Patents
Literature
36 results about "Aminopherase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method and product of germinated wheat rich in gamma-aminobutyric acid
InactiveCN103393013AControl Germination EnvironmentHigh nutritional valueFood preparationNutritive valuesTemperature control
The invention discloses a preparation method and product of germinated wheat rich in gamma-aminobutyric acid, and the method is used for solving the problems of bad existing effect and unstable yield since GABA is enriched by utilizing tea and rice products according to an existing method for preparing gamma-aminobutyric acid based on a biological concentration method. The method comprises the following steps of: raw material selection, pretreatment, imbibition, temperature control training and germination termination. Wheat seeds are dramatically taken as raw materials, the improvement of GAD enzyme activity in wheat seeds is effectively promoted by controlling germination conditions, a cultivated substrate and the pH value, and meanwhile the GAD activity is ensured by controlling the pH value, the activity of GABA aminopherase is inhibited, and the content of gamma-aminobutyric acid in germinated wheat is greatly improved, so that the nutritive value of wheat is greatly improved. Experimental results show that the content of GABA in the prepared germinated wheat is 31.08 mg per 100 g at most, and achieves domestic and international leading level of similar methods.
Owner:SICHUAN AGRI UNIV
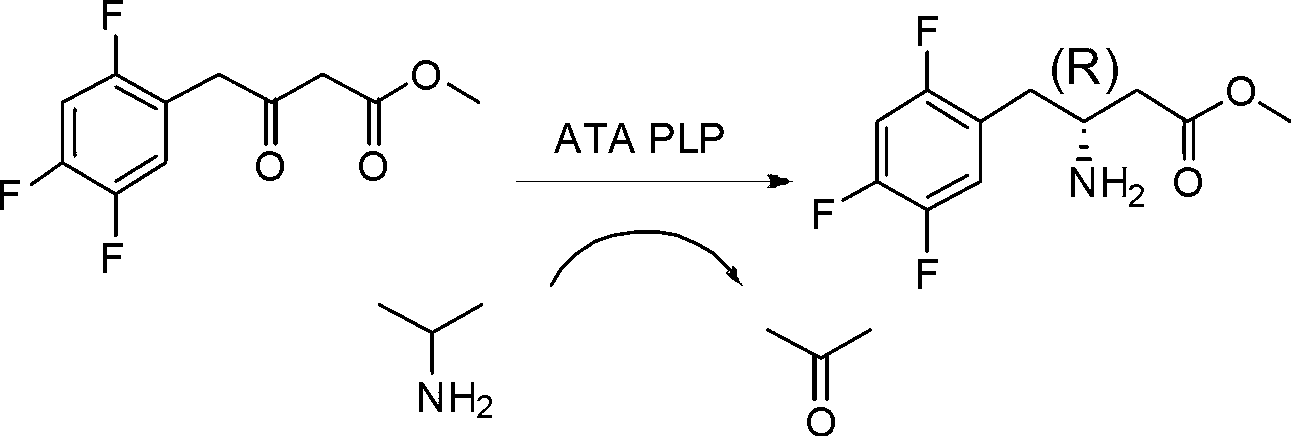
Biological preparation method of 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate
InactiveCN103014081AReduce energy consumptionEasy to operateTransferasesFermentationAminopheraseSolvent
The invention relates to a biological preparation method of 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate. According to the biological preparation method, 3-carbonyl-4-(2,4,5-trifluorophenyl)methyl butyrate is used as a substrate; under the condition that a biocatalyst, a cofactor, an amino donor and a cosolvent exist, the substrate performs the reaction to generate a target product 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate; the biocatalyst is recombination aminopherase; and the reaction is carried out in water-phase buffer solution with the pH of 6.0 to 9.0. The biological preparation method realizes that 3-carbonyl-4-(2,4,5-trifluorophenyl)methyl butyrate is converted into 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate by adopting the recombination aminopherase for the first time; compared with a conventional chemical method, the biological preparation method has low energy consumption, is simple and convenient to operate, generates a small number of by-products; and moreover, the product has high optical activity.
Owner:ENZYMEWORKS
Aminopherase for producing L-2-aminobutyric acid
ActiveCN105441403AOvercoming conversion rateOvercome concentrationBacteriaTransferasesAminopheraseL-2-Aminobutyric Acid
The invention establishes aminopherase through genetic engineering. Compared with ochrobactrum anthropi based wild w-aminopherase coming from ochrobactrum anthropi, the enzyme activity of aminopherase is remarkably improved, and aminopherase can be used for industrially producing L-2-aminobutyric acid.
Owner:湖州颐盛生物科技有限公司
Chinese traditional medicine composition for treating ulcerative colitis and its preparation method
ActiveCN101332284AImprove liver functionLower transaminaseAnthropod material medical ingredientsDigestive systemClinical efficacySide effect
The invention discloses a traditional Chinese medicine composition used for curing chronic hepatitis and a preparation method thereof. The traditional Chinese medicine composition is made of raw material medicines with the weight proportion by parts: 40 to 160 parts of Chinese thorowax root, 40 to 160 parts of debark peony root, 40 to 160 parts of angelica, 40 to 160 parts of India madder root, 40 to 160 parts of largehead atractylodes rhizome, 40 to 160 parts of Tuckahoe, 40 to 160 parts of turtle carapace, 40 to 160 parts of massa medicata fermentata hunanensis, 60 to 240 parts of Codonopsis pilosula nannfeldt, 60 to 240 parts of Lalang Grass Rhizome, 24 to 96 parts of immature orange fruit, 20 to 80 parts of green tangerine peel, 12 to 48 parts of villous amomum fruit, 12 to 48 parts of earthworm and 12 to 48 parts of liguorice. The traditional Chinese medicine composition of the invention has the pharmacological efficacies of antagonizing hepatic damage, protecting liver, regulating immune function, reducing aminopherase, improving liver function and on the like, and the clinical efficacy observation experiments prove that the traditional Chinese medicine composition has the exact efficacies to the chronic hepatitis, in particular to chronic active hepatitis, chronic persisting hepatitis and early cirrhosis; and the clinical application is of safety and no toxicity and side effects.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Artificial pigskin and its production tech. and equipment thereof
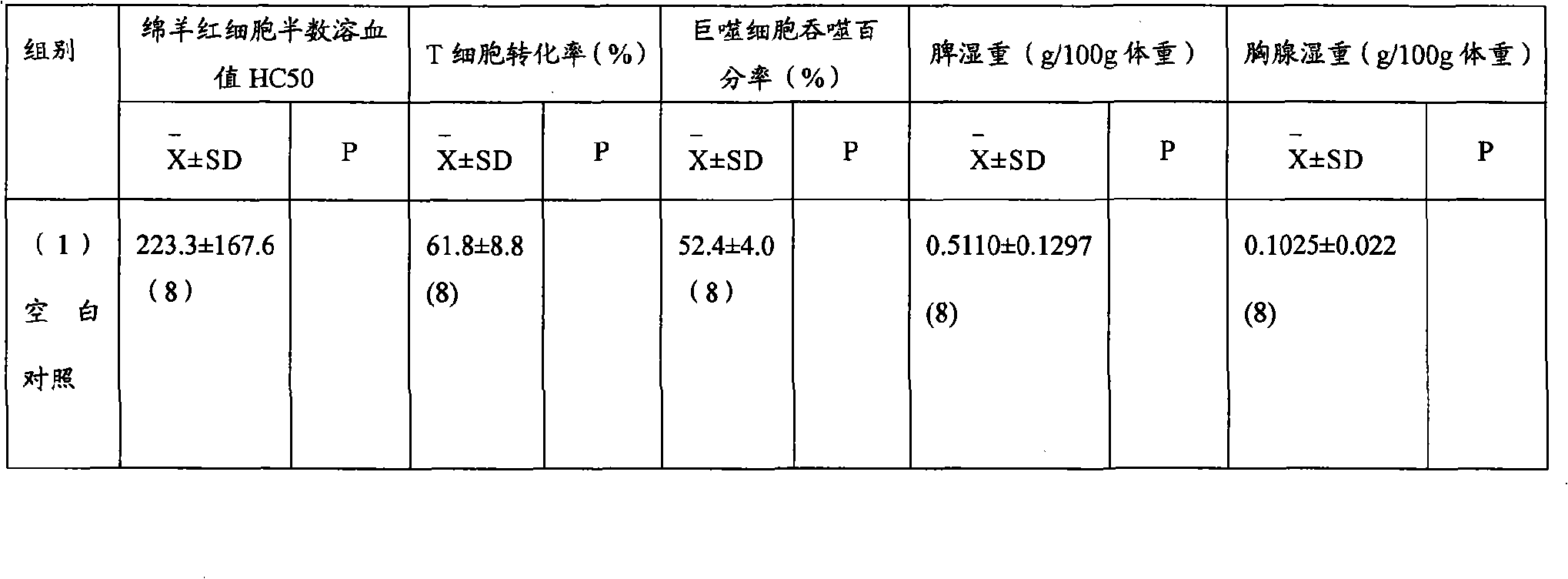
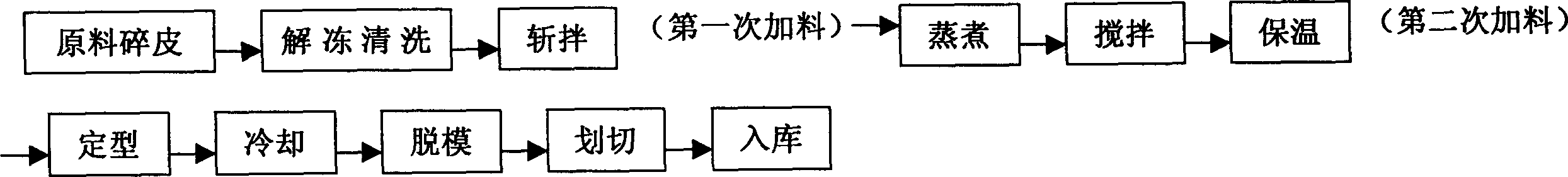
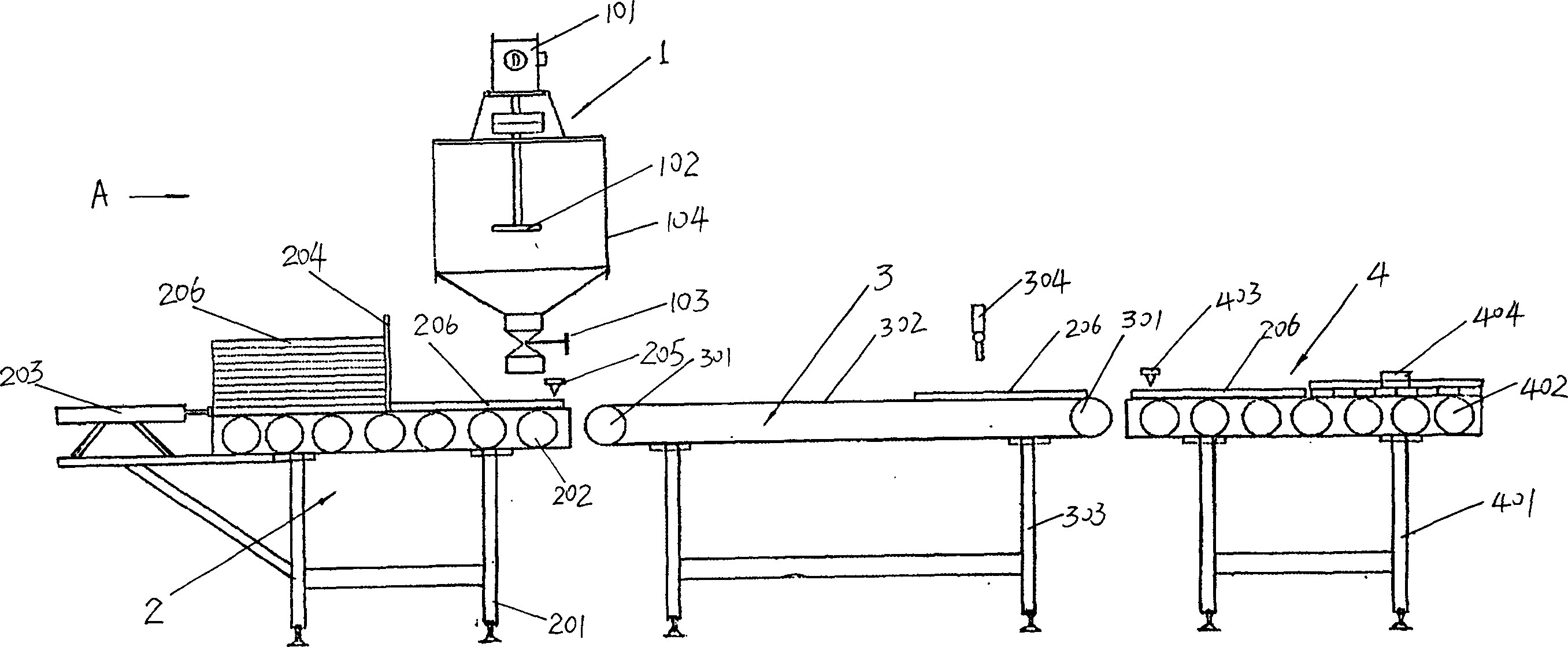
The invention relates to a manufacture method for artificial pigskin and the technology and the device. It is made up from pigskin pieces, ice water, compounding phosphate, salt, sodium acetylide, calcium lactate, glutamyl aminopherase, coloring matter, and spice. It is low cost, easy to operate and has high reality.
