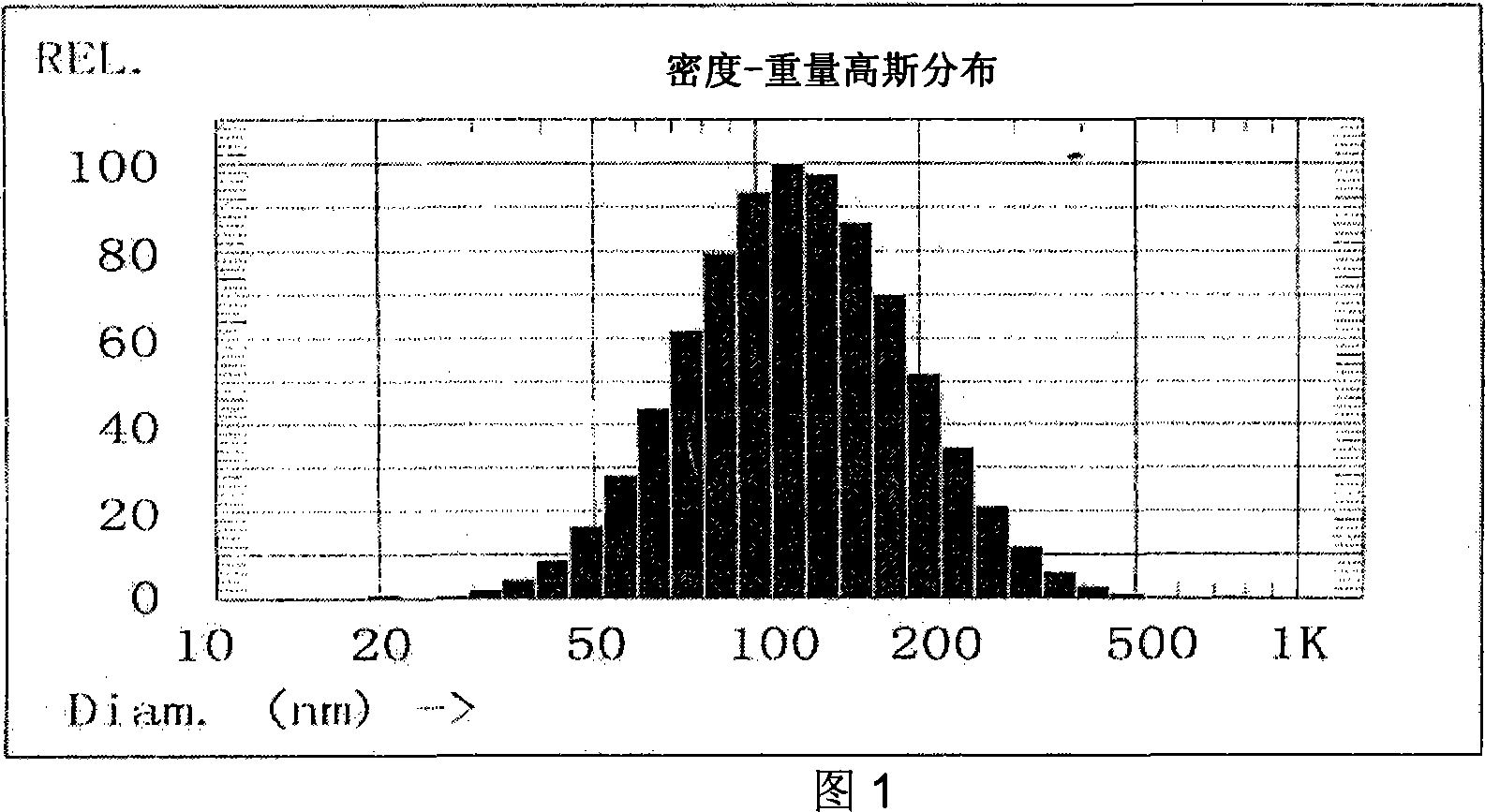
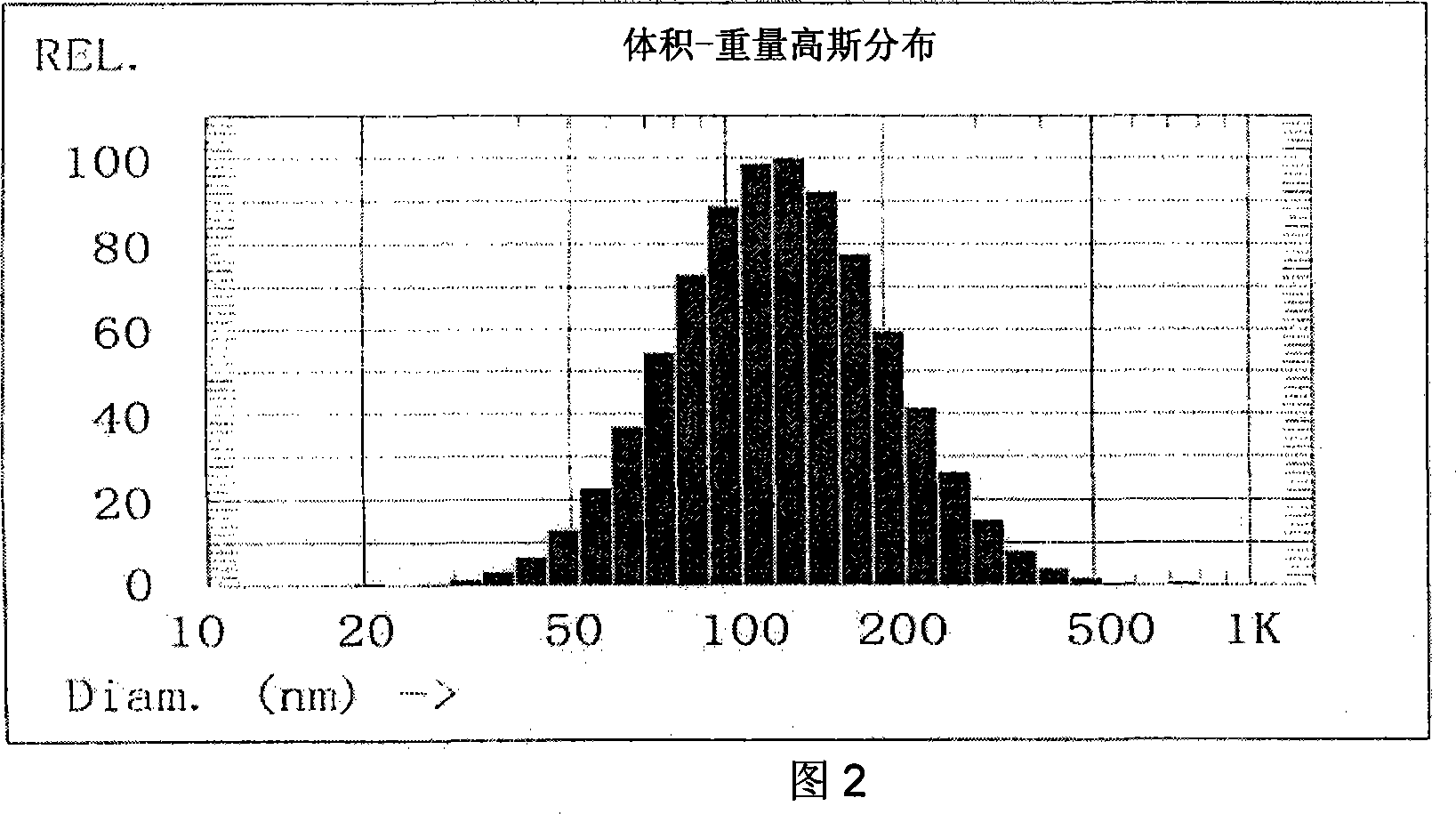
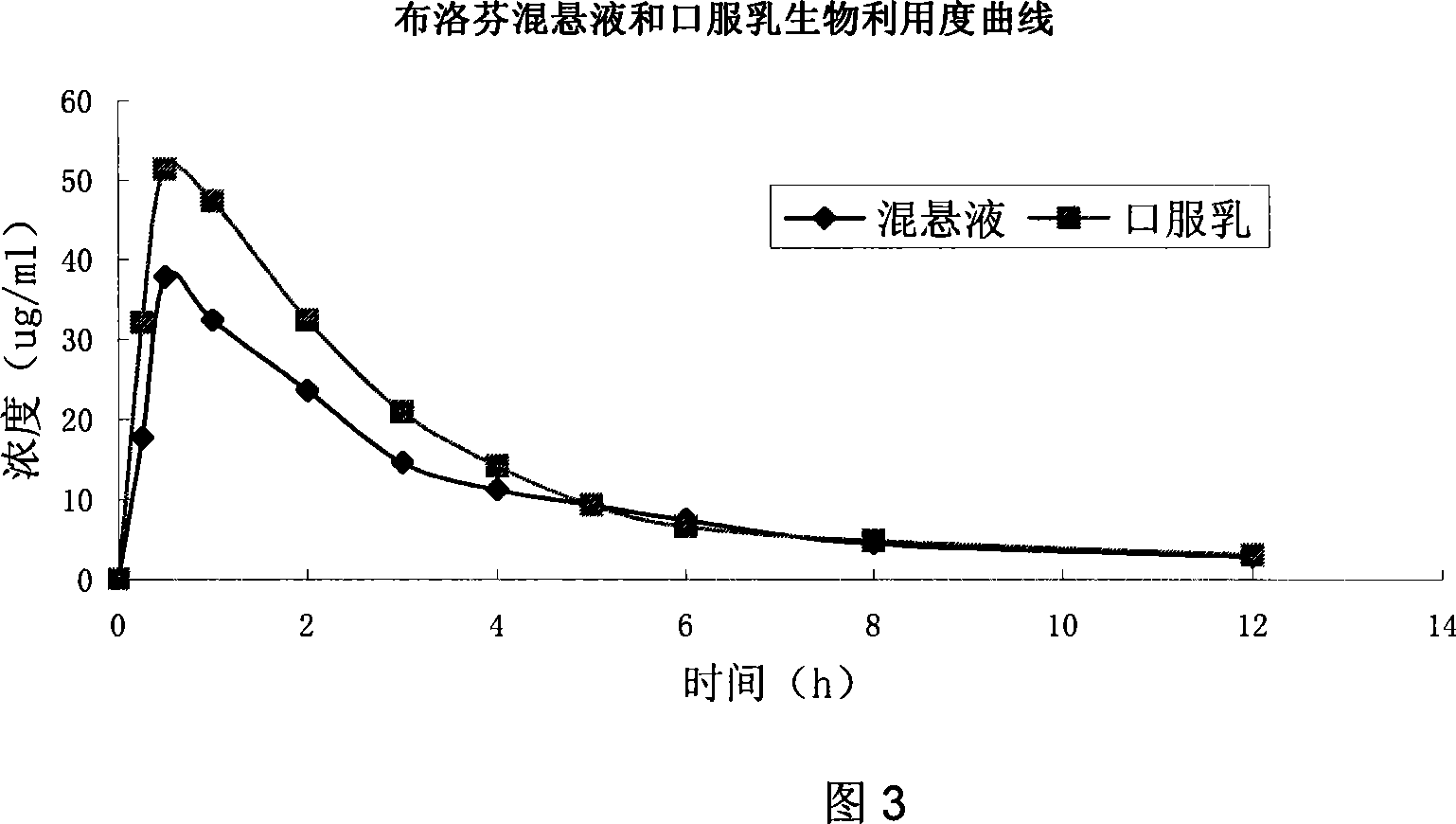
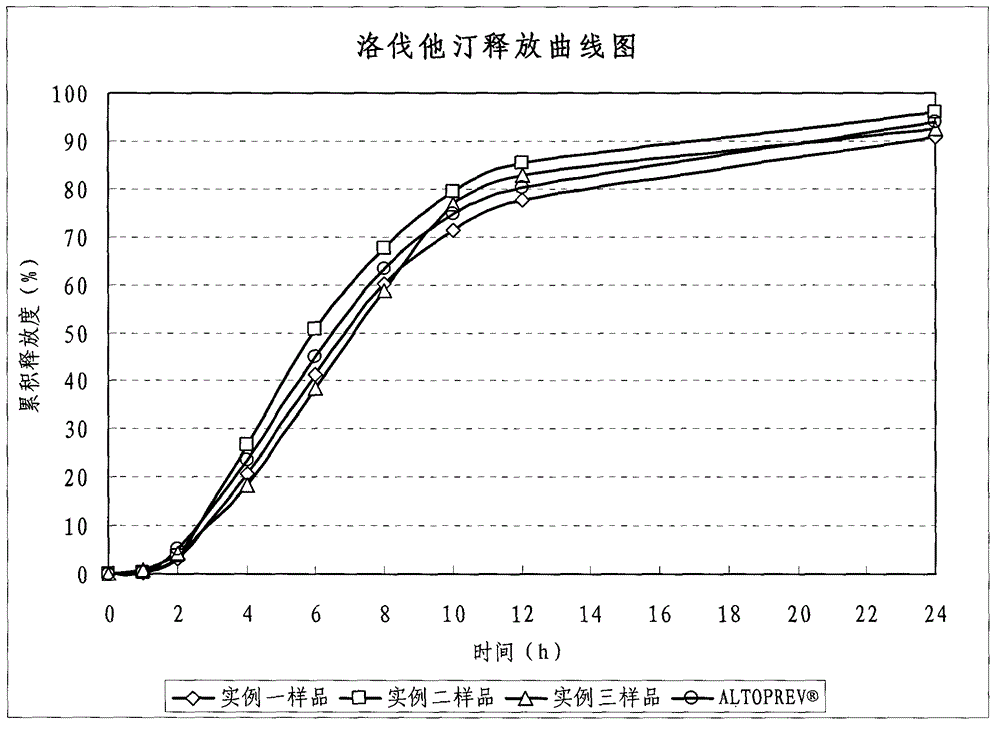
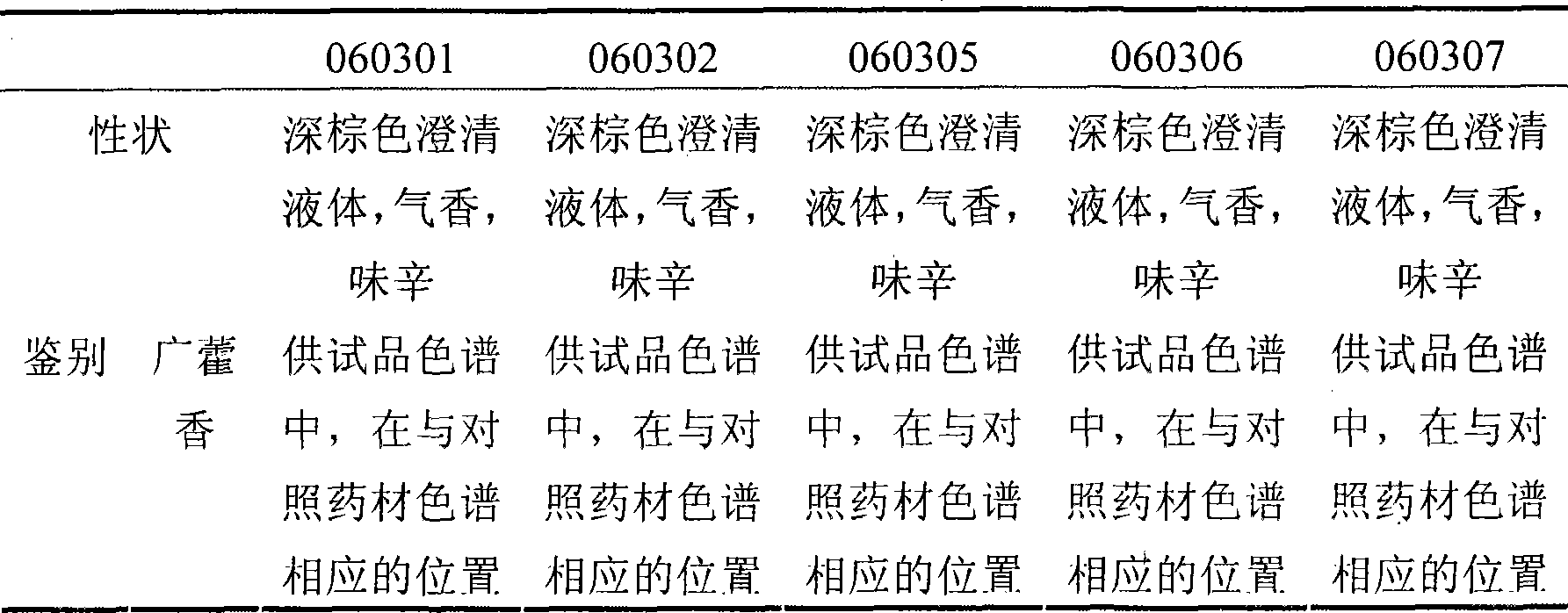
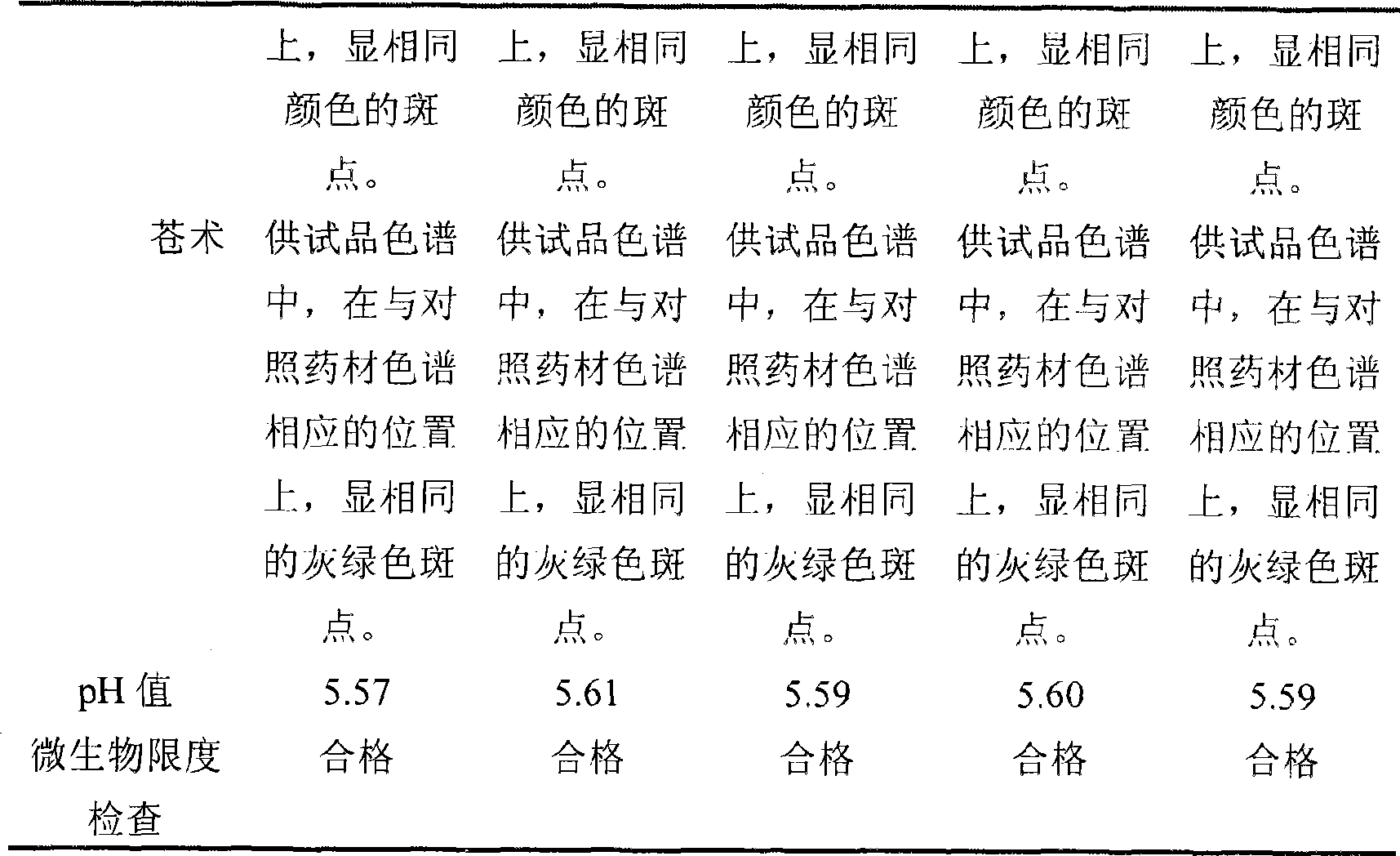
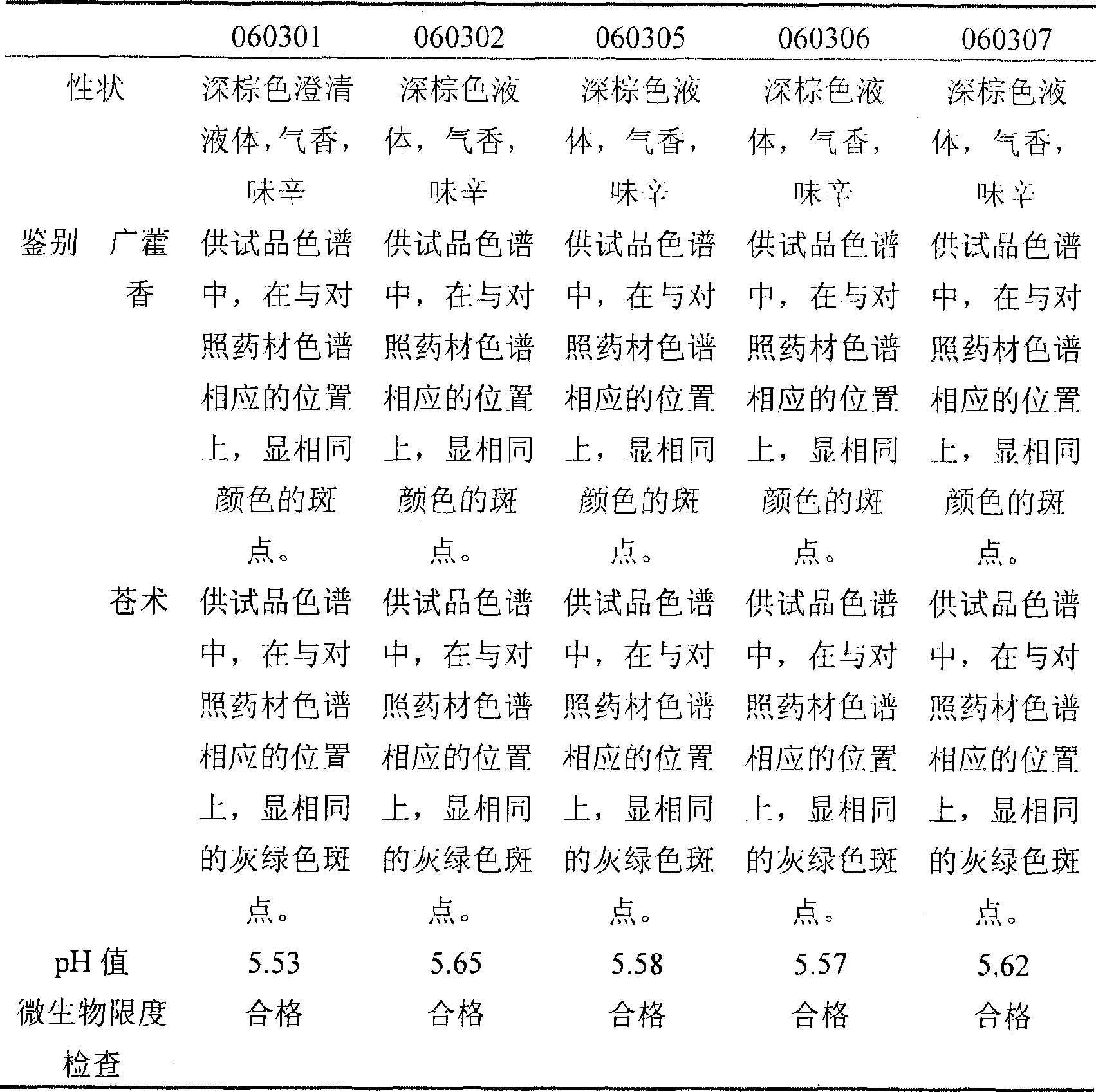
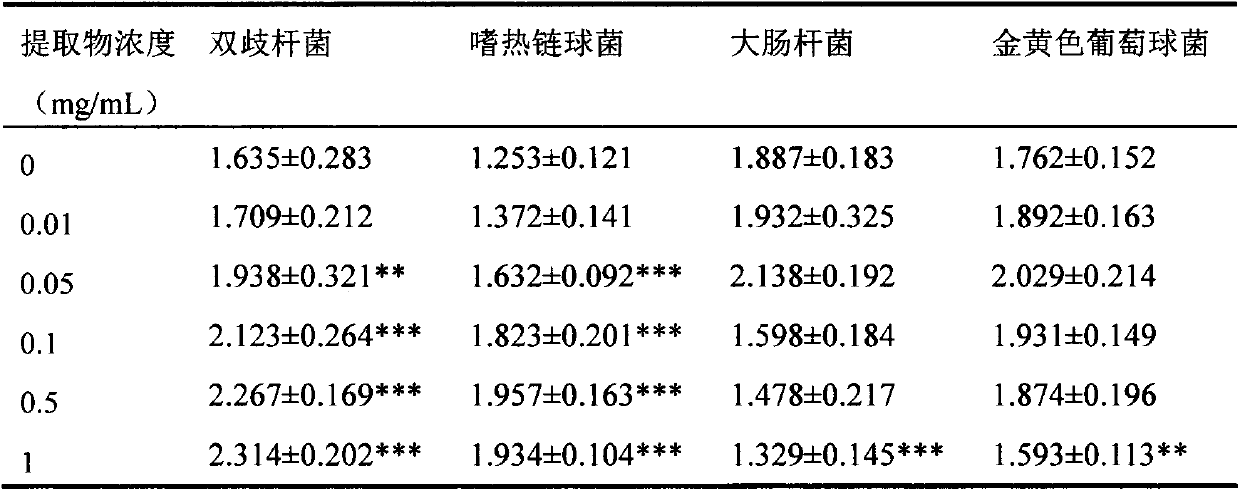
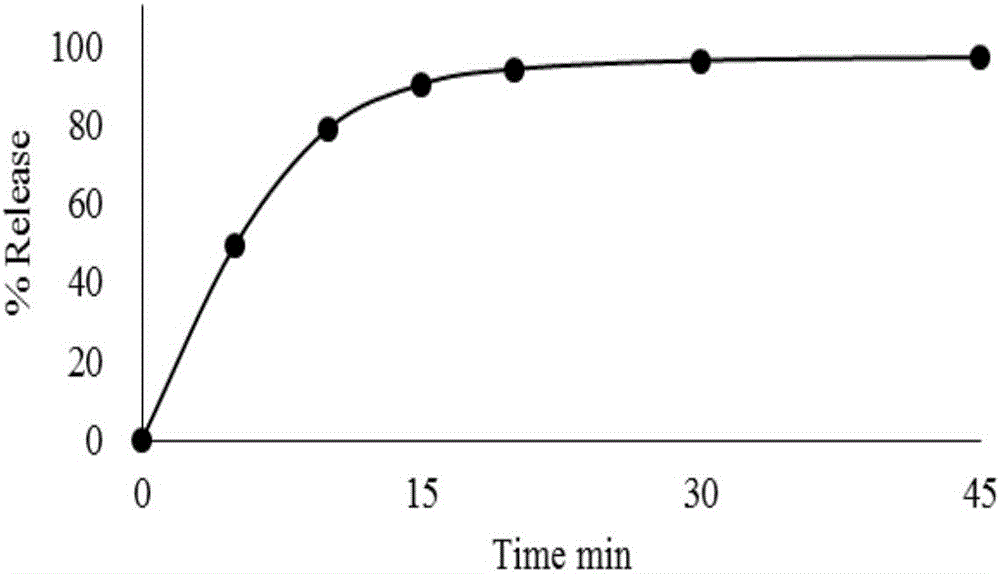
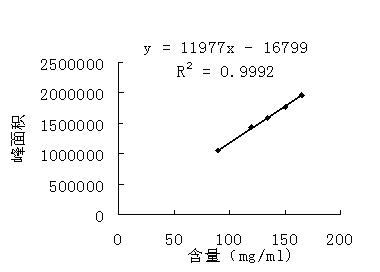
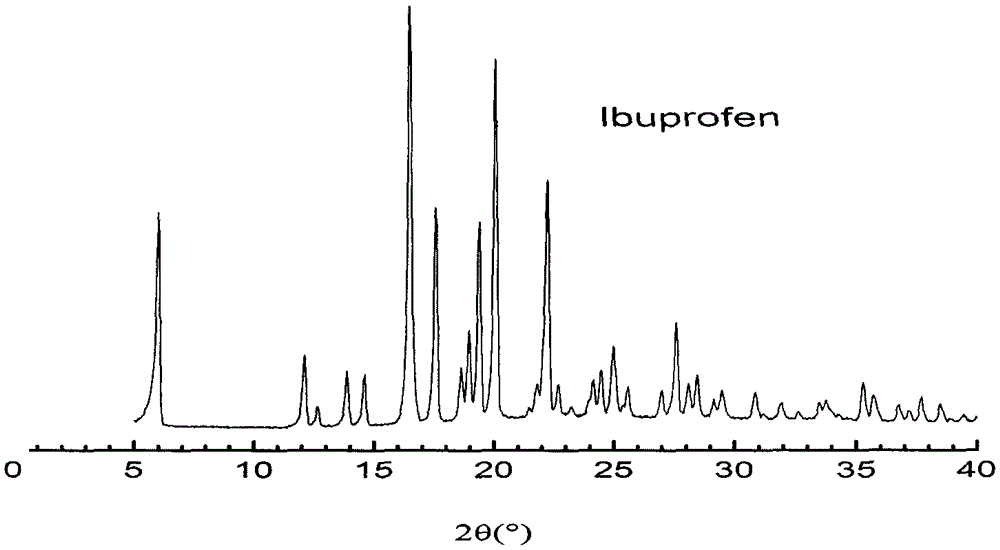
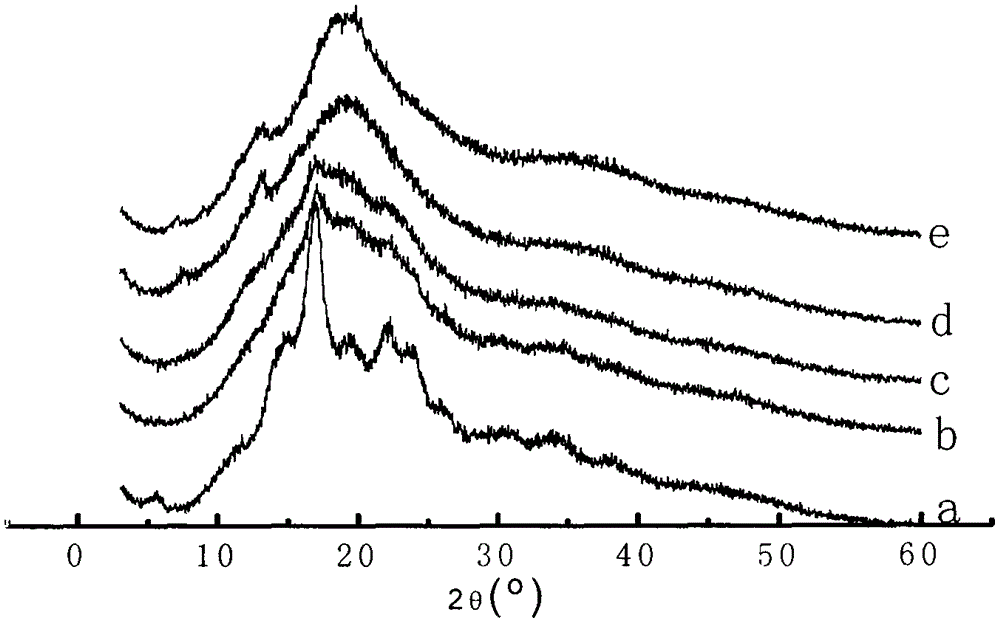
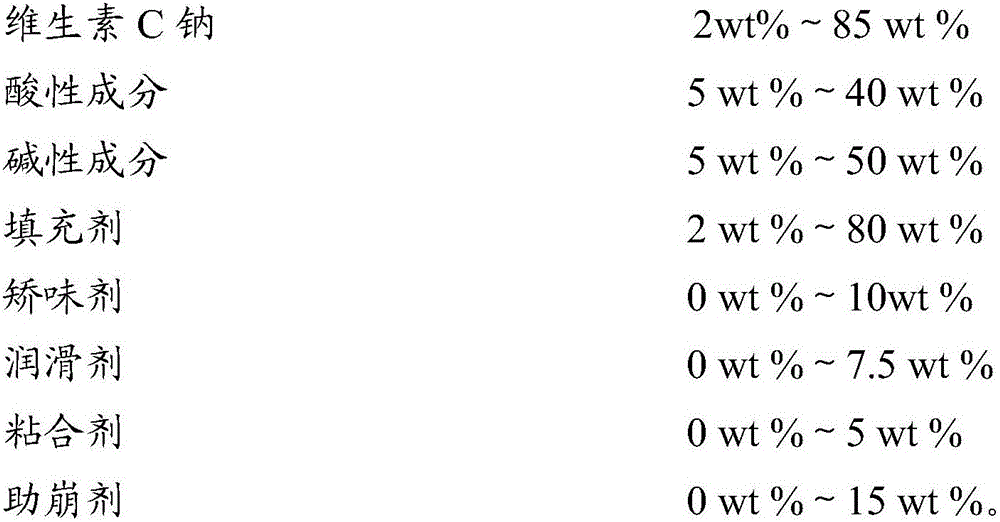Patents
Literature
206 results about "Gastrointestinal irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combined nsaid and acetaminophen formulation and method
InactiveUS20090264530A1Reduce gastrointestinal irritationGood pain reliefBiocideNervous disorderCo administrationGastrointestinal irritation
The present invention is directed to co-administration of a non-steroidal anti-inflammatory agent (NSAID) and acetaminophen for the treatment of pain and inflammation