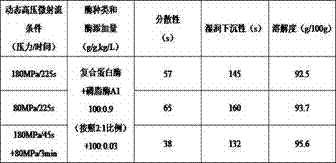
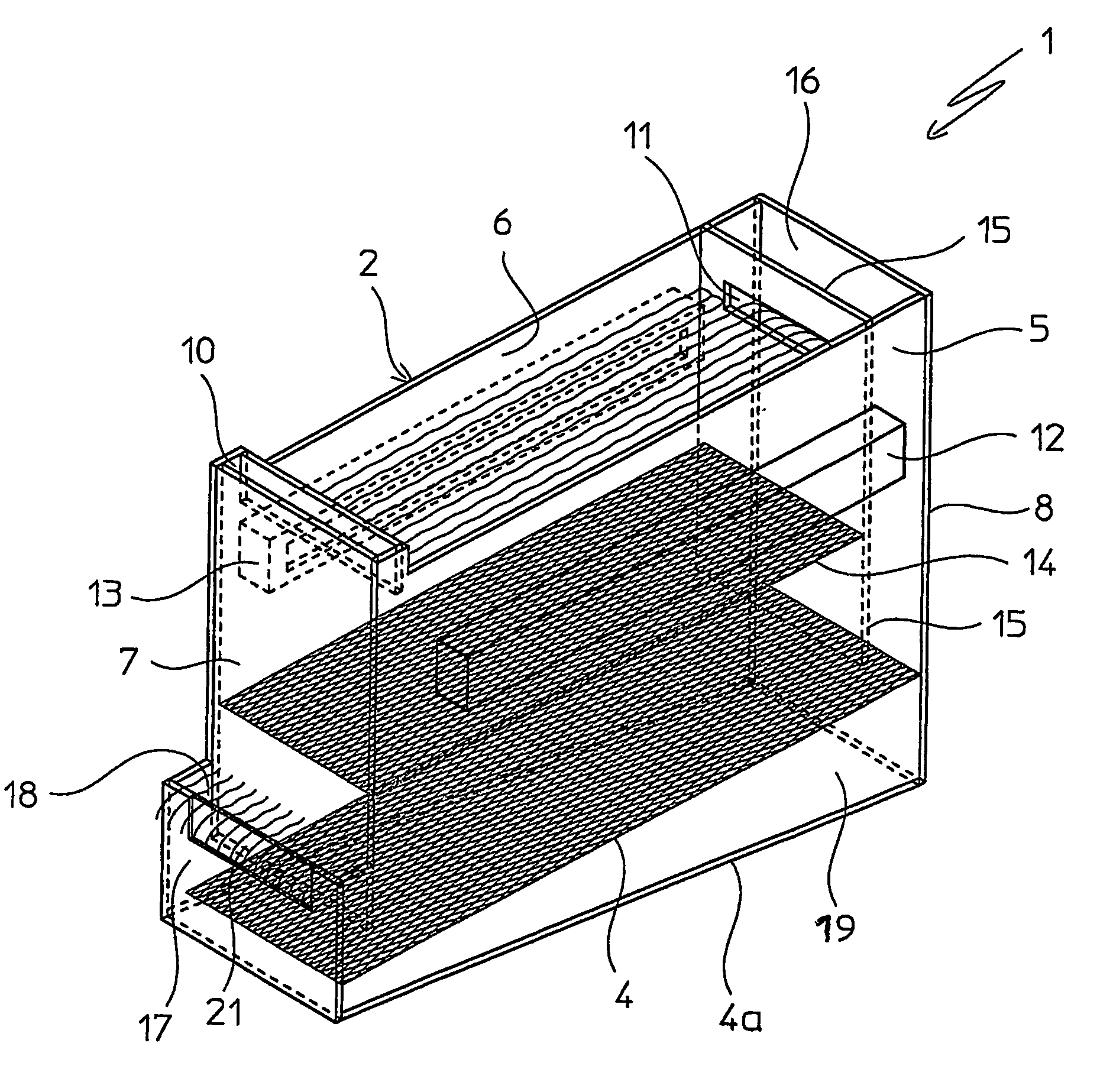
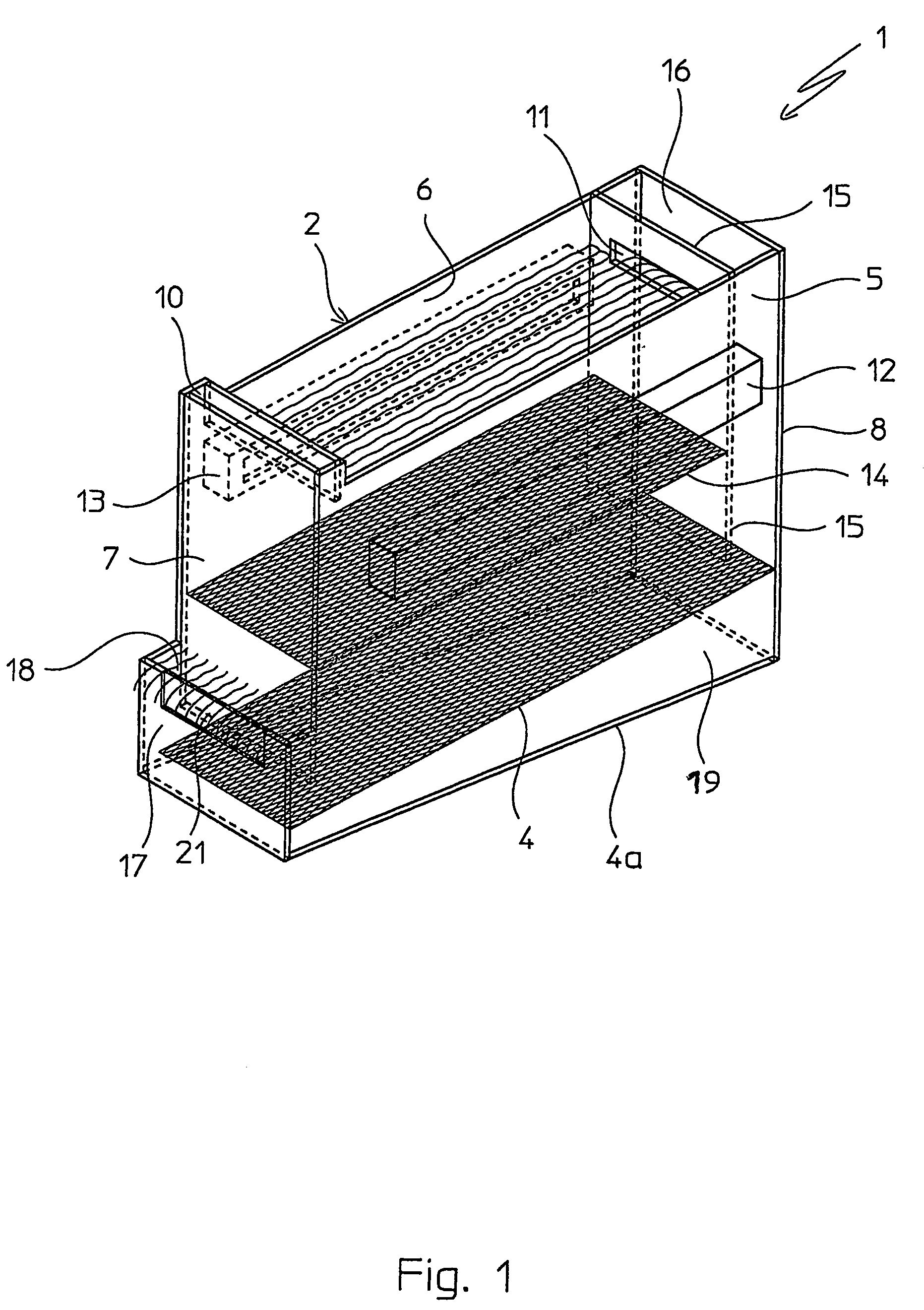
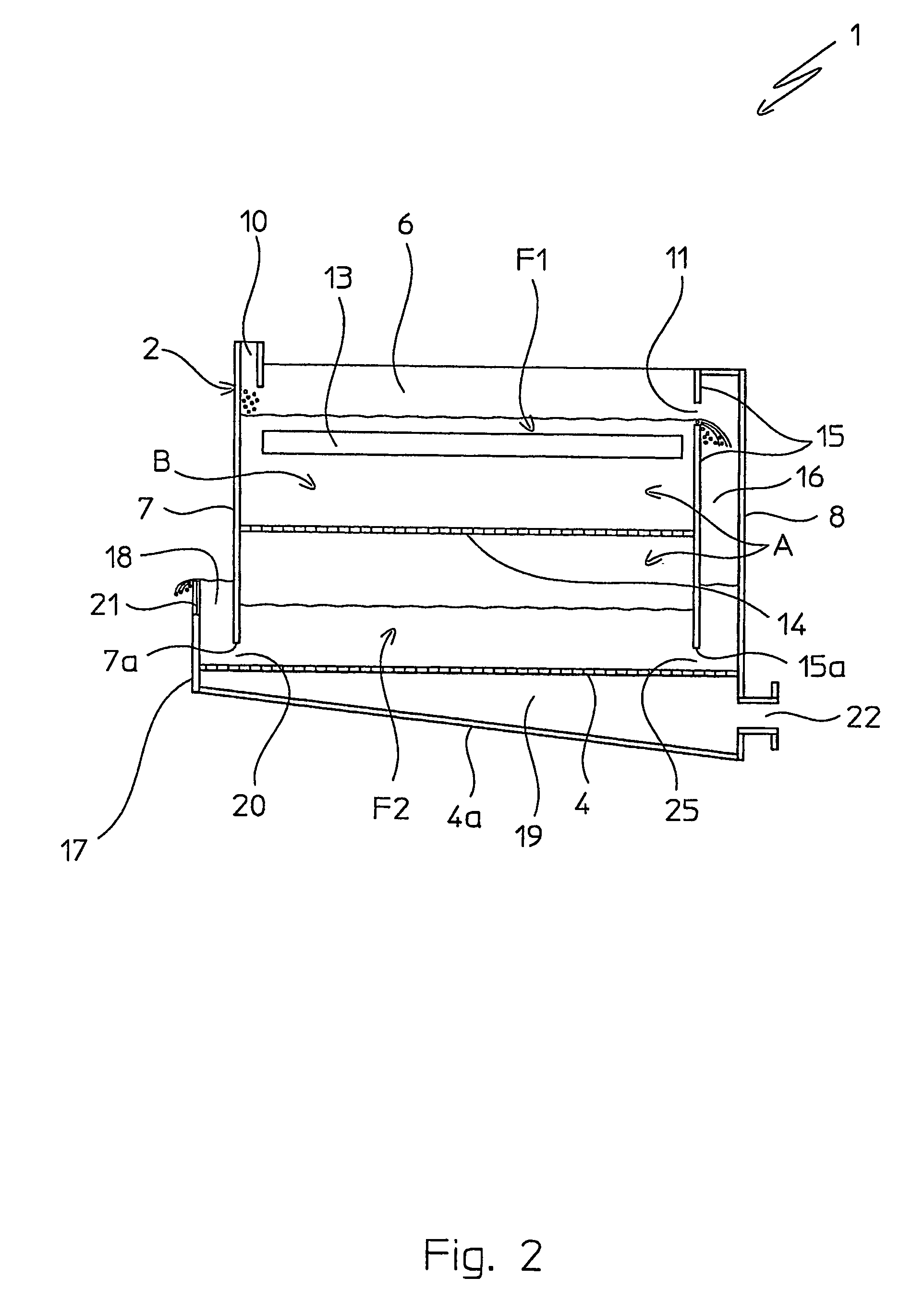
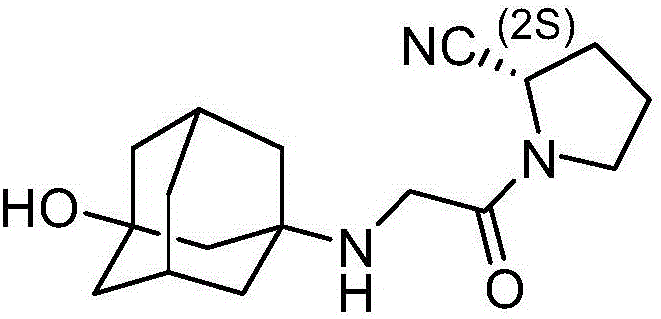
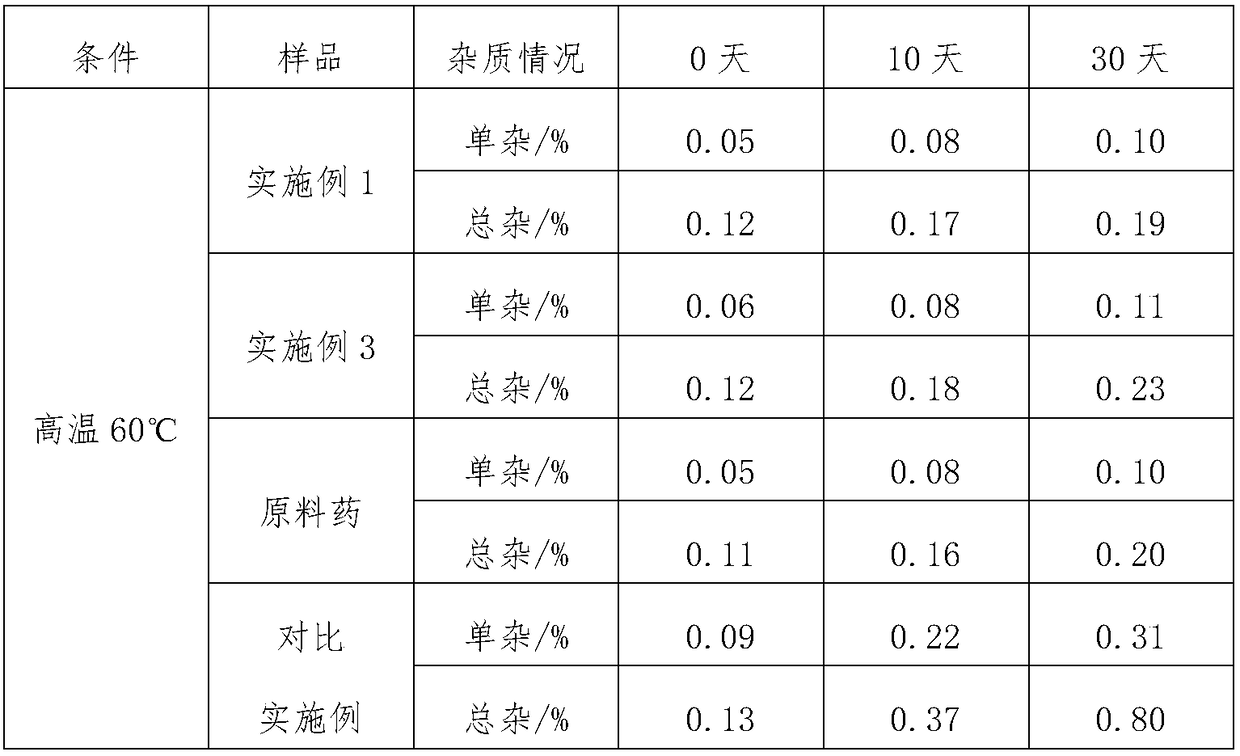
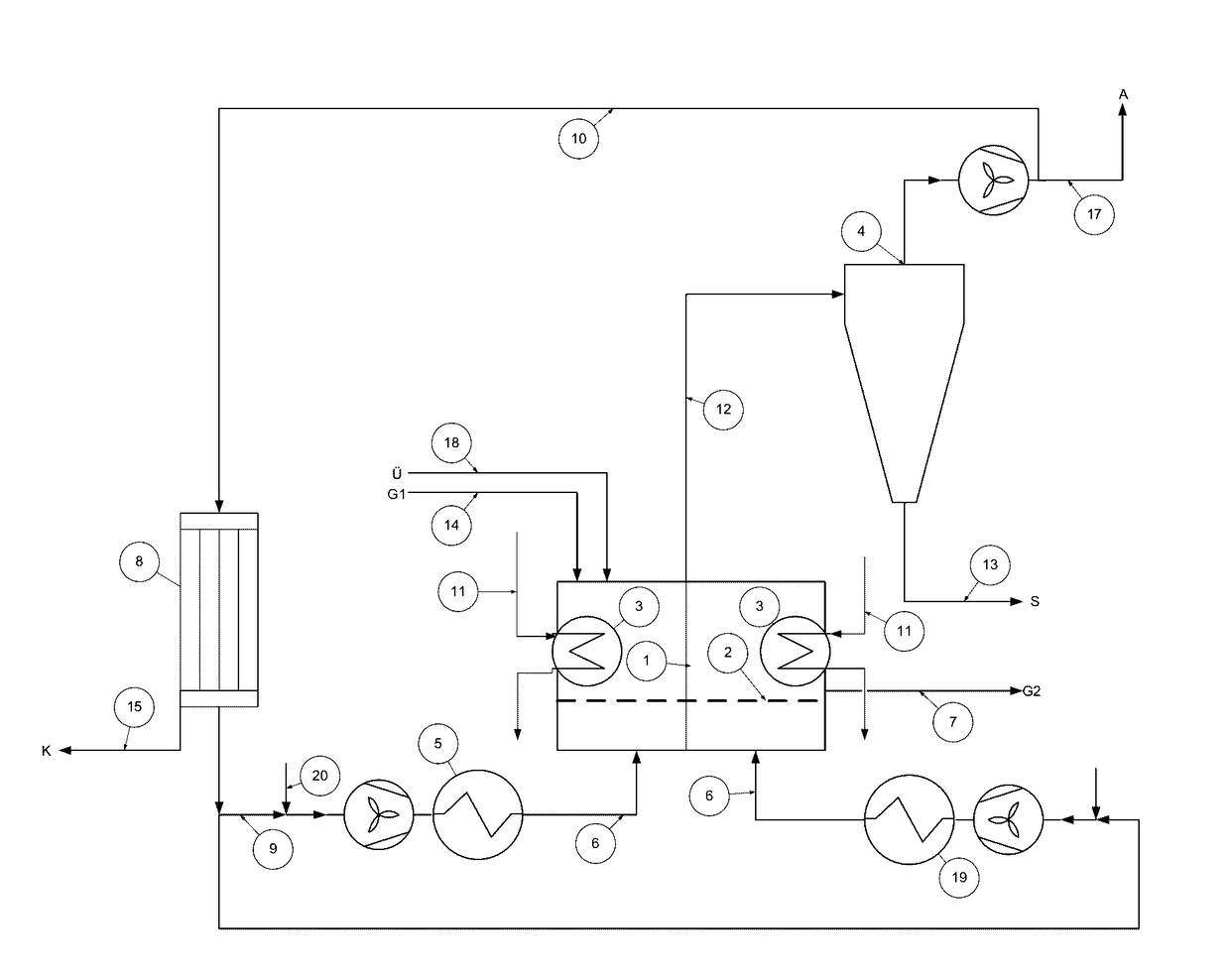
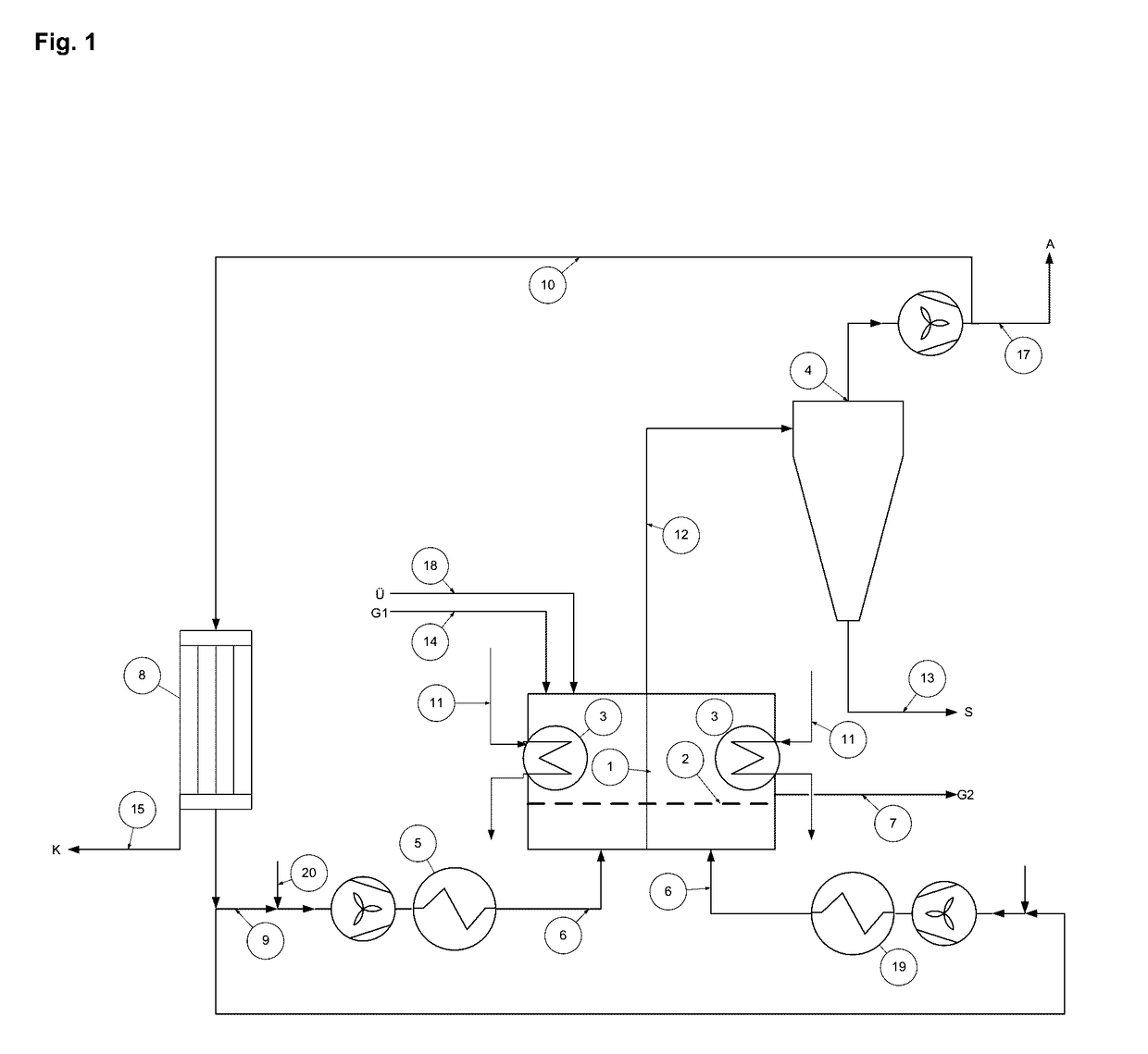
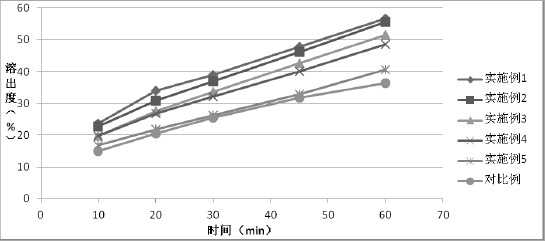
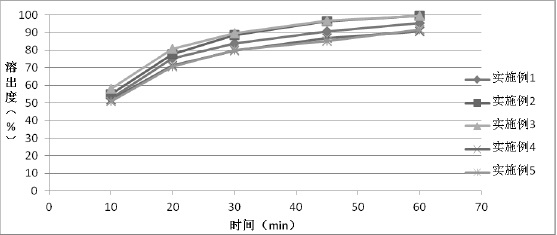
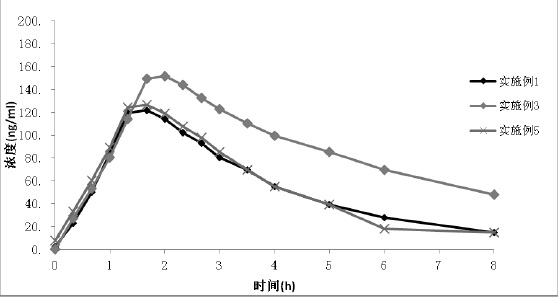
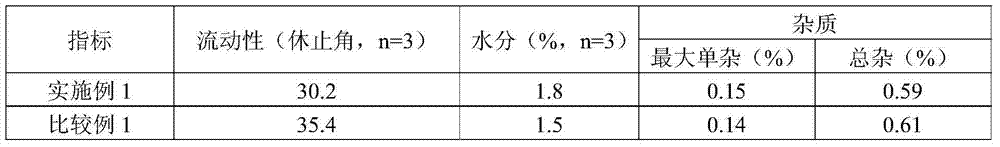
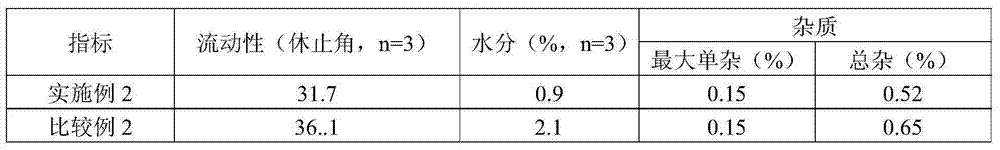
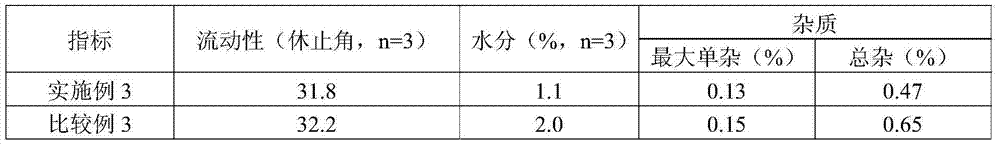
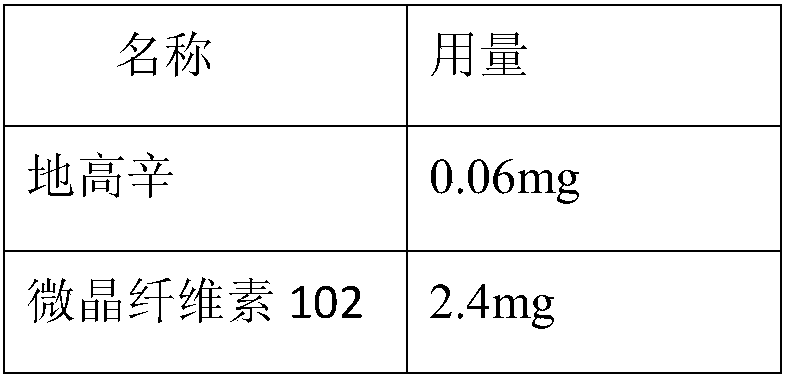
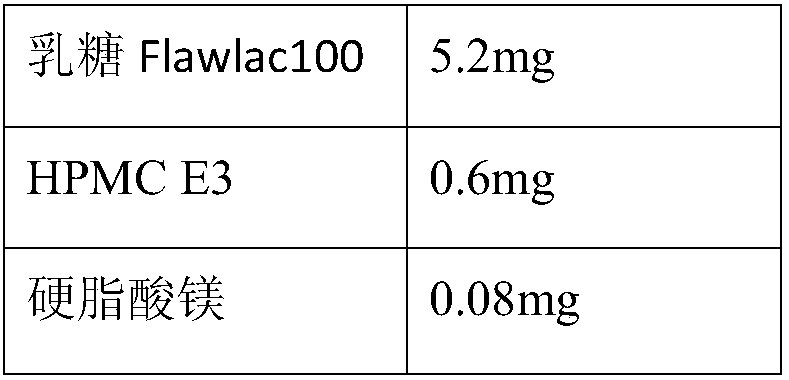
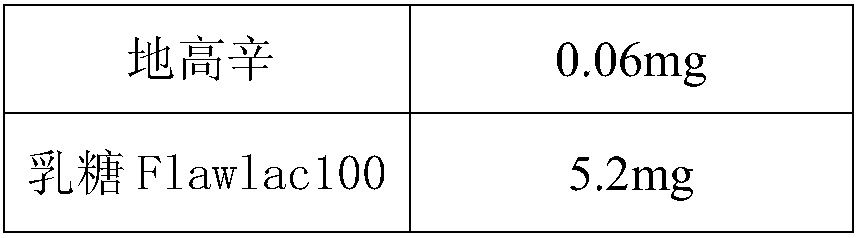
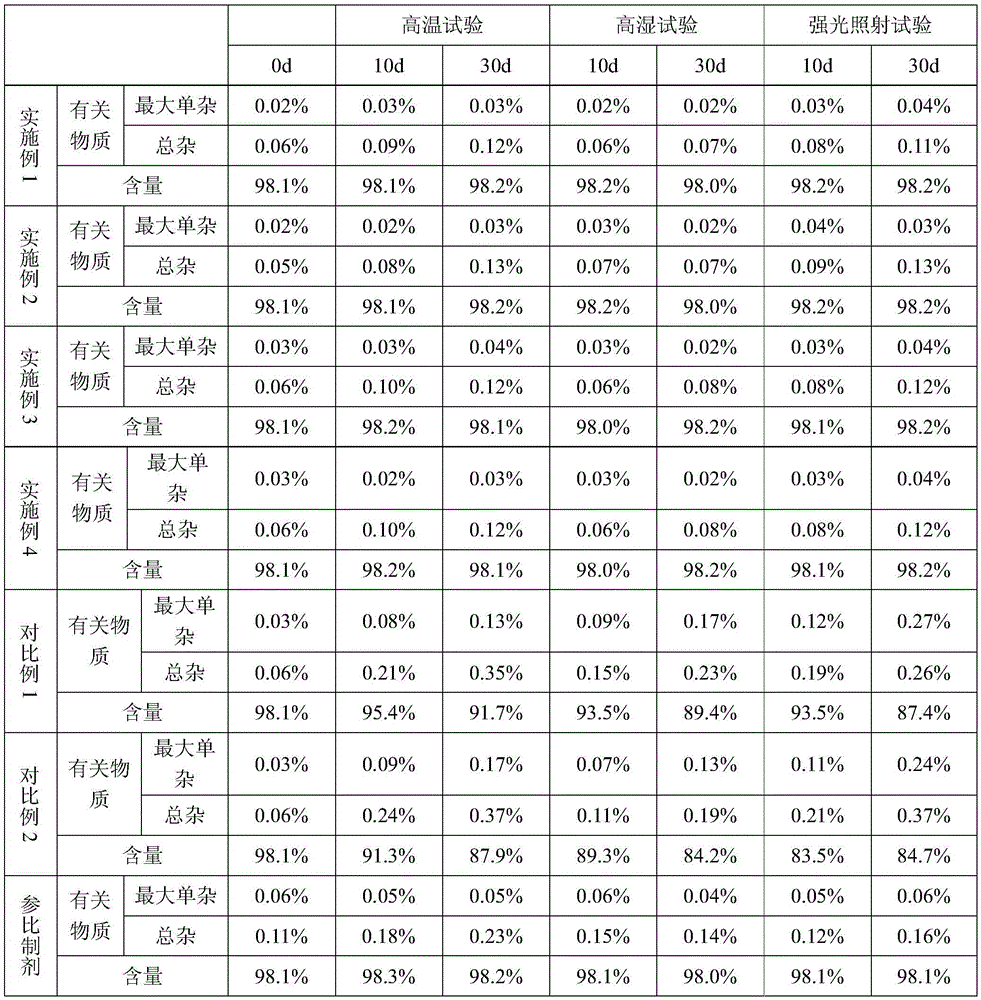
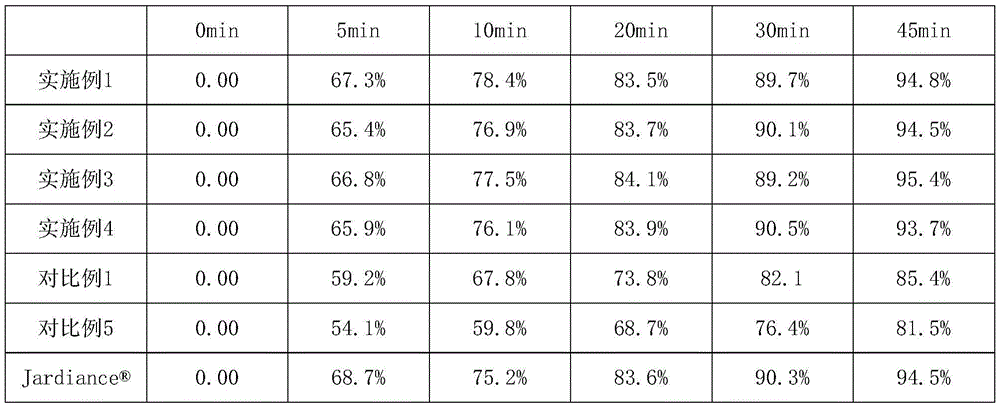
Patents
Literature
153 results about "Fluidized bed granulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beauveria bassiana granular formulation and preparation method thereof
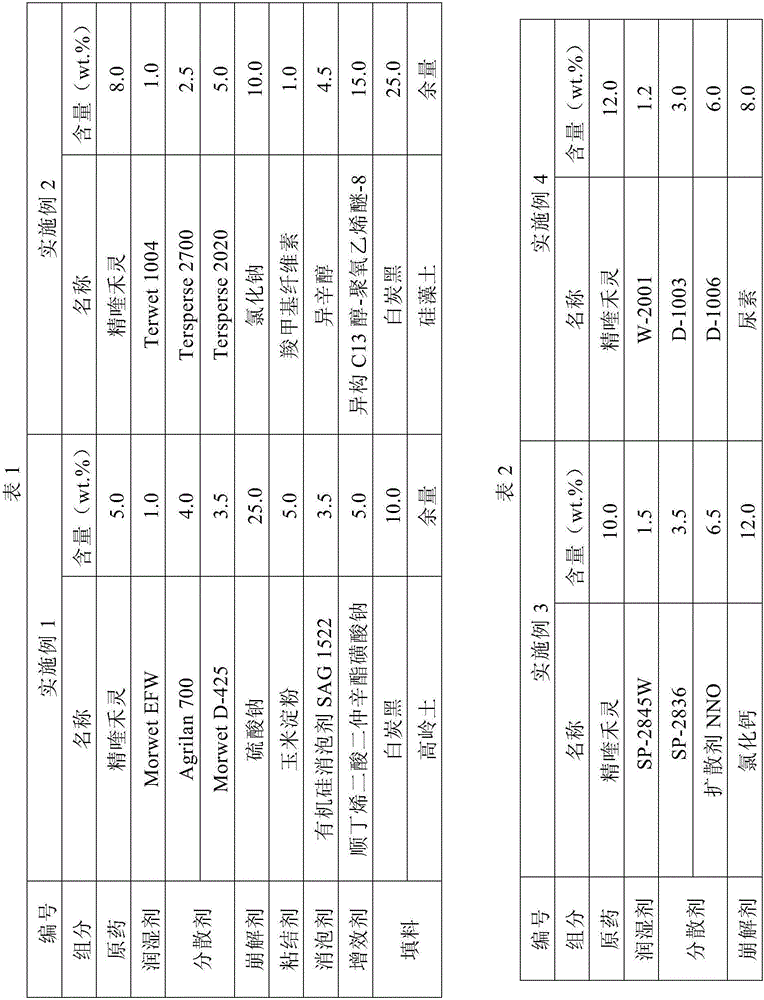
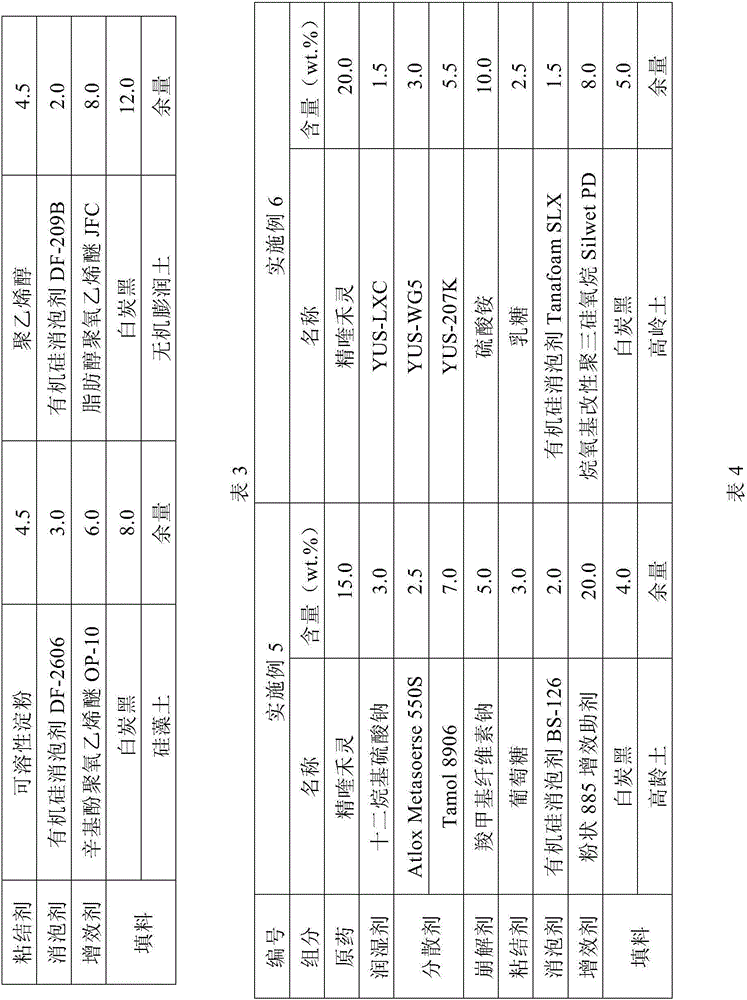
The invention relates to a white muscardine fungus granular formulation and a preparation method thereof. The white muscardine fungus granular formulation is prepared by the following compositions in mass percentage: 1 to 20 percent of white muscardine fungus spore powder, 70 to 95 percent of carrier, 0.05 to 3 percent of an adhesive, 0.5 to 5 percent of a disintegrant, 0 to 2 percent of a disintegration assistant, 0.01 to 1 percent of a dispersant, 0.5 to 5 percent of an anti-ultraviolet agent, 0 to 3 percent of a lubricating agent, and 0 to 2 percent of a coloring agent. The preparation of the white muscardine fungus granular formulation can adopt an extrusion molding granulation method, a fluidized bed granulation method and a coating granulation method. The white muscardine fungus granular formulation has the obvious characteristics that the storage period of white muscardine fungi is between 10 and 12 months, and the white muscardine fungus granular formulation can be directionally and accurately applied, is particularly applicable to killing underground pests, is convenient to pack, store, transport and use, does not pollute the environment, and can also control the release rate of the white muscardine fungi and prolong the lasting period.
