Patents
Literature
32 results about "Idelalisib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
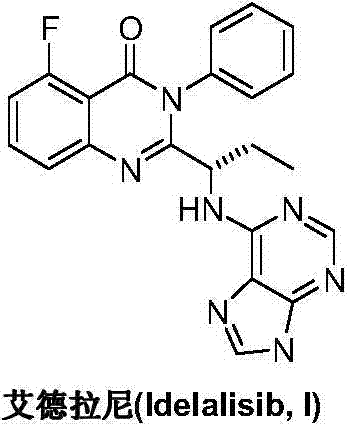
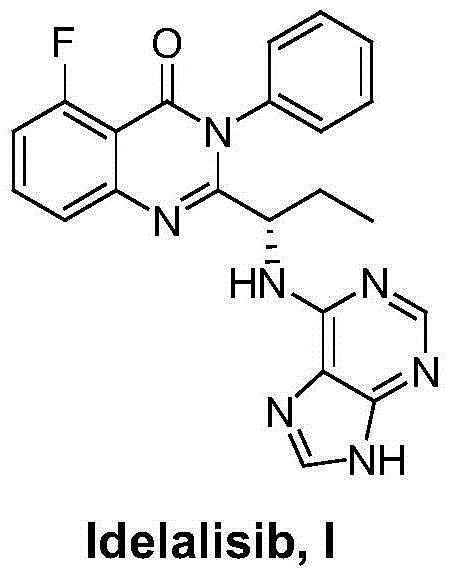
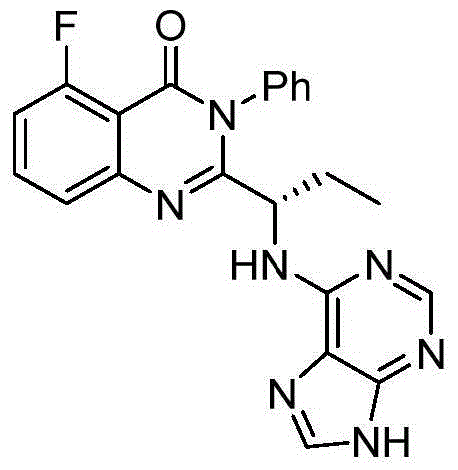
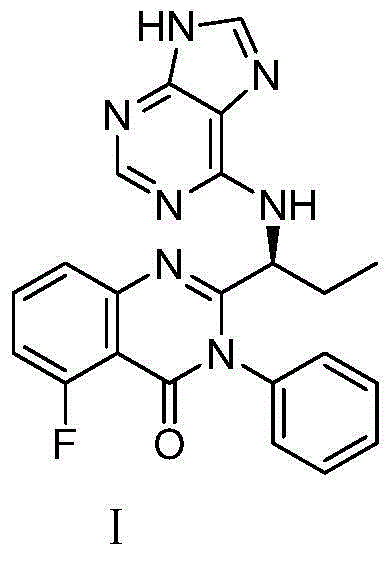
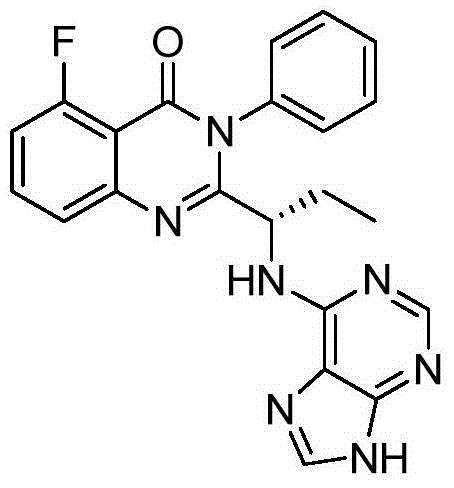
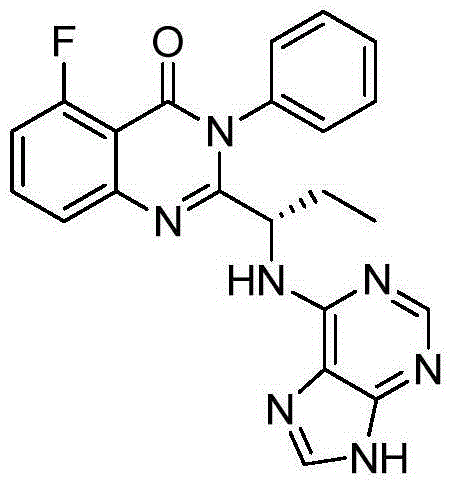
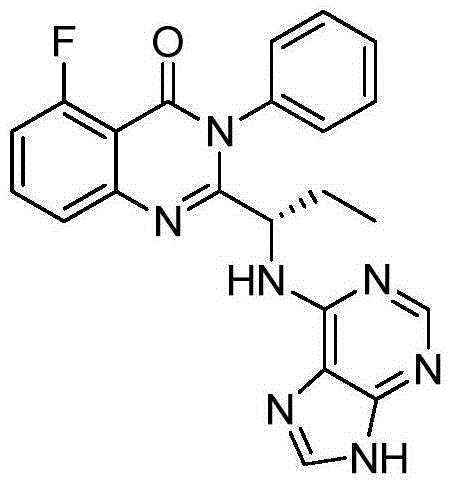
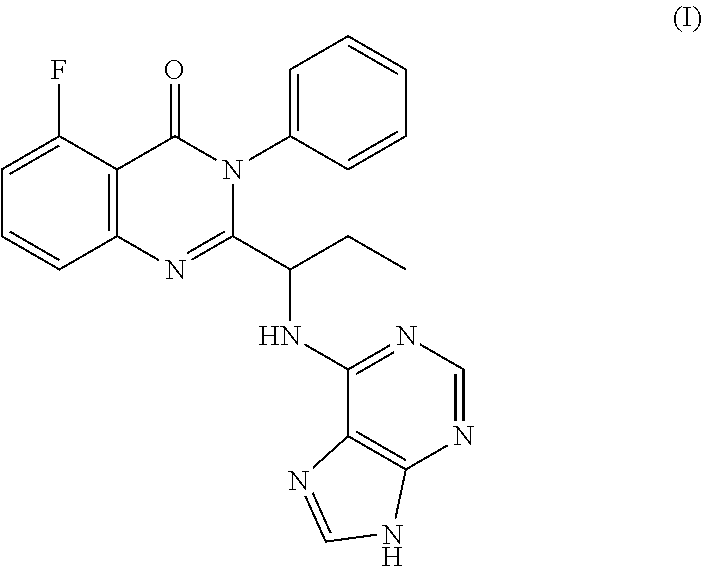
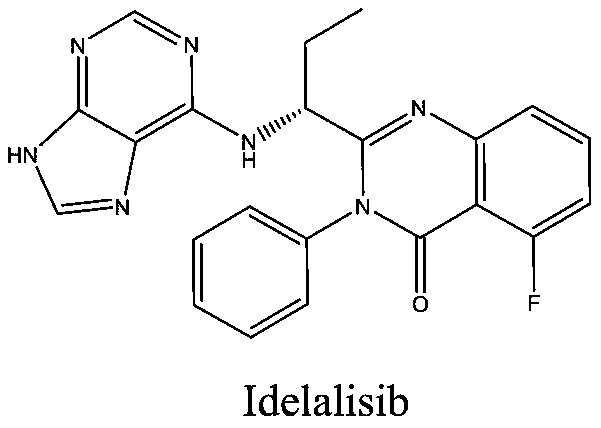
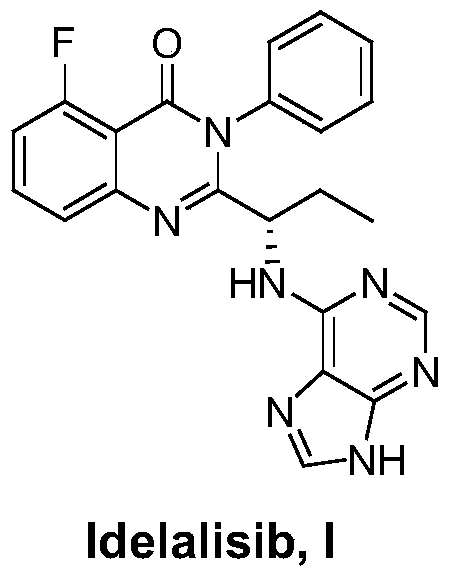
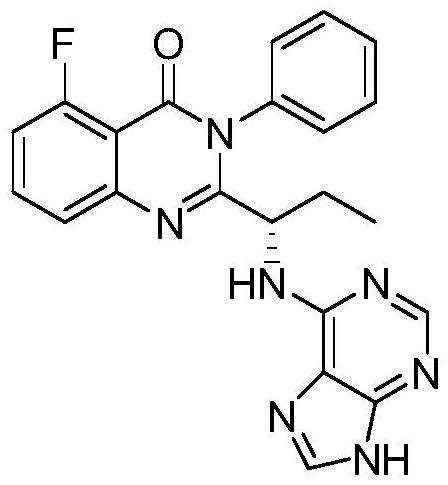
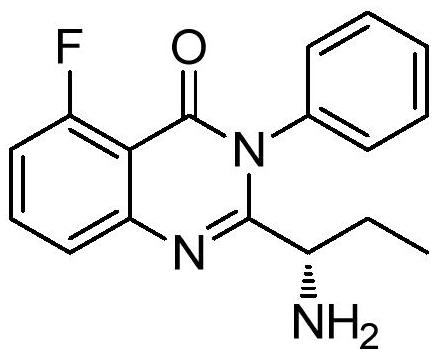
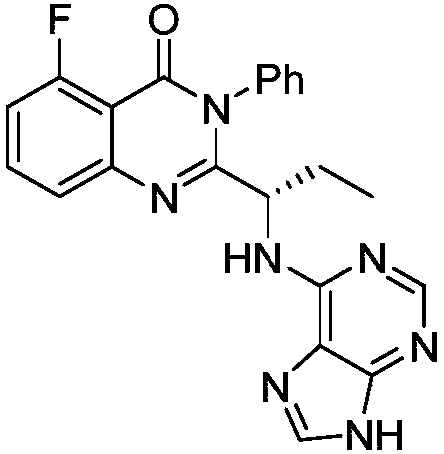
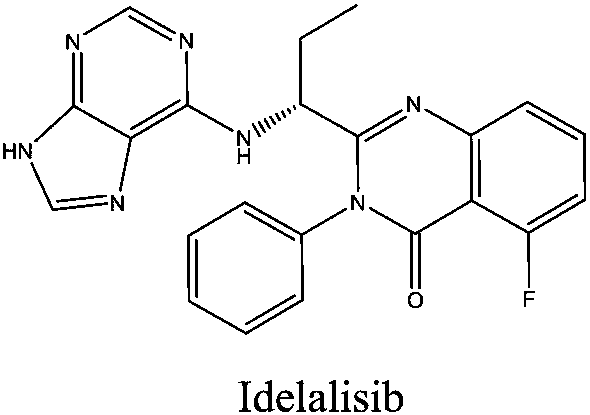
This medication is used to treat certain types of cancer (such as chronic lymphocytic leukemia-CLL, follicular B-cell non-Hodgkin's lymphoma, small lymphocytic lymphoma-SLL).
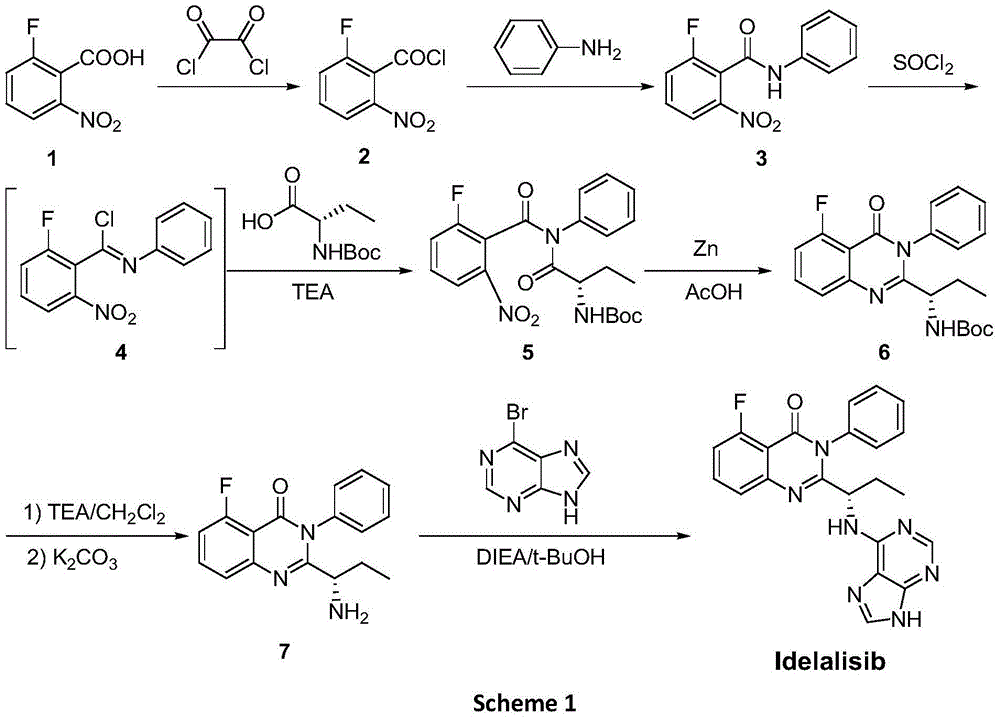
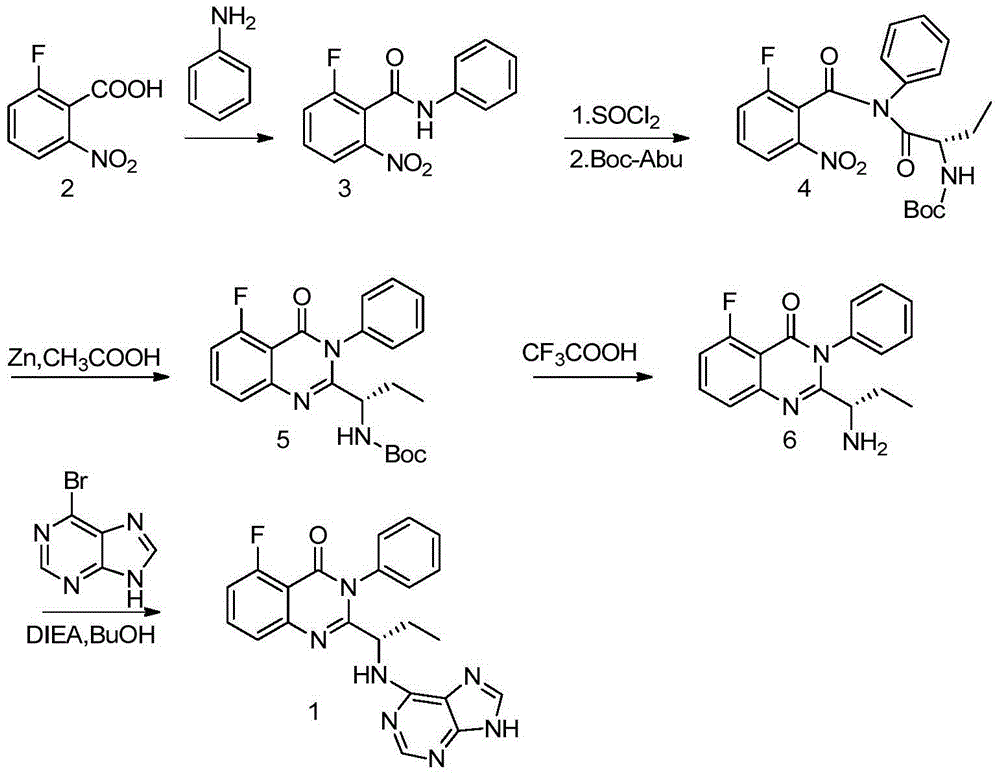
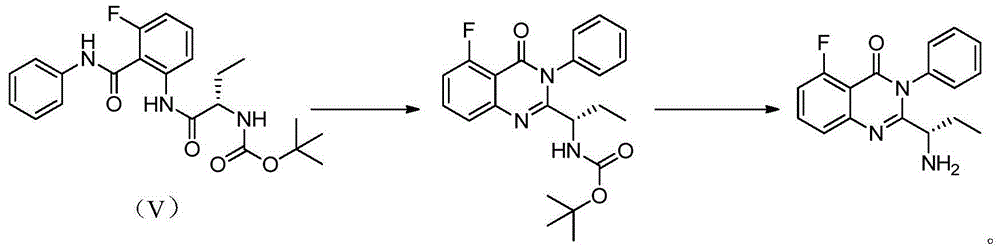
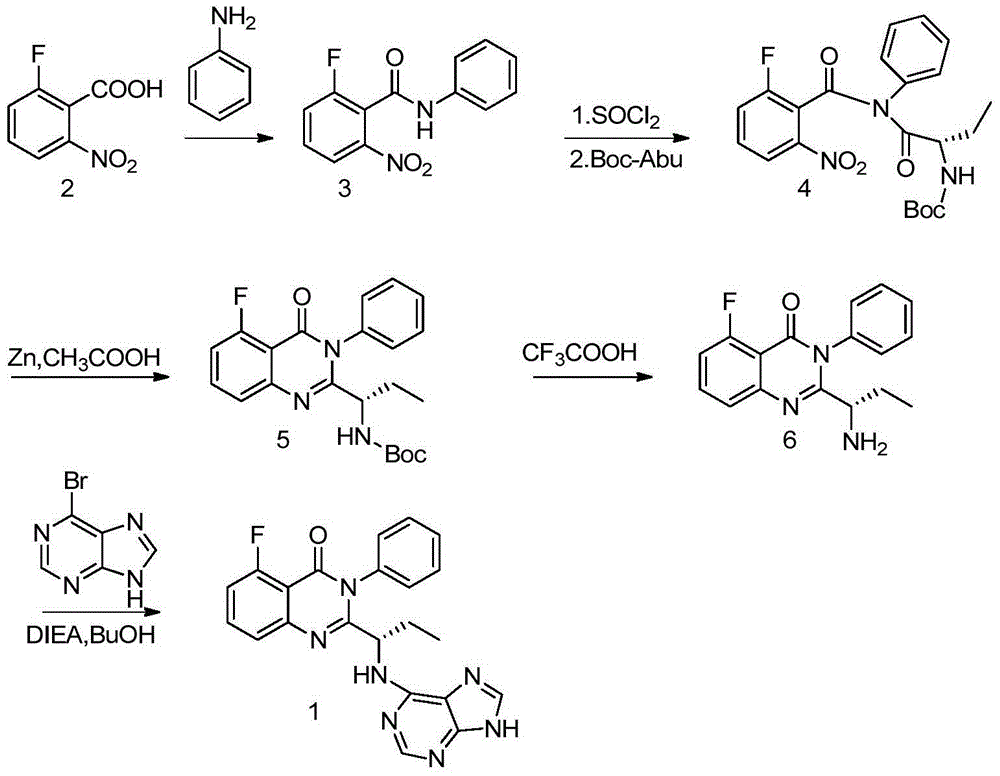
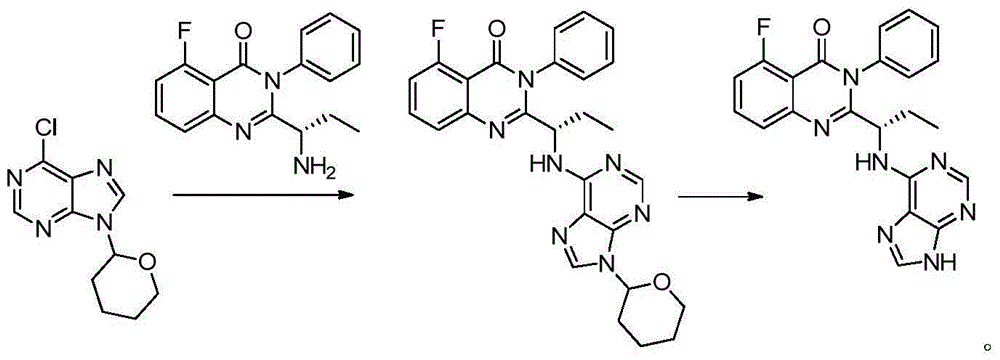
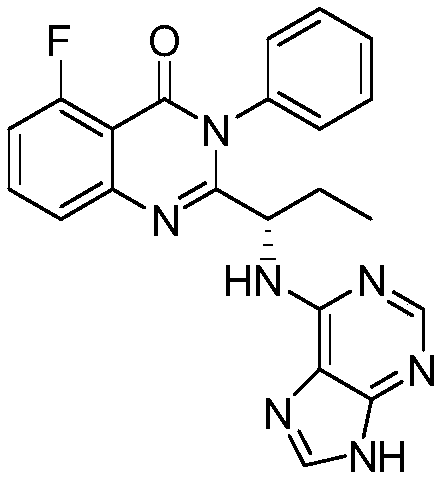
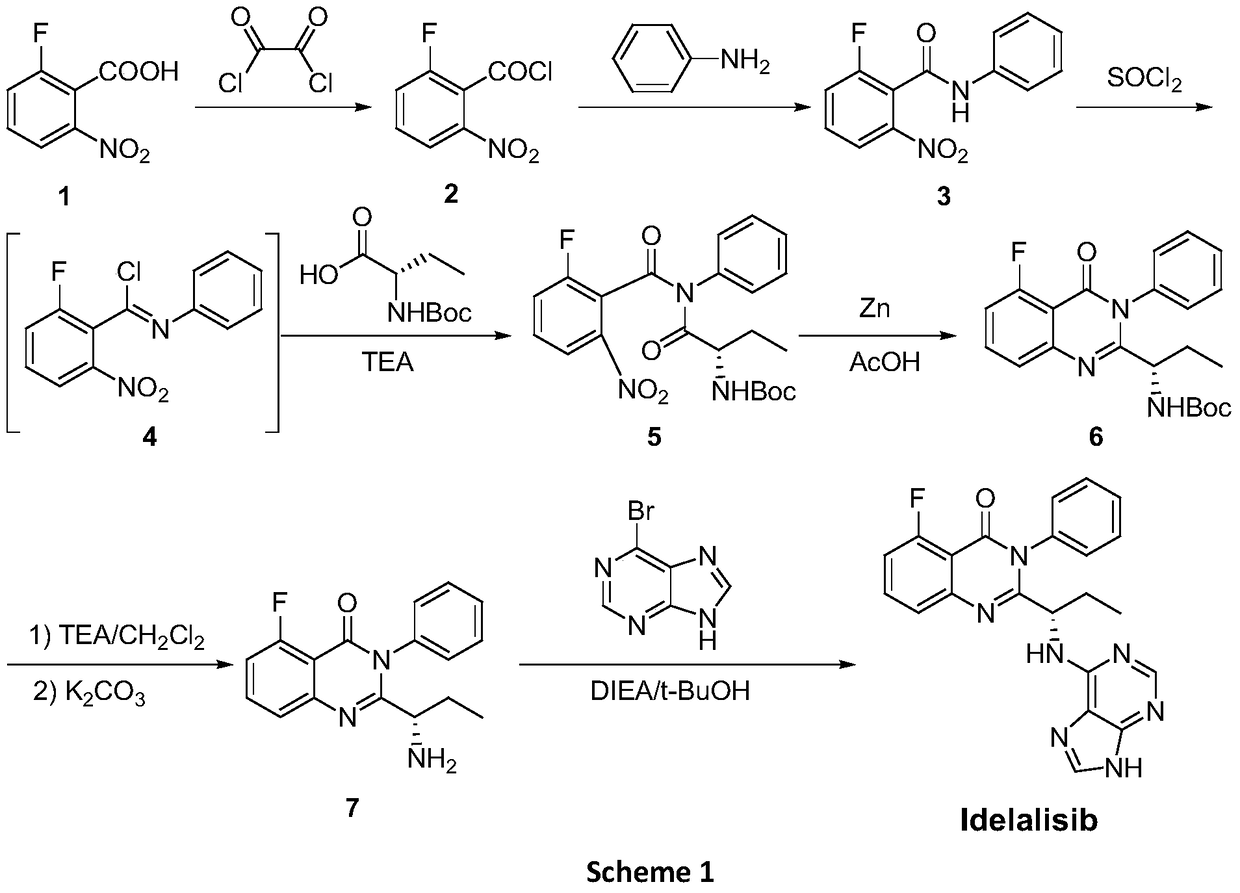
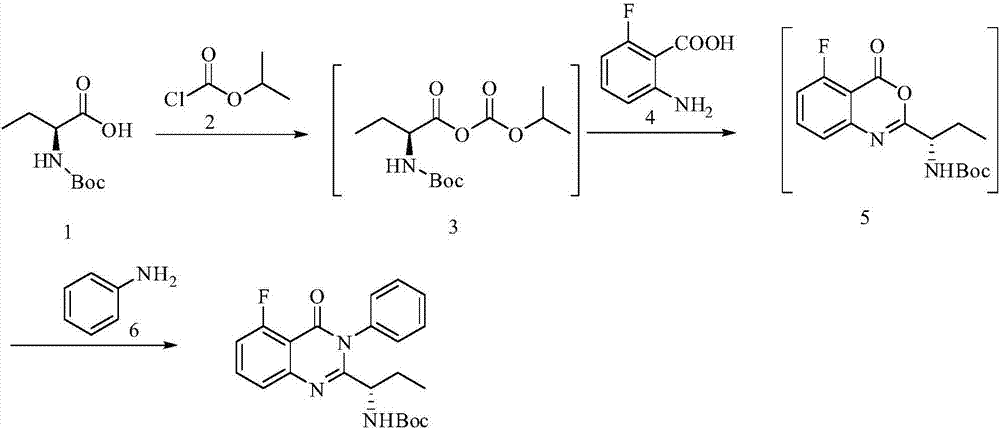
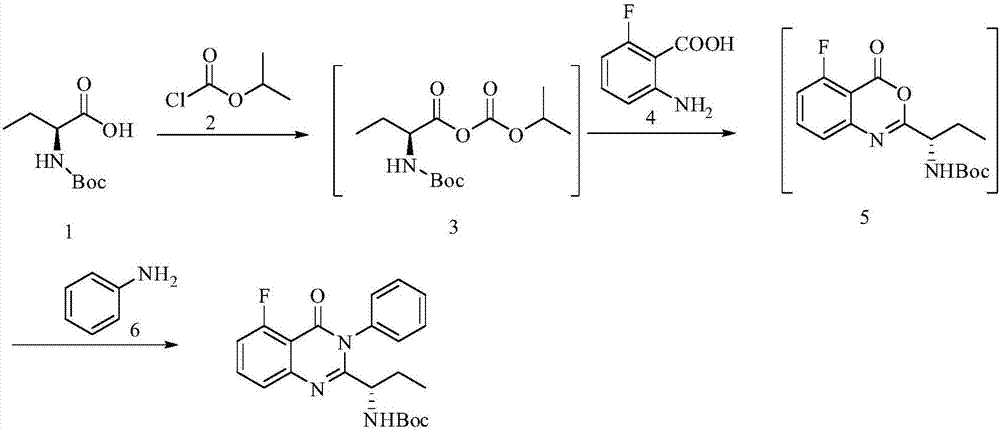
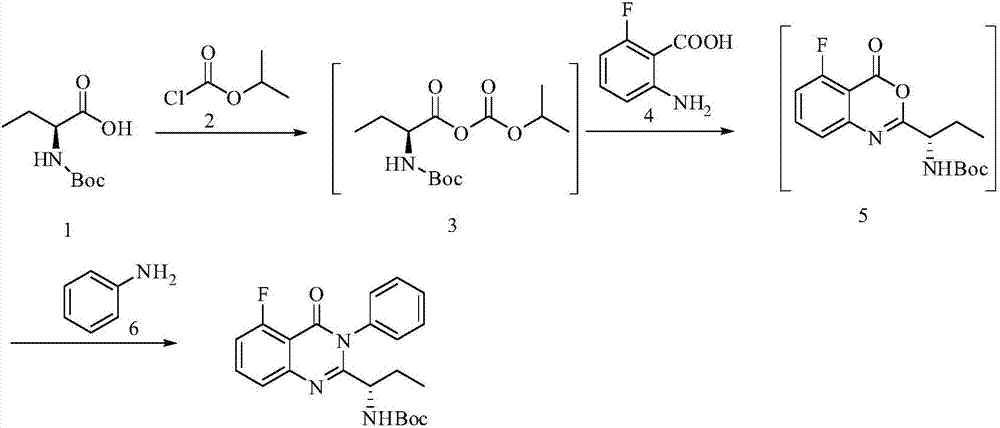
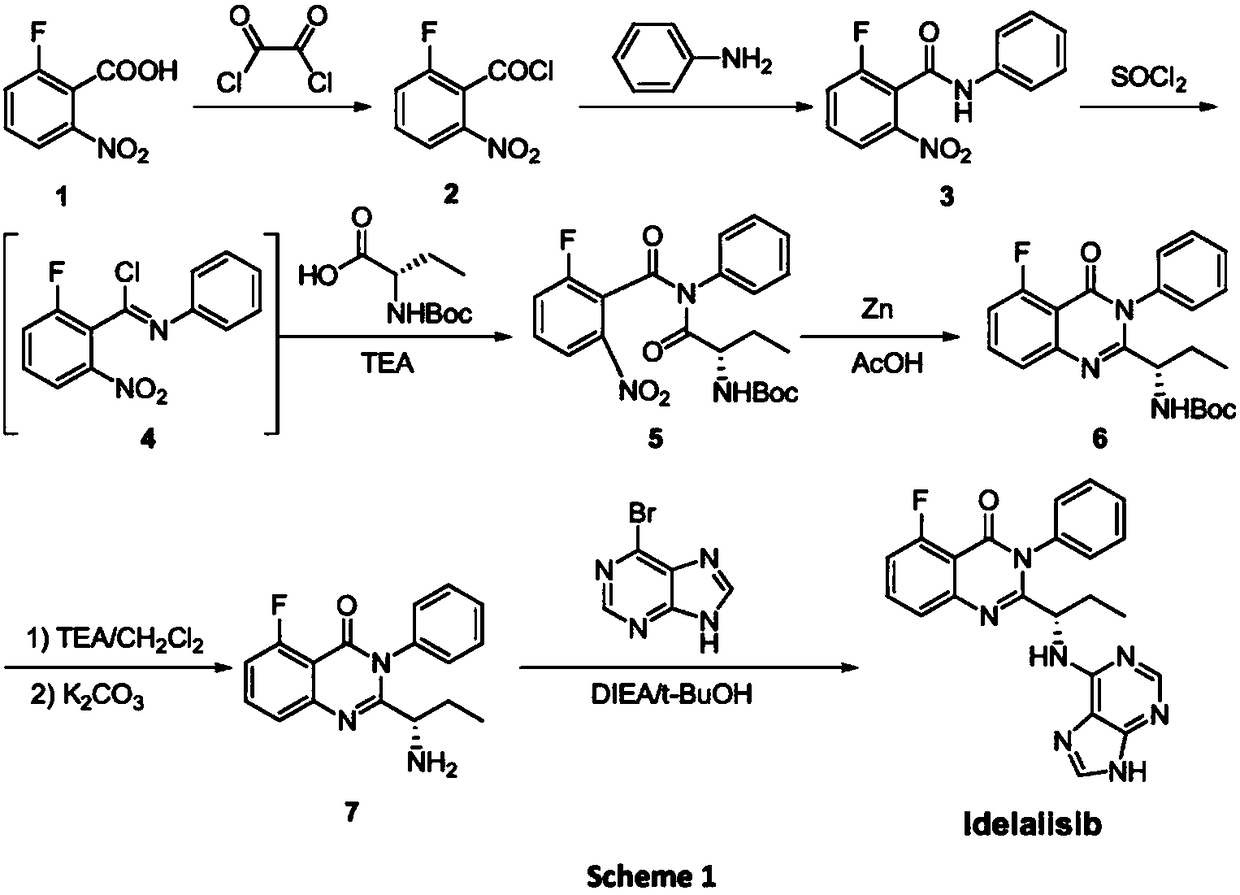
A preparing method of Idelalisib
ActiveCN104262344AEase of industrial productionRaw materials are easy to getOrganic chemistryAcetic anhydrideAminobutyrate
