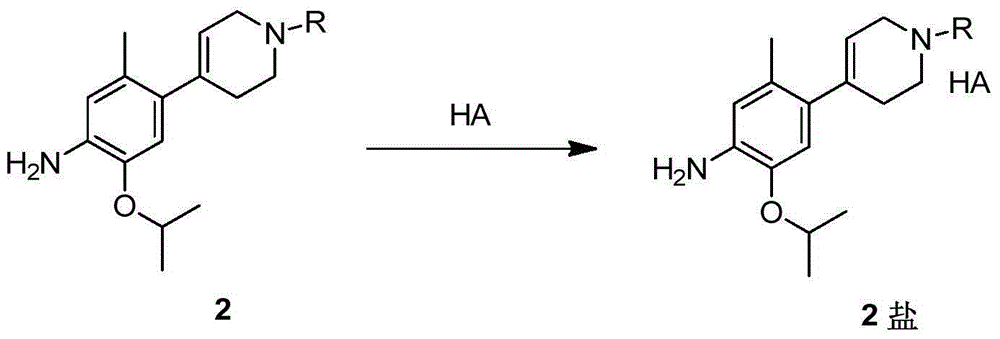
Patents
Literature
268 results about "Transfer hydrogenation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transfer hydrogenation is the addition of hydrogen (H₂; dihydrogen in inorganic and organometallic chemistry) to a molecule from a source other than gaseous H₂. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H₂. One large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.
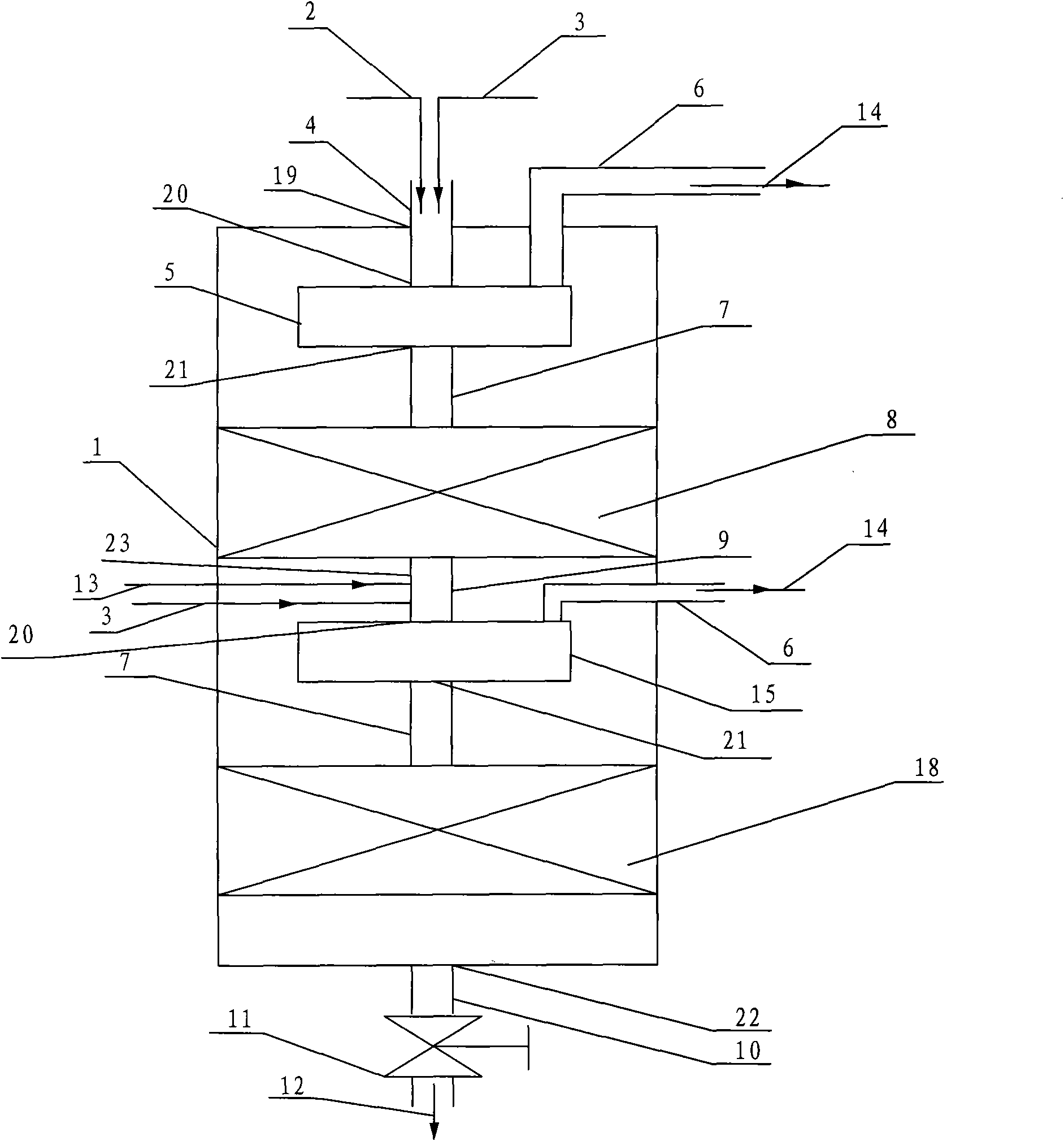
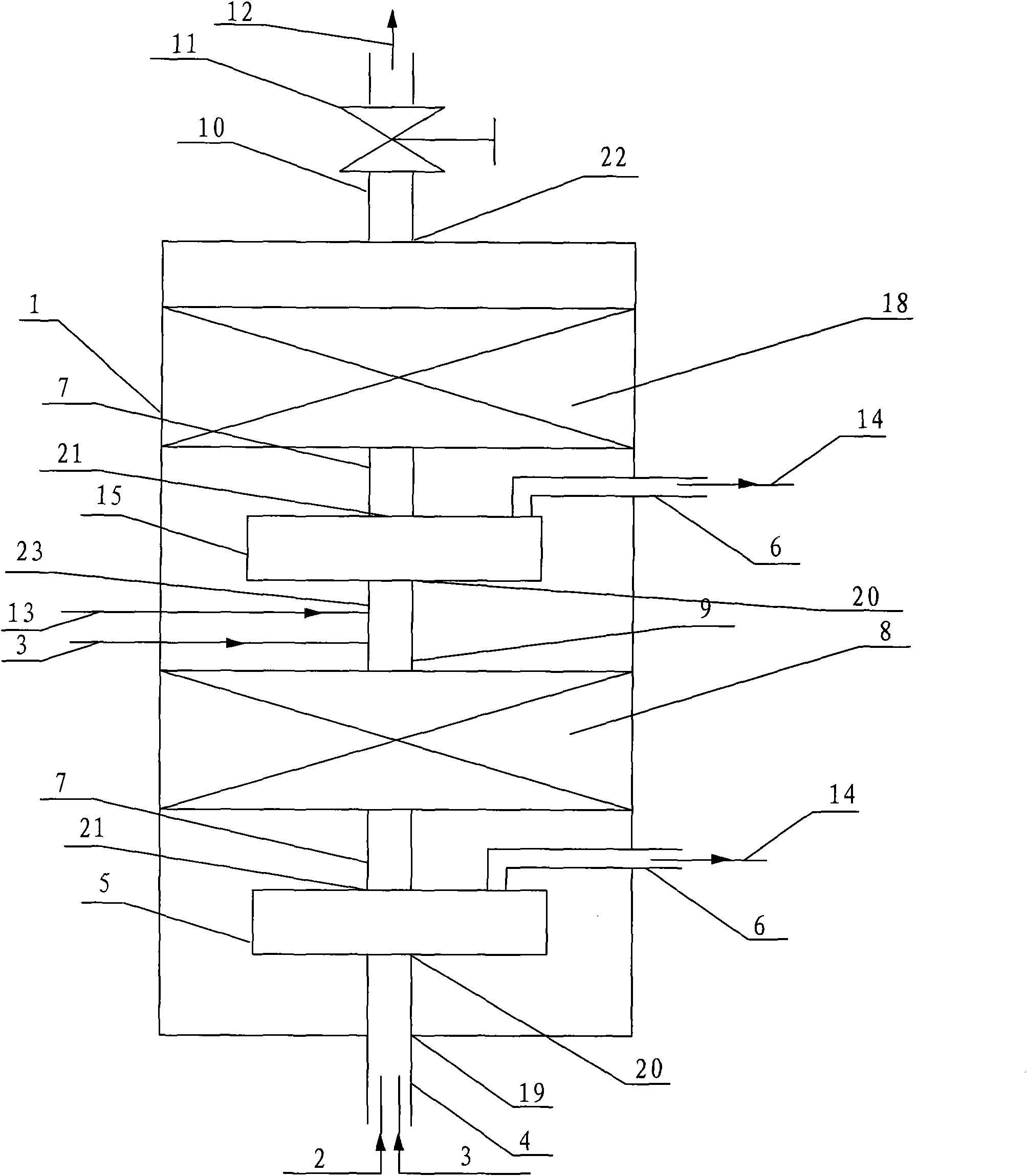
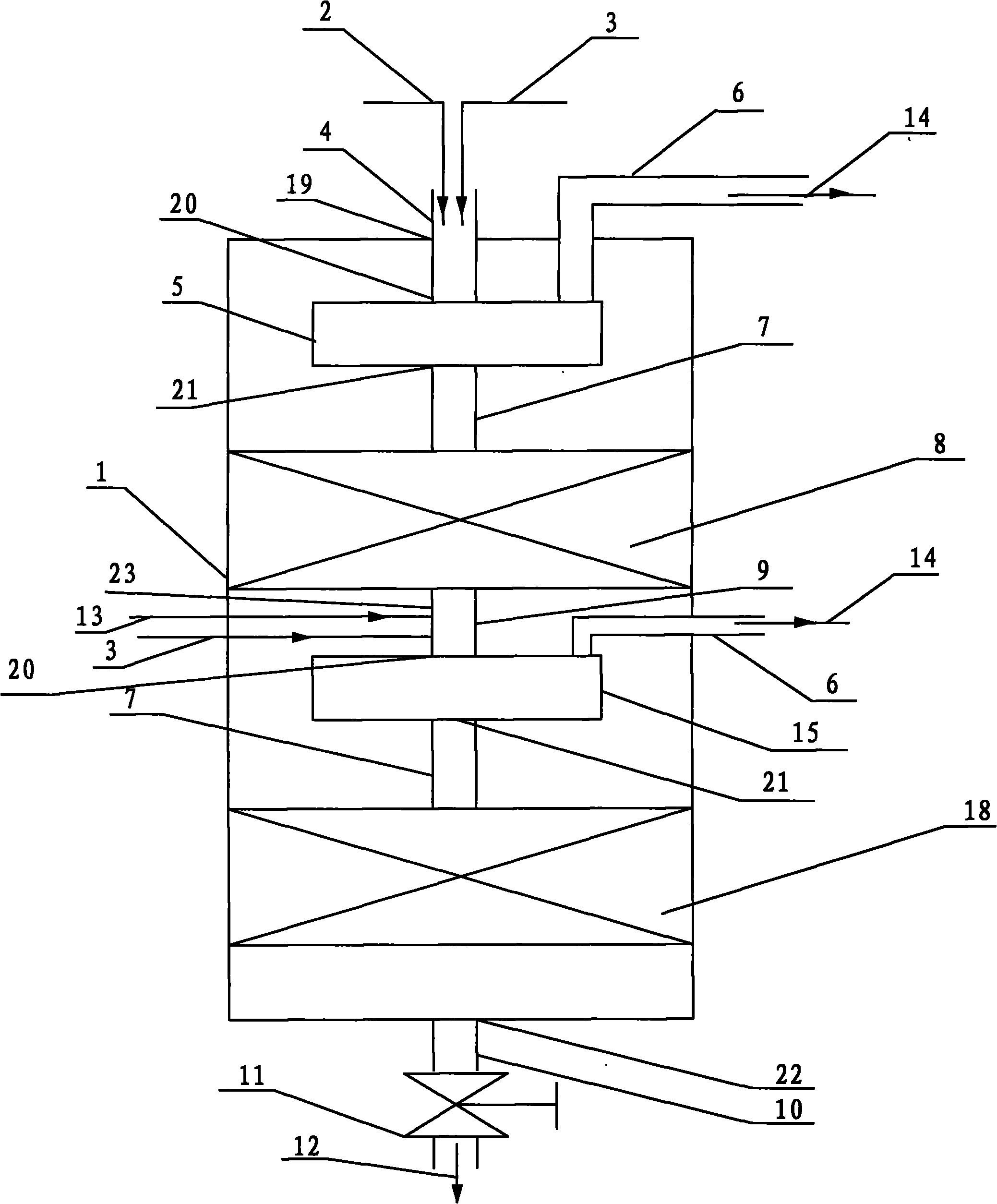
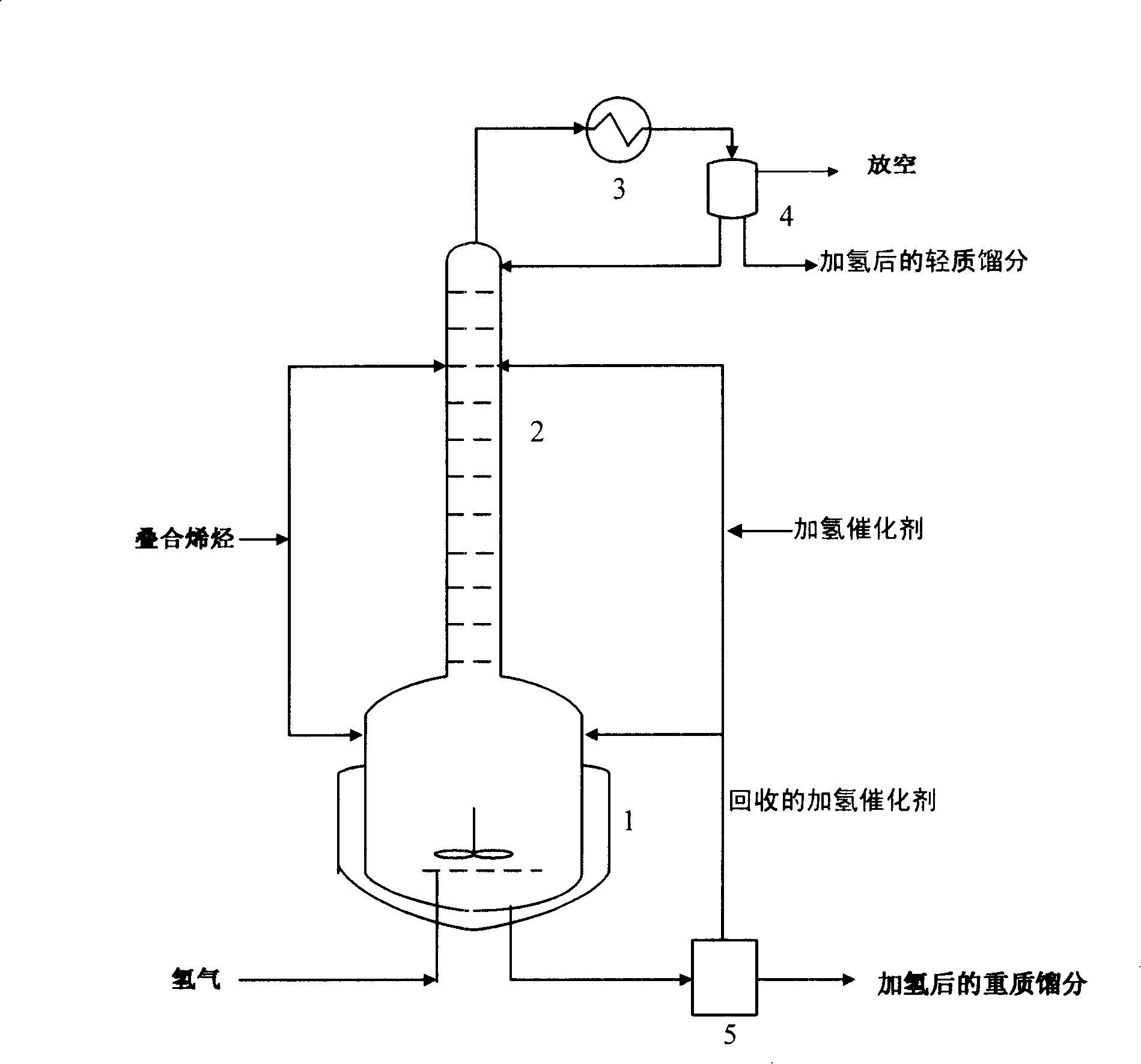
Reactor and application thereof to hydrocarbon oil liquid-solid two-phase hydrogenation
InactiveCN101992048AReduce dosageReduce lossesChemical/physical processesHydrocarbon oils treatmentHydrogenation reactionProduct gas
The invention discloses a hydrocarbon oil hydrogenation reactor, which comprises a reactor barrel body and a catalyst bed layer. An outlet and an inlet are respectively formed at the upper part and lower part of the reactor. The reactor is characterized in that: at least one mixer is arranged in the reactor; the reactor is provided with a raw material mixture and a hydrogen