Owner:ANHUI AGRICULTURAL UNIVERSITY
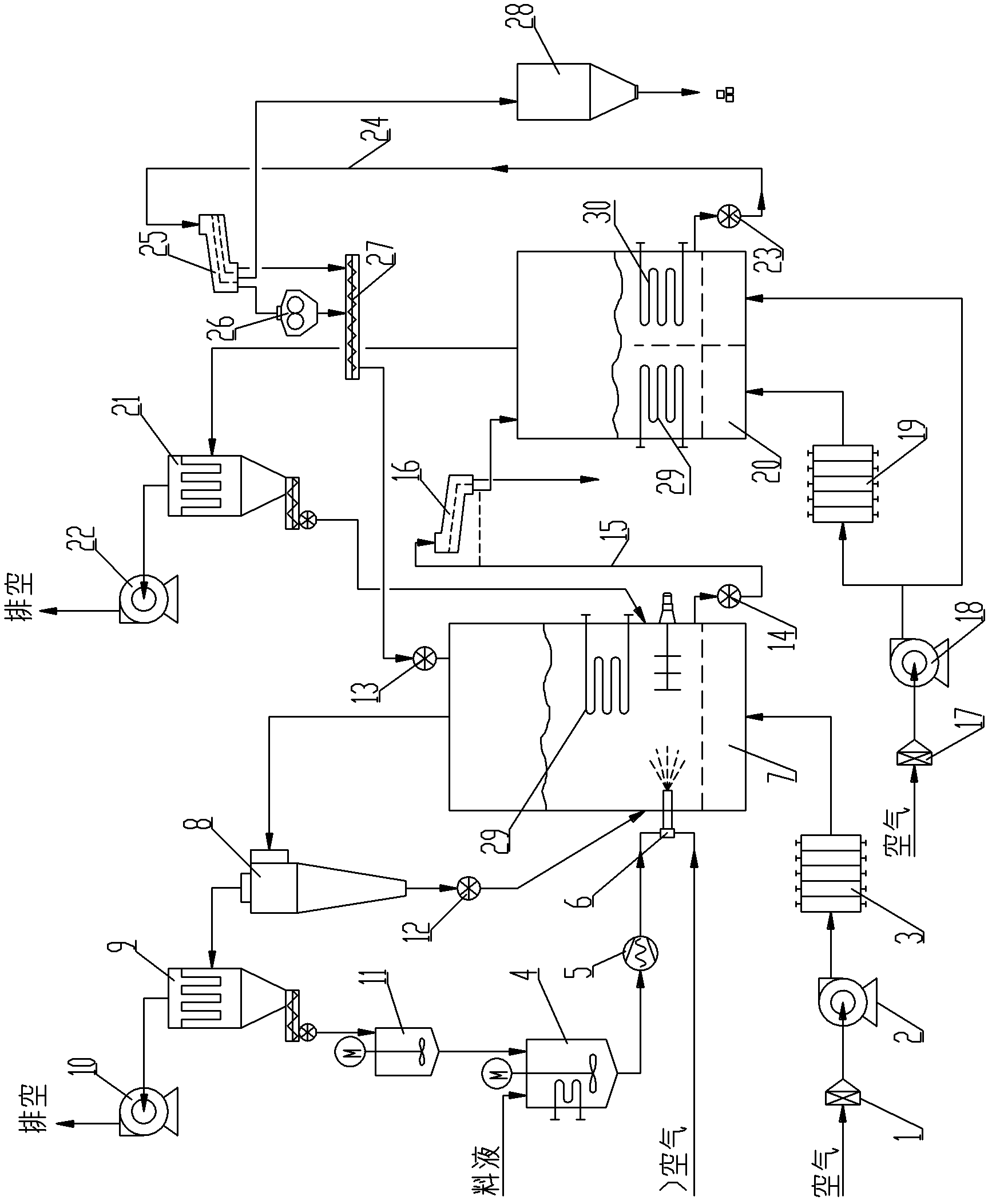
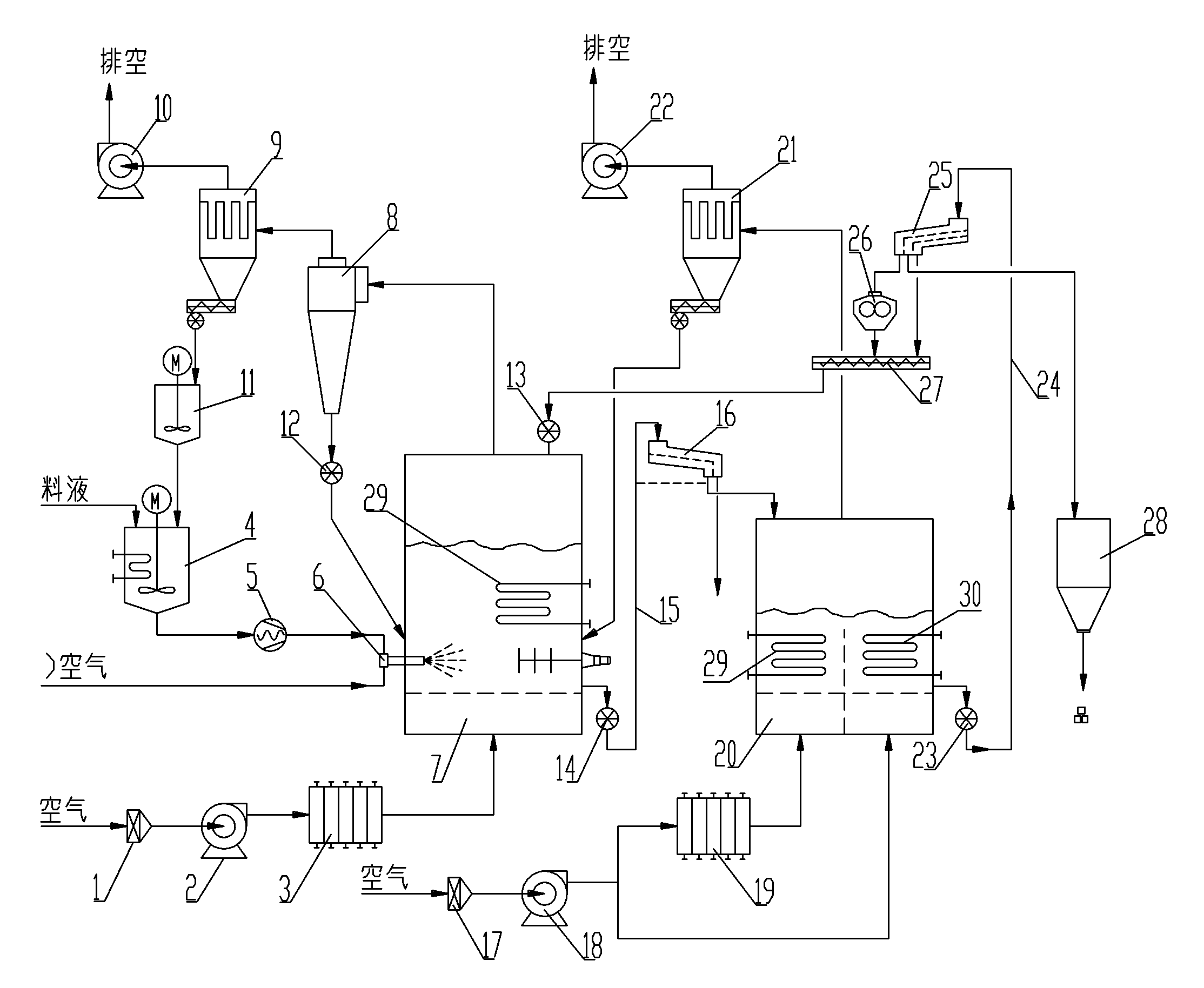
Large-scale continuous energy-saving fluidized bed spray granulation drying process
InactiveCN102274703AAdaptableNo pyrolysis problemsDrying solid materials with heatGranulation by liquid drop formationPrillEngineering
The invention discloses a large-scale continuous energy-saving fluidized bed spray granulation drying process, which belongs to the granulation drying technology. The surface of the seed crystal particles in the granulation fluidized bed is dried under the combined heating of the hot air and the built-in heater. The discharged materials are first screened out of lumps and oversized particles, and then sent to the cooling fluidized bed After the discharged materials are screened, the materials larger than the qualified particle size are crushed by the crusher, and then returned to the granulation fluidized bed together with the material smaller than the qualified particle size as crystal seeds for re-granulation, and the qualified particle size is sent to the into the finished product silo. The invention has strong material adaptability, can obtain granular products with large specific gravity, high strength, good sphericity, uniform particle size, and is not easy to absorb moisture, and realizes continuous large-scale production. Pollution and other characteristics.
Owner:山东奥诺能源科技股份有限公司
Preparation method of Levamlodipine besylate tablet
InactiveCN102846565AHigh dissolution rateConducive to the amount of controlOrganic active ingredientsPill deliveryLevamlodipineDissolution
A preparation method of a Levamlodipine besylate tablet includes pulverizing Levamlodipine besylate, a filler and a disintegrant, sieving, mixing, adding a lubricant, granulating, and tableting. The invention reduces the amount of related substance of the Levamlodipine besylate tablet, and improves stability. The method uses a fluidized bed granulation step to prepare the Levamlodipine besylate tablet, so as to simplify preparation step, shorten time, optimize process parameter, significantly increase the dissolution of the product and improve quality. The inventive method is suitable for industrial production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Process for fluidized bed granulation of amino acid-containing fermentation broths
ActiveUS20150283527A1Reduce disadvantagesOperated economicallyAnimal feeding stuffAccessory food factorsFluidized bed dryingAmino acid fermentation
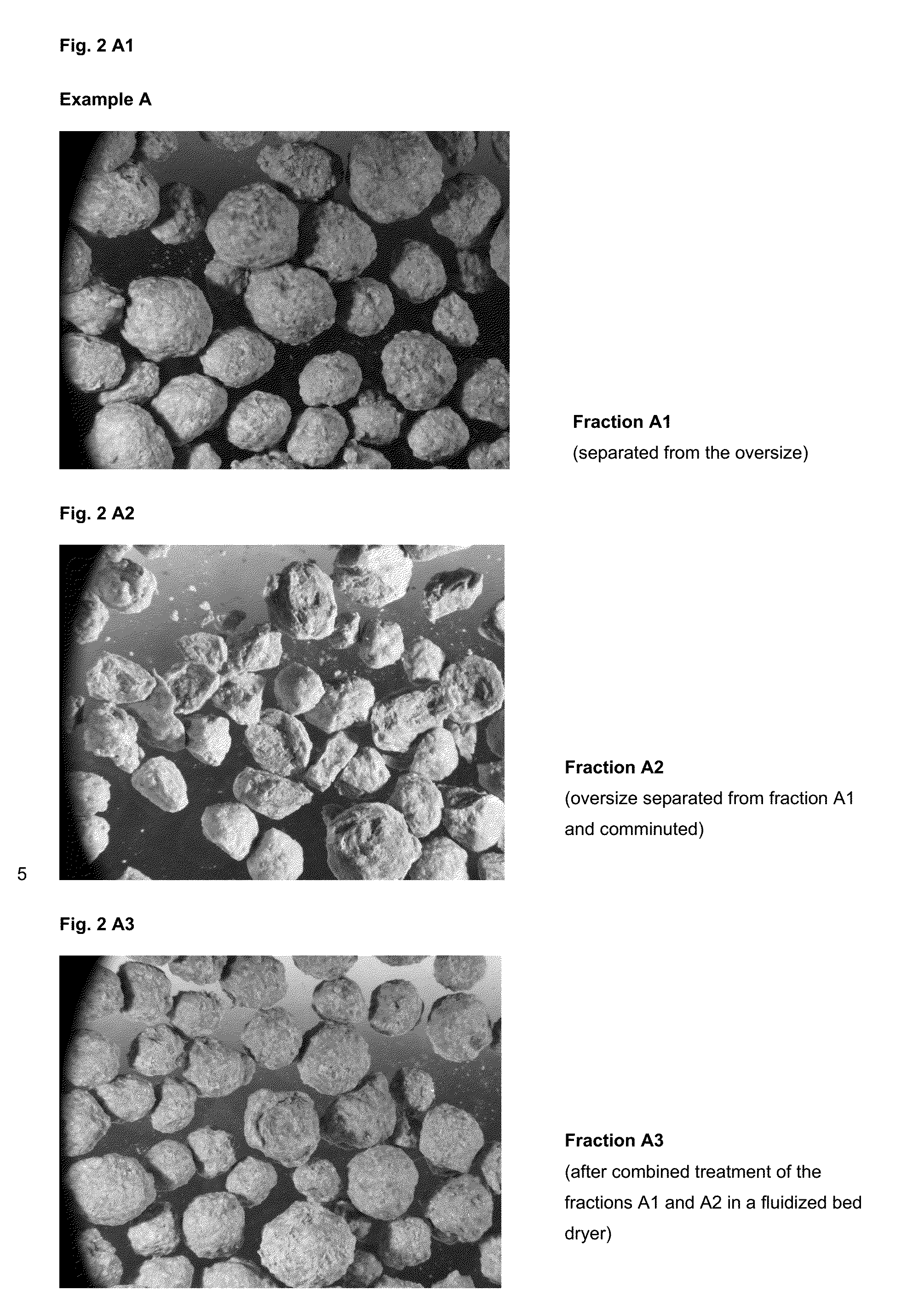
Process for fluidized bed granulation of amino acid-containing fermentation broths comprising the stepsIntroduction of a drying gas with a temperature of 100° C.-450° C. into the fluidized bed granulation chamberSpraying of the amino acid-containing fermentation broth into the fluidized bed granulation chamberDischarge of the granules granulated in the fluidized bed granulation chamber with the drying gas stream, anddrying of the discharged granules in a fluidized bed drying step,wherein the discharged granulated granules are a granule mixture with various particle sizes and contains an oversize fraction, wherein the oversize comprises the particle sizes which lie above a desired particle size, an wherein the oversize fraction is removed from the discharged granule mixture and then comminuted and the comminuted oversize and the granule mixture from which the oversize was separated are fed into the fluidized bed drying step.
