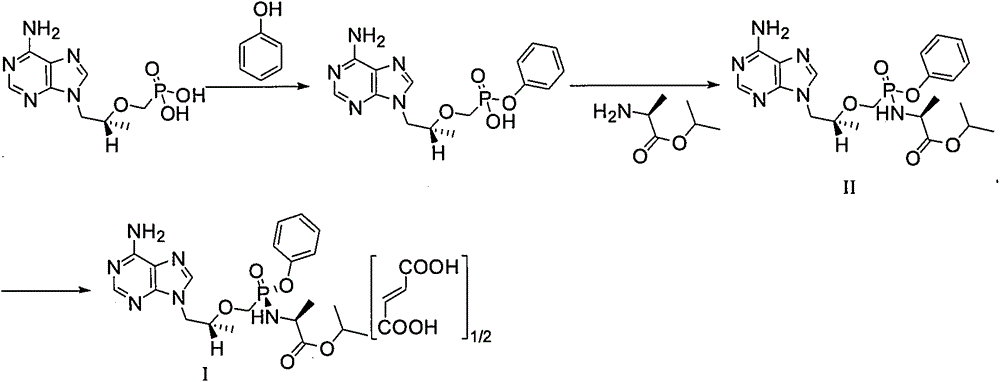
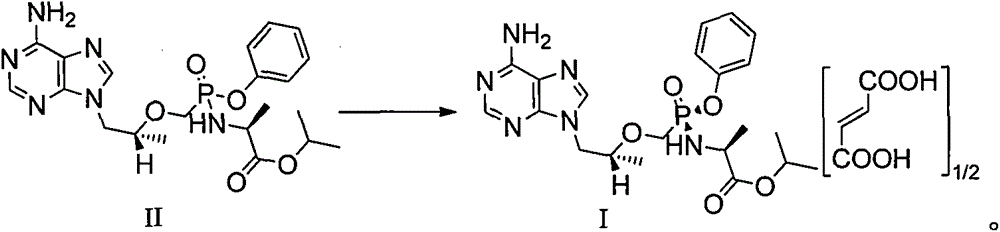
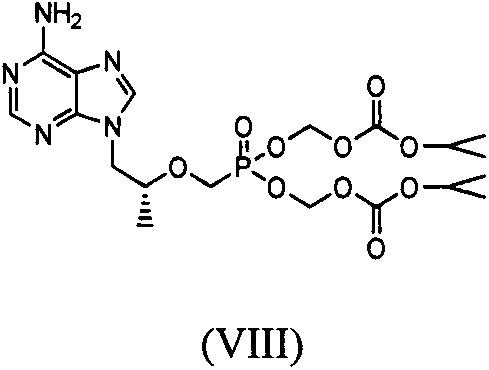
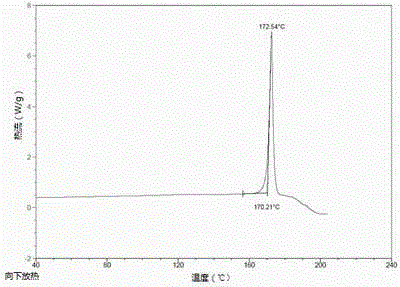
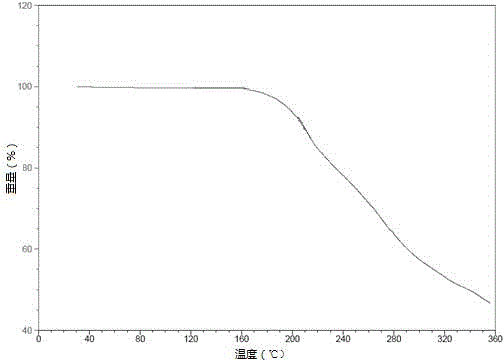
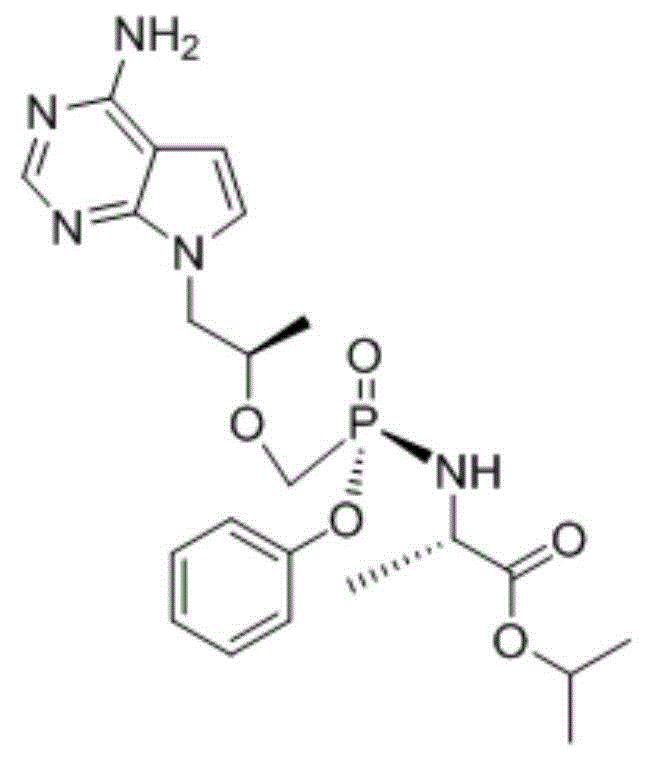
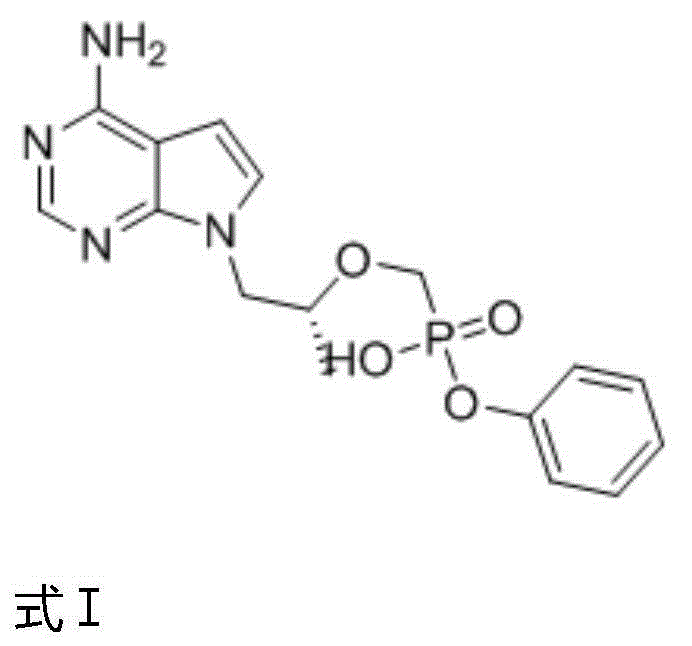
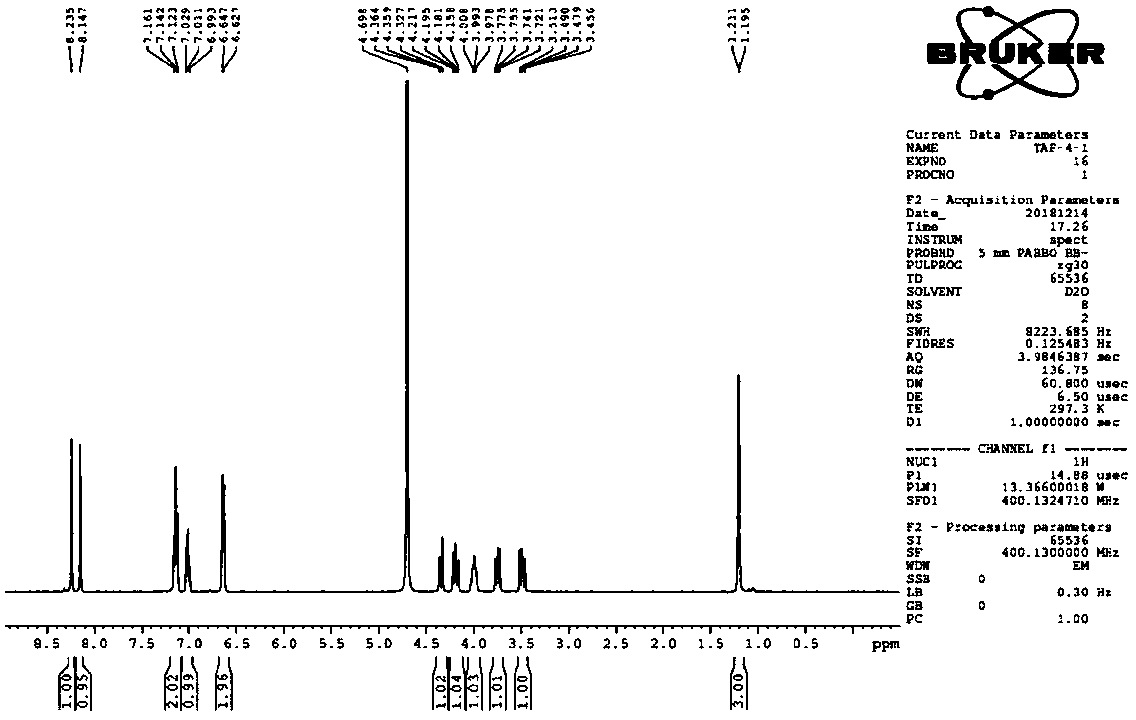
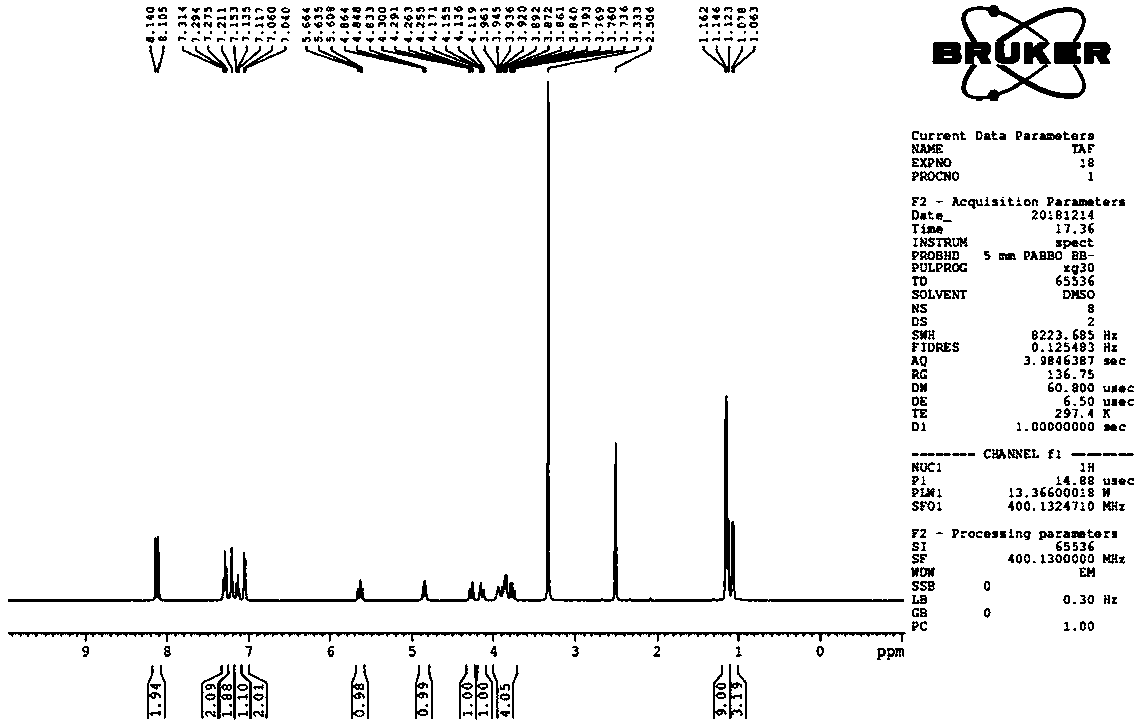
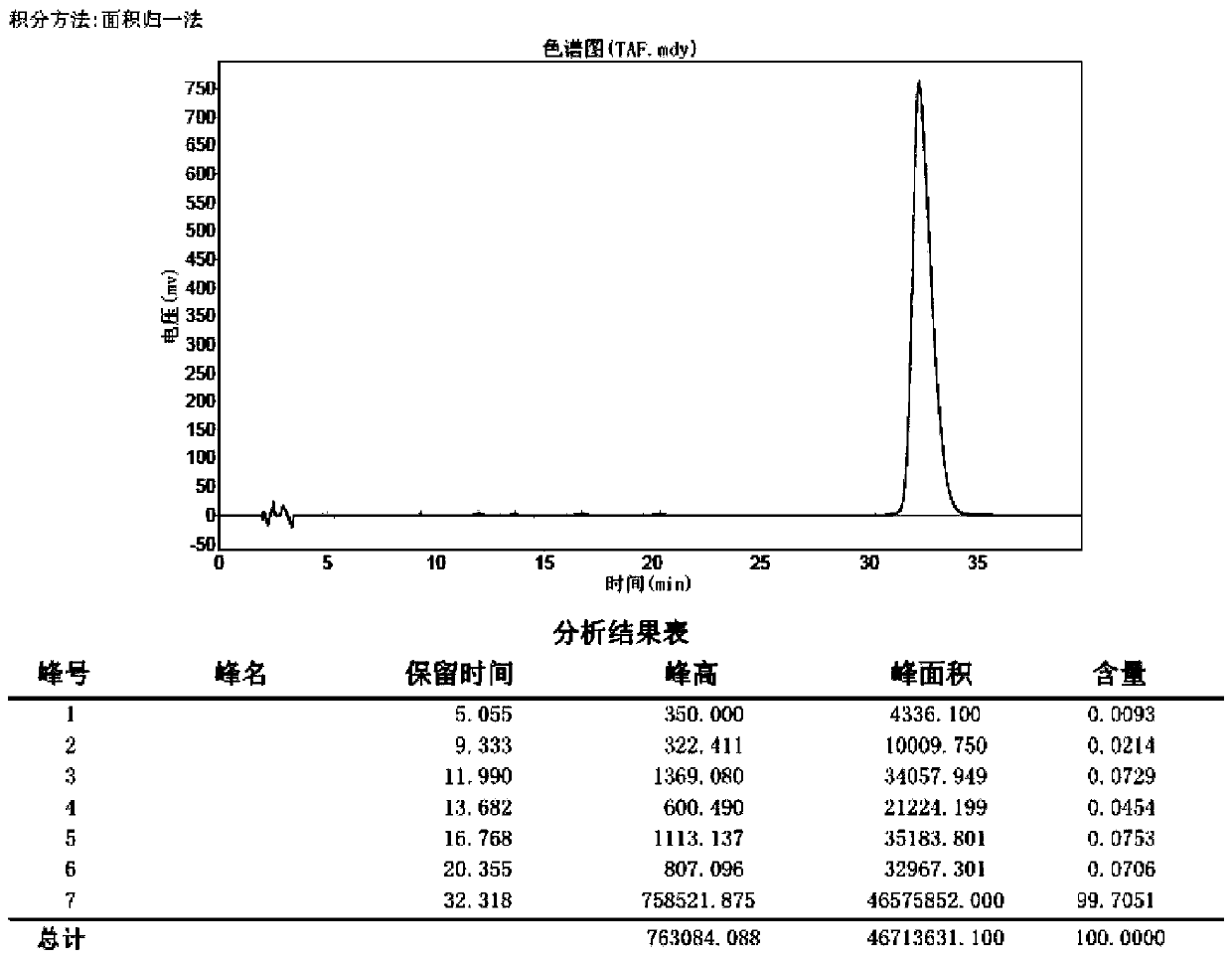
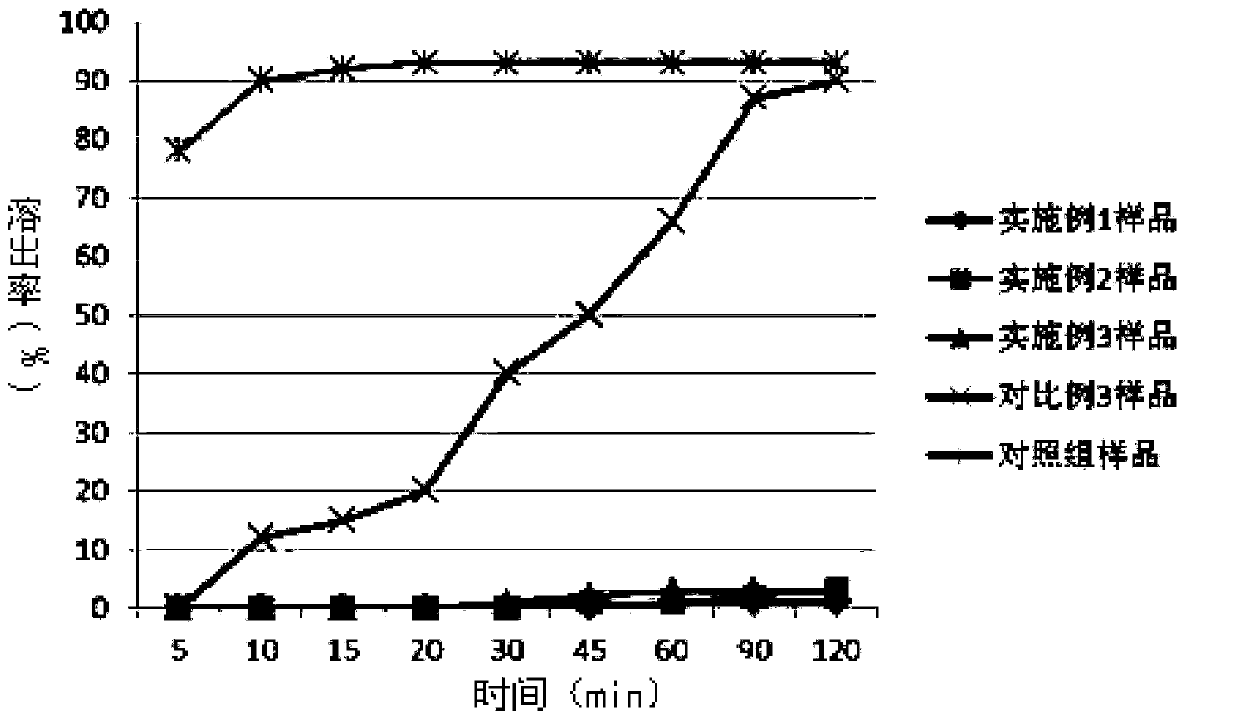
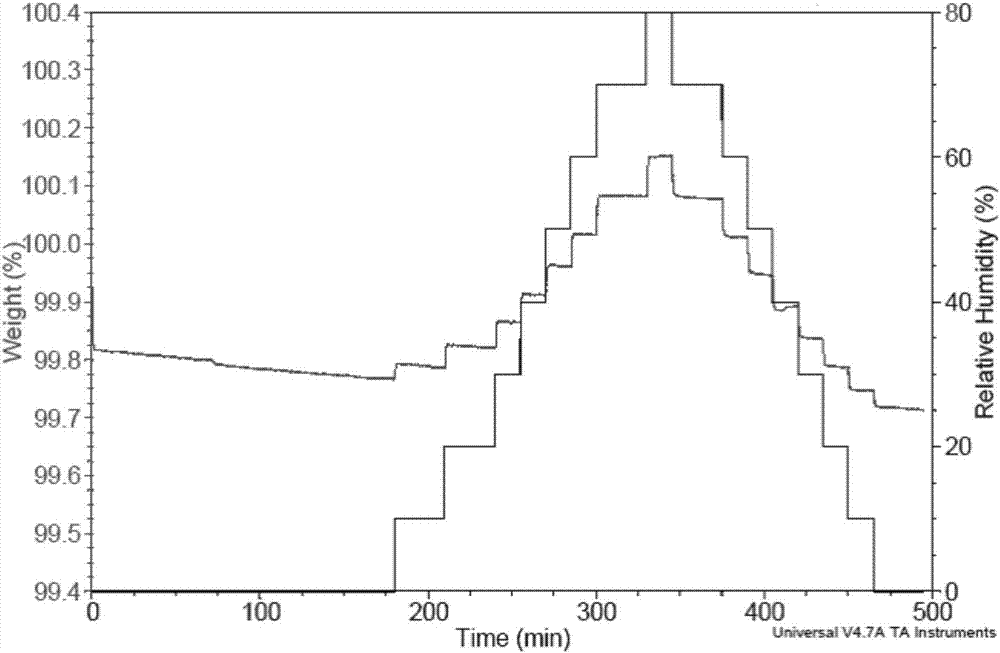
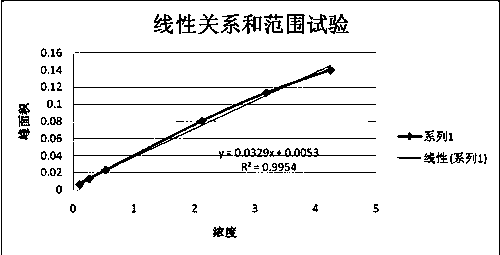
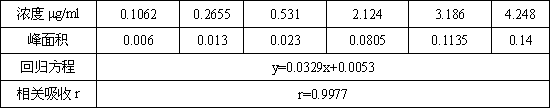
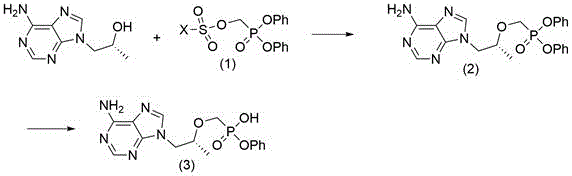
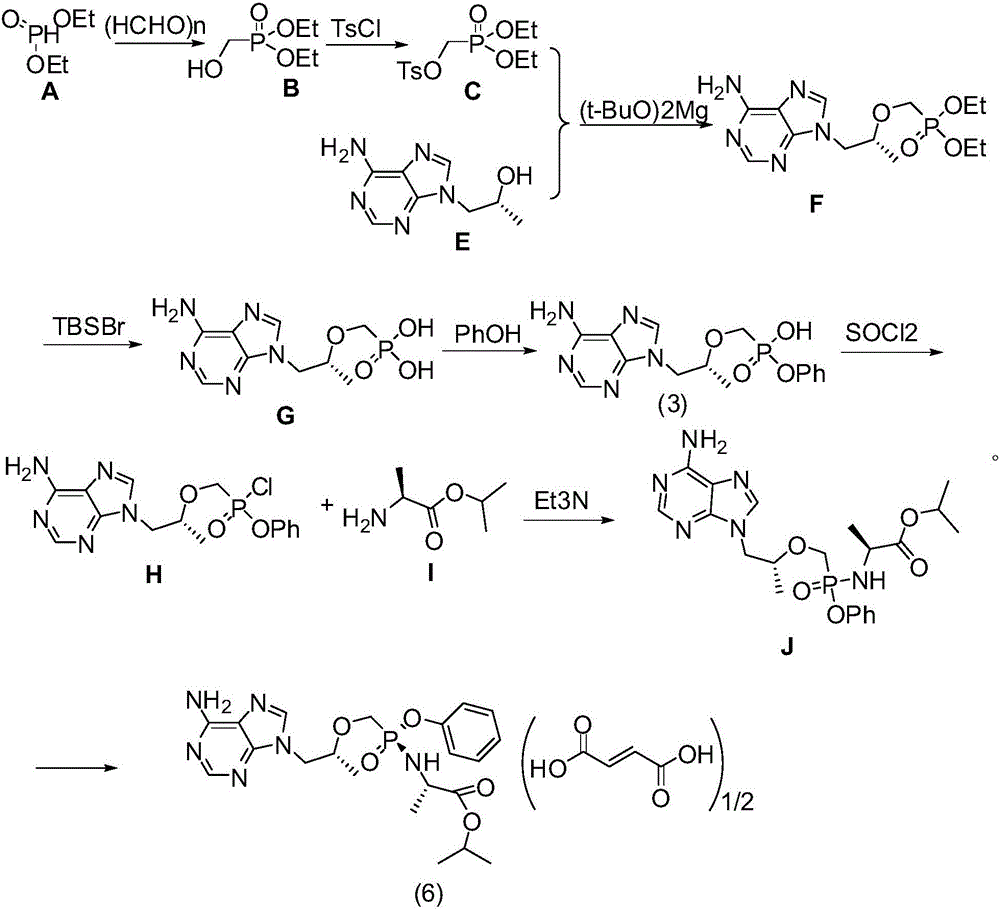
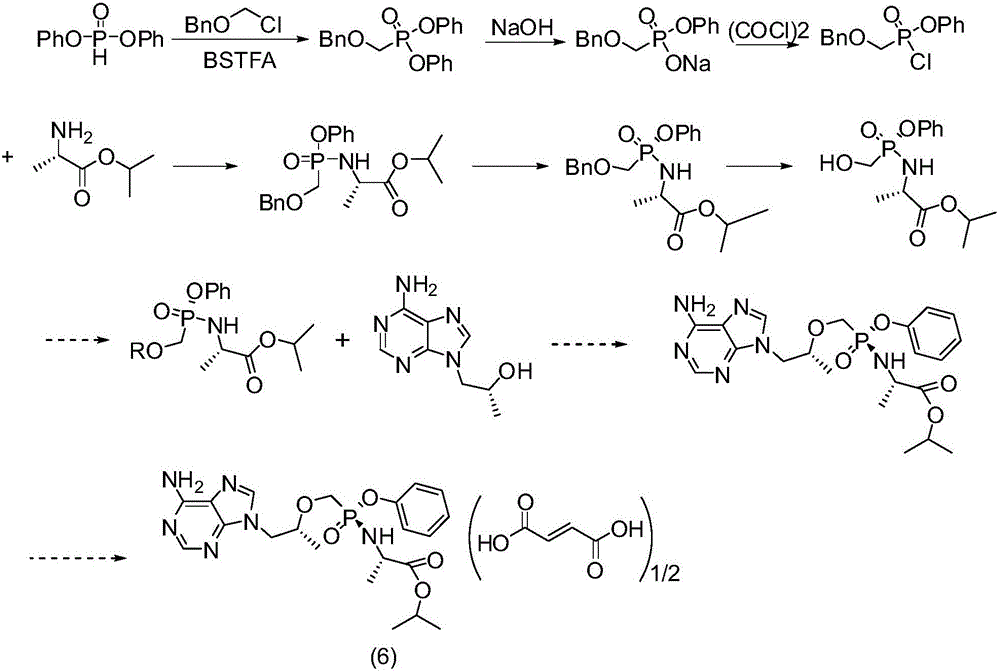
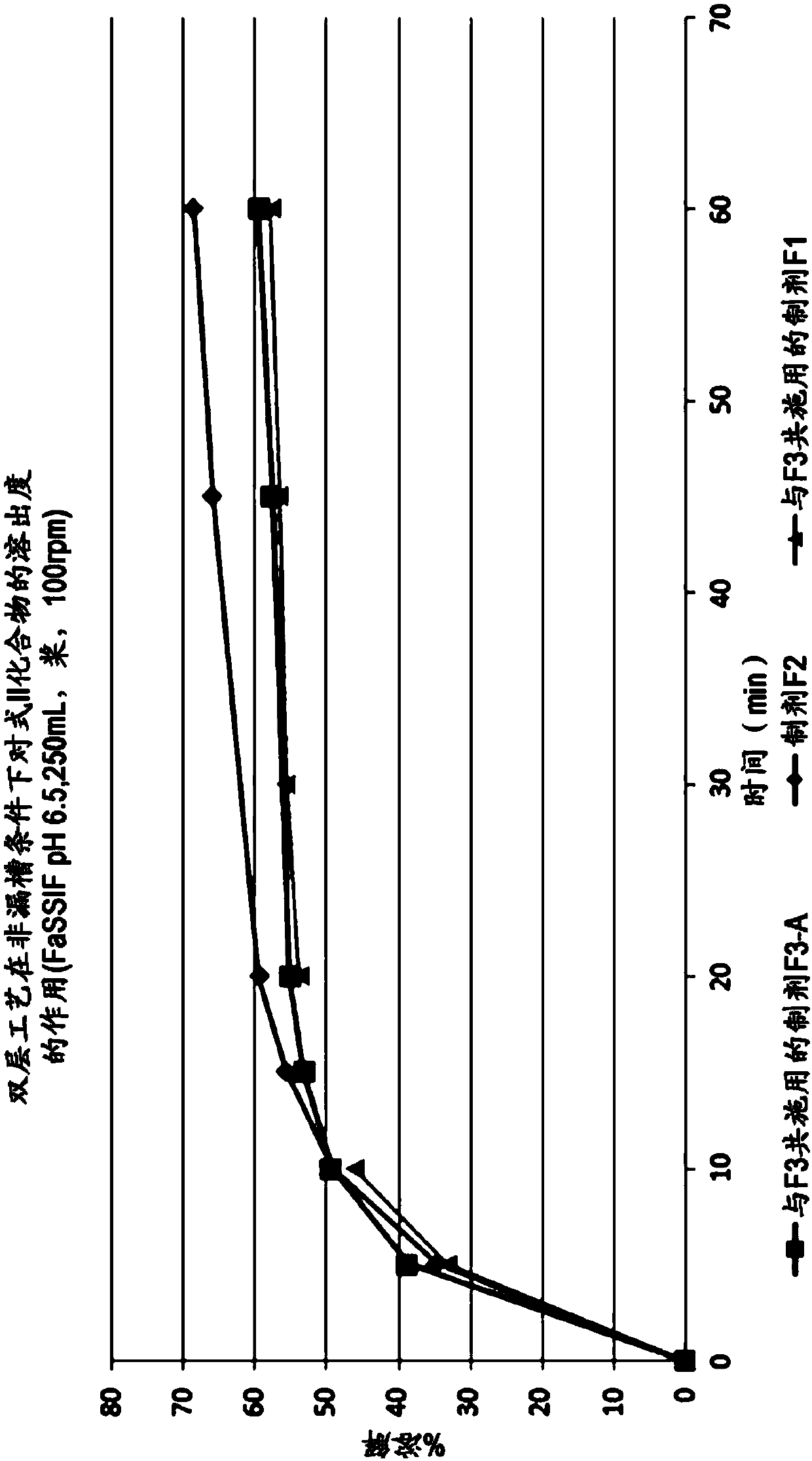
Patents
Literature
107 results about "Tenofovir alafenamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
