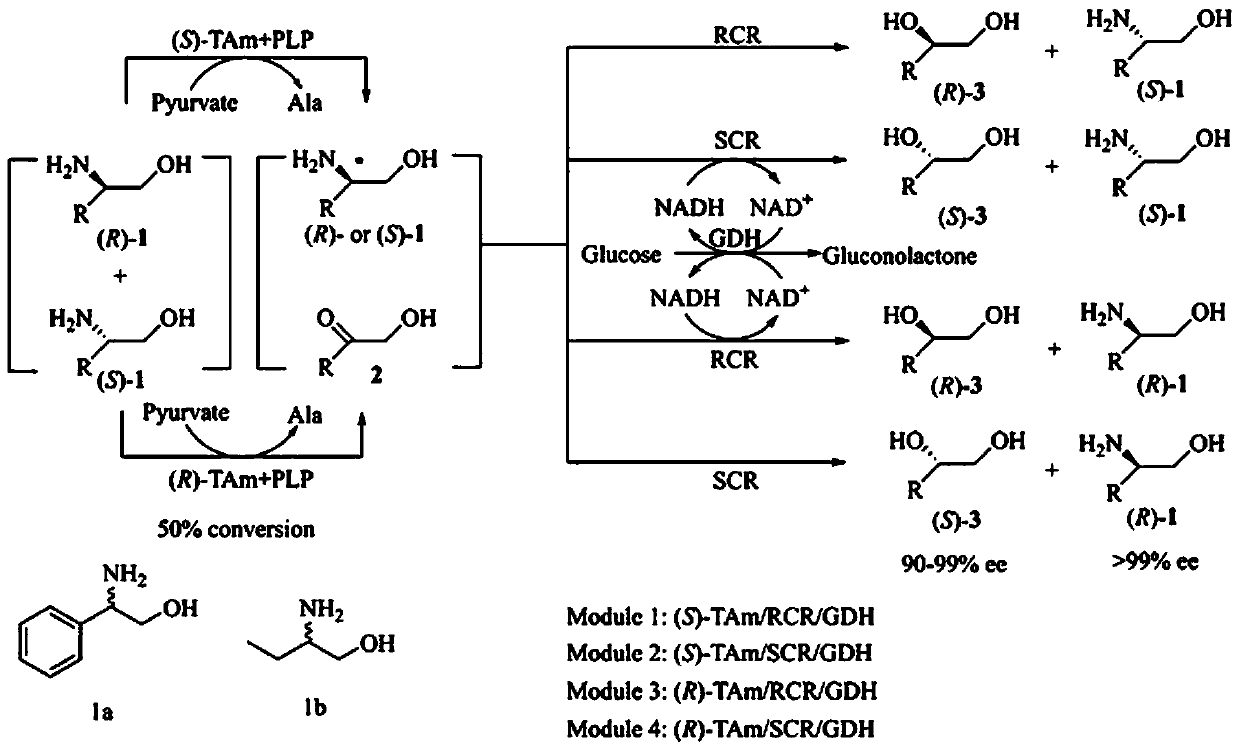
Method for simultaneously preparing chial ortho-alkamine and chial diol through whole-cell biological catalysis
A technology of o-amino alcohol and biocatalysis, applied in the biological field, can solve the problems of substrate cost limitation and low utilization rate of raw materials, and achieve the effects of avoiding the loss of intermediate products, high selectivity and conversion rate, and increasing reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0041] Experimental Example 1: Construction of a recombinant expression vector:
[0042] Design primers according to the gene sequence (PpbauA, CV2025, BDHA, GoSCR, GDH), the transaminase MVTA gene sequence is as shown in SEQ ID NO: 1; the transaminase CV2025 gene sequence is as shown in SEQ IDNO: 2 The nucleotide sequence shown; the transaminase PpbauA gene sequence such as the nucleotide sequence shown in SEQ ID NO: 3; the carbonyl reductase BDHA gene sequence such as the nucleotide sequence shown in SEQ ID NO: 4; the The carbonyl reductase GoSCR gene sequence is the nucleotide sequence shown in SEQ ID NO:5.
