Patents
Literature
41 results about "Vicinal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
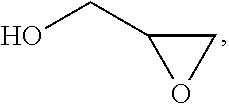
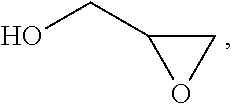
In chemistry the descriptor vicinal (from Latin vicinus = neighbor), abbreviated vic, describes any two functional groups bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). For example, the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not. Mostly, the use of the term vicinal is restricted to two identical functional groups.
Vicinal gallium nitride substrate for high quality homoepitaxy
ActiveUS7118813B2Polycrystalline material growthSemiconductor/solid-state device manufacturingWaferingRms roughness
A III–V nitride, e.g., GaN, substrate including a (0001) surface offcut from the <0001> direction predominantly toward a direction selected from the group consisting of <10-10> and <11-20> directions, at an offcut angle in a range that is from about 0.2 to about 10 degrees, wherein the surface has a RMS roughness measured by 50×50 μm2 AFM scan that is less than 1 nm, and a dislocation density that is less than 3E6 cm−2. The substrate may be formed by offcut slicing of a corresponding boule or wafer blank, by offcut lapping or growth of the substrate body on a corresponding vicinal heteroepitaxial substrate, e.g., of offcut sapphire. The substrate is usefully employed for homoepitaxial deposition in the fabrication of III–V nitride-based microelectronic and opto-electronic devices.
Owner:WOLFSPEED INC
Modulators of peroxisome proliferator activated receptors
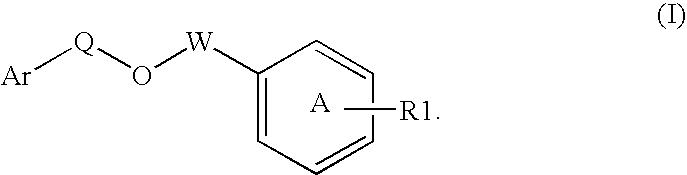
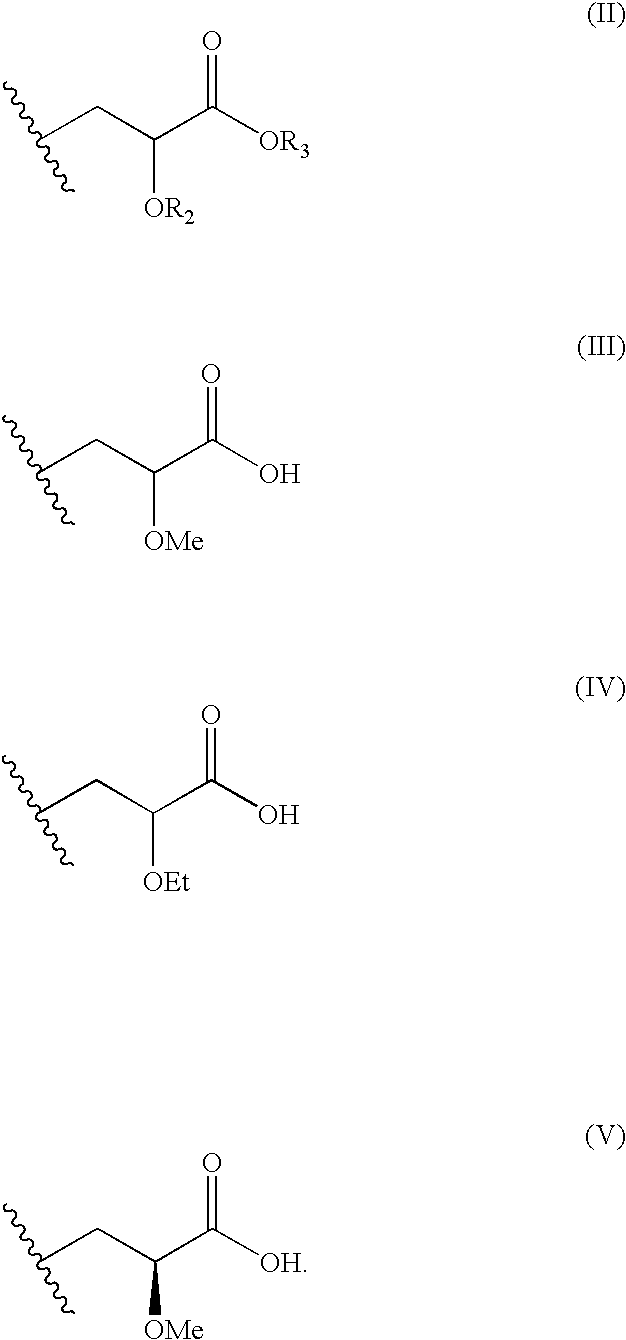
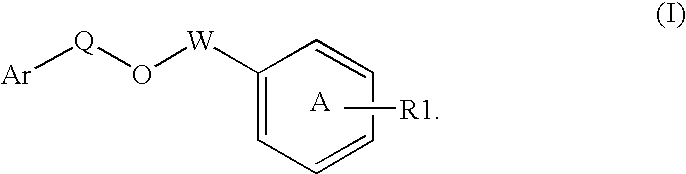
Disclosed is a compound represented by Structural Formula (I): Ar is a substituted or unsubstituted aromatic group. Q is a covalent bond, —CH2— or —CH2CH2—; W is a substituted or unsubstituted alkylene or a substituted or unsubstituted heteroalkylene linking group from two to ten atoms in length, preferably from two to seven atoms in length. Phenyl Ring A is optionally substituted with up to four substituents in addition to R1 and W, R1 is (CH2)n—CH(OR2)—(CH2)mE1, —(CH)═C(OR2)—(CH2)mE, —(CH2)n—CH(Y)—(CH2)mE or (CH)═C(Y)—(CH2)mE; wherein E is COOR3, C1–C3 alkylnitrile, carboxamide, sulfonamide, acylsulfonamide or tetrazole and wherein sulfonamide, acylsulfonamide and tetrazole are optionally substituted with one or more substituents independently selected from: C1–C6 alkyl, haloalkyl and aryl-C0-4-alkyl; R2 is H, an aliphatic group, a substituted aliphatic group, haloalkyl, an aromatic group, a substituted aromatic group, —COR4, —COOR4, —CONR5R6, —C(S)R4, —C(S)OR4 or C(S)NR5R6, R3 is H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. Y is O—, CH2—, —CH2CH2— or CH═CH— and is bonded to a carbon atom in Phenyl Ring A that is ortho to R1. R4–R6 are independently H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. n and m are independently 0, 1 or 2
Owner:ELI LILLY & CO +1
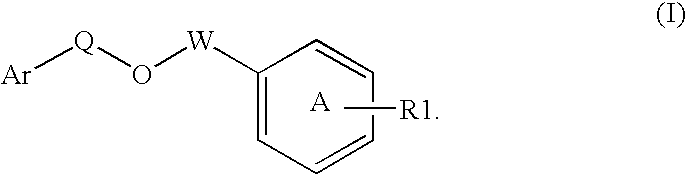
Modulators of peroxisome proliferator activated receptors
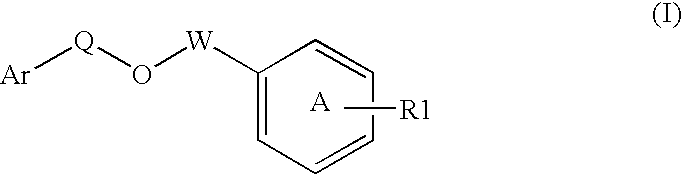
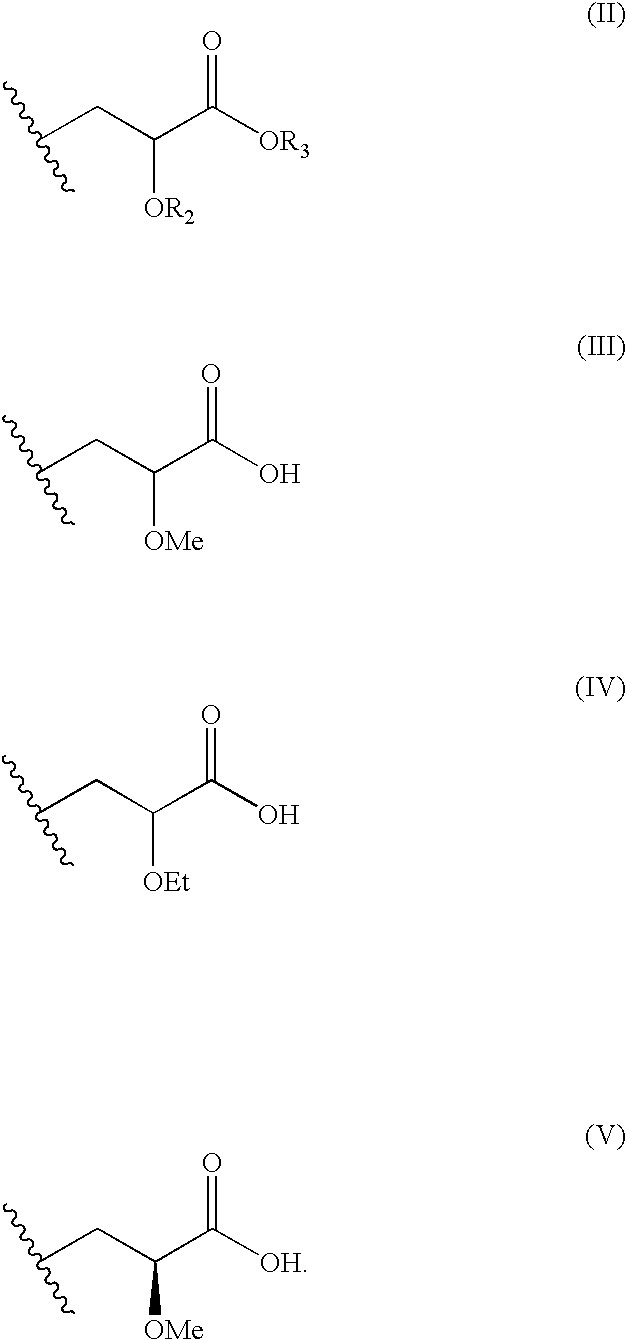
Disclosed is a compound represented by Structural Formula (I): Ar is a substituted or unsubstituted aromatic group. Q is a covalent bond, —CH2— or —CH2CH2—; W is a substituted or unsubstituted alkylene or a substituted or unsubstituted heteroalkylene linking group from two to ten atoms in length, preferably from two to seven atoms in length. Phenyl Ring A is optionally substituted with up to four substituents in addition to R1 and W, R2 is (CH2)n—CH(OR2)—(CH2)nE, —(CH)═C(OR2)—(CH2)nE, —(CH2)n—CH(Y)—(CH2)mE or (CH)═C(Y)(CH2)mE; wherein E is COOR3, C1-C3 alkylnitrile, carboxamide, sulfonamide, acylsulfonamide or tetrazole and wherein sulfonamide, acylsulfonamide and tetrazole are optionally substituted with one or more substituents independently selected from: C1-C6 alkyl, haloalkyl and aryl-Co-4-alkyl; R2 is H, an aliphatic group, a substituted aliphatic group, haloalkyl, an aromatic group, a substituted aromatic group, —COR4, —COOR4, —CONR5R6, —C(S)R4, —C(S)OR4 or C(S)NR5R6, R3 is H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. Y is O—, CH2—, CH2CH2— or CH═CH— and is bonded to a carbon atom in Phenyl Ring A that is ortho to R1. R4-R6 are independently H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. n and m are independently 0, 1 or 2.