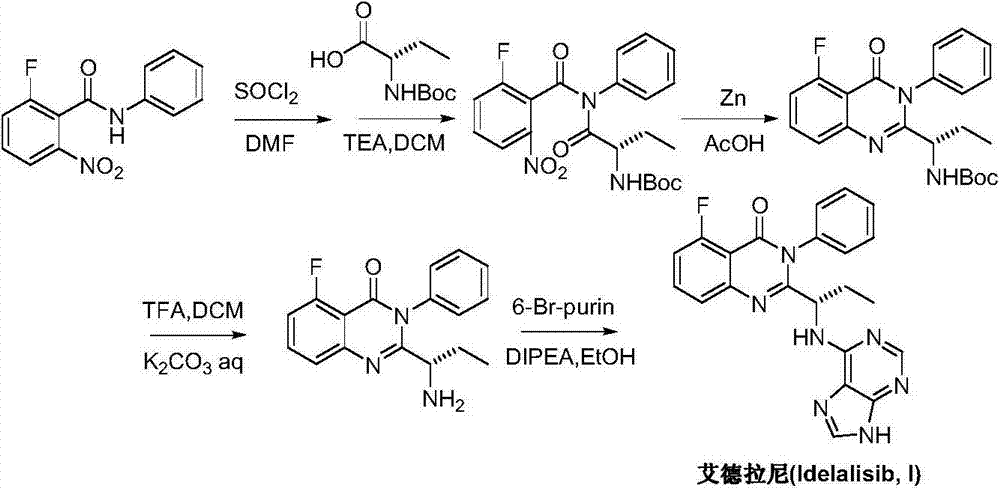
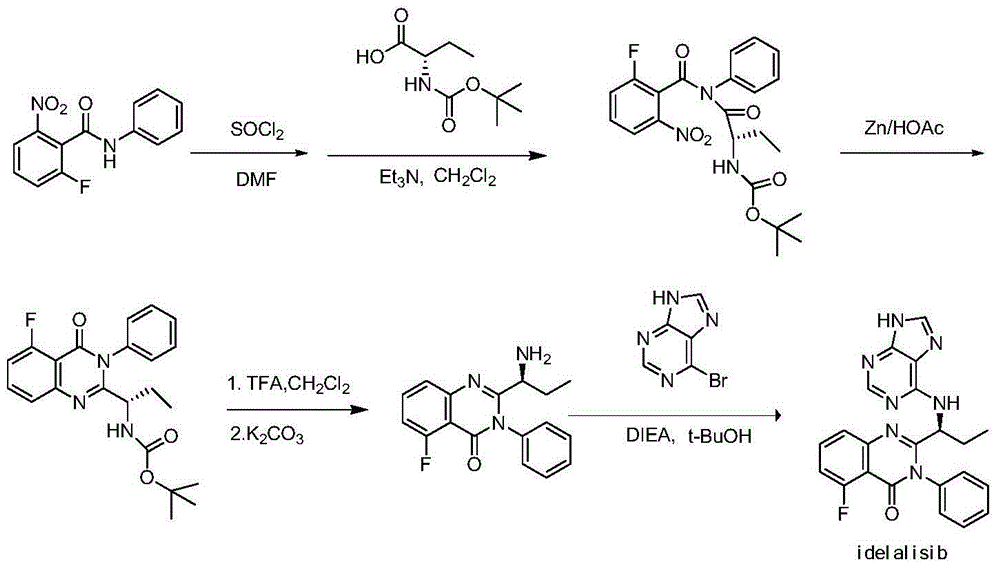
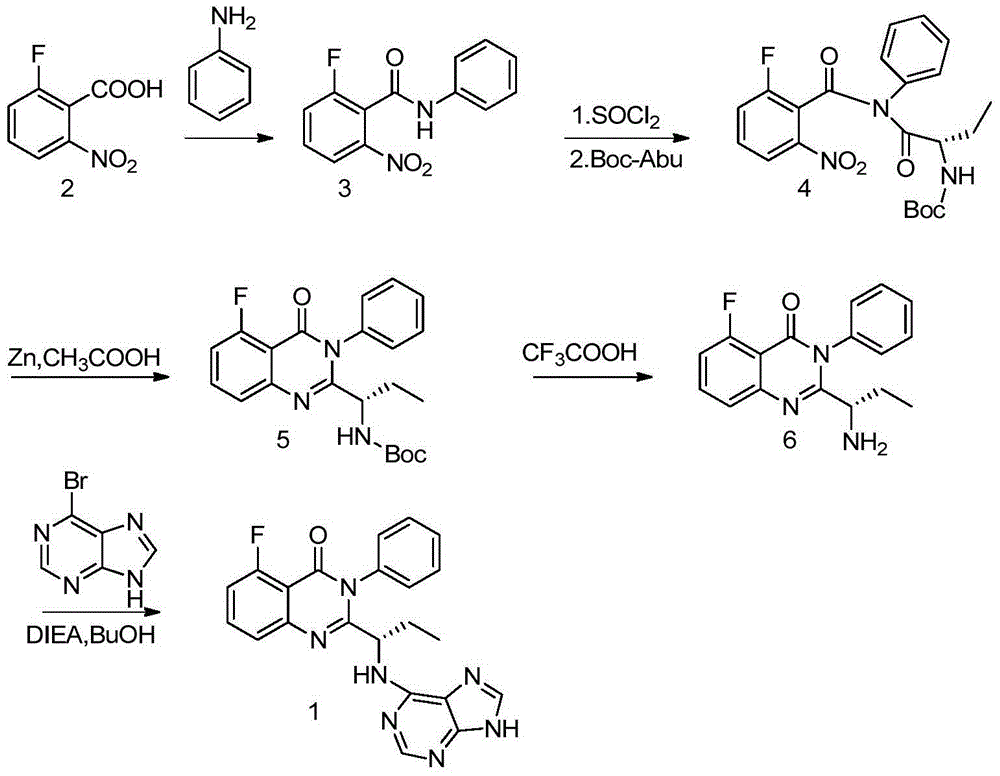
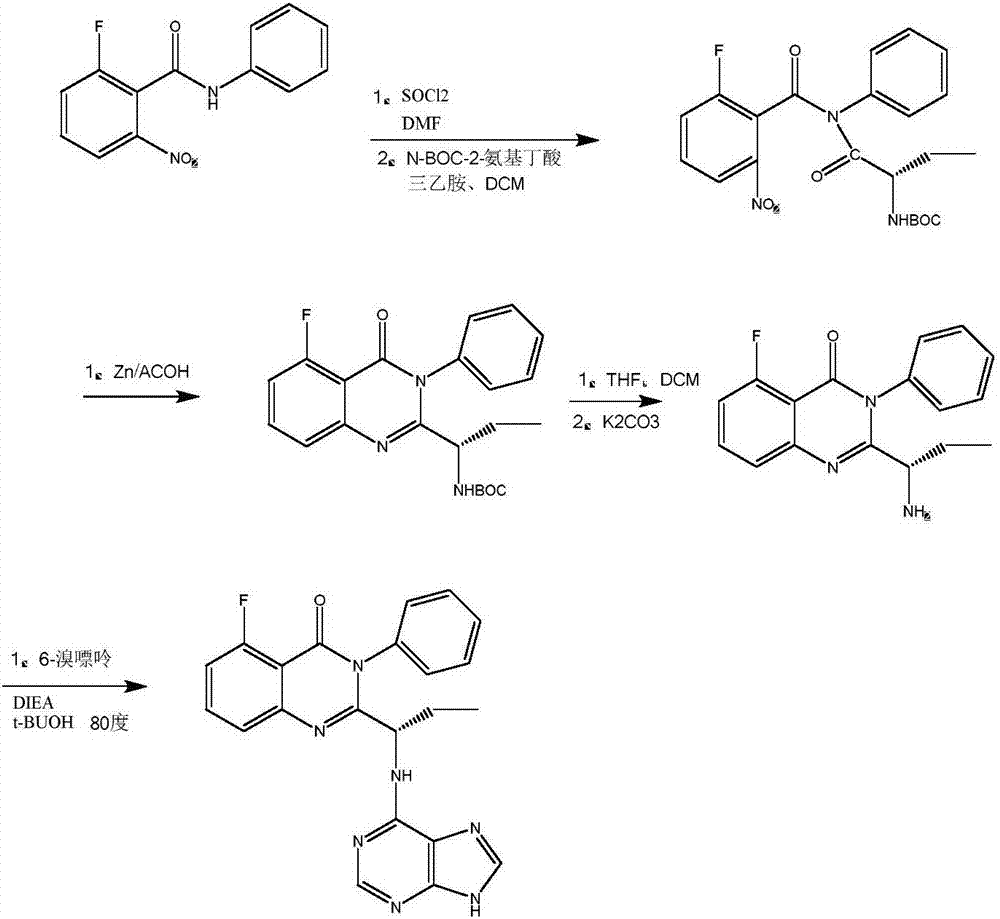
A preparing method of Idelalisib (I) is disclosed. The preparing method includes following steps of: subjecting R-2-hydroxybutyrate (II) and 6-amino-9H-purine to nucleophilic substitution under actions of a leaving agent and an acid-binding agent to produce an intermediate S-2-(N-9H-purin-6-yl)aminobutyrate (III); subjecting the intermediate (III) and 2-amino-6-fluorobenzoic acid to amidation under actions of a catalyst to produce S-2-(N-9H-purin-6-yl)amino-N-(2-carboxyl-3-fluorophenyl)butyramide (IV); subjecting the intermediate (IV) to a cyclization reaction in acetic anhydride; and performing a substitution reaction with phenylamine to obtain the Idelalisib (I). The preparing method has characteristics of easily available raw materials, simple and concise process, capability of being economical and environmental friendly, and suitability for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Idelalisib synthesis method and preparation of intermediate
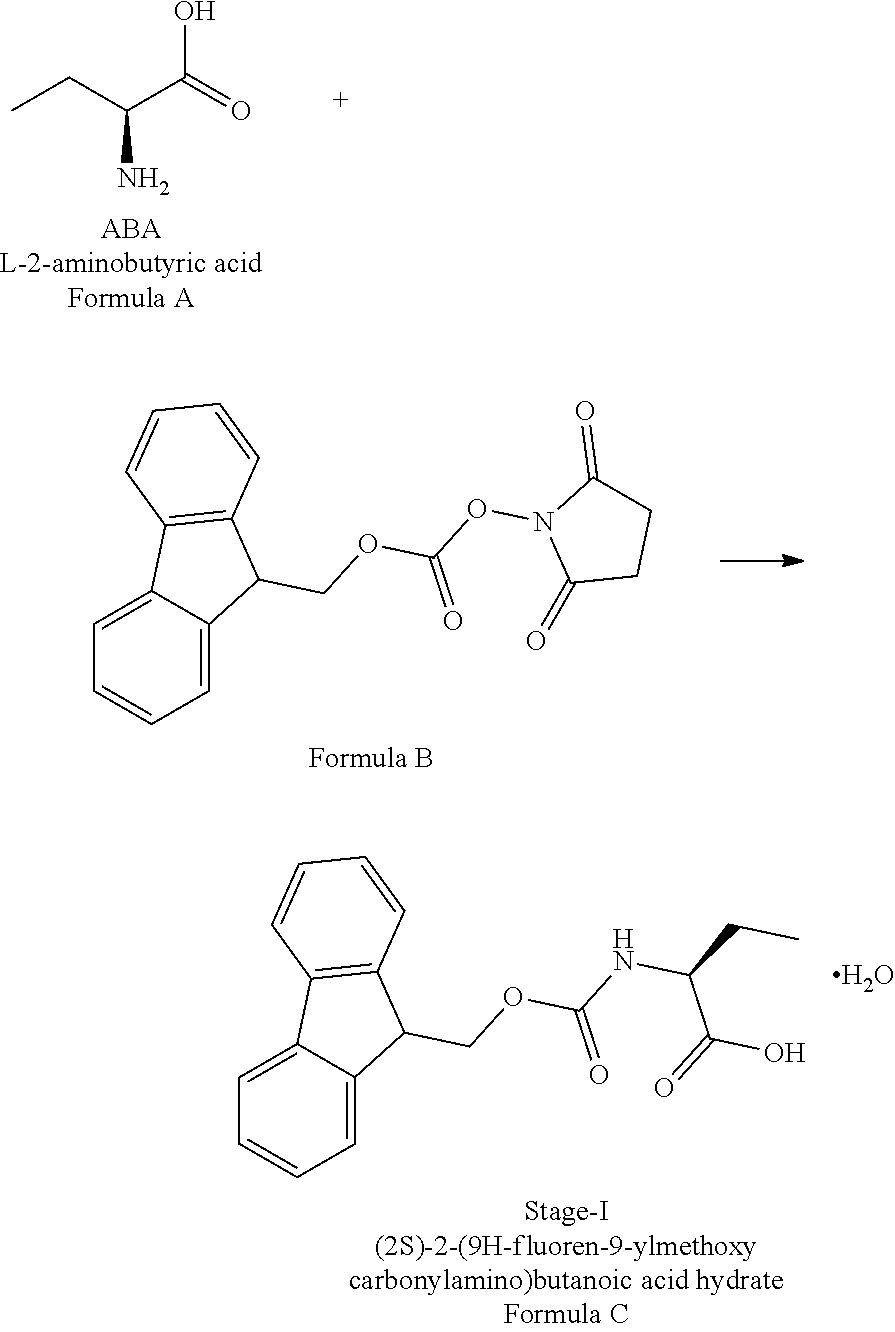
ActiveCN106146502AHigh purityEasy to operateOrganic chemistryBulk chemical productionSynthesis methodsL-2-Aminobutyric Acid