[0043] The upstream primer of MVTA gene is GGGAATTC CATATG GGCATCGACACTGGCACCT, the downstream primer is CCG CTC GAG GTACTGAATCGCTTCAATCAGTG; primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd., the underlined part is Nde I and xho I enzyme cleavage site, then Mycobacterium tuberculosis Mycobacterium vanbaalenii The genome is used as the ...
experiment example 2
[0045] Experimental example 2: Expression of Escherichia coli recombinant bacteria
[0046] For Escherichia coli strains, the following method is used in the present invention: inoculate the strains constructed in Example 1 into the LB medium containing Kana, 37 ° C, 180 rpm shaking culture for 8 h, and inoculate according to the inoculum size of 2%. In a shake flask filled with 50 mL of TB medium, culture at 37°C and 180 rpm, when the OD of the culture medium 600 When it reached 0.6, add IPTG with a final concentration of 0.1 mM, and induce for 12 h at 20°C and 180 rpm. The cells were collected by centrifugation at 8000 rpm for 5 min at 4°C, and washed twice with 100 mM sodium phosphate buffer, pH 7.0. Suspend the obtained bacterial cells with 100 mM sodium phosphate buffer solution with pH 7.0, sonicate them in an ice bath, and collect the supernatant by centrifugation, which is the crude enzyme solution.