Owner:EVONIK OPERATIONS GMBH
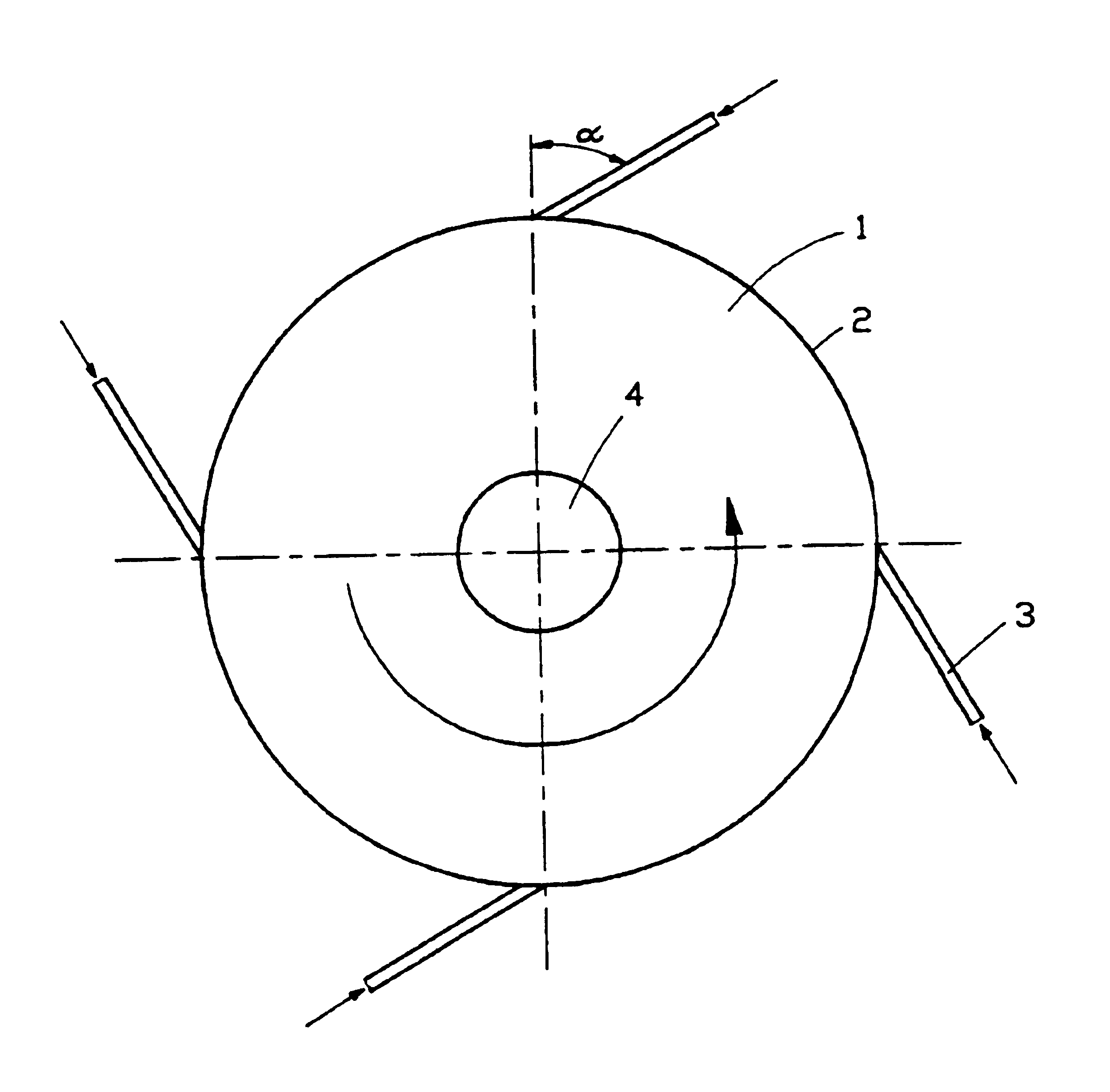
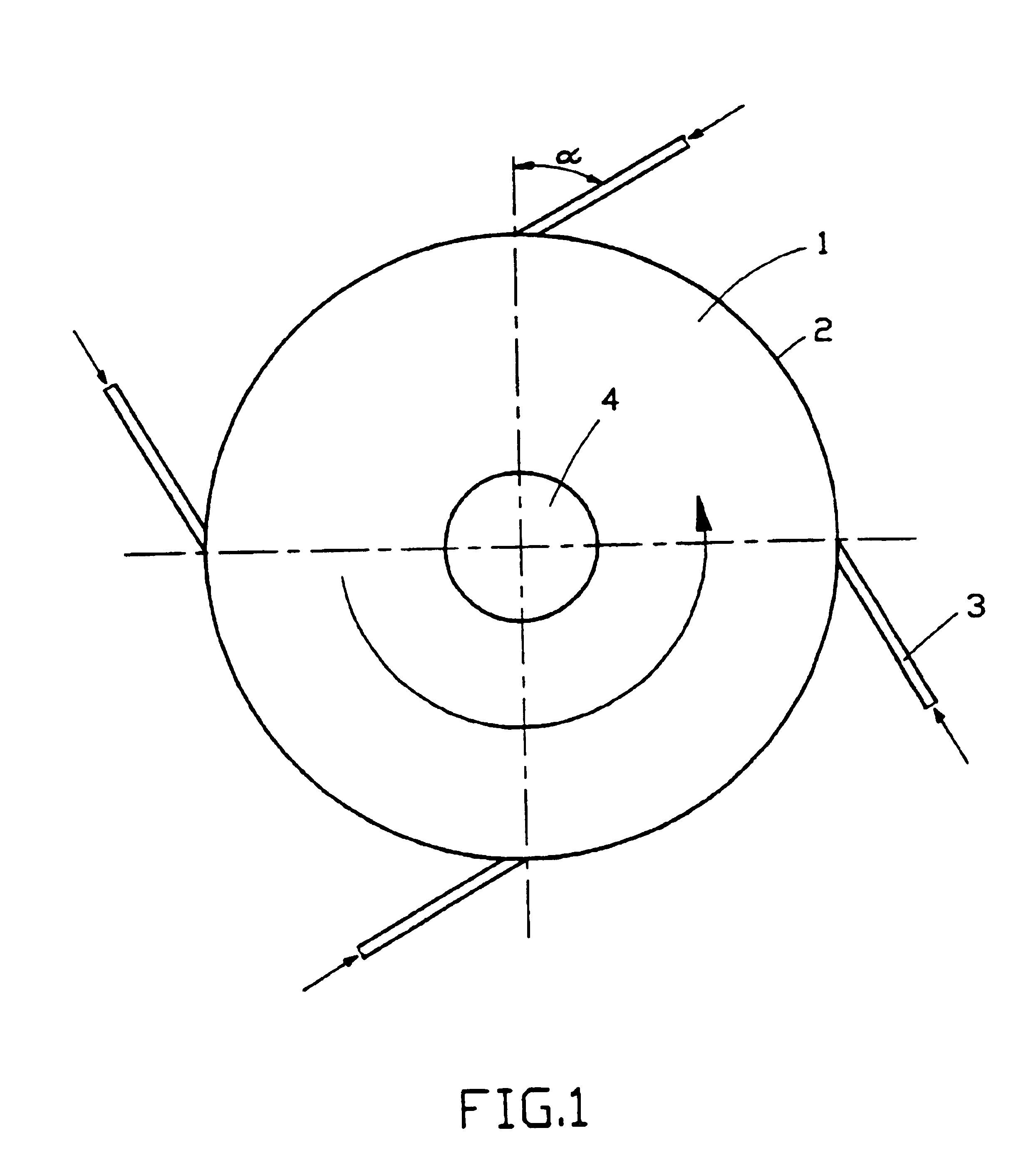
Apparatus for on-line detection of particle properties during fluidized-bed granulation
ActiveCN107870138AImprove monitoring effectEasy to understandParticle size analysisPermeability/surface area analysisPorosityApparent density
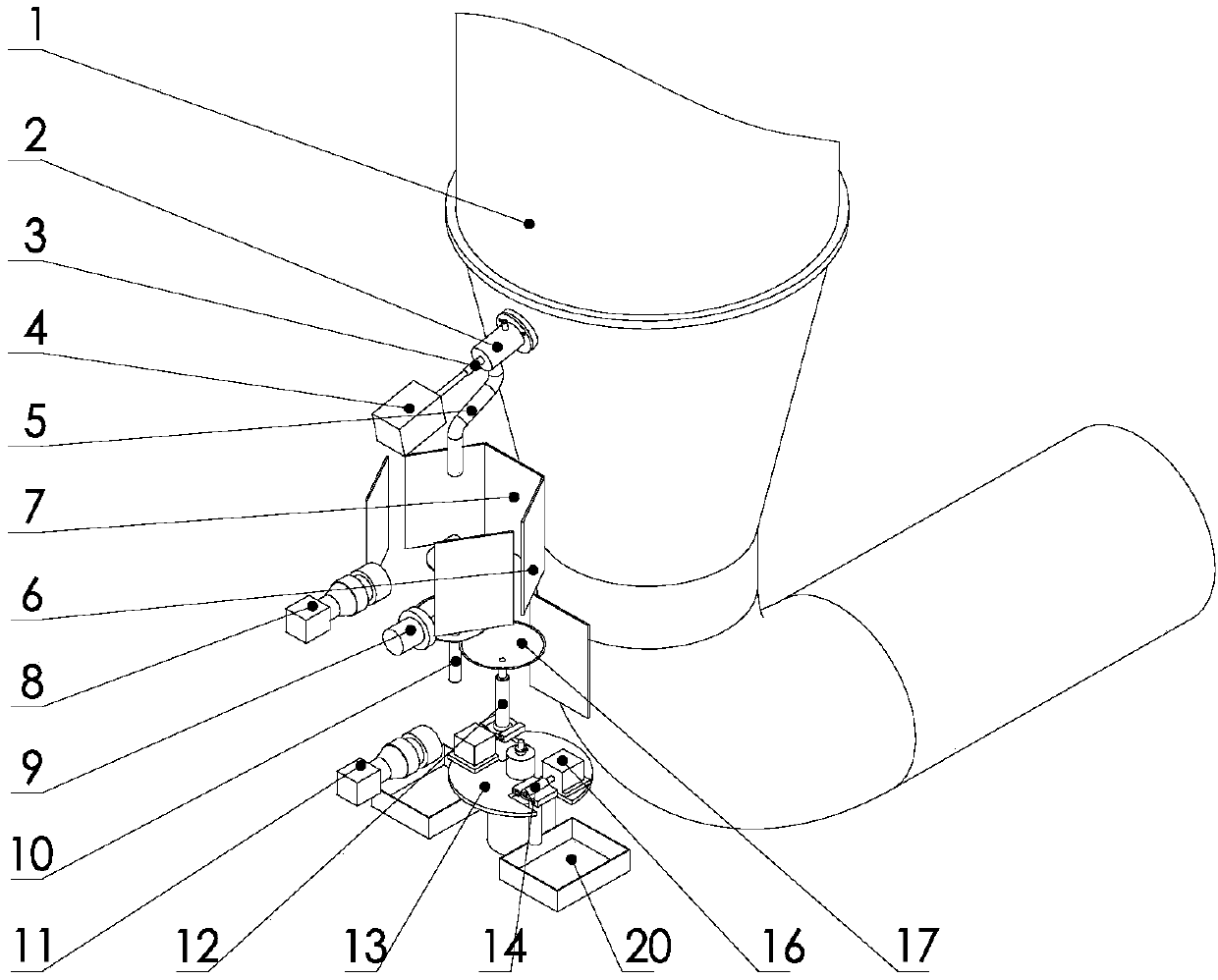
The invention provides an apparatus for on-line detection of particle properties during fluidized-bed granulation. The apparatus comprises a hermetic shell, a sample acquisition mechanism, a first visual inspection unit, a second visual inspection unit and an image processing system, wherein the first visual inspection unit comprises a first camera, a circular tray and a pressure transducer; the image processing system acquires the particle size information of particles from a falling image of a sample and acquires the angle alpha of repose from an image of a sample accumulation; the second visual inspection unit comprises a measuring cylinder, a measuring-cylinder fixing plate, a light source, a vibrating table and a second camera; and the image processing system acquires the apparent density rho of the particles from the image of the sample in the measuring cylinder before starting of the vibrating table and acquires the tap density rho<t> of the particles from the image of the sample in the measuring cylinder during vibration of the vibrating table. The apparatus provided by the invention can realize on-line detection of the particle size distribution, repose angle, apparentdensity, tap density, porosity and Hausner ratio of the particles during fluidized-bed granulation.
Owner:ZHEJIANG UNIV
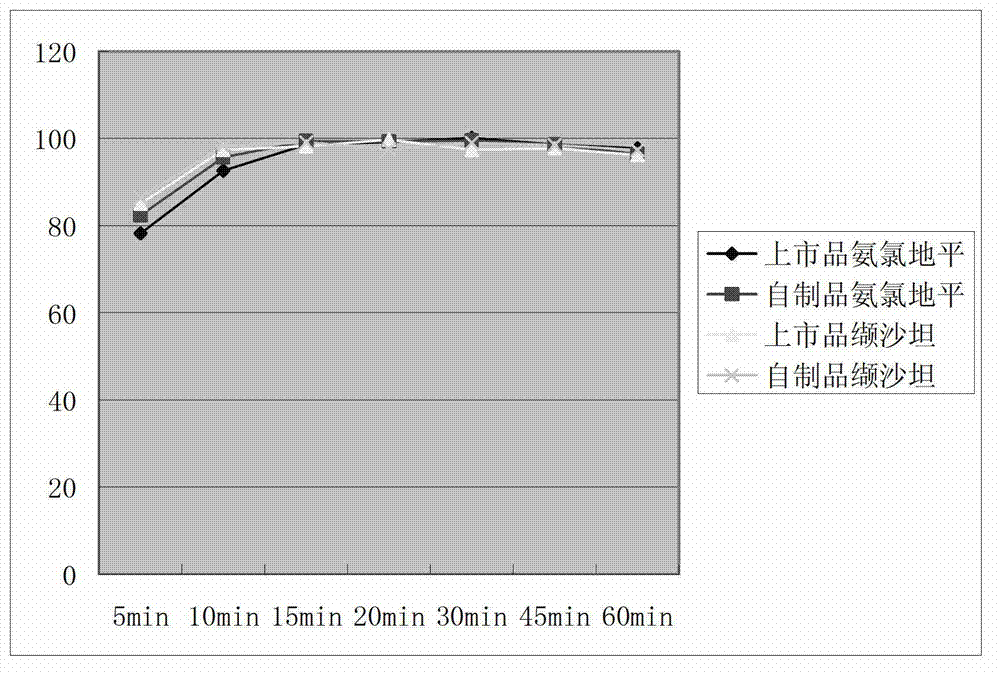
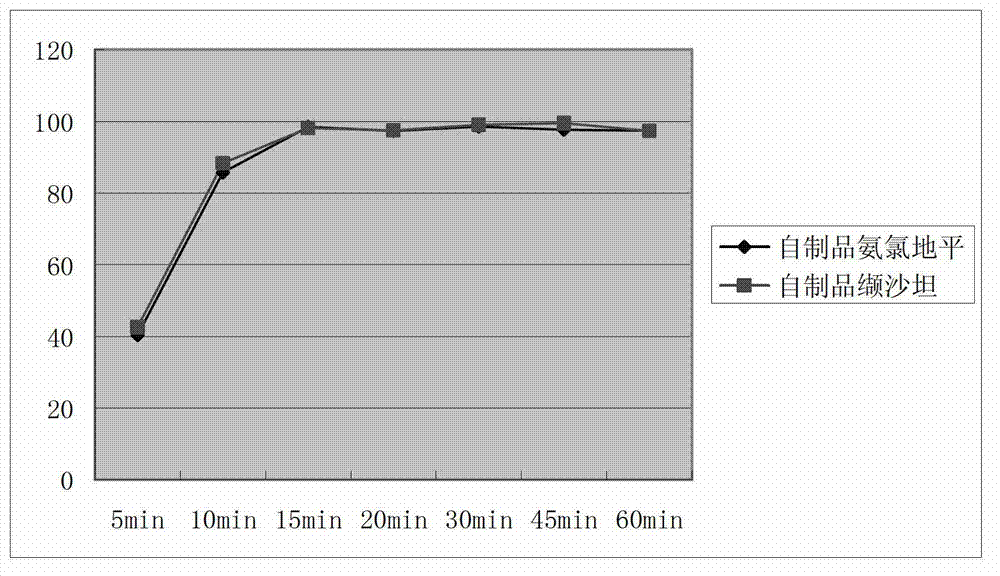
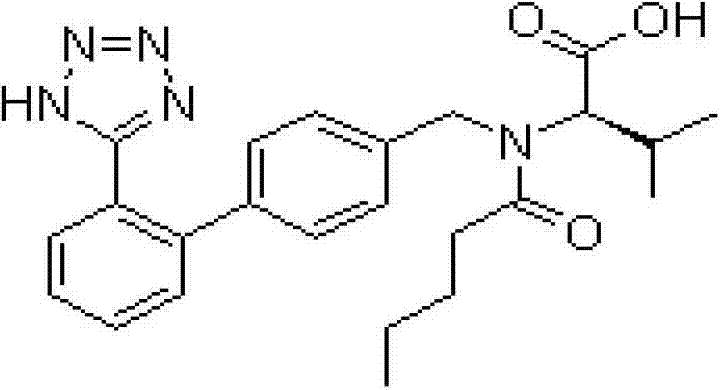
Preparation process of compound valsartan amlodipine solid preparation
ActiveCN103083319AImprove stabilityExtended shelf lifeHeterocyclic compound active ingredientsCardiovascular disorderPelletizingChemistry
The invention belongs to the field of medical technology, and particularly relates to a compound valsartan amlodipine solid preparation, and a preparation process as well as application of the compound valsartan amlodipine solid preparation. The preparation process comprises the following steps of: 1) dissolving amlodipine in an ethanol aqueous solution by adopting a fluidized bed pelletizing technology, atomizing and spraying the solution on a pharmaceutically acceptable auxiliary material which includes the valsartan so as to prepare medicine carrying particles containing the two main medicines; and 2) crushing the valsartan and sieving by using a 200-mesh sieve to increase the density of the raw material.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Method for preparing entecavir tablets
InactiveCN102416003ASolve the uniformity problemImprove practicalityDigestive systemAntiviralsDrug contentMedicine
The invention discloses a method for preparing entecavir tablets, which comprises: preparing an auxiliary material for later use by uniformly mixing a filling agent, a disintegrating agent and a bonding agent in formula amount; dispersing entecavir in a formula amount into injection water to obtain a main medicine for later sue; and uniformly spreading the main medicine into the auxiliary material, adding lubricating agent, uniformly mixing, measuring the content of an intermediate, tabletting and obtaining entecavir tablets. The preparation of the entecavir tablets adopts a spray gun to spray the main medicine into the pre-mixed medicinal auxiliary material, so the uniformity problem of the tablets under a condition of low medicine content is solved. In addition, as the low-water-content microcrystalline cellulose PH-200LM as the filling agent of the entecavir tablets, tabletting can be performed without using a drying process; and the direct tabletting process is simpler and easier to implement than a wet-process granulation, fluidized bed granulation and spray drying processes and other processes, is suitable for industrial production and favorable for product quality control, energy conservation and consumption reduction, can reduce production cost and has high practicality.