The invention provides an Idelalisib synthesis method. The method comprises steps as follows: a compound 3 is processed with hydrogen in the presence of a hydrogenation catalyst and a solvent, nitro is enabled to be reduced, and an amino compound II is obtained; the compound II and N-Boc protected L-2-aminobutyric acid are subjected to condensation in the presence of alkali and a carboxylic acid activator or in the presence of alkali and a condensation agent, and an intermediate I is obtained; the intermediate I is subjected to a ring closing reaction in an appropriate solvent under the action of a hexamethyldisilazane / lewis acid catalytic system, and a compound 7 is obtained; the compound 7 reacts with 6-chloro-9-(2-tetrahydropyran) purine in an appropriate solvent in the presence of an acid-binding agent, and a compound III is obtained; the compound III is subjected to protecting group removal with an appropriate reagent, and Idelalisib is obtained. A reaction path is shown in the specification. The preparation method has the advantages that the reaction conditions of each step are mild, the post-processing is simple and easy to implement and the total yield is high, and the preparation method is environment-friendly and is very suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
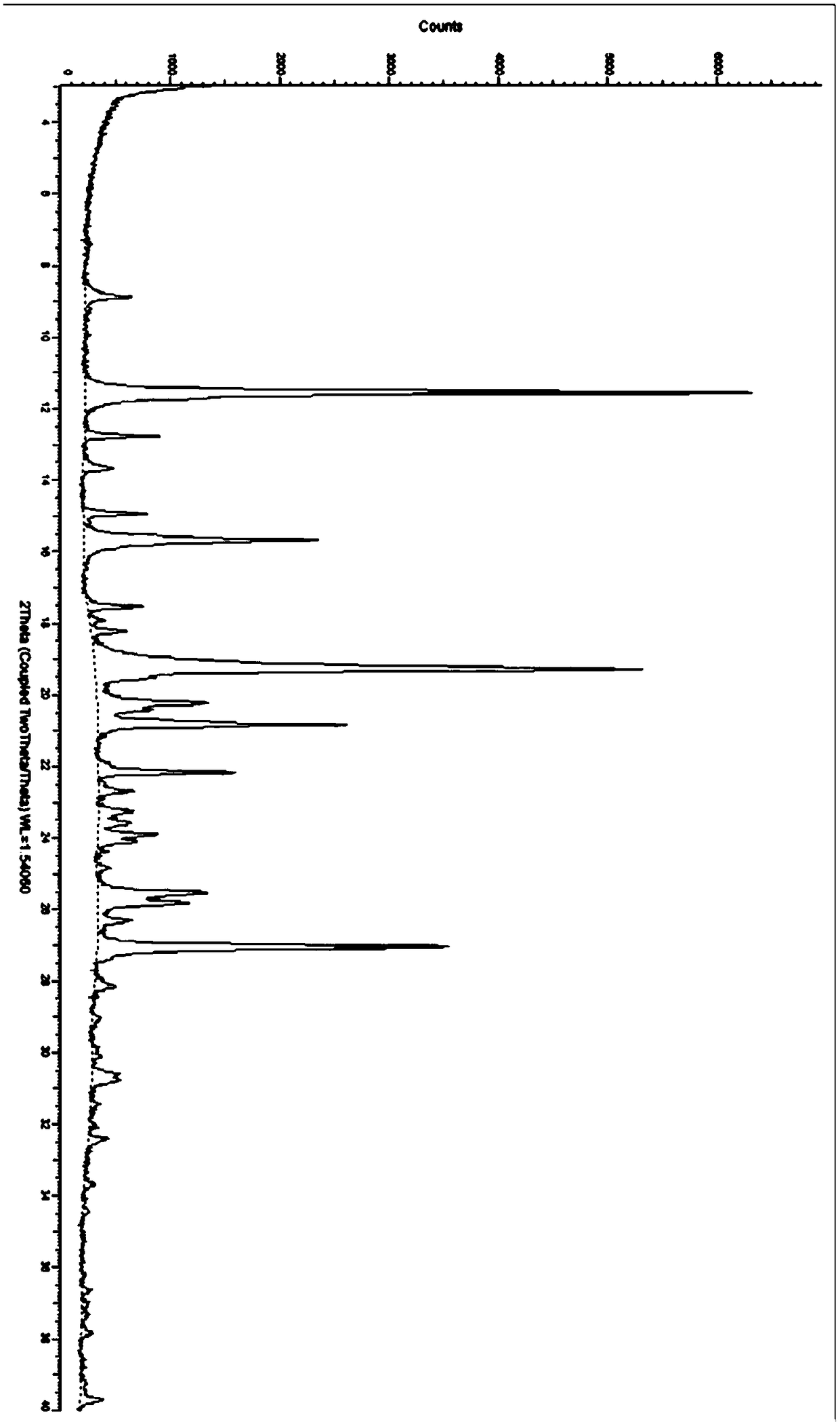
Idelalisib amorphous substance and preparation method thereof
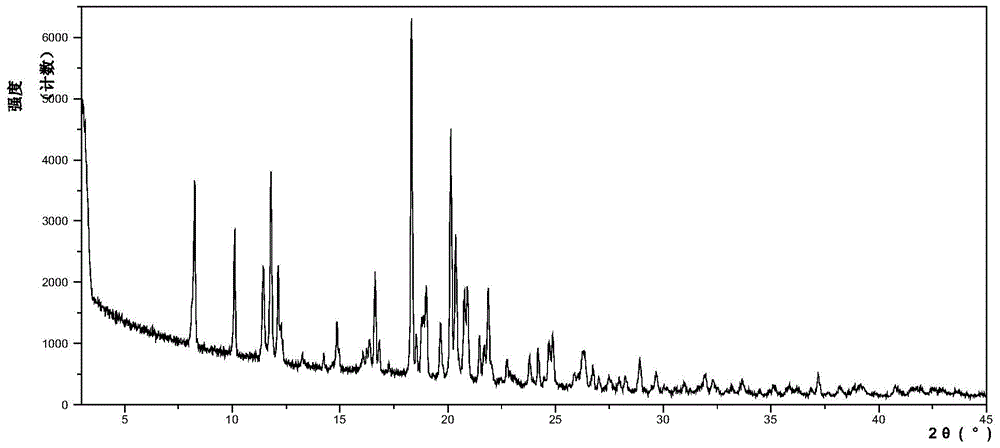
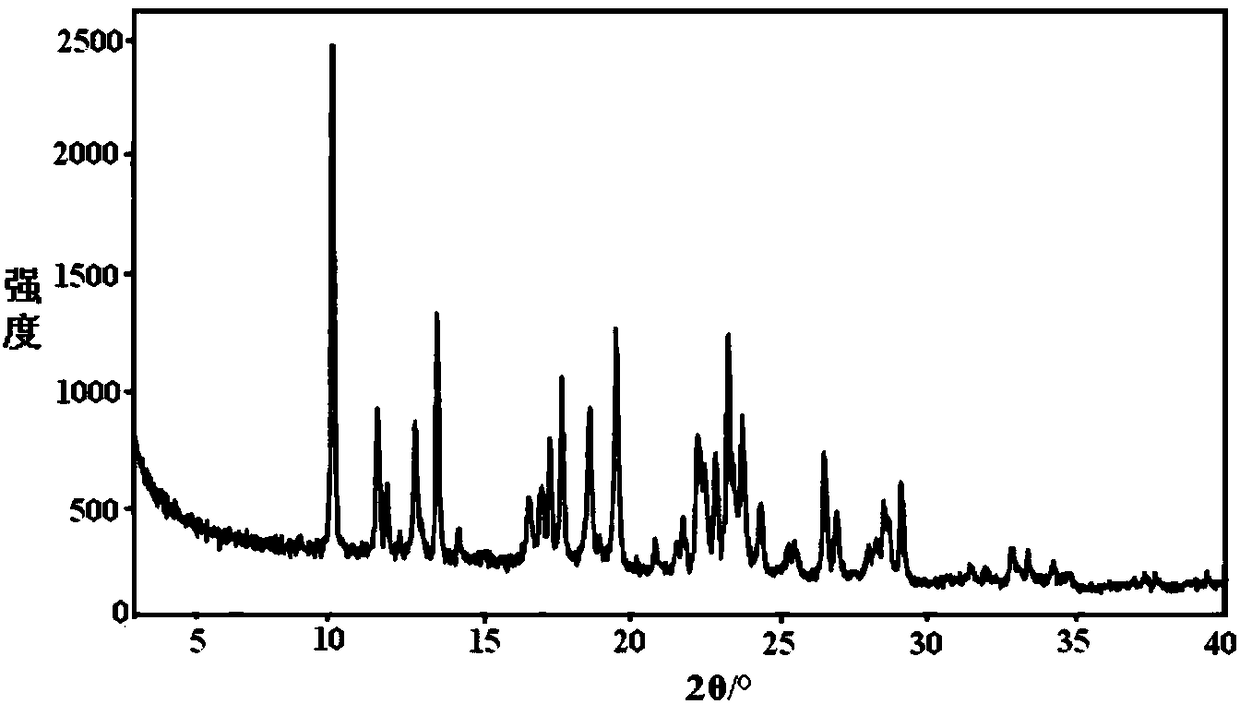
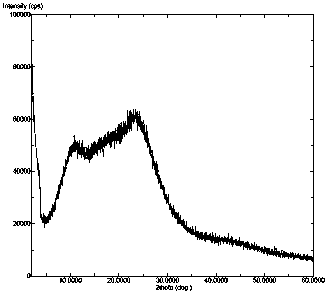
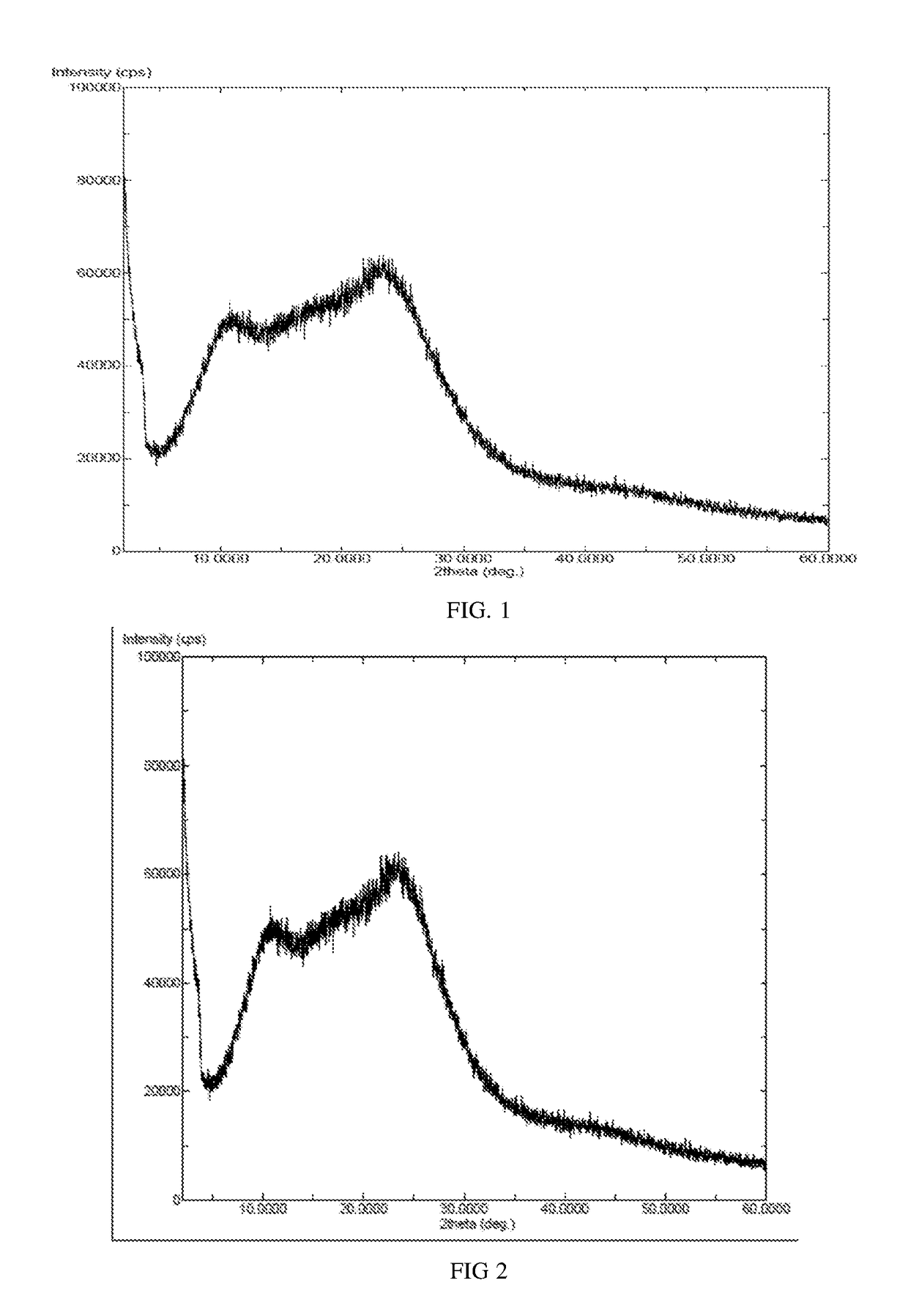
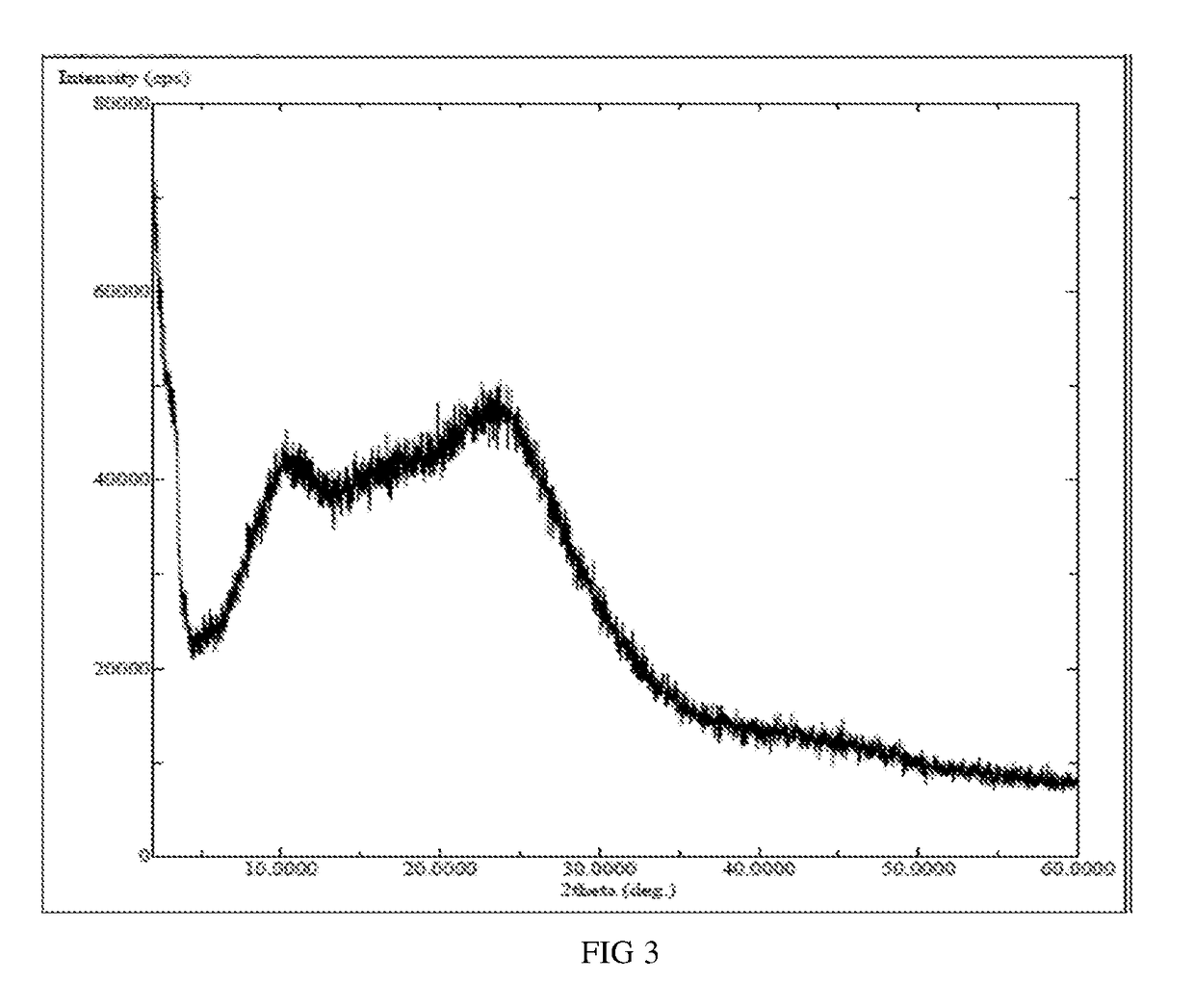
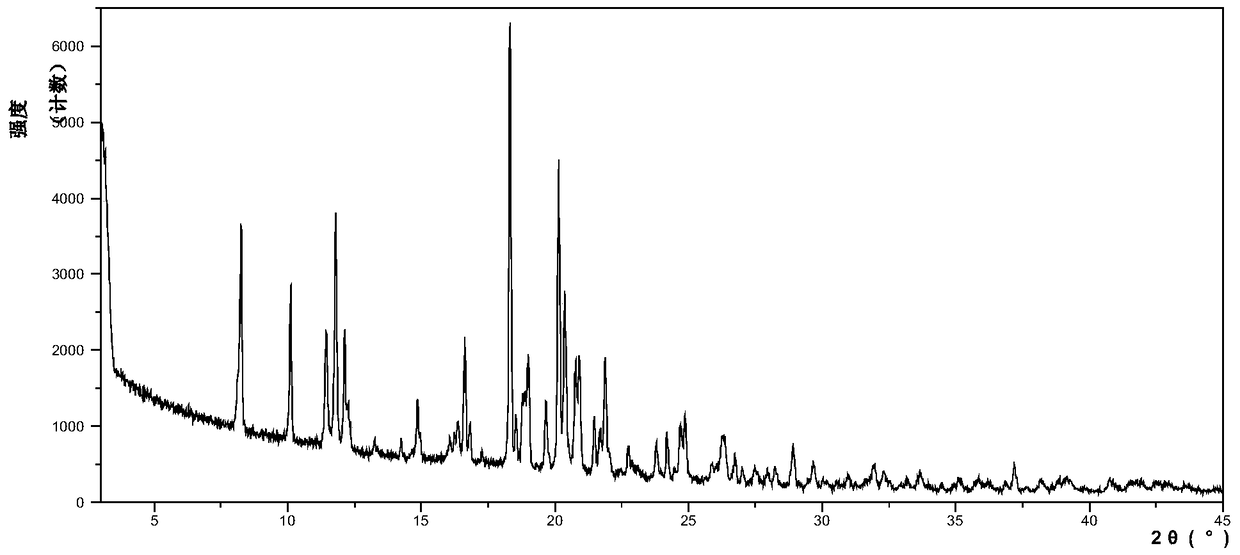
