Therapeutic compositions for treatment of human immunodeficiency virus
A compound and pharmaceutical technology, applied in the solid oral dosage form of emtricitabine and tenofovir alafenamide, including the compound of formula I, can solve the complex decision-making problems of HIV-infected patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0501] Example 1 - Single-dose Tablet of Compound of Formula II
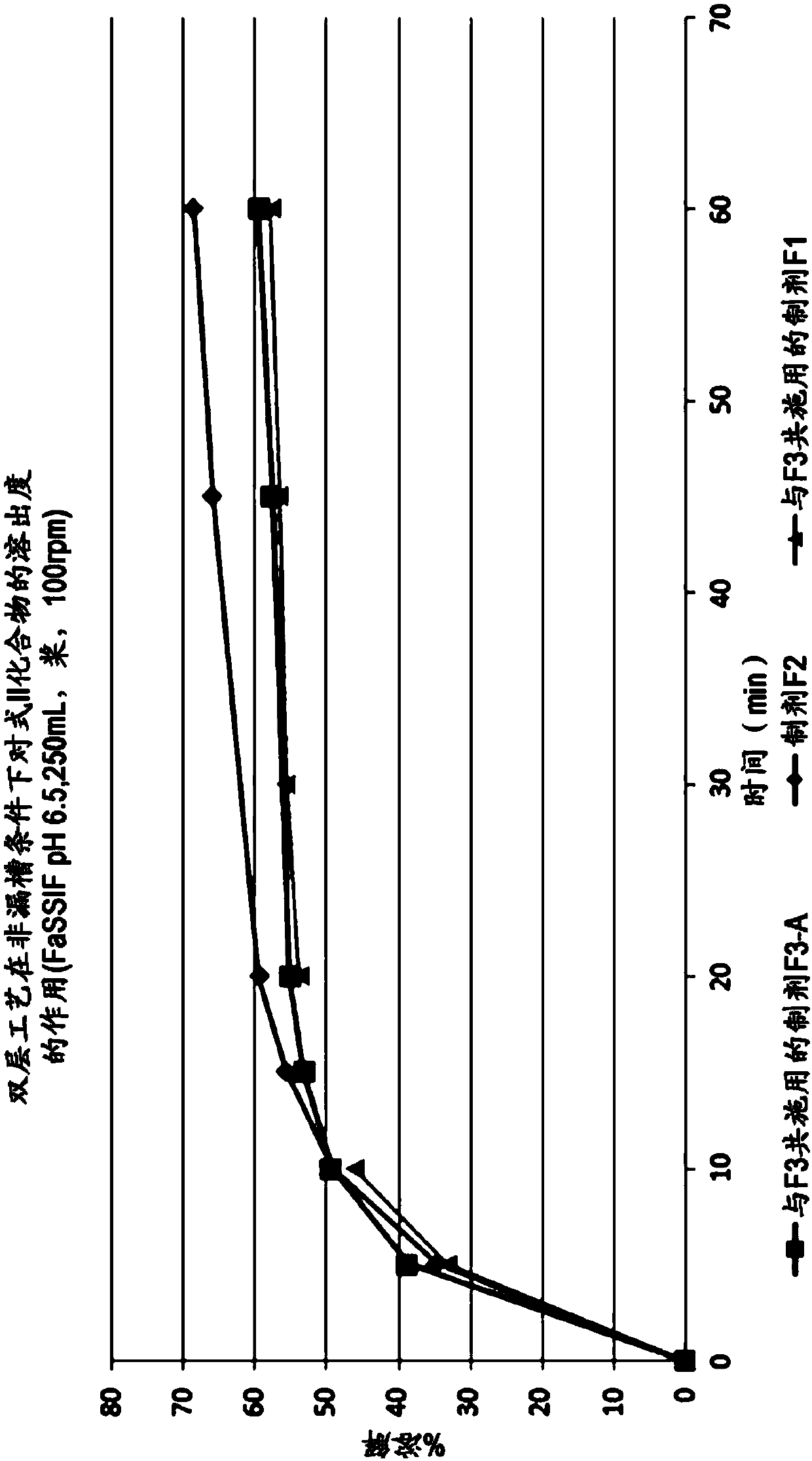
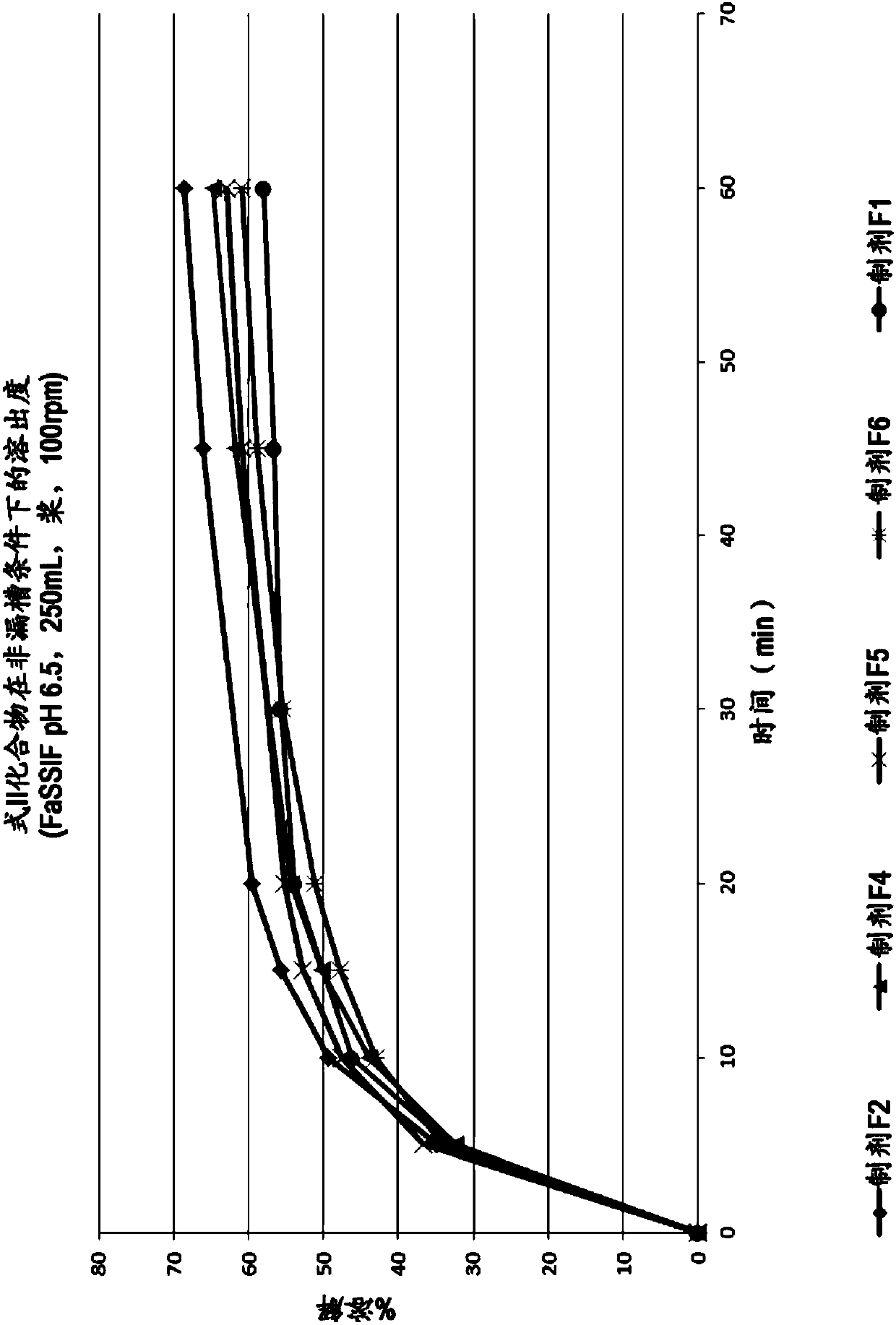
[0502] The formulation of the compound of formula II (tablet F1) is prepared by dry granulation. Figure 5 is a flow chart illustrating the preparation of the formulation. The composition of the single-dose formulation is shown in the table below:
[0503]
[0504]
[0505] *equivalent to 75 mg of compound of formula I
[0506] **Intergranular: 2.25mg (0.75%); Extragranular: 2.25mg (0.75%)
[0507] In the pharmacokinetic study of Example 3, tablets of Formulation F1 were film-coated with 12 mg of Opadry II Yellow 85F92259. The total weight of the film-coated tablet is 312 mg.