inlet and also a hydrogen-containing mixture and a gas outlet; and the inlet of the mixer is connected with the inlet of reactor. Due to the adoption of the reactor, pressure difference of the pressure in the mixer and the pressure outside the mixture is reduced, wall thickness of the reactor is thinned, and equipment investment and operating cost are decreased.
Owner:CHINA PETROCHEMICAL CORP +1
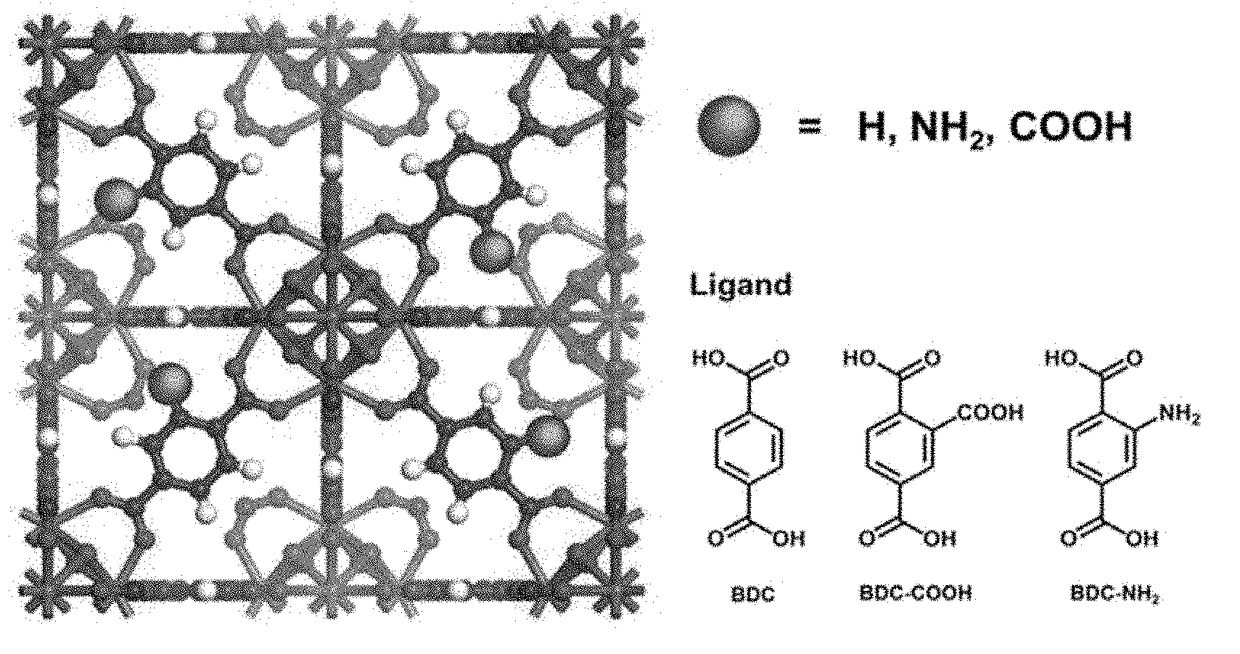
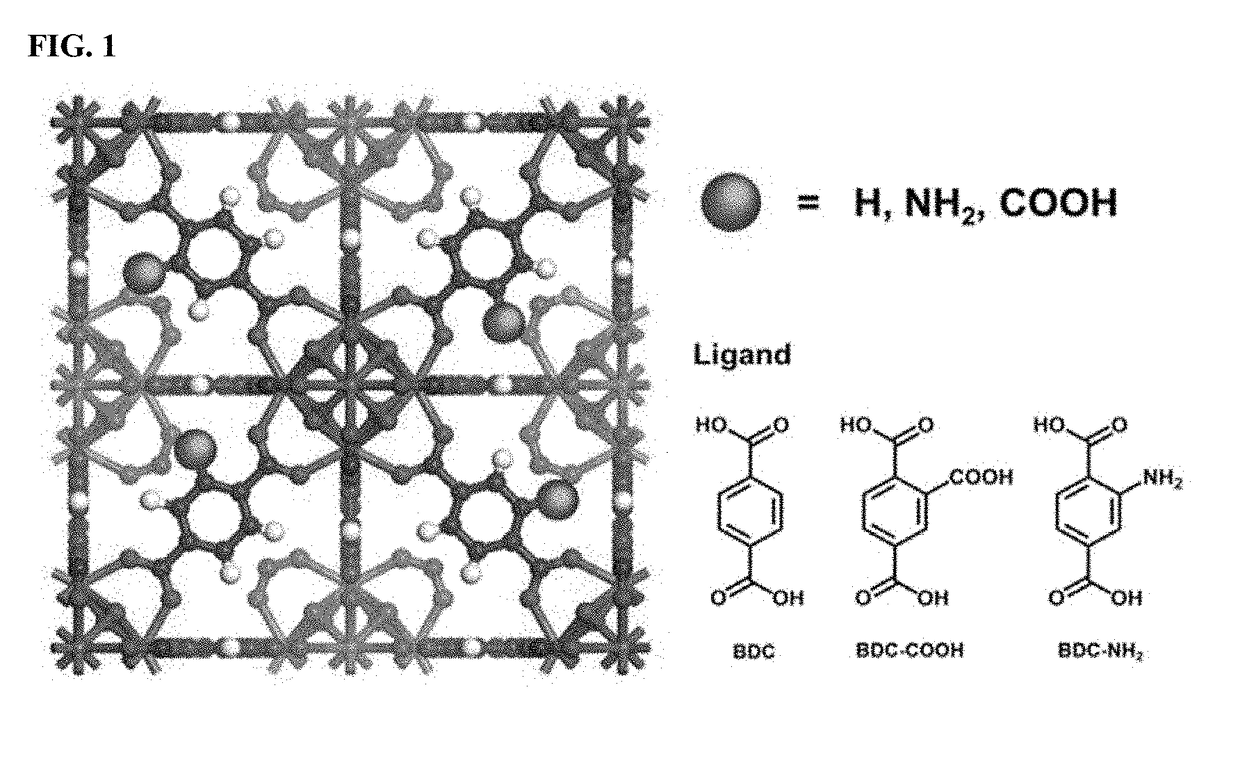
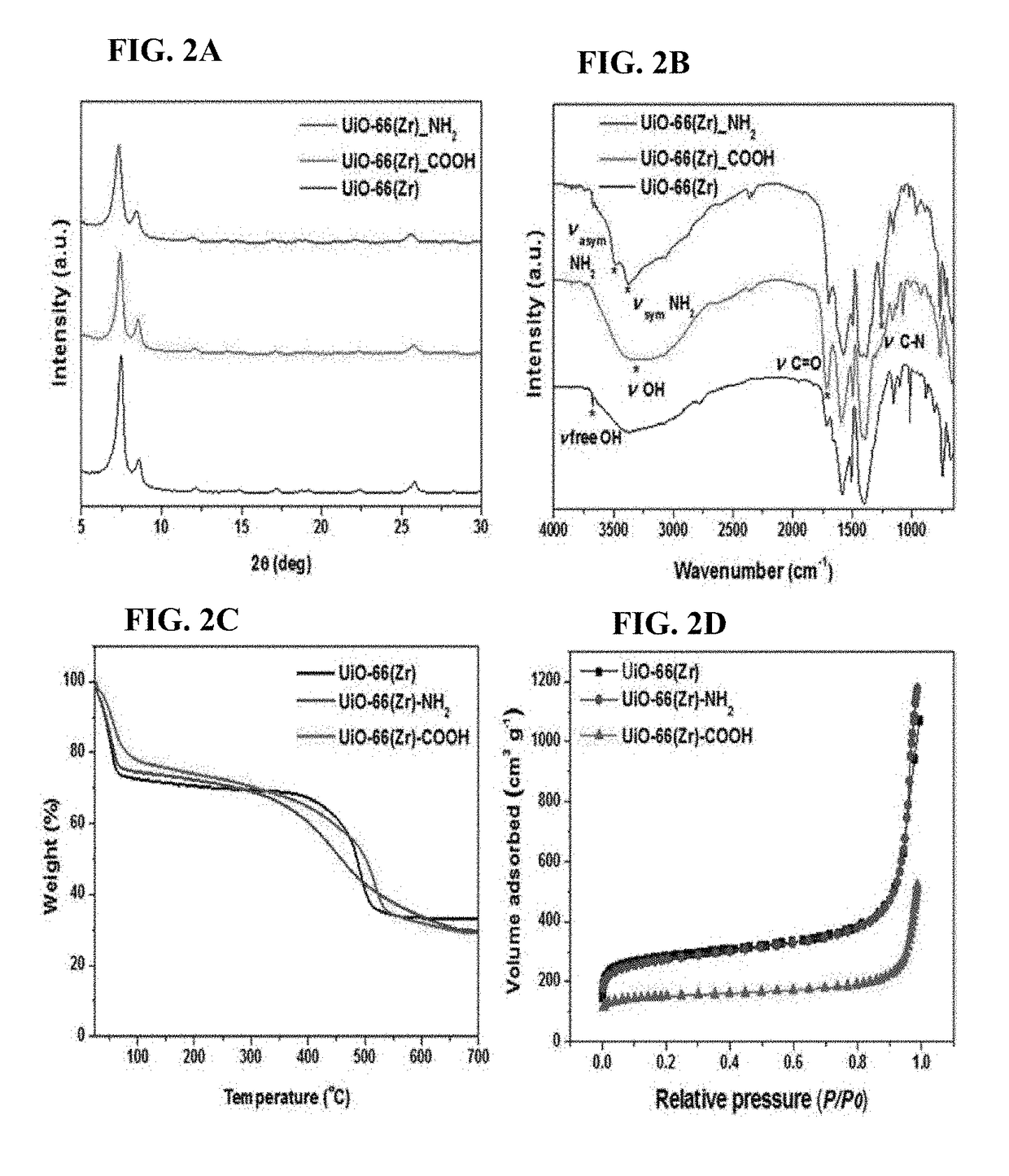
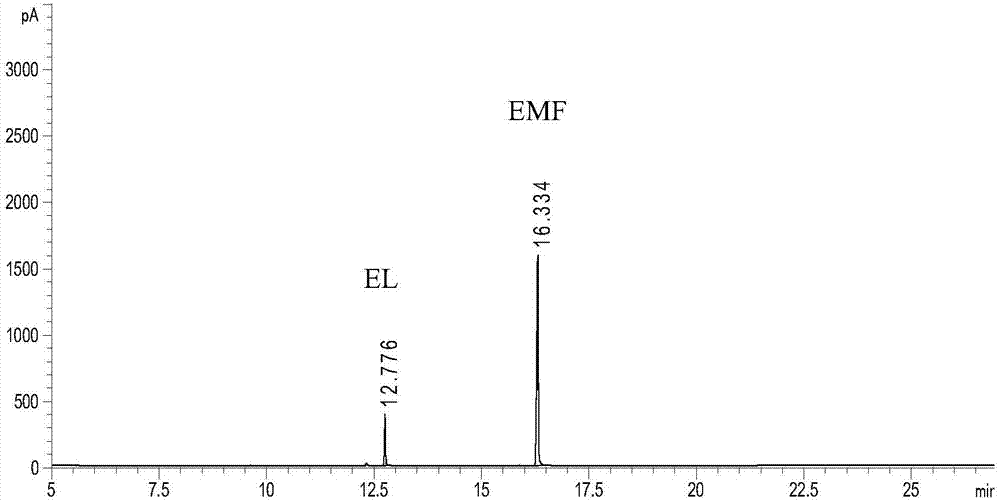
Zirconium-based metal-organic frameworks as catalyst for transfer hydrogenation
ActiveUS20170320790A1Improve performanceOrganic reductionOrganic-compounds/hydrides/coordination-complexes catalystsX-rayMetal-organic framework
The present invention relates to a catalyst for transfer hydrogenation, which is formed of a metal-organic framework having an MOF-808 based X-ray diffraction pattern.A high crystalline porous MOF-808 based metal-organic framework exhibits excellent performance in the transfer hydrogenation of ethyl levulinate (EL) at high and low temperature.
Owner:KOREA RES INST OF CHEM TECH
Organic solvent-free hydrogenation of diene-based polymers