Owner:NANJING YOKO PHARMA GRP CO LTD
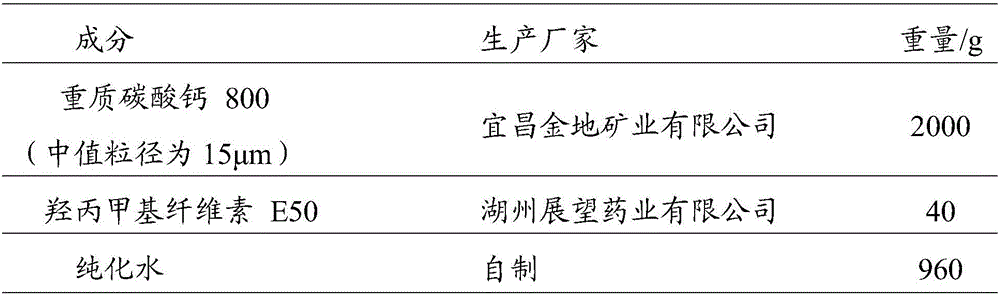
Calcium carbonate particles and preparation method thereof
InactiveCN106389344AReduce flyingReduce the incidence of pneumoconiosisMetabolism disorderConfectionerySucroseMethyl cellulose
The invention relates to calcium carbonate particles and a preparation method thereof. The preparation method comprises the following steps: dissolving or dispersing an adhesive into a solvent to obtain adhesion liquid which is selected from at least one of hydroxypropylmethyl cellulose, high-substituted hydroxyproxyl cellulose, polyvinylpyrrolidone, sucrose, starch, sodium carboxymethylcellulose, methyl cellulose and ethyl cellulose; fluidifying calcium carbonate in a fluidized bed and preheating, wherein the calcium carbonate is ground calcium carbonate of which the median size is 0.1-20 mu m, and the mass ratio of the calcium carbonate and the adhesive is 10-50:1; spraying the adhesion liquid on the preheated calcium carbonate, and pelletizing on the fluidized bed and drying; and granulating to obtain the final product. The calcium carbonate particles which are prepared by the preparation method are good in fluidity, good in compressibility and good in firmness, and can be directly used for vertical tabletting; other filler auxiliary materials are not required to be added during tabletting; and the pressed tablets are small in size and are orally taken conveniently.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Medicinal composition containing cefaclor particles, and preparation method and application thereof
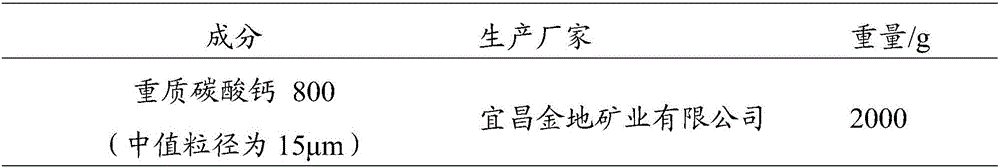
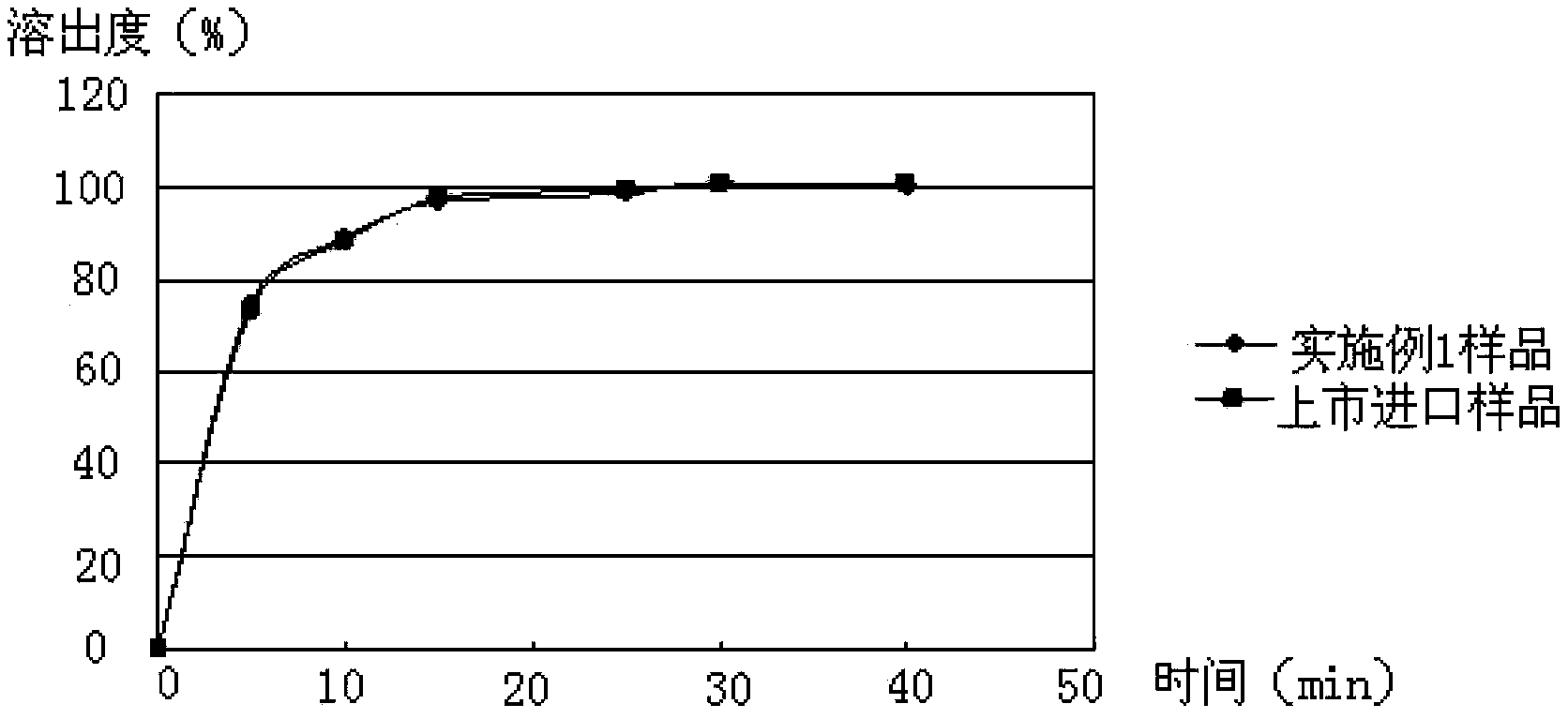
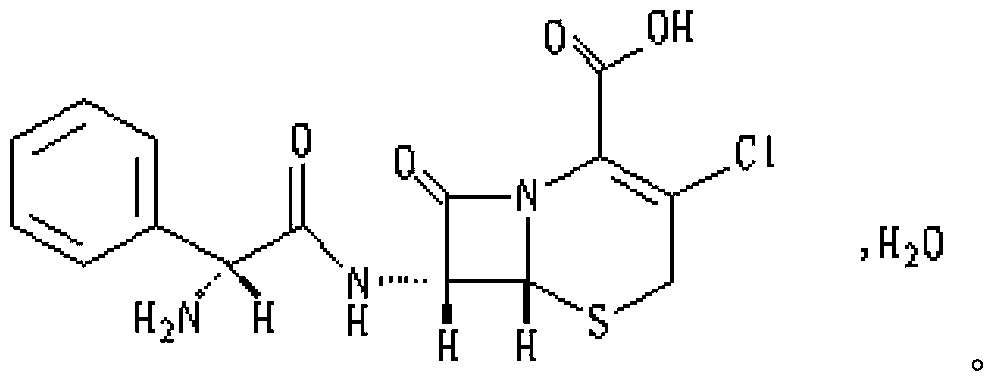
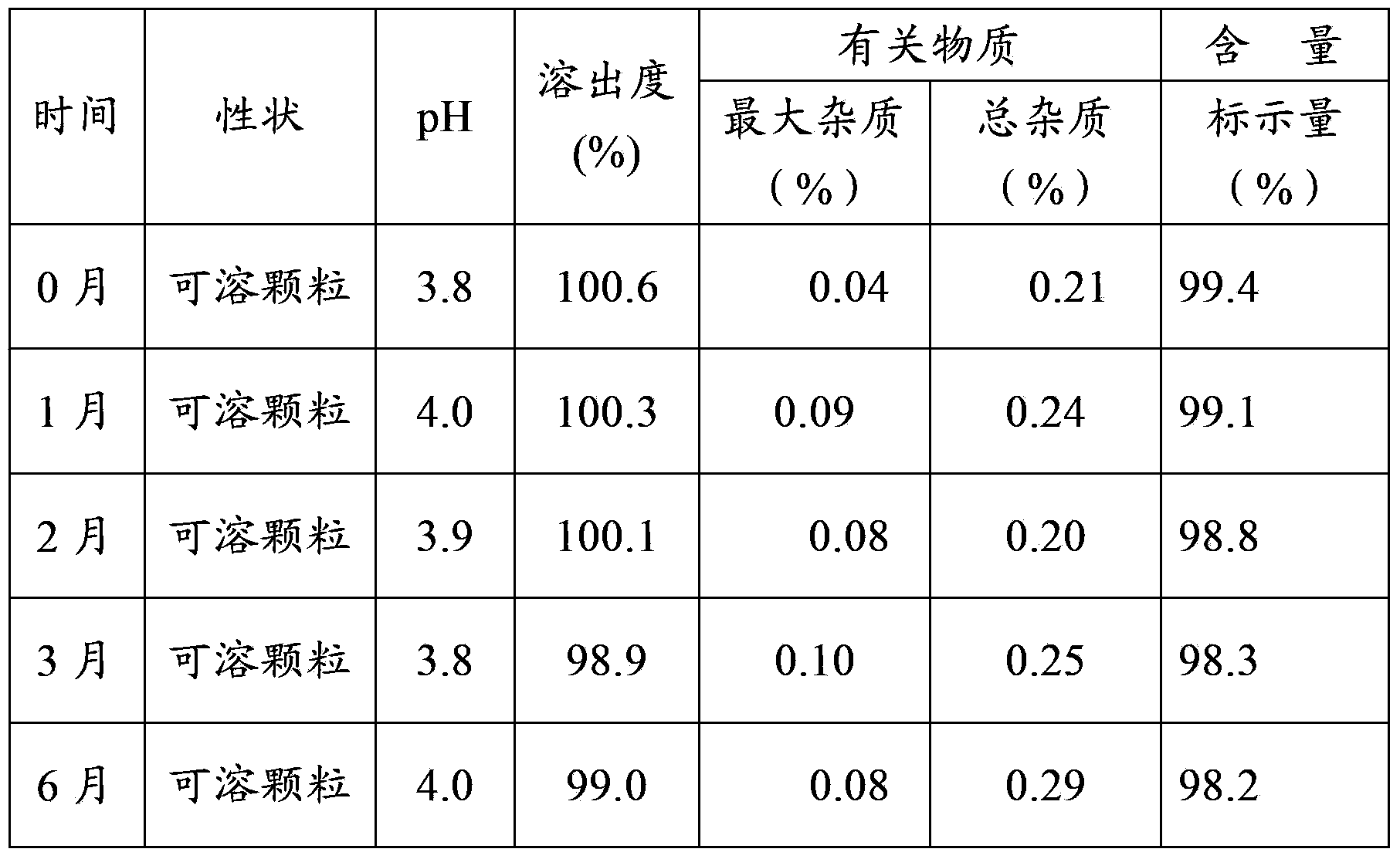
ActiveCN103349646AAvoid breakingShorten production timeAntibacterial agentsOrganic active ingredientsFormularyAqueous solubility
The invention provides a medicinal composition containing cefaclor particles, and a preparation method and an application thereof. The preparation method which adopts a fluidized bed preparation technology to prepare the medicinal composition comprises the following steps: burdening materials preprocessed by a 80 mesh sieve according to a specific formula, placing the obtained mixture in a fluidized bed granulator, and carrying out intake mixing; and rising the intake temperature, spraying an adhesive solution, and mixing and drying under a continuous intake condition to obtain cefaclor particles. The preparation method of the cefaclor particles, which is a one step granulation method, substantially shortens the production time; the whole production process is carried out in the closed environment and accords with medicinal GMP standard requirements; and the prepared cefaclor particles are porous particles having round shapes, have the characteristics of good water solubility, uniform granularity, good fluidity, uniform distribution of a cefaclor raw material in the cefaclor particles, and very good stability and in-vitro dissolution curve, and can be used for treating clinic indications.
Owner:HAIKOU PHARMA FACTORY +1
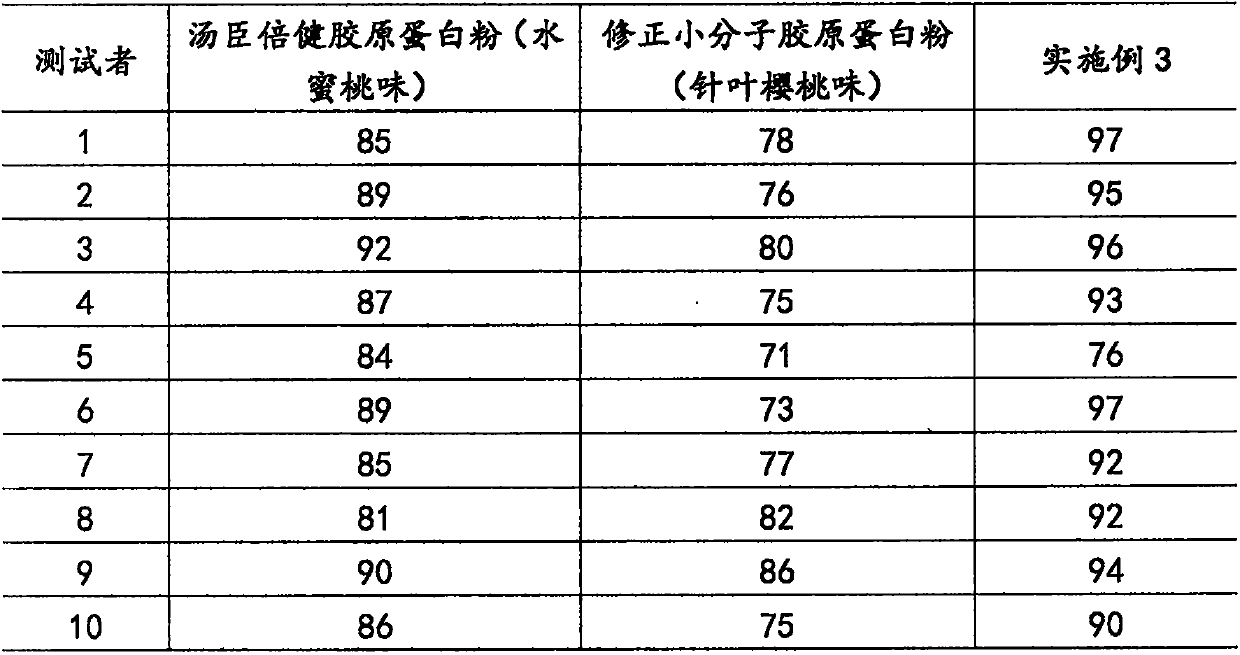
Flavored collagen solid beverage and preparation method thereof
PendingCN109832536AWell mixedImprovement of the single mixing methodFood scienceSolubilityWrinkle skin
The invention discloses a flavored collagen solid beverage and a preparation method thereof. The flavored collagen solid beverage comprises the following components in parts by weight of 0-90 parts ofmarine fish oligopeptide powder, 0-90 parts of collagen peptide powder and 1-90 parts of fruit powder. The flavored collagen solid beverage which is uniform, beautiful and stable in color is obtainedthrough technologies of performing dissolving, performing spray drying, performing fluidized bed granulation and the like. According to the flavored collagen solid beverage disclosed by the invention, re-dissolution, spray drying and granulation with a fluidized bed are united, so that the problems that products prepared through single mixing and granulation are not uniform in appearance color and the products are not uniform to mix can be effectively solved. The flavored collagen solid beverage disclosed by the invention can improve skin moisture, delay senescence, beautify the features andresist wrinkles, and is uniform in color, good in solubility and pleasant in taste.
Owner:北京姿美堂生物技术股份有限公司
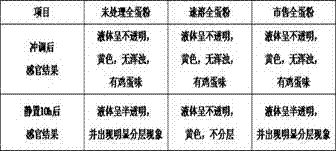
Instant whole egg powder free of bitter taste and fishy smell, and preparation method thereof
The invention relates to a non-bitter fishy smell, instant whole egg powder and a preparation method thereof. The method comprises: (a) adopting two-stage dynamic high-pressure micro-jet treatment; (b) sequentially adding compound protease and phospholipid to the whole egg liquid Enzyme A1; wherein the composite protease is papain and flavor protease, the dosage ratio of the two is 2:1, the amount of composite protease added in the whole egg liquid is whole egg liquid: composite protease=100:0.8-1.2, add The amount is calculated by mass ratio; phospholipase A1 is enzymatically hydrolyzed at room temperature, and the added amount in the whole egg liquid is whole egg liquid: phospholipase A1=100:0.01‑0.05, and the added amount is calculated by kg / L; (c) After spray drying, whole egg powder is fluidized bed granulated with a binder. The invention cooperates with dynamic high-pressure micro-jet homogenization, enzymatic hydrolysis and fluidized bed technology to obtain whole egg powder with good instant solubility, and no stratification is found within 10 hours after brewing.