[0508] The compound of formula II is mixed with intergranular excipients (lactose, microcrystalline cellulose and crospovidone), further blended with the intergranular portion of sodium stearyl fumarate), rolled, ground, and mixed with hard Sodium fatty acyl fumarate is finally blended to produce the final powder mix for compre...
Embodiment 2
[0509] Example 2 - compound of formula II / emtricitabine / tenofovir alafenamide bilayer tablet
[0510] A bilayer formulation of the compound of formula II, emtricitabine and tenofovir alafenamide hemifumarate (tablet F2) was prepared using the method described in Example 8. Figure 6 is a flowchart illustrating the preparation of bilayer tablets. The composition of this formulation is summarized in the table below:
[0511]
[0512] *equivalent to 75 mg of compound of formula I
[0513] **Equivalent to 25mg tenofovir alafenamide
[0514] ***Intergranular: 212.5mg (29.1%); Extragranular: 2.65mg (0.4%)
[0515] Intergranular: 2.65mg (0.4%); Extragranular: 2.65mg (0.4%)
[0516] Intergranular: 2.83mg (0.4%); Extragranular: 2.83mg (0.4%)
[0517] In the pharmacokinetic study of Example 3, tablets of formulation F2 were film coated with 21.9 mg of Opadry II Yellow 85F92259 (representing 3% weight gain). The total weight of the film-coated tablet is 752 mg.