Owner:ELI LILLY & CO +1
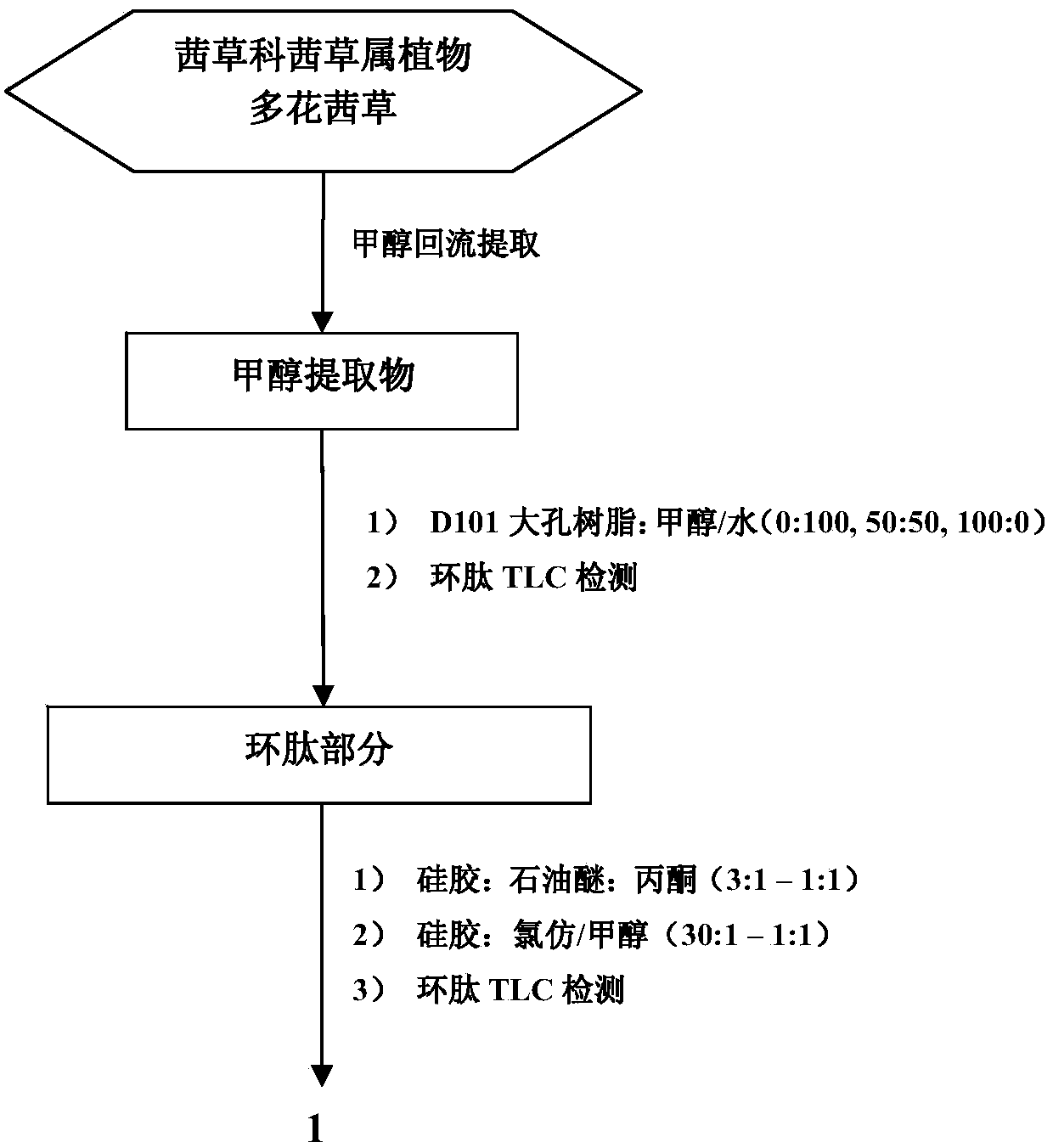
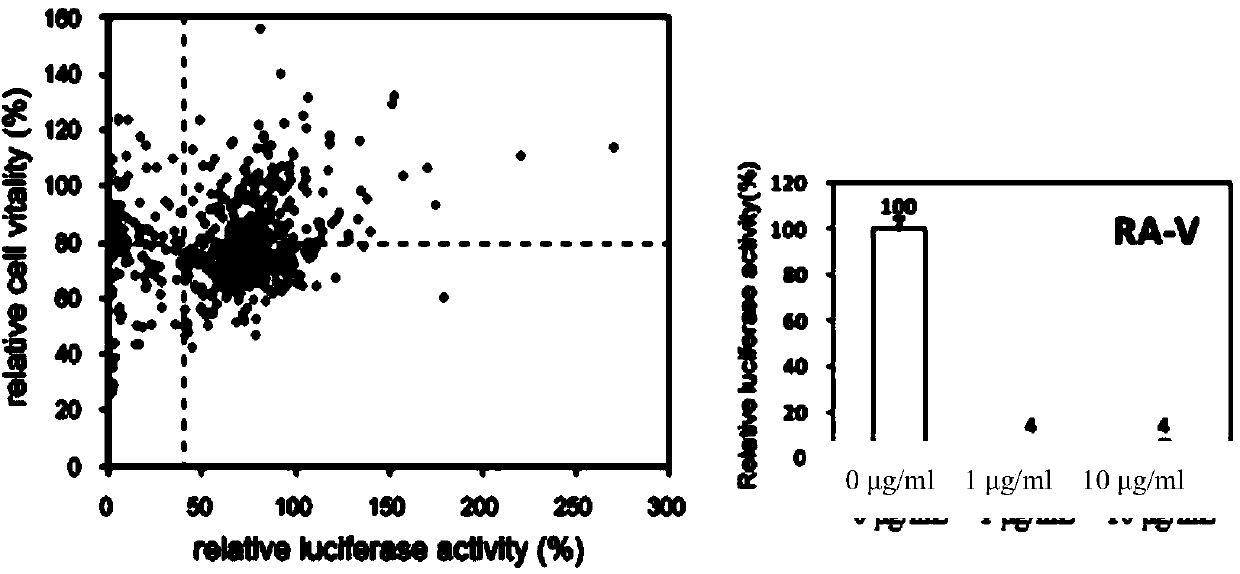
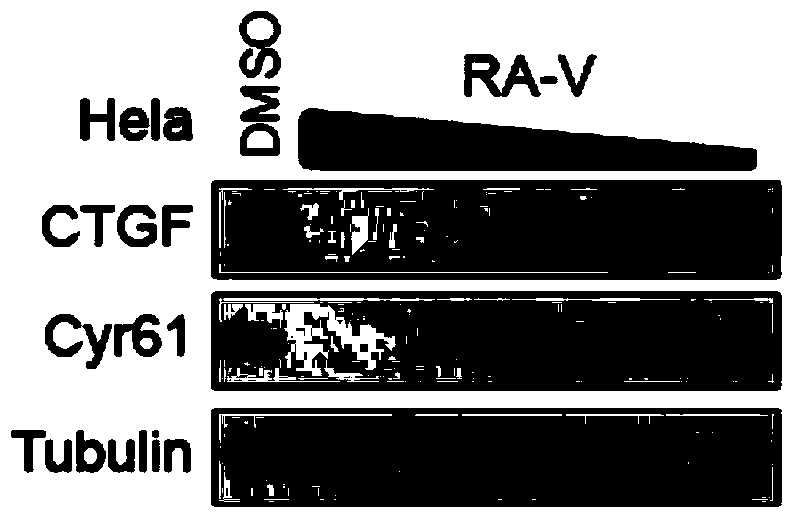
Preparation method of rubiaceous cyclopeptide and application of rubiaceous cyclopeptide as Hippo-YAP signal pathway inhibitor
InactiveCN104193808AEasy to separateImprove efficiencyPeptide preparation methodsCyclic peptide ingredientsCyclic peptideMethyltyrosines
The invention provides an application of rubiaceous cyclopeptide RA-V (1) as a Hippo-YAP signal pathway inhibitor, a preparation method of the rubiaceous cyclopeptide and an application of the rubiaceous cyclopeptide to preparation of drugs for treating and preventing YAP abnormal activation related cancers. The rubiaceous cyclopeptide provided by the invention is formed by condensation of peptide bonds of one alpha-D-alanine, one alpha-L-alanine, three substituted N-methyl-alpha-L-tyrosine and one alpha-L-amino acid of other types, and six amino acids are condensed to form an eighteen-membered ring, wherein a benzene ring between two vicinal tyrosines is connected through an oxygen bridge to form a fourteen-membered ring. Compared with the prior art, the preparation method provided by the invention can be used for rapidly separating and purifying the target compound RA-V (1) and has the advantages of high efficiency, strong purposiveness, few separation step, convenience in operation, good controllability and repeatability, recyclable solvent, low cost and suitability for industrial production.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +2
Ketone compound and synthesis method thereof
ActiveCN107245029ALow toxicityEasy to manufactureOrganic compound preparationCarbonyl compound preparation by oxidationTetrafluoroborateSynthesis methods
The invention discloses a method for generation of a ketone compound from a vicinal diol compound. The method includes: dissolving the vicinal diol compound in a solvent, and then under the action of the oxidizing agent 1-chloromethyl-4-fluoro-1, 4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate), enabling carbon-carbon bond breakage of the vicinal diol compound to generate the ketone compound. The method provided by the invention has the advantages of no need for adding of a metal chelating agent, low reaction temperature, no by-product formation, high conversion rate, and a product yield of 56%-90%, thus being an environment-friendly method.
Owner:GUANGDONG UNIV OF TECH
Multifunctional catalyst useful in the synthesis of chiral vicinal diols and process for the preparation thereof, and process for the preparation of chiral vicinal diols using said multifunctional catalysts
InactiveUS6780810B2Simple procedureEconomic securityOrganic compound preparationOrganic chemistry methodsDiolN oxidation