A process is provided for the hydrogenation of carbon-carbon double bonds in polymers by treatment of the polymers with hydrogen in the presence of rhodium-based catalysts and in the complete absence of organic solvent.
Owner:SHANDONG YUHUANG CHEM CO LTD
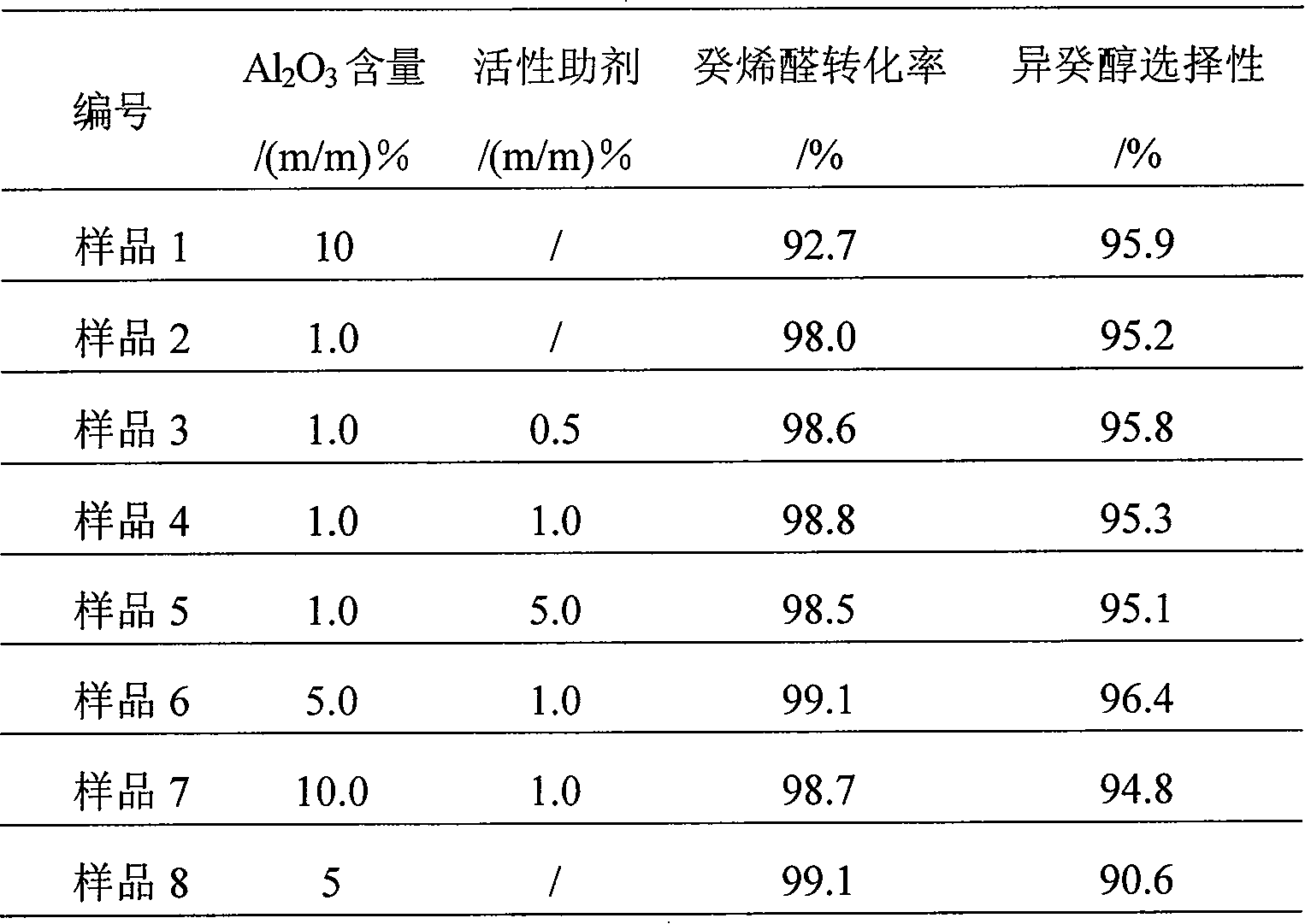
Catalyst for preparation of decyl alcohols by gas-phase hydrogenation of decylenaldehydes and preparation method thereof
InactiveCN101185893AIncrease the outer surface areaReduce resistanceMetal/metal-oxides/metal-hydroxide catalystsGas phaseCopper oxide
A catalyst used for making isodecyl alcohol with decene aldehyde gas phase hydrogenation and a preparation method belong to the technical field of catalyst. The catalyst uses coprecipitation to prepare, and mainly comprises copper oxide, zinc oxide, alumina, the content of which is respectively 20%-70%, 28%-70%, and 1%-10%. The active assistant has the content of 0.1%-2.0% (m / m). The catalyst is used for making isodecyl alcohol with decene aldehyde gas phase hydrogenation, with higher decene aldehyde conversion rate (more than or equal to 99.1%) and isodecyl alcohol selectivity (more than or equal to 96.4%).
