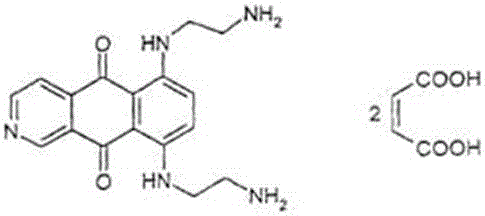
Freeze-dry composition for treating NHL (non-hodgkin lymphoma) and preparation method thereof
A non-Hodgkin, composition technology, applied in the field of medicine, can solve the problems of inconvenience, increase the risk of use by patients, etc., and achieve the effects of improving safety and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
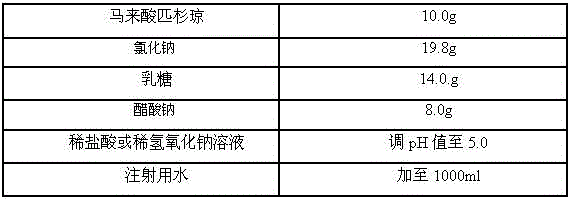
[0017] prescription
[0018]
[0019] Preparation Process
[0020] Weigh the prescribed amount of sodium chloride, lactose and sodium acetate and dissolve them in an appropriate amount of water for injection, weigh the prescribed amount of picantone maleate and dissolve them in the above solution, stir and mix evenly, and dissolve them with an appropriate amount of dilute hydrochloric acid or dilute hydrogen The sodium oxide solution is used to adjust the pH value, and the volume is adjusted to the total amount with water for injection, stirred and mixed evenly, filtered, filled, freeze-dried, and the finished product is obtained by visual inspection.
Embodiment 2
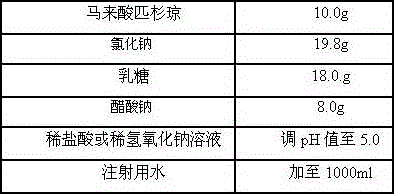
[0022] prescription
[0023]
[0024] Preparation Process:
[0025] Weigh the prescribed amount of sodium chloride, lactose and sodium acetate and dissolve them in an appropriate amount of water for injection, weigh the prescribed amount of picantone maleate and dissolve them in the above solution, stir and mix evenly, and dissolve them with an appropriate amount of dilute hydrochloric acid or dilute hydrogen The sodium oxide solution is used to adjust the pH value, and the volume is adjusted to the total amount with water for injection, stirred and mixed evenly, filtered, filled, freeze-dried, and the finished product is obtained by visual inspection.
Embodiment 3
[0027] prescription
[0028]
[0029] Preparation Process:
[0030] Weigh the prescribed amount of sodium chloride, lactose and sodium acetate and dissolve them in an appropriate amount of water for injection, weigh the prescribed amount of picantone maleate and dissolve them in the above solution, stir and mix evenly, and dissolve them with an appropriate amount of dilute hydrochloric acid or dilute hydrogen The sodium oxide solution is used to adjust the pH value, and the volume is adjusted to the total amount with water for injection, stirred and mixed evenly, filtered, filled, freeze-dried, and the finished product is obtained by visual inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com