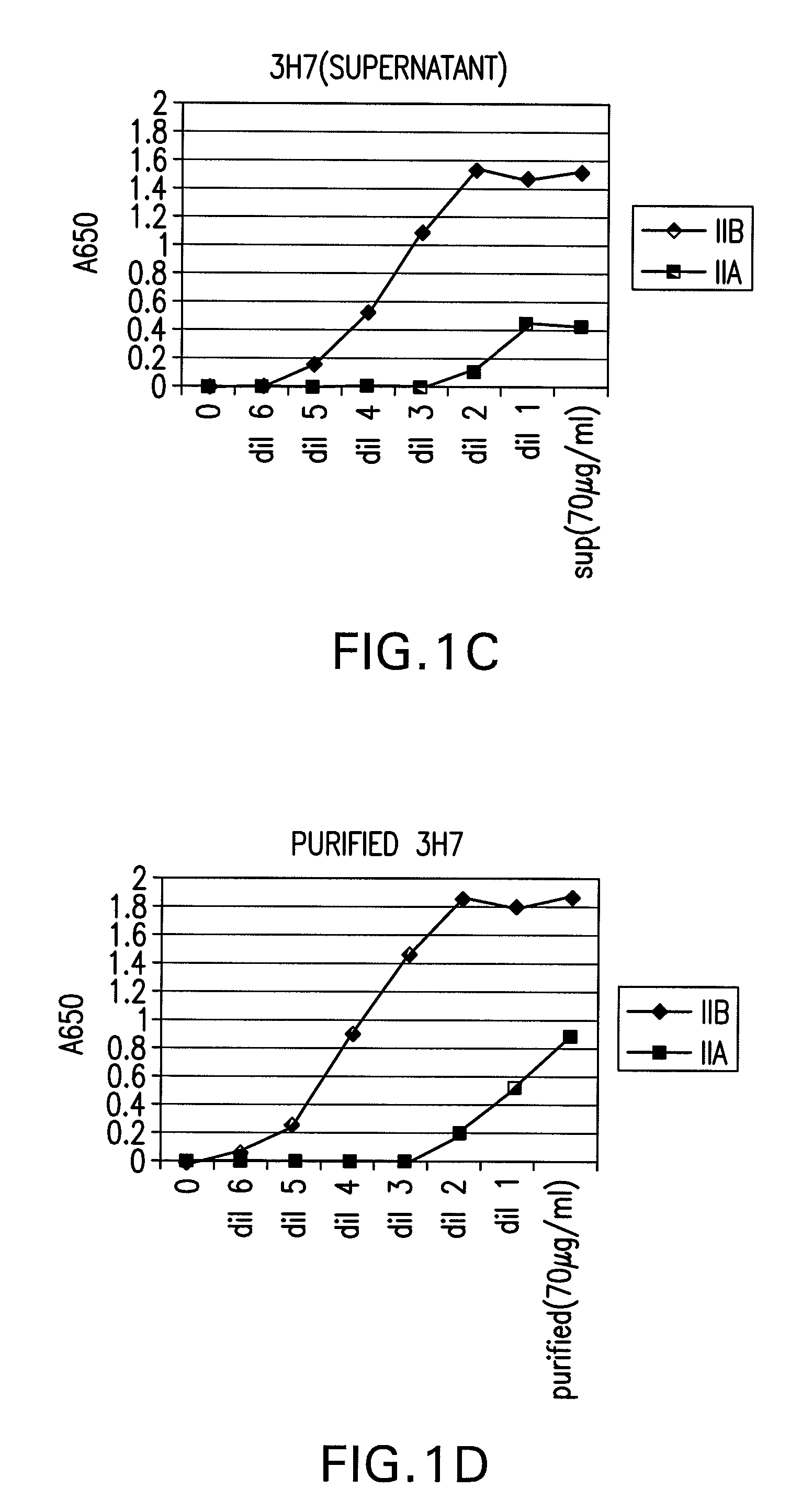
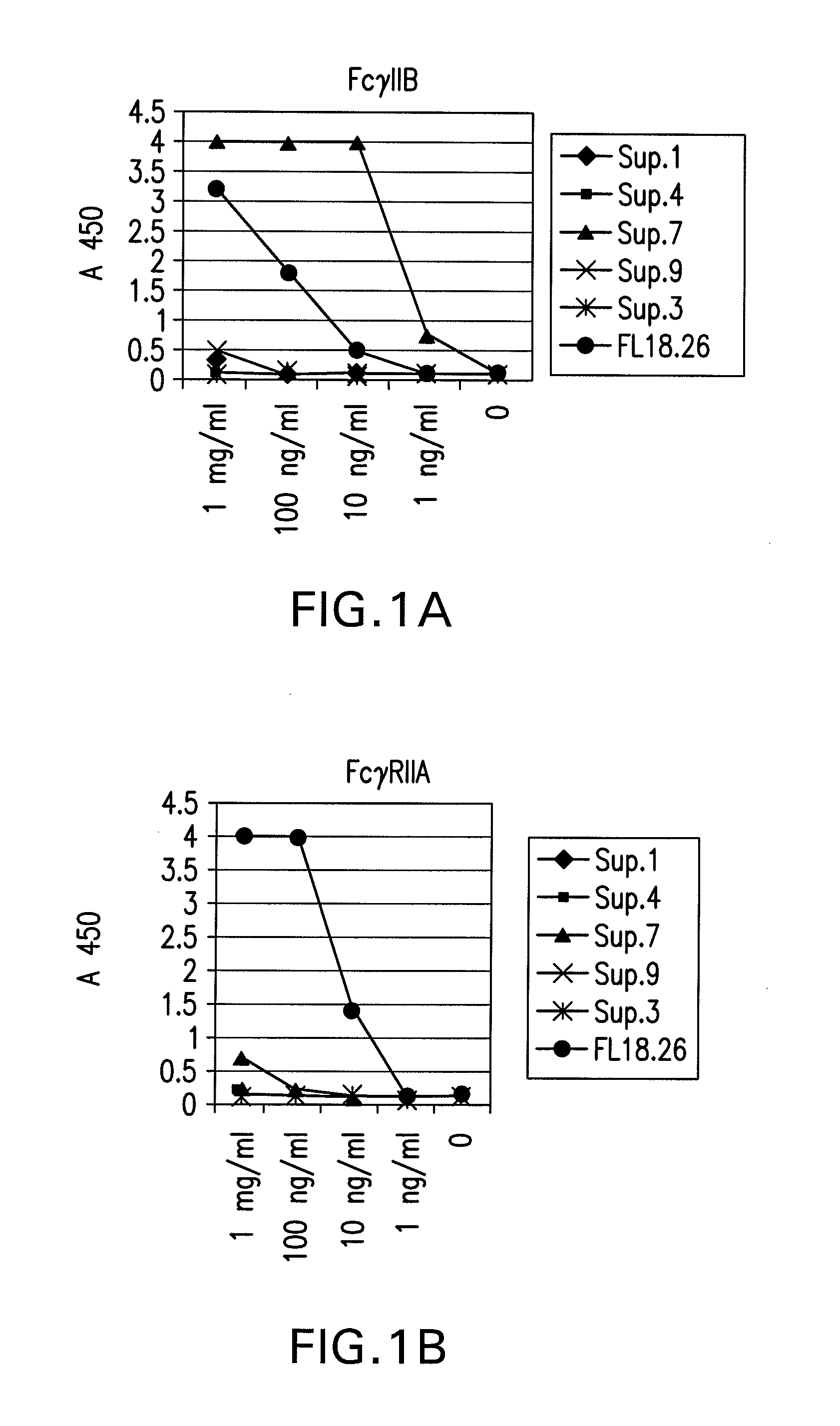
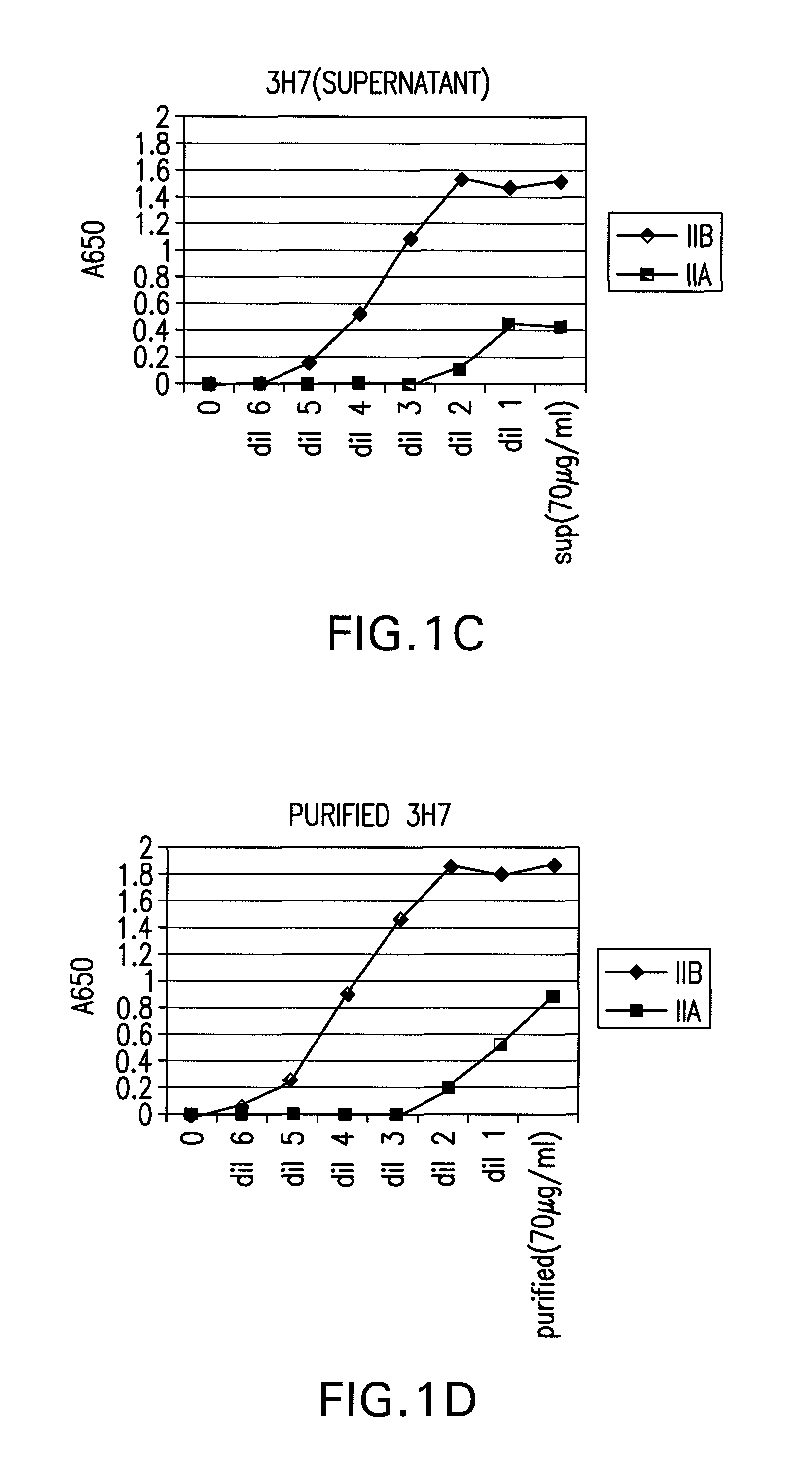
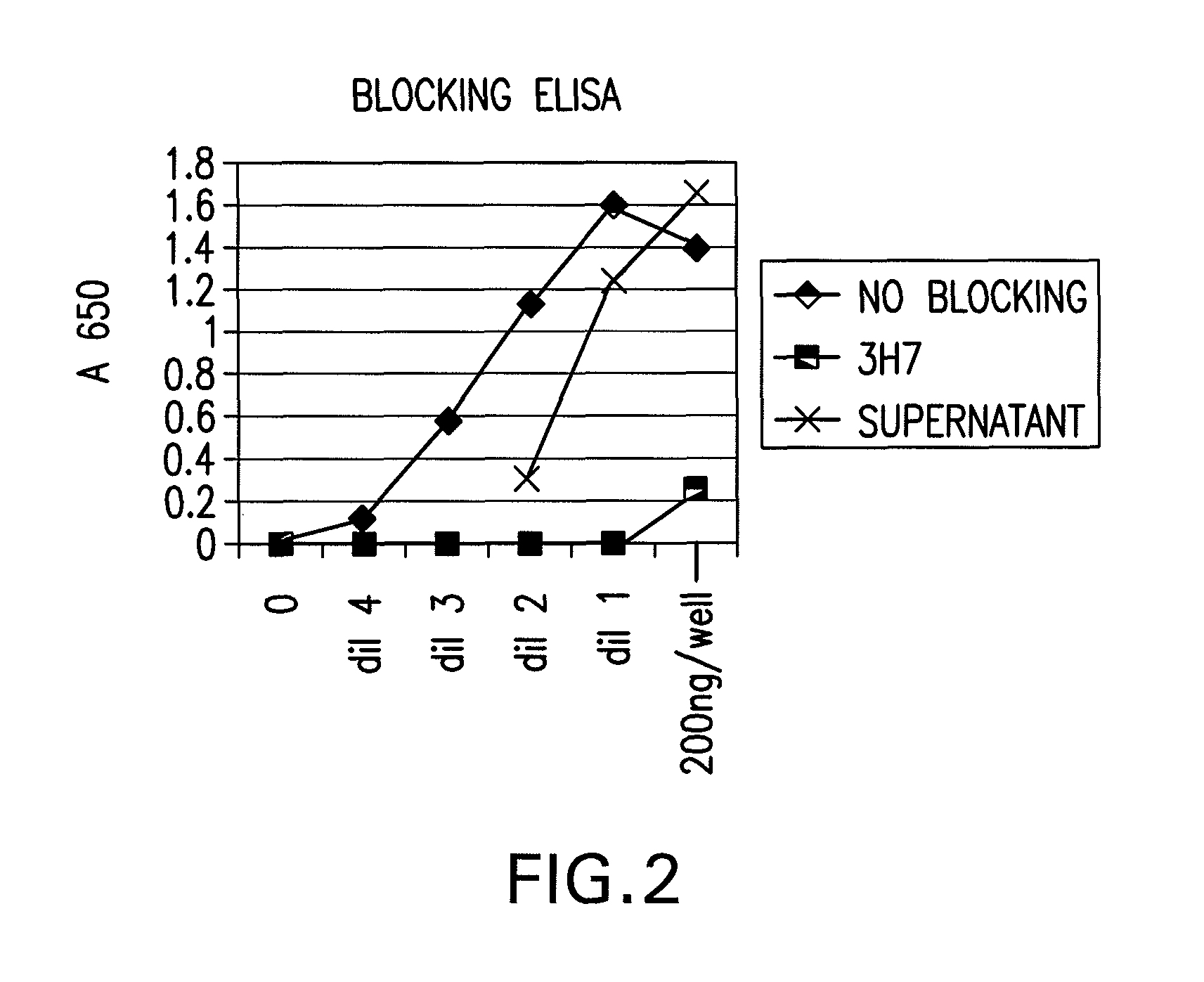
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20020028178A1Prevent and reduce proliferation of cellReduce and prevent proliferationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD20Antiendomysial antibodies
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN MA INC
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
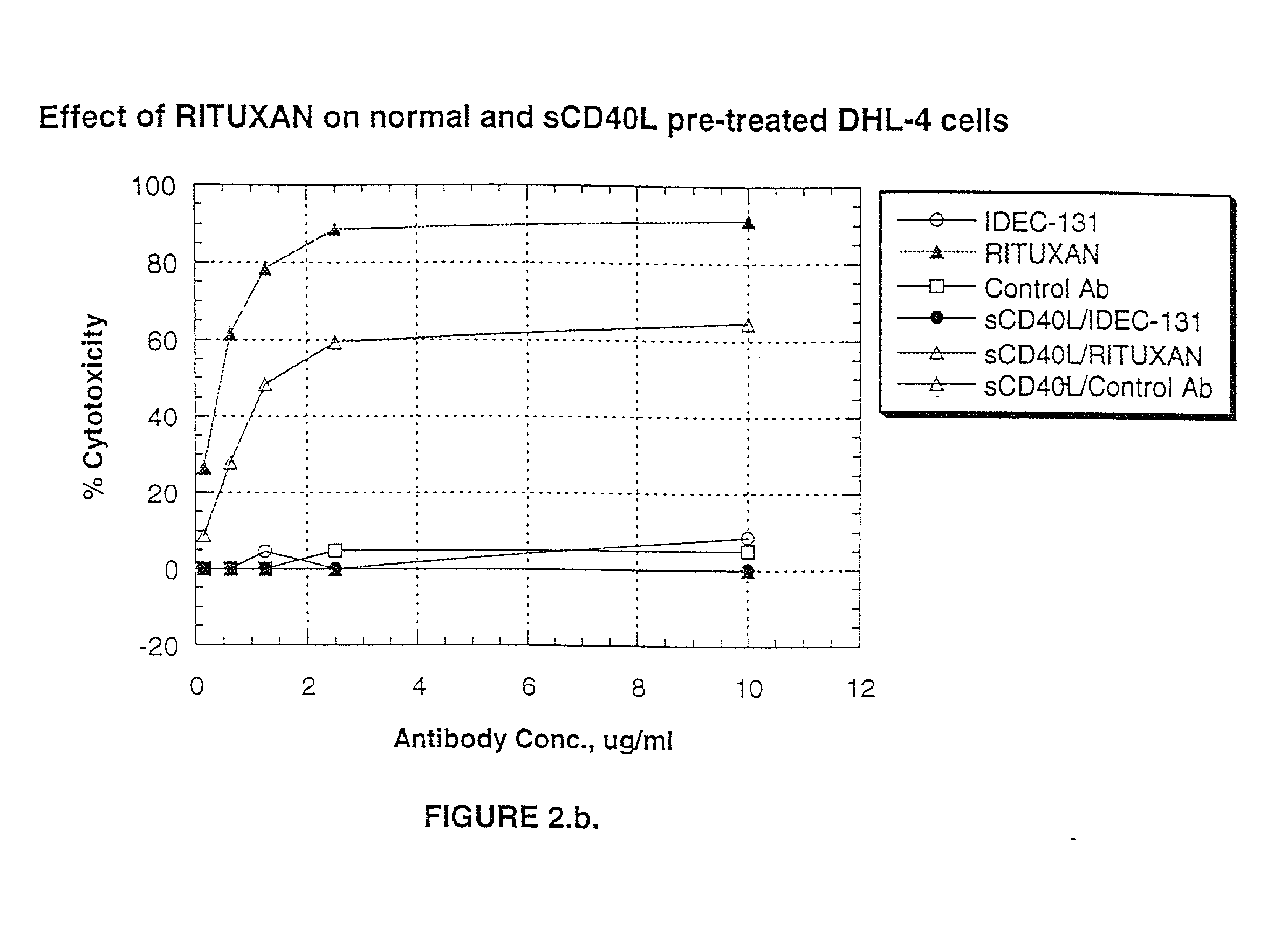
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
Anti-cd20 antibodies and fusion proteins thereof and methods of use
InactiveUS20070020259A1Useful in diagnosisUseful in treatmentAntipyreticAnalgesicsAutoimmune conditionAutoimmune disease
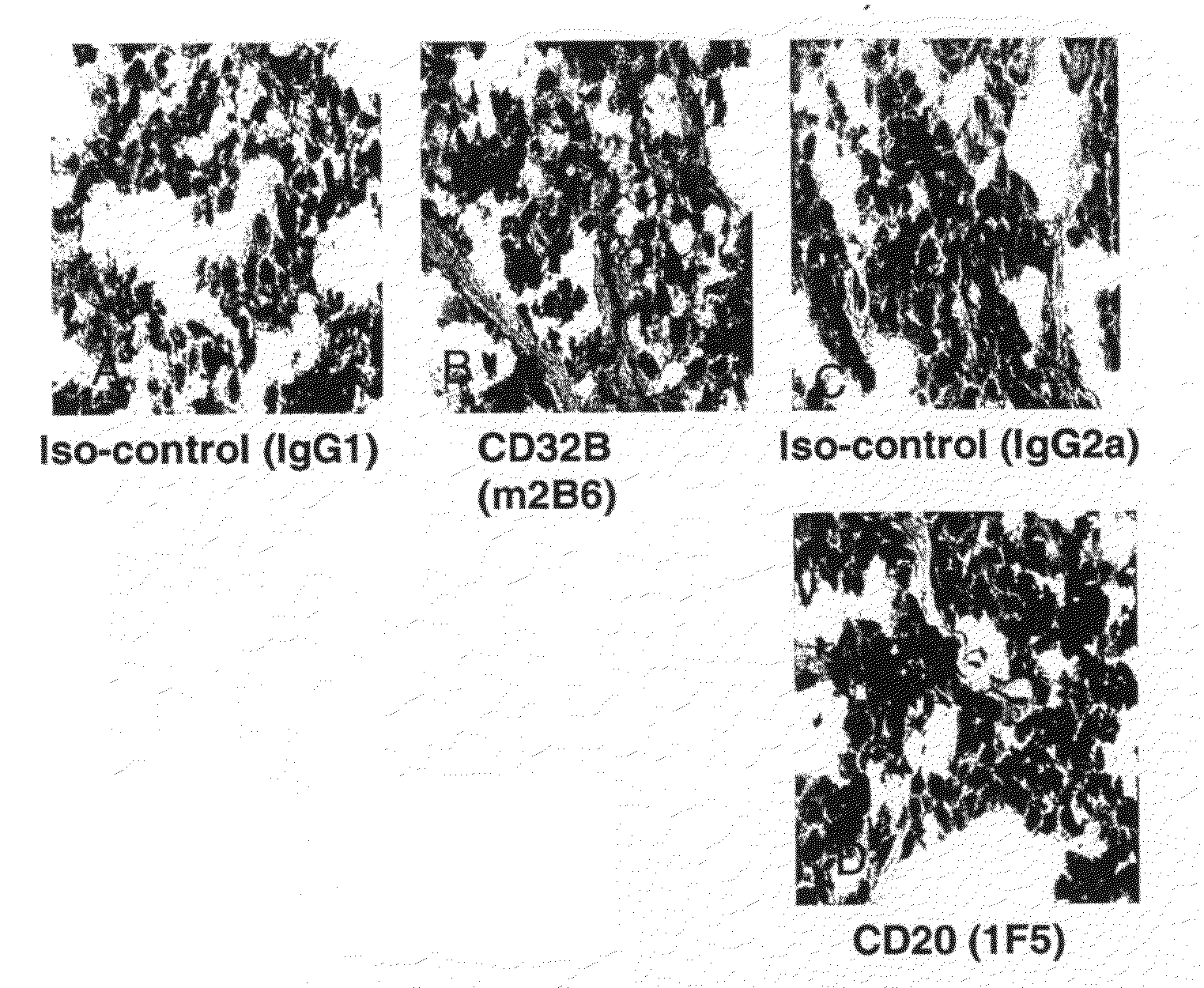
