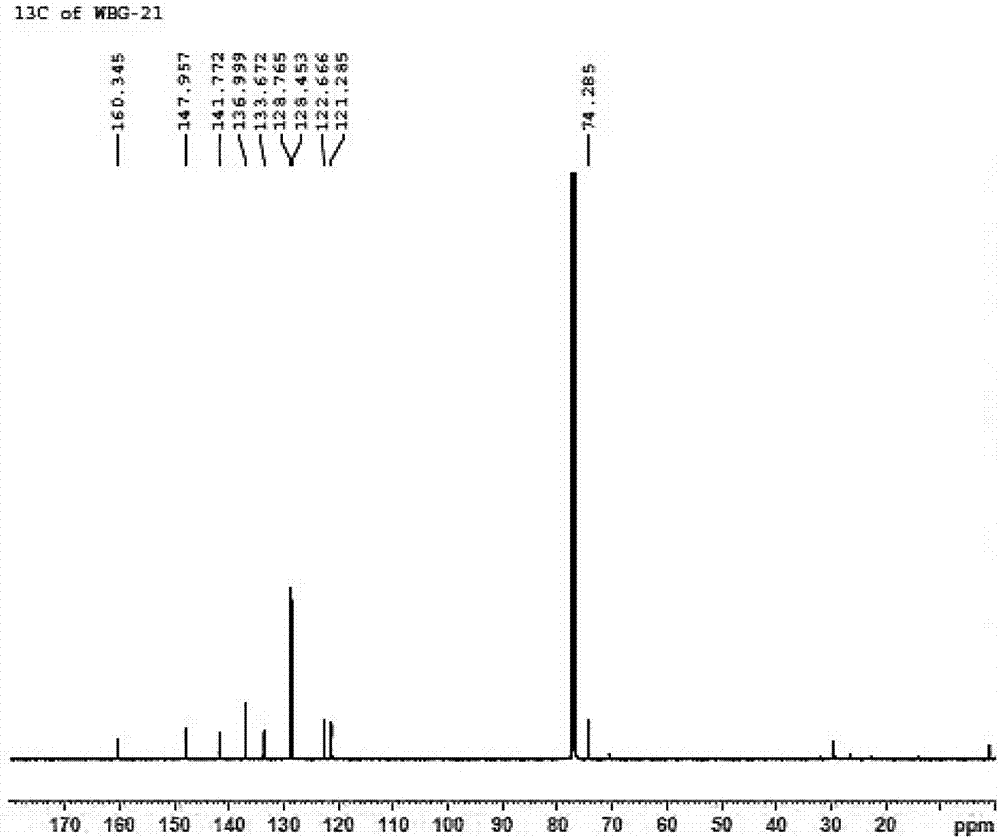
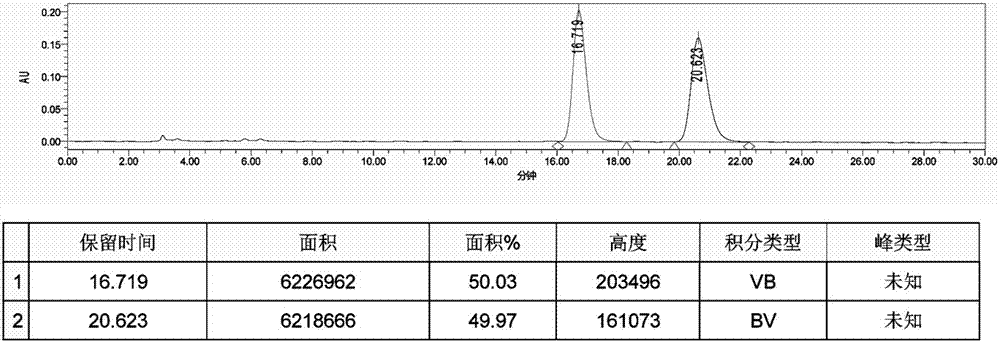
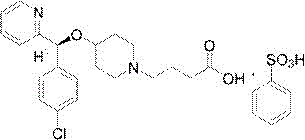
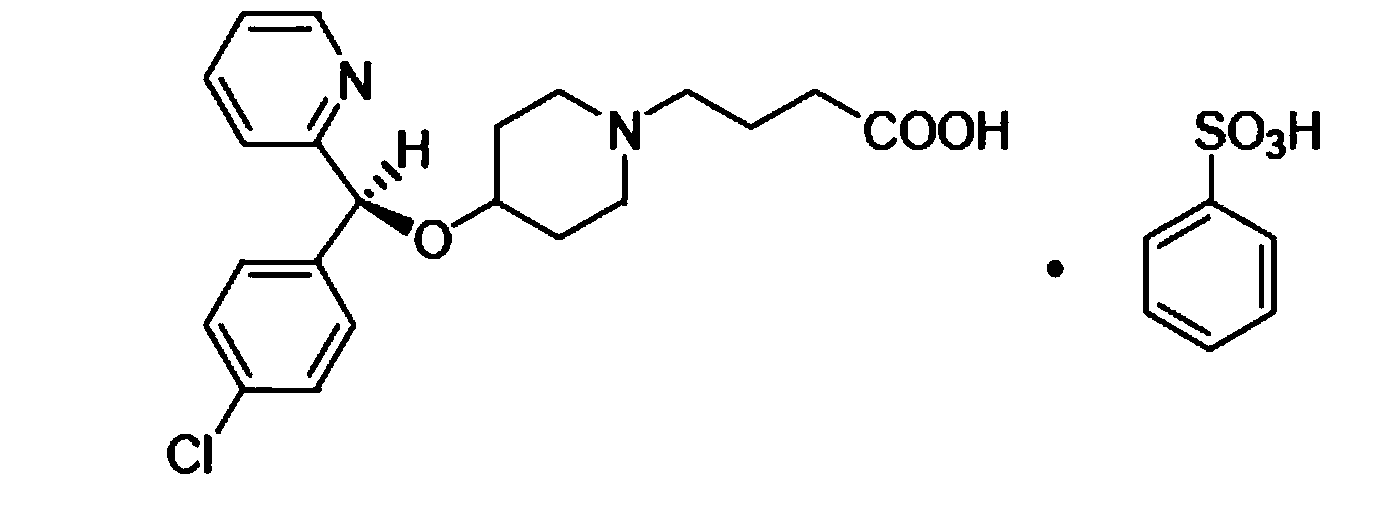
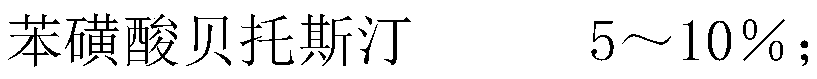Patents
Literature
31 results about "BEPOTASTINE BESILATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
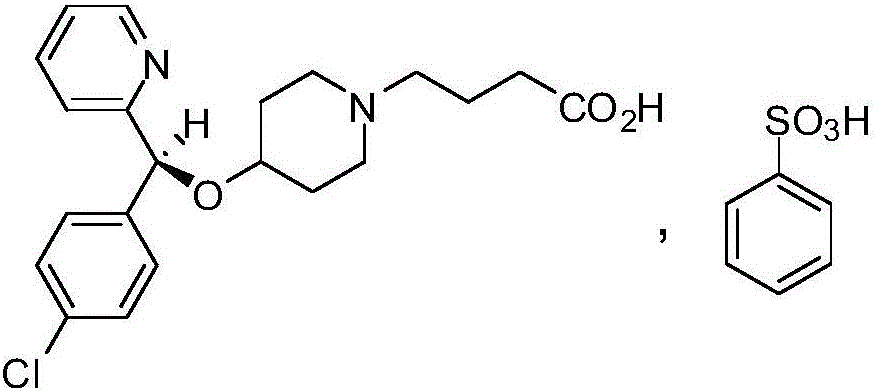
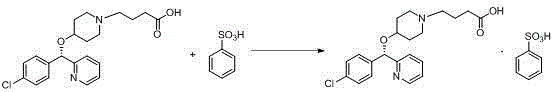
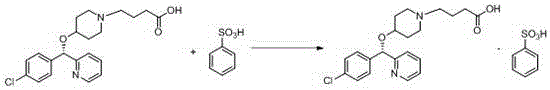
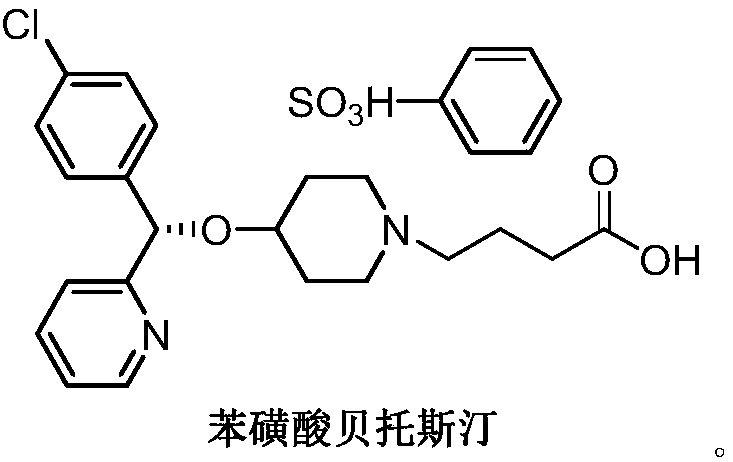
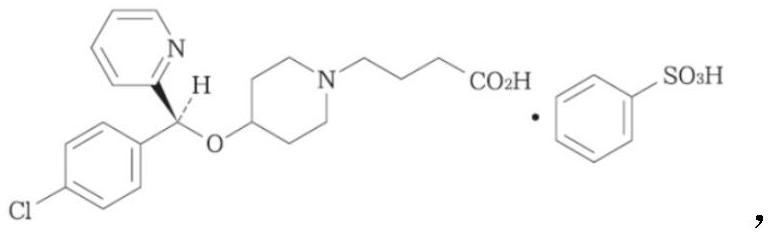
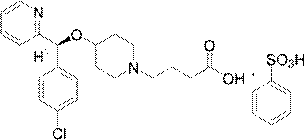
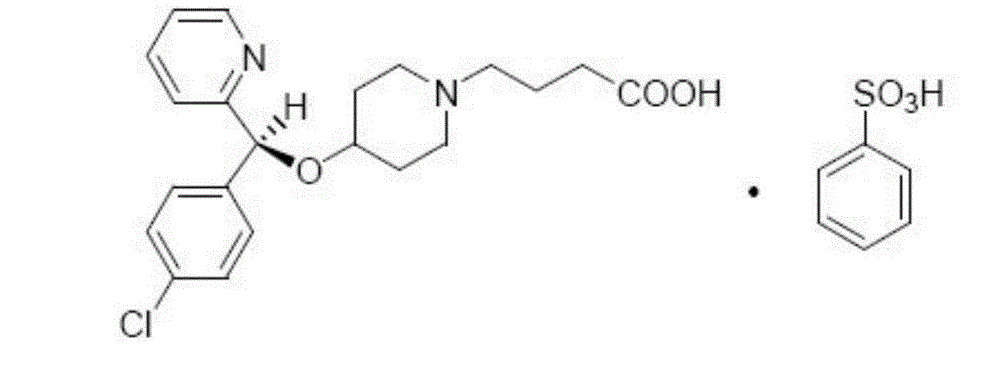
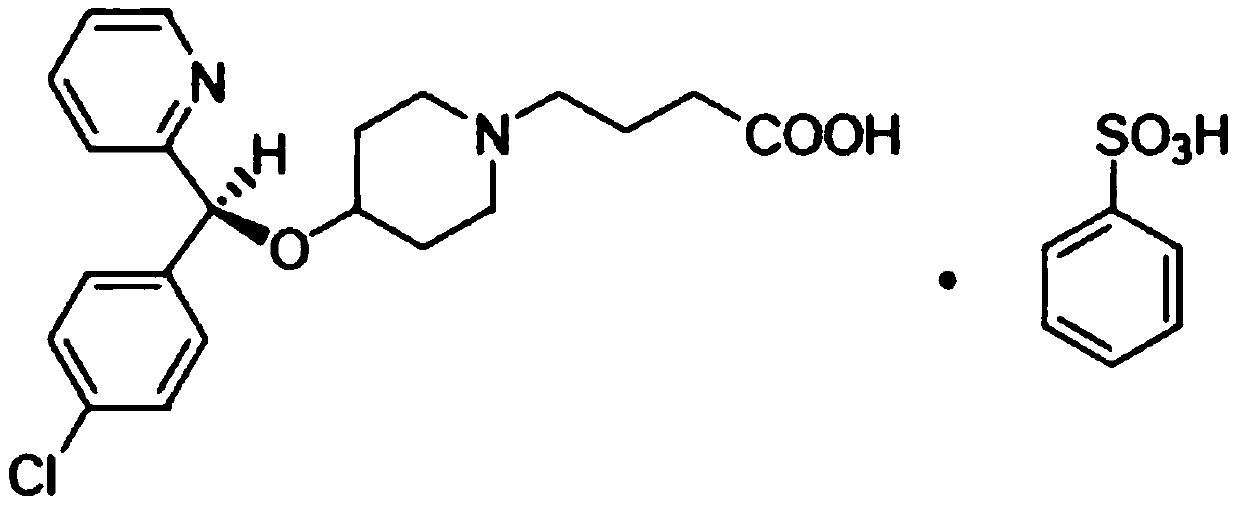
BEPREVE (bepotastine besilate ophthalmic solution) 1.5% is a sterile, topically administered drug for ophthalmic use. Each mL of BEPREVE contains 15 mg bepotastine besilate. Bepotastine besilate is designated chemically as (+) -4-[[(S)-p-chloro-alpha -2pyridylbenzyl]oxy]-1-piperidine butyric acid monobenzenesulfonate.
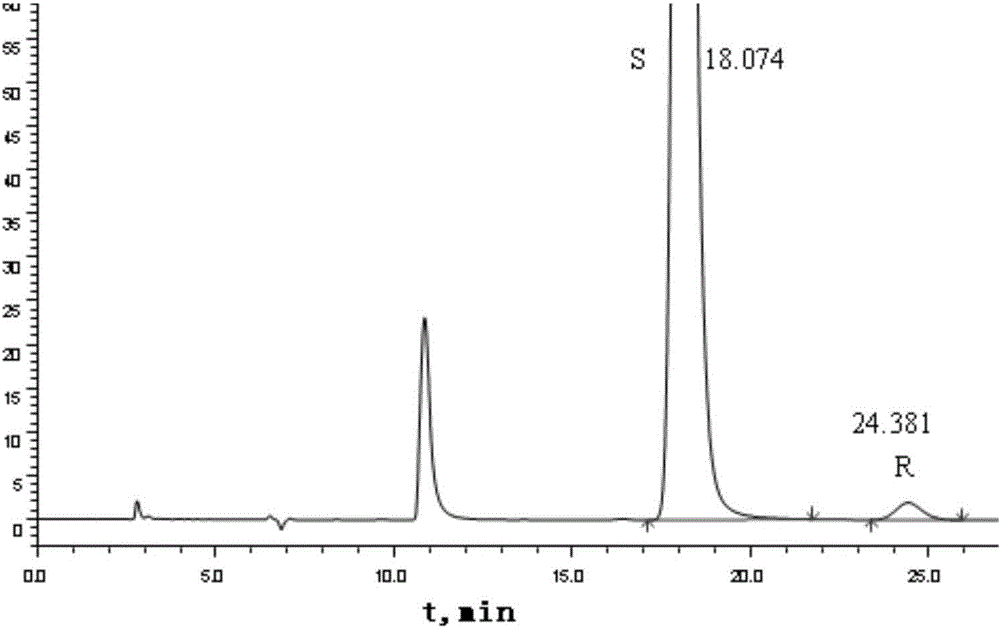
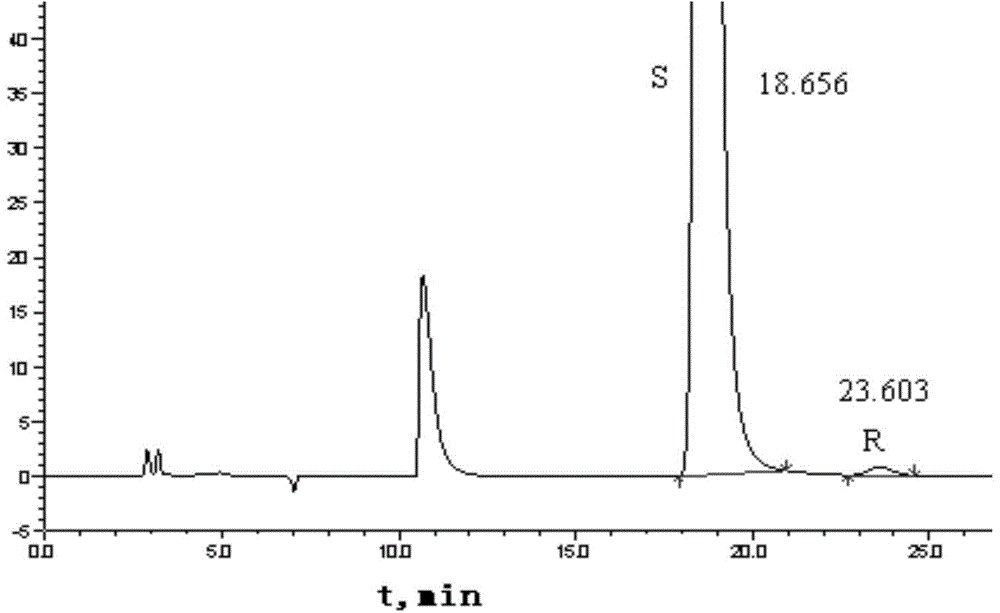
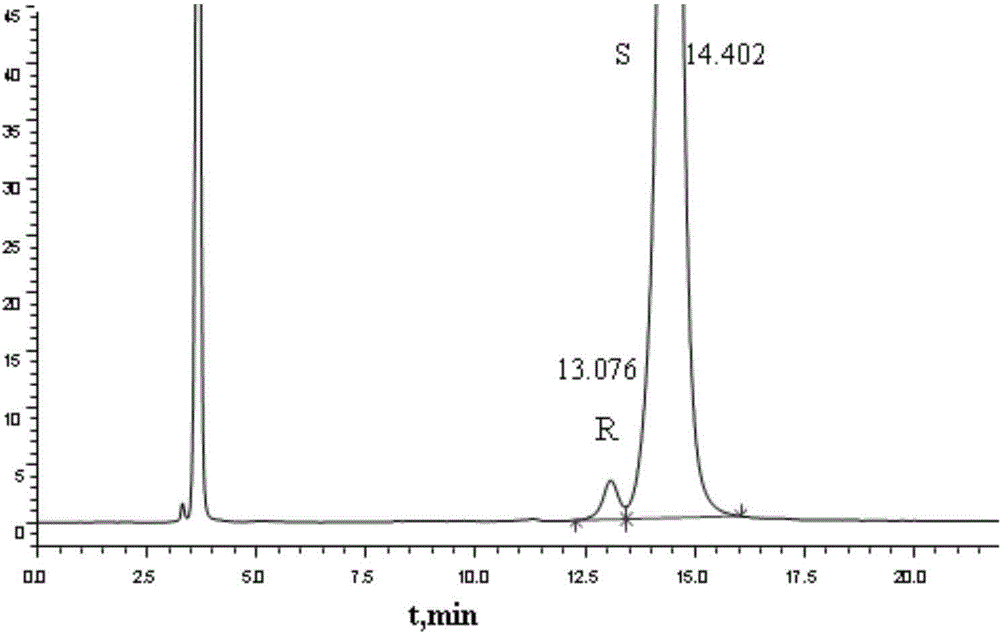
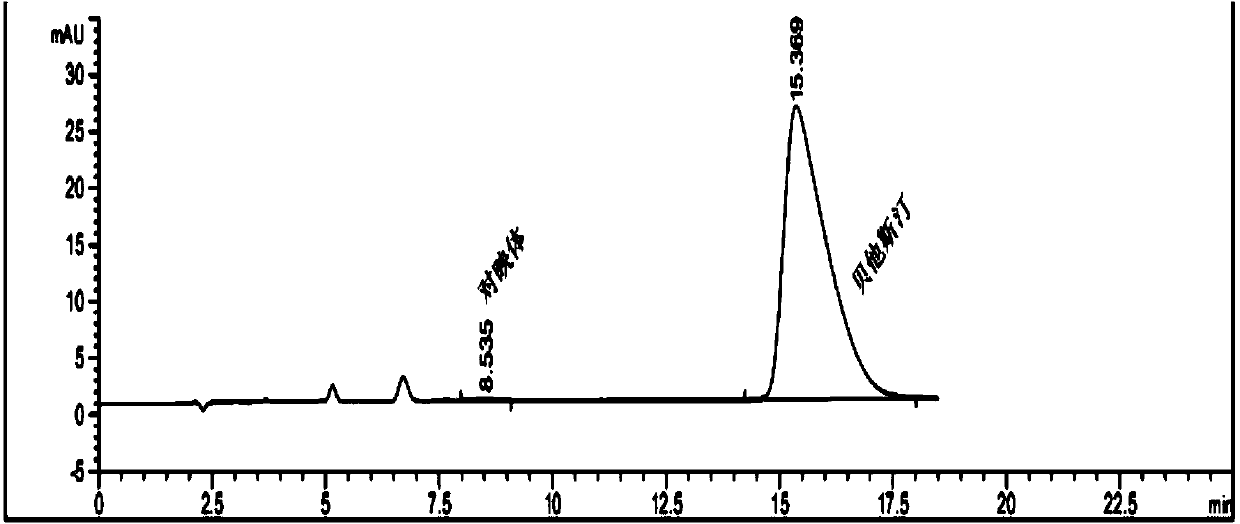
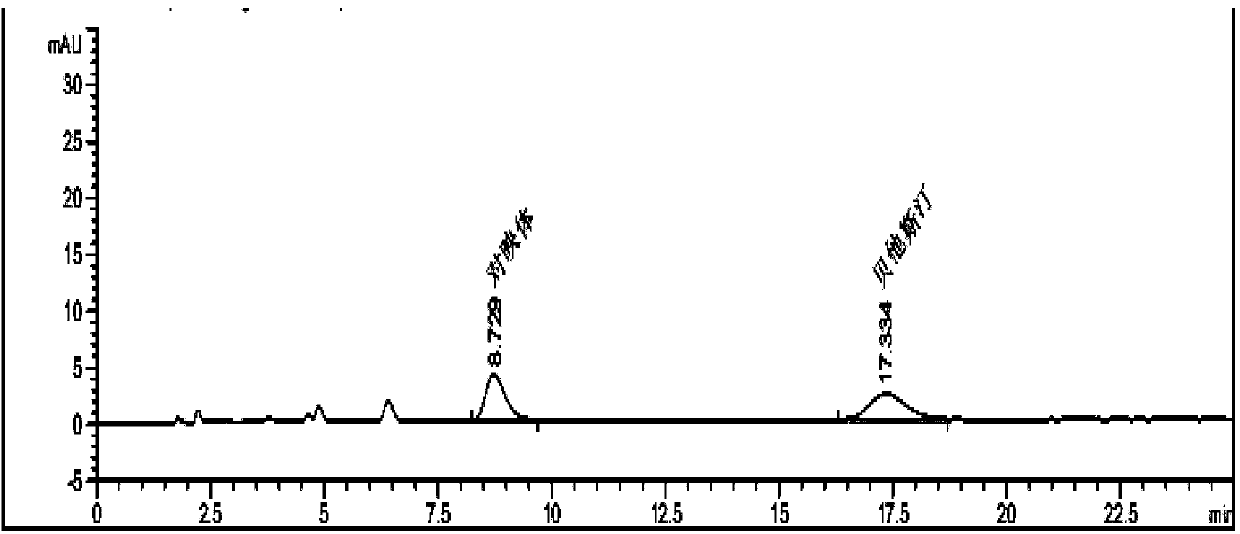
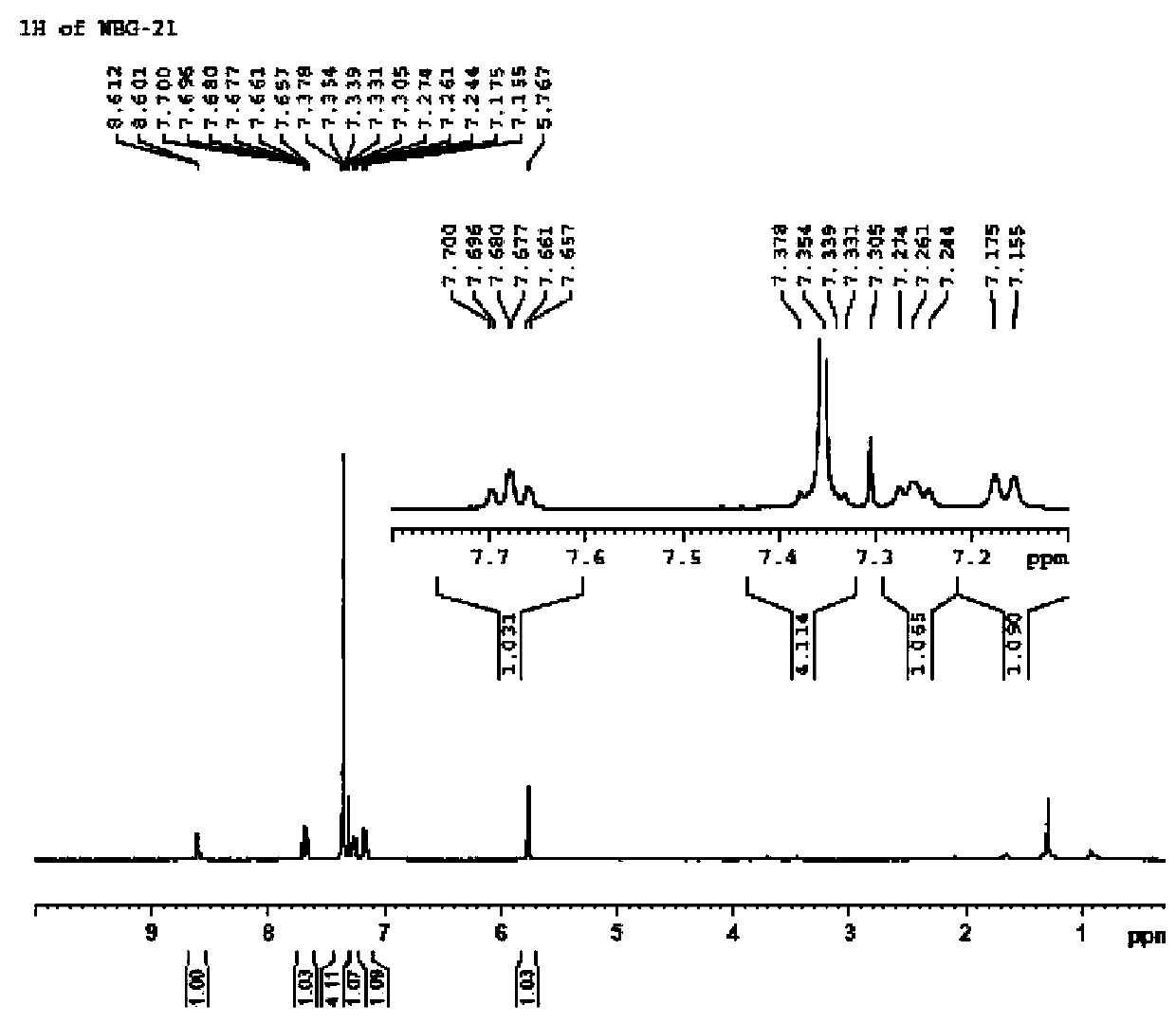
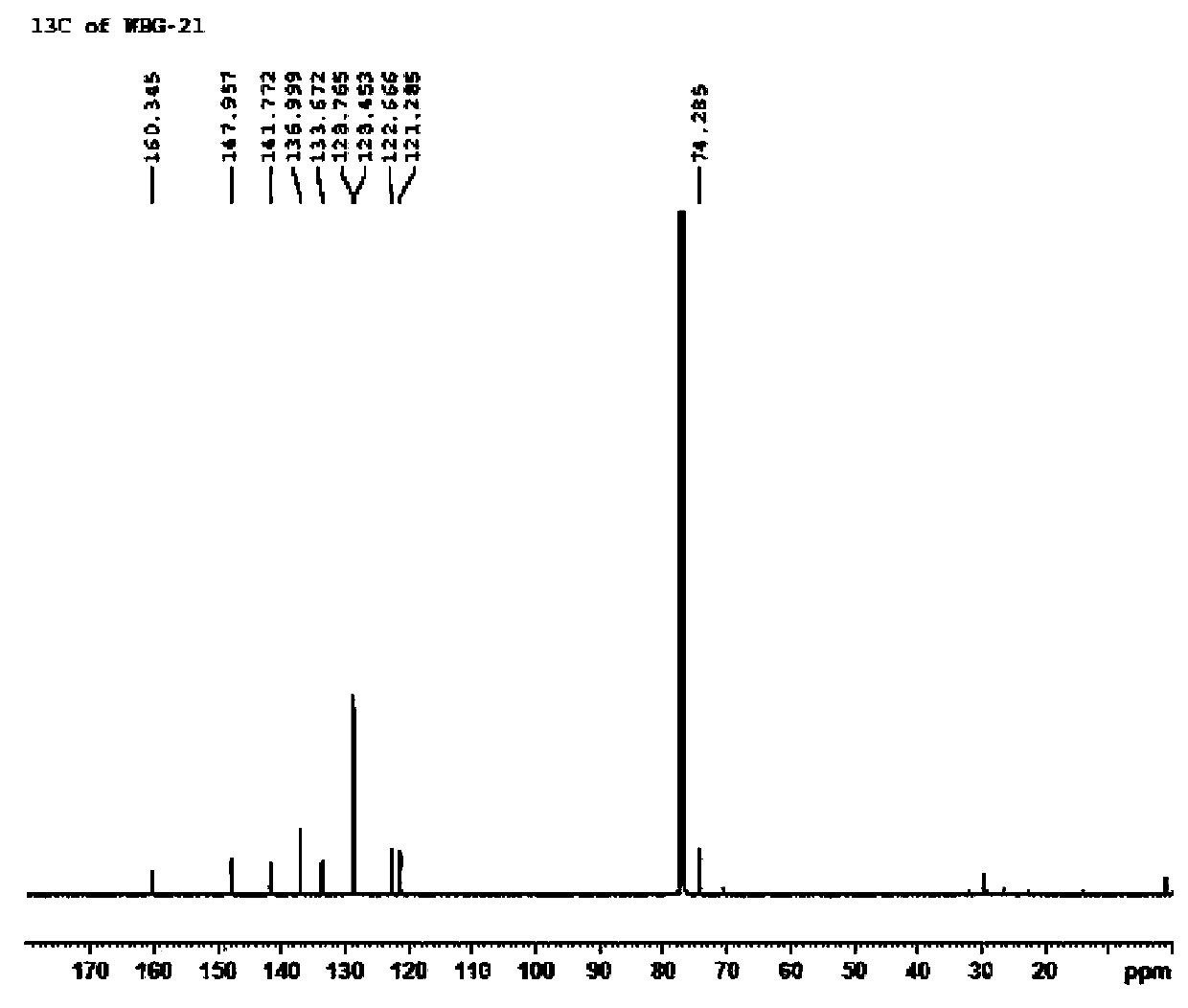
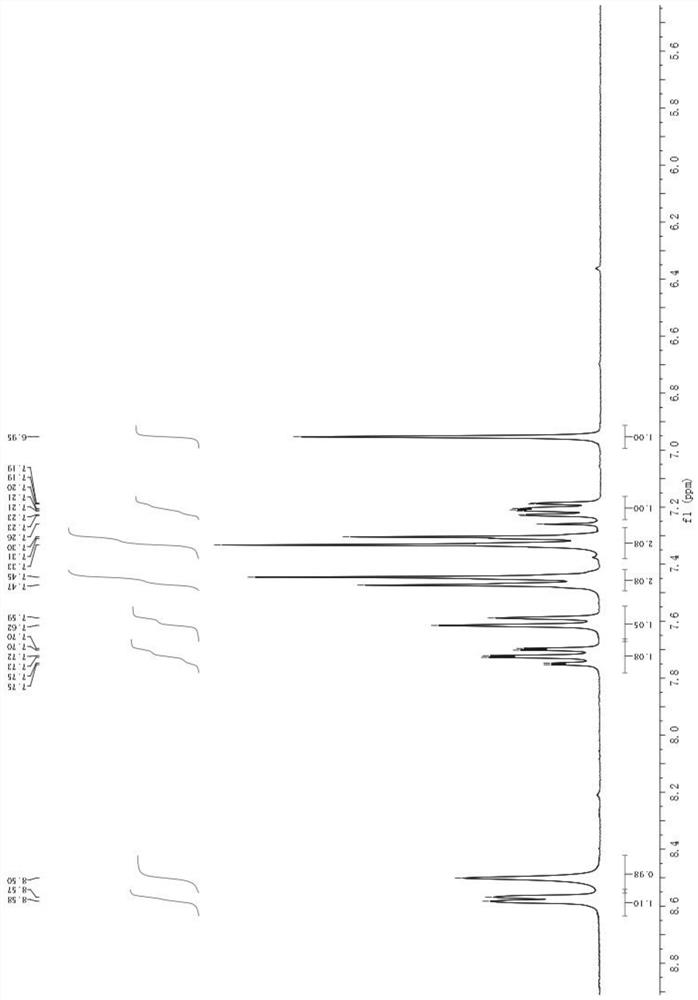
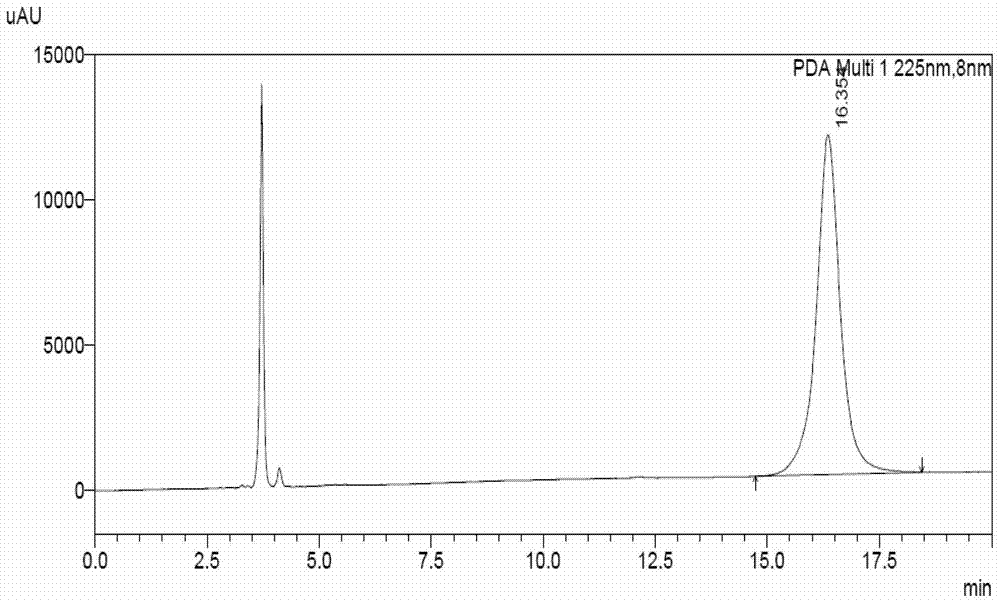
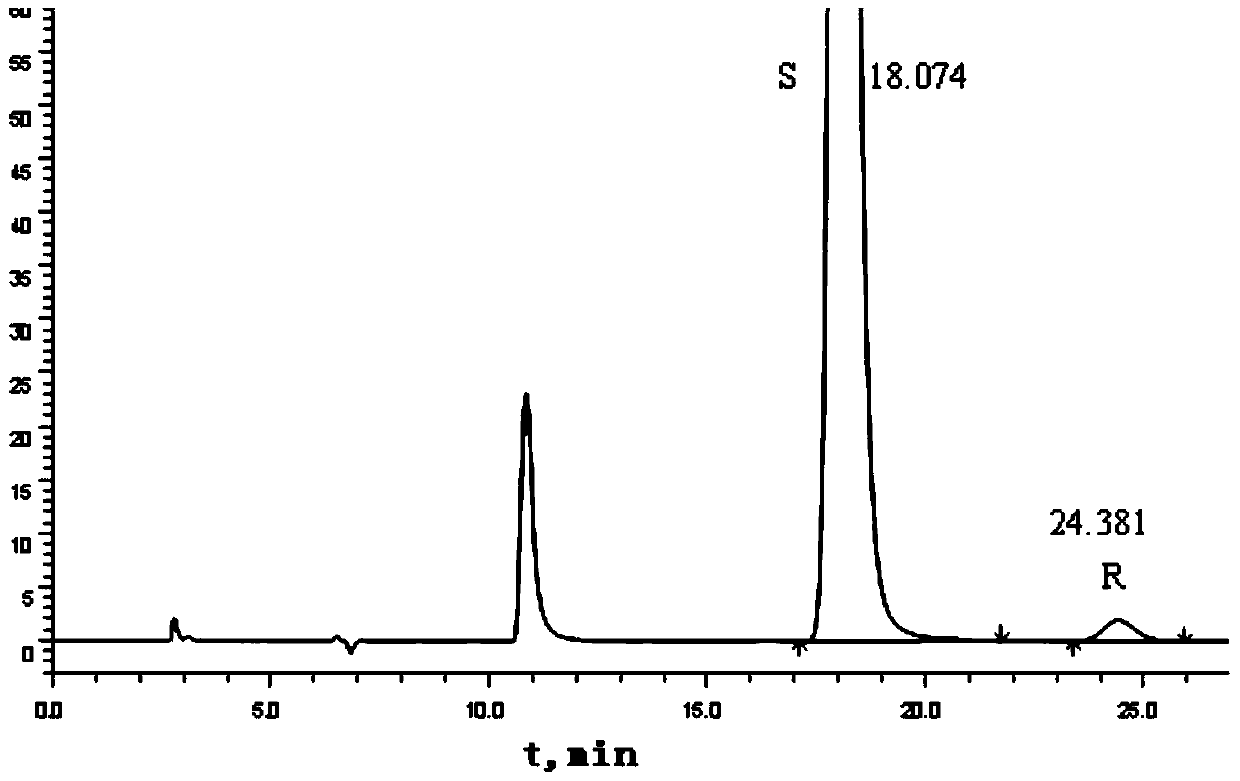
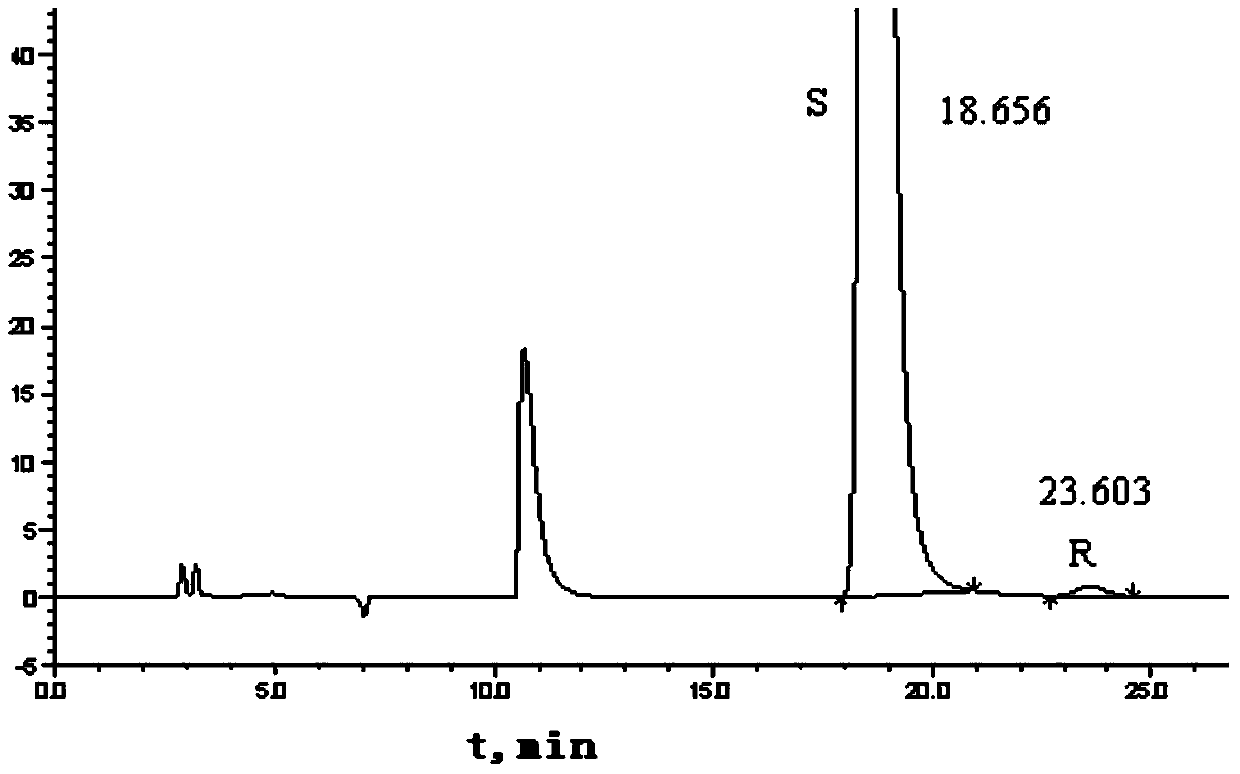
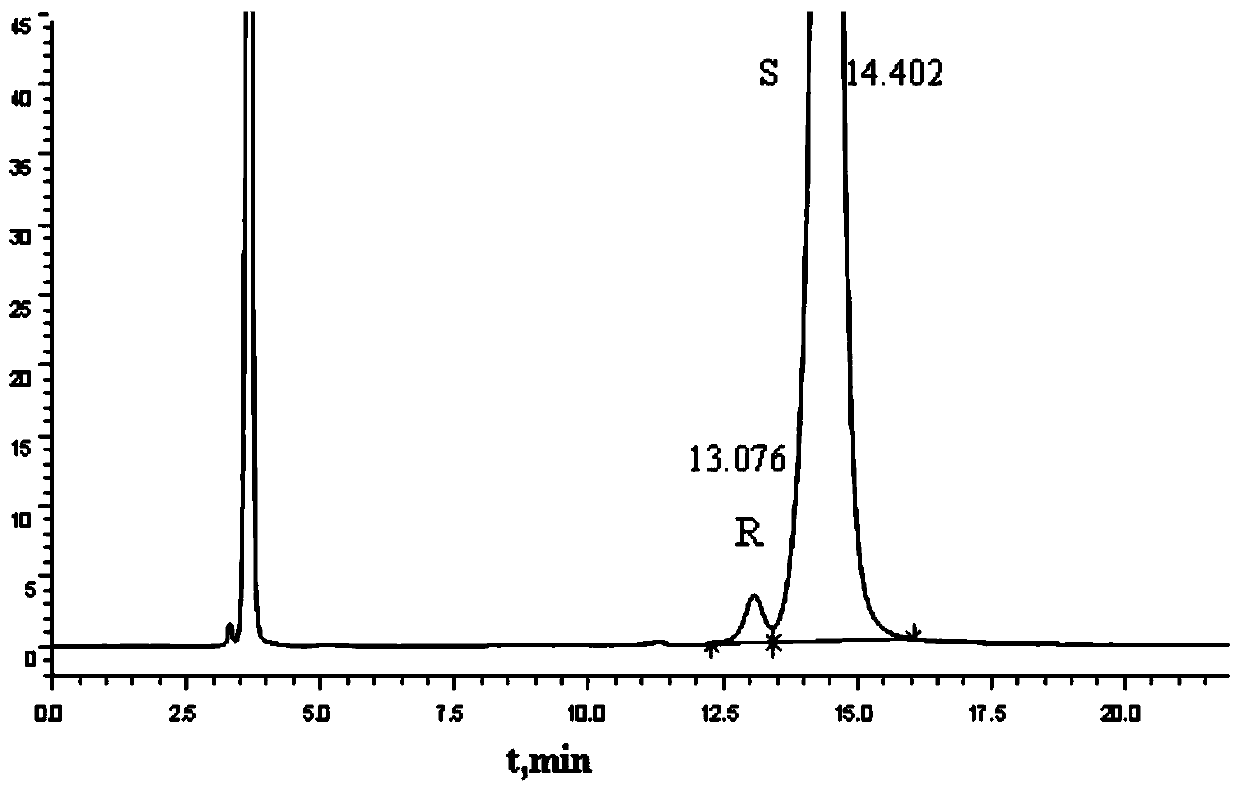
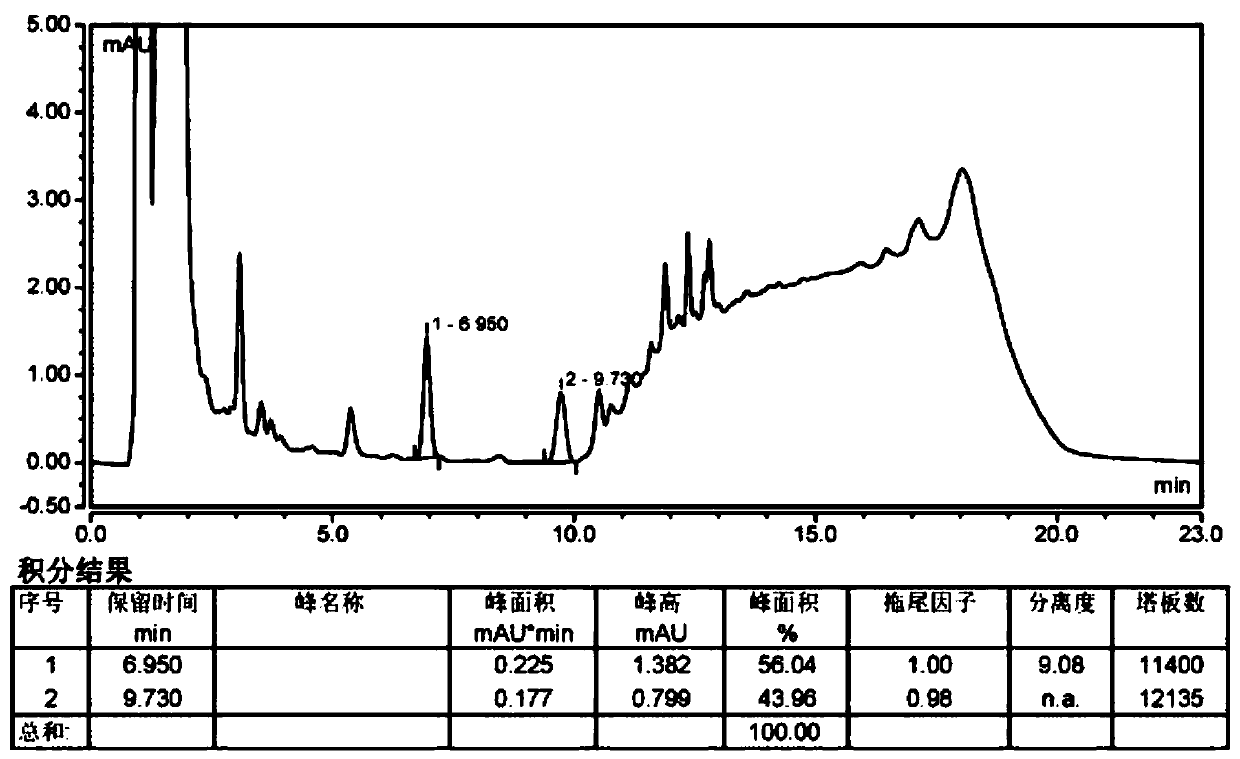
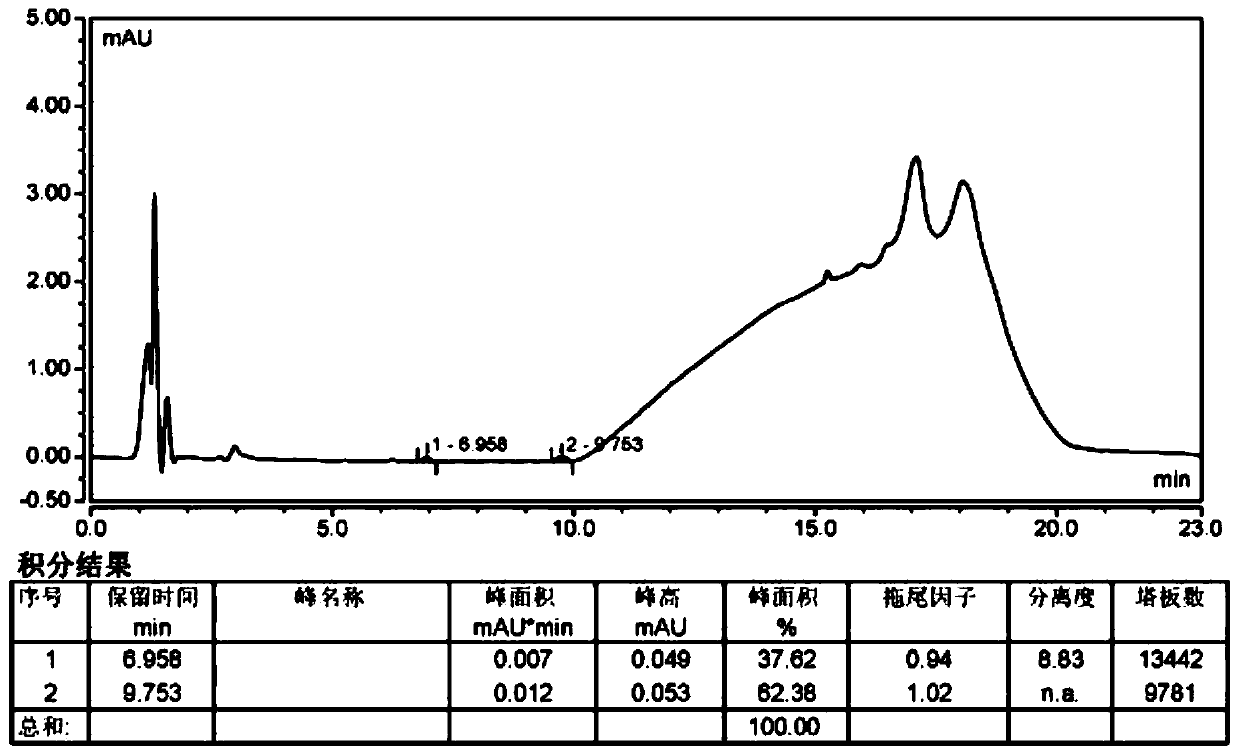