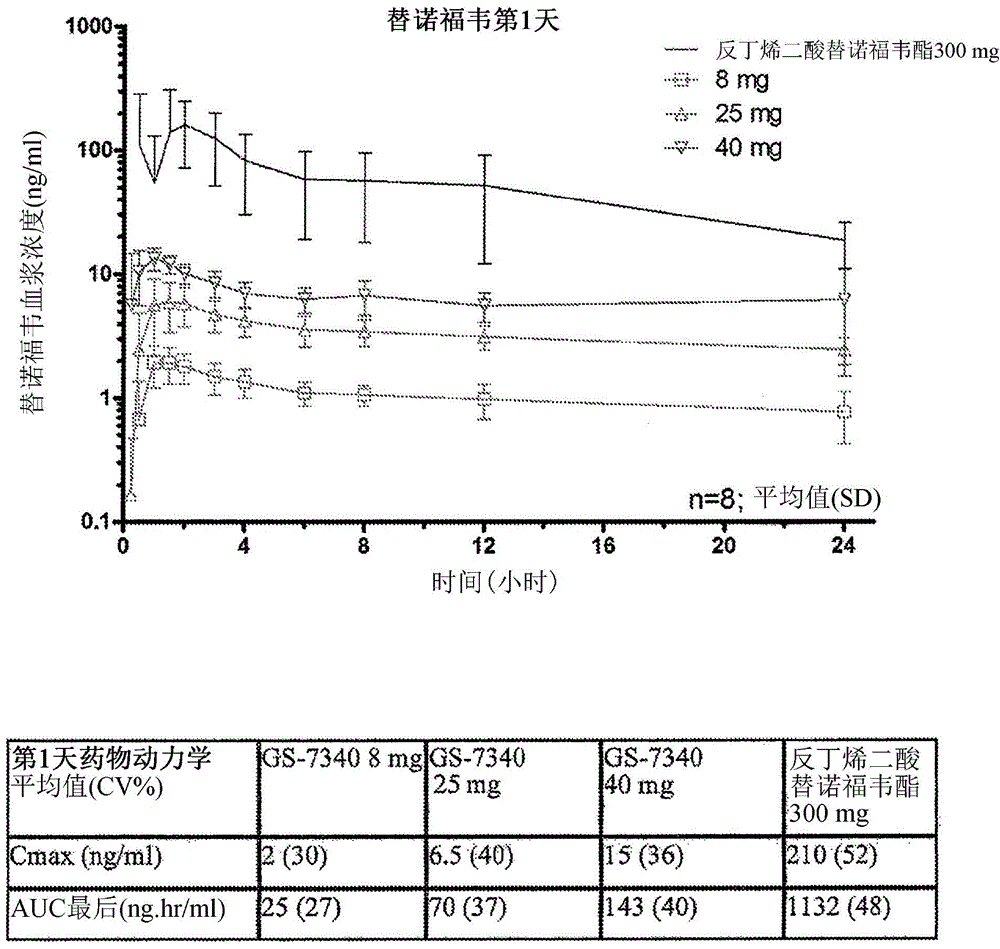
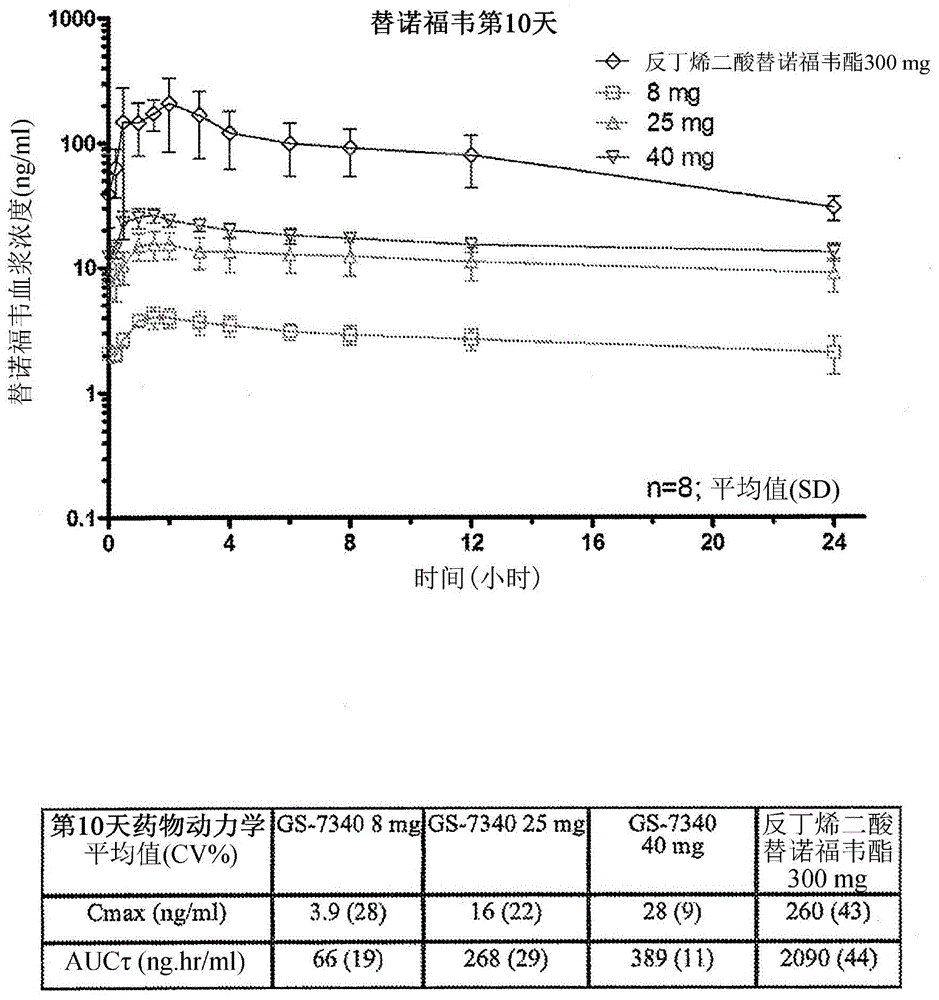
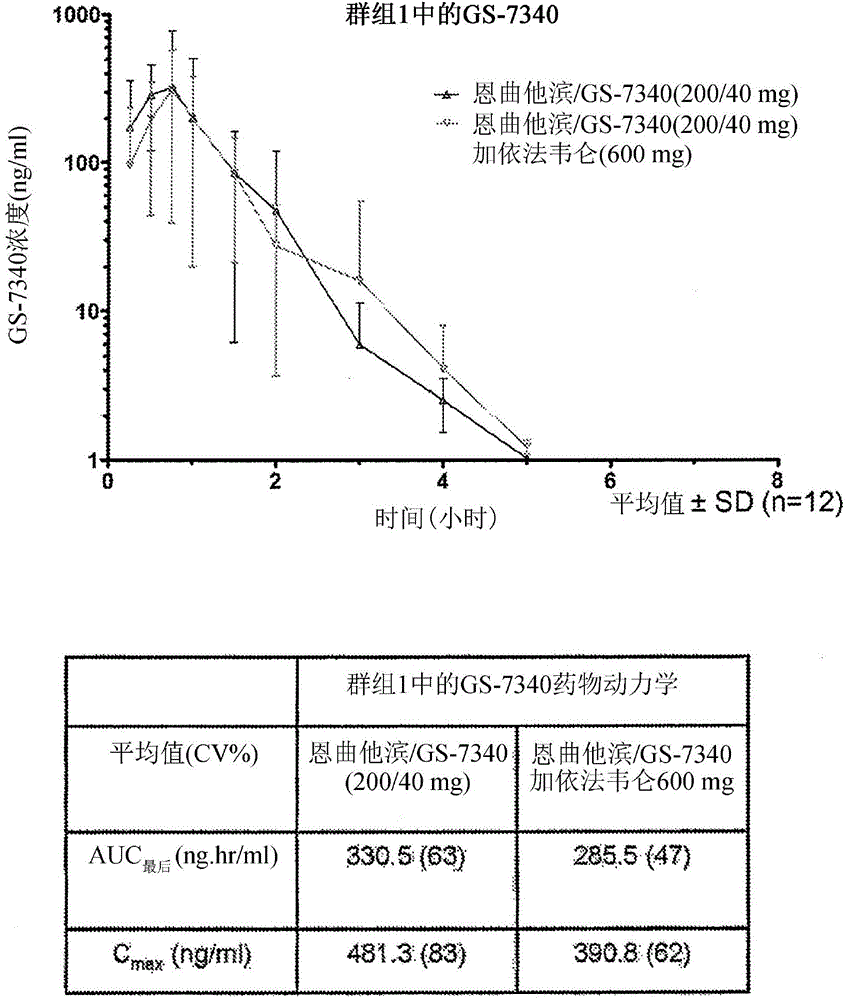
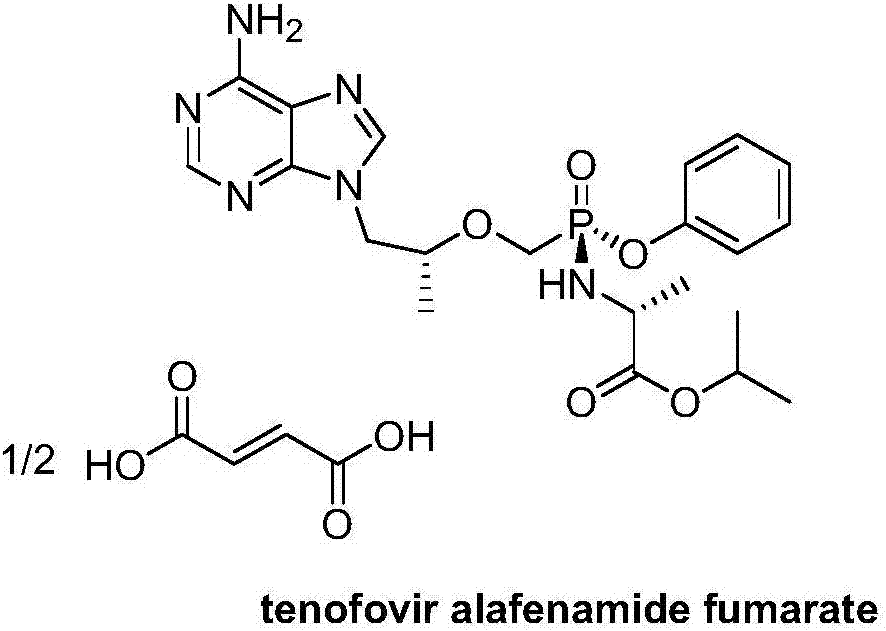
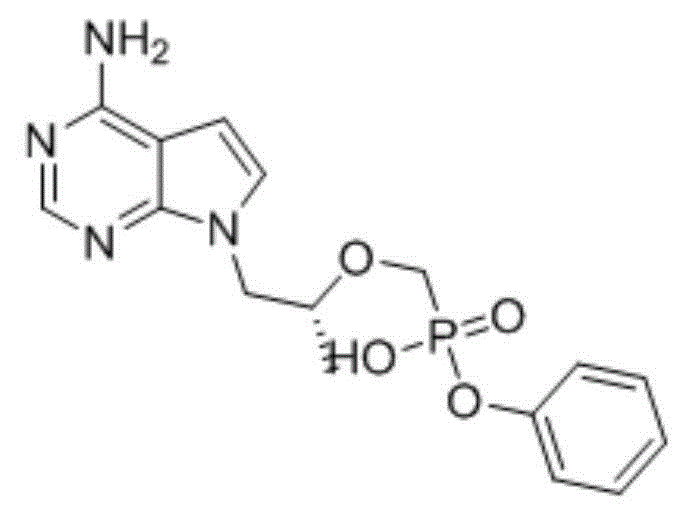
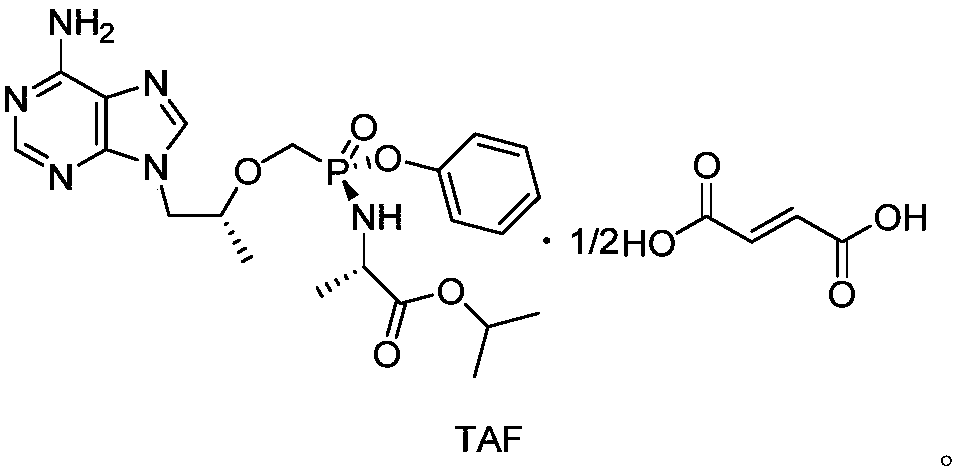
Tenofovir alafenamide (trade name Vemlidy) is a nucleotide reverse transcriptase inhibitor and a prodrug of tenofovir. It was developed by Gilead Sciences for use in the treatment of HIV infection and chronic hepatitis B, and is applied in the form of tenofovir alafenamide fumarate (TAF). Closely related to the commonly used reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF), TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent. Vemlidy was approved by the U.S. Food and Drug Administration (FDA) in November 2016.
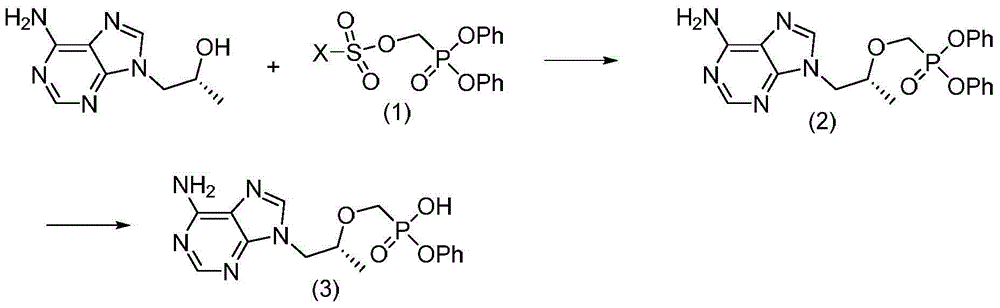
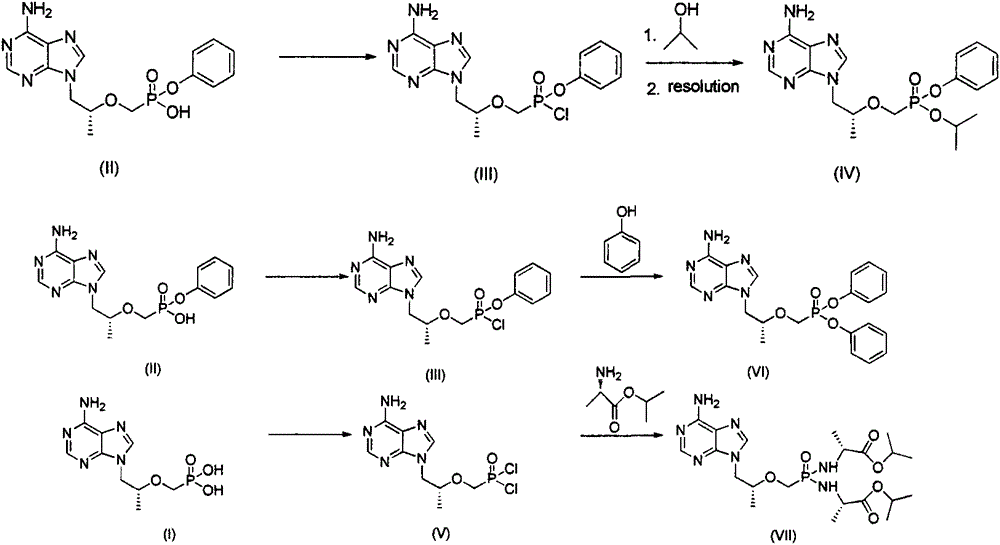
