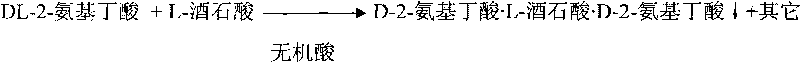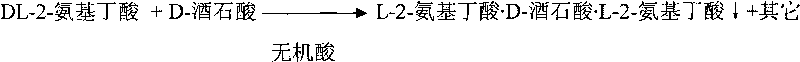Patents
Literature
37 results about "L-2-Aminobutyric Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
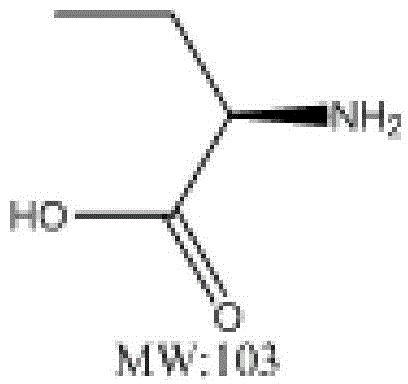
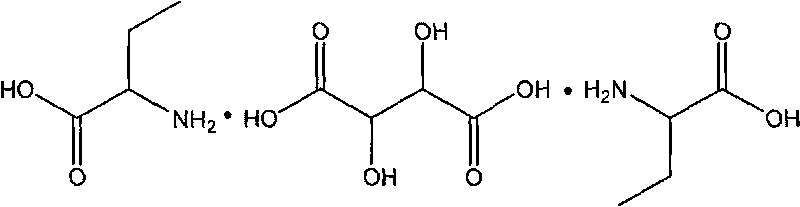
L-2-Aminobutyric acid ≥99% (titration) Synonym: L-α-Aminobutyric acid CAS Number 1492-24-6. Empirical Formula (Hill Notation) C 4 H 9 NO 2. Molecular Weight 103.12 . Beilstein Registry Number 1720935 . EC Number 216-083-3. MDL number MFCD00064415. PubChem Substance ID 24890595
Method for producing L-2-aminobutyric acid
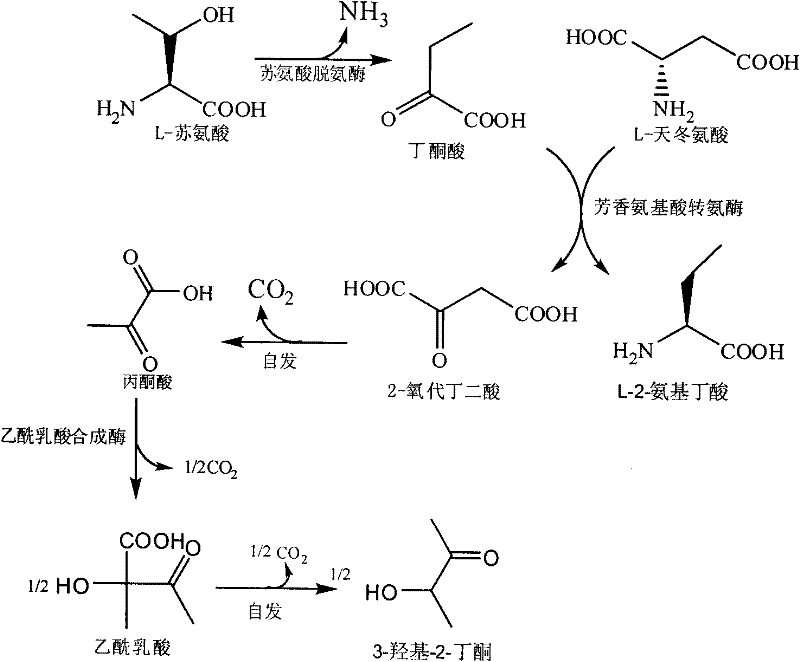
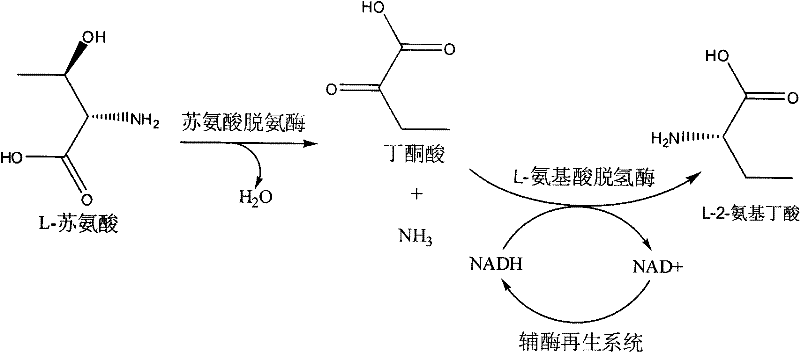
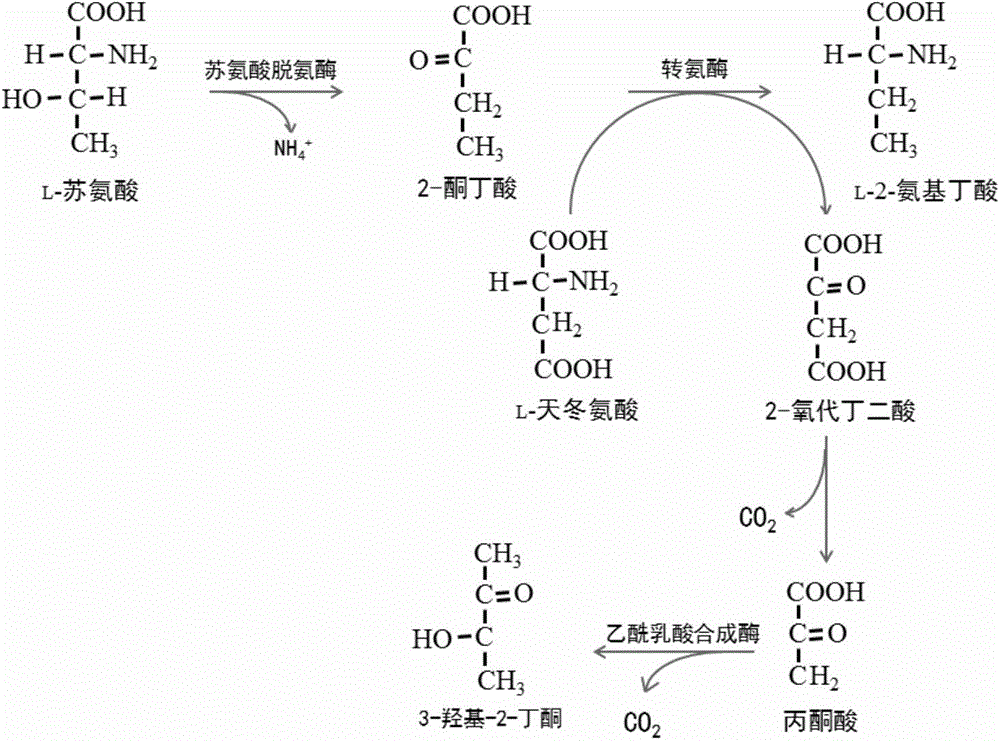
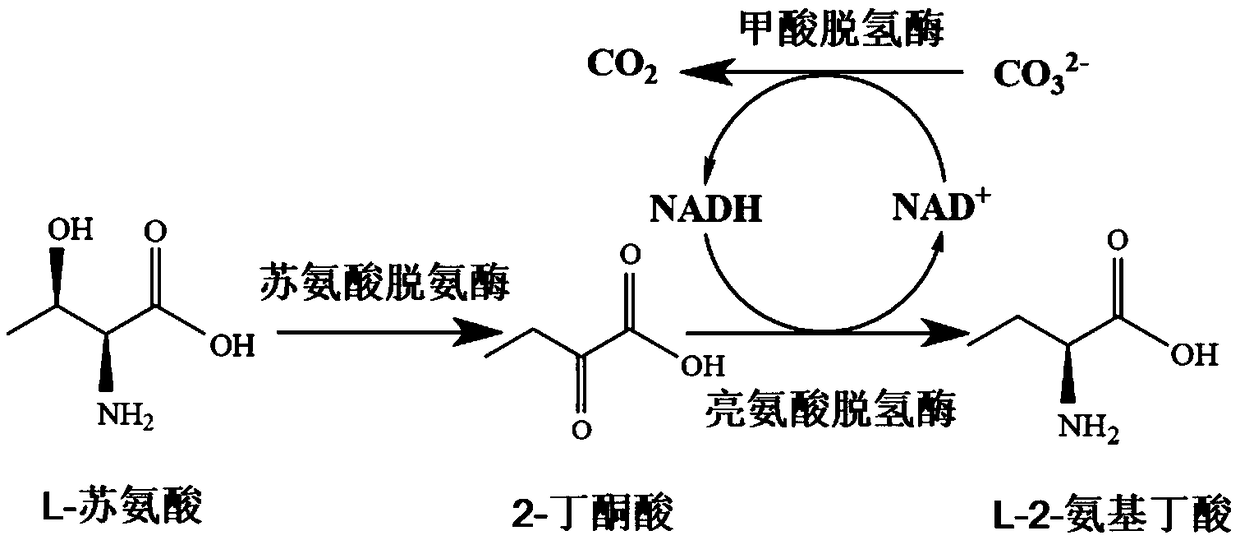
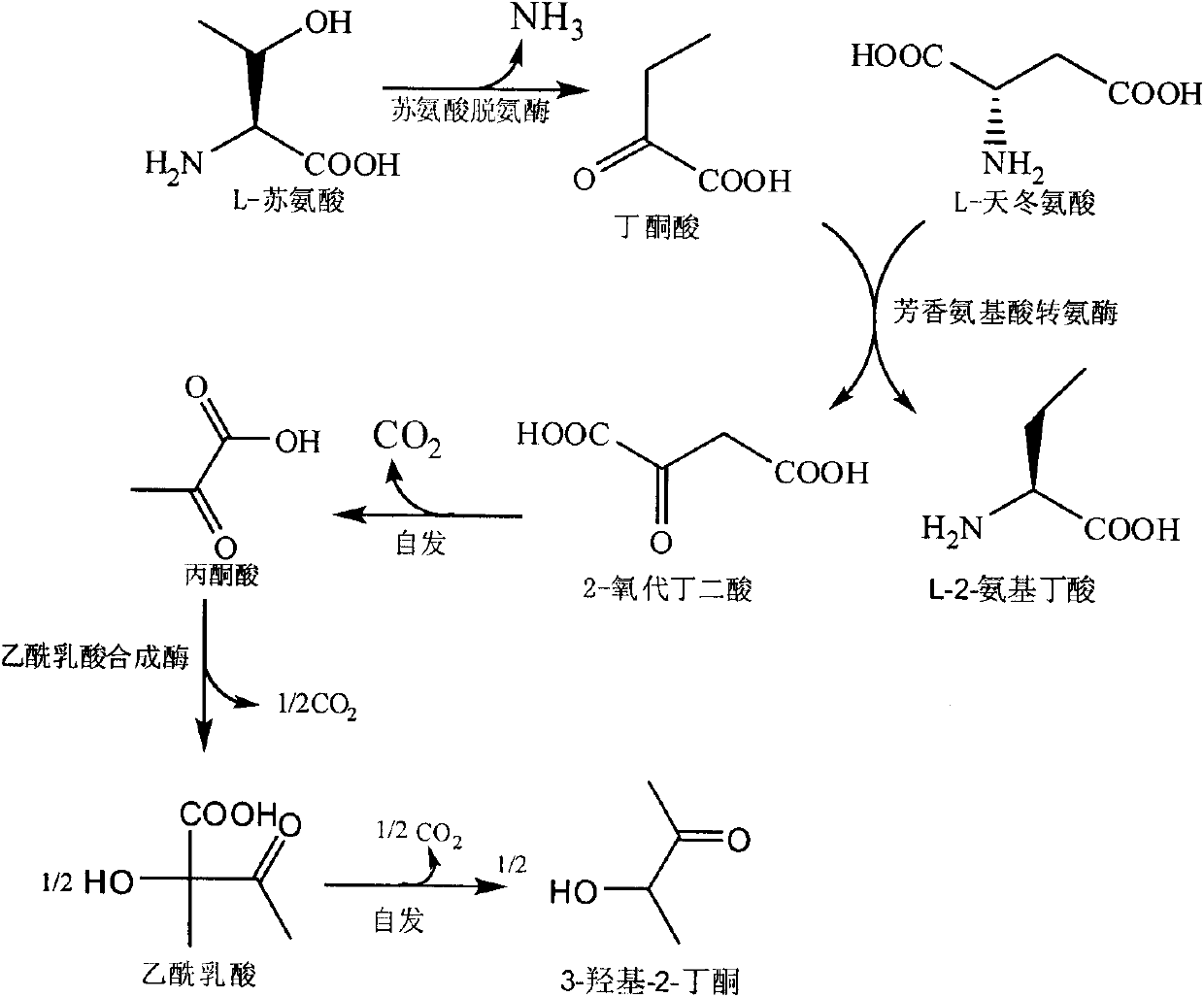
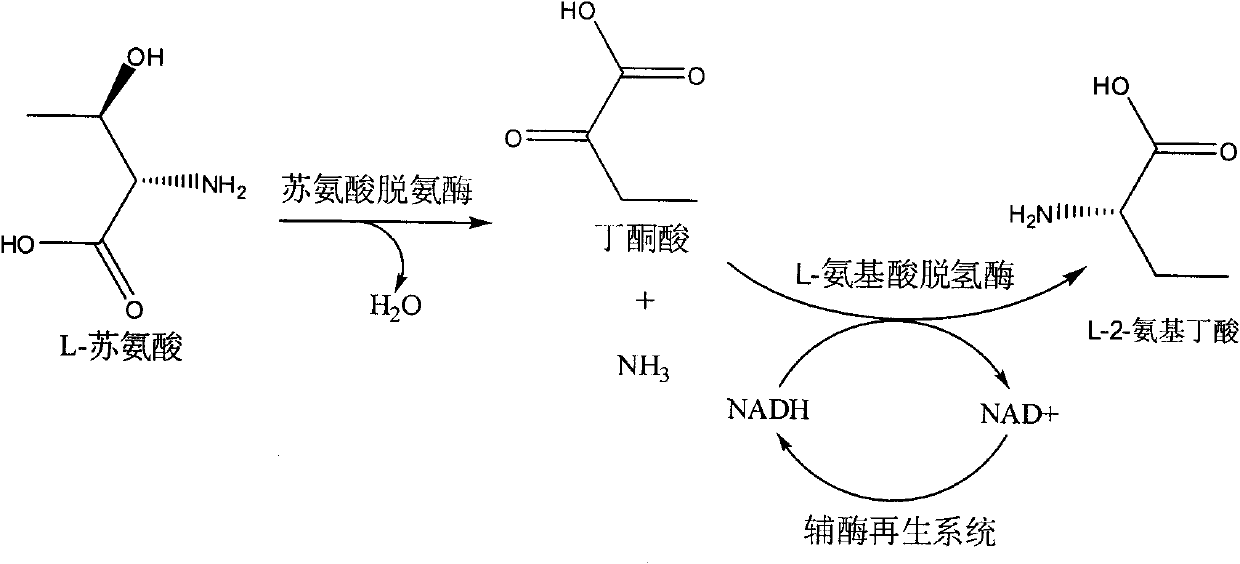
The invention discloses a method for producing L-2-aminobutyric acid, and the method comprises the following step: catalyzing L-threonine utilized as a raw material through an enzyme catalysis system consisting of threonine deaminase, L-amino acid dehydrogenase and coenzyme regeneration systems, thus producing the L-2-aminobutyric acid. The method for producing theL-2-aminobutyric acid has the advantages that the raw material is low in price, the property is stable, and the production cost of the L-2-aminobutyric acid can be greatly lowered, the conversion rate and product concentricity are high, no influence caused by byproducts exists, and the method is suitable for industrialization application.
Owner:湖州颐盛生物科技有限公司
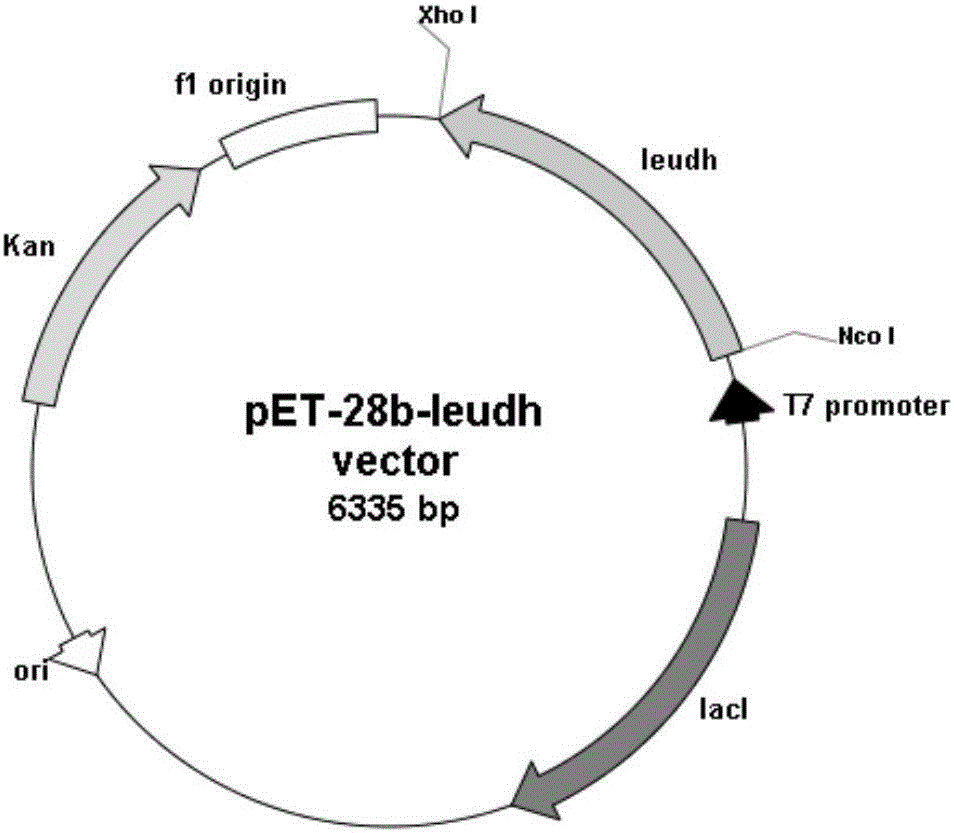
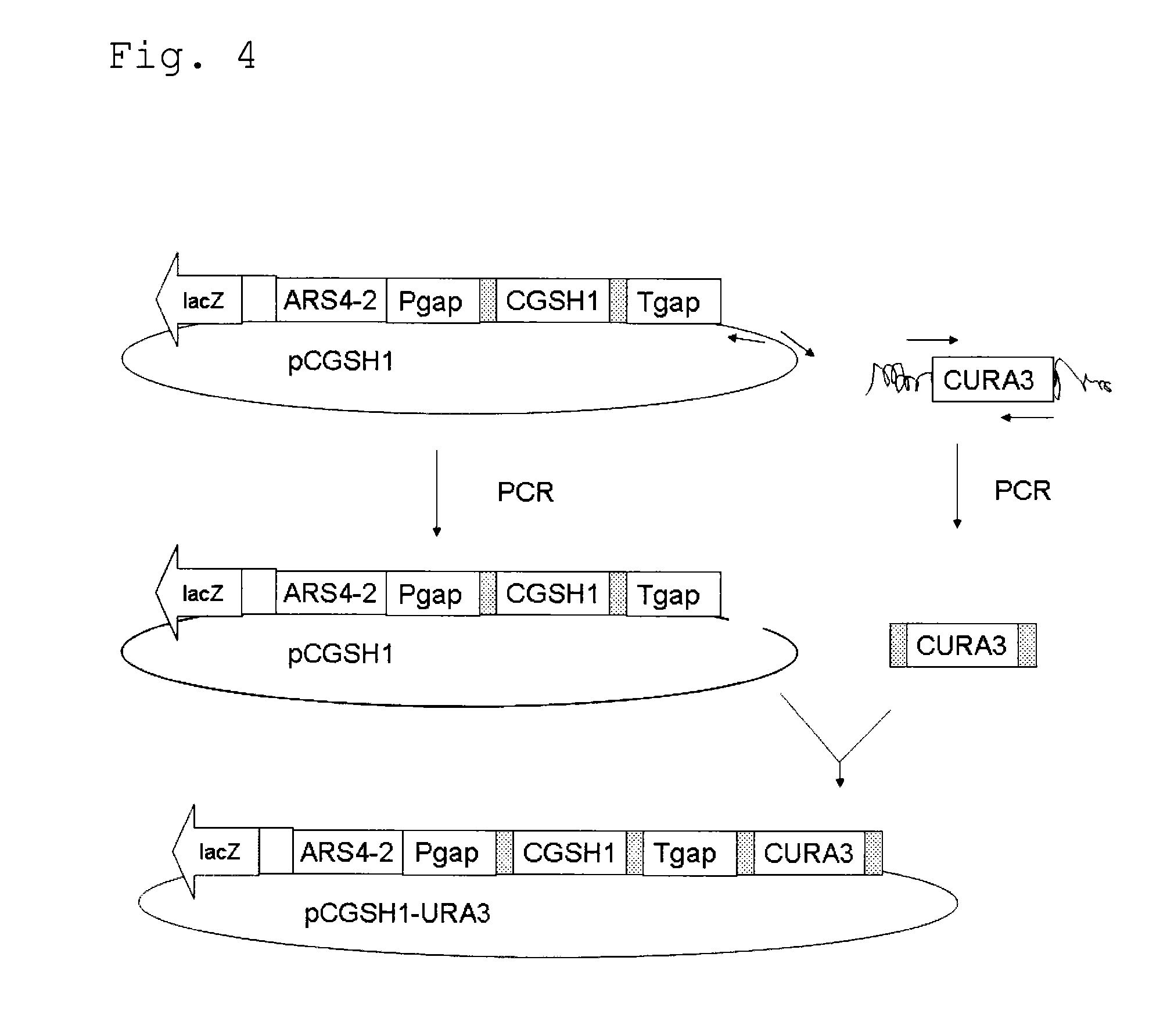
Vector, engineering strain and method for producing L(+)-2-aminobutyric acid
ActiveCN103215291AIncrease contentFermentation properties are stableFungiBacteriaL-2-Aminobutyric AcidEngineered genetic
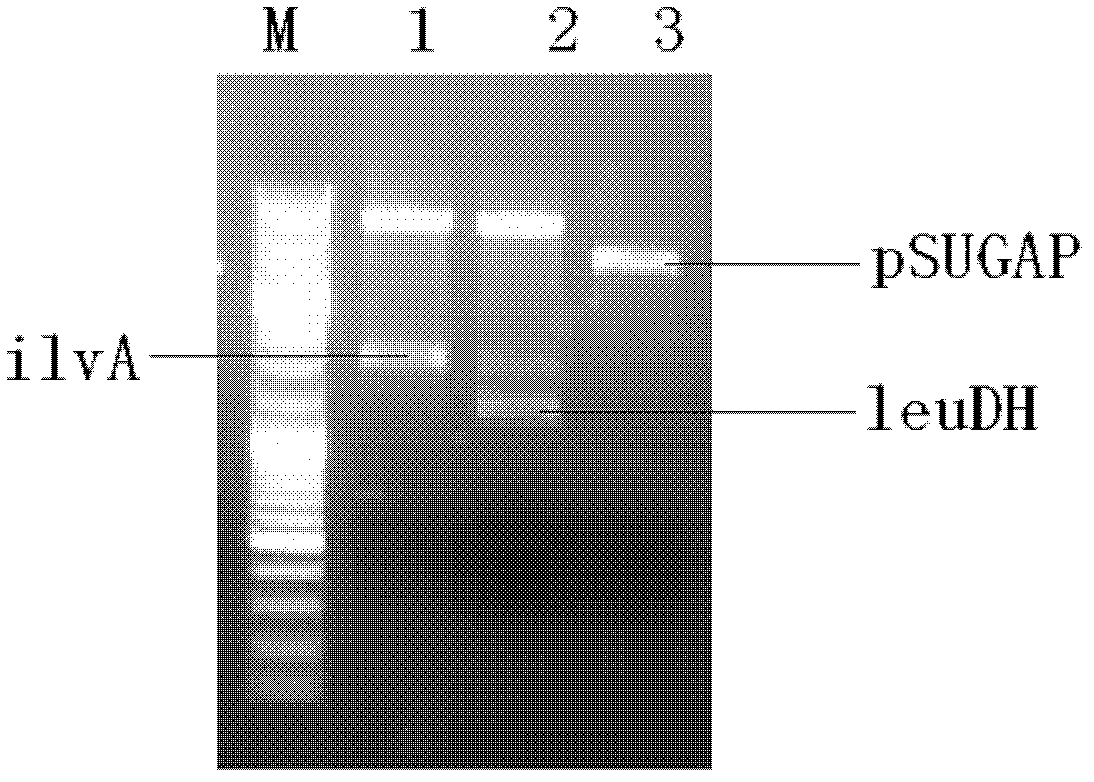
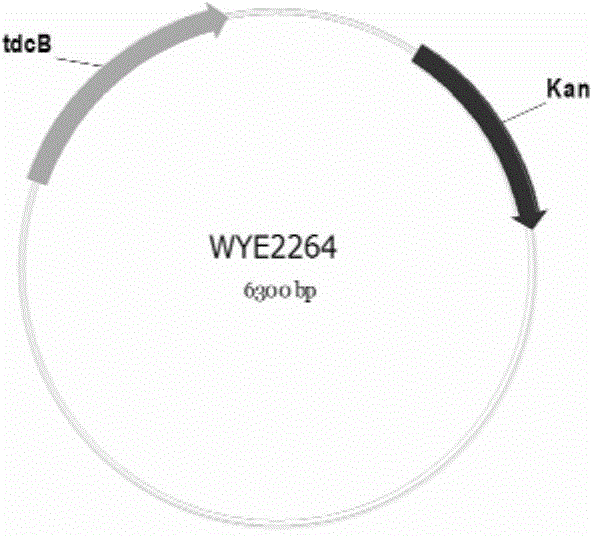
The invention relates to a vector, an engineering strain and a method for producing L(+)-2-aminobutyric acid. The vector is a recombinant vector containing a threonine dehydratase coding gene, an L-amino acid dehydrogenase gene and appropriate vector segments. The engineering strain is obtained by transforming host bacteria by the recombinant vector. The L(+)-2-aminobutyric acid is prepared by fermentation culture of the engineering strain. The engineering strain is fermentation-cultured by glucose as a raw material to produce L(+)-2-aminobutyric acid. The method has the advantages of low cost, high product concentration, no by-product, easy product purification, and good industrial application feasibility.