The present invention relates to a multifunctional reusable catalyst and to a process for the preparation thereof on a single matrix of the support to perform multicomponent reaction in a single pot. The multifunctional catalysts of the invention are useful for the synthesis of chiral vicinal diols by tandem and / or simultaneous reactions involving Heck coupling, N-oxidation and AD reaction of olefins in presence of cinchona alkaloid compounds both as an native one and immobilized one in the said matrix support. This invention also relates to a process for preparing vicinal diols by asymmetric dihydroxylation of olefins in presence of cinchona alkaloid compounds employing reusable multifunctional catalysts as heterogeneous catalysts in place of soluble osmium catalysts.
Owner:COUNCIL OF SCI & IND RES
Compositions and Methods for Imparting a Sunless Tan with a Vicinal Diamine
A composition and method for imparting a sunless tan to skin is described. The composition and method makes use of a sunless tanning agent like dihydroxyacetone in combination with an adjuvant which is a vicinal diamine.
Owner:CONOPCO INC D B A UNILEVER
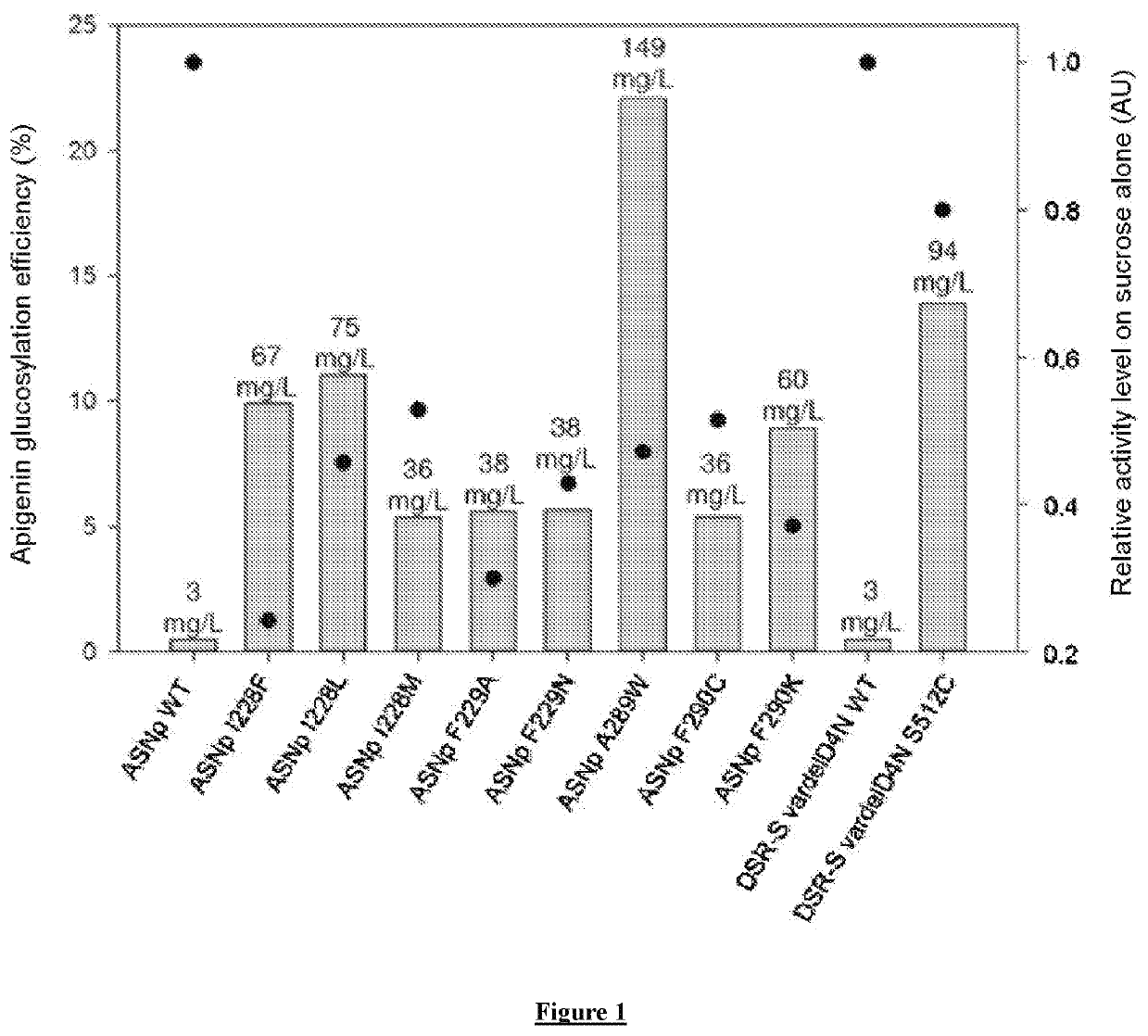
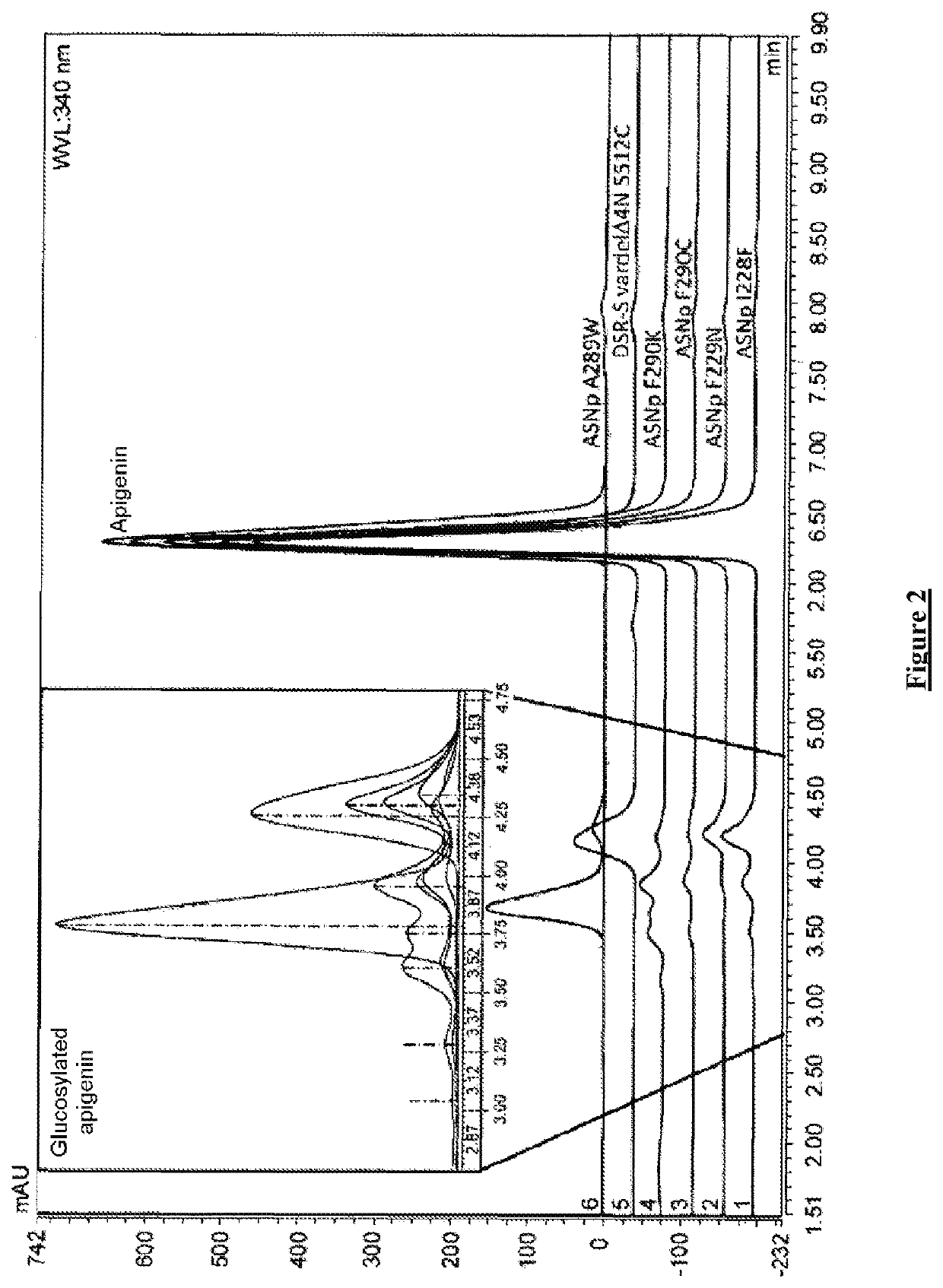
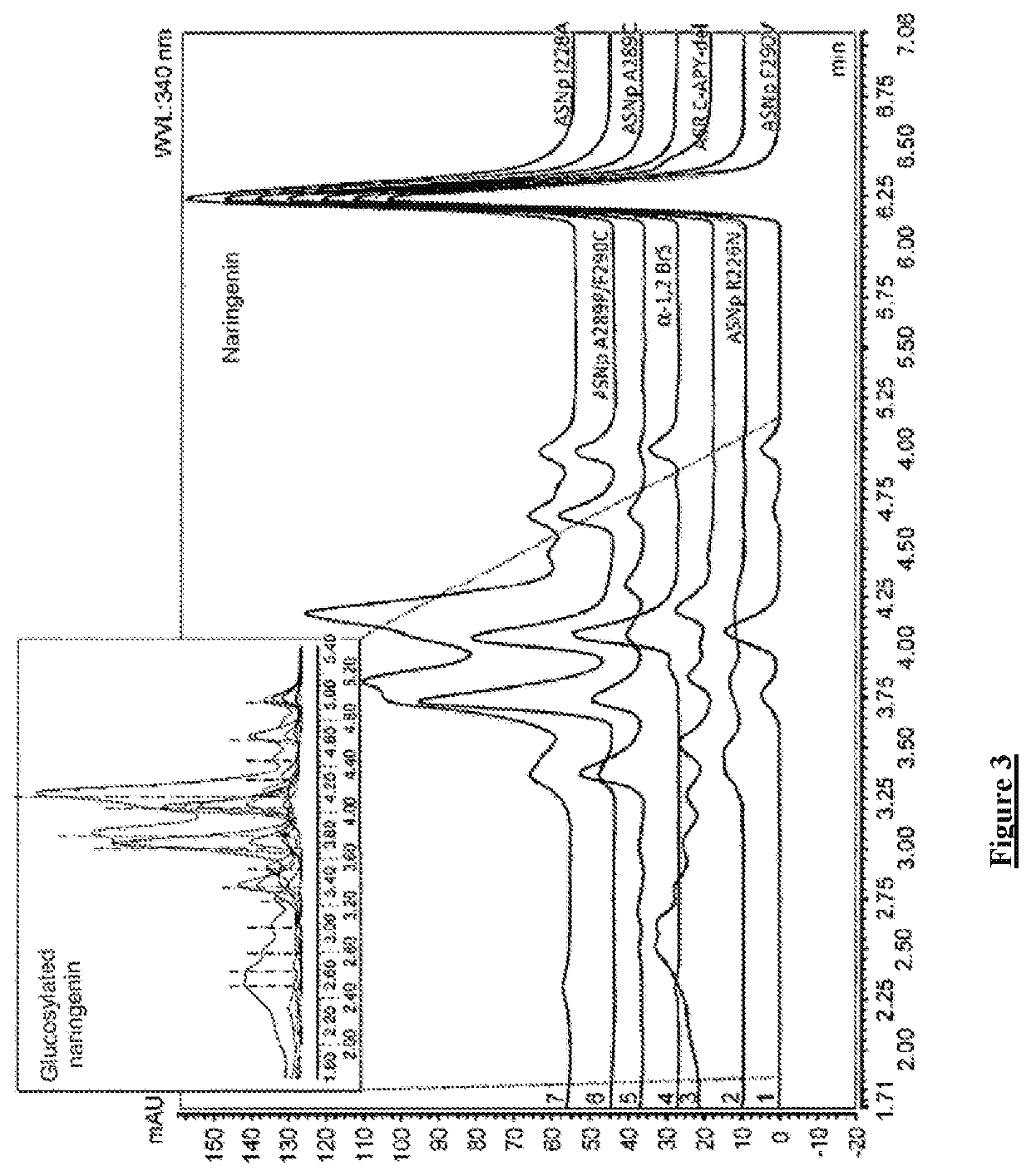
Flavonoids O-A-glucosylated on the B cycle, method for the production thereof and uses
The invention relates to a method for producing derivatives of O-α-glucosylated flavonoid, comprising at least one step of incubating a glucansucrase with a flavonoid and at least one sucrose, the flavonoid being a flavonoid which is monohydroxylated or hydroxylated in a non-vicinal manner on the B cycle. The invention also relates to novel O-α-glucosylated flavonoid derivatives, and to the use thereof.
Owner:INST NAT DES SCI APPLIQUEES DE TOULOUSE +2
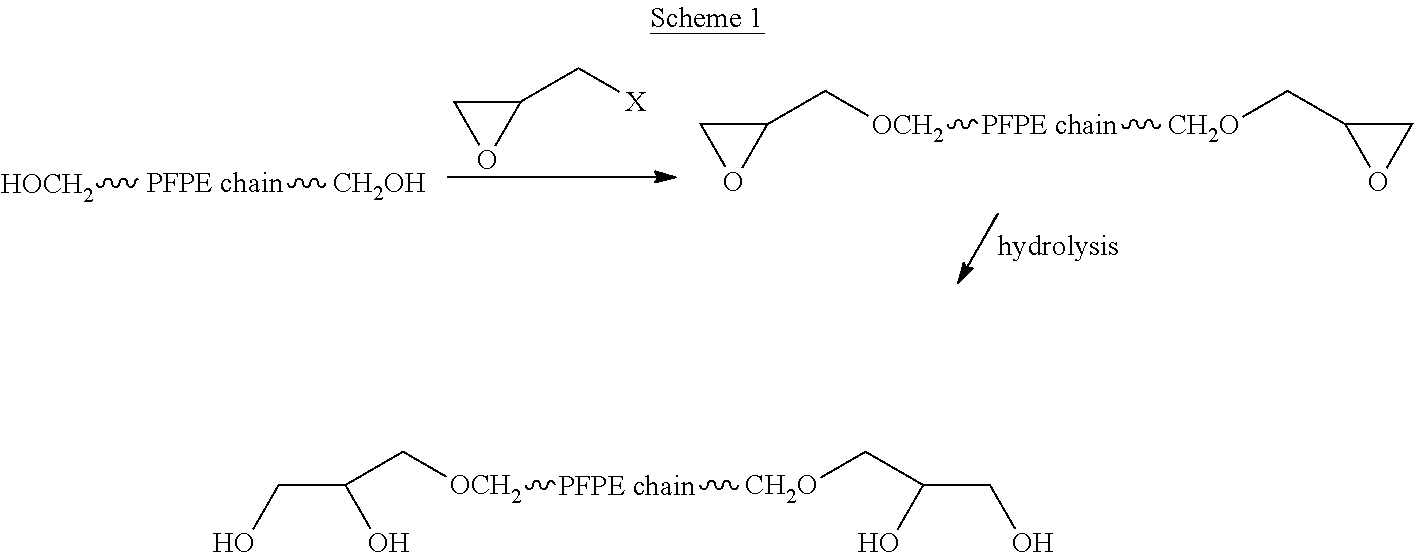
Process for the purification of polyol PFPE derivative
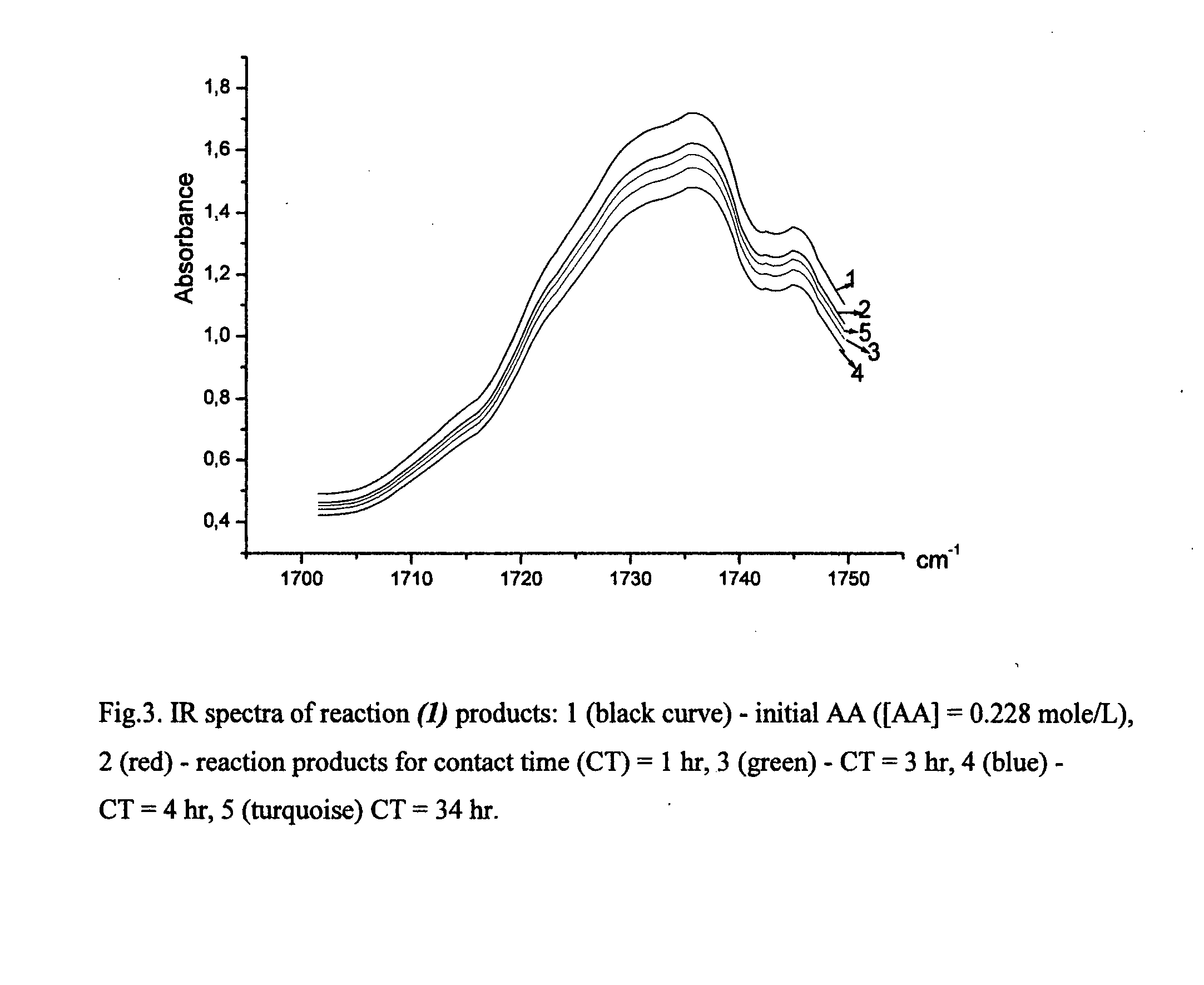
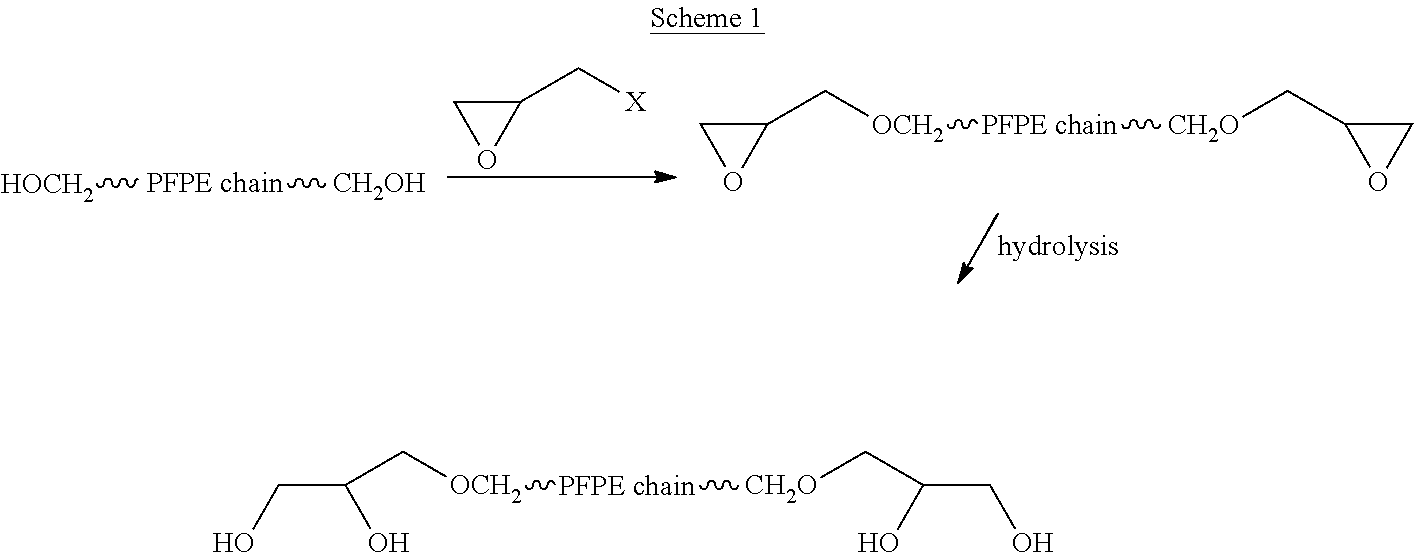
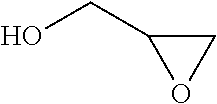
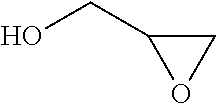
A process for the purification of a tetraol PFPE derivative [tetraol (T)] of formula (I): HO—CH2—CH(OH)—CH2—O—CH2—CF2—O—Rf—CF2—CH2O—CH2—CH(OH)—CH2OH, wherein Rf represents a fluoropolyoxyalkene chain (chain Rf), from a mixture (M) of hydroxyl (per)fluoropolyether derivatives, said mixture comprising said tetraol (T) and at least one hydroxyl (per)fluoropolyether [PFPE (OH)] comprising a chain Rf terminated with at least one end group of formula: —CF2CH2OH; —CF2CH2O—CH(OH)—CH2—O—CH2—CH(OH)—CH2OH; said process comprising: step 1: reacting the mixture (M) with ketones and / or aldehydes so as to selectively protect couples of hydroxyl groups on vicinal carbon atoms by forming corresponding cyclic ketal / acetal derivatives, so as to yield a protected mixture (P); step 2: reacting residual hydroxyl groups of the mixture (P) with suitable functionalizing agents enabling substantial volatility modification, so as to obtain a functionalized protected mixture (Pf); step 3: fractional distillation of the mixture (Pf) so as to isolate the cyclic acetal / ketal derivatives of tetraol (T); and step 4: hydrolyzing the acetal / ketal derivatives of tetraol (T) so as to recover pure tetraol (T).