The present invention provides humanized, chimeric and human anti-CD20 antibodies and CD 20 antibody fusion proteins that bind to a human B cell marker, referred to as CD20, which is useful for the treatment and diagnosis of B-cell disorders, such as B-cell malignancies and autoimmune diseases, and methods of treatment and diagnosis.
Owner:IMMUNOMEDICS INC
Mutated anti-CD22 antibodies with increased affinity to CD22-expressing leukemia cells
ActiveUS7355012B2Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBacteroides
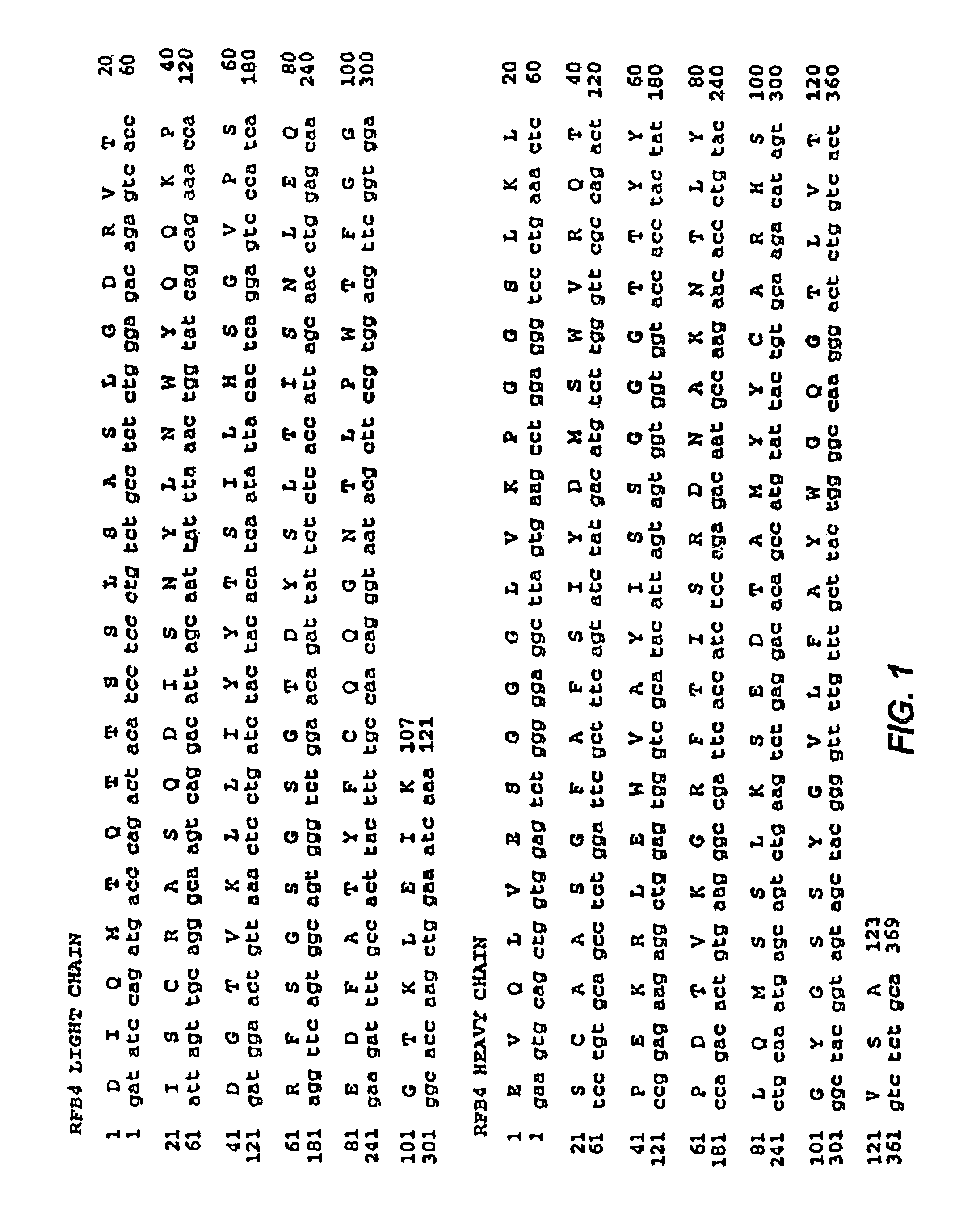
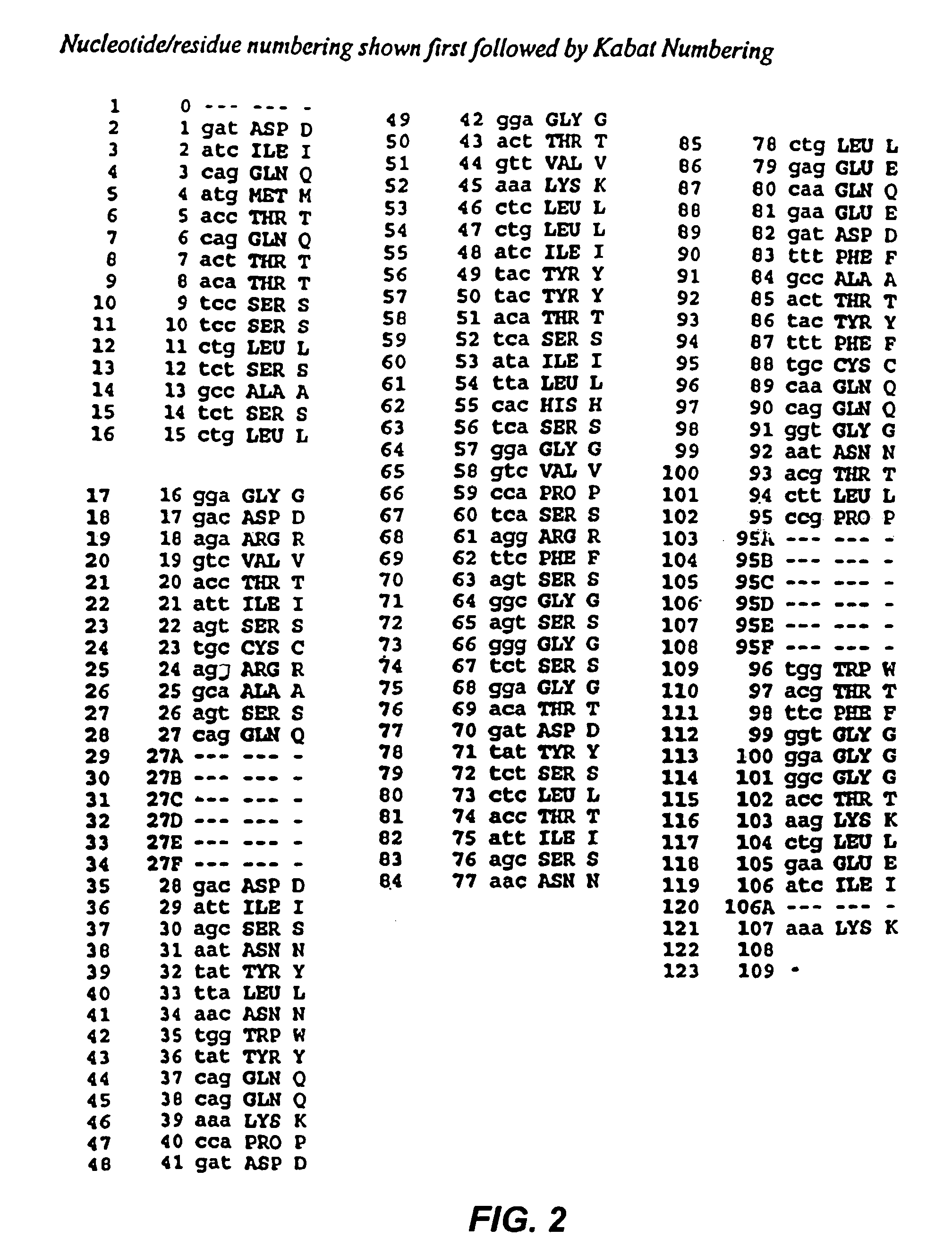
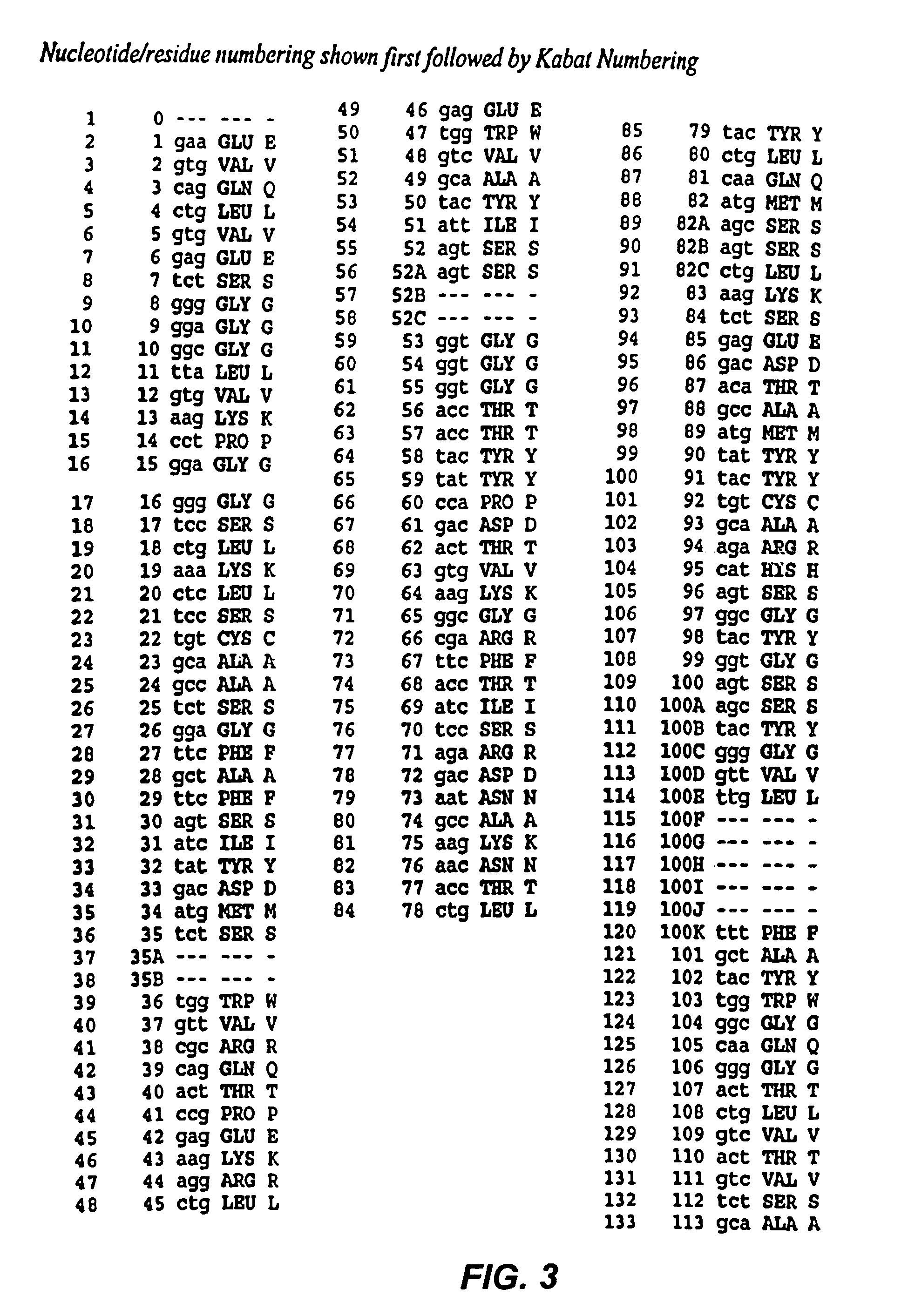
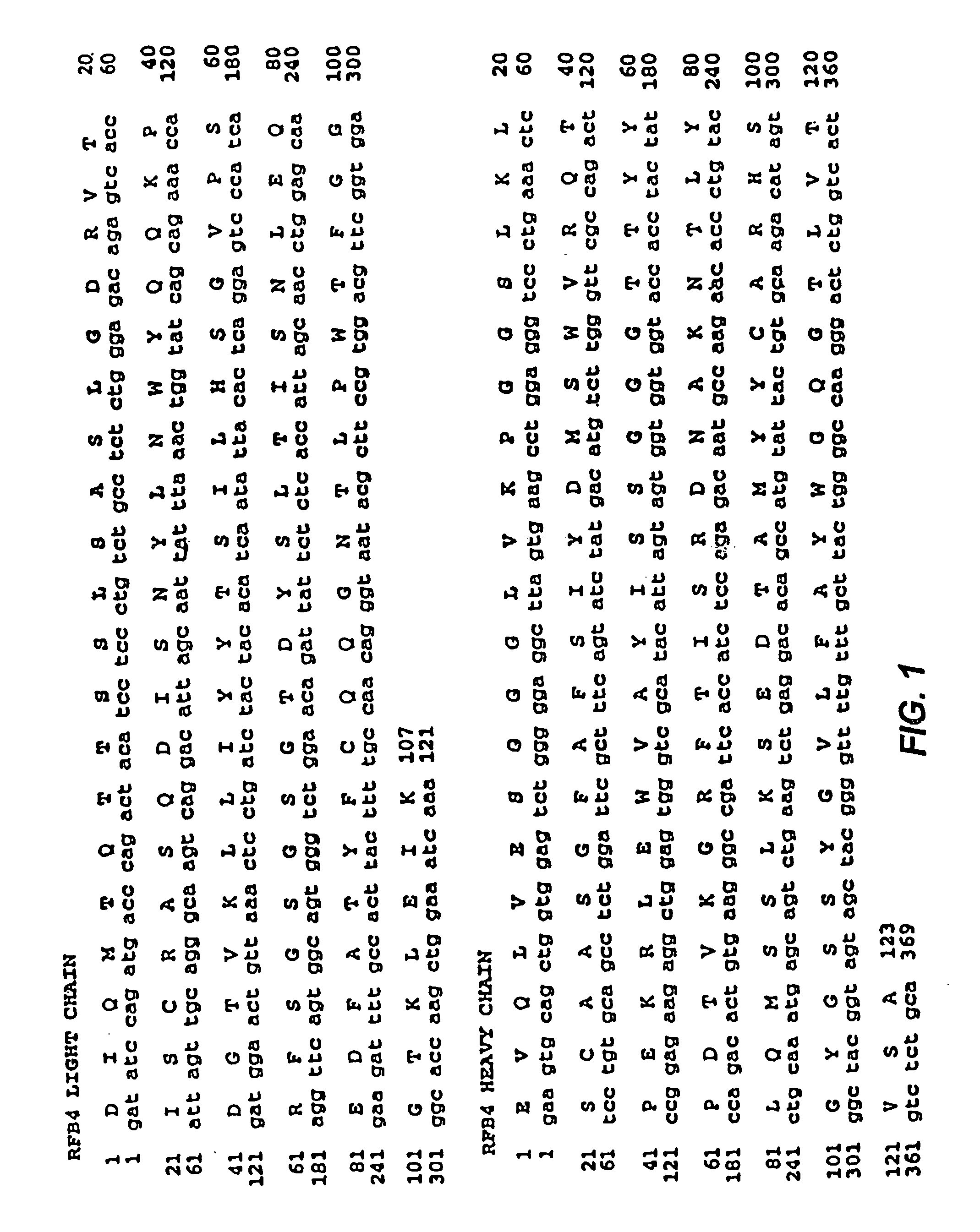
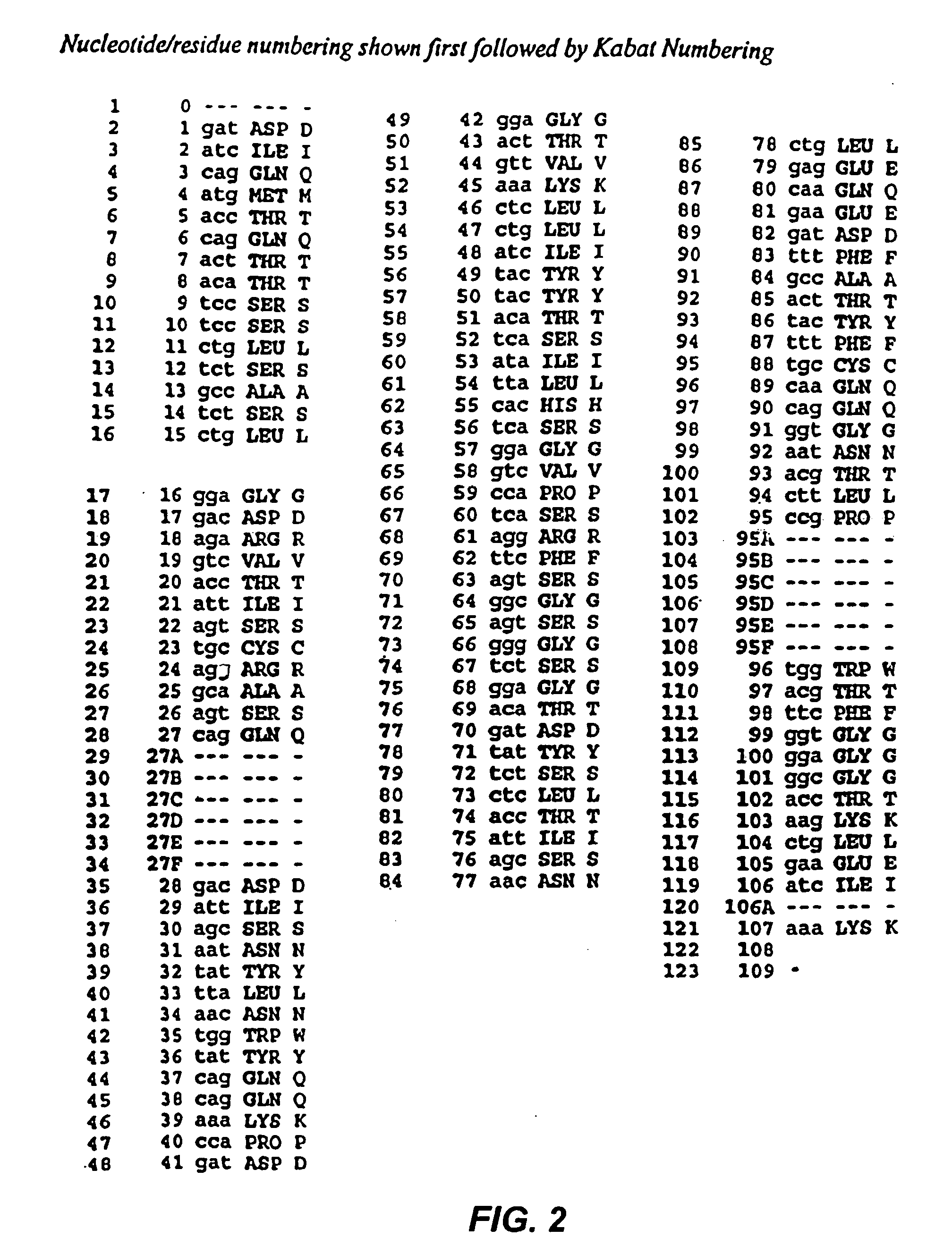
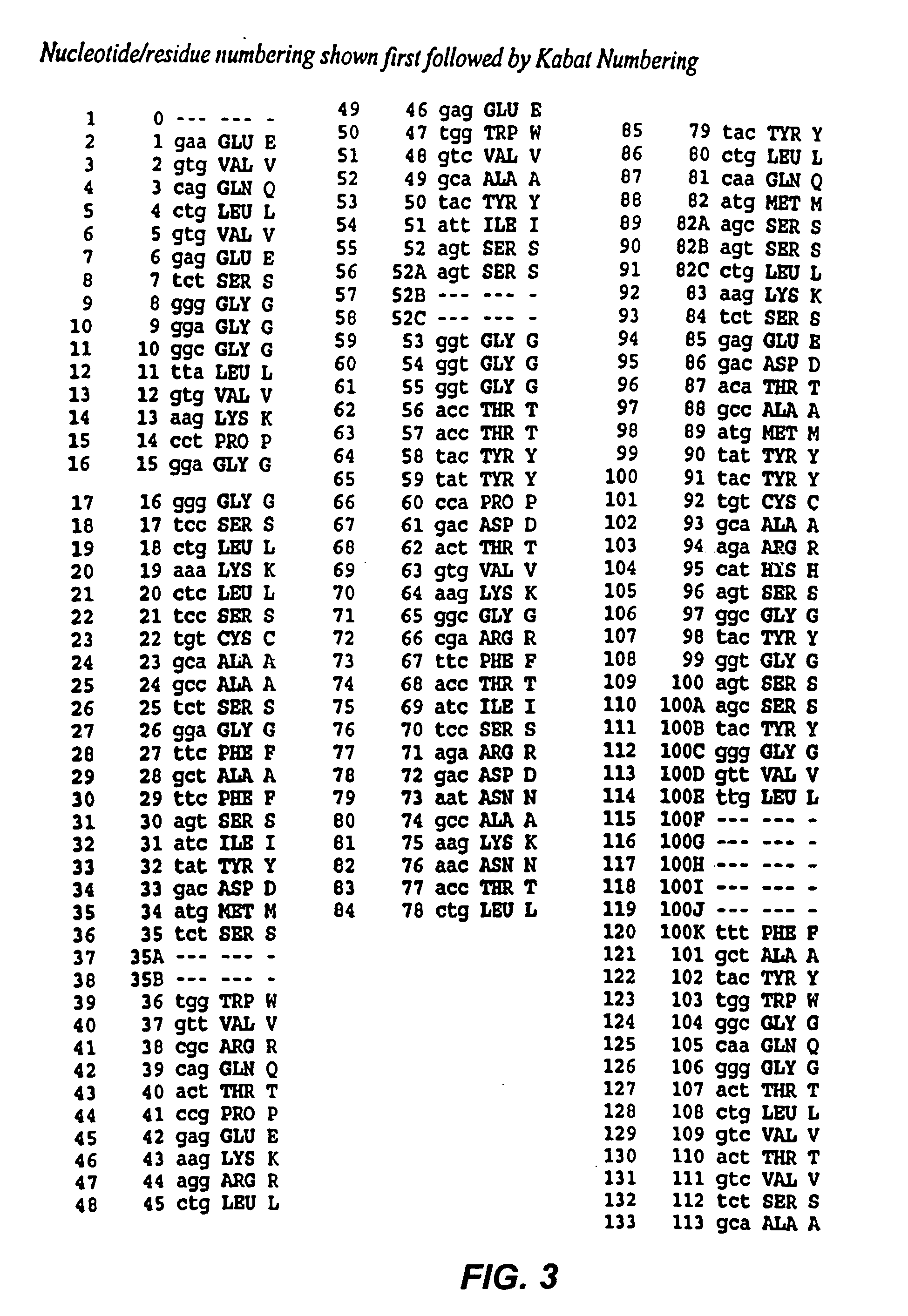
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Antibody variants and uses thereof
The invention provides improved humanized CD20 binding antibodies for treatment of B cell malignancies and autoimmune diseases.
Owner:GENENTECH INC
Anti-CD19 antibodies and uses in oncology
InactiveUS20060233791A1Efficient productionEfficiently depletedAntibody ingredientsImmunoglobulinsDiseaseTherapeutic antibody
The invention relates to immunotherapeutic compositions and methods for the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090053218A1Good curative effectAvoid managementAntibody ingredientsImmunoglobulinsTreatment effectAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
Anti-cd20 therapeutic compositions and methods
InactiveUS20100330089A1Slow tumor growthEliminate the effects ofNervous disorderAntibody mimetics/scaffoldsAntigenDisease
The present invention provides materials and methods for treatment of diseases involving aberrant B-cell activity, using a CD20-specific binding molecule, in particular, antibodies or antigen binding fragment thereof. The compositions disclosed herein is useful for the treatment and diagnosis of B-cell disorders, such as B-cell malignancies and autoimmune diseases.
