Method for separating and detecting tenofovir alafenamide and relevant substances thereof
A technology for tenofovir alafenamide and related substances, which is applied in the field of separation and detection of tenofovir alafenamide and related substances, and can solve the problems of not providing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
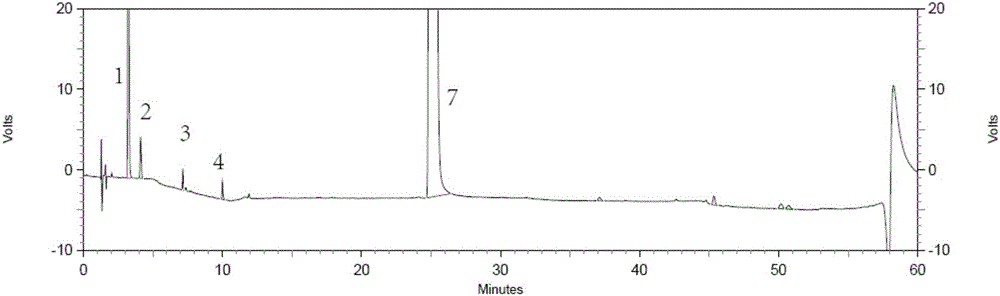
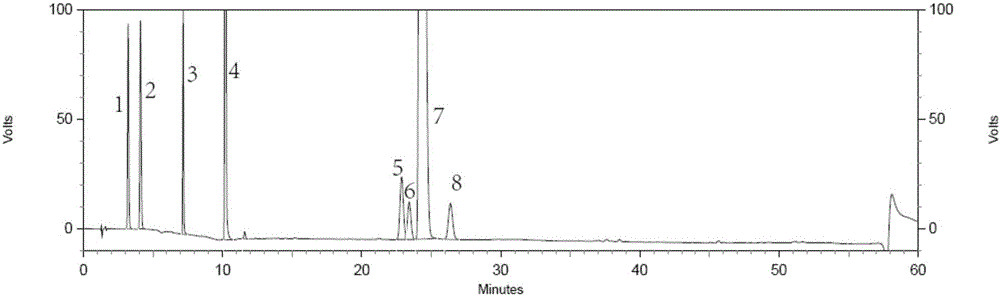
[0028] The invention provides a method for separating and detecting tenofovir alafenamide and its related substances. The method uses an octadecyl-bonded ethylene bridge hybrid silica gel chromatographic column, and the phase A can have a pH value of 1.0 to 1.0. 7.0 buffered saline solution or a mixture of buffered saline solution and a certain proportion of organic solution (such as methanol, ethanol or isopropanol, etc.), phase B is methanol, ethanol or isopropanol, gradient elution samples, using ultraviolet The detector detects.
[0029] In the analysis method provided by the present invention, the octadecyl-bonded ethylene bridged hybrid silica gel chromatographic column can be XBridge BEH C18. Specifically, the specific specifications of the XBridge BEH C18 may be 1 mm to 4.6 mm in diameter, 30 mm to 250 mm in column length, and 1.7 to 10 μm in particle size, such as XBridge BEH C18, 4.6×75 mm, 2.5 μm. According to the common knowledge in the field, those skilled in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com