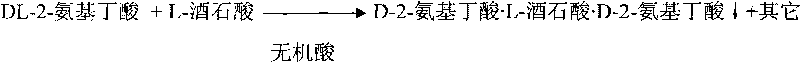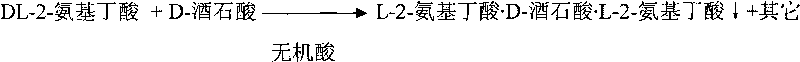Method for preparing D-2-aminobutyric acid or L-2-aminobutyric acid by chemical resolution method
A technology of DL-2-, aminobutyric acid, applied in the field of preparing chiral organic compounds, can solve the problems of large amount of resolving agent, low yield, low market competitiveness, etc., and achieve the effects of cost reduction and yield improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
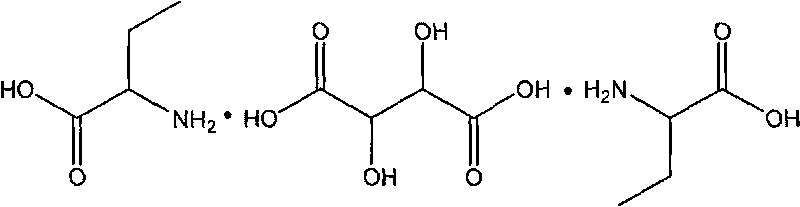
[0027] Embodiment 1 Get 41.25g (0.4 mol) DL-2-aminobutyric acid, and 15.09g (0.1 mol) L-tartaric acid, drop into 500g, 0.1mol / L dilute hydrochloric acid successively, react at 70 ℃ for 3 hours. After cooling to room temperature, a solid mixture was precipitated, and the compound was recrystallized from water, and filtered to obtain 32.15 g of D-2-aminobutyric acid·L-tartaric acid·D-2-aminobutyric acid crystals, with a yield of 90.2%.
Embodiment 2
[0028] Embodiment 2 Get 103.12g (1 mole) DL-2-aminobutyric acid, and 30.18g (0.2 mole) L-tartaric acid, drop into 1000g successively, the dilute hydrochloric acid of 0.1mol / L reacts at 80 ℃ for 2 hours. Other reaction steps and conditions were the same as in Example 1, and 62.87 g of D-2-aminobutyric acid·L-tartaric acid·D-2-aminobutyric acid crystals were obtained with a yield of 88.2%.
Embodiment 3
[0029] Embodiment 3 Get 103.12g (1 mole) DL-2-aminobutyric acid, and 30.18g (0.2 mole) L-tartaric acid, drop into 1000g0.1mol / L dilute sulfuric acid successively and react at 100°C for 2 hours. Other reaction steps and conditions were the same as in Example 1, and 61.32 g of D-2-aminobutyric acid·L-tartaric acid·D-2-aminobutyric acid crystals were obtained with a yield of 86.0%.
[0030] Preparation of L-2-aminobutyric acid·D-tartaric acid·L-2-aminobutyric acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com