Owner:SINOPEC NANJING RES INST OF CHEM IND CO LTD
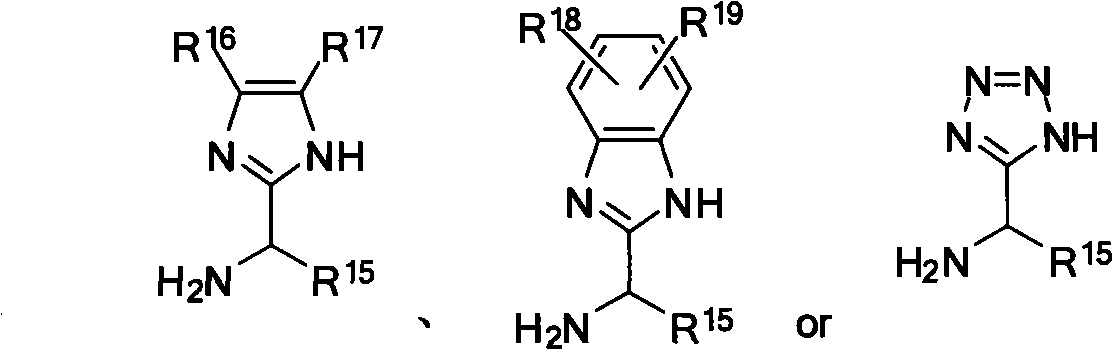
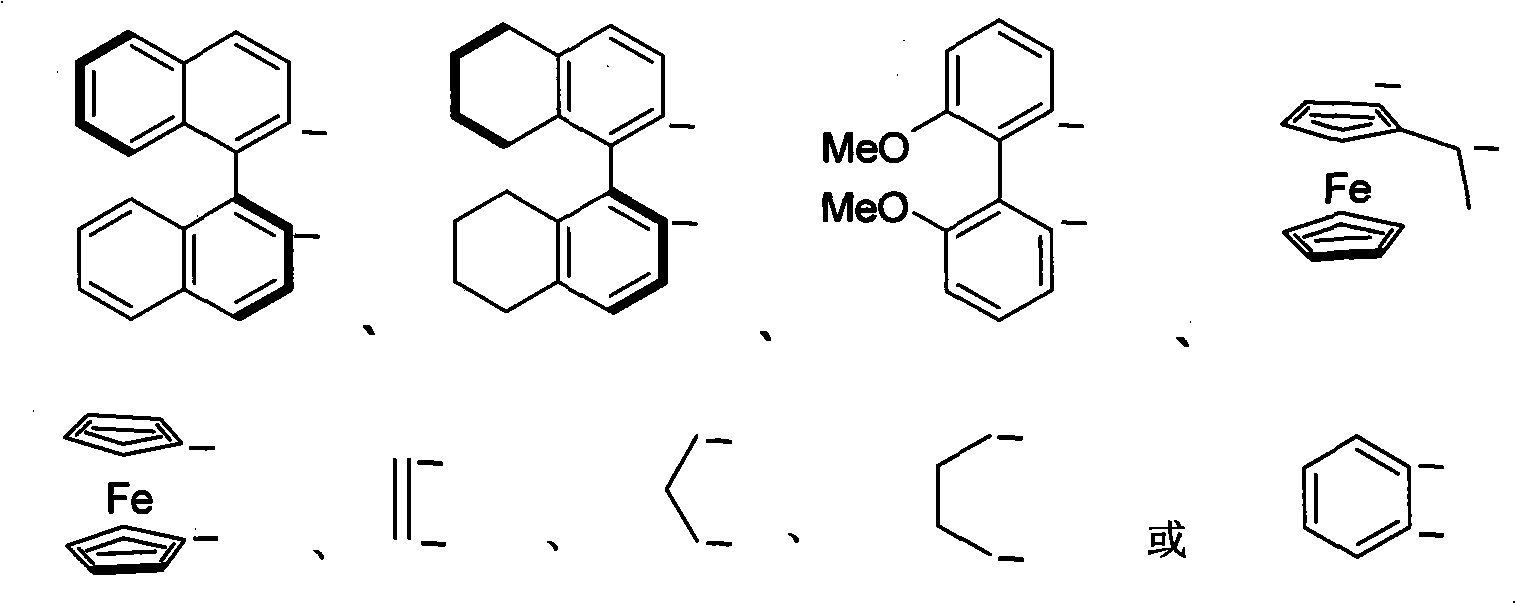
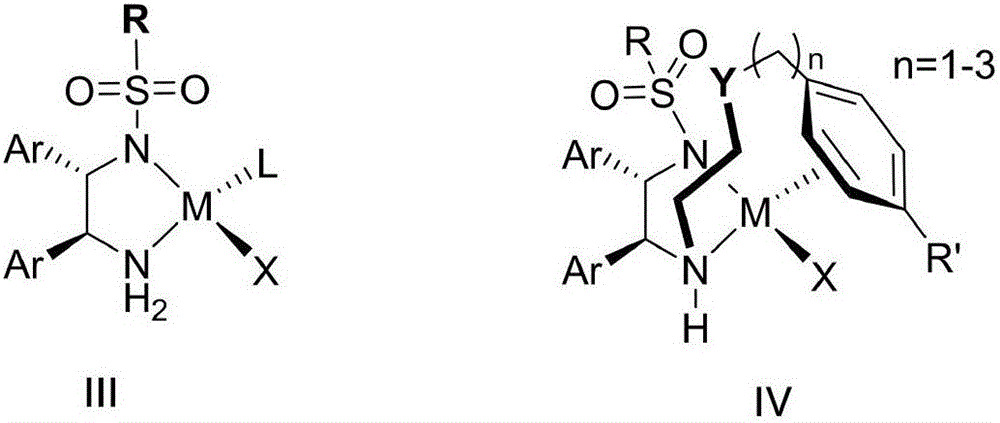
Nitrogen-containing ligand transient metal complex compound , synthetic method and use thereof
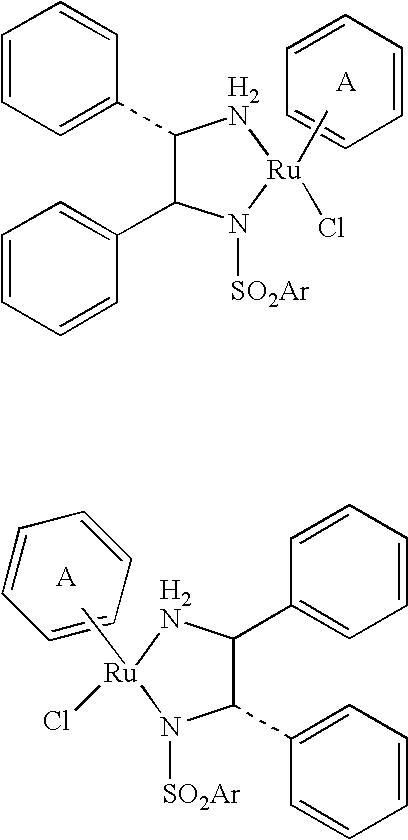
The invention relates to a nitrogenous ligand transition metal complex, a method for synthesizing the same and application of the same, in particular to a metal ruthenium complex with a structural formula shown on the right, other transition metal complexes, a method for synthesizing the same and an application of the same. A diphosphine and dinitrogen transition metal complex which is formed by coordination between a dinitrogen ligand with structural characteristics of NH2-N(sp<2>) and a transition metal can be used for catalytic asymmetrical transfer hydrogenation reaction and also can be used for catalytic hydrogenation reaction, and particularly used for asymmetric catalytic hydrogenation reaction of ketone with large steric hindrance alkyl on the alpha position, hypnone, hypnone derivatives, benzophenone, benzophenone derivatives, beta-N, N-dimethyl amino-alpha hypnone, derivatives of the beta-N, N-dimethyl amino-alpha hypnone, and other ketone compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Method for the production of amines by reductive amination of carbonyl compounds under transfer-hydrogenation conditions
InactiveUS7230134B2Efficiently obtainedOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenMetal catalyst
This application claims the benefit of German priority Application No. 10138140.9, filed on Aug. 9, 2001, and International Application No. PCT / EP02 / 08748, filed on Aug. 6, 2002. The invention relates to the production of amines by the reaction of aldehydes or ketones with ammonia or primary or secondary amines in the presence of a hydrogen-donor and the presence of homogeneous metal catalysts of the eighth sub-group under mild conditions.
