Asymmetric syntheses method of ophthalmologic drug bepotastine besilate
A synthesis method and technology of benzenesulfonic acid, applied in the direction of organic chemistry and the like, can solve problems such as limited industrial application and sensitivity, and achieve the effects of convenient operation, low price and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of (4-chlorophenyl) (2-pyridyl) ketone-N-oxide (formula (III))
[0042] Dissolve 0.2g of (4-chlorophenyl)(2-pyridyl)methanone in 2mL of hydrogen peroxide (30% aqueous solution), add 1.0mL of acetic acid, heat to 85°C for 12h, and check the progress of the reaction by TLC until After the reaction of the raw materials was complete, saturated sodium bicarbonate solution was added, extracted with dichloromethane, washed, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain 0.21 g of a white solid with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ):δ=7.46-7.50(m,5H),7.82(dt,J 1 =4.4Hz,J 2 =2.4Hz, 2H), 8.27-8.29 (m, 1H)ppm.
Embodiment 2
[0043]Embodiment 2: Preparation of (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide (formula (IV))
[0044]
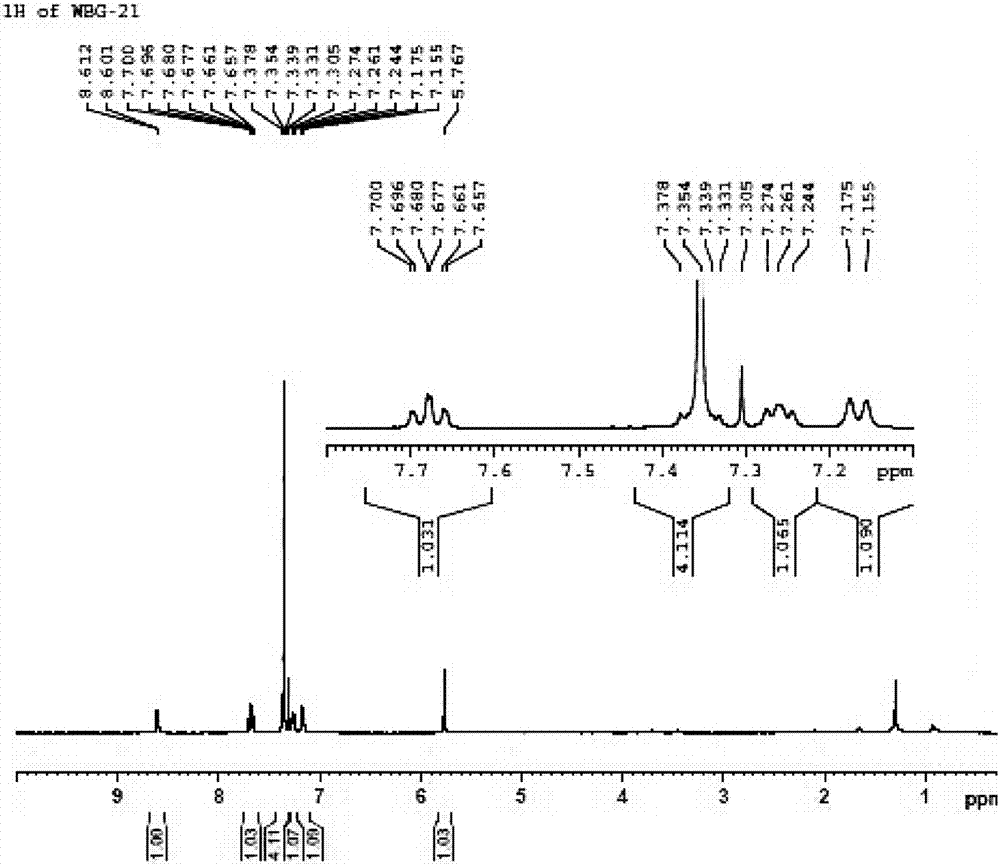
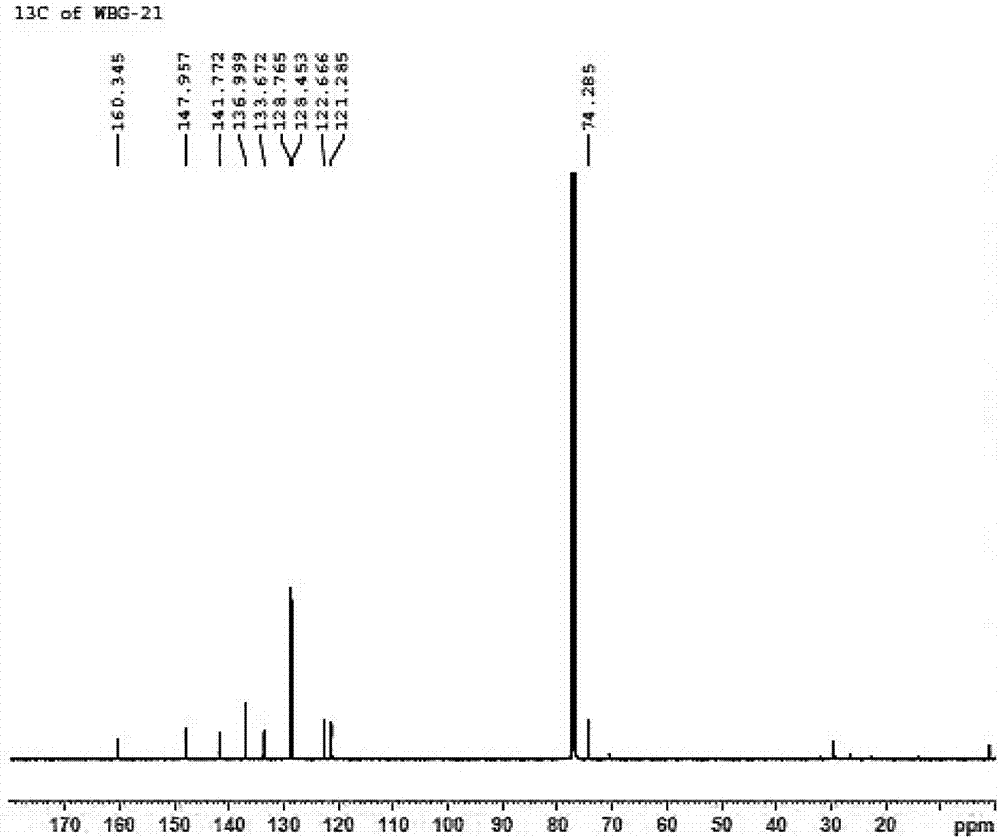
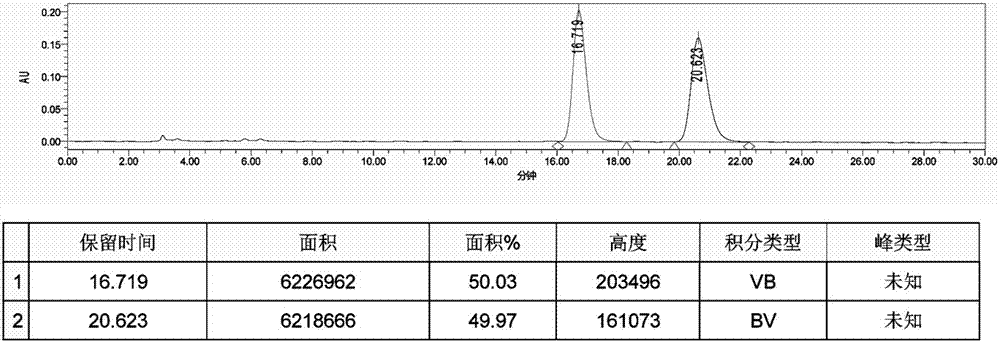