Owner:SOLVAY SOLEXIS
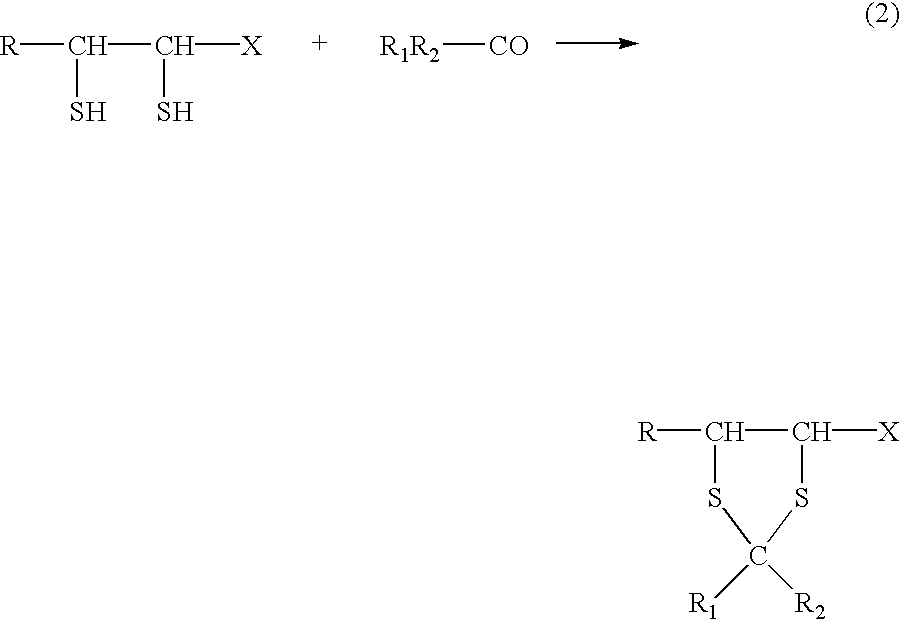
Physiologically active agents containing vicinal dithioglycols and use thereof in various branches of economy
InactiveUS7229637B2Extended shelf lifeImprove immunityCosmetic preparationsOrganic active ingredientsFood additiveActive agent
The invention relates to the food and medical industries, medical cosmetics, dermatology, agriculture and the mixed feed industry. According to the invention vicinal dithioglycole (common formula RCH(SH)CH(SH)R−1 (I)) is used as a food additive, a food product, physiologically-active substances and active ingredients of forage additives and of forage, in cosmetic and / or dermatological and skin-therapeutic remedies. The invention comprises methods for producing such additives, products and remedies. The substance of formula (I) stimulates physiological processes, increases human and animal immunity, inhibits undesirable process in organisms and food products, produces curative and preventive action of skin, hair and nails and after vicinal dithioglycole is administered the intoxication effect of alcohol consumption known as hang-over is completely remored.
Owner:ZENOVICH SERGEI MIKHAILOVICH +1
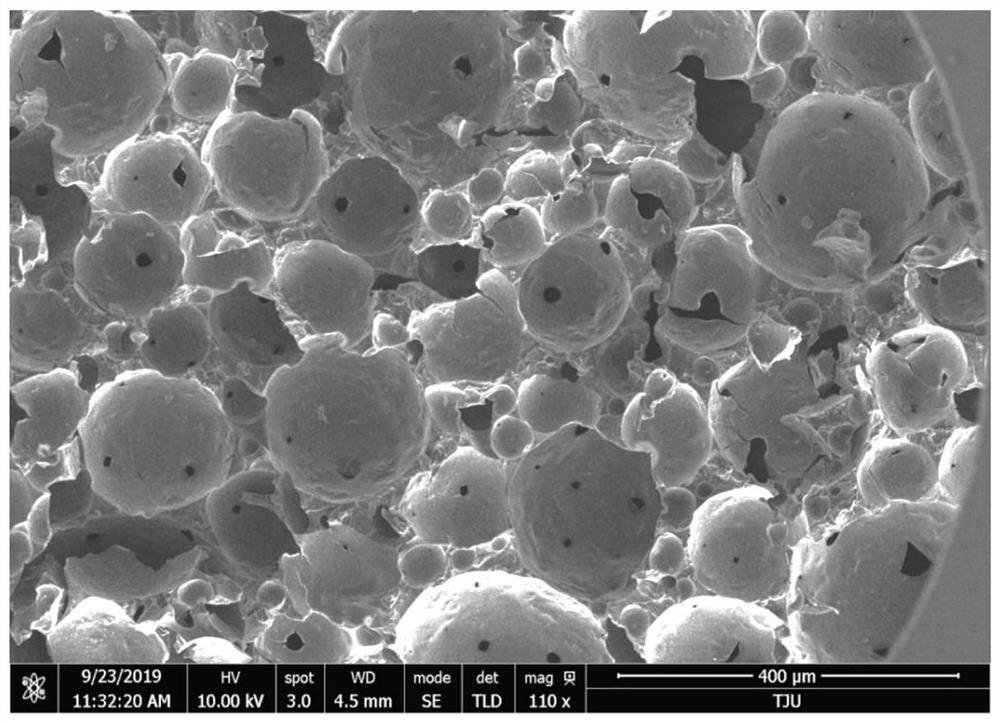
Vicinal diol functionalized macroporous through-hole material as well as preparation method and boric acid adsorption application thereof
ActiveCN111921505AReduce usageAvoid consumptionGeneral water supply conservationOther chemical processesActive agentBoronic acid
The invention discloses a vicinal diol functionalized macroporous through-hole material as well as a preparation method and boric acid adsorption application thereof. The preparation method comprisesthe following steps: (1) adding an anionic surfactant into a polyethyleneimine aqueous solution; (2) adding microcrystalline cellulose into the system as a supporting material, and oscillating at a high speed for several minutes to form an air-in-water emulsion; (3) adding a cross-linking agent into the air-in-water emulsion, and oscillating at a high speed for several minutes to partially cross-link polyethyleneimine to cure the emulsion to form a block material; (4) forming a through-hole material through vacuum dehydration, wherein the microcrystalline cellulose can prevent the porous material from overall great shrinkage in the dehydration process, but local microscopic shrinkage still occurs, so that a porous through-hole structure is formed, and the anionic surfactant adsorbed on thesurface of holes is removed through alkaline washing; and (5) converting amino hydrogen on the surface of the porous material into vicinal diol functional groups through glycidyl ether treatment. Thevicinal diol functionalized macroporous through-hole material has the characteristics of simplicity and convenience in preparation, large adsorption capacity, mild regeneration conditions, repeated regeneration and no powder falling.
Owner:TONGJI UNIV
Leoligin derivatives as smooth muscle cell proliferation inhibitors
InactiveUS20170157301A1Increase stereoselectivityEfficiently prevent restenosisOrganic active ingredientsOrganic chemistryLeiomyocyte2-Methyl-2-butene
A compound of formula (II), and a method of preparation thereof, for use as a smooth muscle cell (SMC) proliferation-inhibiting drug:wherein: R1 to R6 are —H, —F, —CH3, —CF3, —CF2CH3, —OCH3, —COCH3, —C4H9, —COOC2H5, or —C6H5, or two vicinal residues from R1 to R6 form a saturated or unsaturated carbocyclic ring together with the two carbon atoms to which they are attached; R7 is OH, allyloxy, propargyl-oxy, 2,2-dimethylpropanoyloxy (pivaloyloxy), butanoyloxy, 3-methylbutanoyloxy, 2-buten-oyloxy, 2-methyl-2-butenoyloxy, 3-methyl-2-butenoyloxy, isopentanoyloxy, 2-ethylbutanoyl-oxy, 3,3-dimethylbutanoyloxy, cyclopropylcarbonyloxy, cyclobutylcarbonyloxy, cyclo-pentylcarbonyloxy, cyclopentenylcarbonyloxy, cyclohexylcarbonyloxy, cyclo-hexenylcarbonyloxy, adamantylethanoyloxy, 3-phenylpropenoyloxy (cinnamyloyloxy), 2-methylbenzoyloxy, or naphthoyloxy; wherein, in ring A and / or B, one or more carbon ring atoms are optionally replaced by heteroatoms; wherein the compounds of formula (II) are obtained by combining the residues R1 to R7 and which inhibit SMC proliferation at least 50% more effectively than EC proliferation.
Owner:VIENNA UNIVERSITY OF TECHNOLOGY
Composite preservative containing amino acid derivatives and preparation method for composite preservative and cosmetic
PendingCN111135108AGrowth inhibitionIncrease antiseptic and antibacterial effectCosmetic preparationsToilet preparationsBiotechnologyMicroorganism
The invention discloses a composite preservative containing amino acid derivatives. The composite preservative contains the amino acid derivatives, 1,2-vicinal diol and a solvent; the amino acid derivatives are octanoyl amino acid derivatives and / or lauroyl amino acid derivatives. The composite preservative can disturb and inhibit a physiological environment of a cell membrane, namely that the composite preservative is selectively gathered on an oil-water interface in an oil-in-water emulsion system, so that a food source for microorganisms in a region where the composite preservative is gathered is changed to result in being not in favor of growth of the microorganisms, and thereby, the growth of the microorganisms is inhibited; while after the 1,2-vicinal diol is compounded with the octanoyl amino acid derivatives and lauroyl amino acid derivatives dissolved in the solvent, a synergistic effect is generated, so that antisepsis and bacteriostasis effects of octyl chains in the octanoyl amino acid derivatives and lauryl chains in the lauroyl amino acid derivatives can be enhanced to make the composite preservative have a good antisepsis and bacteriostasis effect, and the compositepreservative is very safe and mild to use. The invention further provides a preparation method for the composite preservative and a cosmetic containing the composite preservative.
Owner:广东迪美生物技术有限公司 +1
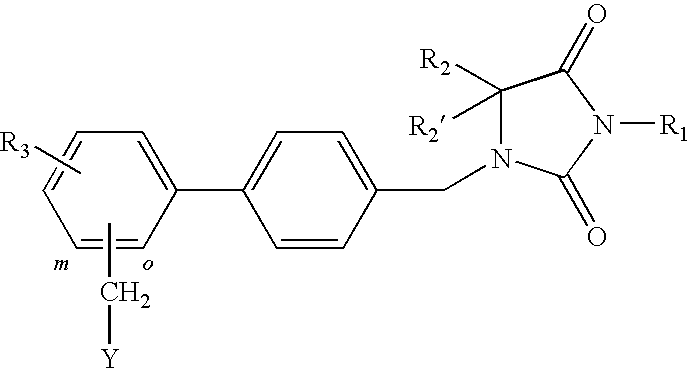
1-(biphenyl-4-ylmethyl)imidazolidine-2,4-dione
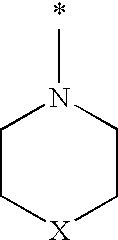
The invention relates to A 1-(biphenyl-4-ylmethyl)imidazolidine-2,4-dione derivative having the general Formula Iwherein R1 is H, (C1-6)alkyl (optionally substituted with oxo, OR4, COOR5, halogen or CN), (C2-6)alkenyl, (C2-6)alkynyl, (C3-6)cycloalkyl or (C3-6)cycloalkyl(C1-3)alkyl; R2 and R2′ are independently H or (C1-3)alkyl; or R2 and R2′ form together with the carbon atom to which they are bound a (C3-5)cycloalkyl group; R3 represents H or 1 to 4 F substituents; Y representsor NR8R9; X represents CHR6, CF2, O, S, SO or SO2; R4 and R5 are (C1-6)alkyl; R6 is H, OR7 or CN; R7 is (C1-3)alkyl; R8 is (C5-7)cycloalkyl comprising a heteroatom selected from O, S, SO and SO2; R9 is H or (C1-4)alkyl; o and m represent the ortho or meta position of the substituent Y—CH2; or a pharmaceutically acceptable salt thereof; as well as to the use of said 1-(biphenyl-4-ylmethyl)imidazolidine-2,4-dione derivatives in the treatment of pain such as for example peri-operative pain, chronic pain, neuropathic pain, cancer pain and pain and spasticity associated with multiple sclerosis.
