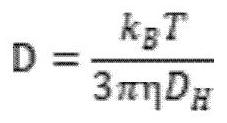Lurasidone self-microemulsion composition and preparation method thereof
A technology of lurasidone and its composition, which is applied in the field of lurasidone self-microemulsion composition and its preparation, can solve the problems of poor water solubility, low solubility, and low oral bioavailability, and achieve high solubility and drug loading The effect of increasing the amount and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
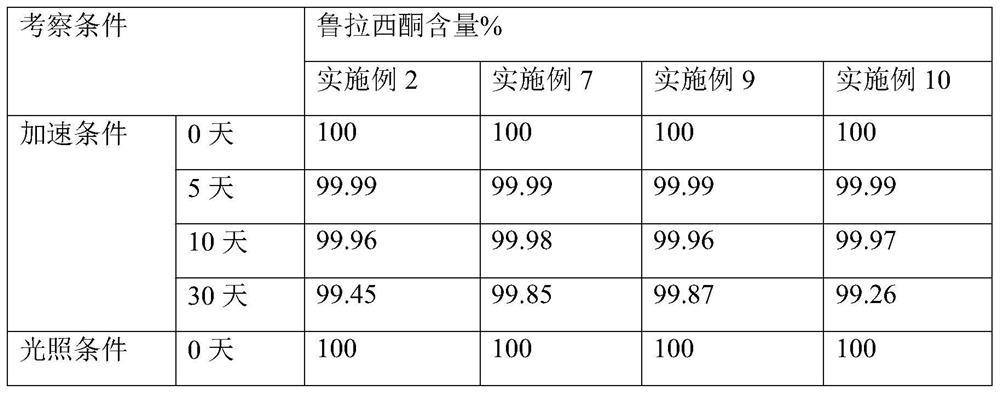
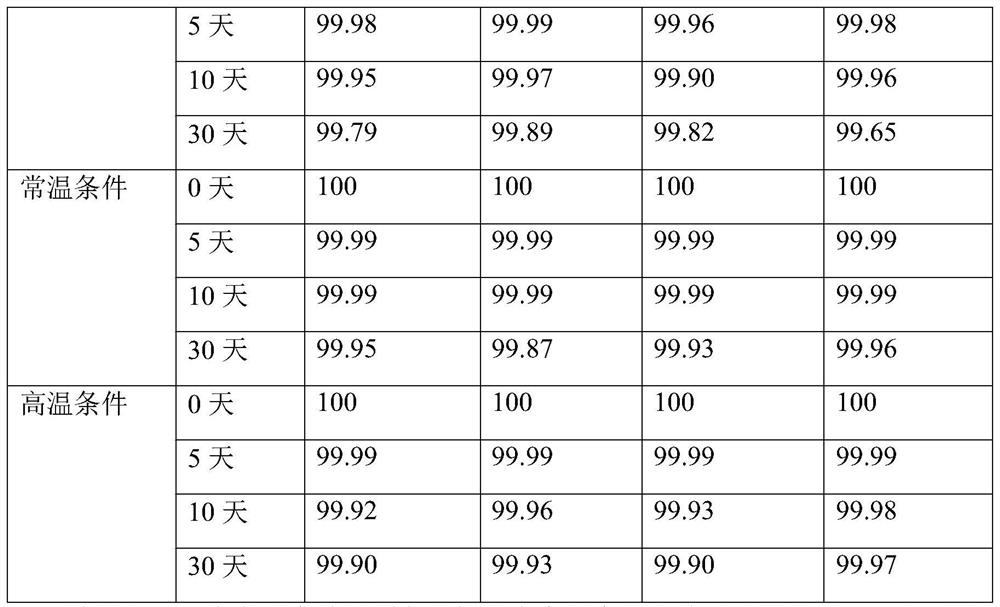
Examples
Embodiment 1
[0038] Lurasidone 20mg
[0039] Caprylic Capric Mono-Diglyceride 400mg
[0040] Tween 80400mg
[0041] Propylene glycol 200mg
[0042] Preparation Process:
[0043] Add lurasidone into caprylic capric acid mono-diglyceride, then add Tween 80 and propylene glycol in turn, stir to mix evenly and then dissolve.
[0044] Particle size: about 500nm.
Embodiment 2
[0046] Lurasidone 20mg
[0047] Glyceryl monocaprylate 200mg
[0048] Polyoxyl 35 castor oil 600mg
[0049] Diethylene glycol monoethyl ether 200mg
[0050] Preparation Process:
[0051] Add lurasidone into glyceryl monocaprylate, then add polyoxyethylene 35 castor oil and diethylene glycol monoethyl ether in turn, stir to mix evenly and then dissolve.
[0052] Particle size: about 15nm.
Embodiment 3
[0054] Lurasidone 20mg
[0055] Glyceryl monocaprylate 165mg
[0056] Oleic acid 165mg
[0057] Polyoxyl 35 castor oil 440mg
[0058] Propylene glycol 230mg
[0059] Preparation Process:
[0060] Add lurasidone into the mixed oil phase of glyceryl monocaprylate and oleic acid, then add polyoxyethylene 35 castor oil and propylene glycol in sequence, stir to mix evenly, and then dissolve.
[0061] Particle size: about 250nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com