Owner:SHANGHAI PUYI CHEM CO LTD
Aminopherase for producing L-2-aminobutyric acid
ActiveCN105441403AOvercoming conversion rateOvercome concentrationBacteriaTransferasesAminopheraseL-2-Aminobutyric Acid
The invention establishes aminopherase through genetic engineering. Compared with ochrobactrum anthropi based wild w-aminopherase coming from ochrobactrum anthropi, the enzyme activity of aminopherase is remarkably improved, and aminopherase can be used for industrially producing L-2-aminobutyric acid.
Owner:湖州颐盛生物科技有限公司
Method for producing L-2-aminobutyric acid by virtue of biological catalysis
ActiveCN104774881AImprove protectionHigh yieldFermentationL-2-Aminobutyric AcidL-alpha-Aminobutyric Acid
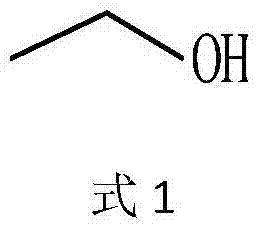
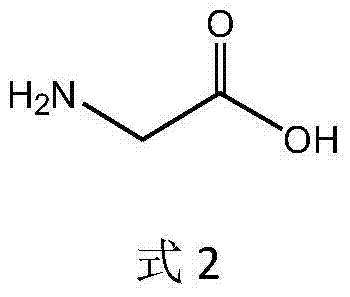
The invention discloses a new method for synthesising L-alpha-aminobutyric acid with a high optical activity. The new method comprehensively applies alcohol dehydrogenase, threonine aldolase, threonine deaminase and L-amino acid dehydrogenase, and ethanol and glycine raw material are directly synthesised into L-alpha-aminobutyric acid by a 'one-pot' method. According to the method disclosed by the invention, L-alpha-aminobutyric acid is synthesised in one pot by virtue of the simple and easily available raw materials; the method has the advantages of being few in operation steps, environment-friendly, good in bio-safety and simple in equipment, and is beneficial to industrial large-scale production for L-alpha-aminobutyric acid with a high optical activity and L-alpha-aminobutyric acid series products.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
Use of peptide for imparting body taste
ActiveCN102481006ALow costExcellent flavor imparting effectFood preparationL-2-Aminobutyric AcidPeptide
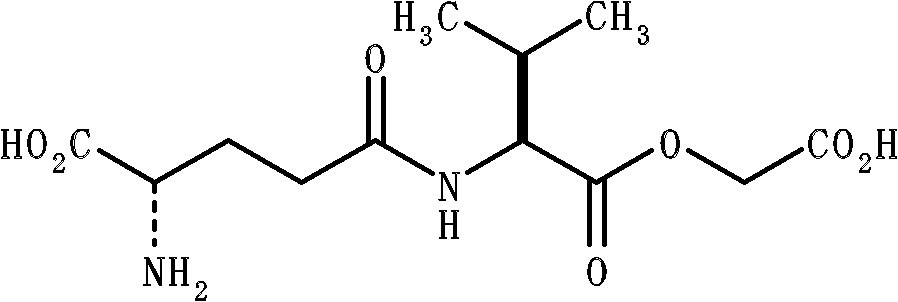
Disclosed are: an agent for imparting body taste comprising a substance, which is obtained by searching for a number of variation compounds having CaSR agonistic activity and finding a substance that has an improved effect of imparting body taste, in particular, an effect of imparting body taste of the first taste-type, and high stability and can be conveniently produced at a low cost; and a composite agent for imparting body taste which comprises said substance together with another substance having CaSR agonistic activity. Specifically disclosed are: an agent for imparting body taste which comprises gamma-Glu-Abu (L-gamma-glutamyl-L-2-aminobutyric acid); and a composite agent for imparting body taste which comprises a combination of said substance with another substance having CaSR agonistic activity.