with reduced gastrointestinal irritation. A pharmaceutical composition suitable for the co-administration contains a therapeutically effective amount of at least one non-steroidal anti-inflammatory agent, and a therapeutically effective amount of acetaminophen or a pharmaceutically acceptable salt thereof. A ratio of the non-steroidal anti-inflammatory agent to acetaminophen in the composition is within a range that provides greater pain relief and reduction of inflammation with less gastrointestinal irritation than that obtainable by the administration of the non-steroidal anti-inflammatory agent or acetaminophen alone. Examples of pharmaceutical compositions for co-administration of the agents are those containing ibuprofen in combination with the acetaminophen.
Owner:NICKELL ROBERT P
Orally taken emulsion and its prepn
The present invention relates to orally taken oil-in-water emulsion with medicine in solid state at normal temperature and its preparation process. The emulsion contains solid state medicine, liquid state edible oil, lecithin, water soluble high molecular matter as assisting emulsifier, and is prepared through regulating emulsion particle size, regulating pH value, and other steps. It has stable physical performance, less stimulation to gastrointestinal tract, good taste and high bioavailability.
Owner:朱芳海
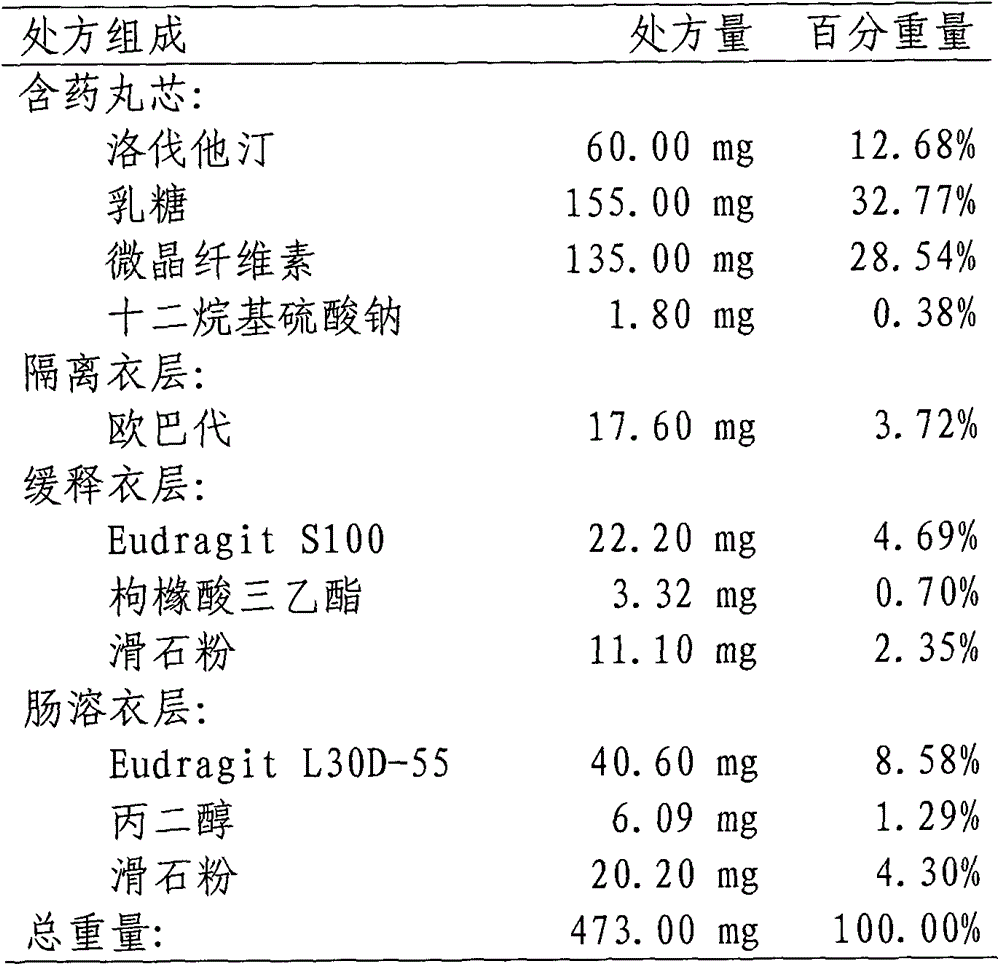
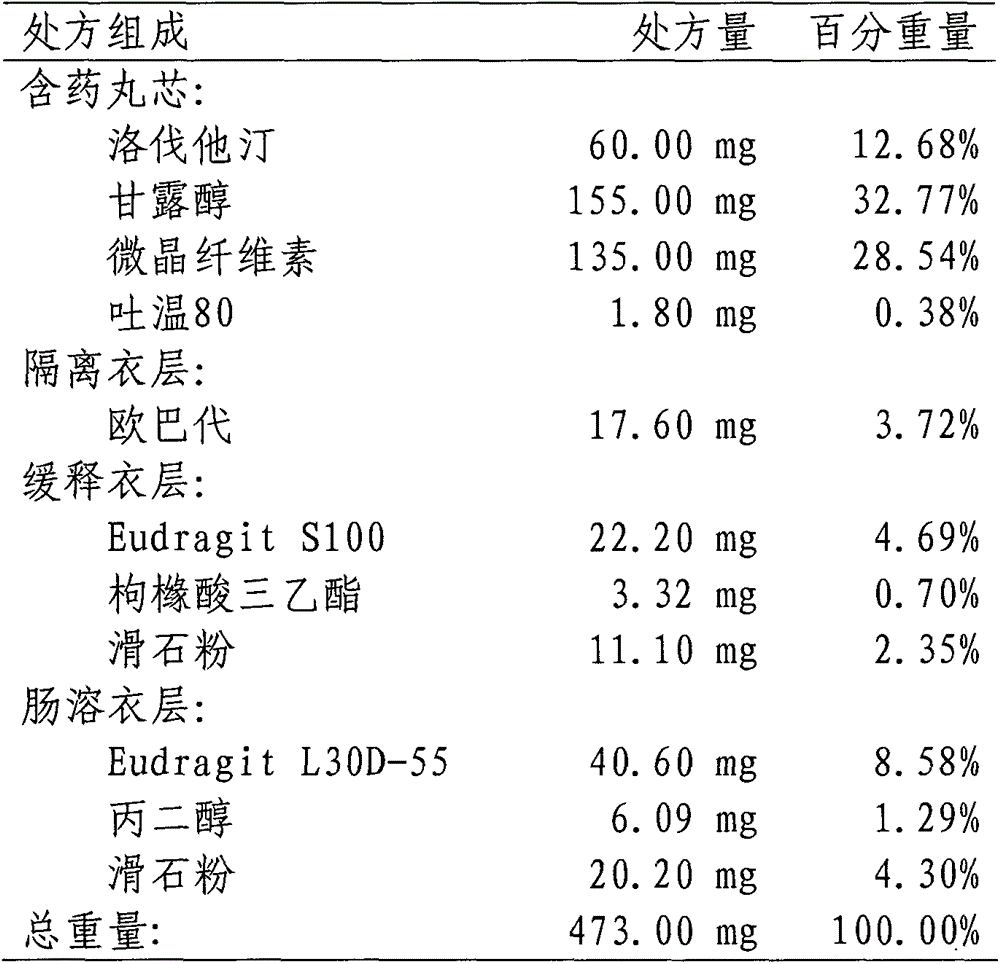
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Chinese patent medicine for treating gynaecologic disease and preparing method
InactiveCN1742993APrevent solidificationImprove liquiditySexual disorderPlant ingredientsDiseaseMedicinal herbs
The Chinese patent medicine for curing gynecopathy is made up by using 30-odd Chinese medicinal materials of ginseng, notoginseng, ass hide glue, velvet deerhorn, Chinese angelica root, cooked rehmannia root and others. Said invention also provides its preparation method, and said Chinese patent medicine can be made into capsule preparation.