[0518] Table...
Embodiment 3
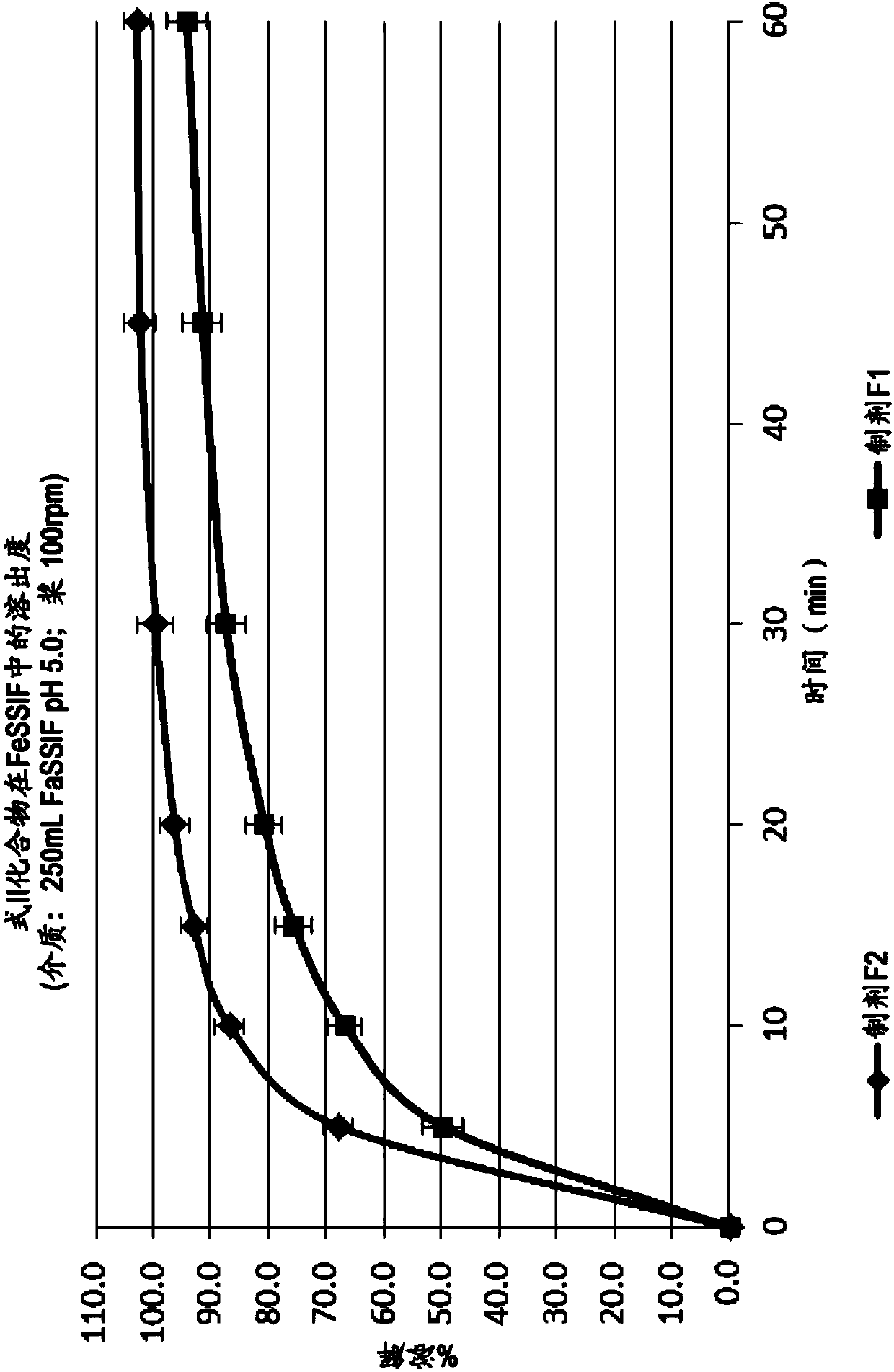
[0524] Example 3-pharmacokinetic studies
[0525] A study was performed to evaluate the pharmacokinetic profiles of Formulations F1, F2 and F3 of Examples 1, 2 and 2A. A randomized, open-label, multi-period, fixed-sequence, crossover study was conducted to evaluate the bilayer tablet formulation F2 relative to a fixed-dose combination tablet containing emtricitabine and tenofovir alafenamide (tablet Formulation F3) Relative bioavailability of coadministered single-dose tablet formulation F1. Bioavailability was assessed in healthy individuals.
[0526] Study Design and Duration of Treatment
[0527] Three single doses of the following tablet formulations were administered orally during a total study duration of up to 21 days:
[0528] (a) Simultaneous administration of a fixed dose combination tablet comprising emtricitabine and tenofovir alafenamide (200 / 25mg-tablet F3) and a single-dose tablet containing a compound of formula I under fasting conditions ( 75mg-tablet F1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com