A kind of crystal form of inhibitor and its preparation method and use
A technology of crystal form and solvent, applied in the field of medicinal chemistry crystallization, can solve problems such as poor stability of crystal form I
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Referring to the method of Example 9 in the patent document WO2005113556A1 to prepare the crystal form I of Idelalisib, the specific operation is: at 80°C, (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl- 3H-quinazolin-4-one (65.6 mmol, 1 equiv), 6-bromopurine (14.6 g, 73.4 mmol, 1.1 equiv) and diisopropylethylamine (24.3 ml, 140 mmol, 2 equiv ) in tert-butanol (40 mL) was stirred for 24 hours. The reaction mixture was concentrated in vacuo and treated with water to give a solid crude product which was collected by concentration in vacuo, washed with water and dried in the air. Half of the crude solid obtained was dissolved in 600 mL methanol, concentrated onto silica gel (300 mL dry), purified by flash chromatography (7.5 x 36 mm, eluted with 10 L 4% methanol / dichloromethane), and concentrated to give a solid. The solid product was then dissolved in 250 mL of ethanol and concentrated in vacuo to obtain a pale yellow solid compound, which was shown by elemental analysis to be...
Embodiment 2
[0209] Take 102.8mg of the crystal form I of Idelalisib in a 10mL glass vial, add 5mL of toluene, ultrasonicate for 5 minutes to obtain a suspension, stir at room temperature for 8 days to crystallize, filter the obtained solid, and vacuum dry at 60°C for 10 hours to obtain the compound of the present invention. The above-mentioned crystal form II. The yield was 94.3 mg; the molar yield was 95.8%.
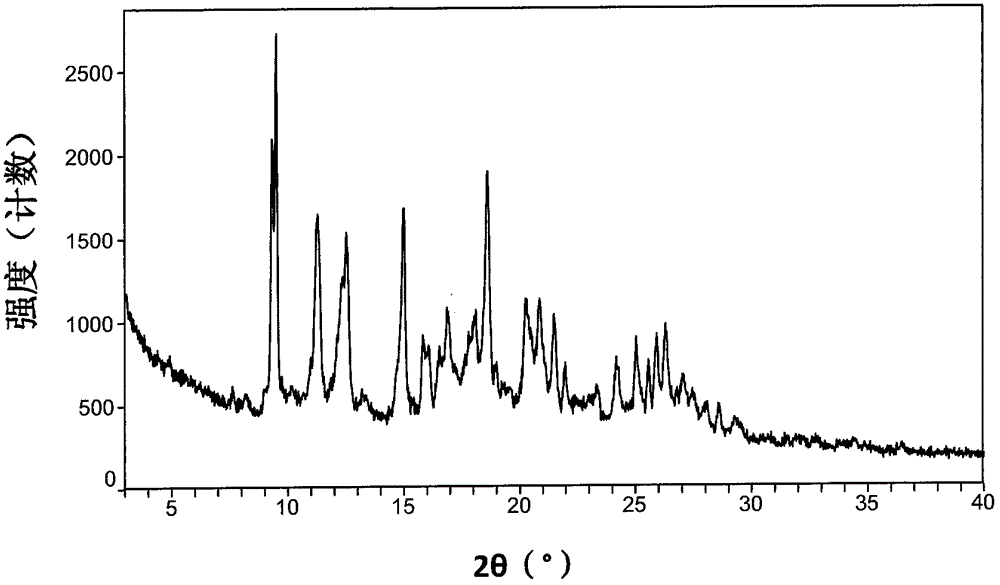
[0210] X-ray powder diffraction spectrum as Figure 5 shown.