Method for separating and measuring Bepotastine Besilate optical isomer impurity
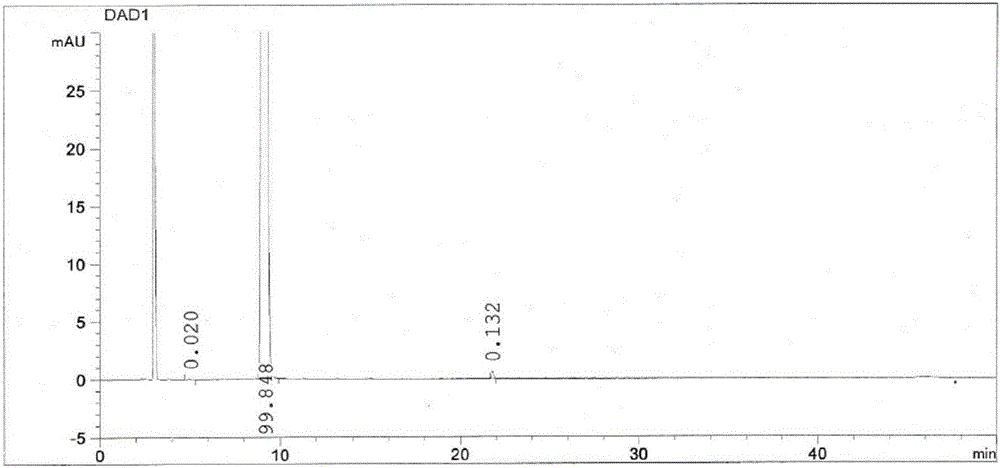
A method for splitting a Bepotastine Besilate optical isomer and quantitatively measuring R-isomer impurity is disclosed. According to the invention, high performance liquid chromatography is adopted. A mobile phase contains n-hexane and ethanol. The method is characterized in that normal-phase liquid chromatography is adopted; packing used in a chromatographic column is silica gel with its surface coated with amylase-tris-(3,5-xylyl carbamate); and the mobile phase also contains an alkaline additive. In comparison with the prior art, the method provided by the invention has advantages as follows: the mobile phase is simple to prepare, and resolutions of two isomers are both within 4.5-5.5. Especially, the chromatographic column of the method has good durability, and the chromatographic column still has high column efficiency after having been used for one year. Thus, analytic determination cost is greatly reduced.
Owner:CHONGQING HUAPONT PHARMA
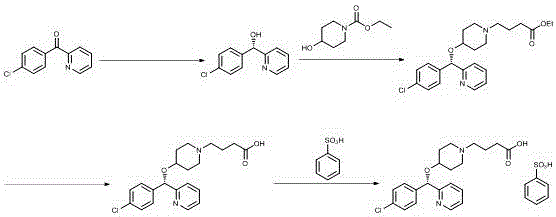
Asymmetric syntheses method of ophthalmologic drug bepotastine besilate
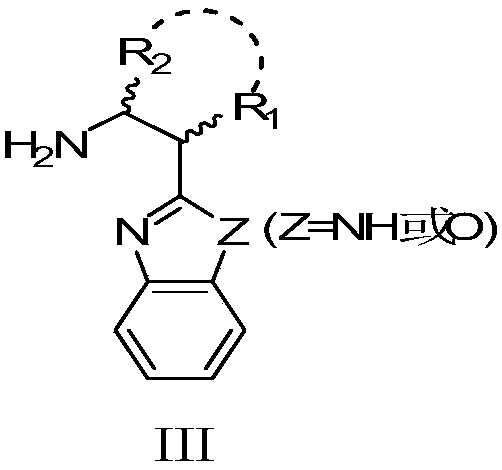
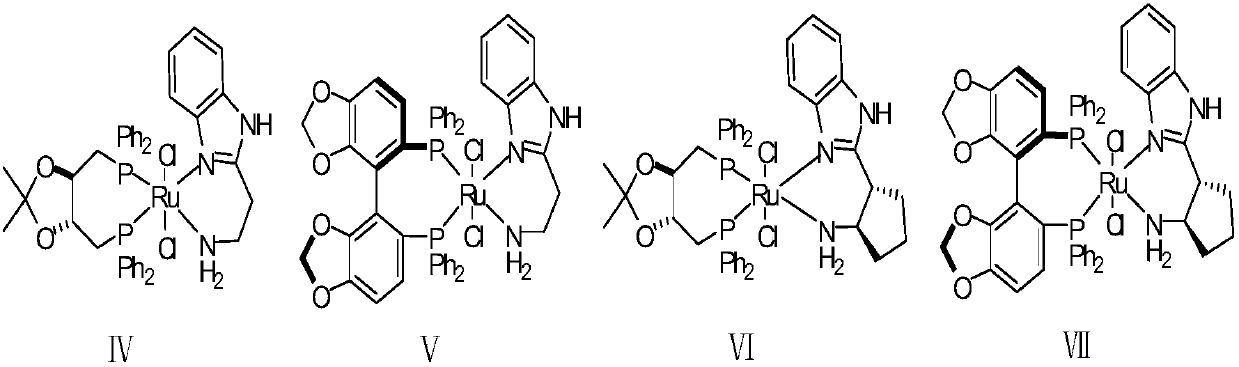
The invention relates to an asymmetric synthesis method of an ophthalmologic drug pylistramine besylate. The method concretely comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)methanone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; carrying out asymmetric transfer hydrogenation reduction on the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through using a complex of monosulfonyl chiral diamine and metallic ruthenium, rhodium and iridium as a catalyst and a sodium formate or formic acid and triethylamine mixture or isopropanol as a hydrogen source in order to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol; and condensing the (S)-(4-chlorophenyl)(2-pyridyl)methanol and ethyl 4-(4-bromopiperidine-1-yl)butyrate to obtain the ophthalmologic drug pylistramine besylate. The total yield is 61.6%.
Owner:YICHANG HUMANWELL PHARMA +1
Improved preparation method of bepotastine besilate
The invention relates to an improved preparation method of bepotastine besilate.The improved preparation method of bepotastine besilate includes the following steps that firstly, 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine and a resolving agent are reacted, and S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalaninate is obtained; secondly, S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine N-acetyl-L-phenylalaninate is added with acid to be extracted, and S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine is obtained; thirdly, S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine and ethyl 4-bromobutyrate are subjected to a coupling reaction, a hydrolysis reaction and a salt-forming reaction in sequence in acetonitrile, and bepotastine besilate is obtained.According to the technical scheme, the yield is greatly increased, the process time is shortened, and the obtained product is high in purity, safe and stable.
