Mycophenolate mofetil capsule and preparation method thereof
A technology of mycophenolate mofetil and capsules, applied in the field of biopharmaceuticals, to achieve the effect of saving time, low strength and low density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
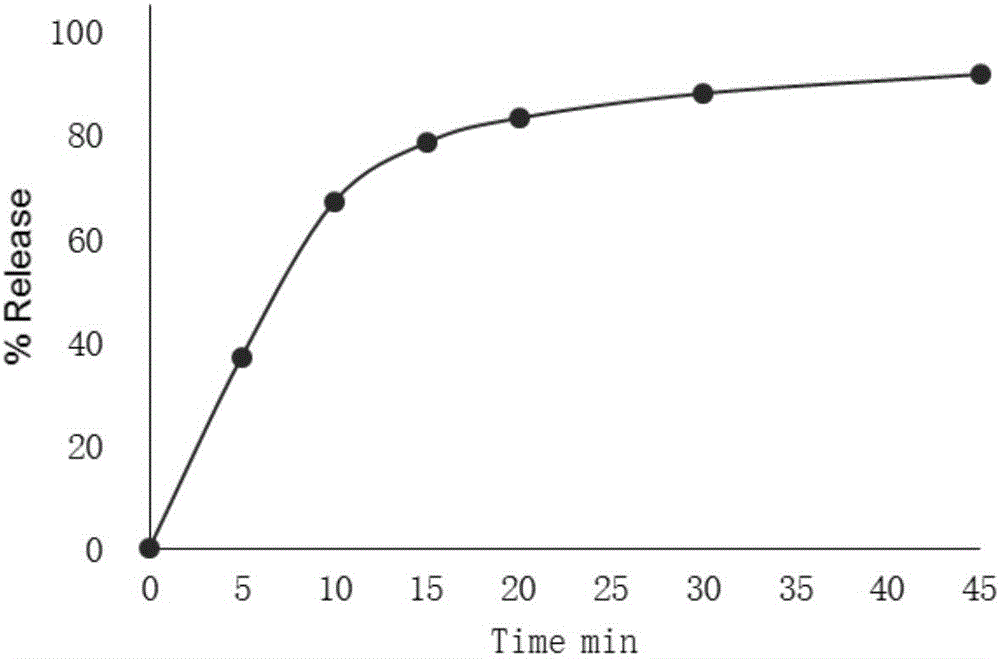
Embodiment 1
[0027] In this embodiment, pregelatinized starch is used as a filler, polyvinylpyrrolidone is used as a binder, croscarmellose sodium is used as a disintegrant, and magnesium stearate is used as a lubricant. In this example, every 2000 mycophenolate mofetil capsules include 1100g mycophenolate mofetil, 150g pregelatinized starch, 40g polyvinylpyrrolidone, 30g croscarmellose sodium, and magnesium stearate. 5g.
[0028] The raw and auxiliary materials are passed through an 80-mesh sieve. Take the above prescription amount of mycophenolate mofetil, pregelatinized starch, polyvinylpyrrolidone and croscarmellose sodium, and then add them to the wet granulator in turn, dry blend for 5-8 minutes, then transfer the mixed powder In the fluidized bed, set the inlet air temperature to 50~60℃ and the inlet air volume to 15~25m 3 / h, atomization pressure 0.1~0.2MPa, add purified water to granulate, measure the moisture after discharging, make the moisture of the granules drop below 2%, pass ...
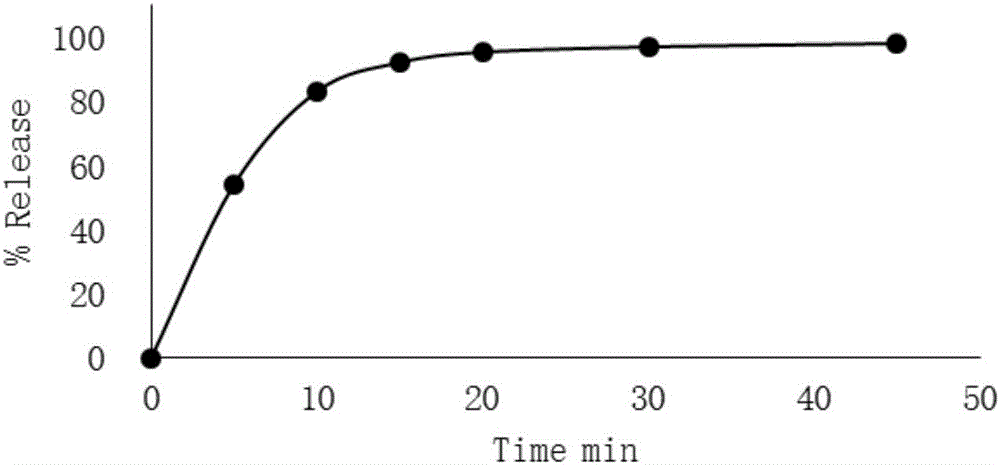
Embodiment 2
[0031] In this embodiment, corn starch is selected as the filler, hypromellose is selected as the binder, crospovidone is selected as the disintegrant, and magnesium stearate is selected as the lubricant. In this embodiment, every 2000 mycophenolate mofetil capsules include 1400g mycophenolate mofetil, 170g pregelatinized starch, 60g polyvinylpyrrolidone, 40g croscarmellose sodium, and 10g talc.