Owner:南京卓润环保科技有限公司
Aminopherase mutant and application thereof in production of L-glufosinate-ammonium
The invention discloses an aminopherase mutant and an application thereof in the production of L-glufosinate-ammonium. The application of the aminopherase mutant in the production of L-glufosinate-ammonium is as follows: a reaction system is formed by using a wet thallus obtained through the fermentation culture of recombinant escherichia coli containing a aminopherase mutant coding gene as a biocatalyst, 2-carbonyl-4 (methyl hydroxyl phosphoryl)-butyrate as a substrate, pyridoxal phosphate as a coenzyme, an amino donor as a cosubstrate, and a buffer solution with the pH value of 6-9 as a reaction medium, and is subjected to biocatalytic reaction at the temperature of 40 to 50 DEG C and the stirring speed of 150 to 250r / min to obtain the L-glufosinate-ammonium. According to the method, thetotal yield is 98%, and the e. e. value of the product reaches 99%.
Owner:ZHEJIANG UNIV OF TECH
Medicament for treating hepatitis
InactiveCN101244215AIndicators are normalLiver function returns to normalDigestive systemAntiviralsAntigenAdditive ingredient
The invention relates to a Chinese herbal medicine, in particular to a medicine for treating hepatitis, which solves the problems of bad hepatitis treating efficacy, low cure rate and others in the prior art. The medicine for treating hepatitis comprises the following active pharmaceutical ingredients: bupleurum, root of herbaceous peony, capillary artemisia, dyers woad root, gardenia, Chinese rhubarb, umbellate pore fungus, astragalus root, pilose asiabell root, bighead atractylodes rhizome, tangerine peel, medicated leaven, germinated barley, red sange root, phellodendron bark, root of baikal skullcap, rhizoma corydalis, madder root and notoginseng. As shown by clinic applications, the medicine has the advantages that the serological biochemical criterions, for example aminopherase, bilirubin and other factors, return to normal; clinic symptoms are improved or eliminated obviously, the removal of virus indicates that the surface antigen in serum turns to negative; the HBV-DNA inspection for liver texture shows negative, the liver functions get right substantially, the effective rate is above 90% and all indicators of human body turn to normal level.
Owner:郭上未
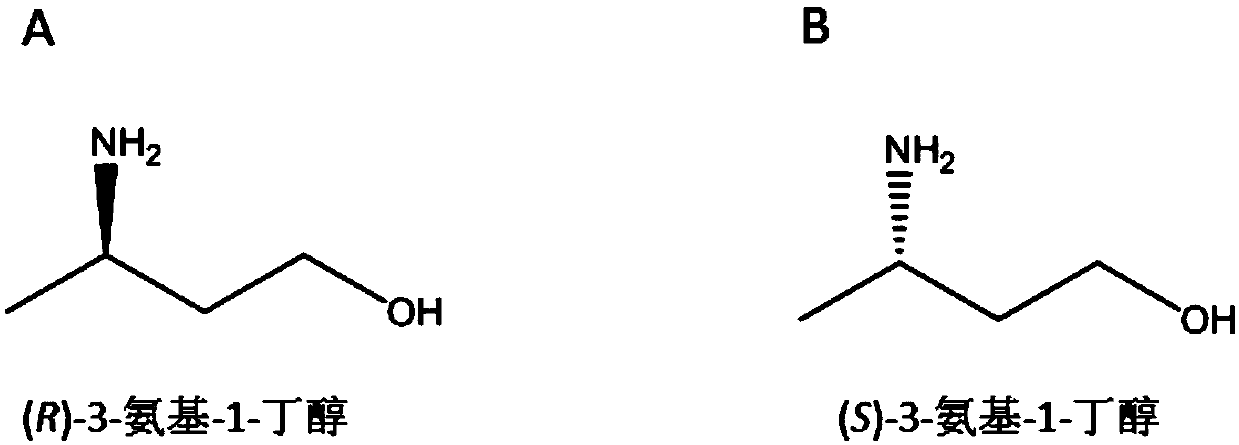
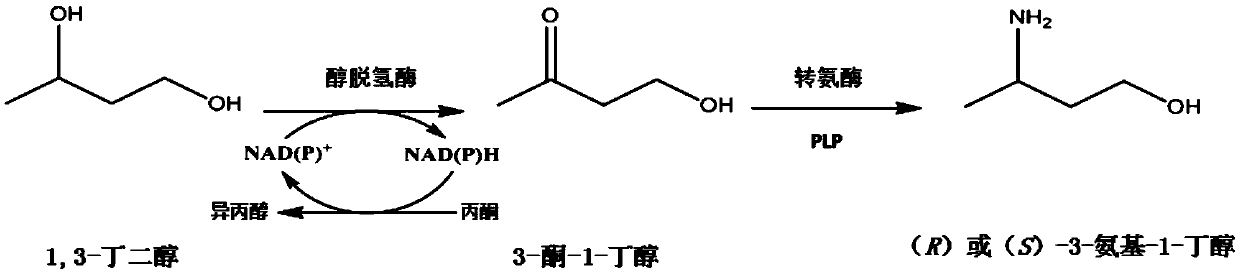
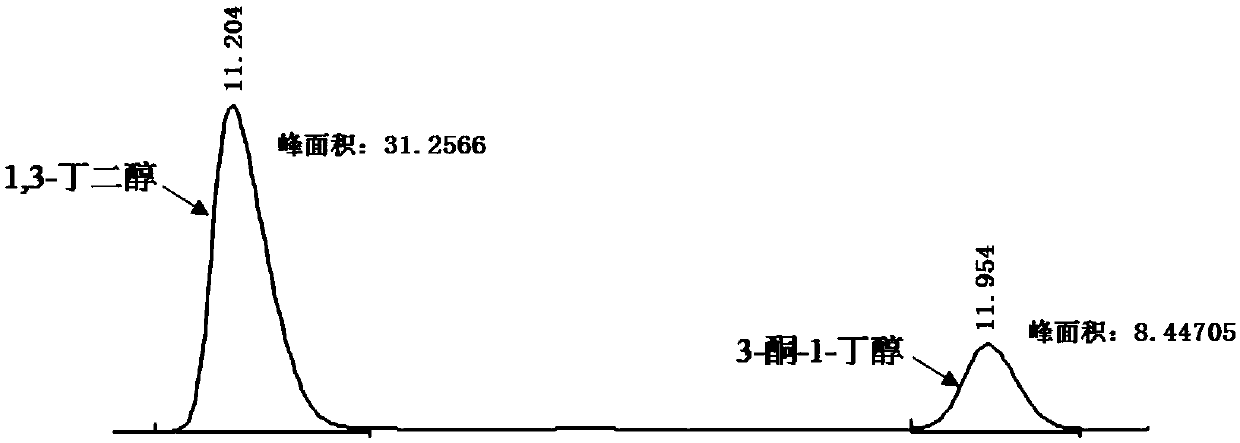
Synthetic method of chiral 3-amino-1-butanol
The invention discloses a synthetic method of chiral 3-amino-1-butanol. The method comprises the following steps of with 1, 3-butanediol as a substrate, performing a catalytic reaction through an enzyme A and a coenzyme thereof to generate 3-ketone-1-butanol, and with the 3-ketone-1-butanol as a substrate, performing a catalytic reaction through an enzyme B and a coenzyme thereof to generate the chiral 3-amino-1-butanol, wherein the enzyme A is selected from alcohol dehydrogenase or an alcohol dehydrogenase mutant, and the enzyme B is selected from aminopherase or an aminopherase mutant. According to the synthetic method disclosed by the invention, a bran-new green biosynthetic route is provided, the cheap 1, 3-butanediol is used as a raw material, and through multi-enzyme co-expression orcascade or multiple-step catalysis, the chiral 3-amino-1-butanol namely (R)-3-amino-1-butanol and (S)-3-amino-1-butanol can be synthetized.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
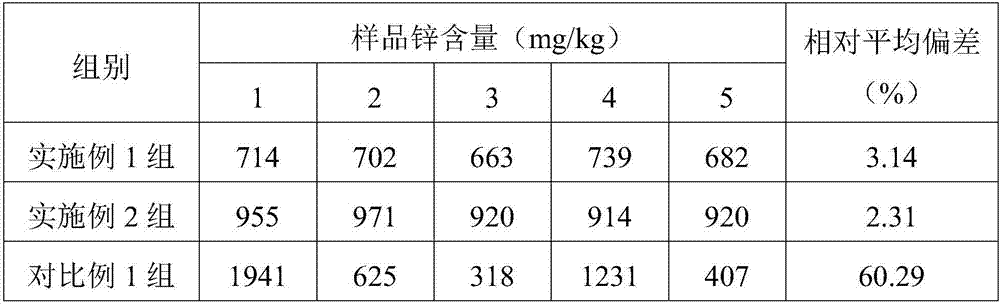
Beef balls and preparation method thereof
ActiveCN106923207AReasonable supplementAdded evenness improvementFood ingredientsSodium bicarbonateMonosodium glutamate
The invention relates to the fields of foods and food processing, in particular to beef balls and a preparation method thereof. According to the beef balls disclosed by the invention, beef, butter, isolated protein, starch, zinc gluconate, docosahexenoic acid, a yeast extract, onion powder, olive oil, table salt, white granulated sugar, a dark soy sauce, cooking wine, pricklyash peel powder, chili powder, pepper powder, monosodium glutamate, sodium bicarbonate, phosphate, glutamine aminopherase and D-erythorbic are used as raw materials; and the beef balls are prepared through the technologies of treating the raw materials, performing injection, performing superfine crushing, performing mixing, performing pickling, performing shaping, performing cooking, performing quick-freezing and the like. Through the adoption of the technology disclosed by the invention, zinc and the docosahexenoic acid are uniformly added to the beef balls, the total intaking quantity of human bodies to the docosahexenoic acid can be accurately calculated and controlled through the eating quantity of the beef balls, and finally the purpose of reasonably complementing the zinc and the docosahexenoic acid is realized.