Owner:SHIJIAZHUANG GERUI PHARMA CO LTD
Bepotastine besilate nasal spray and preparation method thereof
ActiveCN103816121AOrganic active ingredientsAerosol deliveryMucous membrane inflammationPolyethylene glycol
The invention discloses a bepotastine besilate nasal spray and a preparation method thereof. For the nasal spray, 1-10 g of bepotastine besilate and 3-15 g of solubilizing composition are added in 100 mL of water; the solubilizing composition comprises components in percentage by weight as follows: 80%-95% of propylene glycol, polyethylene glycol 300 or polyethylene glycol 400 and the balance of caprylocaproyl macrogolglycerides; and pH of a nasal spray solution ranges from 6 to 8. In the composition, bepotastine besilate does not exist in a solid particle mode and cannot be crystallized during a long-term storage process, so that not only is rapid medicine absorption facilitated, but also a spray pump port is not blocked easily, and anaphylactic rhinitis and mucous membrane inflammation related to rhinitis can be cured effectively.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Preparation method of bepotastine besilate pharmaceutical composition
ActiveCN107753448AImprove stabilityProlong clotting timeOrganic active ingredientsPharmaceutical non-active ingredientsProduction lineExcipient
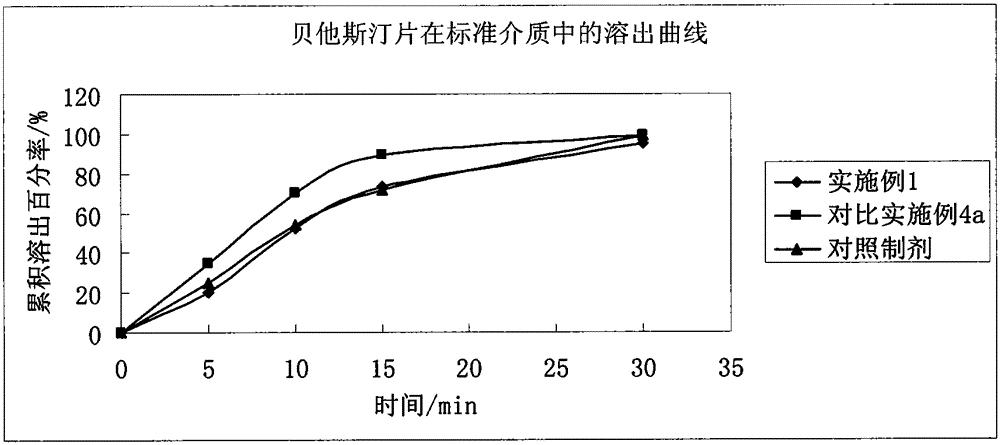
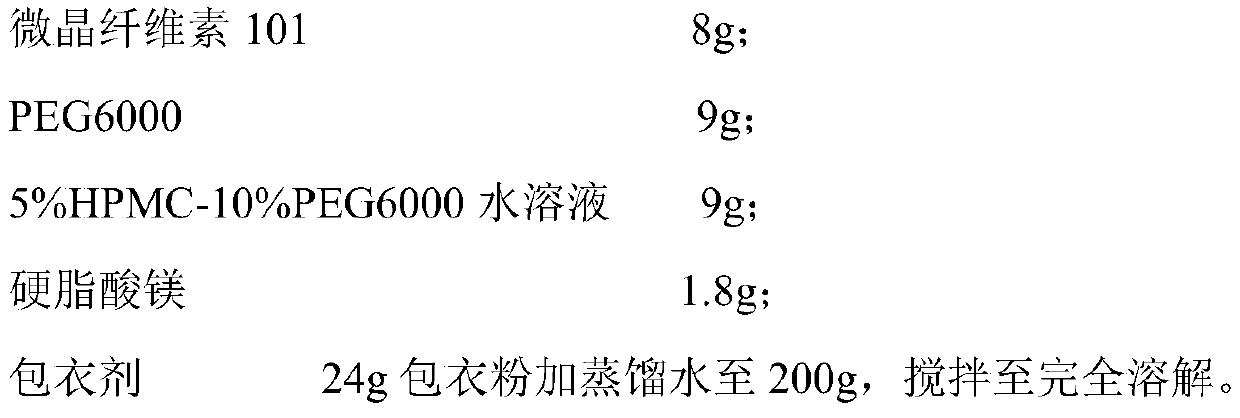
The invention belongs to the technical field of medicines, and relates to a preparation method of a bepotastine besilate pharmaceutical composition. Wet process granulation is carried out by adoptingconventional wet process granulation equipment; polyethyleneglycol 6000 and water are mixed according to a mass ratio of (2 to 1) to (20 to 1) and are heated to obtain a mixture; the mixture is addedinto a material containing bepotastine besilate and an excipient and then granulation is carried out so as to prepare the bepotastine besilate pharmaceutical composition. Compared with an original triturate, the preparation method disclosed by the invention has the advantages that requirements on equipment and processes are lower, the preparation method has a mature production line in China and issuitable for industrial production; prepared bepotastine besilate tablets are good in stability, and a stripping curve is consistent with a reference formulation.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Chiral synthesis method of bepotastine besilate intermediate
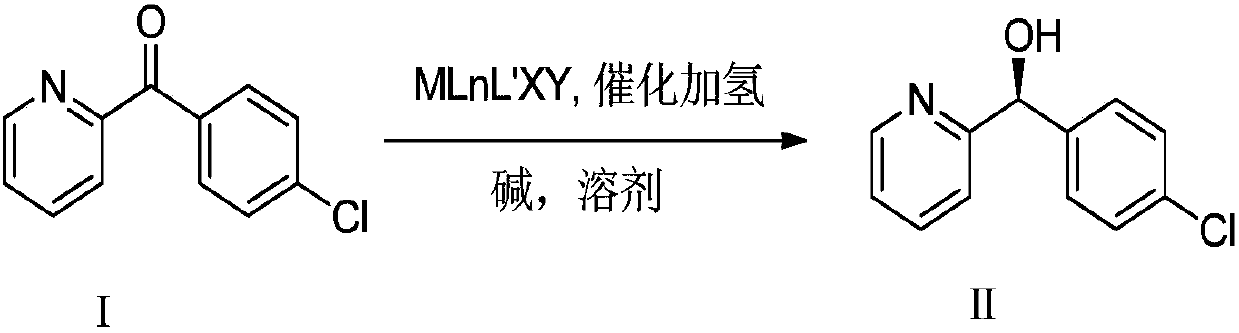
The invention discloses a chiral synthesis method of a bepotastine besilate intermediate. The chiral synthesis method comprises the following step: by taking (4-chlorphenyl) (pyridine-2-radical) ketone as a raw material, under the action of alkali and a catalyst, carrying out hydrogenation stirring reaction in a solvent, so as to obtain (S)-(4-chlorphenyl) (pyridine-2-radical) methanol. Accordingto the method disclosed by the invention, a high-purity product can be obtained only by one-step catalytic hydrogenation reaction of a production process, so that the process period is greatly shortened; the chiral synthesis method has the characteristics of simple synthesis line, small use amount of the catalyst, stable reaction process, environmental friendliness, economy and the like, and has an extremely industrial application value in the aspect production and preparation of the (S)-(4-chlorphenyl) (pyridine-2-radical) methanol.
Owner:ENANTIOTECH CORP
Refining method for high-purity bepotastine besilate
ActiveCN106117183AEfficient removalEasy to operateOrganic chemistryOrganic compound preparationAcetonitrileSolvent
Owner:HEFEI JIUNUO MEDICAL TECH
Stable bepotastine besilate crystal and preparation method thereof
ActiveCN104119314AReduce the overall heightImprove stabilityOrganic active ingredientsOrganic chemistryFt ir spectraActive ingredient
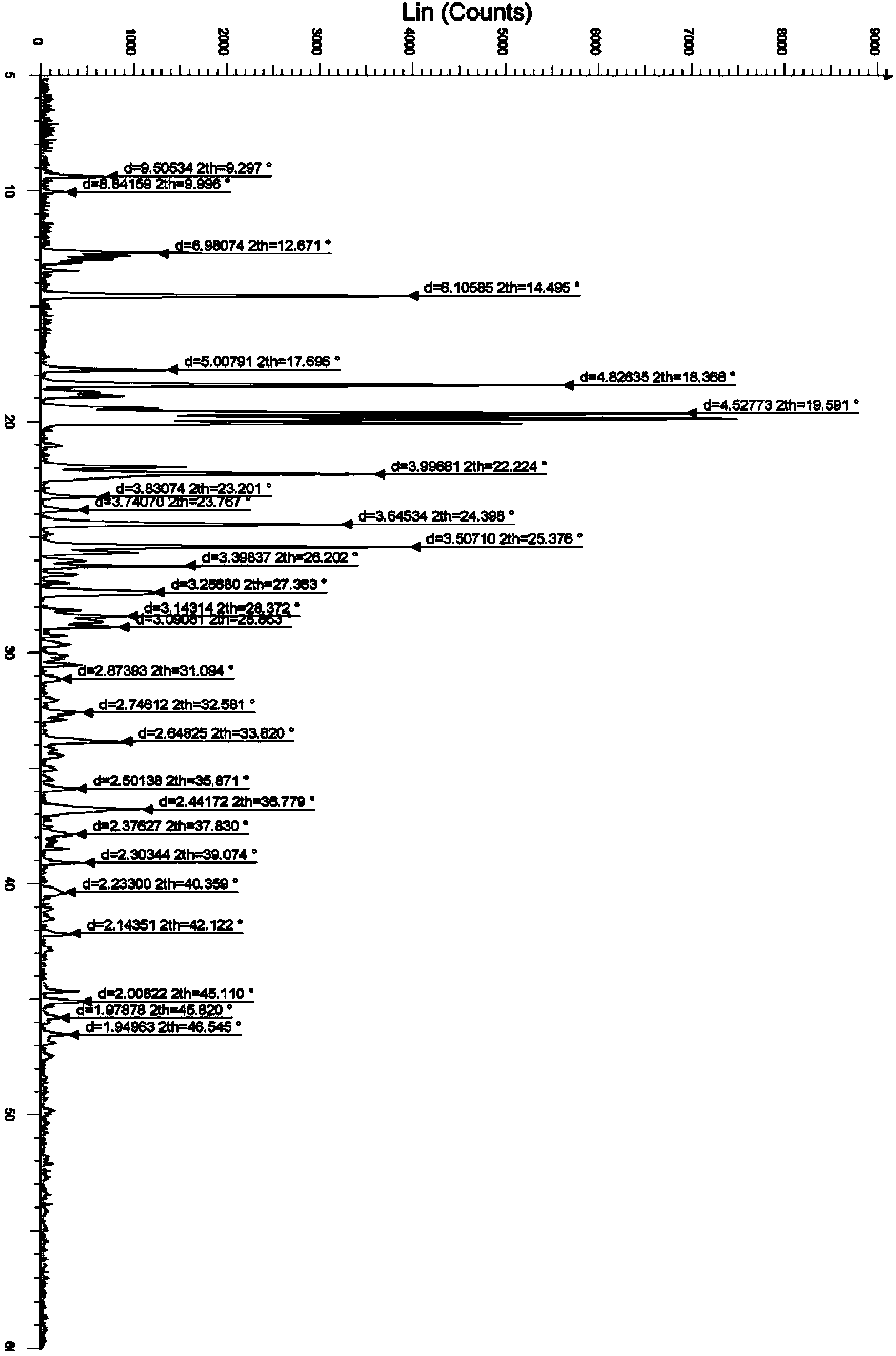
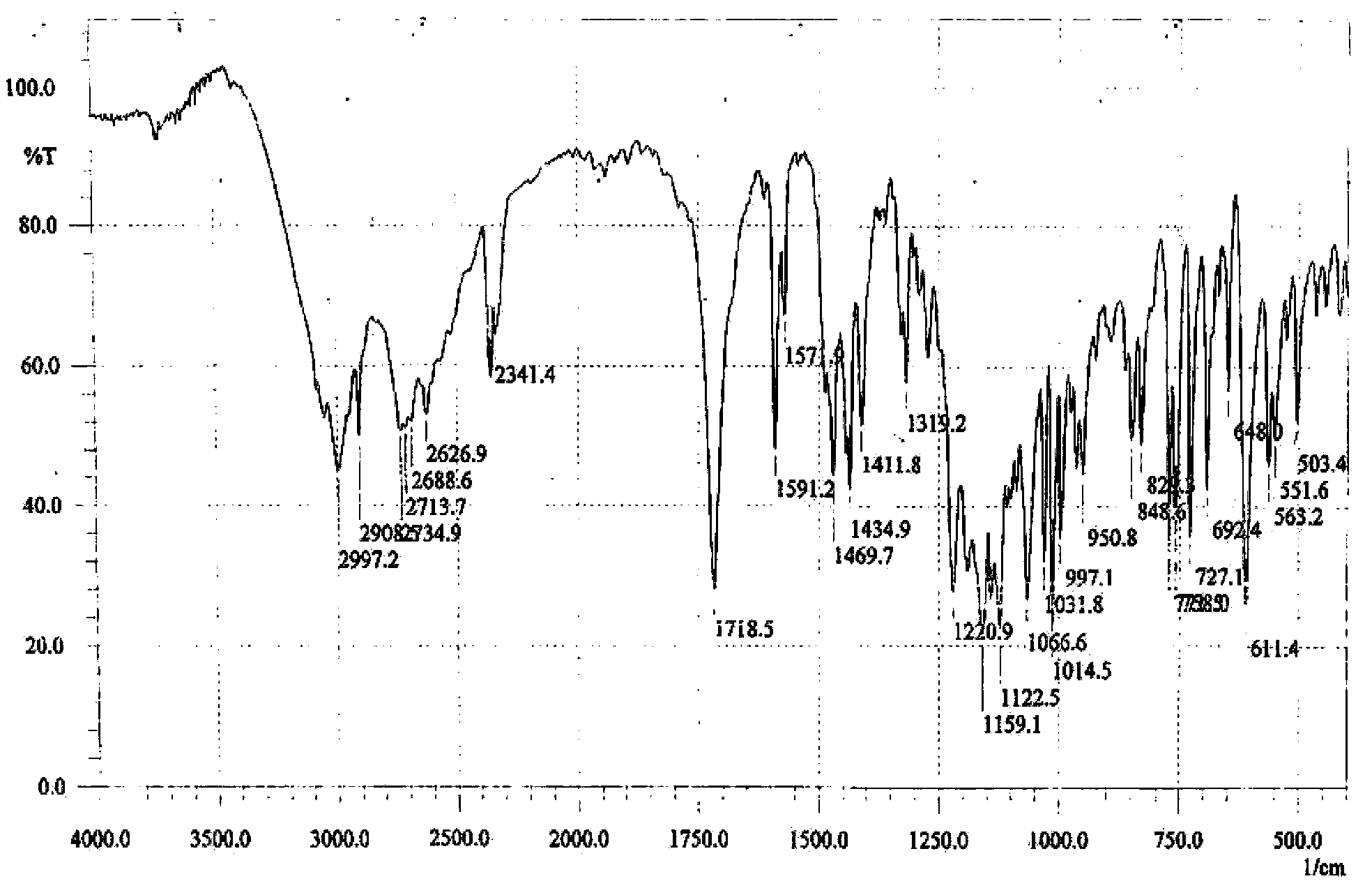
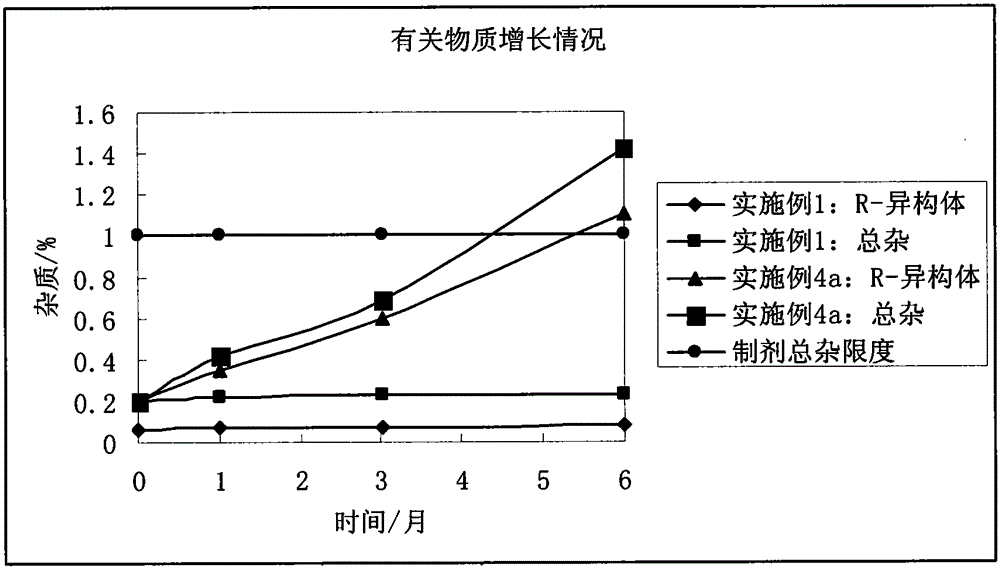
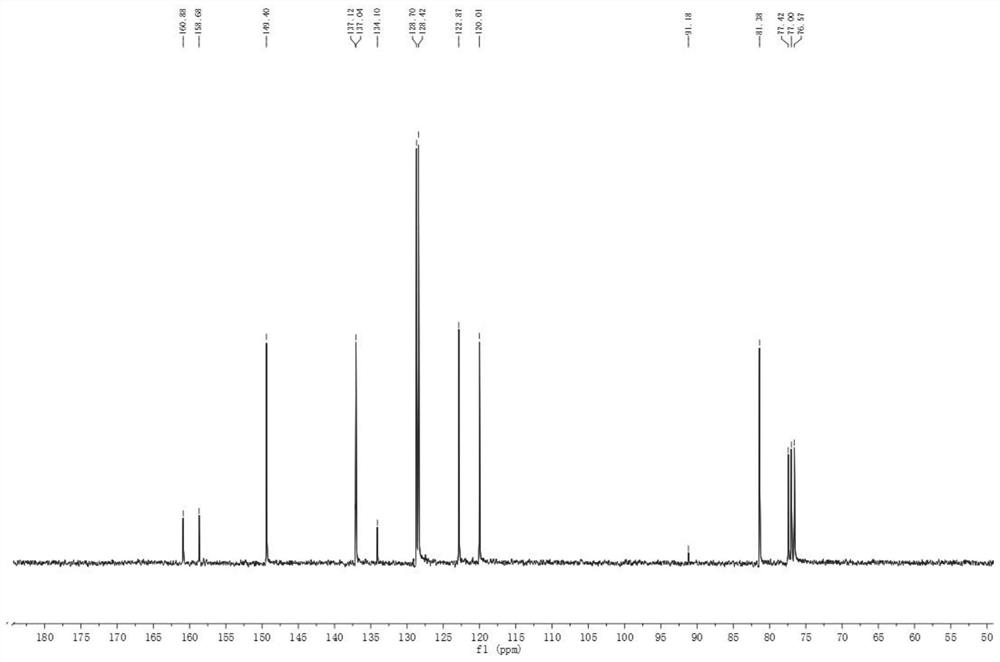
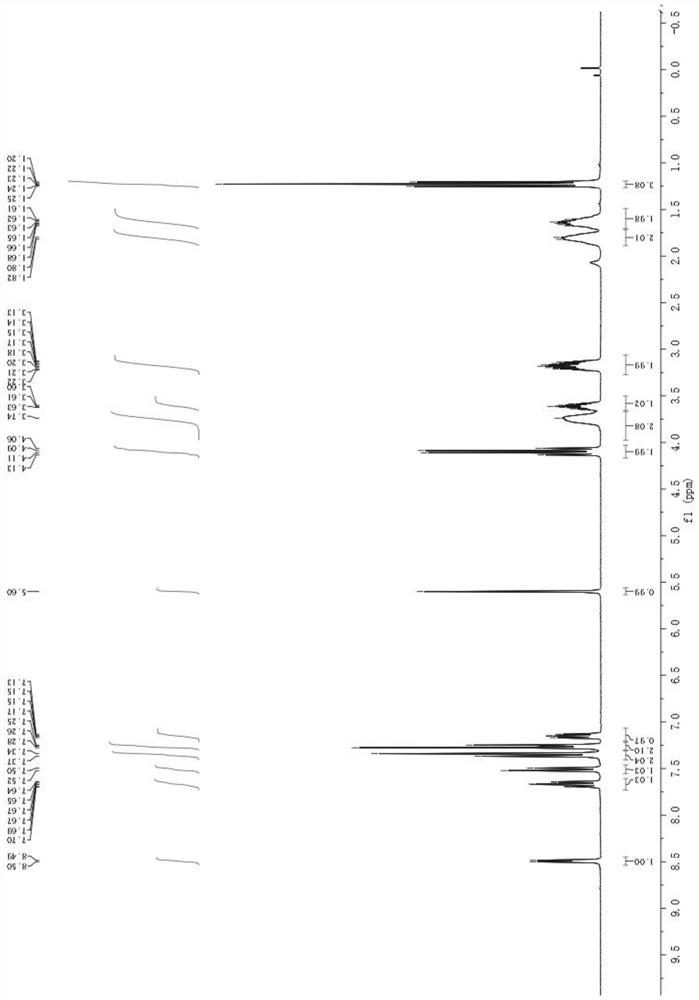
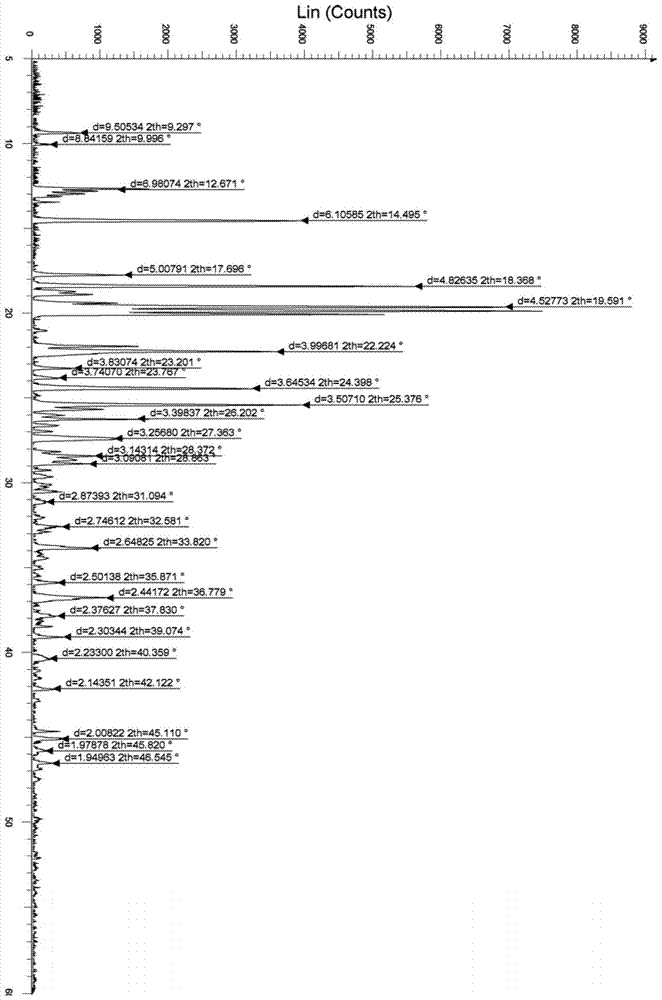
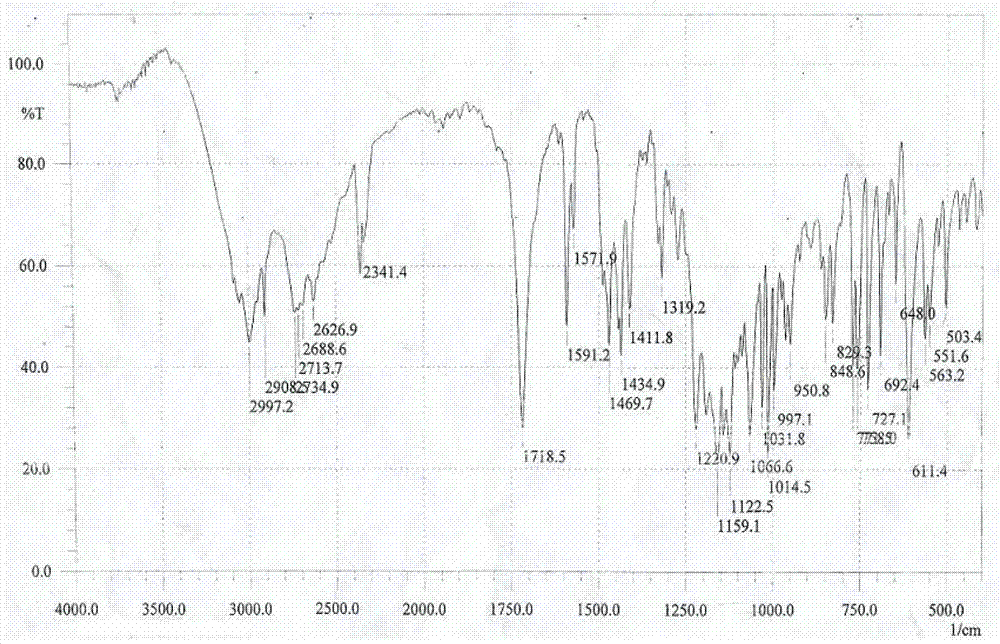
The invention discloses a stable bepotastine besilate crystal in novel crystal form through a recrystallization method. The obtained bepotastine besilate crystal is confirmed through X-ray powder diffraction and infrared spectrum. Stability contrast experiments prove that the provided bepotastine besilate crystal has extremely high stability, and both bulk drug and preparation of the bepotastine besilate crystal have stability obviously better than that of existing bepotastine besilate crystals.