Owner:EMERGENT PRODUCTS DEVELOPMENT SEATTLE LLC +1
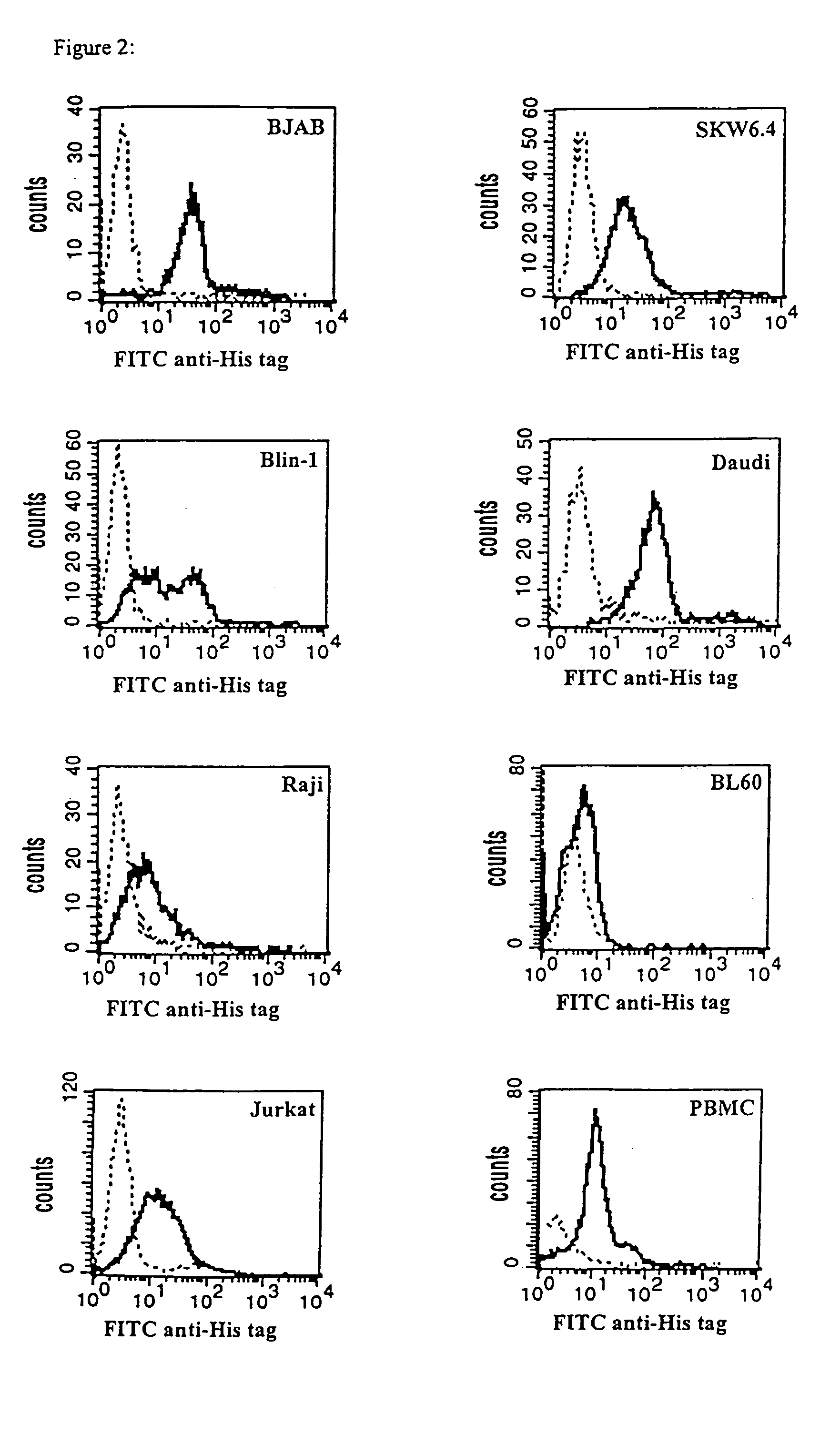
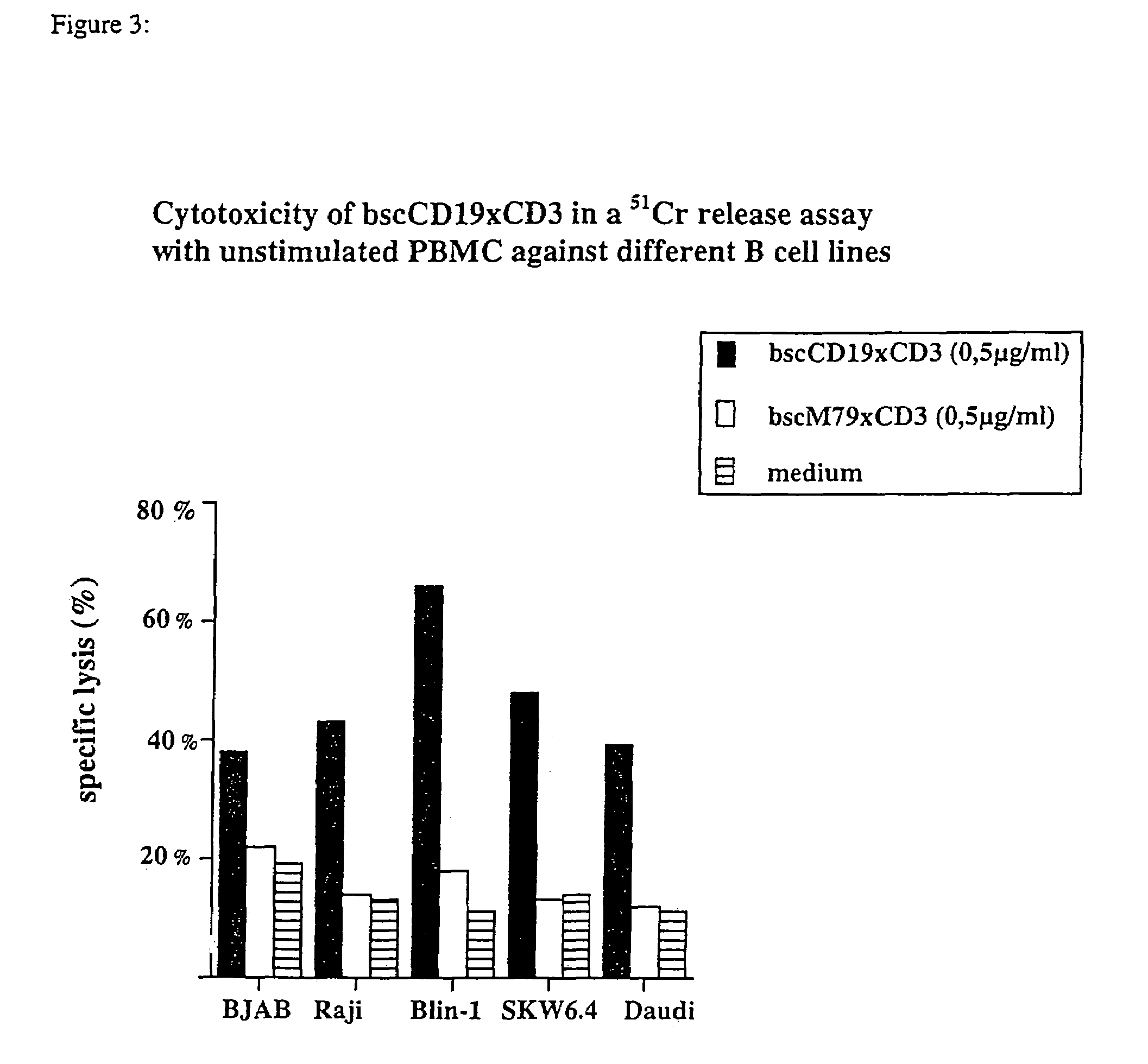
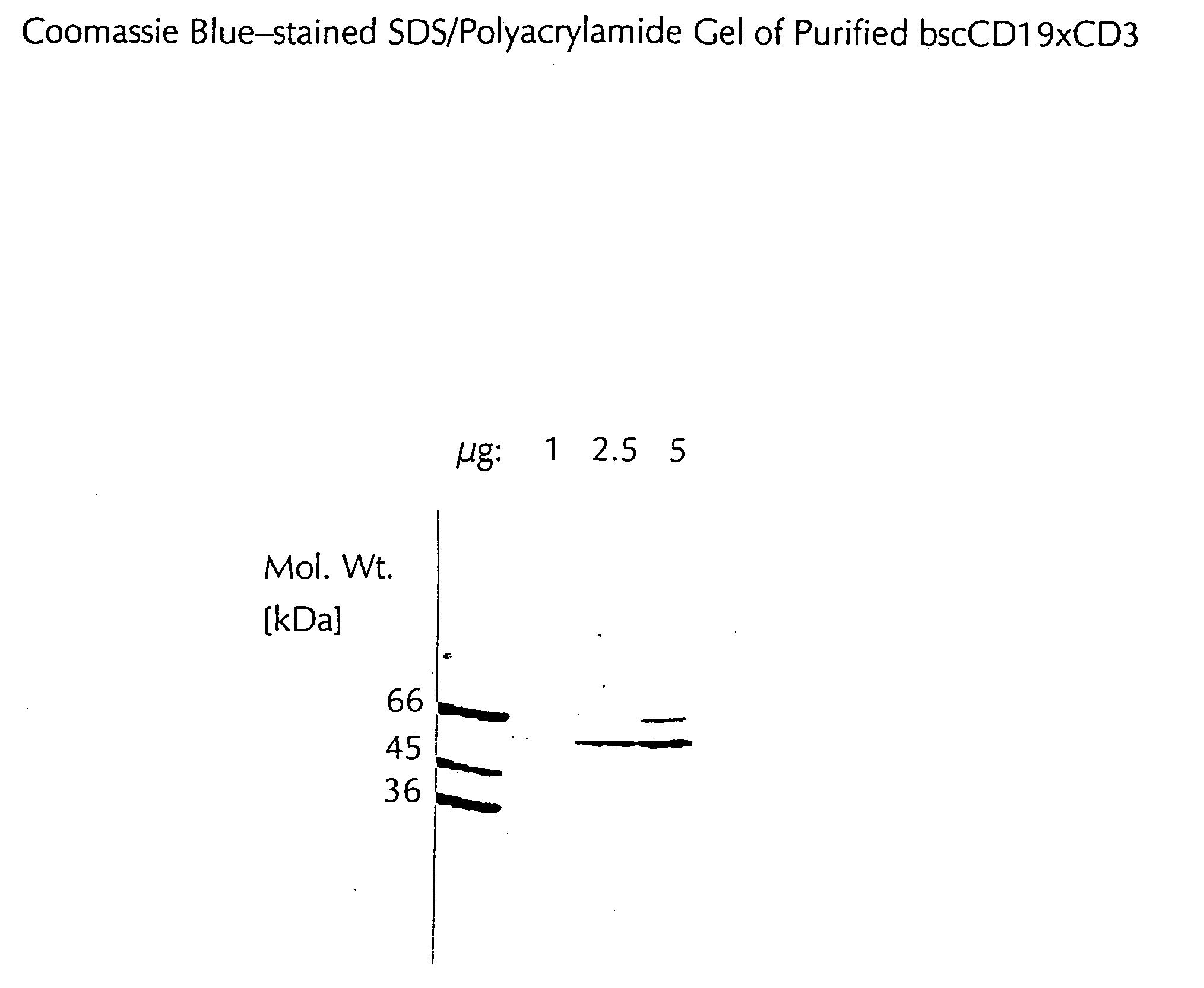
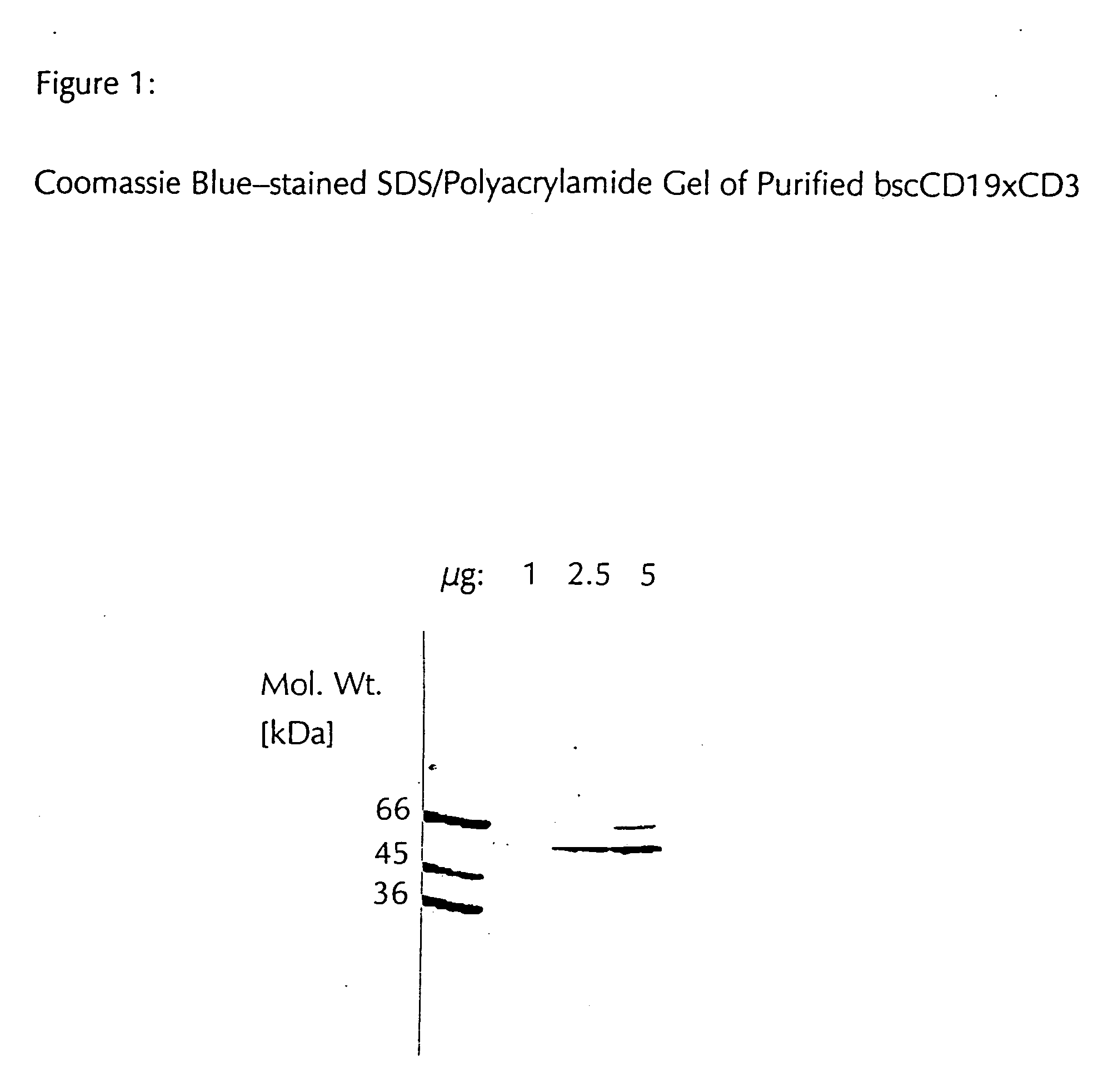
CD19xCD3 specific polypeptides and uses thereof
Described are novel single-chain multifunctional polypeptides comprising at least two binding sites specific for the CD19 and CD3 antigen, respectively. Further provided are polypeptides, wherein the above-described polypeptide comprises at least one further domain, preferably of pre-determined function. Furthermore, polynucleotides encoding said polypeptides as well as to vectors comprising said polynucleotides and host cells transformed therewith and their use in the production of said polypeptides are described. In addition, compositions, preferably pharmaceutical and diagnostic compositions are provided comprising any of the afore-described polypeptides, polynucleotides or vectors. Described is also the use of the afore-mentioned polypeptides, polynucleotides and vectors for the preparation of pharmaceutical compositions for immunotherapy, preferably against B-cell malignancies such as non-Hodgkin lymphoma.
Owner:AMGEN RES (MUNICH) GMBH
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
Immunotherapy of B cell malignancies and autoimmune diseases using unconjugated antibodies and conjugated antibodies and antibody combinations and fusion proteins
ActiveUS7534427B2Easy to manageSimilar response ratePeptide/protein ingredientsAntipyreticDiseaseMammal
The invention is directed to a method for treating a treating and diagnosing a B cell-related disease, T cell-related disease or an autoimmune disease in a mammal by concurrently or sequentially administering to the mammal a therapeutic composition that comprises a pharmaceutically acceptable vehicle and at least one conjugated antibody, wherein predosing with a non-radiolabeled antibody is not performed.