Owner:山东如康清真食品有限公司
Alpha-aminopherase and mutant and application of alpha-aminopherase and mutant to asymmetric synthesis of L- glufosinate-ammonium
ActiveCN109609478AHigh catalytic activityImprove conversion rateTransferasesGenetic engineeringMulti siteReaction temperature
The invention discloses an application of novel aminopherase and a high-vitality mutant thereof to preparation of L-glufosinate-ammonium. The amino acid sequence of the aminopherase is as shown in SEQID:5, and the mutant of the aminopherase is prepared through performing single-site mutation or multi-site mutation on one or a plurality of 124th, 144th, 237th, 250th and 328th in the amino acid sequence as shown in the SEQ ID:5. The high-efficiency expression of vitality mutant genes of high-conversion rate aminopherase can be realized, and the highest enzyme activity is 840U / mg. The highest optimum reaction temperature of the aminopherase mutant can reach 67 DEG C, and during asymmetric synthesis of the L-glufosinate-ammonium by catalyzing 800mM of glufosinate-ammonium precursor ketone atthe temperature through inorganic amine and n-butylamine, the conversion rate is as high as 100%. The AcTA mutant solves the technical difficult problems that few enzyme sources exist, the enzyme activity is low, substrate tolerance is bad and amino donors are expensive in a current L-glufosinate-ammonium preparation technology from the aminopherase, and has better application prospects.
Owner:ZHEJIANG UNIV OF TECH
Omega-aminopherase double mutant and application thereof
ActiveCN110144335AIncreased half-inactivation temperatureRaise the thermal unfolding temperatureTransferasesFermentationAminopheraseHalf-life
The invention discloses an omega-aminopherase double mutant and an application thereof. The amino acid sequence of the omega-aminopherase double mutant is as shown in SEQID NO.1. The omega-aminopherase double mutant disclosed by the invention is obtained in a manner than the 115th phenylalanine of wild type aspergillus terreus (Aspergillus terreus) omega-aminopherase is mutated into leucine and the 118th leucine is mutated into threonine. The semi-inactivated temperature of the omega-aminopherase double mutant is 8.8 DEG C higher than that of wild type omega-aminopherase, the thermolysis collapse temperature of the omega-aminopherase double mutant is increased by 7.7 DEG C than that of the wild type omega-aminopherase, and the half-life period of the omega-aminopherase double mutant at 40DEG C is prolonged by 59.0min than that of the wild type omega-aminopherase and is 9.55 times of that of the wild type omega-aminopherase.
Owner:山东聚鸿生物科技研究院有限公司
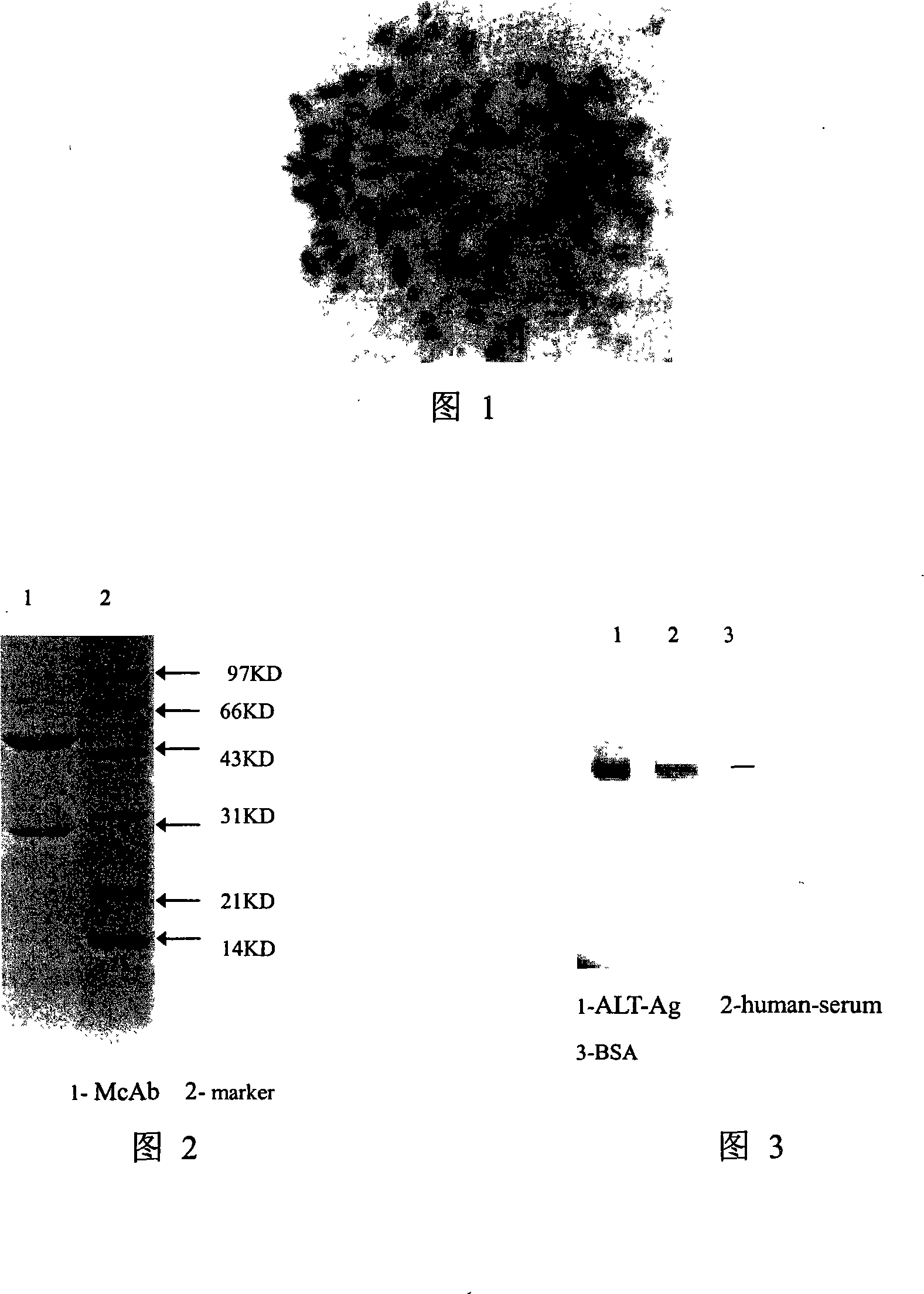
Alanine aminopherase monoclonal antibody and use thereof
InactiveCN101250229AHigh affinityTissue cultureImmunoglobulins against enzymesBALB/cAlanine aminotransferase
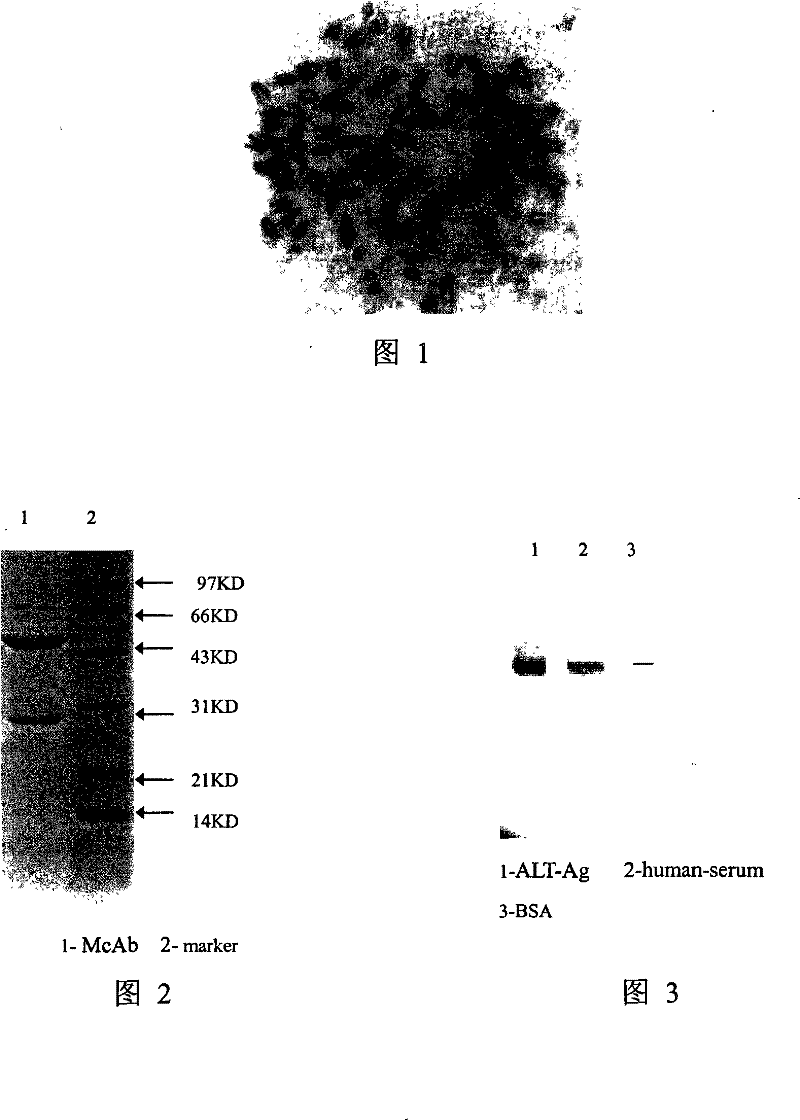
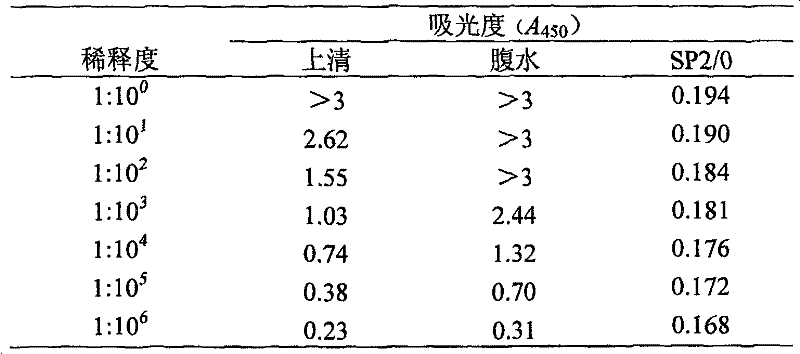
The invention discloses an alanine aminotransferase monoclonal antibody and an application, belonging to the medicine product containing antibody or the preparation of monoclonal antibody. The invention has the specificity of anti human alanine aminotransferase, which is generatd by hybridoma cell whose preservation number is CGMCC No2232. The invention uses purified ALT antigen to immunize BALB / C mice, fuses spleen cells and SP2 / 0 myeloma cells, uses indirect ELISA to screen hybridoma cell, grafts in mice abdominal, collects and purifies McAb ascetic fluid, to obtain anti human ALT specific ALT monoclonal antibody. The inventive product can be used to check alanine aminotransferase of serum. The product an specifically recognize ALT in serum without cross reaction with other proteins in serum, but with better infinity, thereby providing necessary condition for simple, quick and specific ALT immunity analysis.
