Vector, engineering strain and method for producing L(+)-2-aminobutyric acid
An amino acid, recombinant vector technology, applied in genetic engineering, biochemical equipment and methods, and the use of vectors to introduce foreign genetic material, etc., can solve the problems of low yield, high production cost and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] For the preparation and transformation methods of competent cells, refer to Scheme 25 in Chapter 1 of "Molecular Cloning Experiment Guide (Third Edition)" (Science Press, 2002).
[0089] For the measurement method of glucose content, refer to "Zhou Yaxuan et al., Enzymatic Determination of Glucose Content in Wine, Chinese Journal of Health Inspection, 2005, 12(5): 194-221".
[0090] Advantages of the invention
[0091] 1. Fermentation and cultivation The genetically engineered bacterial strain constructed by the present invention is used to produce L-2-aminobutyric acid, and the content of L-2-aminobutyric acid in the obtained fermentation liquid is high, and the fermentation property is stable;
[0092] 2. The cost of the method of the present invention is greatly reduced, and the process is stable;
[0093] 3. The conversion rate of the method of the present invention is high, there is no influence of by-products, the product is easy to purify, and is very suitable f...
Embodiment 1
[0096] Construction of L-threonine high-yielding strain and fermentation of L-threonine
[0097] 1. Construction of high-yield L-threonine metabolic engineering bacteria
[0098] 1.1 Genetic modification of gene cluster thrABC in Escherichia coli W3110
[0099] With reference to the method disclosed in the literature of Lee KH et al. (Lee KH et al. Systems metabolic engineering of Escherichia coli for L-threonine production. Molecular systems biology. 2007, 3: 149), genes ThrABC, rhtC, rhtA, rhtB; thrA encodes aspartokinase I, rhtA, rhtB encode threonine and homoserine transporters, transport threonine out of the cell, rhtC encodes threonine transporter, transport threonine out of the cell Inside. The 1034th base C of gene thrA was site-directedly mutated into base T (Ser345→Phe) by overlapping PCR to obtain ThrA*BC'.
[0100]ThrA*BC was connected to the plasmid pKK223-3 with a tac promoter, and then the rop gene and rhtC, rhtA, rhtB genes of the size of 1.6kb on pBR322 wer...
Embodiment 2
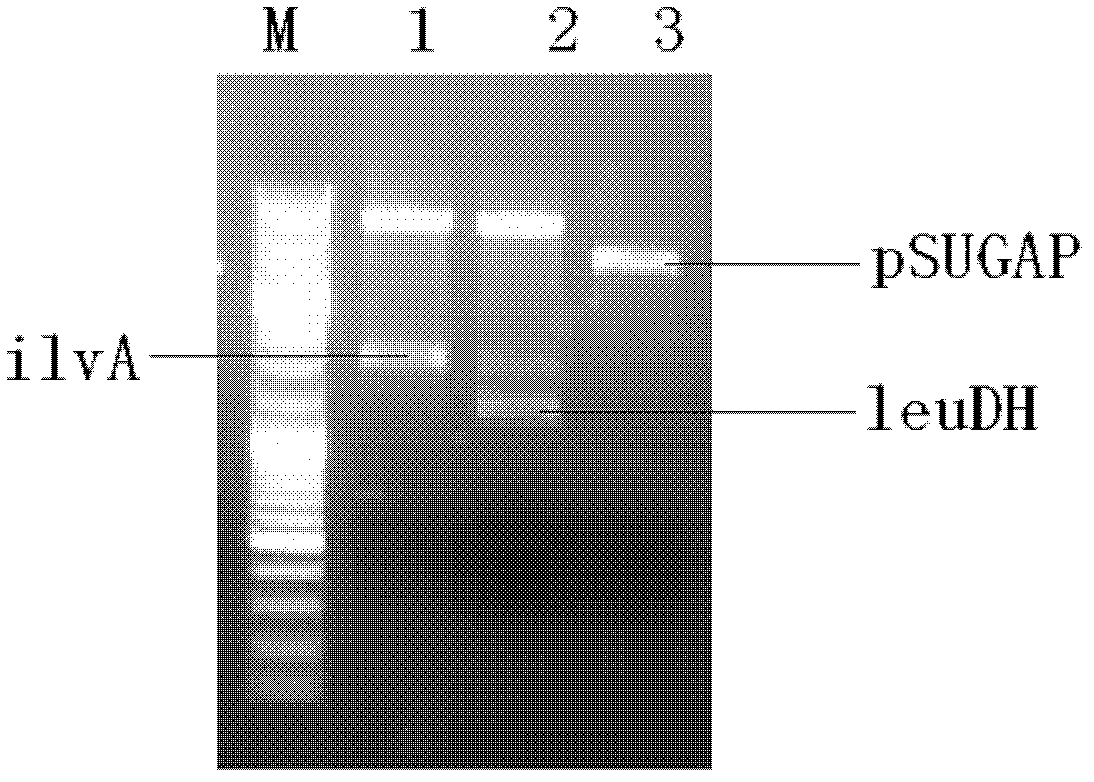
[0120] Construction of Genetic Engineering Strains THR / pSUGAP-leuDH-ilvA and THR / pSUGAP-ilvA-leuDH
[0121] Threonine deaminase gene ilvA is from Escherichia coli W3110 (NCBI accession number: AP009048); leucine dehydrogenase gene leuDH is from Bacillus cereus (NCBI accession number: AE016877).