Owner:IMMUNOMEDICS INC
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcγRIIB specific antibodies and methods of use thereof
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
Anti-CD19 Antibodies
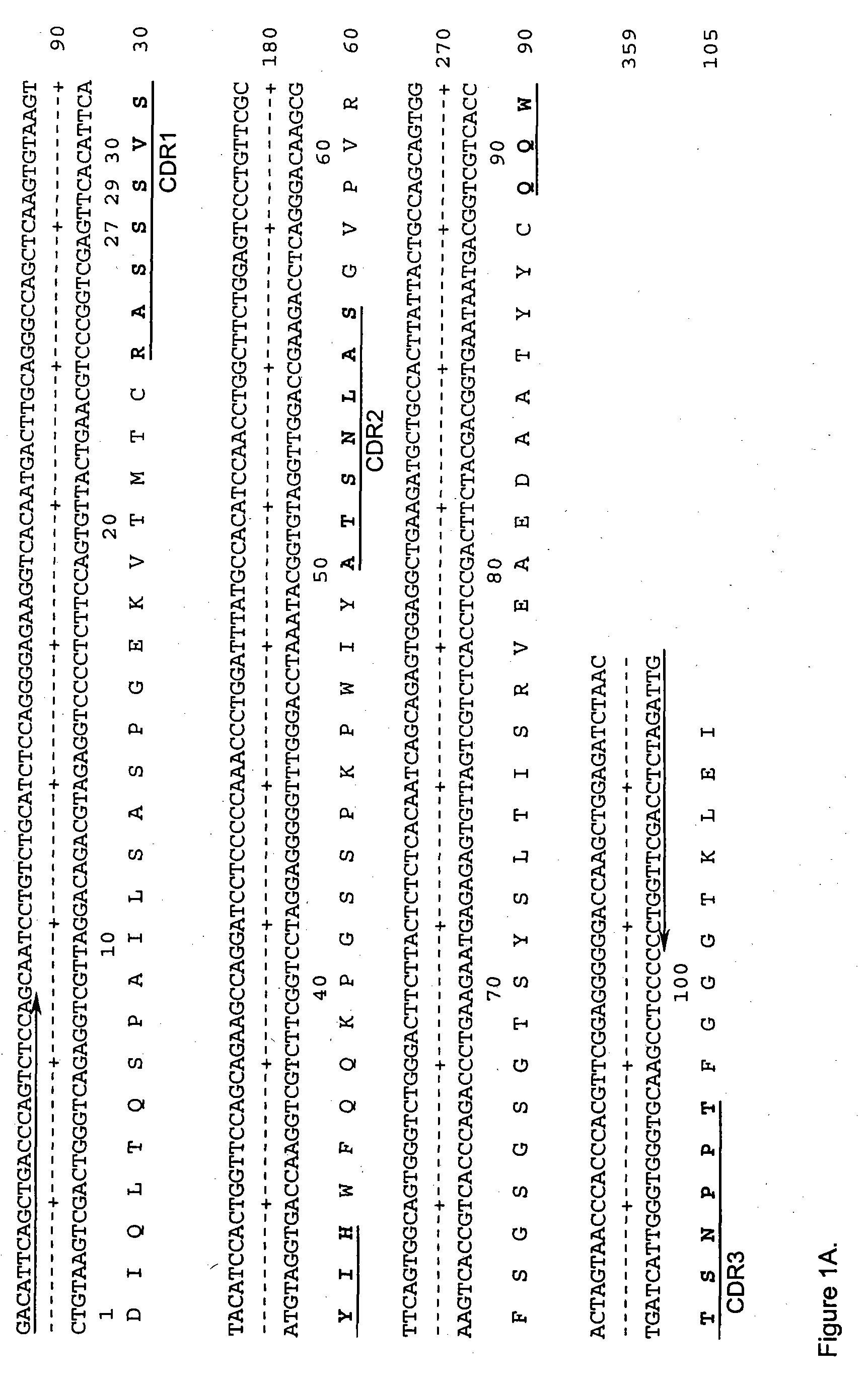
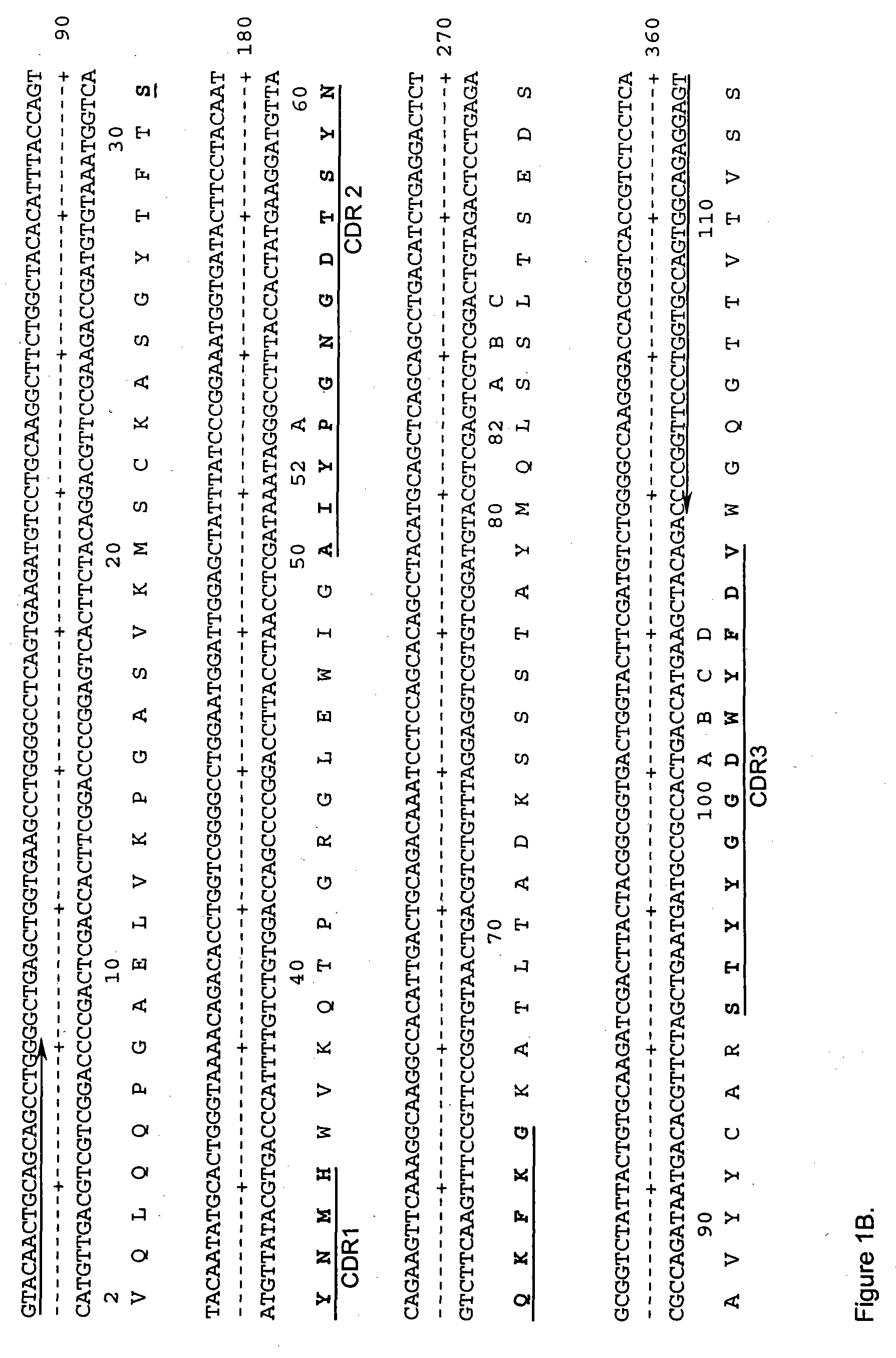
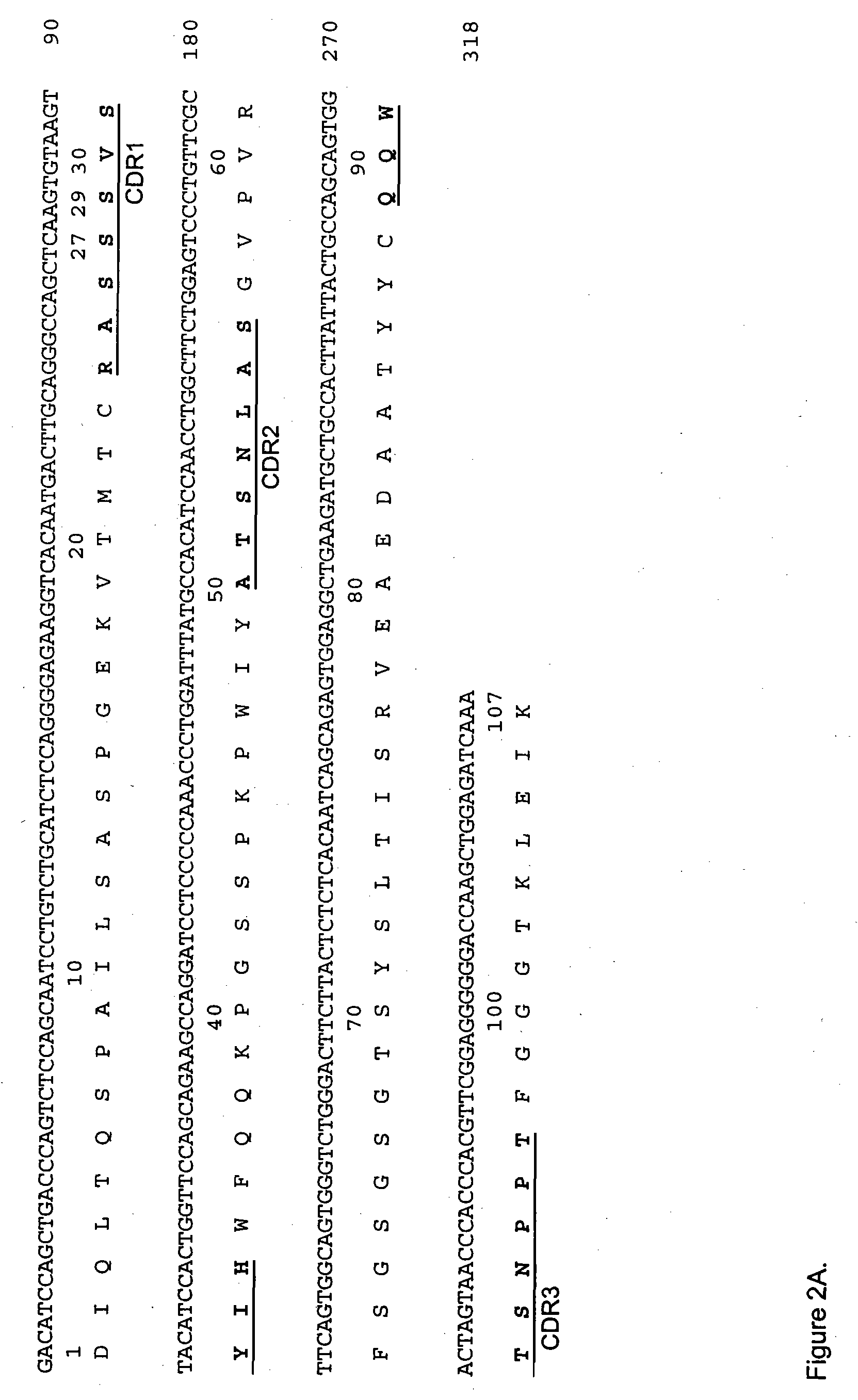
The present invention provides humanized, chimeric and human anti-CD19 antibodies, anti-CD19 antibody fusion proteins, and fragments thereof that bind to a human B cell marker. Such antibodies, fusion proteins and fragments thereof are useful for the treatment and diagnosis of various B-cell disorders, including B-cell malignancies and autoimmune diseases. In more particular embodiments, the humanized anti-CD19 antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels. In a particularly preferred embodiment, the substitutions comprise a Ser91Phe substitution in the hA19 VH sequence.