Owner:南京中农食品科技有限公司
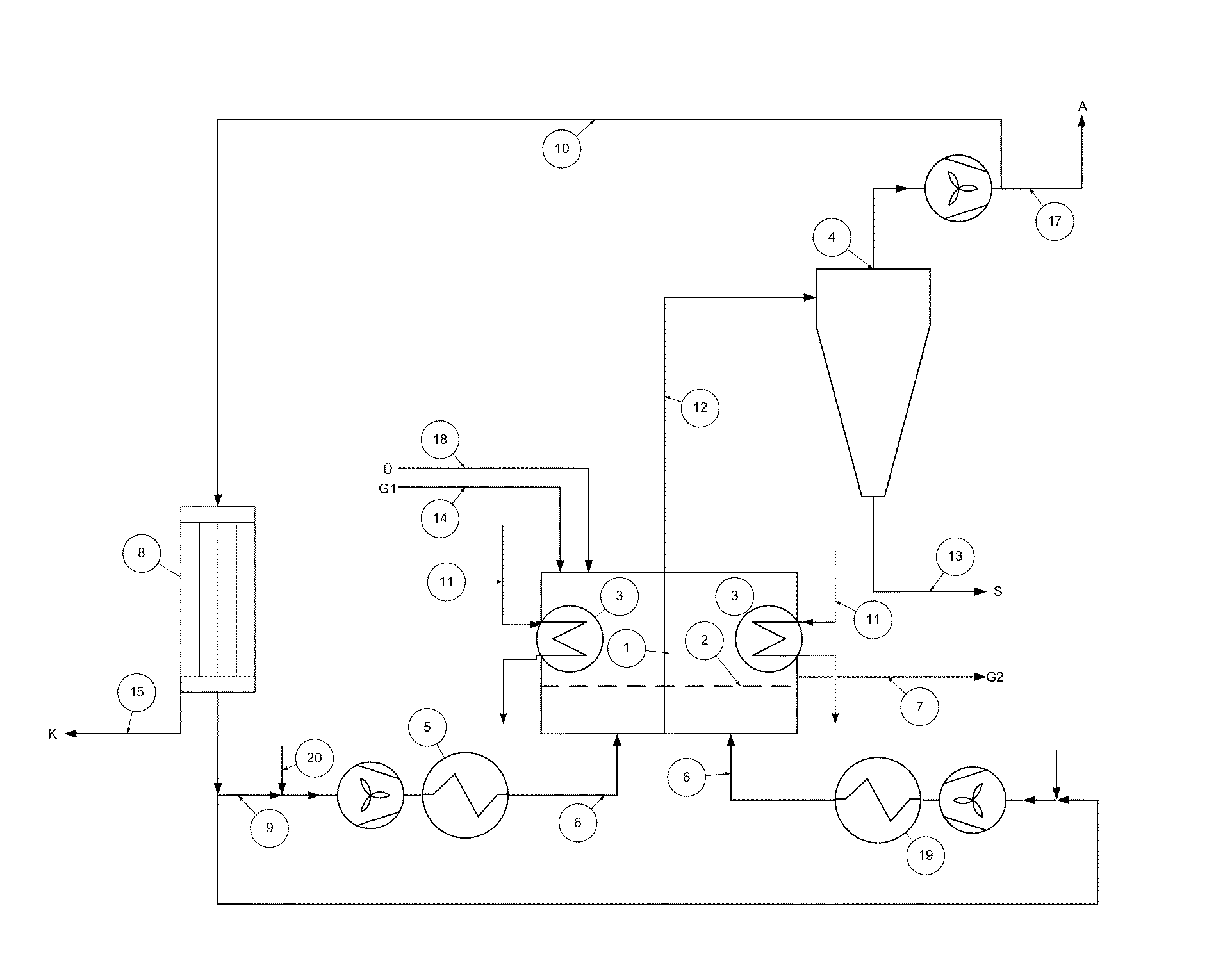
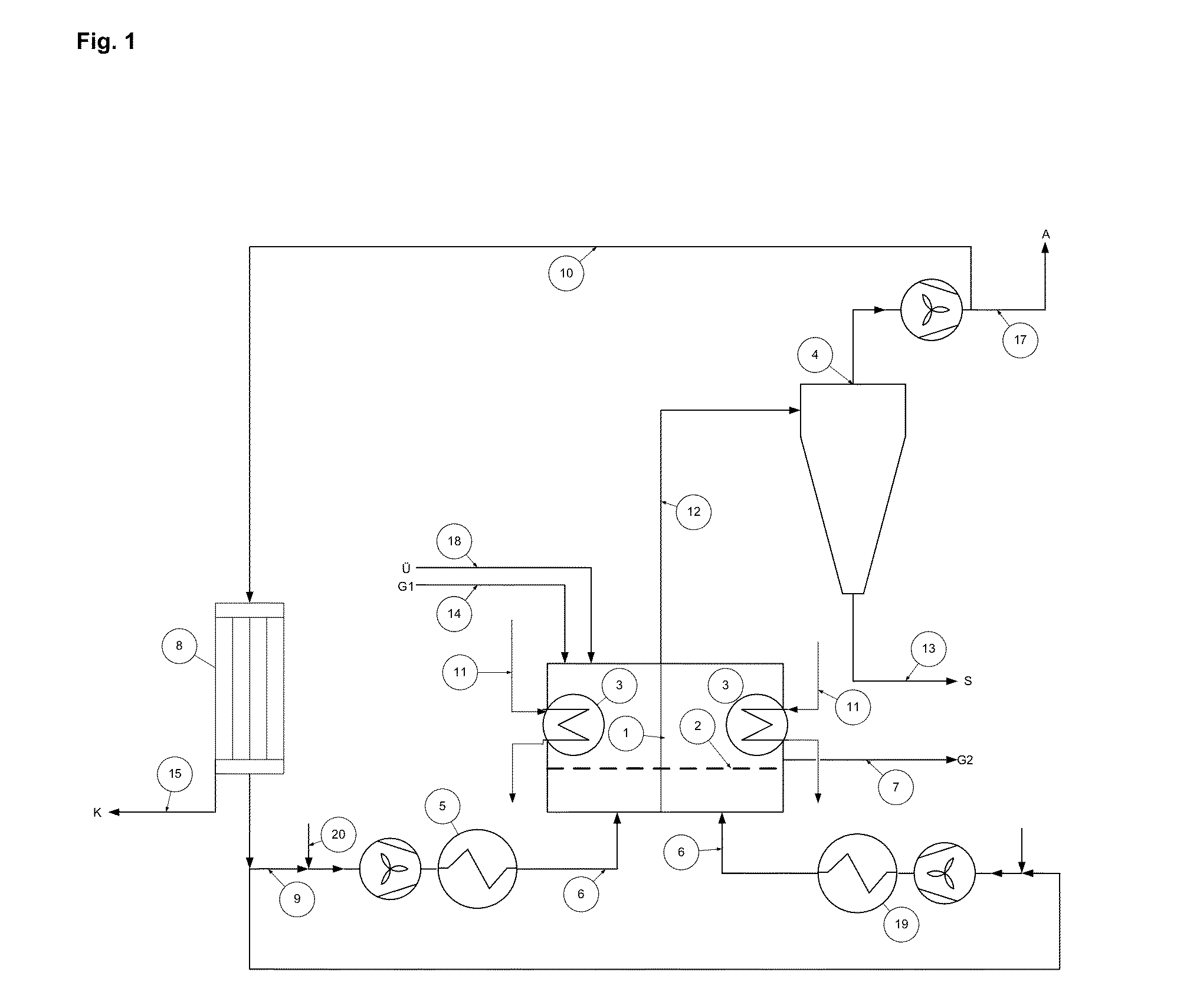
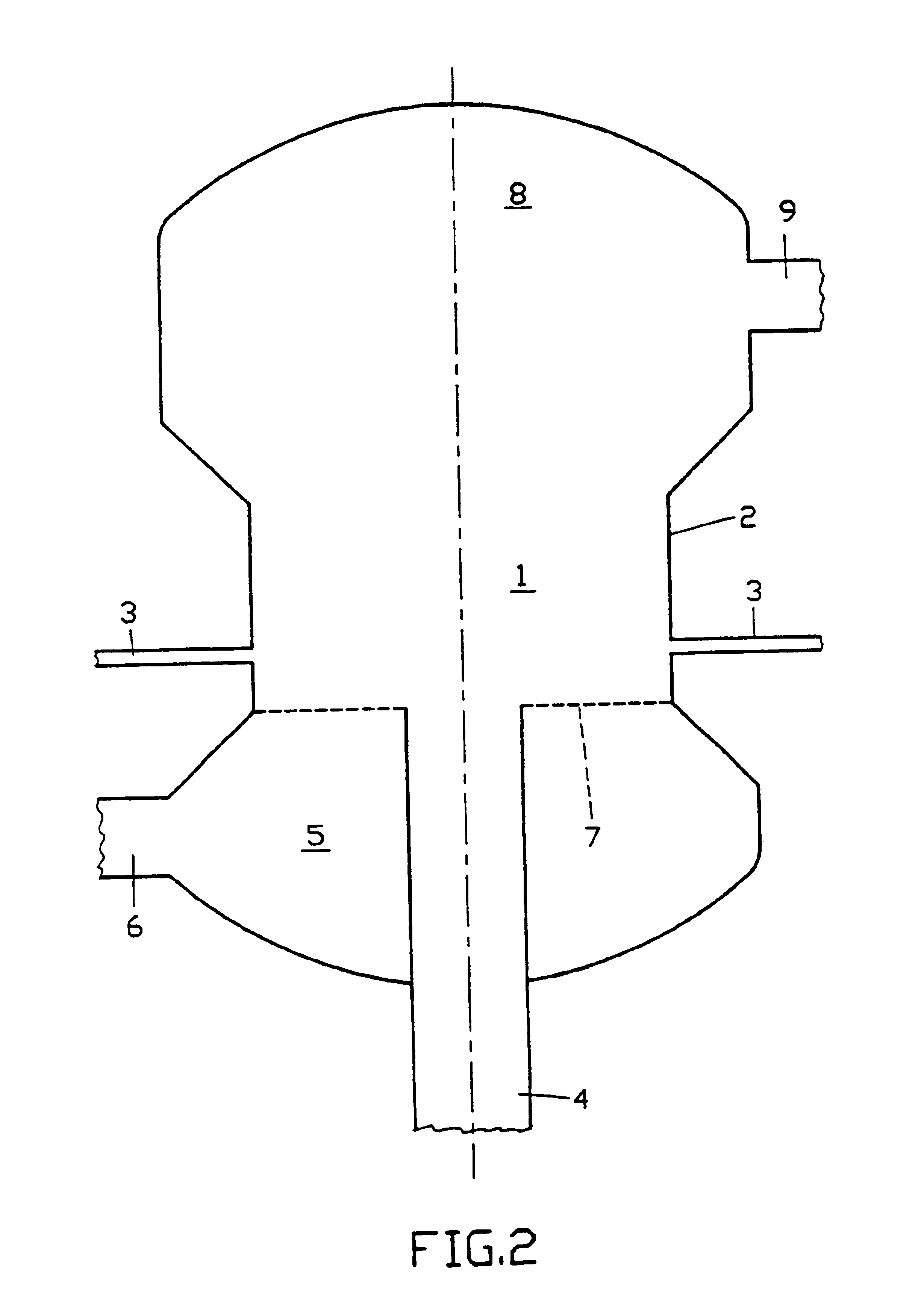
Fluid bed granulation process and apparatus
ActiveUS7966745B2Fully processedDrying using combination processesLiquid surface applicatorsEngineeringCooling fluid
Fluid bed granulation process comprising the step of cooling the granules in a cooling fluid bed (F2). At least part of the fluidizing air coming out from said cooling fluid bed (F2) is fed into the granulation fluid bed (F1).
Owner:CASALE SA
Fluidized bed granulation coating device and fluidized bed granulation coating method
InactiveUS7112244B2Guaranteed continuous performanceFully dryLiquid surface applicatorsGranule coatingForming gasEngineering
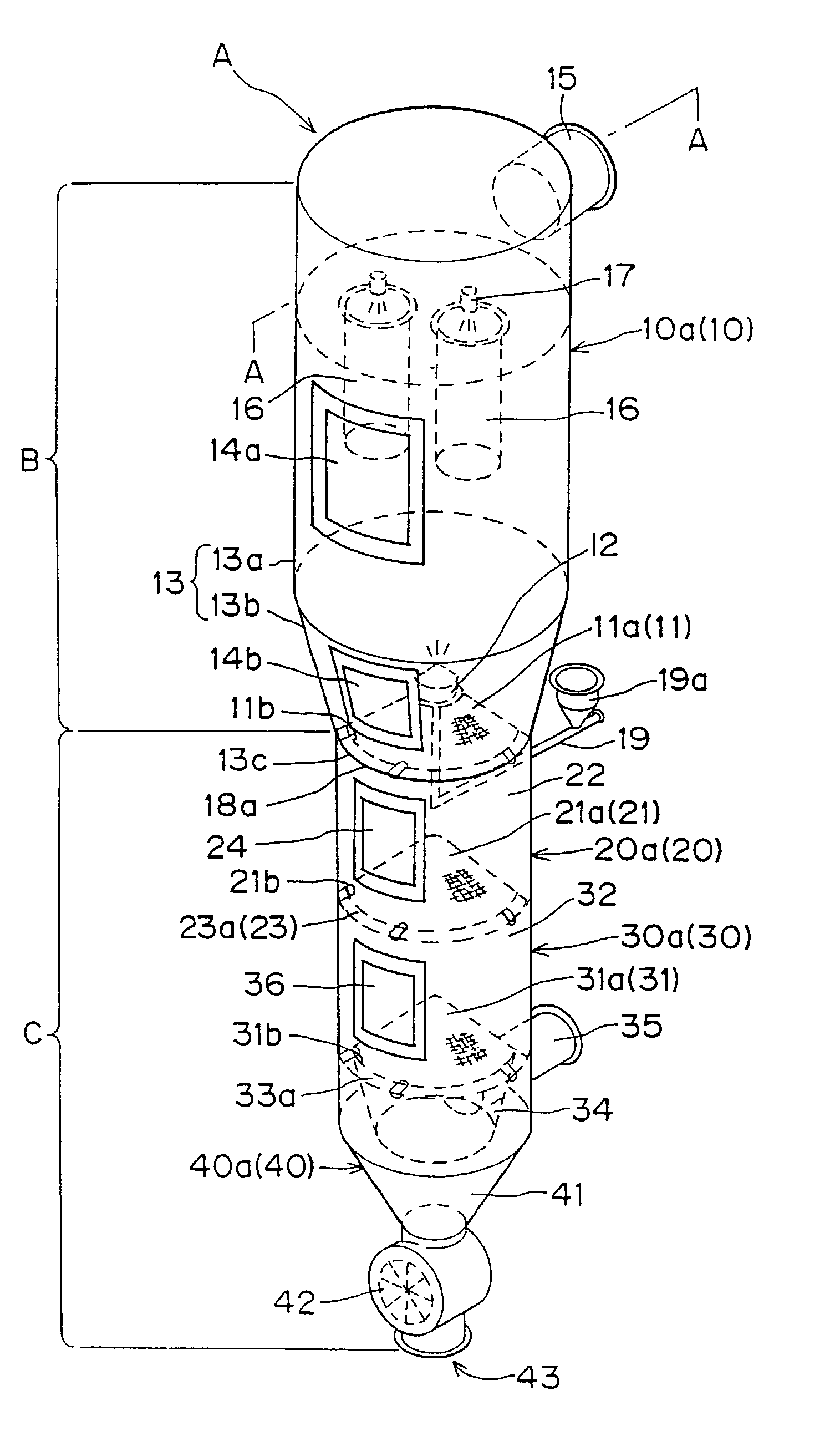
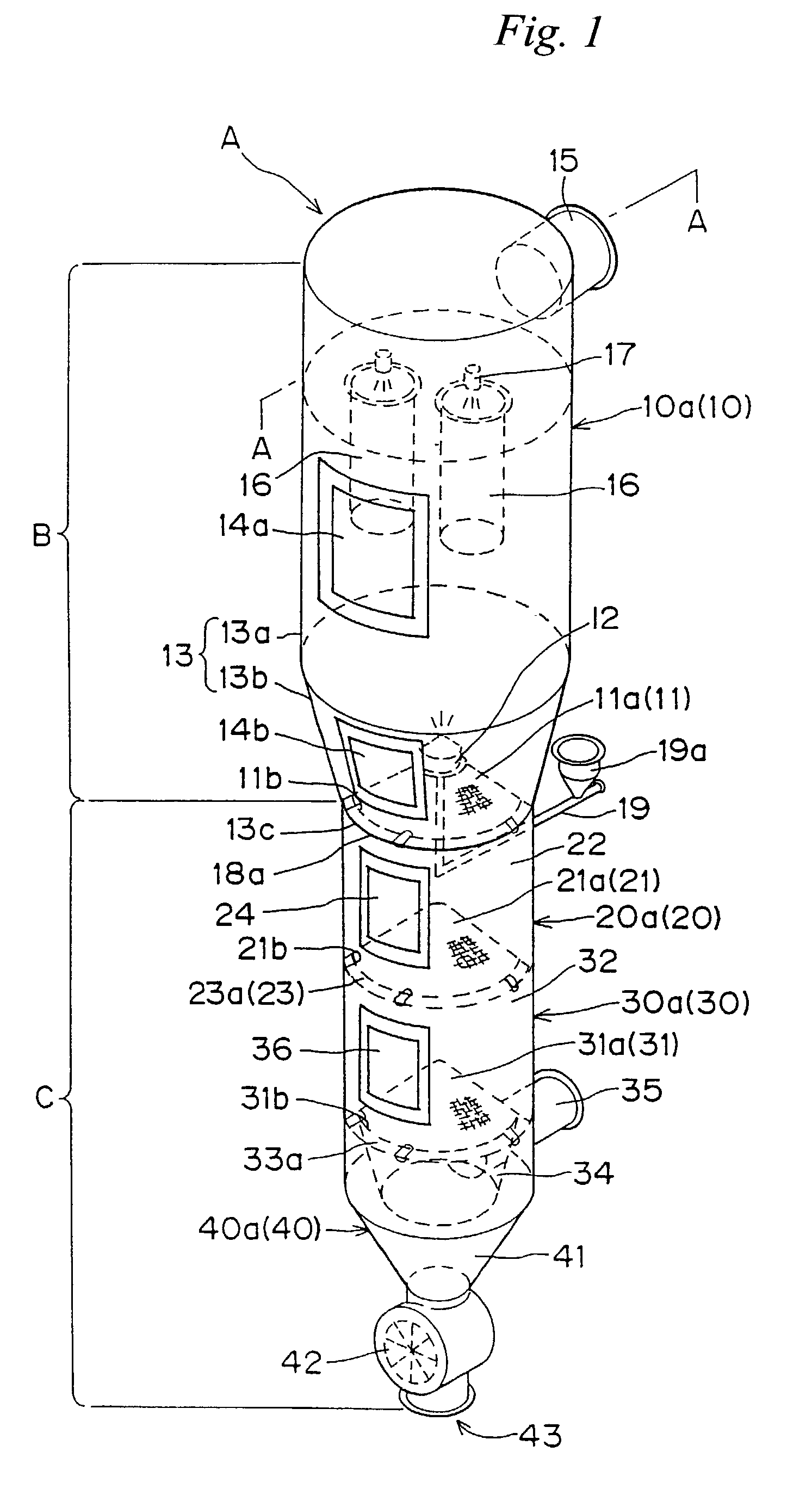
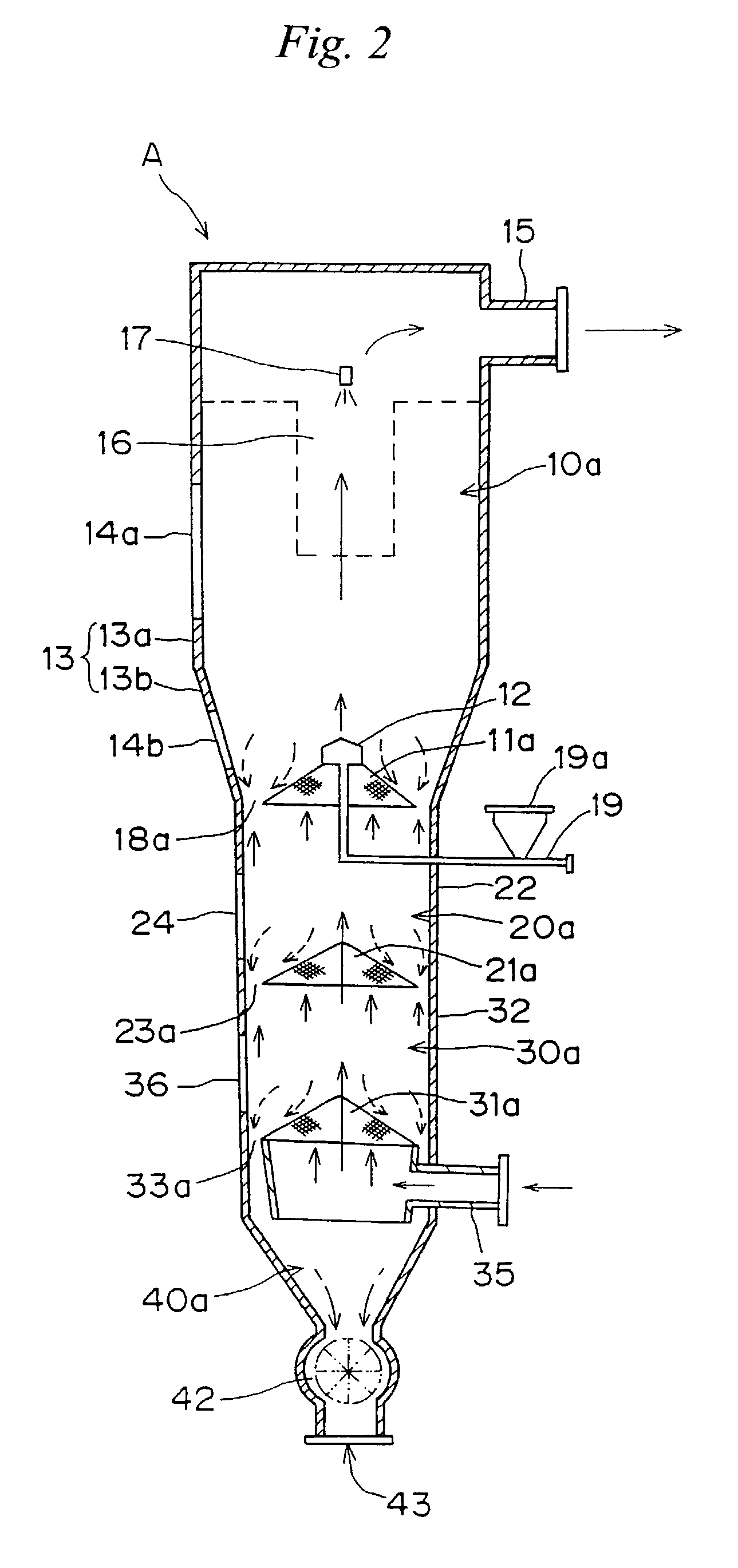
Under the granulating section 10a for performing fluidized-bed granulation coating of powder grains, a drying section 20a for drying powder grains granulated is provided. A drying section 30a for drying the powder grains that are not sufficiently dried is provided under the drying section 20a. A product discharging section 40a for discharging a dried product is provided under the drying section 30a. A fluidized-bed forming-gas is supplied downward to upward, and granulation and dryness are continuously performed by the fluidized-bed forming-gas, and the powder grains moved from the granulating section to the drying section located immediately thereunder are pneumatic classified.