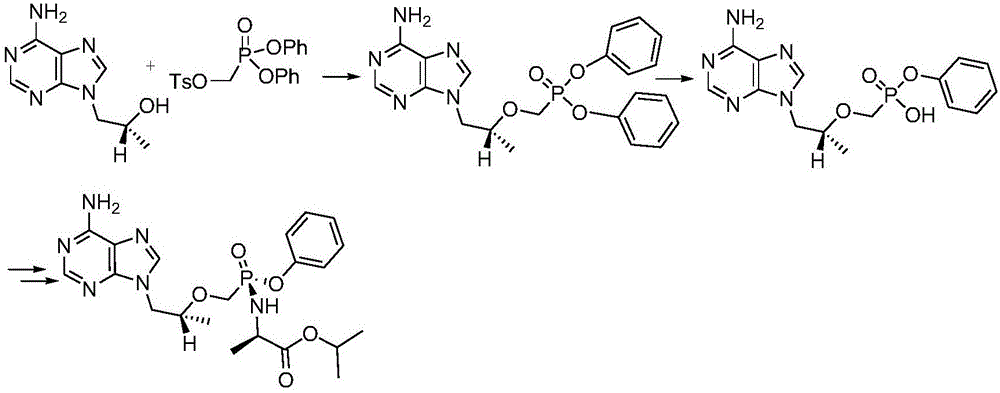
Tenofovir alafenamide compound, preparation method and purpose thereof
InactiveCN105085571AOrganic active ingredientsGroup 5/15 element organic compoundsTenofovir alafenamideHepatitis B virus
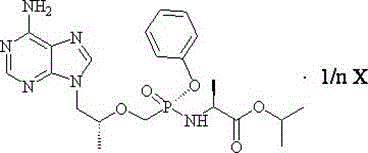
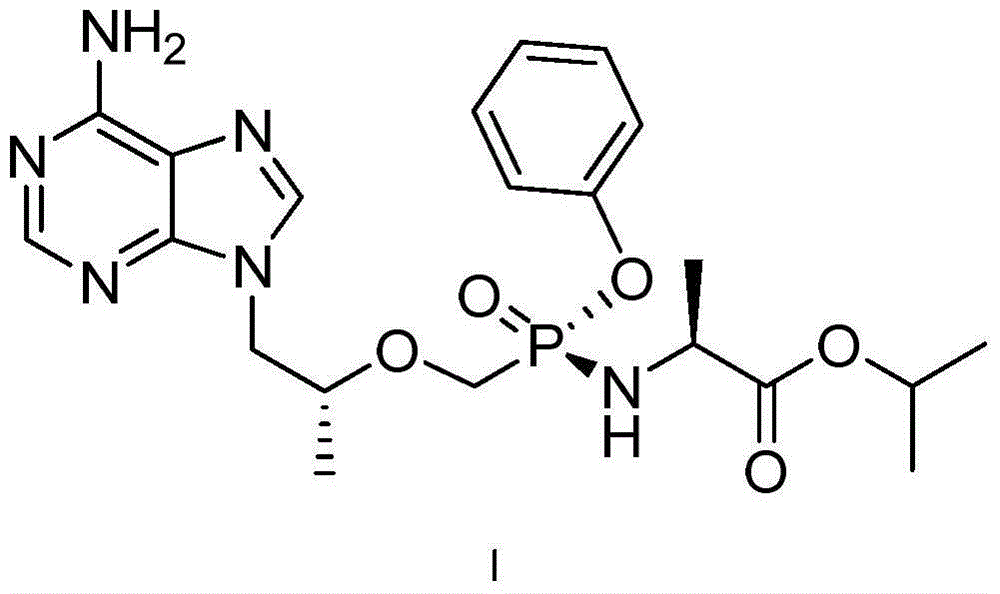
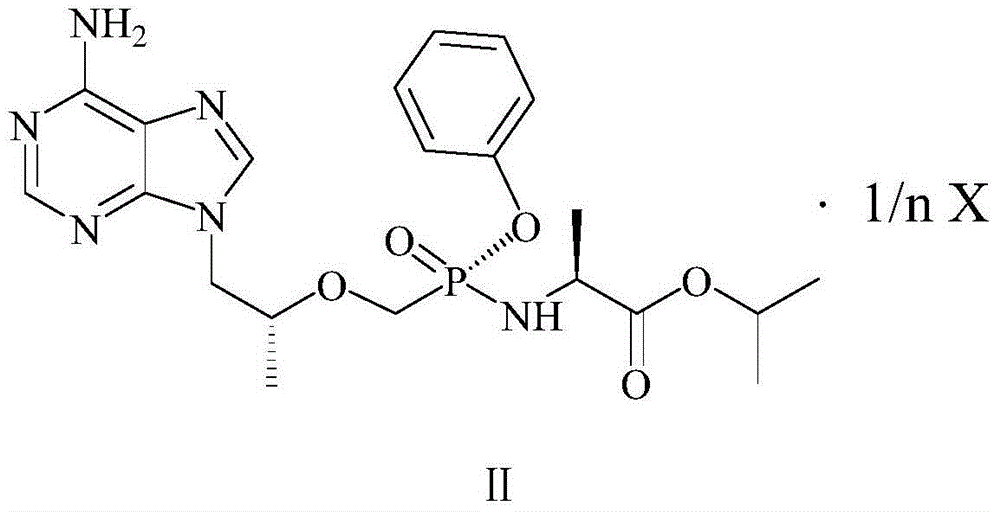
The present invention relates to tenofovir alafenamide complex represented by formula II. The present invention also relates to a preparation method for said tenofovir alafenamide complex, pharmaceutical compositions containing the tenofovir alafenamide complex, and uses of the tenofovir alafenamide complex in preparing medicines for preventing and / or treating virus infection, especially Hepatitis B Virus (HBV) and / or Human Immunodeficiency Virus (HIV) infection.