Owner:AJINOMOTO CO INC
Recombinant strain for producing L-2-aminobutyric acid and preparing method and application thereof
ActiveCN106148259AIncrease productionHigh substrate conversion rateBacteriaMicroorganism based processesL-2-Aminobutyric AcidThreonine
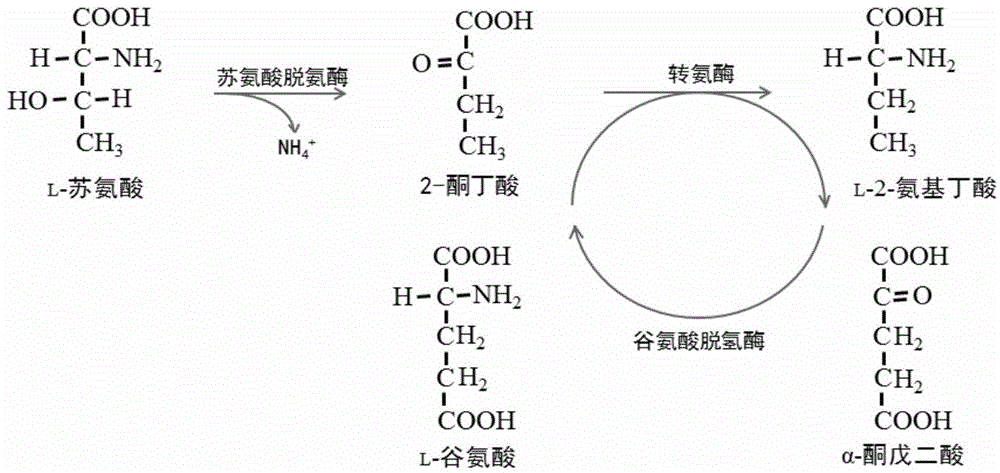
The invention discloses a recombinant strain for producing L-2-aminobutyric acid and a preparing method and application thereof. The recombinant strain is a strain obtained in the mode that threonine dehydratase, transaminase and glutamate dehydrogenase are subjected to overexpression at the same time. Due to the recombinant strain for producing the L-2-aminobutyric acid with threonine serving as a substrate, the yield of the L-2-aminobutyric acid and the substrate conversion ratio are remarkably increased, accumulation of by-products is effectively reduced, and the production technology is simplified.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Construction and application of recombinant strain converting L-threonine to L-2-aminobutyric acid
ActiveCN109266595ALow costEasy to scale up industrial productionCarbon-nitrogen lyasesBacteriaL-threonineSal ammoniac
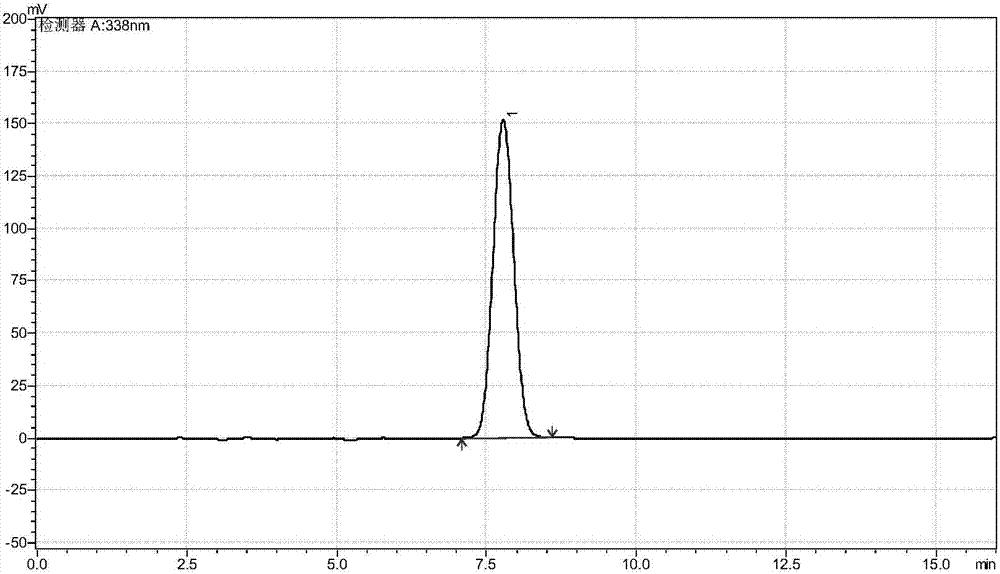
The invention discloses a construction and application of a recombinant strain converting L-threonine to L-2-aminobutyric acid, belonging to the field of bioengineering technology. The production method of the invention utilizes a recombinant bacterium expressing two plasmids to simultaneously realize the high-efficient expression of three enzymes, and comprises the steps of: conversion of L-threonine to L-2-aminobutyric acid, coupled with a coenzyme regeneration system, converts NAD+ into NADH, so that the concentration of NADH in the system is relatively stable, and the conversion can be carried out efficiently. Furthermore, the CO<2> converted from ammonium formate can be dissolved in ammonia water, which has little environmental pollution and industrial application value. The method has the advantages of mild conversion condition, strong specificity, low cost and short conversion time. The L-2-aminobutyric acid is prepared adopting the method, 40g / L L-threonine is added, The concentration of the obtained product L-2-aminobutyric acid is 43.3 g / L, and the conversion ratio was over 99.9%.