[0047] co-expressed recombinant bacteria E. coli (BDHA–GDH) and ...
Embodiment 3
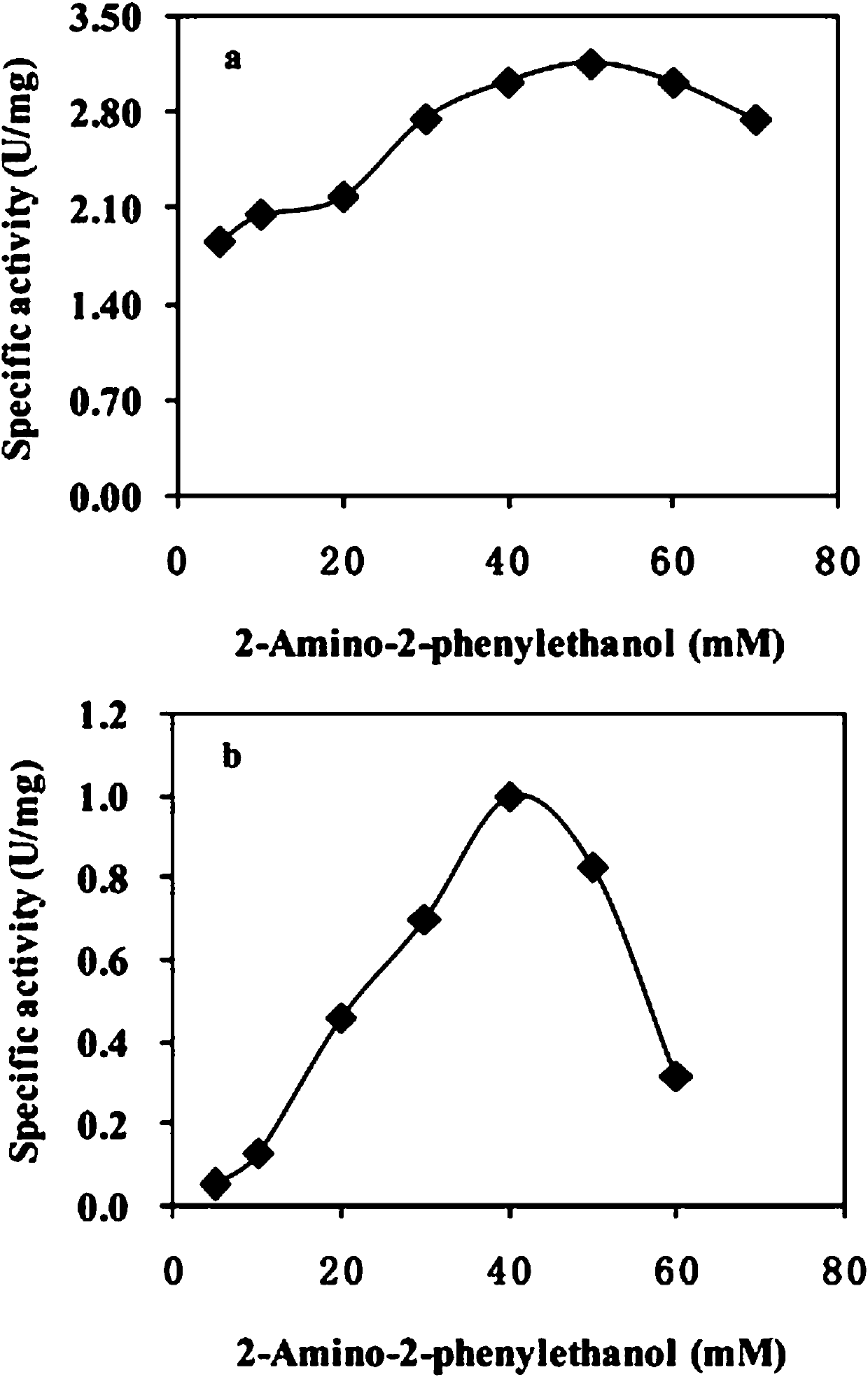
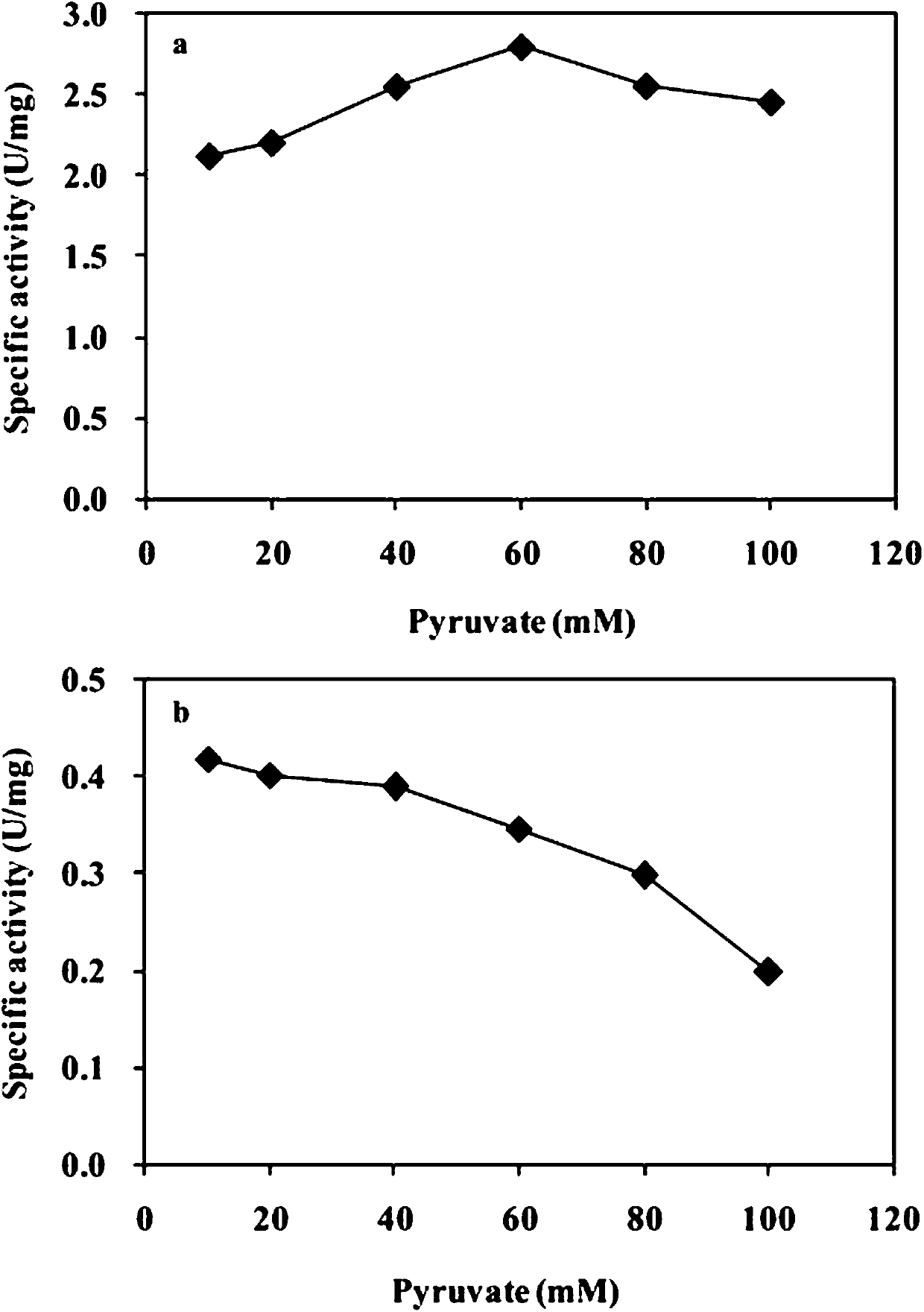
[0048] Embodiment 3: The influence of substrate and product in the reaction on enzyme activity
[0049] (1) Determination of the effect of phenylglycinol on the activity of transaminase: take different concentrations of phenylglycinol (10–100 mM) and 50 mM sodium pyruvate respectively, draw the curve of enzyme activity with the concentration of phenylglycinol, and compare The enzyme activity of transaminase under different phenylglycine concentration conditions determines the optimum catalytic substrate concentration of transaminase. Experimental results such as figure 2 shown. figure 2 It shows that for MVTA, as the concentration of phenylglycine increases, the enzyme activity also increases. When the concentration of phenylglycine is 50 mM, the enzyme activity begins to be inhibited. After the concentration exceeds 50 mM, the enzyme activity of MVTA begins decreased, but the decline was not obvious, and the enzyme activity was still 80% of the highest activity when the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com