Owner:天津市血液中心
Method of inhibiting proteolytic enzyme in enzyme preparation obtained in glutamin transaminase fermentation method
InactiveCN1724658AInhibition of activityAvoid lostChemical treatment enzyme inactivationProteinase activityAminopherase
The invention relates to a method to make protease in enzyme by restrain glutamine aminopherase fermenting method that belongs to enzyme technology field. The invention uses glutamine aminopherase fermenting to make enzyme. And during the manufacturing process, the metal ion inhibitor would be added and the activity of protease would be restrained. The invention is easy to operate and high efficiency.
Owner:JIANGNAN UNIV
Conditioning device of solar photoelectrical generation system applied to purple sweet potato refrigeration
InactiveCN101630168AEmission reductionProtect the safety of the environmentBatteries circuit arrangementsElectric powerNew energySilicon solar cell
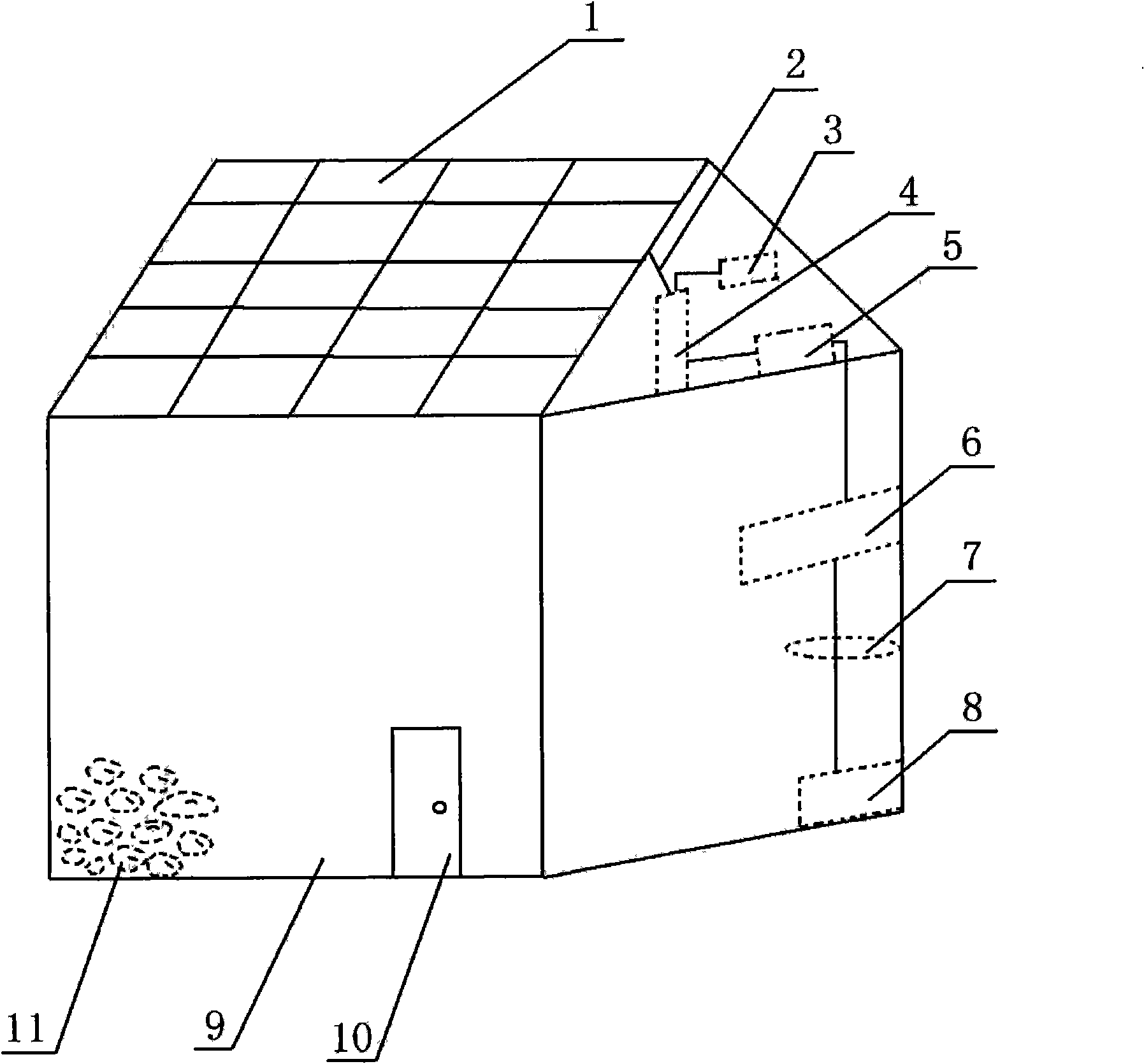
The invention relates to a conditioning device of a solar photoelectrical generation system applied to purple sweet potato refrigeration, which belongs to the technical field of new energy application. A purple sweet potato contains anthocyanin and selenium, has very strong antioxidation function, can reduce aminopherase in blood serum, has the function of preventing cardiovascular diseases of hyperpiesis and the like, and tumor; however, the fresh purple sweet potato is difficult to store because of thin peel and much juice. In recent years, the purple sweet potato has rapid planting development and growing consumption market. Sunlight irradiates a silicon solar panel arranged on the roof of a purple sweet potato refrigeration warehouse to generate direct current, and the direct current passes through a lead, a controller and a converter to be converted into alternating current, the alternating current is input to an air conditioner, a temperature controlling device and a humidity controlling device so as to enable the purple sweet potato refrigeration warehouse to keep a proper storage temperature of 10-15 DEG C and a proper humidity so that the fresh purple sweet potato can be stored for a long time and can be supplied for the market continuously. The current input to a lithium ion battery through the lead from the controller can be stored for preservation.
Owner:WUXI MEICUN TONGCHUN SOLAR ENERGY PV TECH AGRI PLANTATION
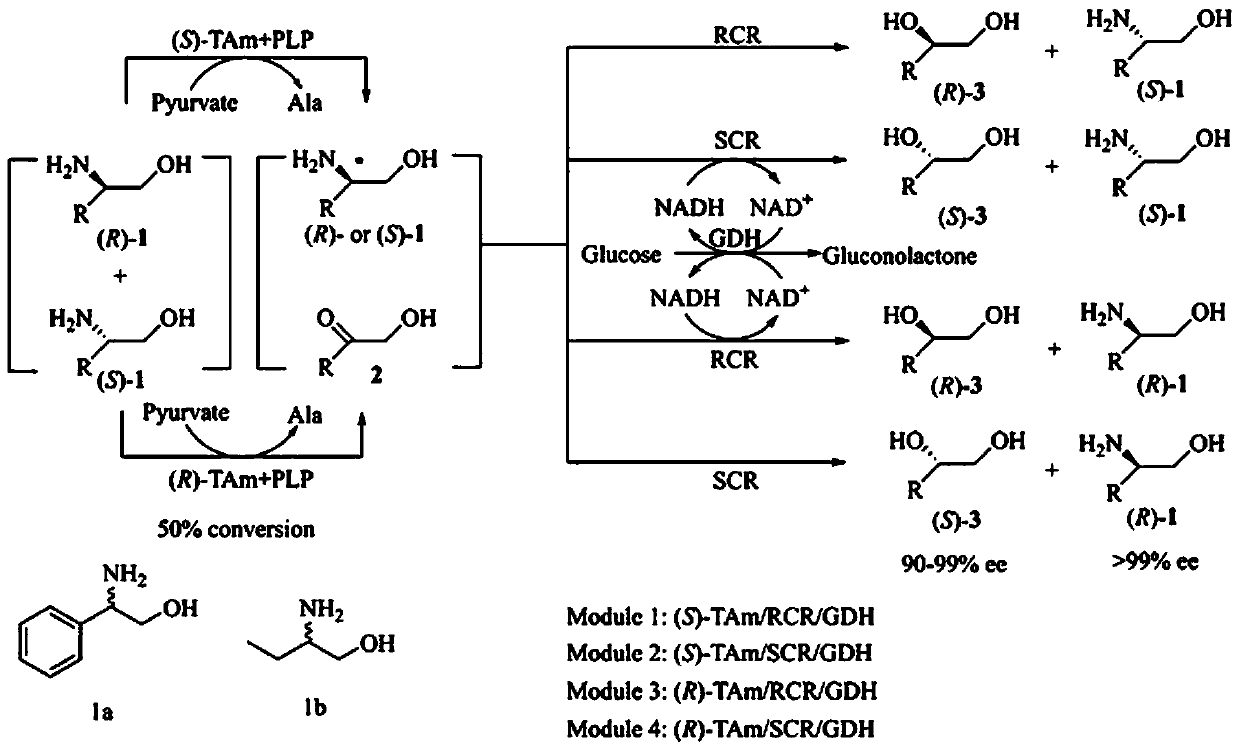
Method for simultaneously preparing chial ortho-alkamine and chial diol through whole-cell biological catalysis
InactiveCN109666715AAvoid lostSuitable for industrial applicationsTransferasesMicroorganism based processesEscherichia coliKetone
The invention belongs to the technical field of biology, and provides a method for simultaneously preparing chial ortho-alkamine and chial diol through whole-cell biological catalysis. According to the method, recombinant escherichia coli E.coli(TAm), E.coli(CR) and E. coli(GDH) whole cells serve as biological catalysts, racemization ortho-alkamine serves as a substrate, a reaction is carried outin a phosphate buffering solution, and meanwhile, and chial pure diol and ortho-alkamine are simultaneously synthesized. The cascading catalysis reaction adopts two enzyme catalysts for two catalyzingsteps, wherein in the first step, through aminopherase, racemization ortho-alkamine is subjected to kinetic resolution to obtain chial pure ortho-alkamine and hydroxyl ketone; in the second step, through carbonyl reductase, hydroxyl ketone is subjected to asymmetric reduction to synthesize chial pure diol. By means of the method, racemization ortho-alkamine is catalyzed, chial pure diol and ortho-alkamine can be simultaneously prepared, and the method has the advantages of being high in catalysis efficiency, mild in reaction condition, simple in reaction process, low in energy consumption andthe like, and conforms to the principle of green chemistry.