Owner:CHONGQING HUAPONT PHARMA
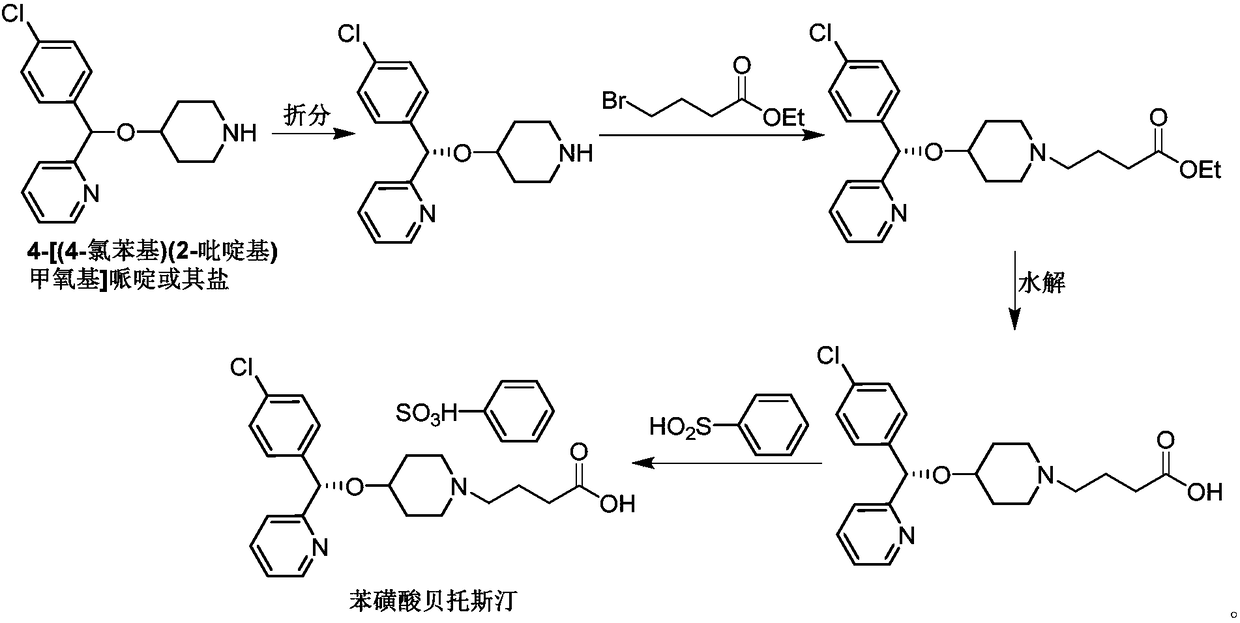
Industrial preparation method for bepotastine besilate or racemoid of bepotastine besilate
The invention provides an industrial preparation method for bepotastine besilate or racemoid of the bepotastine besilate. The method includes the following steps of firstly, dissolving 2-4-[(S)-(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine or racemate of the 2-4-[(S)-(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in an organic solution; secondly, adding an alkaline acid-binding agent and ethyl 4-bromobutyrate, conducting stirring, temperature rising and backflow, and conducting the condensation reaction; thirdly, conducting cooling and filtering after the reaction ends, and conducting organic layer vacuum concentration to obtain grease; fourthly, adding purified water to the grease, conducting stirring, ethyl acetate extraction and skimming to obtain an organic layer, washing the organic layer with alkaline liquid, drying the organic layer, and conducting reduced pressure distillation after filtering out a drying agent to obtain an intermediate; fifthly, adding alkaline liquid in the intermediate for hydrolyzing, adjusting to acid after hydrolyzing, conducting stirring, filtering and concentrating, adding dichloromethane for stirring and drying, and then conducting filtering and reduced pressure concentration again to obtain the bepotastine besilate or the racemoid of the bepotastine besilate. By means of the method, the operation procedure is simplified, reaction time is shortened, no racemization phenomena can occur, and the method has the advantages of being high in yield, easy to operate and the like.
Owner:JIANGSU LIANHUAN PHARMA
Bepotastine besilate composition and preparation prepared thereby
InactiveCN106309389AImprove liquidityGood compressibilityOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLPolymer science
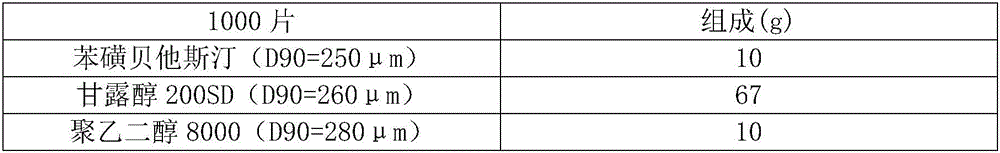
The invention belongs to the technical field of medicines, and relates to a bepotastine besilate composition and a preparation prepared thereby, and the preparation particularly relates to a bepotastine besilate tablet. The composition is prepared from bepotastine besilate, mannitol and solid-state polyethylene glycol polymer, and the partical sizes of bepotastine besilate, mannitol and solid-state polyethylene glycol polymer are all controlled at D90 of 200 micrometers to 300 micrometers. The preparation is a bepotastine besilate preparation containing the bepotastine besilate composition. In particular, the bepotastine besilate tablet is prepared from the bepotastine besilate composition and a pharmaceutically acceptable carrier. The composition has good flowability and pressing ability, and is used for preparing the bepotastine besilate tablet and other preparations which are easy to mix evenly and shape by tablet compressing, thus a finished product with uniform content and stable quality is obtained. The preparation method is easy, operation is convenient, requirements for production equipment are low, and it is easy to realize industrialization.
Owner:CHONGQING HUAPONT PHARMA
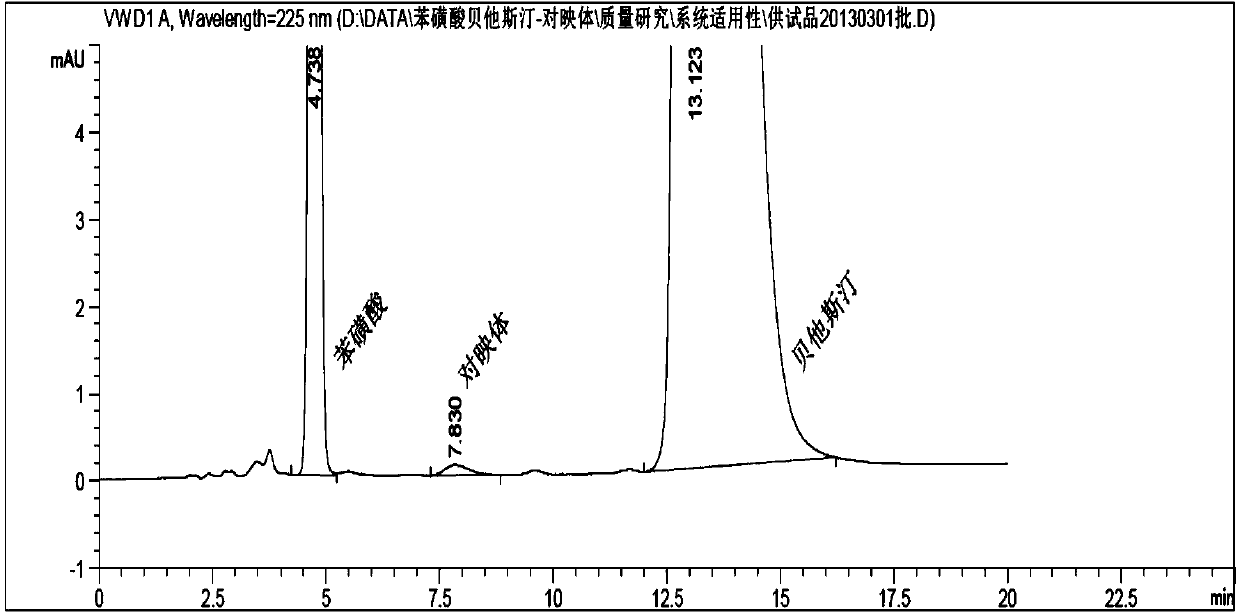
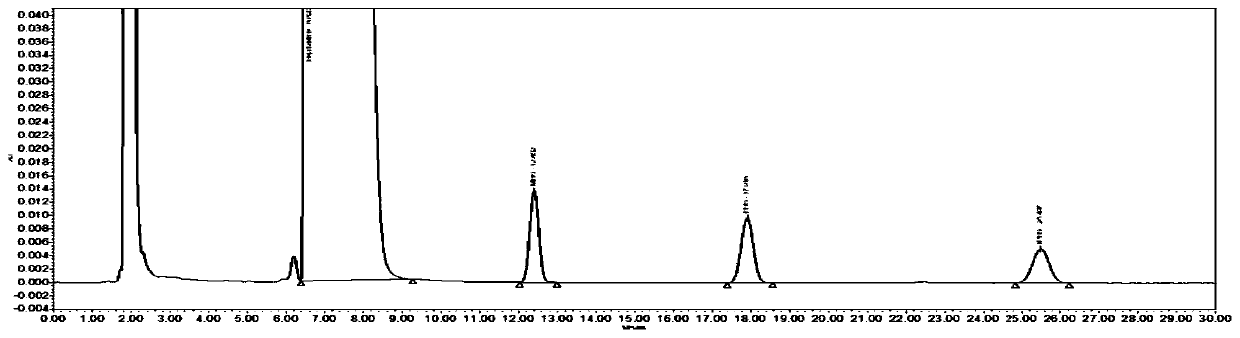
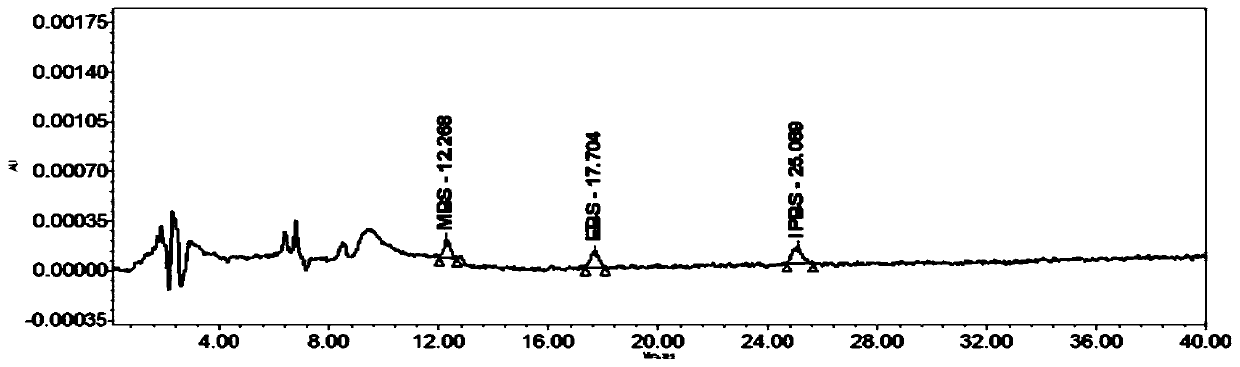
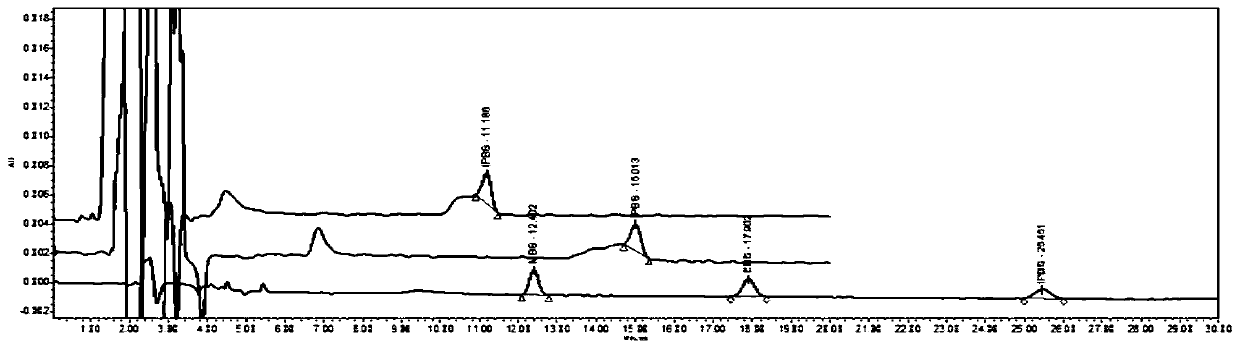
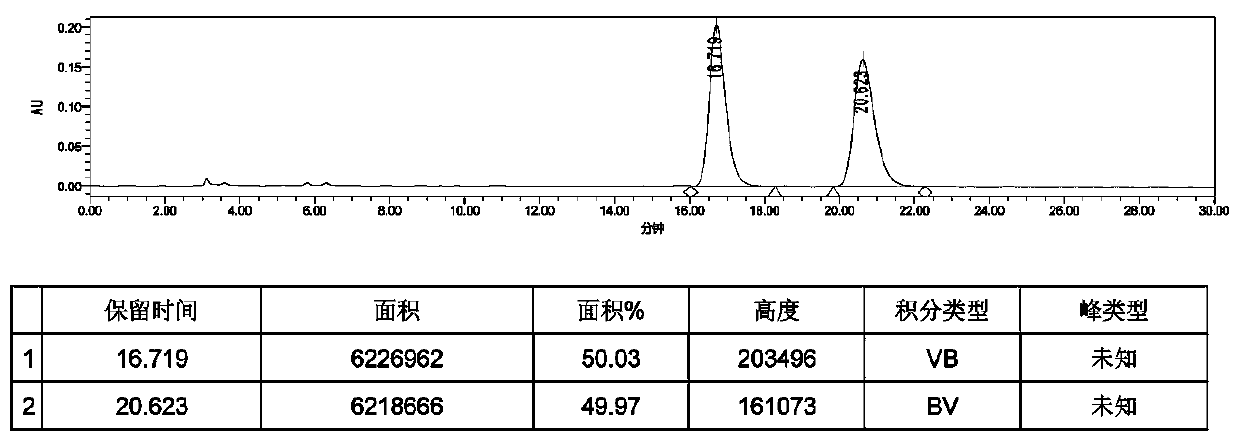
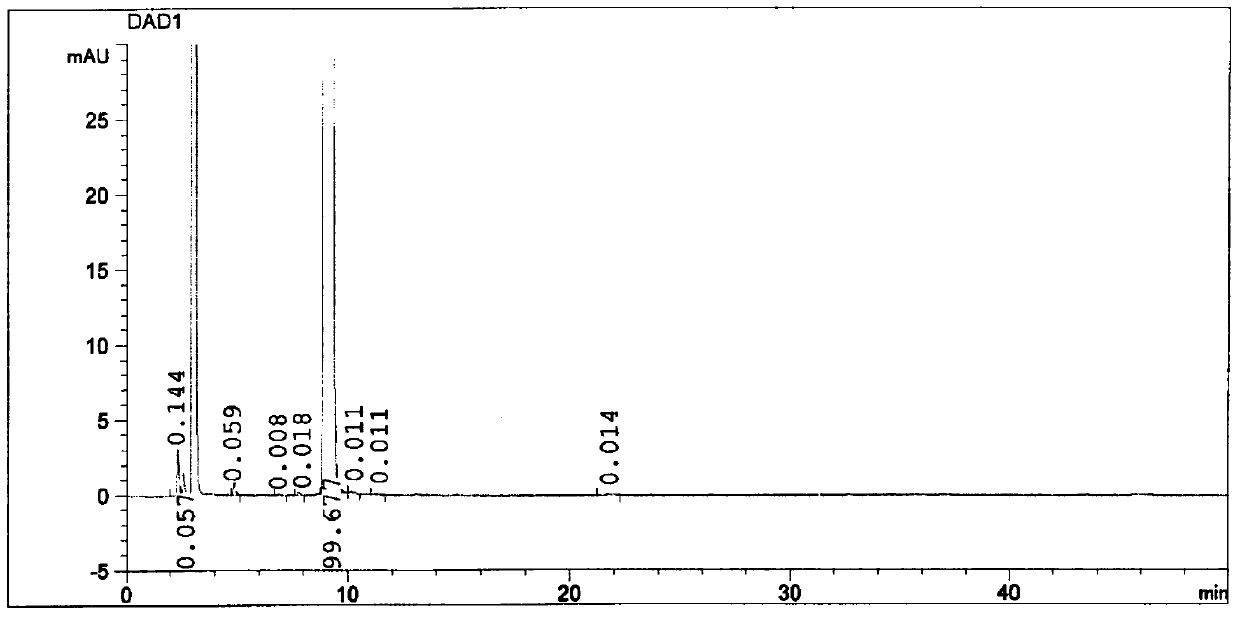
Method for separating and measuring bepotastine besilate and potential genotoxic impurities thereof with HPLC (High Performance Liquid Chromatography) method
ActiveCN107782832ASeparation assay is validHigh sensitivityComponent separationPhosphoric acidGradient elution