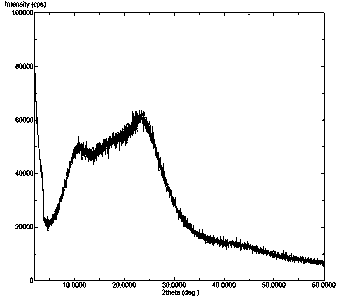
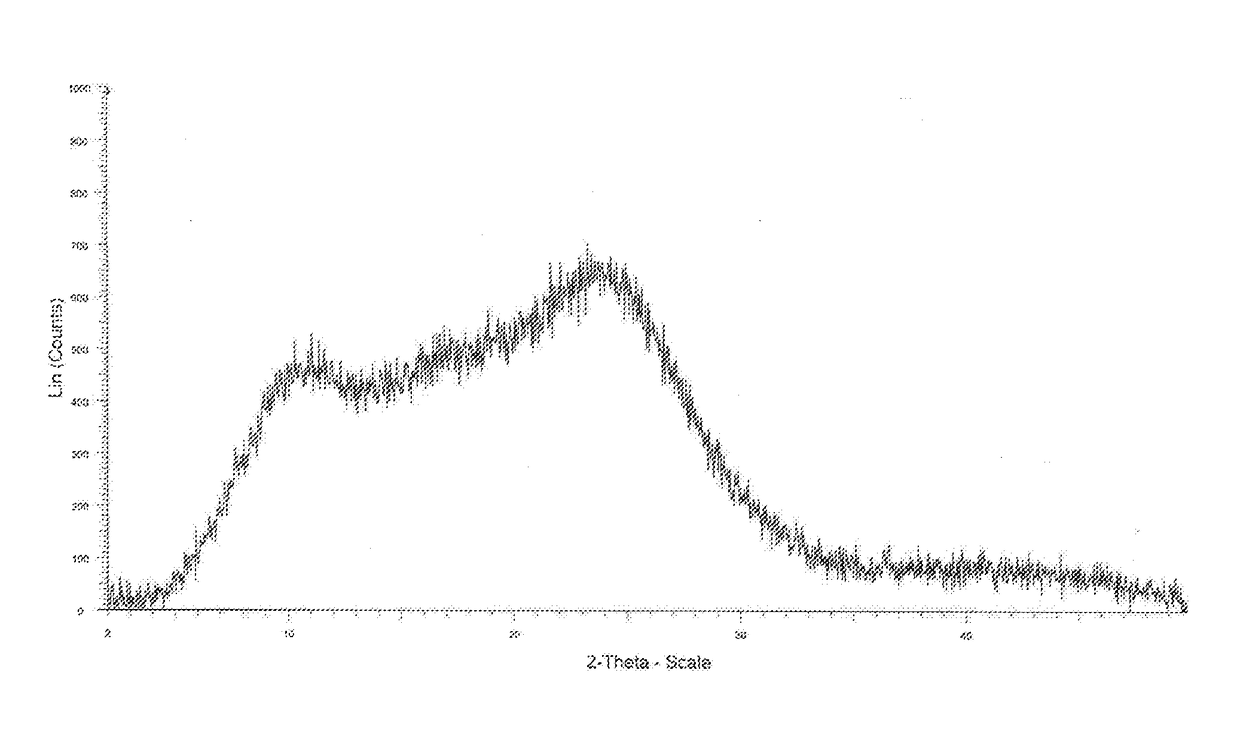
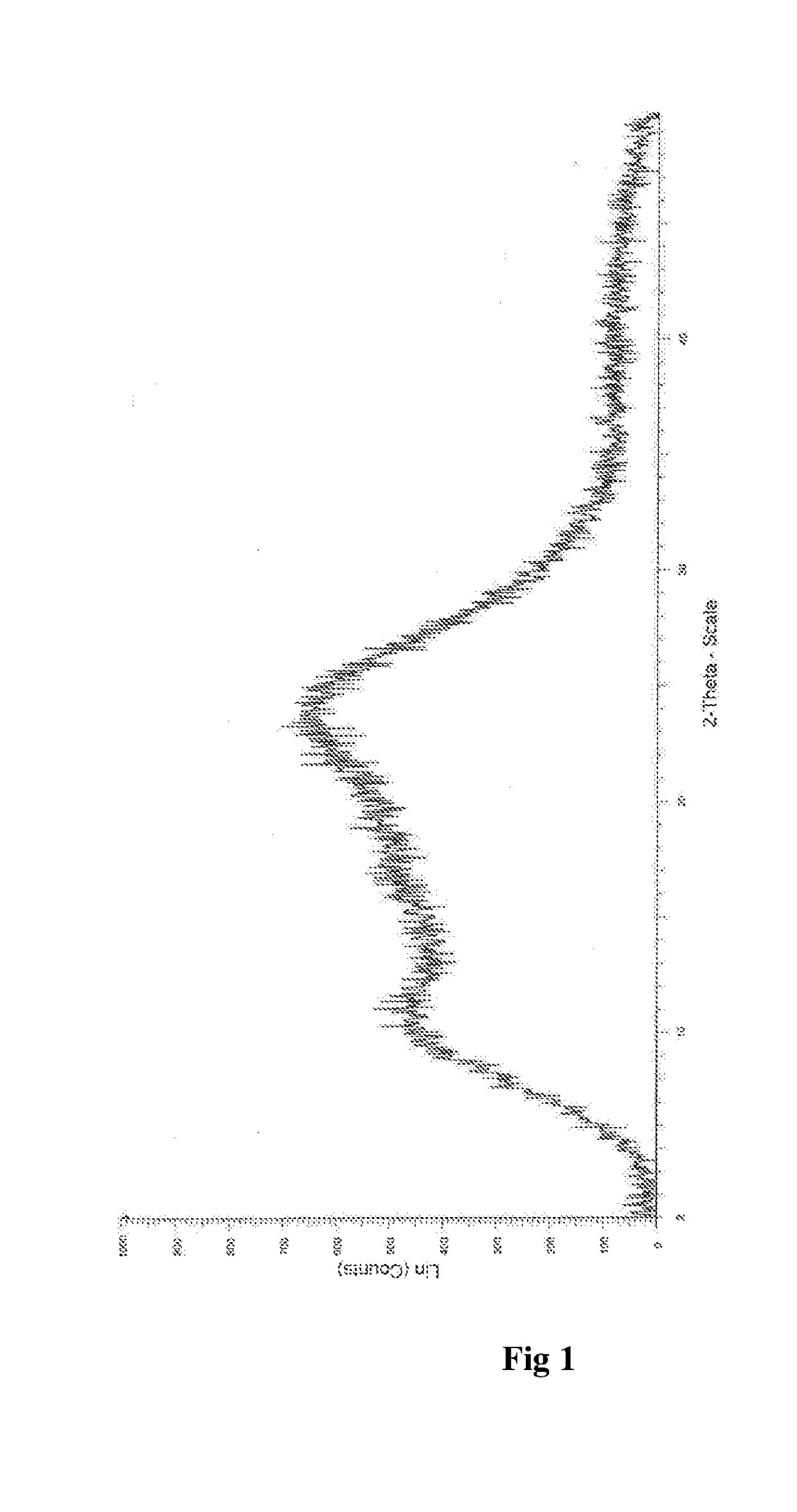
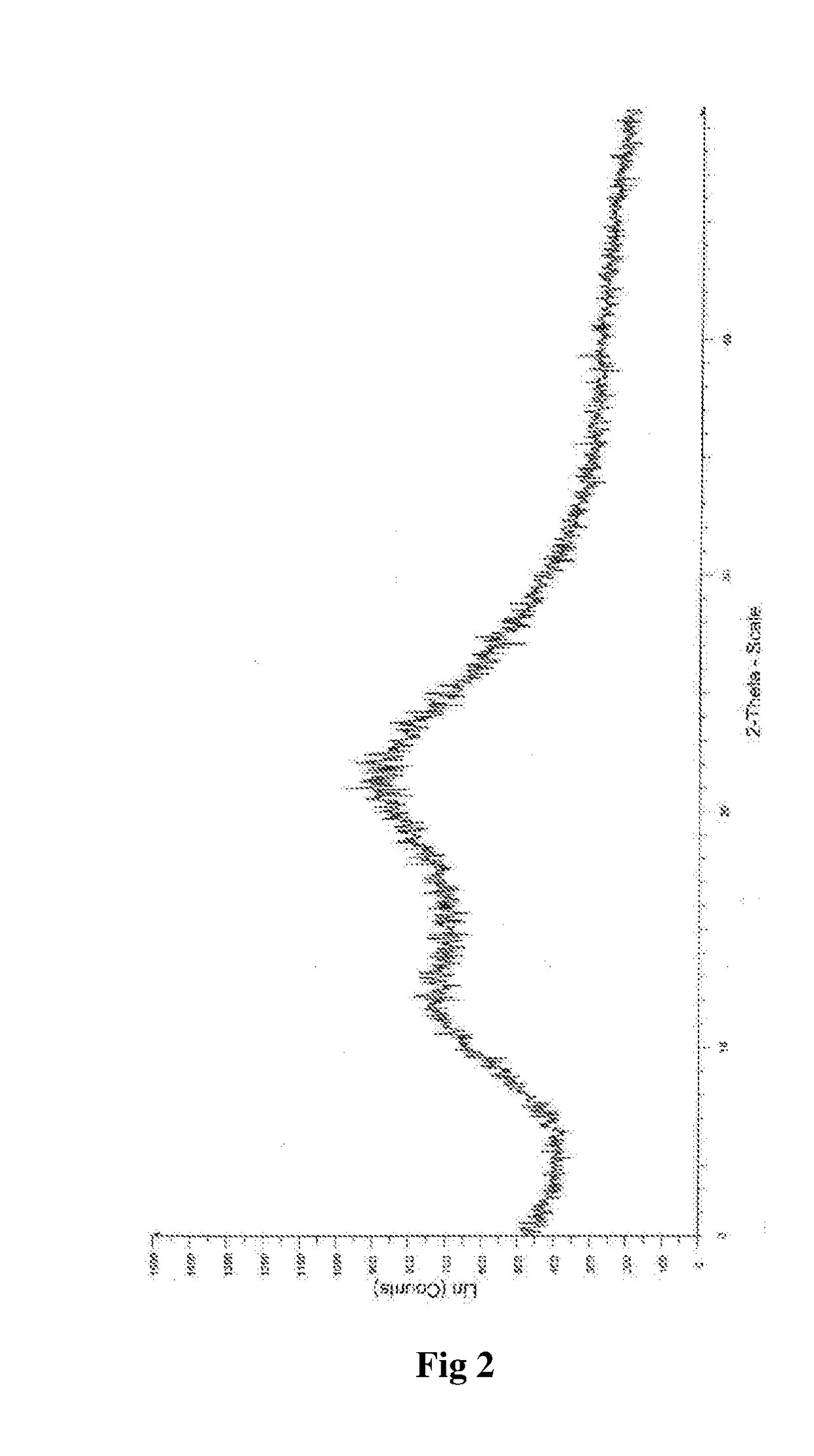
InactiveCN104892612AImprove bioavailabilityImprove solubilityOrganic chemistryAntipyreticSolubilityX-ray
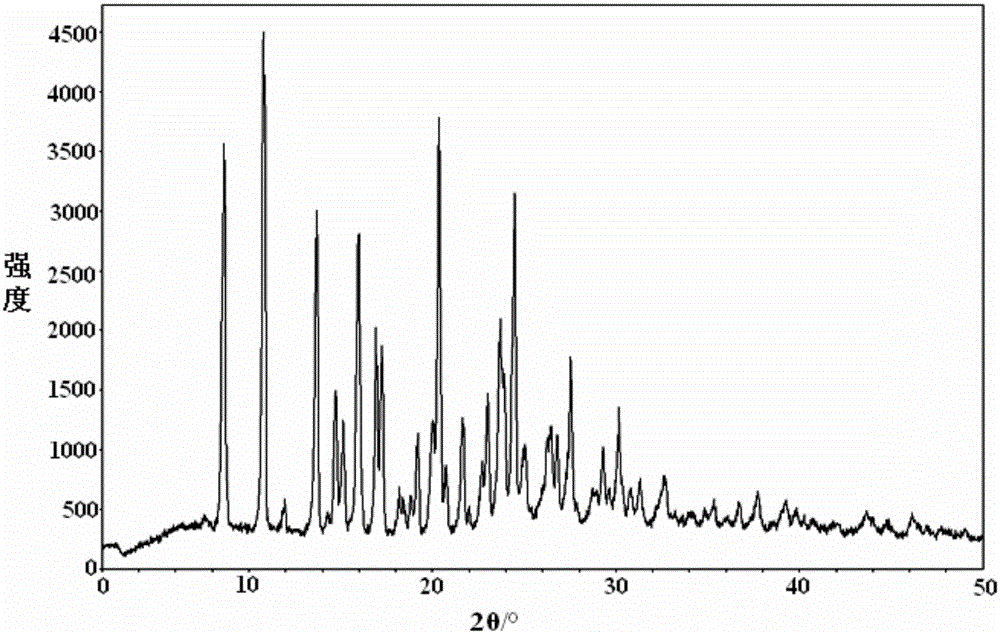
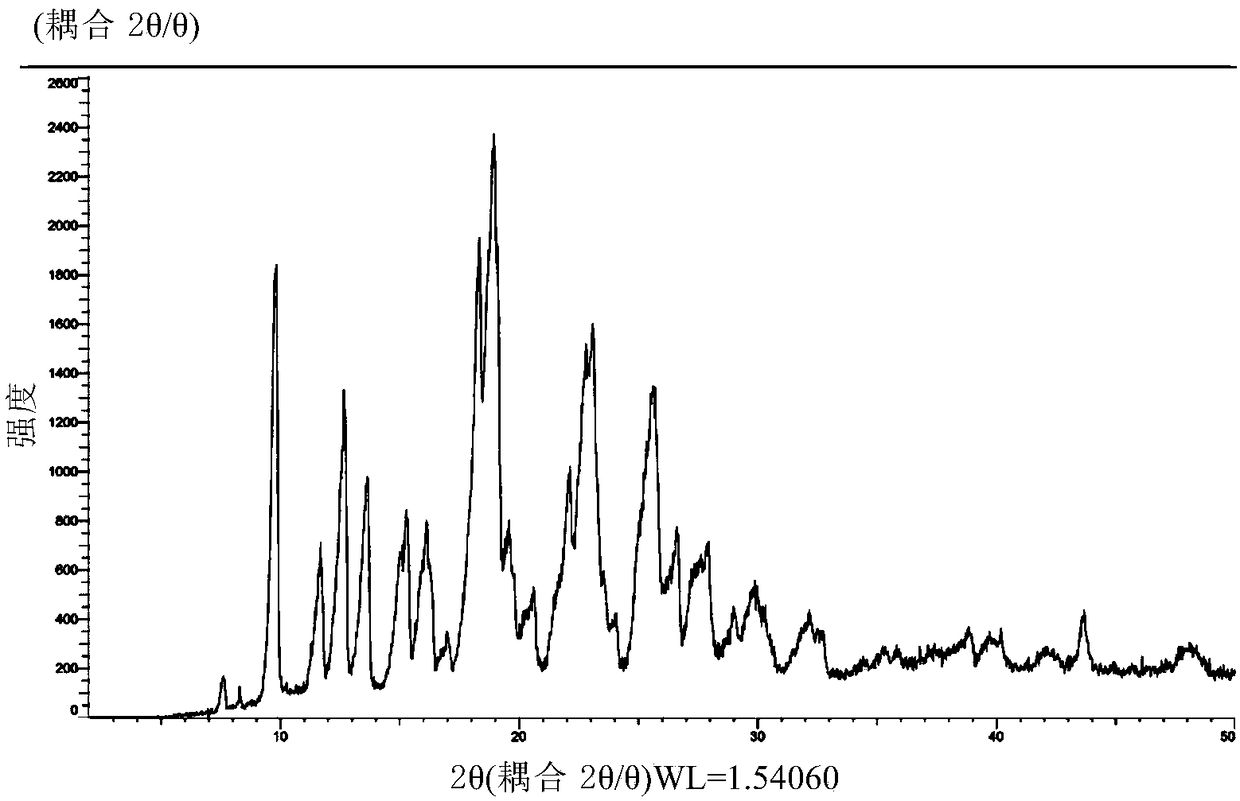
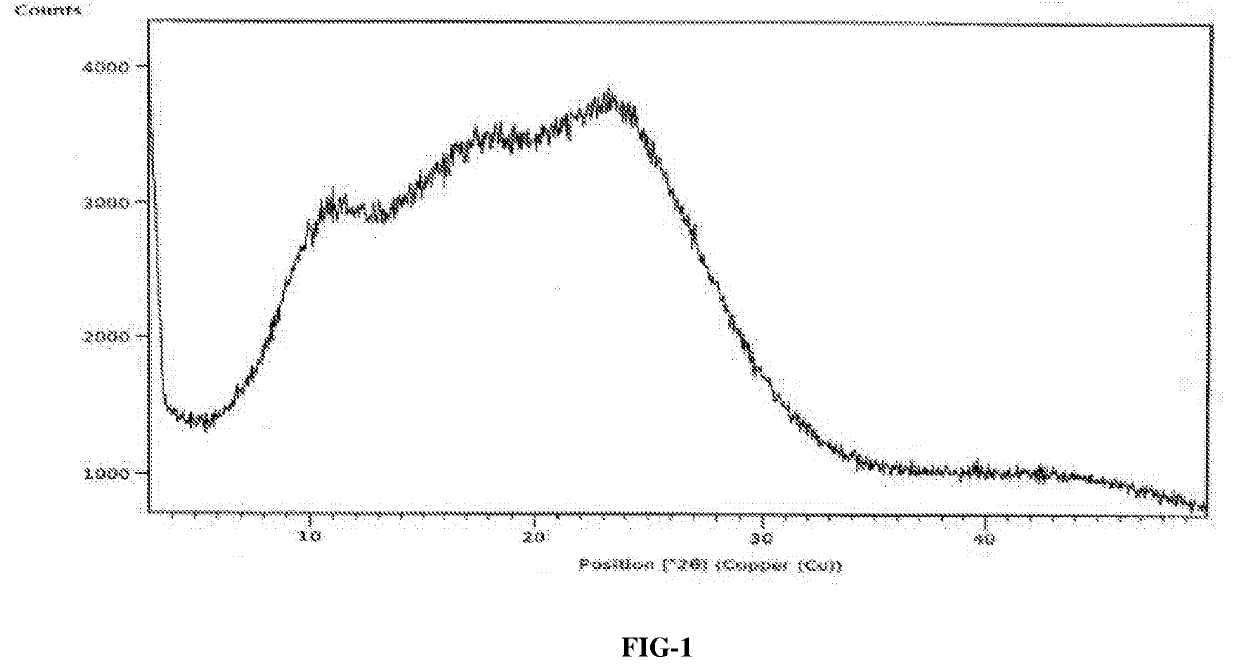
The invention relates to an idelalisib amorphous substance and a preparation method thereof. According to the invention, Cu-Kalpha radiation is used, no sharp diffraction peak in a spectrum is diffracted by X-ray powder expressed by degree 2 theta, wherein X-ray powder diffraction is shown in a graph 1. The invention also provides a preparation method of the idelalisib amorphous substance. The idelalisib amorphous substance increase the dissolvability of idelalisib and is in favor of increasing the biological utilization degree of a medicinal preparation, compared with current crystal form idelalisib, solubility is obviously increased, the idelalisib amorphous substance is in favor of absorbing the medicines by the body, so that medicine can better perform clinical disease treatment effect. Under the condition of accelerated testing on the idelalisib amorphous substance, good physical stability and chemical stability can be kept. The preparation method of the idelalisib amorphous substance has the advantages of simple operation and easy realization.