Owner:SICHUAN HAISCO PHARMA CO LTD
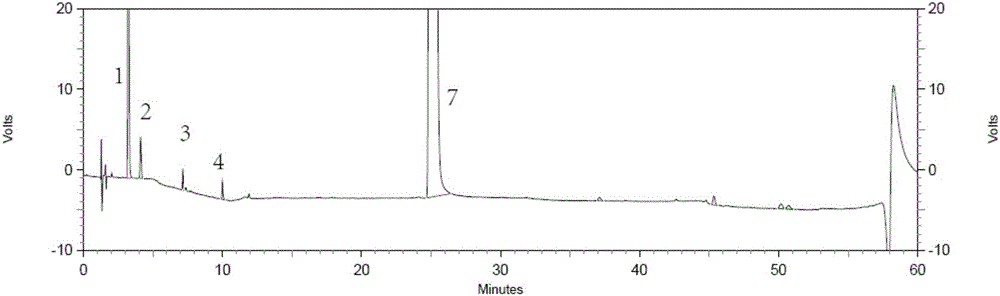
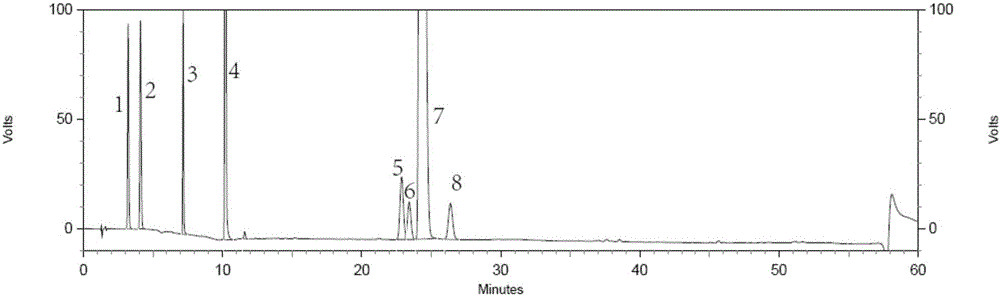
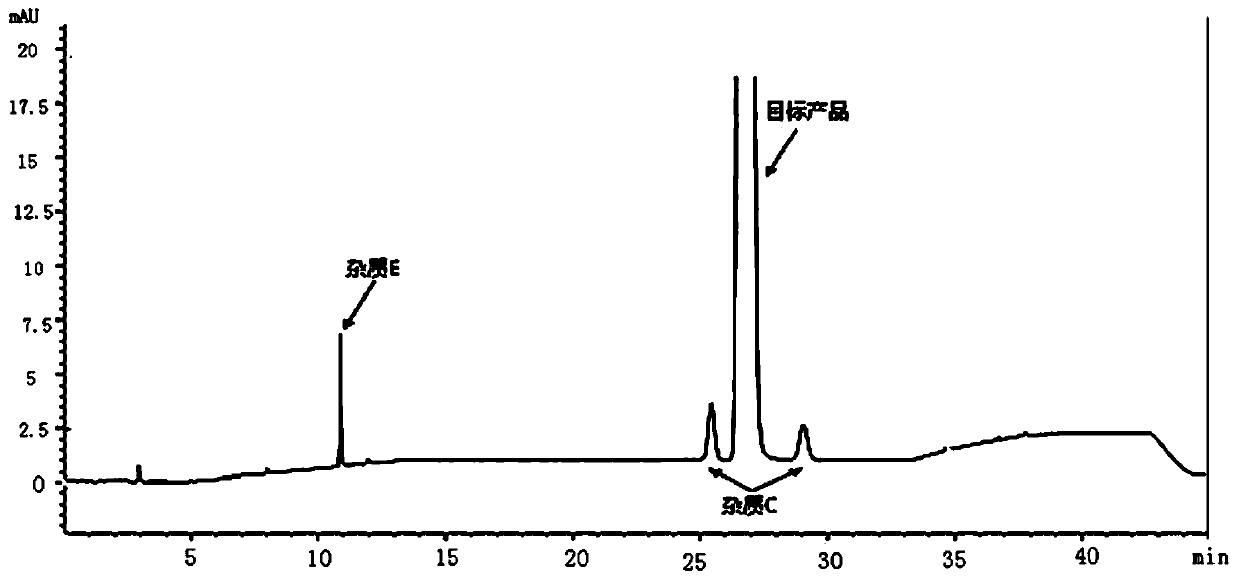
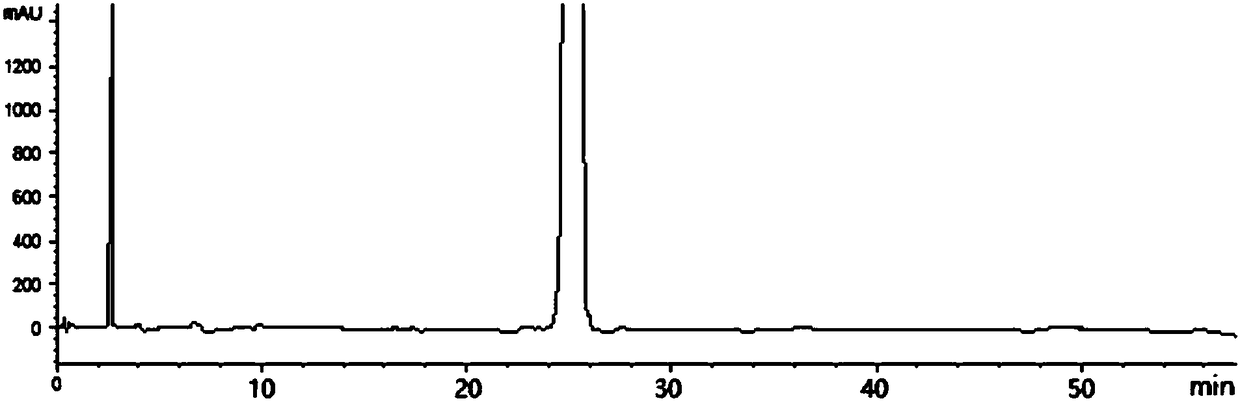
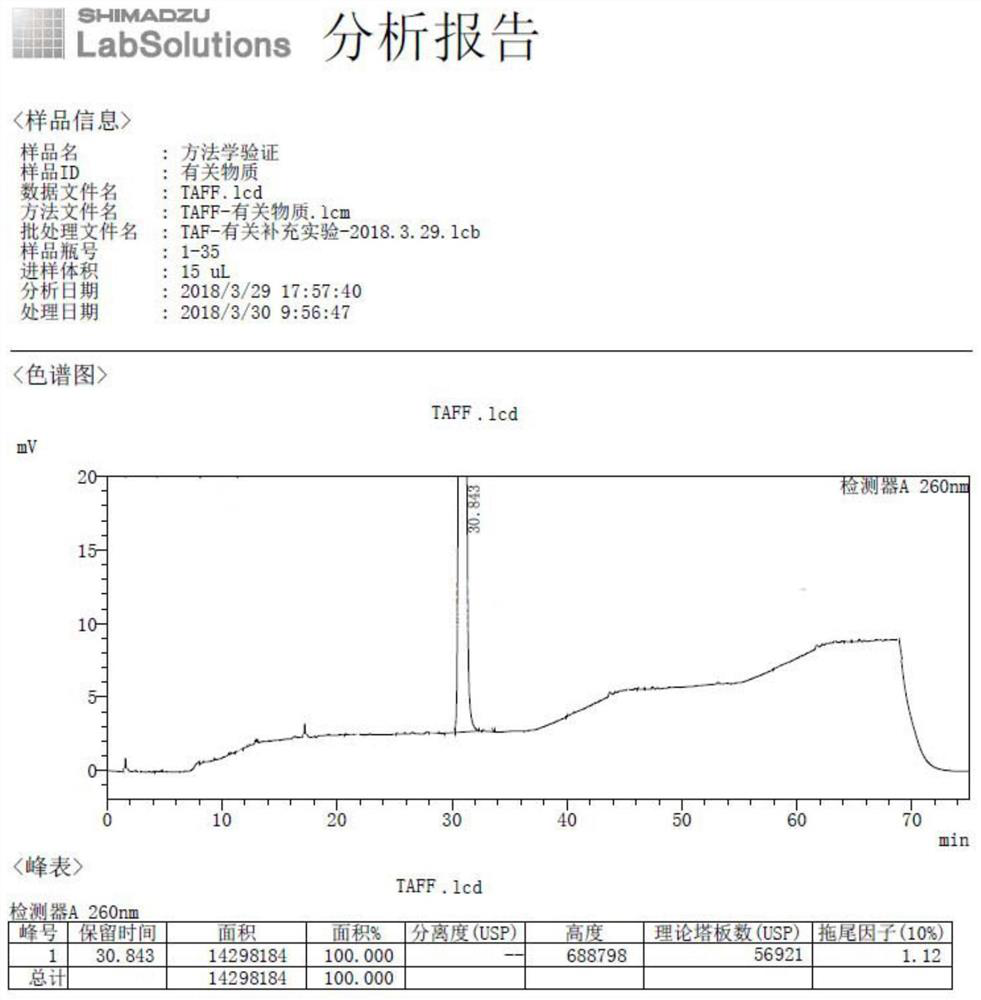
Method for separating and detecting tenofovir alafenamide and relevant substances thereof
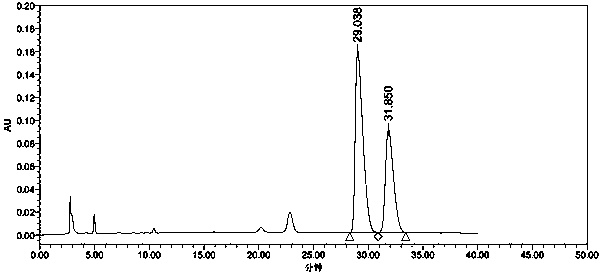
The invention relates to a method for separating and detecting tenofovir alafenamide and relevant substances thereof. The method can be used for detecting a tenofovir alafenamide sample by adopting an octadecyl-bonded ethylene bridge hybridization silica gel chromatographic column. According to the separating and detecting method disclosed by the invention, diastereomers and other relevant substances of the tenofovir alafenamide can be effectively separated and detected. The method has the characteristics of simplicity, convenience, accuracy and reliability, and is suitable for controlling the quality of tenofovir alafenamide products in industry.
Owner:SUNSHINE LAKE PHARM CO LTD
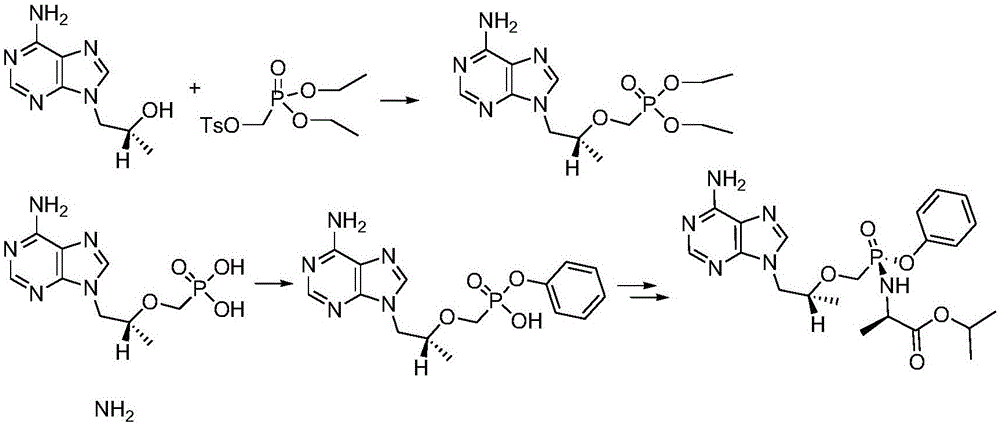
Synthetic process of key intermediate of hemifumarate tenofovir alafenamide
ActiveCN104817593AAvoid harsh conditionsThe reaction steps are simpleGroup 5/15 element organic compoundsTenofovir alafenamideReaction step
The invention discloses a synthetic process of key intermediate of hemifumarate tenofovir alafenamide. A synthetic route is shown below. The synthetic process has the advantages that the strict conditions of the prior art are avoided, the reaction steps are simplified, the raw materials are easy to obtain, reaction is moderate, the cost is lower and the synthetic process is suitable for industrial production.
Owner:GUANGZHOU TROJAN PHARMATEC LTD
Preparation method and application for high-purity tenofovir alafenamide fumarate intermediate
ActiveCN106478725ASimple and safe operationHigh yieldGroup 5/15 element organic compoundsOrganic chemistry methodsTenofovir alafenamideSolvent
The invention discloses a preparation method and an application for a high-purity tenofovir alafenamide fumarate intermediate. The preparation method for the tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II provided by the invention comprises the following steps: subjecting crude tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II to recrystallization in a mixed solvent of a nitrile solvent and water or a mixed solvent of the nitrile solvent and an aromatic hydrocarbon solvent so as to obtain the high-purity tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II. The high-purity tenofovir alafenamide II has an optical purity larger than 99.50% and a chemical purity larger than 99.60%; and the crude tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II has the optical purity in a range of 60.00% to 99.00% and the chemical purity in a range of 60.00% to 99.00%. The preparation method provided by the invention has the advantages of simple and safe operation, high yield, high product purity, low production cost, and applicability to industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Tenofovir alafenamide fumarate impurity preparing method
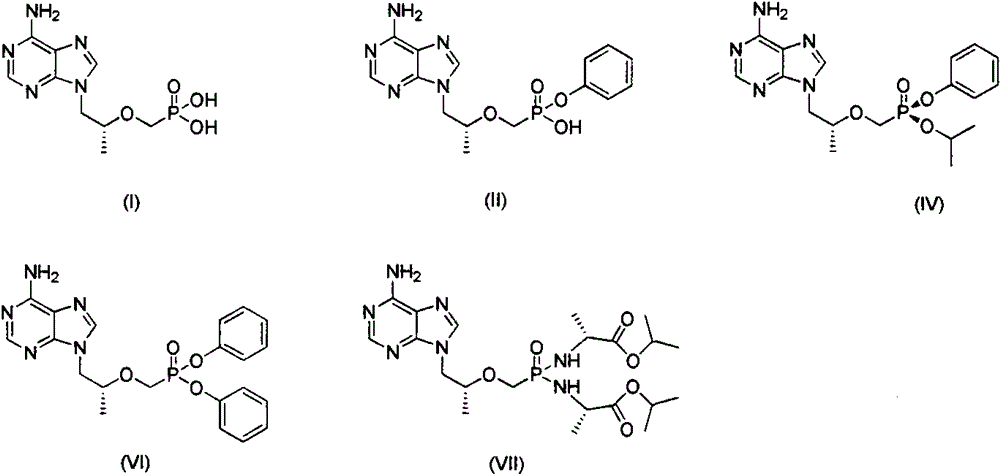
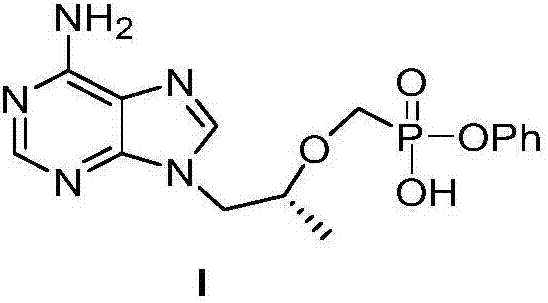
The invention relates to a novel synthetic method of three tenofovir alafenamide fumarate impurities. The synthetic method has important significance in synthesis of high-quality tenofovir alafenamide fumarate. The invention mainly aims at studying synthesis of a tenofovir alafenamide isopropyl ester impurity 9-[(R)-2-[[(S)-[[(S)-1-isopropoxy phenoxyl phosphinyl] methoxyl] propyl] adenine (IV), a tenofovir alafenamide diphenyl ester impurity 9-[(R)-2-[[(S)-[bi-(phenoxyl) phosphinyl] methoxyl] propyl] adenine (VI) and a tenofovir alafenamide diamide impurity 9-[(R)-2-[[bi-[[(S)-1-(isopropoxy carbonyl) ethyl] amino] phosphinyl] methoxyl] propyl] adenine (VII), and their specific synthesis routes are shown in the description.
Owner:CHINA PHARM UNIV
Combination therapy comprising tenofovir alafenamide hemifumarate and cobicistat for use in the treatment of viral infections
InactiveCN104105484AAntiviralsHeterocyclic compound active ingredientsEmtricitabineTenofovir alafenamide
The use of the hemifumarate form of {9-[(R)-2-[[(S)-[[(S)-l- (isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine} (tenofovir alafenamide hemifumarate) in combination with cobicistat is disclosed. In addition, the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and elvitegravir, and the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and darunavir, are disclosed.
Owner:GILEAD SCI INC
Preparation method of TAF (tenofovir alafenamide fumarate) nucleoside derivative and intermediate of TAF nucleoside derivative
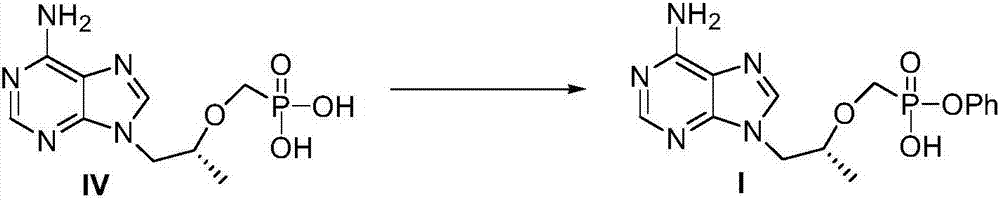
The invention discloses a preparation method of a TAF (tenofovir alafenamide fumarate) nucleoside derivative and an intermediate of the TAF nucleoside derivative. The method comprises the steps that in a solvent of a compound II, in the presence of alkali, stirring is performed until the reaction is completed, and a required compound I is obtained. Compared with the prior art, the preparation method has the advantages that the raw materials are cheap and are easily obtained; the reaction conditions are mild; the side reactions are few; the yield is high; the environment pollution is little; the preparation method is applicable to industrial production; a new path is provided for the preparation of the TAF key intermediate. The formulas are shown in the specification.
Owner:FUJIAN COSUNTER PHARMA CO LTD
HPLC detecting method for tenofovir alafenamide and isomer thereof
ActiveCN107655987AEffective separation assayEasy to separateComponent separationIsocratic elutionFractionation
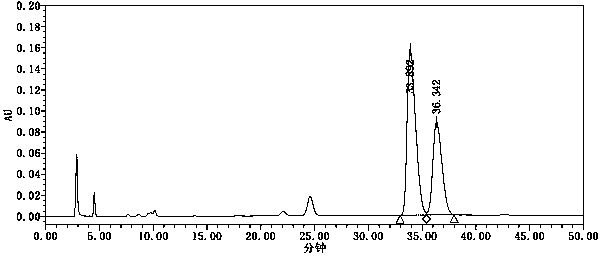
The invention relates to a fractionation detection method for tenofovir alafenamide and isomer thereof, and belongs to the technical field of fractionation detection. The detection method comprises the following steps: taking a chromatographic column filled with octadecylsilane chemically bonded silica; and carrying out isocratic elution by taking methanol and an acidic aqueous solution as mobilephases under certain conditions. By the method, the tenofovir alafenamide and the isomer thereof can be effectively detected in a fractionation manner, the peak pattern of chromatographic peaks is good, the appearance time is short, and the degree of fractionation is good.
