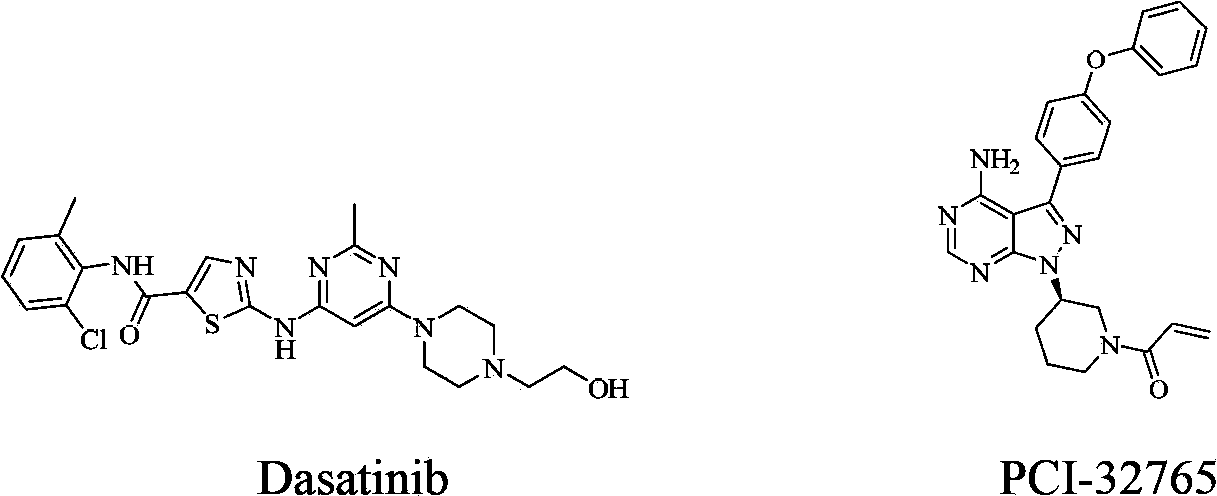
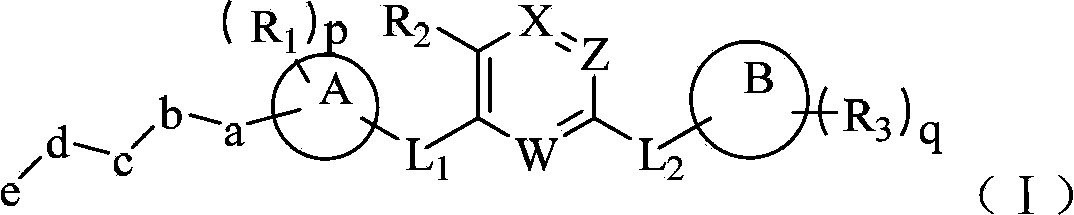
Hetero-aromatic ring and derivative type tyrosine kinase inhibitor thereof
A technology of heteroaryl and amino groups, applied in the field of preparation and treatment of blood cancer related to B cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] The preparation of step 1 intermediate 1
[0142] Mix 5-bromouracil (1 equivalent) with raw material 1 (at least 5 equivalents), without solvent, or add a small amount of polar solvent (water, ethanol, n-butanol, etc.) and heat (100-150 degrees) and stir for several hours Until 5-bromouracil disappears. After cooling, a solid precipitated out and was filtered to obtain Intermediate 1. When the raw material 1 is mercaptan, it is necessary to add an equivalent amount of alkali (such as sodium hydroxide) during the reaction.
[0143] Step 2 Preparation of Intermediate 2
[0144] Mix Intermediate 1 (1 equivalent) with the solvent amount of phosphorus oxychloride (at least 10 equivalents), add about two equivalents of N,N-dimethylaniline, heat (90-110 degrees) and stir for several hours to reach Intermediate 1 disappear. Cooled, poured into ice water, precipitated solid, filtered and dried to obtain Intermediate 2. Or concentrate the reaction system and perform column c...
