Tenofovir alafenamide diastereoisomer, preparation method and application of diastereoisomer
A technology of tenofovir alafenamide and diastereoisomers, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, measuring devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
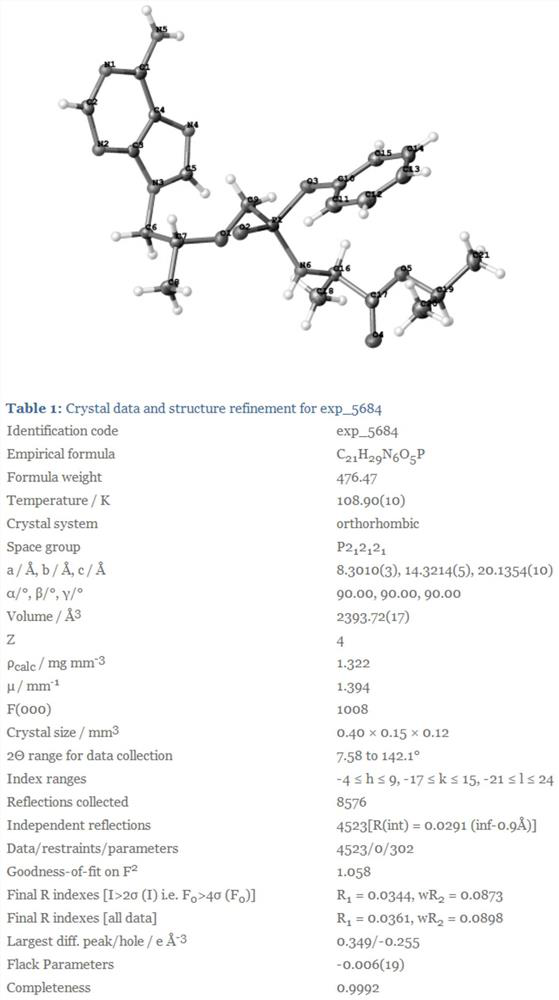
[0049] The preparation of embodiment 1 formula I diastereoisomer
[0050] Step 1: Preparation of formula 2 compound
[0051] Add 6g of commercially available compound of formula 1, 3.9g of phenol, 50g of acetonitrile, 4.6g of triethylamine and 10g of dicyclohexylcarbodiimide into a 250mL reaction flask, and react at 70°C for 18h. After adding water to quench, filter, and extract the filtrate with ethyl acetate 2-3 times. Collect the aqueous phase and adjust the aqueous solution to about 3.0 with hydrochloric acid, stir and crystallize for about 2 hours, filter, and dry to obtain 3.7 g of the brown yellow compound of formula 2.
[0052] Step 2: Preparation of formula I diastereoisomers