Owner:SHANGHAI FANGNAN PHARMA
Preparation method of Idelalisib
InactiveCN106279171AReagents obtainedEasy to getOrganic chemistryBulk chemical productionCarbamateIodine
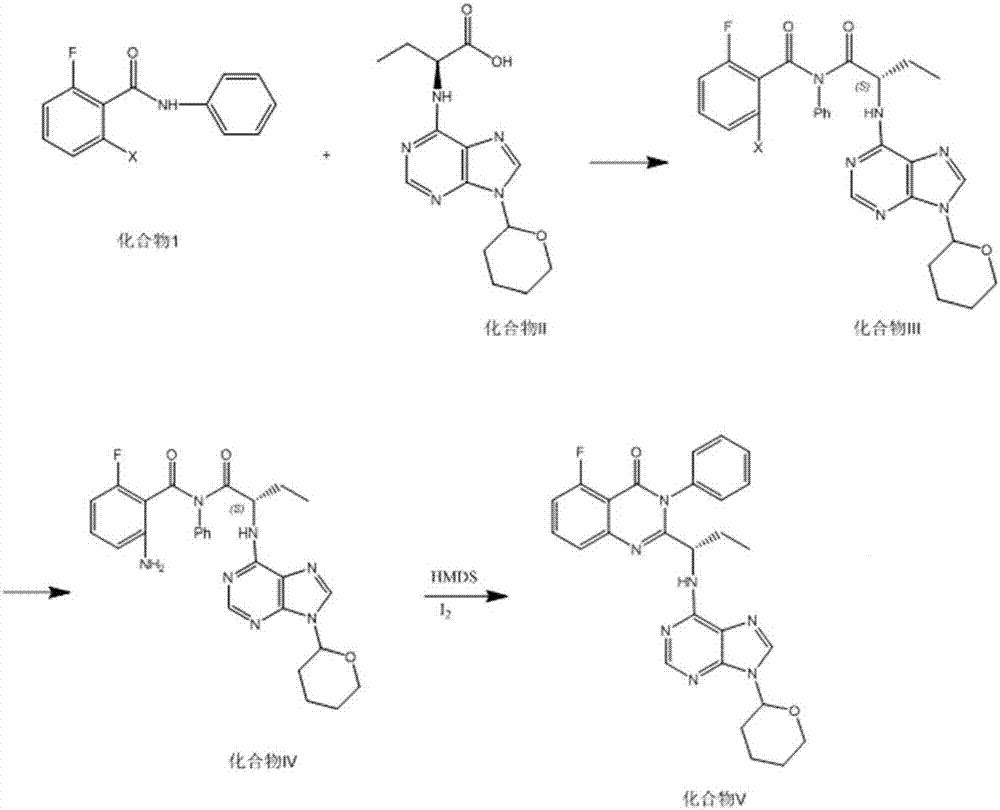
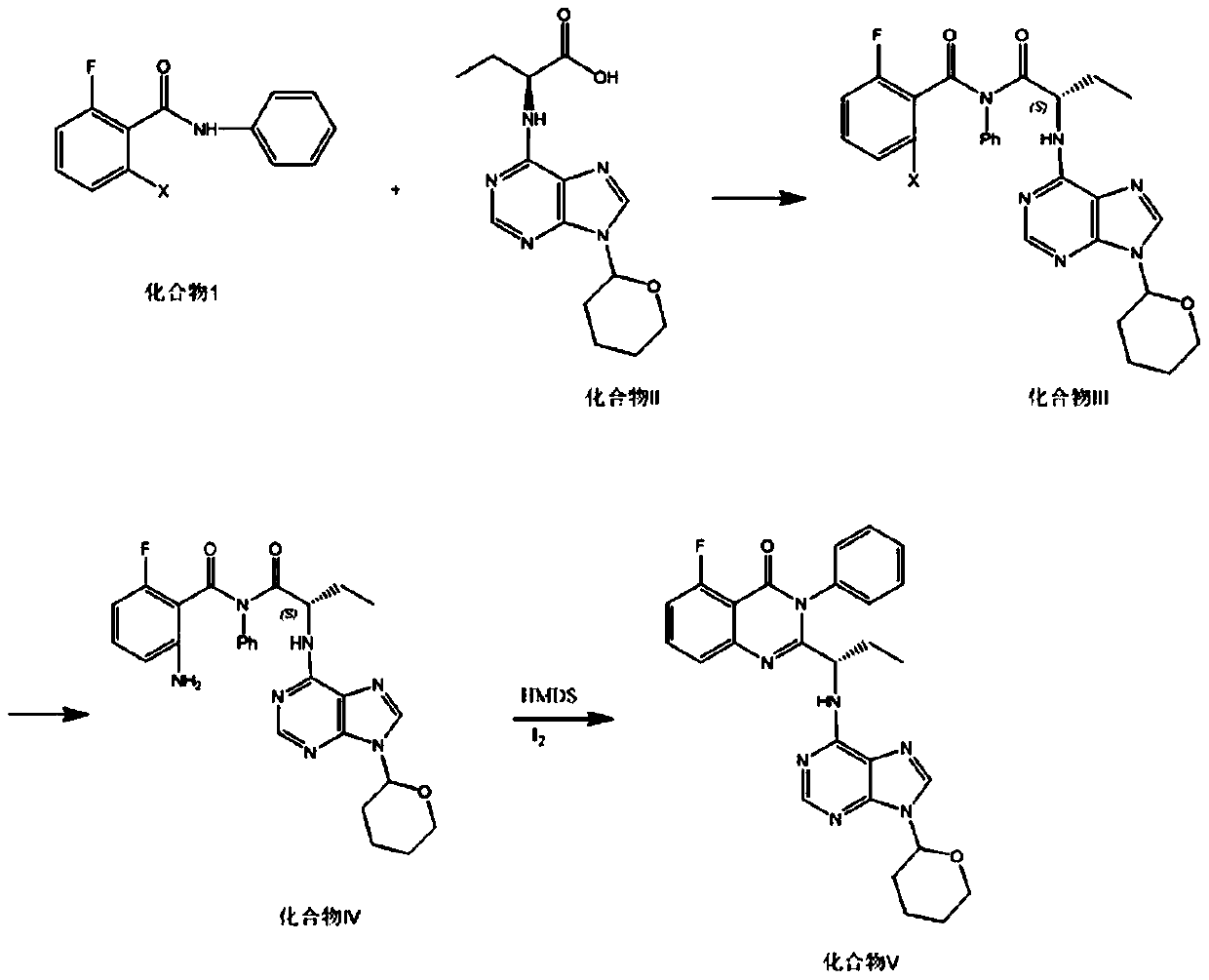
The invention belongs to the field of pharmaceutical and chemical engineering and specifically relates to a preparation method of Idelalisib. According to the preparation method, in the presence of hexamethyldisilazane and iodine, (S)-N-[2-[[3-fluoro-2-[(phenylamino)carbonyl]phenyl]amino]-1-ethyl-2-oxoethyl]-tert-butyl carbamate undergoes a reaction to prepare a key intermediate (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H-quinazoline-4-ketone; and the key intermediate undergoes two-step reaction to finally obtain Idelalisib. The reaction reagents are easily available; reaction conditions are mild; operation is simple and the product is easy to prepare; production efficiency is high; the prepared intermediate and the product have high yield and high purity; and in comparison with the prior art, impurities are not easy to produce. Thus, the preparation method of the invention is especially suitable for industrial production.
Owner:NANJING ANYUAN BIO PHARMA TECH CO LTD +1
Idelalisib crystal form A and preparation method thereof
ActiveCN106565716ASimple preparation processEasy to operateOrganic chemistry methodsSolubilityThermal insulation
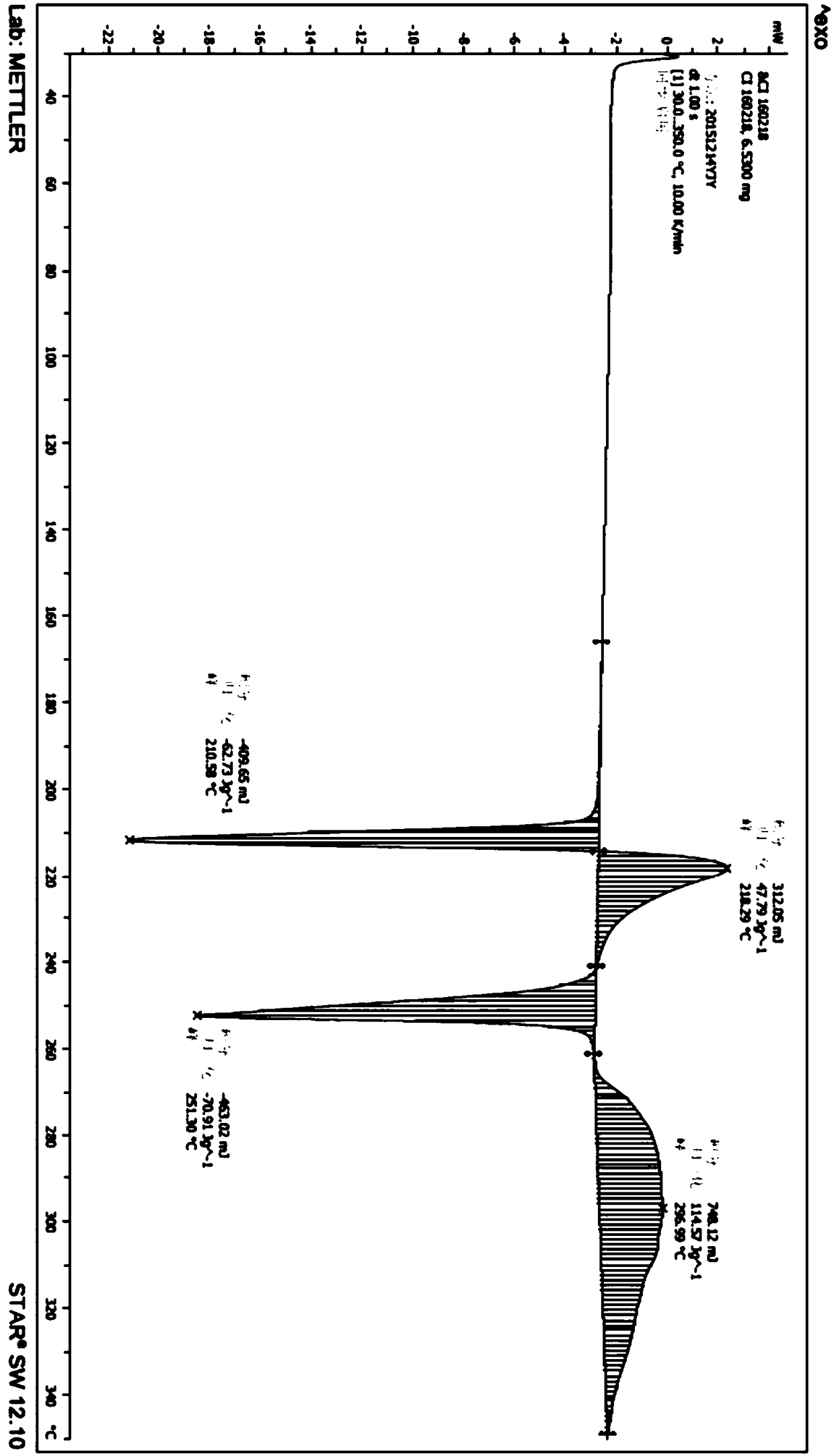
The present invention discloses an Idelalisib crystal form A and a preparation method thereof. According to the present invention, the Idelalisib crystal form A is a single molecule hydrate, and has the characteristic peak having a relative intensity of more than 50% at a 2[theta] angle of 8.5+ / -0.2 DEG, 10.7+ / -0.2 DEG, 13.6+ / -0.2 DEG, 15.8+ / -0.2 DEG, 20.2+ / -0.2 DEG, 23.7+ / -0.2 DEG and 24.3+ / -0.2 DEG under powder X-ray diffraction; the preparation method comprises: dissolving an Idelalisib raw material in a suitable solvent to obtain a clarified solution, adding an antisolvent to the obtained solution in a dropwise manner until the turbidity initially appears, carrying out thermal insulation stirring to crystallize, cooling to a room temperature, filtering, and drying to prepare the Idelalisib crystal form A; and the Idelalisib crystal form A has advantages of excellent solubility, excellent thermal stability, excellent high-humidity stability and excellent pressure stability, and can be used as the pharmaceutically active ingredient of the preparation.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
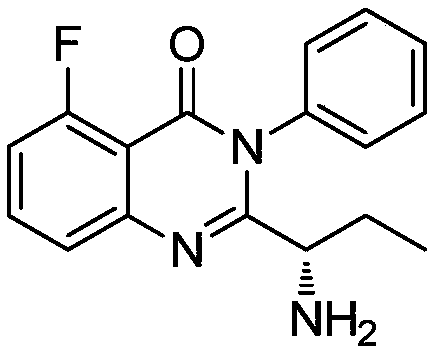
Preparation method of (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H-quinazoline-4-one
InactiveCN106146411AMild reaction conditionsSmooth responseOrganic chemistryTrimethylsilyl chlorideCarbamate
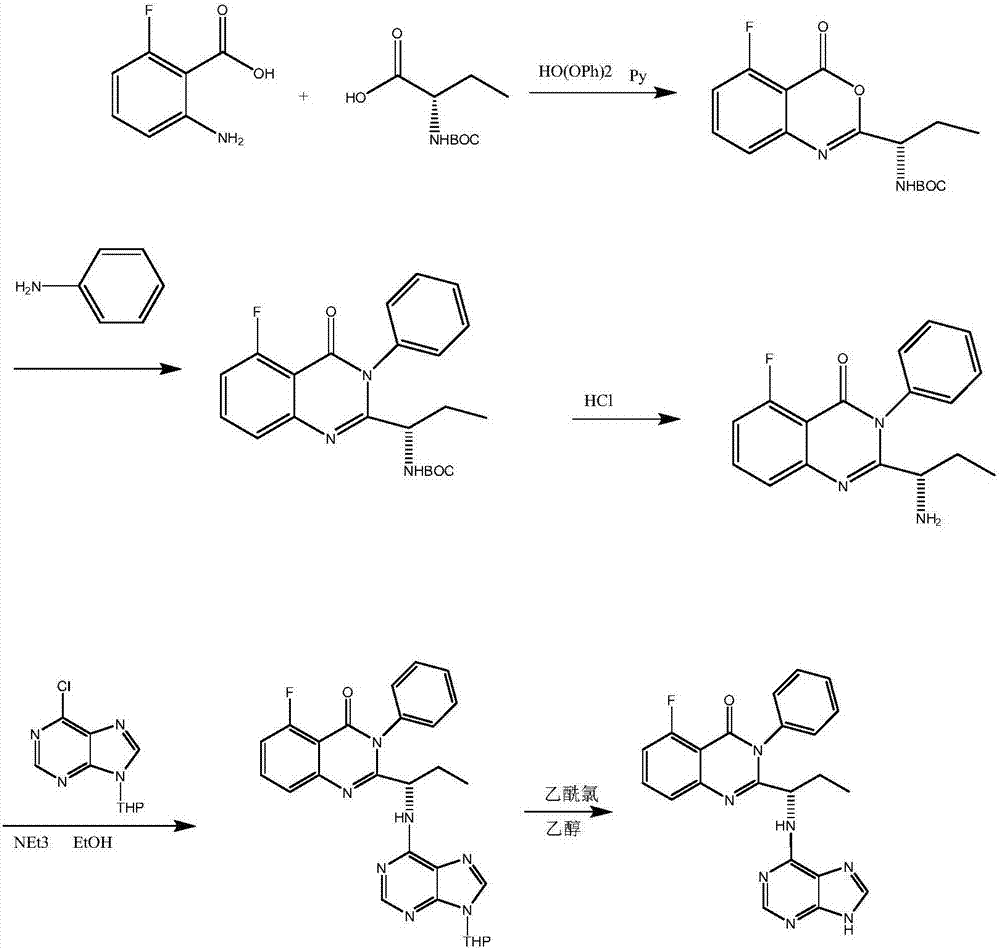
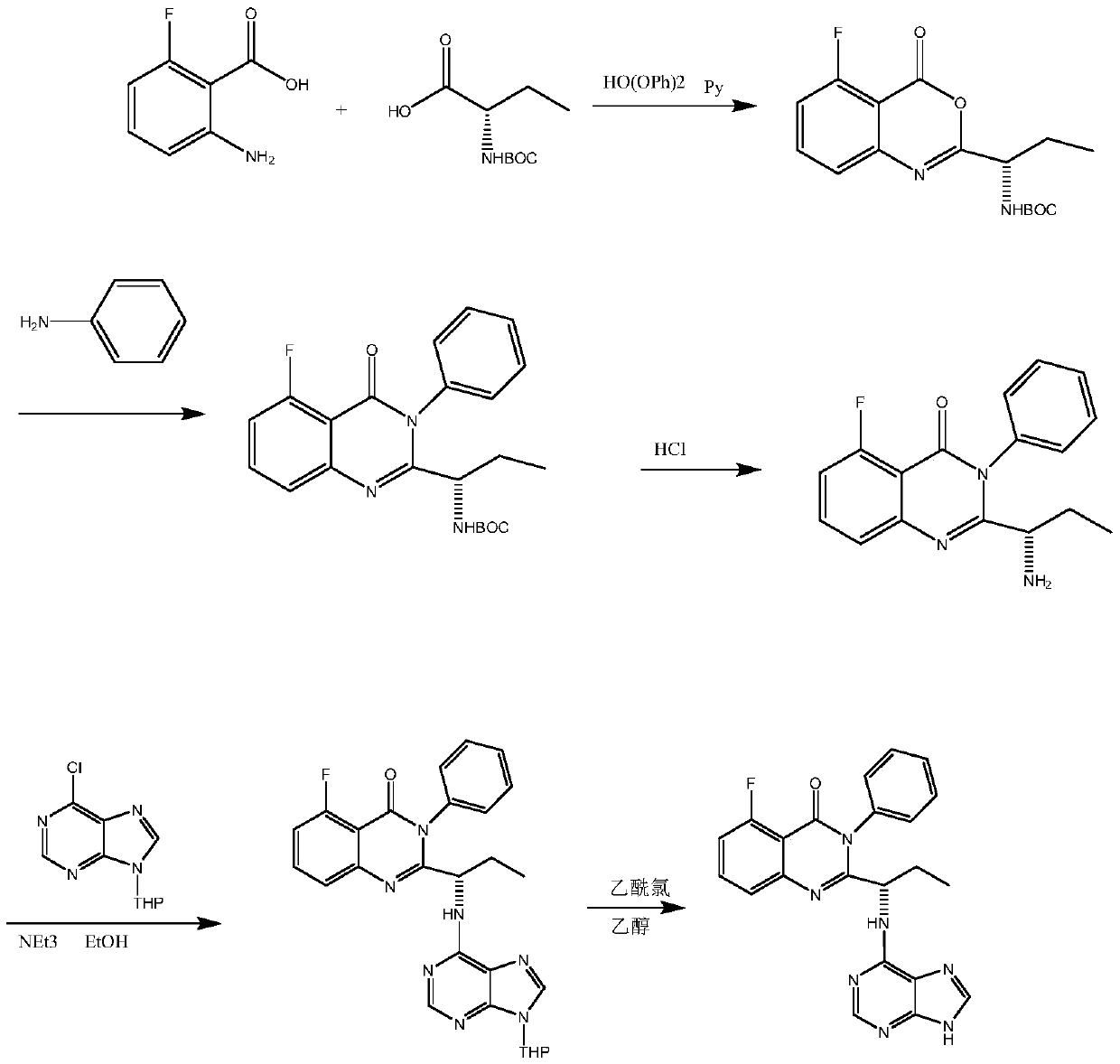
The invention discloses a preparation method of (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H-quinazoline-4-one. According to the method, a compound represented as a formula (V) is subjected to cyclization under the action of trimethylchlorosilane, (S)-[1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydro-quinazoline-2-yl) propyl]-isobutyl carbamate is obtained and subjected to BOC (t-butyloxycarboryl) de-protection through acid, and a product is obtained. A synthetic route provided by the invention overcomes defects in the prior art, has the advantages of being mild in reaction condition of each step, stable in reaction and high in yield, and is particularly applicable to industrial large-scale production of a key intermediate 6 of Idelalisib.