Owner:厦门蔚扬药业有限公司
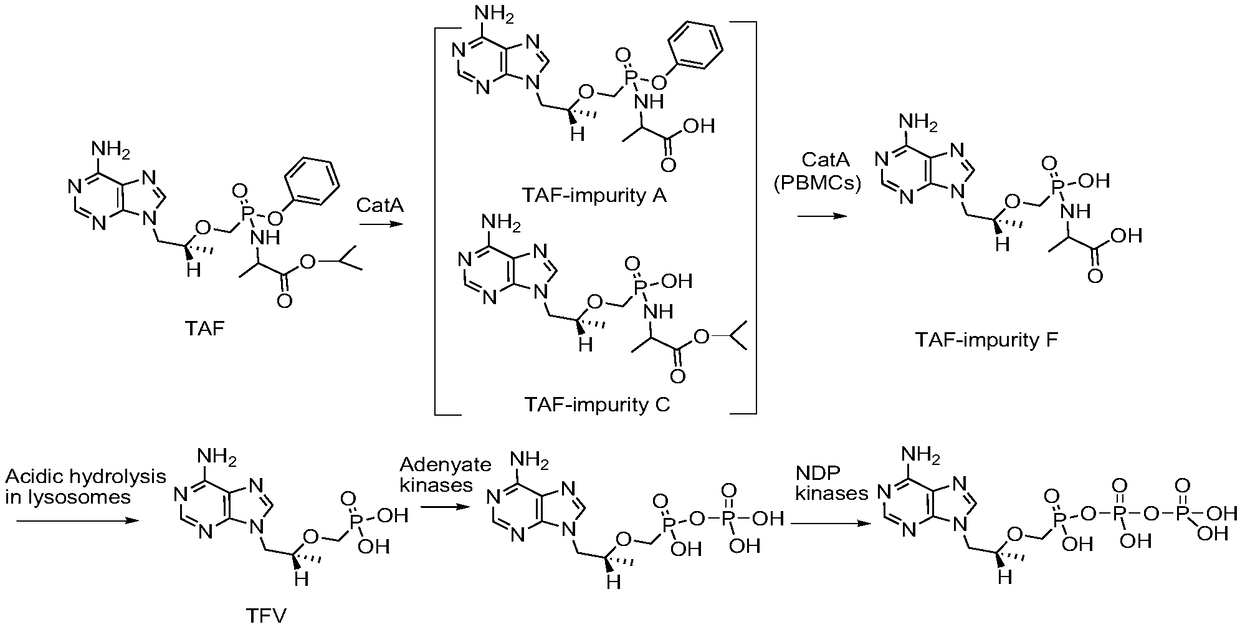
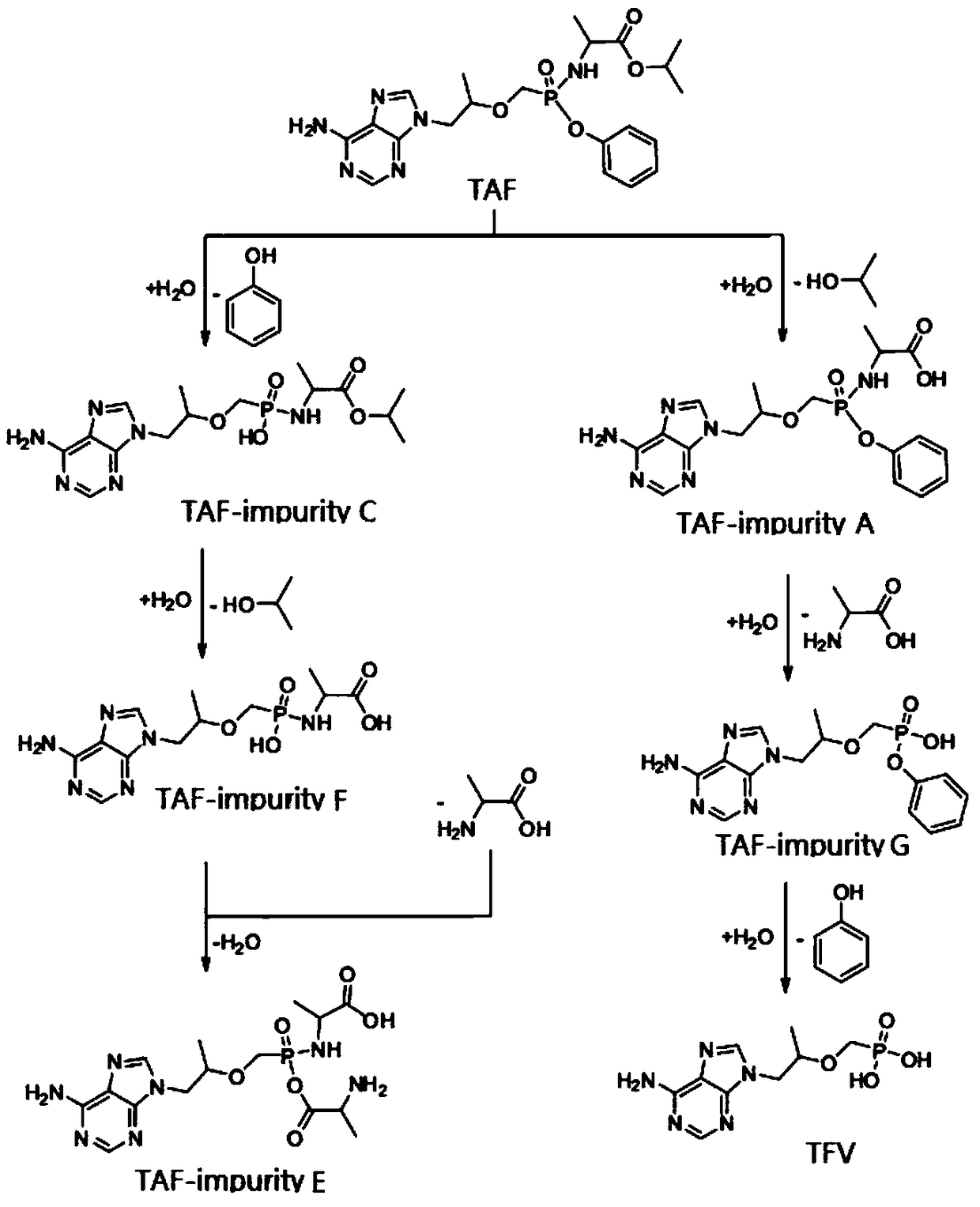
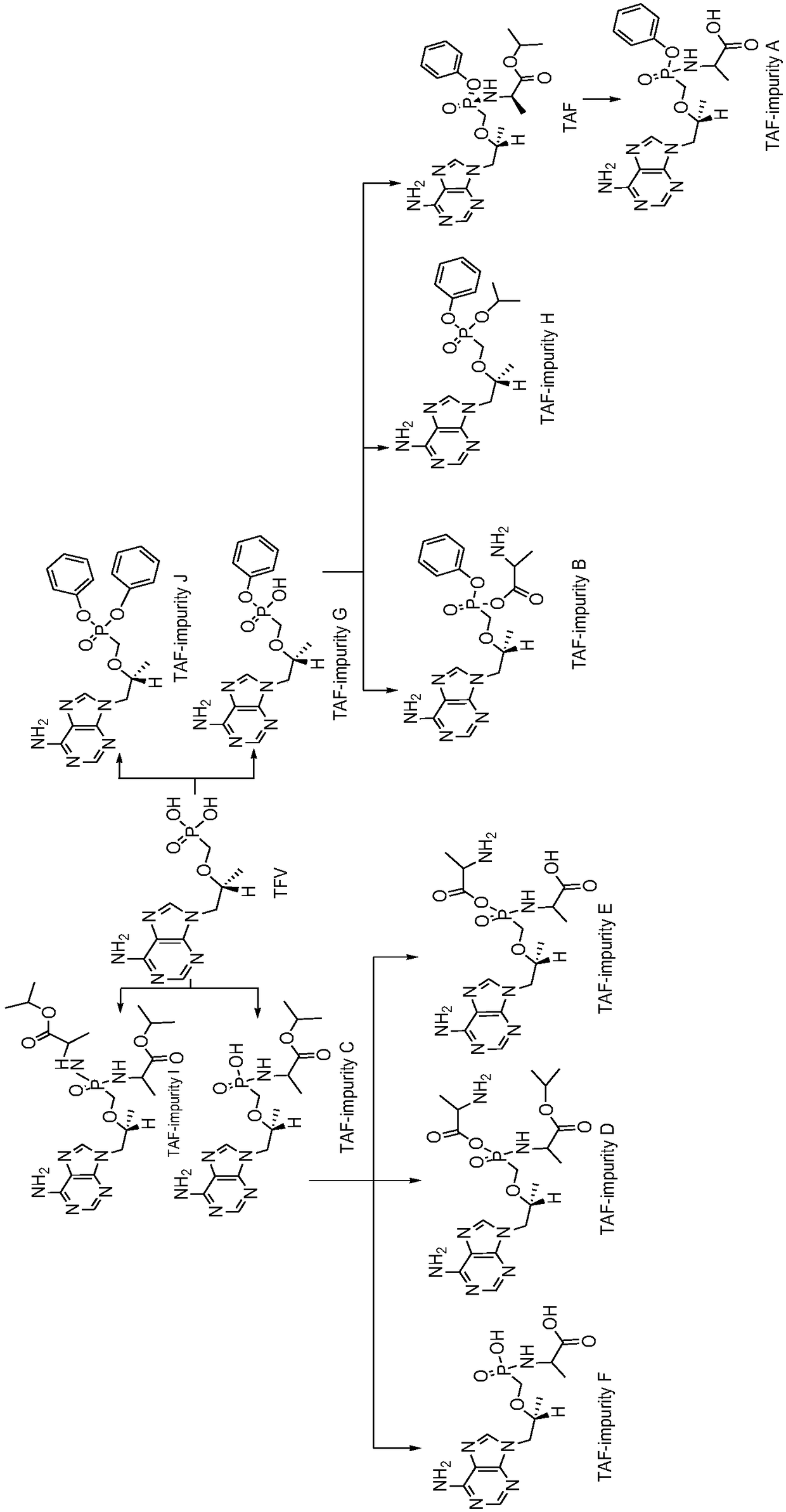
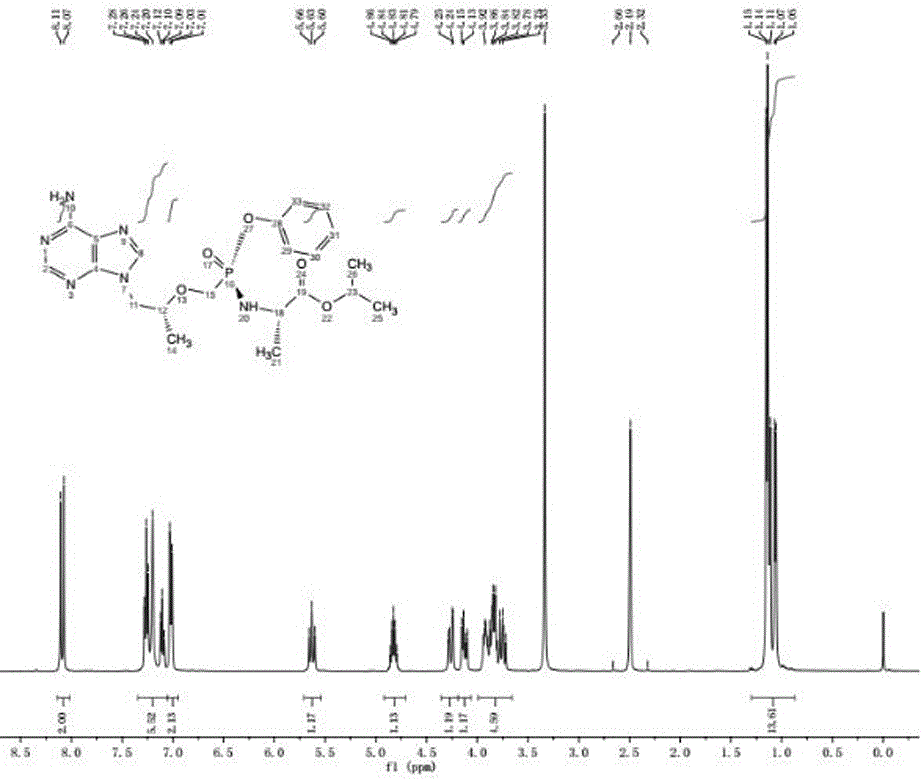
Tenofovir alafenamide series impurities and synthesis method thereof
InactiveCN110256494AQuality Research FacilitationComponent separationOrganic chemistry methodsSynthesis methodsTenofovir alafenamide
The present invention provides tenofovir alafenamide series impurities and a synthesis method thereof. The series impurities are found in the process of synthesizing tenofovir alafluamine by the inventor of the invention, and are new impurities not reported in the prior art, so the synthesis method of the series impurities is provided, the studies of the tenofovir alafenamide impurities are benefited, and great convenience can be brought for the development of analytical methods and the quality studies of medicines.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Preparation method and application of process impurity of tenofovir alafenamide fumarate
InactiveCN110092803AComponent separationGroup 5/15 element organic compoundsCompound aTenofovir alafenamide
The present invention relates to a process impurity of tenofovir levamide amine fumarate 9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine hemifumarate, and a preparation method thereof. The impurity is selected from a compound A (S)- isopropyl-2-(((R)-((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy)methyl)(methoxy)phosphinyl)amino)propanoate fumarate andthe like.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
Industrialized continuous production method of hemifumarate tenofovir alafenamide
InactiveCN107522743AInhibition of diastereomersHigh purityGroup 5/15 element organic compoundsCarboxylic acid salt preparationEnantiomerTenofovir alafenamide
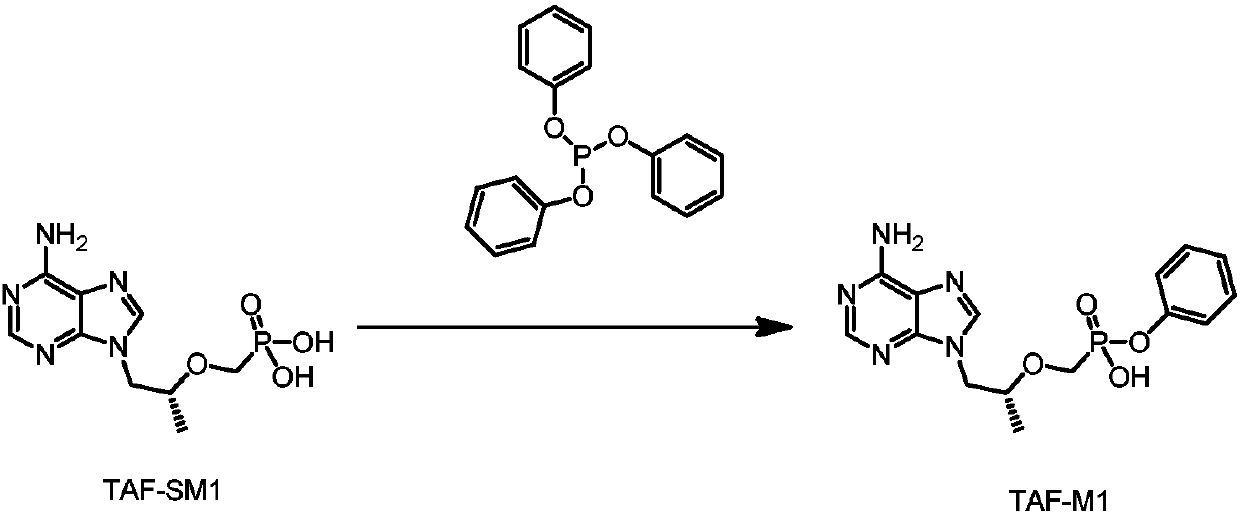
The invention discloses an industrialized continuous production method of tenofovir alafenamide and hemifumarate. The method comprises the following steps: firstly, in the presence of an acid-binding agent, reacting tenofovir with triphenyl phosphate to obtain a TAF-I M shown as a formula II; secondly, continuously preparing a TAF-II M by using the TAF-I M; thirdly, carrying out a salt forming reaction on the TAF-II M to obtain the hemifumarate tenofovir alafenamide. According to the industrialized continuous production method disclosed by the invention, a key compound tenofovir alafenamide is obtained by continuous production; the hemifumarate tenofovir alafenamide is obtained by accurately feeding fumaric acid and the tenofovir alafenamide; and in addition, a diastereoisomer of the tenofovir alafenamide is inhibited by using high catalytic enantioselectivity of a proline catalyst, and industrial production of optically-pure tenofovir alafenamide of which the purity is greater than 99.9 percent is realized by primary crystallization. The industrial production method disclosed by the invention has the advantages of simplicity, safety and low production cost; and besides, a high-purity product is obtained.
Owner:SHENZHEN KEXING PHARM CO LTD
Tenofovir alafenamide semi-tartrate
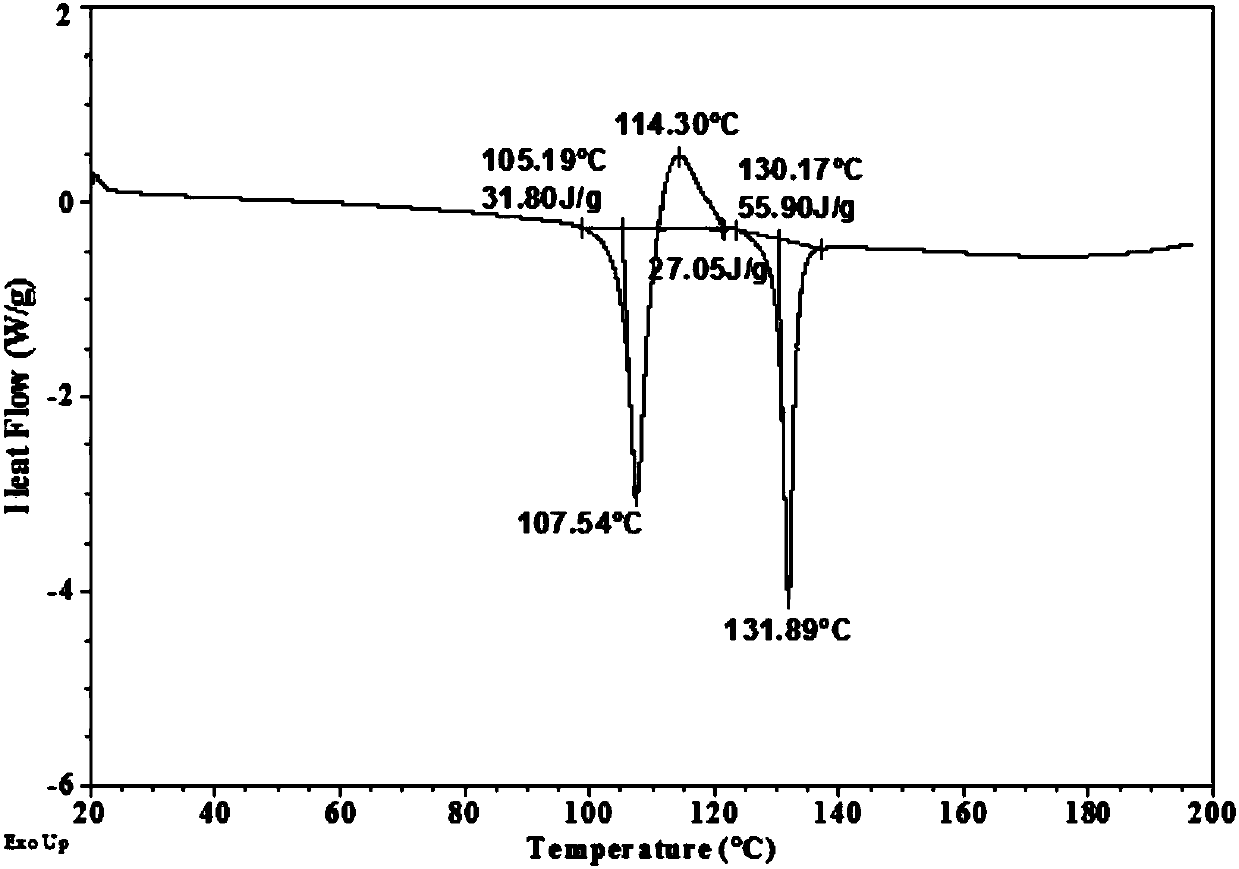
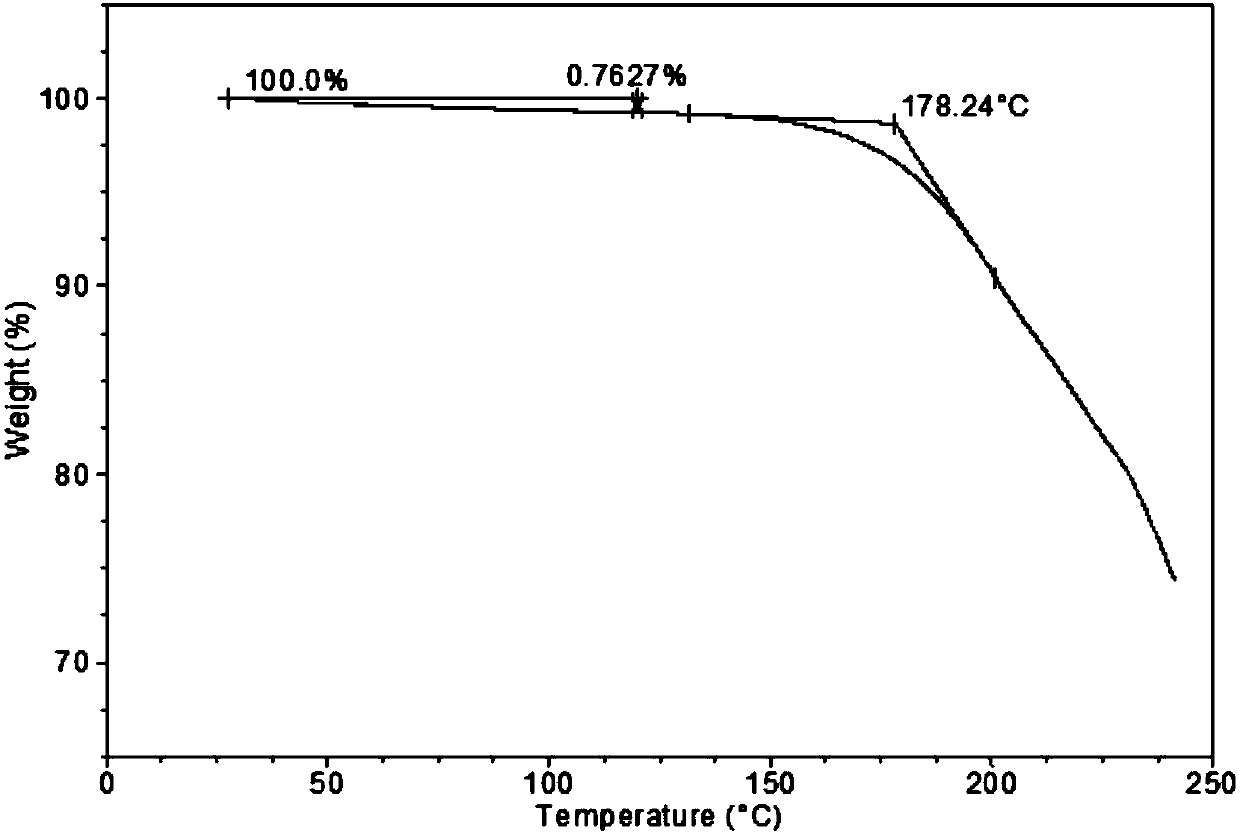
ActiveCN104926872AGood chemical stabilityImprove thermal stabilityOrganic active ingredientsGroup 5/15 element organic compoundsTenofovir alafenamideCrystallinity
The invention provides a novel pharmaceutically acceptable salt of tenofovir alafenamide, and the 9-[(R)-2-[[(S)-[[(S)-1-(isopropionic carbonyl)ethyl]amino]phenoxylphosphinyl]methoxy]propyl]adenine semi-tartrate. The salt has modified chemical stability and thermal stability, and has a relatively high melting point. The salt is more suitable to be used as a raw materials medicine. Also, the salt has excellent crystallinity. The salt can be prepared with a convenient method, and purity and yield are further improved. The salt is suitable for large-scale industrialized productions.
