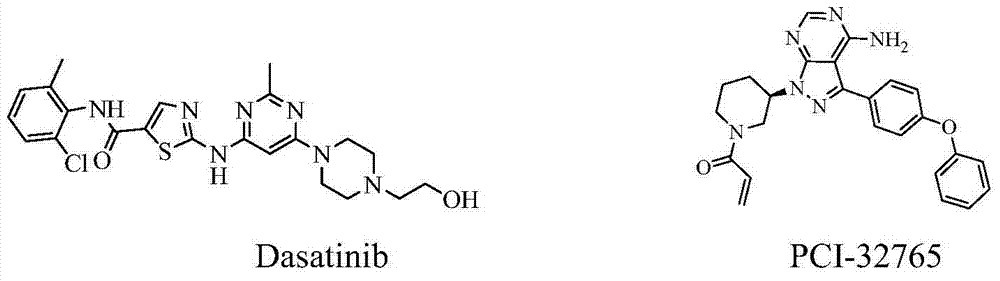
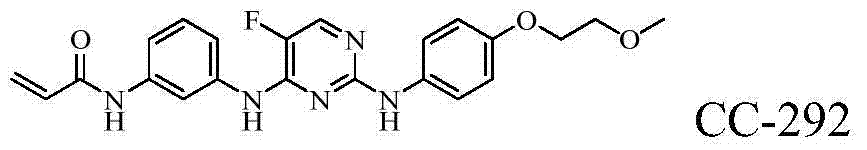
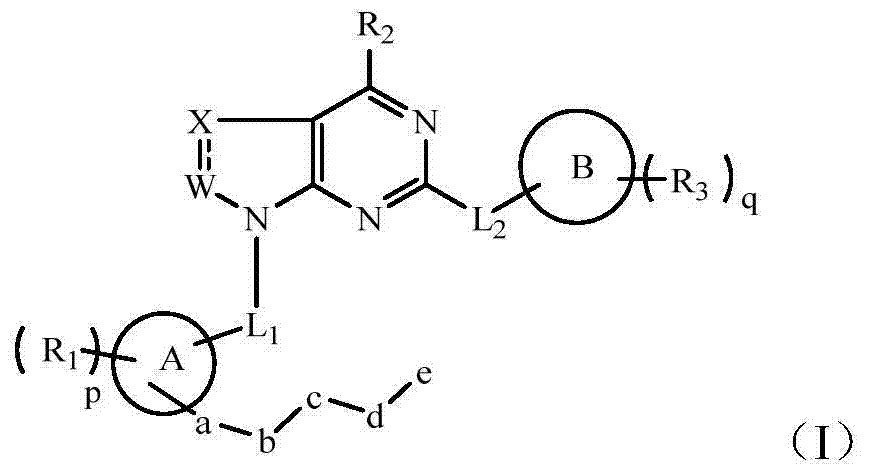
Heterocyclic nitrogen compound acting as tyrosine kinase inhibitor
A compound, amino technology, applied in the direction of active ingredients of heterocyclic compounds, anti-inflammatory agents, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0270] Example 14-(4-(8-(3-acrylamidophenyl)-6-methyl-7-oxo-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidine -2-base Preparation of amino)phenoxy)-N-methylpyridine-2-carboxamide (compound 1)
[0271]
[0272] (1) Preparation of 5-(methylamino)pyrimidine-2,4(1H,3H)-dione
[0273]
[0274] 5-Bromouracil (10g, 52.4mmol) was added to 50mL of 27% methanol solution, and the reaction was refluxed for 16h after addition. The system was cooled and suction filtered to obtain 4.7 g of a light yellow solid with a yield of 63.5%.
[0275] (2) Preparation of 2,4-dichloro-N-methylpyrimidin-5-amine
[0276]
[0277] Add 5-(methylamino)pyrimidine-2,4(1H,3H)-dione (4.7 g, 33.3 mmol) to 50 mL POCl 3 Add 2mL of N,N-dimethylaniline dropwise, and react at 110°C for 24h after the addition. Cooling, concentrating the system under reduced pressure, adding 20mL toluene and spinning the system again, the obtained oil was poured into ice water, and washed with Na 2 CO 3 Neutralized to neutr...
Embodiment 2
[0295] Example 24-(4-(7-(3-acrylamidophenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-ylamino)phenoxy)-N-methyl Preparation of pyridine-2-carboxamide (compound 2)
[0296]
[0297] (1) Preparation of 2-chloro-7-(3-nitrophenyl)-7H-pyrrolo[2,3-d]pyrimidine
[0298]
[0299] 2-Chloro-7H-pyrrolo[2,3-d]pyrimidine (1.54 g, 10.0 mmol) and 3-nitrophenylboronic acid (2.51 g, 15.0 mmol) were dissolved in dry dichloromethane (60 mL) . Add anhydrous copper acetate (3.64g, 20.0mmol) and pyridine (1.58g, 20.0mmol) to it, react at room temperature for 24 hours, filter, wash the solid with dichloromethane several times, concentrate the filtrate and perform silica gel column chromatography (Petroleum ether: ethyl acetate = 5:1), 1.42 g of white solid was obtained with a yield of 51.7%.
[0300] (2) Preparation of 3-(2-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)aniline
[0301]
[0302] Dissolve 2-chloro-7-(3-nitrophenyl)-7H-pyrrolo[2,3-d]pyrimidine (1.42g, 5.17mmol) in a mixed solution of tetr...
Embodiment 3
[0311] Example 3N-(3-(2-(4-(2-methoxyethoxy)anilino)-7-methyl-8-oxo-7H-purin-9(8H)-yl)phenyl ) C Preparation of Enamide (Compound 3)
[0312]
[0313] (1) Preparation of 4-(2-methoxyethoxy)aniline
[0314]
[0315] Add 1-(2-methoxyethoxy)-4-nitrobenzene (1.97g, 10mmol) to 50mL of methanol solution, add 10% Pd / C197mg, add Biton H 2 Reaction at room temperature for 16h. The solid was removed by suction filtration, and the filtrate was concentrated to obtain 1.6 g of a light red oil, with a yield of 95.7%.
[0316] (2) Preparation of tert-butyl 3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylamino)pyrimidin-4-ylamino)phenylcarbamate
[0317]
[0318] Dissolve 4-(2-methoxyethoxy)aniline (668mg, 4.0mmol) in 6mL of ethanol, then add 3-(2-chloro-5-(methylamino)pyrimidin-4-ylamino)benzene tert-butyl carbamate (1.4g, 4.0mmol) was added and microwaved at 100°C for 3h. After cooling, the system was spin-dried, and column chromatography (DCM:MeOH=100:1—4:1) obtained 900 mg of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com