[0032] The raw and auxiliary materials are passed through an 80-mesh sieve. Take the above prescription amount of mycophenolate mofetil, cornstarch, hypromellose and crospovidone into the wet granulator in turn, dry blend for 5-8 minutes, then transfer the mixed powder to the fluidized bed , Set the inlet air temperature to 50~60℃, and the inlet air volume to 15~25m 3 / h, atomization pressure 0.1~0.2MPa, add purified water to granulate, measure the moisture after discharging, make the moisture of the granules fall below 2%, pass through a 30-mesh sieve, and add talc powder after the yie...
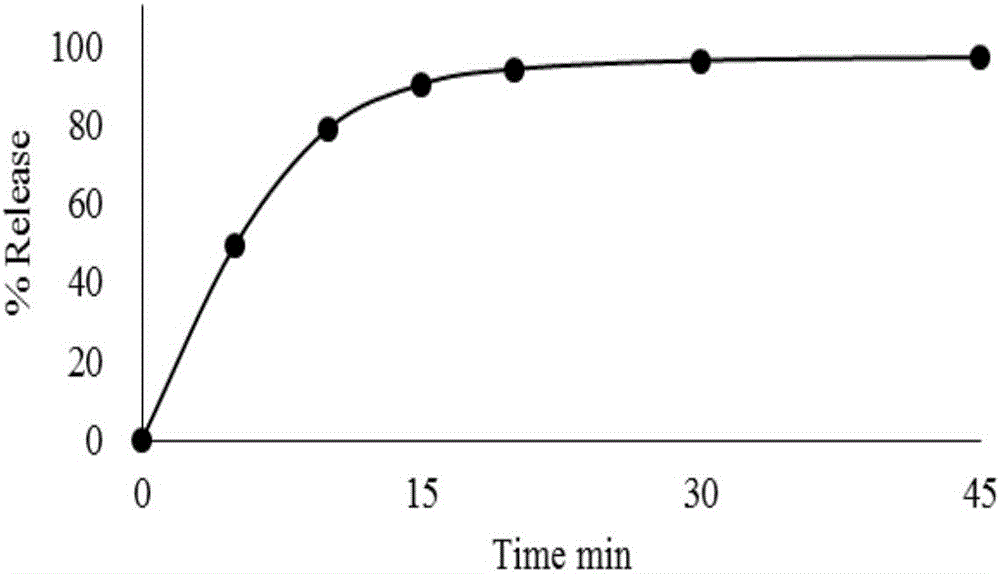
Embodiment 3
[0035] In this embodiment, lactose is used as a filler, maltose is used as a binder, sodium carboxymethyl starch is used as a disintegrant, and magnesium stearate is used as a lubricant. In this embodiment, every 2000 mycophenolate mofetil capsules include 1300g mycophenolate mofetil, 160g lactose, 50g maltose, 35g sodium carboxymethyl starch, and 8g magnesium stearate.
[0036] The raw and auxiliary materials are passed through an 80-mesh sieve. Take the above-mentioned prescription amount of mycophenolate mofetil, lactose, maltose and sodium carboxymethyl starch into the wet granulator in sequence, dry-mix for 5-8 minutes, transfer the mixed powder to the fluidized bed, set the air inlet The temperature is 50~60℃, the inlet air volume is 15~25m 3 / h, atomization pressure 0.1~0.2MPa, add purified water to granulate, measure the moisture after discharging, make the moisture of the granules drop below 2%, pass through a 30-mesh sieve, and then add magnesium stearate and mix Evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com