Owner:TAIYUAN UNIV OF TECH
Chinese traditional medicine composition for treating ulcerative colitis and its preparation method
ActiveCN101332284BAnthropod material medical ingredientsDigestive systemRhizomeChinese traditional medicine
The invention discloses a traditional Chinese medicine composition used for curing chronic hepatitis and a preparation method thereof. The traditional Chinese medicine composition is made of raw material medicines with the weight proportion by parts: 40 to 160 parts of Chinese thorowax root, 40 to 160 parts of debark peony root, 40 to 160 parts of angelica, 40 to 160 parts of India madder root, 40 to 160 parts of largehead atractylodes rhizome, 40 to 160 parts of Tuckahoe, 40 to 160 parts of turtle carapace, 40 to 160 parts of massa medicata fermentata hunanensis, 60 to 240 parts of Codonopsis pilosula nannfeldt, 60 to 240 parts of Lalang Grass Rhizome, 24 to 96 parts of immature orange fruit, 20 to 80 parts of green tangerine peel, 12 to 48 parts of villous amomum fruit, 12 to 48 parts of earthworm and 12 to 48 parts of liguorice. The traditional Chinese medicine composition of the invention has the pharmacological efficacies of antagonizing hepatic damage, protecting liver, regulating immune function, reducing aminopherase, improving liver function and on the like, and the clinical efficacy observation experiments prove that the traditional Chinese medicine composition has the exact efficacies to the chronic hepatitis, in particular to chronic active hepatitis, chronic persisting hepatitis and early cirrhosis; and the clinical application is of safety and no toxicity and side effects.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Traditional Chinese medicine for treating chronic active hepatitis
InactiveCN101152394ACompatibility is simpleLow costDigestive systemAntiviralsDiseaseAdditive ingredient
A Chinese traditional medicine for curing chronic active hepatitis belongs to the Chinese traditional medicine for curing hepatitis. The ingredients of the medicine (with weights) are milkvetch root 30g, root of red salvia 30g, codonopsis pilosula 15g, angelica 15g, white peony root 15g, cornel 15g, nutgrass galingale rhizome 15g, red peony root 15g, rhizome of giant knotweed 15g, turtle shell 25g, liquorice 5g; the auxiliary ingredients include sanxiange 15g, radish seed 20g and areca 10g under the situation of obvious abdominal distension; rhizoma Corydalis 15g and szechwan chinaberry fruit 10g under the situation of hypochondriac pain; virgate wormwood herb 50g under the