Owner:SHANGHAI INST OF PHARMA IND +1
Idelalisib preparation method
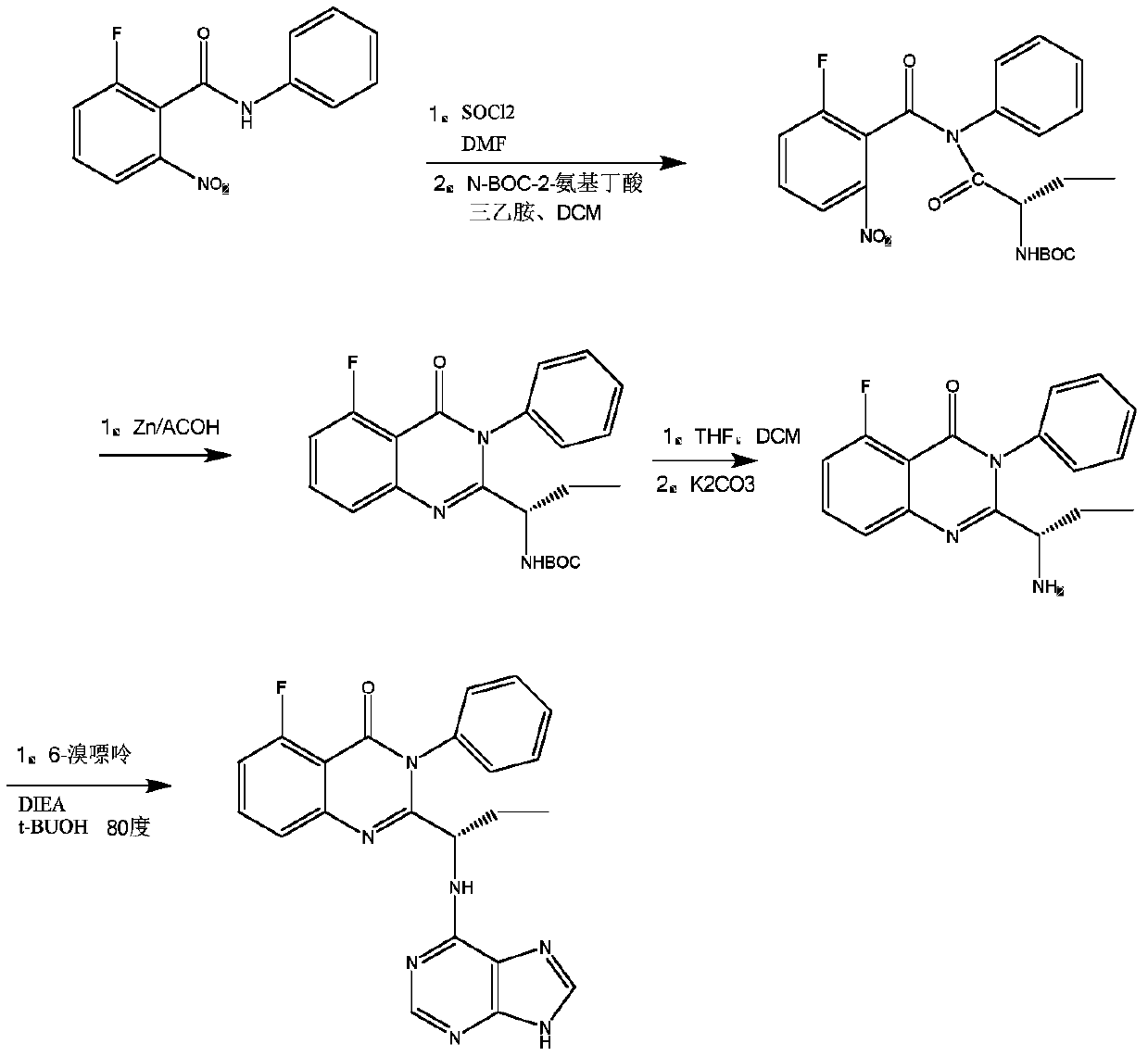
The invention discloses a novel Idelalisib preparation method. The method includes the steps that firstly, 6-chloro-9-(tetrahydro-2-pyranyl)-purine and (S)-2-(1-aMinopropyl)-5-fluoro-3-phenylquinazolin-3H-quinazoline-4-ketone have a nucleophilic substitution reaction to obtain (S)-5-fluoro-3-phenylquinazolin-2-{1-[(9-tetrahydro-2H-pyran-2-yl)-9H-purin-6-ylamino]propyl}-3H-4-hydroxyquinazoline; secondly, the product in the first step is subjected to deprotection by means of hydrochloric acid and ethyl alcohol to obtain Idelalisib. The yield of the method is much higher than that of the prior art, post-treatment is easy, pure Idelalisib can be obtained only through extraction and re-crystallization, and the method is suitable for industrial production. Please see the molecular formula in the description.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Idelalisib intermediate and preparation method thereof
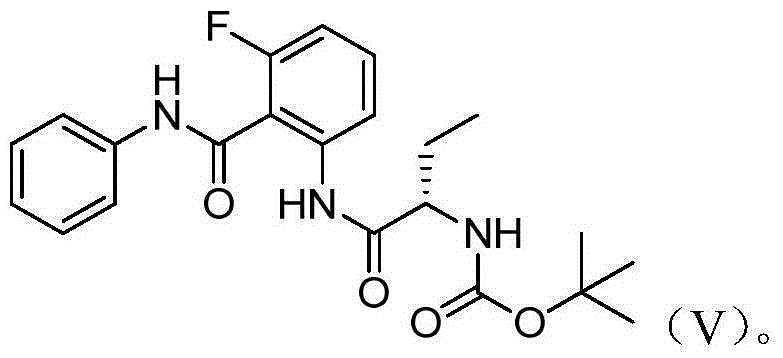
InactiveCN106146352ASmooth responseHigh yieldCarbamic acid derivatives preparationOrganic compound preparationAmino acidStereochemistry
The invention discloses an Idelalisib new intermediate compound (V)(S)-2-{[3-fluoro-2-[(phenyl amino) carbonyl] phenyl] amino-1-ethyl}-amino acid butyl ester. According to the Idelalisib prepared by the intermediate, compared with the existing disclosed methods, the reaction is stable, the yield is high, the reaction condition is mild, the defects in the prior art are overcome, the Idelalisib intermediate is very suitable for industry large scale production, and the yield is higher than that of the existing method. (Please see the formula of the compound in the description.).
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Method for detecting residual of solvents in idelalisib
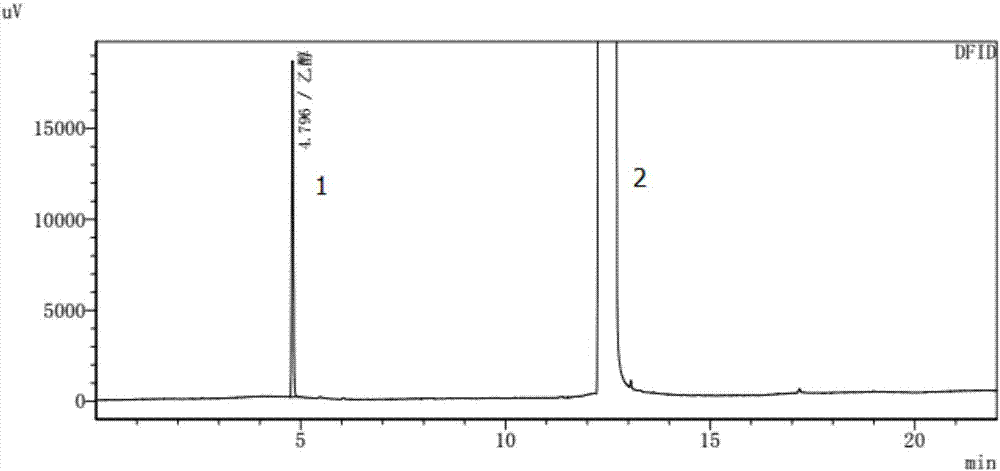
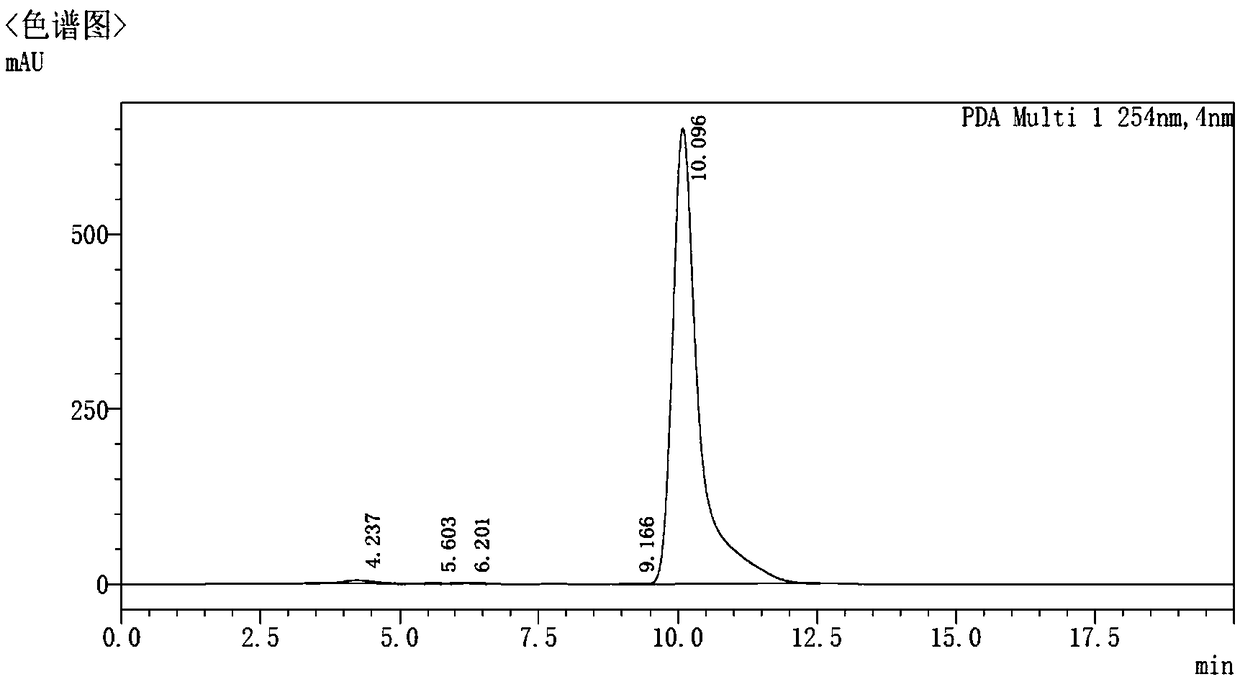
ActiveCN107966498AAccurate detectionImprove stabilityComponent separationN dimethylformamideOrganic solvent
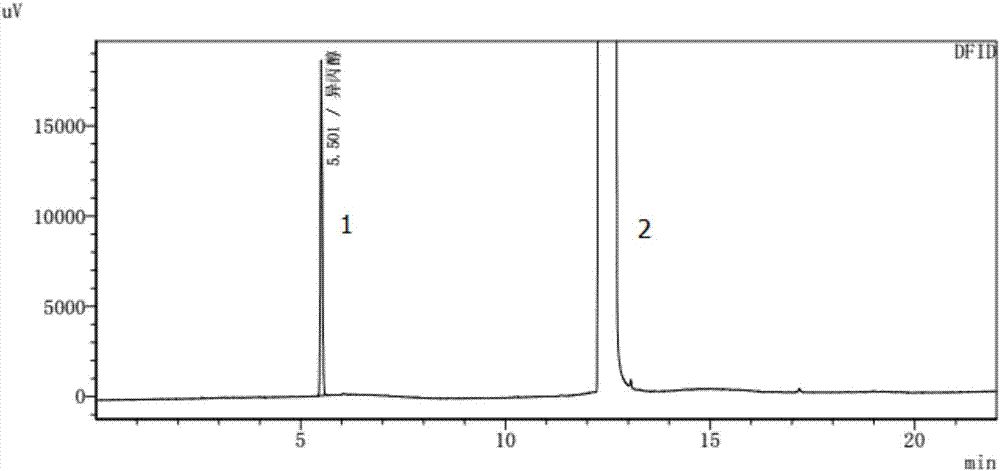
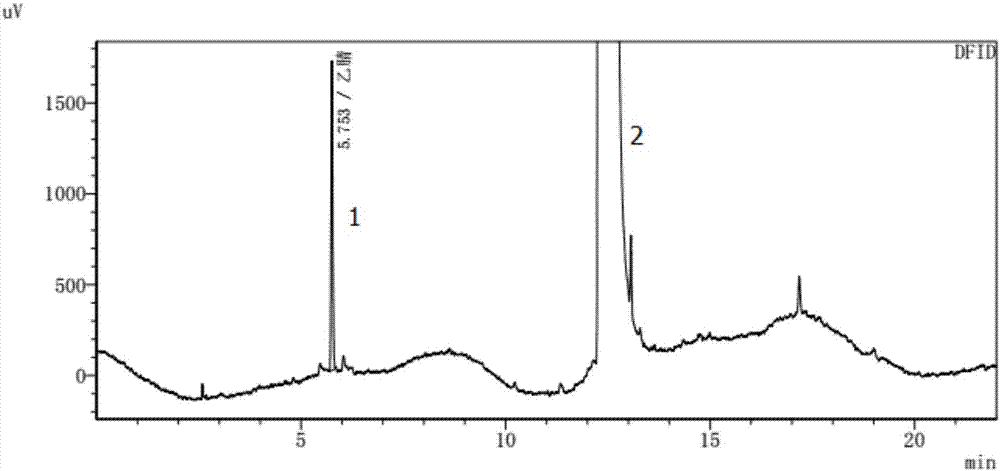
The invention relates to a method for detecting the residual of solvent in idelalisib, and concretely relates to a method for detecting the residual of various organic solvents in idelalisib by gas chromatography. The method comprises the following steps: 1) preparing an N,N-dimethylformamide solution of idelalisib, used as a test solution; 2) mixing all organic solvents to be detected with the N,N-dimethylformamide to form a mixed reference substance solution; 3) respectively carrying out gas chromatography detection on the test solution obtained in step 1) and the mixed reference substance solution obtained in step 2) to obtain chromatograms; and 4) calculating the content of every organic solvent to be detected in idelalisib according to peak areas through an external standard technology. The detection method can achieve easy, fast and accurate detection of the residual amounts of the organic solvents to be detected in the idelalisib. The detection method also has the advantages ofgood applicability, good durability, good precision, good sensitivity, good accuracy, good repeatability, good recovery rate, good stability of the test solution and improvement of the quality controlmethod of the above medicine.
Owner:HUBEI BIO PHARMA IND TECHCAL INST +2
Idelalisib crystal form C and preparation method thereof
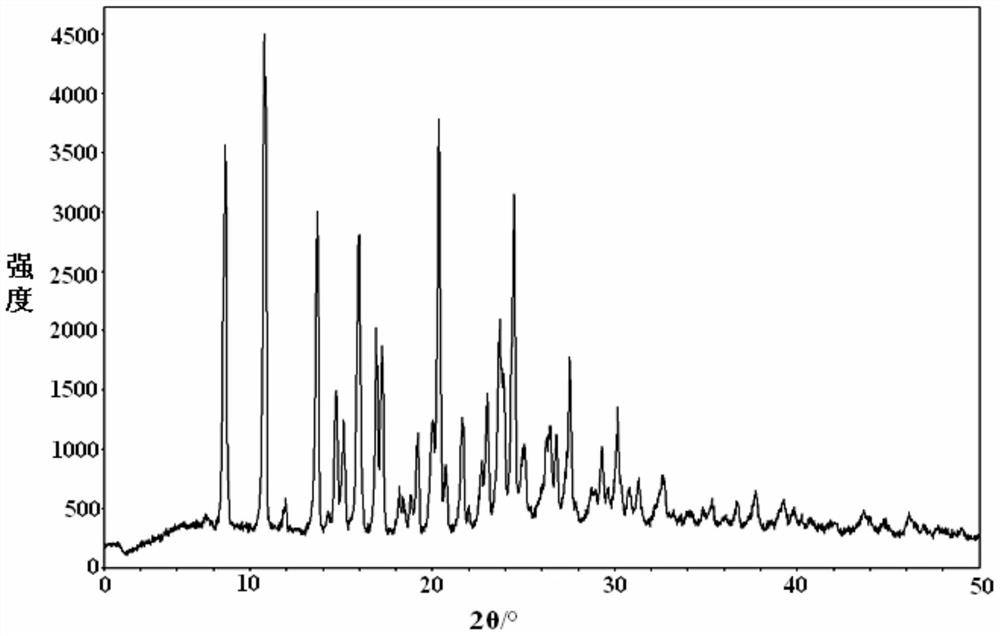
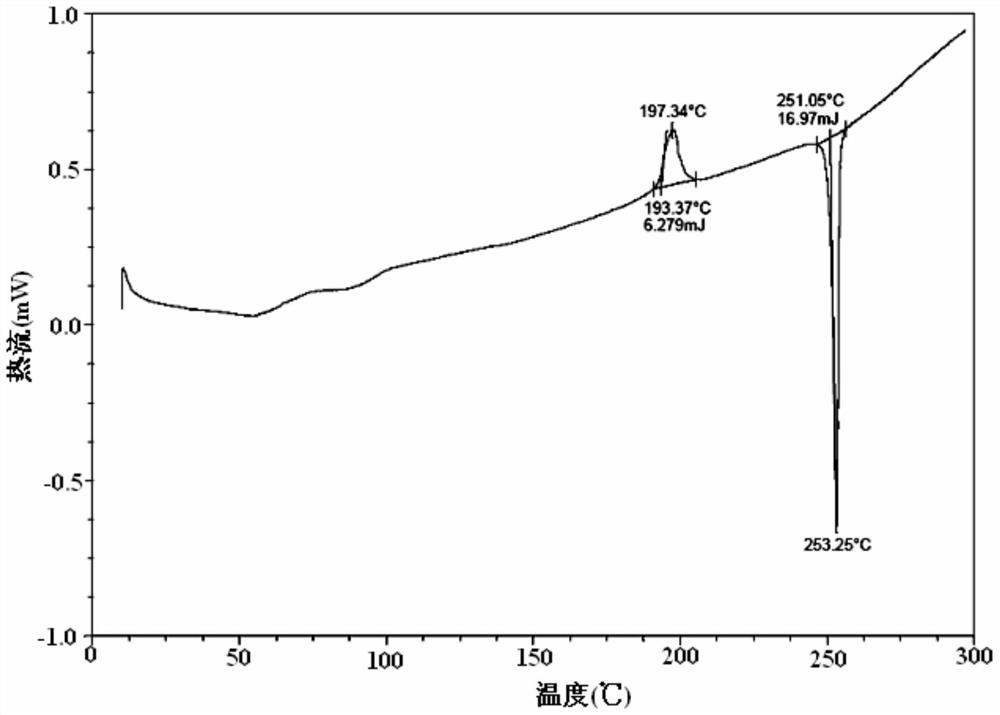
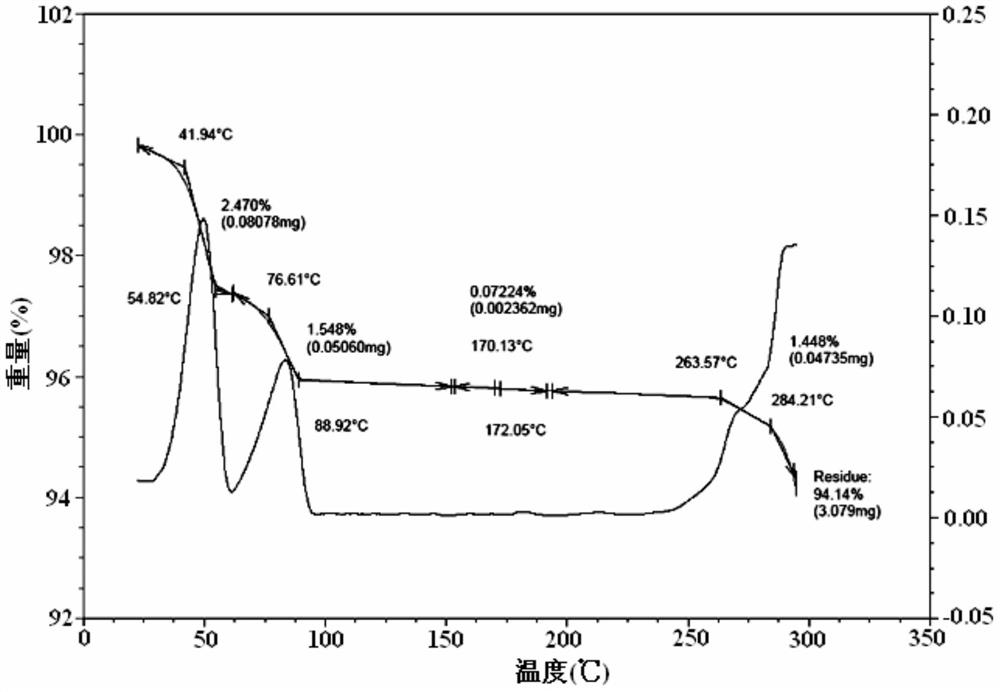
InactiveCN108218872ANo solvent toxicity issuesImprove thermal stabilityOrganic chemistry methodsAntineoplastic agentsHeat stabilityOperability
The invention relates to an idelalisib crystal form C and a preparation method thereof. Particularly, the idelalisib crystal form C has the advantages that the purity is high; compared with 4-moleculehydrate and 0.7-molucule hydrate, the heat stability is better, and the wet stability and pressure stability are higher; the electrostatic function is small; the idelalisib crystal form C is suitablefor the industrialized preparation of preparations, and meets the property requirements of the preparations; the preparation technology is simple, the operability is strong, the technology conditionsare mild, the yield rate is high, the quality is stable, the cost is low, the production cycle is short, and the scale production can be easily realized.