Owner:四川泰华堂制药有限公司
Composition of medication for treating anemia, and application
ActiveCN1759877AIncrease stimulationImprove symptoms of anemiaPill deliveryCapsule deliveryMedicineIrritation
A composite medicine for treating the hemorrhagic anemia, iron-deficiency anemia and nutritional anemia is prepared from the arnono acid chelated iron, one or more Chinese-medicinal materials for nourishing blood, and pharmacologically, acceptable salt. Its advantages are high curative rate and biologic utilization rate, and low irritation to enterogastric tract.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
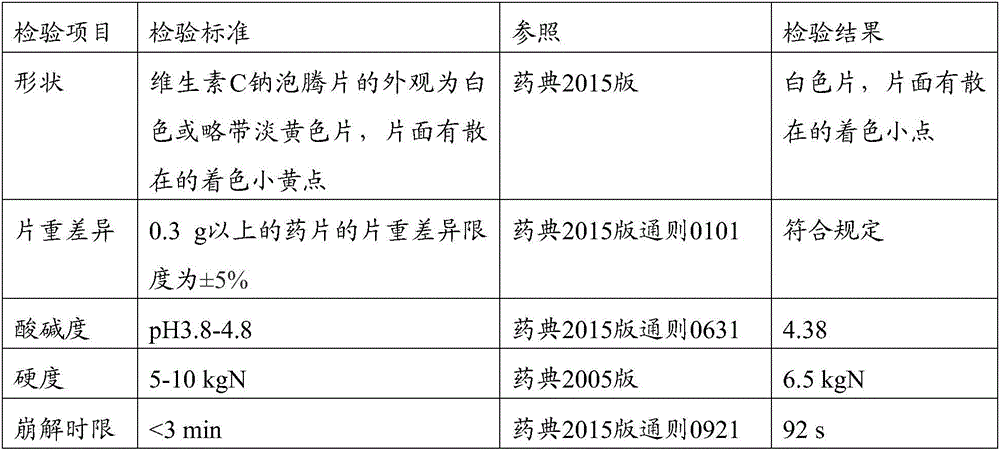
Vitamin C sodium effervescence formulation and preparation method thereof
InactiveCN106387918AImprove stabilityNo gastrointestinal irritationFood shapingFood ingredient functionsVitamin CAdhesive
The invention belongs to the field of health-care products, and particularly relates to a vitamin C sodium effervescence formulation and a preparation method thereof. The vitamin C sodium effervescence formulation provided by the invention comprises acidic components, alkaline components, a filling agent, a correctant, a lubricant, an adhesive and a disintegration auxiliary. The effervescence formulation is an effervescent tablet agent or effervescence solid particles, is good in stability and free from irritant to the stomach and the intestinal tracts, and can be effectively used as a supplementing agent of vitamin C. The invention further discloses the preparation method of the effervescence formulation. The preparation method is simple to operate. The prepared vitamin C sodium effervescence formulation is smooth, clean and level in appearance, free from bubbling phenomena, large in effervescence particle size and low in hygroscopicity.
Owner:GUANGDONG UNIV OF TECH
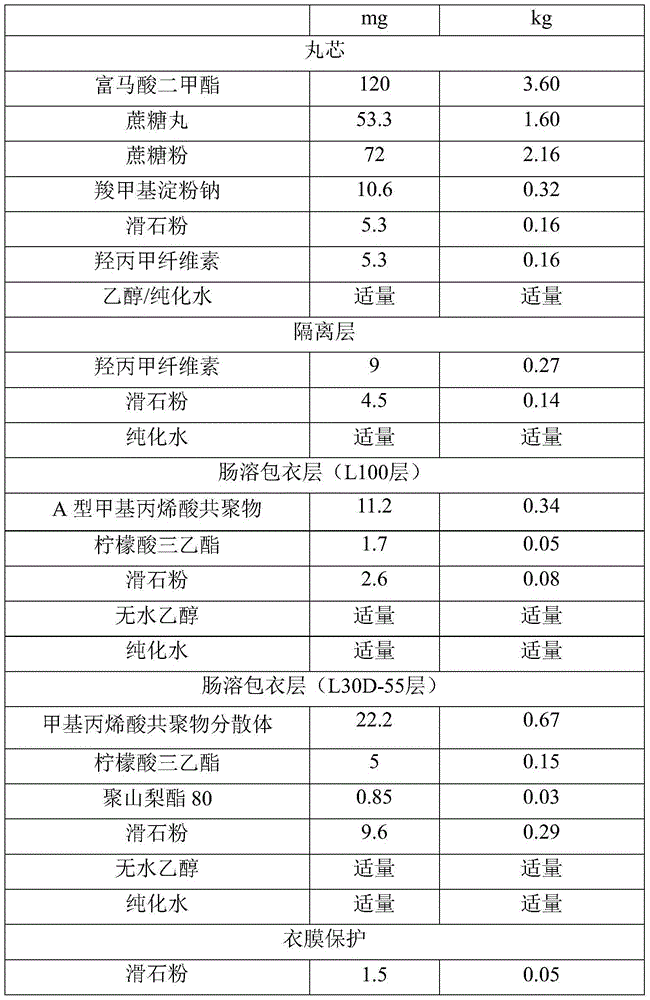
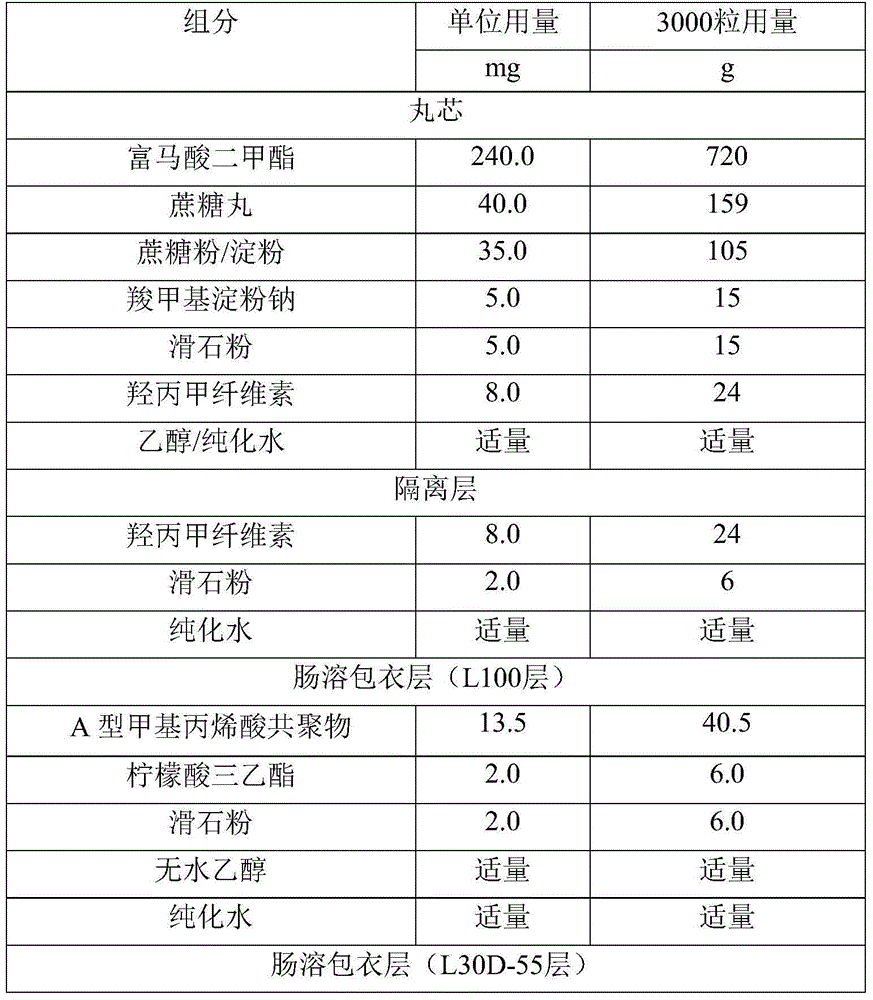
Dimethyl fumarate enteric-coated pellets and preparation method thereof
ActiveCN104971048AReduce stimulationPromotes even distributionOrganic active ingredientsGranular deliveryMedicineEnteric coated pellets
The invention discloses dimethyl fumarate enteric-coated pellets and a preparation method thereof. Each enteric-coated pellet comprises a blank pellet core, an active drug layer wrapping the blank pellet core, an isolating layer, an inner layer enteric-coated coating layer and an outer layer enteric-coated coating layer from inside to outside. The enteric-coated pellets are simple in process, complete in release, good in reproducibility, small in gastrointestinal tract irritation, safe and effective.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD
Selenium-rich shiitake mushroom production process
InactiveCN104521551AImprove qualityEfficient absorption and utilizationCultivating equipmentsMushroom cultivationBiotechnologyEdible mushroom
The invention relates to a selenium-rich shiitake mushroom production process, and relates to the technical field of fungus cultivation. According to the invention, a shiitake mushroom thallus production process is carried out with the steps of material pretreatment, material preparation, pot sterilization, blending with shiitake mushroom cultivar, shiitake mushroom stick preparation and bag removing, and mushroom fruiting management. During the production process, an organic selenium source is added, and the selenium source is pretreated. According to the invention, the organic selenium source is added to a shiitake mushroom cultivation medium, such that shiitake mushroom quality is improved. Toxic and side effects and gastrointestinal irritation to human bodies caused by direct use of chemical selenium are eliminated, and selenium in the shiitake mushrooms can be more highly efficiently and more safely absorbed and utilized by human bodies. Greater economic benefit can be brought to mushroom farmers, such that the edible fungus industry can be promoted to a large-scale, normalized and standardized level.
Owner:YILIANG COUNTY LIANZHONG VEGETABLES & FLOWERS PLANTING PROFESSIONAL COOP
Medlar polysaccharide chewable tablet and preparation method thereof
The invention discloses a medlar polysaccharide chewable tablet and a preparation method thereof. The medlar polysaccharide chewable tablet is prepared from a medlar extract, dextrin, menthol and magnesium stearate. The chewable tablet has the advantages that the chewable tablet is sour and sweet in flavor, good in taste, easy to chew, easy to absorb, and free of thrill on gastrointestinal tracts, can achieve the effect of immune adjustment in people taking the chewable tablet, can play the roles of resisting aging, tumors, fatigue, and oxygen deficit, reducing blood sugar and blood pressure, improving the immunity of the organism and the like, and is suitable for all peoples and any sensitive people. The medlar polysaccharide chewable tablet does not contain micromolecule sugar, and is suitable for diabetics.
Owner:HANGZHOU ZHONGYING TECH GRP
Progesterone sustained-release microcapsule preparation and preparation method thereof
ActiveCN106063783ASmall toxicityImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
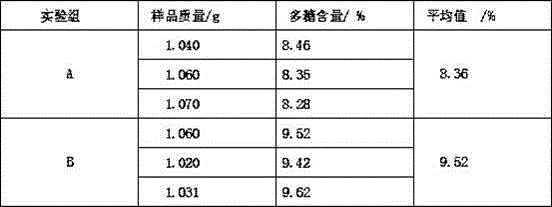
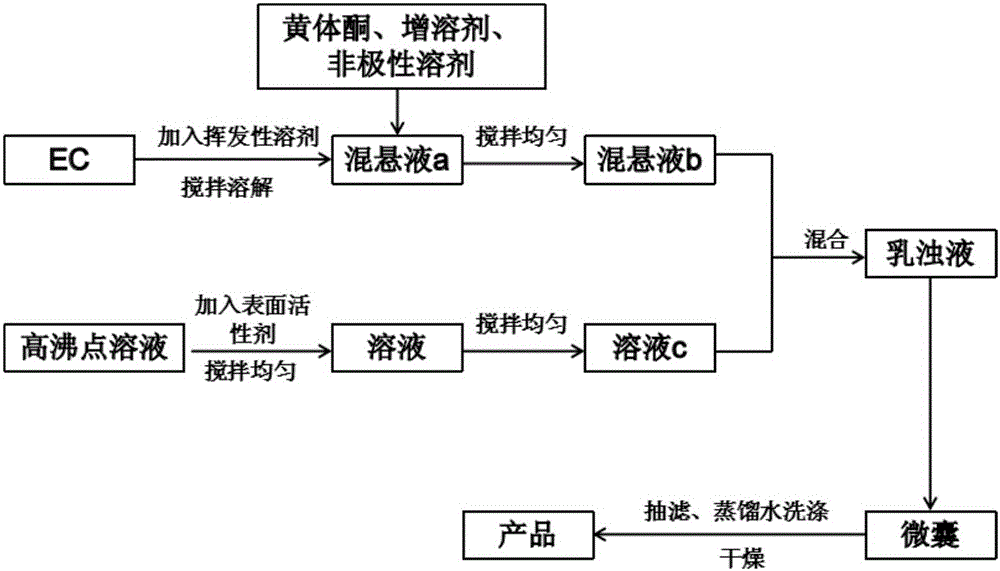
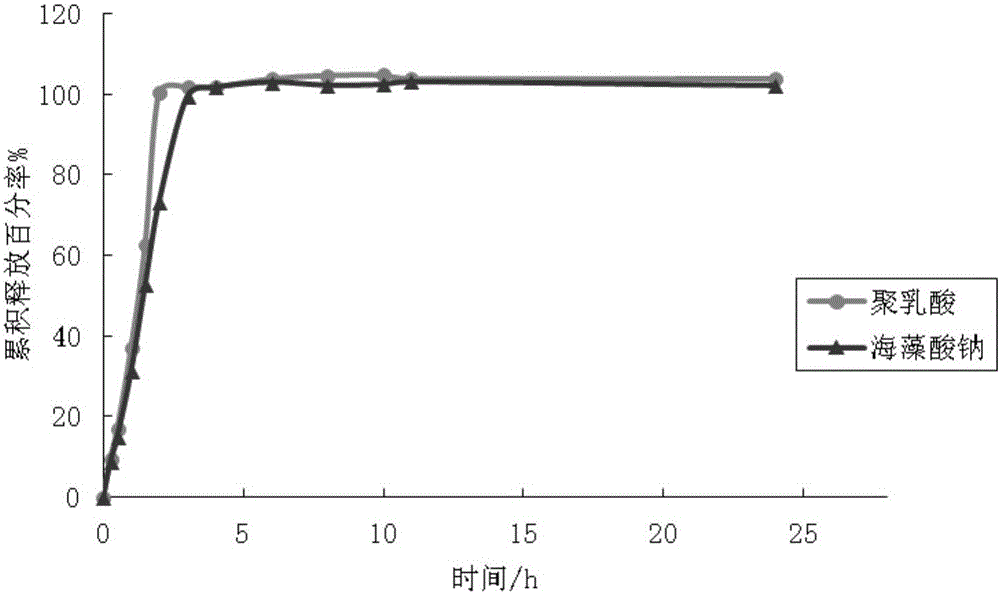
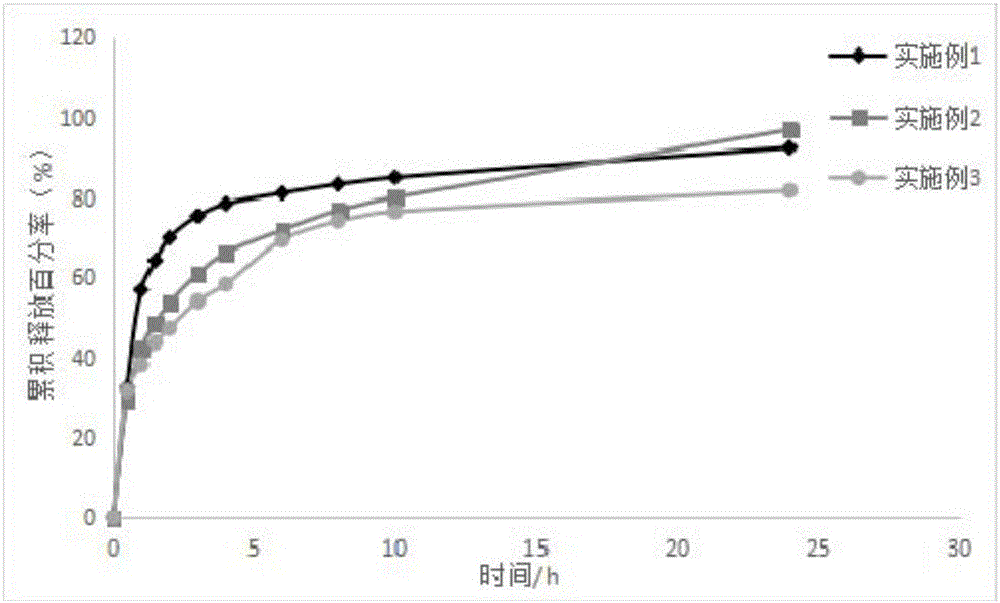
The invention relates to a progesterone sustained-release microcapsule preparation and a preparation method thereof and belongs to the technical field of drugs. An oil solution is prepared from oil-soluble progesterone powder and the progesterone sustained-release microcapsule is prepared through an emulsification-solvent diffusing method. The progesterone powder is processed to form the oil solution so that solubility is improved. Through selection of a wall material, release in vitro and in vivo is improved. The prepared progesterone-ethyl cellulose sustained-release microcapsule has a drug loading ratio of 10%, particle sizes less than 150 microns and a large gastrointestinal tract distribution area, improves progesterone dissolvability, improves bioavailability, reduces gastrointestinal tract irritation and reduces drug toxic or side effects.