Owner:JIANGNAN UNIV
Leucine dehydrogenase mutant, coding gene, vector, engineering bacterium and application thereof
ActiveCN106497895AIncrease productionIncrease enzyme activityBacteriaMicroorganism based processesEscherichia coliRecombinant escherichia coli
The invention discloses a leucine dehydrogenase mutant, a coding gene, a recombinant vector, a gene engineering bacterium and application thereof. The recombinant Escherichia coli strain with efficiently expressed leucine dehydrogenase has the advantages of high yield, simple technique and the like, is convenient for industrialized application, and can be directly used for producing L-2-aminobutyric acid. After the LeuDH-Q358T mutant fermentation finishes, the total enzyme activity for 2-ketobutyric acid at 35 DEG C is up to 965.7 U / g, and the conversion rate of 2-ketobutyric acid is up to 99%, thereby providing favorable technical supports for large-scale production of L-2-aminobutyric acid.
Owner:ZHEJIANG UNIV OF TECH
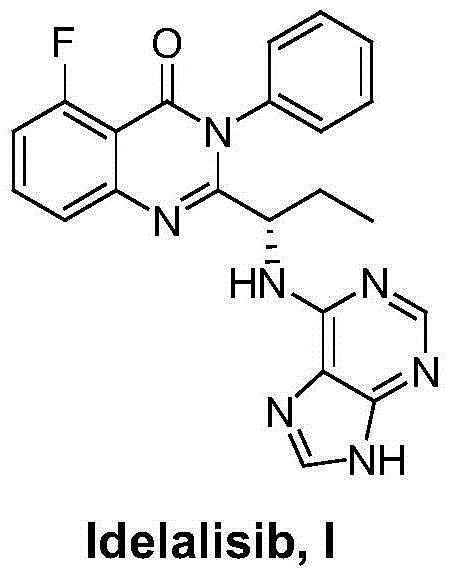
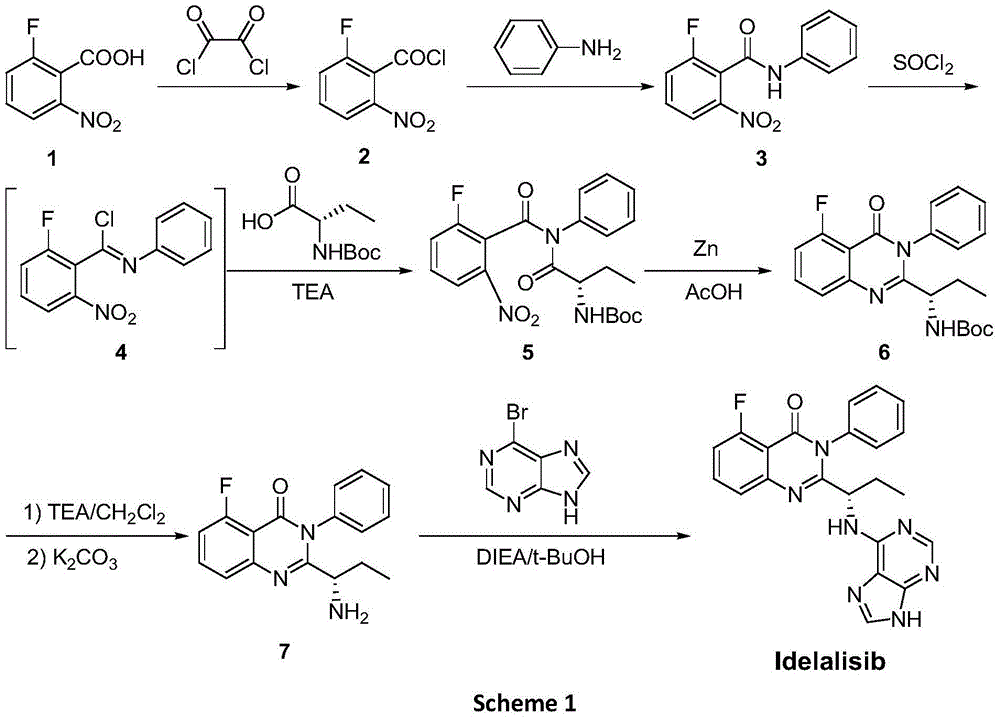
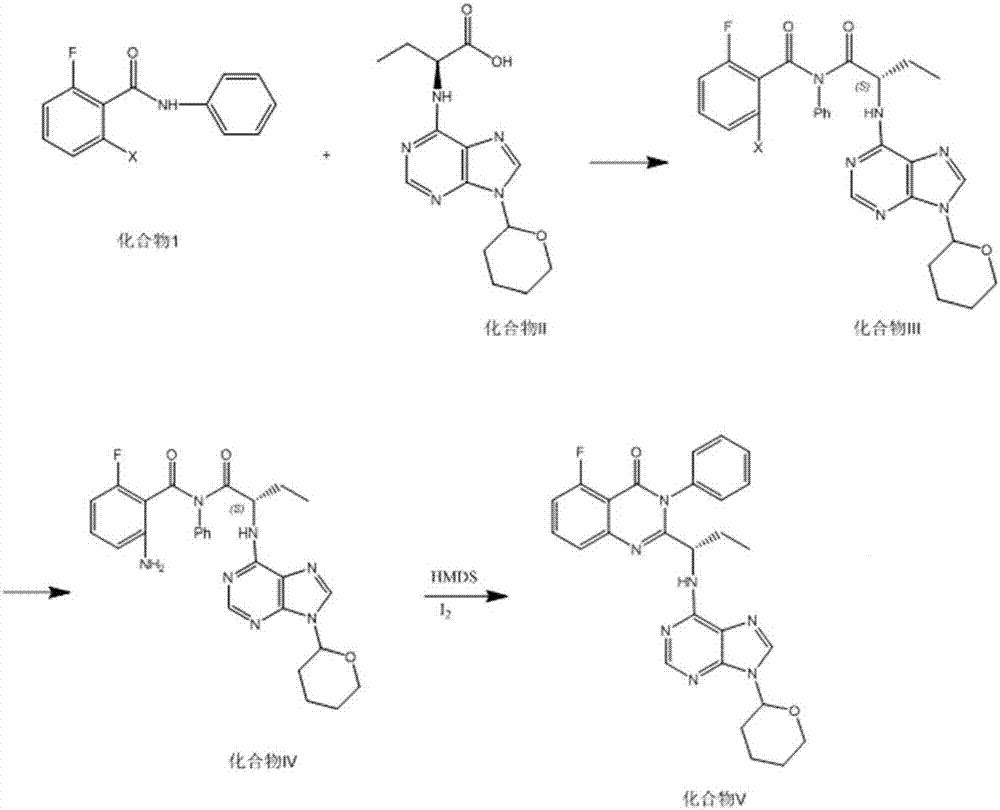
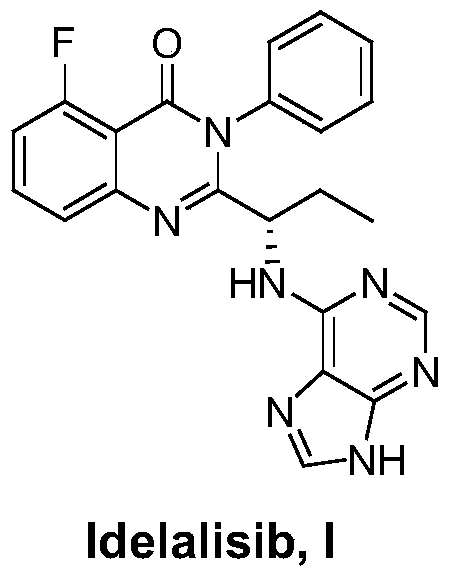
Idelalisib synthesis method and preparation of intermediate
ActiveCN106146502AHigh purityEasy to operateOrganic chemistryBulk chemical productionSynthesis methodsL-2-Aminobutyric Acid
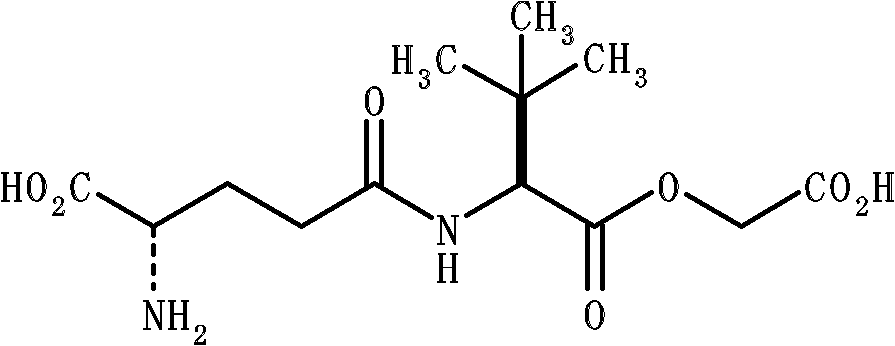
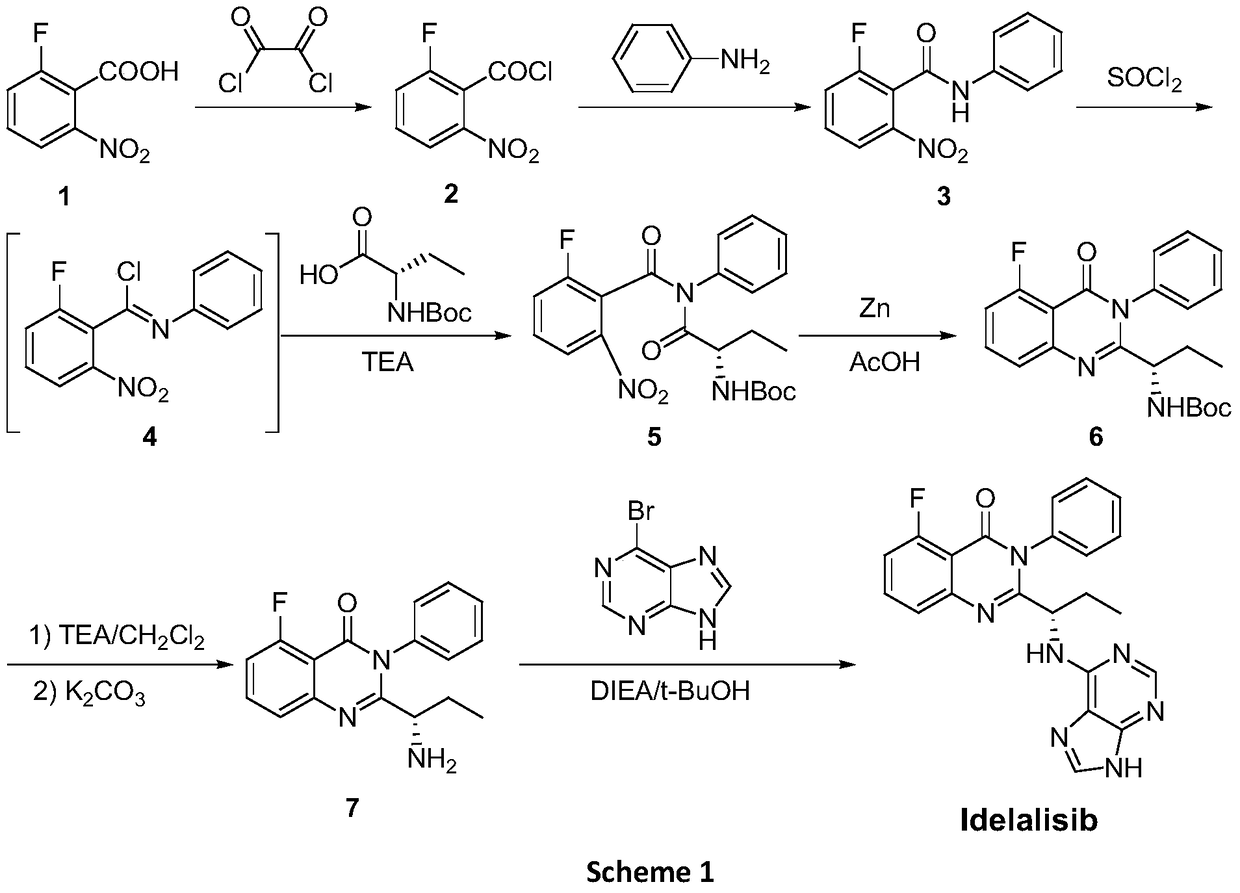
The invention provides an Idelalisib synthesis method. The method comprises steps as follows: a compound 3 is processed with hydrogen in the presence of a hydrogenation catalyst and a solvent, nitro is enabled to be reduced, and an amino compound II is obtained; the compound II and N-Boc protected L-2-aminobutyric acid are subjected to condensation in the presence of alkali and a carboxylic acid activator or in the presence of alkali and a condensation agent, and an intermediate I is obtained; the intermediate I is subjected to a ring closing reaction in an appropriate solvent under the action of a hexamethyldisilazane / lewis acid catalytic system, and a compound 7 is obtained; the compound 7 reacts with 6-chloro-9-(2-tetrahydropyran) purine in an appropriate solvent in the presence of an acid-binding agent, and a compound III is obtained; the compound III is subjected to protecting group removal with an appropriate reagent, and Idelalisib is obtained. A reaction path is shown in the specification. The preparation method has the advantages that the reaction conditions of each step are mild, the post-processing is simple and easy to implement and the total yield is high, and the preparation method is environment-friendly and is very suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Preparation method of S-(+)-2-aminobutanamide hydrochloride
InactiveCN103045667AAvoid lostHigh yieldMicroorganism based processesFermentationChemical synthesisEnvironmental resistance
The invention provides a production method of S-(+)-2-aminobutanamide hydrochloride which is a key intermediate necessary for synthesizing anti-epileptic chiral drug, namely levetiracetam. The method comprises the following steps of: using L-threonine as an initial raw material, preparing L-2-aminobutyric acid by using a biotransformation method, and then performing esterification and ammonolysis reaction to obtain a target compound. According to the method provided by the invention, biotransformation and chemical synthesis are combined, and the good optical selectivity of the biotransformation is utilized for generating the L-2-aminobutyric acid, the reaction condition is mild and simple, the raw material cost is low, an organic solvent is not necessary, the process is environment-friendly, the purity of an isomer is high, and both the conversion rate and the yield are high; and therefore, the S-(+)-2-aminobutanamide hydrochloride is suitable for industrial production.
Owner:姚强
Method for producing L-2-aminobutyric acid by fermentation process
InactiveCN105671098AIncrease production capacityImprove efficiencyFermentationActivated carbonUltrafiltration
The invention relates to a method for producing L-2-aminobutyric acid by fermentation, comprising the following steps: (1), inserting the strain into a seed tank to obtain a mature strain; (2), adding the mature strain Connect it into a fermenter for fermentation and cultivation; (3) After the fermentation is over, the fermentation liquid is first sterilized by a ceramic membrane, then passed through an ultrafiltration membrane with a molecular weight of 2-4Kd, and decolorized by activated carbon, and the 732 ion exchange resin is used Adsorbed and eluted with ammonia water, evaporated and concentrated to obtain the crude product of L-2-aminobutyric acid. The method for producing L-2-aminobutyric acid of the present invention, through the improvement of fermentation formula and process control, microbial enzyme conversion scheme and control process, extraction scheme and control process, makes the extraction process simple and the product purity can reach more than 95%, The production capacity and efficiency of L-2-aminobutyric acid are greatly improved, which can meet the large-scale industrial production of L-2-aminobutyric acid, and have higher social and economic benefits.
Owner:HENAN JULONG BIOLOGICAL ENG CO LTD
Method for synthesizing L-2-aminobutyric acid by enzymatic method
ActiveCN107012178ANo effectSuitable for industrial productionFermentationHydrogenL-2-Aminobutyric Acid