Owner:FREUNT IND
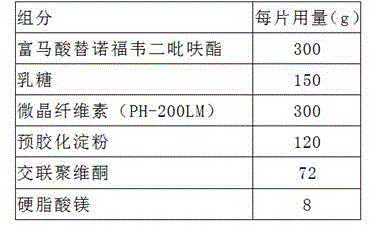
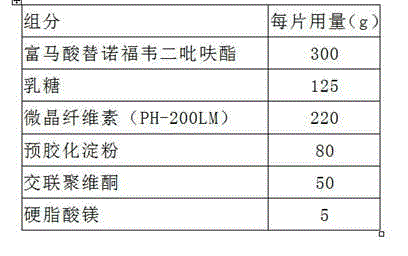
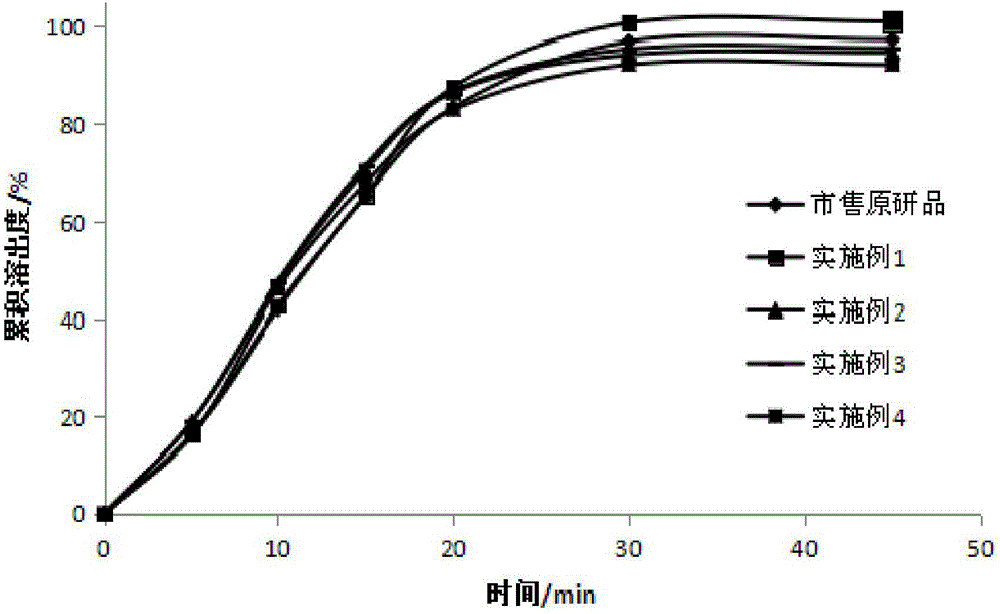
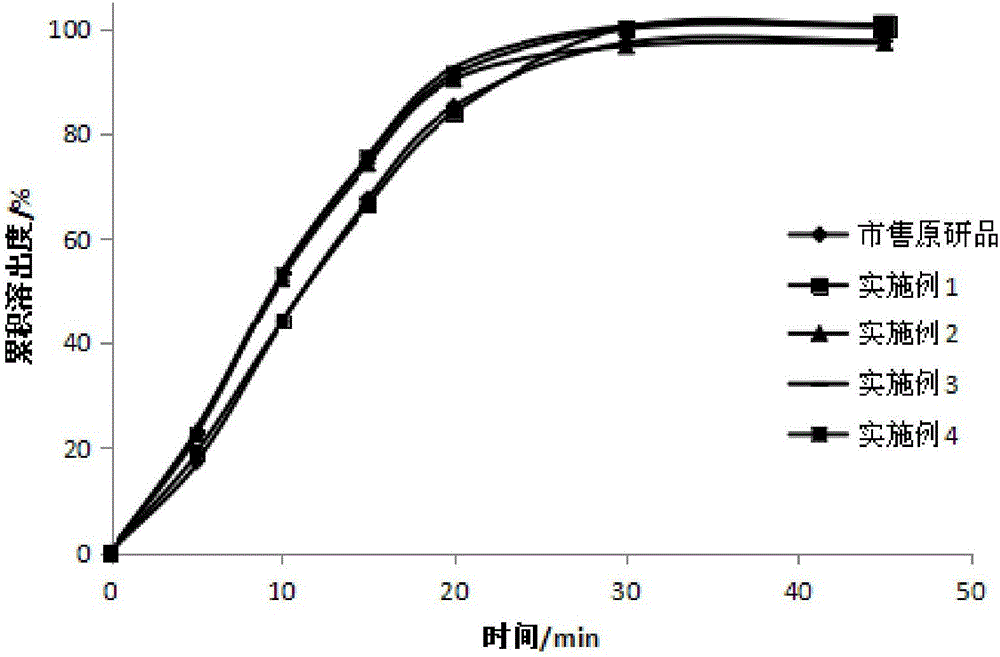
Readily soluble tenofovir disoproxil fumarate tablets and preparation method thereof
ActiveCN104688700AIntermediate fluidityRound particlesOrganic active ingredientsAntiviralsDissolutionChemistry
The invention belongs to the technical field of medicinal preparations and in particular relates to readily soluble tenofovir disoproxil fumarate tablets and a preparation method thereof. The readily soluble tenofovir disoproxil fumarate tablets are prepared by taking tenofovir disoproxil fumarate as a raw material and a pharmaceutically acceptable auxiliary component as an auxiliary material, and adopting a fluidized bed granulation tabletting process and a wet activity dry granulation tabletting process, wherein the auxiliary material comprises microcrystalline cellulose, pregelatinized starch, lactose, crosslinking carboxymethyl cellulose sodium, Cellactose 80 and magnesium stearate. The readily soluble tenofovir disoproxil fumarate tablets provided by the invention have the advantages of relatively good intermediate fluidity, mellow and full granules, uniform size, high dissolution rate and the like, and can obviously improve the dissolution rate of a medicament in water, and by virtue of significantly improving the dissolution rate of the medicament, the biological utilization degree of an oral administration medicament is improved.
Owner:SHANDONG WEIFANG PHARMA FACTORY
Pharmaceutical composition containing vildagliptin and metformin and preparation method of pharmaceutical composition
ActiveCN106265641AGood content uniformityImprove liquidityMetabolism disorderPill deliveryFiller ExcipientAdhesive
The invention provides a pharmaceutical composition containing vildagliptin and metformin. In the pharmaceutical composition, vildagliptin is good in content uniformity, high in dissolution rate and stable in product. A preparation method of the pharmaceutical composition comprises the steps that metformin hydrochloride and an adhesive are prepared into granules through a fluidized bed granulation technology; the granules, vildagliptin and a filler are mixed to be uniform, and then dry granulation is conducted; the mixture is mixed with a lubricant, and tablets are prepared. By means of the fluidized bed granulation technology, the fluidity and compressibility of metformin hydrochloride are greatly improved, the granularity of vildagliptin is controlled to be within a certain range, direction contact of metformin hydrochloride and vildagliptin is reduced, and degradation of vildagliptin is reduced; by means of dry granulation, the content uniformity, a tablet weight difference and the like of the granules all meet the requirements. By means of the preparation process, compound preparations with small differences between batches can be obtained, the friability, content uniformity and stability of the tablets all meet the requirements, and the pharmaceutical composition can be subjected to scale-up production in a workshop.
Owner:QILU PHARMA CO LTD
Montelukast sodium granules and preparation method thereof
ActiveCN108057021AFast absorptionImprove solubilityPharmaceutical non-active ingredientsGranular deliveryAdhesiveLactose
The invention disclose montelukast sodium granules and a preparation method thereof, and relates to the field of a montelukast sodium preparation. The montelukast sodium granules provided by the invention are prepared from a raw material by dry granulation or fluidized bed granulation; the raw material comprises the following components in percentage by weight: 0.5% to 1.5% of montelukast sodium,35% to 65% of mannitol, 15% to 45% of lactose anhydrous, 10 to 30% of microcrystalline cellulose, 0% to 20% of adhesive used when a fluidized bed is adopted to carry out granulation, 0.5% to 3.5% of corrigent and 0.5% to 5% of lubricant. The montelukast sodium granules not only have the characteristics of rapid absorption, good dissoluvability, rapid response, convenience and comfort for taking, good taste and the like, but also have excellent stability.
Owner:南京康舟医药科技有限公司
Quizalofop-p-ethyl containing water dispersible granule and preparation method thereof
InactiveCN106305729AAvoid undersupplyGuarantee normal performanceBiocideAnimal repellantsDispersityWater dispersible
The invention discloses a quizalofop-p-ethyl containing water dispersible granule and a preparation method thereof. The quizalofop-p-ethyl containing water dispersible granule is prepared from the following components in percent by weight: 5 to 20 percent of quizalofop-p-ethyl, 1 to 20 percent of a synergist, 0.5 to 3 percent of a wetting agent, 1 to 12 percent of a dispersing agent, 1 to 30 percent of a disintegrating agent, 0.1 to 5 percent of an adhesive, 0.1 to 5 percent of a defoaming agent and the balance of filler. By the adoption of a fluidized bed granulation process, the obtained quizalofop-p-ethyl containing water dispersible granule is high in dispersity and high in disintegrating speed; the suspension rate is above 90 percent; annual gramineous weeds in various broadleaf crop fields can be effectively prevented; furthermore, the product is high in heat stability; no organic solvent is used, and thus the product is little in environmental pollution and safe to a worker; and the use of the worker is facilitated to a larger extent.
Owner:JIANGSU FENGSHAN GROUP
Tobacco extractive particle and preparation method thereof
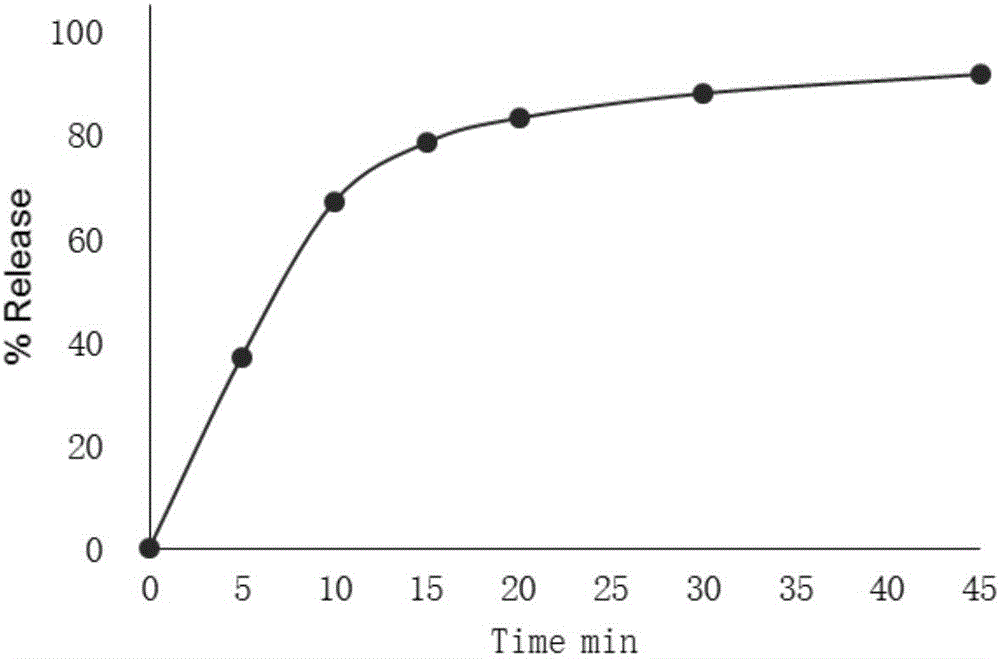
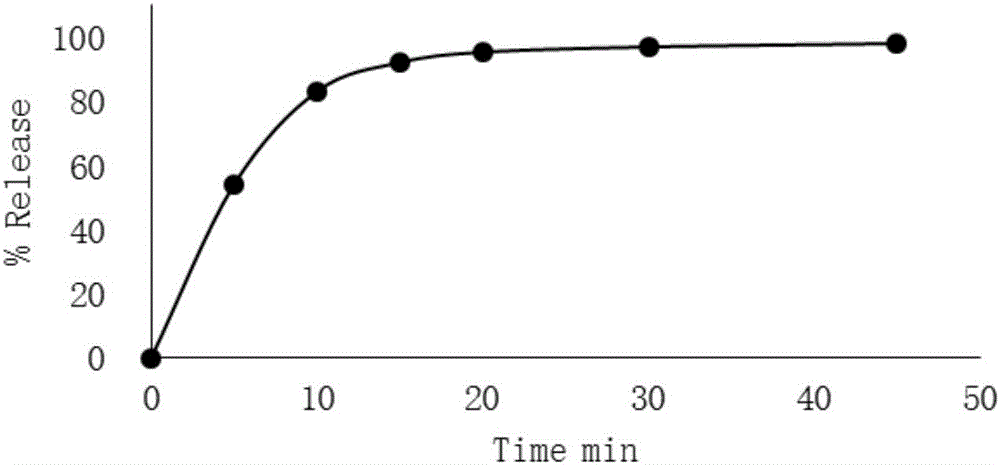
InactiveCN104997160ASmooth releaseUniform release rateTobacco treatmentBiotechnologyFluidized bed granulation
The invention belongs to the field of tobacco, and discloses a tobacco extractive particle and a preparation method thereof. The tobacco extractive particle comprises at least two substrate layers with different tobacco extractive contents, wherein the substrate layers are wrapped layer by layer, the surfaces of the substrate layers are wrapped by coating layers, and the substrate layers internally contain excipients; and the tobacco extractive contents of the substrate layers from, the inside to the outside, of the tobacco extractive particle are gradually decreased. Tobacco extractives employ water and ethanol as extractants, and through low-temperature ultrasonic extraction by use of two-step method, the extraction amounts of effective components are ensured. The preparation method of the tobacco extractive particle comprises an extruding-rolling granulation method and a fluidized bed granulation method. The prepared particle has a multilayer structure, is uniform in structure and guarantees the mouthfeel and local flavor of a novel tobacco product with the tobacco extractive particle added thereto.
Owner:GUANGDONG BRANCH OF CHINA TOBACCO GENERAL
Mycophenolate mofetil capsule and preparation method thereof
InactiveCN106389379AReduce intensityLow densityOrganic active ingredientsSkeletal disorderIrritationAdhesive
The invention provides a mycophenolate mofetil capsule and a preparation method thereof. The mycophenolate mofetil capsule comprises, by weight, 1100-1400 parts of mycophenolate mofetil, 150-170 parts of filler and 75-110 parts of pharmaceutical acceptable auxiliary materials; the filler is selectively prepared from a mixture of one or more optional proportions of pregelatinized starch, corn starch or lactose; the pharmaceutical acceptable auxiliary materials include adhesive, disintegrant and lubricant. A fluidized-bed granulation technology is adopted, the process is simplified, time is saved, labor intensity is low, obtained particles are porous soft particles, low density and low strength are achieved, the particles are evenly distributed in particle size, and good fluxility is achieved; the mycophenolate mofetil capsule has the advantages of high disintegration speed, good absorptivity, convenience in taking, small in intestinal irritation, stable in long-term storage quality and the like; the advantages of both simpleness in production equipment for ordinary capsules and convenience in package storage and transportation as well as carrying are achieved, and the mycophenolate mofetil capsule is convenient for patients to take.