Owner:JIANGSU CHUANGUO PHARMA CO LTD +2
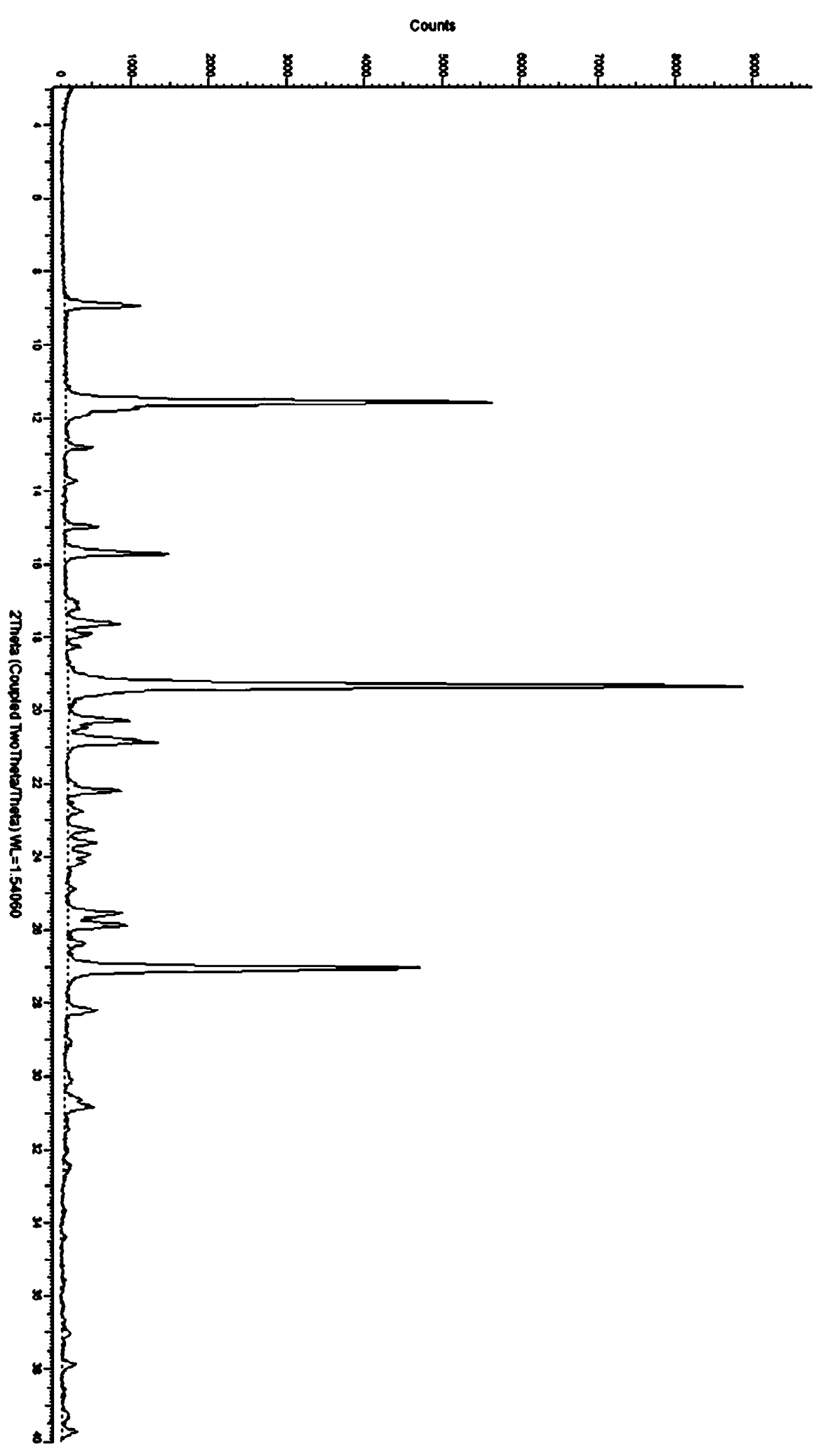
Amorphous substance of Idelalisib and preparation method therefor
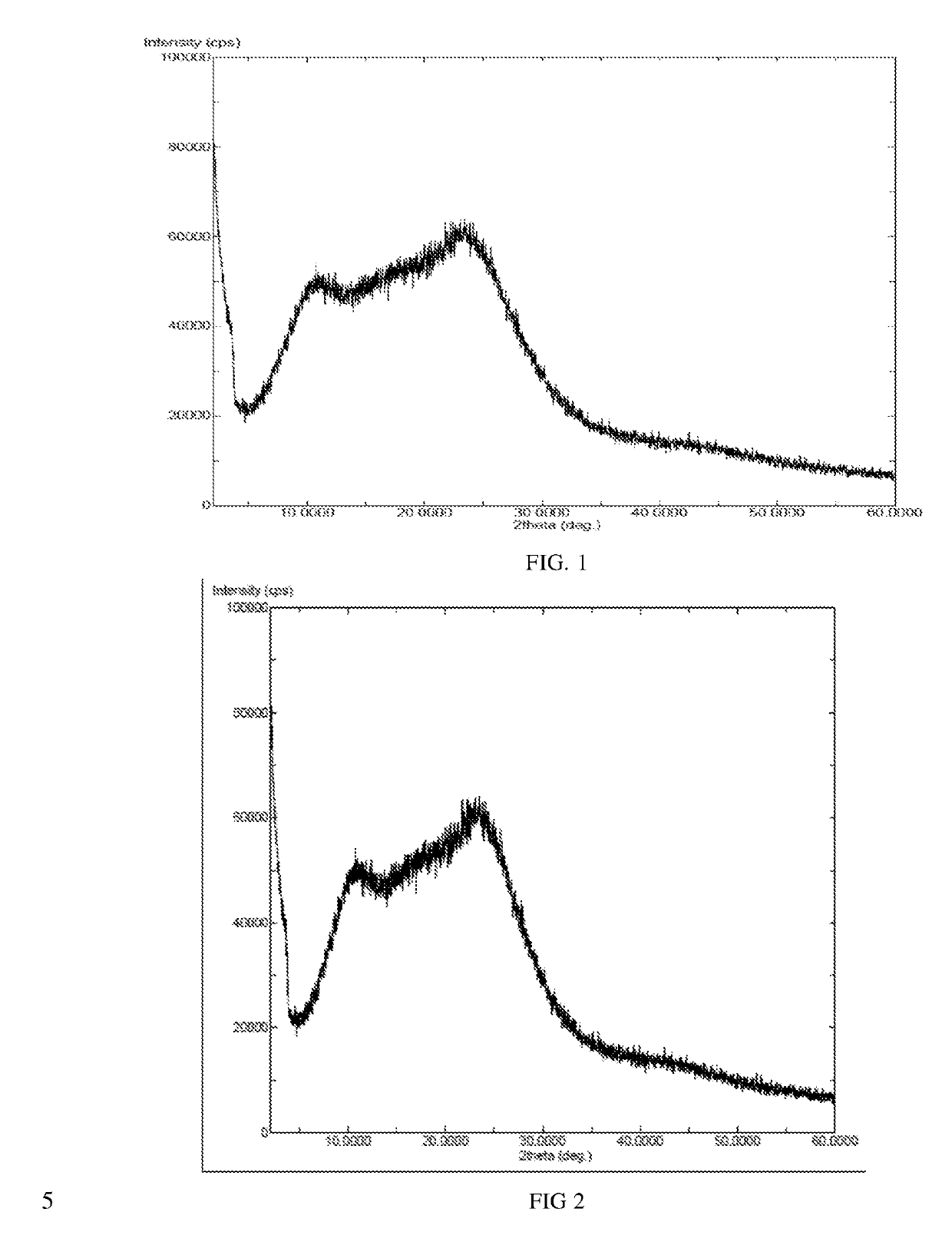
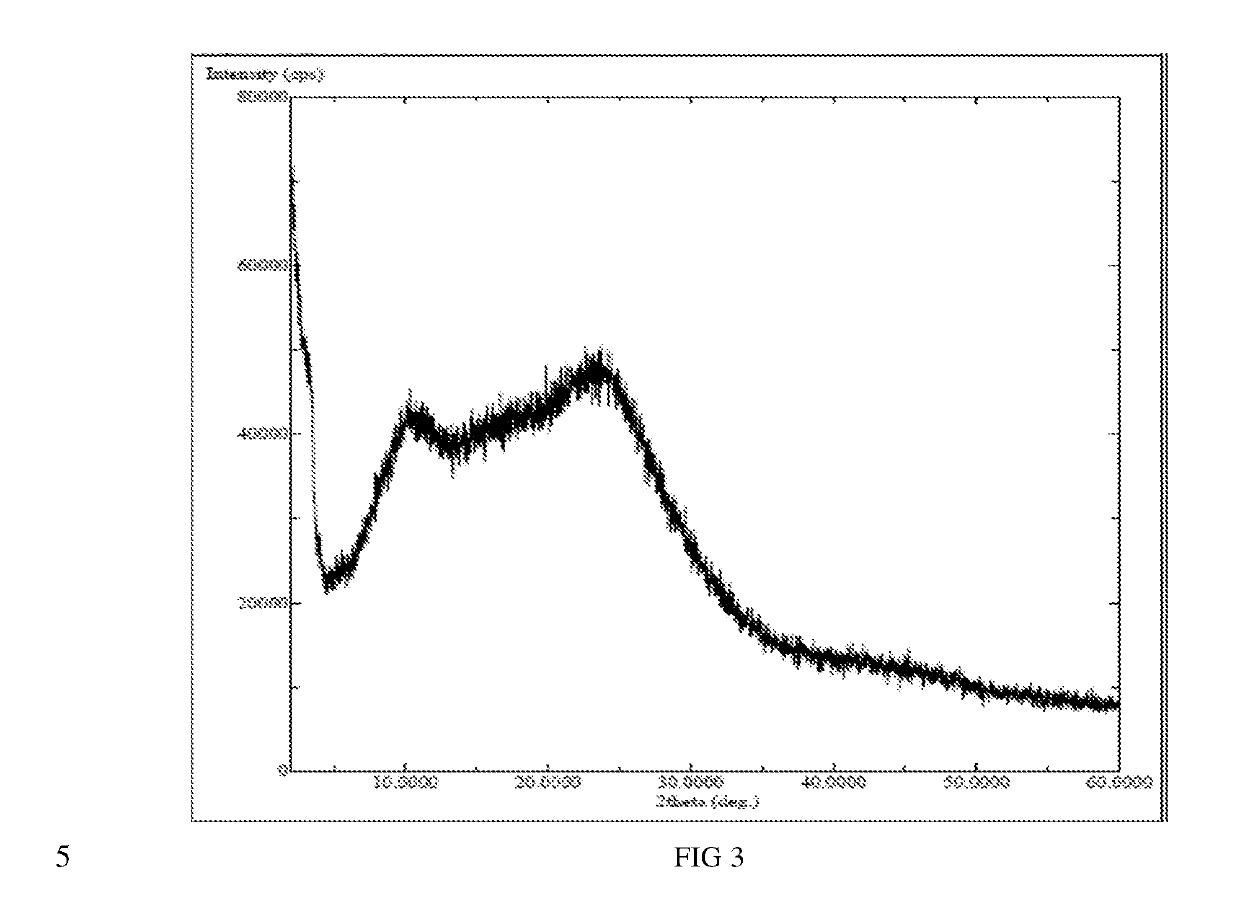
InactiveUS10370376B2Improves body 's absorptionEffective treatmentOrganic chemistryAntipyreticDiseaseSolubility
The present invention relates to an amorphous form of Idelalisib and its methods of preparation. Using a Cu-Kα radiation, the X-ray powder diffraction (XRPD) pattern does not contain sharp diffraction peaks at a diffraction angle expressed in degrees 2θ, and the X-ray powder diffraction pattern is shown in FIG. 1. The present invention also provides a preparation method for the amorphous form of Idelalisib. The amorphous form of Idelalisib of the present invention increases the solubility of Idelalisib and improves bioavailability of the drug product. As compared to the existing crystalline forms of Idelalisib, its solubility increases significantly, which improves body's absorption of the drug and makes it more efficacious in the clinical therapeutic treatment of diseases. Under the stress test conditions, the amorphous material can maintain good physical and chemical stabilities. The preparation method of amorphous Idelalisib according to the present invention is simple to operate and easy to implement.
Owner:SHANGHAI FANGNAN PHARMA
Preparation method of amorphous substance of idelalisib
The invention relates to a preparation method of an amorphous substance of idelalisib. The amorphous substance of idelalisib can greatly reduce usage amount of a solvent and improve industrial production efficiency, reduces generation of waste water, and further greatly reduces production cost of the product. Under an acceleration test condition at 40 + / - 2 DEG C under humidity of 75% + / - 5%, theamorphous substance maintains excellent physical and chemical stabilities.
Owner:宁波爱诺医药科技有限公司
The preparation method of edranil
ActiveCN104262344BEase of industrial productionRaw materials are easy to getOrganic chemistryAcetic anhydridePurine
A preparing method of Idelalisib (I) is disclosed. The preparing method includes following steps of: subjecting R-2-hydroxybutyrate (II) and 6-amino-9H-purine to nucleophilic substitution under actions of a leaving agent and an acid-binding agent to produce an intermediate S-2-(N-9H-purin-6-yl)aminobutyrate (III); subjecting the intermediate (III) and 2-amino-6-fluorobenzoic acid to amidation under actions of a catalyst to produce S-2-(N-9H-purin-6-yl)amino-N-(2-carboxyl-3-fluorophenyl)butyramide (IV); subjecting the intermediate (IV) to a cyclization reaction in acetic anhydride; and performing a substitution reaction with phenylamine to obtain the Idelalisib (I). The preparing method has characteristics of easily available raw materials, simple and concise process, capability of being economical and environmental friendly, and suitability for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Amorphous substance of idelalisib and preparation method therefor
InactiveUS20180037584A1Improves body 's absorptionEffective treatmentOrganic chemistryAntipyreticDiseaseSolubility
The present invention relates to an amorphous form of Idelalisib and its methods of preparation. Using a Cu-Kα radiation, the X-ray powder diffraction (XRPD) pattern does not contain sharp diffraction peaks at a diffraction angle expressed in degrees 2θ, and the X-ray powder diffraction pattern is shown in FIG. 1. The present invention also provides a preparation method for the amorphous form of Idelalisib. The amorphous form of Idelalisib of the present invention increases the solubility of Idelalisib and improves bioavailability of the drug product. As compared to the existing crystalline forms of Idelalisib, its solubility increases significantly, which improves body's absorption of the drug and makes it more efficacious in the clinical therapeutic treatment of diseases. Under the stress test conditions, the amorphous material can maintain good physical and chemical stabilities. The preparation method of amorphous Idelalisib according to the present invention is simple to operate and easy to implement.