The invention discloses a method for synthesizing L-2-aminobutyric acid by an enzymatic method. According to the method, 2-ketobutyric acid is catalyzed by the aid of alanine dehydrogenase and formate dehydrogenase to produce the L-2-aminobutyric acid. The method particularly includes the steps: uniformly mixing 0.5-1.5mol of 2-ketobutyric acid, 0.5-1.5mol of ammonium formate, o-0.5g / L of NAD (nicotinamide adenine dinucleotide) and 20g / L of formate dehydrogenase and alanine dehydrogenase co-expression wet cells (or 10-30g / L of formate dehydrogenase wet cells, 10-30g / L of alanine dehydrogenase wet cells) in a rector; adjusting a pH (potential of hydrogen) value to be 7.0-9.0; performing catalytic reaction for 16-20h at the temperature ranging from 30 DEG C to 37 DEG C to obtain the L-2-aminobutyric acid. The method is rapid in reaction, reverse oxidation deamination activity is low, and product loss is greatly reduced.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
Method for preparing L-2-aminobutyric acid through whole-cell bioconversion
ActiveCN104531793AAvoid purification processEasy to solveMicroorganism based processesFermentationEscherichia coliL-2-Aminobutyric Acid
The invention relates to a method for preparing L-2-aminobutyric acid through whole-cell bioconversion. The method disclosed by the invention comprises the following steps of: carrying out tandem expression on threonine deaminase and leucine dehydrogenase, and cloning on a chlorampenicol resistant expression vector so as to realize co-expression of the two enzymes in the same bacterium; cloning formilase on a kalamycin resistant pET-28a vector to realize expression, and carrying out tandem expression with a pcnB gene in a rate-limiting step of coenzyme I metabolic pathways in escherichia coli so as to realize high expression of the two enzymes; converting the expression vector having two resistances into the same bacterium to carry out co-expression so as to realize expression of four enzymes in the same host bacterium; and carrying out fermentation expression of whole-cell for escherichia coli containing the four enzymes by using escherichia coli so as to realize biological reduction conversion from threonine to L-2-aminobutyric acid. According to the invention, high-efficiency conversion of 2-ketobutyric acid can be realized without addition of coenzyme; the cost is low; and the method is simple.
Owner:NANJING Y BIO PHARMA CO LTD
Method for producing L-2-aminobutyric acid
The invention discloses a method for producing L-2-aminobutyric acid, and the method comprises the following step: catalyzing L-threonine utilized as a raw material through an enzyme catalysis system consisting of threonine deaminase, L-amino acid dehydrogenase and coenzyme regeneration systems, thus producing the L-2-aminobutyric acid. The method for producing theL-2-aminobutyric acid has the advantages that the raw material is low in price, the property is stable, and the production cost of the L-2-aminobutyric acid can be greatly lowered, the conversion rate and product concentricity are high, no influence caused by byproducts exists, and the method is suitable for industrialization application.
Owner:湖州颐盛生物科技有限公司
Threonine deaminase mutant as well as preparation method and application thereof
ActiveCN109182319AImprove thermal stabilityRealize industrializationCarbon-nitrogen lyasesBacteriaBiotechnologyL-2-Aminobutyric Acid
The invention discloses a threonine deaminase mutant as well as a preparation method and application thereof. The mutant is prepared by carrying out single-point mutation or multi-point mutation on amino acids on a site 14, a site 323, a site 344, a site 449 and a site 510 in an amino acid sequence represented by SEQ ID NO.1. Compared with wild threonine deaminase, threonine deaminase provided bythe invention has the advantages that the stability is extremely high, and the service life of threonine deaminase in the industrial production can be greatly prolonged. A G323D / F510L / T344A mutant isapplied to the production of L-2-aminobutyric acid, the conversion rate of L-threonine can reach 99%, and the addition amount of NAD<+> can be decreased to 0.04g / L, so that a good technical support isprovided for the large-scale production of L-2-aminobutyric acid.
Owner:ZHEJIANG UNIV
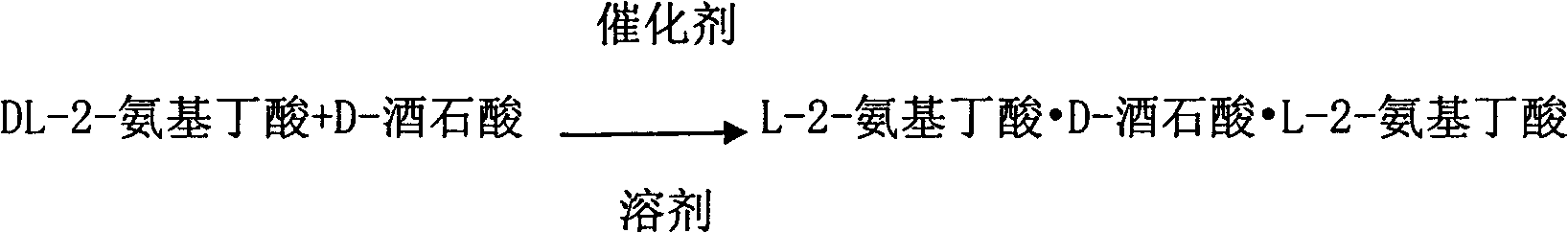
Method for preparing L-2-aminobutyric acid by asymmetric conversion method
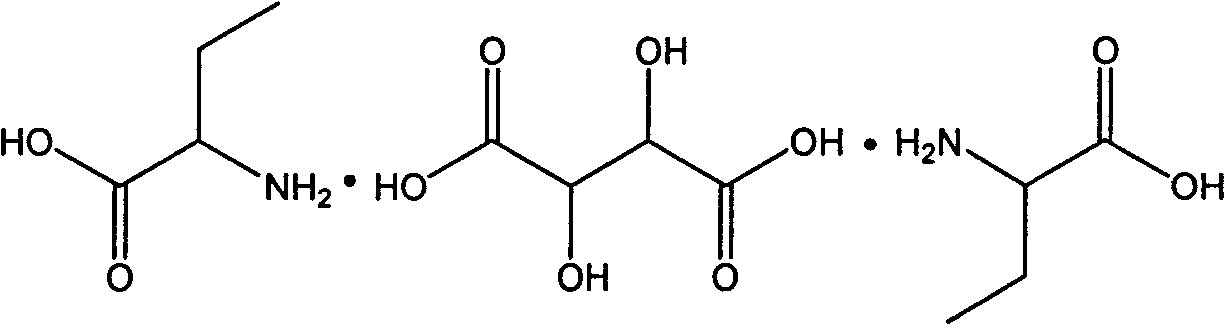
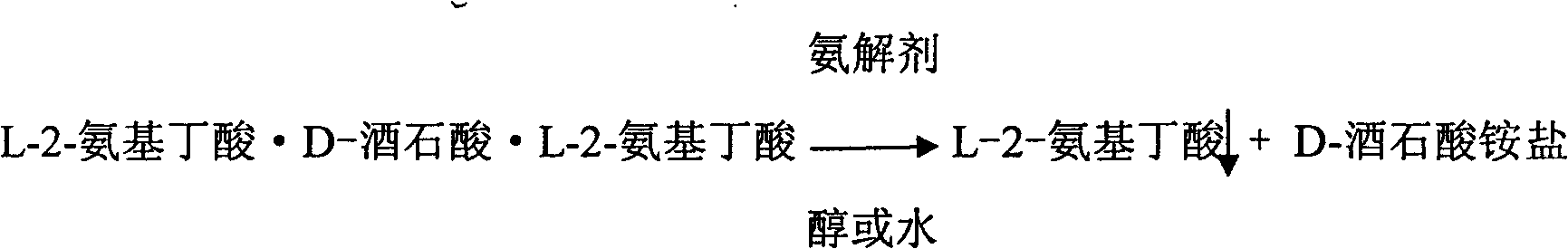
InactiveCN102060721AAvoid entrained precipitationReduce usageOrganic compound preparationAmino-carboxyl compound preparationL-2-Aminobutyric AcidReaction intermediate
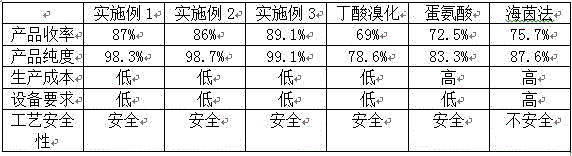
The invention discloses a method for preparing L-2-aminobutyric acid by an asymmetric conversion method. The method comprises the following steps: mixing DL-2-aminobutyric acid with D-tartaric acid; in the presence of aromatic aldehyde as catalyst, reacting in acid solvent; cooling, filtering, and re-crystallizing to obtain a reaction intermediate product; mixing the reaction intermediate product with an aminolysis agent to be subjected to aminolysis reaction; and then cooling, filtering and washing to obtain the L-2-aminobutyric acid. The L-2-aminobutyric acid prepared by the method has the advantages of high yield, higher optical purity and chemical purity, lower cost and suitability for large-batch industrial production.