[0122] 1. Construction of pSUGAP plasmid
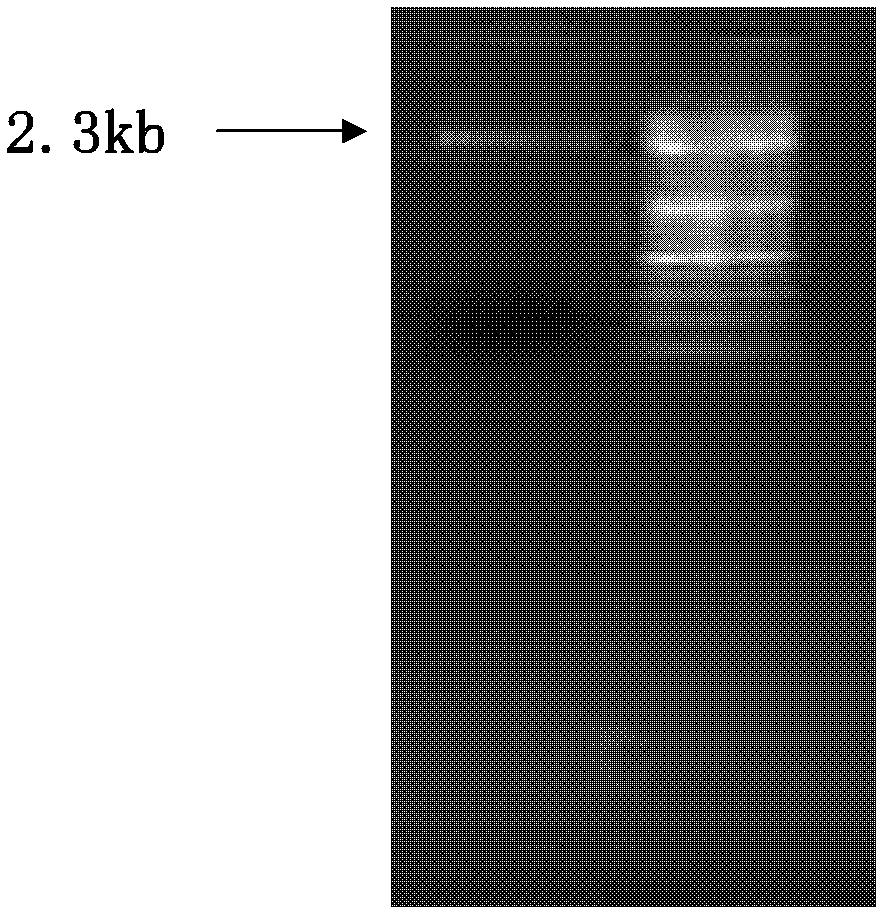
[0123] Plasmid pSU2718 was extracted with a plasmid extraction kit (Martinez et al., 1988), and the plasmid was digested with Sac I / SmaI double enzymes at 37°C for 2 hours. The enzyme digestion system was: 76 μl of plasmid, 20 μl of 10×Tango buffer, Sac I 2μl, Sma I 2μl, electrophoresis to recover the 2.3Kb pSU2718 fragment, such as figure 1 .
[0124] A 0.2 Kb fragment containing the GAP promoter was isolated from Escherichia coli (NCBI accession number: CP001509) by PCR using the ecgapup and ecgapdn primers in Table 3.
[0125] PCR conditions: ddH 2 O 33μl, 10×KOD buffer 5μl, template 2μl, 25mM MgCl 2 3 μl, 1 μl each primer, 4 μl dNTP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com