Owner:EVONIK DEGUSSA GMBH
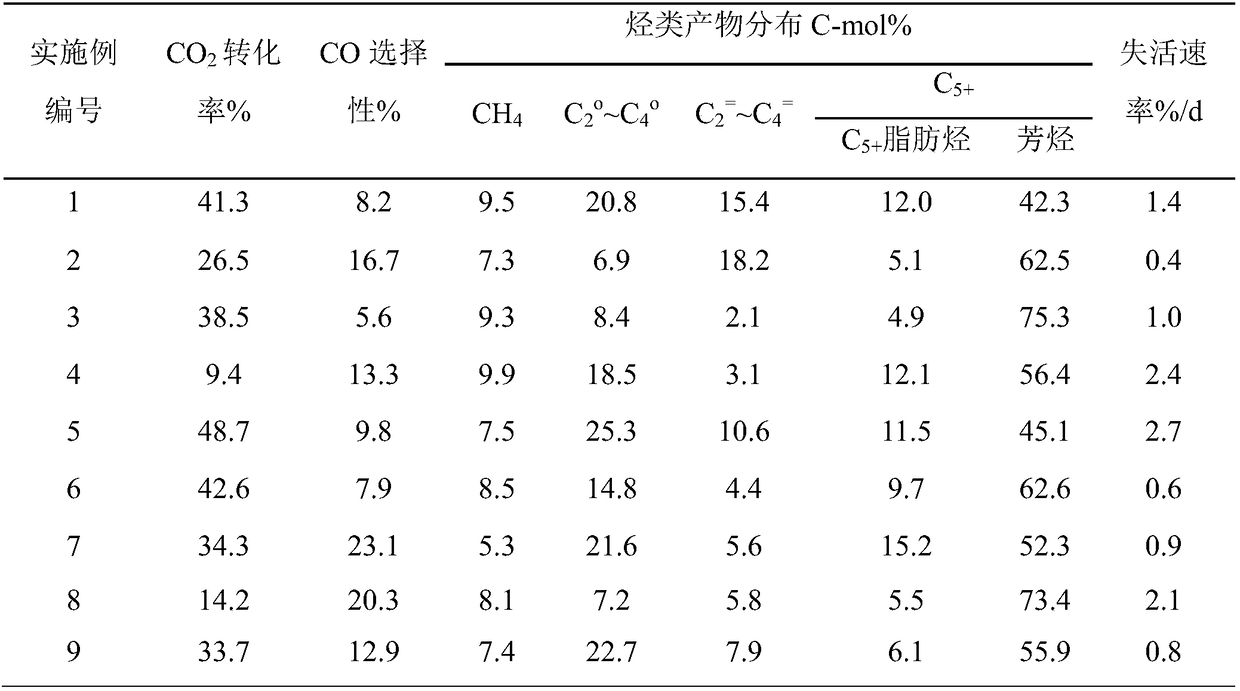
Catalyst for one-step hydrogenation of carbon dioxide to prepare aromatic hydrocarbons, and preparation method and application thereof
ActiveCN108160104ASmall sizeLarge specific surface areaMolecular sieve catalystsLiquid hydrocarbon mixtures productionMolecular sieveAromatic hydrocarbon
The invention provides a catalyst for one-step hydrogenation of carbon dioxide to prepare aromatic hydrocarbons, and a preparation method and an application thereof. The catalyst comprises, by mass, 10-90% of nano-metal oxide and 10-90% of a ZSM-5 molecular sieve. The catalyst obtained in the invention has the advantages of excellent catalysis performance, good reaction stability and high target product selectivity; and the highest selectivity of C5+ in hydrocarbon products reaches 80% or more, and the highest selectivity of aromatic hydrocarbons reaches 75%.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Method for preparing sugar alcohol from monosaccharide
ActiveCN105859522AHigh yieldSimple processPreparation by oxygen reductionReaction temperatureAlcohol sugars
The invention discloses a method for preparing sugar alcohol from monosaccharide. The method comprises the following steps: mixing the monosaccharide with deionized water, adding the obtained mixture to a reaction kettle, and stirring the mixture under normal pressure and nitrogen atmosphere conditions under the action of a metal catalyst and a hydrogen donor to carry out a transfer hydrogenation reaction in order to obtain corresponding sugar alcohol. The hydrogen donor is used as a hydrogen source to replace hydrogen, the initial reaction pressure decreases to normal pressure from 3.0-12.0MPa, the reaction temperature decreases to 85-95DEG C from 120-150DEG C, the mass yield of the sugar alcohol in the final product under mild reaction conditions can reach 70% or above, and the used hydrogen donor is acidified by formic acid to realize reutilization, so the method is green and environmentally-friendly. The method has the advantages of simple process, mild reaction conditions, low energy consumption in the production process, low production difficulty, high operating safety, high greenness, high safety, low cost, and promotion facilitation.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
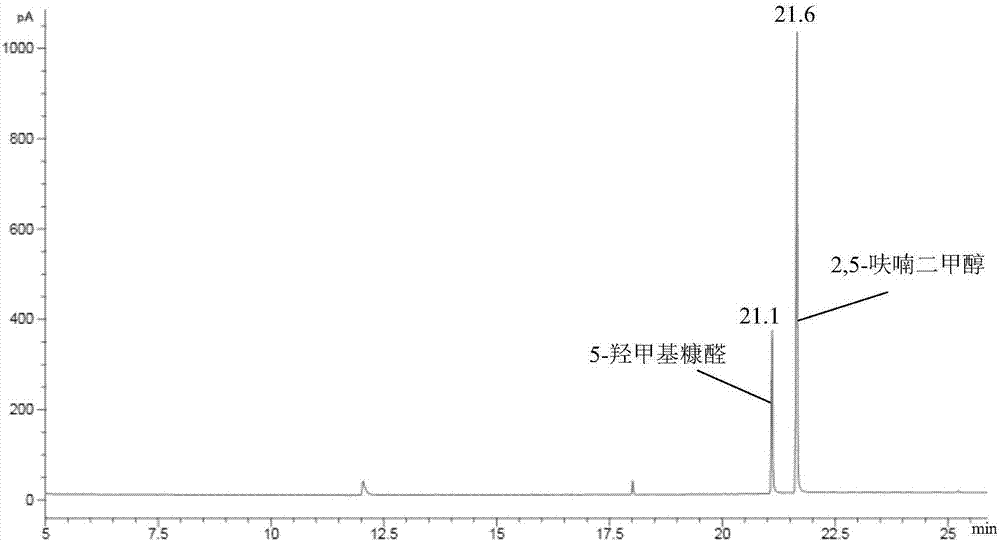
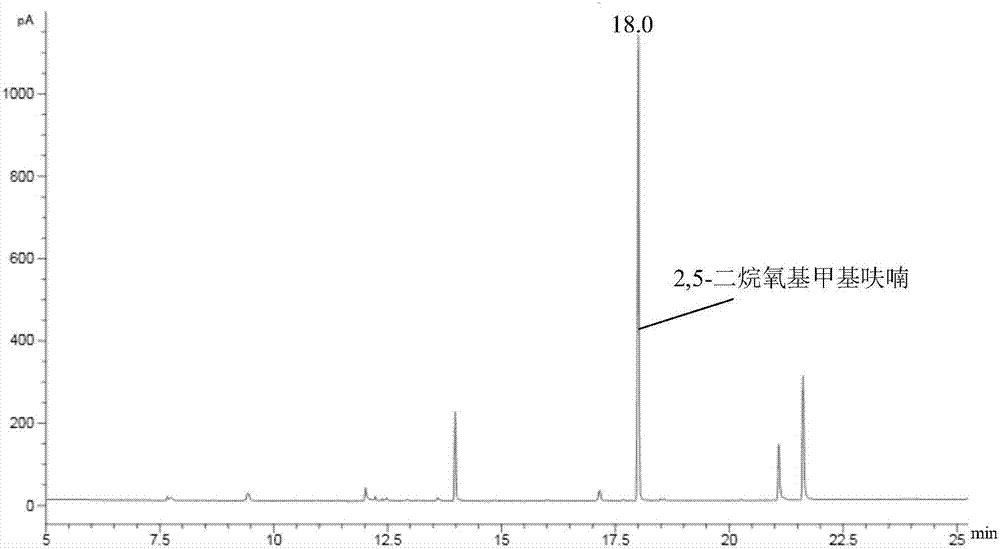
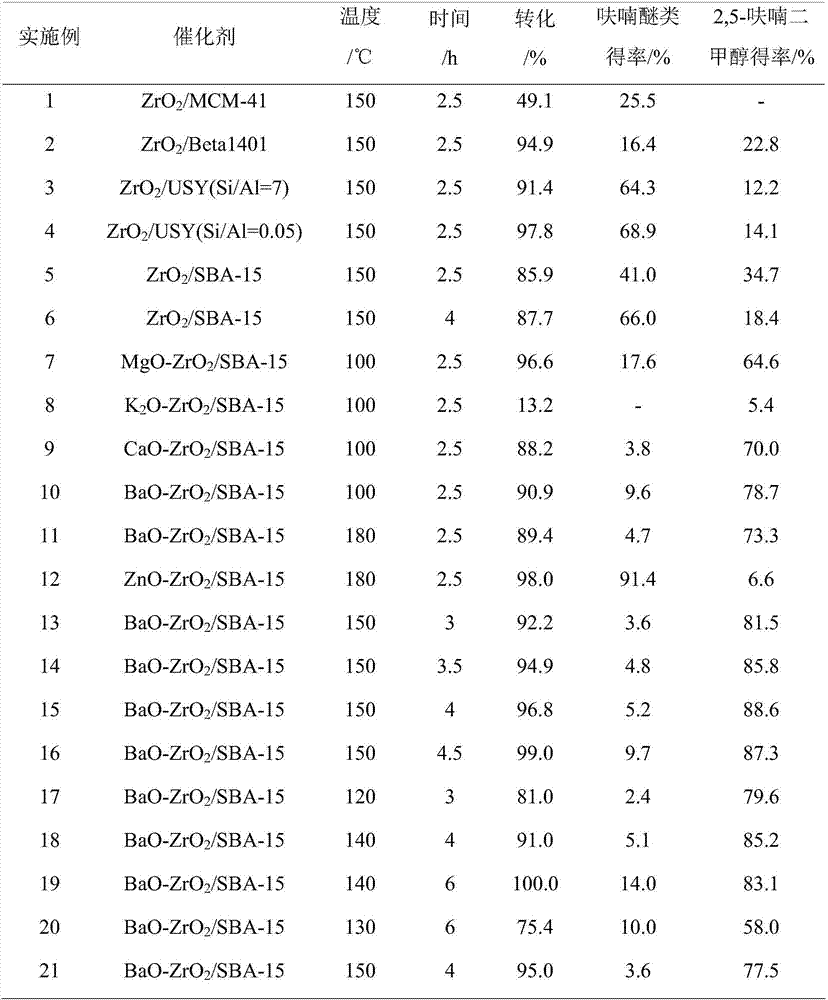
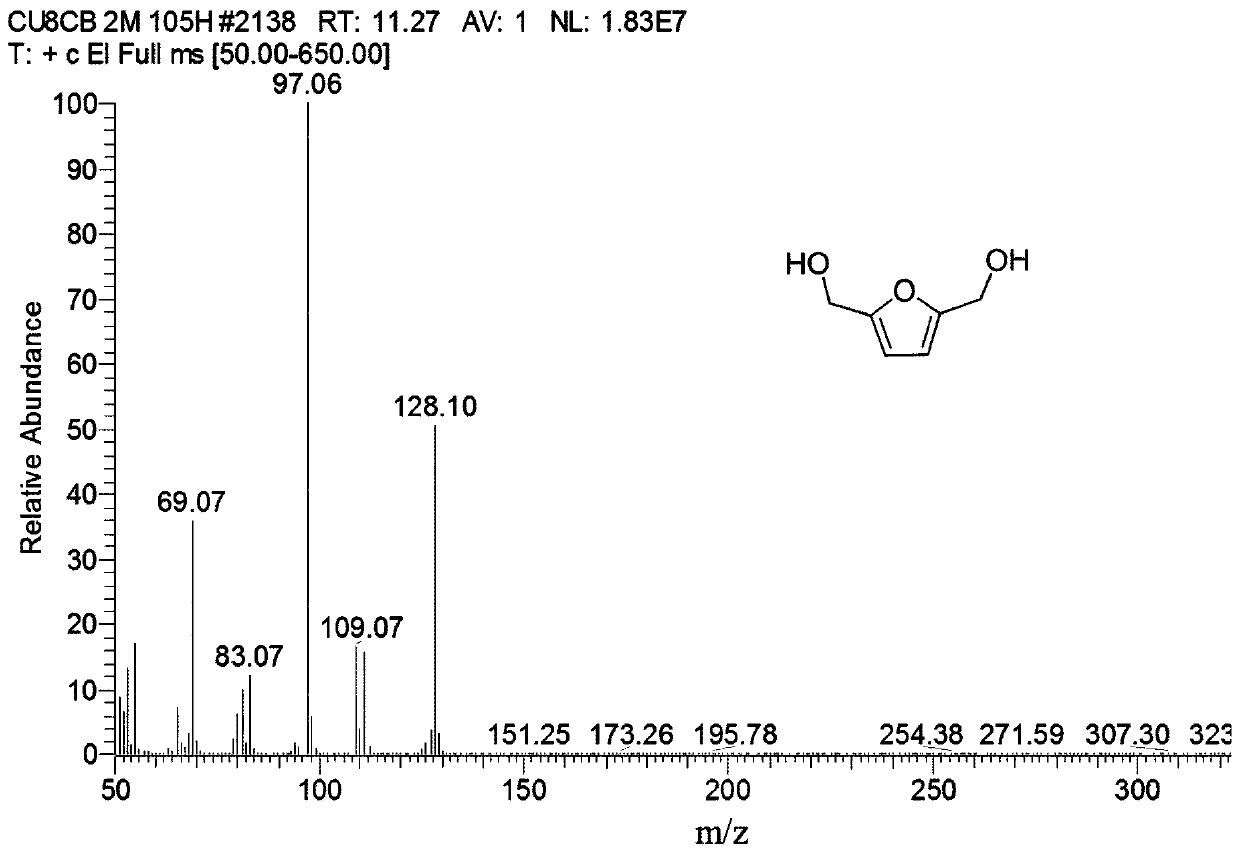
Synthetic method of 2,5-furan dimethanol and etherified product of 2,5-furan dimethanol
The invention discloses a synthetic method of 2,5-furan dimethanol and an etherified product of 2,5-furan dimethanol and relates to 2,5-furan dimethanol. The synthetic method comprises the following steps of: adding organic alcohol into a reaction substrate; putting the obtained mixture as a raw material in a high pressure reaction kettle; adding a certain amount of a molecular sieve supported catalyst; and performing a heating reaction to obtain 2,5-furan dimethanol and the etherified product of 2,5-furan dimethanol. By using alcohol as a hydrogen donor and a reaction substrate, no external hydrogen source does not needed. By adjusting the acid-base property of a catalyst through an active metal component, 2,5-furan dimethanol can be obtained by selectively catalyzing transfer hydrogenation of 5-hydroxymethylfurfural or 2,5-dialkoxyl methyl furan is obtained by further etherification. The catalyst is cheap and easily available and good in repeatability, no excessively hydrogenated product exists, and the whole reaction is hydrogenated in a hydrogen environment, and the synthetic method has relatively strong operating safety of industrial production and very good industrial application potential.
Owner:XIAMEN UNIV
Method for producing 1,2-pentadiol in one-step hydrogenation by furaldehyde
InactiveCN102924232ARich sourcesLow costOrganic compound preparationHydroxy compound preparationFuraldehydeReaction temperature
The invention discloses and provides a method for producing 1,2-pentadiol in one-step hydrogenation by furaldehyde with wide source, low price, high development yield, simple process and small environmental contamination. The method comprises the following steps of: catalyzing furaldehyde serving as a raw material and compound oxide containing copper oxide serving as a catalyst on a continuous fixed bed; and hydrogenating to prepare the 1,2-pentadiol at a temperature of between 100 and 200 DEG C and pressure of between 4.0 and 10.0MPa, wherein the furaldehyde is high in conversion rate and the 1,2-pentadiol is high in selectivity. According to the method, the 1,2-pentadiol is produced by utilizing the renewable resource furaldehyde, the raw materials is rich in source and low in cost; a base metal is adopted as the catalytic material, so that the catalyst has low price; and the selective hydrogenation technology with short process flow, easy operation and no pollution is adopted.
