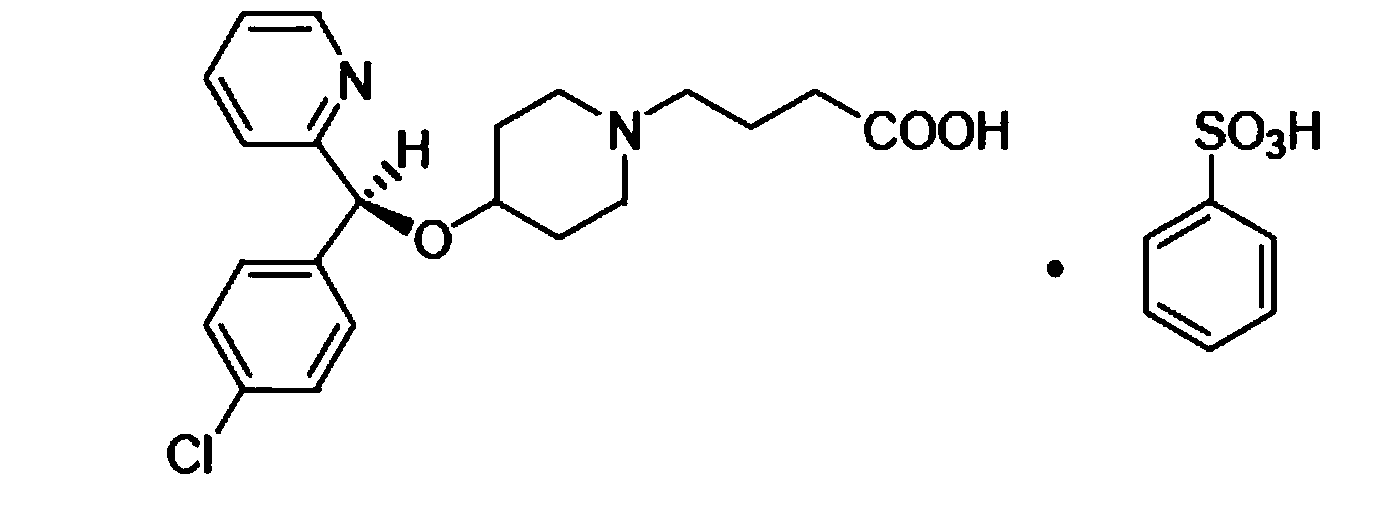
Bepotastine besilate nasal spray and preparation method thereof
A technology of bepotastine besylate and nasal spray, which is applied in the preparation field of bepotastine besylate and its preparation field, and can solve the problems of rapid absorption of unfavorable drugs, easy precipitation of crystallization, easy blockage of spray pump ports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037]
[0038] Preparation:
[0039] Dissolve bepotastine benzenesulfonate, disodium edetate, disodium hydrogen phosphate, anhydrous glucose, propylene glycol, caprylic capric macrogol glyceride, and benzalkonium chloride in 80ml of pure water, mix, Add 1mol / L sodium hydroxide to adjust the pH to 6.5, add 20ml of pure water, mix well, filter, fill, and add a spray pump to obtain a solution-type nasal spray.
[0040] The above-mentioned nasal spray was subjected to three cycles of low temperature test, each cycle was at 2°C for 2 days, and then at 40°C for 2 days under accelerated conditions, sampling and testing showed that the solution was clear and no crystallization was observed.
[0041] The above-mentioned nasal spray was subjected to three cycles of freeze-thaw test, each cycle was subjected to -10°C for 2 days, and then inspected under accelerated conditions of 40°C for 2 days, and samples were taken for testing. As a result, the solution was clear and ...
Embodiment 2
[0043]
[0044]
[0045] The preparation method is the same as in Example 1. Get a solution nasal spray.
[0046] The above-mentioned nasal spray was subjected to three cycles of low temperature test, each cycle was at 8°C for 2 days, and then at 40°C for 2 days under accelerated conditions, sampling and testing showed that the solution was clear and no crystallization was observed.
[0047] The above-mentioned nasal spray was subjected to three cycles of freeze-thaw test, each cycle was at -20°C for 2 days, and then inspected at 40°C for 2 days under accelerated conditions, and samples were taken for testing. As a result, the solution was clear and no crystals were precipitated.
Embodiment 3
[0049]
[0050] The preparation process is the same as in Example 1. Get a solution nasal spray.
[0051] The above-mentioned nasal spray was placed in three cycles of low temperature and freeze-thaw as in Example 1, and the solution was clear without crystallization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com