Owner:MERCK SHARP & DOHME BV
Physiologically active agents containing vicinal dithioglycols and use thereof in various branches of economy
InactiveUS20070298081A1Extended shelf lifeImprove immunityCosmetic preparationsOrganic active ingredientsFood additivePhysiology
The invention relates to the food and medical industries, medical cosmetics, dermatology, agriculture and the mixed feed industry. According to the invention vicinal dithioglycole (common formula RCH(SH)CH(SH)R−1 (I)) is used as a food additive, a food product, physiologically-active substances and active ingredients of forage additives and of forage, in cosmetic and / or dermatological and skin-therapeutic remedies. The invention comprises methods for producing such additives, products and remedies. The substance of formula (I) stimulates physiological processes, increases human and animal immunity, inhibits undesirable process in organisms and food products, produces curative and preventive action of skin, hair and nails and after vicinal dithioglycole is administered the intoxication effect of alcohol consumption known as hang-over is completely remored.
Owner:ZENOVICH SERGEI MIKHAILOVICH +1
Process for the purification of polyol PFPE derivative
A process for the purification of a tetraol PFPE derivative [tetraol (T)] of formula (I): HO—CH2—CH(OH)—CH2—O—CH2—CF2—O—Rf—CF2—CH2O—CH2—CH(OH)—CH2OH, wherein Rf represents a fluoropolyoxyalkene chain (chain Rf), from a mixture (M) of hydroxyl (per)fluoropolyether derivatives, said mixture comprising said tetraol (T) and at least one hydroxyl (per)fluoropolyether [PFPE (OH)] comprising a chain Rf terminated with at least one end group of formula: —CF2CH2OH; —CF2CH2O—CH(OH)—CH2—O—CH2—CH(OH)—CH2OH; said process comprising: step 1: reacting the mixture (M) with ketones and / or aldehydes so as to selectively protect couples of hydroxyl groups on vicinal carbon atoms by forming corresponding cyclic ketal / acetal derivatives, so as to yield a protected mixture (P); step 2: reacting residual hydroxyl groups of the mixture (P) with suitable functionalizing agents enabling substantial volatility modification, so as to obtain a functionalized protected mixture (Pf); step 3: fractional distillation of the mixture (Pf) so as to isolate the cyclic acetal / ketal derivatives of tetraol (T); and step 4: hydrolyzing the acetal / ketal derivatives of tetraol (T) so as to recover pure tetraol (T).
Owner:SOLVAY SOLEXIS
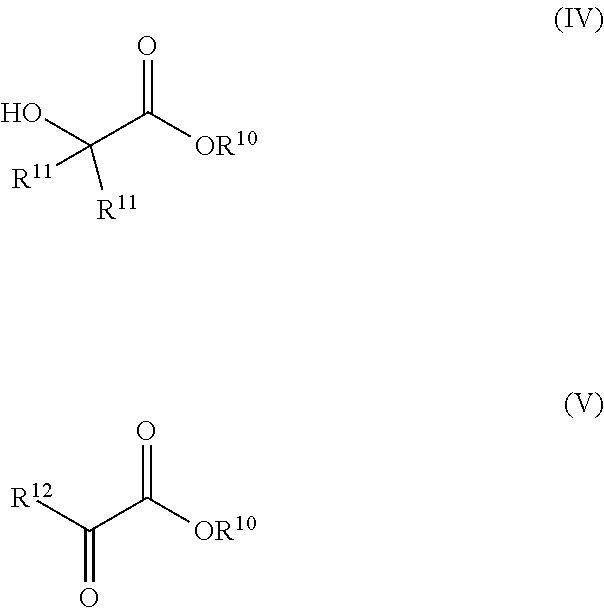
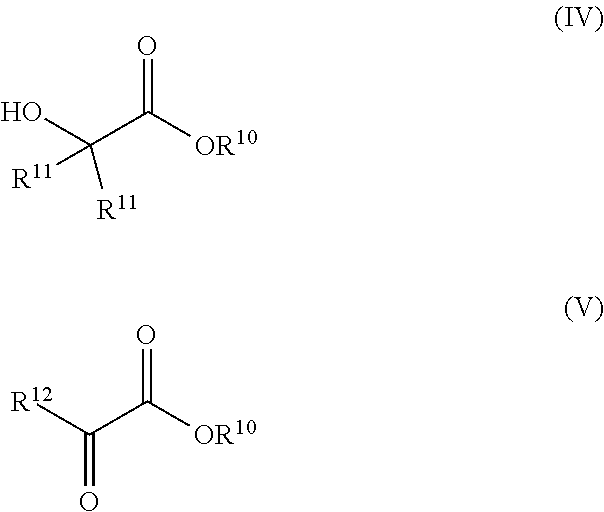
Synthesis of glycols via transfer hydrogenation of alpha-functional esters with alcohols
ActiveUS10266467B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSimple Organic CompoundsAlcohol
A transfer hydrogenation process for forming vicinal diols by hydrogenating 1,2-dioxygenated organic compounds using alcohols as the reducing agent instead of the traditional H2 gas. The transfer hydrogenation is carried out under milder conditions of temperature and pressure than is typical for ester hydrogenation with H2. The milder conditions of operation provide benefits, such as lower operating and capital costs for industrial scale production as well as savings in product purification due to the avoidance of by-products from exposure of reaction mixtures and products to high temperatures.
Owner:EASTMAN CHEM CO
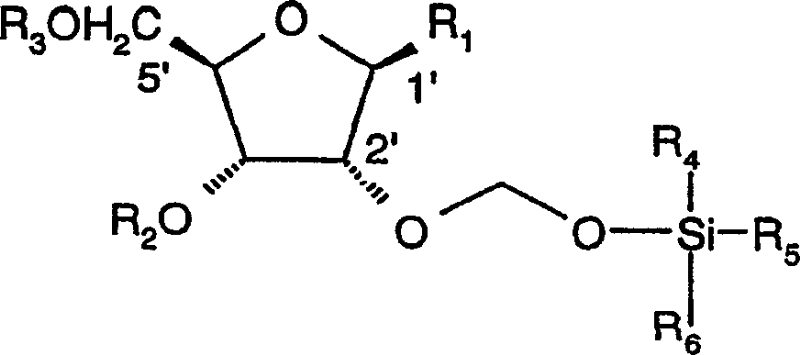
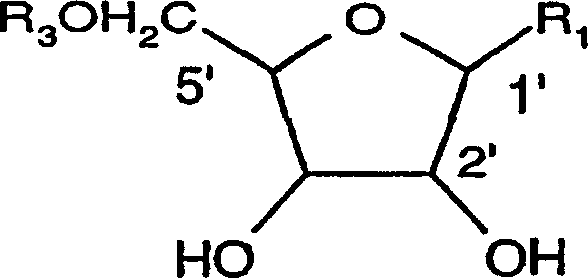
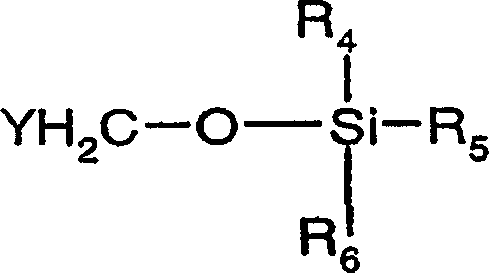
Organic compounds of 2'-o-trisubstituted siloxy methyl ribonucleoside derivatives and their preparation
The invention provides ribonucleoside derivatives with novel protecting groups and methods for the preparation of such ribonucleoside derivatives. The general formula (I) of the ribonucleoside derivatives is: wherein R1 is a base of the purine- or pyrimidine-family or a derivative of such a base or any other residue with serves as a nucleobase surrogate, R2 is a proton or a substituted derivative of phosphonic acid, R3 is a proton or a protection-group for the oxygen atom in 5'-position, R4, R5 and R6 are independently alkyl or aryl or a combination of alkyl and aryl or heteroatom, R4, R5 or R6 may also be cyclically connected to each other; and wherein at least one of the R4, R5 or R6 substituents comprises a tertiary C-atom or a heteroatom vicinal to the Si-atom.
Owner:NOVARTIS AG
Macrocyclic poly(alkane)s and poly(alkane-co-alkene)s
Macrocyclic polyalkene homopolymers and copolymers can be formed and converted to macrocyclic polyalkanes or macrocyclic poly(alkane-co-alkene) upon hydrogenation or, when the macrocyclic polyalkene is reacted with an alkene in the presence of an olefin metathesis catalyst, to a macrocyclic poly(alkane-co-alkene) comprising vicinal -C(=CR2)-'s. Upon hydrogenation of a macrocyclic poly(alkane-co-alkene) comprising vicinal -C(=CR2)- 's, macrocyclic poly(alkane)s or poly(alkane-co-alkene)s with isolated -C(=CR2)- groups can be provided, depending on the degree of hydrogenation. The poly(alkane-co-alkene)s with isolated -C(=CR2)- units can be used to form poly(macrocyclic poly(alkane-co-alkene))s, poly(macrocyclic poly(alkane))s, and / or bi-, tri-, and / or multi-macrocyclic poly(alkane-co- alkene)s or bi-, tri-, and / or multi-macrocyclic poly(alkane)s.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Formaldehyde free microspheres and encapsulation
Processes for producing polymer microcapsules using vicinal functional oligomers are also described. The vicinal functional oligomers can be made by polymerizing an acrylate monomer, a styrene monomer, or both in the presence of a chain transfer agent. The vicinal functional oligomers can be reacted with epichlorohydrin to form vicinal epoxies. The vicinal epoxies can be reacted with polyamines to form epoxy polymer microspheres. The vicinal epoxies can be reacted with carbon dioxide in the presence of a catalyst to form vicinal cyclic carbonates. The vicinal cyclic carbonates can be reacted with polyamines to form isocyanate-free polymer microspheres. Polymer microspheres made by the processes are also described.