Owner:ZHEJIANG ASEN PHARMA
Berberine hydrochloride oral pill dried gel as well as preparation method and applications thereof
ActiveCN106511260AGuaranteed efficacyImprove complianceOrganic active ingredientsAerosol deliverySide effectIrritation
The invention provides berberine hydrochloride oral pill dried gel as well as a preparation method and applications of the berberine hydrochloride oral pill dried gel. According to the berberine hydrochloride oral pill dried gel as well as the preparation method and the applications of the berberine hydrochloride oral pill dried gel, firstly, the applications of berberine hydrochloride in preparing the medicines for treating heart diseases are defined, and secondly, the condition that the berberine hydrochloride oral pill dried gel comprises berberine hydrochloride pills uniformly distributed in gel matrix is defined, and the gel matrix comprises a gel forming agent, a curing agent, a corrigent, an aromatic, a preservative and a pH regulator. The berberine hydrochloride provided by the invention can be used for preparing the medicines for treating heart diseases, the treatment effects are obvious, and the side effects can be effectively reduced; for the berberine hydrochloride, through the pill coating technology, the stability of the medicine is improved, the bad smell of the medicine is masked, and the side effects are reduced; meanwhile, after the pill is added into the gel matrix, the slow release effect can be effectively achieved, so that the medicine taking times is reduced, the irritation to the gastrointestinal tract is reduced, the mouthfeel of the preparation is completely improved, the fearful mental state generated when children patients take the medicine is overcome, and the compliance is improved.
Owner:黑龙江童医生儿童生物制药有限公司
Preparation method of no-alcohol type Shexiang Qushu liquid oral preparation
The invention relates to a preparation method of a nonalcoholic ageratum summer-heat clearing liquid oral preparation. The raw materials are chosen according to the raw materials which are specified by 'ageratum summer-heat clearing water' recorded by ministerial traditional Chinese medicine standard and the dosage proportion thereof. The particular method of the invention is as follows: patchouly, elsholtzia haichowensis, angelica dahurica, perilla leaf, rhizoma atractylodis, clove, dried orange peel, shell of areca nut, rhizoma pinellinae praeparata, tuckahoe, ginger and liquorice are chosen; firstly, the patchouly, the elsholtzia haichowensis, the angelica dahurica, the perilla leaf, the rhizoma atractylodis, the clove, the dried orange peel and the ginger which contain volatile oil are extracted to obtain the volatile oil; solubilizer is added into distilment to lead the volatile oil and aromatic water to be well blended; the water part is steamed with water, and clear paste is remained; the residue after the volatile oil is extracted, shell of areca nut, rhizoma pinellinae praeparata, tuckahoe, and liquorice are extracted by ethanol; after the ethanol is volatilized, the two clear pastes are combined and are blended with the volatile oil; finally flavouring agent and specified amount of water are added to prepare nonalcoholic ageratum summer-heat clearing liquid oral preparation. Compared with ageratum summer-heat clearing liquid which contains 40-45% of ethanol recorded by ministered standard, the invention does not contain ethanol, thus avoiding the side effect such as irritating gastrointestinal tract and the like, being accepted by patients easily, better taking the healing effect, expanding the scope of treated subjects. In addition, compared with the original ageratum summer-heat clearing water, the amount of the ethanol is reduced in the course of production, and the cost is reduced.
Owner:SINOMEDICINE BEIJING PHARMA SCI
Aceclofenac in extended-released tablets and method of manufacturing the same
ActiveCN101108170AReduce stimulationSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticSustained Release TabletAdditive ingredient
The invention relates to a drug sustained-release preparation and its preparation method, in particular to an aceclofenac sustained-release tablet and its preparation method, which comprises the following ingredients according to the weight percentage: aceclofenac of 60 to 95 per cent, skelecton retarder of 3 to 30 per cent, adhesive of 1 to 10 per cent and lubricant of 0.5 to 15 per cent. The hydroxypropylmethyl cellulose and carboxyvinyl polymer are adopted as optimized skelecton retarder. With such a technical proposal in the invention, the one aceclofenac sustained-release tablet can be taken a day to effectively reduce the fluctuation of blood drug level, prolong the maintenance duration of effective blood drug level and lower down the incitement to gastrointestinal tract. Besides, the invention also provides the preparation method of the aceclofenac sustained-release tablet.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
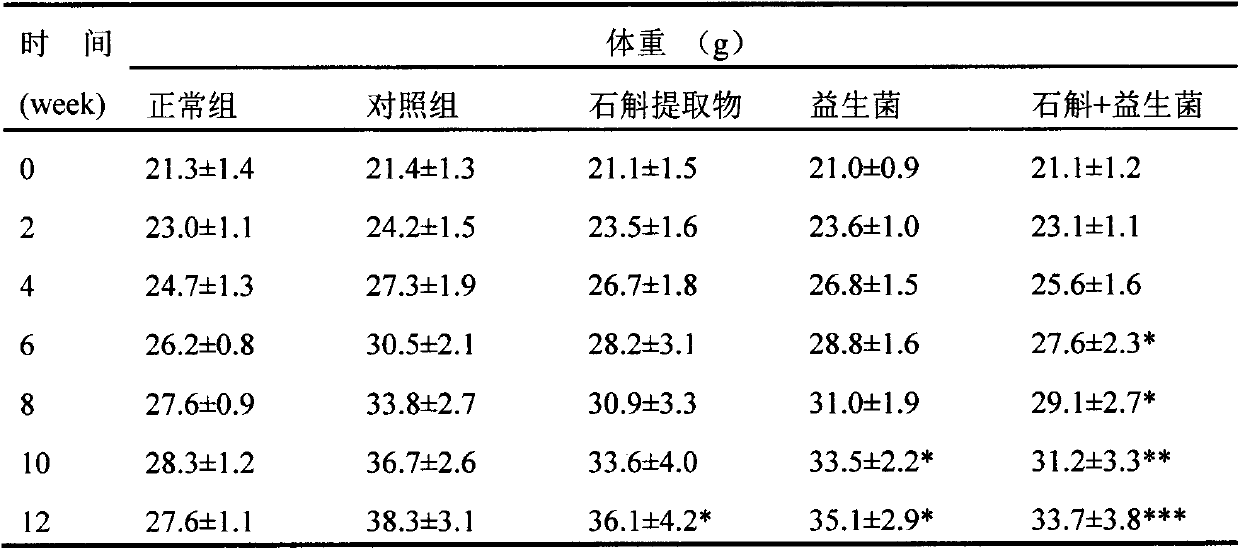
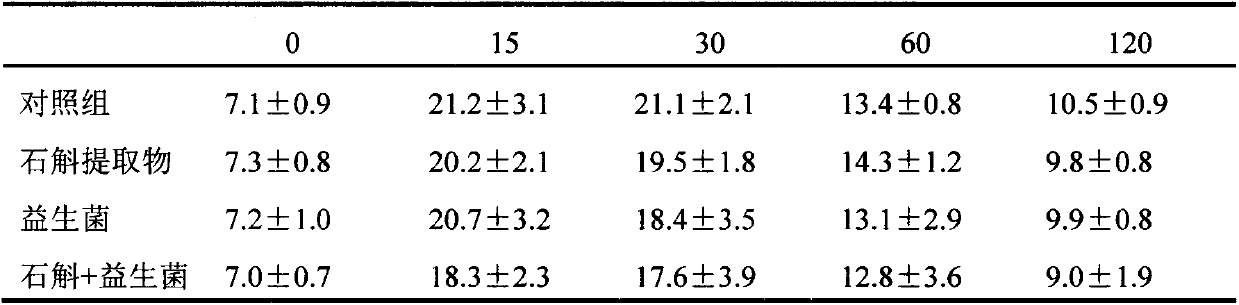
Application of dendrobium officinale and extract thereof to improvement of diabetes and metabolic syndrome in combination with probiotics
PendingCN110604789AReduce cholesterolLow densitySenses disorderNervous disorderDiseaseGlucose uptake
The invention relates to the technical field of biology, in particular to dendrobium officinale and a dendrobium officinale extract combined probiotics for regulating blood sugar and blood fat, and improving diabetes and metabolic syndrome and alleviating occurrence of related complications so as to maintain metabolic stability of organisms. The dendrobium officinale has the effects of regulatingand controlling blood sugar, enhancing body immunity and the like; the probiotics is an active microorganism beneficial to a host, can regulate intestinal flora balance, and can generate an exact health effect so as to improve the micro-ecological balance of the host and play a beneficial role. According to the invention, the dendrobium officinale exerts the effects of reducing blood sugar and blood fat, prebiotics can promote the growth of probiotics and regulate glucose uptake and improve the gastrointestinal irritation caused by taking the probiotics alone, so that the probiotics and dendrobium officinale can better play the role in reducing blood sugar, complications are reduced, and a pharmaceutical composition for preventing or treating diabetes mellitus and metabolic syndromes can be prepared by the probiotics and dendrobium officinale. Therefore, the product provides a new application for improving diabetes and metabolic syndrome related diseases.
Owner:NANJING UNIV
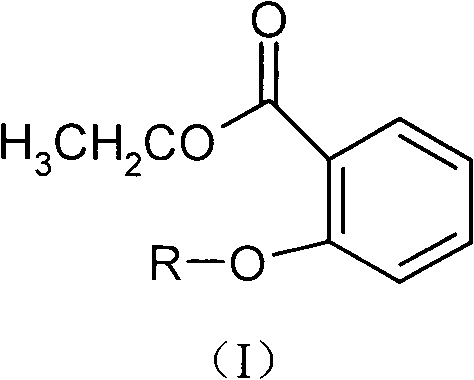
Ethyl salicylate glycosides and synthetic method and application thereof
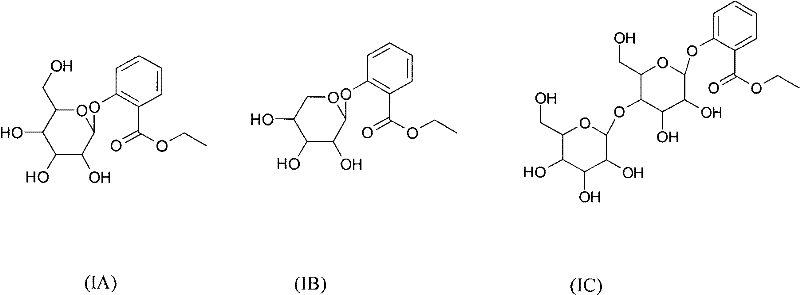
ActiveCN102453060AThe synthesis method is simpleLow costOrganic active ingredientsSugar derivativesEthyl salicylateSalicylic acid
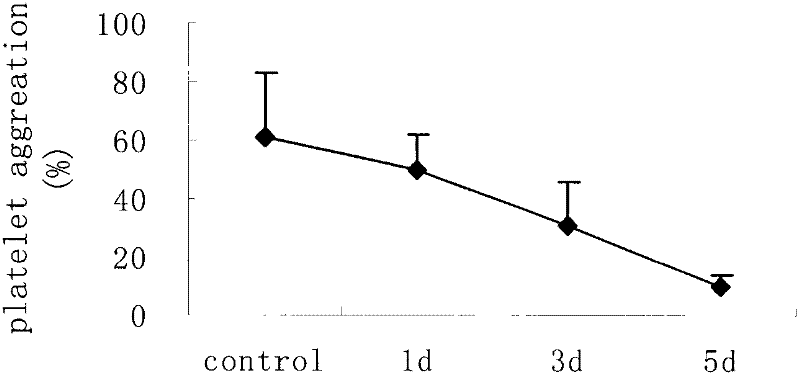
The invention relates to new ethyl salicylate glycosides, a synthetic method thereof, pharmaceutical compositions containing the ethyl salicylate glycosides, and applications of ethyl salicylate glycosides as medicaments, in particular antipyretic, analgesic, antiphlogistic and thrombosis inhibiting medicaments. The structural formula of the ethyl salicylate glycosides is as shown in the specification, wherein R is monosaccharide or disaccharide. The synthetic method provided by the invention has the advantages of simplicity, low synthesis cost and small environmental pollution; shown by activity evaluation, the ethyl salicylate glycosides have obvious antiphlogistic, analgesic and anti-platelet agglutination effects, the action time is prolonged, the adverse reaction is obviously reduced compared with salicylic acid medicaments, and mainly the stimulation to the gastrointestinal tract is reduced, therefore, the ethyl salicylate glycosides can be hopefully developed into new analgesic, antiphlogistic and thrombosis inhibiting medicaments with competitive power.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
TDF (tenofovir disoproxil fumarate) pellets and preparation method thereof
InactiveCN105147628AImprove stabilityHigh dissolution rateOrganic active ingredientsAntiviralsAdhesiveIrritation
The invention belongs to the field of medicines and discloses TDF (tenofovir disoproxil fumarate) pellets and a preparation method thereof. The TDF pellets are prepared from raw materials comprising 70-90 parts of TDF pellets by weight and 6-32 parts of a pharmaceutic adjuvant by weight through pressing, wherein the TDF pellets are prepared from raw materials comprising components in parts by weight as follows: 20-80 parts of TDF, 10-75 parts of filler, 0.5-2 parts of a disintegrating agent and 1-5 parts of a moistening adhesive. Compared with tablets in the traditional process, the prepared TDF pellets are convenient to take, good in stability, high in dissolution rate, quick and uniform to release and small in irritation to gastrointestinal tracts, significantly improve the bioavailability of medicines and have great clinical significance.
