Preparation method of idelalisib intermediate
A technology for ederalix and intermediates, which is applied in the field of preparation of idelalix intermediates, can solve problems such as potential safety hazards and serious pollution, and achieve the effects of high reaction efficiency, less three wastes and fewer reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
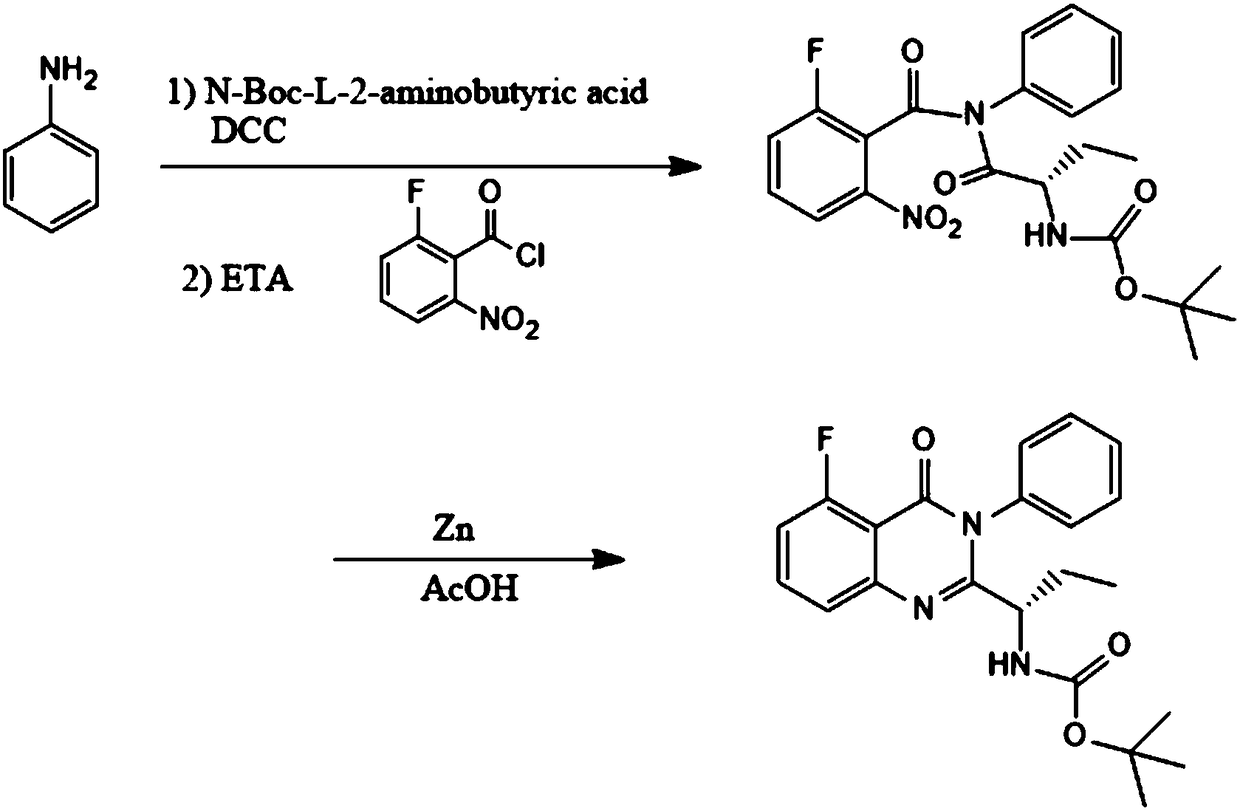
[0024] A kind of preparation method of idelaris intermediate, such as figure 1 shown, including the following steps:
[0025] Step 1: Add 1L of dichloromethane to the reaction bottle, then add 100g of aniline and stir for 5 minutes, then add 225g of DCC, and then add 220g of N-Boc-L-2-aminobutyric acid and stir at 0°C to carry out the intermediate control reaction. After the reaction, add 300g of triethylamine, dropwise add 230g of 2-fluoro-5-nitrobenzoyl chloride, after the dropwise addition, stir and react for 5 hours, add the reaction solution into water, separate the liquid, concentrate the organic phase, Crystallization, filtration, and drying yielded 445 g of the intermediate (S)-N-(2-Boc-aminobutyryl)-2-fluoro-6-nitro-N-phenylbenzamide.
Embodiment 1
[0027] Add 400g (S)-N-(2-Boc-aminobutyryl)-2-fluoro-6-nitro-N-phenylbenzamide in the reaction flask, add 3L acetic acid in the reaction flask, and then batch Add 85g of zinc powder, stir for 30min, raise the temperature to 100°C, and carry out the central control reaction. After the reaction is completed, it is concentrated under reduced pressure, cooled, filtered, washed with water, and dried to obtain 292g of the product idelaris intermediate 5-fluoro -3-Phenyl-2-[(S)-1-Boc-aminopropyl]-4-(3H)-quinazolin-4-one.
Embodiment 2
[0029] Add 400g (S)-N-(2-Boc-aminobutyryl)-2-fluoro-6-nitro-N-phenylbenzamide in the reaction flask, add 3L acetic acid in the reaction flask, and then batch Add 32g of magnesium powder, stir for 30min, raise the temperature to 80°C, and carry out the central control reaction. After the reaction is completed, it is concentrated under reduced pressure, cooled, filtered, washed with water, and dried to obtain 286g of the product idelaris intermediate 5-fluoro -3-Phenyl-2-[(S)-1-Boc-aminopropyl]-4-(3H)-quinazolin-4-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com