situation of obvious jaundice; schisandra fruit 10g under the situation of high-content aminopherase. The medicines used in the prescription are all natural herbs and the Chinese traditional medicine is processed with traditional method; the fetching of material is convenient and the prescription and manufacture method are simple; the expense for manufacturing the medicine is low. The composition of the Chinese herbal medicine is simple and the medicines used in the prescription are all natural ones and the fetching and use are convenient; the manufacture method is simple and the effect is excellent; the price is low; in this way, the herbal medicine is especially fit for people living in remote villages far away from counties and towns; the curing expense for patients with the chronic active hepatitis is low which solves the problem that the household income is low and the life is poor and the medical conditions is deficient locally.
Owner:李英
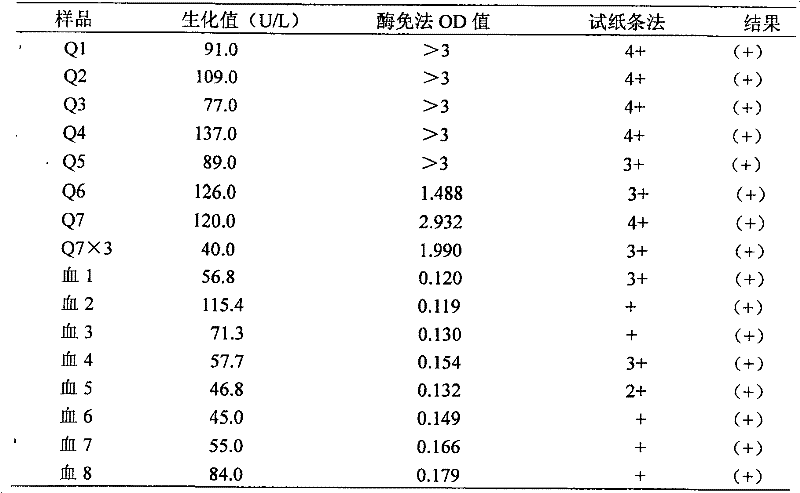
Method and kit for scaling investigating angiotensin aminopherase
InactiveCN1693878AAvoid defectsReduce mistakesMicrobiological testing/measurementColor/spectral properties measurementsReference rangeOrganism
Buttery's(1993) velocity of FAPGG method for measuring the activity of hypertension transformation enzyme on the clinical chemistry examination is applied widely. However, Buttery's method lacks a generally accepted standard method to mark the samples, thus the test accuracy often descends. Moreover, different laboratories apply dissimilar biochemical analysis instruments, and the normal reference data scope for hypertension transformation enzyme is usually confused and hardly compared with others. The invention uses the Hurst's method(1981) to examine the activity of the standard hypertension transformation enzyme. Then, following the standard activity, it applies the Buttery's method to measure the activity of hypertension transformation enzyme in living creature samples quantificationally. The invention has solved or reduced the blemishes of the Buttery's method. The invention also involves a reagent box used for implementing the method mentioned above.
Owner:ZHEJIANG YAKE SCI & TECH +1
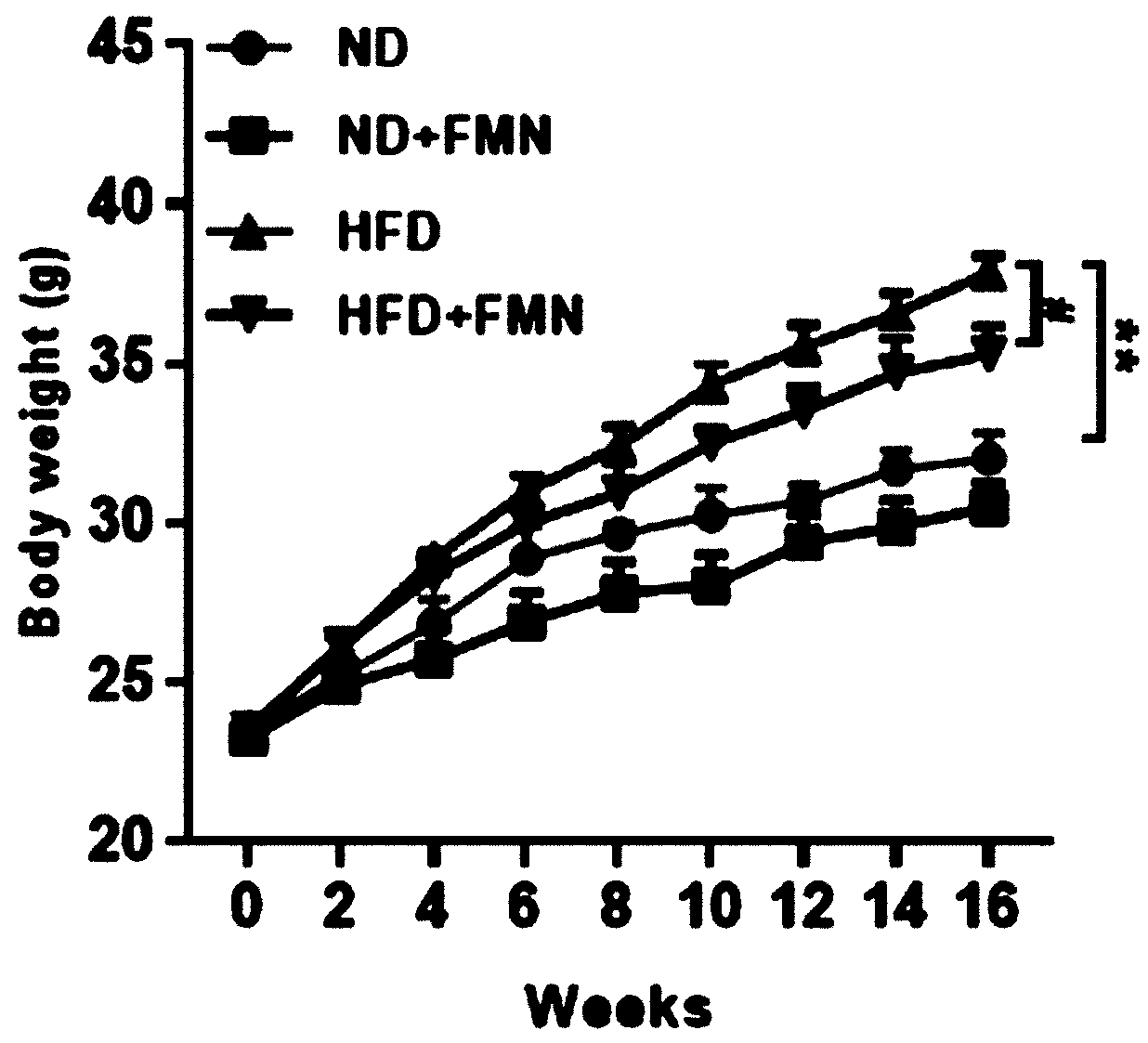
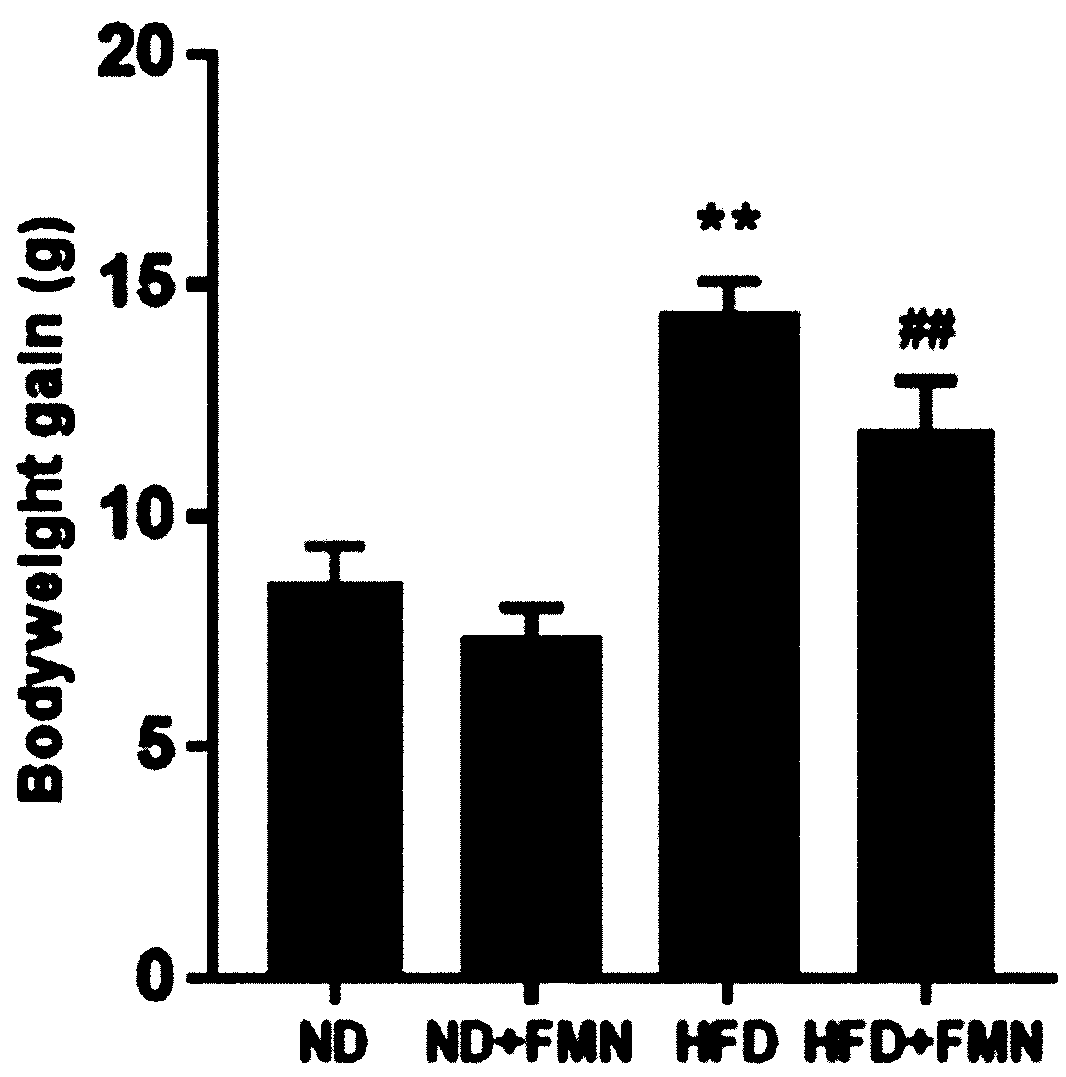
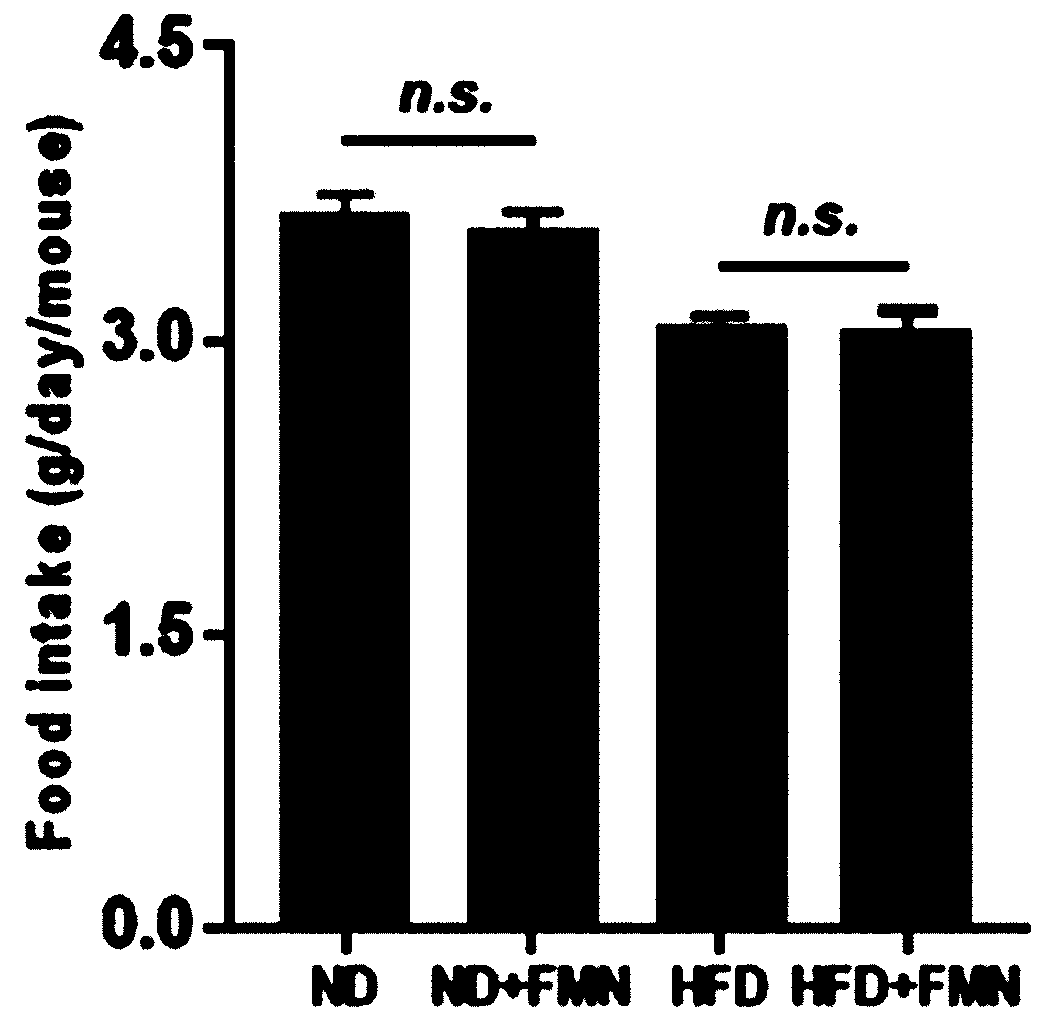
Application of formononetin to treatment of nonalcoholic fatty liver disease
InactiveCN110090209ALose weightReduce liver weight/body mass indexOrganic active ingredientsDigestive systemAlanine aminotransferaseLiver triglyceride