Owner:BATTELLE MEMORIAL INST
Novel mycobacterial inhibitors
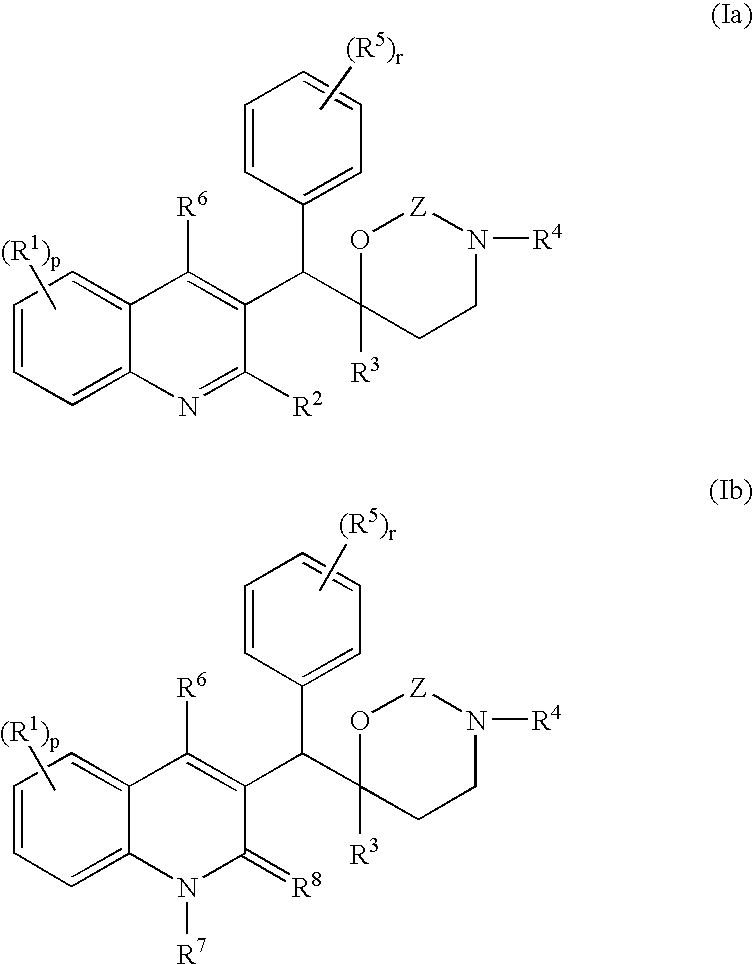
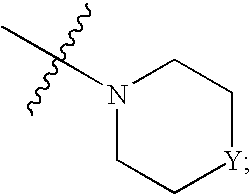
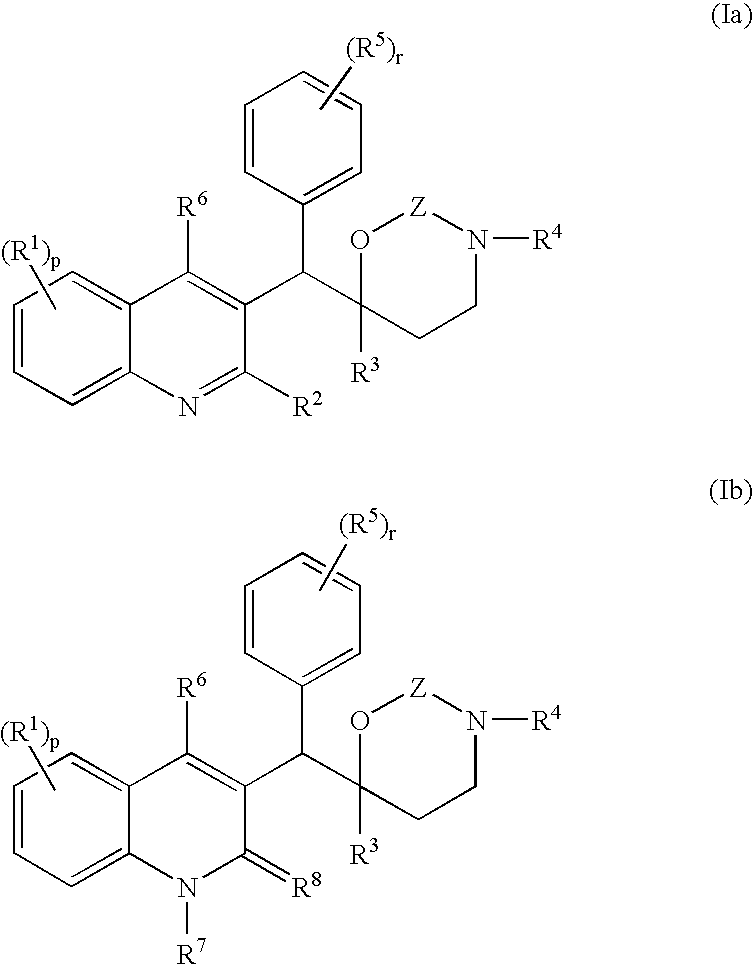
The present invention relates to novel substituted quinoline derivatives according to the general formula (Ia) or the general formula (Ib) salts, quaternary amines, stereochemically isomeric forms, tautomeric forms and N-oxide forms thereof, wherein R1 is hydrogen, halo, haloalkyl, cyano, hydroxy, Ar, Het, alkyl, alkyloxy, alkylthio, alkyloxyalkyl, alkylthioalkyl, Ar-alkyl or di(Ar)alkyl; p is 1, 2, 3 or 4; R2 is hydrogen, hydroxy, thio, alkyloxy, alkyloxyalkyloxy, alkylthio, mono or di(alkyl)amino or a radical of formula R3 is alkyl, Ar, Ar-alkyl, Het or Het-alkyl; R4 is hydrogen, alkyl or benzyl; R5 is hydrogen, halo, haloalkyl, hydroxy, Ar, alkyl, alkyloxy, alkylthio, alkyloxyalkyl, alkylthioalkyl, Ar-alkyl or di(Ar)alkyl; or two vicinal R5 radicals may be taken together to form together with the phenyl ring to which they are attached a naphthyl; r is 1, 2, 3, 4 or 5; R6 is hydrogen, alkyl, Ar or Het; R7 is hydrogen or alkyl; R8 is oxo; or R7 and R8 taken together form the radical CH═CH—N═; Z is CH2 or C(═O). The claimed compounds are useful for the treatment of mycobacterial diseases, particularly those diseases caused by pathogenic mycobacteria such as M. tuberculosis, M. bovis, M. avium, M. smegmatis and M. marinum. Also claimed is a pharmaceutical composition containing a compound of the present invention, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of mycobacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
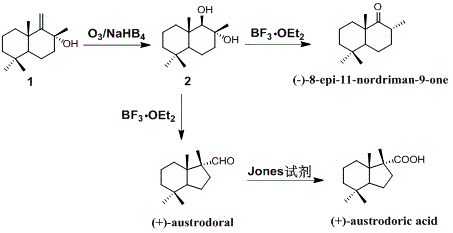
Synthetic method of austrodoral and austrodoric acid
ActiveCN105669440AFew stepsHigh yieldPreparation by oxidation reactionsOrganic compound preparationChemical synthesisNatural product
The invention relates to a synthetic method of an ocean terpene natural product austrodoral, austordoric acid and a land terpene natural product 8-epi-11-nordriman-9-one and belongs to the field of chemical synthesis. Sesquiterpenes vicinal diol is prepared by selective ozonization reaction of sesquiterpenes enol, the land terpene natural product 8-epi-11-nordriman-9-one and the ocean terpene natural product austrodoral are synthesized by the selective rearrangement reaction of the sesquiterpenes vicinal diol, and the ocean terpene natural product austrodoric acid is synthesized by oxidation reaction of the austrodoral. The method has the characteristics of low production cost, simple process conditions, easiness in control, suitability for industrial production, high overall yield and the like.
Owner:HARBIN INST OF TECH AT WEIHAI
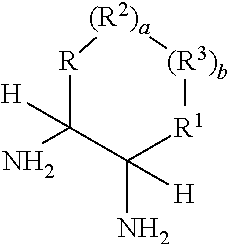
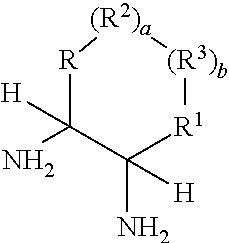
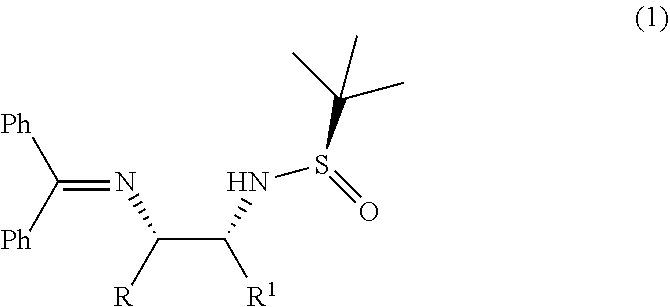
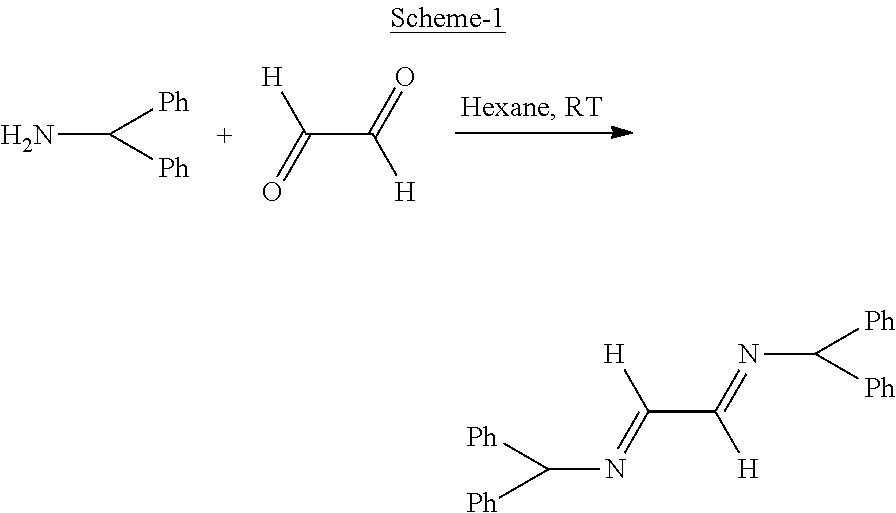
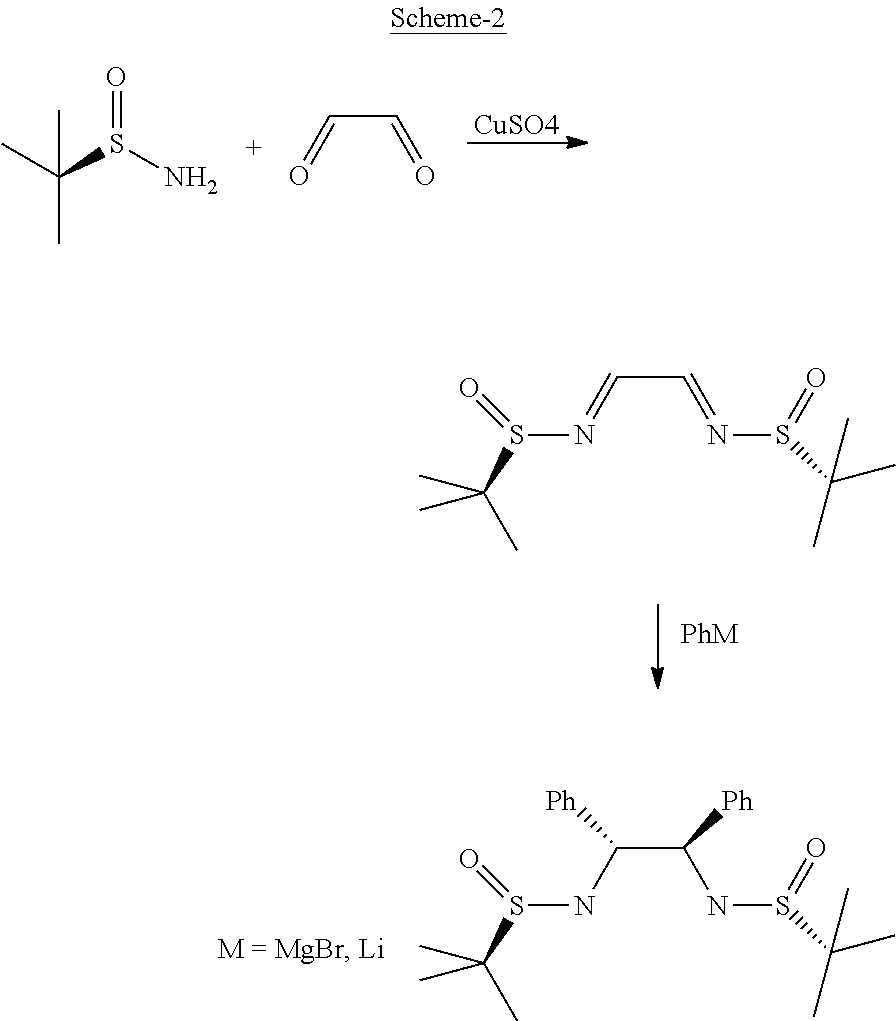
Process for diastereoselective synthesis of vicinal diamines
ActiveUS11299455B2Organic chemistry methodsSulfonic acid amide preparationImideEnantioselective synthesis
Owner:PIRAMAL PHARMA LTD
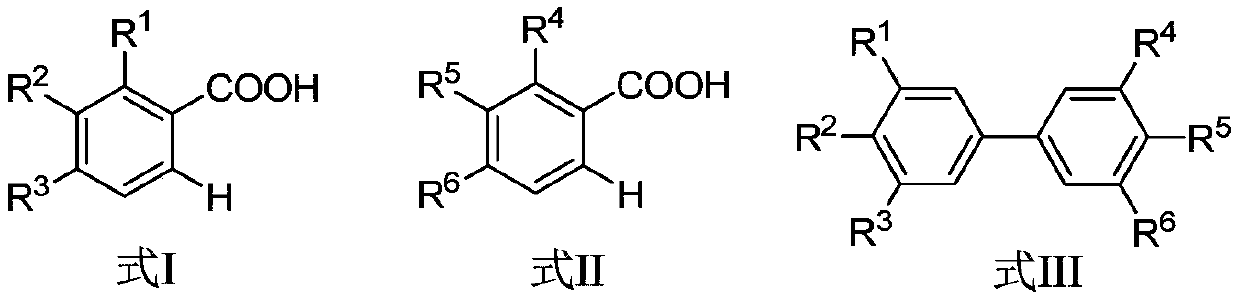
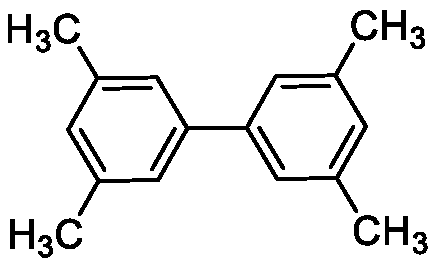
A kind of synthetic method of biphenyl derivatives
ActiveCN107324964BEfficient synthesisEasy to operateOrganic compound preparationCatalystsBiphenyl derivativesCarboxyl radical
The invention discloses a synthesis method of biphenyl derivatives. According to the method, transition metal palladium is taken as a catalyst, aromatic carboxylic acid is subjected to a carboxyl-vicinal crossed dehydrogenate coupling and decarboxylic reaction, and a series of biphenyl derivatives are synthesized in one step simply, conveniently and efficiently. The synthesis method has the advantages that raw materials are simple and easy to obtain, operation is simple, environment-friendliness is realized and the like.
Owner:SHAANXI NORMAL UNIV
Synthetic method of 1,2-o-diol compounds
ActiveCN109384641BHigh selectivityHigh yieldOrganic compound preparationPreparation by hydrolysisInorganic saltsPtru catalyst
The invention relates to a method for synthesizing 1,2-o-diol compounds. The synthesis method comprises the following steps: (1) using a terminal olefin as a raw material, potassium hydrogen persulfate compound salt as an oxidizing agent, an inorganic salt as a catalyst, mixing water and a ketone organic solvent as a reaction solvent, and reacting at 30-100° C.; (2) After the reaction, add an inorganic base at 0-40°C to make the pH of the reaction system 10-14; (3) continue the reaction at 30-100°C, after the reaction, separate and purify to obtain 1,2-o-diol class of compounds. The synthesis method has mild reaction system, cheap and easy-to-obtain raw materials, environment-friendly oxidant, good reaction selectivity and high conversion rate.