Owner:SUZHOU HOMESUN PHARMA
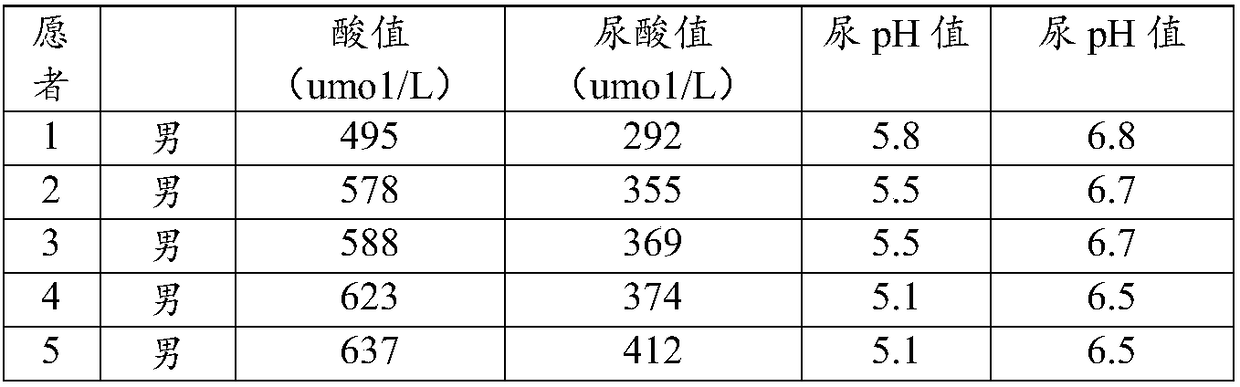
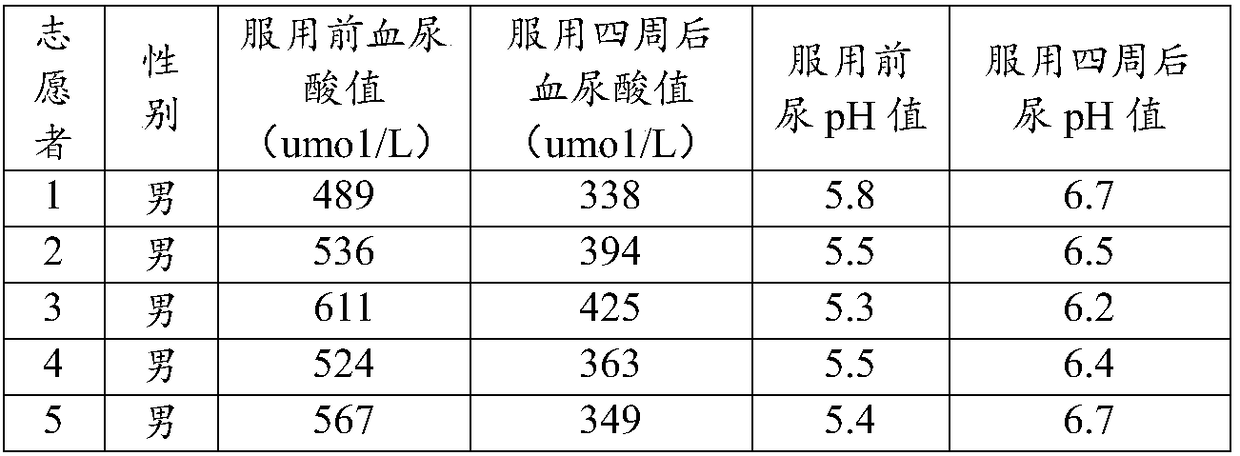
Alkaline effervescent tablets for treating gout and hyperuricemia
InactiveCN108295101AEasy to takeQuick resultsOrganic active ingredientsInorganic non-active ingredientsBacteriuriaEffervescent tablet
The invention relates to alkaline effervescent tablets for treating gout and hyperuricemia, and a preparation method of alkaline effervescent tablets. The alkaline effervescent tablets are prepared from the following raw materials by mass percent: 45-55% of citric acid, 30-40% of an alkali source, 7-15% of citrate, 2-4% of an acerola cherry extract, 3-8% of a filling agent, 0.8-1.4% of a lubricant, 0.5-0.8% of sucralose and 1-2% of lemon essence; the preparation method comprises the following steps: mixing the citric acid and the filling agent with a right amount of the lubricant to prepare first granules; preparing second granules by using the alkali source, the citrate and a right amount of the lubricant; then, evenly mixing the first granules and the second granules with the rest components, and tabletting to obtain the alkaline effervescent tablets. The alkaline effervescent tablets have the advantages that the alkaline effervescent tablets for treating the gout and the hyperuricemia are provided for the first time, and are convenient to take, less in gastrointestinal irritation and easy to absorb; due to the cooperation of the citrate and the acerola cherry extract in the alkaline effervescent tablets, the alkaline effervescent tablets are good in urine alkalization effect.
Owner:光谷迈德(武汉)慢性病研究院有限公司
Levofloxacin hydrochloride micro-pill capsule and its preparing method
InactiveCN1813758AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsLevofloxacinGastrointestinal irritation
The present invention relates to a levofloxacin hydrochloride micropill capsule and its preparation method. It is composed of capsule external shell and micropill, the micropill is formed from levofloxacin hydrochloride, blank pill core and adhesive, every pill contains (by wt%) 10%-80% of levofloxacin hydrochloride, 15%-60% of medicine micropill pill core and 5%-30% of adhesive. Besides, said invention also provides the concrete steps of its preparation method.
Owner:范敏华
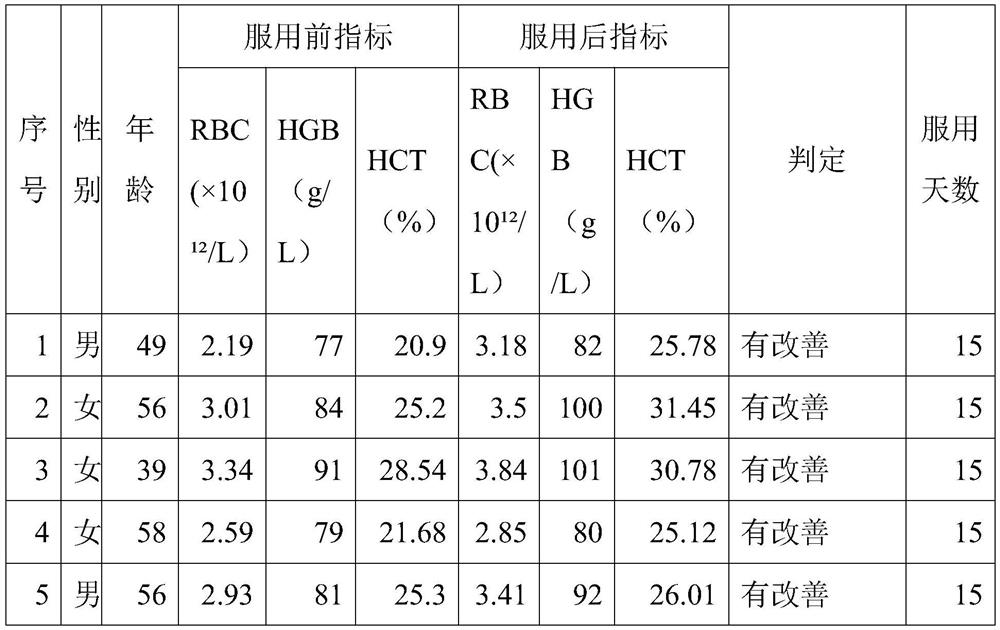
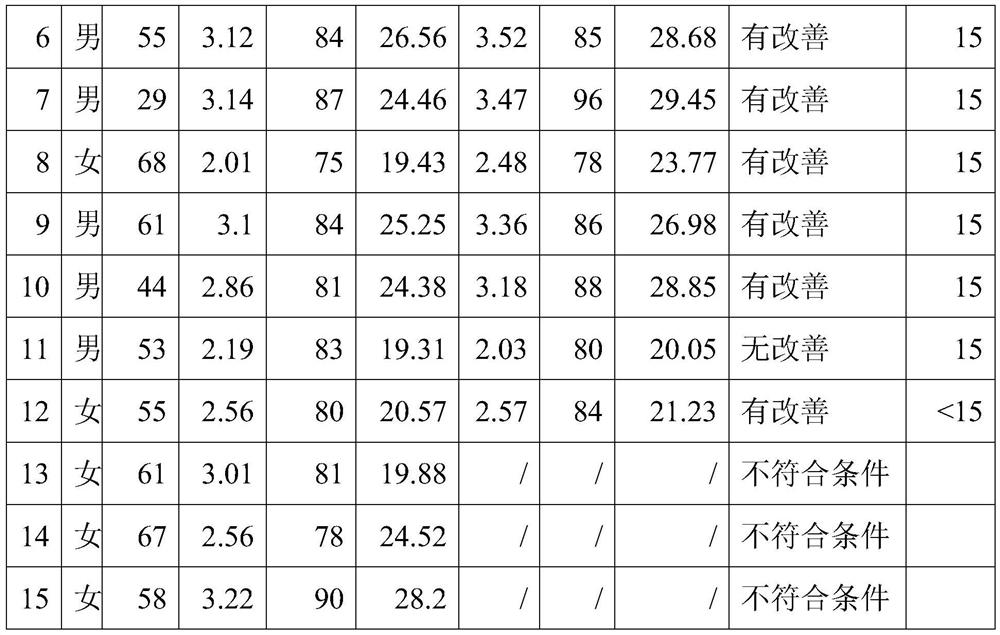
Preparation for improving anemia symptoms and preparation method thereof
PendingCN114010762AImprove absorption rateHigh activityPeptide/protein ingredientsHaemoglobins/myoglobinsSucroseTaurine
The invention discloses a preparation for improving anemia symptoms and a preparation method of the preparation, belonging to the technical field of blood protein polypeptides. The preparation for improving anemia symptoms comprises the following components in parts by weight: 3 to 10 parts of blood protein polypeptide powder, 3 to 10 parts of collagen peptide powder, 3 to 10 parts of red date extract, 0.1 to 0.3 part of taurine, 0.01 to 0.04 part of sucralose, 0.015 to 0.025 part of stevioside, 0.1 to 0.3 part of erythritol, 0.6 to 0.8 part of citric acid, 0.05 to 0.15 part of pectin, 0.15 to 0.18 part of edible essence, 0.1 to 0.2 part of ginseng powder and 0.1 to 0.2 part of donkey-hide gelatin peptide powder. The preparation is high in absorptivity, free of accumulation, high in safety and free of gastrointestinal irritation, and effectiveness and safety of the preparation are proved by rat experiments. The preparation can effectively improve anemia indexes for anemia, especially tumor-related anemia, and has a very remarkable erythropoiesis effect under the condition that a patient does not receive a hemopoietin promotion injection during a test taking period.