The invention discloses an application of formononetin to treatment of nonalcoholic fatty liver disease. An in vitro and an in vitro cell model are adopted, formononetin is used for treating animals,and the improvement effect of the formononetin on metabolic syndromes of nonalcoholic fatty liver, obesity, insulin resistance, lipid disorders and the like can be observed. Experiment proves that theformononetin can notably reduce weight, liver weight / body mass indexes, liver triglyceride(TG), liver lipidoses, liver function damage criterion-millet straw aminopherase (AST) and alanine aminotransferase (ALT) of organisms suffering from nonalcoholic fatty liver, and besides, can improve insulin resistance. Blood biochemistry detection indicates that the formononetin can also reduce the level of triglyceride (TG), cholesterol (TC) and low intensity lipoprotein (LDL-C) of HFD mice, and can improve the lipid disorders. The formononetin has important effects on treatment of nonalcoholic fattyliver.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
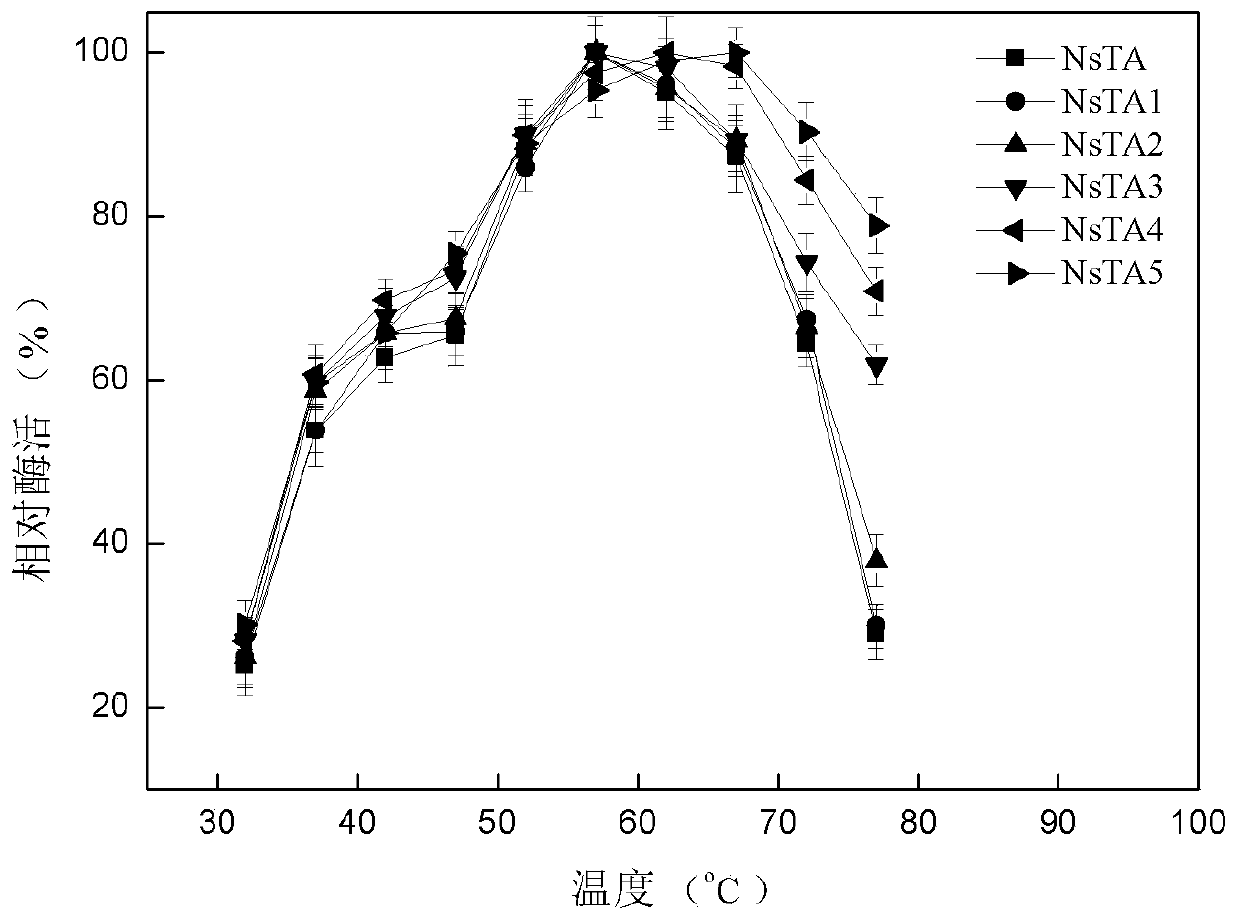
α-Transaminases and mutants and their application in asymmetric synthesis of l-glufosinate
ActiveCN109609476BHigh catalytic activityTransferasesGenetic engineeringAminopheraseReaction temperature
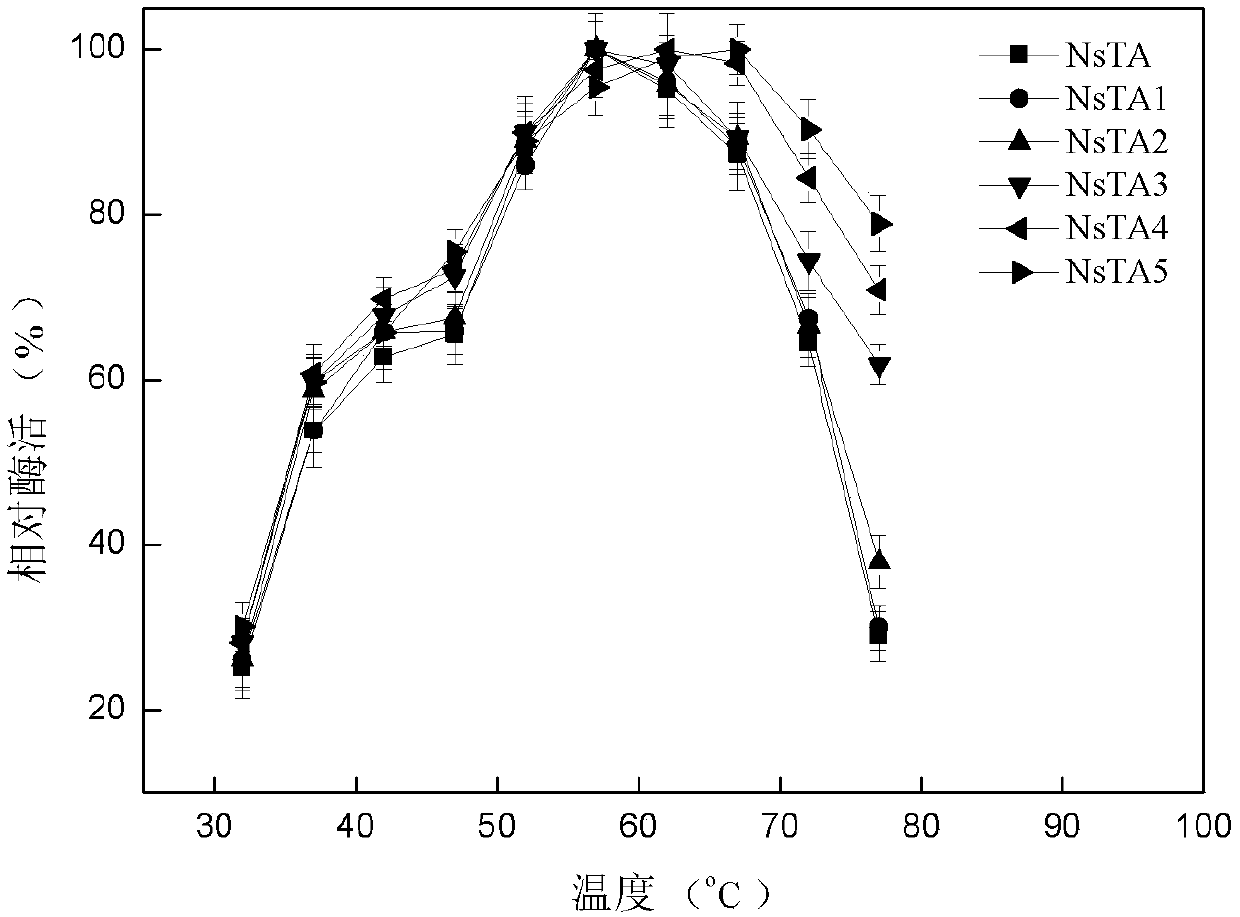
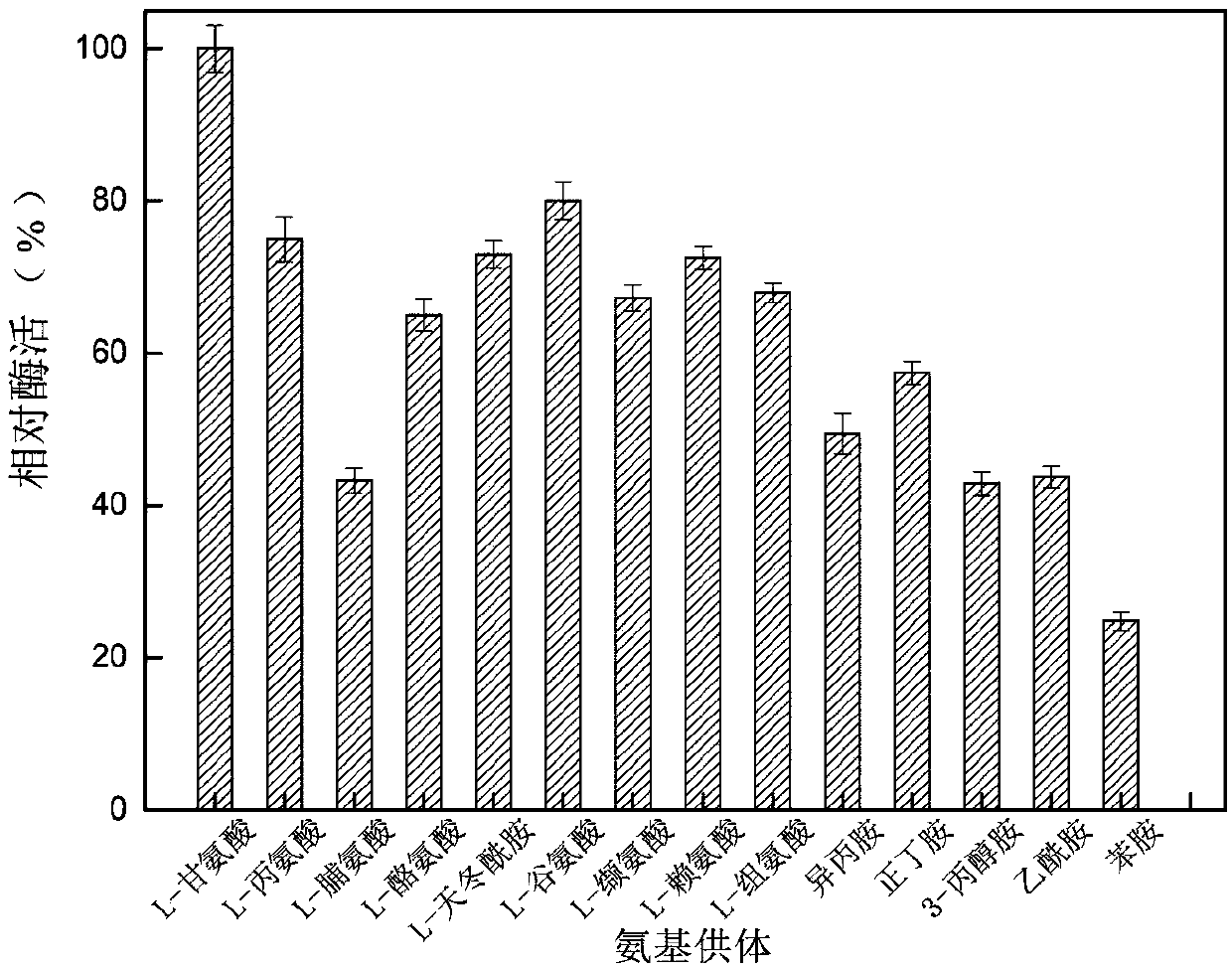
The invention discloses an application of novel aminopherase and a high-vitality mutant thereof to asymmetric synthesis of L-glufosinate-ammonium. The amino acid sequence of the aminopherase is as shown in SEQ ID:1, and the mutant of the aminopherase is prepared through performing single-site mutation or multi-site mutation on one or a plurality of 87th, 108th, 167th, 304th and 357th in the aminoacid sequence as shown in the SEQ ID:1. The high-efficiency expression of vitality mutant genes of high-conversion rate aminopherase can be realized, and the highest enzyme activity is 818.4U / mg. Thehighest optimum reaction temperature of the aminopherase vitality mutant can reach 67 DEG C, and during asymmetric synthesis of the L-glufosinate-ammonium through catalyzing 800mM of glufosinate-ammonium precursor ketone at the temperature, the conversion rate is as high as 100%. The NsTA mutant solves the technical difficult problems that few enzyme sources exist, the enzyme activity is low, substrate tolerance is bad and the conversion rate is low in a current L-glufosinate-ammonium preparation technology from the aminopherase, and has better application prospects.