Owner:IMMUNOMEDICS INC
FcγRIIB specific antibodies and methods of use thereof
InactiveUS8946387B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090076251A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
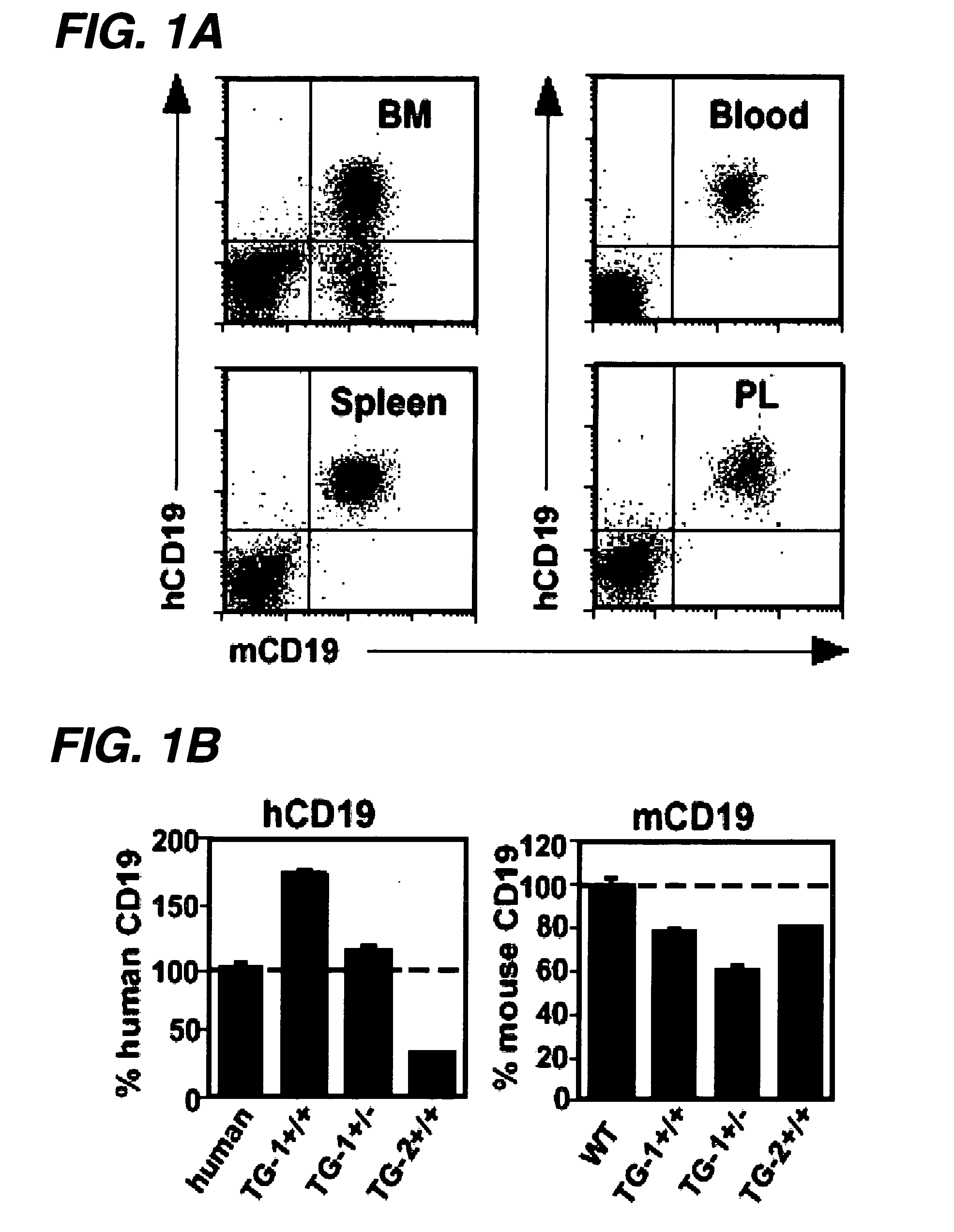
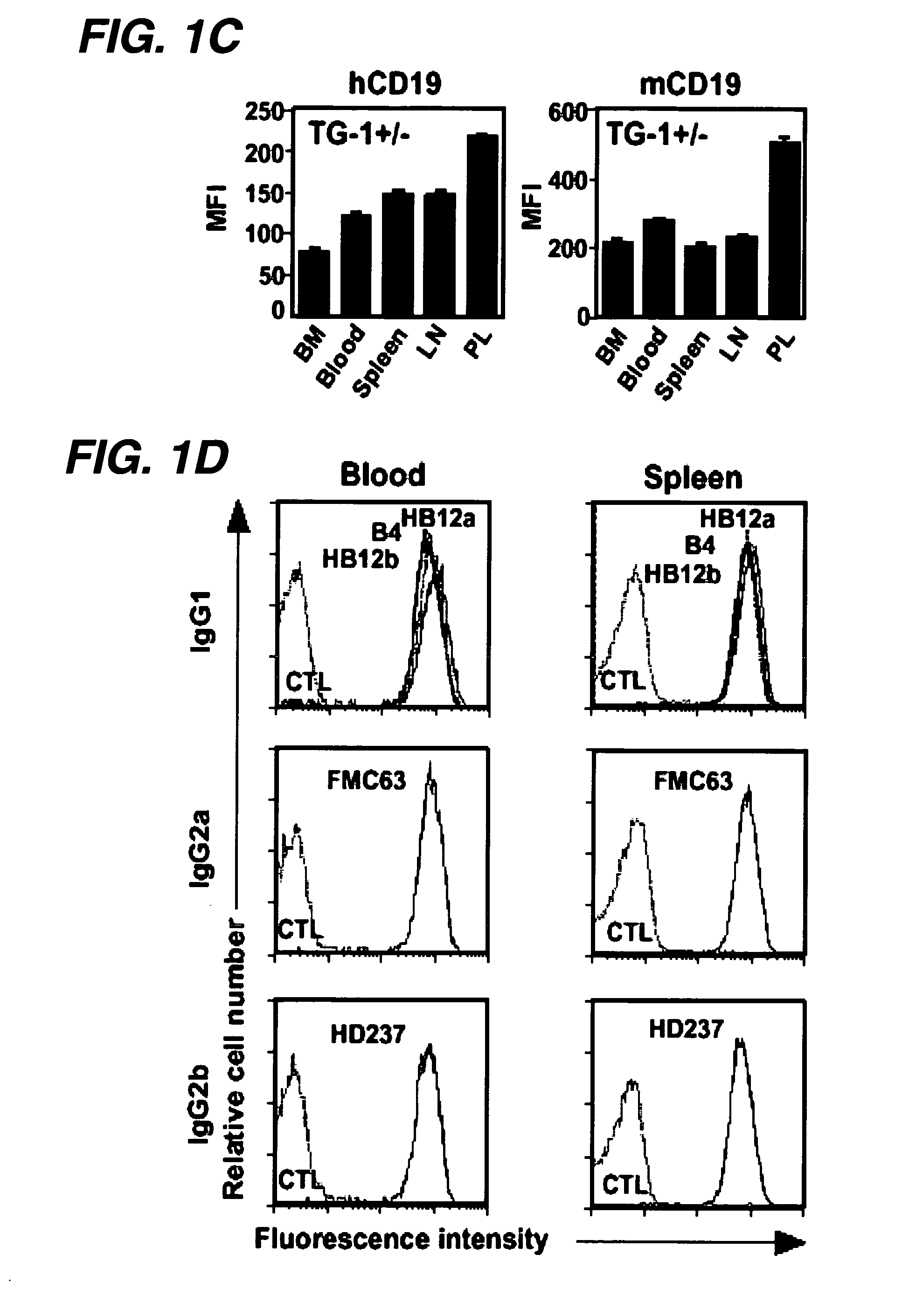
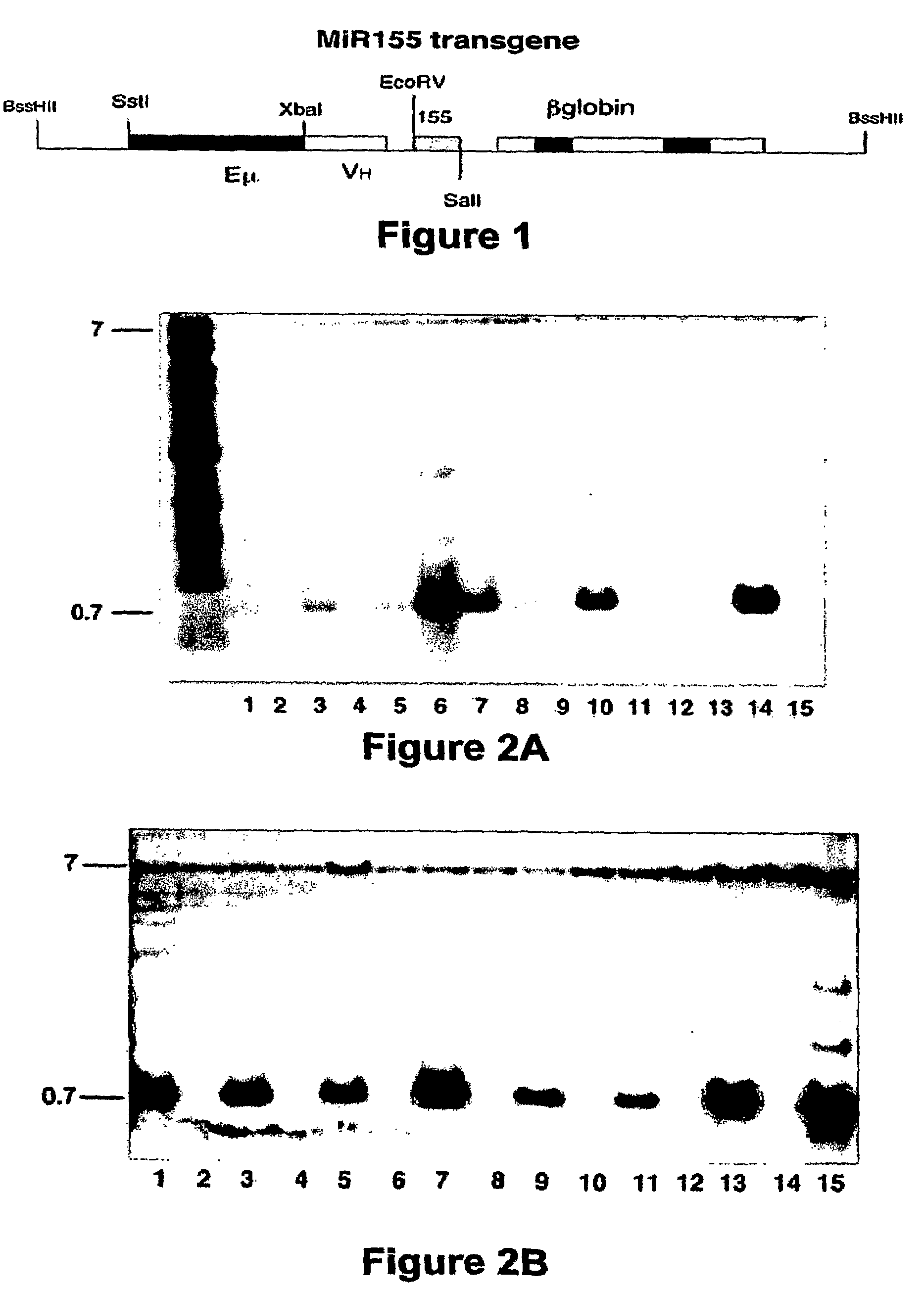
Transgenic mouse model of B cell malignancy
A transgenic non-human animal, such as a mouse, has a genome that include a nucleic acid construct having at least one transcriptional regulatory sequence capable of directing expression in B cells of the animal, wherein the transcriptional regulatory sequence is operably linked to a nucleic acid encoding a miR155 gene product. A method of testing the therapeutic efficacy of an agent in treating or preventing a lymphoproliferative condition includes assessing the effect(s) of the agent on a transgenic non-human animal.
Owner:THE OHIO STATE UNIV RES FOUND
Mutated anti-cd22 antibodies with increased affinity to cd22-expressing leukemia cells
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090202537A1Balanced functionImmune responseSenses disorderNervous disorderFc(alpha) receptorImmunologic disorders
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer (preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma), autoimmune disease, inflammatory disease or IgE-mediated allergic disorder. The present invention also encompasses the use of a humanized FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
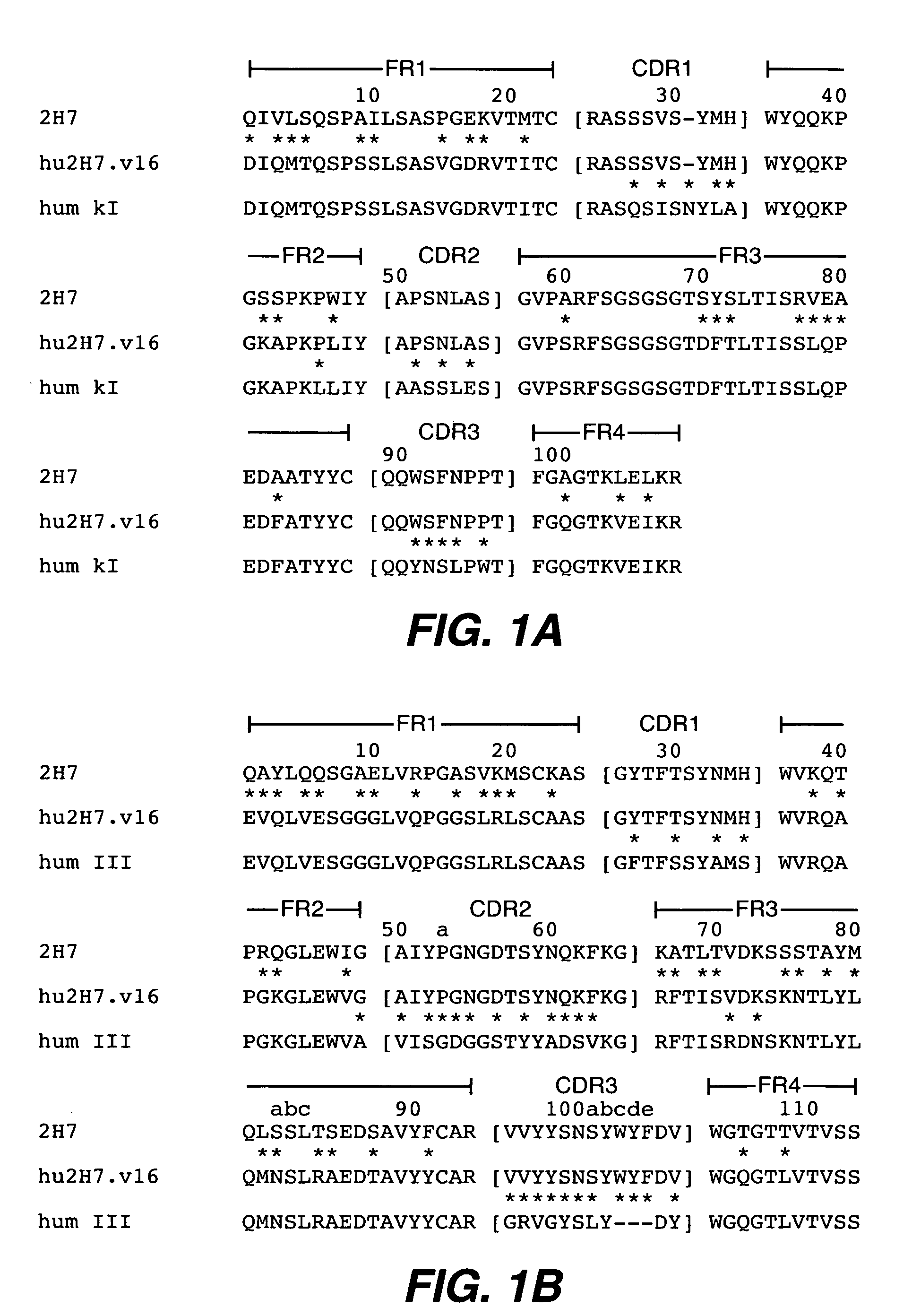
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Anti cd37 antibodies
InactiveUS20100189722A1Lower levelImprove the level ofAntipyreticAnalgesicsDiseaseAntiendomysial antibodies
Owner:BOEHRINGER INGELHEIM INT GMBH
Mutated anti-cd22 antibodies and immunoconjugates
ActiveUS7982011B2Immunoglobulin superfamilyAntibody mimetics/scaffoldsAntiendomysial antibodiesCancer cell
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of such cancers. Additionally, the invention provides a method of increasing the cytotoxicity of forms of Pseudomonas exotoxin A (“PE”) with the mutation of a single amino acid, as well as compositions of such mutated PEs, nucleic acids encoding them, and methods for using the mutated PEs.