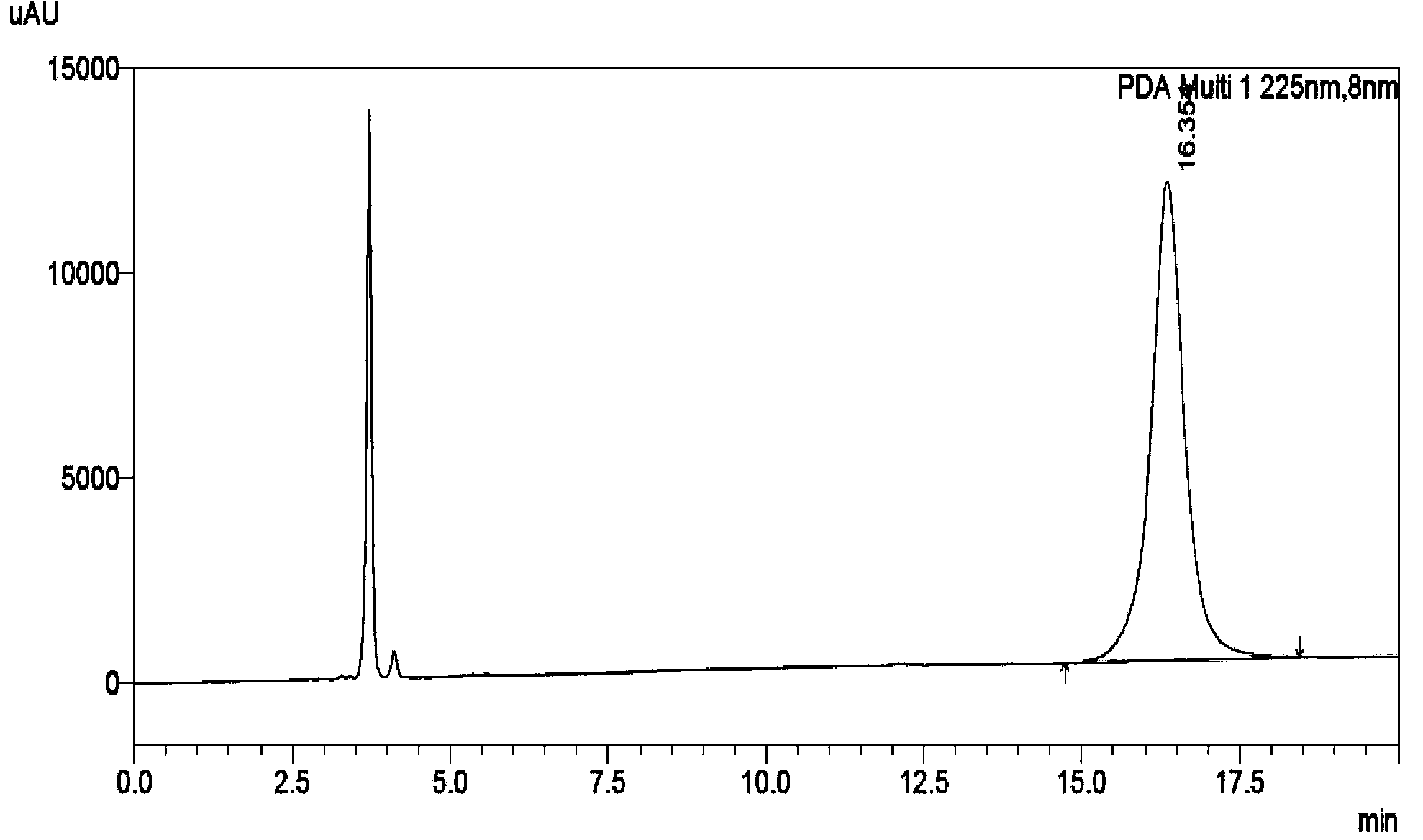
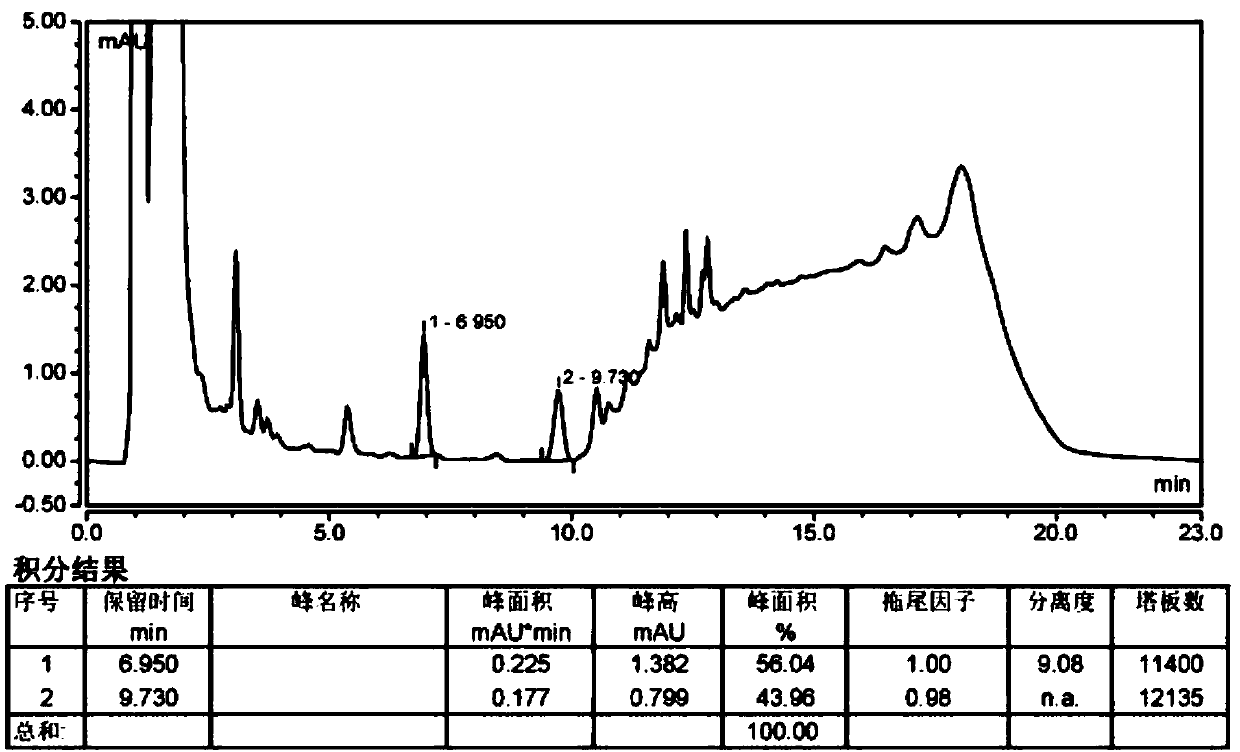
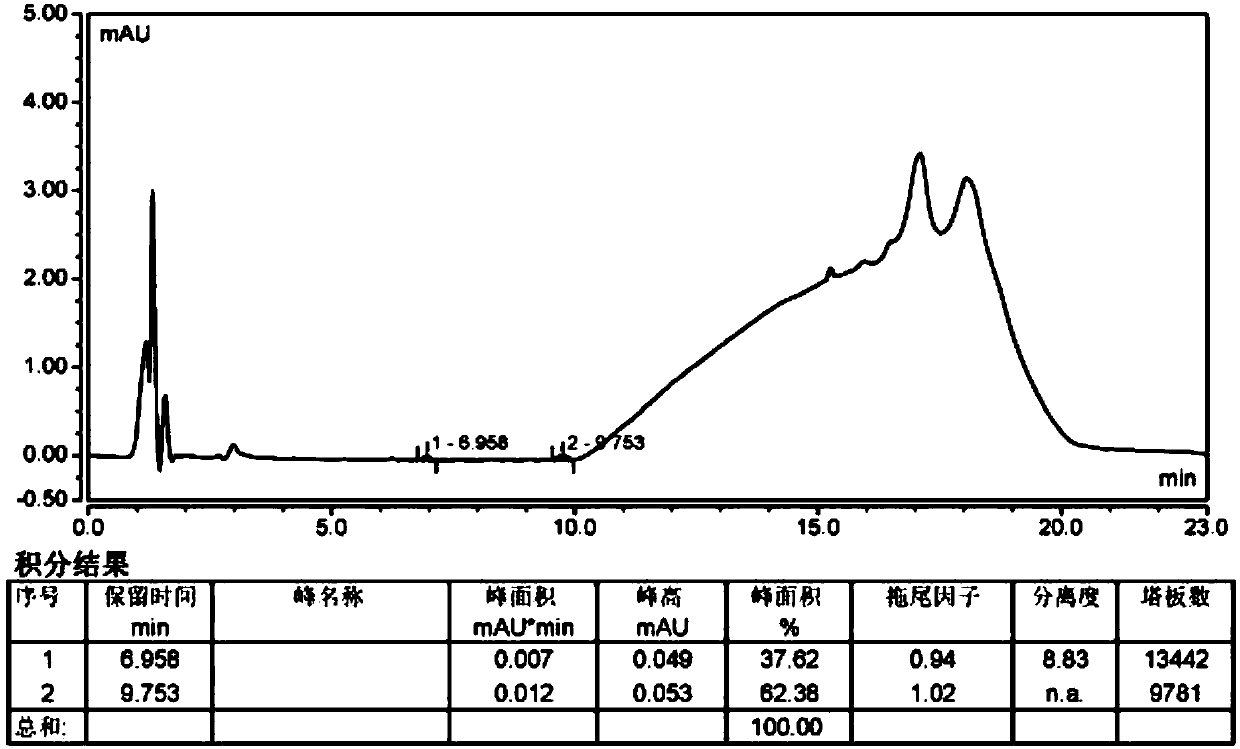
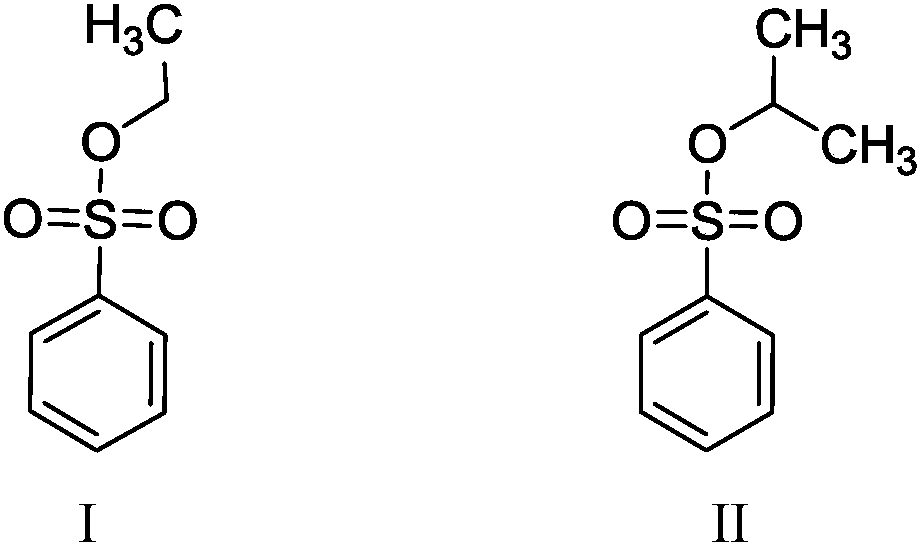
The invention belongs to the field of analytical chemistry, in particular to a method for separating and measuring bepotastine besilate and potential genotoxic impurities thereof with a HPLC (High Performance Liquid Chromatography) method. According to the method, an adopted chromatographic column is characterized in that octadecylsilane chemically bonded silica is taken as a filler, a flowing phase A and a flowing phase B are adopted for performing gradient elution, and enter a detector for detecting; the potential genotoxic impurities includes ethyl benzenesulfonate and isopropyl benz-enesulfonate; the flowing phase A is a phosphoric acid solution, and the flowing phase B is an acidic acetonitrile solution. By adopting the method, the contents of the bepotastine besilate and the potential genotoxic impurities thereof can be effectively separated and measured; high sensitivity, specificity and repeatability are achieved; the measurement is not interfered by a solvent peak during detection, and a detection result is accurate and reliable, so that the method has an extremely important significance in realizing quality control for the bepotastine besilate and a preparation thereof.
Owner:CHONGQING HUAPONT PHARMA
Salt-forming method of bepotastine besilate
ActiveCN106045974AIncrease productivityShort crystallization timeOrganic chemistryN-Butyric acidAlcohol
The invention discloses a salt-forming method of bepotastine besilate, wherein the method includes the steps of: 1) adding benzenesulfonic acid monohydrate to organic alcohol, stirring the solution until the benzenesulfonic acid is completely dissolved for later use; 2) dissolving (+)-(S)-4-{4-[(4-chlorophenyl)(2-pyridyl)methoxyl]piperidyl}n-butyric acid in organic alcohol, controlling the temperature at -20 - 20 DEG C and stirring speed at 50-200 rpm, adding a less amount of bepotastine besilate crystal seeds, dropwisely adding the benzenesulfonic acid organic alcohol solution, and then continuously stirring the solution with temperature maintained; and 3) performing crystallization for 1-5 h and filtering the solution, pour-washing a filter cake with the organic alcohol, and performing pressure reduced drying to obtain the bepotastine besilate. The method can form uniform granules in the product, is high in yield, has good impurity removal effect and excellent operability, is low in production cost and high in efficiency, and is suitable for industrial production.
Owner:HANGZHOU HEZE PHARMA TECH
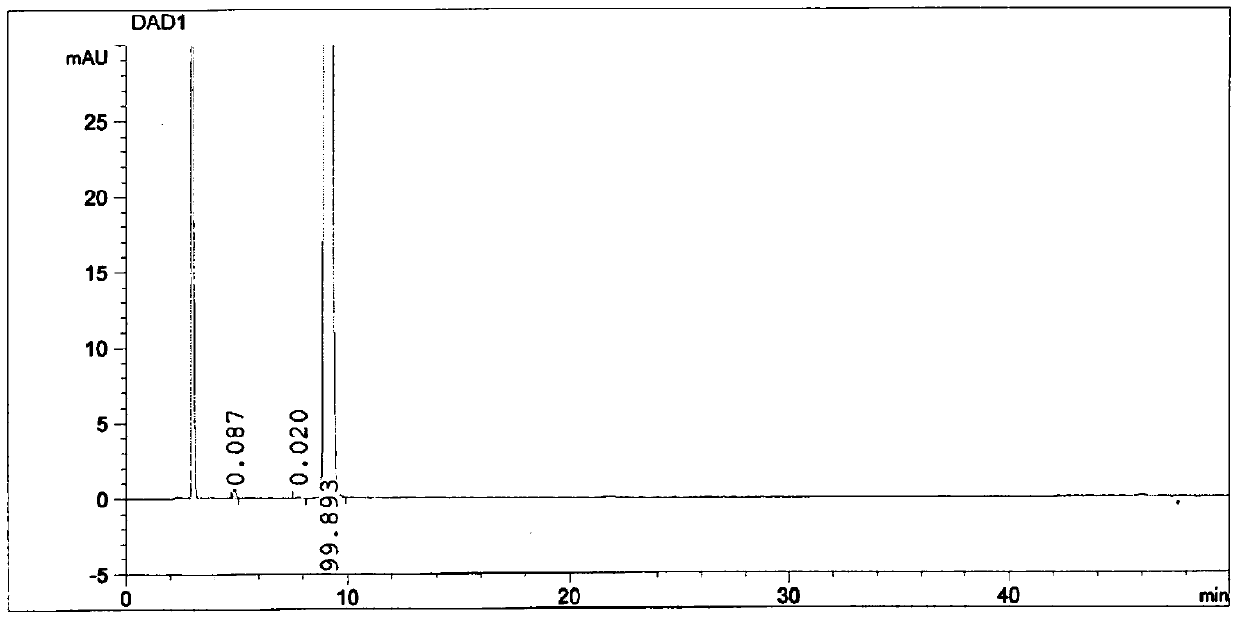
Method for detecting genotoxic impurities in bepotastine besilate
InactiveCN111307958AImprove stabilityEasy to separateComponent separationFluid phasePhysical chemistry
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to a method for detecting genotoxic impurities in bepotastine besilate. According to the detection method, high performance liquid chromatography is adopted for determination; the high performance liquid chromatography is reversed-phase liquid chromatography; and chromatographic conditions include that a chromatographic column with the carbon loading capacity smaller than or equal to 15% and the specific surface area larger than or equal to 450 m < 2 > / g is adopted, and acetonitrile serves as a solvent of a to-be-detected sample solution. The detection method has a good separation effect on genotoxic impurities in bepotastine besilate, and is high in sensitivity, good in stability and good inpracticability.