Owner:HANGZHOU HEZE PHARMA TECH +2
TAF(tenofovir alafenamide fumarate) preparation method
InactiveCN105131038AReduce pollutionOptimizationGroup 5/15 element organic compoundsEtherPropylene carbonate
The invention provides a TAF(tenofovir alafenamide fumarate) preparation method. The method comprises taking adenine as an initial raw material, employing propylene carbonate to add isopropanol at 1 position, then under the effect of magnesium isopropoxide, reacting with diethyl (tosyloxy)methylphosphonate, then removing ether, then reacting with phenol for condensation, and performing chlorination, condensation, resolution and salification, so as to obtain the product. The technology route is subjected to optimization and improvement, and pollution discharge is reduced.
Owner:ZHEJIANG TIANSHUN BIOTECH
Preparation method of tenofovir alafenamide hemifumarate
InactiveCN107793452AAddressing StereoselectivitySolve control problemsOrganic compound preparationGroup 5/15 element organic compoundsTenofovir alafenamidePhenol
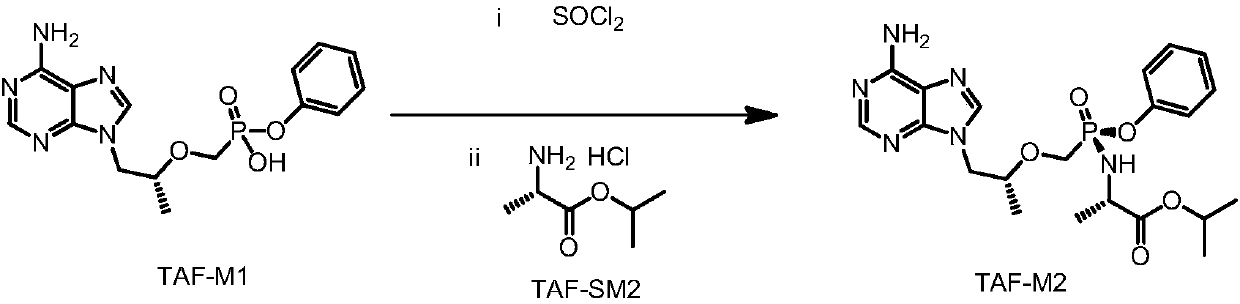
The invention discloses a preparation method of tenofovir alafenamide hemifumarate. The preparation method comprises the following steps of: reacting tenofovir with triphenyl phosphite under the condition of alkali catalysis to prepare tenofovir phenol ester; preparing tenofovir alafenamide through acylating chlorination and a reaction with L-alanine isopropyl ester; preparing tenofovir alafenamide hemifumarate by a special salt-forming process with fumaric acid. The tenofovir alafenamide hemifumarate prepared by the process is accurate in control of a salt type ratio, and simple in process operation, and avoids complex process operation and purification means such as chiral resolution, the total purity can reach 99% or more, and indexes of related substances are qualified.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Efficient synthesis technology of tenofovir alafenamide
InactiveCN110105392AHigh purityShort conversion timeGroup 5/15 element organic compoundsIsomerizationReaction temperature
The invention discloses an efficient synthesis technology of tenofovir alafenamide. The efficient synthesis technology comprises the steps that A, tenofovir reacts with triphenyl phosphite under an alkali catalysis condition to prepare tenofovir monophenyl ester, wherein the initial reaction temperature is 50-70 DEG C, the reaction temperature rises according to the gradient of 5-10 DEG C / h, and the total reaction duration is 6-10 h; B, the tenofovir monpohenyl ester is subjected to acylating chlorination to prepare tenofovir phenyl ester phosphoryl chloride; C, the tenofovir phenyl ester phosphoryl chloride is isomerized, then the isomerized tenofovir phenyl ester phosphoryl chloride reacts with an L-alanine isopropyl ester compound to prepare a target compound, wherein methylbenzene andother non-polar solvents are adopted for an isomerization solvent. The prepared tenofovir alafenamide has high purity and high yield, the technology operation is simple, the cost is low, complicated technology operation and purification means like using chiral resolution agents are omitted, and the efficient synthesis technology is very suitable for industrial production.
Owner:石家庄凯赛医药科技有限公司
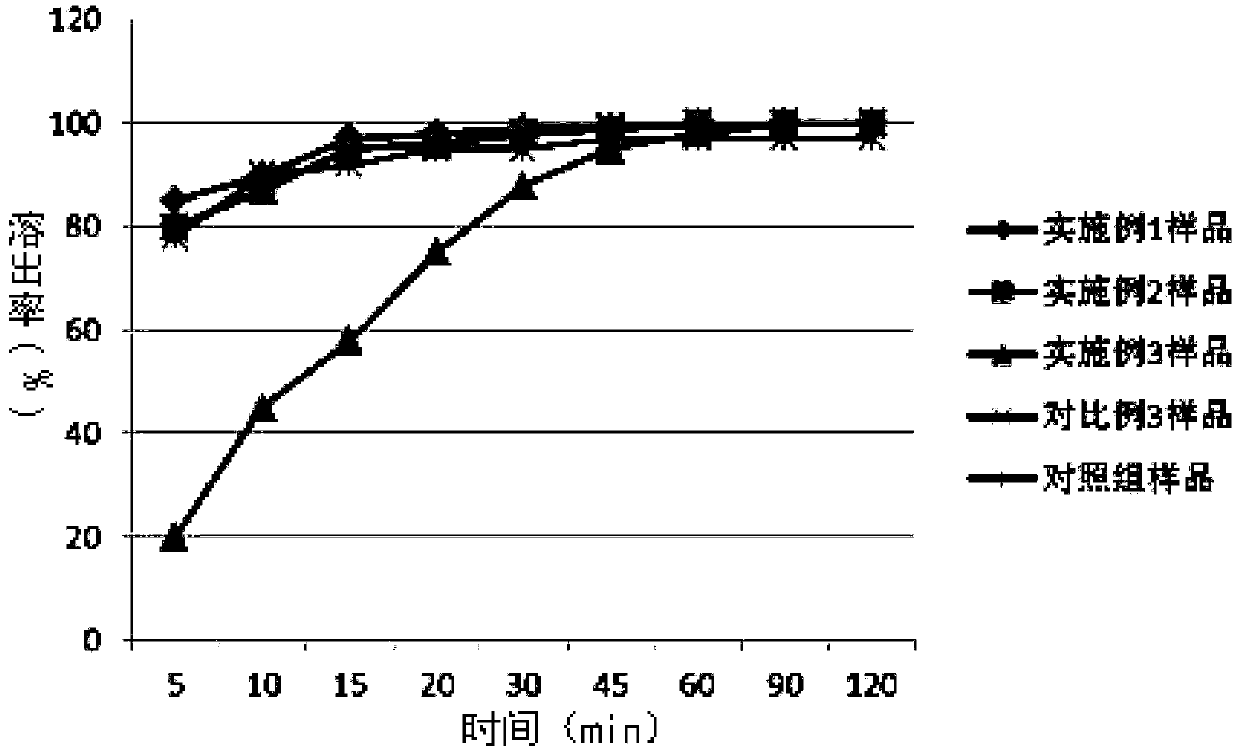
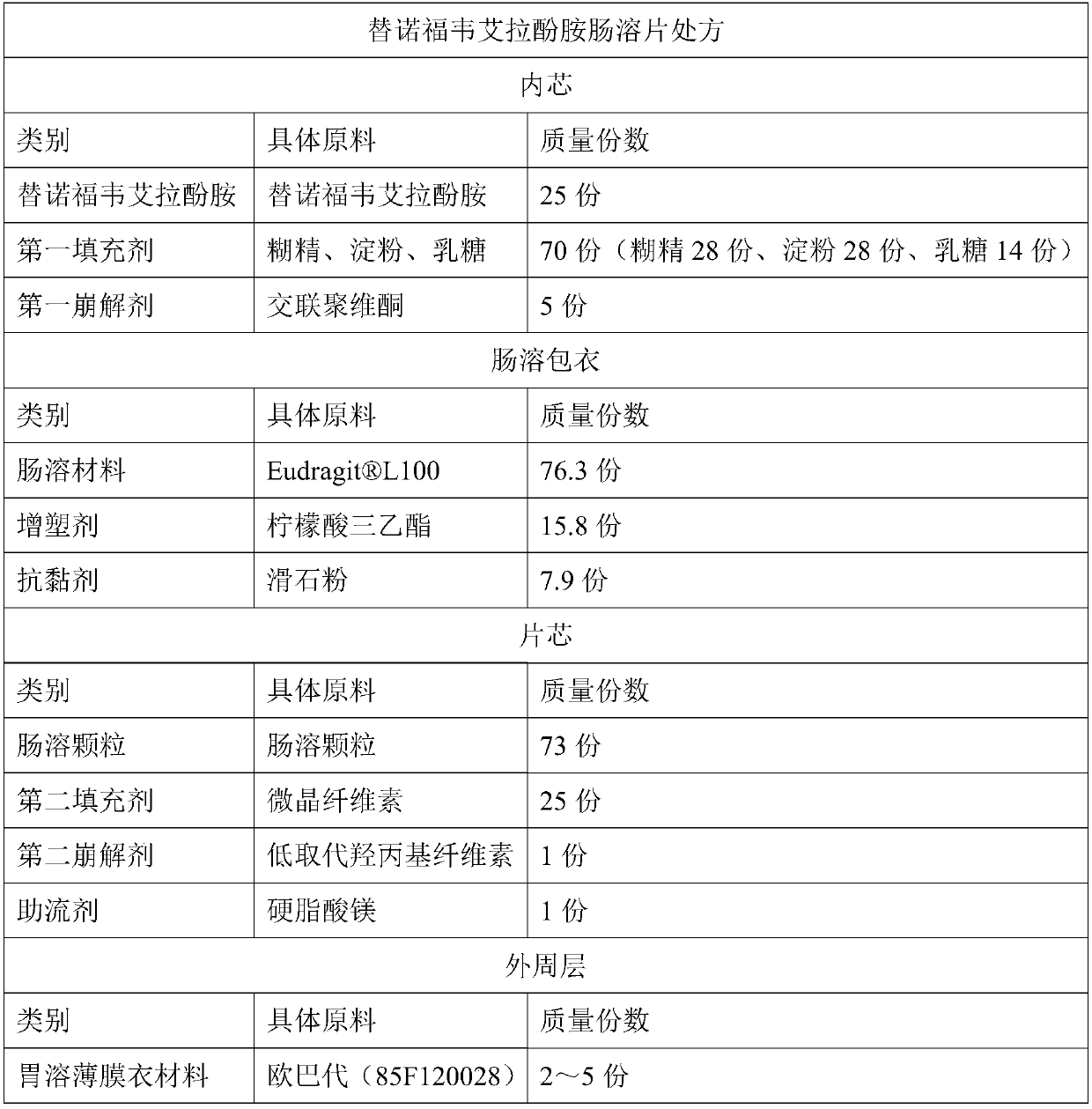
Tenofovir alafenamide enteric-coated tablet and preparation method thereof
ActiveCN109568284AFlexible way of takingImprove securityOrganic active ingredientsDigestive systemMulti unitCoated tablets
The invention discloses a tenofovir alafenamide enteric-coated tablet and a preparation method thereof. The enteric-coated tablet comprises a tablet core and a peripheral layer, wherein the tablet core contains enteric particles, a second filler, a second disintegrating agent and a glidant; the enteric particles contain an inner core and an enteric coating; and the inner core contains tenofouvir alafenamide, a first filler and a first disintegrating agent. The tenofovir alafenamide enteric-coated tablet is prepared by the preparation method successfully, and the limitation that the tablet mustbe taken with food is broken; and the bioavailability and the efficacy can not be affected by the type and amount of the food, and the safety and effectiveness of medication are improved through theenteric-coated tablet. The enteric-coated tablet is prepared by the process of enteric-coated multi-unit granule tabletting, so that the tablet is uniformly distributed after being taken orally, and the stimulation to the gastrointestinal tract caused by the excessive local concentration of drug is avoided. At the same time, the problems of poor compressibility of the tenofovir alafenamide, much fine powder from dry granulation, and uneven subsequent enteric-coated coating are overcame through the tenofovir alafenamide enteric-coated tablet. The preparation process is simple and the preparation conditions are controllable.