Owner:ZHEJIANG UNIV OF TECH
A low-temperature active treatment process for flavored freshwater fish and its treatment equipment
Owner:湖北允泰坊食品有限公司
Medicine for treating hepatitis, and preparation method and application thereof
InactiveCN106727864ARepair damageObvious reliefOrganic active ingredientsDigestive systemSide effectCirrhosis
The invention discloses a medicine for treating hepatitis, and a preparation method and application thereof. The medicine is prepared from the following raw materials: 1 to 5 parts of radix platycodonis, 11 to 19 parts of citric acid, 7 to 15 parts of scamonia grease, and 3 to 7 parts of stachyose. The preparation method comprises the steps of mixing and grinding the radix platycodonis and the scamonia grease, screening through a 150-mesh sieve, adding deionized water with the mass being 4.3 times of the total mass of the radix platycodonis and the scamonia grease, then warming to 76 to 78 DEG C, stirring for 40 to 45min at the temperature, and preparing a mixture A; putting the citric acid and the stachyose into the mixture A, carrying out ultrasonic treatment at the temperature of 60 DEG C, stirring at the temperature of 55 DEG C until dry, and pelleting. The medicine provided by the invention has a better curative effect on various hepatitis, and can be beneficial to reducing aminopherase action, repairing damaged hepatocyte, and particularly relieving and treating hepatitis B, icterohepatitis, liver cirrhosis and the like. The medicine for treating hepatitis provided by the invention is good in curative effect, fast in effect, free of toxic and side effect, relapse-free after cured, beneficial to protecting liver and lowering transaminase, low in cost, simple to prepare, and suitable to be produced and popularized in large scale.
Owner:ZHENGZHOU ZHANGMENG NETWORK TECH CO LTD
Schizandrol dripping pills for decreasing aminopherase and method for preparing the same
InactiveCN100375613CIncrease surface areaHas a wetting effectDigestive systemAntiviralsAminopheraseMedicine
Owner:北京旷博生物技术股份有限公司
Medicine for treating liver cirrhosis
InactiveCN106511436ARepair damageObvious reliefHydroxy compound active ingredientsDigestive systemSide effectGlycerol
The invention discloses a medicine for treating liver cirrhosis. The medicine is prepared from the following raw materials: 1-5 parts of calculus bovis, 11-19 parts of glycerol, 7-15 parts of convovulus scammonia and 3-7 parts of inulin. A preparation method comprises the following steps: mixing and grinding the calculus bovis and convovulus scammonia; adopting a 150-mesh sieve for screening; adding deionized water of which the weight is 4.3 times of the weight of calculus bovis and convovulus scammonia, and then increasing the temperature to 76-78 DEG C, stirring under the temperature for 40-45min, thereby acquiring a mixture A; and adding the glycerol and inulin into the mixture A, ultrasonically treating at 60 DEG C, then stirring at 55 DEG C till the mixture is dry, and pelletizing to acquire the medicine. The medicine for treating liver cirrhosis has excellent curative effects for all types of liver cirrhosis, can effectively reduce aminopherase effect and repair the injured hepatic cells, and especially has obvious relieving and treating effects on hepatitis B cirrhosis, jaundice liver cirrhosis, liver cirrhosis, and the like. The medicine for treating liver cirrhosis has the advantages of good curative effect, fast effect, no toxic side effect, no relapse after healing, protection of liver and reduction of enzyme, low cost, simple preparation and suitability for large-scale production and popularization.
Owner:HENAN BALING ELECTRONICS TECH CO LTD
Alpha-aminopherase and mutant and application of alpha-aminopherase and mutant to asymmetric synthesis of L- glufosinate-ammonium
ActiveCN109609476AHigh catalytic activityTransferasesGenetic engineeringMulti siteGlufosinate-ammonium
The invention discloses an application of novel aminopherase and a high-vitality mutant thereof to asymmetric synthesis of L-glufosinate-ammonium. The amino acid sequence of the aminopherase is as shown in SEQ ID:1, and the mutant of the aminopherase is prepared through performing single-site mutation or multi-site mutation on one or a plurality of 87th, 108th, 167th, 304th and 357th in the aminoacid sequence as shown in the SEQ ID:1. The high-efficiency expression of vitality mutant genes of high-conversion rate aminopherase can be realized, and the highest enzyme activity is 818.4U / mg. Thehighest optimum reaction temperature of the aminopherase vitality mutant can reach 67 DEG C, and during asymmetric synthesis of the L-glufosinate-ammonium through catalyzing 800mM of glufosinate-ammonium precursor ketone at the temperature, the conversion rate is as high as 100%. The NsTA mutant solves the technical difficult problems that few enzyme sources exist, the enzyme activity is low, substrate tolerance is bad and the conversion rate is low in a current L-glufosinate-ammonium preparation technology from the aminopherase, and has better application prospects.
Owner:ZHEJIANG UNIV OF TECH
Low-temperature activity treatment technology of flavor freshwater fish and treatment equipment of treatment technology
ActiveCN103431443AAccelerate evaporationReduce processing timeFood preparationAdditive ingredientEvaporation
The invention discloses a low-temperature activity treatment technology of flavor freshwater fish, which comprises the steps of fresh fish selection and pretreatment, fish meat rinsing and draining, stirring of fish meat and ingredients, dewatering and drying in a drying warehouse, vacuum packing and the like. According to the treatment technology, a specially-made system, and control and adjustment of a technological parameter allow fresh fish to be dewatered and dried at a lower drying temperature (5-10 DEG C) quickly, so that a unique flavor is formed, and a sanitary condition is improved greatly; a centrifugal fan in the system exchanges air quickly, and a dryer dries and dehumidifies the air, so that the indoor air can be kept in a compulsory circulation state all the time, and water evaporation of a material is facilitated; the freshwater fish is subjected to protein crosslinking by glutamoyl aminopherase, so that the flavor and a taste of the freshwater fish are further improved; and the machining time of the freshwater fish is shortened to be 30h from the existing approximate 80h, so that a production cycle is shortened, and the production efficiency is improved, and a good economic benefit is exerted.
Owner:湖北允泰坊食品有限公司
Prodrug, preparation method therefor, pharmaceutical compositions and use thereof
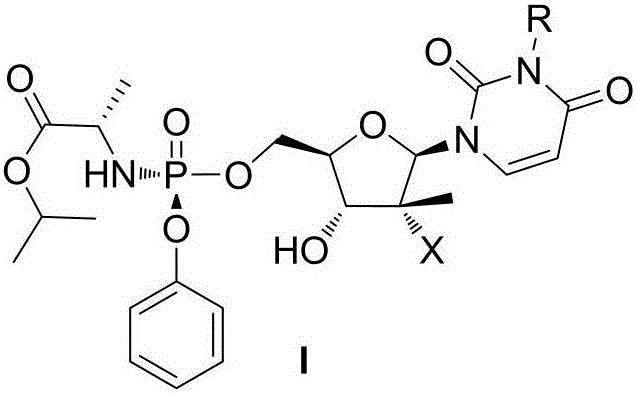
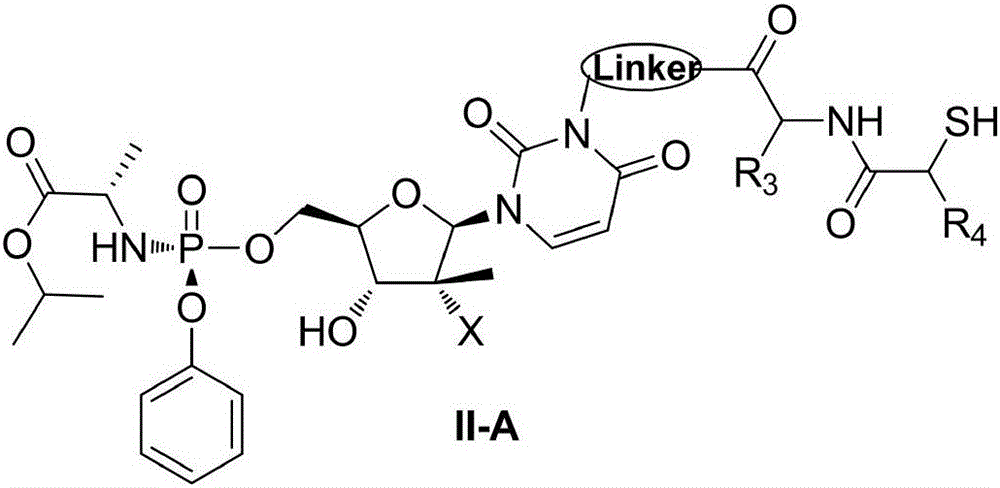
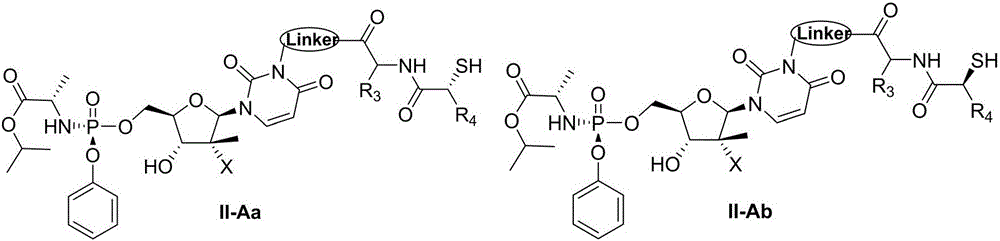
ActiveCN106883280AImprove oral bioavailabilityGood metabolic propertiesOrganic active ingredientsSugar derivativesAminopherasePharmaceutical drug
The invention discloses a nucleoside structure containing prodrug represented by a formula I shown in the description or tautomers, stereomers, stereomer mixtures or pharmaceutically-acceptable solvates thereof and simultaneously discloses preparation methods for the prodrug or the tautomers, stereomers, stereomer mixtures or pharmaceutically-acceptable solvates thereof, pharmaceutical compositions prepared from the prodrug or the tautomers, stereomers, stereomer mixtures or pharmaceutically-acceptable solvates thereof and use of the pharmaceutical compositions as hepatitis C resisting drugs. The compounds or pharmaceutical compositions thereof can be applied to the treatment of hepatitis C and meanwhile play roles in protecting hepatic tissue and hepatic cells, improving hepatic functions, reducing aminopherase, and the like.
Owner:HANGZHOU HERTZ PHARMA
Mutton squid roll and preparation method thereof
A mutton squid roll and a preparation method thereof. The mutton squid roll comprises mutton and is characterized in that the mutton squid roll is in a structure that the mutton is rolled-up by a squid roll, wherein proteins in the mutton and the squid roll are crosslinked together by glutamine aminopherase.
Owner:谈丽娜
Method of inhibiting proteolytic enzyme in enzyme preparation obtained in glutamin transaminase fermentation method
InactiveCN1318583CInhibition of activityAvoid lostChemical treatment enzyme inactivationProteinase activityAminopherase
The invention relates to a method to make protease in enzyme by restrain glutamine aminopherase fermenting method that belongs to enzyme technology field. The invention uses glutamine aminopherase fermenting to make enzyme. And during the manufacturing process, the metal ion inhibitor would be added and the activity of protease would be restrained. The invention is easy to operate and high efficiency.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com