Owner:HUAIHUA UNIV
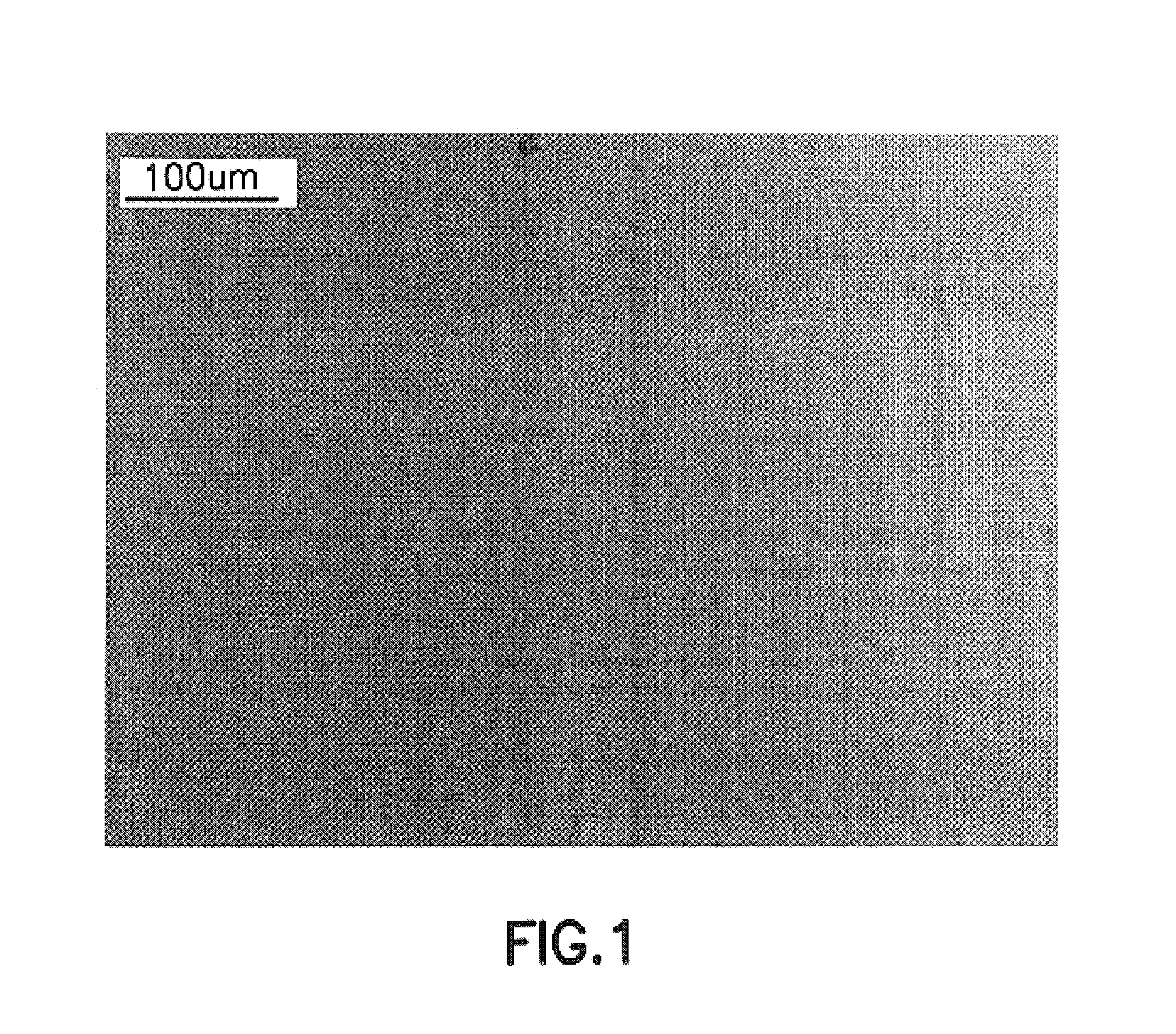
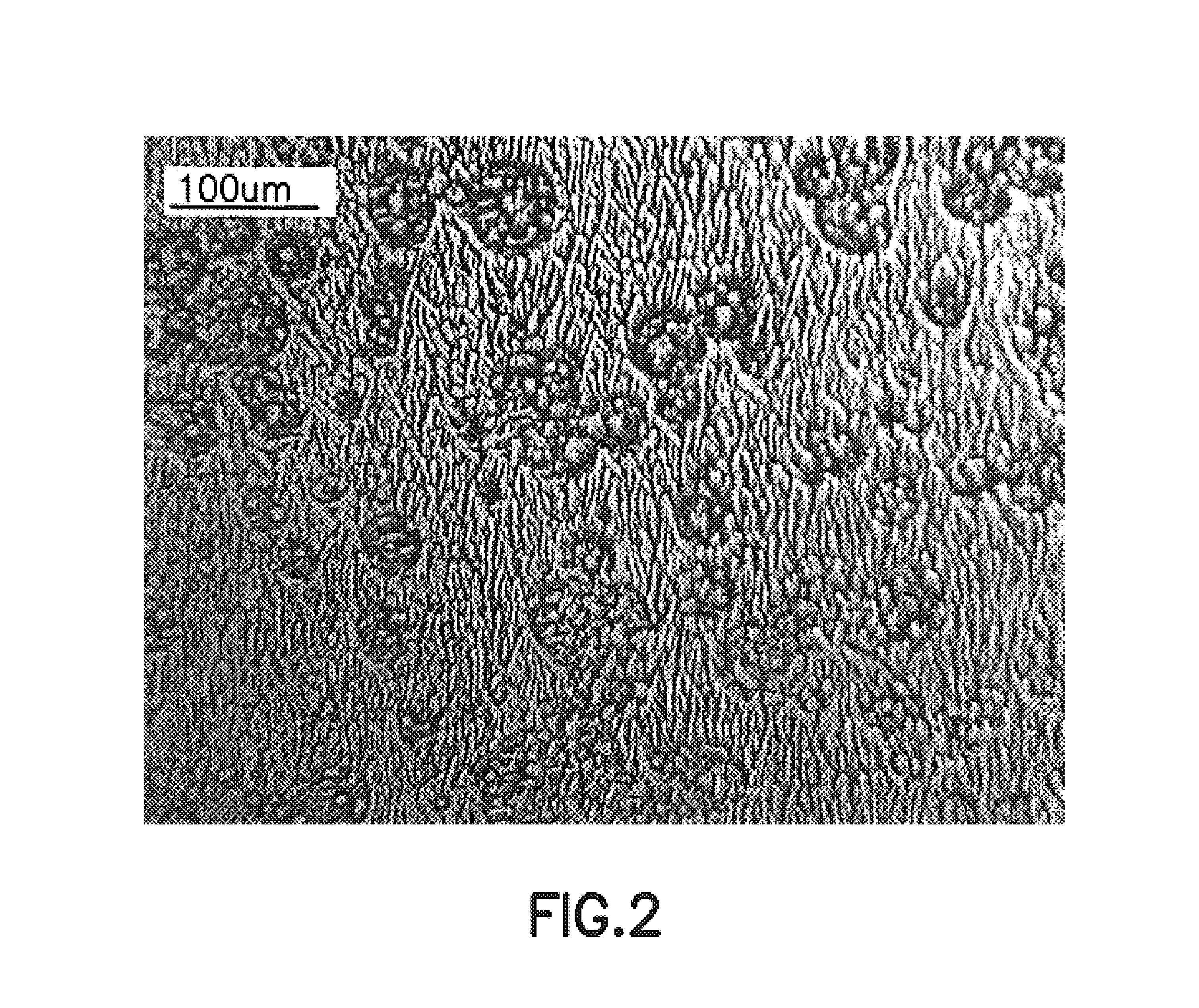
Process for epitaxying gallium selenide on a [111]-oriented silicon substrate
ActiveUS11342180B2Reduce defectsReduce formationPolycrystalline material growthSemiconductor/solid-state device manufacturingCrystallographyBi layer
A process for epitaxying GaSe on a [111]-oriented silicon substrate, includes a step of selecting a [111]-oriented silicon substrate resulting from cutting a silicon bar in a miscut direction which is one of the three [11-2] crystallographic directions, the miscut angle (α) being smaller than or equal to 0.1°, the obtained surface of the substrate forming a vicinal surface exhibiting a plurality of terraces and at least one step between two terraces; a passivation step consisting of depositing an atomic bilayer of gallium and of selenium on the vicinal surface of the silicon substrate so as to form a passivated vicinal surface made of silicon-gallium-selenium (Si—Ga—Se), said passivated vicinal surface exhibiting a plurality of passivated terraces and at least one passivated step between two passivated terraces; a step of forming a layer of two-dimensional GaSe by epitaxy on the passivated surface, said formation step comprising a step of nucleation from each passivated step and a step of lateral growth on the passivated terraces from the nuclei obtained in the nucleation step. A structure obtained by means of the epitaxying process is also provided.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +2
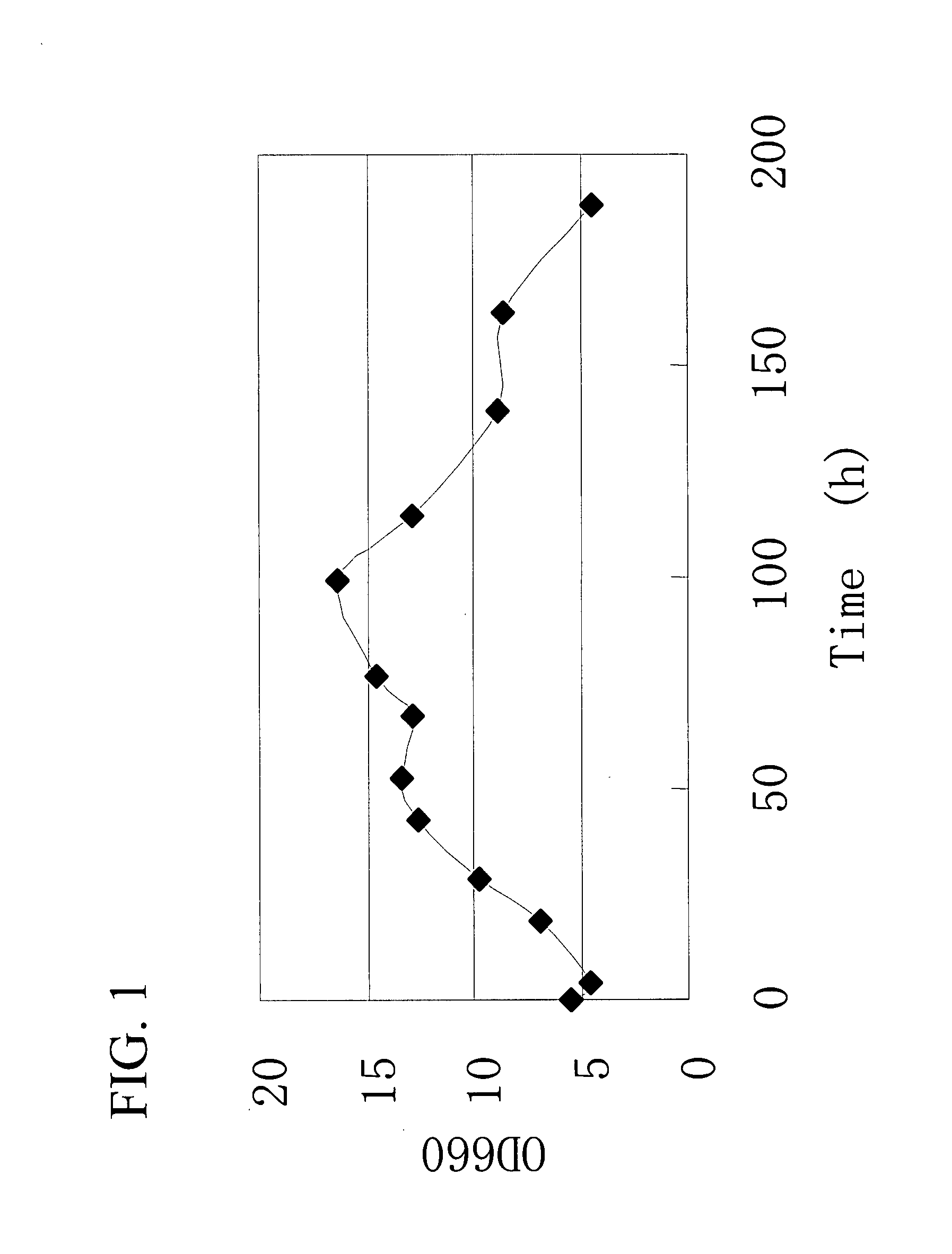
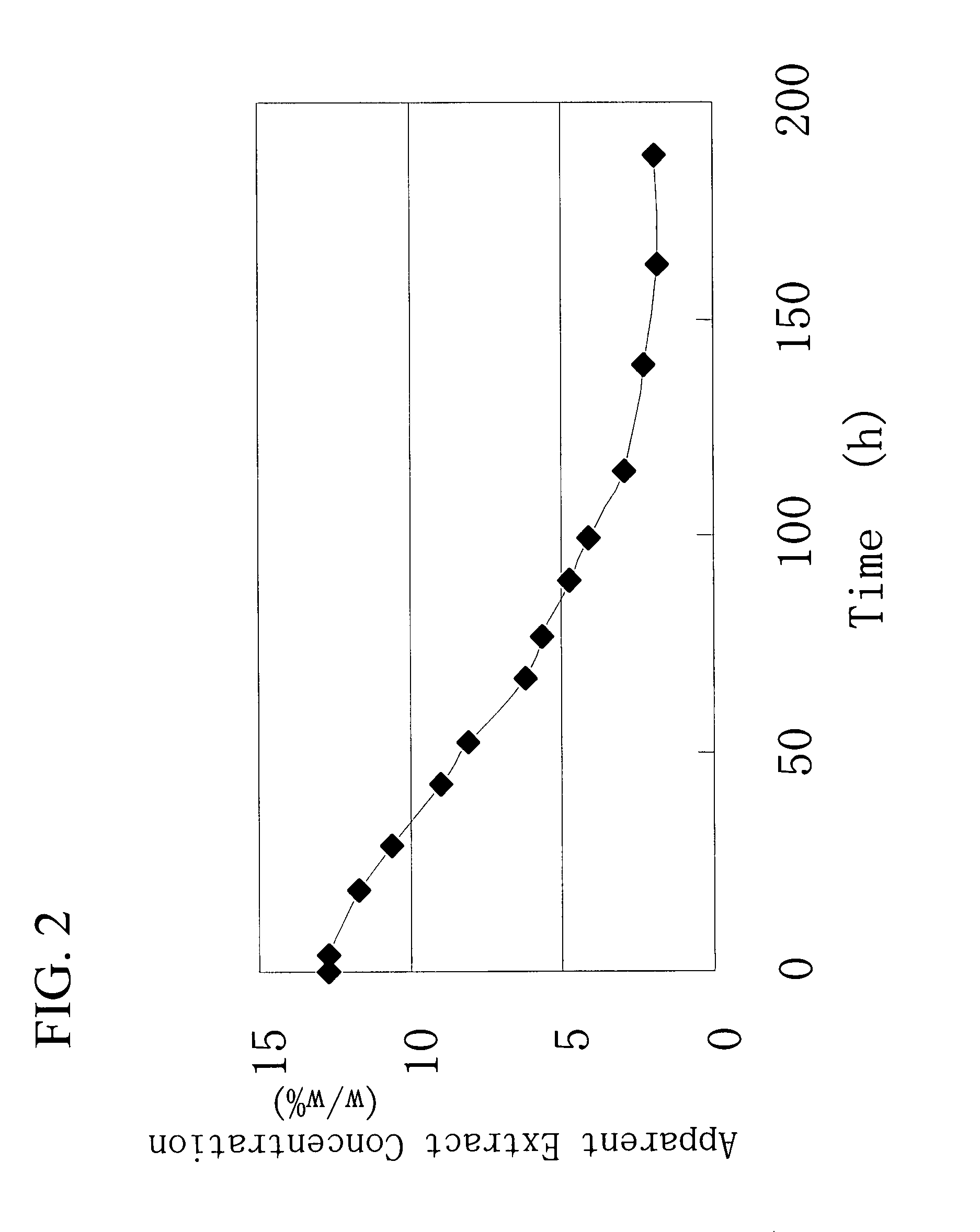
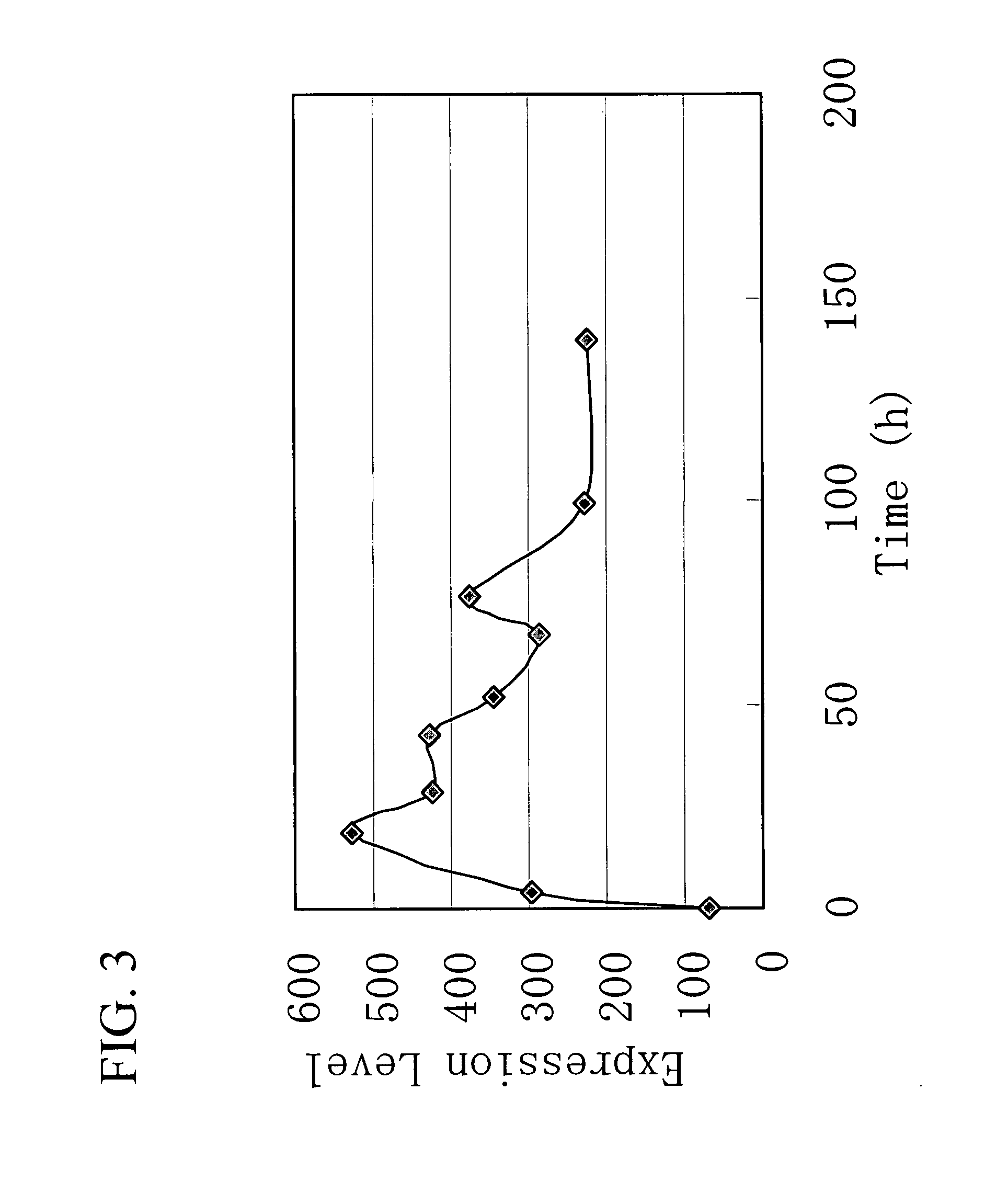
Gene Encoding Protein With Vicinal Diketone or Diacetyl-Reducing Activity and Use Thereof
The present invention relates to a gene encoding a protein having a vicinal diketone or diacetyl-reducing activity and use thereof, in particular, a brewery yeast for producing alcoholic beverages with superior flavor, alcoholic beverages produced with said yeast, and a method for producing said beverages. More particularly, the present invention relates to a yeast, whose capability of producing vicinal diketones, especially diacetyl, that are responsible for off-flavors in products, is reduced by amplifying expression level of MMF1 gene encoding a protein (Mmflp) having a vicinal diketone or diacetyl-reducing activity, especially the non-ScMMF1 gene or ScMMF1 gene specific to a lager brewing yeast, and to a method for producing alcoholic beverages with said yeast.