Owner:杭州佰倍优生物科技有限公司 +1
Preparation method of celecoxib preparation
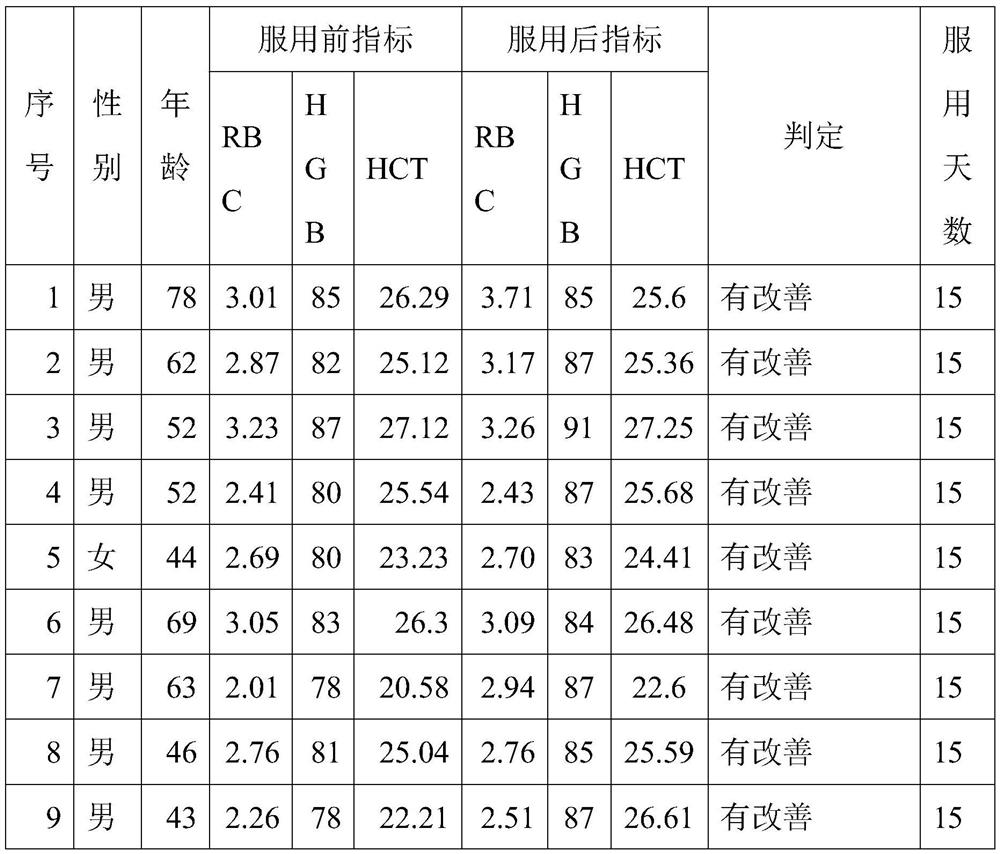
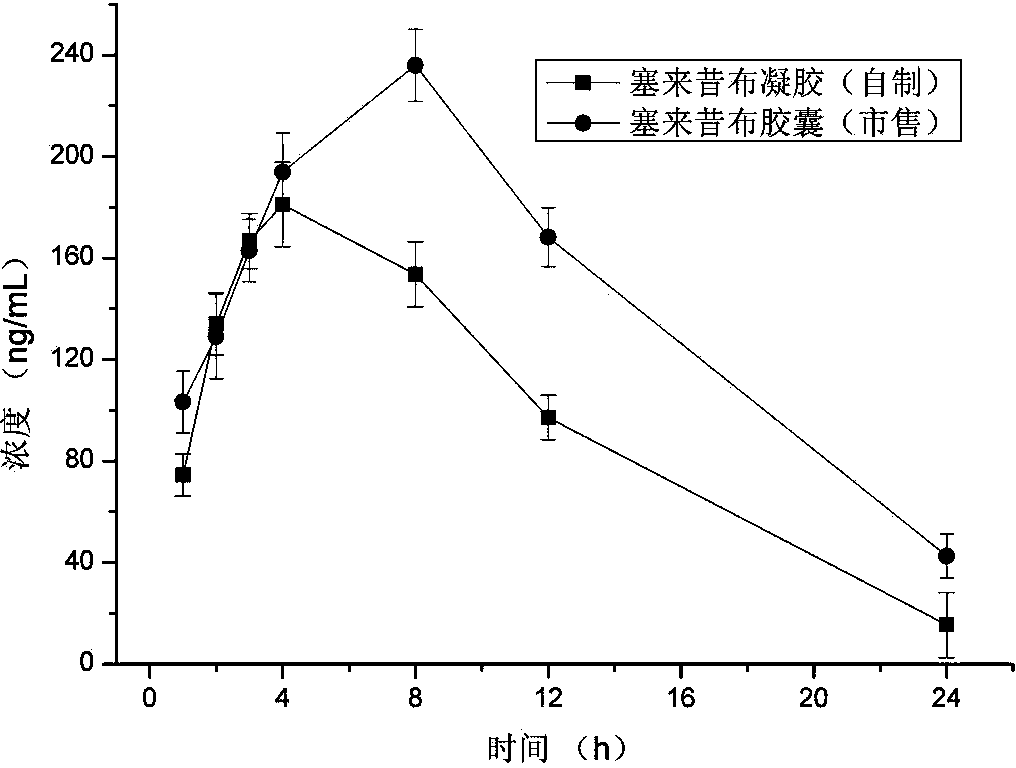
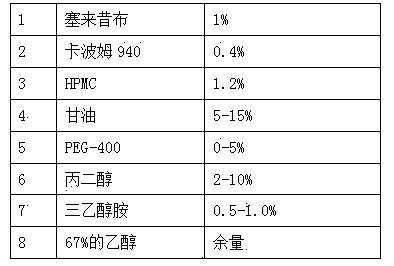
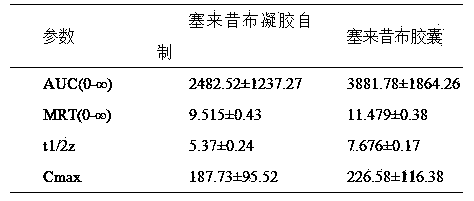
The invention provides a preparation method of a celecoxib gel preparation. The gel preparation comprises 1 to 5% of celecoxib, 1 to 5% of gel substrate, 5 to 30% of permeation accelerant, 0.5 to 1.0% of pH regulator, and 50 to 80% of 67% alcohol. The gel preparation is in a form of semi-solid gel for transdermal absorption, which avoids nausea, emesis, gastrointestinal irritation, anabrosis and other side effects caused by oil administration, and also avoids gastrointestinal reaction and drug first-pass effect; and in addition, the concentration of a sick part is raised, the adverse reaction is reduced, and pain can be relieved for a patient. The preparation is simple in preparation process, and stable and reliable in quality.
Owner:JIANGSU QINGJIANG PHARMA
Cetirizine and pseudoephedrine sustained-release capsule and preparation method thereof
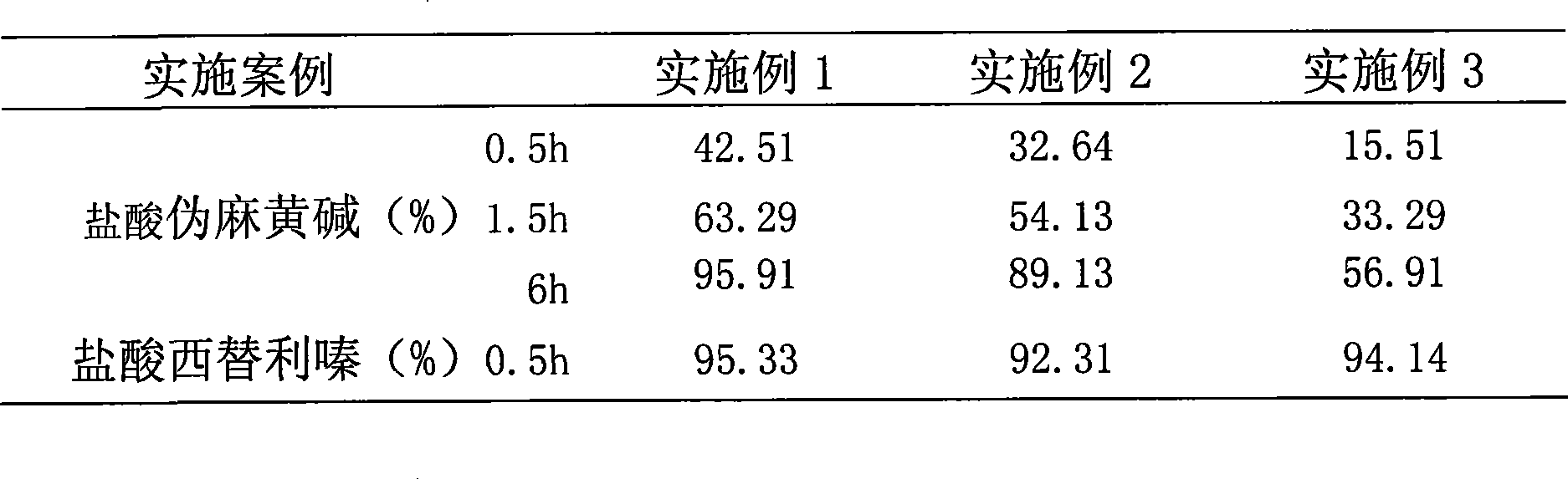
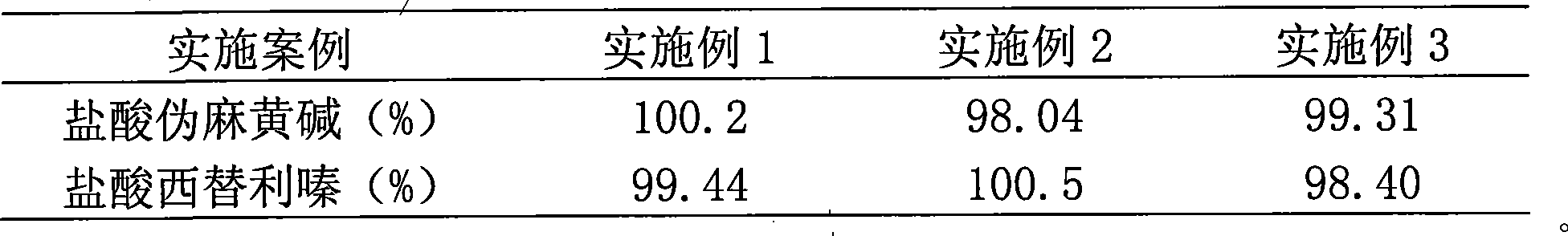
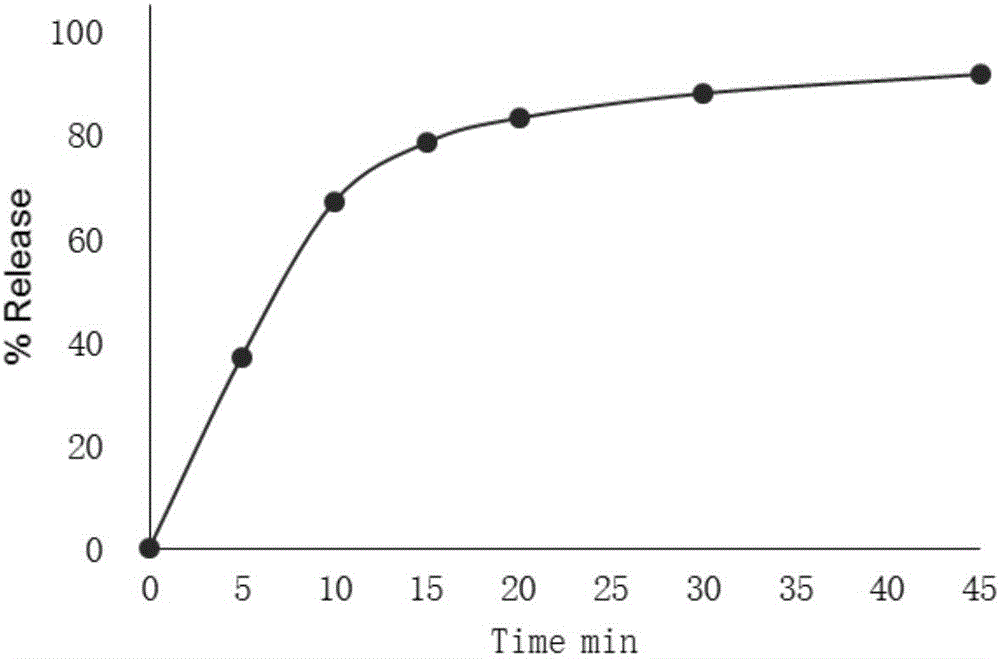
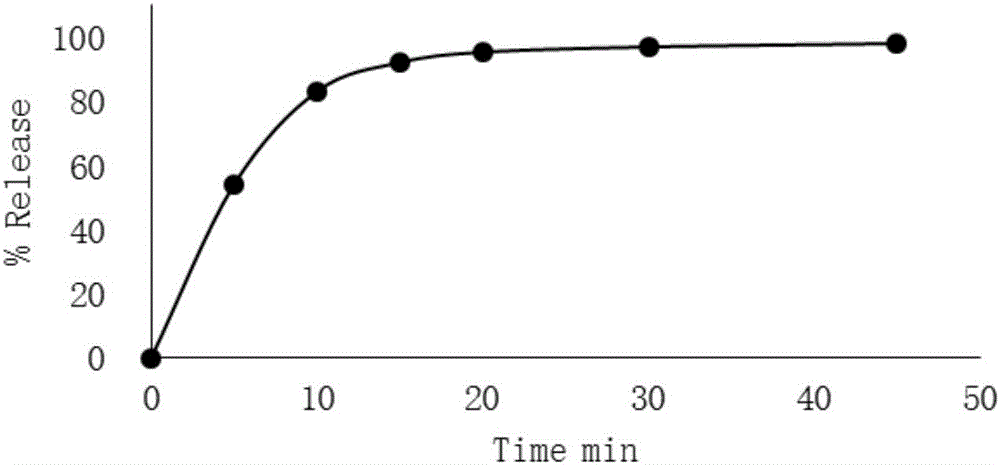
InactiveCN101474166AWell mixedQuick effectOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideSustained Release Capsule
The invention discloses a cetirizine pseudoephedrine slow release capsule. Cetirizine hydrochloride is prepared to quick release pellets and pseudoephedrine hydrochloride is prepared to slow release pellets, in accordance with the weight proportion that each 1000 preparations contain 5g of cetirizine hydrochloride and 120g of pseudoephedrine hydrochloride, the two pellets are uniformly mixed and encapsulated; or the two pellets are encapsulated respectively in proportion. The quick release part of the cetirizine hydrochloride and the slow release part of the pseudoephedrine hydrochloride are synthesized together subsequent to being prepared to the pellets respectively, so as to prepare the capsule with two different medicine-releasing speeds, the medicine-releasing speed of each pellet is uniform and the medicine effect is steady. The capsule has the advantages of even and extensive distribution of the medicine in vivo subsequent to the administering, sufficient absorption of the medicine, small stimulation to gastrointestinal tracts and good reproducibility of the medicine-releasing rule, and improves, while maintaining the properties of rapid effecting and long half-life of the cetirizine, the pharmacokinetic properties of the pseudoephedrine hydrochloride to administer twice per day instead of having to administer four times per day, so that both reach the optimal cooperative curative effect.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Mycophenolate mofetil capsule and preparation method thereof
InactiveCN106389379AReduce intensityLow densityOrganic active ingredientsSkeletal disorderIrritationAdhesive
The invention provides a mycophenolate mofetil capsule and a preparation method thereof. The mycophenolate mofetil capsule comprises, by weight, 1100-1400 parts of mycophenolate mofetil, 150-170 parts of filler and 75-110 parts of pharmaceutical acceptable auxiliary materials; the filler is selectively prepared from a mixture of one or more optional proportions of pregelatinized starch, corn starch or lactose; the pharmaceutical acceptable auxiliary materials include adhesive, disintegrant and lubricant. A fluidized-bed granulation technology is adopted, the process is simplified, time is saved, labor intensity is low, obtained particles are porous soft particles, low density and low strength are achieved, the particles are evenly distributed in particle size, and good fluxility is achieved; the mycophenolate mofetil capsule has the advantages of high disintegration speed, good absorptivity, convenience in taking, small in intestinal irritation, stable in long-term storage quality and the like; the advantages of both simpleness in production equipment for ordinary capsules and convenience in package storage and transportation as well as carrying are achieved, and the mycophenolate mofetil capsule is convenient for patients to take.