Owner:澳美制药(苏州)有限公司
Stable bepotastine besilate tablet and preparation method thereof
The invention relates to a tablet containing bepotastine besilate or benzene sulfonate thereof and a preparation method of the tablet, and belongs to the technical field of pharmaceutical preparations. The tablet consists of the bepotastine besilate or the benzene sulfonate thereof, a diluent, an adhesive, a disintegrant and a lubricant; and the preparation method of the tablet containing the bepotastine besilate or the benzene sulfonate thereof comprises the following steps: 1) obtaining blank particles, specifically, uniformly mixing the diluent, the adhesive and / or the disintegrant, and adding proper amount of moistening agent and / or binding agent for performing granulation; 2) obtaining granules for tabletting, specifically, uniformly mixing the blank granules with crude drugs, the lubricant and / or the disintegrant; and 3) conducting compression molding, specifically, conducting compression molding on the granules for tabletting by virtue of proper equipment. The bepotastine besilate tablet prepared by the invention has the advantages of being good in repeatability, good in dissolution, good in drug stability, simple and convenient in technological operation and the like.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Preparation method of bepotastine besilate
InactiveCN113173913AEfficient removalReduce detectionOrganic chemistryBenzenesulfonic acidAqueous solution
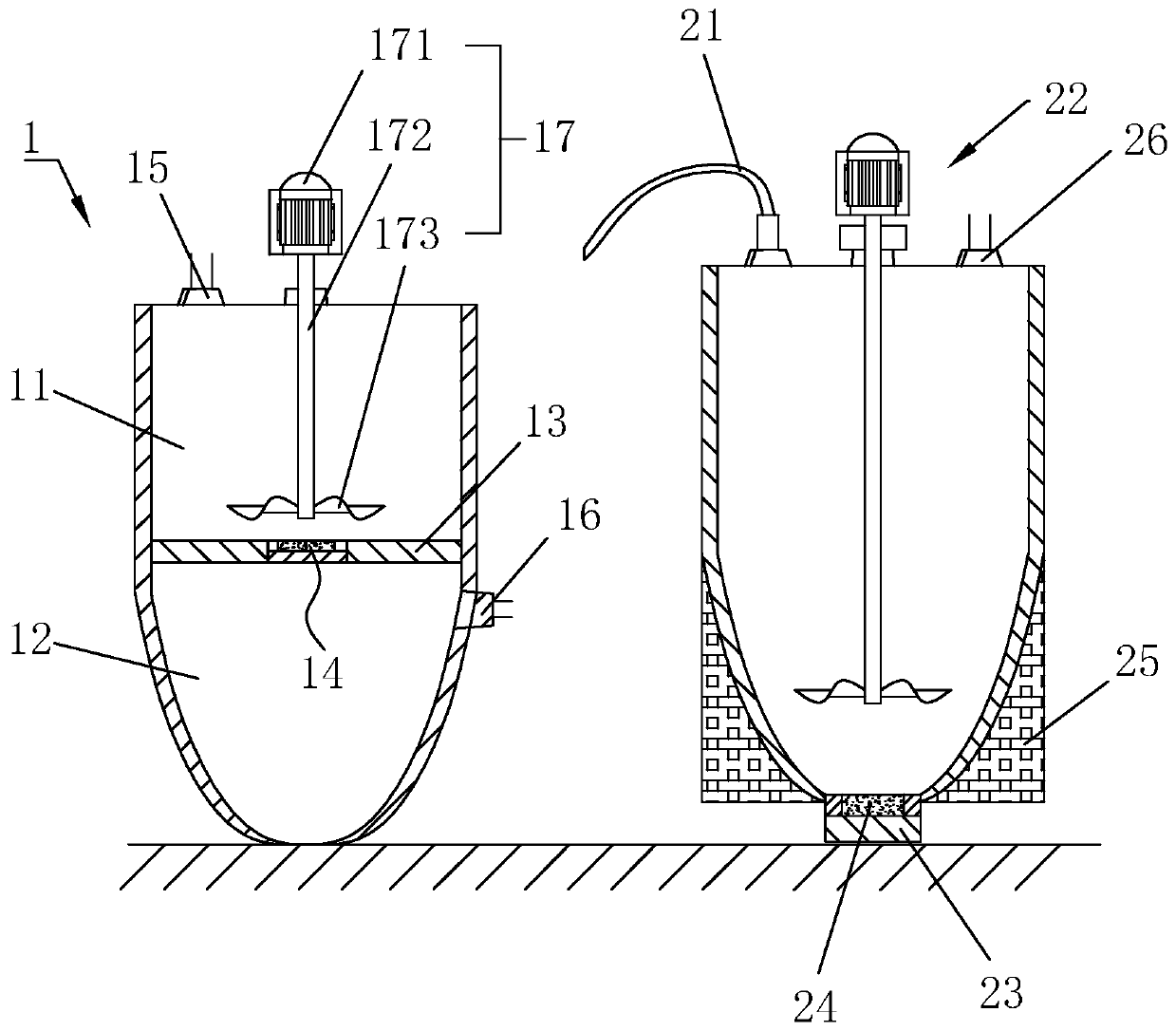
The invention provides a preparation method of bepotastine besilate. According to a technical scheme in the invention, the preparation method of bepotastine besilate is characterized by comprising the following step: S1, putting an aqueous inorganic alkali solution into a first reaction cavity of a first reaction kettle, then adding a crude bepotastine besilate product and starting a first stirrer for stirring so as to fully mix and disperse the crude bepotastine besilate product and the aqueous inorganic alkali solution. The preparation method has the beneficial effects that bepotastine besylate is convenient and rapid to prepare and high in stability.
Owner:浙江天顺药业有限公司
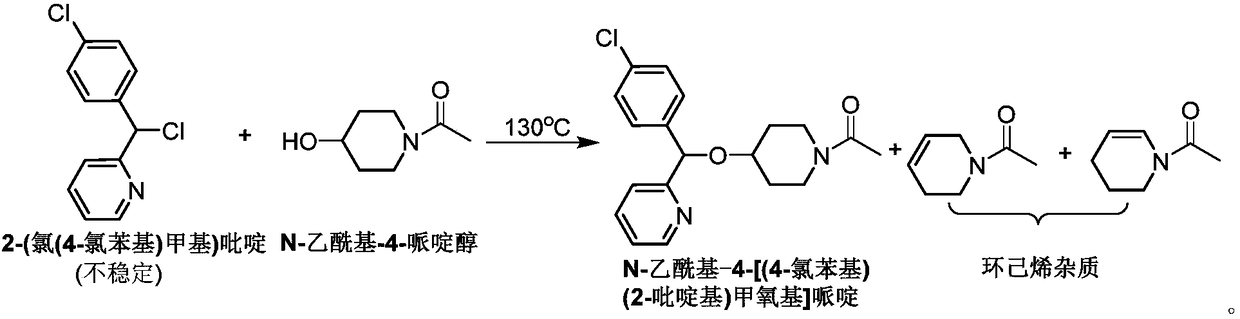
Method for preparing bepotastine besilate key intermediate
The invention relates to a method for preparing a bepotastine besilate key intermediate, in particular to 4-[(4-chlorphenyl)(2-pyridyl)methoxyl]piperidine-1-formate prepared from alpha-(4-chlorphenyl)-2-pyridinemethanol and 4-halogenated piperidine-1-carboxylate through a reaction.
Owner:WISDOM PHARM CO LTD
Pharmaceutical composition as well as preparation method and application thereof
ActiveCN112121013AAvoid gastrointestinal irritationAvoid degradationOrganic active ingredientsAerosol deliveryNasal passagesIrritation
The invention discloses a pharmaceutical composition as well as a preparation method and application thereof. The pharmaceutical composition comprises bepotastine besilate, a tackifier, a tension agent, a preservative and a pH regulator, wherein the granularity D90 of the bepotastine besilate is 2-10 [mu]m. The preparation method of the pharmaceutical composition comprises the following steps of (1) pre-treating the bepotastine besilate; and (2) preparing the pharmaceutical composition. The preparation method is simple and easy to implement, and the prepared pharmaceutical composition enters the nasal cavity through a portable spraying device, is convenient to administrate, takes effect quickly, can release drugs slowly, is high in bioavailability, stable in product quality and small in irritation to the nasal cavity, and can be used as an antihistamine drug.
Owner:南京科默生物医药有限公司
A kind of preparation method of improved bepotastine besilate
The present invention relates to an improved preparation method of bepotastine bepotastine, which comprises the following steps: the first step, combining 2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine with Resolving agent reaction, obtain S-2-[(4-chlorophenyl) (4-piperidinyloxy) methyl] pyridine N-acetyl-L-phenylalanine salt, the second step, S ‑2‑[(4‑chlorophenyl) (4‑piperidinyloxy) methyl] pyridine N‑acetyl‑L‑phenylalanine salt plus acid extraction to obtain S‑2‑[(4‑chlorobenzene Base) (4-piperidinyloxy)methyl]pyridine, the third step, S-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine sequentially in acetonitrile Coupling reaction with ethyl 4-bromobutyrate, hydrolysis reaction and salt-forming reaction to obtain bepotastine besilate. The technical scheme of the invention greatly improves the yield, shortens the process time, and the obtained product has high purity, safety and stability.
Owner:SHIJIAZHUANG GERUI PHARMA CO LTD
Bepotastine besilate
InactiveCN110368368AGood water solubilityImprove bioavailabilityOrganic active ingredientsPill deliveryMedicinePlasticizer
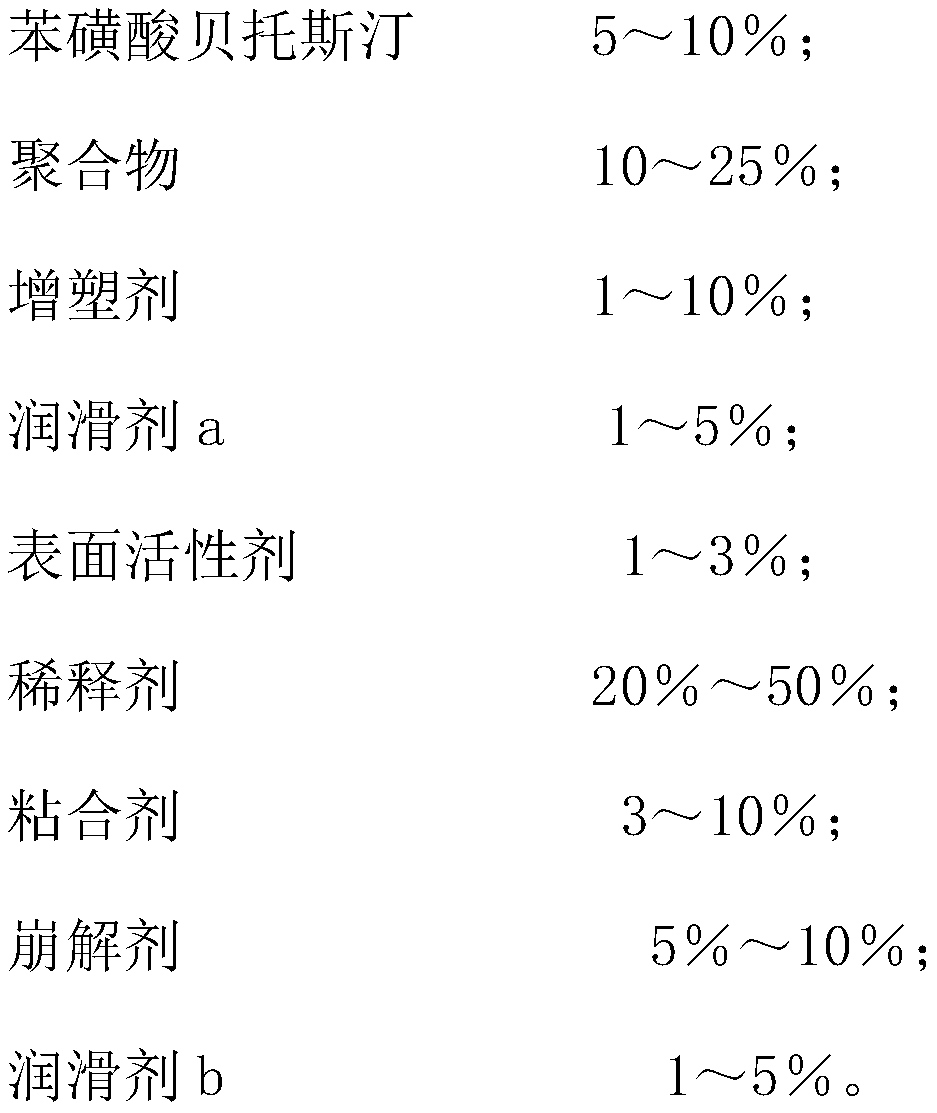
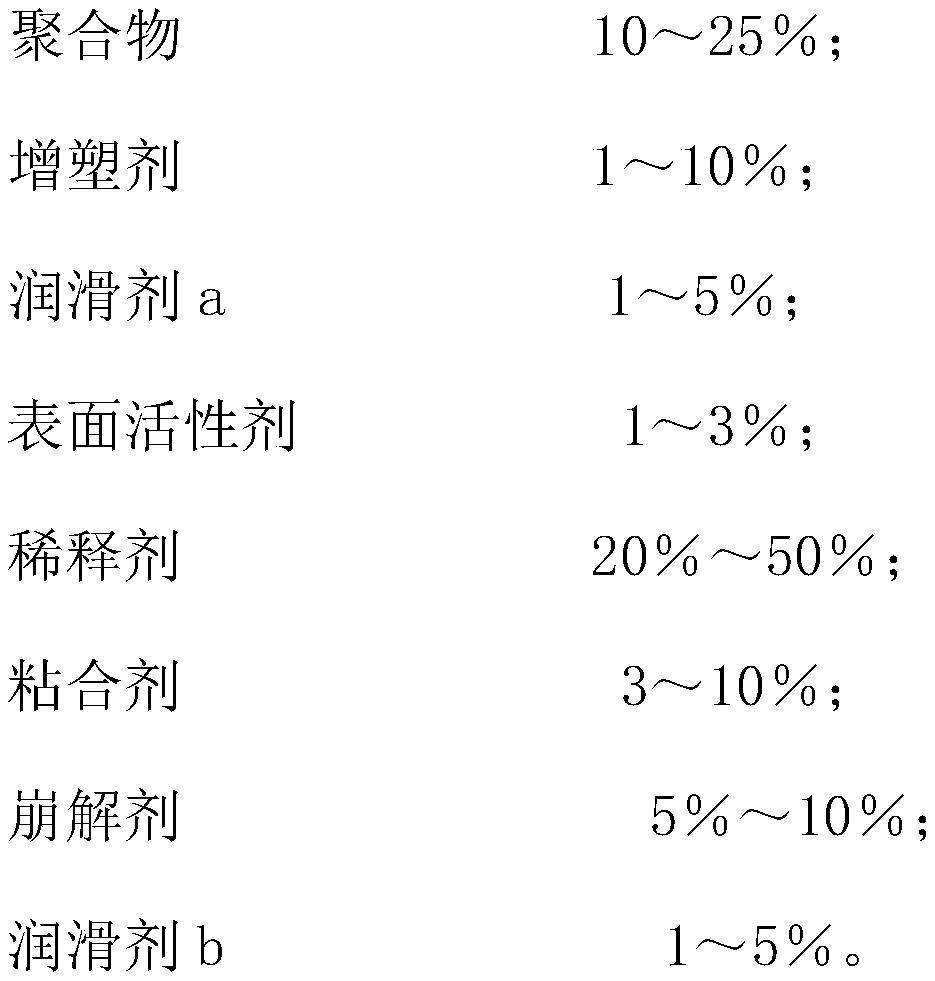
The invention discloses bepotastine besilate, and belongs to the field of organic chemistry and medicinal chemistry. The technical scheme mainly includes that the bepotastine besilate, polymers, plasticizers, lubricating agents a, surface active agents, diluting agents, binding agents, disintegrating agents and lubricating agents b are combined to prepare bepotastine besilate tablets by a hot-meltextrusion technique. The tablets have stability and further have the advantages of high drug dissolution and bioavailability.
