Pharmaceutical composition as well as preparation method and application thereof
A technology of composition and medicine, which is applied in the field of pharmaceutical composition and its preparation, can solve the problems of weak therapeutic effect on allergic rhinitis and inability to quickly suppress rhinitis, and achieve avoidance of liver first-pass effect, rapid and complete nasal absorption, good compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Prescription
[0039]
[0040]
[0041] 2. Preparation method
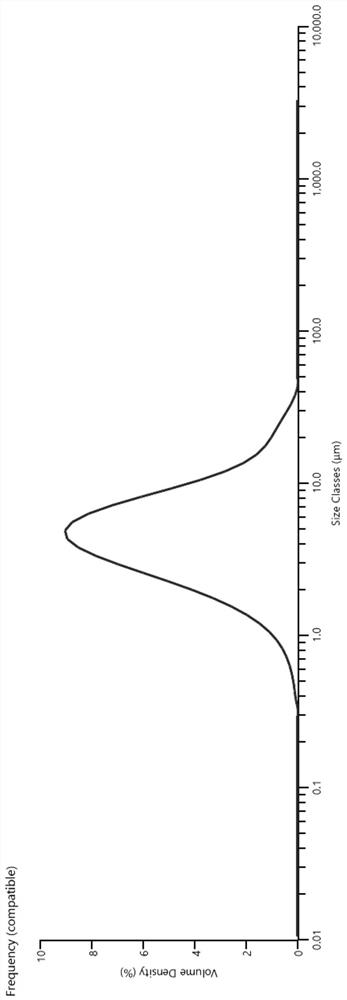
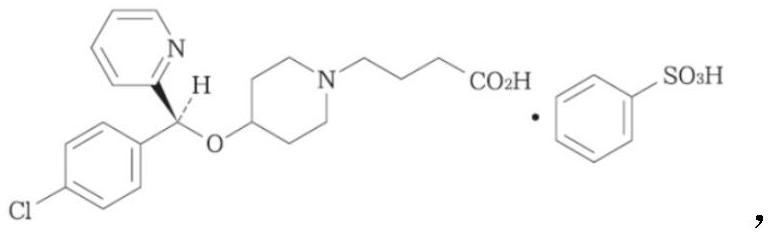
[0042] Crush bepotastine besilate to particle size D 90 At 2-10μm, then fully mix the raw and auxiliary materials (except pH adjusters and preservatives) and water in the prescribed amount, adjust the pH of the mixed solution to 6.5 with 0.1mol / L sodium hydroxide, and finally add benzalkonium chloride to mix Evenly, fill, and add a spray pump to obtain a suspension nasal spray.
Embodiment 2
[0044] 1. Prescription
[0045]
[0046] 2. Preparation method
[0047] Crush bepotastine besilate to particle size D 90 At 2-10 μm, then fully mix the raw and auxiliary materials (except pH adjusters and preservatives) and water in the prescribed amount, adjust the pH of the mixed solution to 5.0 with 0.1mol / L sodium hydroxide, and finally add benzalkonium chloride and mix well , filling, and adding a spray pump to obtain a suspension nasal spray.
Embodiment 3
[0049] 1. Prescription
[0050]
[0051] 2. Preparation method
[0052] Crush bepotastine besilate to particle size D 90 At 2-10 μm, then fully mix the raw and auxiliary materials (except pH regulator and preservative) and water in the prescribed amount, adjust the pH of the mixed solution to 6.0 with sodium hydroxide, and finally add benzalkonium chloride to mix evenly, fill, Add a spray pump for a nasal spray suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com