Owner:SUNTORY HLDG LTD
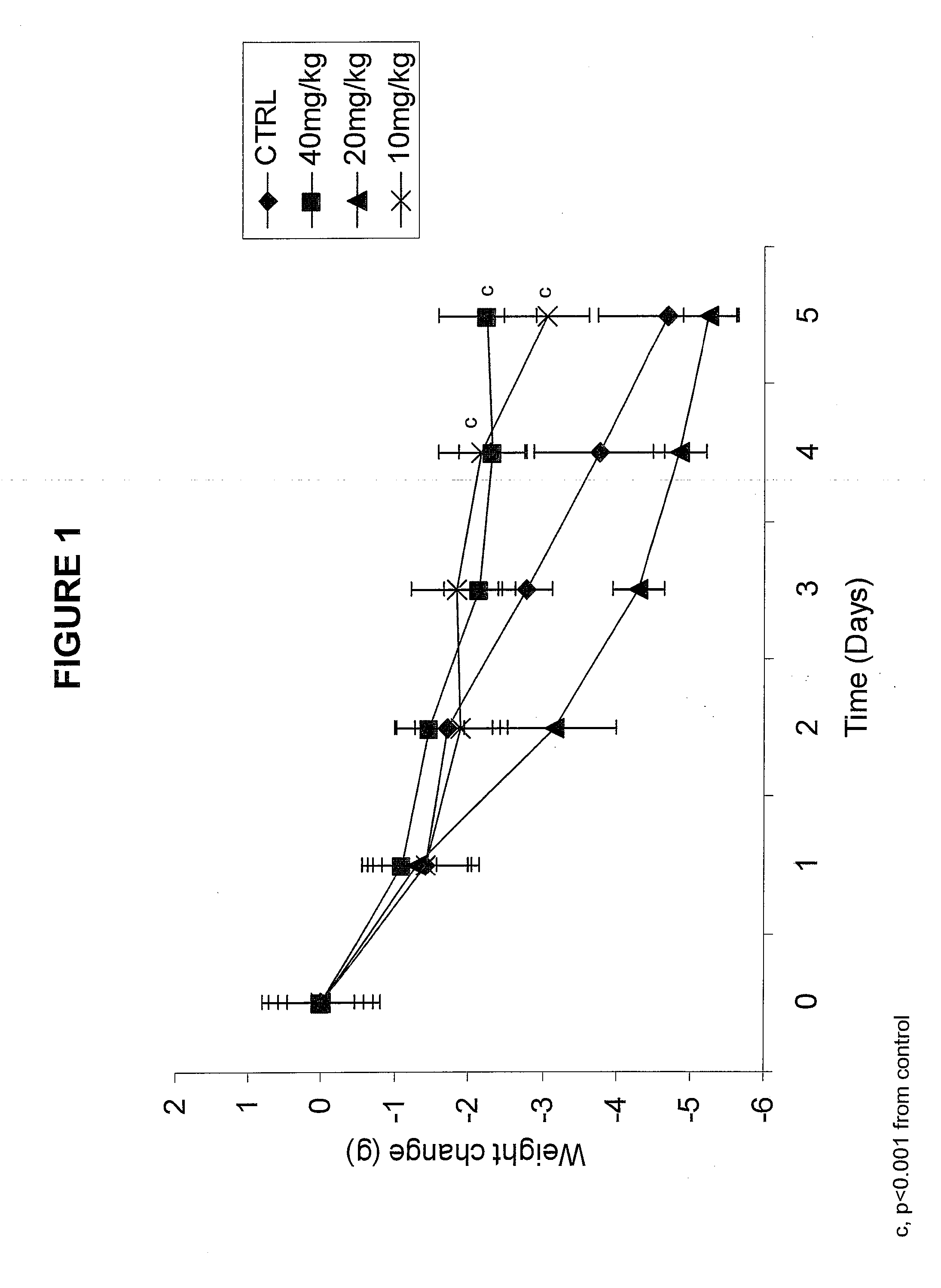
Method and Means for the Treatment of Cachexia
InactiveUS20100261682A1Reduce enzyme activityPreventing and alleviating and conditionBiocideMetabolism disorderBiochemistryPharmacology
The present invention relates to the treatment of cachexia in a mammal by the use of a compound comprising a high density negatively charged domain of vicinally oriented radicals.
Owner:BIONERIS
Method for preparing urea-functional siloxanes
Urea-functional organopolysiloxanes containing units of the formulaRnSiO(4-n) / 2 (I),where R is a radical R1 or a radical —OR2 or a radical Q, Q is a urea-functional radical of the formula—R5—[NR4—R6—]xNR4R3 (II),where the radicals R4 are identical or different and are each a radical R4′ or a radical Ru, where R4′ is hydrogen or a monovalent C1-C6-hydrocarbon radical where Ru is radical of the formula —C(═O)—NH2, and where at least one urea-functional radical Q having a radical Ru is present per molecule are prepared by reacting amino-functional organopolysiloxanes comprising units of the formulaR′nSiO(4-n) / 2 (IV),where A is an amino-functional radical of the formula—R5—[NR4′—R6—]xNR4′R3 (V),with the proviso that at least one amino-functional radical A is present per molecule in the organopolysiloxanes made up of units of the formula (III),with ortho-substituted aryl carbamates of the formulaR7—Ar—O—C(═O)—NH2 (VI),where Ar is an ortho-substituted aryl radical.
Owner:WACKER CHEM GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
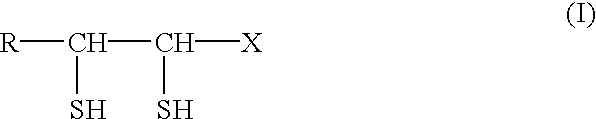
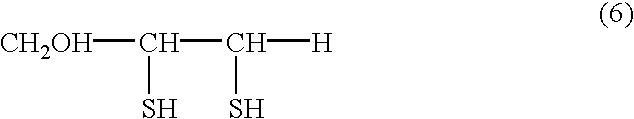

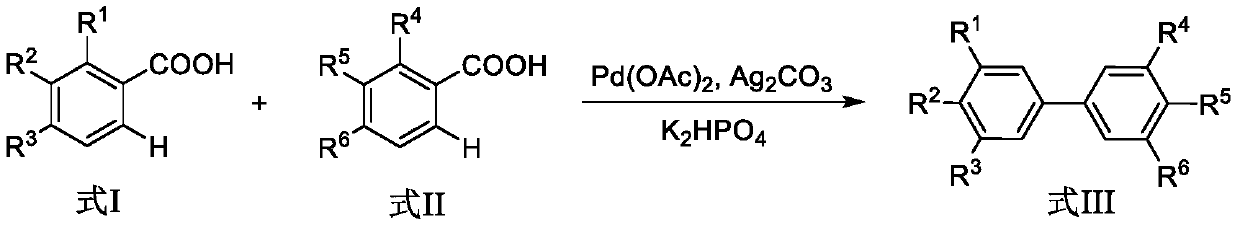
![Process for epitaxying gallium selenide on a [111]-oriented silicon substrate Process for epitaxying gallium selenide on a [111]-oriented silicon substrate](https://images-eureka.patsnap.com/patent_img/7f96b98c-6328-45ec-8e62-b8b0bc5dc2f3/US11342180-D00001.png)
![Process for epitaxying gallium selenide on a [111]-oriented silicon substrate Process for epitaxying gallium selenide on a [111]-oriented silicon substrate](https://images-eureka.patsnap.com/patent_img/7f96b98c-6328-45ec-8e62-b8b0bc5dc2f3/US11342180-D00002.png)
![Process for epitaxying gallium selenide on a [111]-oriented silicon substrate Process for epitaxying gallium selenide on a [111]-oriented silicon substrate](https://images-eureka.patsnap.com/patent_img/7f96b98c-6328-45ec-8e62-b8b0bc5dc2f3/US11342180-D00003.png)