Owner:ZHUHAI KAIMEI TECH
2-methylfuran preparation catalyst by gas-phase hydrogenation of furfural
InactiveCN101422731AHigh activityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsMicrowave methodGas phase
The invention discloses a catalyst that is used for preparing 2-dimethylfuran by furfural gas phase hydrogenation, and is characterized in that the catalyst consists of an active component CuO, a metallic oxide addition agent ZnO and a carrier Al2O3 that are mixed; wherein, the weight percentage of the active component CuO is 18 to 22 percent, the weight percentage of the metallic oxide addition agent ZnO is 3 to 7 percent, and the weight percentage of the carrier Al2O3 is 73 to 77 percent. The preparation method of the catalyst adopts a coprecipitation and microwave method, which comprises the steps of: (1) solution preparation, (2) precipitate generation, (3) precipitate rinsing, (4) precipitate drying, (5) precipitate baking and (6) microwave processing. The invention has the advantages that the catalyst has extremely high activity and selectivity, can achieve the furfural transformation rate of 100 percent and the 2-dimethylfuran selectivity of over 90 percent, and the catalyst does not contain Cr that is harmful to human body and has less environmental pollution.
Owner:CHINA NAT ACAD NANOTECH & ENG
Catalyst for preparing 2-methylfuran by gas-phase hydrogenation of furaldehyde and its preparation method
InactiveCN101143324AHigh activityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsAlkaline earth metalEnvironment effect
The invention provides a catalyst to prepare furfural gas 2-methylfuran and the preparation method. Silica is used as the carrier, copper oxide is used as the main active component and alkali metal or alkaline earth metal is used as the promoter. The preparation method of the catalyst adopts impregnation method. the catalyst has the advantages of high product yield, few by-products, little influence on environment, and long service life, etc, and is widely applicable in production of 2-methylfuran.
Owner:CHINA NAT ACAD NANOTECH & ENG
Chromium-free hydrogenation of hydroformylation mixtures
ActiveUS9567276B2High strengthLower cost of capitalOrganic compound preparationHeterogenous catalyst chemical elementsChromium freeDouble bond
The invention relates to a process for the preparation of alcohols by hydrogenation of aldehydes, in which use mixture comprising at least one aldehyde and at least one accompanying component is brought into contact, in the presence of hydrogen, with a heterogeneous catalyst, giving a product mixture which comprises at least the alcohol corresponding to the hydrogenated aldehyde, and at least one by-product, where the catalyst comprises a support material, and nickel and copper applied thereto. The invention also includes a chromium-free catalyst suitable for hydrogenating aldehyde mixtures with different chain lengths, in particular those which originate from different hydroformylations and can also comprise substances with C═C double bonds.
Owner:EVONIK OXENO GMBH & CO KG
Preparation method and application of glycopyrronium bromide chiral antipode
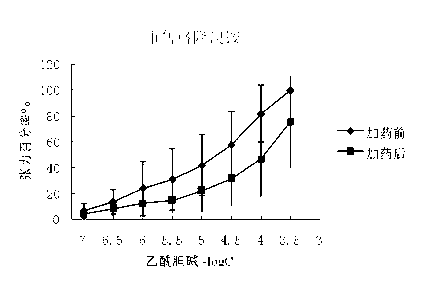
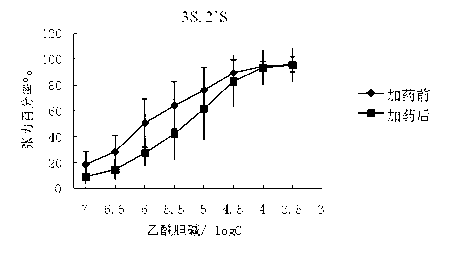
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
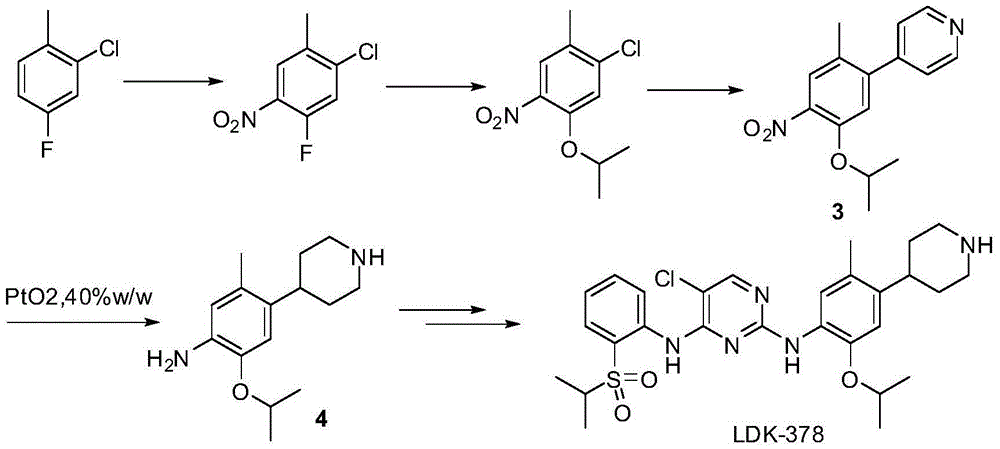
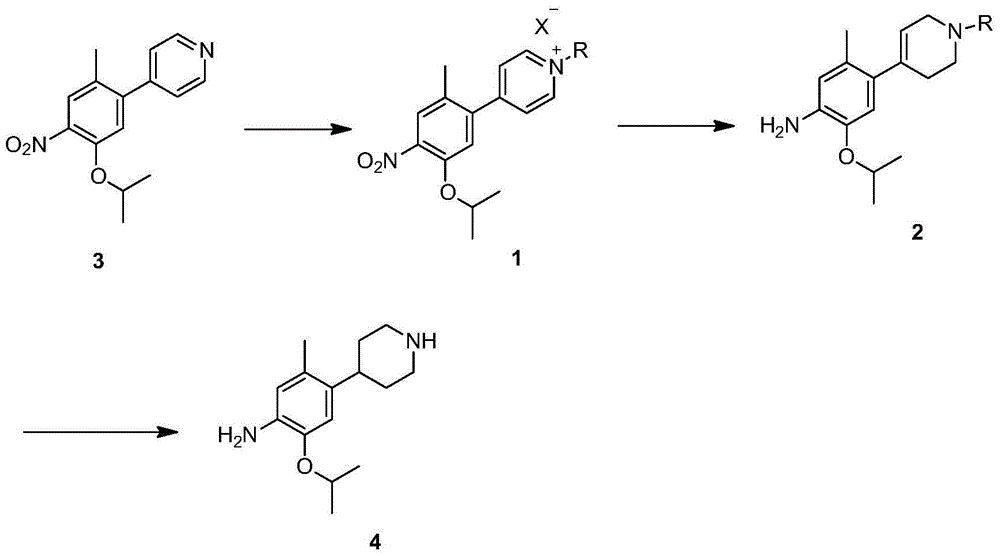
New intermediates for preparing ceritinib and preparation method of intermediate
The invention relates to intermediates, namely a compound 1 of a formula (1) as shown in the specification and a compound 2 of a formula (2) as shown in the specification, for preparing ceritinib, or a chemically acceptable salt of the compound 2, wherein R represents the benzylic group of saturated or unsaturated aromatic ring methylene or heteroaromatic ring methylene, and X represents a halogen. The invention relates to a method for preparing a compound 4 by use of the new intermediates, namely the compound 1 and the compound 2, wherein the step of reduction from the compound 1 to the compound 2 is performed by use of a hydroboron or a composition thereof and an alcohol solvent; the compound 2 is reduced by use of a catalytic hydrogenation or transfer hydrogenation method to generate the compound 4. The route of preparing the compound 4 by use of the compound 1 and the compound 2 has the advantages that the chemical reduction step is combined with a catalytic hydrogenation, the use of expensive platinum dioxide is avoided and the cost of synthesizing the intermediate 4 of the ceritinib is effectively reduced.