Owner:WUXI FORTUNE PHARMA
Betahistine hydrochloride liposome and preparation method thereof
ActiveCN102631320AImprove efficacyImprove antioxidant capacityOrganic active ingredientsSenses disorderRotary evaporatorMethylene Dichloride
The invention relates to a betahistine hydrochloride liposome and a preparation method of the betahistine hydrochloride liposome. The preparation method comprises the steps of: firstly dissolving lipide ingredients in methylene dichloride, and carrying out reduced-pressure evaporation in water bath until a film is formed; dissolving the film in diethyl ether, and then adding phosphate buffer containing betahistine into the solution; carrying out ultrasonic treatment on the mixed suspension liquid, placing the treated liquid on a rotary evaporator, and carrying out reduced-pressure vaporization until all the organic reagent is evaporated; and finally, obtaining the betahistine hydrochloride liposome. The betahistine hydrochloride is made into liposome, so that the medicine effect is obviously improved, the permeability of blood capillary can be improved, and the dosage of the medicine reaching blood vessels of the brain can be increased; the betahistine hydrochloride liposome is used for improving the treatment effect of Meniere syndrome, reduces the gastrointestinal irritation and the adverse drug reaction, and is beneficial to reducing the side effects such as stomach upset, nausea, dizziness and the like.
Owner:黑龙江亿达鸿药业有限公司
Cetirizing hydrochloride cataplasm preparation
InactiveCN1457781AIncrease concentrationImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochlorideOral medication
The present invention relates to medicine technology and is cataplasm as one new form of Cetirizine hydrochloride. There are available oral forms of Cetirizine hydrochloride, such as tablet, capsule, solution preparation, etc. The present invention prepares the cataplasm with Cetirizine hydrochloride as main medicine component and different supplementary material, and the cataplasm is administrated via skin. The present invention can avoid stimulation to gastrointestinal tract of the medicine, reduce medicine concentration in blood, reduce central nerve suppressing effect, raise the medicine concentration in local affected part, decrease the administration times and raise patient's compatibility.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Chinese-medicinal compound lotion for treating dermatitis eczema
InactiveCN102204981AImprove complianceAvoid gastrointestinal irritationHydroxy compound active ingredientsPharmaceutical delivery mechanismSide effectIrritation
The invention, relating to the technical field of medicine, provides a Chinese-medicinal compound lotion for treating dermatitis eczema and a preparation method thereof. The component proportion of the lotion is: 50-70g of sophora flavescens, 40-60g of common cnidium fruit, 20-40g of phellodendron, 25-35g of Chinese angelica, 15-25g of Ligusticum wallichii, 15-30g of rheum officinale, 3-6g of borneol, and 0.1wt% of antiseptic ethylparaben. The preparation method comprises the following steps: 1) preparing an extract of sophora flavescens, common cnidium fruit and phellodendron; 2) preparing an extract of Chinese angelica, Ligusticum wallichii and rheum officinale; 3) mixing the extracts obtained by step 1) and 2), adding borneol according to the proportion and 0.1wt% of antiseptic ethylparaben, adding water until the amount of the mixture reaches to 1000ml for dilution, and sub-packaging. The lotion can avoid the irritation on gastrointestinal tracts by oral administration, reduce the blood concentration of body and reduce side effects through the transdermal drug delivery. The preparation method is simple, and the lotion is easy to operate and improves the compliance of medicine. The invention provides a novel medicinal preparation for treating dermatitis eczema.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
In-situ preparation method for starch-ibuprofen clathrate compound of V-type crystal structure
InactiveCN105288651AControlled release rateReduce pretreatment processOrganic active ingredientsAntipyreticSide effectPotato starch
The invention belongs to the field of pharmaceutical technologies, and particularly relates to an in-situ preparation method for a starch-ibuprofen clathrate compound of a V-type crystal structure. The method includes the steps that with potato starch as the raw material, ibuprofen is dissolved in a diluted ethanol solution, a certain amount of potato starch is added, an NaOH solution is slowly added, mixing and sufficient constant-temperature stirring are performed, and the mixed solution is kept reacting for certain time; then, the mixed solution is titrated through a hydrochloric acid ethanol solution to have the pH of 7.0 and slowly stirred for certain time, and extraction, washing, drying and smashing are performed to obtain the starch-ibuprofen clathrate compound. The starch-ibuprofen clathrate compound is structurally characterized by being of the V-type crystal structure, the preparation process is simple, and cost is low. The accumulative release rate of the starch-ibuprofen clathrate compound in simulated gastric fluid (pH 1.2) is smaller than 7%, and an active compound is slowly released in simulated intestinal fluid (pH 7.4) under the action of enzyme to take effect, so that drug bioavailability is improved, and the side effect of stimulating the gastrointestinal tract is avoided.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Cornel extract oral medicine compositions low irritative to gastrointestinal tract and its preparation
The invention relates to a cornel extract oral medicine compositions low irritative to gastrointestinal tract and its preparation, the oral pharmaceutical composition and preparation of its active constitution, the invention also relates to the process for preparing the oral pharmaceutical composition and preparation.
Owner:HUTCHISON MEDIPHARMA ENTERPRISES LTD
Enteric quick-dissolving tablets contg. aconitine, and its prepn. method
ActiveCN1903181AEnhance analgesic and anti-inflammatory activityImprove bioavailabilityOrganic active ingredientsAntipyreticPlasticizerFast release
Owner:YUNNAN HAOPY PHARM LTD
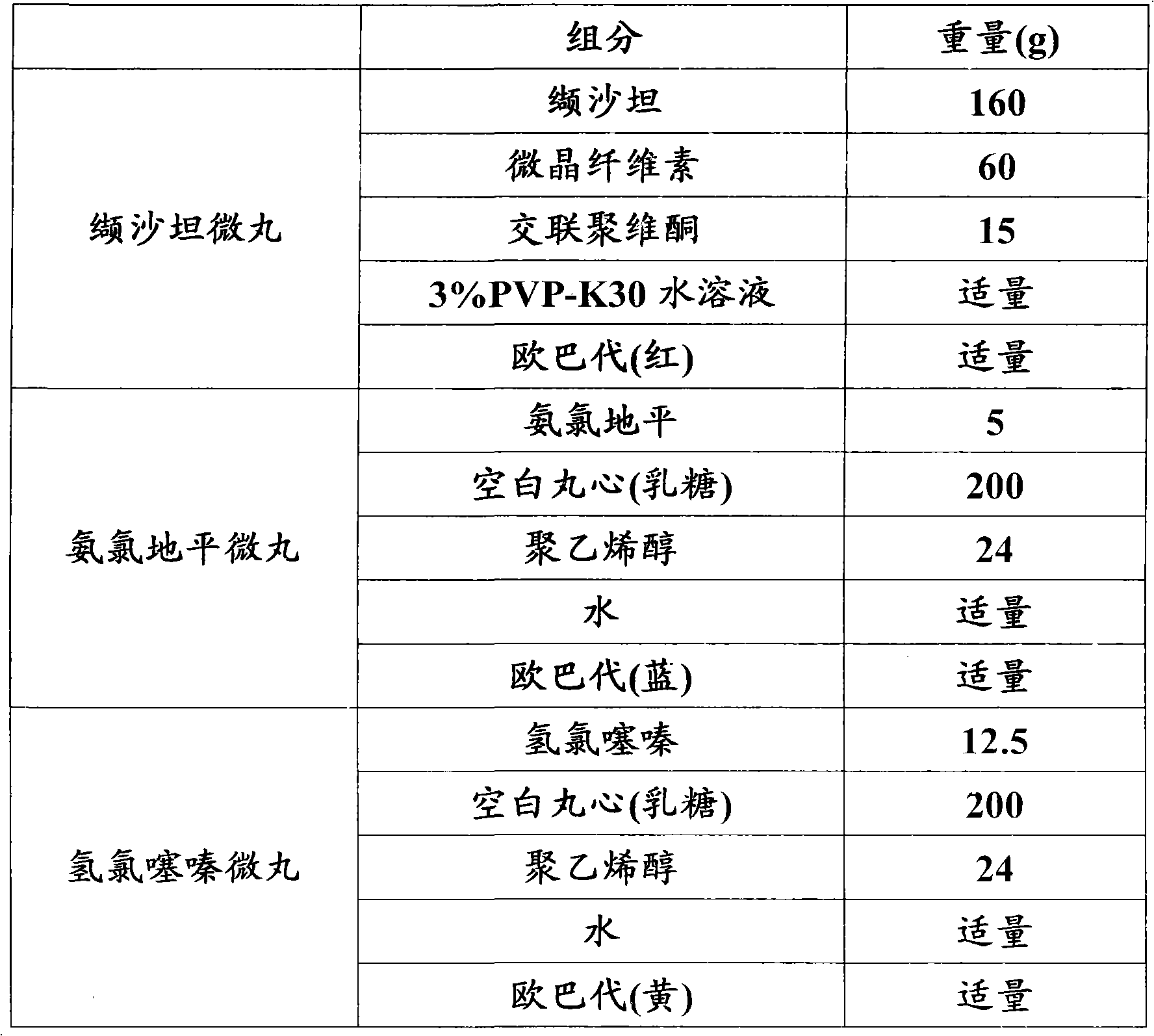
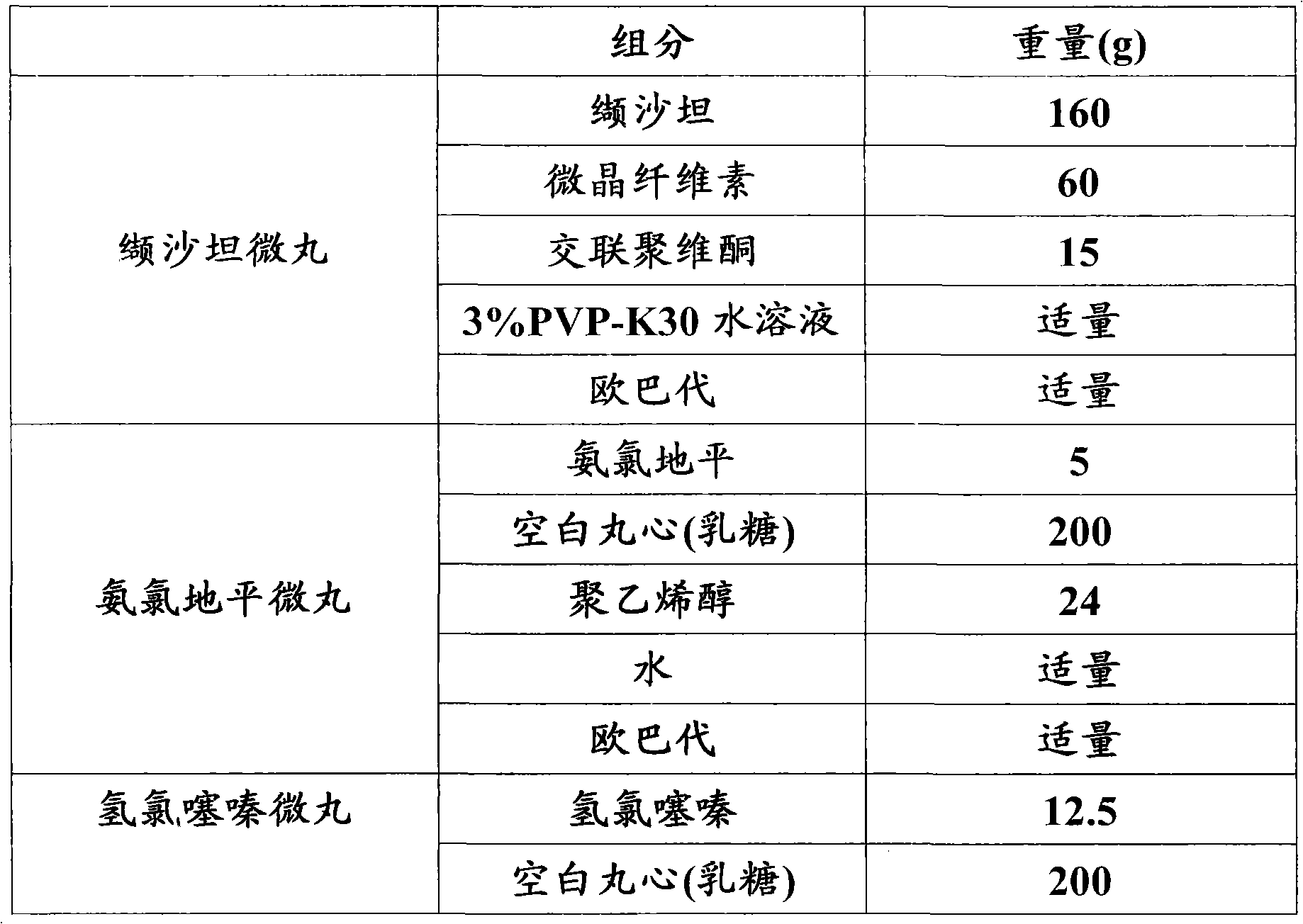
Pellet medicine combination containing valsartan, amlodipine and hydrochlorothiazide
ActiveCN102614189ACapsule deliveryHeterocyclic compound active ingredientsHydrochlorothiazideValsartan
The invention relates to a pellet medicine combination containing valsartan, amlodipine and hydrochlorothiazide, which is characterized by simultaneously containing three active ingredients, namely valsartan, amlodipine and hydrochlorothiazide. The active ingredients of valsartan, amlodipine and hydrochlorothiazide and medicine acceptable additives are together prepared into pellets, and the pellets containing medicines are coated with coating layers with different colors. Each pallet is composed of a pellet core and a coating layer, and the pellet combination can be further made into pellet capsules. The pellet medicine combination has the advantages that the medicine can be scattered in gastrointestinal tract fast, has low thrill on the gastrointestinal tract and is even and complete in absorption and high in bioavailability, a pellet capsule casing is free of bitter taste and the like. The obtained preparation can be controlled in quality easily and is good in stability and suitable for industrial production.
Owner:BEIJING MEDISAN TECH +1
Multifunctional high-efficient calcium replenishing agent and preparation thereof
The invention relates to a nutrient supplement for calcium supplementation, in particular to a multi-functional high-efficient calcium supplemental agent and a preparation method thereof which belong to the field of nutritious and health care products. The product of the invention selects inorganic calcium salt, L-ascorbic acid, L-aspartic acid for carrying out the reaction to prepare L-ascorbic acid-L-aspartic acid calcium; and the L-ascorbic acid-L-aspartic acid calcium can be prepared into health care foods with different forms such as tablets, granules, capsules, oral liquor and other after being added with necessary excipients. The product has good taste, no bitter taste and no gastrointestinal tract stimulation; the product has easy absorption, good safety and multiple nutritious health care functions. The product can more easily enter various parts of the human body through the passive diffusion, the transport or active transport and other different modes when in lack of calcium of the human body particularly, thereby meeting the needs on the calcium element by various tissues of the human body.
Owner:辽宁美天佳生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com