Embodiment 1
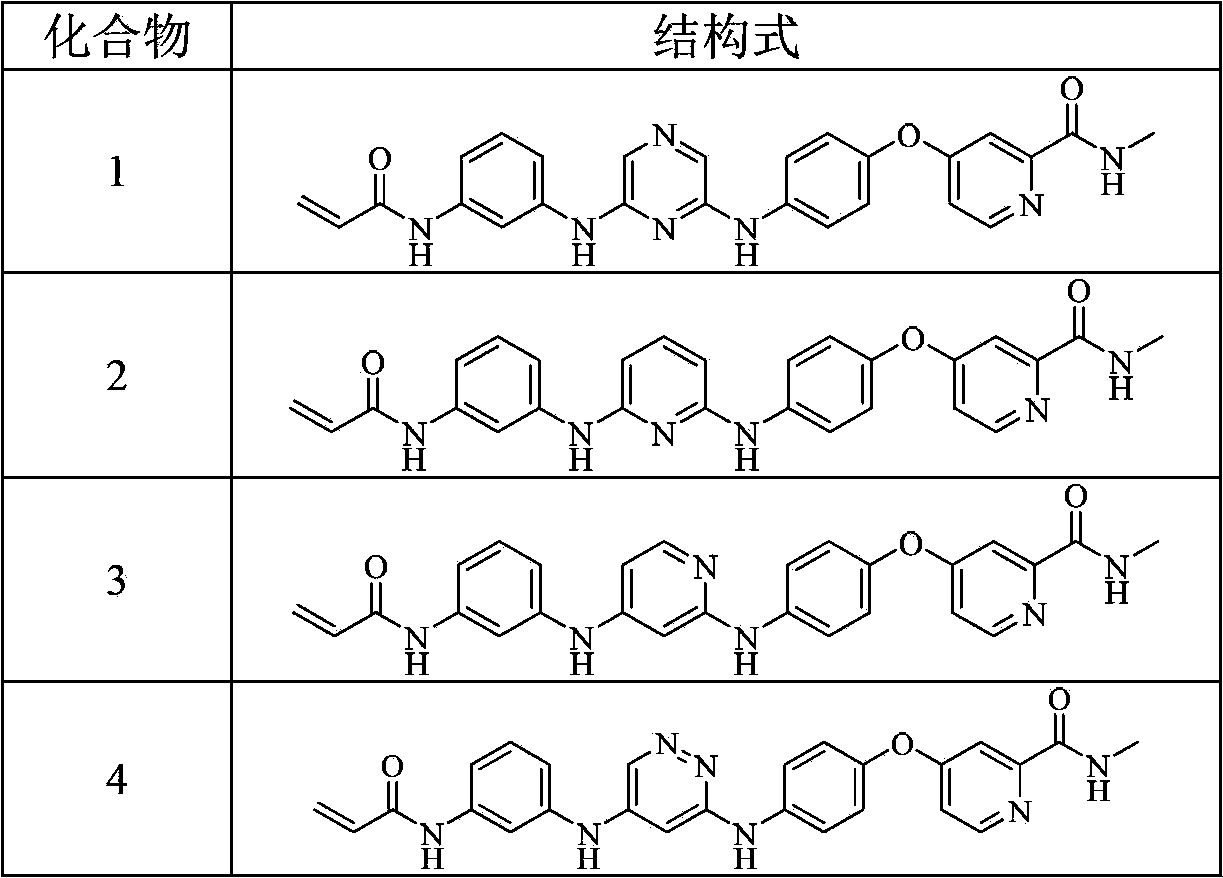
[0217] Preparation of Example 14-(4-(6-(3-acrylamidoanilino)pyrazin-2-ylamino)phenoxy)-N-methylpyridine-2-carboxamide (compound 1)
[0218]
[0219] (1) Preparation of tert-butyl 3-(6-chloropyrazin-2-ylamino)phenylcarbamate
[0220]
[0221] Weigh 2,6-dichloropyrazine (3.0g, 20mmol), tert-butyl 3-aminophenylcarbamate (4.165g, 20mmol), tris(dibenzylideneacetone) dipalladium (0.183g, 0.2mmol ), 1,1′-binaphthyl-2,2′-bisdiphenylphosphine (0.436g, 0.7mmol), potassium tert-butoxide (4.49g, 40mmol), add 50mL of toluene as solvent, and protect under nitrogen at 90°C Light reaction for 4 hours, after stopping the reaction, cool down, directly measure 1 / 2 of the reaction solution and directly put it into the next reaction.
[0222] (2) Preparation of tert-butyl 3-(6-(4-(2-(methylcarbamoyl)pyridin-4-yloxy)anilino)pyrazin-2-ylamino)phenylcarbamate
[0223]
[0224] Measure the reaction solution of 1 / 2 of the previous step, add 4-(4-aminophenoxy)-N-methylpyridine-2-carboxamide...
Embodiment 2
[0233] Example 2 Preparation of 4-(4-(6-(3-acrylamidoanilino)pyridin-2-ylamino)phenoxy)-N-methylpyridine-2-carboxamide (compound 2)
[0234]
[0235] (1) Preparation of tert-butyl 3-(6-bromopyridin-2-ylamino)phenylcarbamate
[0236]
[0237] Weigh 2,6-dibromopyridine (3.0g, 12.7mmol), tert-butyl 3-aminophenylcarbamate (3.17g, 15.2mmol), palladium acetate (57mg, 0.25mmol), 1,1′-linked Naphthalene-2,2′-bisdiphenylphosphine (0.16g, 0.26mmol), potassium carbonate (6.0g, 43.4mmol), add 20mL of toluene as solvent, microwave reaction at 116°C for 0.5 hours, stop the reaction, concentrate, silica gel Column chromatography (petroleum ether-petroleum ether:ethyl acetate=5:1) gave 1.9 g of a yellow solid with a yield of 41.1%.
[0238] (2) Preparation of tert-butyl 3-(6-(4-(2-(methylcarbamoyl)pyridin-4-yloxy)anilino)pyridin-2-ylamino)phenylcarbamate
[0239]
[0240]Weigh 3-(6-bromopyridin-2-ylamino)phenylcarbamate tert-butyl ester (1.9g, 5.22mmol), 4-(4-aminophenoxy)-N-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com