Owner:药源生物科技(启东)有限公司
Transient metal complex compound, synthetic method and use thereof
InactiveCN101323630AThe synthesis method is simpleOrganic reductionOrganic compound preparationKetoneAcetophenone
The invention relates to a metal ruthenium complex with the general formula MXYLnL', other transition metal complexes, a synthetic method thereof and an application thereof. Dinitrogen ligand with active hydrogen reacts with transition metal under the action of alkali to generate a transition metal complex which has novel structure, contains phosphine and nitrogen and can be used for catalytic hydrogenation reaction with asymmetric transferring and also for the catalytic hydrogenation reaction, especially used for the catalytic asymmetric hydrogenation reaction of hypnone and derivatives, benzophenone and derivatives, Beta-N, N-dimethylamino-Alpha-hypnone and derivatives, and other ketone chemical compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Catalytic hydrogenation method for olefin
ActiveCN101209951AImprove hydrogenation efficiencyEasy to controlHydrocarbon by hydrogenationHydrocarbon oils refiningHydrogenation reactionAlkene
The invention relates to a catalytic hydrogenation method of alkene. Under the conditions of alkene hydrogenation and the existence of hydrogenation catalysts, the alkene is reacted with hydrogen in contact way in a reactor; wherein, the reactor comprises a reacting tower and a reacting vessel, and the reacting tower and the reacting vessel are communicated with each other; the reaction tower is positioned above the reacting vessel; the hydrogen is sent into the reacting vessel from the bottom of the reacting vessel; the alkene is sent into from the middle-upper part of the reacting tower and / or the reacting vessel and contacts hydrogen in a countercurrent way; during the hydrogenation, the production separation is carried out; the light fraction exacted from the reacting tower after hydrogenation and the heavy fraction left on bottom of the reacting vessel after hydrogenation are reclaimed. The method of the invention saves the separation step and separation equipment of the ordinary alkene hydrogenation, thus greatly simplifying technology process. Besides, by directly applying reaction heat to separation of products, the energy consumption is decreased. In addition, the catalysis is carried out by taking the way of suspending and dispersing the hydrogenation catalyst into the reaction compound containing hydrogen and alkene so as to greatly improve catalysis effect and efficiency.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for preparing 1, 4-cyclohexanedimethanol by catalyzing dimethyl terephthalate through one-step hydrogenation and preparation method of catalyst
InactiveCN102580732AHigh yieldAvoid separationPreparation by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsPlatinumDimethyl terephthalate
The invention discloses a catalyst for preparing 1, 4-cyclohexanedimethanol by catalyzing dimethyl terephthalate through one-step hydrogenation and a preparation method of the catalyst, belonging to the technical field of catalyst preparation. The catalyst is a supported catalyst active ingredients of which are loaded on carriers Al2O3, wherein the active ingredients include three metals, namely, ruthenium, platinum and tin. The catalyst is prepared by adopting a co-impregnation method. According to the invention, the 1, 4-cyclohexanedimethanol is prepared by performing one-step hydrogenation on dimethyl terephthalate by only using a hydrogenation catalyst and a singe reaction solvent, and higher product yield is obtained; the separation of an intermediate product and the consumption of the solvent are avoided; and the consumption of energy and resources through the traditional process is reduced.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing 1,6-hexylene glycol through dimethyl adipate gas-phase hydrogenation
ActiveCN107118076AImprove conversion rateHigh selectivityMolecular sieve catalystsOrganic compound preparationDispersityGas phase
The invention provides a method for preparing 1,6-hexylene glycol through dimethyl adipate gas-phase hydrogenation. The method comprises the following steps: by taking a hierarchical pore SiO2 molecular sieve as a carrier, loading a copper-based catalyst, mixing gasified dimethyl adipate with hydrogen, putting the mixture into a fixed bed reactor for reaction under a certain condition, a 1,6-hexylene glycol-containing liquid phase product is obtained. According to the method provided by the invention, by taking the SiO2 molecular sieve with ordered meso pores and micro pores as the carrier, the dispersity of copper serving as an active component is substantially promoted, and the number of effective active sites of the catalyst is increased; moreover, due to the adoption of an ammonia distillation method, the copper is loaded on the mesoporous silicon oxide molecular sieve, so that high dispersion of the catalyst is realized; furthermore, the ordered meso pores of the hierarchical pore SiO2 molecular sieve is retained, and the catalysis capacity is improved.
Owner:TIANJIN UNIV
Method for synthesizing dihydrogen phenanthridine
InactiveCN102952073ARaw materials are easy to getFew reaction stepsOrganic chemistryMetallolePhenanthridine
The invention relates to a method for synthesizing dihydrogen phenanthridine. According to the invention, transition metals [Ru (II), Rh (I), and Ir (I)] are adopted as catalyst for realizing phenanthridine catalytic hydrogenation. Also, according to the invention, 9,10-dihydrogen phenanthridine in-situ regeneration is applied in asymmetric transfer hydrogenation of unsaturated imine. Only a catalytic amount of phenanthridine is needed to be added, and chiral amine can be synthesized with high enantioselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing 2,5-furandimethanol by transfer hydrogenation of 5-hydroxymethylfurfural
ActiveCN110204519AThe preparation process is simple and environmentally friendlyHigh selectivityOrganic chemistryPhysical/chemical process catalystsHydrogenSucrose
The invention discloses a method for preparing 2,5-furandimethanol by transfer hydrogenation of 5-hydroxymethylfurfural. The 5-hydroxymethylfurfural, a catalyst MnO@C-N and a lower alcohol are added into a stainless steel closed reactor for reaction at 150-200 DEG C for 1-30 h, and the materials are stirred at a rate of 300-900 rpm during the reaction. For the first time, the method of the invention uses the MnO@C-N as a catalyst to catalyze aldehyde to alcohol. The catalyst MnO@C-N used by the method uses renewable sucrose and urea as raw materials, and the preparation process is simple and environmentally friendly. The method uses the cheap lower alcohol as a hydrogen source for a reduction reaction, has the advantages of safe reaction process, simple operation and high product selectivity, and has great industrial application value.
Owner:XIAMEN UNIV
Method for preparing allyl alcohol compounds from alpha,beta-unsaturated aldehyde ketones
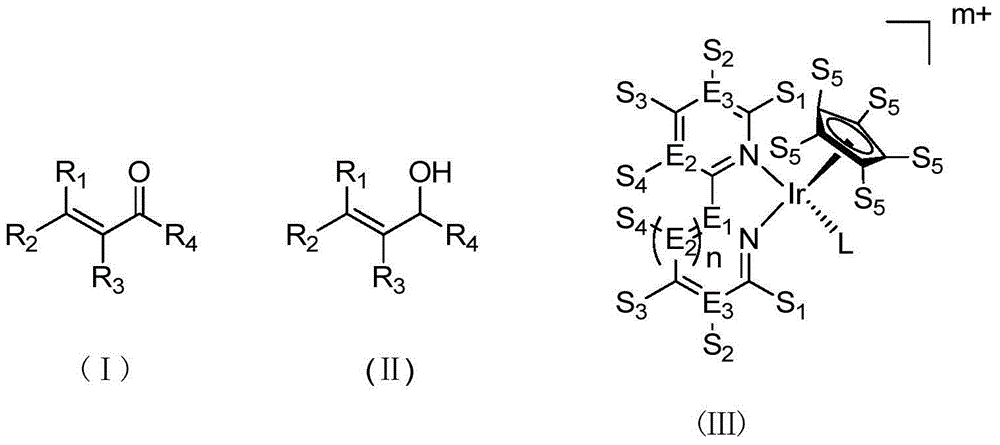
InactiveCN104945208AHigh purityBroaden the reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationIridiumKetone
The invention belongs to the field of allyl alcohol compounds, and relates to a method for allyl alcohol compounds (II) from alpha,beta-unsaturated aldehyde ketones (I) by transfer hydrogenation, which comprises the following step: by using a metal iridium coordination compound (III) as a catalyst, carrying out transfer hydrogenation reaction on the alpha,beta-unsaturated aldehyde ketones in a formic acid / sodium formate water phase solution. The method provided by the invention is characterized in that the allyl alcohol compounds are prepared by catalyzing the alpha,beta-unsaturated aldehyde ketones in the formic acid / sodium formate water phase. Compared with the reported allyl alcohol compound preparation method, the method provided by the invention uses water as the solvent, and thus, is green, safe, environment-friendly and pollution-free; and meanwhile, the method has the advantages of mild conditions and high product purity, and is simple to operate. The obtained allyl alcohol compounds have very high economic value in the aspects of synthesis of aroma regulators, food flavor additives and drug intermediates, and the like.