Owner:SHANGHAI FANGNAN PHARMA
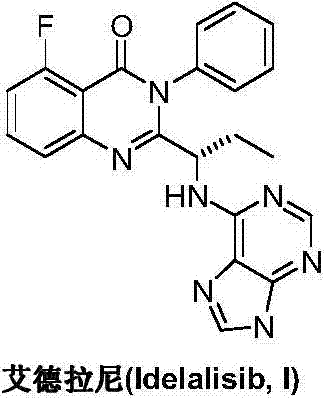
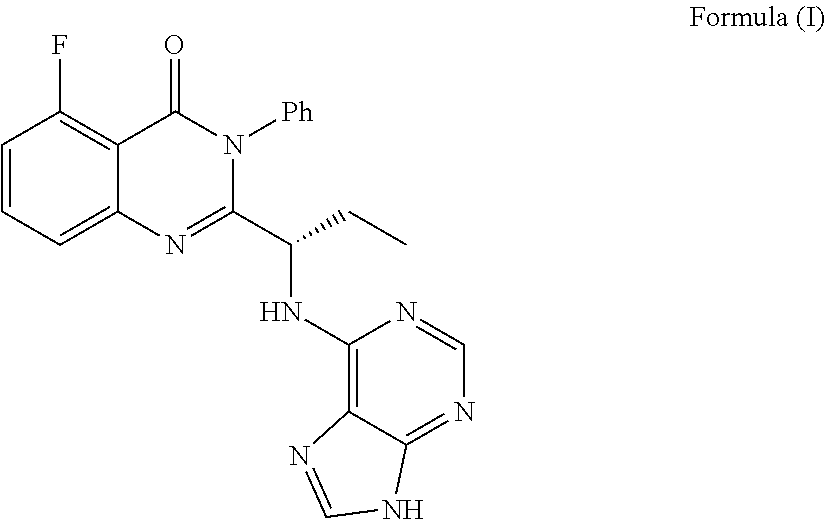
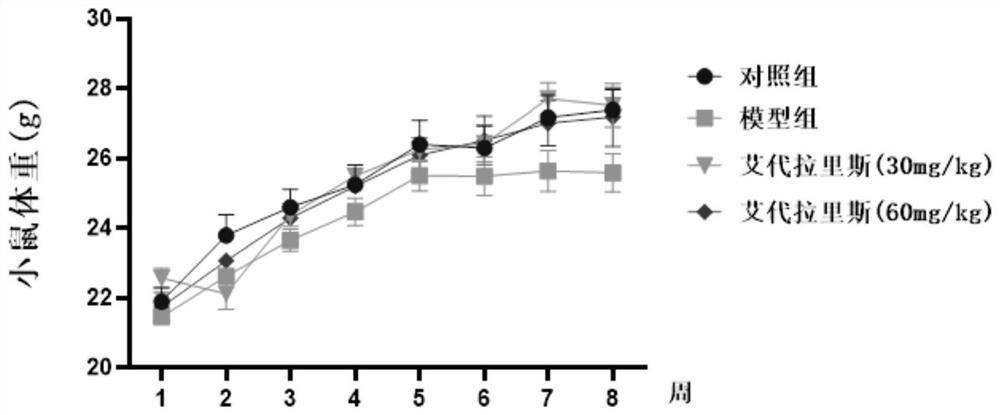
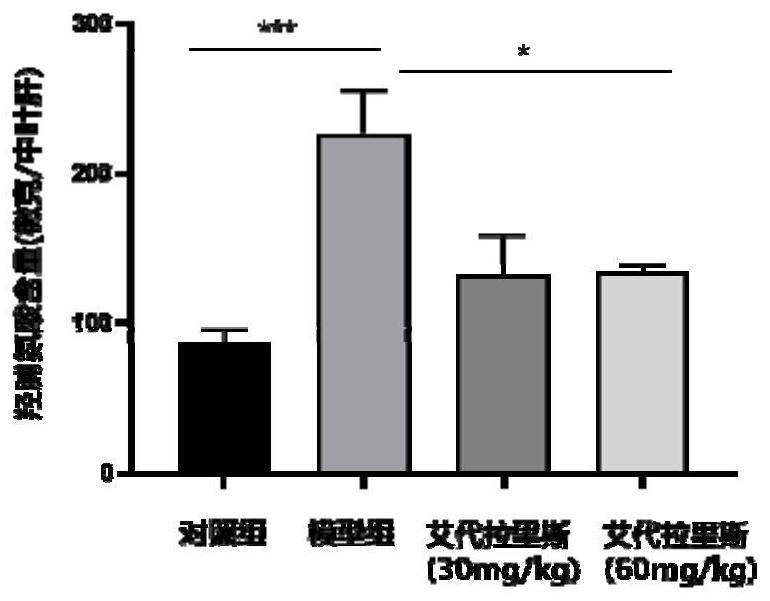
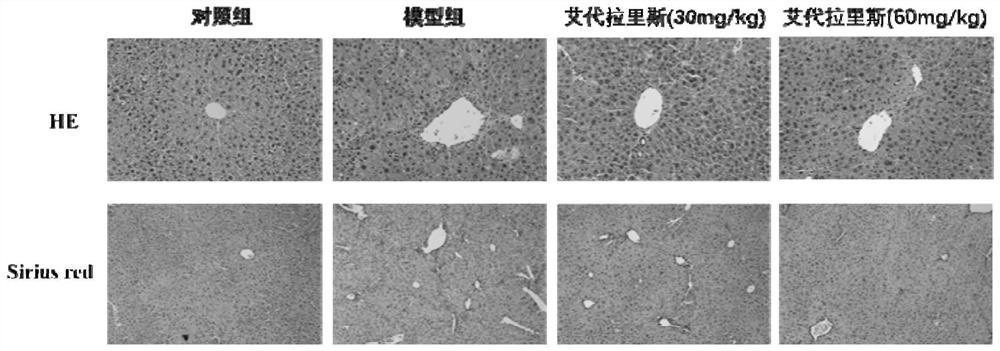
Application of idelalisib in preparation of medicine for treating hepatic fibrosis diseases
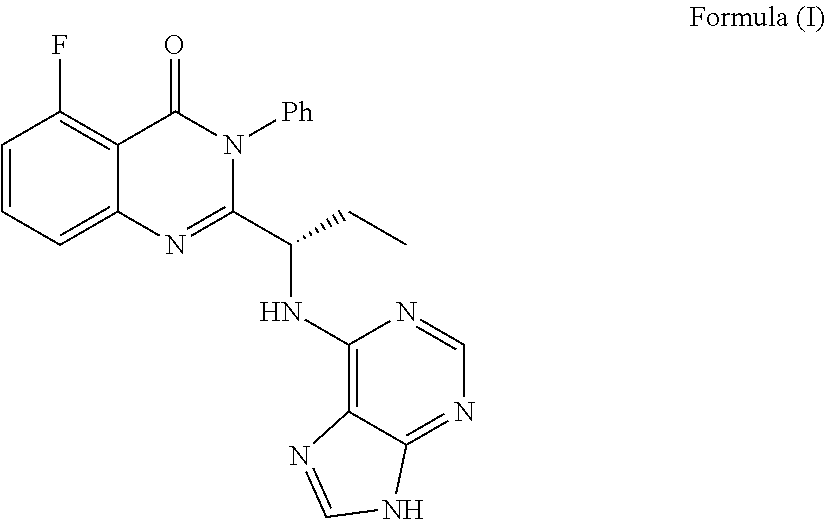
ActiveCN111840297AGood effectNo adverse reactionDigestive systemHeterocyclic compound active ingredientsDiseaseEfficacy
The invention provides an application of idelalisib in preparation of a medicine for treating hepatic fibrosis diseases. Wherein the structure of the idelalisib is shown as the following formula (I),and the research shows that the idelalisib has a good effect on liver fibrosis, has no adverse reaction, can slow down mouse liver fibrosis induced by carbon tetrachloride, and has a good applicationprospect in the aspects of treating, relieving or improving liver fibrosis diseases.
Owner:TIANJIN JIKUN MEDICAL TECH CO LTD
Preparation method of Idelalisib and intermediate thereof
ActiveCN107573345AAvoid pollutionReduce solid wasteOrganic chemistryL-2-Aminobutyric AcidZinc Acetate Dihydrate
The invention discloses a preparation method of Idelalisib and an intermediate thereof. The method comprises the following steps: using 2-halogeno-6-fluorine-N-phenyl benzamide as a raw material, performing a reaction on the 2-halogeno-6-fluorine-N-phenyl benzamide and N-(9'-tetrahydropyrane-6'-purinyl)-L-2-aminobutyric acid, performing a reaction on a reaction product and ammonium carbonate or liquid ammonia to replace 2-halogeno with ammonia by cuprous oxide, copper and potassium carbonate or cesium carbonate, performing a ring-closure reaction under the existence of hexamethyl disilazane and iodine, and performing a reaction on a reaction product and hydrochloric acid solution to remove pyran finally, so as to generate an Idelalisib finished product. According to the process, the novelmethod is provided for synthesizing the Idelalisib; in the process, the easily available 2-halogeno-6-fluorobenzoic acid is used as a starting material, and zinc acetate pollution and the like can beprevented from being generated through reduction of nitro and the like with zinc powder in a synthesis process.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Process for the preparation of amorphous idelalisib
The present invention relates to a process for the preparation of stable and 5 pure amorphous form of Idelalisib. Further, the present process is simple, more economical, cost effective and efficient method of manufacturing that is suitable for industrial scale-up having a high degree of chromatographic purity.
Owner:NATCO PHARMA LTD
Preparation method of idelalisib intermediate
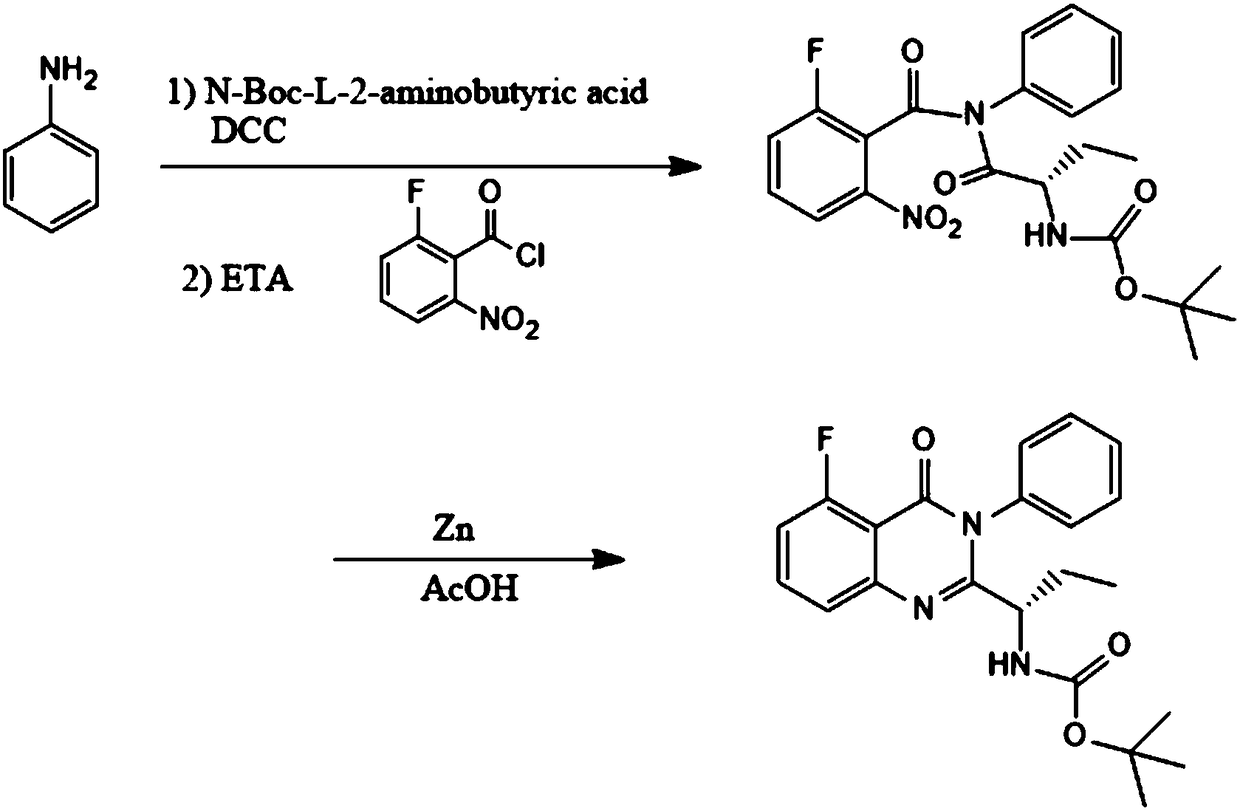
InactiveCN108409674ANo need to separateNovel reaction routeOrganic chemistryL-2-Aminobutyric AcidChloride
The invention discloses a preparation method of an idelalisib intermediate, the idelalisib intermediate (S)-N-(2-Boc-aminobutyryl)-2-fluoro-6-N-phenyl benzamide is prepared from aniline, N-Boc-L-2-aminobutyric acid, DCC, triethylamine and 2-fluoro-5-nitrobenzoyl chloride as raw materials by a three-step reaction of condensation reaction, amidation reaction and ring closure reaction for one-pot synthesis, and the condensation reaction and the amidation reaction do not require separation. The preparation method is a preparation method with the advantages of high yield, low cost, less waste, easyoperation and suitable industrialization.
Owner:南京法恩化学有限公司
O-fluorine o-imidogen benzoic acid intermediate compound as well as preparation method and application thereof
ActiveCN109503430AAvoid disordered activationAvoid it happening againCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidOrganic solvent
The invention relates to an o-fluorine o-imidogen benzoic acid intermediate compound as well as a preparation method and application thereof, and belongs to the technical field of medicine intermediate synthesis. To solve the problems of hard separation and poor activity, the invention provides the o-fluorine o-imidogen benzoic acid intermediate compound as well as the preparation method and application thereof. The preparation method comprises the following steps: dissolving a compound shown by a formula II as shown in the specification with an organic solvent, then adding pyridine and triphenyl phosphate, stirring to activate carboxyl, and adding 2-amino-6-fluorobenzoic acid for a condensation reaction to obtain the corresponding o-fluorine o-imidogen benzoic acid intermediate compound.The intermediate compound provided by the invention is high in reaction activity and easy to activate, can be effectively applicable to synthesis of key intermediate raw materials of Idelalisib, and is relatively high in reaction stability, and an obtained corresponding intermediate product has the effects of high quality and high purity.
Owner:浙江东亚药业股份有限公司
Crystal form, pharmaceutical composition, preparation method and use of Idelalis
InactiveCN106632337BIncrease dissolution rateEasy to prepareOrganic chemistry methodsAntineoplastic agentsDrug compoundChemical compound
Owner:HUBEI BIO PHARMA IND TECHCAL INST +1
A kind of idelalisib crystal form a and preparation method thereof
ActiveCN106565716BSimple preparation processEasy to operateOrganic chemistry methodsSolubilityHigh humidity
The invention discloses an Idelalisib crystal form A and a preparation method thereof. The Idelalisib crystal form A is a molecular hydrate of Idelalisib, and under powder X-ray diffraction, the 2θ is 8.5±0.2°, 10.7±0.2°, 13.6±0.2°, 15.8±0.2°, 20.2±0.2°, 23.7±0.2°, 24.3±0.2° have characteristic peaks with relative intensities greater than 50%, and a clear solution can be obtained by dissolving the Idelalisib raw material in a suitable solvent , and then add the anti-solvent dropwise to the obtained solution until the initial turbidity, and then heat and stir to crystallize, cool down to room temperature, filter, and dry to prepare. The Idelalisib crystal form A provided by the present invention has excellent solubility, thermal stability, high humidity stability and pressure stability, and is more suitable as a pharmaceutically active component of a preparation.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
A kind of preparation method of ederaris
ActiveCN108409740BMild reaction conditionsThe reaction conditions are mild and easy to controlOrganic chemistryAntineoplastic agentsCompound aChemical compound
The invention belongs to the field of medicine synthesis and provides a novel idelalisib preparation method. The novel idelalisib preparation method includes steps: in an appropriate solvent, subjecting a compound A to nucleophilic substitution reaction with B in existence of an acid-binding agent to obtain an intermediate C; hydrolyzing the compound C into an intermediate D under appropriate alkali action; subjecting the intermediate D and a compound E to condensation to obtain an intermediate F; in an appropriate solvent, subjecting the intermediate F to ring closing reaction under a catalytic system of HMDS (hexamethyl disilazane) / lewis acid to obtain a final product namely idelalisib.
Owner:YANCHENG TEACHERS UNIV +1
Synthetic method and preparation intermediate of Adelaris
ActiveCN106146502BHigh purityEasy to operateOrganic chemistryBulk chemical productionHydrogenSynthesis methods
The invention provides an Idelalisib synthesis method. The method comprises steps as follows: a compound 3 is processed with hydrogen in the presence of a hydrogenation catalyst and a solvent, nitro is enabled to be reduced, and an amino compound II is obtained; the compound II and N-Boc protected L-2-aminobutyric acid are subjected to condensation in the presence of alkali and a carboxylic acid activator or in the presence of alkali and a condensation agent, and an intermediate I is obtained; the intermediate I is subjected to a ring closing reaction in an appropriate solvent under the action of a hexamethyldisilazane / lewis acid catalytic system, and a compound 7 is obtained; the compound 7 reacts with 6-chloro-9-(2-tetrahydropyran) purine in an appropriate solvent in the presence of an acid-binding agent, and a compound III is obtained; the compound III is subjected to protecting group removal with an appropriate reagent, and Idelalisib is obtained. A reaction path is shown in the specification. The preparation method has the advantages that the reaction conditions of each step are mild, the post-processing is simple and easy to implement and the total yield is high, and the preparation method is environment-friendly and is very suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
A kind of o-fluoro-o-iminobenzoic acid intermediate compound and its preparation method and application
ActiveCN109503430BHigh reactivityImprove reaction stabilityCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidPhosphorous acid
The invention relates to an o-fluoro-o-iminobenzoic acid intermediate compound and a preparation method and application thereof, belonging to the technical field of pharmaceutical intermediate synthesis. In order to solve the existing problems of difficult separation and poor activity, an intermediate compound of o-fluoro-o-iminobenzoic acid and its preparation method and application are provided. The method comprises dissolving the compound of formula II in an organic solvent, and then adding pyridine Stir with triphenyl phosphite to activate the carboxyl group, add 2-amino-6-fluorobenzoic acid for condensation reaction, and obtain the corresponding intermediate compound of o-fluoro-o-iminobenzoic acid. The intermediate compound of the present invention has high reactivity and is easy to be active, can be effectively applied to the key intermediate raw material for synthesizing Idelalisib, and has high reaction stability, and the obtained corresponding intermediate product also has the effect of high quality and purity.
Owner:浙江东亚药业股份有限公司
A kind of preparation method of idelaris and its intermediate
The invention discloses a preparation method of Idelalisib and an intermediate thereof. The method comprises the following steps: using 2-halogeno-6-fluorine-N-phenyl benzamide as a raw material, performing a reaction on the 2-halogeno-6-fluorine-N-phenyl benzamide and N-(9'-tetrahydropyrane-6'-purinyl)-L-2-aminobutyric acid, performing a reaction on a reaction product and ammonium carbonate or liquid ammonia to replace 2-halogeno with ammonia by cuprous oxide, copper and potassium carbonate or cesium carbonate, performing a ring-closure reaction under the existence of hexamethyl disilazane and iodine, and performing a reaction on a reaction product and hydrochloric acid solution to remove pyran finally, so as to generate an Idelalisib finished product. According to the process, the novelmethod is provided for synthesizing the Idelalisib; in the process, the easily available 2-halogeno-6-fluorobenzoic acid is used as a starting material, and zinc acetate pollution and the like can beprevented from being generated through reduction of nitro and the like with zinc powder in a synthesis process.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Preparation method of Idelalisib intermediate
The invention relates to a preparation method of an Idelalisib intermediate. The method is a novel method for preparing a tert-butyl (S)-(1-(5-fluoro-4-carbonyl-3-phenyl-3, 4-dihydroquinazolin-2-yl)propyl)carbamate. The preparation method has the advantages of simple reaction steps, high yield, high optical purity and mass production.
Owner:山东轩德医药科技有限公司
Idelalisib effervescent tablet and preparation method thereof
InactiveCN106074430AImprove bioavailabilityIncrease blood concentrationInorganic non-active ingredientsPill deliveryEffervescent tabletSide effect
The invention relates to an idelalisib effervescent tablet for treating recurrent follicular B-cell non-hodgkin lymphoma (FL) and recurrent small lymphotic lymphoma (SLL), and a preparation method of the idelalisib effervescent tablet. The invention aims at providing a novel preparation, namely, the idelalisib effervescent tablet which is rapid in disintegration, rapid in absorption, high in bioavailability, convenient to take, little in intestinal tract residue and small in side effects for vast patients and medical workers. According to the idelalisib effervescent tablet, idelalisib is taken as a raw material, auxiliary materials with certain varieties and ratios are added, and the idelalisib effervescent tablet is prepared by adopting the technical means provided by the invention; the idelalisib effervescent tablet is sweet in taste, has fragrance, is rapid in taking effect, and is high in bioavailability, and the medication compliance of the patients can be easily improved.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
Idelalisib amorphous substance and preparation method thereof
The invention relates to a preparation method of an Idelalisib amorphous substance. The Idelalisib amorphous substance prepared by the method can effectively reduce solvent residue; and through a conventional vacuum drying means, the amorphous substance of which the solvent residue conforms to the specifications of ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) can be obtained. The amorphous substance can keep favorable physical stability and chemical stability under accelerated test conditions (the temperature is 40+ / -2 DEG C, and the humidity is 75%+ / -5%).The preparation method of the Idelalisib amorphous substance is simple to operate and easy to realize industrial production.
Owner:宁波爱诺医药科技有限公司
Process for the Preparation of Amorphous Idelalisib and its Premix
Processes for the preparation of amorphous idelalisib are provided. Processes for the preparation of a premix of amorphous idelalisib are also provided.
Owner:MYLAN LAB
Novel idelalisib preparation method
ActiveCN108409740AMild reaction conditionsThe reaction conditions are mild and easy to controlOrganic chemistryAntineoplastic agentsDrugs synthesisSolvent
The invention belongs to the field of medicine synthesis and provides a novel idelalisib preparation method. The novel idelalisib preparation method includes steps: in an appropriate solvent, subjecting a compound A to nucleophilic substitution reaction with B in existence of an acid-binding agent to obtain an intermediate C; hydrolyzing the compound C into an intermediate D under appropriate alkali action; subjecting the intermediate D and a compound E to condensation to obtain an intermediate F; in an appropriate solvent, subjecting the intermediate F to ring closing reaction under a catalytic system of HMDS (hexamethyl disilazane) / lewis acid to obtain a final product namely idelalisib.
Owner:YANCHENG TEACHERS UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com