Owner:NANJING INST OF TECH
Method for preparing L-2-aminobutyric acid through biocatalysis
ActiveCN104946694AEfficient conversion preparationOxidoreductasesFermentationL-2-Aminobutyric AcidGenetically engineered
The invention provides a method for preparing L-2-aminobutyric acid through biocatalysis. The method comprises the following steps: establishing genetically engineered bacteria for simultaneously expressing FDH (Formate Dehydrogenase) and LDH (Leucine Dehydrogenase) to obtain an FDH / LDH co-expression crude enzyme solution containing the FDH and the LDH through fermentation; preparing a reaction system in a buffer solution, and performing biocatalytic reaction to prepare L-2-aminobutyric acid, wherein the reaction system comprises threonine, ammonium formate, a TD enzyme solution, the FDH / LDH co-expression crude enzyme solution, NAD<+> and pyridoxal phosphate. Through the adoption of the method, the preparation of L-2-aminobutyric acid can be simple, efficient and low in cost.
Owner:ABA CHEM CORP
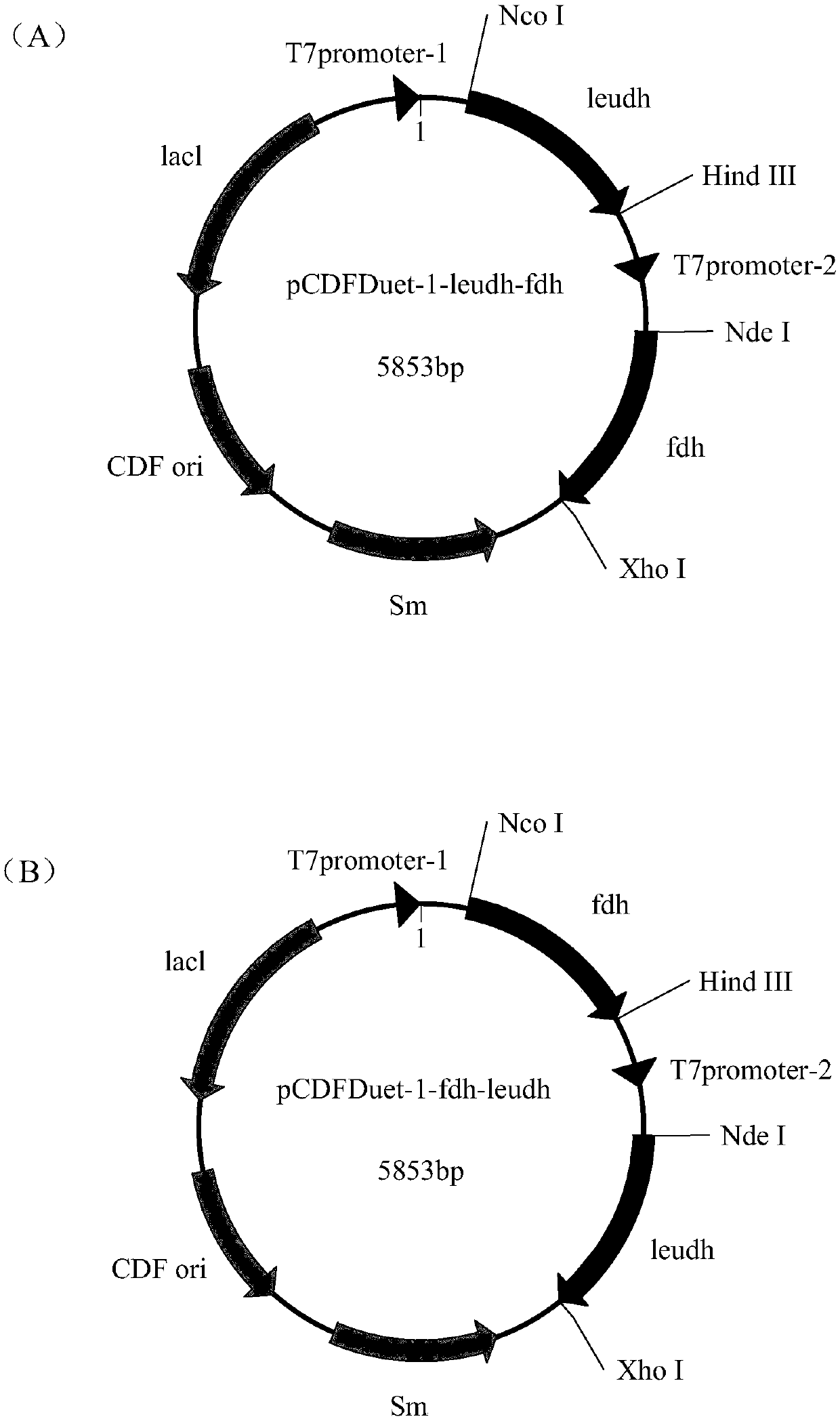
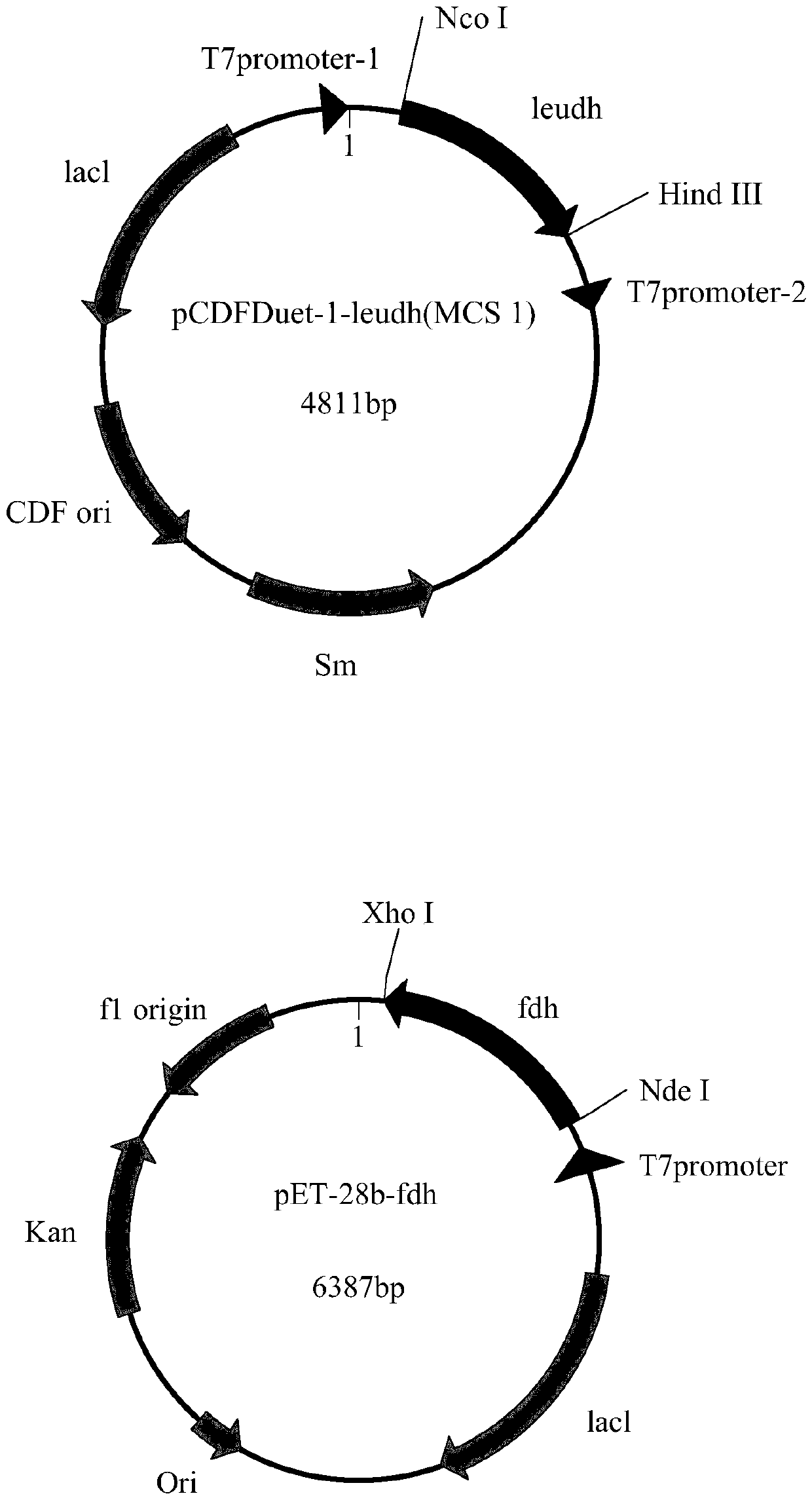
Recombination co-expression system for preparing L-2-aminobutyric acid and application thereof
ActiveCN109679978ASmall steric hindranceAchieve recyclingBacteriaMicroorganism based processesL-2-Aminobutyric AcidGene coexpression
The invention discloses a recombination co-expression system for preparing L-2-aminobutyric acid and application thereof and belongs to the technical field of genetic engineering. The recombination co-expression system is a single-plasmid double-gene expression carrier or a double-plasmid co-expression system, wherein the single-plasmid double-gene expression carrier takes pCDFDuet-1 or pETDuet-1as a foundation and a formate dehydrogenase gene and a leucine dehydrogenase gene are inserted into a polycloning site; the double-plasmid co-expression system takes any two of the pCDFDuet-1, the pETDuet-1 and pET-28b as foundations and comprises the leucine dehydrogenase gene and the formate dehydrogenase gene. The double-gene co-expression system is constructed and is transformed to obtain recombination genetic engineering bacteria; fermented and cultured recombination genetic engineering bacterium living cells are used as a catalyst and are applied to a catalytic reaction system for preparing the L-2-aminobutyric acid; the production efficiency is high, a coenzyme does not need to be added in a reaction process and the cost is saved.