Owner:DALIAN UNIV OF TECH
Method for synthesizing glycolic acid ester by gas-phase hydrogenation of oxalate
ActiveCN104926657AImprove technical effectOrganic compound preparationCarboxylic acid esters preparationOxalateHydrogen
The invention relates to a method for synthesizing glycolic acid ester by gas-phase hydrogenation of oxalate, and is used for mainly solving the technical problem of low yield of glycolic acid ester in the prior art. The problem is better solved through adopting the technical scheme that the method comprises the steps: taking oxalate as a raw material, pre-mixing with hydrogen gas containing a dilution gas, then under conditions of the temperature of 120 DEG C-250 DEG C, the pressure of 0.5-5 MPa, the oxalate weight space velocity of 0.1-6 h<-1> and the hydrogen gas-oxalate molar ratio of 10-150, carrying out a contact reaction with a catalyst, and generating an effluent containing glycolic acid ester. The method can be used in industrial production of glycolic acid ester.
Owner:CHINA PETROLEUM & CHEM CORP +1
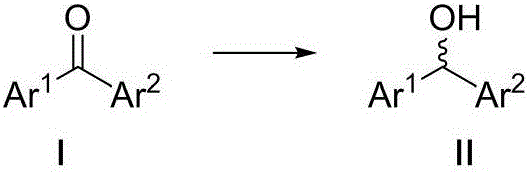
Optical activity di(heteto)aryl methanol and asymmetric synthesis method thereof
InactiveCN106831550AEasy to synthesizeLow priceOrganic compound preparationHydroxy compound preparationIridiumSynthesis methods
The invention relates to optical activity di(heteto)aryl methanol and an asymmetric synthesis method thereof. The method comprises the following steps: taking mono-sulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as catalysts, taking sodium formate or formic acid / triethylamine or isopropanol as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction of di(heteto)aryl ketone first, thereby obtaining the optical activity di(heteto)aryl methanol. The method is mild in reaction conditions, easy and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspects of synthesis of antihistamine chiral drugs such as diphenhydramine, methyldiphenhydramine, carbinoxamine and bepotastine.
Owner:CHINA THREE GORGES UNIV
Porous carbon aerogel catalyst, and preparation method and application thereof
ActiveCN108745333AGood dispersionImprove efficiencyHydroxy group formation/introductionCatalyst activation/preparationCellulosePorous carbon
The invention belongs to the technical field of preparation of catalysts, and concretely relates to a porous carbon aerogel catalyst, and a preparation method and an application thereof. The method comprises the following steps: dissolving cellulose under a high-speed stirring condition, mixing the dissolved cellulose with a transition metal salt solution, and sequentially solidifying, drying, carbonizing and washing the obtained mixed solution to simultaneously achieve the preparation of aerogel and the loading of a transition metal in order to obtain the porous carbon aerogel with highly dispersed catalyst active sites. The catalyst has a good stability and a high catalytic activity, and can efficiently catalyze the transfer hydrogenation reaction of aldehydes, ketones or levulinates andalcoholic organic substances, wherein the conversion rate of aldehydes to alcohols reaches up to 98-100%, and the selectivity of alcohols reaches up to 81-99%. The catalyst is a solid aerogel monolith catalyst, so the catalyst can be well dispersed in a solution without stirring, and is easy to separate and recover. Additionally, the catalyst adopts cellulose as a raw material, so the catalyst has the advantages of low cost, greenness, environmental protection, renewability, simple preparation process, and easiness in industrialization.
Owner:SOUTH CHINA UNIV OF TECH
Two-stage hydrogenation based direct coal liquefaction process
ActiveCN104419439AReduced hydrocracking reaction efficiencyReduce effective reaction spaceLiquid hydrocarbon mixture productionGas phaseReaction temperature
The invention discloses a two-stage hydrogenation based direct coal liquefaction process. The process comprises the following steps: subjecting coal slurry obtained by mixing pulverized coal with a hydrogen-donor solvent, hydrogen and a catalyst to reaction in a primary reactor at a reaction temperature of 420-460 DEG C, sending a primary reaction product to the upper part of a secondary reactor, enabling a gas phase component in the primary reaction product to flow out from the top of the secondary reactor, enabling solid and liquid components in the primary reaction product to flow down in the secondary reactor to carry out countercurrent contact reaction with hydrogen flowing up at a reaction temperature of 430-480 DEG C, and separating the product after the reaction ends. The process has the beneficial effects that the gas content of gases which do not participate in reaction in the secondary reactor can be reduced below 15%, thus obviously improving the production efficiency of direct coal liquefaction. The process is suitable for the large-scale direct coal liquefaction industry.
Owner:任相坤
An enzymatic citral asymmetric reduction method capable of increasing optical purity of (R)-citronellal
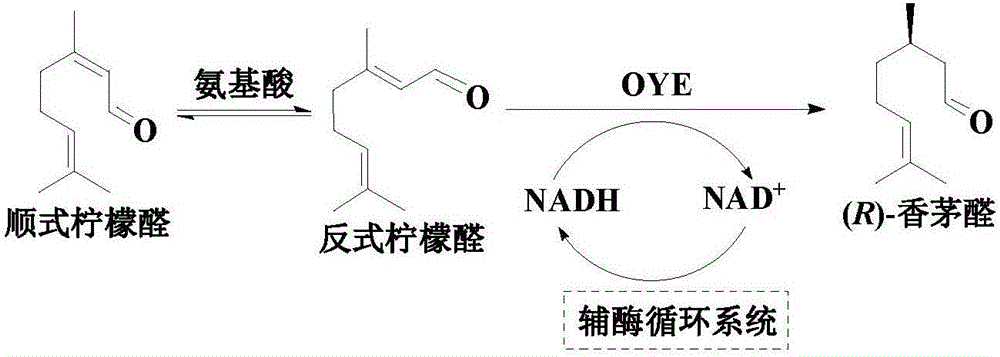
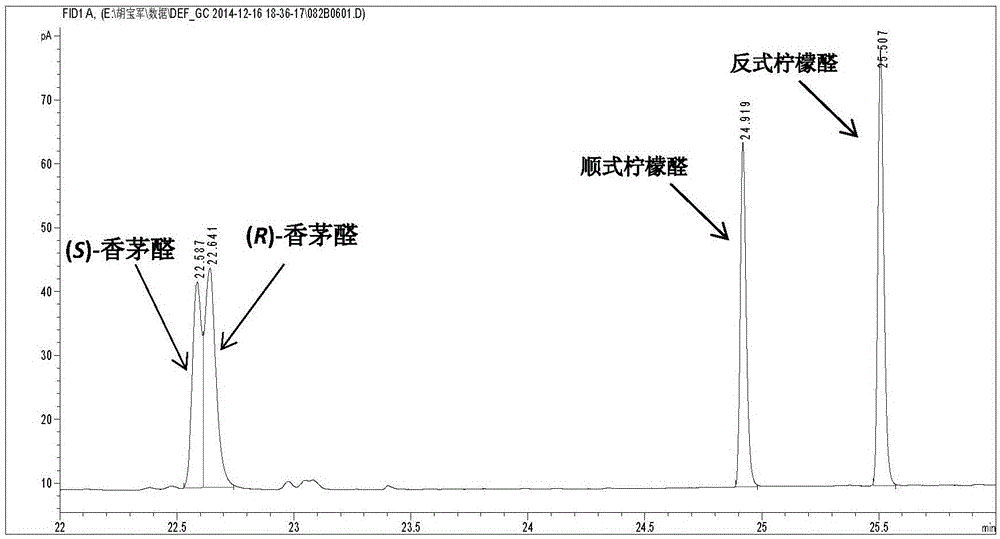
An enzymatic citral asymmetric reduction method capable of increasing optical purity of (R)-citronellal is disclosed, namely a method coupling an amino-acid-catalyzed citral cis-trans isomerization reaction and a citral asymmetric hydrogenation reaction catalyzed by a saccharomyces cerevisiae enol reductase OYE1 to increase the optical purity of the (R)-citronellal that is a product of citral hydrogenation. When citral cis-trans isomers are subjected to the asymmetric hydrogenation reaction catalyzed by the saccharomyces cerevisiae enol reductase OYE1 to synthesize the (R)-citronellal, the (R)-citronellal is derived from trans-citral, (S)-citronellal is derived from cis-citral, and the catalysis speed for the trans-citral is higher than that for the cis-citral. Through coupling with the amino-acid-catalyzed citral cis-trans isomerization reaction, a part of the cis-citral is converted into the trans-citral, thus greatly increasing the ee value of the product that is the (R)-citronellal. In a catalytic system having a volume of 10 mL, 100 mg / mL of glycine is added, after 50 mM citral is subjected to a catalytic reaction for 4 h, the ee value of the (R)-citronellal is 65.4%, and is increased by 48.7% when being compared with the ee value of (R)-citronellal when the cis-trans isomerization reaction is not coupled.
Owner:ZHEJIANG UNIV OF TECH
Asymmetric syntheses method of ophthalmologic drug bepotastine besilate
The invention relates to an asymmetric synthesis method of an ophthalmologic drug pylistramine besylate. The method concretely comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)methanone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; carrying out asymmetric transfer hydrogenation reduction on the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through using a complex of monosulfonyl chiral diamine and metallic ruthenium, rhodium and iridium as a catalyst and a sodium formate or formic acid and triethylamine mixture or isopropanol as a hydrogen source in order to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol; and condensing the (S)-(4-chlorophenyl)(2-pyridyl)methanol and ethyl 4-(4-bromopiperidine-1-yl)butyrate to obtain the ophthalmologic drug pylistramine besylate. The total yield is 61.6%.
Owner:YICHANG HUMANWELL PHARMA +1
Method for producing optically active amines
ActiveUS20100160636A1Easy to produceOrganic compound preparationSulfonic acid amide preparationIridiumHydrogen
The present invention provides a method for producing chiral amines, comprising asymmetric transfer hydrogenation of imine compounds in the presence of a hydrogen donor compound and an iridium(III) complex having a chiral prolinamide compound as a ligand. The present invention is useful for production of chiral amines in an efficient manner in terms of their optical and chemical yields.
Owner:HAMARI CHEM LTD
Method for preparing furan ethers from carbohydrates by one-pot in-situ catalysis
ActiveCN106957289ASimple reaction systemSimple processOrganic compound preparationCarboxylic acid esters preparationFuranDecomposition
A method for preparing furan ethers from carbohydrates by one-pot in-situ catalysis, which relates to furan ethers. An organic alcohol is added to a reaction substrate to obtain a mixed liquid which is put into a high-pressure reaction kettle as a raw material, and then a metal salt is added thereto with heating carried out to cause in-situ form in-situ decomposition to form a composite catalyst of a metal hydroxide and an inorganic acid. After the reaction, a mixed furan ether product is obtained. The reaction substrate is glucose or fructose. With a cheap metal salt as a catalyst precursor, the carbohydrates are directly converted to prepare furan ethers by a one-pot method. The alcohol is used as both a hydrogen donor and a reaction medium with not need for an external hydrogen source and other solvents. The method is simple in reaction system, simple in process and easy to operate, and has a promising industrialization prospect. The metal hydroxide and the inorganic acid formed in situ are used to catalyze carbohydrate hydrolyzation, transfer hydrogenation reduction and etherification through a one-pot process to synthesize furan ethers.
Owner:XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com