Owner:WUXI FORTUNE PHARMA
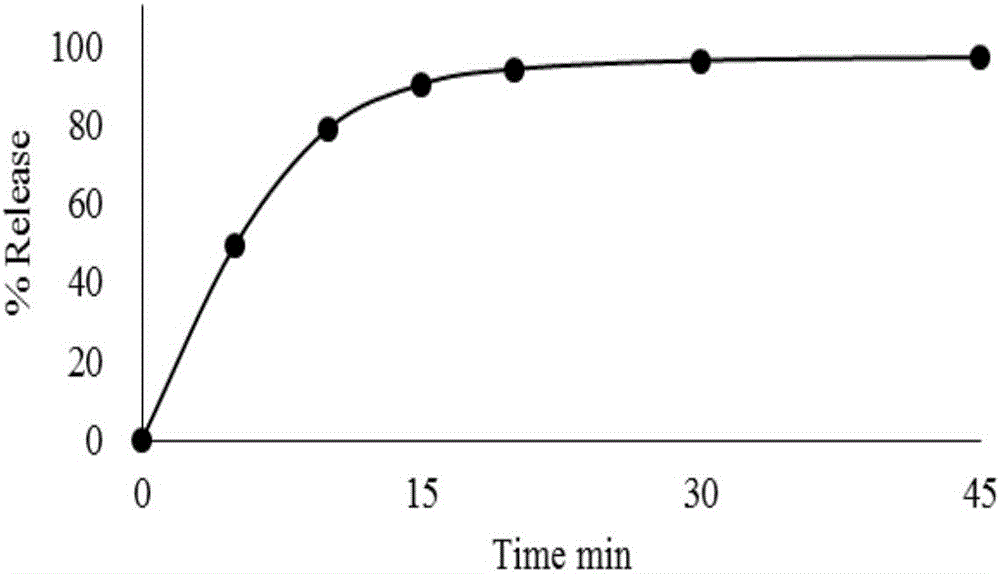
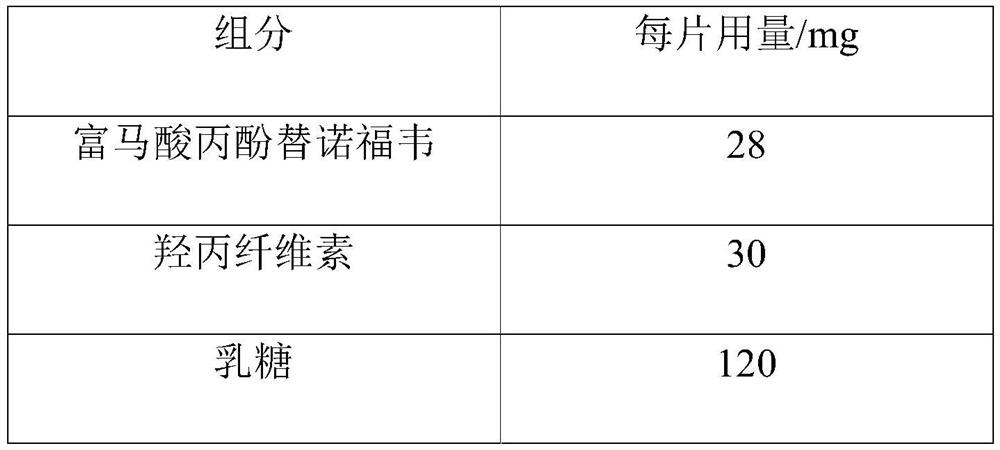
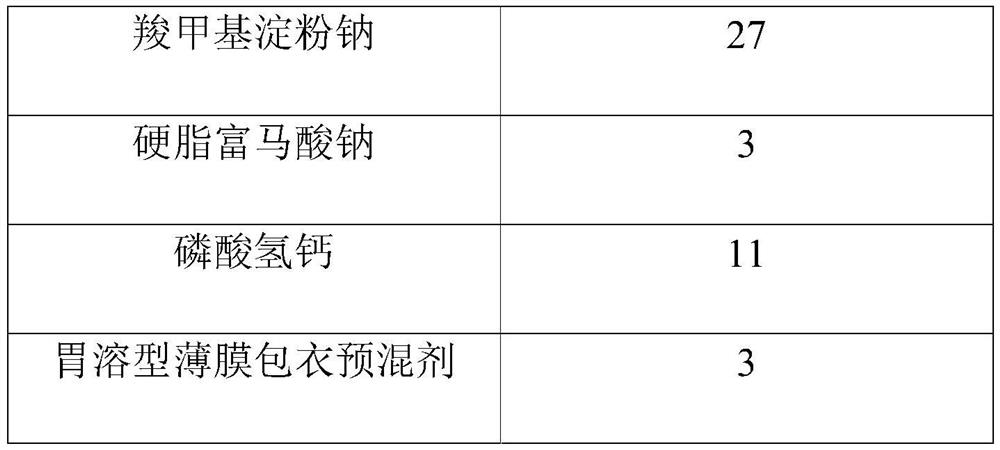
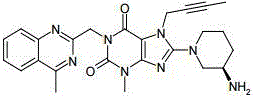
Tenofovir alafenamide fumarate tablet, preparation method thereof and detection method of related substances
ActiveCN112336695AImprove roundnessNarrow particle size distributionOrganic active ingredientsComponent separationDrugs preparationsTenofovir alafenamide
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a tenofovir alafenamide fumarate tablet and a preparation method thereof. The tenofovir alafenamide fumarate tablet comprises the following components in percentage by weight: 12%-14% of tenofovir alafenamide fumarate, 10%-15% of a cross-linking agent, 45%-58% of a diluent, 5%-12% of a disintegrating agent, 1%-5% of sodium stearyl fumarate and 5%-15% of calcium hydrophosphate. The tenofovir alafenamide fumarate tablet is prepared from the raw materials and auxiliary materials with a fluidized bed granulation method. The tenofovir alafenamide fumarate tablet prepared by steps of selecting the auxiliary materials and matching the optimized auxiliary material proportion with the fluidized bed granulation process is high in in-vitro dissolution rate, low in impurity content and good in stability, the safety of clinical application is improved, the tenofovir alafenamide fumarate tablet can be consistent with an original ground product in four dissolution media, besides, the preparation process is simple, and the tablet is suitable industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
Linagliptin active component-containing composition and preparation method thereof
The invention discloses a linagliptin active component-containing composition and a preparation method thereof. The composition contains the active component linagliptin, and also comprises the following auxiliary materials, by weight, 80-150 parts of mannitol, 10-60 parts of pregelatinized starch, 1-30 parts of corn starch, 1-10 parts of polyvinylpyrrolidone and 1-5 parts of magnesium stearate. Fluidized bed granulation integrates mixing, granulation and drying, so the bare link in the granulation process is reduced, and the operation is simple and controllable. Linagliptin is added in a suspending injection manner, so uniform mixing of linagliptin is facilitated.
Owner:合肥远志医药科技开发有限公司
Epinastine hydrochloride granule, and preparation method thereof
ActiveCN105708808ASolve the extremely painful shortcomingsImprove compliancePharmaceutical non-active ingredientsGranular deliveryEpinastine HydrochloridePrill
The invention discloses an epinastine hydrochloride granule, and a preparation method thereof. 1000 weight parts of the epinastine hydrochloride granule comprise 10 parts of epinastine hydrochloride, 945 parts of a filler, 40 parts of a composite flavoring agent, 3 parts of polyvinylpyrrolidone, and 2 parts of an essence. The preparation method comprises two times of granulation. According to the preparation method, epinastine hydrochloride, the filler, and the composite flavoring agent are uniformly mixed; an appropriate amount of a polyvinylpyrrolidone solution is added for wet granulation; drying is carried out; and an appropriate amount of polyvinylpyrrolidone is added for fluidized bed granulation; granule shaping is carried out; and an obtain product is uniformly mixed with the essence so as to obtain a finished product. The preparation method is capable of solving problems that epinastine hydrochloride granule particle size distribution range is wide, and much particle fine powder is generated; stable and uniform granules are obtained via the preparation method; and the preparation method is suitable for industrialized production.
Owner:重庆瑞泊莱医药科技有限公司
Polymer granules produced by fluidized bed granulation
InactiveUS6841614B1Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsPolymer scienceFluidized bed granulation
The invention relates to a method of producing soluble polymer granules which are suitable for use in washing and / or cleaning surfactants. According to said method, an aqueous preparation of the polymer which contains at least 30% by weight polymer, optionally in admixture with other washing and / or cleaning surfactant ingredients, is granulated and at the same time dried batchwise or continuously, preferably continuously, in a circular fluidized bed. In said fluidized bed, an eddy flow about the vertical axis of the apparatus is produced above the ground of the fluidized bed by means of an air inlet. The invention also relates to soluble, spherical polymer granules with a polymer content of 50 to 95% by weight and to washing or cleaning surfactants containing the inventive granules.
Owner:HENKEL KGAA
Process for fluidized bed granulation of amino acid-containing fermentation broths
ActiveUS9649609B2Reduce disadvantagesOperated economicallyAnimal feeding stuffAccessory food factorsFluidized bed dryingFluidized bed granulation
Process for fluidized bed granulation of amino acid-containing fermentation broths comprising the stepsIntroduction of a drying gas with a temperature of 100° C.-450° C. into the fluidized bed granulation chamberSpraying of the amino acid-containing fermentation broth into the fluidized bed granulation chamberDischarge of the granules granulated in the fluidized bed granulation chamber with the drying gas stream, anddrying of the discharged granules in a fluidized bed drying step,wherein the discharged granulated granules are a granule mixture with various particle sizes and contains an oversize fraction, wherein the oversize comprises the particle sizes which lie above a desired particle size, an wherein the oversize fraction is removed from the discharged granule mixture and then comminuted and the comminuted oversize and the granule mixture from which the oversize was separated are fed into the fluidized bed drying step.
Owner:EVONIK OPERATIONS GMBH
A preparing method of novel multifunctional diatomite ceramsite
The invention relates to a preparing method of novel multifunctional diatomite ceramsite. The method includes weighing certain amounts of diatomite, ultrafine tourmaline powder and activated carbon to form a mixture, mixing the mixture with a dispersant, an adhesive and water, adding into a ball mill, grinding and mixing. The particle size distribution of ground slurry is that D50 is not more than 10 [mu]m. The mixture comprises 70-90 parts by weight of the diatomite, 5-25 parts by weight of the ultrafine tourmaline powder and 1-5 parts by weight of the activated carbon. 100 parts by mass of the mixture, 0.1-2 parts by mass of the dispersant and 100-200 parts by mass of the water are mixed. The diatomite ceramsite is prepared through filter pressing, drying, scattering, fluidized-bed granulation, sieving and calcination. The prepared diatomite ceramsite has the characteristics of high strength, a plurality of micropores, high adsorbability, capability of being moisture-proof, odor-removing and thermal-insulating, high negative ion release capability, and far-infrared treatment environmental-friendly healthcare effects.
Owner:LIAONING SILICATE RES INST
Medicinal solid preparation of tolvaptan and preparation method
PendingCN111888335AImprove bioavailabilityMetabolism disorderPill deliveryMagnesium stearateStearic acid
The invention discloses a medicinal solid preparation containing tolvaptan and a preparation method. The preparation method comprises steps of converting active pharmaceutical ingredients of tolvaptaninto amorphous forms by spray-drying. The preparation method is characterized by controlling the amorphous solid dispersible powder to be 35-80 [mu]m in particle size D90. The preparation method alsocomprises steps of adding lactose, corn starch, microcrystalline cellulose, hydroxypropyl cellulose, low-substituted hydroxypropyl cellulose, magnesium stearate and other auxiliary materials, and conducting granulation through a fluidized bed to obtain the medicinal solid preparation of tolvaptan with good in-vivo solubility and high bioavailability.
Owner:FUAN PHARMA LYBON PHARMA TECH
Imidafenacin film-coated tablet and preparation method thereof
InactiveCN103479594AImprove uniformityGuarantee of good quality uniformityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineImidafenacin
The invention provides an imidafenacin film-coated tablet. The imidafenacin film-coated tablet comprises raw and auxiliary materials in parts by weight as follows: 1-5 parts of imidafenacin, 700-850 parts of pregelatinized starch, 600-700 parts of microcrystalline cellulose, 3-6 parts of lubricants and appropriate coating agents. The invention further provides a preparation method of the film-coated tablet. According to research findings, the imidafenacin can dissolve completely even in 5min in a specific prescription ratio of the tablet; meanwhile, a specific fluidized bed granulation process is adopted, so that the uniformity of the content of the imidafenacin in the preparation can be remarkably improved; and the content of main drugs in the imidafenacin tablet is quite low, so that the quality uniformity of drugs is well guaranteed with the preparation method.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
Digoxin micro tablet and preparation method thereof
InactiveCN108261400AEasy to swallowGuaranteed curative effectOrganic active ingredientsPharmaceutical non-active ingredientsCompressibilityDigoxin
The invention discloses a novel digoxin preparation and a method of preparing the novel digoxin preparation. Digoxin, a bonding agent, a filling agent and the like are used as raw materials, and through methods such as dry granulation tablet compression, wet granulation tablet compression and top jet fluidized bed granulation tablet compression, the novel digoxin preparation with good compressibility is prepared. According to the novel digoxin preparation, for patients who need to take a precise amount of medicine, a small dose of medicine can be applied to the patients multiple times, particularly for people with large individual metabolism difference, counting can be conducted during medicine taking, for people who have difficulty in taking common preparations, the adaptability of medicine taking can be improved, the adopted method is simple, the raw materials are easily obtained, and industrial production is facilitated.
Owner:COSCI MED TECH CO LTD
Preparation method of succinic acid Trelagliptin tablets
ActiveCN105476974AQuality improvementGood in vitro dissolution behaviorOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseTrelagliptin
The invention provides a preparation method of succinic acid Trelagliptin tablets. The method includes: taking succinic acid Trelagliptin, adding sorbitol, microcrystalline cellulose and crosslinking sodium carboxymethyl cellulose, mixing, placing mixture under a condition with certain temperature and relative humidity, moving into a vacuum kettle, rising the temperature under a vacuum condition, adding binder, performing wet granulation, drying obtained granules in a fluidized bed granulation coating machine to obtain dried granules, adding lubricant into the dry granules, mixing, tableting, and coating to obtain the succinic acid Trelagliptin tablets. The preparation method has the advantages that the succinic acid Trelagliptin tablets prepared by the method is stable in quality and good in in-vitro dissolution performance, the method is simple in production process, and large-scale industrial production can be achieved.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
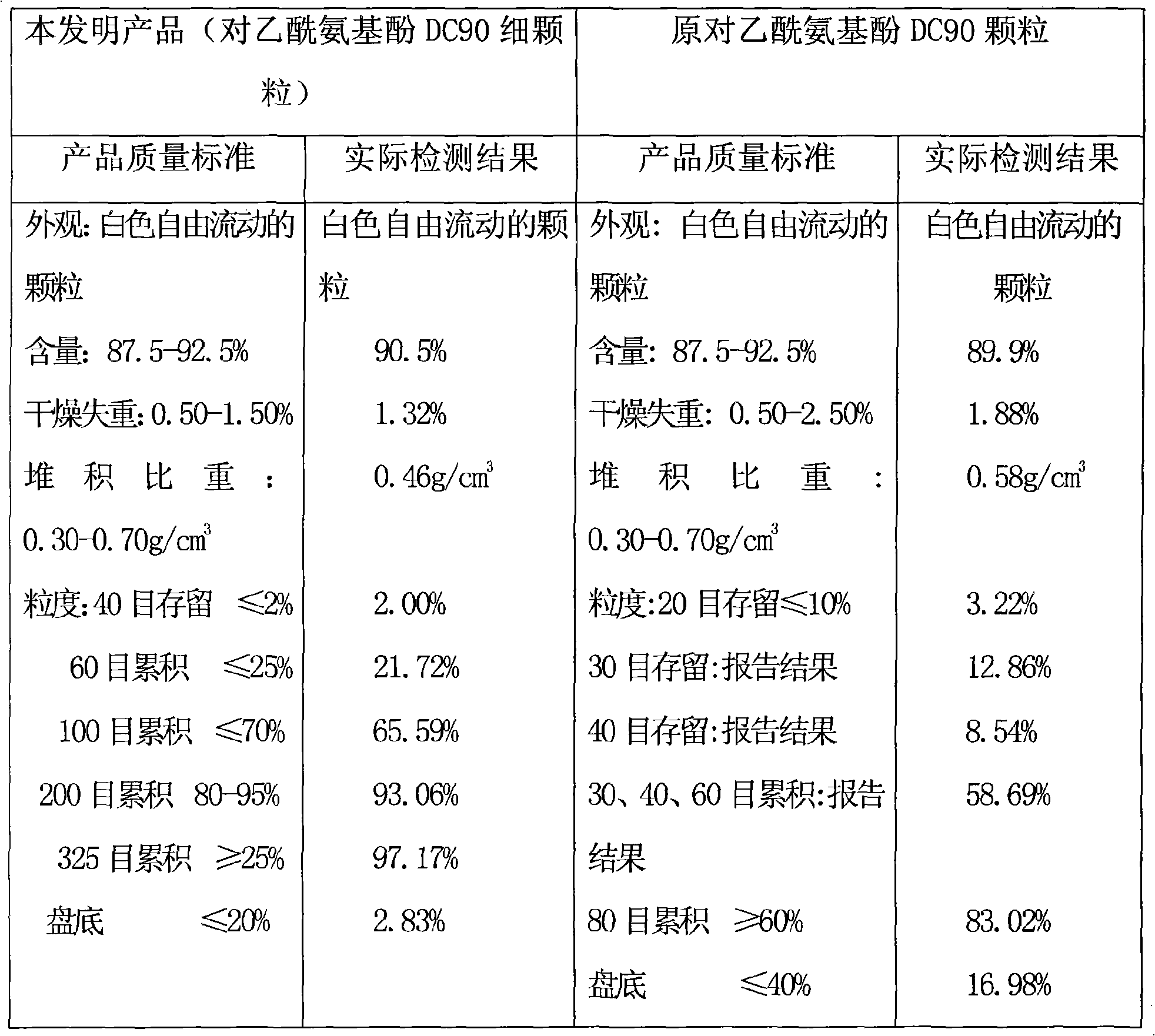
Acetaminopher DC90 fine particle and preparation method thereof
ActiveCN101342144AFine and uniform particlesWell mixedOrganic active ingredientsPowder deliveryCrospovidonesPrill
The invention discloses paracetanol DC90 fine particles and a preparation method thereof. The paracetanol DC90 comprises following materials by weight ratio: 90 percent of paracetanol, 3.50 percent of cornstarch, 3.50 percent of pre gelatinized starch, 2.00 percent of povidone, 0.5 percent of crospovidone and 0.5 percent of stearic acid. Firstly, the paracetanol and the cornstarch are mixed evenly, then the pre gelatinized starch and the povidone are made into starch slurry to be used as bond which is evenly sprayed on the mixture of the fluidied paracetanol and cornstarch, fluidized bed granulation is carried out in the inlet air temperature, the wet particles are dried under the temperature of 80 DEG C and then are cooled at the temperature of 50 DEG C till water conforms to the requirement, the sizes of the particles are adjusted, the stearic acid and the crospovidone are added after the particle sizes are adjusted to be mixed evenly to obtain the paracetanol DC90 fine particles. The invention adopts the above technical proposal to inspect the product, and the particles are fine and uniform and have favorable fluidity, thereby being well and evenly mixed with other small quantities of active components powder.
Owner:ANQIU LUAN PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com