[0045] In a 20 mL Schlenk test tube, add 33 mg catalyst 1a, 0.23 g (4-chlorophenyl) (2-pyridyl) ketone-N-oxide, 5 mL formic acid / triethylamine mixture (formic acid / triethylamine molar ratio 1.1 / 1), seal the test tube, replace the gas with nitrogen for 3 times, react at 40°C for 24 hours, add water after the reaction, extract 3 times with ethyl acetate, combine and concentrate to dryness, and purify to obtain 0.22 g of white solid with a yield of 95%. The enantiomeric excess ee value of the product (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide determined by HPLC was 92%. HPLC separation conditions: OD-H chiral column, mobile phase: n-hexane / isopropanol=90:10 (volume ratio), flow rate: 1.0mL / min, wavelength: 220nm, column temperature: 30°C, retention time: t R =9.0min,t S = 11.5min; 1 H NMR (400MHz, CDCl 3 ): δ=6.08(d, J=4.4Hz, 1H), 6.42(d, J=4.8Hz, 1H), 7.00(t,...
Embodiment 3
[0046] Embodiment 3: Preparation of (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide (formula (IV))
[0047]
[0048] In a 20 mL Schlenk test tube, add 67 mg of catalyst 2a, 0.45 g of (4-chlorophenyl)(2-pyridyl)methanone-N-oxide, 3.35 g of sodium formate, seal the test tube, replace the gas with nitrogen 3 times, and add by syringe 5mL EtOH / H 2 O(EtOH / H 2 (2 volume ratio is 1:1) mixed solvent, reacted for 24 hours at 50 DEG C, extracted 3 times with ethyl acetate after the completion of the reaction, combined and concentrated to dryness, purified to give white solid 0.43g, productive rate 92%, HPLC assay product (S )-(4-Chlorophenyl)(2-pyridyl)methanol-N-oxide has an enantiomeric excess ee of 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com