Owner:浙江天顺药业有限公司
A kind of tablet of bepotastine besilate and preparation method thereof
ActiveCN108309947BEasy to getReduce security risksOrganic active ingredientsSenses disorderAdhesivePolyethylene glycol
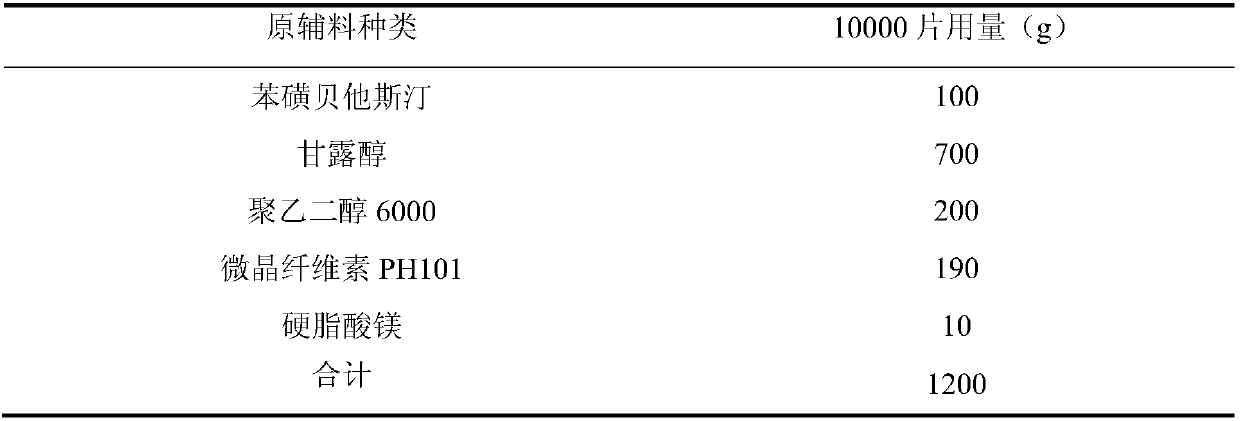
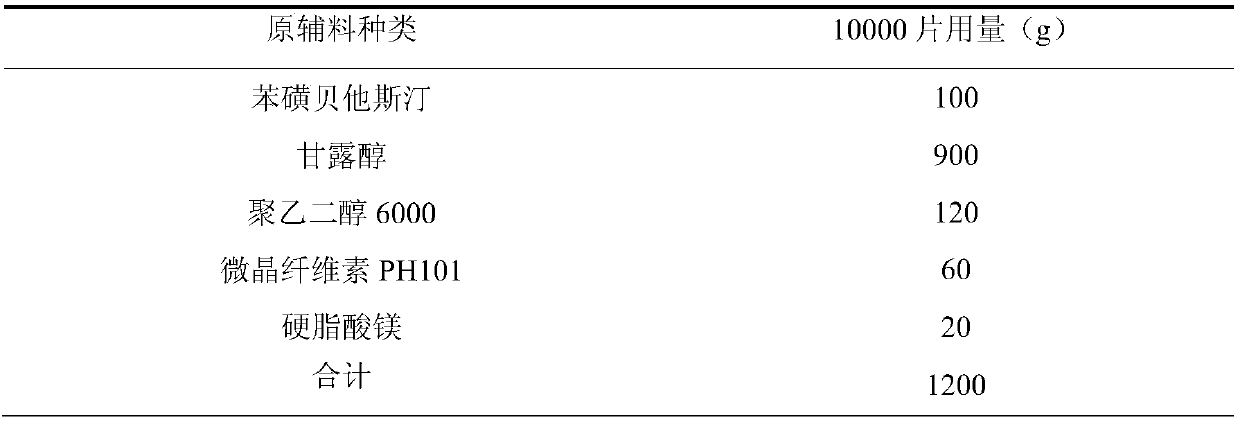
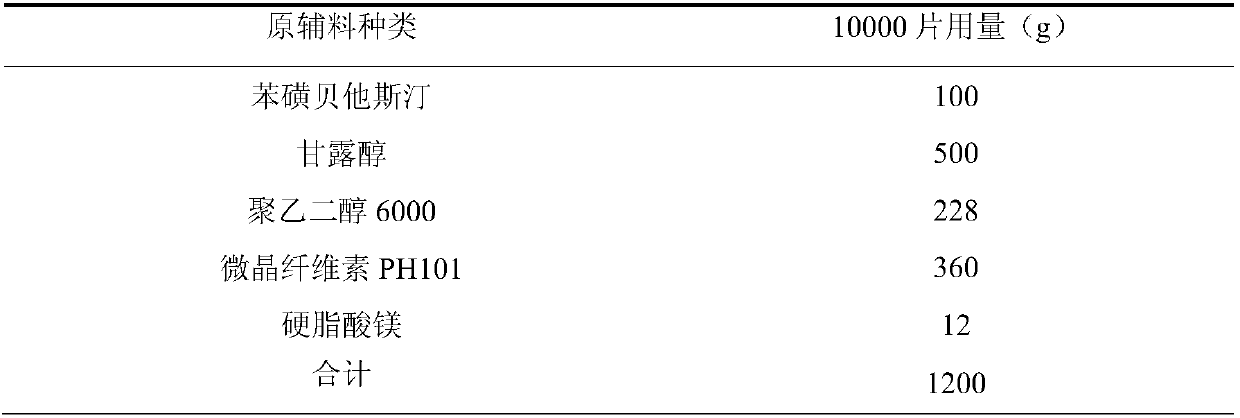
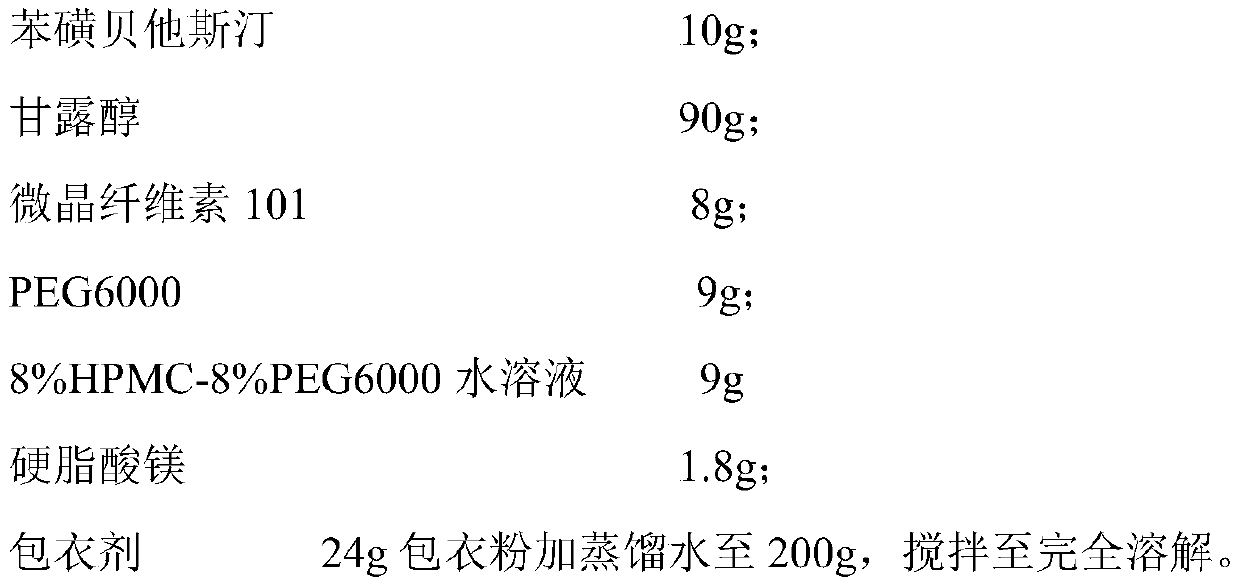
The invention relates to a bepotastine besilate tablet and a preparation method thereof, and belongs to the technical field of medicament. The bepotastine besilate tablet is mainly prepared from the following components in parts by weight: 5 to 10 parts of bepotastine besilate, 50 to 90 parts of mannitol, 6 to 12 parts of PEG (Polyethylene Glycol) 6000, 0.2 to 2 parts of hydroxypropyl methyl cellulose, 5 to 10 parts of microcrystalline cellulose and 1 to 3 parts of magnesium stearate. The preparation method is characterized by during a preparation process, firstly, mixing active components, afilling agent and an adhesive, and then adding an adhesive solution prepared from the adhesive and water, thus preparing a soft material; secondly, pelleting; finally, carrying out tabletting and coating on granules with an anti-adhesion agent and a lubricating agent. A preparation technology comprises the following steps of pretreatment of auxiliary materials, mixing, wet granulation, drying, grain finishing, total mixing, tabletting and coating. The bepotastine besilate tablet prepared by adopting the preparation method has good dissolubility and is stable in quality, simple in preparation technology and suitable for large-scale production.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
Preparation method of bepotastine besilate tablets
InactiveCN108057026AConducive to the role of disintegrantSimple processOrganic active ingredientsPharmaceutical non-active ingredientsHeating timeBEPOTASTINE BESILATE
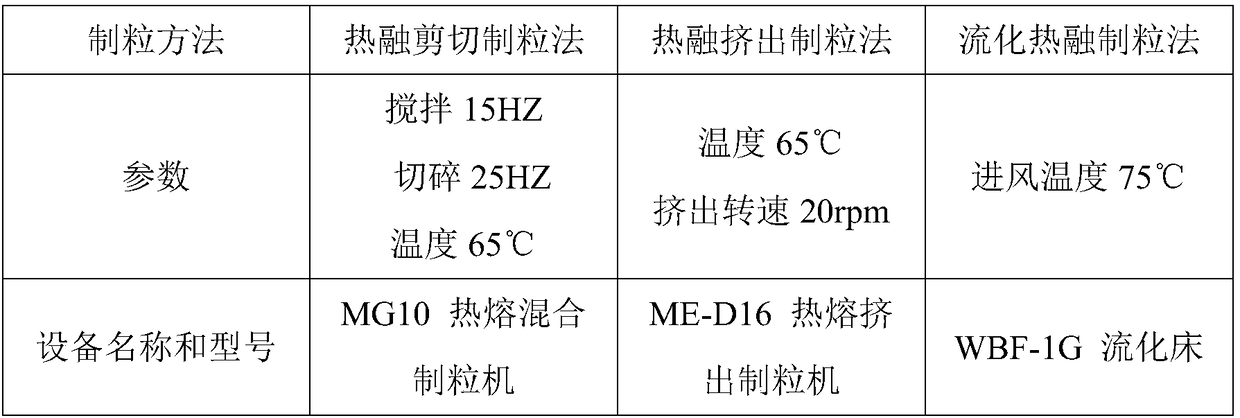
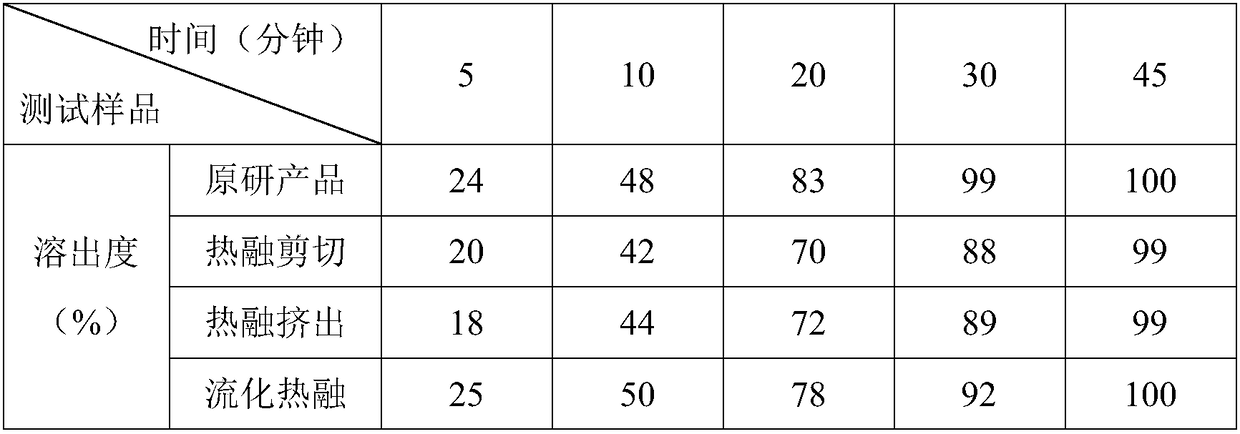
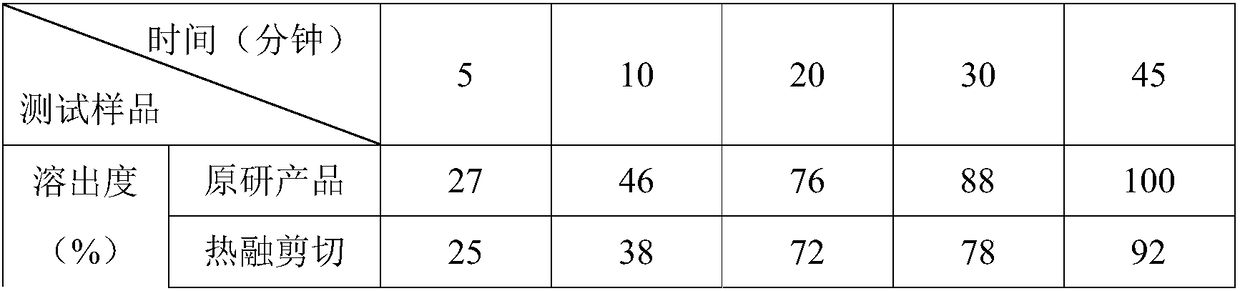
The invention discloses a preparation method of bepotastine besilate tablets. The bepotastine besilate tablets are molded in one step by adopting a hot melting granulation method. The preparation method of the bepotastine besilate tablets, disclosed by the invention, one-step molding is realized by adopting the hot melting granulation method and the preparation method has the advantages of simpletechnology and short heating time; a sample is more easily stabilized and the quality of a product is ensured. Compared with an existing product, three different granulation methods are adopted, so that the effect of an additional auxiliary agent, i.e., a disintegrating agent, is more easily expressed; the disintegrating agent can be corroded and also can be partially disintegrated; products withdissolution similar with an original product can be produced and the preparation method has very good practicability.
Owner:JIANGSU LIANHUAN PHARMA
Preparation method of bepotastine besilate
InactiveCN110372668AEfficient removalReduce detectionSulfonic acids salts preparationChemical/physical/physico-chemical stationary reactorsOrganic solventFiltration
The invention discloses a preparation method of bepotastine besilate, and belongs to the fields of organic chemistry and medicinal chemistry. The preparation method comprises the following steps: performing alkali dissolution by using crude bepotastine besilate as a raw material, performing organic solvent extraction, performing acid crystallization, performing beating, performing filtration, andperforming drying to obtain the high-purity bepotastine besilate. The method provided by the invention effectively solves the technical problems of a high content of impurities and low purity of the crude bepotastine besilate, and has the advantages of a simple process and low costs.
Owner:浙江天顺药业有限公司
Bepotastine besilate nasal spray and preparation method thereof
ActiveCN103816121BOrganic active ingredientsAerosol deliveryMucous membrane inflammationPolyethylene glycol
The invention discloses a bepotastine besilate nasal spray and a preparation method thereof. For the nasal spray, 1-10 g of bepotastine besilate and 3-15 g of solubilizing composition are added in 100 mL of water; the solubilizing composition comprises components in percentage by weight as follows: 80%-95% of propylene glycol, polyethylene glycol 300 or polyethylene glycol 400 and the balance of caprylocaproyl macrogolglycerides; and pH of a nasal spray solution ranges from 6 to 8. In the composition, bepotastine besilate does not exist in a solid particle mode and cannot be crystallized during a long-term storage process, so that not only is rapid medicine absorption facilitated, but also a spray pump port is not blocked easily, and anaphylactic rhinitis and mucous membrane inflammation related to rhinitis can be cured effectively.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Asymmetric synthesis method of ophthalmic drug bepotastine besilate
The invention relates to an asymmetric synthesis method of an ophthalmologic drug pylistramine besylate. The method concretely comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)methanone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; carrying out asymmetric transfer hydrogenation reduction on the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through using a complex of monosulfonyl chiral diamine and metallic ruthenium, rhodium and iridium as a catalyst and a sodium formate or formic acid and triethylamine mixture or isopropanol as a hydrogen source in order to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol; and condensing the (S)-(4-chlorophenyl)(2-pyridyl)methanol and ethyl 4-(4-bromopiperidine-1-yl)butyrate to obtain the ophthalmologic drug pylistramine besylate. The total yield is 61.6%.
Owner:YICHANG HUMANWELL PHARMA +1
Total synthesis method of bepotastine besilate
PendingCN113480521ARaw materials are cheap and easy to getThe synthesis method is simpleOrganic chemistry methodsSulfonic acids salts preparationChlorobenzenePtru catalyst
The invention discloses a total synthesis method of bepotastine besilate, belonging to the technical field of organic synthesis. Thetotal synthesis methodcomprises the following steps: with (4-chlorphenyl)(2-pyridyl)methanol as an initial raw material, successively conducting an addition reaction, an etherification reaction, a deprotecting group reaction, chiral resolution, a condensation reaction, a hydrolysis reaction and the like. According to the synthesis method, raw materials are cheap and easy to obtain, an expensive chiral catalyst is not needed, the synthesis method is simpler, conditions are mild, side reactions are few, cost is low, environmental protection is realized, and the synthesis method is suitable for large-scale production of the optical pure anti-allergic medicine bepotastine besilate.
Owner:CHENGDU LIKAI CHIRAL TECH
A kind of stable bepotastine besilate crystal and preparation method thereof
ActiveCN104119314BReduce the overall heightImprove stabilityOrganic active ingredientsOrganic chemistryPowder diffractionCrystallization
The invention discloses a stable bepotastine besilate crystal in novel crystal form through a recrystallization method. The obtained bepotastine besilate crystal is confirmed through X-ray powder diffraction and infrared spectrum. Stability contrast experiments prove that the provided bepotastine besilate crystal has extremely high stability, and both bulk drug and preparation of the bepotastine besilate crystal have stability obviously better than that of existing bepotastine besilate crystals.
Owner:CHONGQING HUAPONT PHARMA
Method for separation and determination of optical isomer impurities of bepotastine besilate
A method for splitting a Bepotastine Besilate optical isomer and quantitatively measuring R-isomer impurity is disclosed. According to the invention, high performance liquid chromatography is adopted. A mobile phase contains n-hexane and ethanol. The method is characterized in that normal-phase liquid chromatography is adopted; packing used in a chromatographic column is silica gel with its surface coated with amylase-tris-(3,5-xylyl carbamate); and the mobile phase also contains an alkaline additive. In comparison with the prior art, the method provided by the invention has advantages as follows: the mobile phase is simple to prepare, and resolutions of two isomers are both within 4.5-5.5. Especially, the chromatographic column of the method has good durability, and the chromatographic column still has high column efficiency after having been used for one year. Thus, analytic determination cost is greatly reduced.
Owner:CHONGQING HUAPONT PHARMA
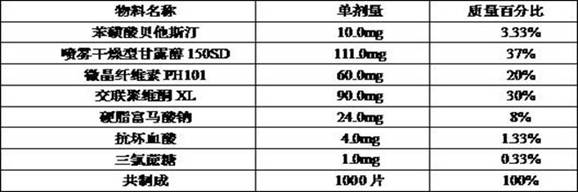
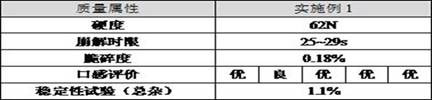
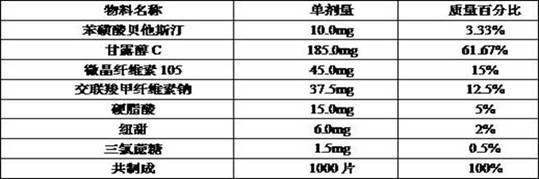
Beta-potastine besilate orally disintegrating tablet and preparation method thereof
ActiveCN114668732AImprove convenienceGood reproducibilityOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletTraditional medicine
The invention discloses a bepotastine besilate orally disintegrating tablet and a preparation method thereof, the orally disintegrating tablet comprises the following components by mass: 2%-20% of bepotastine besilate, 35%-80% of a filler, 2%-35% of a disintegrating agent, 1%-10% of a lubricant, and 1-6% of a flavoring agent, the orally disintegrating tablet has a smooth and beautiful surface and appropriate hardness, can be rapidly disintegrated in the oral cavity, and can be rapidly absorbed; the orally disintegrating tablet has the advantages of fast effect taking, simple process, excellent friability, fast disintegration and excellent taste, and solves the problem of residual aftertaste and the problem of insufficient bitter taste covering effect.
Owner:HANGZHOU HEZE PHARMA TECH
Method for separation and determination of bepotastine besilate and its potential genotoxic impurities by HPLC
ActiveCN107782832BSeparation assay is validHigh sensitivityComponent separationHplc methodPhosphoric acid
The invention belongs to the field of analytical chemistry, in particular to a method for separating and measuring bepotastine besilate and potential genotoxic impurities thereof with a HPLC (High Performance Liquid Chromatography) method. According to the method, an adopted chromatographic column is characterized in that octadecylsilane chemically bonded silica is taken as a filler, a flowing phase A and a flowing phase B are adopted for performing gradient elution, and enter a detector for detecting; the potential genotoxic impurities includes ethyl benzenesulfonate and isopropyl benz-enesulfonate; the flowing phase A is a phosphoric acid solution, and the flowing phase B is an acidic acetonitrile solution. By adopting the method, the contents of the bepotastine besilate and the potential genotoxic impurities thereof can be effectively separated and measured; high sensitivity, specificity and repeatability are achieved; the measurement is not interfered by a solvent peak during detection, and a detection result is accurate and reliable, so that the method has an extremely important significance in realizing quality control for the bepotastine besilate and a preparation thereof.
Owner:CHONGQING HUAPONT PHARMA
A kind of purification method of high-purity bepotastine besylate
ActiveCN106117183BHigh purityEasy to operateOrganic chemistryOrganic compound preparationAcetonitrileSolvent
The invention discloses a refining method for high-purity bepotastine besilate. The refining method is a solvent crystallization method adopting a crude bepotastine besilate product as a raw material and comprises the following steps: firstly, the crude bepotastine besilate product is heated and dissolved with acetonitrile and filtered, a filtrate is cooled, stirred and crystallized, crystals are filtered and dried, and primary refined product is obtained; the primary refined product is heated, stirred, dissolved in water and filtered, a filtrate is cooled, stirred and crystallized, crystals are filtered and dried, and a pure bepotastine besilate product is obtained. The method has the advantages that the operation is simple and convenient, the yield is high, the product is pure, the cost is saved and the like, the total yield is 80% or higher, the purity of the pure bepotastine besilate product is 99.9% or higher proved by HPLC detection, the isomer impurity content is reduced to 0.1% or lower from 0.4%, and other single impurity content is reduced to 0.1% or lower from 0.2%.
Owner:HEFEI JIUNUO MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com