Owner:中润药业有限公司
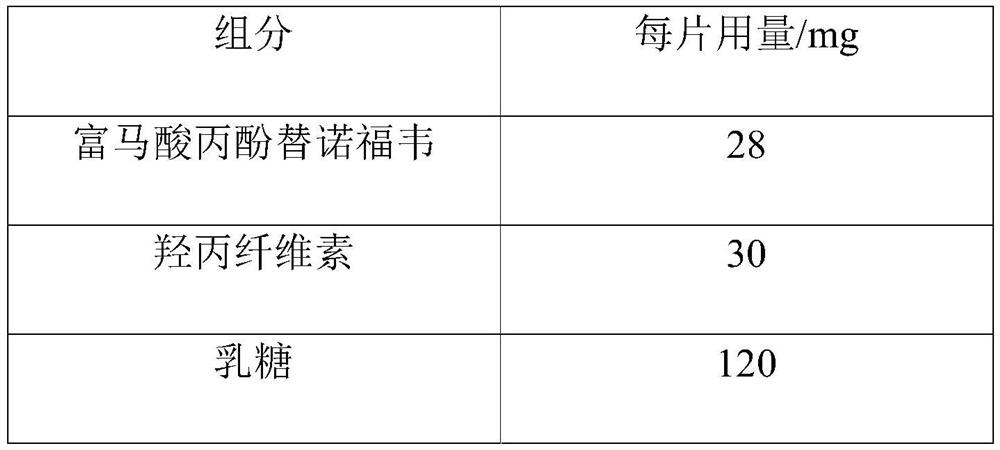
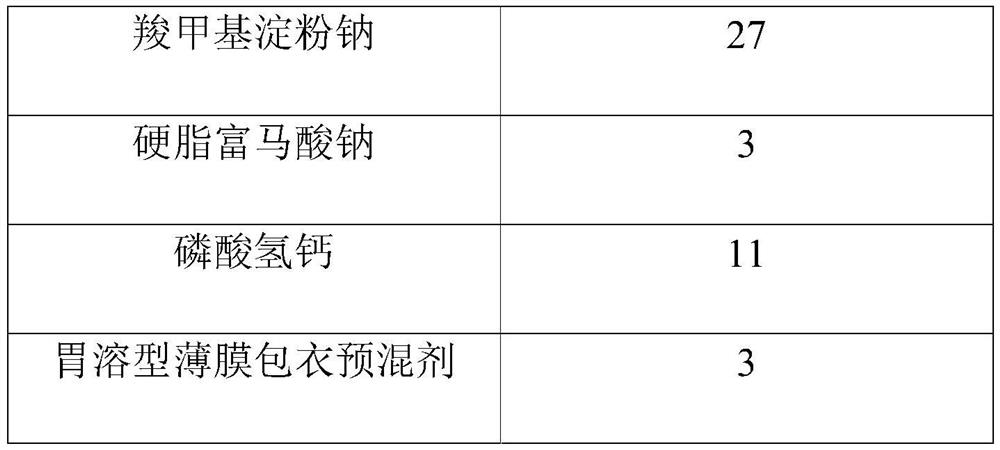
Tenofovir alafenamide fumarate tablet, preparation method thereof and detection method of related substances
ActiveCN112336695AImprove roundnessNarrow particle size distributionOrganic active ingredientsComponent separationDrugs preparationsTenofovir alafenamide
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a tenofovir alafenamide fumarate tablet and a preparation method thereof. The tenofovir alafenamide fumarate tablet comprises the following components in percentage by weight: 12%-14% of tenofovir alafenamide fumarate, 10%-15% of a cross-linking agent, 45%-58% of a diluent, 5%-12% of a disintegrating agent, 1%-5% of sodium stearyl fumarate and 5%-15% of calcium hydrophosphate. The tenofovir alafenamide fumarate tablet is prepared from the raw materials and auxiliary materials with a fluidized bed granulation method. The tenofovir alafenamide fumarate tablet prepared by steps of selecting the auxiliary materials and matching the optimized auxiliary material proportion with the fluidized bed granulation process is high in in-vitro dissolution rate, low in impurity content and good in stability, the safety of clinical application is improved, the tenofovir alafenamide fumarate tablet can be consistent with an original ground product in four dissolution media, besides, the preparation process is simple, and the tablet is suitable industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
Synthesis method of potential impurities in production of tenofovir alafenamide hemifumarate
ActiveCN108101942ASimple production processImprove internal control qualityGroup 5/15 element organic compoundsSynthesis methodsTenofovir alafenamide
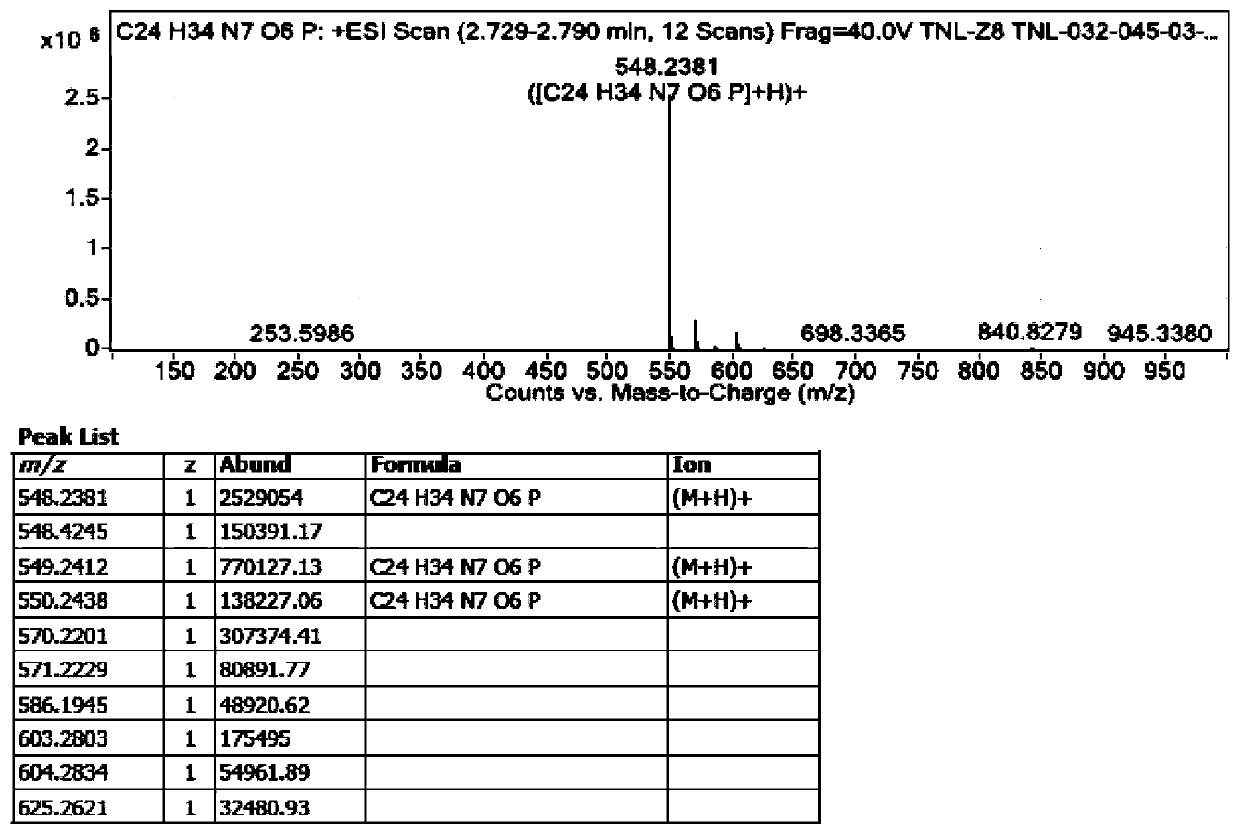
The invention discloses a preparation method of potential impurities and degradation impurities in the production technology of tenofovir alafenamide hemifumarate. Illustrating the impurity spectrogram of the tenofovir alafenamide hemifumarate plays a guiding role in improving the production technology and enhancing the internal control quality of the product.
Owner:SHENZHEN KEXING PHARM CO LTD
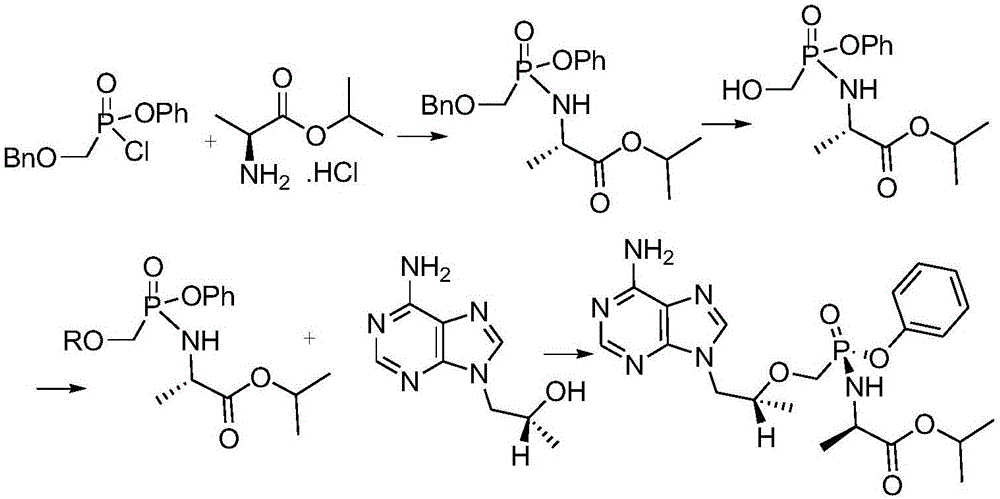
Preparation method of tenofovir alafenamide
ActiveCN106632484AAvoid operating proceduresThe reaction steps are simpleGroup 5/15 element organic compoundsTenofovir alafenamidePhenol
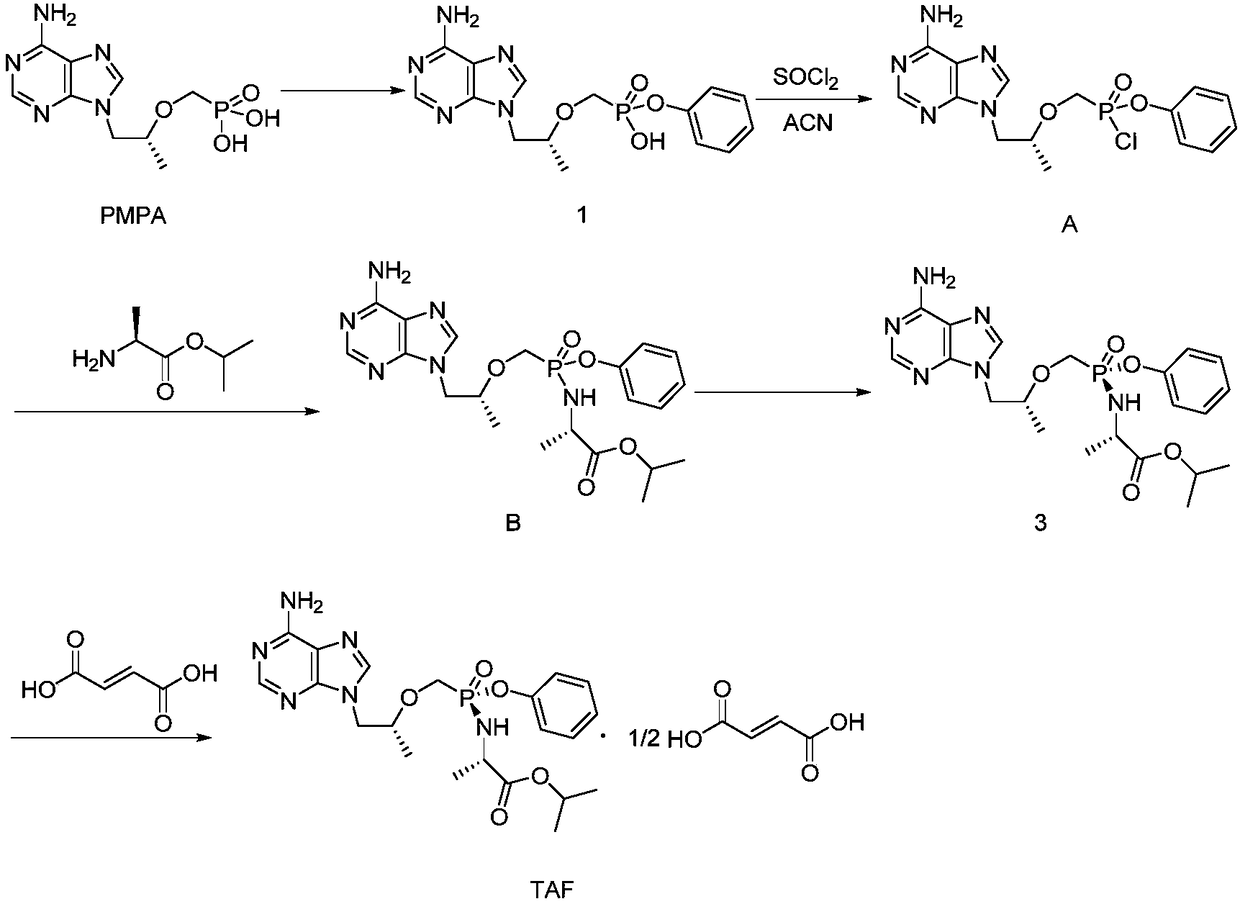
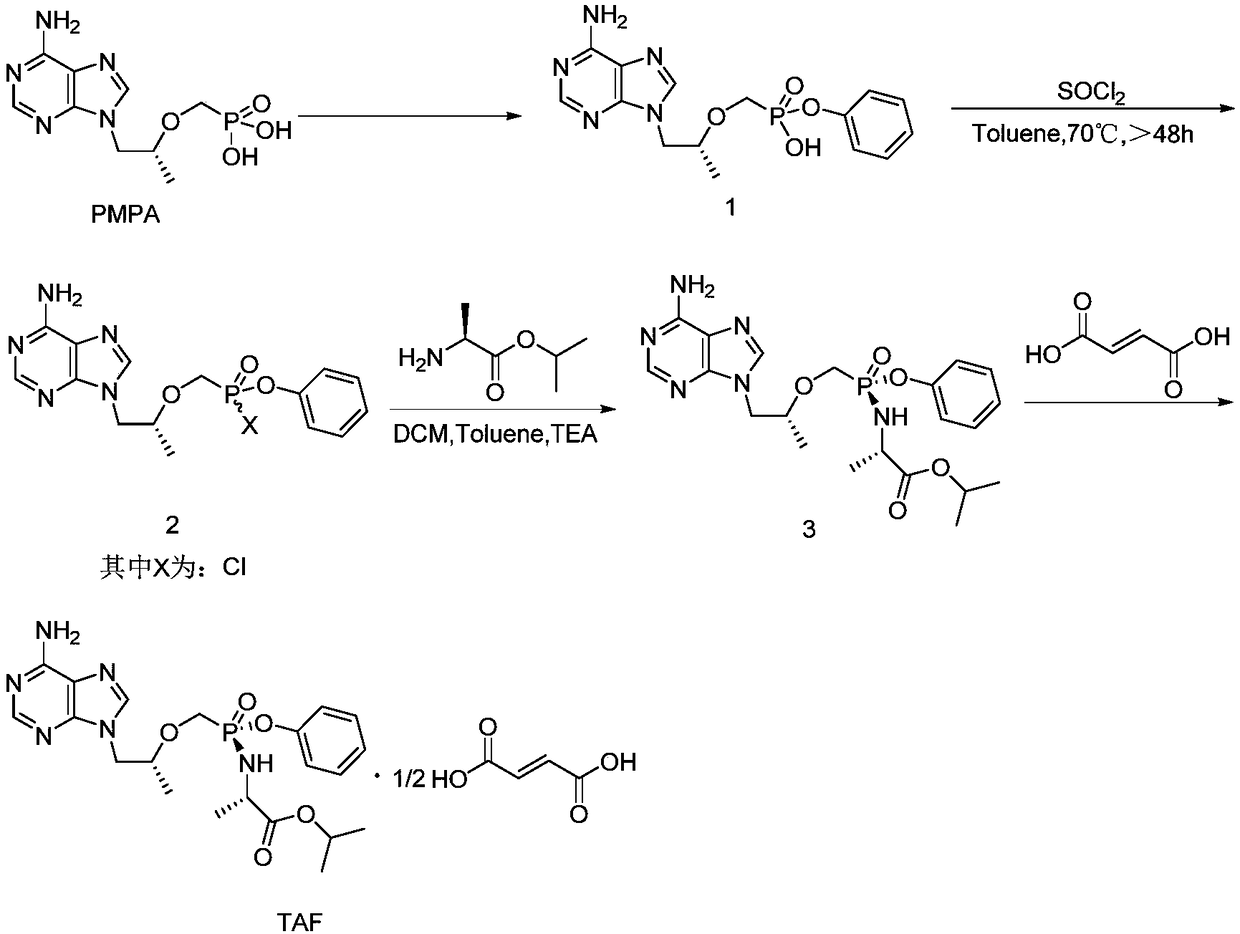
The invention discloses a preparation method of tenofovir alafenamide. The preparation method comprises the following specific steps: carrying out heating reaction on PMPA and a chlorination agent to obtain PMPA-2Cl; enabling the PMPA-2Cl to react with phenol and L-alanine isopropyl ester in sequence through a one pot method to obtain TAF-RS; purifying the TAF-RS to obtain the tenofovir alafenamide, wherein the chlorination agent is one of sulfoxide chloride, phosphorus chloride, phosphorus pentachloride and oxalyl chloride; the one pot method is to enable the PMPA-2Cl to react with the phenol first in an organic solvent under a condition of -30 to -20 DEG C under existence of organic alkali, and then add the L-alanine isopropyl ester for reaction. According to the synthesis process provided by the invention, the complicated operation process is avoided, the reaction steps are simplified; furthermore, as the raw materials are readily available, the reactions are mild, and the cost is relatively low, the preparation method is suitable for industrial production.
Owner:安庆多辉生物科技有限公司
Novel preparation process of tenofovir alafenamide fumarate
ActiveCN108440596AOrganic compound preparationGroup 5/15 element organic compoundsChemistryTenofovir alafenamide
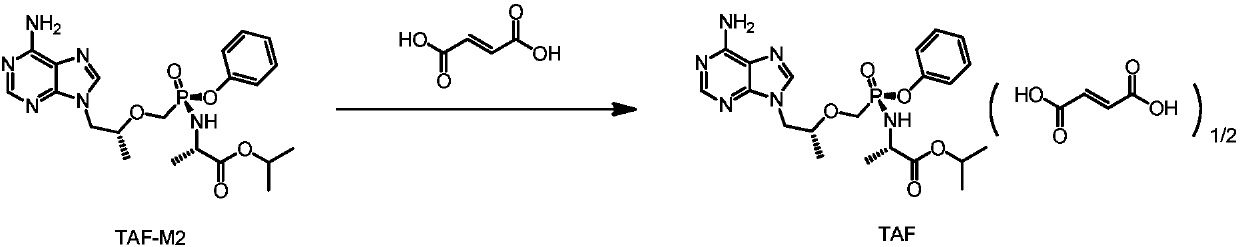
The invention discloses a novel preparation process of tenofovir alafenamide fumarate, and in particular provides a preparation method of the tenofovir alafenamide fumarate. The method comprises the steps of adding a crude tenofovir alafenamide product into isobutanol, dissolving by heating, and then adding a mixed solution of acetonitrile and n-hexane for refining; then, adding the obtained tenofovir alafenamine refined product and fumaric acid into acetonitrile, salifying and crystallizing to obtain the tenofovir alafenamide fumarate. After the method provided by the invention is adopted, diastereomer impurities in crude tenofovir alafenamide product can be effectively removed, and the high-quality tenofovir alafenamide fumarate can be obtained.
Owner:科兴生物制药股份有限公司
Preparation method of fumaric acid tenofovir alafenamide
ActiveCN108409788AOrganic compound preparationGroup 5/15 element organic compoundsTenofovir alafenamideEsterification reaction
The invention provides a preparation method of fumaric acid tenofovir alafenamide. By esterification reaction, halogenating reaction, condensation reaction, refining and salt forming reaction, the fumaric acid tenofovir alafenamide which is high in yield, easy to purify and suitable for industrial mass production is obtained.