Owner:杭州优泽生物科技有限公司
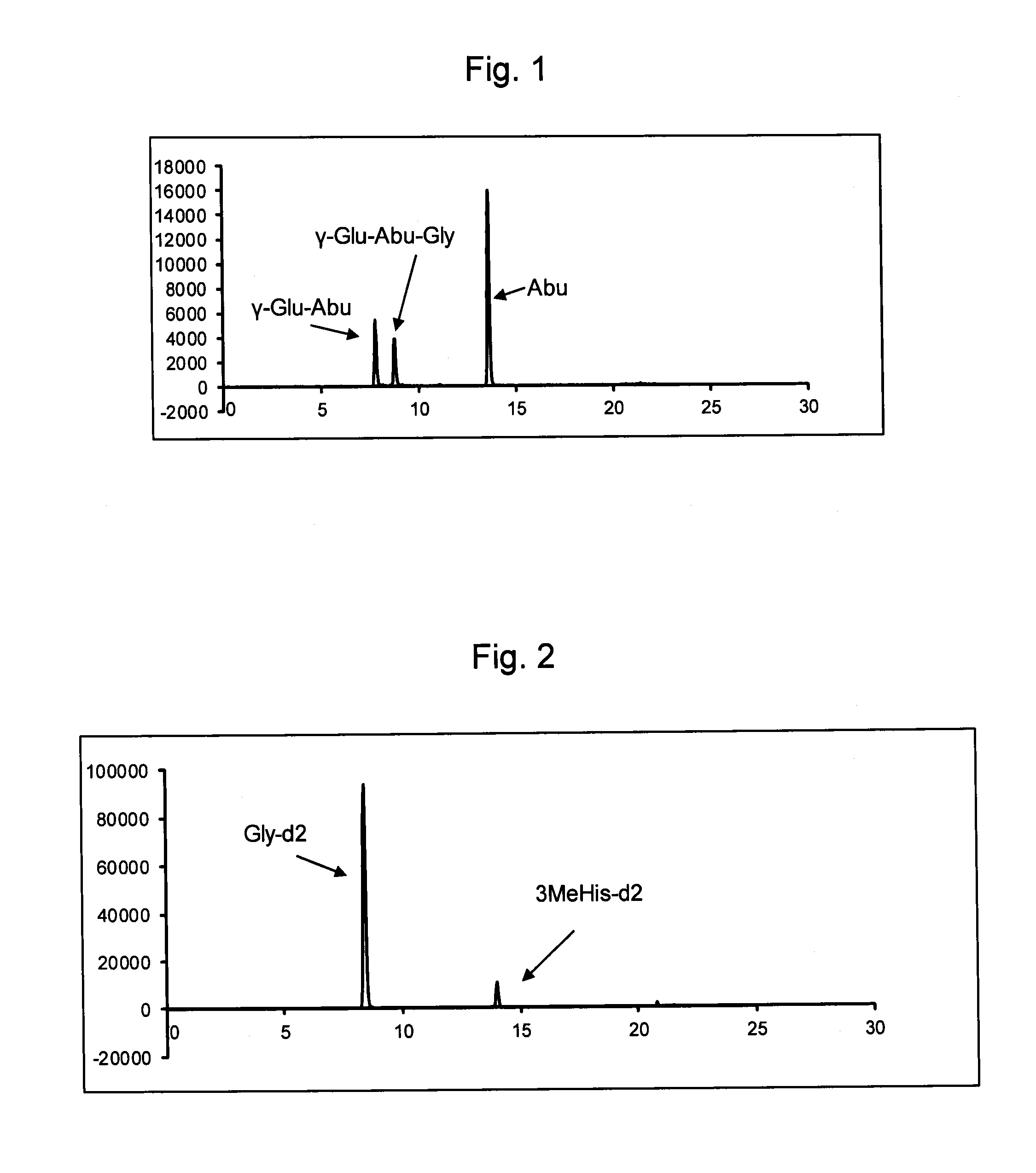
Yeast, yeast extract containing gamma-glu-abu, and a method for producing the same
InactiveUS20130280381A1High activityGood effectFungiSolid waste disposalBiotechnologyL-2-Aminobutyric Acid
A yeast extract containing 0.2% or more of γ-Glu-Abu based on dry weight of the yeast extract is produced by culturing a yeast, such as Saccharomyces cervisiae or Candida utilis, in a medium containing a compound selected from Abu (L-2-aminobutyric acid) and γ-Glu-Abu (L-γ-glutamyl-L-2-aminobutyric acid), and preparing a yeast extract from the obtained cells.
Owner:AJINOMOTO CO INC
Processing technique of L-2-amino butyramide hydrochloride
InactiveCN106187801AHigh response rateIncreased process safetyOrganic compound preparationCarboxylic acid amides preparationSal ammoniacHexamethylenetetramine
The invention relates to a processing technique of L-2-amino butyramide hydrochloride. The processing technique includes: using 2-chlorobutyric acid as a raw material and hexamethylenetetramine as a catalyst to prepare 2-aminobutyric acid; using L-tartaric acid to resolve 2-aminobutyric acid to obtain L-2-aminobutyric acid, acrylating L-2-aminobutyric acid to obtain L-2-aminobutyryl compound, and obtaining L-2-amino butyramide hydrochloride under the condition of ammonia water. The processing technique has the advantages that by the processing technique, reaction yield is increased, and byproducts are few. In addition, the processing technique is mild in reaction condition, easy in reaction control, low in cost, high in yield, high in product purity, low in equipment requirement and suitable for industrial production, and technique safety is improved greatly.
Owner:ABA CHEM NANTONG
Method for preparing D-2-aminobutyric acid or L-2-aminobutyric acid by chemical resolution method
InactiveCN101691334ALow costHigh yieldOrganic compound preparationAmino-carboxyl compound preparationL-2-Aminobutyric AcidIndustrial scale
The invention discloses a method for preparing D-2-aminobutyric acid or L-2-aminobutyric acid by a chemical resolution method, comprising the following steps: carrying out reaction between the DL-2-aminobutyric acid and the L-dihydroxysuccinic acid or D-dihydroxysuccinic acid in inorganic acid, cooling, precipitating the solid intermediate compound, recrystallizing the compound with water, carrying out ammonolysis reaction on the recrystallized intermediate compound and the ammonolysis agent, cooling, filtering, and washing to obtain D-2-aminobutyric acid or L-2-aminobutyric acid. The D-2-aminobutyric acid or L-2-aminobutyric acid prepared by the method in the invention has high purity and high yield, and the cost is substantially reduced, thus being applicable to industrial scale production.
Owner:NANJING INST OF TECH
Preparation method of Idelalisib and intermediate thereof
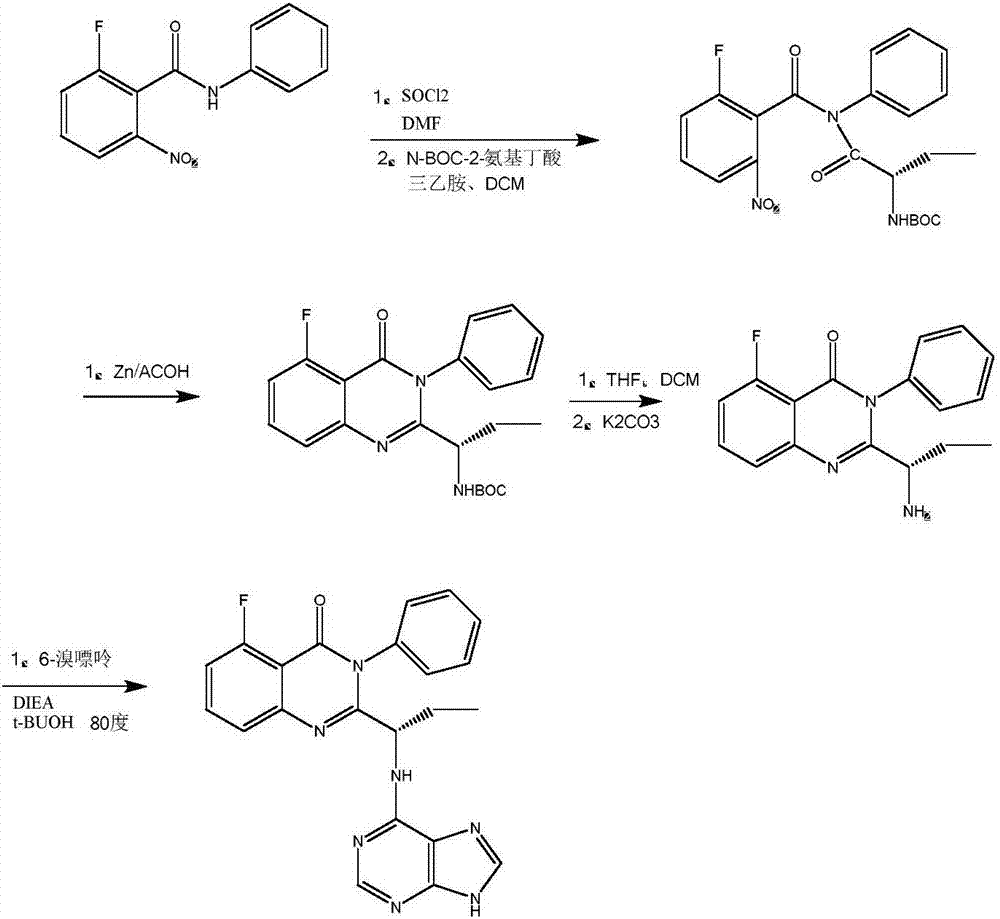
ActiveCN107573345AAvoid pollutionReduce solid wasteOrganic chemistryL-2-Aminobutyric AcidZinc Acetate Dihydrate
The invention discloses a preparation method of Idelalisib and an intermediate thereof. The method comprises the following steps: using 2-halogeno-6-fluorine-N-phenyl benzamide as a raw material, performing a reaction on the 2-halogeno-6-fluorine-N-phenyl benzamide and N-(9'-tetrahydropyrane-6'-purinyl)-L-2-aminobutyric acid, performing a reaction on a reaction product and ammonium carbonate or liquid ammonia to replace 2-halogeno with ammonia by cuprous oxide, copper and potassium carbonate or cesium carbonate, performing a ring-closure reaction under the existence of hexamethyl disilazane and iodine, and performing a reaction on a reaction product and hydrochloric acid solution to remove pyran finally, so as to generate an Idelalisib finished product. According to the process, the novelmethod is provided for synthesizing the Idelalisib; in the process, the easily available 2-halogeno-6-fluorobenzoic acid is used as a starting material, and zinc acetate pollution and the like can beprevented from being generated through reduction of nitro and the like with zinc powder in a synthesis process.