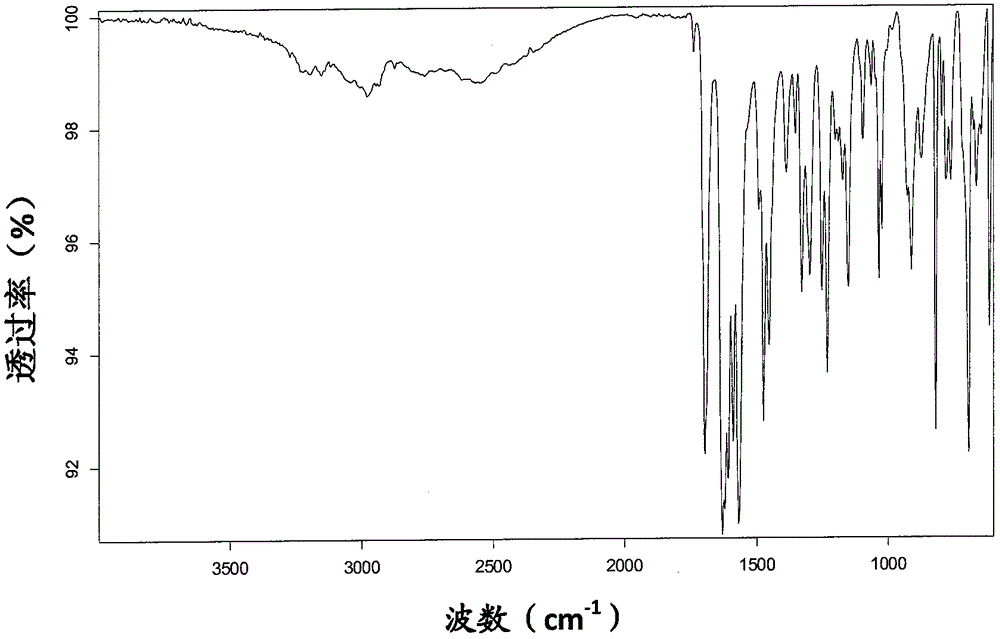
[0211] IR spectrum such as Figure 6 shown.
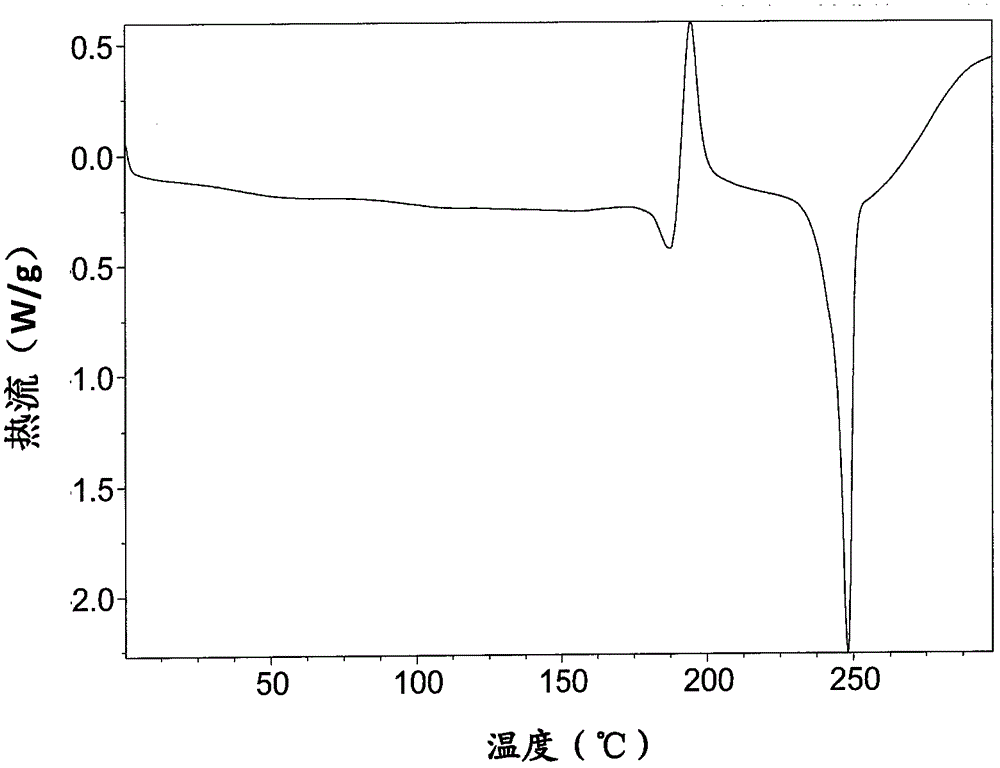
[0212] DSC spectrum such as Figure 7 shown. Display: The melting range is 244-249°C.
[0213] TGA spectrum as Figure 8 shown. It shows that there is about 0.41% weight loss (anhydrous matter) before 150°C, and the decomposition temperature is 271°C.
[0214] DVS shows: 0.46% weight change in the range of 20%-80% relative humidity.
Embodiment 3
[0216] Take 102.8mg of Idelalisib crystal form I in a 15mL glass vial, add 5mL of water and 5mL of tetrahydrofuran, sonicate for 5 minutes to obtain a suspension, stir at 40°C for 1 day to crystallize, filter the obtained solid, and dry under vacuum at 40°C for 24 hours to obtain this Said crystalline form II was invented. The yield was 80.2 mg; the molar yield was 81.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com