Owner:科兴生物制药股份有限公司
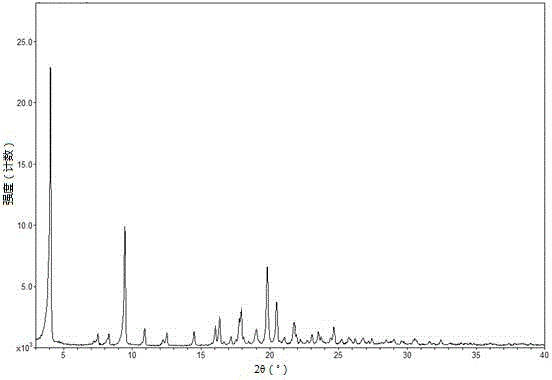
Tenofovir alafenamide hemifumarate compound, and pharmaceutical composition thereof
InactiveCN107793451AEasy to prepareSuitable for preparationOrganic active ingredientsGroup 5/15 element organic compoundsTenofovir alafenamidePowder diffraction
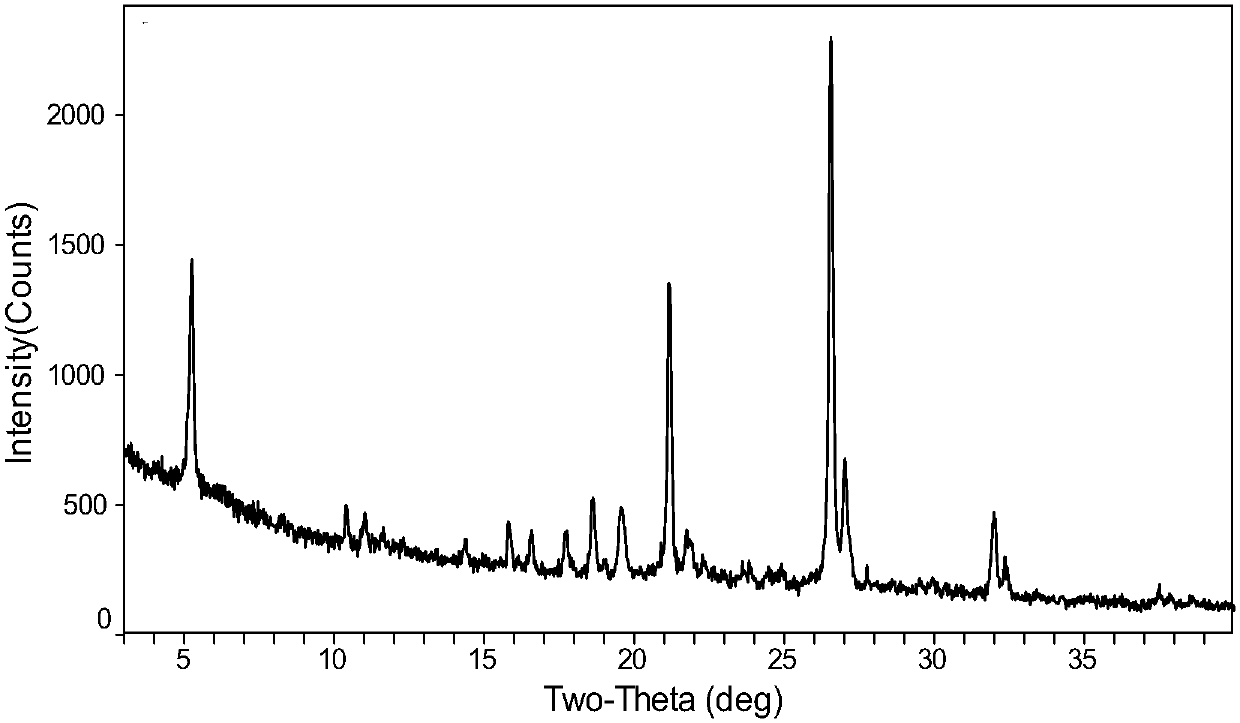
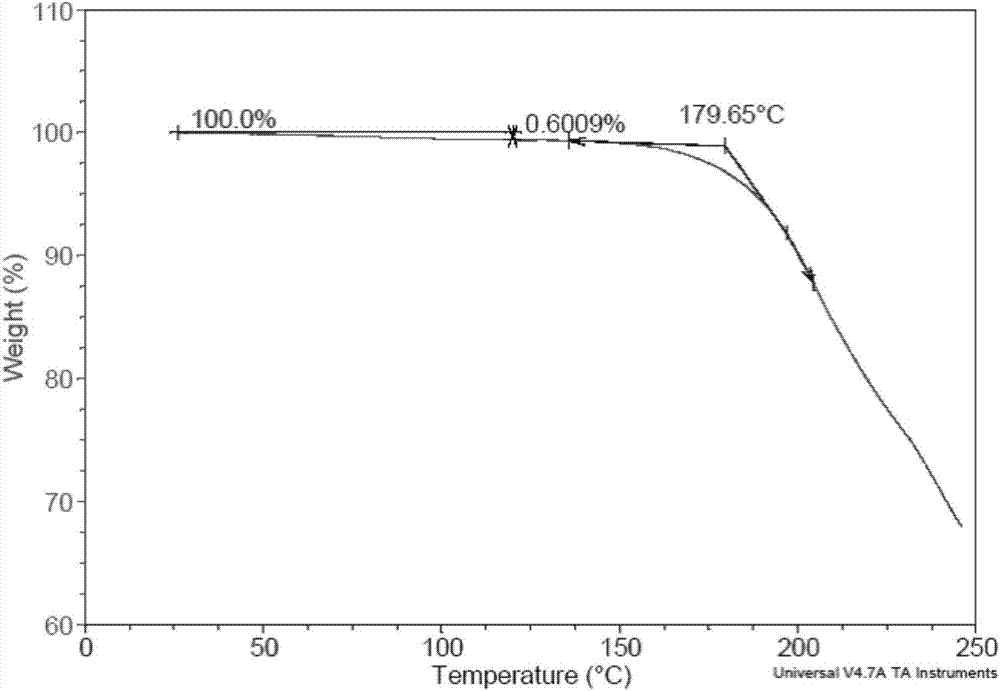
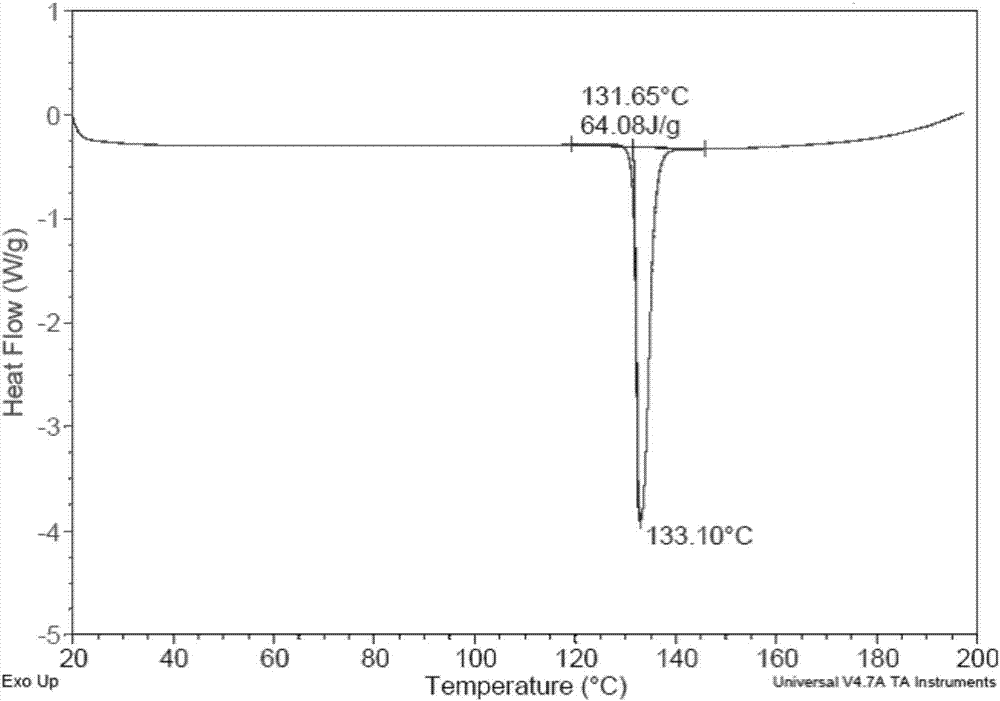
The invention provides a plurality of crystal forms of a tenofovir alafenamide hemifumarate compound, and especially a crystal form I of the tenofovir alafenamide hemifumarate. The tenofovir alafenamide hemifumarate crystal form I is excellent in stability, and is suitable for preparation of drugs. Characteristic peaks are observed at 2theta of 5.28+-0.2, 6.88+-0.2, 10.95+-0.2, 16.19+-0.2, 19.55+-0.2, 20.67+-0.2, 21.27+-0.2, and 26.59+-0.2 in an X-ray powder diffraction spectrum under Cu target radiation. Operation of a preparation of the crystal form I is simple, and the preparation method issuitable for industrialized production and applications.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of key intermediate TAF (tenofovir alafenamide fumarate)
ActiveCN108822149AEasy to operateHigh yieldGroup 5/15 element organic compoundsTenofovir alafenamideCombinatorial chemistry
The invention belongs to the field of pharmaceutical synthesis, relates to a preparation method of a drug intermediate and particularly relates to a preparation method of a key intermediate TAF (tenofovir alafenamide fumarate). The invention provides a method for preparing a key intermediate TAF 2 with high diastereoisomer purity; the method has the characteristics of being short in reaction time,less in by-products, simple in operation, good in product quality and high in reaction recovery, being suitable for industrial production and the like.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Tenofovir alafenamide fumarate compound and pharmaceutical composition thereof
InactiveCN107226826AEasy to prepareImprove stabilityOrganic active ingredientsGroup 5/15 element organic compoundsX-rayTenofovir alafenamide
The invention provides a plurality of crystal forms of a tenofovir alafenamide fumarate compound, which comprise crystal forms I, II, III, IV and V, wherein the crystal forms I, II and V have higher stability, and especially the crystal form I has the highest stability and is very suitable for producing preparations. Under the Cu-Kalpha radiation, the crystal form I has characteristic peaks in an X-ray powder diffraction spectrum when the 2theta angle is 6.724+ / -0.2, 8.347+ / -0.2, 10.785+ / -0.2, 16.068+ / -0.2, 17.446+ / -0.2, 18.150+ / -0.2, 20.028+ / -0.2 and 20.606+ / -0.2 degrees. The invention also provides preparation methods of the crystal forms. The preparation methods are simple to operate and applicable to industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
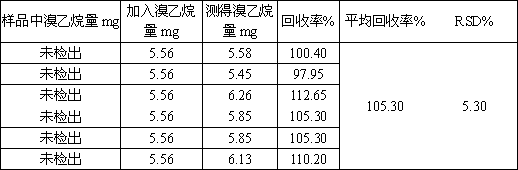
Method for determining bromoethane in tenofovir alafenamide
InactiveCN110895264AEasy to operateHigh sensitivityComponent separationTenofovir alafenamideBiochemistry
The invention provides a method for determining bromoethane in tenofovir alafenamide by adopting a direct solution sampling method. The method comprises the following steps: preparing tenofovir alafenamide into a certain solution, measuring trace bromoethane by using a capillary column taking 6% cyanopropylphenyl-94% dimethyl polysiloxane as a stationary liquid, and calculating the content of bromoethane in tenofovir alafenamide by using an external standard method. According to the method disclosed by the invention, the tenofovir alafenamide is prepared into the solution only by using a proper solvent, the pretreatment is simple, the operation is easy, and the content of bromoethane in the tenofovir alafenamide can be better determined.
Owner:HENAN TIANSHENG TAIFENG PHARM TECH CO LTD
Tenofovir alafenamide fumarate raw material medicine and production process thereof
InactiveCN108299500AExcellent anti-hepatitis B effectStrengthen the anti-hepatitis B effectGroup 5/15 element organic compoundsCarboxylic acid salt preparationTenofovir alafenamideEthyl acetate
The invention discloses a tenofovir alafenamide fumarate raw material medicine and a production process thereof. The tenofovir alafenamide fumarate raw material medicine includes anhydrous tenofovir,triphenyl phosphite, 4-dimethylaminopyridine, triethylamine, acetonitrile, ethyl acetate, methanol, concentrated hydrochloric acid, thionyl chloride, toluene, dichloromethane, L-alanine isopropyl ester hydrochloride, potassium hydrogencarbonate, fumaric acid and isopropanol. Production raw material excipients for the tenofovir alafenamide fumarate raw material medicine include lactose monohydrate,microcrystalline cellulose, croscarmellose sodium and magnesium stearate. The new manufacturing raw materials and the production raw material excipients are selected, so that the anti-hepatitis B effect of the medicine is more excellent, mass production can be performed through the novel production method, and the anti-hepatitis B effect is strengthened to some extent.
Owner:安徽安科恒益药业有限公司
Synthesis process of key intermediate of tenofovir alafenamide hemifumarate
ActiveCN104817593BAvoid harsh conditionsThe reaction steps are simpleGroup 5/15 element organic compoundsState of artTenofovir alafenamide
The invention discloses a synthetic process of key intermediate of hemifumarate tenofovir alafenamide. A synthetic route is shown below. The synthetic process has the advantages that the strict conditions of the prior art are avoided, the reaction steps are simplified, the raw materials are easy to obtain, reaction is moderate, the cost is lower and the synthetic process is suitable for industrial production.
Owner:GUANGZHOU TROJAN PHARMATEC LTD
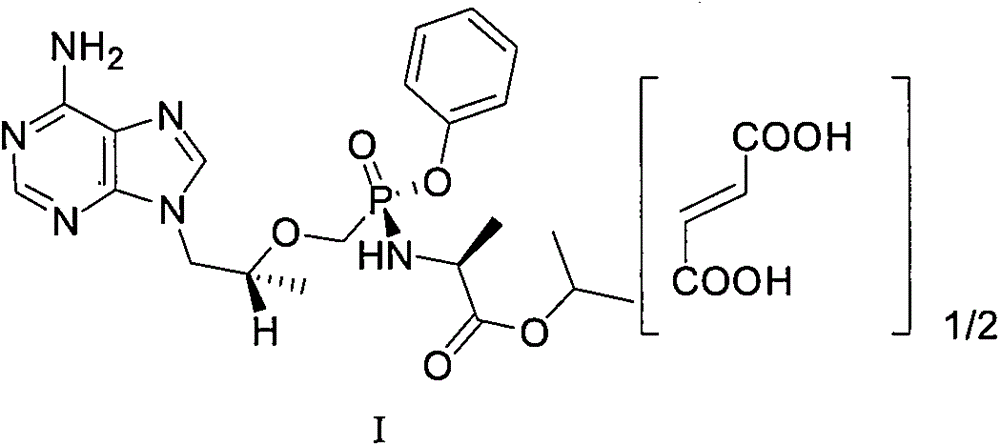
Therapeutic compositions for treatment of human immunodeficiency virus
A solid oral dosage form is provided, comprising a compound of Formula I or a pharmaceutically acceptable salt thereof, tenofovir alafenamide or a pharmaceutically acceptable salt thereof, and emtricitabine or a pharmaceutically acceptable salt thereof.
Owner:GILEAD SCI INC
Tenofovir alafenamide diastereoisomer, preparation method and application of diastereoisomer
InactiveCN111620908AContent calculationComponent separationGroup 5/15 element organic compoundsCombinatorial chemistryTenofovir alafenamide
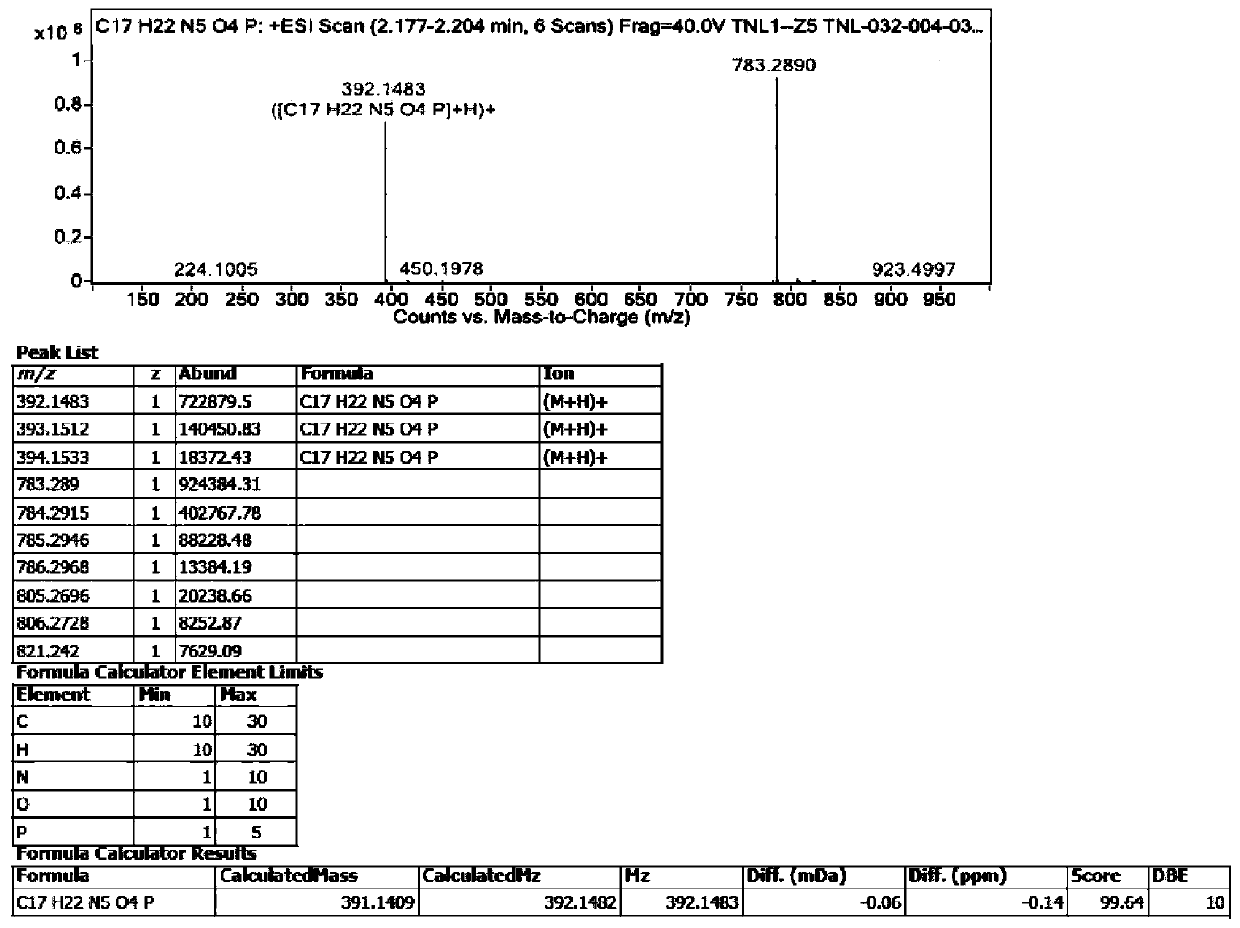
The invention relates to a tenofovir alafenamide diastereomer as shown in a formula I. The invention also relates to a preparation method of the tenofovir alafenamide diastereoisomer as shown in the formula I, and application of the tenofovir alafenamide diastereomer in detection of purity of the tenofovir alafenamide and salt thereof, detection of content of optical isomers in the tenofovir alafenamide and salt thereof, and quality control in production or preparation of the tenofovir alafenamide and salt thereof. The tenofovir alafenamide diastereomer disclosed by the invention has importantsignificance for quality monitoring and standard research in industrial production of tenofovir alafenamide.
Owner:北京华睿鼎信科技有限公司 +1
Preparation method of tenofovir alafenamide semifumarate
InactiveCN108546274ASolve difficult problemsEasy to operateOrganic chemistry methodsGroup 5/15 element organic compoundsRoom temperatureSingle crystal
The invention provides a preparation method of tenofovir alafenamide semifumarate monocrystal. The preparation method comprises the following steps of dissolving tenofovir alafenamide and fumaric acidinto a solvent of the tenofovir alafenamide and the fumaric acid with the total mass time being 20-50 times at room temperature according to a molar ratio being 2 to 1, containing an obtained mixed liquid in a vessel, enabling the solvent to be volatilizable with the vessel not being sealed or partly sealed, then putting the vessel in the environment with a single crystal cultivation temperaturebeing 40-60 DEG C, and enabling the solvent to volatilize until a cuboid or rectangular colorless and transparent monocrystal is obtained. By adopting the preparation method, a tenofovir alafenamide semifumarate monocrystal can be relatively easily prepared, and structure confirmation of the tenofovir alafenamide semifumarate is facilitated.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com