Owner:ZHEJIANG LEPU PHARMA CO LTD
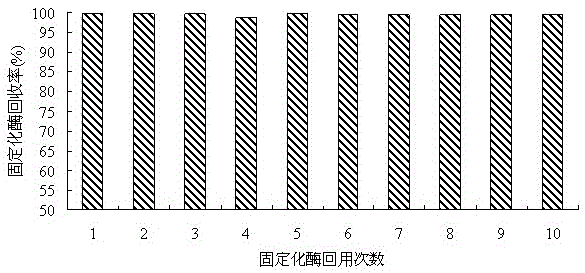
A method for producing l-2-aminobutyric acid by a double-immobilized multi-enzyme system
InactiveCN102517351BImprove recycling ratesImprove regeneration efficiencyChemical industryFermentationL-2-Aminobutyric AcidAlcohol oxidase
The invention discloses a method for producing L-2-aminobutyric acid by double immobilized multi-enzyme systems. The method provided by the invention comprises the following steps that 1, threonine dehydrogenase and leucine dehydrogenase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized multi-enzyme system is obtained; and 2, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized coenzyme regeneration system is obtained. The method for producing L-2-aminobutyric acid by the double immobilized multi-enzyme systems utilizes dissolution reversibility of co-immobilized enzymes, realizes effective separation of the co-immobilized enzymes and products, improves accessibility between the co-immobilized enzymes and reactants, improves recovery and utilization rates of threonine dehydrogenase, leucine dehydrogenase, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase, improves coenzyme regeneration efficiency, reduces follow-up separation purification processes, simplifies a process flow and reduces a production cost.
Owner:李鑫
The preparation method of high quality s-2-chlorobutanol
ActiveCN104478663BReduce processing costsSuitable for industrializationOrganic compound preparationMicroorganism based processesL-2-Aminobutyric AcidSodium borohydride
The invention relates to a preparation method of high-quality S-2-chlorobutanol. The method comprises the following steps: with L-2-aminobutyric acid prepared by a biological reduction transformation method as a raw material, preparing the S-2-chlorobutanol by adopting a diazotization chlorination method; further esterifying, and reducing with sodium borohydride / titanium tetrachloride, so as to obtain the product. According to the prepared high rotary S-2-chlorobutanol, the EE value is over 99%; and the S-2-chlorobutanol is good in repeatability and stable in process.
Owner:上海弗凯生物科技有限公司
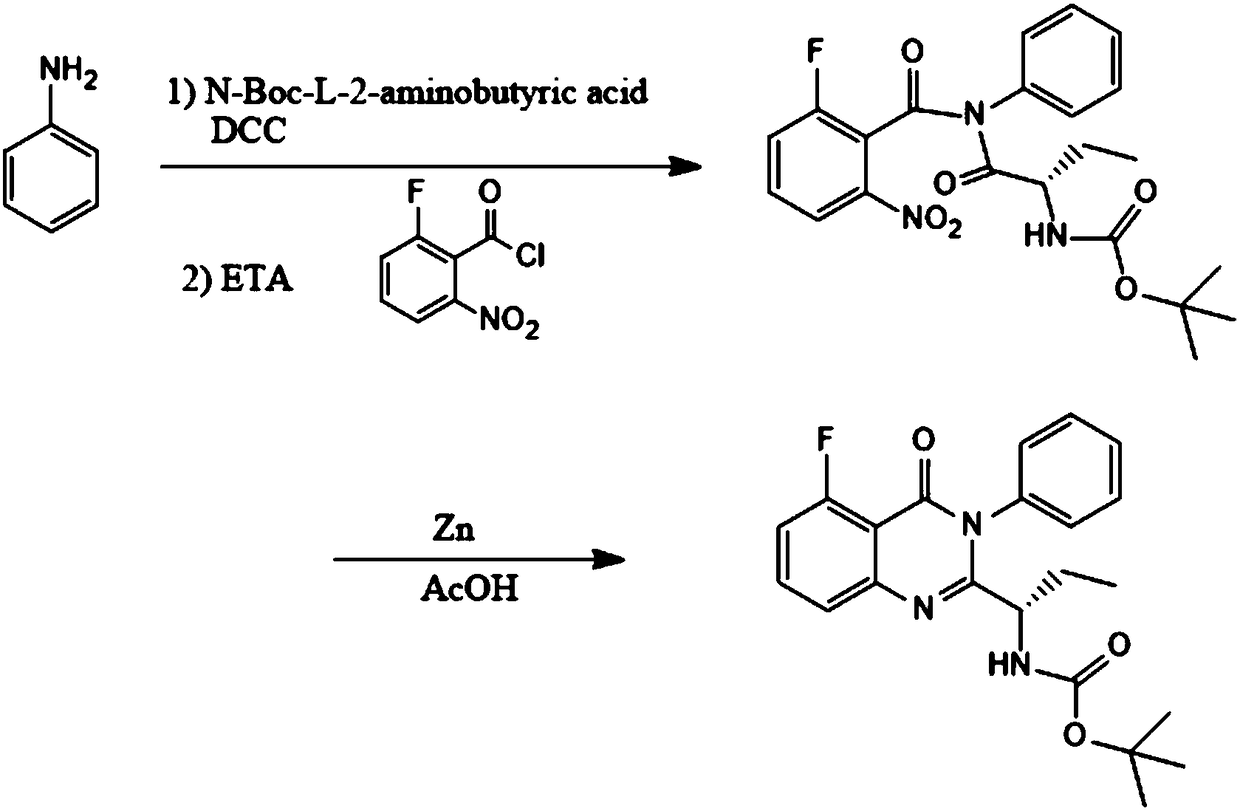
Preparation method of idelalisib intermediate
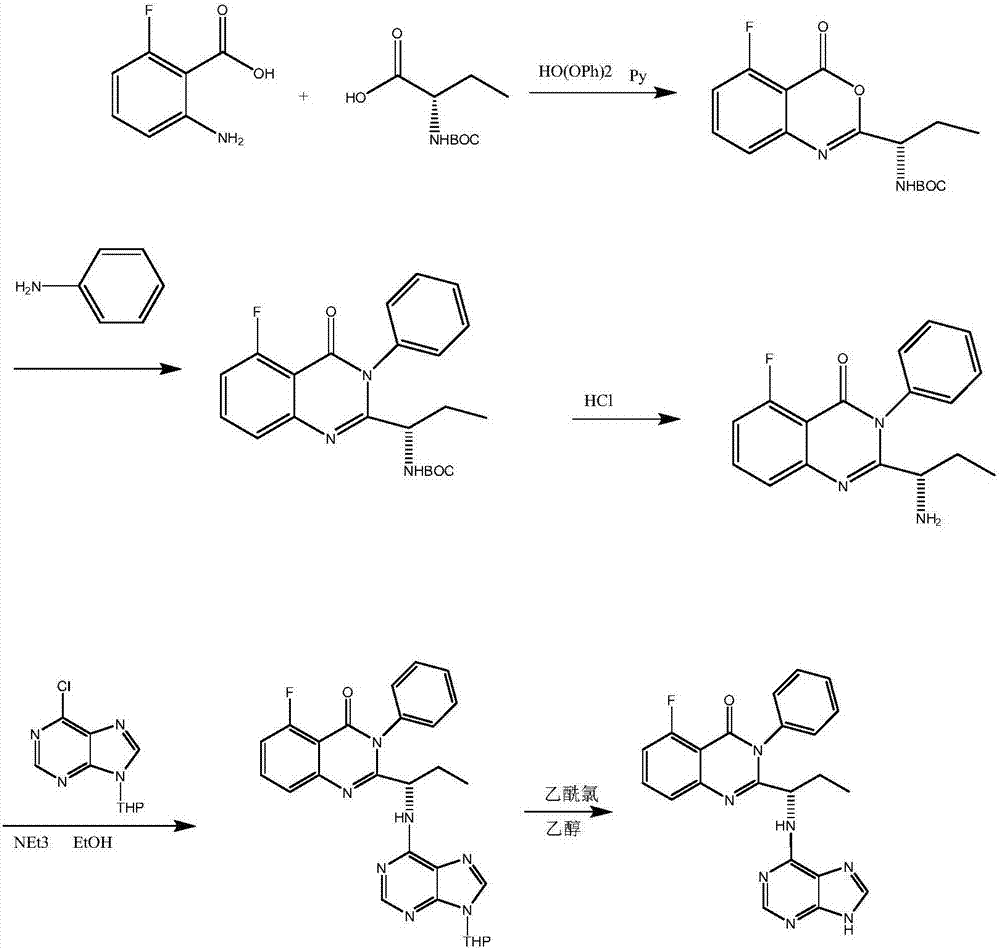
InactiveCN108409674ANo need to separateNovel reaction routeOrganic chemistryL-2-Aminobutyric AcidChloride
The invention discloses a preparation method of an idelalisib intermediate, the idelalisib intermediate (S)-N-(2-Boc-aminobutyryl)-2-fluoro-6-N-phenyl benzamide is prepared from aniline, N-Boc-L-2-aminobutyric acid, DCC, triethylamine and 2-fluoro-5-nitrobenzoyl chloride as raw materials by a three-step reaction of condensation reaction, amidation reaction and ring closure reaction for one-pot synthesis, and the condensation reaction and the amidation reaction do not require separation. The preparation method is a preparation method with the advantages of high yield, low cost, less waste, easyoperation and suitable industrialization.
Owner:南京法恩化学有限公司
Yeast, yeast extract containing gamma-glu-abu, and a method for producing the same
A yeast extract containing 0.2% or more of γ-Glu-Abu based on dry weight of the yeast extract is produced by culturing a yeast, such as Saccharomyces cervisiae or Candida utilis, in a medium containing a compound selected from Abu (L-2-aminobutyric acid) and γ-Glu-Abu (L-γ-glutamyl-L-2-aminobutyric acid), and preparing a yeast extract from the obtained cells.
Owner:AJINOMOTO CO INC
Synthetic method and preparation intermediate of Adelaris
ActiveCN106146502BHigh purityEasy to operateOrganic chemistryBulk chemical productionHydrogenSynthesis methods
The invention provides an Idelalisib synthesis method. The method comprises steps as follows: a compound 3 is processed with hydrogen in the presence of a hydrogenation catalyst and a solvent, nitro is enabled to be reduced, and an amino compound II is obtained; the compound II and N-Boc protected L-2-aminobutyric acid are subjected to condensation in the presence of alkali and a carboxylic acid activator or in the presence of alkali and a condensation agent, and an intermediate I is obtained; the intermediate I is subjected to a ring closing reaction in an appropriate solvent under the action of a hexamethyldisilazane / lewis acid catalytic system, and a compound 7 is obtained; the compound 7 reacts with 6-chloro-9-(2-tetrahydropyran) purine in an appropriate solvent in the presence of an acid-binding agent, and a compound III is obtained; the compound III is subjected to protecting group removal with an appropriate reagent, and Idelalisib is obtained. A reaction path is shown in the specification. The preparation method has the advantages that the reaction conditions of each step are mild, the post-processing is simple and easy to implement and the total yield is high, and the preparation method is environment-friendly and is very suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Vector, engineering strain and method for producing l-2-aminobutyric acid
ActiveCN103215291BIncrease contentFermentation properties are stableFungiBacteriaBiotechnologyL-2-Aminobutyric Acid
The invention relates to a vector, an engineering strain and a method for producing L(+)-2-aminobutyric acid. The vector is a recombinant vector containing a threonine dehydratase coding gene, an L-amino acid dehydrogenase gene and appropriate vector segments. The engineering strain is obtained by transforming host bacteria by the recombinant vector. The L(+)-2-aminobutyric acid is prepared by fermentation culture of the engineering strain. The engineering strain is fermentation-cultured by glucose as a raw material to produce L(+)-2-aminobutyric acid. The method has the advantages of low cost, high product concentration, no by-product, easy product purification, and good industrial application feasibility.
Owner:SHANGHAI PUYI CHEM CO LTD
Method for preparing l-2-aminobutyric acid through whole cell biotransformation
ActiveCN104531793BAvoid purification processEasy to solveMicroorganism based processesFermentationEscherichia coliFormate
Owner:上海弗凯生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com