[0053] Add 3g of compound of formula 2, 25g of acetonitrile, and 2.8g of thionyl chloride into a 100mL reaction flask, heat to 85°C for 6 hours, evaporate to dryness under reduced pressure, add 25g of acetonitrile, and dropwise add 3.2g of D- A solution of isopropyl alanine dissolved in 20 g...
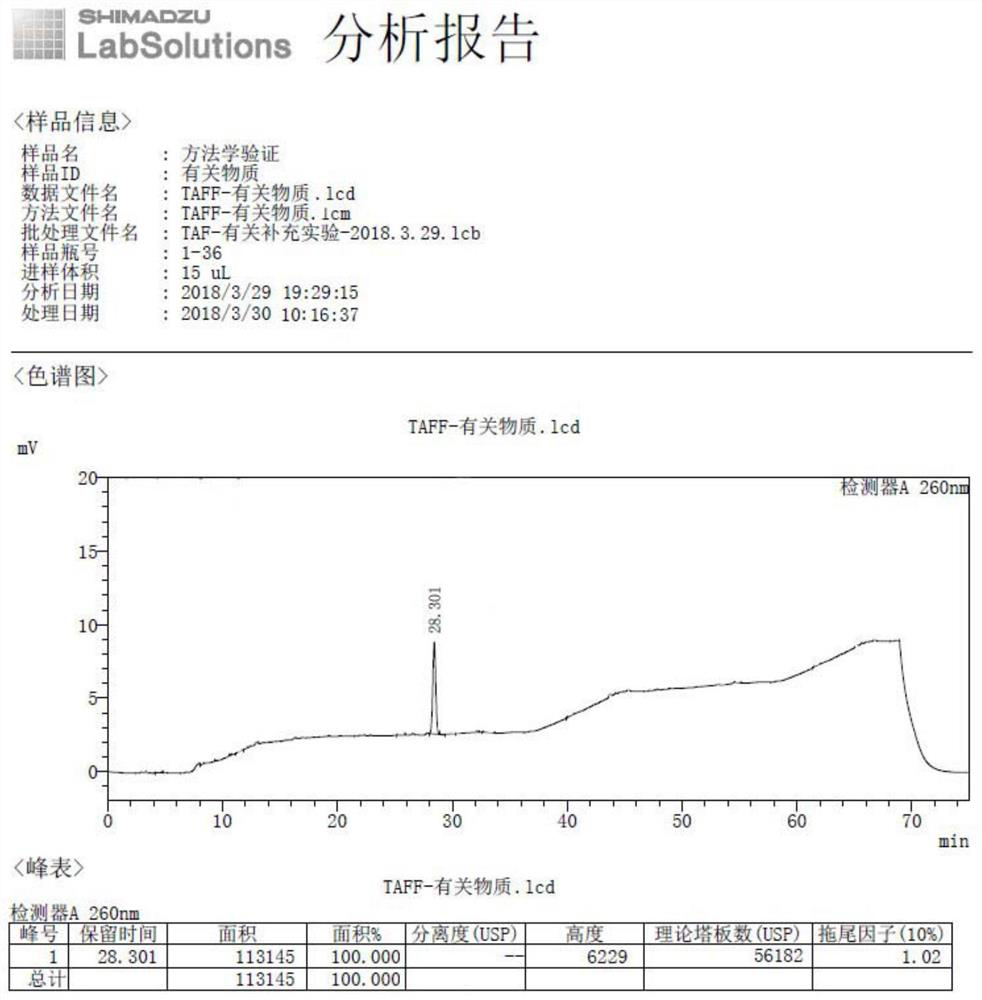
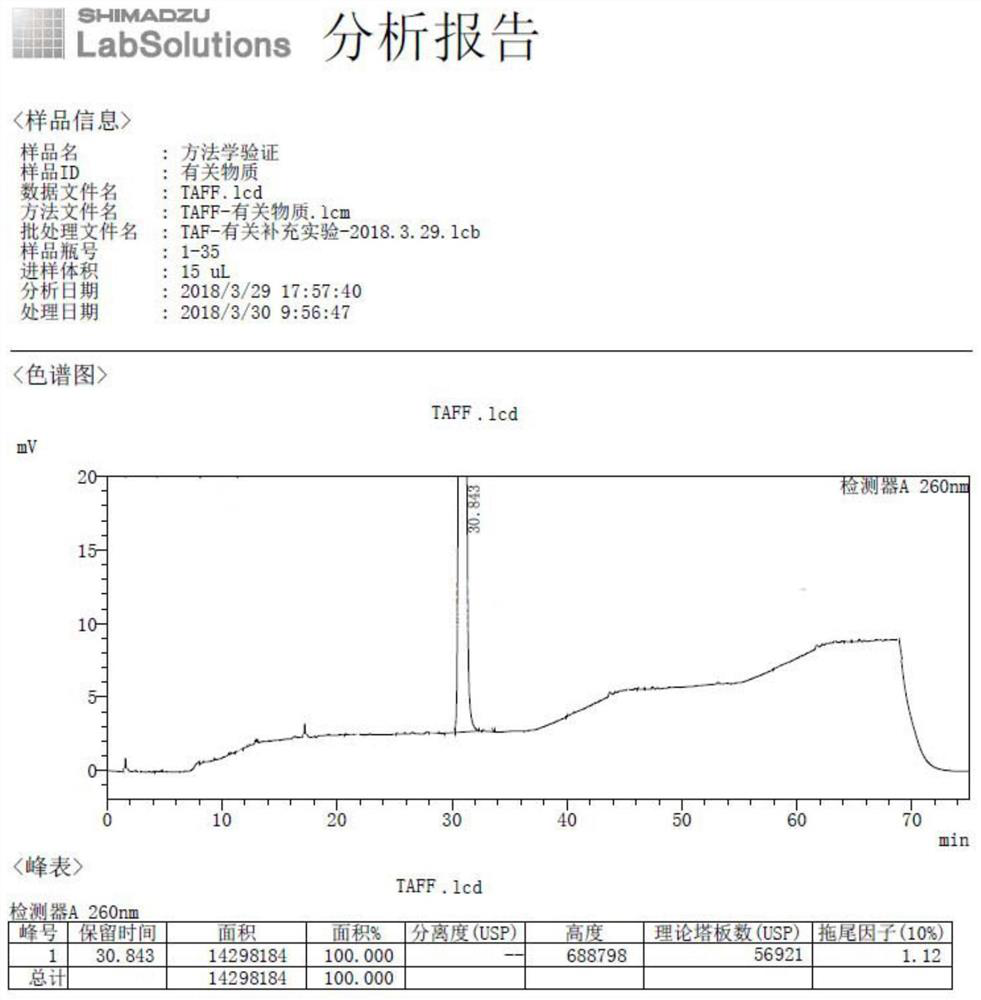
Embodiment 2
[0056] Example 2 The external standard method in high performance liquid chromatography detects the content of optical isomers in tenofovir alafenamide High performance liquid chromatography: Shimadzu 2030C
[0057] Column: Waters, Shield RP18, 4.6×150mm, 3.5μm
[0058] Mobile phase: mobile phase A is 0.02mol / L potassium dihydrogen phosphate buffer (pH6.0)-[tetrahydrofuran-acetonitrile (7:3)] (99:1), mobile phase B is 0.02mol / L dihydrogen phosphate Potassium buffer (pH6.0)-[tetrahydrofuran-acetonitrile (7:3)] (50:50), eluted according to the gradient elution program in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com