Owner:UNITED STATES OF AMERICA
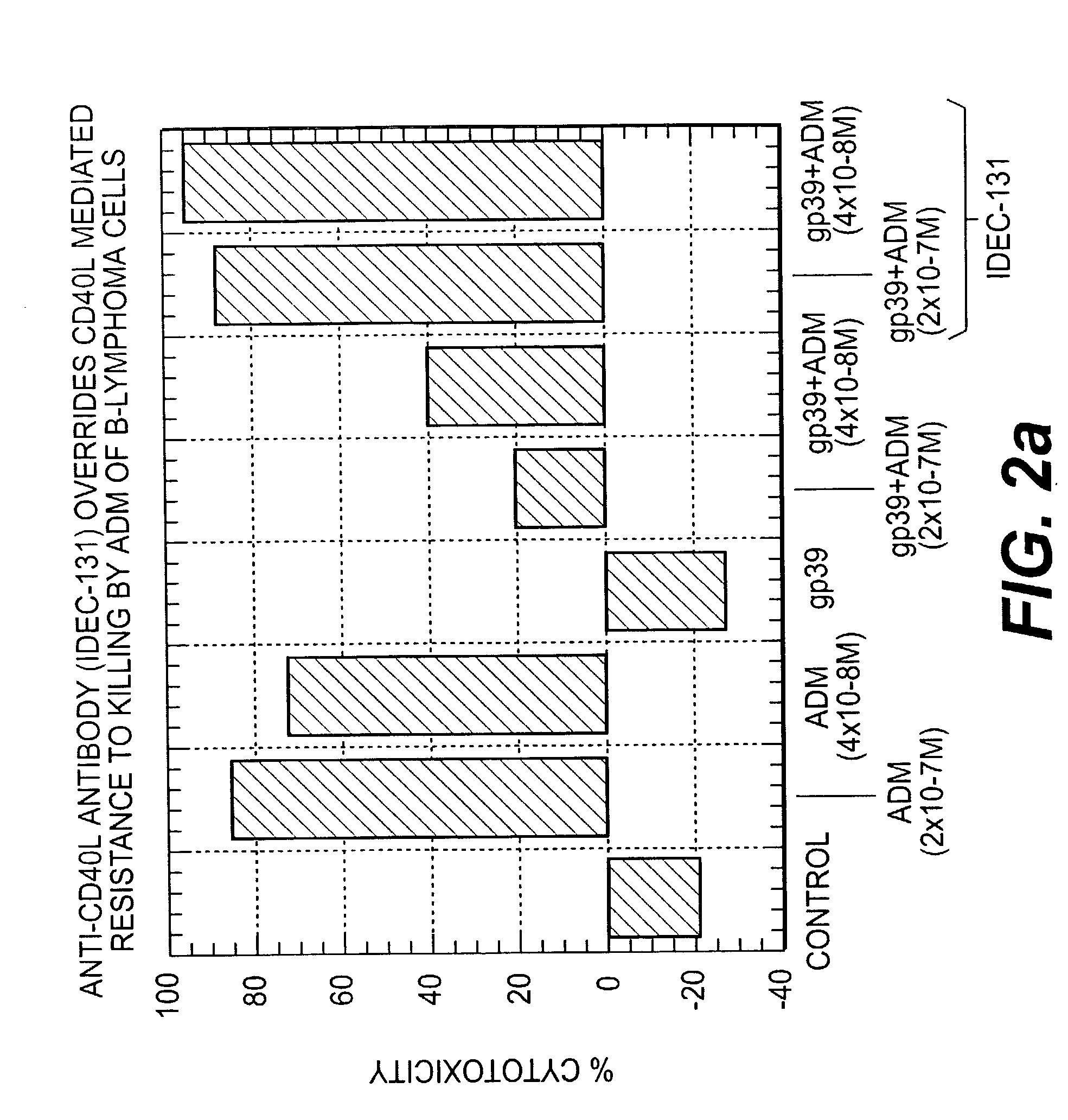
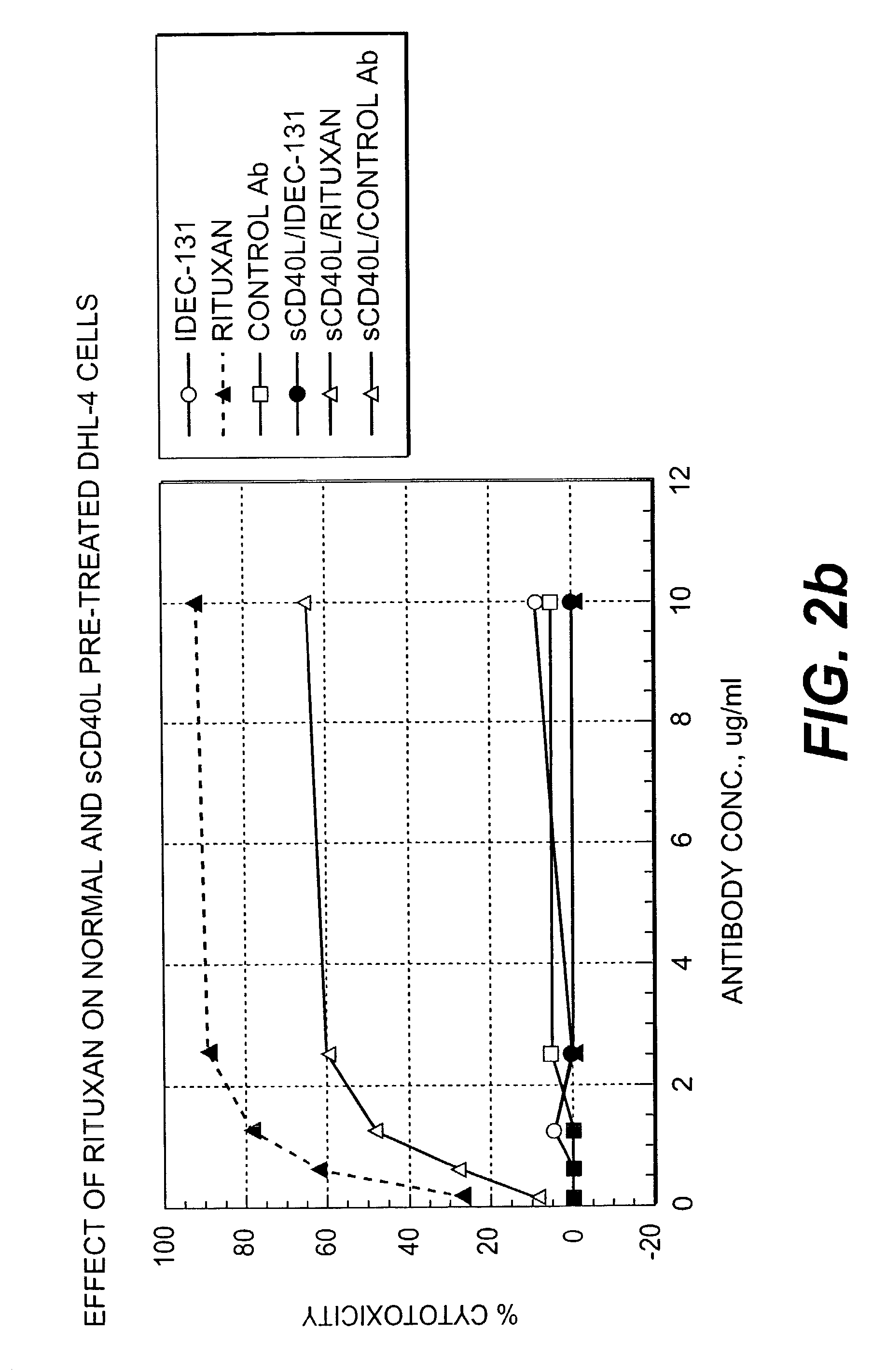
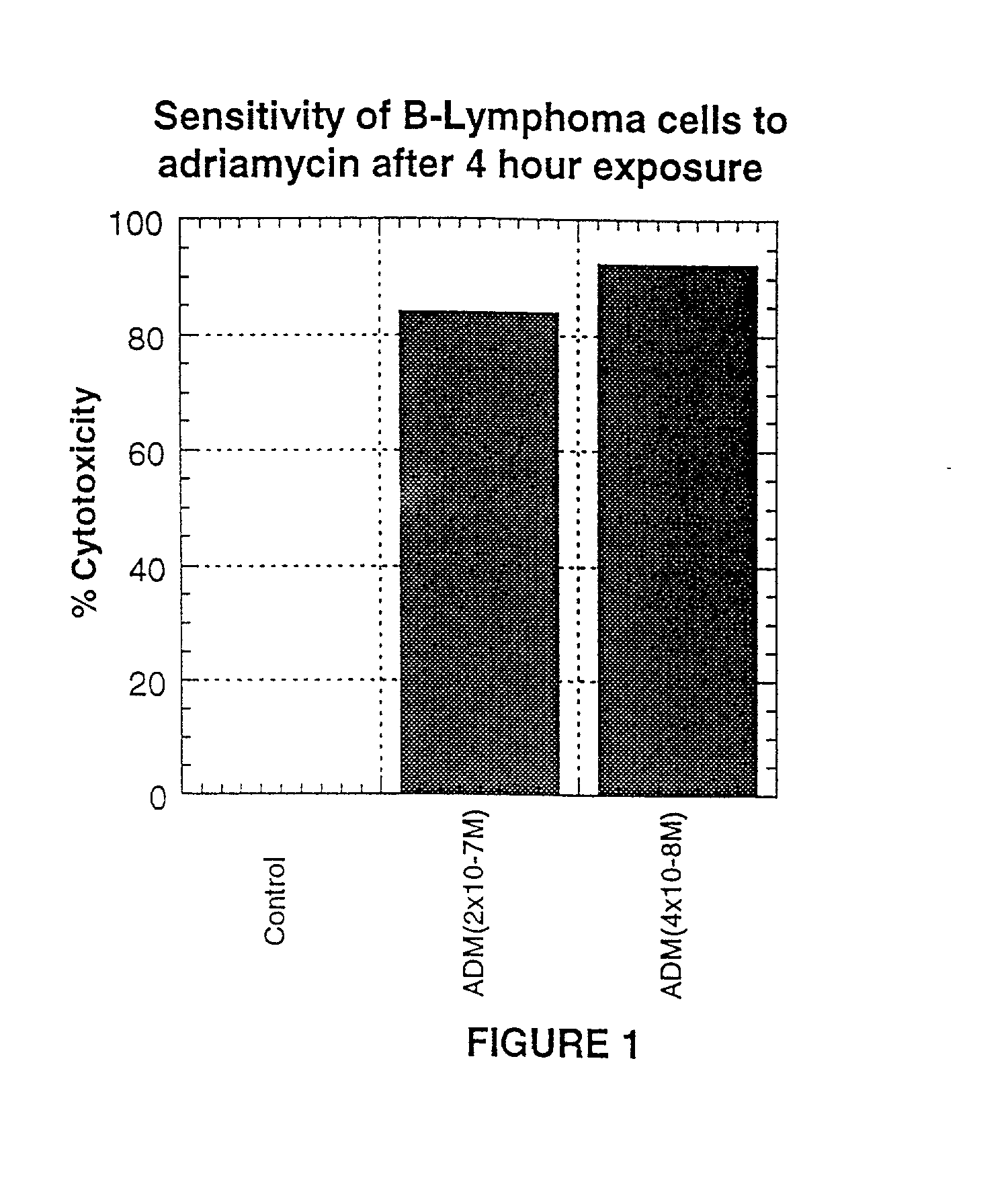
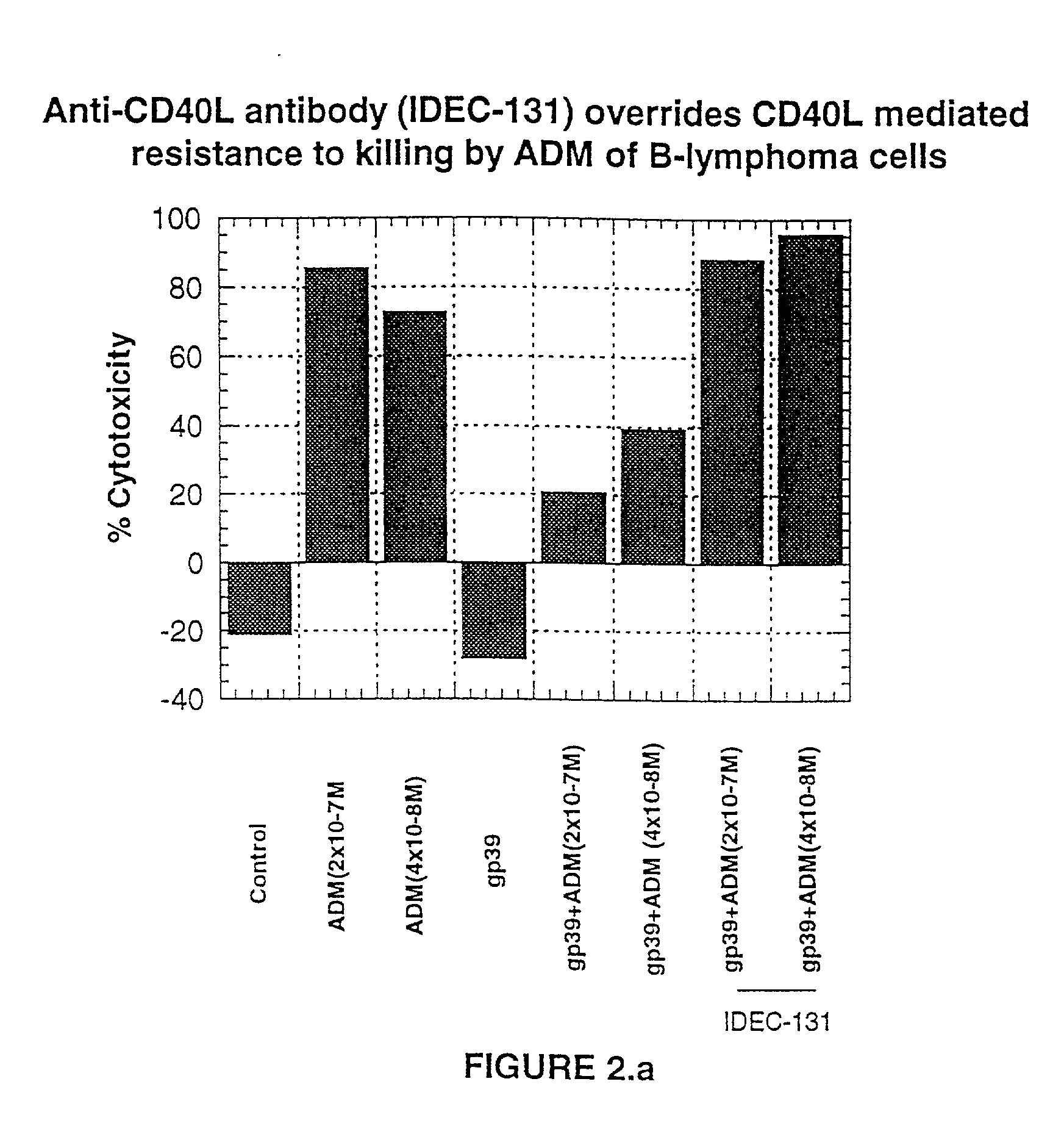
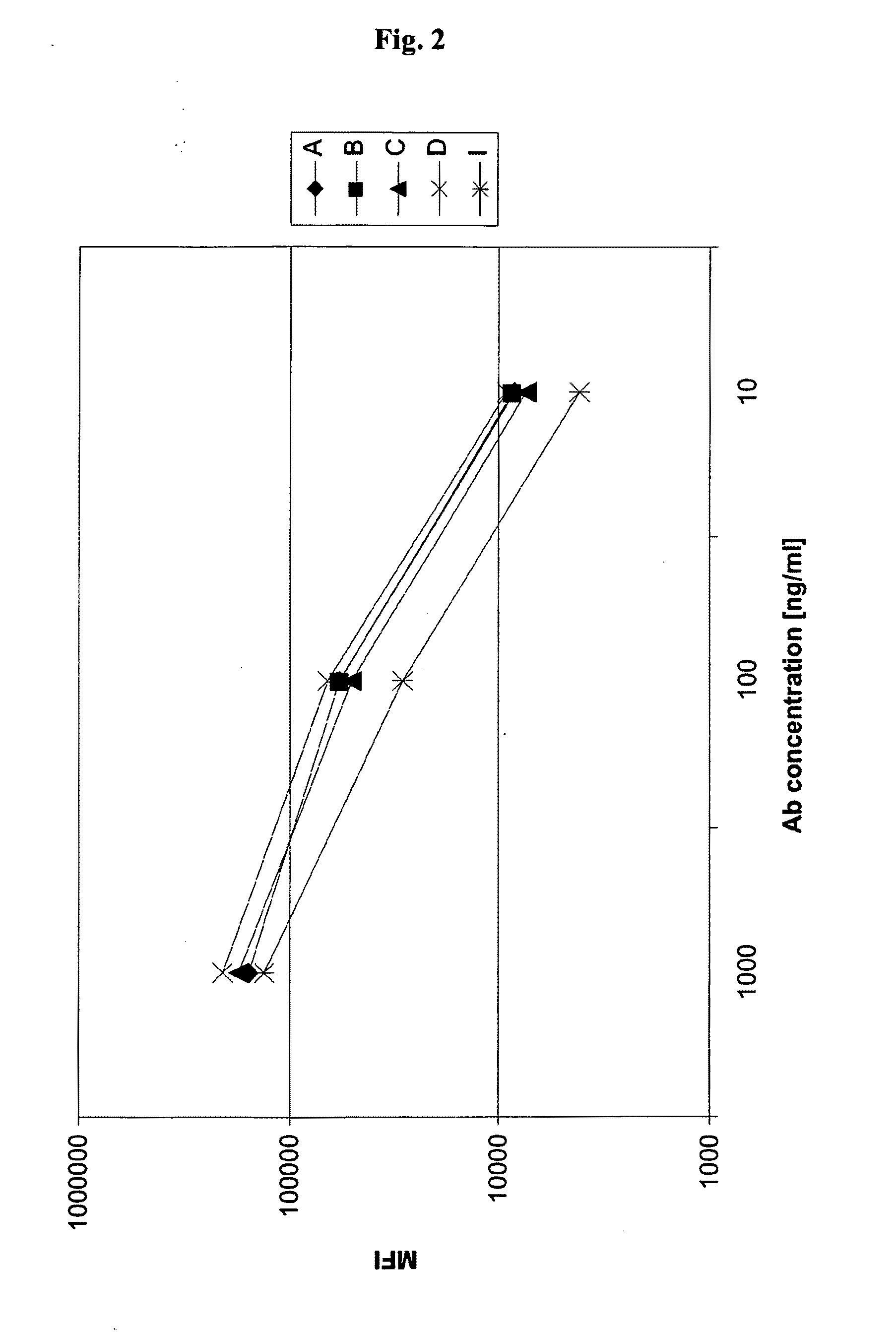
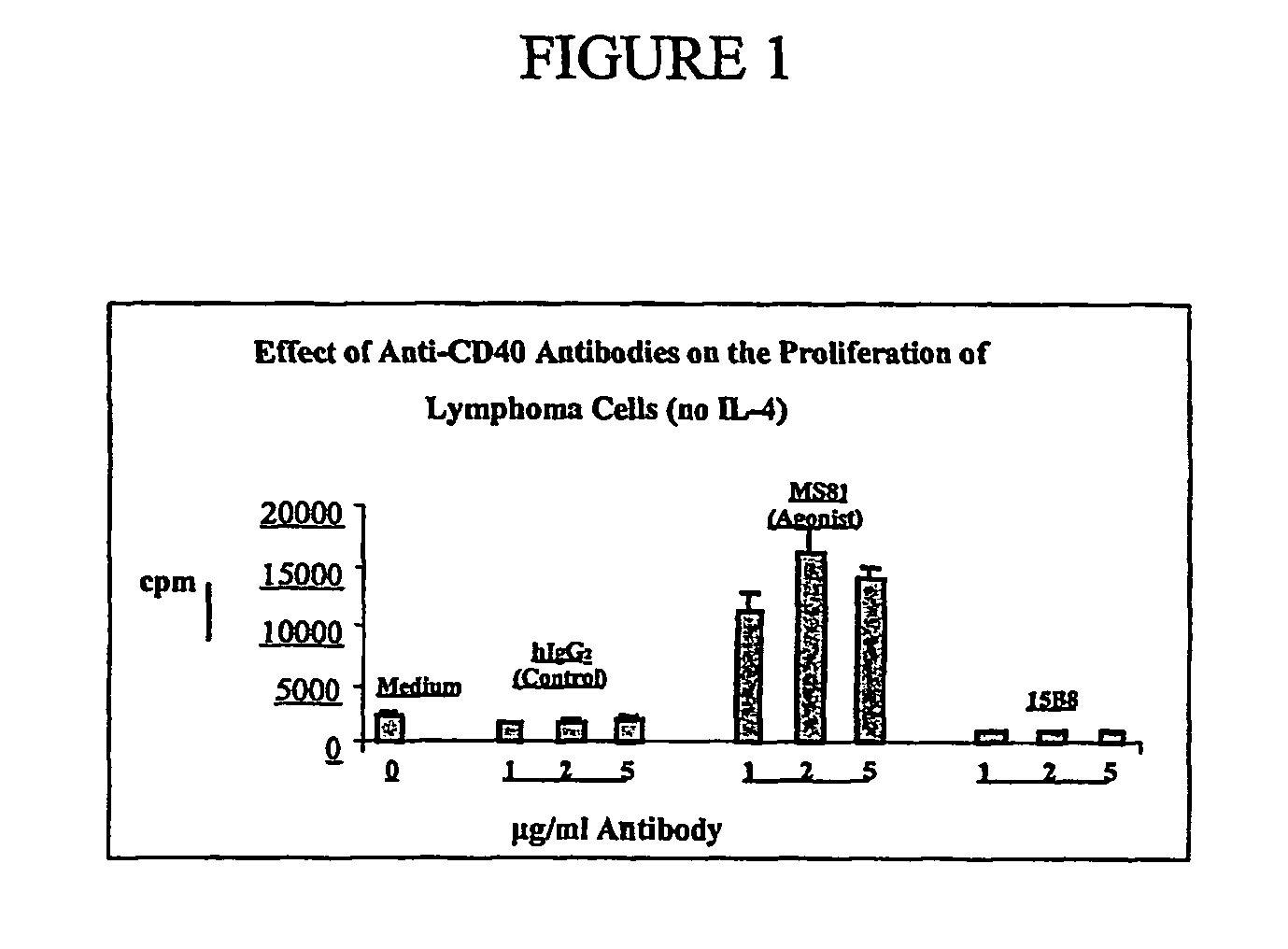
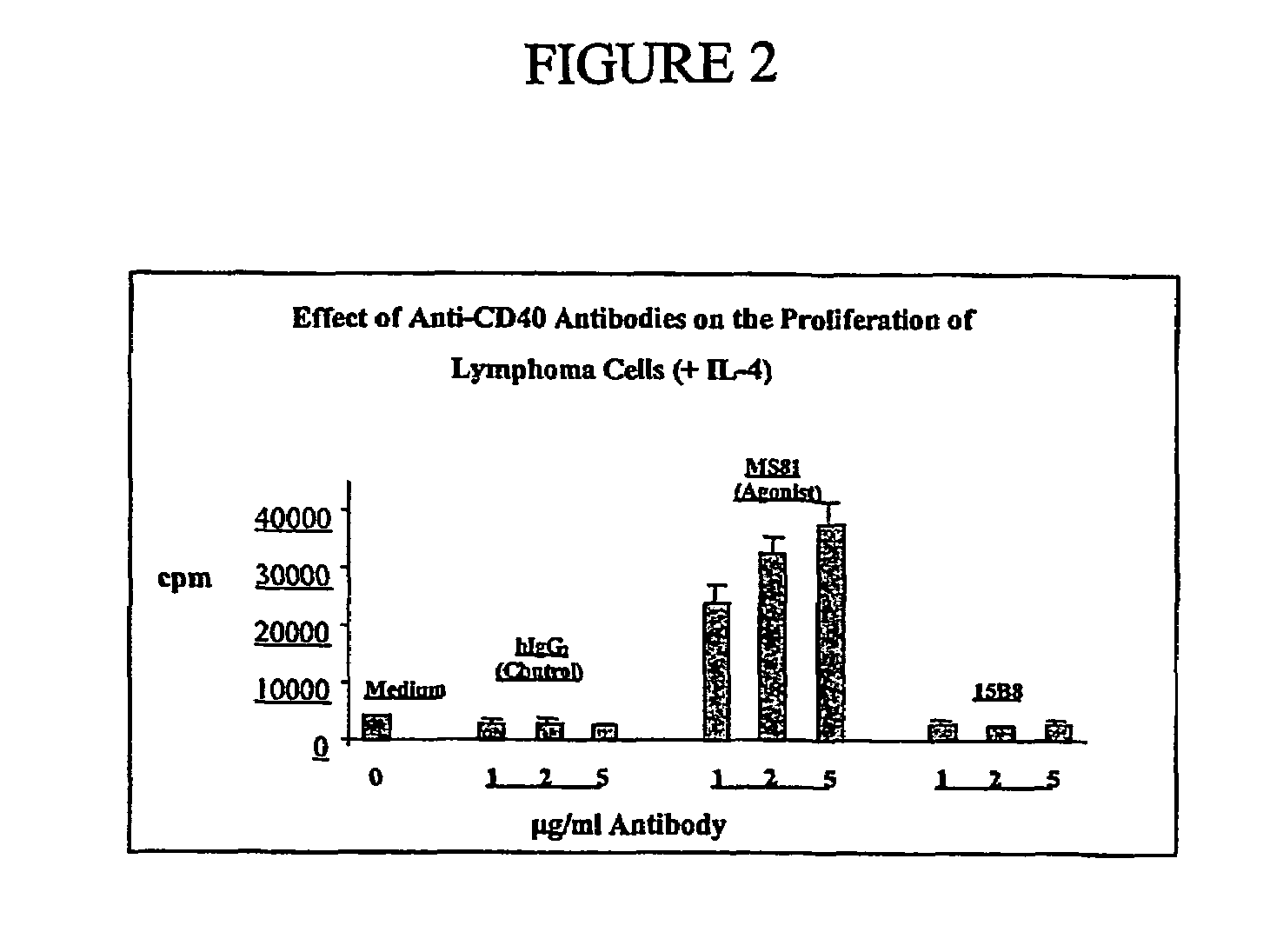
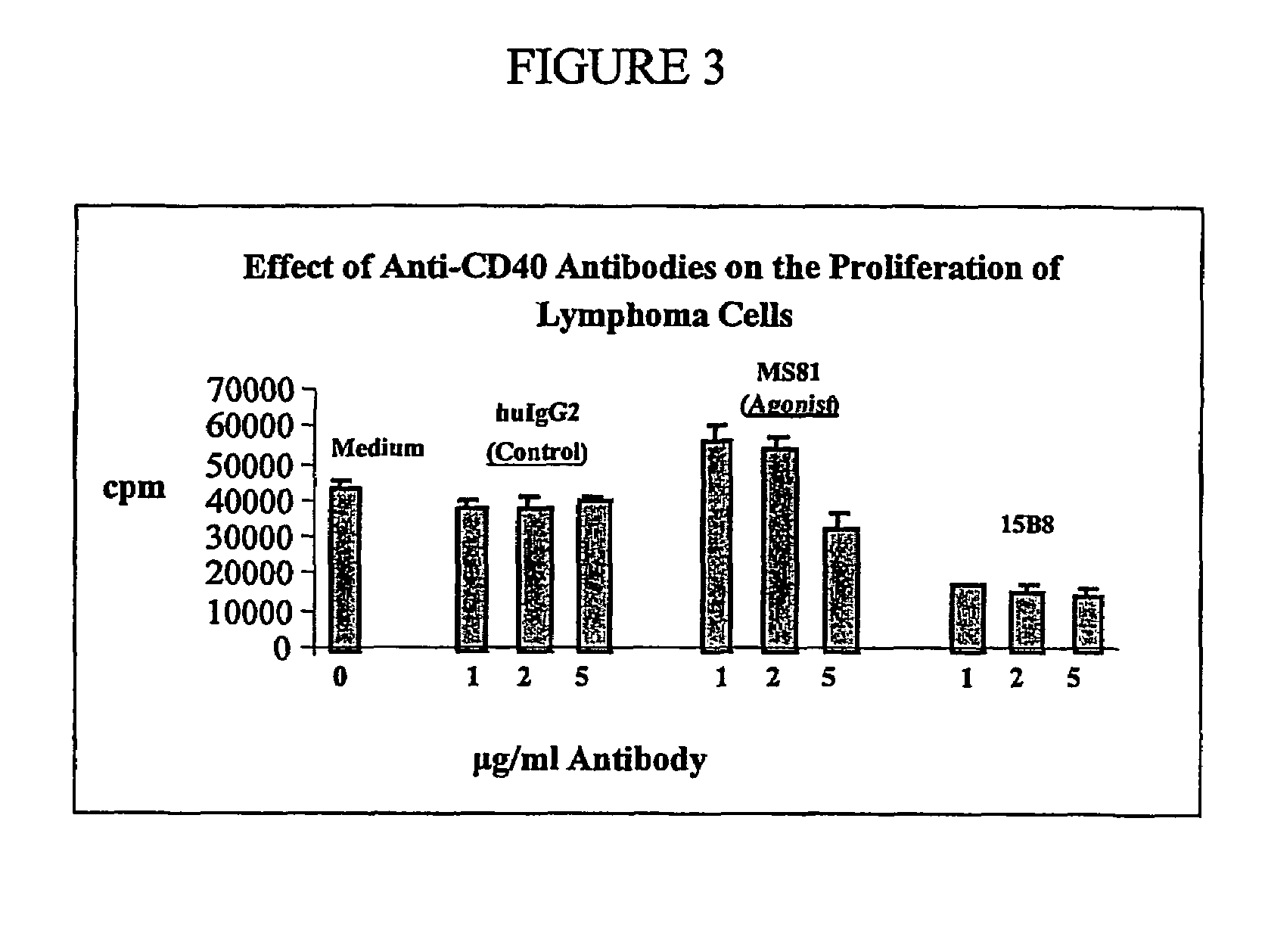
Methods of therapy for B-cell malignancies using antagonist anti-CD40 antibodies
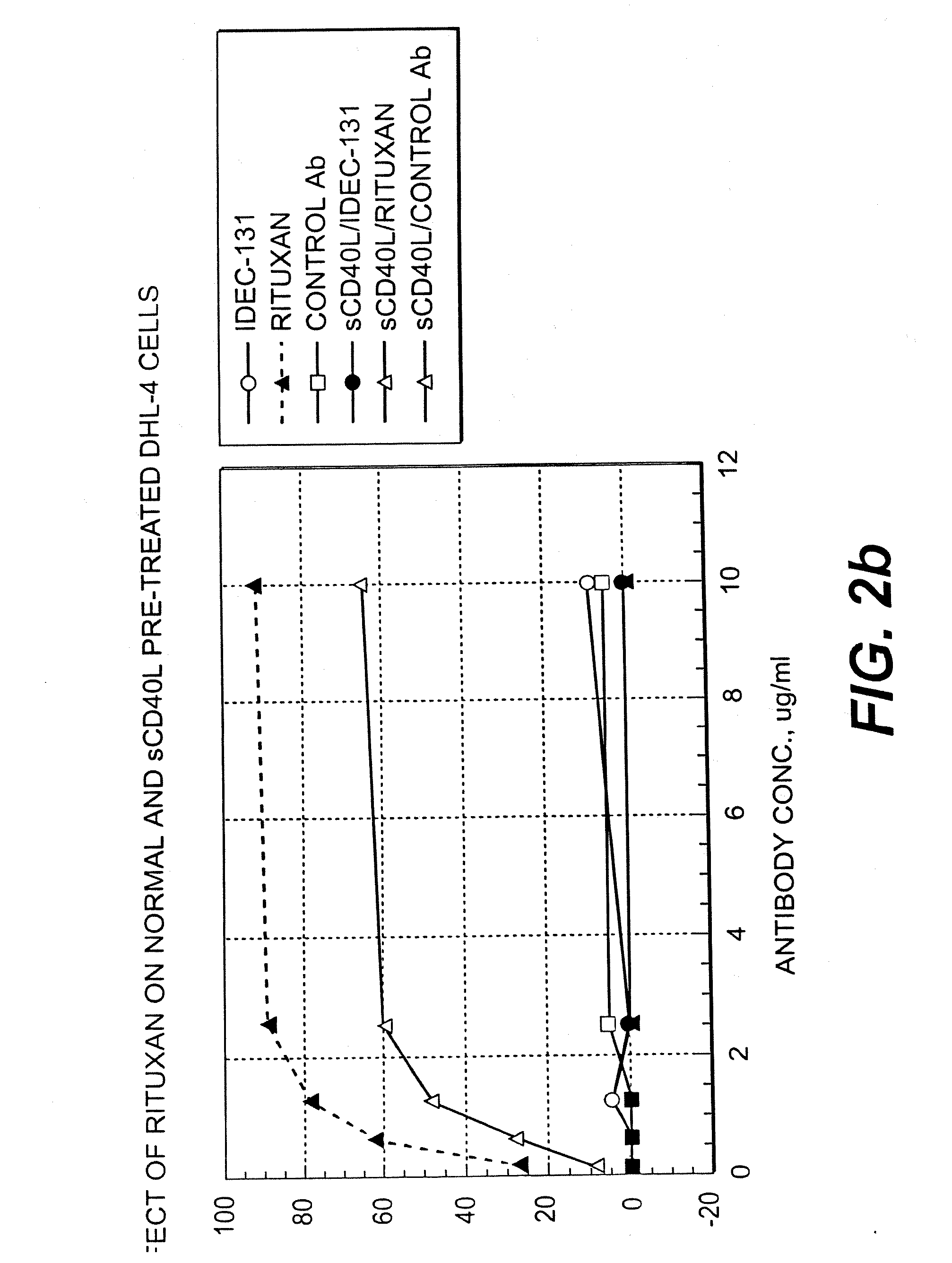
InactiveUS7288252B2Little or no agonist activityNervous disorderVirusesAntigenAntigen Binding Fragment
Methods of therapy for B-cell malignancies are provided. The methods comprise administering a therapeutically effective amount of an antagonist anti-CD40 antibody or antigen-binding fragment thereof to a patient in need thereof. The antagonist anti-CD40 antibody or antigen-binding fragment thereof is free of significant agonist activity when the antibody binds a CD40 antigen on a normal human B cell, exhibits antagonist activity when the antibody binds a CD40 antigen on a malignant human B cell, and can exhibit antagonist activity when the antibody binds a CD40 antigen on a normal human B cell. Antagonist activity of the anti-CD40 antibody or antigen-binding fragment thereof beneficially inhibits proliferation and / or differentiation of malignant human B cells.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Novel CD19xCD3 specific polypeptides and uses thereof
Described are novel single-chain multifunctional polypeptides comprising at least two binding sites specific for the CD19 and CD3 antigen, respectively. Further provided are polypeptides, wherein the above-described polypeptide comprises at least one further domain, preferably of pre-determined function. Furthermore, polynucleotides encoding said polypeptides as well as to vectors comprising said polynucleotides and host cells transformed therewith and their use in the production of said polypeptides are described. In addition, compositions, preferably pharmaceutical and diagnostic compositions are provided comprising any of the afore-described polypeptides, polynucleotides or vectors. Described is also the use of the afore-mentioned polypeptides, polynucleotides and vectors for the preparation of pharmaceutical compositions for immunotherapy, preferably against B-cell malignancies such as non-Hodgkin lymphoma.
Owner:AMGEN RES (MUNICH) GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com